Abstract
In March 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-based infections were declared ‘COVID-19 pandemic’ by the World Health Organization. Pandemic raised the necessity to design and develop genuine and sensitive tests for precise specific SARS-CoV-2 infections detection. Nanotechnological methods offer new ways to fight COVID-19. Nanomaterials are ideal for unique sensor platforms because of their chemically versatile properties and they are easy to manufacture. In this context, selected examples for integrating nanomaterials and distinct biosensor platforms are given to detect SARS-CoV-2 biological materials and COVID-19 biomarkers, giving researchers and scientists more goals and a better forecast to design more relevant and novel sensor arrays for COVID-19 diagnosis.
Keywords: SARS-CoV-2, COVID-19 biomarkers, Colloidal nanoparticles, Interfaces
Graphical abstract

Introduction
Coronaviruses (CoVs) are large enveloped nonsegmented single-stranded RNA viruses responsible for causing respiratory tract disorders in humans. A new coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) named (COVID-19) emerged in Wuhan, China, in 2019 [1]. SARS-CoV-2 is a beta-CoV family member and causes mild upper respiratory diseases together with four ubiquitous family members, namely 229E, OC43, NL63, and HKU1 [2]. SARS-CoV-2 pathogenic strain is a combination of SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) viruses. The metagenomic analysis revealed that the SARS-CoV-2 has approximately 80% similarity with SARS-CoV and two other SARS-like CoVs recognized in bats (bat-SL-CoVZC45 and bat-SL-CoVZXC21) [2]. The appearance of new CoVs in humans depends on primary factors for the frequent emergence of the CoVs related to the wide distribution, high prevalence, high genetic diversity, frequent genome recombination, and human–animal interface activities [3].
Although COVID-19 showed a low fatality rate in humans, it was highly infectious, with raw reproduction number (R0) ranging from 1.4 to 6.47 [4]. The primary clinical symptoms in these studies are fever, cough, shortness of breath, and myalgia [5]. Soon after infection, acute respiratory distress syndrome was observed in patients, followed by a severe cytokine storm, which frequently accounted for COVID-19-related deaths [3]. Owing to the severity of the infection as a pandemic, highly sensitive, precise, distinctive, and specific biosensor platforms should be developed by the researchers.
Nanotechnology offers various in vitro and in vivo solutions to combat SARs-CoV-2-based infections. Nanomaterials are a critical component in these technologies and crucial in detecting or transforming biochemical interactions [6]. In this review, various nanomaterial-based particular biosensor arrays are given in a select platform.
Biological materials and indicators of COVID-19 diagnosis
Biological materials for SARS-CoV-2 testing
The whole SARS-CoV-2 itself, as well as its structural proteins, such as spike (S) glycoprotein, nucleocapsid (N), matrix (M), and a small envelope (E), are used as antigens in several testing methods. According to previous reports, S-protein is crucial for the adhesion to host cells, in which the S-protein mediates the interaction of the angiotensin-converting enzyme-2 (ACE2) with its receptor-binding domain. S and N proteins are invaluable antigen biomarkers for serological testing in the detection of COVID-19. The produced IgM, IgG, and IgA against S and N proteins are mostly used to develop various serological assay methods [7].
Typically, preferred targets for SARS-CoV-2 genetic assays are spike glycoprotein S gene, envelope protein E gene, nucleocapsid protein N genes, nonstructural RNA-dependent RNA polymerase gene, and open reading frame of the 1a/bc ORF1a/b gene. E and ORF1a/b genes are most frequently used in molecular tests to detect SARS-CoV-2 infections [8].
Indicators of COVID-19
According to Ponti and his coworkers (2020), many biochemical, hematological, and inflammatory indicators are found to be accompanying COVID-19 infections [9]. The leading hematological indicators in COVID-19 infections are counts of lymphocytes and neutrophils and their ratios [9]. Several studies reported lymphopenia in COVID-19 patients in severe cases [10,11]. As other biomarkers, several biochemical indicators such as troponin, creatine kinase, and D-dimer are present. According to Yao and coworkers (2020) and Tersalvi and coworkers (2020), the disease severity is directly related to the D-dimer levels [12,13]. Garg and coworkers (2021) postulated that elevated levels of inflammatory biomarkers such as procalcitonin (PCT), C-reactive protein (CRP), ferritin (FT), and interleukin-6 (IL-6) are indicators of the severity of COVID-19 infections [14].
Standard test methods for SARS-CoV-2
Detection of the virus is a crucial weapon in combating the COVID-19 pandemic and plays a decisive role in isolating infected individuals early to avoid the risk of transmission. Early, affordable, and accurate diagnosis of SARS-CoV-2 is an urgent need to ease the early intervention efforts and slowing down the transmission of this pandemic disease. The standard test for COVID-19 is quantitative reverse transcription-polymerase chain reaction (qRT-PCR) [15] as a gold standard. Also, nanoparticles (NPs) get their importance in nucleic acid–based methods with their usage in RNA purification techniques. With this in mind, a simple and modern magnetic NP (MNP) protocol is proposed for assisted SARS-CoV-2 RNA extraction by using zinc ferrite MNPs with carboxyl-containing polymers functionalized surface [16]. In another approach, a simplified three-step method for the production of large quantities of MNPs for RNA extraction in response to developing countries’ needs was used. Poly-NH2-MNPs are optimized and scaled for high-quality SARS-CoV-2 RNA extraction [17]. Owing to the simple and inexpensive nature of MNP-based extraction methods, these can be a suitable substitute for traditional techniques.
The neutralizing antibody (IgM and IgG) detection based on enzyme-linked immunosorbent assay, which depends on their interactions with antigenic compounds of virus ligand, is another traditional technique for the detection of COVID-19 neutralizing antibody testing. However, unwanted false-negative results, long response time, and low sensitivity are their main drawbacks. The development of serological tests to accurately show the previous infection and the following immunity to SARS-CoV-2 will be critical for epidemiological research and monitoring tests [18].
Owing to the need for fast, sensitive, and low-consumption diagnosis of COVID-19, nanotechnology can play a crucial role in detecting SARS-CoV-2. Mechanical, electronic, and magnetic properties of NPs are fundamental in diagnostics endeavors [19].
Nanoparticle-based detection strategies of COVID-19
Diagnostic methods are essential strategies that could be used to tackle COVID-19, isolating the infected patients, and prevent dissemination [20]. Several techniques are available to detect SARS-CoV-2, such as enzyme-linked immunosorbent assay or RT-PCR from nasopharyngeal swabs obtained from noses and throats of patients [7]. These techniques rely on their interaction with the detection of ligand complementary strands. The main disadvantage of these methods is their false-negative results and low sensitivity [21].
Nanomaterials are the most current components in SARS-CoV-2 testing because they have distinct properties, such as their large surface-to-volume ratios. Owing to the extensive surface interactions of the nanomaterials and the sensor and the analyte, these materials allow rapid and reliable detections with high sensitivity [19].
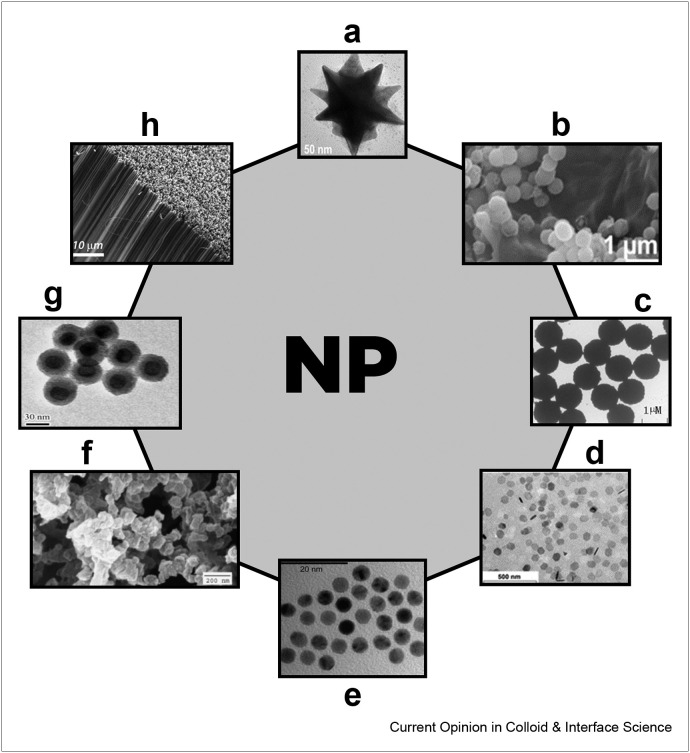
SARS-CoV-2 virus infection triggers many inflammatory, biochemical, and hematological biomarkers. Various inflammation markers, such as PCT, CRP, FT, and IL-6 have been intricately linked to this infection. Detection of these markers can also help to understand the level of disease. By monitoring these biomarkers, infections with low SARS-CoV-2 RNA are also predicted in cases where traditional tests miss. In this area, NPs are also used to develop several COVID-19 diagnosis methods [14]. Several types of NPs used in SARS-CoV-2 detection methods are shown in Figure 1 .
Figure 1.
Distinctive colloids and nanoparticles used in SARS-CoV-2 detection and COVID-19 diagnosis. (a) Transmission electron micrographs of gold nanospike; reprinted with permission from [22]. (b) Scanning electron microscopy (SEM) images for CuWO4@rGO hybrids; reprinted with permission from [23]. (c) Transmission electron microscopy (TEM) of the superparamagnetic beads; reprinted with permission from [24]. (d) TEM image of single-crystalline graphene quantum dots; reprinted with permission from [25]. (e) TEM image of size distributed gold nanoparticles (AuNPs); reprinted with permission from [26]. (f) SEM image of aggregated cellulose nanoparticles with an average diameter of 80 nm; reprinted with permission from [27]. (g) TEM image of lanthanide nanoparticles with lanthanide complexes doped in the silica shell; reprinted with permission from [28]. (h) Field emission-scanning electron microscopy (FE-SEM) image of a single-walled carbon nanotubes (SWCNTs) forest; reprinted with permission from [29].
Gold nanoparticles
Within NPs, gold NPs are universally used in testing strategies because of their unique features. Their advantages include easy to synthesize, stability in time, size variability, biocompatibility, enhancement of catalytic size by silver deposition, conductivity, electrochemical properties, and ability to quench fluorescence. Therefore, NPs become highly effective materials for developing fluorescent sensors [30]. Gold NPs have peculiar optical properties, which is named localized surface plasmon resonance (LSPR). LSPR is used in biosensors because of its light adjustability and scattering of wavelengths in the visible spectrum. LSPR spectra of gold NPs change as a function of the refractive index of their surrounding media. Thus, NP aggregation degree is considered a decisive factor for their applicability in biological detection [31]. Alafeef and his coworkers (2020) reported a fast, inexpensive, easy-to-apply, and quantitative paper-based electrochemical sensor chip that enables digital detection of SARS-CoV-2 genetic material by using gold NPs coated with highly specific antisense oligonucleotides that target the viral nucleocapsid phosphoprotein gene with a limit of 6.9 copies/μl [32]. Sensor probes are immobilized on a paper-based electrochemical platform to provide a nucleic acid tester with a screen that can be registered with a simple handheld reader [32].
Broughton and coworkers (2020) used the powerful genome editing techniques of CRISPR/CAS9 for detecting viral RNA of SARS-CoV-2 from nasopharyngeal swabs in a lateral flow assay (LFA) using gold nanoparticles (AuNPs) as a label [33]. Ventura and coworkers (2020) recently developed a colorimetric biosensor for the mass testing of COVID-19 with a sensitivity and specificity higher than 95% for more than 90 samples [34]. In another application, conjugated colloidal gold NPs with streptavidin are used for an reverse transcription loop-mediated isothermal amplification (RT-LAMP) combined with a flow-through assay for CoV detection [35]. Biotin/FITC-labeled amplicons bind to streptavidin-functionalized gold NPs, and by using an antibody-coated strip, the color formation can be detected. The assay takes 35 min to detect limit of detection (LOD) 10 copies/μl of MERS-CoV RNA. Also, this approach produced high specificity without cross-reactivity for other CoVs [35]. In another research, gold NPs were conjugated with thiol-modified antisense oligonucleotides to detect the RNA coding for the N-protein of the SARS-CoV-2, calorimetrically. Results were seen within 10 min with an LOD less than 0.18 ng/μl [36].
One of the most frequently used techniques to detect neutralizing antibodies of SARS-CoV-2 is the lateral flow immunoassay technique. The technique is used in several tests developed for COVID-19 antibodies, virus antigens, and nucleic acid–based methods [37]. Roda and his coworkers (2021) developed a dual optical/chemiluminescent type of an lateral flow immunoassay (LFIA) immunosensor for IgA in serum and saliva, which depends on capturing SARS-CoV-2 antibodies with a recombinant nucleocapsid antigen [38]. A simple device using a smartphone camera measures the color signal provided by anti-human IgA labeled nanogold particles. For ultrasensitive chemiluminescence transduction, they used a portable contact imaging device based on cooled charged-coupled device (CCD) and measured the light signal that resulted from the Horseradish peroxidase-labeled anti-human IgA reaction with an H2O2/luminol/enhancer substrate [38].
Gold nano-spikes are a promising class of molded NPs because these multibranched NPs with sharp tips have exciting plasmonic properties with a broad plasmon band in the near-infrared region [22]. Funari and coworkers (2020) used the LSPR to design an opto-microfluidic sensor with gold nano-spikes for detecting SARS-CoV-2 spike protein in human plasma in less than 30 min. This label-free method achieves the LOD of ~0.08 ng mL−1 [39].
Gold NPs are also used in the detection of COVID-19 biomarkers several times. For this purpose, an ultrasensitive sandwich electrochemical immunosensor was developed for PCT detection [40]. The immunosensor is formed using delaminated-sulfur doped MXene (dS-Ti3C2TX MXene)-modified carbon electrode containing the PCT antibody-conjugated AuNPs used as an immunosensor array. Carboxylated graphitic carbon nitride was then conjugated with PCT Ab2 as a signal amplifier. Electrochemical impedance spectroscopy and cyclic voltammetry (CV) are performed to detect carboxylated graphitic carbon nitride catalytic activity against H2O2 and are used directly as a redox probe. The linearity range and LOD results were calculated as 0.01–1.0 pg ml−1 and 2.0 fg ml−1, respectively [40].
An optical assay–based aggregation of AuNPs coated with two complementary aptamers for different IL-6 target fragments in a sandwich type was developed for IL-6 detection. Recognition and binding of the complementary pair of aptamers caused the corresponding functionalized NPs to be aggregate. The aggregation of AuNPs gives a visible color, which is changed from red to pink with a difference in absorbance 520–540 nm. The test works in a concentration range from 3.3 to 125 μg ml−1 IL-6, and the detection limit is 1.95 μg ml−1 [41].
An origami paper–based electrochemical immunoassay for detecting CRP was developed with multiple electrode modification steps onto a graphene-modified screen-printed carbon electrode (G/SPCE). Gold NPs electrodeposited a self-assembled monolayer of l-cysteine modifying G/SPCE. A capture anti-CRP does CRP measurement on the modified electrode by using hexacyanoferrate as a redox probe. The increase in impedance is found as a concentration range of 0.05–100 μg ml−1 CRP with 15 ng ml−1 LOD [42].
Magnetic nanoparticles
MNPs are indispensable tools for the development of several diagnosis and analysis methods. MNPs have been used in several methods in COVID-19 diagnosis and development of detection tests against SARS-CoV-2. The prominent use of a MNP is to separate the viral RNA for diagnostic purposes, of which iron oxide NPs are mostly used because of their high magnetic capability and easy manufacturing procedure [43]. It was recently demonstrated that a carboxyl group–attached polyaminoester-coated MNP could detect SARS-CoV-2 RNA with high purity performance within 30 min. The polyaminoester-coated MNP proved high binding capacity with viral RNA, and the pcMNP−RNA complexes were amplified by real time PCR [44].
Direct virus detections are also shown recently [45]. The study shows the detection of SARS-CoV-2 by measuring the magnetic particle spectroscopy (MPS) signal of MNPs functionalized with the SARS-CoV-2 spike protein-antibody with a detection limit of 0.084 nM (5.9 fmol). An MPS system was used to measure the MPS signal from functionalized MNPs. Also, AC sensitivity spectra were measured with a rotating magnetic field system to evaluate the Brownian relaxation time [45].
A carbon-based screen-printed electrode (SPE) as a sensor combined with an MB-based electrochemical test with a 19-ng mL−1 detection limit was reported for SARS-CoV-2 in saliva. SARS-CoV-2 proteins, S and N, were used as the target analyte to develop a sandwich experiment with antibodies immobilized on MBs for S or N protein from nasopharyngeal swab samples [46].
On the other hand, magnetic bead–based methods are developed for detecting COVID-19 biomarkers. A new electrochemical lab-on-chip magnetoimmunoassay was proposed for the determination of PCT. The method depends on the online presence of the biorecognition event and detection on a microfluidic thin-film gold electrode chamber operating at E = −0.20 V, compared to Au in less than 15 min with a detection limit and quantification at 0.02 ng ml−1 and 0.05 ng ml−1, respectively [47]. The same group, this time, developed an electrochemical immunoassay based on magnetic beads in a sandwich format with two different approaches: The first approach consisted of disposable SPCE and microfluidic chips (EMC-Au) with integrated Au electrodes. This approach offers good sensitivity with LOD of 0.1 and 0.04 ng mL−1 for SPE-C and EMC-Au, respectively, with a total test time less than 20 min [48].
Quantum dots
Quantum dots (QDs) possess unique optical and electrical properties, so they have been used to detect several viruses [49]. As the QDs also have an excellent plasmonic property, a star-shaped chiroplasmonic gold NP is conjugated with it to develop an optical biosensor to detect the influenza virus [50]. For this anti-HA and anti-NA, the influenza virus antibodies are immobilized on the gold NP on QDs' surfaces. The recombinant protein of the respective virus is mixed to form a nanohybrid complex with the gold NP and QDs, and after that, a plasmon-excitation interaction is created on the nanohybrids, which enhances the chiral optical response of the solution. In this way, the viral recombinant protein is detected with the measured circular dichroism response of the solution. By these techniques, a group of researchers achieved an LOD of less than 1 pg ml−1 for influenza virus and CoV [50]. A similar method was developed to aid a magnetoplasmonic fluorescent biosensor depending on the zirconium QDs and Fe3O4–Au core−shell magnetoplasmonic NPs. Once the virus is added to the mixture, the conjugated zirconium QDs and magnetoplasmonic NPs formed magnetoplasmonic−fluorescent nanohybrid structures. The virus presence is showed by the nanohybrids complexes' photoluminescence properties in which LOD was 79.15 EID/50 μl in blood samples [51]. The possibility of QDs ability to increase the sensitivity of surface plasmon resonance (SPR)-based fluoroimmunoassay for the detection of norovirus was established [52]. Such types of novel fluorescent-based QDs can be used to develop a practical method of COVID-19 detection [53].
A spike recombinant receptor-binding domain conjugated with fluorescent QDs was used to develop an imaging probe. Energy transfer quenching with ACE2-conjugated gold NPs in solution is used to monitor the interaction. Neutralizing antibodies and recombinant human ACE2 block quenching with a specific binding interaction within nanomolar potency. The QD probe can then be used to study the inhibitors of SARS-CoV-2 spike and ACE2 receptor binding proteins in human cells [54].
In a novel study, QDs from biosurfactant stabilized/functionalized tungsten disulfide (WS2–B) and their effect on a FT immune sensor were described [55]. Extensive characterization is performed using analytical techniques to investigate further possible SPEs applications, functionalized with WS2–B-QDs. CV and differential pulse voltammetry techniques are combined for electrochemical immunosensing of FT with detection limits of 3800 ng ml−1 in differential pulse voltammetry and 6048 ng ml−1 in CV [55]. The same group also tested FT detection by hexagonal boron nitride QDs. Electrochemical impedance spectroscopy is used in a platform where hexagonal boron nitride QDs are a functionalized detection platform for screen-printed electrodes. The developed immune sensor has a linear range from 10 to 2000 ng/ml−1 FT concentration with a detection limit of 1.306 ng/ml−1 [56].
Wu and his coworkers (2018) report an LFIA for quantitative and rapid detection of CRP based on CdSe/ZnS QDs by measuring the fluorescence intensity immediately afterward with a fluorescence immunoassay analyzer. QDs are synthesized using the ‘green’ phosphine-free method with a wide detection range between 0.5 ng ml−1 and 1 μg ml−1, and the analytical detection limit is 0.3 ng ml−1 [57].
Graphene nanoparticles
The two-dimensional hexagonally arranged carbon has a promising future because of its high ultrasensitive characteristic of surface area and electrical and ionic mobility [58]. A field-effect transistor (FET) sensor to detect SARS-CoV-2 in human nasopharyngeal swabs was reported recently [59]. In this wet transfer method, graphene is placed onto the SiO2/Si substrate using the photolithographic method. The graphene surface is coated with SARS-CoV-2 spike protein antibody to prepare the FET-based analytical system. The clinical samples of COVID-19 patients are applied on the FET-based biosensing device, and LOD is reported as 1 fg/mL [59].
Carbon nanotubes
In a new approach, reactive oxygen species (ROS), harmful to many viruses, including CoVs, are used to detect COVID-19 diagnosis [46]. Recently, metal-decorated SWCNTs adsorbed with hydrogen peroxide (H2O2) were investigated for their possible use in the design of inactivation of viruses on surfaces [60]. Pt, Pd, Ni, Cu, Rh, or Ru decorated SWCNTs are tested for detecting SARS-CoV-2. The detection of separate H2O2 molecule adsorption on bare and metal-functionalized SWCNTs is based on the density functional theory [60]. Based on H2O2 production and adsorption on the CNTs, an electrochemical sensor to detect the ROS concentration in the sputum sample was developed [61]. The accuracy and sensitivity of the sensor are found at 97% and 95%, respectively. The COVID-19 ROS detection approach contains an electrochemical ROS/H2O2 system. It consists of a portable automatic electrochemical reader and a single-use sensor for detection. The sensor is made by growing multiwalled carbon nanotubes on the tip of steel needles [61].
An optical nanosensor based on noncovalently functionalized SWCNTs with ACE2 proteins was developed recently [62]. The nanosensor principle depends on the SARS-CoV-2 spike protein presence, which leads to an increase in nanosensor fluorescence within 90 min of exposure to the spike protein. Sensor responded in the salivary samples and virus transport environments. It is shown that ACE2-SWCNT nanosensors retain detection capability in a modified surface with a 73% fluorescence turn-on response within 5 s of exposure to 35 mg/L SARS-CoV-2 virus-like particles [62].
Specialized nanoparticles
Lanthanide complexes and NPs are excellent fluorescent probes in a wide variety of biomedical research because of their unique fluorescence emission peaks, bright and monochromatic emissions, high quantum efficiency, large Stokes drift, and long fluorescence lifetimes [63]. Immense research has been conducted to improve the credibility and sensitivity of point-of-care tests for SARS-CoV-2 by using lanthanide NPs. A recent study reported the utilization of lanthanide-doped polystyrene NPs and detection with a portable fluorescence reader obtained from RT-PCR [64].
Wang and coworkers (2020) offered an LFA test kit based on SARS-CoV-2 nucleoprotein modified with selenium NPs to detect anti-SARS-CoV-2 IgM and IgG in human serum [65]. The results were visible to naked eyes within 10 min. Selenium NPs are synthesized by l-ascorbic acid reduction of selenic acid and conjugated with the nucleoprotein. The IgM and IgG detection limits are 20 ng mL−1 and 5 ng mL−1, respectively. No cross-reaction with other antinuclear antibodies and influenza viruses is observed using the designed test kit [65].
A specific LFIA-based biosensor for COVID-19 was developed using a cellulose nanosphere platform. SARS-CoV-2 nucleocapsid protein–specific single-chain variable fragment crystallizable fragment fusion antibodies are generated by phage display technology. Single-chain variable fragment crystallizable fragment antibodies are specific to SARS-CoV-2-NP antigen with high affinity, and three specific antibody pairs are used on the cellulose nanosphere–based LFIA kit. The detection limits for the best pair are found as 2 ng of antigen protein and 2.5 × 104 pfu of the cultured virus [66].
Individual NPs are also used in COVID-19 biomarker detections. A conductive nano-hybrid material consisting of Au-NPs and molybdenum disulfide, functionalized with an ionic liquid (Au-NPs/IL-MoS2), was produced and used to immobilize primary CRP antibodies. Later, 1,5-diaminonaphthalene was adsorbed via p-p stacking on graphene oxide, which is modified with iridium NPs as a label for labeling secondary CRP antibodies. Immunosensor shows a linear detection ranging from 0.01 to 100 ng ml−1 and LOD of 3.3 pg ml−1 [67].
A photoelectrochemical PCT immunosensor platform based on photoactive CuWO4 nanospheres, grown on reduced graphene oxide layers (CuWO4 @ rGO), was constructed [23]. The immunosensor is used for PCT detection based on an electrocatalytic mechanism. The recorded reduction of the photocatalytic oxidation signal was observed within the concentration range varying from 10 pg mL−1 to 50 ng mL−1 [23].
A label-free electrochemical immunosensor was developed to detect PCT using toluidine blue-functionalized-NiFe-Prussian blue analog nanocubes (NiFe PBA-nanocubes @ TB) as signal amplifiers. NiFe PBA nanocubes were synthesized with a selective method. The electrochemical performance of nanocubes is increased by functionalization with TB. The developed immune sensor showed a linear detection range of 0.001–25 ng ml−1 and a LOD of 3 × 10−4 ng ml−1 [68].
A quantitative Eu–Np combined LFIA method for the detection of IL-6 is created by Huang and his coworkers (2020) in serum [69]. To develop the quantitative IL-6 detection kit, a double antibody sandwich immunofluorescence assay is tested on LFIA with a wide linear range (2–500 pg/ml and good sensitivity (0.37 pg/ml) [69].
Recently developed exclusive interfaces-based biosensors for SARS-CoV-2 testing and COVID-19 diagnosis
Nanobiosensors are a valuable alternative to conventional laboratory devices for clinical and environmental analysis. They can combine the unique electrical and optical properties of nanomaterials with biological or synthetic molecules that are used as receptors for the selective detection of all types of analytes [70]. In Figure 2 , distinct nanobiosensor platforms are given in the detection of SARS-CoV-2.
Figure 2.
Fascinating nanobiosensor platforms in the detection of SARS-CoV-2. (a) Lateral flow test platform; reprinted with permission from [71]. (b) Paper-based electrochemical impedance spectroscopy nanobiosensor; reprinted with permission from [72]. (c) Nanoplasmonic sensor in generic microplate reader and point-of-care device; reprinted with permission from [73]. (d) Real-time optomagnetic detection of SARS-CoV-2; reprinted with permission from [74]. (e) Field-effect transistor-based biosensor for SARS-CoV-2 detection; reprinted with permission from [59]. (f) Exhaled breath sampling and analysis procedure; reprinted with permission from [75]. (g) The COVID-19 ROS diagnosis system with three electrodes coated by functionalized multiwall carbon nanotubes; reprinted with permission from [61].
Breath analyzers
Two recent reports showed that the potential volatile organic compounds (VOCs) marker contain higher ethyl butyrate levels than healthy control [76,77]. The markers used by breath analyzers are reported to be related to COVID-19 disruption of respiratory biochemistry resulting from ketosis, inflammatory processes, and gastrointestinal effects [78]. A multiplex sensor was designed based on nanomaterials linked with organic ligands, which created a versatile sensor layer that can change its structural properties when exposed to VOCs and cause electrical resistance changes [79]. Inorganic nanomaterials were responsible for electrical conductivity within the sensor, and the organic film element provided sites for VOC adsorption. When exposed, VOCs spread to the sensor layer and reacted with functional groups covering organic segments or inorganic nanomaterials. The interactions caused a change in volume via swelling or shrinkage in the nanomaterial film. The nanomaterial layer's exposure to VOCs caused a rapid charge transfer to/from the inorganic nanomaterial, resulting in fluctuations in the measured conductivity even if no structural changes occurred in the sensor layer. The presence of geonosis was used for distinguishing COVID-19 surgery patients from noninfected participants based on VOC patterns in two exhaled samples [80].
Plasmonic nanosensors
Nanoplasmonic biosensor technologies based on SPR and LSPR are now available and commercialized in different areas [81]. Also, the acquisition of real nanoplasmonic point-of-care biosensors includes and enhances microfluidic systems that minimize or automate sample processing and easy-to-use readings SARS-CoV-2 detections [81].
Recently, a fast and online nanoplasmonic resonance sensor for detecting SARS-CoV-2 without sample preparation was developed in a single step. Within 15 min, only 370 vp/ml is detected, and the linearity is ranging from 0 to 107 vp/ml. The measurements obtained from a generic microplate reader and a handset combined with a smartphone showed that cheap and fast detection methods could be applied quickly in normal clinical conditions and resource-constrained settings [73].
As an alternative to RT-PCR, a plasmonic biosensor combined with a plasmonic photothermal effect and SPR was recently developed [71]. Gold nanomaterials linked with complementary DNA sequences to detect hybridized cDNAs of SARS-CoV-2 are used. This device can detect cells using a cell surface protein reaction with specific antibodies conjugated to AuNPs [82].
A multilayer grid-linked fluorescent plasmonic biosensor platform is being developed to measure antibodies against COVID-19 in human blood serum and dried bloodstain samples [83]. The array gives antibody-antigen binding interactions by showing 100% selectivity and 86.7% sensitivity in serum IgG levels against Spike S1, Spike S1S2, and N protein COVID-19 antigens. The test was repeated on other sample matrices, such as dried bloodstain samples, to demonstrate the effectiveness of the test. The test also successfully detects IgM, IgG, and IgA antibody-antigen interactions and multiple immunoglobulin isotypes [83].
Novel plasmonic biosensor platforms are also developed to detect COVID-19 biomarkers. A novel plasmonic biosensor platform was developed [84]. The study presented a label-free method for the quantitative detection of IL-6. Cobalt ferrite and magnetite NPs are combined in a hydrogel matrix by applying an external magnetic field, and detected antibodies are conjugated on their surfaces. The interaction of IL-6 with the antibody created a blue shift in the resonance wavelength after cobalt ferrite addition and magnetite-based MPC at a 50 pg/ml concentration, and the reflection intensity increased up to 50% and 44%, respectively [84].
PCT detection is achieved with an uncoated segment of glass fiber conjugated with an anti-PCT captured antibody to obtain a fiber sensor, and then the anti-PCT detection antibody is conjugated to AuNPs to provide nanoplasmonic probes. A comprehensive linear response range is achieved from 1 pg/ml to 100 ng/ml and LOD of 7.3 fM for PCT within 15 min [85].
Electrochemical sensors
Various non-SARS-CoV-2 electrochemical biosensors were developed using potentiometric or amperometric readings. An SPCE biosensor device (eCovSens) using screen printing and compared to a commercial fluorine-doped tin oxide electrode consisted of a potentiostat [86]. Researchers tested their new device for sensitivity, specificity, detection time, sample volume, portability, and COVID-19 antigen detection. The ECovSens device consists of a biosensor element (nCovid-19 Ab), a transducer (carbon electrode), and an internal device to detect voltage changes. Conjugated gold NPs recognize virus particles and catalyze the electrochemical signal by improving the electrical conductivity. COVID-19 particles trapped on the modified electrode caused current changes proportional to the interested analyte concentration. Virus particles were successfully captured and detected at 90 fM (LOD) using prickly saliva samples and found within 10–30 s [86].
A fast and sensitive method for detecting and measuring D-dimer, a COVID-19 biomarker, present in high concentrations in patients, was developed [87]. The method relies on an immunosensor, based on a single-chain antibody immobilized on the transducer surface. The redox activity of an N-alpha-bis (carboxymethyl)-1-lysine/Cu2+ complex bound to a polypyrrole skeleton is taken as the signal. D-dimer presence is monitored with a linear ranging from 0.1 ng mL−1 to 500 ng mL−1. An LOD of 100 pg mL−1 in PBS is measured by electrochemical impedance spectroscopy [87].
A simple, inexpensive, and label-free electrochemical immunoassay was developed to measure serum CRP concentrations with an immunosensor strip put on an anti-CRP functionalized AuNPs modified SPCE. The measurement is based on the reduction in the redox indicator Fe3+/Fe2+ oxidation current, resulting from the immune reaction between CRP and anti-CRP with a linear range of 0.4–200 with a detection limit of 0.15 nM [88].
An entropy-based enhanced electrochemiluminescence method was developed by Fan and coworkers (2021) to detect the RNA-dependent RNA polymerase gene of SARS-CoV-2 [89]. DNA tetrahedron on the surface of the electrode is modified for the design of the biosensor. Biosensor shows an ‘electrochemiluminescence on’ state with LOD up to 2.67 fM [89].
Field-effect transistors
FET biosensors show high sensitivity and selectivity through biorecognition on the conducting channel. An FET device is functionalized with an anti-SARS-CoV-2 spike protein antibody to detect the SARS-CoV-2 where graphene sheets were used, with a detection limit of 1 fg/mL in phosphate-buffered saline and 100 fg/mL in clinical transport medium. Changes in surface charge upon binding lead to transducable differences in source-drain current measurements [59].
A label-free immunosensor, based on a SWCNT FET device, was developed to detect CRP. The immune reaction principle relied on direct adsorption of CRP-specific antibodies (anti-CRP) onto SWCNT networks with a linear range of 10−4-102 μg/ml [90].
Conclusion
Unlike the traditional sensing platforms, nanomaterial-based biosensors offer much-required selectivity, sensitivity, reliability, reproducibility, and robustness while being cost-effective in sample measurements. Biosensor platforms based on nanomaterials are becoming more critical to a clinical diagnostic approach. Extensive studies are dedicated to displaying the applicability of various nanomaterial-based sensors against a wide variety of RNA viruses. Based on these findings, it is safe to say that nanotechnology will play an efficient and particular role in the effective surveillance of COVID-19 in the future. Therefore, it is clear that nanomaterials can be used more effectively in the management of SARS-CoV-2, which can be achieved by developing various nanomaterial-based sensor arrays such as plasmonic-based, paper-based, FET-based, and electrochemical-based, LFA-based methods. Also, significant challenges are not given for the usage of QDs and VOC biosensors. This can be a real future perspective of the nanomaterial and distinct interface-based nanotechnological methods for the detection of SARS-CoV-2 and COVID-19 diagnosis. In future, reliable and cost-effective biomedical devices are expected to provide affordable, practical, and easy-to-use products accessible to all countries. The internet of telephone and smart technologies are outstanding tools that must be used to monitor, control, and predict the evolution of COVID-19 pandemics.
Author contribution
Both authors contributed equally.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This review comes from a themed issue on Hot Topic: COVID-19
Edited by Reinhard Miller and Libero Liggieri
References
- 1.Lu R., Wu X., Wan Z., Li Y., Jin X., Zhang C. A novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Int J Mol Sci. 2020;21:2826. doi: 10.3390/ijms21082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Xing-Lou Y., Xian-Guang W., Ben H., Zhang L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microb Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (Lond Engl) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Hitzky E., Darder M., Wicklein B., Ruiz-Garcia C., Martín-Sampedro R., et al. Nanotechnology responses to COVID-19, dv. Healthcare Mater. 2020;9:2000979. doi: 10.1002/adhm.202000979. [DOI] [PubMed] [Google Scholar]
- 7.Saatçi E. Newly developed diagnostic methods for SARS-CoV-2 detection. Turk J Biochem. 2020;45:465–474. [Google Scholar]
- 8.Cui F., Zhou H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens Bioelectron. 2020;165:112349. doi: 10.1016/j.bios.2020.112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020:1–11. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article gives an valuable information on COVID-19 related biomarkers in a particular order.
- 10.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:1–10. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanshan C., Zhou M., Dong X., Qu J., Gong F., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y., Cao J., Wang Q., Shi Q., Liu K., et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8:1–11. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tersalvi G., Vicenzi M., Calabretta D., Biasco L., Pedrazzini G., Winterton D. Elevated troponin in patients with Coronavirus Disease 2019 (COVID-19): possible mechanisms. J Card Fail. 2020;26:470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Sharma A.L., Singh S. Advancement in biosensors for inflammatory biomarkers of SARS-CoV-2 during 2019–2020. Biosens Bioelectron. 2021;171:112703. doi: 10.1016/j.bios.2020.112703. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article is related to critical COVID-19 biomarkers detection methods in newly developed biosensors, which is a perfect reference for the development of related biosensors.
- 15.Barreto H.G., de Pádua Milagres F.A., de Araújo G.C., Daúde M.M., Benedito V.A. Diagnosing the novel SARS-CoV-2 by quantitative RT-PCR: variations and opportunities. J Mol Med (Berl) 2020;17:1–10. doi: 10.1007/s00109-020-01992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somvanshia B.S., Prashant B.K., Sarafa T.S., Somwanshic S.B., Shejulc S.B., Jadhava K.M. Multifunctional nano-magnetic particles assisted viral RNA-extraction protocol for potential detection of COVID-19. Mater Res Innovat. 2020:1–6. [Google Scholar]
- 17.Chacón-Torres J.C., Reinoso C., Navas-León D.G., Briceño1 S., González G. Optimized and scalable synthesis of magnetic nanoparticles for RNA extraction in response to developing countries' needs in the detection and control of SARS-CoV-2. Nature Res, Scientific Reports. 2020;10:19004. doi: 10.1038/s41598-020-75798-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng M.P., Papenburg J., Desjardins M., Kanjilal S., Quach C. Diagnostic testing for severe acute respiratory syndrome–related coronavirus 2. A narrative review. Ann Intern Med. 2020;172:727–735. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokhtarzadeh A., Eivazzadeh-Keihan R., Pashazadeh P., Hejazi M., Gharaatifar N., et al. Nanomaterial-based biosensors for detection of pathogenic virus. Trends Anal Chem. 2017;97:445–457. doi: 10.1016/j.trac.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J., Kupferschmidt K. Labs scramble to produce new coronavirus diagnostics. Science. 2020;14:727. doi: 10.1126/science.367.6479.727. [DOI] [PubMed] [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]; This helpful article compares standard test methods according to their sensitivity and specificity.
- Rahman D.S., Chatterjee H., Ghosh S.K. Excess surface energy at the tips of gold nanospikes: from experiment to modeling. J Phys Chem C. 2015;119:14326–14337. [Google Scholar]; The article gives excessive information about gold nanospikes structural properties.
- 23.Abbas Z., Soomro R.A., Kalwar N.H., Tunesi M., Willander M., et al. In situ growth of CuWO4 nanospheres over graphene oxide for photoelectrochemical (PEC) immunosensing of clinical biomarker. Sensors. 2020;20:148. doi: 10.3390/s20010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C., Liu Z., Wang D., Li L., Hu P., et al. Generation of internal-image functional aptamers of okadaic acid via magnetic-bead SELEX. Mar Drugs. 2015;13:7433–7445. doi: 10.3390/md13127066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.H., Kim D.Y., Lee J., Lee S.B., Han H., et al. Synthesis of single-crystalline hexagonal graphene quantum dots from solution chemistry. Nano Lett. 2019;19:5437–5442. doi: 10.1021/acs.nanolett.9b01940. [DOI] [PubMed] [Google Scholar]
- 26.Raliya R., Saha D., Chadha T.S., Raman B., Biswas P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci Rep. 2017;7:44718. doi: 10.1038/srep44718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Elder T.J., Pu Y., Ragauskas A.J. Facile synthesis of spherical cellulose nanoparticles. Carbohydr Polym. 2007;69:607–611. [Google Scholar]
- 28.Lia Z., Zhang Y. Facile synthesis of lanthanide nanoparticles with paramagnetic, down and up-conversion properties. Nanoscale. 2010;2:1240–1243. doi: 10.1039/c0nr00073f. [DOI] [PubMed] [Google Scholar]
- 29.Miller T.S., Ebejer N., Guell A.G., Macpherson J.V., Unwin P.R. Electrochemistry at carbon nanotube forests: sidewalls and closed ends allow fast electron transfer. Chem Commun. 2012;48:7435–7437. doi: 10.1039/c2cc32890a. [DOI] [PubMed] [Google Scholar]
- 30.Khan M.S., Vishakante G.D., Siddaramaiah H. Gold nanoparticles: a paradigm shift in biomedical applications. Adv Colloid Interface Sci. 2013;199:44–58. doi: 10.1016/j.cis.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Fong K.E., Yung L.Y.L. Localized surface plasmon resonance: a unique property of plasmonic nanoparticles for nucleic acid detection. Nanoscale. 2013;5:12043–12071. doi: 10.1039/c3nr02257a. [DOI] [PubMed] [Google Scholar]
- Alafeef M., Dighe K., Moitra P., Pan D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano. 2020;14:17028–17045. doi: 10.1021/acsnano.0c06392. [DOI] [PMC free article] [PubMed] [Google Scholar]; The manuscript consists of an attractive, efficient and rapid method to detect SARS-CoV-2 RNA with an electrochemical biosensor chip with AuNP usage.
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article includes the niche technique between the several detection methods of SARS-CoV-2 nucleic acids with high sensitivity and rapid detection.
- 34.Ventura B.D., Cennamo M., Minopoli A., Campanile R., Censi S.B., et al. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sens. 2020;5:3043–3048. doi: 10.1021/acssensors.0c01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hang P., Wang H., Cao Z., Jin H., Chi H., et al. A rapid and specific assay for the detection of MERS-CoV. Front Microbiol. 2018;9:1101. doi: 10.3389/fmicb.2018.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moitra P., Alafeef M., Dighe K., Frieman M., Pan D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped Plasm∗onic nanoparticles. ACS Nano. 2020;14:7617–7627. doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S., Ma L., Zhou M., Li Y., Xia Y., et al. New opportunities for emerging 2D materials in bioelectronics and biosensors. Curr Opin Biomed Eng. 2020;13:32–41. [Google Scholar]
- Roda A., Cavalera S., Di Nardo F., Calabria D., Rosati S., et al. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens Bioelectron. 2021;172:112765. doi: 10.1016/j.bios.2020.112765. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study includes a simple device utilizing a smartphone camera that measures the color signal provided nanogold particles.
- Funari R., Chu K., Shen A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens Bioelectron. 2020;169:112578. doi: 10.1016/j.bios.2020.112578. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article has unique content for the usage of gold nanospikes in the detection of SARS-CoV-2 in less than 30 min.
- Medetalibeyoglu H., Beytur M., Akyıldırım O., Atar N., Yol M.L. Validated electrochemical immunosensor for ultra-sensitive procalcitonin detection: carbon electrode modified with gold nanoparticles functionalized sulfur doped MXene as sensor platform and carboxylated graphitic carbon nitride as signal amplification. Sensor Actuator B Chem. 2020;319:128195. [Google Scholar]; The study includes unique colloidal nanoparticle- distinctly modified carbon electrode usage in developing an electrochemical sensing of PCT.
- 41.Giorgi-Coll S., Marín M.J., Sule O., Hutchinson P.J., Carpenter Keri L.H. Aptamer-modified gold nanoparticles for rapid aggregation-based detection of inflammation: an optical assay for interleukin-6. Microchimica Acta. 2020;187:13. doi: 10.1007/s00604-019-3975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boonkaew S., Chaiyo S., Jampasa S., Rengpipat S., Siangproh W., Chailapakul O. An origami paper-based electrochemical immunoassay for the C-reactive protein using a screen-printed carbon electrode modified with graphene and gold nanoparticles. Mikrochim Acta. 2019;186:153. doi: 10.1007/s00604-019-3245-8. [DOI] [PubMed] [Google Scholar]
- 43.Ma C., Li C., Wang F., Ma N., Li X., et al. Magnetic nanoparticles-based extraction and verification of nucleic acids from different sources. J Biomed Nanotechnol. 2013;9:703–709. doi: 10.1166/jbn.2013.1566. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.02.22.961268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong J., Roesch E.L., Viereck T., Schilling M., Ludwig F. 2020. Rapid and sensitive detection of SARS-CoV-2 with functionalized magnetic nanoparticles. arXiv:2010.03886 [physics.med-ph] [DOI] [PubMed] [Google Scholar]
- 46.Fabiani L., Saroglia M., Galat`a G., De Santis R., Fillo S., et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens Bioelectron. 2021;171:112686. doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinero-Fernández Á., López M.Á., Escarpa A. An on-chip microfluidic-based electrochemical magneto-immunoassay for the determination of procalcitonin in plasma obtained from sepsis diagnosed preterm neonates. Analyst. 2020;145:5004–5010. doi: 10.1039/d0an00624f. [DOI] [PubMed] [Google Scholar]; The study contains a niche technique consisting lab-on-chip magnetoimmunoassay and gold NPs.
- 48.Molinero-Fernández Á., Moreno-Guzmán M., Ángel López M., Escarpa A. Magnetic bead-based electrochemical immunoassays on-drop and on-chip for procalcitonin determination: disposable tools for clinical sepsis diagnosis. Biosensors. 2020;10:66. doi: 10.3390/bios10060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H., Xu T., Li C.-W., Yang M. A microfluidic device with microbead array for sensitive virus detection and genotyping using quantum dots as fluorescence labels. Biosens Bioelectron. 2010;25:2402–2407. doi: 10.1016/j.bios.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed S.R., Nagy É., Neethirajan S. Self-assembled star-shaped chiroplasmonic gold nanoparticles for an ultrasensitive chiro-immunosensor for viruses. RSC Adv. 2017;7:40849–40857. [Google Scholar]
- 51.Ahmed S.R., Kang S.W., Oh S., Lee J., Neethirajan S. Chiral zirconium quantum dots: a new class of nanocrystals for optical detection of coronavirus. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashiba H., Sugiyama Y., Wang X., Shirato H., Higo-Moriguchi K., et al. Detection of norovirus virus-like particles using a surface plasmon resonance-assisted fluoroimmunosensor optimized for quantum dot fluorescent labels. Biosens Bioelectron. 2017;93:260–266. doi: 10.1016/j.bios.2016.08.099. [DOI] [PubMed] [Google Scholar]
- Manivannan S., Ponnuchamy K. Quantum dots as a promising agent to combat COVID-19. Appl Organomet Chem. 2020:e5887. doi: 10.1002/aoc.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]; The review gives a future perspective about the usage of QDs in COVID-19 diagnosis.
- 54.Gorshkov K., Susumu K., Chen J., Xu M., Pradhan M., et al. Quantum dot-conjugated SARS-CoV-2 spike pseudo-virions enable tracking of angiotensin converting enzyme 2 binding and endocytosis. ACS Nano. 2020;14:12234–12247. doi: 10.1021/acsnano.0c05975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg M., Chatterjee M., Sharmaa A.L., Singh S. Label-free approach for electrochemical ferritin sensing using biosurfactant stabilized tungsten disulfide quantum dots. Biosens Bioelectron. 2020;151:111979. doi: 10.1016/j.bios.2019.111979. [DOI] [PubMed] [Google Scholar]
- 56.Garg M., Rani R., Sharma A.L., Singh S. White graphene quantum dots as electrochemical sensing platform for ferritin. Faraday Discuss. 2021 doi: 10.1039/c9fd00111e. [Advance Article] [DOI] [PubMed] [Google Scholar]
- Wu R., Zhou S., Chen T., Li J., Shen H., Chai Y., Li L.S. Quantitative and rapid detection of C-reactive protein using quantum dot-based lateral flow test strip. Anal Chim Acta. 2018;1008:1–7. doi: 10.1016/j.aca.2017.12.031. [DOI] [PubMed] [Google Scholar]; The article represents a unique quantitative CdSe/ZnS QDs-based LFIA method for rapid detection of CRP.
- 58.Peña-Bahamonde J., Nguyen H.N., Fanourakis S.K., Rodrigues D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J Nanobiotechnol. 2018;16:75. doi: 10.1186/s12951-018-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S., Choi M., et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]; The article is a fundamental study when a researcher wants to work with FET sensors.
- 60.Aasi A., Aghaei S.M., Moore M.D., Panchapakesan B. Pt-, Rh-, Ru-, and Cu-Single-Wall carbon nanotubes are exceptional candidates for design of anti-viral surfaces: a theoretical study. Int J Mol Sci. 2020;21:5211. doi: 10.3390/ijms21155211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miripour Z.S., Sarrami-Forooshani R., Sanati H., Makarem J., Taheri M.S., et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens Bioelectron. 2020;165:112435. doi: 10.1016/j.bios.2020.112435. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study gives ROS-COVID-19 interaction in an electrochemical system, which is very narrative for the future studies.
- 62.Pinals R.L., Ledesma F., Yang D., Navarro N., Jeong S., et al. Rapid SARS-CoV-2 detection by carbon nanotube-based near-infrared nanosensors. medRxiv. 2020 Nov 4 doi: 10.1021/acs.nanolett.1c00118. 2020.11.02.20223404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li R., Li R., Liu X., Changa X., Feng X. Lanthanide complexes based on a conjugated pyridine carboxylate ligand: structures, luminescence and magnetic properties. RSC Adv. 2020;10:6192–6199. doi: 10.1039/c9ra10975g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z., Zhang Z., Zhai X., Li Y., Lin L., et al. Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal Chem. 2020;92:7226–7231. doi: 10.1021/acs.analchem.0c00784. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z., Zheng Z., Hu H., Zhou Q., Liu W., et al. A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab Chip. 2020;20:4255–4261. doi: 10.1039/d0lc00828a. [DOI] [PubMed] [Google Scholar]
- 66.Kim H., Lee J., Kim M.J., Park S.C., Choi M., et al. Development of a SARS-CoV-2-specific biosensor for antigen detection using scFv-Fc fusion proteins. Biosens Bioelectron. 2021;175:112868. doi: 10.1016/j.bios.2020.112868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Yang J., Yang T., Deng Y., Gu M., et al. Electrochemical detection of C-reactive protein using functionalized iridium nanoparticles/graphene oxide as a tag. RSC Adv. 2020;10:9723–9729. doi: 10.1039/c9ra10386d. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article combines novel modification methods on iridium NPs with GO to develop an immunosensor.
- 68.Gao Z., Li Y., Zhang C., Zhang S., Jia Y., et al. An enzyme-free immunosensor for sensitive determination of procalcitonin using NiFe PBA nanocubes@TB as the sensing matrix. Anal Chim Acta. 2020;1097:169–175. doi: 10.1016/j.aca.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Huang D., Ying H., Jiang D., Liu F., Tian Y., Du C., Zhang L., Pu X. Rapid and sensitive detection of interleukin-6 in serum via time-resolved lateral flow immunoassay. Anal Biochem. 2020;588:113468. doi: 10.1016/j.ab.2019.113468. [DOI] [PubMed] [Google Scholar]
- 70.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14:6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 71.O׳Farrell B. Lateral flow technology for field-based applications—basics and advanced developments. Top Companion Anim Med. 2015;30:139–147. doi: 10.1053/j.tcam.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 72.Li X., Qin Z., Fu H., Li T., Peng R., et al. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: an experimental approach. Biosens Bioelectron. 2020:112672. doi: 10.1016/j.bios.2020.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang L., Ding L., Zhou J., Chen S., Chen F., et al. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens Bioelectron. 2021;171:112685. doi: 10.1016/j.bios.2020.112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian B., Gao F., Fock J., Dufva M., Hansen M.F. Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens Bioelectron. 2020;165:112356. doi: 10.1016/j.bios.2020.112356. [DOI] [PubMed] [Google Scholar]
- 75.Nardi-Agmon I., Abud-Hawa M., Liran O., Gai-Mor N., Ilouze M., et al. Exhaled breath analysis for monitoring response to treatment in advanced lung cancer. J Thorac Oncol. 2016;11:827–837. doi: 10.1016/j.jtho.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Chen H., Qi X., Ma J., Zhang C., Feng H., Yao M. Breath-borne VOC biomarkers for COVID-19. medRxiv. 2020 doi: 10.1101/2020.06.21.20136523. [DOI] [Google Scholar]
- Lamote K., Janssen E., Schillebeeck E., Lapperre T.S., De Winter B.Y., Van Meerbeeck J.P. The scent of COVID-19: viral (semi-) volatiles as fast diagnostic biomarkers? J Breath Res. 2020;14 doi: 10.1088/1752-7163/aba105. [DOI] [PubMed] [Google Scholar]; The article gives a primary, fast and sensitive method for SARS-CoV-2 virus particles with VOC specimens.
- 78.Ruszkiewicz D.M., Sanders D., O'Brien R., Hempel F., Reed M.J., et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry-a feasibility study. EClinicalMedicine. 2020:100609. doi: 10.1016/j.eclinm.2020.100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B., Broza Y.Y., Li W., Wang Y., Wu S., et al. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano. 2020;14:12125–12132. doi: 10.1021/acsnano.0c05657. [DOI] [PubMed] [Google Scholar]; The article shows the crucial role of VOCs in the determination of SARS-CoV-2 virus particles with a versatile sensor layer.
- 80.Wintjens A.G.W.E., Kim F.H.H., Engelen S.M.E., Lubbers T., Savelkoul P.H.M., et al. Applying the electronic nose for pre-operative SARS-CoV-2 screening. Surg Endosc. 2020:1–8. doi: 10.1007/s00464-020-08169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M., Estevez M.C., Cardenosa-Rubio M., Astua A., Lechuga L.M. How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sens. 2020;5:2663–2678. doi: 10.1021/acssensors.0c01180. [DOI] [PubMed] [Google Scholar]; The study is a fast and online nanoplasmonic resonance sensor for detecting SARS-CoV-2 without sample preparation.
- 82.Dalal A., Mohan H., Prasad M., Pundir C.S. Detection methods for influenza A H1N1 virus with special reference to biosensors: a review. Biosci Rep. 2020;40 doi: 10.1042/BSR20193852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady N.C., Tokranova N., Minor A., Nikvand N., Strle K., et al. Multiplexed detection and quantification of human antibody response to COVID-19 infection using a plasmon enhanced biosensor platform. Biosens Bioelectron. 2021;171:112679. doi: 10.1016/j.bios.2020.112679. [DOI] [PMC free article] [PubMed] [Google Scholar]; In the article, a multilayer grid-linked fluorescent plasmonic (GC-FP) biosensor platform is being developed to measure antibodies against COVID-19 in human blood serum and dried bloodstain samples.
- 84.Khan M.A., Mujahid M., Looa S.C.J., Chamundeswaria V.N., et al. Nanophotonics based label free detection mechanism for real-time monitoring of interleukin-6. Nanoscale. 2020;12:9194–9207. doi: 10.1039/d0nr01151g. [DOI] [PubMed] [Google Scholar]
- 85.Chiang C., Huang T., Wang C., Huang C., Tsai T., et al. Fiber optic nanogold-linked immunosorbent assay for rapid detection of procalcitonin at femtomolar concentration level. Biosens Bioelectron. 2020;151:111871. doi: 10.1016/j.bios.2019.111871. [DOI] [PubMed] [Google Scholar]
- 86.Mahari S., Roberts A., Shahdeo D., Gandhi S. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.04.24.059204. [DOI] [Google Scholar]
- 87.Chebil S., Hafaiedh I., Sauriat-Dorizona H., Jaffrezic-Renault N., Errachid A. Electrochemical detection of d-dimer as deep vein thrombosis marker using single-chain d-dimer antibody immobilized on functionalized polypyrrole. Biosens Bioelectron. 2010;26:736–742. doi: 10.1016/j.bios.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 88.Thangamuthu M., Santschi C., Martin O.J.F. Label-free electrochemical immunoassay for C-reactive protein. Biosensors. 2018;8:34. doi: 10.3390/bios8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan Z., Yao B., Ding Y., Zhao J., Xie M., Zhang K. Entropy-driven amplified electrochemiluminescence biosensor for RdRp gene of SARS-CoV-2 detection with self-assembled DNA tetrahedron scaffolds. Biosens Bioelectron. 2021;178:113015. doi: 10.1016/j.bios.2021.113015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Justino C.I.L., Freitas A.C., Amaral J.P., Rocha-Santos T.A.P., Cardoso S., Duarte Armando C. Disposable immunosensors for C-reactive protein based on carbon nanotubes field effect transistors. Talanta. 2013;108:165–170. doi: 10.1016/j.talanta.2013.03.007. [DOI] [PubMed] [Google Scholar]