Abstract
Coronavirus disease 2019 (COVID-19) is one of the worst pandemics to have hit the humanity. The manifestations are quite varied, ranging from severe lung infections to being asymptomatic. Hence, there is an urgent need to champion new tools to accelerate the end of this pandemic. Compromised immunity is a primary feature of COVID-19. Allium sativum (AS) is an effective dietary supplement known for its immune-modulatory, antibacterial, anti-inflammatory, anticancer, antifungal, and anti-viral properties. In this paper, it is hypothesized that carbon dots (CDs) derived from AS (AS-CDs) may possess the potential to downregulate the expression of pro-inflammatory cytokines and revert the immunological aberrations to normal in case of COVID-19. CDs have already been explored in the world of nanobiomedicine as a promising theranostic candidates for bioimaging and drug/gene delivery. The antifibrotic and antioxidant effects of AS are elaborated, as demonstrated in several studies. It is found that the most active constituent of AS, allicin has a highly potent antioxidant and reactive oxygen species (ROS) scavenging effect. The antibacterial, antifungal, and anti-viral effects along with their capability of negating inflammatory effects and cytokine storm are discussed. The synthesis of theranostic CDs from AS may provide a novel weapon in the therapeutic armamentarium for the management of COVID-19 infection and, at the same time, could act as a diagnostic agent for COVID-19.
Keywords: Coronavirus, COVID-19, Anti-inflammatory, Garlic, Allicin, Carbon dots
1. Introduction
Coronavirus disease 2019 or COVID-19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has substantially compromised the human civilization and became one of the most deadly diseases globally [[1], [2], [3], [4]]. Further, the treatment of COVID-19 with corticosteroids in its wake has brought other superinfections to the fore thus, emphasizing the need for novel strategies. Pharmacologic or non-pharmacologic interventions and their combinations have emerged for the interruption of its spread [5]. Recently, mRNA-based two vaccines of Pfizer-BioNTech and Moderna have been given emergency authorization for use in many countries [6]. Moreover, other vaccines like Sputnik V, Covaxin, Covishield, Sinovac, and others are also being used in various countries to curb the spread of COVID-19 infection. The gap between the demand to supply is widening and is hampered by slower production rate in contrast to the world population. So, there is a dire need to explore more therapeutic options which can aid in the management of COVID-19 [[7], [8], [9], [10]]. A large number of established drugs, including chloroquine, hydroxychloroquine, remdesivir, faviparavir, ivermectin, and others, have been tried as the immediate option for short-term prophylaxis [11]. However, it has been reported that hydroxychloroquine failed as a promising therapeutic drug for SARS-CoV-2 because of lack of efficacy and severe side effects [12]. In contrast, other pharmacological interventions still need more assessment before being considered as potential treatment option [13]. As a matter of fact, early diagnosis and treatment of SARS-CoV-2 have become a crucial step in preventing the further spread of the virus. The development of a promising theranostic agent, which is safe and of natural origin with diagnostic capability, might be advantageous [[14], [15], [16]]. Over the last decade, different biosensing platforms accompanied by functional materials have been developed for various infectious and non-infectious diseases [9,[17], [18], [19], [20], [21], [22], [23]]. Among different functional materials, carbon dots (CDs) have gained enormous scientific attention in view of their unique physiochemical properties.
CDs are extremely important among the newly emerging class of fluorescent nanomaterials tested in nanomedicine and biomedical applications [24]. In recent years, CDs have emerged as a promising theranostic agent with broad biological applications including biotherapy, biosensing, biolabeling, bioimaging, drug/gene delivery owing to their exciting properties such as ease of synthesis, tunable fluorescence emission, and excitation, good photochemical stability, excellent biocompatibility, surface tenability, high water solubility and low cytotoxicity [[25], [26], [27], [28]]. Interestingly, CDs are helpful for cell labeling, tracking cellular events, targeting specific cells due to their intrinsic fluorescent properties, and a vehicle for intracellular transport of drugs/genes [29,30]. Different nanomaterials such as graphene quantum dots (GQDs), carbon quantum dots (CQDs), CDs partially graphitized core-shell carbon nanoparticles, and amorphous carbon nanoparticles are included in the family of fluorescent carbon materials [29,[31], [32], [33], [34]]. The surface functional groups of CDs provide easy conjugation with numerous therapeutic agents, prevailing in anti-viral applications [[35], [36], [37]]. In addition, the surface engineering of CDs helps in providing low cytotoxicity and specific anti-viral properties [38]. These fluorescent materials have emerged as efficient and predominant candidates to modulate the viral infection cycle. The virus attaches with host cells through multivalent interactions; therefore CDs treatment might prevent viral entry into the host cells [39]. The CDs possess low cytotoxicity and indicate specific anti-viral activities due to the surface functional groups via different mechanisms, making their spectrum broad [40]. Additionally, the ease of surface functionalization and their small size helps in providing easy passage through the cell membrane and subsequent delivery of the anti-viral drug [41]. Since the origin of CDs by Xu et al., different methods have been employed for synthesizing CDs using abundant, inexpensive green precursors including sugarcane juice, onion peel, milk, pomelo peel, bamboo leaves, honey, flowers, apple juice, potatoes, sweet and green tea etc. [[42], [43], [44]].
Allium sativum L. (AS) has been widely used as a prophylactic and therapeutic agent all over the world [45]. AS possesses strong antimicrobial, anticancer, antifungal, antibacterial, antioxidant, and anti-viral properties [[46], [47], [48], [49]], used in various diseases such as common cold, influenza, and cardiac diseases, smallpox, etc., since ages [47,[50], [51], [52], [53]]. AS possesses an anti-inflammatory effect by restricting the activation of nuclear factor-kappa B (NFκB) in enzymes, namely cyclooxygenase-II (COX-II) and inducible nitric oxide synthase (iNOS) [54]. Angiotensin-converting enzyme 2 (ACE2) is a functional glycoprotein found in the heart, lungs, endothelium, and kidneys and is shared by SARS as well as SARS-CoV-2 as the host-cell receptor [[55], [56], [57], [58]]. The inhibition of ACE2 protein can help in reducing the complications associated with SARS-CoV-2 infection [59]. Organosulfur compound allicin is obtained from AS and was first studied by Bailey and Cavallito in 1944 [60]. Yang et al., probed the anti-inflammatory and antioxidant properties of AS-CDs in vitro in lipopolysaccharide (LPS) stimulated Raw 264.7 macrophages. Induction of macrophages with LPS caused the release of pro-inflammatory cytokines and increased the expression of inflammatory transcription factor NFκB. Moreover, it leads to an increase in iNOS activity which generates free radicals causing nitrosative damage in the tissues. In contrast, incubation of LPS induced macrophages with AS-CDs abrogated these changes and showed their remarkable anti-inflammatory and anti-oxidant potential [61]. Furthermore, in a significant number of studies, it has been demonstrated that AS attenuated the inflammation by downregulating the expression of pro-inflammatory cytokines like interleukin (IL)-1, IL-6, IL-17 and tumor necrosis factor-alpha (TNF-α) [62,63]. It has been anticipated that allicin has strong interactions with the amino acids of the ACE2 protein and could serve as a dual strategy theranostic agent if formulated in the form of CDs.
AS is one of the highly efficient naturally occurring antimicrobial agent against a wide array of viruses and bacterias. Beside allicin, other organosulfur constituents, S-allyl-l-cysteine (SAC), caffeic acid (CA), uracil, diallyltrisulfide (DATS), diallyl disulfide (DADS) and flavonoids such as quercetin are responsible phytoconstituents of AS for their potent immunomodulatory and anti-inflammatory effects. It may attenuate immune system-related dysfunctions seen in patients with COVID-19 infection [64]. Aqueous extracts and essential oils of AS showed interaction with Mpro protease of SARS-CoV-2 and reduced the rate of viral infection caused by SARS-CoV-2 [65,66]. The encapsulation of these active constituents by using different methods can improve their oxidative stability and bio-functionality and may offer targeted drug delivery. The consumption of these encapsulated/free bioactive compounds of AS may help in reducing the incidence rate of COVID-19 [67].
2. Hypothesis
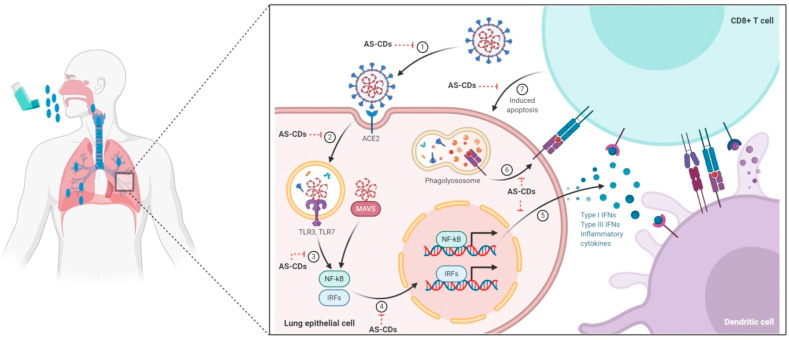
We hypothesize AS-derived carbon dots (AS-CDs) as a potential theranostic agent to combat the COVID-19 crisis. By virtue of its versatile therapeutic potential to modulate the activity of NF-κB, MAPKinase, scavenging ROS species, and capability to activate the nuclear factor erythroid 2-related factor 2 (Nrf2) protein expression, AS-CDs may be helpful to abrogate the coronavirus liaised inflammation and cytokine storm (Fig. 1 ). Moreover, AS-based carbon dots can be used as a promising theranostic agent.
Fig. 1.
Acute Immune Responses to Coronaviruses: To infect the cell, coronavirus gets entry inside the cell by binding to the ACE2 receptor. After entry, they sensitize the MMAVS & TLR3/7 receptors that upregulate the replication of pro-inflammatory genes (NF-κB), leading to increased cytokines and interferon production. Dendritic cells recognize the antigen and transfer to the lymphoid tissues to prime adaptive immunity. After recognizing the antigen on DCs or infected cells surface, CD8-T cells stimulate apoptosis. This mechanistic figure illustrates different steps where AS-CDs might produce theranostic activity. 1. Entry of coronavirus into the cell through binding ACE2 receptor. 2. Coronavirus initiate replication cycle inside the host cell. 3. Triggers the cytokine regulatory proteins (NF-κB, IRFs). 4. Induced protein incites the production of inflammatory cytokines. 5. Release of inflammatory cytokines. 6. Degradation of the cellular receptor. 7. Induction of cell apoptosis. The figure was created with BioRender.com.
3. Justification of hypothesis
3.1. Allium sativum derived carbon dots (AS-CDs) and their significance
Fluorescent nanoparticles, especially CDs, are getting increased interest for various biomedical applications because of their exciting properties, including ease of functionalization, low cytotoxicity, high biocompatibility, and exceptional cell membrane permeability. CDs have been utilized as a theranostic agent for chemiluminescence, in vitro as well as in vivo bioimaging, anti-viral agent, and drug delivery applications in view of their biocompatibility and no cell toxicity. In line with this, Das et al. [68], utilized the AS peel (garlic husk) toward the synthesis of sulphur and nitrogen co-doped CDs through a one-step pyrolysis method. The obtained CDs exhibited negative surface charge (∼-2.0 mV), low cytotoxicity, high water dispersibility and good photostability. It was observed that increasing the concentration of CDs does not cause a decrease in cell viability. The cell viability was found to be more than 90% for the concentration of these CDs varying from 0 to 0.6 mg mL−1. Further, the bio-labeling potential was investigated through the two-photon imaging by treating the mesenchymal stem cells (ADMSCs) with these CDs and injecting to rabbit ear skin. A stable and bright green fluorescent signal was exhibited by ADMSCs under the excitation of 800 nm, indicating the photo-theranostic capability of these CDs. In a similar manner, Zhao et al. [69], reported the green synthesis of bifunctional CDs via the hydrothermal method using garlic as the precursor material. The blue fluorescent CDs were found resistant to a high ionic strength environment, the addition of biomolecules, and metal ions. The cytotoxicity of these garlic CDs was explored on A549 cell lines via MTT assay. These CDs revealed around 100% cell viability up to 0.5 mg mL−1concentration. Besides, multicolor cellular imaging potential was also demonstrated by utilizing these CDs as a fluorescent probe. Furthermore, the radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals was estimated by determining the reduction of DPPH caused by these CDs. A dose-dependent scavenging was observed for the concentration of CDs varying from 0 to 200 μg/mL. On the other hand, Yang et al. [61], revealed a microwave-assisted method for the synthesis of multi-colored bioactive garlic CDs for therapeutic applications. The cell viability was measured by incubating macrophage cells with different concentrations of garlic CDs for a duration of 24 h. Even at the highest concentration of 2 mg mL−1, there was no decrease in cell viability, revealing low cytotoxicity and higher biocompatibility of these CDs. Further, it was observed that CDs were present in the cytoplasmic regions of the cells indicated by red, green, and blue fluorescent images of the treated cells. Besides, the anti-oxidative effect of garlic CDs toward macrophages was explored. The pre-incubation of LPS activated macrophages with garlic CDs considerably reduced LPS-induced nitric oxide (NO) production, whereas macrophages treated with LPS alone indicated increased NO production. Zhang et al. [70], reported the synthesis of nitrogen (N) doped CDs using ethylenediamine as the N dopant and garlic as the green precursor for Fe3+ ions detection. The obtained CDs revealed excellent optical properties along with remarkable stability in high NaCl concentrations and wide pH ranges. In another study, Lee et al. [71], reported the microwave synthesis of garlic-derived CDs for exploring their potential as antiplatelet agent. The obtained CDs inhibited p38 mitogen-activated protein kinase (MAPK) phosphorylation, c-Jun N-terminal kinase (JNK), Akt (protein kinase B), and collagen-activated protein kinase C (PKC) activation. Besides, CDs revealed an effective inhibition in collagen-stimulated human platelet aggregation.
3.2. AS-CDs as antifibrotic and antioxidant
Whole AS and its aqueous extract exhibits potent antioxidant effects & raise the levels of glutathione peroxidase and catalase in the serum [72,73]. AS and its major active constituent allicin efficiently scavenge the free radicals in a dose-dependent manner. Other active constituents, such as SAC, also showed remarkable antioxidant potential in the in-vitro experimental model [74]. In various animal models, AS has been reported to protect the tissue against oxidative stress-mediated damage and improves organ performance. Treatment with AS extract for 21 days in male Wistar rats showed increased glutathione levels and decreased malondialdehyde activity in the heart, aorta kidney, and urinary bladder [75]. The aqueous extract of AS protected the tissues against the nicotine-stimulated oxidative injury and improved renal function. DATS present in the AS is an efficient antioxidant at (10 mmol/kg) and extensively increases the activities of quinone reductase and GST [76,77]. AS as an anti-oxidative and antifibrotic agent has been reported in L-arginine induced chronic pancreatitis rat model, with increased GSH activity and decreased lipid peroxidation. It also reduced the mRNA expression of TGF-β1, collagen-α1, and fibronectin, resulting in reduced inflammation and fibrosis [78,79]. A herbal composite containing AS, with other herbal drugs like white horehound, boneset, aniseed, fennel, licorice, and thyme, reduced the clinical symptoms of recurrent airway obstruction [80]. Besides these research postulates, the synthesis of theranostic CDs from AS could provide a solution [61,69]. These CDs also possess strong cationic surface charge characteristics, making them viable to interact with the negative RNA strand of SARS-CoV-2, resulting in ROS generation and disable the S-protein in the virus [41,81]. Further, the replication of the virus could be suppressed by inducing interferon-α (IFN-α), and the genomic replication in SARS-CoV-2 can be significantly altered by functionalizing the CDs with required surface functional groups [82].
3.3. 3AS-CDs as an antimicrobial and anti-viral agent
AS oil and its allyl alcohol constituent significantly halts the growth of yeast [83]. AS also inhibited the diarrhoeagenic Gram-negative pathogens, as observed in stool samples [84]. In vitro study of AS exhibited antimicrobial properties towards streptococci bacteria and anticariogenic activity against oral microorganisms [85]. A broad category of organosulfur compounds, whether natural or synthetic, exhibits antibacterial properties. Interestingly, AS contains organosulfur groups that react as strong nucleophiles, electrophiles, and potent metal chelates based on the surrounding milieu wherein a specific reaction takes place [86]. AS is also known as ‘Russian penicillin’ for its prevalent use as a systemic antimicrobial and topical agent [87]. At room temperature, crude extracts of AS revealed antimicrobial activity towards the Gram-negative as well as Gram-positive bacteria. Aged AS extract showed a dose-dependent antimicrobial action against three different strains of H. pylori at concentration ranges of 2-5 mg mL−1; though, heat treatment diminished the anti-bacterial activity [88]. AS with a proton pump inhibitor has been reported to be synergistic against H. pylori infection [89]. AS subdued the augmentation of twenty different kinds of intracellular Mycobacterium avium strains in patients having no AIDS [90]. In the pre-clinical rabbit model, aqueous AS extract and its active constituent allicin had potent antibacterial activity against Shigella flexneri [91].
In conjunction with the above, the anti-viral effect of AS and its sulphur derivatives have been identified in a number of infectious viruses which cause respiratory and flu infections covering adenovirus, Newcastle disease virus (NDV), measles virus (MeV), porcine reproductive and respiratory syndrome virus (PRRSV), pseudorabies virus (PRV), influenza, rhinovirus, parainfluenza, SARS-CoV, and coronavirus through pre-clinical studies [49]. The recalcitrant multiple common warts (RMCW), infection of human papillomavirus (HPV), have no treatment that could eradicate RMCW, except for intralesional immunotherapy. In 2014, Kenawy et al., probed the lipid garlic extract (LGE) role to purge the RMCW [92].
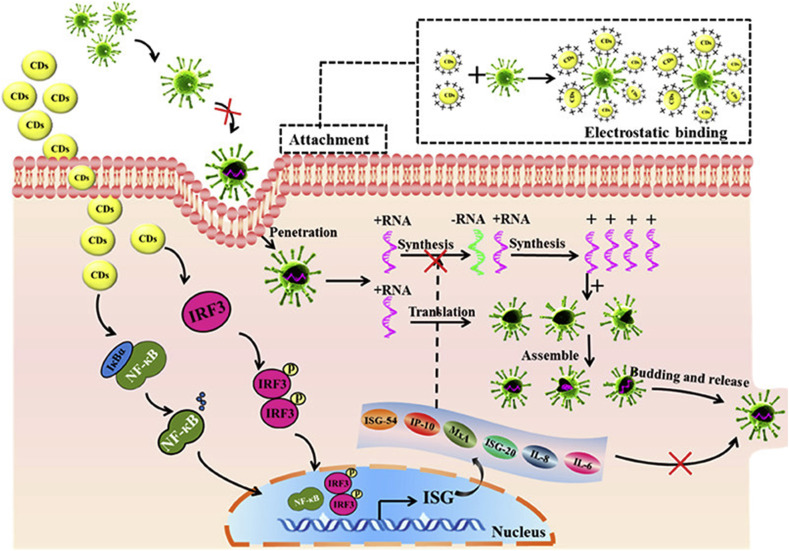
CDs have emerged as a potential theranostic agent against different stages of viral infection in view of their unique physiochemical properties. Besides, heteroatom doping can impart advantageous properties in CDs, reinforcing their antiviral potential. Moreover, surface functionalization also provides multifarious peculiarities, enabling them to perturb virus-host cell interaction [93]. Generally, viral infection includes four critical stages, viz. attachment, penetration, replication, and, lastly budding [94]. The foremost step of viral infection is attachment to the host cells. Therefore, the inhibition of virus attachment by altering their surface protein might be an efficient approach for managing the viral infection. In this context, Huang et al. [35], reported the anti-viral ability of benzoxazine monomer derived carbon dots (BZM-CDs) inhibiting the entry of the adeno-associated virus (AAV), porcine parvovirus (PPV) as well as different flaviviruses such as dengue viruses, Zika, and Japanese encephalitis virus. The hydrothermally synthesized BZM-CDs were found to restrain the viral entry in a concentration-dependent manner, investigated through transmission electron microscopy (TEM) and plaque reduction assay. Similarly, Barras et al. [95], reported the entry inhibition potential of 4-aminophenylboronic acid hydrochloride functionalized CDs against herpes simplex type 1 virus (HSV-1). It was observed that functionalized CDs specifically interact with the HSV-1 virus at the early stage, inhibiting their entry into the cells. In another work, Ting et al., reported the hydrothermal synthesis of highly stable and uniform cationic carbon dots (CCM-CDs) using curcumin and citric acid as precursors. These CDs were utilized to study the anti-viral effect of the porcine epidemic diarrhea virus (PEDV) as the coronavirus model, and entry of virus was found to be restrained by CCM-CDs altering the structure of surface proteins in viruses, leading to virus aggregation. This results in subsequent suppression of the negative-strand RNA synthesis and reactive oxygen species (ROS) accumulation. Moreover, the CCM-CDs stimulate the production of pro-inflammatory cytokines and interferon-stimulating genes (ISGs), thereby inhibit viral replication (Fig. 2 ) [41]. Similarly, CDs helped in restricting the viral interaction to RD cell membranes, and the ROS could be scavenged by inhibiting the production of PGE2 [40]. Moreover, according to the most recent studies, it has been analyzed that the CDs either synthesized from the boronic acid as a precursor or functionalized with boronic acid can lead to the prohibition of human coronaviruses (HCoV) and impede their attachment [40,96]. In another study, Tong and co-workers [97], demonstrated the hydrothermal synthesis of highly biocompatible CDs named Gly@CDs using Chinese herbal medicine to investigate their antiviral activity against PRRSV. The Gly@CDs inhibited the propagation of intracellular ROS induced by PRRSV infection. Besides, the replication and invasion of PRRSV were also inhibited by stimulating cells for regulating the expression of anti-viral genes (NOS3 and DDX53). Moreover, the anti-viral potential of Gly@CDs was investigated against PEDV (a coronavirus model) through immunofluorescence assay.
Fig. 2.
CCM CDs viral infection mechanism. Reprinted with permission from Ref. [41] Copyright (2018) American Chemical Society.
However, suppose the virus invades the host cell by interacting with specific cell membrane receptors. In that case, the viral infection can only be managed either by inhibiting the virus replication or by preventing budding. The replication of the virus might be inhibited by tailoring the enzymes required for replicating the viral genetic material. In line with this, Ting et al., reported that CCM-CDs result in suppressing the negative-strand RNA synthesis. Compared to the control group, the replication of PEDV in CCM-CDs treated Vero cells indicated reduced virus titers and decreased plaque numbers [41]. In another study, Du et al. [36], demonstrated the potential of CDs in inhibiting the replication of both RNA (PRRSV) and DNA (PSV) viruses, validated by the results of viral protein expression and virus titers. Besides, it was mentioned that CDs treatment induces the production of IFN as well as IFN stimulated genes (ISGs), leading to the inhibition of viral replication. Compared to untreated controls, the expressions of ISG-54, ISG-15, IP-10, and IFN-α, were 4.6, 11.8, 24.8, and 5 fold higher, respectively. Loczechin et al. [39], reported the synthesis of seven different functional CQDs and investigated their anti-viral activity against human coronavirus (HCoV-229E). These CQDs were divided into two different generations depending upon the precursors and functional groups. First-generation includes CQDs 1-4, synthesized utilizing citric acid and ethylenediamine as precursors. These CQDs were further functionalized with different boronic acid ligands and exhibited concentration-dependent virus inactivation behavior. On the other hand, second-generation includes CQDs 5-7, synthesized utilizing 4-aminophenyl boronic acid. Interestingly, both viral replication, as well as HCoV-229E entry inhibition, were observed with these CQDs. Recently, Garg et al. [93], reported the anti-viral potential of heteroatom co-doped and triazole functionalized CQDs against human coronaviruses. The authors proposed that these CDs might help in blocking the viral entry by disturbing the virus-host cell interaction. Additionally, viral enzymes like 3CLpro and helicase required for viral replication might be blocked, thereby controlling the viral infection. Therefore, it can be envisaged that CDs may be an efficient anti-viral agent. Complying with the study of CDs, functional AS-CDs can also be synthesized as a dual-purpose therapeutic agent. Altogether different pre-clinical and clinical trials revealed the anti-viral property of AS against viral fever, cold and flu infections, which strongly appraise its use in clinical settings.
3.4. AS-CDs abrogates inflammation and cytokine storm
Cytokine storm is the main culprit in making the COVID-19 more complicated and dreadful [98]. Reducing the expression of cytokines is a challenging task [99]. It can be very constructive to consider the application of phytotherapy-based approaches in the modulation of cytokine storms [100]. Numerous reports support the use of CDs as an anti-inflammatory agent and their capability to modulate the cytokine levels. In line with this, Zhang et al. [101], demonstrated the green synthesis of CDs through the pyrolysis method using Phellodendri chinensis Cortex (PCC) as the starting material for investigating acute kidney injury. The obtained CDs were found to be efficient in reducing the levels of anti-inflammatory cytokine (IL-10) and inflammatory cytokines (IL-1β). The proposed AS-based CDs can be used to tackle coronavirus-mediated inflammation and cytokine storms and as an imaging agent for diagnosis. In recent times, Fabián et al., assessed the effects of allicin in LPS stimulated (100 ng/mL) 3T3-L1 adipocytes, where it prevented the rise in the expression of pro-inflammatory cytokine genes such as IL-6, MCP-1, and Egr-1. It also reduced the phosphorylation of ERK1/2 protein, and gene expression profiling through microarray shows upregulation of genes involved in immunological responses and downregulation of cancer-associated genes. In fact, SAC, CA, uracil, DATS, DADS, and other AS compounds can restrain the activity of cellular transcription factor NFκB, which is a master regulator of cytokine related genes. Thus, inhibiting the transcription of numerous cytokine genes such as, IL-1β, IL-6, IL-12, TNF-α, and MCP-1 which are implicated in pro-inflammatory responses [102]. Thus, employing AS-CDs in the COVID-19 could reduce the burden of cytokine storm.
3.5. AS-CDs as a potent immunomodulator
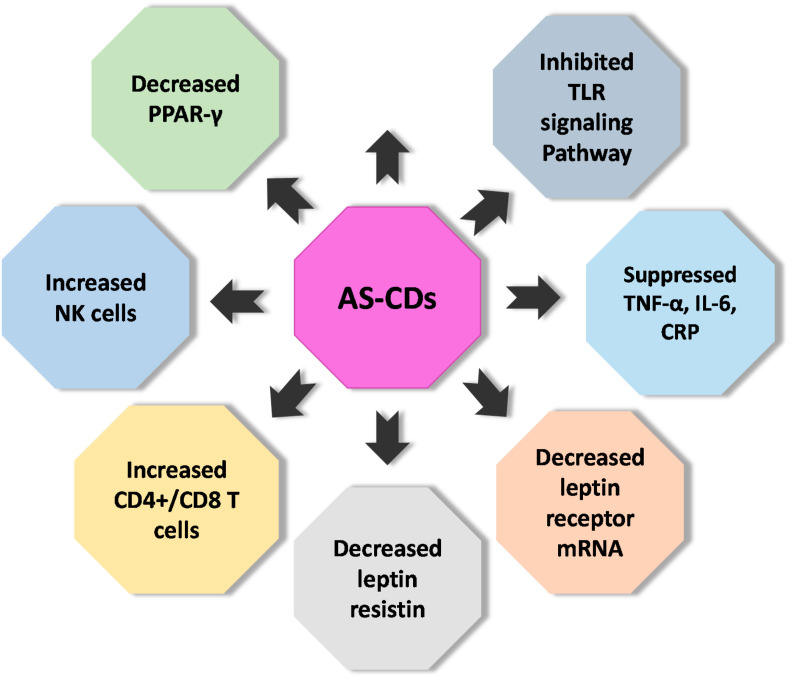
AS is a potent immunomodulator, and reports suggest that dietary meals having AS can modulate immunity. Recently in a clinical trial, it was reported that meal or diet having aged AS extract can strongly alter adult inflammatory status and immune system with obesity. Nantz et al., performed a double-blinded randomized clinical trial. Diminution of the severity of cold & flu, duration of symptoms, antioxidant levels, defined innate-like lymphocytes (γδ-T cell and NK cell), number of incidences, and work/school missed were assessed. AS capsule daily showed a remarkable reduction in the above parameters (Fig. 3 ). The findings showed significant proliferation of NK cells and γδ-T cells, antioxidant levels in serum, cytosolic GSH, and drop in the secretion of inflammatory cytokines in AS consuming cohort [49,64,103].
Fig. 3.
The pharmacological effects of AS-CDs on different immune system-related cells and biomolecules.
3.6. AS-CDs as a diagnostic agent
The employability of CDs as a diagnostic agent relies on their rapid and easy traceability at a particular wavelength. The inherent fluorescence imaging capability of CDs allows them to be utilized as an imaging probe in studying the lifecycle and structures of different microorganisms including viruses in a variety of biosensors and chemosensors. In this context, the visual and rapid detection of influenza A virus, including the H3N2 and H1N1 strains, has been reported through carbon nanoparticles (CNPs) when incorporated with lateral flow immunoassay. The CNPs have been utilized as reporters, which accumulate in the test zone because of sandwich immunocomplex formation (antibody-antigen-CNPs-antibody), resulting in the direct visual of analytical signals [104]. In another work by Liu et al., different colored CDs (blue and cyan) were obtained from young barley leaves as precursors to utilize them in selective cell imaging. The blue-colored CDs were found to enter selectively into the cytoplasm of PK-15 cells and indicated the anti-viral activity towards the pseudorabies virus. However, the cyan-colored CDs were found to disperse all over the cells, including the nucleus [105]. Apart from that, few research groups have also addressed the role of CDs derived from AS in a significant way for cellular imaging through its distinct characteristics [61,69]. Interestingly, the shape and size of these AS-CDs can be controlled to a great extent during synthesis to provide unique features to the targeted CDs, which might help eradicate the infectious viruses, including SARS-CoV-2. In general, different top-down and bottom-up methods can be employed for synthesizing the CDs. Among them, hydrothermal carbonization method is widely utilized because of nontoxic, low temperature environmentally friendly process with minimal cost.
4. Implications of the hypothesis
The theranostic outcome of any reliable disease management system relies on the formulation of a suitable dosage form and its administration route. With the advent of nanotechnology, the development of an effective theranostic agent could be scoped to curb the menace of infectious diseases. Considering the case of COVID-19, that spreads rapidly through the respiratory tract (biofluids, droplets, cough, etc.) and primarily targets the human lungs. This crisis could be managed by adopting the following measures; (a) impeding the dissemination of COVID-19 by decontaminating the surrounding surfaces and, (b) developing a promising theranostic agent with known safety and of natural origin with an imaging modality. In this context, the proposed AS-CDs could be employed as an agent that has the capabilities to damage the viral membrane as well as decompose the constituent organic components. Another postulate of the study is the targeted delivery of the anti-viral drug to the lungs along with the diagnostic capability for the effective treatment of COVID-19. In this progression, AS-CDs could be suggested as a theranostic agent owing to their anti-viral and inherent bioimaging properties. The bioimaging capability of AS-CDs might be helpful in providing diagnostic utilities by fluorescing the SARS-CoV-2 virus inside the lungs. The delivery of these theranostic CDs can be practiced in the form of colloidal dispersions through nebulization, or a solid powdered form of AS-CDs can be inhaled by means of pressurized metered-dose inhalers. Such practices might be helpful in providing the direct delivery of AS-CDs to the lungs and capable enough to suppress the progression of COVID-19 infection.
5. Conclusions
AS has been known to be a potent antimicrobial agent for a very long time. The recent studies contributed to the additional anti-inflammatory and immune-modulatory effects of AS to the list. AS extracted compounds have participated in cytokine secretion modulation, which is the primary basis of its therapeutic modulatory effects. While the two main concerns for the introduction of nanobiomedicine in food science are biocompatibility and safety, it also has been studied that the AS-derived nanocompounds or AS-derived CDs may be highly cytocompatible. Besides, the CDs are considered for their higher suitability for bioimaging. They may effectively be taken up by the macrophages, where they may be able to monitor and reduce the inflammation concurrently. In a nutshell, the proposed hypothesis could be practiced as a pre-clinical measure for the deterrence of COVID-19 infection. The AS-CDs can act as an efficient theranostic agent and capable of attenuating the progressive symptoms of COVID-19 by enhancing the respiratory functionalities. Based on the above discussion, it might be suggested that AS-CDs may be helpful in providing prevention measures by blocking the transmission of infection. The proposed hypothesis can be utilized as a futuristic approach in providing a stepping-stone towards the development of a novel theranostic tool that might be highly efficient in managing the ongoing crisis of COVID-19.
Declaration of competent interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
A.K and R.P are thankful to the Ministry of Education, Government of India, for the fellowship. GP acknowledge the Department of Biotechnology, Government of India (BT/PR 25095/NER/95/1011/2017), Shastri Institutional Collaborative Research Grant (SICRG) 2020-21 and the Science & Engineering Research Board (SERB), Government of India (grant number: TAR/2019/000245).
References
- 1.Lai C.-C., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V., et al. Critical neurological features of COVID-19: role of imaging methods and biosensors for effective diagnosis. Sens. Int. 2021:100098. doi: 10.1016/j.sintl.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S., et al. Indians vs.COVID-19: the scenario of mental health. Sens. Int. 2020;1:100038. doi: 10.1016/j.sintl.2020.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allawadhi P., et al. Medical Hypotheses; 2021. Decorin as a Possible Strategy for the Amelioration of COVID-19; p. 110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun W., et al. vol. 11. 2020. (Management of Immunity Alteration-Induced Chronic Pain during the Coronavirus Disease-2019 (COVID-19) Pandemic). 2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khurana A., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38:101142. doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangayasami A., et al. Influence of nanotechnology to combat against COVID-19 for global health emergency: a review. Sens. Int. 2021:100079. doi: 10.1016/j.sintl.2020.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das K., et al. Sensitivity and elasticity analysis of novel corona virus transmission model: a mathematical approach. Sens. Int. 2021;2:100088. doi: 10.1016/j.sintl.2021.100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukkitgar S.D., Shetti N.P., Aminabhavi T.M. Electrochemical investigations for COVID-19 detection-A comparison with other viral detection methods. Chem. Eng. J. 2020:127575. doi: 10.1016/j.cej.2020.127575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shetti N.P., et al. Invasion of novel corona virus (COVID-19) in Indian territory. Sens. Int. 2020;1:100012. doi: 10.1016/j.sintl.2020.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan Z., et al. New Microbes and New Infections; 2020. Diagnostic Approaches and Potential Therapeutic Options for Coronavirus Disease (COVID-19) p. 100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection. Ann. Intern. Med. 2021;174(3):344–352. doi: 10.7326/M20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khurana I., et al. Can bilirubin nanomedicine become a hope for the management of COVID-19? Med. Hypotheses. 2021;149:110534. doi: 10.1016/j.mehy.2021.110534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrahari R., et al. Update vision on COVID-19: structure, immune pathogenesis, treatment and safety assessment. Sens. Int. 2021;2:100073. doi: 10.1016/j.sintl.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purohit B., et al. Biosensor nanoengineering: design, operation, and implementation for biomolecular analysis. Sens. Int. 2020;1:100040. [Google Scholar]
- 16.Sharma S., et al. Current treatment protocol for COVID-19 in India. Sens. Int. 2020;1(2666–3511):100013. doi: 10.1016/j.sintl.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S., et al. Effect of Brownian motion on reduced agglomeration of nanostructured metal oxide towards development of efficient cancer biosensor. Biosens. Bioelectron. 2018;102:247–255. doi: 10.1016/j.bios.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan R., et al. Optimization, fabrication, and characterization of four electrode-based sensors for blood impedance measurement. Biomed. Microdevices. 2021;23(1):9. doi: 10.1007/s10544-021-00545-4. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan R., et al. Four electrode-based impedimetric biosensors for evaluating cytotoxicity of tamoxifen on cervical cancer cells. RSC Adv. 2021;11(2):798–806. doi: 10.1039/d0ra09155c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro B.V., et al. Biosensors for the detection of respiratory viruses: a review. Talanta Open. 2020;2:100007. doi: 10.1016/j.talo.2020.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo-Henríquez L., et al. Biosensors for the detection of bacterial and viral clinical pathogens. Sensors. 2020;20(23) doi: 10.3390/s20236926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suleman S., et al. Point of care detection of COVID-19: advancement in biosensing and diagnostic methods. Chem. Eng. J. 2021;414:128759. doi: 10.1016/j.cej.2021.128759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shetti N.P., et al. Conventional and nanotechnology-based sensing methods for SARS coronavirus (2019-nCoV) ACS Appl. Bio Mater. 2021;4(2):1178–1190. doi: 10.1021/acsabm.0c01545. [DOI] [PubMed] [Google Scholar]
- 24.Shen C.-L., et al. Chemiluminescent carbon dots: synthesis, properties, and applications. Nano Today. 2020;35:100954. [Google Scholar]
- 25.Boakye-Yiadom K.O., et al. Carbon dots: applications in bioimaging and theranostics. Int. J. Pharm. 2019;564:308–317. doi: 10.1016/j.ijpharm.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 26.Mishra V., et al. Carbon dots: emerging theranostic nanoarchitectures. Drug Discov. Today. 2018;23(6):1219–1232. doi: 10.1016/j.drudis.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Du J., et al. Carbon dots for in vivo bioimaging and theranostics. Small. 2019;15(32):1805087. doi: 10.1002/smll.201805087. [DOI] [PubMed] [Google Scholar]
- 28.Ge J., et al. Carbon dots with intrinsic theranostic properties for bioimaging, red-light-triggered photodynamic/photothermal simultaneous therapy in vitro and in vivo. Adv. Healthc. Mater. 2016;5(6):665–675. doi: 10.1002/adhm.201500720. [DOI] [PubMed] [Google Scholar]
- 29.Chandra S., et al. Synthesis, functionalization and bioimaging applications of highly fluorescent carbon nanoparticles. Nanoscale. 2011;3(4):1533–1540. doi: 10.1039/c0nr00735h. [DOI] [PubMed] [Google Scholar]
- 30.Gopinath P., et al. Cancer Nanotheranostics. Springer; 2015. Cancer nanotheranostics; pp. 1–93. [Google Scholar]
- 31.Sciortino A., Cannizzo A., Messina F. vol. 4. 2018. p. 67. (Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response). 4. [Google Scholar]
- 32.Georgakilas V., et al. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015;115(11):4744–4822. doi: 10.1021/cr500304f. [DOI] [PubMed] [Google Scholar]
- 33.Kalkal A., et al. Biofunctionalized graphene quantum dots based fluorescent biosensor toward efficient detection of small cell lung cancer. ACS Appl. Bio Mater. 2020;3(8):4922–4932. doi: 10.1021/acsabm.0c00427. [DOI] [PubMed] [Google Scholar]
- 34.Kadian S., et al. Effect of sulfur doping on fluorescence and quantum yield of graphene quantum dots: an experimental and theoretical investigation. Nanotechnology. 2019;30(43):435704. doi: 10.1088/1361-6528/ab3566. [DOI] [PubMed] [Google Scholar]
- 35.Huang S., et al. Benzoxazine monomer derived carbon dots as a broad-spectrum agent to block viral infectivity. J. Colloid Interface Sci. 2019;542:198–206. doi: 10.1016/j.jcis.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Du T., et al. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon. 2016;110:278–285. [Google Scholar]
- 37.Ju E., et al. Specific inhibition of viral MicroRNAs by carbon dots-mediated delivery of locked nucleic acids for therapy of virus-induced cancer. ACS Nano. 2020;14(1):476–487. doi: 10.1021/acsnano.9b06333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W., et al. Carbon dots: surface engineering and applications. J. Mater. Chem. B. 2016;4(35):5772–5788. doi: 10.1039/c6tb00976j. [DOI] [PubMed] [Google Scholar]
- 39.Łoczechin A., et al. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl. Mater. Interfaces. 2019;11(46):42964–42974. doi: 10.1021/acsami.9b15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Innocenzi P., Stagi L. Carbon-based antiviral nanomaterials: graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020;11(26):6606–6622. doi: 10.1039/d0sc02658a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ting D., et al. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1(10):5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- 42.Sharma V., Tiwari P., Mobin S.M. Sustainable carbon-dots: recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B. 2017;5(45):8904–8924. doi: 10.1039/c7tb02484c. [DOI] [PubMed] [Google Scholar]
- 43.Chatzimitakos T.G., Stalikas C.D. In: Handbook of Nanomaterials in Analytical Chemistry. Mustansar Hussain C., editor. Elsevier; 2020. 1 - carbon nanodots from natural (re)sources: a new perspective on analytical chemistry; pp. 3–28. [Google Scholar]
- 44.Meng W., et al. Biomass-derived carbon dots and their applications. Energy Environ. Mater. 2019;2(3):172–192. [Google Scholar]
- 45.Omar S.H., Al-Wabel N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharmaceut. J. 2010;18(1):51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris J.C., et al. Antimicrobial properties of Allium sativum (garlic) Appl. Microbiol. Biotechnol. 2001;57(3):282–286. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- 47.El-Saber Batiha G., et al. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 2020;12(3):872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fratianni F., et al. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.) J. Funct. Foods. 2016;21:240–248. [Google Scholar]
- 49.Rouf R., et al. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: a systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020;104:219–234. doi: 10.1016/j.tifs.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lissiman E., Bhasale A.L., Cohen M. Garlic for the common cold. Cochrane Database Syst. Rev. 2014;2014(11) doi: 10.1002/14651858.CD006206.pub4. CD006206-CD006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayaz E., Alpsoy H.C. [Garlic (Allium sativum) and traditional medicine] Turk. Parazitoloji Derg. 2007;31(2):145–149. [PubMed] [Google Scholar]
- 52.Rawat P.S., et al. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021;139:111708. doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- 53.Anchi P., et al. The role of plant-derived products in pancreatitis: experimental and clinical evidence. Phytother Res. 2017;31(4):591–623. doi: 10.1002/ptr.5792. [DOI] [PubMed] [Google Scholar]
- 54.Ide N., Lau B.H.S. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-κB activation. J. Nutr. 2001;131(3):1020S–1026S. doi: 10.1093/jn/131.3.1020S. [DOI] [PubMed] [Google Scholar]
- 55.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2) doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khurana A., et al. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine. 2019;14(14):1805–1825. doi: 10.2217/nnm-2018-0318. [DOI] [PubMed] [Google Scholar]
- 58.Khurana A., et al. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation. Nanomed. Nanotechnol. Biol. Med. 2019;18:54–65. doi: 10.1016/j.nano.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 59.Paraskevis D., et al. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020;79:104212. doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavallito C.J., Bailey J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944;66(11):1950–1951. [Google Scholar]
- 61.Yang C., et al. Theranostic carbon dots derived from garlic with efficient anti-oxidative effects towards macrophages. RSC Adv. 2015;5(118):97836–97840. [Google Scholar]
- 62.Moutia M., et al. Allium sativum L. regulates in vitro IL-17 gene expression in human peripheral blood mononuclear cells. BMC Compl. Alternative Med. 2016;16(1):1–10. doi: 10.1186/s12906-016-1365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sung J., et al. Garlic (Allium sativum) stimulates lipopolysaccharide-induced tumor necrosis factor-alpha production from J774A. 1 murine macrophages. Phytother Res. 2015;29(2):288–294. doi: 10.1002/ptr.5253. [DOI] [PubMed] [Google Scholar]
- 64.Donma M.M., Donma O. The effects of allium sativum on immunity within the scope of COVID-19 infection. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109934. 109934-109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khubber S., et al. Garlic (Allium sativum L.): a potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020;19(1):124. doi: 10.1186/s12937-020-00643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thuy B.T.P., et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5(14):8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Saber Batiha G., et al. Chemical constituents and pharmacological activities of garlic (allium sativum L.): a review. Nutrients. 2020;12(3):872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Das P., et al. Converting waste Allium sativum peel to nitrogen and sulphur co-doped photoluminescence carbon dots for solar conversion, cell labeling, and photobleaching diligences: a path from discarded waste to value-added products. J. Photochem. Photobiol. B Biol. 2019;197:111545. doi: 10.1016/j.jphotobiol.2019.111545. [DOI] [PubMed] [Google Scholar]
- 69.Zhao S., et al. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl. Mater. Interfaces. 2015;7(31):17054–17060. doi: 10.1021/acsami.5b03228. [DOI] [PubMed] [Google Scholar]
- 70.Sun C., et al. Synthesis of nitrogen and sulfur Co-doped carbon dots from garlic for selective detection of Fe3+ Nanoscale Res. Lett. 2016;11(1):110. doi: 10.1186/s11671-016-1326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee T.-Y., et al. Carbon dot nanoparticles exert inhibitory effects on human platelets and reduce mortality in mice with acute pulmonary thromboembolism. Nanomaterials. 2020;10(7) doi: 10.3390/nano10071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumaraguruparan R., et al. Attenuation of N-methyl-N'-nitro-N-nitrosoguanidine induced genotoxicity and oxidative stress by tomato and garlic combination. Life Sci. 2005;76(19):2247–2255. doi: 10.1016/j.lfs.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Khurana A., et al. It's all about the spaces between cells: role of extracellular matrix in liver fibrosis. Ann. Transl. Med. 2020;9(8):728. doi: 10.21037/atm-20-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imai J., et al. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60(5):417–420. doi: 10.1055/s-2006-959522. [DOI] [PubMed] [Google Scholar]
- 75.Sener G., et al. Aqueous garlic extract alleviates ischaemia-reperfusion-induced oxidative hepatic injury in rats. J. Pharm. Pharmacol. 2005;57(1):145–150. doi: 10.1211/0022357055209. [DOI] [PubMed] [Google Scholar]
- 76.Augusti K.T. Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.) Indian J. Exp. Biol. 1996;34(7):634–640. [PubMed] [Google Scholar]
- 77.Fukao T., et al. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem. Toxicol. 2004;42(5):743–749. doi: 10.1016/j.fct.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 78.Sharma S., et al. Beneficial effect of garlic (anna sativum) on L-arginine induced chronic pancreatitis in rats. Pancreas. 2013;42:1381–1382. [Google Scholar]
- 79.Gedik N., et al. Long-term administration of aqueous garlic extract (AGE) alleviates liver fibrosis and oxidative damage induced by biliary obstruction in rats. Life Sci. 2005;76(22):2593–2606. doi: 10.1016/j.lfs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 80.Pearson W., et al. Pilot study investigating the ability of an herbal composite to alleviate clinical signs of respiratory dysfunction in horses with recurrent airway obstruction. Can. J. Vet. Res. 2007;71(2):145–151. [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L., Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C. 2020;112:110924. doi: 10.1016/j.msec.2020.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iannazzo D., et al. Graphene quantum dots based systems as HIV inhibitors. Bioconjugate Chem. 2018;29(9):3084–3093. doi: 10.1021/acs.bioconjchem.8b00448. [DOI] [PubMed] [Google Scholar]
- 83.Chung I., et al. Synergistic antiyeast activity of garlic oil and allyl alcohol derived from alliin in garlic. J. Food Sci. 2007;72(9):M437–M440. doi: 10.1111/j.1750-3841.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 84.Eja M.E., et al. A comparative assessment of the antimicrobial effects of garlic (Allium sativum) and antibiotics on diarrheagenic organisms. Southeast Asian J. Trop. Med. Publ. Health. 2007;38(2):343–348. [PubMed] [Google Scholar]
- 85.Groppo F.C., et al. Antimicrobial activity of garlic against oral streptococci. Int. J. Dent. Hyg. 2007;5(2):109–115. doi: 10.1111/j.1601-5037.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- 86.Konaklieva M.I., Plotkin B.J. Antimicrobial properties of organosulfur anti-infectives: a review of patent literature 1999-2005. Recent Pat. Anti-Infect. Drug Discov. 2006;1(2):177–180. doi: 10.2174/157489106777452683. [DOI] [PubMed] [Google Scholar]
- 87.Amin M., Kapadnis B.P. Heat stable antimicrobial activity of Allium ascalonicum against bacteria and fungi. Indian J. Exp. Biol. 2005;43(8):751–754. [PubMed] [Google Scholar]
- 88.Cañizares P., et al. Allyl-thiosulfinates, the bacteriostatic compounds of garlic against Helicobacter pylori. Biotechnol. Prog. 2004;20:397–401. doi: 10.1021/bp034143b. [DOI] [PubMed] [Google Scholar]
- 89.Sivam G.P., et al. Helicobacter pylori--in vitro susceptibility to garlic (Allium sativum) extract. Nutr. Cancer. 1997;27(2):118–121. doi: 10.1080/01635589709514512. [DOI] [PubMed] [Google Scholar]
- 90.Cellini L., et al. Inhibition of Helicobacter pylori by garlic extract (Allium sativum) FEMS Immunol. Med. Microbiol. 1996;13(4):273–277. doi: 10.1111/j.1574-695X.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 91.Chowdhury A.K., et al. Efficacy of aqueous extract of garlic & allicin in experimental shigellosis in rabbits. Indian J. Med. Res. 1991;93:33–36. [PubMed] [Google Scholar]
- 92.Kenawy S., et al. Evaluation of TNF-α serum level in patients with recalcitrant multiple common warts, treated by lipid garlic extract. Dermatol. Ther. 2014;27(5):272–277. doi: 10.1111/dth.12136. [DOI] [PubMed] [Google Scholar]
- 93.Garg P., et al. Exploring the role of triazole functionalized heteroatom co-doped carbon quantum dots against human coronaviruses. Nano Today. 2020;35:101001. doi: 10.1016/j.nantod.2020.101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kotta S., et al. vol. 7. 2020. (Exploring the Potential of Carbon Dots to Combat COVID-19). 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barras A., et al. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces. 2016;8(14):9004–9013. doi: 10.1021/acsami.6b01681. [DOI] [PubMed] [Google Scholar]
- 96.Khanal M., et al. Boronic acid-modified lipid nanocapsules: a novel platform for the highly efficient inhibition of hepatitis C viral entry. Nanoscale. 2015;7(4):1392–1402. doi: 10.1039/c4nr03875d. [DOI] [PubMed] [Google Scholar]
- 97.Tong T., et al. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16(13):1906206. doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allawadhi P., et al. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35:100982. doi: 10.1016/j.nantod.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allawadhi P., et al. Potential of electric stimulation for the management of COVID-19. Med. Hypotheses. 2020;144:110259. doi: 10.1016/j.mehy.2020.110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Antonelli M., et al. Phytotherapic compounds against coronaviruses: possible streams for future research. Phytother Res. 2020;34(7):1469–1470. doi: 10.1002/ptr.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang M., et al. Protective effects of carbon dots derived from Phellodendri chinensis Cortex carbonisata against deinagkistrodon acutus venom-induced acute kidney injury. Nanoscale Res. Lett. 2019;14(1):377. doi: 10.1186/s11671-019-3198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quintero-Fabián S., et al. Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3T3-L1 adipocytes. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/381815. 381815-381815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allawadhi P., et al. Isoproterenol-induced cardiac ischemia and fibrosis: plant-based approaches for intervention. Phytother Res. 2018;32(10):1908–1932. doi: 10.1002/ptr.6152. [DOI] [PubMed] [Google Scholar]
- 104.Wiriyachaiporn N., et al. Carbon nanotag based visual detection of influenza A virus by a lateral flow immunoassay. Microchim. Acta. 2017;184(6):1827–1835. [Google Scholar]
- 105.Liu H., et al. Blue and cyan fluorescent carbon dots: one-pot synthesis, selective cell imaging and their antiviral activity. RSC Adv. 2017;7(45):28016–28023. [Google Scholar]