Abstract
Cases of cutaneous melanoma and controls were enrolled in a New Mexico population-based study; subjects were administered questionnaires concerning ultraviolet (UV) and inorganic arsenic (iAs) exposure. Historical iAs exposure was estimated. UV exposure estimates were also derived using geo-spatial methods. Drinking water samples were collected for iAs analysis. Blood samples were collected for DNA repair (Comet) and DNA repair gene polymorphism assays. Arsenic concentrations were determined in urine and toenail samples. UV exposures during the previous 90 days did not vary significantly between cases and controls. Mean (±SD) current home iAs drinking water was not significantly different for cases and controls (3.98 μg/L (±3.67) vs. 3.47 μg/L (±2.40). iAs exposure showed no effect on DNA repair or association with melanoma. Results did not corroborate a previously reported association between toenail As and melanoma risk. Arsenic biomarkers in urine and toenail were highly significantly correlated with iAs in drinking water. A UV-DNA repair interaction for UV exposure over the previous 7 to 90 days was shown; cases had higher DNA damage than controls at low UV values. This novel finding suggests that melanoma cases may be more sensitive to low level UV exposure than are controls. A UV-APEX1 interaction was shown. Subjects with the homozygous rare APEX1 DNA repair gene allele had a higher risk of early melanoma diagnosis at low UV exposure compared with those with the homozygous wild type or the heterozygote. Notably, a UV-arsenic interaction on inhibition of DNA repair was not observed at iAs drinking water concentrations below 10 ppb (μg/L).
Keywords: Arsenic, iAs, ultraviolet, melanoma, DNA repair, biomarkers, drinking water
1. Introduction
Chronic exposure to inorganic arsenic (iAs) has long been associated with increased incidence of non-melanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) (Platanias, 2009; Tokar et al., 2010; Chervona et al., 2012). Cutaneous malignant melanoma incidence is rising with the annual incidence rate increase world-wide for melanoma for Caucasians estimated at 3–7%)(Parkin et al., 2000); for 2002–2005, New Mexico ranked third among all US states in melanoma incidence for non-Hispanic whites (NAACCR, 2007). Increased melanoma incidence in Asian and Indian populations has not been detected in extensive studies in those areas of the world (Taiwan, Bangladesh) in which very high iAs exposure occur. These populations are known to be more resistant to ultra-violet (UV) radiation-induced melanoma than are Caucasians perhaps due to differences in skin color and genetic background. Evidence for the association of arsenic (As) exposure with increased risk for melanoma in Caucasian populations is less well established than that for non-melanoma skin cancers. Nonetheless, a suggestive, but not statistically significant, association between melanoma and ever use of arsenical pesticides has been reported (Dennis et al., 2010). An earlier study among white Iowans found a two-fold increase increased risk of melanoma for participants with elevated toenail total As (tAs) content (Beane-Freeman et al., 2004). In addition, a slight increase in melanoma risk was reported to be associated with soil As exposure in areas of economic disadvantage in Australia (Pearce et al., 2012).
UV exposure is a known etiological factor associated with the development of non-melanoma and melanoma skin cancers (IARC, 1992; Pfeifer and Besaratinia, 2012). Arsenic is a recognized in vivo co-carcinogen with UV in exacerbating development of non-melanoma (SCC) skin cancer in mice (Rossman et al. 2001; Rossman et al. 2002; Rossman et al. 2004; Rossman and Klein, 2011). Epidemiology evidence is available describing a positive association between solar UV and non-melanoma skin cancer in populations exposed to arsenic (Watanabe et al., 2001; Chen et al., 2003, 2006; Melkonian et al. 2011). Exposure to As and UV is known to induce oxidative stress (Wiencke et al., 1997; Yager and Wiencke, 1997; Cooper et al. 2009) and to inhibit repair of UV-induced DNA damage (Ding et al., 2008; Zhou et al., 2011). Inhibition of one of many DNA repair genes, poly(ADP-ribose)polymerase (PARP)-1 by As has been substantiated in a number of in vitro studies (Wiencke et al., 1997; Yager and Wiencke, 1997; Cooper et al., 2009; Ding et al. 2009). Activity of the base excision repair PARP-1 pathway is important in resolving oxidative lesions and DNA strand breaks associated with oxidative DNA damage associated with exposure to UV and As.
Many studies have shown that chronic intake of iAs in drinking water above 100–200 μg/L are associated with induction of a number of cancers including non-melanoma skin cancer (Tseng et al., 1968; Chen et al., 1985; Chen et al. 1986; Chen et al., 2009). However the relationship at low doses is currently unclear. No significant increase in a biomarker of DNA repair was seen in urine from 124 non-smoking individuals exposed below 40 μg/L As in drinking water (Burgess et al., 2008). A meta-analysis of bladder cancer indicated that low-level As exposure in drinking water alone did not appear to be a significant independent risk factor for bladder cancer (Mink et al., 2008).
A recent quantitative analysis of bladder cancer studies focusing on low level As drinking water exposures corroborated these findings (Tsuji et al. 2014). Baastrup et al. (2008) found no significant positive association between low exposure to arsenic in drinking water (0.05 to 25 μg/L) and risk for cancers of the lung, bladder, liver, kidney, prostate, colorectal, or melanoma skin cancer in a prospective study of 57,053 persons in Denmark.
Whereas UV levels are low in Denmark, New Mexico has very high ground UV levels due to high elevation (1350 – 2130 m for the four largest cities of the state) and low latitude. In addition, New Mexico has a history of naturally occurring As in drinking water sources at levels greater than 50 μg/L. Although New Mexico community water supplies are commonly in compliance with the 2006 US EPA As drinking water standard of 10 μg/L, private wells that serve approximately 10% of the New Mexico population are not required to be compliant under the Federal standard. Given that As is a known co-carcinogen with UV in exacerbating development of non-melanoma skin cancer, additional information is needed to clarify the effect of the potential interface of exposure to As and UV on cutaneous melanoma. This population-based case-control study provided an important opportunity to obtain new information on whether historically higher As exposures in addition to current exposures (principally via water consumption) contribute to melanoma initiation through DNA repair inhibitory mechanisms and possible DNA repair genetic susceptibility factors in a New Mexico non-Hispanic white population.
2. Materials and Methods
2.1. Subjects
Incident cases of melanoma were identified through the statewide New Mexico Tumor Registry which is part of the National Cancer Institute Surveillance Epidemiology and End Results (SEER) program. Cases were selected for enrollment and contacted after they were identified as state residents, aged 19–95, newly diagnosed with cutaneous melanoma (ICDO C44.0–9) between January 1, 2009 and December 31, 2011. All cases had confirmed pathological diagnosis and detailed information on their melanoma subtypes (most common were superficial spreading and nodular). Controls were selected from among individuals attending dermatology outpatient clinics and identified as having a benign nevus confirmed by a dermatopathologist. Subjects participated in the study under signed informed consent as approved by the University of New Mexico Health Sciences Center Human Research Protection Office. Subjects were administered a validated questionnaire that covered family history of melanoma, UV exposure patterns over their lifetime and a residential history (from which past arsenic and ambient UV exposure were derived). As described in the following sections, questionnaires were administered and home drinking water, blood, urine and toenail samples were collected from participants for subsequent analyses.
2.2. Questionnaires
A health and UV history exposure questionnaire was administered to cases and controls to determine demographic characteristics, phenotypic characteristics, family history, lifetime sun exposure history, residential history, outdoor activities and holidays all known to contribute to traditional risk for developing malignant melanoma. This questionnaire has been validated for assessment of lifetime personal sun exposure (Kricker et al., 2005). Residential location was recorded for each subject and used as described in later sections for historical UV and As exposure data reconstruction. An environmental questionnaire adapted from the New Mexico Department of Health Biomonitoring Project (unpublished) addressing personal arsenic exposure history and including current water source(s) (well or community water supply), as well as other potential environmental and occupational exposures was also administered to each subject.
2.3. Estimation of Ultra-Violet (UV) Exposure
Residential history by decade of life was ascertained via questionnaire. UV exposure was assessed both by questionnaire information on sun behavior and by estimating annual ground level UVA, UVB and erythemal UV (kJ/m2) adjusted for cloud cover at the residence location of each subject by decade using results from the Tropospheric Ultraviolet-Visible (TUV) model developed at the National Center for Atmospheric Research in Boulder, Colorado (Madronich et al. 2010). Erythemal UV estimates for the 1979–2000 period were used in participant exposure index calculations. The primary UV exposure index used was annual UV irradiance averaged over the lifetime to age at first melanoma diagnosis (Kricker et al. 2007). Current UV exposure data were obtained from the Integrated Surface Irradiance Study (ISIS) Network instrument located at the Albuquerque International Airport. UV flux readings were aggregated to daily values and averaged over 90, 60, 30, 14 and 7 days before blood was drawn from participants. ISIS data are calibrated to be representative of erythemally-weighted UV.
2.4. Arsenic Exposure Assessment
Current inorganic arsenic (iAs) exposure was assessed by measure of iAs in the drinking water, urine and toenail clippings of subjects as described in the following sections. Estimates of past iAs drinking water exposures were based on association of self-reported New Mexico residential history with past documented iAs drinking water levels in those geographic areas as available. Drinking water concentrations at past residences by decade outside of NM were estimated when feasible using US Geological Survey data and secondary databases compiled by the Environmental Working Group (EWG, 2009) and the Natural Resources Defense Council (NRDC, 2001) arsenic in community water supplies as described in the following sections.
2.4.1. Determination of iAs Concentration in Drinking Water
Current drinking water iAs exposure of study participants was based upon measured iAs in their domestic water supply. Approximately 1 L of water was collected from the home water supply at point-of-use at the time of interview. Samples were immediately analyzed for total iAs at the certified EPA-approved Hall Environmental Analysis Laboratory, Albuquerque, NM, by inductively coupled plasma-mass spectrometry (ICP-MS) using EPA Method 200.7–200.8. Turbid samples were analyzed by pretreatment as described in EPA Method 200.2.
2.4.2. Assessment of Historical Levels of iAs in Drinking Water
Estimates of past arsenic water concentrations (predominantly pre-2004) were obtained for public water systems (Albuquerque Bernalillo County Water Utility Authority (ABCWUA, 2013), the Safe Drinking Water Information System (SDWIS) (EHPT, 2013) and the US Geological Survey (Plummer et al., 2004) and used in assigning geographic exposure strata based on concentrations before 2006 regulations were in place. Ascertained candidate addresses were geocoded and locations were compared to estimates of historic iAs in public water supplies and well concentrations. iAs exposures associated with drinking water levels outside of New Mexico were estimated from tabulations of iAs concentrations in public drinking water systems in the geographic areas identified from residential histories of study subjects obtained from questionnaires. Current drinking water source (well or community water supply) was obtained from questionnaires; drinking water source information from previous residences was not available. Data sources included databases compiled by the Environmental Working Group (EWG, 2009), the Natural Resources Defense Council (NRDC, 2001), the US Geological Survey (Ryker, 2001; USGS, 2013) and a review of water quality reports and Consumer Confidence Reports (CCR) for individual public water systems available from the USEPA (http://water.epa.gov/lawsregs/rulesregs/sdwa/ccr/index.cfm). In cases where the USEPA database did not include the CCR, they were found by using the Yahoo search engine to locate them by city name. (See, for example, http://www.cityoforange.org/depts/publicworks/water_services/water_consumer_confidence_report.asp, and http://www.sandiego.gov/water/quality/reports.shtml for the CCRs for the cities of Orange and San Diego, California.) A drinking water arsenic exposure index was calculated by averaging estimated water concentrations for each year of life to the age of benign nevi removal for controls and first melanoma diagnosis for cases. The index was based on home iAs measured by this study for years residing at the current address and on historic estimates from public water systems during previous years. Historic estimates for iAs in public water systems were assumed to be equally representative of exposure for cases and controls. Although historic estimates had greater uncertainty than current iAs exposure estimates, we did not find any systematic biases for cases or controls due to changes in detection limits.
2.5. Biomarkers
2.5.1. Comet Assay
Damage reflecting DNA single-strand breakage and base excision repair was assessed in lymphocytes isolated from freshly drawn whole blood via the single cell gel electrophoresis assay (Comet assay) (Tice et al., 2000; Schmezer et al., 2001). Specifically, venous blood samples were obtained from each subject by a certified phlebotomist at the time of the home visit and interview. Lymphocytes were separated and harvested on Ficoll and washed with Hanks Balanced Salt Solution. The pellet was resuspended in Recovery Cell Culture Media (Gibco, Grand Island, NY) containing 10% dimethyl sulfoxide (DMSO), Dulbecco’s Modified Eagle Medium (High Glucose), fetal bovine serum and calf sera, then aliquoted into cryotubes and stored in liquid nitrogen at −80° C. On the day of assay, lymphocytes were quick thawed and washed in 100 μl ice-cold phosphate buffered saline (PBS, Sigma D8537) and centrifuged (5 min. @ 800 rpm) to pellet cells. Cells were resuspended in 500 μl PBS and counted. Approximately 1 × 105 cells were added to low-melt agarose and mounted on commercially prepared CometSlides (Trevigen, Gaithersburg, MD). Slides were prepared for analysis using the CometAssay reagent kit (Trevigen, Gaithersburg, MD) following the manufacturer’s instructions. Samples were assayed in duplicate with each 20-well slide including negative and positive control cells and at least 2 samples from the control population. DNA was stained with SybrGreen (Trevigen, Gaithersburg, MD) and imaged using an Olympus America IX70 fluorescent microscope equipped with a DP72 digital camera. A minimum of 50 cells per sample were then analyzed by CometScore software by TriTek (autocomet.com/freeware) with Tail Moment and percent Tail DNA calculated for each sample and control.
2.5.2. DNA Repair Gene Polymorphisms
Genotyping was performed using PCR technique and commercially available TaqMan® assays (Applied Biosystems, Carlsbad, California) amplified on an ABI 7900 HT genotyping machine (Applied Biosystems, Carlsbad, California). The following genetic polymorphisms were obtained for common DNA repair genes for which variant frequencies among Caucasians are known to be above 11%: XPD312, XRCC1, ERCC2, APEX1, and PARP1. The Gentra® Puregene® kit (Gentra, Valencia, CA) was used to extract DNA from whole blood samples. Extracted DNA concentration was determined (ng/μl) and checked for purity using a Nanodrop spectrophotometer 2.0 (TheroFisher Scientific, Waltham, MA). A measured sample’s quality was acceptable if its 260/280 ratio was between 1.7 to 1.9. The genotyping assay employs patented non-labeled forward and reverse primers along with two fluorescent TaqMan® oligonucleotide probes (the allele 1-specific probe was labeled with FAM and the allele 2-specific probe labeled with VIC dyes). The probes differentially bind to amplicons generated during PCR and selectively indicate the respective alleles. For absolute quantification, 2 μl of DNA was combined with 0.125 μl 40 X assay mix (including primers and FAM- and VIC-labeled probes) (supplied by Applied Biosystems) and 2.5 μl of TaqMan Universal Genotyping Master Mix in a 5μl total reaction volume. Samples were subjected to 40 cycles of amplification. Results for the TaqMan® assay were calculated by using the 99.9% confidence level settings in the 7900HT Sequence Detection System software. An allelic discrimination (AD) plot was also generated to detect the variants of a single nucleic acid sequence. Control samples consisted of a no template control and human genomic DNA (Roche, Pleasanton, CA) and were included on every plate; 10% of samples were repeated.
2.5.3. Determination of Arsenic Species in Urine
At the time of interview, a fresh sample of urine was collected from cases and controls, immediately frozen at −80° C. and shipped to the service laboratory at the University of North Carolina School of Public Health, Clinical Nutrition Research Center for determination of speciated urinary arsenic concentrations. Urine samples were analyzed as described (Matousek et al., 2008) with modification (Hernandez-Zavala et al., 2008). Briefly, arsines are generated from arsenicals within the samples using sodium borohydride. Arsines are flushed into a U-tube packed with 15% OV-3 (Supelco, Inc., Bellefonte, PA, USA) and immersed in liquid nitrogen. Arsines are trapped cryogenically for quantification of iAs and methylated metabolites monomethylarsonic acid (MMA) and dimethylarsenic acid (DMA). The sum of iAs, and methylated metabolites MMA and DMA is determined by pretreatment of the sample with 2% L-cysteine.
2.5.4. Determination of Total Arsenic in Toenail Clippings
Toenail clippings were collected in pre-labeled paper envelopes provided at the time of the interview from both melanoma cases and controls. Approximately 100 mg (10 – 800) of toenail clippings from each subject were submitted to the Research Reactor Center, University of Missouri-Columbia, MO for digestion and analysis by graphite furnace atomic absorption spectrophotometry with appropriate internal standards (Karagas et al. 1996; Karagas et al. 2000).
2.5.5. Determination of Serum Vitamin D
Total serum Vitamin D (OH)25 was determined in case and control samples at a certified commercial laboratory utilizing the standard Liaison chemiluminescent immunoassay (van Helden and Weiskirchen, 2014). The detection limit range for this assay was 2.5 – 150 ng/ml. This laboratory’s reference range was 30 – 80 ng/ml.
2.6. Data Management and Statistical Methods
Data were entered in the Research Electronic Data Capture (REDCap) data capture system hosted and supported by the University of New Mexico Clinical and Translational Science Center (CTSC) (Harris et al. 2009). REDCap is a secure web-based application designed to support research data capture. All questionnaire information was entered by trained data entry personnel and then double-entered and quality-controlled by one of the authors (EE) over 10% of all cases and controls.
Univariate analyses were carried out by applying parametric (Pearson’s) and non-parametric (Spearman’s) correlation to current and past UV and iAs exposures with the Comet assay biomarker results. Non-parametric Kruskal-Wallis or Wilcoxon tests were used to assess case and control status of iAs exposure measures (water, urine and toenail concentrations) and UV exposure indices. Fisher Exact Tests were used to assess whether categorical variables from the home survey were associated with case and control status. Multivariable regression was used to assess the joint effects of household and patient characteristics on iAs exposure measures, Vitamin D, and Comet assay results. The relation between genetic polymorphisms, time-varying UV exposure and age to melanoma case diagnosis or home survey of controls was assessed by Cox model regression methods. Statistical analyses and reporting functions were performed using SAS v9.3.
3. Results and Discussion
This study attained a total of 101 participants of which 66 were population-based cases of pathologically confirmed cutaneous malignant melanoma and 35 were healthy controls with histologically confirmed benign skin nevi (Table 1). Approximately forty-five percent of cases and thirty percent of referents contacted by mail and telephone agreed to participate in the study. Acceptance rates of males and females in both groups were approximately equal. Fifty-five percent of participants were located in Albuquerque with the remaining participants in suburbs and outlying towns. Administration of questionnaires and collection and analysis of biological and environmental samples were completed for nearly all subjects enrolled in the study; two case enrollees were excluded from sections of the analyses due to failure to complete one or more study elements. Although non-Hispanic whites were targeted for enrollment in the study as earlier described, 5 of the 101 individuals identified themselves as Hispanic white during the interview portion of the study. Males and females were represented in approximately equal numbers in both groups.
Table 1.
Subject Characteristics
| Characteristic | Case | Control | Total | P-valuea | |||
|---|---|---|---|---|---|---|---|
| N | 64 | 35 | 99 | ||||
| Males, N (%) | 30 (47)b | 17 (49) | 47 (48) | 0.87 | |||
| Females, N (%) | 34 (53) | 18 (51) | 52 (53) | ||||
| Age, Mean ± SD | 63 ± 14 | 50 ± 15 | 58 ± 16 | <0.001 | |||
| Current Smoker, N (%) | 3 (5) | 5 (14) | 8 (8) | 0.13 | |||
| Smoker in home, N (%) | 7 (11) | 7 (20) | 14 (14) | 0.24 | |||
| Tanning index: easy to moderate tanning, N (%) | 27 (44) | 20 (57) | 47 (49) | 0.29 | |||
| Tanning index: occasionally to no tanning/freckle, N (%) | 29 (48) | 11 (31) | 40 (42) | 0.14 | |||
| Vitamin D (ng/ml), Mean ± SD | 29 ± 9 | 25 ± 8 | 28 ± 9 | 0.01 | |||
| Well (N) Drinking Water Source and As Cone. (μg/L), Mean ± SD | 11 | 4.0 ± 6.9 | 2 | 2.7 ± 2.8 | 13 | 3.8 ± 6.4 | 0.77 |
| Municipal Drinking Water Source (N) Source and As Cone. (μg/L), Mean ± SD | 53 | 3.9 ± 2.6 | 33 | 3.5 ± 2.4 | 86 | 3.7 ± 2.5 | 0.50 |
| As (μg/L) home drinking water concentration, Mean ± SD | 3.9 ± 3.6 | 3.5 ± 2.4 | 3.7 ± 3.2 | 0.79 | |||
| Drinking water As concentration average lifetime to age first diagnosis (μg/L), Mean ± SD | 5.0 ± 3.3 | 6.0 ± 3.2 | 5.3 ± 3.3 | 0.09 | |||
| Drinking water Arsenic Average Concentration (μg/L) Age 10, Mean ± SD | 3.3 ± 3.8 | 3.8 ± 4.2 | 3.5 ± 4.0 | 0.64 | |||
| Reverse Osmosis filters in home (whole house or drinking/cooking), N (%) | 5 (8) | 5 (14) | 10 (10) | 0.31 | |||
| Urinary iAs (ng/L), Mean ± SD | 0.51 ± 0.71 | 0.54 ± 0.54 | 0.52 ± 0.65 | 0.48 | |||
| Urinary MMA (ng/L), Mean ± SD | 0.83 ± 0.86 | 0.82 ± 0.54 | 0.82 ± 0.76 | 0.30 | |||
| Urinary DMA (ng/L), Mean ± SD | 4.86 ± 5.58 | 4.79 ± 3.49 | 4.83 ± 4.92 | 0.29 | |||
| Urinary ƹ As species (ng/L), Mean ± SD | 6.33 ± 7.35 | 6.14 ± 4.40 | 6.26 ± 6.44 | 0.29 | |||
| Toenail As (total ug/g), Mean ± SD | 0.09 ± 0.06 | 0.11 ± 0.06 | 0.10 ± 0.06 | 0.11 | |||
| Comet Assay Tail Moment, Mean ± SD | 58.3 ± 51.7 | 54.5 ± 46.9 | 57.0 ± 49.8 | 0.93 | |||
| Comet Assay % DNA in Tail, Mean ± SD | 47.3 ± 20.3 | 40.4 ± 21.6 | 44.9 ± 20.9 | 0.17 | |||
| UV erythemal irradiance previous 90d (kJ/m2/d),Mean ± SD | 4.20 ± 1.22 | 3.46 ± 1.16 | 3.94 ± 1.24 | 0.001 | |||
| UV erythemal irradiance previous 7d (kJ/m2/d),Mean ± SD | 4.22 ± 1.66 | 4.56 ± 1.66 | 4.34 ± 1.66 | 0.33 | |||
| UV erythemal annual average irradiance lifetime to age first diagnosis (kJ/m2), Mean ± SD | 1187 ± 204 | 1215 ± 160 | 1197 ± 189 | 0.75 | |||
| UV erythemal annual average irradiance Age 10 (kJ/m2), Mean ± SD | 1110 ± 305.5 | 1094 ± 277.0 | 1104 ± 294.4 | 0.77 | |||
| Homozygous Rare | 3 (4.7) | 6 (17.1) | 9 (9.1) | ||||
| Homozygous Rare | 8 (12.5) | 6 (17.1) | 14 (14.1) | ||||
| Homozygous Rare | 14 (21.9) | 7 (20.0) | 21 (21.2) | ||||
| Homozygous Rare | 1 (1.6) | 0 (0.0) | 1 (1.0) | ||||
| Homozygous Rare | 4 (6.3) | 7 (20.0) | 11 (11.1) | ||||
Statistical tests were Fisher Exact tests for categorical variables and Wilcoxon exact tests for continuous variables.
Standard deviation.
Table 1 shows mean values and standard deviation (SD) for each of the principal study endpoints for cases and controls separately and for the group as a whole along with p-values for case vs. control attained by Fisher Exact tests for categorical variables and Wilcoxon exact tests for continuous variables. Melanoma cases were statistically significantly older (63 ± 14 years) than controls; the average age of controls was 50 ± 15 years. Based on questionnaire responses, UV erythemal irradiance during the 90 days previous to the home survey was significantly higher for cases than for controls. Mean (± SD) current home As in drinking water was not significantly different for cases and controls (3.98 μg/L (± 3.67) vs. 3.47 μg/L (± 2.40). Individuals with residential wells did not have significantly higher iAs concentrations than participants whose homes were on municipal supplies. No differences in drinking water iAs concentrations were observed for these water sources between cases and controls. Assessment of individual historical arsenic exposure showed no significant differences between cases and controls (p = 0.09). There was no difference between cases and controls in the average percent of homes that stated presence of a reverse osmosis water filter (known to be effective in reducing iAs concentrations) for their home or drinking and cooking water supplies.
3.1. Biomarkers
There was no significant difference between cases and controls for the following group average As biomarker endpoints: urinary iAs, MMA, DMA and sum of As species, tAs in toenails or Comet assay endpoints (Table 1). Comet assay measures that reflect increased DNA damage (which indicates less DNA repair) were not significantly correlated with either current or historical As exposure estimates.
iAs in drinking water and As biomarkers in urine and toenails were highly significantly correlated for both cases and controls. Pearson’s correlations (r): for iAs in drinking water vs. urinary biomarkers were: iAs: r = 0.62, p<0.0001; MMA: r = 0.46, p < 0.0001; DMA: r = 0.50, p < 0.0001. iAs in drinking water correlation with toenail total arsenic (tAs) (μg/g) was r = 0.49, p < 0.0001; toenail tAs correlations with urinary As biomarkers were: iAs: r = 0.36, p = 0.002; MMA: r = 0.29, p = 0.0028; and DMA: r = 0.29, p = 0.0031. Results from Spearman’s correlation analyses were very similar. These results indicate a consistent ability to quantify internal exposure to these very low levels of environmental exposure to iAs in drinking water. Toenail As concentration was also significantly correlated with the major urinary As species. When controlling for UV exposure for cases and controls, iAs concentrations in drinking water and internal biomarkers of iAs exposure were not significantly correlated with either of the two Comet assay endpoints. These results suggest that at these low As drinking water exposure levels, there may be little, if any, in vivo effect of arsenic exposure on DNA repair inhibition. Further, no association was seen between toenail tAs concentration and risk of melanoma, therefore our study was unable to confirm an association between toenail As concentration and risk of melanoma in white Iowans aged 40 and older earlier reported by Beane-Freeman et al. (2004).
The non-parametric Kruskal-Wallace statistical test on As biomarkers associated with exposures determined from the environmental questionnaire showed the following statistically significant differences (p ≤ 0.05): the concentration of iAs urinary methylated metabolites MMA and DMA was significantly higher in seafood consumers (n=22); urinary DMA was higher in homeopathic remedy users (n=21); home water iAs and toenail (tAs) were higher in dietary supplement users (n=7); urinary MMA was higher with soldering or welding exposure (n=9); toenail (tAs) was lower in home pesticide users (n=26); and home water iAs and urinary iAs are higher in sport fishers (n=4) who are also seafood consumers. These findings, particularly the known association between exposure to As and ingestion of seafood, in this small population-based study points toward the usefulness of sensitive internal arsenic exposure (biomarker) methods possessing high analytical sensitivity (e.g., low limits of detection).
3.2. UV and Comet assay
Mean UV exposure in the 90 days prior to diagnosis (±SD) of cutaneous melanoma (cases) or benign nevus (controls) UV erythemal exposure was significantly higher for cases (4.20 kJ/m2/d ± 1.22) than controls (3.46 kJ/m2/d ± 1.16, p = 0.001) (Table 1). UV exposure in the previous 7 days prior to diagnosis was not different for cases (4.22 ± 1.66) and controls (4.56 ± 1.66, p = 0.33). However, examination of UV exposure over previous time increments (7 to 90 days) and DNA repair as measured by Comet assay endpoints indicates a UV-Comet assay interaction such that cases have higher Comet Tail Moment (Figure 1a) and percent DNA in Tail (Figure 1b) with both endpoints indicating significantly more DNA damage in cases than controls at low UV values (slopes for cases and controls were significantly different for each measure, p < 0.001). However, as indicated in Figure 1a and b, case results converge with control values (no difference between cases and controls for Comet assay endpoints) at higher UV exposures. A significant Case - UV interaction was found for UV averaged over the previous 7 to 60 days for both measures; Figure 1a and b show results of the 7 day analysis only. We assessed whether the significant UV x case status interaction was due to confounding with age by adjusting for age and by testing whether there was a UV x age interaction. We also tested whether UV exposure was associated with age. UV exposures were not correlated with age and neither of the multivariable analyses indicated that age was responsible for differential responses for cases and controls. The UV x case status interaction is a new finding that suggests that melanoma cases are more sensitive (e.g., experience more DNA damage and less DNA repair) to low level UV exposure than are controls.
Figure 1.
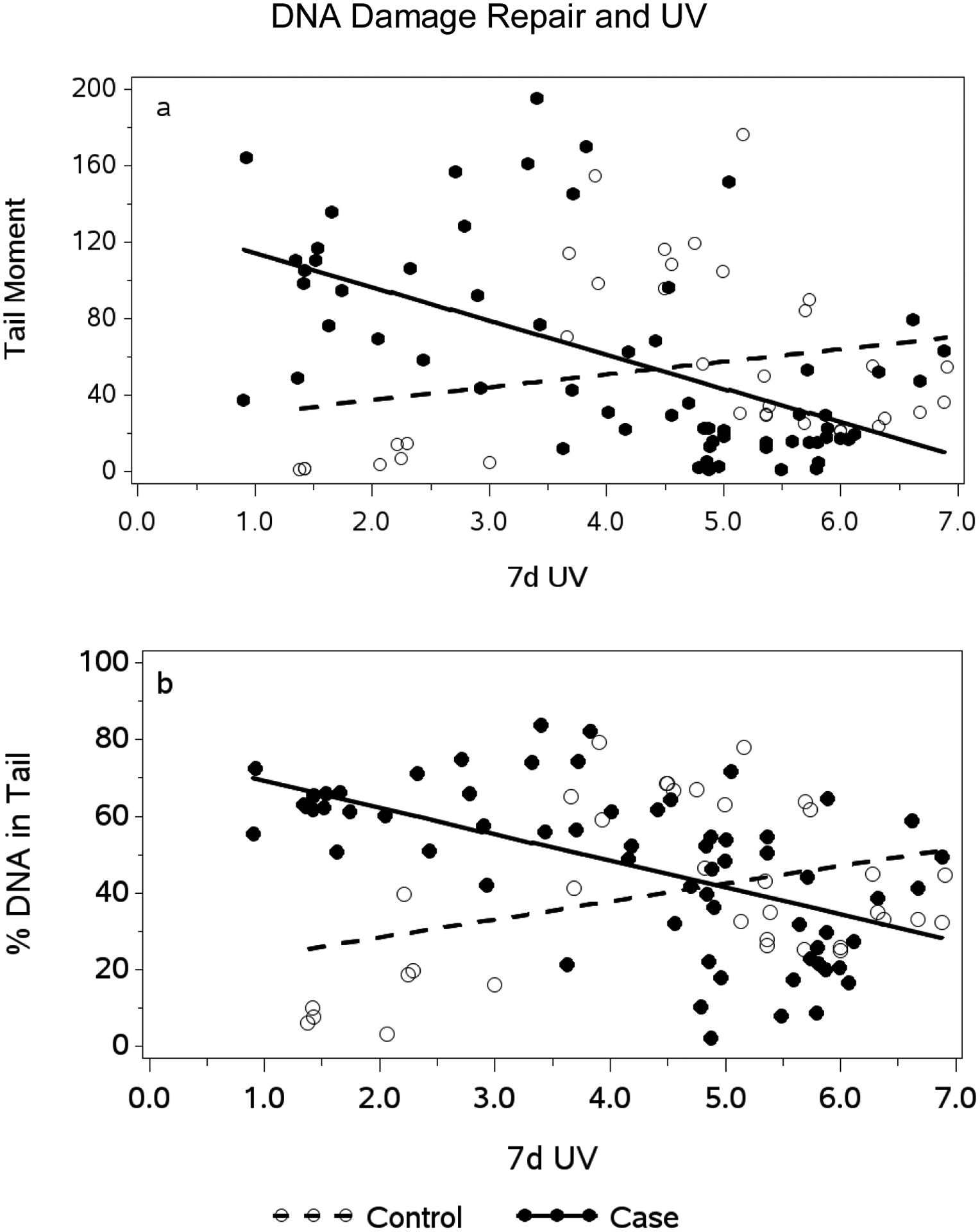
Association between erythemal UV Exposure in the previous 7 days (kJ/m2/d) and DNA Repair as reflected in Comet Tail Moment (a) and percent DNA in Comet tail (b) for Melanoma Cases and Controls
x-axis: UV erythemal irradiance in the previous 7 days measured in kilojoules per meter squared per day (kJ/m2/d).
y-axis (a): Comet tail moment defined as the product of comet tail length and fraction of total DNA in the comet tail (unitless). Tail moment reflects a measure of both the smallest detectable size of migrating DNA and the number of relaxed/broken DNA strands as a measure of unrepaired DNA. Open circles are Controls; closed circles are Cases.
y-axis (b): Per cent DNA in Comet Tail reflecting the number of relaxed/broken DNA strands as a measure of unrepaired DNA. Open circles are Controls; closed circles are Cases.
3.3. Joint Analysis (Current and Historical) of Arsenic in Drinking Water and UV on Melanoma Outcome
The association between UV and As drinking water exposure indices and melanoma was assessed using time-to-event Cox model regression. Controls were treated as censored at their blood draw date. For cases, age at first melanoma diagnosis was the time-to-event measure. Arsenic and UV exposure variables were calculated as baseline covariates and as time-variable covariates for analyses. In the time-variable analyses, exposure values were calculated for each event time. As shown in Table 1, UV at age 10, arsenic exposure at age 10, and time-variable UV were associated with an increased hazard of a melanoma diagnosis. Childhood UV at age 10 and time-variable UV to first melanoma diagnosis had similar Akaike Information Criteria (AIC) values. Smaller AIC values indicate a better fit to the model. However, joint models with UV and arsenic did not reveal any significant UV-As interactions. When both As in drinking water and UV were included in analyses, UV was the singular significant predictor of melanoma. Thus exposure to drinking water As, either alone or in conjunction with UV exposure, was not associated with increased hazard of a melanoma diagnosis in this study population. The known positive association between UV radiation exposure and increased risk of having a malignant melanoma diagnosis (IARC, 1992; Pfeifer and Besaratinia, 2012) detected here provide additional positive affirmation of validity for this population-based study.
A corroborative in vitro study of keratinocytes and normal melanocytes was conducted to examine the joint effect of exposure to iAs and UV radiation on DNA repair in each cell type (Cooper et al., 2014). Melanocytes were markedly more resistant to UV radiation induced cytotoxicity. Each cell type was exposed to increasing concentrations of iAs for 24 hrs prior to a single exposure to 3 kJ/m2 UV radiation for keratinocytes or 10 kJ/m2 UV for melanocytes. DNA base excision repair activity was determined 1 hour following exposure by determination of PARP activity. iAs treatment followed by UV exposure caused a significant dose-dependent decrease in PARP-1 activity in both cell types starting at 0.1 μM iAs (7.5 μg/L), the lowest concentration tested. Exposure levels experienced by subjects found that mean iAs drinking water concentrations for both cases and controls was < 4 μg/L (0.05 μM) while mean UV exposure in the previous 7 days for both groups was about 4.4 kJ/m2 per day. Although it is not possible to directly compare in vitro – in vivo findings due to obvious study design differences, taken together, results are suggestive that a joint effect of iAs drinking water exposure and exposure to UV on inhibition of DNA repair was not observed in the present study due to low concentrations of iAs in the drinking water.
3.4. Gene-Environment Interactions
The two groups showed no significant differences for mean percent of DNA repair gene polymorphisms in XPD312, XRCC1, APEX1, PARP1, and ERCC2 (Table 1). Specific genetic polymorphisms in the DNA repair genes were not significantly associated with melanoma. However, a significant effect modification of the time-variable UV exposure association with risk of melanoma by APEX1 was detected (APEX1 x UV interaction, p = 0.004) (Figure 2). APEX1 (apurinic/apyrimidinic endonuclease) is a DNA base excision repair gene. We found that individuals with two copies of the variant APEX1 allele (rs 1130409, G/1) had an increased risk of melanoma even at low UV exposure compared with those with no variant copies or one copy of the variant allele at the same UV exposure level. We found no effect modification between exposure to As and any other DNA repair gene polymorphisms examined.
Figure 2.
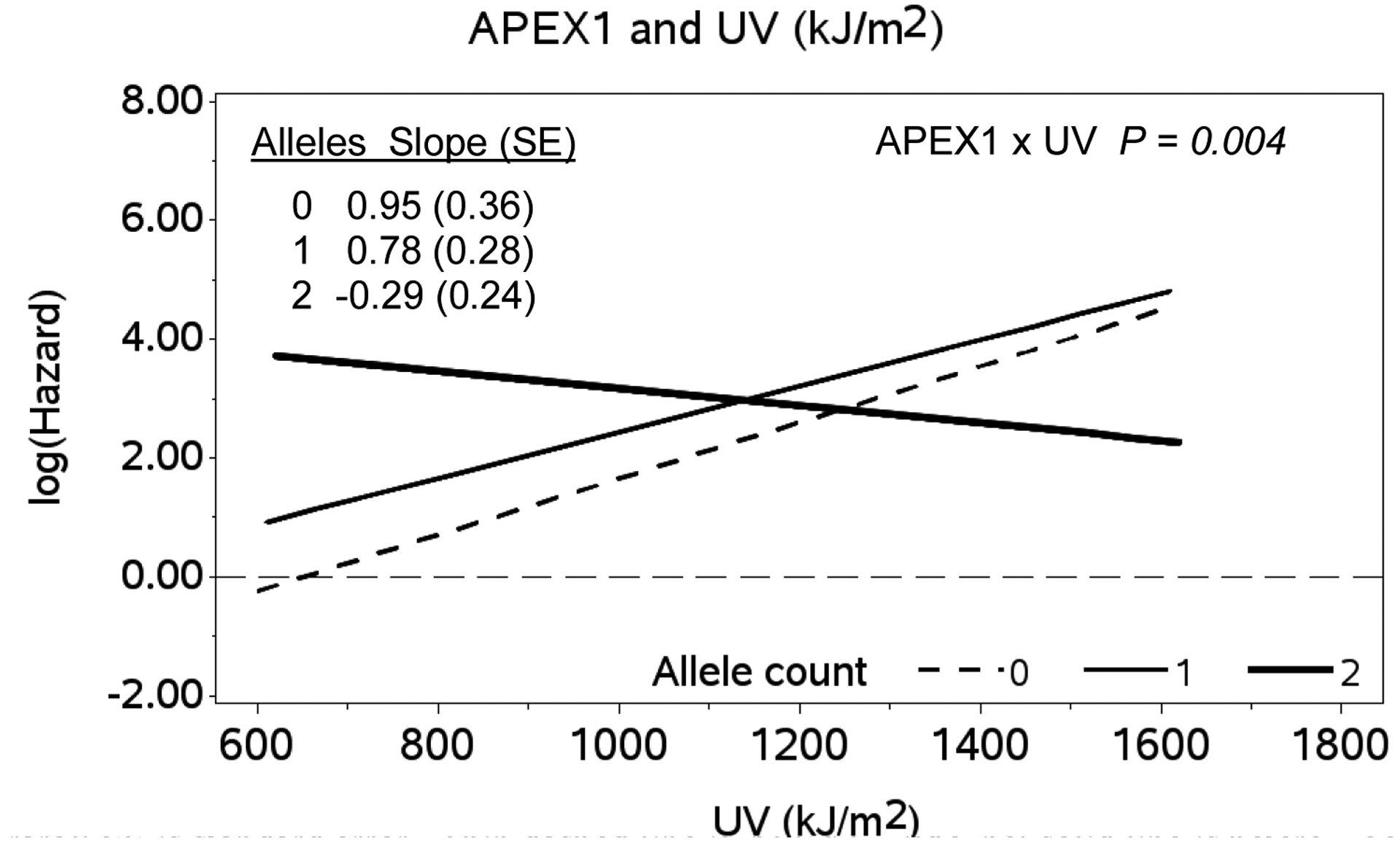
Association between Log-hazard of Melanoma Diagnosis and Time-variable ambient UV Exposure from Age One to Age at first Melanoma Diagnosis by APEX1 Genotype (Referent UV exposure = 650 kJ/m2)
x-axis: Time-variable ambient UV (UV(t)) in kilojoules per meter squared (kJ/m2) erythemal weight.
y-axis: Log-hazard of melanoma diagnosis; APEX1 genotypes: (0= wild type, 1 = heterozygote, 2 = homozygous rare); SE is standard error. Thin dashed line is APEX1 wild type; solid line is heterozygote; heavy solid line is homozygote. APEX1 x UV interaction, p = 0.004.
In a previous literature report, a population exposed to iAs in drinking water was examined for expression of ERCC1 (excision repair cross-complementing repair deficiency gene, complementation group 1) (Andrew et al., 2006). Subjects included New Hampshire residents who were exposed to an average of 0.7 μg/L (range 0.007 – 5.3) (low exposure group) or an average of 32 μg/L (range 10.4 – 74.7) (high exposure group) as well as subjects (total n = 16) from Colonia Allende, Mexico exposed to 5.5 ± 0.20 μg/L As (low exposure group) and Esperanza, Mexico exposed to 43.3 ± 8.4 μg/L As (high exposure group) in drinking water. All subjects were subsequently assigned to either a low exposure group (≤ 6 μg/L As in drinking water; n=42) or a high exposure group (> 6 μg/L As in drinking water; n=11). Individuals in the high exposure group were found to have significantly lower ERCC1 gene expression (p < 0.05). In addition, lymphocytes from a subset of New Hampshire residents were assessed for DNA repair efficiency using the Comet assay. Higher levels of DNA damage as reflected in the Comet assay were detected in those subjects exposed to ≥ 13–93 μg/L As compared with those exposed to < 0.7 μg/L As in drinking water. From these observations, it appears that iAs in drinking water can be associated with decreased DNA repair capacity and ERCC1 gene expression at higher exposure levels than were seen in this present study.
Surdu et al. (2014) examined the risk of non-melanoma skin cancer and the association between XRCC1 (X-ray repair cross-complementing group 1) polymorphisms and exposure to sunlight and As. Results indicated an increased risk of SCC for the homozygous variant genotype of XRCC1. Likewise, Applebaum et al. (2007) examined polymorphisms in nucleotide excision repair genes XPA and XPD in relation to As exposure and risk of non-melanoma skin cancer finding an increased risk of BCC associated with high As exposure among those with the XPA homozygous variant. Hung et al. (2005) conducted a meta-analysis of genetic polymorphisms in the base excision repair pathway and risk of cancer associated with tobacco use focusing on the 8-oxoguanine DNA glycosylase (OGG1), apurinic/apyrimidinic endonuclease (APEX1) and x-ray repair cross-complementing group 1(XRCC1). No association was found between cancer risk in this study and APEX1 and XRCC1 polymorphisms. No studies were located that assessed DNA repair gene polymorphisms in relation to risk of malignant melanoma skin cancer.
3.5. Vitamin D
Vitamin D is produced in vivo mainly by UVB in sunlight; higher levels of vitamin D may have positive effects in controlling melanoma lesion formation and aggressiveness related to improved survival (Berwick et al., 2005) although the role of vitamin D (25-OH-D) in relation to melanoma risk and mortality is not yet clearly understood (Berwick and Erdei, 2012).
Case and control vitamin D levels were determined in this study (Table 1). Interestingly, cases had significantly higher levels of vitamin D than controls (P = 0.01). Results show that serum vitamin D levels were not correlated with UV exposure (kJ/m2/d) erythemally-weighted levels. The association between UV exposure and vitamin D levels was confounded by an interaction between UV exposure and supplement use. Vitamin D increased with 60-d and 90-d UV exposure in subjects not taking nutritional supplements (P = 0.04 and P = 0.02, respectively) and did not increase in patients reporting that they were using supplements. The effects of vitamin D on overall health status and various cancer incidences are extremely complex. A recent clinical practice guidance article draws attention to this issue (Manson and Bassuk, 2015). Among our principally Caucasian subjects, there is a possibility that UV exposure levels had not fully induced vitamin D production due to lack of UVB-specific exposures and time spent outdoors (noon hour exposures are the most preferable) (Terushkin et al., 2010). Furthermore, other adaptive skin protection responses are possible if short and long-term UV exposures are considered that were not evaluated in our study (Desotelle et al., 2012).
4. Conclusions
Internal exposure to iAs was detected by multiple biomarkers in both cases and controls that were all significantly correlated with iAs concentrations in drinking water in spite of the fact that mean drinking water concentrations were very low at < 4 μg/L for both groups. No significant associations were observed between iAs exposure and inhibition of DNA repair as reflected in the sensitive Comet assay. No interaction between exposure to iAs and UV on inhibition of DNA repair was seen. A UV-genetic polymorphism interaction was shown in that individuals with two copies of the variant APEX1 allele have an increased risk of melanoma even at low UV exposure compared with those with no variant copies or one copy of the variant allele at the same UV exposure level. A UV- Comet assay (DNA repair) interaction was observed such that melanoma cases had more DNA damage than controls at low UV exposures. These new findings suggest that melanoma cases may be more sensitive to low level UV exposure than are controls.
Results from this study indicate that arsenic at currently low levels in drinking water in New Mexico does not have adverse effects in increasing the risk of development of malignant melanoma and that arsenic at currently low levels does not have an effect on inhibition of DNA repair either as a single exposure entity or in combination with UV exposure. From a public health standpoint, it is encouraging that a UV- arsenic interaction on inhibition of DNA repair was not observed at arsenic drinking water concentration exposures below the current allowable Maximum Concentration Limit (MCL) of 10 μg/L.
The strengths of this study lie in the robust measurements of UV and iAs exposure and the application of a number of sensitive internal biomarkers of iAs exposure as well as use of questionnaires that obtained comprehensive information on environmental UV and occupational exposures, as well as health conditions. A constraint on the study is that results may be limited by relatively small sample size. A larger population-based study would be important to validate these current results.
5. Acknowledgments
This study was supported by National Institute of Environmental Health Sciences (NIEHS) Grant #1R21ES018705. The University of North Carolina National Obesity Research Center (NORC) laboratory under the direction of Dr. Miroslav Styblo is independently supported by NIH Grant #DK56350; NORC was reimbursed for performance of urinary arsenic speciation analyses for this study.
The authors are especially indebted to Ms. Adriana Sanchez and Ms. Natalia Gurule for their impeccable performance of multiple complex tasks critical to the field portion of the study, to Dr. Karen Cooper, Ph.D., for skilled performance and analysis of the Comet assays, to Ms. Miranda Cajero for expertise in formatting study variables in REDCap, to Mrs. Elizabeth Harris for precise REDCap data entry, and to Laboratory Manager, Ms. Kirsten White and the dedicated staff and students of the Berwick Molecular Epidemiology Laboratory for excellent record and sample logistics organization and proficiency in carrying out genetic polymorphism assays. The authors declare they have no conflicts of interest.
References
- ABCWUA, 2013. Your Drinking Water, Albuquerque Bernalillo County Water Utility Authority. http://www.abcqua.org/content/view/36/31/ (accessed 2001 report March 2013; reports for 2002, 2007, 2008 are no longer retrievable).
- Andrew AA, Burgess JL, Meza MM, Demidenko E, Waugh MG, Hamilton JW, Karagas MR (2006) Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ Health Perspect 114 (8), 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum KM, Karagas MR, Hunter DJ, Catalano PJ, Byler SH, Morris S et al. (2007) Polymorphisms in nucleotide excision repair genes, arsenic exposure and non-melanoma skin cancer in New Hampshire. Environ Health Perspect 115 (8), 1231–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baastrup R, Sorensen M, Balstrom T, Fredericksen K, Larsen CL, Tjonneland A et al. (2008) Arsenic in drinking –water and risk for cancer in Denmark. Environ Health Perspect 116 (2), 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane-Freeman LE, Dennis LK, Lynch CF, Thorne PS, Just CL (2004) Toenail arsenic content and cutaneous melanoma in Iowa. Amer J Epidemiol 160, 679–687. [DOI] [PubMed] [Google Scholar]
- Berwick M, Erdei EO (2012) Vitamin D and melanoma incidence and mortality. Pigment Cell Melanoma Res 26, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick M, Armstrong BK, Ben-Porat L, Fine J, Kricker A, Eberie C et al. (2005) Sun exposure and mortality from melanoma. J Natl Cancer Inst 97(3), 195–9. [DOI] [PubMed] [Google Scholar]
- Burgess JL, Meza MM, Josyula AB, Poplin GS, Kopplin MJ, McClellen HE et al. (2008) Environmental arsenic exposure and urinary 8-OHdG in Arizona and Sonora. Clinical Toxicol 45, 490–498. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Chuang YC, Lin TM, Wu HY (1985) Malignant neoplasms among residents of a blackfoot disease endemic area in Taiwan: high arsenic artesian well water and cancer. Canc Res 45, 5895–5899. [PubMed] [Google Scholar]
- Chen CJ, Chugan YC, You SI, Lin TM, Wu HY (1986) A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. Br J Cancer 53(3), 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Chiou HY, Hsu LI, Hsueh YM, Wu MM, Chen CJ (2009) Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan. Environ Res doi: 10.1016/j.envres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Chervona Y, Arita A, Costa M (2012) Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics 4, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KL, Liu KJ, Hudson LG (2009) Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase. Free Radic Biol Med 47, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KL, Yager JW, Hudson LG (2014) Melanocytes and keratinocytes have distinct and shared responses to ultraviolet radiation and arsenic. Toxicol Letters 224, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis LK, Lynch CF, Sandler DP, Alavanja MC (2010) Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural health study. Environ Health Perspect 118, 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desotelle JA., Wilking MJ, Ahmad N(2012) The circadian control of skin and cutaneous photodamage. Photochem Photobiol 88(5),1037–47. doi: 10.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Hudson LG, Sun X, Feng C, Liu KJ (2008) As(III) inhibits ultraviolet radiation-induced cyclobutane pyrimidine dimer repair via generation of nitric oxide in human keratinocytes. Free Radic Biol Med 45, 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ (2009) Inhibition of poly(ADP-ribose)polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem 284, 6809–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHPT (2013) New Mexico Environmental Health Tracking Program Community Drinking Water Data Table. https://nmtracking.org/en/environ_exposure/water-qual/community-drinking-water-data/#download. (accessed March 8, 2013).
- EWG, 2009. National Drinking Water Database. Environmental Working Group, Washington, DC. 20009. (http://www.ewg.org/tap-water/whats-in-your-water) (accessed March 10, 2013). [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap) – A meta-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 42(2), 377–81. http://project-redcap.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Zavala A, Matousek T, Drobna Z, Paul DS, Walton F, Adair BM et al. (2008). Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz atomizer (multiatomizer). Journal of Analytical Atomic Spectrometry 23, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung RJ, Hall J, Brennan P, Boffetta P (2005) Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am J Epidemiol 162(10), 925–42. [DOI] [PubMed] [Google Scholar]
- IARC, 1992. Solar and Ultraviolet Radiation. International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- Karagas MR, Morris JS, Weiss JE, Spate V, Baskett C, Greenberg ER, et al. (1996) Toenail samples as an indicator of drinking water arsenic exposure. Cancer Epidemiology Biomarkers and Prevention 5(10), 849–52. [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Blum J, Klaue B, Weiss JE, Stannard V, et al. (2000) Measurement of low levels of arsenic exposure: a comparison of water and toenail concentrations. Amer J Epidemiol 152(1), 84–90. [DOI] [PubMed] [Google Scholar]
- Kricker A, Vajdic CM, Armstrong BK (2005) Reliability and validity of a telephone questionnaire for estimating lifetime personal sun exposure in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 14, 2427–32. [DOI] [PubMed] [Google Scholar]
- Kricker A, Armstrong BK, Goumas C, Litchfield M, Begg CB, Hummer AJ, et al. (2007) Ambient UV, personal sun exposure and risk of multiple primary melanomas. Cancer Causes and Control 18(3), 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madronich W, Flocke S, Zeng J, Petropavlovskikh I, Lee-Taylor J (2010) Atmospheric Chemistry Division, January 10, 2010. National Center for Atmospheric Research, P.O. Box 3000, Boulder, Colorado 80307. [Google Scholar]
- Manson JE, Bassuk SS (2015) Vitamin D research and clinical practice – At a crossroad. JAMA, published online February 19, 2015. [DOI] [PubMed] [Google Scholar]
- Matousek T, Hernandez-Zavala A, Suoboda M, Langrova L, Adair BM, Drobna Z et al. (2008). Oxidation state specific generation of arsines from methylated arsenicals based on L-cysteine treatment in buffered media for speciation of analysis by hydride-generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz atomizer (multiatomizer). Spectrochimica Acta Part B 63, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink PJ, Alexander DD, Barraj LM, Kelsh MA, Tsuji JS (2008) Low-level arsenic exposure in drinking water and bladder cancer: a review and meta-analysis. Regul Toxicol Pharmacol 52(3), 299–310. [DOI] [PubMed] [Google Scholar]
- NAACCR, 2007. North American Association of Central Cancer Registries (http://www.naaccr.org)
- NRDC, 2001. Arsenic and Old Laws: A Scientific and Public Health Analysis of Arsenic Occurrences in Drinking Water, Its Health Effects, and EPA’s Outdated Arsenic Tap Water Standard. Natural Resources Defense Council, New York, NY. (http://www.nrdc.org/water/drinking/arsenic/aolinx.asp) accessed March 2013. [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan. Int J Cancer 94(2),153–156. [DOI] [PubMed] [Google Scholar]
- Pearce DC, Dowling K, Sim MR (2012) Cancer incidence and soil arsenic exposure in a historical gold mining area in Victoria, Australia: a geospatial analysis. J Expos Sci Environ Epidemiol 22, 248–257. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Besaratinia A (2012) UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci 11, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC (2009) Biological responses to arsenic compounds. J Biol Chem 284, 18583–18587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer NL, Bexfield LM, Anderholm SK, Sanford WE, Busenber E (2004) Geochemical characterization of ground-water glow in the Santa Fe Group Aquifer System, Middle Rio Grande Basin, New Mexico. Water-Resources Investigation Report 03–4131. US Geological Survey, Reston, VA. [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC (2001) Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol 176, 64–71. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC (2002) Arsenite cocarcinogenesis: an animal model derived from genetic toxicology studies. Environ Health Perspect 110 (Suppl 5), 749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ (2004) Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol 198, 394–404. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Klein CB (2011) Genetic and epigenetic effects of environmental arsenicals. Metallomics 3, 1135–1141. [DOI] [PubMed] [Google Scholar]
- Ryker SJ (2001) Mapping arsenic in groundwater – A real need, but a hard problem: Geotimes Newsmagazine of the Earth Sciences, 46 (11), 34–36. http://water.usgs.gov/nawqa/trace/pubs/geo_v46n11/index/html (accessed March 7, 2013). [Google Scholar]
- Schmezer P, Rajaee-Behbahan N, Risch A, Thiel S, Rittgan W, Drings P et al. (2001) Rapid screening assay for mutagen sensitivity and DNA repair capacity in human peripheral blood lymphocytes. Mutagenesis 16, 25–30. [DOI] [PubMed] [Google Scholar]
- Surdu S, Fitzgerald EF, Bloom MS, Boscoe FP, Carpenter DO, Haase RF et al. (2014) Polymorphisms in DNA repair genes XRCC1 and XRCC3, occupational exposure to arsenic and sunlight, and the risk of non-melanoma skin cancer in a European case-control study. Environ Res 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Terushkin V, Bender A, Psaty EL, Engelsen O, Wang SQ, Halpern AC (2010) Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol 62(6), 929.e1–9. doi: 10.1016. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, et al. (2000) Single cell gel-comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environmental and Molecular Mutagenesis 35(3), 206–21. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Benbrahim-Tallaa L, Ward JM, Lunn R, Sams RL 2nd, Waalkes MP (2010) Cancer in experimental animals exposure to arsenic and arsenic compounds. Crit Rev Toxicol 40, 912–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WP, Chu HM, How SW, Fong JM, Lin CS, Yeh S (1968) Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Nation Canc Inst 40(3), 453–463. [PubMed] [Google Scholar]
- Tsuji JS, Alexander DD, Perez V, Mink PJ (2014) Arsenic exposure and bladder cancer: quantitative assessment of studies in human populations to detect risks at low doses. Toxicol 317, 17–30. [DOI] [PubMed] [Google Scholar]
- USGS (2013) Datasets obtained from USGS National Water Information Systems in 2001 and modified in 2007. (http://water.usgs.gov/nawqa/trace/data/arsenic_nov2001.txt) accessed March 2013.
- Van Helden J, Weiskirchen R (2014) Experience with the fully automated chemiluminescence immunoassay for the quantification of 1a, 25-dihydoxy-vitamin D. Clin. Chem Lab Med doi: 10.1515/cclm-2014-0698. [DOI] [PubMed] [Google Scholar]
- Wiencke JK, Yager JW, Varkonyi A, Hultner M, Lutze LH (1997) Study of arsenic mutagenesis using the plasmid shuttle vector pZ189 propagated in DNA repair proficient human cells. Mutat Res 386, 335–344. [DOI] [PubMed] [Google Scholar]
- Yager JW, Wiencke JK (1997) Inhibition of poly(ADP-ribose)polymerase by arsenite. Mutat Res 386, 345–351. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG (2011) Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J Biol Chem 286, 22855–22863. [DOI] [PMC free article] [PubMed] [Google Scholar]


