Abstract
Background:
Tamoxifen is one of the medicines for adjuvant endocrine therapy of hormone-dependent breast cancer. However, development of resistance to tamoxifen occurs inevitably during treatment. This study aimed to determine whether sensitivity of tamoxifen-resistant breast cancer cells (TAM-R) could be reinstated by tetrandrine (Tet).
Methods:
All experiments were conducted in TAM-R cells derived from the MCF-7 breast cancer cell line by long-term tamoxifen exposure. Cell growth, apoptosis, and autophagy were end-points that evaluated the effect of Tet (0.9 μg/ml, 1.8 μg/ml, and 3.75 μg/ml) alone or in combination with TAM (1 μM). Cell apoptosis was determined by an ELISA assay and autophagy was determined by fluorescent staining using the Enzo autophagy detection kit. Immunoblotting was used to evaluate markers for apoptosis, autophagy, and related signal pathway molecules.
Results:
Growth of TAM-R cells was significantly inhibited by Tet. Combination of Tet with tamoxifen induced a greater inhibition on cell growth than tamoxifen alone, which was predominantly due to enhancement of pro-apoptotic effect of TAM by Tet. Autophagy was significantly inhibited in TAM-R cells treated with Tet plus TAM as shown by increased autophagosomes and the levels of LC3-II and p62. At 0.9 μg/ml, Tet increased the levels of both apoptosis and autophagy markers. Among them increase in p53 levels was more dramatic.
Conclusions:
Tet as a monotherapy inhibits TAM-R cells. Tet potentiates the pro-apoptotic effect of TAM via inhibition of autophagy.
Keywords: tetrandrine, tamoxifen, tamoxifen-resistant breast cancer cells, apoptosis, autophagy
Introduction
Breast cancer is one of the most common malignancies in women with high mortality. So far, medication and surgery remain the primary choice for breast cancer.1 Tamoxifen (TAM) is an important drug used for endocrine therapy for breast cancer. It can inhibit the growth of breast tumor by altering the tumor hormone environment via an antiestrogenic effect.2 TAM-based endocrine therapy is the standard treatment for breast cancer and proves to be highly effective. However, secondary resistance to TAM during treatment has limited its benefits.3 Understanding the mechanism of secondary resistance and developing new strategies to delay or prevent TAM-resistance has been the focus of preclinical research. One strategy is the combination therapy that could enhance the efficacy of tamoxifen in TAM-R cells.4
Tetrandrine [(1b)-6,6′,7,12-tetramethoxy-2,2′-dimethyl-berbaman] (Tet) is an extract from the root tuber of Stephania tetrandra, and the active constituent is bisbenzylisoquinoline alkaloid. Tet has been widely used as an effective agent to treat patients with hypertension, arthritis, arrhythmia, inflammation, and silicosis in traditional Chinese medicine.5 Much research has shown that Tet has great potential in cancer therapy.6 Tet inhibits proliferation and induces apoptosis in a number of cancer cells.7-10 Tet could also enhance sensitivity of drug-resistant cancer cells to chemotherapy in combination treatment.11-14
Our previous studies have indicated that Tet could reverse tamoxifen resistance of TAM-R cells.15 But the mechanism is still unclear. We also found that autophagy might be a major cause of tamoxifen resistance in breast cancer cells (MCF-7).16 This study discussed the effect of Tet on the apoptosis and autophagy of TAM-R cells cultured in vitro and assessed if Tet can be an agent to overcome TAM resistance so as to provide reference for clinical treatment.
Materials and Methods
Cell Lines
TAM-R cells were developed by culturing MCF-7 cells in the medium containing 10−7 M tamoxifen for at least 1 year (Figure 1).17 The addition of TAM was discontinued 1 week before the experiment. The cells were then cultured in IMEM medium (Corning, USA) supplemented with 5% fetal bovine serum (Invitrogen, USA) and 1% penicillin/streptomycin (Invitrogen, USA).
Figure 1.
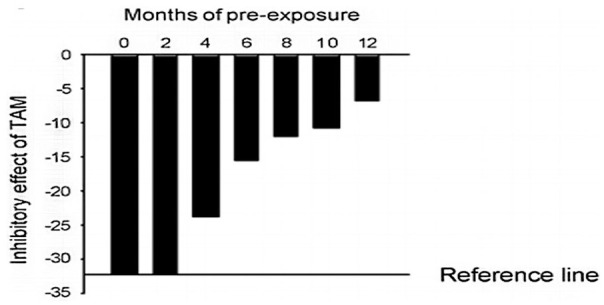
Response of MCF-7 cells to tamoxifen after long term tamoxifen exposure. MCF-7 cells were continuously cultured in phenol-red and FBS containing medium with 10−7 M tamoxifen. Characteristically, 5-day treatment of MCF-7 cells with tamoxifen (10−7 M) in the medium containing phenol red and 5% FBS causes 30% to 35% reduction in cell number. The inhibitory effect of TAM was gradually diminished in the cells continuously exposed to TAM over a period of 12 months (TAM-R).
Determination of Cell Growth
TAM-R cells were inoculated to a 6-well plate at a density of 6 × 104 cells per well in IMEM supplemented with 5% FBS. The culture medium was discarded 2 days later and Tet or TAM at different concentrations or control was added. The culture medium was replaced with fresh culture medium on day 3. After culture, TAM-R cells were washed with PBS. Sequentially, the nuclei were prepared by continuous addition of 2 ml HEPES-MgCl2 solution (0.01 mol/l HEPES and 1.5 mol/l MgCl2) and 0.2 ml ZAP solution (0.13 mol/l ethylhexadecyldimethylammonium bromide, 3% glacial acetic acid [v/v]). Then, the nuclei were counted by using a Z1 Coulter Counter.
Determination of Apoptosis
Apoptosis activity was assayed using the Cell Death ELISA kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instruction. Briefly, cells were inoculated to a 12-well plate at a density of 8 × 104 cells per well. Two days later, the cells were treated with testing compounds for 3 days. After cultivation, the cells were incubated with 0.5 ml lysis buffer at room temperature at 4°C for the preparation of cell lysates. In the meantime, a parallel plate with the same treatment was prepared for cell counting. Apoptosis was expressed by absorbance at OD405nm per 10 000 cells.
Immunoblotting
TAM-R cells were cultured in 60 mm culture dishes in 5% FBS-IMEM. When the cells were approximately 80% confluent, corresponding medium was added into the culture dish for each treatment. After culture for 24 hours, the cells were washed once with PBS and then treated with 0.5 ml of lysis buffer containing protease inhibitor on ice for 5 minutes. The cells were harvested with a scraper into a 1.5 ml centrifuge tube and centrifuged at 4°C and 14 000 r/min for 10 minutes. Then, cell lysate was stored at −80°C until quantitative analysis. A Standard Bradford assay was performed to determine total protein content in the lysate using Bio-Rad reagent (Hercules, CA, USA). On the 10% SDS-PAGE, 50 μg of total proteins were isolated and transferred to the nitrocellulose membrane. The membrane was incubated with primary antibody in TBS supplemented with 5% bovine fetal serum at room temperature for 2 hours followed by 1 hour incubation with Irdye-conjugated secondary antibody (LiCor, Lincoln, NE, USA). The membranes were scanned using the Odyssey imager (LiCor Inc., Lincoln, NE, USA) and protein bands quantitated.
Cell Autophagy Detection
TAM-R cells were placed on cover slips in 6-well plates in IMEM containing 5% FBS. When the cells became adherent, fresh culture mediums containing treating agents were added and incubated for 24 hours. Cell autophagy was detected using CYTO-ID Autophagy Detection kit (Enzo Life Sciences Inc., Farmingdale, NY, USA) according to the manufacturer’s instruction. Autophagosomes were stained with green dye, and the nuclei were stained with Hoechst 33342 dye. Images were captured under the Olympus IX81 microscope.
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 software. Measurements were expressed as mean ± standard deviation (x ± s), and intergroup comparisons were performed by t-test. Counts were represented as percentages (%), and intergroup comparisons were performed by using the chi-square test. P < .05 indicated significant difference.
Results
Effects of TAM Alone or in Combination with Tet on the Growth of TAM-R Cells
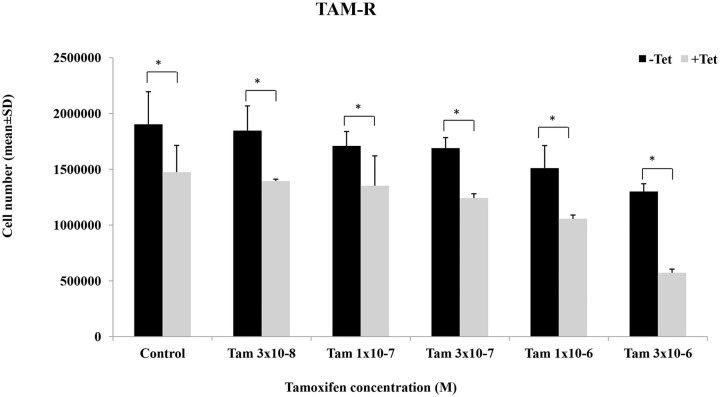
Effects of TAM alone and in combination with Tet on cell growth were examined in TAM-R cells. Treatment with various concentrations of TAM alone for 5 days caused dose-dependent inhibition of cell growth but the extent of inhibition was small (Figure 2). Cell number was reduced by ~30% in the cells treated with the highest concentration of TAM. Tet also induced dose-dependent inhibition of growth of TAM-R cells (data not shown). We have chosen a concentration of Tet (1.8 µg/ml) that slightly inhibited cell growth. When combined with Tet, the inhibitory effect was greater than TAM alone (P < .05). These results indicate that Tet has a potential to increase the sensitivity of TAM-R cells to tamoxifen.
Figure 2.
Effects of Tet and TAM on the growth of TAM-R cells. Cell growth assay of TAM-R cells treated with Tam ± Tet (1.8 µg/ml) for 5 days. Seeding cells number was 6 × 104 cells/well in 6-well plate in IMEM containing 5% FBS.
*P < .05.
Effects of Tet and TAM on the Apoptosis of TAM-R Cells
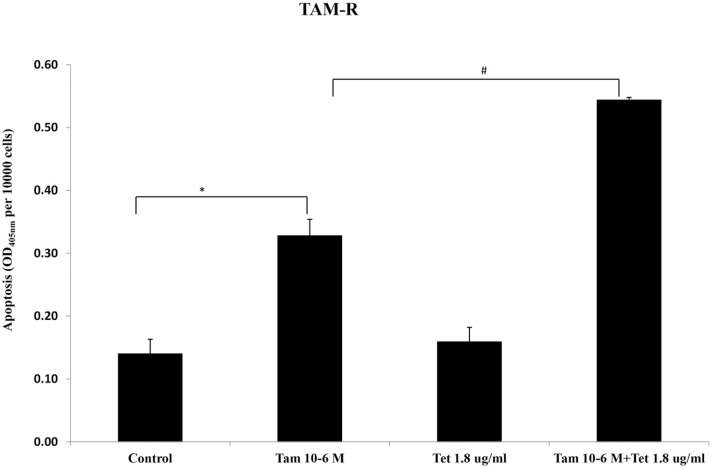
To further investigate mechanism of cell growth inhibition by Tet and TAM, the effect of these agents on apoptosis was examined by an ELISA assay. As shown in Figure 3, TAM alone (10−6 M) induced a 2-fold increase in apoptosis of TAM-R cells. While Tet alone at 1.8 µg/ml had no effect on apoptosis, combination of Tet and TAM dramatically increased apoptosis (P < .05) (Figure 3).
Figure 3.
Effects of Tet and TAM on the number of apoptotic TAM-R cells. TAM-R cells were inoculated to a 12-well plate at a density of 8 × 104 cells/well in IMEM containing 5% FBS. Two days later, the cells were treated with Tam (10−6 M) ± Tet (1.8 µg/ml) for 3 days.
*P < .05, compared with control group.
#P < .05, compared with Tam (10−6 M) + Tet (1.8 µg/ml) group.
Effects of Tet on the Apoptotic Markers in TAM-R Cells
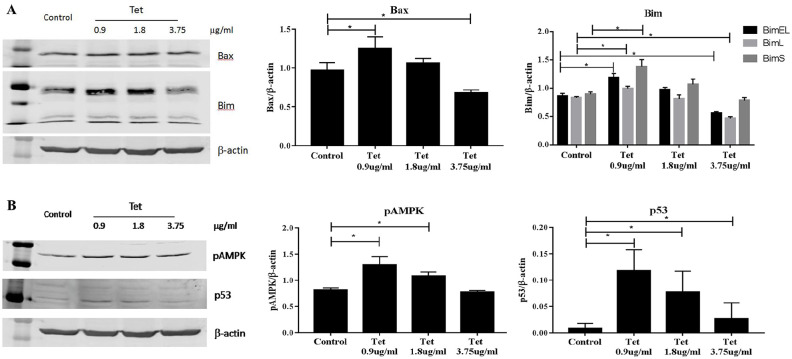
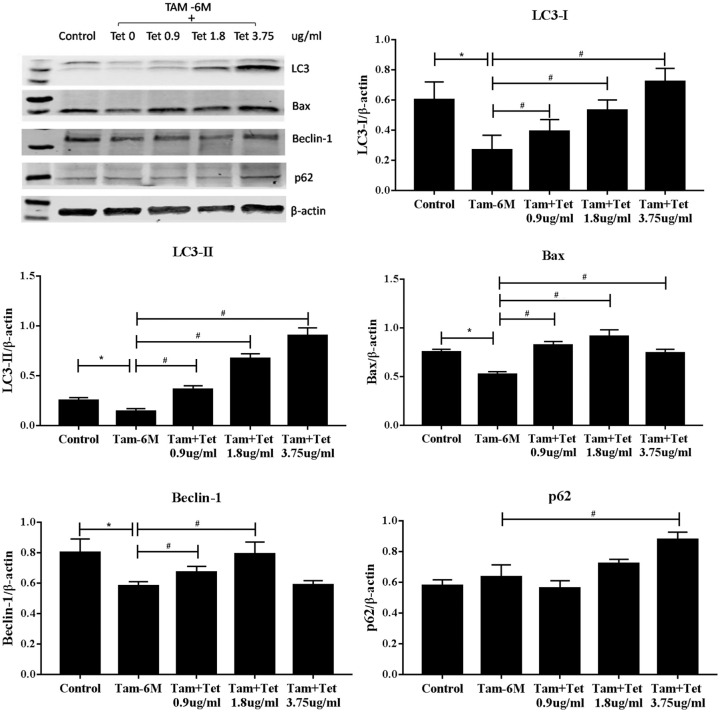
We then determined the effect of different doses of Tet on the expressions of apoptotic markers in the TAM-R cells. Three doses of Tet, 0.9 μg/ml, 1.8 μg/ml, and 3.75 μg/ml were used. Compared with the control group, the expressions of apoptotic markers Bax and Bim exhibited bi-phasic changes, for example. Both markers were higher in the cells treated with 0.9 μg/ml Tet but lower than the control at higher concentration (Figure 4A). These were consistent with the apoptosis assay (Figure 3). Our previous studies have demonstrated that Bax and Bim expressions are regulated by AMPK/FoxO3 pathway.18 The levels of phosphorylated AMPK also increased at low concentration of Tet and reduced to the control level at 3.75 µg/ml (Figure 4B). p53 is another factor that is involved in DNA repair and regulation of apoptosis and autophagy. Treatment with Tet induced significant increases of p53 but the effect was inversely correlated with Tet concentration (Figure 4B).
Figure 4.
Effects of Tet on the apoptotic markers in TAM-R cells. TAM-R cells were plated in 60 mm dishes in IMEM containing 5% FBS. When cells were about 80% of confluence, the cells were treated with Tet (0.9 µg/ml, 1.8 µg/ml, 3.75 µg/ml) for 24 hours in the same culture medium. (A) Effects of Tet on the apoptotic markers Bax and Bim in TAM-R cells. (B) Effects of Tet on the expressions of pAMPK and p53 in TAM-R cells.
*P < .05, compared with corresponding control group.
Effects of Tet Alone or in Combination with TAM on the Autophagy Markers in TAM-R Cells
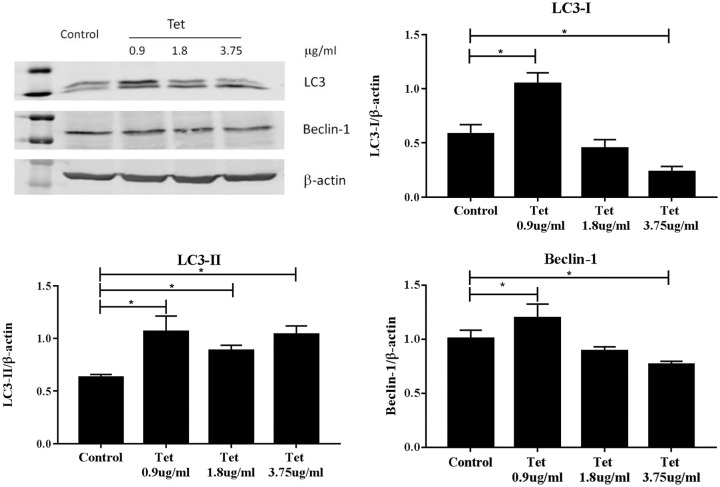
Autophagy is an important survival mechanism of mammalian cells especially in the situation of nutrient shortage. It has been reported that autophagy and apoptosis are mutually regulated in cancer cells. To explore whether the effect of Tet on TAM-R cell growth and apoptosis had to do with autophagy, we first examined the effect of Tet alone on autophagy markers LC3 and Beclin-1. These 2 proteins are incorporated into autophagosomes during autophagy. LC3-I was reduced in a dose-dependent fashion in TAM-R cells treated with Tet. In contrast, LC3-II was higher in all treated cells (Figure 5). Increase in Beclin-1 was only seen in the cells exposed to 0.9 μg/ml Tet.
Figure 5.
Effects of Tet on the expressions of autophagy markers LC3 and Beclin-1 in TAM-R cells. TAM-R cells were plated in 60 mm dishes in IMEM containing 5% FBS. When cells were about 80% of confluence, the cells were treated with Tet (0.9 µg/ml, 1.8 µg/ml, 3.75 µg/ml) for 24 hours in the same culture medium.
*P < .05, compared with control group.
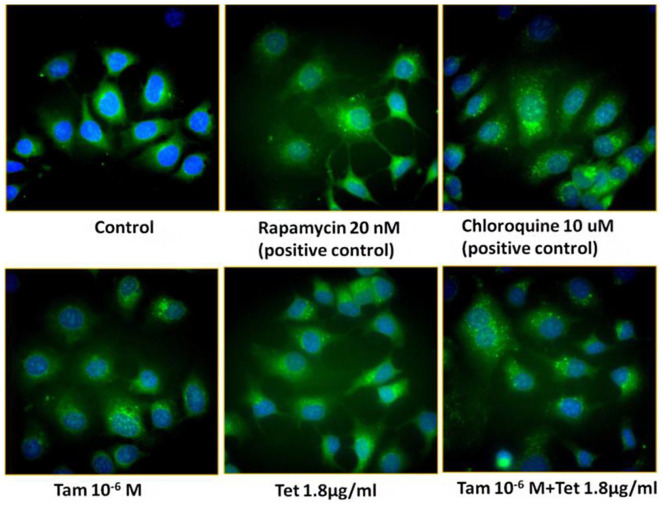
Combination of TAM (10−6 M) with different concentrations of Tet dose-dependently increased LC3-I, more significantly with LC3-II (Figure 6). While increases in LC3-II levels usually indicate enhanced autophagy, it also happens when the process of autophagy is blocked at later stage. For this reason, we also looked at p62, an indicator of autophagic flux (Figure 6). p62 levels are inversely correlated with autophagy activity, for example, the lower the p62 levels the higher the autophagy flux. Blockade of autophagy at the step of lysosome degradation will increase the levels of p62 as well as LC3-II which is the situation seen in the cells treated with chloroquine, a lysosome protease inhibitor (Figure 7). Consistently with LC3-II, p62 levels were also increased in a dose-dependent fashion in the cells treated with TAM + Tet (Figure 6). The level of Beclin-1 was lower in TAM-treated cells than the control but addition of Tet (2 lower concentrations) increased Beclin-1 (Figure 6). These data suggested that Tet had a trend to inhibit autophagy that was more significant when combined with TAM. This impression was confirmed by fluorescent microscopy of autophagy (Figure 7). In this experiment, 2 positive controls were included: rapamycin, an autophagy inducer and chloroquine, an autophagy inhibitor acting at the autophagolysosome. As shown in Figure 7, rapamycin increased the number of green punctate autophagosomes. Green punctate autophagosomes were more numerous and coarser in chloroquine-treated cells because autophagic flux was blocked. There was a moderate increase in the number of green punctate autophagosomes in the cells treated with TAM or Tet alone. A further increase was seen when TAM and Tet were combined. These results suggest that Tet might inhibit autophagy via a mechanism similar to chloroquine; however, further study is needed to confirm this. The Bax expression increased in all TAM + Tet groups, and the difference was the largest in the TAM + 1.8 μg/ml Tet group (P < .05).
Figure 6.
Effects of Tet combined with TAM on the expressions of LC3, Bax, Beclin-1, and p62 in TAM-R cells. TAM-R cells were plated in 60 mm dishes in IMEM containing 5% FBS. When cells were about 80% of confluence, the cells were treated with Tam (10−6 M) ± Tet (0.9 µg/ml, 1.8 µg/ml, 3.75 µg/ml) for 24 hours in the same culture medium.
*P < .05, compared with control group.
#P < .05, compared with Tam (10−6 M) + Tet group.
Figure 7.
Fluorescence microscopy of autophagy of TAM-R cells. TAM-R cells were grown on cover slip in 6-well plates in IMEM containing 5% FBS. When the cells were attached, fresh medium with treatment agents were added and incubated for 24 hours.
Discussion
TAM has been a first-line medicine for endocrine therapy of hormone-dependent breast cancer, which blocks the binding of estrogen to its receptor, thus exerting an antiestrogenic effect. TAM resistance of breast cancer cells has severely impaired the efficacy of treatment. Therefore, overcoming TAM resistance represents a major direction of clinical study. It has been indicated that TAM has an anti-cancer effect mainly by inhibiting the growth of breast cancer cells instead of directly killing the cancer cells.19,20 Mechanisms for acquired resistance to tamoxifen appear very complex and are dominated by crosstalk between ER and growth factor signaling pathways, the presence of ER-negative undifferentiated cells, cell fate regulation through autophagy or apoptosis, antioxidant protein-gene regulation, and genetic polymorphisms of a specific tamoxifen-metabolizing enzyme.21
Autophagy and apoptosis are 2 important pathways that regulate cell fate in response to stresses. Autophagy plays dual roles in regulating cell fates; it can act as a cell survival mechanism when extracellular nutrients or growth factors are limited, or as an alternative cell death pathway to apoptosis when stress level is over the threshold. When the stress level is low, the autophagy pathway prevails to promote cell survival. If the stress level remains high and cannot be corrected, apoptosis pathway will be activated. Autophagy and apoptosis are mutually regulated. In most circumstances, the crosstalk of these 2 pathways is inhibitory and autophagy usually precedes apoptosis.22 A number of studies have shown that autophagy is a mechanism of TAM resistance,23 and inhibition of autophagy has a potential to restore TAM sensitivity.24,25
Induction of autophagy occurs in breast cancer cells which might be 1 mechanism responsible for acquired tamoxifen resistance. Suppressing autophagy could be a novel strategy to overcome or delay development of tamoxifen resistance. Our TAM-R cells showed increased basal levels of LC3 and Beclin-1 compared with wild type MCF-7 cells. TAM treatment accelerate the progress of autophagic flux that is manifested by reduction of LC3-II, Beclin-1, and p62 because of lysozyme digestion of autophagy cargos.16 Our result has shown that combination with Tet significantly increased the apoptotic effect of TAM (Figure 3).
Enhanced apoptosis in the cells treated with Tam plus Tet is not an additive effect of these 2 drugs because Tet alone at the concentration used did not induce apoptosis. Compared to TAM-treated cells 1 significant change in TAM + Tet (1.8 µg/ml) treated cells is the increase in LC3-II and Beclin-1 (Figure 6). Increases in the levels of these 2 autophagy markers could represent 2 opposite situations: increased autophagy activity or inhibition of autophagy. To distinguish these 2 situations, a third marker indicating autophagic flux is necessary. Sequestosome 1 (SQSTM1, p62), is such a marker. p62 is an ubiquitin-binding protein involved in protein degradation of the ubiquitin-proteasome system (UPS) and endolysosome-autophagic system. The levels of p62 reduce with increased autophagy activity and vise versa.26 In TAM + Tet (1.8 µg/ml) treated cells, p62 is also increased which indicates autophagy was inhibited rather than increased. Inhibition of autophagy by Tet apparently occurred in late stage via interrupting the process of enzymatic digestion of the autophagosomes. Qiu et al27 reported that Tet is a potent lysosomal deacidification agent and therefore blocks autophagic flux. This effect is similar to that of chloroquine, a known inhibitor of lysozyme (Figure 7).
Other mechanisms may also involve. p53 expression which was dramatically increased by Tet (Figure 4). This molecule participates in regulation of many cellular activities including apoptosis and autophagy. Cytosolic p53 can repress autophagy by interacting with autophagy protein FIP200 and reduction of autophagosome formation.22 On the other hand, the transcriptional function of p53 up-regulates pro-apoptotic factors such as Bax, Bim, and PUMA.22 It also induces cell cycle arrest by induction of p21 expression and CDK2 inhibition.28 Therefore, enhanced expression of p53 by Tet might be an important mechanism promoting apoptosis of TAM-R cells.
Bcl-2 family comprises a group of important regulatory proteins of cell apoptosis, including survival-promoting factor Bcl-2 and pro-apoptotic factors Bax and Bim. In the present study, Tet as monotherapy increased expression of pro-apoptotic factors Bax and Bim in TAM-R cells. Up-regulation of Bax and Bim by Tet is the result of activation of AMPK and p53. The pro-apoptotic effect of Tet plus its inhibition of autophagy facilities the TAM-R cells in the direction of apoptotic death when combined with TAM. Increased Bim could also bind to Beclin-1 to inhibit autophagy, a mechanism attributable to the effect of Tet in TAM-R cells. Our results suggest that Tet has a potential to re-sensitize TAM-R cells to tamoxifen. Tet was reported to inhibit proliferation of chemo-resistant breast cancer cells when combined with doxorubicin.29
Tet inhibits growth of many different kind of cancer cells including breast cancer. The present study provides experimental evidence that Tet has a potential to re-sensitize tamoxifen resistant MCF-7 cells to the inhibitory effect of tamoxifen. Our data indicate that targeting autophagy could be new strategy to enhance efficacy and therapeutic duration of tamoxifen.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Young Scientists Fund of the National Natural Science Foundation of China (No. 81503302) and the Dongfang Hospital affiliated to Beijing University of Traditional Chinese Medicine, 1166 Development Program for Junior Scientists (No. 030903010332).
ORCID iD: Haiyan Chen  https://orcid.org/0000-0002-5829-5783
https://orcid.org/0000-0002-5829-5783
References
- 1. Carter P, Alifrangis C, Cereser B, et al. Molecular profiling of advanced breast cancer tumors is beneficial in assisting clinical treatment plans. Oncotarget. 2018;9:17589-17596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ye P, Fang C, Zeng H, et al. Differential microRNA expression profiles in tamoxifen-resistant human breast cancer cell lines induced by two methods. Oncol Lett. 2018;15:3532-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferreira AR, Palha A, Correia L, et al. Treatment adoption and relative effectiveness of aromatase inhibitors compared to tamoxifen in early breast cancer: a multi-institutional observational study. Breast. 2018;37:107-113. [DOI] [PubMed] [Google Scholar]
- 4. Liang Y-K, Zeng D, Xiao Y-S, et al. MCAM/CD146 promotes tamoxifen resistance in breast cancer cells through induction of epithelial–mesenchymal transition, decreased ERα expression and AKT activation. Cancer Lett. 2017;386:65-76. [DOI] [PubMed] [Google Scholar]
- 5. Shen Y-C, Chou C-J, Chiou W-F, Chen C-F. Anti-inflammatory effects of the partially purified extract of radix Stephaniae tetrandrae: comparative studies of its active principles tetrandrine and fangchinoline on human polymorphonuclear leukocyte functions. Mol Pharmacol. 2001;60:1083-1090. [PubMed] [Google Scholar]
- 6. Liu T, Liu X, Li W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget. 2016;7:40800-40815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lien J-C, Lin M-W, Chang S-J, et al. Tetrandrine induces programmed cell death in human oral cancer CAL 27 cells through the reactive oxygen species production and caspase-dependent pathways and associated with beclin-1-induced cell autophagy. Environ Toxicol. 2017;32:329-343. [DOI] [PubMed] [Google Scholar]
- 8. Singh K, Dong Q, TimiriShanmugam PS, Koul S, Koul HK. Tetrandrine inhibits deregulated cell cycle in pancreatic cancer cells: differential regulation of p21Cip1/Waf1, p27Kip1 and cyclin D1. Cancer Lett. 2018;425:164-173. [DOI] [PubMed] [Google Scholar]
- 9. Yu VW, Ho WS. Tetrandrine inhibits hepatocellular carcinoma cell growth through the caspase pathway and G2/M phase. Oncol Rep. 2013;29:2205-2210. [DOI] [PubMed] [Google Scholar]
- 10. Liu W, Kou B, Ma Z-K, et al. Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells. Asian J Androl. 2015;17:850-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao X, Luo J, Gong T, Zhang Z-R, Sun X, Fu Y. Coencapsulated doxorubicin and bromotetrandrine lipid nanoemulsions in reversing multidrug resistance in breast cancer in vitro and in vivo. Mol Pharm. 2015;12:274-286. [DOI] [PubMed] [Google Scholar]
- 12. Ye L-Y, Hu S, Xu H-E, et al. The effect of tetrandrine combined with cisplatin on proliferation and apoptosis of A549/DDP cells and A549 cells. Cancer Cell Int. 2017;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu Y, Li F, Xu T, Sun J. Tetrandrine prevents multidrug resistance in the osteosarcoma cell line, U-2OS, by preventing Pgp overexpression through the inhibition of NF-κB signaling. Int J Mol Med. 2017;39:993-1000. [DOI] [PubMed] [Google Scholar]
- 14. Wang K, Ma L, Bu GB, et al. Tetrandrine reverses the drug resistance of colon cancer to 5-fluorouracil. Biomed Res. 2017;28:4843-4848. [Google Scholar]
- 15. Chen HY, Chen XY. Tetrandrine reversed the resistance of tamoxifen in human breast cancer MCF-7/TAM cells: an experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi [Chinese journal of integrated traditional and Western medicine]. 2013;33:488-491. [PubMed] [Google Scholar]
- 16. Liu JX, Yue W, Chen HY. The correlation between autophagy and tamoxifen resistance in breast cancer. Int J Clin Exp Pathol. 2019;12:2066-2074. [PMC free article] [PubMed] [Google Scholar]
- 17. Yue W, Fan P, Wang J, Li Y, Santen RJ. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen HY, Wang JP, Santen RJ, Yue W. Adenosine monophosphate activated protein kinase (AMPK), a mediator of estradiol-induced apoptosis in long-term estrogen deprived breast cancer cells. Apoptosis. 2015;20:821-830. [DOI] [PubMed] [Google Scholar]
- 19. Zhang H-Y, Liang F, Zhang J-W, Wang F, Wang L, Kang X-G. Effects of long noncoding RNA-ROR on tamoxifen resistance of breast cancer cells by regulating microRNA-205. Cancer Chemother Pharmacol. 2017;79:327-337. [DOI] [PubMed] [Google Scholar]
- 20. Bui QT, Im JH, Jeong SB, et al. Essential role of Notch4/STAT3 signaling in epithelial–mesenchymal transition of tamoxifen-resistant human breast cancer. Cancer Lett. 2017;390:115-125. [DOI] [PubMed] [Google Scholar]
- 21. Chang M. Tamoxifen resistance in breast cancer. Biomol Ther (Seoul). 2012;20:256-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samaddar JS, Gaddy VT, Duplantier J, et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7:2977-2987. [DOI] [PubMed] [Google Scholar]
- 24. Qadir MA, Kwok B, Dragowska WH, et al. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res Treat. 2008;112:389-403. [DOI] [PubMed] [Google Scholar]
- 25. Crawford AC, Riggins RB, Shajahan AN, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PloS One. 2010;5:e8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi YK, Cho S-G, Choi Y-J, et al. SH003 suppresses breast cancer growth by accumulating p62 in autolysosomes. Oncotarget. 2016;8:88386-88400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu W, Su M, Xie F, et al. Tetrandrine blocks autophagic flux and induces apoptosis via energetic impairment in cancer cells. Cell Death Dis. 2014;5:e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roy S, Banerjee S, Chakraborty T. Vanadium quercetin complex attenuates mammary cancer by regulating the P53, Akt/mTOR pathway and downregulates cellular proliferation correlated with increased apoptotic events. Biometals. 2018;31:647-671. [DOI] [PubMed] [Google Scholar]
- 29. Luo J-W, Zhang T, Zhang Q, et al. A novel injectable phospholipid gel co-loaded with doxorubicin and bromotetrandrine for resistant breast cancer treatment by intratumoral injection. Colloids Surf B Biointerfaces. 2016;140:538-547. [DOI] [PubMed] [Google Scholar]