Abstract
Background & aims
COVID-19 has emerged as a global pandemic affecting millions of people. Vitamin D deficiency is one of the risk factors for increased susceptibility to COVID-19. This study aimed to examine the correlation between the prevalence of vitamin D deficiency and COVID-19 infection and mortality rates among the adult population in European and Asian continents.
Methods
Prevalence of vitamin D deficiency in each country was retrieved through literature searching on PubMed® database for the last ten years. As of December, 31st 2020, COVID-19 infections and mortalities per million population were extracted from the ‘real time’ statistics of the Worldometer website. The association between both vitamin D deficiency and COVID-19 infections and mortalities were explored.
Results
Forty seven countries were included in the analysis. The prevalence of vitamin D deficiency ranged from 6.9 to 81.8% in European countries and 2.0–87.5% in Asian countries. Significantly positive correlations were observed for both COVID-19 infection (r = 0.76; p < 0.001) and mortality rates (r = 0.75; p < 0.001) in the Asian continent. The correlation values for the infections and mortality rates in the European continent were (r = 0.37; p = 0.08) and (r = 0.43; p = 0.04) respectively. When both the continents were combined, the correlation results for both infection (r = 0.42; p = 0.003) and mortality (r = 0.35; p = 0.016) rates with vitamin D deficiency values remained significant.
Conclusion
Prevalence of vitamin D deficiency was significantly associated with the mortality rate of COVID-19 in Europe and Asia. The association between the infection rate and prevalence of vitamin D deficiency was significant for Asia only. Both the associations were significant when the two continents were combined in the analysis. Therefore we suggest that vitamin D supplementation could play a key role in the prevention and/or treatment of the COVID-19 patients.
Keywords: Vitamin D, Deficiency, COVID-19, Infections, Mortalities, Europe, Asia
1. Introduction
The novel coronavirus infection (COVID-19) which was originated in Wuhan, China [1], in late 2019 is continuing its spread across the world affecting more than 100 million people and claiming nearly 2.4 million lives in 221 countries and territories [2]. The control and prevention of the pandemic has become challenging due to the rapid transmission and relatively high mortality rates specially among vulnerable populations [3]. The disease has now spread rapidly to almost every region of the world causing significant loss of life, disrupting livelihoods, and threatening the recent advances in health.
A significant variation in the number of infections and as well as the severity and mortality of the disease have been observed due to several state level and individual level predisposing factors [4]. The individual factors include age, sex, ethnicity, social conditions, malnutrition, pre-existing conditions and immunity status [5], while state level factors include, but not limited to, a country's level of preparedness, policies and interventions of governments, health infrastructure, timing of lockdown, rapid border closures, implementation of social distancing and socioeconomic status [4].
Vitamin D deficiency is a major public health problem worldwide in all age groups [6]. A number of factors are known to influence vitamin D levels and contribute to risk of impaired vitamin D status. These factors include variation in the sun exposure due to latitude, season, time of day, atmospheric components, clothing, sunscreen use, skin pigmentation, as well as age, obesity [7], nutrition and supplements [8]. Epidemiological studies have exhibited a strong relationship between seasonal differences in nutrient D levels and the occurrence of varied infectious diseases, such as sepsis [9], respiratory infection [10], and seasonal influenza [11]. In addition to classic actions related to mineral homeostasis, vitamin D has pleiotropic physiological actions including immune system regulation [12]. Vitamin D can modulate the innate and adaptive immune responses [13]. Deficiency in vitamin D is associated with decreased autoimmunity and an increased susceptibility to infections.
Several mechanisms have been reported in reducing the risk of viral infection and mortality by vitamin D [14]. To reduce the risk of common cold, vitamin D uses three pathways: physical barrier, cellular natural immunity, and adaptive immunity [15]. It improves the body's physical immunity barrier by regulating the production of proteins for tight junctions, adherens junctions, and gap junctions, which can be disturbed by infection by microorganisms, including viruses [16]. In addition vitamin D enhances cellular immunity by decreasing the cytokine storm which causes immunogenic damage to the endothelium and alveolar membrane [17]. It also regulates adaptive immunity through inhibiting T helper cell type 1(Th1) responses and stimulating Th2 responses [18]. It is evident that vitamin D deficiency might increase the risk of respiratory tract infections including COVID-19.
Therefore this study aimed to investigate the association between the prevalence of vitamin D deficiency and the infection and mortality rates of COVID-19 in two major continents specifically Asia and Europe, where the infection first spread prior to other parts of the world. Besides, these two continents were specially selected due to their rich ethnic and cultural diversity among populations with Caucasian and Asian backgrounds.
2. Methods
2.1. Data sources and inclusion/exclusion criteria
The number of reported COVID-19 cases and deaths per one million population as of December 31st, 2020, by country were obtained from the Worldometer website [2]. This is a reference webpage providing real-time world statistics of COVID-19 directly from official Government's communication channels of individual countries or through local media sources when deemed reliable.
The prevalence of vitamin D deficiency data among the countries were extracted by conducting a comprehensive electronic search in the PubMed® database. An advanced search was performed at the level of title/abstract by using the keywords such as “Vitamin D″ OR “25-hydroxyvitamin D3”, combined with “Deficiency”, “Prevalence” OR “Status” and the names of each European and Asian countries. In addition, some of the published work was taken from references and other relevant websites. The search was limited to the articles published in the last ten years (As of January 31st, 2021). Among the suggestions resulted from each search, the following inclusion criteria were applied to screen the articles: a) Population-based studies; b) studies reporting non-institutional adults; c) studies defining the vitamin D deficiency as the serum levels <20 ng/ml or <50 nmol/L; d) studies reporting the vitamin D deficiency as the prevalence of the sample population. In addition, conference proceedings, editorials, commentaries, book chapters/book reviews and studies confined to selective sample of community-dwelling people such as pregnant women, elderly people aged ≥60 years and patients with diagnosed illnesses were excluded. Finally, out of the screened articles, the prevalence of vitamin D deficiency data were retrieved from the most recently published article with the most representative study sample for each country.
2.2. Data extraction
Information on both COVID-19 infections and mortalities per one million (1M) population as of December, 31st 2020, were extracted from the Worldometer website and rounded off to the nearest whole number. From the selected articles reporting vitamin D deficiency among these countries, name of the first author, published year, sample size, age range of the study population and prevalence of vitamin D deficiency were retrieved. All data were extracted by two reviewers (PS and DTJ) using a standardized form and were checked for accuracy by a third reviewer (RJ). Any discrepancies in the data extracted in this manner were re-checked and resolved by discussion.
2.3. Data analysis
The relationship between the prevalence of vitamin D deficiency and other dependent variables such as total number of COVID-19 infections and mortalities per 1million (M) population were explored with correlation coefficients. The number of COVID-19 infections and mortalities per 1M population of each country were converted to log10 prior to statistical analysis because the variances in the data were not normally distributed [19]. Scatter plots and regression lines were used to visually demonstrate the correlations and to evaluate the outcome for each continent and for both combined.
3. Results
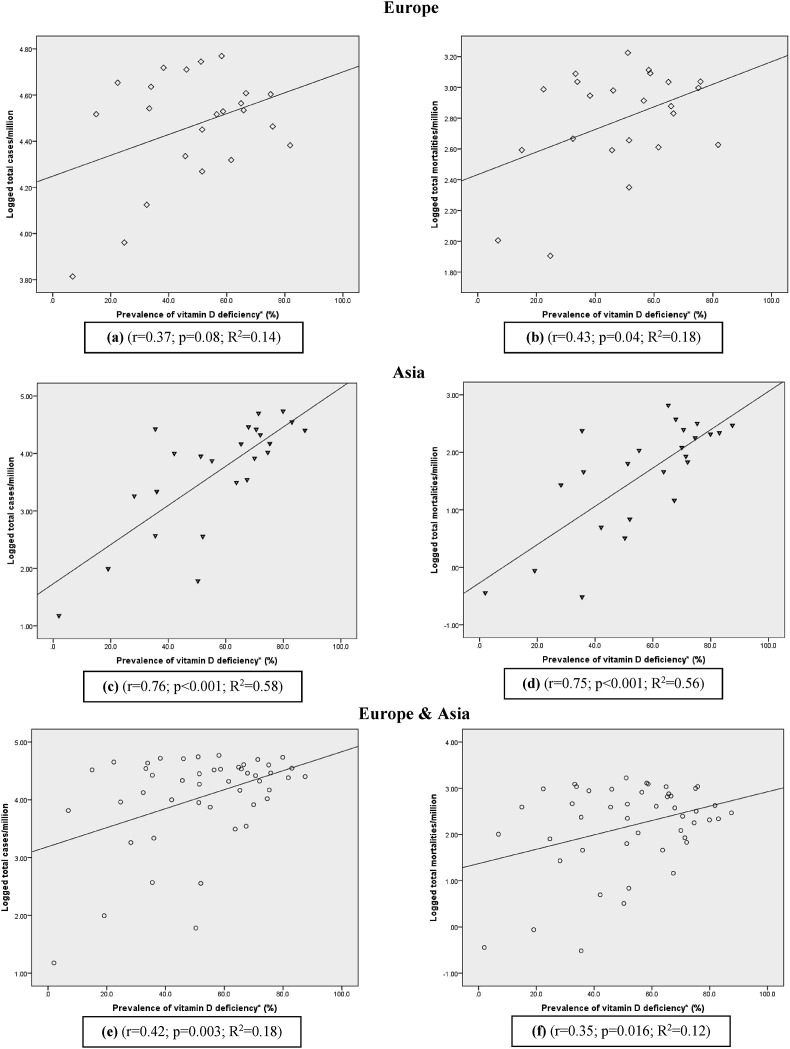
A total of 23 countries in the European continent (Table 1 ) and 24 countries of the Asian continent (Table 2 ), fulfilling the inclusion/exclusion criteria were included in the analysis. Among European countries the highest and the lowest vitamin D deficiency rates were observed in Ukraine and Finland respectively with vitamin D deficiency values ranging from 6.9 to 81.8%. In most of the European countries (n = 13) more than the half of the adult population was vitamin D deficient. The size of the population used for testing vitamin D deficiency among the countries varied from 125 (Slovenia) to 74,235 (Italy). Study participants were adults except in one which included subjects from 5 to 18 years. The highest and the lowest number of COVID-19 infections per 1M of the total population were from Slovenia (58,779 cases/M) and Finland (6517 deaths/M) accordingly. With respect to the COVID-19 mortalities per 1M population, the highest number, was documented in Belgium (1677 deaths/1M), whereas the lowest number was reported in Norway (80 deaths/1M). The results of the correlation analysis in the European continent for vitamin D deficiency with COVID-19 cases per 1M of population demonstrated a weak positive correlation which is close to being statistically significant (r = 0.37; p = 0.08) (Fig. 1 a). Similarly, COVID-19 mortality per 1M population was also significant and positively correlated with the prevalence of vitamin D deficiency (r = 0.43; p = 0.04) (Fig. 1b).
Table 1.
The prevalence of vitamin D deficiency among European countries.
| Country | Author, Year | Sample size (M/F) | Age (years) | Prevalence of vit D deficiency∗ (%) | COVID-19 cases/M | COVID-19 mortalities/M |
|---|---|---|---|---|---|---|
| Belgium [20] | Hoge et al., 2015 | 697 (378/319) | 20–69 | 51.1 | 55587.8 | 1677.4 |
| Bosnia and Herzegovina [21] | Sokolovic et al., 2016 | 2483 (603/1880) | >18 | 58.7 | 33828.4 | 1234.4 |
| Bulgaria [22] | Borissova et al., 2013 | 2016 (948/1068) | 20–80 | 75.8 | 29109.5 | 1090.3 |
| Croatia [23] | Barić et al., 2016 | 791 (660/131) | 45.5 | 46.1 | 51357.7 | 954.9 |
| Denmark [24] | Hansen et al., 2018 | 2565 (1048/1517) | 18–69 | 51.5 | 28224.0 | 224.1 |
| Finland [25] | Adebayo et al., 2020 | 798 (352/446) | 30–64 | 6.9 | 6516.7 | 101.3 |
| France [26] | Deplanque et al., 2017 | 297 (127/170) | 18–65 | 75.1 | 40145.3 | 992.4 |
| Germany [27] | Rabenberg et al., 2018 | 6995 (3360/3635) | 18–79 | 61.5 | 20833.6 | 408.1 |
| Greece [28] | Dimakopoulos et al., 2019 | 1084 (410/674) | ≥18 | 32.4 | 13321.4 | 464.2 |
| Ireland [29] | Griffin et al., 2020 | 17,590 | ≥18 | 51.5 | 18587.1 | 453.0 |
| Italy [30] | Giuliani et al., 2019 | 74,235 (18,811/55,424) | >18 | 33.3 | 34851.2 | 1226.5 |
| Norway [31] | Petrenya et al., 2020 | 4465 (2041/2424) | 40–69 | 24.7 | 9143.1 | 80.4 |
| Poland [32] | Pludowski et al., 2016 | 5775 (1311/4464) | 16–90 | 65.8 | 34210.9 | 754.5 |
| Portugal [33] | Duarte et al., 2020 | 3092 (1907/1995) | ≥18 | 66.6 | 40569.8 | 677.3 |
| Romania [34] | Niculescu et al., 2017 | 812 | >21 | 56.5 | 32865.8 | 819.6 |
| Russia [35] | Karonova et al., 2016 | 1544 (205/1294) | 18–75 | 45.7 | 21648.7 | 390.7 |
| Slovakia [36] | Sebekova et al., 2016 | 578 (254/324) | 5–81 | 15.0 | 32885.5 | 391.6 |
| Slovenia [37] | Hribar et al., 2020 | 125 | 18–64 | 58.2 | 58779.0 | 1297.3 |
| Spain [38] | González-Molero et al., 2011 | 1262 | 20–83 | 33.9 | 43246.9 | 1087.3 |
| Sweden [39] | Nälsén et al., 2020 | 268 (124/144) | 18–80 | 22.4 | 45029.5 | 971.8 |
| Switzerland [40] | Guessous et al., 2012 | 367 (167/200) | 25–70 | 38.2 | 52260.6 | 883.3 |
| Ukrain [41] | Povoroznyuk et al., 2012 | 1575 | 20–95 | 81.8 | 24124.3 | 423.8 |
| United Kingdom [42] | Jolliffe et al., 2016 | 222 (89/133) | 48–94 | 64.9 | 36661.2 | 1082.9 |
F-Female; M-Male.
∗Prevalence of Vitamin D deficiency; Serum 25-hydroxyvitamin D3 level <20 ng/ml or <50 nmol/L; cases/M-cases per million population; mortalities/M-mortalities per million population.
Table 2.
The prevalence of vitamin D deficiency among Asian countries.
| Country | Author, Year | Sample size (M/F) | Age (years) | Prevalence of vit D deficiency∗ (%) | COVID-19 cases/M | COVID-19 mortalities/M |
|---|---|---|---|---|---|---|
| Bahrain [43] | Almesri et al., 2020 | 314 (164/150) | >30 | 79.9 | 54464.2 | 206.9 |
| Bangladesh [44] | Acherjya et al., 2019 | 160 (69/91) | ≤70 | 63.7 | 3118.1 | 45.9 |
| Brunei [45] | Leong et al., 2016 | 446 (77/331) | >18 | 52.0 | 358.9 | 6.9 |
| China [46] | Jiang et al., 2020 | 14,302 (3002/11,299) | 18–65 | 50.3 | 60.5 | 3.2 |
| India [47] | Mechenro et al., 2018 | 424 (179/245) | >18 | 55.2 | 7453.8 | 108.0 |
| Iran [48] | Esmaeili et al., 2019 | 7504 (3552/3952) | 18–65 | 65.3 | 14586.3 | 657.5 |
| Iraq [49] | K.A Al-Hilali et al., 2016 | 300 (120/180) | 25–70 | 75.3 | 14800.0 | 318.6 |
| Japan [50] | Asakura et al., 2020 | 107 (53/54) | 20–69 | 28.2 | 1820.9 | 27.0 |
| Jordan [51] | Khasawneh et al., 2018 | 3007 (710/2297) | <83 | 67.9 | 28863.1 | 375.8 |
| Kazakhstan [52] | Gromova et al., 2019 | 1347 (528/819) | >18 | 70.0 | 8240.0 | 121.7 |
| Kuwait [53] | Zhang et al., 2016 | 960 (436/524) | ≥20 | 83.0 | 35260.9 | 218.7 |
| Lebanon [54] | Saad et al., 2020 | (46,099/96,032) | >19 | 35.5 | 26592.1 | 237.1 |
| Malaysia [55] | Shafinaz and Moy et al., 2016 | 858 (77/781) | ≥18 | 67.4 | 3491.6 | 14.6 |
| Mongolia [56] | Bromage et al., 2016 | 320 (160/160) | 20–58 | 35.5 | 370.6 | 0.3 |
| Nepal [57] | Sherchand et al., 2018 | 300 (109/191) | ≥18 | 51.3 | 8943.8 | 63.7 |
| Oman [58] | Abiaka et al., 2013 | 206 (101/105) | 18–55 | 87.5 | 25235.3 | 293.5 |
| Pakistan [59] | Kandhro et al., 2019 | 1255 (519/725) | ≤84 | 36.0 | 2171.7 | 45.7 |
| Qatar [60] | Zainel et al., 2019 | 102,342 (34,946/67,393) | 18–65 | 71.4 | 49924.1 | 85.0 |
| Saudi Arabia [61] | Altowijri et al., 2018 | 350 (150/200) | 20–40 | 74.6 | 10419.4 | 178.8 |
| Singapore [62] | Bi et al., 2016 | 114 (59/55) | 21–100 | 42.1 | 10016.3 | 5.0 |
| Thailand [63] | Rajatanavin et al., 2019 | 120 (56/64) | 25–60 | 19.1 | 98.6 | 0.9 |
| Turkey [64] | Gotkas et al., 2020 | 11,734 (2592/9142) | ≥18 | 70.6 | 26187.8 | 247.6 |
| UAE [65] | Al Zarooni et al., 2019 | 12,346 (4561/7785) | ≥18 | 72.0 | 21012.5 | 67.6 |
| Vietnam [66] | Ho-Pham et al., 2011 | 637 (205/432) | 18–87 | 2.0 | 15.1 | 0.4 |
F-Female; M-Male.
∗Prevalence of Vitamin D deficiency; Serum 25-hydroxyvitamin D3 level <20 ng/ml or <50 nmol/L; cases/M-cases per million population; mortalities/M-mortalities per million population.
Fig. 1.
Scatter diagrams of the prevalence of vitamin D deficiency against COVID-19 infections and mortality rates.
Among the countries from Asian continent, the vitamin D deficiency values varied from 2.0 to 87.5% with the lowest and highest values recorded in Vietnam and Oman respectively. Similar to Europe, half of the Asian countries (n = 17) also had vitamin D deficiency rates exceeding 50% among adults. The population sizes included in studies ranged from 107 to 142,131 consisting of all adults and elderly participants.
As of December, 31st 2020, among the countries in the Asian region, Bahrain (954,464) and Vietnam [15] reported the highest and lowest COVID-19 infections per 1M of the total population, while Iran (658) and Mongolia (0.3) had the highest and lowest mortalities per 1M population. The correlation analysis in the Asian region displayed a significant, strong positive correlation (r = 0.76; p < 0.001) with the prevalence of vitamin D deficiency and cases of COVID-19 infections per 1M population. In addition 58% of the variation (R2 = 0.58) in total infections was attributed to the high prevalence of vitamin D deficiency (Fig. 1c). As illustrated by the (Fig. 1d) COVID-19 mortality per 1M population also had a strong positively significant correlation with the prevalence rates of vitamin D deficiency (r = 0.75; p > 0.001). Majority of the countries were scattered around the regression line, indicating 56% variability (R2 = 0.56) among the mortalities due to the high prevalence of vitamin D deficiency. China was positioned as an outlier in both Fig. 1c and Fig. 1d graphs and the correlation was increased markedly when it was removed from the analysis. After removing China from the analysis the correlation values were increased for both infection and (r = 0.81; p < 0.001; R2 = 0.65) and mortality (r = 0.76; p < 0.001; R2 = 0.57) rates.
When both the continents were combined, the correlation analysis between vitamin D deficiency and cases of COVID-19 infections per 1M population, yielded a significant, moderately positive correlation (r = 0.42; p = 0.003; R2 = 0.18) (Fig. 1e). Similarly, in the analysis between the mortalities per 1M population and vitamin D deficiency, a positive, significant correlation could be observed (r = 0.35; p = 0.016; R2 = 0.12) (Fig. 1f).
4. Discussion
To the best of our knowledge, this is the first study reporting the correlation of vitamin D and COVID-19 in two major continents. We have identified a positive association between the vitamin D deficiency levels and COVID-19 infections and mortality rates among populations in European and Asian continents. Prior research work also has reported similar results [[67], [68], [69]]. A study by Pugach et al., investigated the association between vitamin D deficiency and COVID-19 severity, revealed that severe cases of COVID-19 present 65% more vitamin D deficiency compared with mild cases of the disease (OR = 1.64; 95% CI = 1.30–2.09) [70].
Vitamin D deficiency is a common public health problem affecting individuals of all ages worldwide. 75% of countries had more than 50% of the adult population suffering from vitamin D deficiency. Vitamin D status can be highly varied between different countries of these regions due to several reasons such as different exposure to sunshine, dietary intake of vitamin D and the use of supplements [71].
According to our analysis, the Asian countries demonstrated a strong positive correlation with both the number of COVID 19 infections as well as mortalities than the countries in the European region. Although some countries in Asia are at similar latitude to countries in Europe, and receive sunlight throughout the year unlike European countries, the vitamin D deficiency is much more prevalent [72]. This could be due to the lower dietary intake of vitamin D in Asian countries in comparison to European countries where vitamin D fortified food and supplements are readily available in the market [73]. Indeed, the use of vitamin D supplements and cod liver oil is common in Northern European countries and has a strong positive influence on vitamin D status [73]. In addition, skin pigmentation and cultural behaviors such as clothing and avoidance of outdoor activities in Mediterranean countries can contribute to vitamin D insufficiency [74].
Although the correlation analysis between the prevalence of vitamin D deficiency and COVID-19 infections and mortality rates resulted higher values for European and Asian continents separately, the association became modest after combing the both continents. The regression line depicting the association between vitamin D deficiency and number of infections for all the countries indicated 18% variability (R2 = 0.18) which infers approximately 18% of death rate from COVID-19 can be explained by prevalence of vitamin D deficiency. Therefore, the causes of high prevalence of vitamin D deficiency and increased deaths from COVID-19 are multifactorial. The identification of vitamin D deficiency as a contributor provides a useful target for treatment and prevention.
Since sufficient information on vitamin D deficiency could not be found for African and Latin American countries, we limited our study only to the continents of Europe and Asia. However, the majority of the data on prevalence of vitamin D deficiency among these countries were not from national level surveys. Due to the lack of national survey information in some countries, the sample sizes of studies were heterogeneous and were not representing the whole nation. This is a main limitation of this study as it could affect the power of the analysis. Therefore, to resolve this issue the most recently published studies with the most representative sample of each country population were carefully selected for the analysis. In addition, the overall prevalence of vitamin D deficiency was considered in the adult population, although there were substantial differences in the prevalence between different age groups. However, the younger population was hardly affected by the COVID-19 pandemic. Furthermore, serum vitamin D level fluctuates seasonally, due to climate, and possibly diet, especially in European countries [75]. Therefore, when different prevalence values were identified across different seasons, the mean prevalence of vitamin D deficiency throughout the year was considered. Furthermore, COVID-19 cases and mortality numbers only up to 31st of December were considered. Because the vaccination seemed to be reducing infections and death rates in vaccinated individuals thereafter [76]. This study included almost half the number of both European and Asian countries. Therefore, we believe that the results of this study can be generalized to the whole Asian and the European continents.
From the result of this study it is now evident that there is an association between vitamin D deficiency and incidence or mortality of the COVID-19 pandemic. Therefore, supplementation with vitamin D might be recommended to vitamin D deficient and insufficient patients suffering from COVID-19 [77]. In addition introducing mandatory fortification of products to the public such as dairy with vitamin D [67] and also promoting supplementation to individuals belonging to high risk groups of deficiency [78] could be effective strategies to reduce the prevalence of vitamin D deficiency. Moreover, vitamin D status ‘by country’ should be screened as part of standard health practices, as these results could provide valuable insights into how public health initiatives can better protect the population's health during public health emergencies, such as infectious disease pandemics [79].
5. Conclusion
We observed strong, positively significant correlations for both COVID-19 infection (r = 0.76; p < 0.001) and mortality rates (r = 0.75; p < 0.001) with vitamin D deficiency prevalence values in the Asian continent. Comparatively lower, near marginal and significant correlation values were observed for both COVID-19 infection (r = 0.371; p = 0.08) and mortality rates (r = 0.43; p = 0.04) for the European continent respectively. The correlation values for both COVID-19 cases (r = 0.42; p = 0.003) and mortalities (r = 0.35; p = 0.016) still remained positive and significant, when the two continents were combined in the analysis.
Funding statement
None.
Authors’ contributions
RJ devised the conceptual idea. PS and DTJ searched databases. PS and DTJ were involved in retrieving data and analysis. PS and RJ drafted the manuscript. NK revised the manuscript. All authors provided critical feedback on the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Not applicable.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
None.
References
- 1.Alanagreh La, Alzoughool F., Atoum M. The human coronavirus disease COVID-19: its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens. 2020;9(5):331. doi: 10.3390/pathogens9050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometer . 2021. COVID-19 coronovirus pandemic.https://www.worldometers.info/coronavirus/ [updated January 28, 2021. Available from: [Google Scholar]
- 3.Clemente-Suárez V.J., Hormeño-Holgado A., Jiménez M., Benitez-Agudelo J.C., Navarro-Jiménez E., Perez-Palencia N., et al. Dynamics of population immunity due to the herd effect in the COVID-19 pandemic. Vaccines. 2020;8(2):236. doi: 10.3390/vaccines8020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry R., Dranitsaris G., Mubashir T., Bartoszko J., Riazi S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine. 2020;25:100464. doi: 10.1016/j.eclinm.2020.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin J., Agarwala N., Kundu P., Harvey B., Zhang Y., Wallace E., et al. medRxiv; 2020. Assessment of individual-and community-level risks for COVID-19 mortality in the US and implications for vaccine distribution. [Google Scholar]
- 6.Van Schoor N., Lips P. Global overview of vitamin D status. Endocrinol Metabol Clin. 2017;46(4):845–870. doi: 10.1016/j.ecl.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Tsiaras W.G., Weinstock M.A. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115–124. doi: 10.2340/00015555-0980. [DOI] [PubMed] [Google Scholar]
- 8.van Schoor N.M., Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metabol. 2011;25(4):671–680. doi: 10.1016/j.beem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Danai P.A., Sinha S., Moss M., Haber M.J., Martin G.S. Seasonal variation in the epidemiology of sepsis. Crit Care Med. 2007;35(2):410–415. doi: 10.1097/01.CCM.0000253405.17038.43. [DOI] [PubMed] [Google Scholar]
- 10.Grant W.B. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 2008;27(9):853. doi: 10.1097/INF.0b013e3181817bc1. [DOI] [PubMed] [Google Scholar]
- 11.Cannell J., Vieth R., Umhau J., Holick M., Grant W., Madronich S., et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimitrov V., Barbier C., Ismailova A., Wang Y., Dmowski K., Salehi-Tabar R., et al. Vitamin D-regulated gene expression profiles: species-specificity and cell-specific effects on metabolism and immunity. Endocrinology. 2021;162(2):bqaa218. doi: 10.1210/endocr/bqaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olliver M., Spelmink L., Hiew J., Meyer-Hoffert U., Henriques-Normark B., Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J Infect Dis. 2013;208(9):1474–1481. doi: 10.1093/infdis/jit355. [DOI] [PubMed] [Google Scholar]
- 14.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maggini S., Wintergerst E.S., Beveridge S., Hornig D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(S1):S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 16.Bae M., Kim H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules. 2020;25(22) doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu F., Zhu Y., Zhang J., Li Y., Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ open. 2020;10(7) doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantorna M.T., Snyder L., Lin Y.-D., Yang L. Vitamin D and 1, 25 (OH) 2D regulation of T cells. Nutrients. 2015;7(4):3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoit K. vol. 22. London School of Economics; London: 2011. pp. 23–36. (Linear regression models with logarithmic transformations). 1. [Google Scholar]
- 20.Hoge A., Donneau A.F., Streel S., Kolh P., Chapelle J.P., Albert A., et al. Vitamin D deficiency is common among adults in Wallonia (Belgium, 51°30' North): findings from the Nutrition, Environment and Cardio-Vascular Health study. Nutr Res. 2015;35(8):716–725. doi: 10.1016/j.nutres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Sokolovic S., Alimanovic-Alagic R., Dzananovic L., Cavaljuga S., Beslic N., Ferhatbegovic-Opankovic E. Vitamin D status in Bosnia and Herzegovina: the cross-sectional epidemiological analysis. Osteoporos Int. 2017;28(3):1021–1025. doi: 10.1007/s00198-016-3831-0. [DOI] [PubMed] [Google Scholar]
- 22.Borissova A.M., Shinkov A., Vlahov J., Dakovska L., Todorov T., Svinarov D., et al. Vitamin D status in Bulgaria--winter data. Arch Osteoporos. 2013;8:133. doi: 10.1007/s11657-013-0133-4. [DOI] [PubMed] [Google Scholar]
- 23.Colić Barić I., Keser I., Bituh M., Rumbak I., Rumora Samarin I., Beljan K., et al., editors. Vitamin D status and prevalence of inadequacy in Croatian population. Book of Abstracts of 4th International congress of nutritionists; 2016. [Google Scholar]
- 24.Hansen L., Tjønneland A., Køster B., Brot C., Andersen R., Cohen A.S., et al. Vitamin D status and seasonal variation among Danish children and adults: a descriptive study. Nutrients. 2018;10(11):1801. doi: 10.3390/nu10111801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adebayo F.A., Itkonen S.T., Lilja E., Jaaskelainen T., Lundqvist A., Laatikainen T., et al. Prevalence and determinants of vitamin D deficiency and insufficiency among three immigrant groups in Finland: evidence from a population-based study using standardised 25-hydroxyvitamin D data. Publ Health Nutr. 2020;23(7):1254–1265. doi: 10.1017/s1368980019004312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deplanque X., Wullens A., Norberciak L. Prevalence and risk factors of vitamin D deficiency in healthy adults aged 18-65 years in northern France. Rev Med Interne. 2017;38(6):368–373. doi: 10.1016/j.revmed.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Rabenberg M., Scheidt-Nave C., Busch M.A., Thamm M., Rieckmann N., Durazo-Arvizu R.A., et al. Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Publ Health. 2018;18(1):1–14. doi: 10.1186/s12889-018-5769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimakopoulos I., Magriplis E., Mitsopoulou A.-V., Karageorgou D., Bakogianni I., Micha R., et al. Association of serum vitamin D status with dietary intake and sun exposure in adults. Clin Nutr ESPEN. 2019;34:23–31. doi: 10.1016/j.clnesp.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Griffin T.P., Wall D., Blake L., Griffin D.G., Robinson S., Bell M., et al. Higher risk of vitamin D insufficiency/deficiency for rural than urban dwellers. J Steroid Biochem Mol Biol. 2020;197:105547. doi: 10.1016/j.jsbmb.2019.105547. [DOI] [PubMed] [Google Scholar]
- 30.Giuliani S., Barbieri V., Di Pierro A.M., Rossi F., Widmann T., Lucchiari M., et al. LC–MS/MS based 25 (OH) D status in a large Southern European outpatient cohort: gender-and age-specific differences. Eur J Nutr. 2019;58(6):2511–2520. doi: 10.1007/s00394-018-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrenya N., Lamberg-Allardt C., Melhus M., Broderstad A.R., Brustad M. Vitamin D status in a multi-ethnic population of northern Norway: the SAMINOR 2 clinical survey. Publ Health Nutr. 2020;23(7):1186–1200. doi: 10.1017/S1368980018003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Płudowski P., Ducki C., Konstantynowicz J., Jaworski M. Vitamin D status in Poland. Pol Arch Med Wewn. 2016;126(7–8):530–539. doi: 10.20452/pamw.3479. [DOI] [PubMed] [Google Scholar]
- 33.Duarte C., Carvalheiro H., Rodrigues A.M., Dias S.S., Marques A., Santiago T., et al. Prevalence of vitamin D deficiency and its predictors in the Portuguese population: a nationwide population-based study. Arch Osteoporos. 2020;15(1):36. doi: 10.1007/s11657-020-0695-x. [DOI] [PubMed] [Google Scholar]
- 34.Niculescu D.A., Capatina C.A.M., Dusceac R., Caragheorgheopol A., Ghemigian A., Poiana C. Seasonal variation of serum vitamin D levels in Romania. Arch Osteoporosis. 2017;12(1):1–7. doi: 10.1007/s11657-017-0407-3. [DOI] [PubMed] [Google Scholar]
- 35.Karonova T., Andreeva A., Nikitina I., Belyaeva O., Mokhova E., Galkina O., et al. Prevalence of Vitamin D deficiency in the North-West region of Russia: a cross-sectional study. J Steroid Biochem Mol Biol. 2016;164:230–234. doi: 10.1016/j.jsbmb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Sebekova K., Krivosikova Z., Gajdos M., Podracka L. Vitamin D status in apparently healthy medication-free Slovaks: association to blood pressure, body mass index, self-reported smoking status and physical activity. Bratisl Lek Listy. 2016;117(12):702–709. doi: 10.4149/BLL_2016_135. [DOI] [PubMed] [Google Scholar]
- 37.Hribar M., Hristov H., Gregorič M., Blaznik U., Zaletel K., Oblak A., et al. Nutrihealth study: seasonal variation in vitamin D status among the slovenian adult and elderly population. Nutrients. 2020;12(6) doi: 10.3390/nu12061838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González-Molero I., Morcillo S., Valdés S., Pérez-Valero V., Botas P., Delgado E., et al. Vitamin D deficiency in Spain: a population-based cohort study. Eur J Clin Nutr. 2011;65(3):321–328. doi: 10.1038/ejcn.2010.265. [DOI] [PubMed] [Google Scholar]
- 39.Nälsén C., Becker W., Pearson M., Ridefelt P., Lindroos A.K., Kotova N., et al. Vitamin D status in children and adults in Sweden: dietary intake and 25-hydroxyvitamin D concentrations in children aged 10-12 years and adults aged 18-80 years. J Nutr Sci. 2020;9:e47. doi: 10.1017/jns.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guessous I., Dudler V., Glatz N., Theler J.M., Zoller O., Paccaud F., et al. Vitamin D levels and associated factors: a population-based study in Switzerland. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13719. 0. [DOI] [PubMed] [Google Scholar]
- 41.Povoroznyuk V., Balatska N., Muts V., Klymovytsky F., Synenky O. Vitamin D deficiency in Ukraine: a demographic and seasonal analysis. Gerontol. 2012;13(4):191–198. [Google Scholar]
- 42.Jolliffe D.A., Hanifa Y., Witt K.D., Venton T.R., Rowe M., Timms P.M., et al. Environmental and genetic determinants of vitamin D status among older adults in London, UK. J Steroid Biochem Mol Biol. 2016;164:30–35. doi: 10.1016/j.jsbmb.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Almesri N., Das N.S., Ali M.E., Gumaa K., Giha H.A. Gender-dependent association of vitamin D deficiency with obesity and hypercholesterolemia (LDLC) in adults. Endocrine, metabolic & immune disorders-drug targets (formerly current drug targets-immune. Endocrine & Metabolic Disorders) 2020;20(3):425–436. doi: 10.2174/1871530319666191009154528. [DOI] [PubMed] [Google Scholar]
- 44.Acherjya G., Ali M., Tarafder K., Akhter N., Chowdhury M., Islam D., et al. Study of vitamin D deficiency among the apparently healthy population in Jashore, Bangladesh. Mymensingh Med J. 2019;28(1):214–221. [PubMed] [Google Scholar]
- 45.Leong J.F., Yakob M., Fung E.C., Pande K.C. High prevalence of vitamin D insufficiency and deficiency in a mixed sample of patients in Brunei Darussalam. Brunei Int Med J. 2016;12(4):134–139. [Google Scholar]
- 46.Jiang W., Wu D.-B., Xiao G.-B., Ding B., Chen E.-Q. An epidemiology survey of vitamin D deficiency and its influencing factors. Med Clínica. 2020;154(1):7–12. doi: 10.1016/j.medcli.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Mechenro J., Venugopal G., Kumar M.B., Balakrishnan D., Ramakrishna B.S. Vitamin D status in kancheepuram district, Tamil nadu, India. BMC Publ Health. 2018;18(1):1–8. doi: 10.1186/s12889-018-6244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esmaeili S.A., Mohammadian S., Radbakhsh S., Momtazi-Borojeni A.A., Kheirmand Parizi P., Atabati H., et al. Evaluation of vitamin D3 deficiency: a population-based study in northeastern Iran. J Cell Biochem. 2019;120(6):10337–10341. doi: 10.1002/jcb.28317. [DOI] [PubMed] [Google Scholar]
- 49.Al-Hilali K. Prevalence of hypovitaminosis D in adult Iraqi people including postmenopausal women. Sci Res J. 2016;4:53–62. [Google Scholar]
- 50.Asakura K., Etoh N., Imamura H., Michikawa T., Nakamura T., Takeda Y., et al. Vitamin D status in Japanese adults: relationship of serum 25-hydroxyvitamin D with simultaneously measured dietary vitamin D intake and ultraviolet ray exposure. Nutrients. 2020;12(3):743. doi: 10.3390/nu12030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khasawneh R., Hiari M., Khalaileh M., Khasawneh H., Alzghoul B., Almomani A. Frequency of vitamin D deficiency and insufficiency in a Jordanian cohort: a hospital based study. J Royal Med Services. 2018;102(5938):1–4. [Google Scholar]
- 52.Gromova O., Doschanova A., Lokshin V., Tuletova A., Grebennikova G., Daniyarova L., et al. Vitamin D deficiency in Kazakhstan: cross-sectional study. J Steroid Biochem Mol Biol. 2020;199:105565. doi: 10.1016/j.jsbmb.2019.105565. [DOI] [PubMed] [Google Scholar]
- 53.Zhang F.F., Al Hooti S., Al Zenki S., Alomirah H., Jamil K.M., Rao A., et al. Vitamin D deficiency is associated with high prevalence of diabetes in Kuwaiti adults: results from a national survey. BMC Publ Health. 2016;16(1):1–9. doi: 10.1186/s12889-016-2758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saad R.K., Akiki V.C., Rahme M., Ajjour S., Assaad M., Fuleihan G.A.E.-H. Time trends and predictors of hypovitaminosis D across the life course: 2009–2016. Metabolism. 2020;105:154138. doi: 10.1016/j.metabol.2020.154138. [DOI] [PubMed] [Google Scholar]
- 55.Shafinaz I., Moy F. Vitamin D level and its association with adiposity among multi-ethnic adults in Kuala Lumpur, Malaysia: a cross sectional study. BMC Publ Health. 2016;16(1):1–9. doi: 10.1186/s12889-016-2924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bromage S., Rich-Edwards J.W., Tselmen D., Baylin A., Houghton L.A., Baasanjav N., et al. Seasonal epidemiology of serum 25-hydroxyvitamin D concentrations among healthy adults living in rural and urban areas in Mongolia. Nutrients. 2016;8(10):592. doi: 10.3390/nu8100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherchand O., Sapkota N., Chaudhari R.K., Khan S.A., Baranwal J.K., Pokhrel T., et al. Association between vitamin D deficiency and depression in Nepalese population. Psychiatr Res. 2018;267:266–271. doi: 10.1016/j.psychres.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 58.Abiaka C., Delghandi M., Kaur M., Al-Saleh M. Vitamin D status and anthropometric indices of an Omani study population. Sultan Qaboos University Med J. 2013;13(2):224. doi: 10.12816/0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kandhro F., Dahot M.U., Ahmed Naqvi S.H., Ujjan I.U. Study of Vitamin D deficiency and contributing factors in the population of Hyderabad, Pakistan. Pak J Pharm Sci. 2019;32(3) [PubMed] [Google Scholar]
- 60.Zainel A.-J.A.L., Qotba H., Al Nuaimi A., Syed M. Vitamin D status among adults (18–65 years old) attending primary healthcare centres in Qatar: a cross-sectional analysis of the Electronic Medical Records for the year 2017. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2019-029334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Altowijri A., Alloubani A., Abdulhafiz I., Saleh A. Impact of nutritional and environmental factors on vitamin D deficiency. Asian Pac J Cancer Prev APJCP: Asian Pac J Cancer Prev APJCP. 2018;19(9):2569. doi: 10.22034/APJCP.2018.19.9.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bi X., Tey S.L., Leong C., Quek R., Henry C.J. Prevalence of vitamin D deficiency in Singapore: its implications to cardiovascular risk factors. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0147616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajatanavin N., Kanokrungsee S., Aekplakorn W. Vitamin D status in Thai dermatologists and working-age Thai population. J Dermatol. 2019;46(3):206–212. doi: 10.1111/1346-8138.14742. [DOI] [PubMed] [Google Scholar]
- 64.Göktaş O., Ersoy C., Ercan I., Can F.E. Vitamin D status in the adult population of Bursa-Turkey. Eur J Gen Pract. 2020;26(1):156–162. doi: 10.1080/13814788.2020.1846712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al Zarooni A.A.R., Al Marzouqi F.I., Al Darmaki S.H., Prinsloo E.A.M., Nagelkerke N. Prevalence of vitamin D deficiency and associated comorbidities among Abu Dhabi Emirates population. BMC Res Notes. 2019;12(1):1–6. doi: 10.1186/s13104-019-4536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ho-Pham L.T., Nguyen N.D., Lai T.Q., Eisman J.A., Nguyen T.V. Vitamin D status and parathyroid hormone in a urban population in Vietnam. Osteoporos Int. 2011;22(1):241–248. doi: 10.1007/s00198-010-1207-4. [DOI] [PubMed] [Google Scholar]
- 67.Laird E., Rhodes J., Kenny R.A. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J. 2020;113(5):81. [PubMed] [Google Scholar]
- 68.Radujkovic A., Hippchen T., Tiwari-Heckler S., Dreher S., Boxberger M., Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12(9):2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jayawardena R., Jeyakumar D.T., Francis T.V., Misra A. Diabetes & Metabolic Syndrome: Clinical Research & Reviews; 2021. Impact of the vitamin D deficiency on COVID-19 infection and morality in Asian countries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pugach I.Z., Pugach S. Strong correlation between prevalence of severe vitamin D deficiency and population mortality rate from COVID-19 in Europe. Wien Klin Wochenschr. 2021;133(7):403–405. doi: 10.1007/s00508-021-01833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spiro A., Buttriss J. Vitamin D: an overview of vitamin D status and intake in E urope. Nutr Bull. 2014;39(4):322–350. doi: 10.1111/nbu.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraser D.R. Vitamin D-deficiency in Asia. J Steroid Biochem Mol Biol. 2004;89:491–495. doi: 10.1016/j.jsbmb.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 73.Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103(3–5):620–625. doi: 10.1016/j.jsbmb.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 74.Scharla S. Prevalence of subclinical vitamin D deficiency in different European countries. Osteoporos Int. 1998;8:S7. doi: 10.1007/pl00022726. [DOI] [PubMed] [Google Scholar]
- 75.Kull M., Kallikorm R., Tamm A., Lember M. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Publ Health. 2009;9(1):1–8. doi: 10.1186/1471-2458-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mallapaty S. Vaccines are curbing COVID: data from Israel show drop in infections. Nature. 2021;590(7845):197. doi: 10.1038/d41586-021-00316-4. [DOI] [PubMed] [Google Scholar]
- 77.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes & Metabolic Syndrome: Clin Res Rev. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lips P., Cashman K.D., Lamberg-Allardt C., Bischoff-Ferrari H.A., Obermayer-Pietsch B., Bianchi M.L., et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 79.Castillo M.E., Costa L.M.E., Barrios J.M.V., Díaz J.F.A., Miranda J.L., Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]