Abstract
Purpose:
Morphine is often used for the treatment of moderate and severe cancer pain, but long-term use can lead to morphine tolerance. Methods for effectively inhibiting morphine tolerance and the related mechanism of action are of great significance for the treatment of cancer pain. Previous studies have shown that electroacupuncture (EA) can inhibit the occurrence of morphine tolerance, but the mechanism is not yet clear. The aim of the present study was to explore the signaling pathway by which EA attenuates the development of bone cancer pain (BCP)-morphine tolerance (MT).
Materials and methods:
Changes in the paw withdrawal threshold (PWT) of rats with bone cancer pain-morphine tolerance were observed in a study of EA combined with intrathecal injection of a PI3K inhibitor (LY294002) or agonist (insulin-like growth factor-1 [IGF-1]). We also tested the protein expression of phosphorylated phosphatidylinositol 3-kinase (p-PI3K), phosphorylated protein kinase B (p-Akt), phosphorylated c-Jun NH2-terminal kinase 1/2 (p-JNK1/2), and β-arrestin2 in the L4-6 spinal dorsal horn of rats.
Results:
The protein expression of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 was upregulated in the L4-6 spinal dorsal horn of rats with bone cancer pain and bone cancer pain-morphine tolerance. EA delayed the occurrence of morphine tolerance in rats with bone cancer pain and downregulated the protein expression of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the L4-6 spinal dorsal horn of rats with bone cancer pain-morphine tolerance. Intrathecal injection of LY294002 attenuated the development of morphine tolerance and downregulated the protein expression of p-Akt, p-JNK1/2, and β-arrestin2 in the spinal dorsal horn of rats with bone cancer pain-morphine tolerance. In addition, the inhibitory effect of EA on morphine tolerance was reversed by IGF-1.
Conclusion:
The mechanism underlying the ability of EA to attenuate morphine tolerance may be associated with inhibition of the PI3K/Akt/JNK1/2 signaling pathway.
Keywords: electroacupuncture, bone cancer pain, morphine tolerance, LY294002, IGF-1, PI3K/Akt, JNK1/2
Introduction
Morphine is an opioid analgesic that is effective for treating many kinds of pain, particularly moderate and severe cancer pain. However, long-term morphine treatment can lead to several negative effects, such as addiction, physical dependence, nausea, constipation, dysuria, lethargy, and potentially life-threatening respiratory depression.1-3 Furthermore, it also results in morphine tolerance, defined the gradual loss of the potency or efficacy of the drug and the shortening of its duration of action,4 which leads to the need for increased doses to achieve the same analgesic effect.5-7 Morphine tolerance leads to severe challenges for clinical pain management. Identifying ways to reduce the development of morphine tolerance is a pivotal issue in clinical pain treatment.
Acupuncture is a safe and effective treatment for various types of acute and chronic pain, including sciatica, migraine, dysmenorrhea, cancer pain, osteoarthritis, etc. Acupuncture is a first-line treatment that is recommended for pain before opioids are prescribed and may reduce opioid use or opioid addiction.8 Electroacupuncture (EA), which is based on traditional acupuncture combined with electric pulse stimulation, has been used as an adjuvant therapy for many clinical conditions to reduce adverse drug reactions or side effects and improve the effectiveness of treatment.9 Studies have shown that opioid peptides (µ-, δ-, and κ-receptors), glutamate, cholecystokinin octapeptide, noradrenalin, and 5-hydroxytrypatamine are involved in acupuncture analgesia. Therefore, opioid peptides and their receptors in arcuate nucleus-periaqueductal gray-nucleus raphe magnus-spinal dorsal horn pathway play an essential role in mediating acupuncture analgesia.10 Han11 found that EA at 2 Hz accelerated the release of endorphin, β-endorphin, and enkephalin, while EA at 100 Hz selectively increased the release of dynorphin. EA at 2/100 Hz induces the simultaneous release of all 4 opioid peptides, generating the greatest therapeutic effect. Our and other research teams have observed that EA intervention can reduce the development of morphine tolerance, which is associated with an increase in μ-opioid receptor (MOR) expression and a decrease in calcitonin-gene-related peptide (CGRP) expression.12,13 It has been reported that activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)-mitogen-activated protein kinase (MAPK) signaling pathway in the spinal cord underlies the development of morphine tolerance.14 c-Jun NH2-terminal kinase 1/2 (JNK1/2), a member of the MAPK family, plays an important role in mediating antinociception and chronic tolerance to morphine in acute, inflammatory, and neuropathic pain states.15 However, whether the effect of EA on morphine tolerance is involved in modulating the function of the PI3K/Akt/JNK1/2 signaling pathway is still not clear. In this paper, we explored whether the PI3K/Akt/JNK1/2 signaling pathway is involved in the effect of EA on morphine tolerance.
Material and Methods
Experimental Animals
Adult female Sprague-Dawley rats weighing 140 to 180 g (Shanghai Slac Laboratory Animal Co., Ltd., China) were raised in the animal experimental center of Zhejiang Chinese Medical University. The rats were provided free access to food and water and caged under comfortable conditions (12-hour light/dark cycle, temperature of 24°C ± 1°C, and humidity of 40%-60%). The rats were habituated for 1 week before the experimental operation. All experimental procedures were performed in accordance with the Guidelines for the Humane Treatment of Laboratory Animals issued by The Ministry of Science and Technology of the People’s Republic of China. The experiments were approved by the Animal Ethics Committee of the university. All in vivo experiments strictly complied with animal ethics requirements (No. 20180319-12).
Intrathecal Catheterization
A total of 59 rats underwent surgery under gaseous anesthesia with an isoflurane mixture (RWD, Shenzhen, Guangdong, China; 3% for induction and 1.5% for maintenance) at a 1:1 flow ratio of air/O2 (1 L/min for induction and 0.5 L/min for maintenance).16 Intrathecal catheterization was carried out according to the methods of Bian Juhua and others.17 The rats were placed in a prone position. A cylinder (diameter of 3-5 cm) was placed under the abdomen to support the back. The fur was shaved from the skin prior to skin disinfection. After skin disinfection, a longitudinal incision approximately 1 cm in length was made along the L6 spinous process, and then the skin and fascia were cut. The L5-L6 spinous processes were exposed by bluntly separating the muscle. A PE-10 catheter (Smiths Medical, Hangzhou, Zhejiang, China) was implanted between the L5 and L6 spinous processes by a guide cannula.18 The cannula was inserted until there was no longer a sense of breakthrough or resistance and there was a flick of the rat’s tail, which indicated that the cannula had entered the canalis vertebralis. The guide cannula was removed, and the PE-10 catheter was slowly inserted into the cavum subarachnoidale; cerebrospinal fluid clearly spilled out, and the depth of the catheter was determined to be 2.5 cm. The proximal catheter outside the muscle was wound into a small ring of “lying 8,” and the left and right sides were sutured and fixed on the muscle and fascia, respectively. Then, the skin was sutured. The other end of the catheter was guided to the back of the neck through a subcutaneous tunnel with an epidural puncture needle. The catheter was sealed with hot glue to prevent leakage of cerebrospinal fluid, and the distal catheter was fixed to the skin. The muscle and skin were sutured. Then, penicillin (200 000 U) was injected intramuscularly. Each rat was housed in a single cage after the operation. Sustained-release buprenorphine (orb61125, Biorbyt, UK) was injected intramuscularly (0.6 mg/kg) to alleviate surgical pain. The behavior of the rats was observed for 24 hours after they awoke from surgery, and rats that were paralyzed or showed disability were eliminated from the experiment. A total of 3 rats were disabled after intrathecal catheterization. The success of intrathecal catheterization was determined by the paralysis of both hind limbs of the rats after intrathecal injection of 20 μl of 2% lidocaine followed by 10 μl of normal saline for flushing and by a lack of foot retraction after acupuncture or clamping.18 The total success rate of intrathecal catheterization was 79.66% (47/59).
Experimental Groups
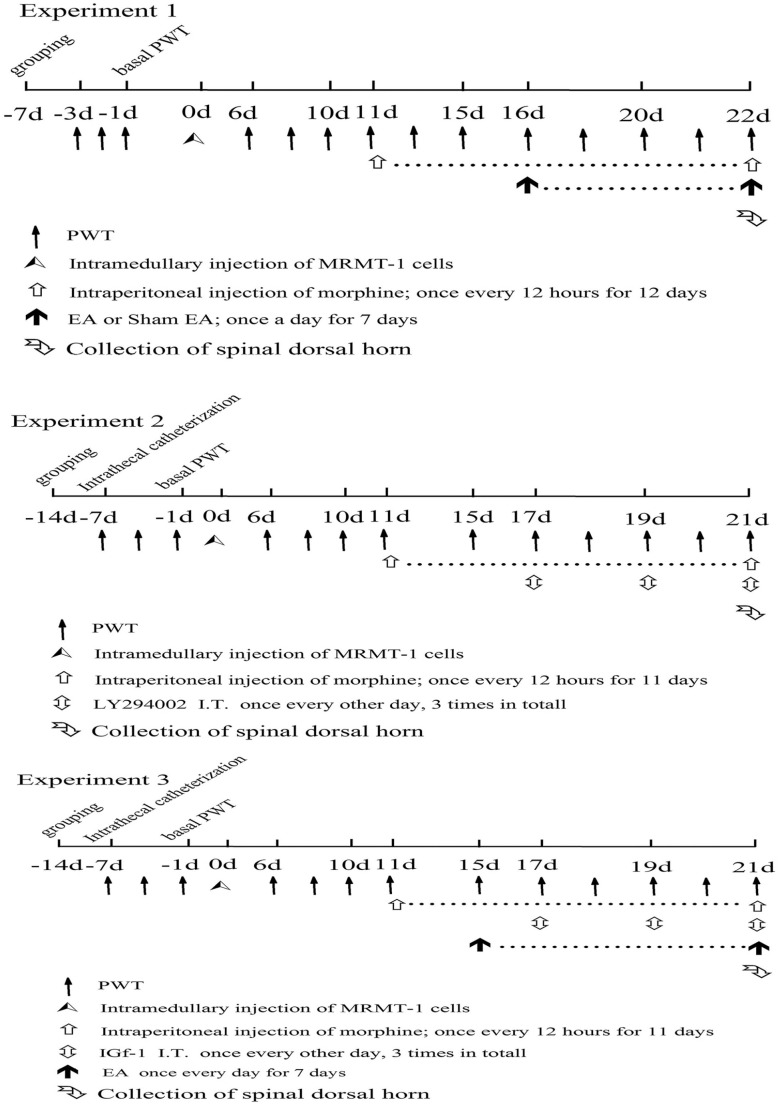
In experiment 1, the effect of EA on bone cancer pain-morphine tolerance was observed. The rats were randomly divided into 5 groups, with 10 rats in each group: the sham operation (sham) group, bone cancer pain (BCP) group, bone cancer pain-morphine tolerance (BCP + MT) group, bone cancer pain-morphine tolerance + EA (BCP + MT + EA) group, and bone cancer pain-morphine tolerance + sham EA (BCP + MT + sham EA) group (Figure 1). There were 10 rats in which surgical modeling failed, and those rats were excluded.
Figure 1.
The experimental protocol.
In experiment 2, the effect of a PI3K inhibitor (LY294002) on bone cancer pain-morphine tolerance was observed. The rats that underwent intrathecal catheterization were randomly allocated into 2 groups: the bone cancer pain-morphine tolerance + dimethyl sulfoxide (BCP + MT + DMSO) group (n = 14) and the bone cancer pain-morphine tolerance + PI3K inhibitor (BCP + MT + LY294002) group (n = 15) (Figure 1). The rats in which surgical modeling failed were excluded.
In experiment 3, the effect of a PI3K agonist (insulin-like growth factor-1 [IGF-1]) on the preventative effect of EA against bone cancer pain-morphine tolerance was observed. The rats that underwent intrathecal catheterization were randomly allocated into 2 groups, with 15 rats in each group: the bone cancer pain-morphine tolerance + PBS (BCP + MT + EA + PBS) group (n = 15) and the bone cancer pain-morphine tolerance + PI3K agonist (BCP + MT + EA + IGF-1) group (n = 15) (Figure 1). The rats in which surgical modeling failed were excluded.
Establishment of a Bone Cancer Pain-Morphine Tolerance Model
Cell culture
MRMT-1 rat mammary gland carcinoma cells (American Type Culture Collection [ATCC], Manassas, VA, USA) frozen in liquid nitrogen were thawed and subcultured. The cells were cultured in medium containing 89% RPMI-1640 (Gibco, Grand Island, NY, USA), 1% penicillin-streptomycin (Gibco), and 10% fetal bovine serum (FBS; Gibco) in humidified 95% air and 5% CO2 at 37°C.19 The medium was refreshed every 3 days. To harvest the cells, the culture medium was poured out, and the cells were detached from the culture bottle by brief exposure to 2 ml of 0.25% (w/v) trypsin (Gibco).20 Then, 8 ml of phosphate-buffered saline (PBS; Gibco) was added to the culture bottle to prevent cell destruction by trypsin. After repeatedly pipetting and washing them with a Pasteur pipette, the cells were first collected by centrifuging 10 ml of medium for 3 minutes at 1200 r/min.19,21 The collected cell pellet was washed twice with 10 ml of PBS. Finally, the cell pellet was resuspended in 1 ml of PBS, and the cells were counted using a cell counting plate (BIO-RAD, Hercules, CA, USA). The cells were diluted to achieve a final concentration of 1 × 107 cells/ml. The cell suspension was kept on ice until it was injected into the tibias of the rats.21
Model of bone cancer pain
Rats in the BCP, BCP + MT, BCP + MT + EA, BCP + MT + Sham EA, BCP + MT + DMSO, BCP + MT + LY294002, BCP + MT + EA + PBS, and BCP + MT + EA + IGF-1 groups were established as the bone cancer pain model. Rats were anesthetized with 2% isoflurane and placed in the supine position. A model of bone cancer pain was established as described in previous studies.20,21 The left hind limb of each rat was shaved, and the skin was disinfected with 75% alcohol. The left knee joint of the rat was fixed with the left hand. A 0.5 cm rostrocaudal incision was made over the top half of the tibia. The tibia was carefully exposed with minimal damage. Using a 20-gauge needle, the bone was pierced 5 mm below the knee joint (the edge of the skeletal ligament) for 1 cm along the tibial longitudinal axis to the distal end of the tibia.21,22 The needle was inserted at an angle to allow it to be pushed into the intramedullary canal of the bone.21 A microinjector was used to inject 3 μl of MRMT-1 mammary gland carcinoma cells (3 × 104 cells) into the marrow cavity of the left tibia.20,21 After 1 minute of injection, the microinjector was pulled out, and the hole was immediately sealed with sterile bone wax (Ethicon, Cincinnati, OH, USA) until there was no bleeding. The skin was sutured, and 200 000 U of penicillin was injected intramuscularly to prevent infection.
Rats in the sham group were injected with 3 μl of PBS into the marrow cavity of the left tibia, and the other procedures were the same as those performed in the bone cancer pain model.
Induction of bone cancer pain-morphine tolerance
The rats in the BCP + MT, BCP + MT + EA, BCP + MT + Sham EA, BCP + MT + DMSO, BCP + MT + LY294002, BCP + MT + EA + PBS, and BCP + MT + EA + IGF-1 groups were established as morphine tolerance models. After establishment of the bone cancer pain (BCP) model, the rats were injected with morphine hydrochloride (10 mg/kg, intraperitoneal injection; Shenyang First Pharmaceutical Factory, Liaoning, China)22 once every 12 hours (at 8:00 and 20:00) for 11 days to induce morphine tolerance.
The sham and BCP groups did not receive other interventions after the operation.
Intervention Method
EA intervention
The sham and BCP groups did not receive any interventions after the operation. The rats in the BCP + MT + EA, BCP + MT + EA + PBS, and BCP + MT + EA + IGF-1 groups were treated with EA (once a day in the morning) 30 minutes after morphine injection. Previous research had shown that bilateral acupoints selection was better than unilateral acupoint selection for analgesia.23 Therefore, we took bilateral acupoints for electroacupuncture treatment. The “Hou-san-li” acupoint is located at the lateral edge of the knee joint, 5 mm below the small head of the fibula. The “Gen-duan” acupoint is located in the depression between the lateral malleolus and the Achilles tendon. Stainless steel acupuncture needles (0.13 mm × 0.25 mm) were inserted vertically 5 mm into the bilateral “Hou-san-li” acupoint and obliquely 3 mm into the “Gen-duan” acupoint at an approximately 15° angle and connected to a HANS acupuncture point nerve stimulator (HANS-100B, Nanjing Jisheng Medical Technology Co., Ltd., Jiangsu, China). The stimulus parameters were as follows: stimuli at alternating frequencies of 2 and 100 Hz (automatically shifting between 2- and 100-Hz stimulation for 3 seconds each) and an intensity ranging from 0.5 to 1.5 mA (10 minutes each, a total of 30 minutes). Sham EA was also given 30 minutes after the injection of morphine hydrochloride. The bilateral “Hou-san-li” and “Gen-duan” acupoints were used. Stainless steel acupuncture needles were inserted subcutaneously, and the electrodes were connected, but the power switch was not turned on.
Intrathecal injection of LY294002
On the 17th, 19th, and 21st days after MRMT-1 cell implantation, the rats in the BCP + MT + LY294002 group were intrathecally injected with 20 μl of LY294002 (MCE, Monmouth Junction, NJ, USA) (dissolved in 5% DMSO, 0.25 μg/μl) 30 minutes before morphine injection.18,24 The rats in the BCP + MT + DMSO group were intrathecally injected with 20 μl of vehicle (saline containing 5% DMSO [Sigma, St Louis, MO, USA]) at the same time.24
Intrathecal injection of IGF-1
On the 17th, 19th, and 21st days after MRMT-1 cell implantation, the rats in the BCP + MT + EA + IGF-1 group were intrathecally injected with 20 μl of IGF-1 (dissolved in PBS, 0.36 μg/μl) 30 minutes before injection of morphine.25 The rats in the BCP + MT + EA + PBS group were intrathecally injected with 20 μl of PBS at the same time.
Paw Withdrawal Threshold
The pain behavior assessments were performed by a researcher blinded to the treatment group. Before testing, the rats were placed in large transparent plastic cages on metal mesh for 15 to 30 minutes to acclimate to the environment. Paw withdrawal threshold (PWT) was tested by a dynamic plantar aesthesiometer (Ugo Basile, 37450, Italy). When the rats were calm (stopped grooming and exploring), filaments with a diameter of approximately 0.5 mm were applied to the center of the left plantar pad of the rats, and the stimulus increased to a rate of 2.5 g/sec. When the animal withdrew its hind paw, the force of the stimulation, as recorded by the device, was considered the PWT. The maximum stimulation force was 50 g. Each rat was tested 5 times with at least 3 to 5 minutes intervals. The maximum and minimum values were excluded, and the average value was taken as the final PWT.26
Western Blotting
All rats were deeply anesthetized with pentobarbital sodium (100 mg/kg, I.P.) and transcardially perfused with 200 ml of 0.9% NaCl (4°C). The left dorsal horn of the L4-6 spinal cord was collected. Each rat’s dorsal horn was encapsulated separately and stored at −80°C. Western blotting was performed according to previously described methods.14 The spinal cord tissues were homogenized and lysed in RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing phenylmethylsulfonyl fluoride (PMSF) and phosphatase and protease inhibitor cocktails, and the supernatant was obtained after centrifugation at 14000 × g for 5 minutes. After protein quantification by the BCA method, 20 µg of protein from each sample was separated by electrophoresis on a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane.27 The PVDF membrane was blocked with 5% nonfat milk for 2 hours at room temperature and then incubated with the following primary antibodies overnight at 4°C: anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Proteintech Group, Chicago, IL, USA), anti-p-PI3K, anti-p-Akt, anti-p-JNK1/2, and anti-β-arrestin2 (Affinity, USA).28 The membrane was washed 3 times with 0.1% Tris-buffered saline/Tween-20 (TBST) for 10 minutes each and incubated with the corresponding secondary antibody (Jackson, West Grove, PA, USA) for 2 hours at room temperature. The blots were visualized with ECL reagent (Beyotime, Shanghai, China) and analyzed with Image Quant LAS4000 (GE, Chicago, IL, USA).28 The above procedures were carried out by personnel other than those in charge of treatment.
Statistical Analysis
All data are presented as the mean ± SEM. SPSS and Origin software were used for statistical analysis and plotting. Student’s t-test was used to compare parametric data between 2 samples. Comparisons of more than 2 groups were performed by one-way ANOVA followed by the LSD or Games-Howell test. Two-way repeated-measures ANOVA was used for PWT to determine the significance of differences between time points in the same group, and multifactor ANOVA was used for comparisons between groups at the same time point, followed by LSD post hoc tests. P < .01 or P < .05 was considered statistically significant.
Results
EA Delayed the Occurrence of Bone Cancer Pain-Morphine Tolerance
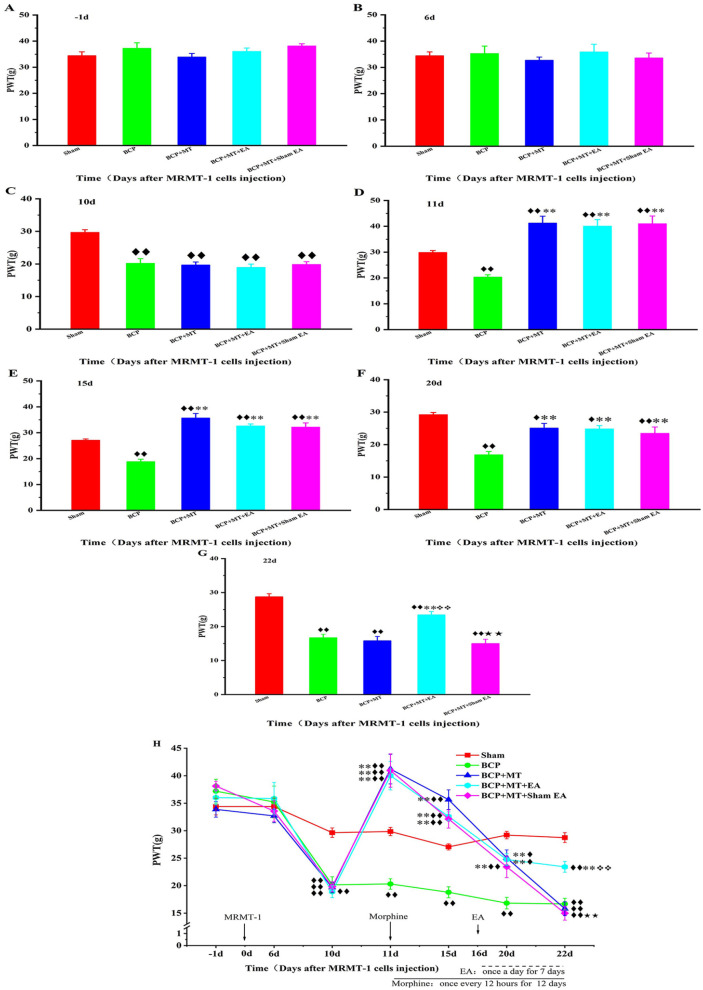
There was no significant difference in the basal (−1 day) PWT among the 5 groups before MRMT-1 cell implantation (P > .05, Figure 2A). In the intragroup comparison, the PWT of the BCP, BCP + MT, BCP + MT +EA, and BCP + MT + sham EA groups on the 10th day after the intramedullary injection of MRMT-1 cells was significantly lower than the basal PWT (Figure 2B and H). Compared with that of the sham group, the PWT of the BCP, BCP + MT, BCP + MT + EA, and BCP + MT + sham EA groups decreased significantly on the 10th day after intramedullary injection of MRMT-1 cells (P < .01, Figure 2C), and there was no significant difference among the 4 groups (P > .05). In addition, X-ray imaging showed bone destruction in the left tibia in rats with bone cancer pain. These results suggested that the bone cancer pain model was established successfully. Compared with that of the BCP group, the PWT of the BCP + MT, BCP + MT + EA and BCP + MT + sham EA groups increased significantly on the first day (11 days after the intramedullary injection of MRMT-1 cells) after morphine injection (P < .01), indicating that morphine injection had an obvious analgesic effect on bone cancer pain in rats (Figure 2D). After repeated morphine injection, the PWT of the BCP + MT, BCP + MT + EA, and BCP + MT + sham EA groups decreased gradually (Figure 2H). In the intragroup comparison, there were no significant differences in the PWT of BCP + MT and BCP + MT + sham EA groups between the 12th day of morphine injection (the 22nd day after MRMT-1 cell implantation) and the 10th day after MRMT-1 cell implantation (Figure 2H). The PWT of the BCP + MT and BCP groups was not significantly different (P > .05) until the 12th day of morphine injection (the 22nd day after MRMT-1 cell implantation), indicating that morphine tolerance developed at this point (Figure 2E, F and G). Compared with that of the BCP + MT group, the PWT of the BCP + MT + EA group increased after 7 days of EA treatment (the 22nd day after the operation and the 12th day after the injection of morphine) (P < .01, Figure 2G). However, the PWT of the BCP + MT + sham EA group was not different than that of the BCP + MT group (Figure 2G and H).
Figure 2.
Changes of PWT before and after bone cancer pain modeling and morphine injection in each group of rats.
The PWT have not different amonge the Sham, BCP+MT, BCP+MT+EA, BCP+MT+Sham EA groups at -1d and 6d (A, and B). A bone cancer pain model was established successfully on the 10th day after MRMT-1 cell implantation (C). The PWT of the BCP + MT, BCP + MT + EA, and BCP + MT + sham EA groups increased significantly on the first day after morphine injection (the 11th day after MRMT-1 cell implantation) (D). EA intervention was performed once a day for 7 days beginning on the 16th day after cell implantation. Morphine has an analgesic effect (E, and F). After 12 days of repeated morphine injection (the 22nd day after MRMT-1 cell implantation), morphine tolerance developed in the BCP + MT and BCP + MT + sham EA groups but not in the BCP + MT + EA group (G). All the data of PWT (H). The data are presented as the mean ± SEM (sham, n = 10; BCP, n = 7; BCP + MT, n = 8; BCP + MT + EA, n = 7; BCP + MT + sham EA, n = 8). Compared with the sham group, P < .01, P < .05; compared with the BCP group, P < .01; compared with the BCP + MT group, ❖❖P < .01; compared with the BCP + MT + EA group,P < .01.
Effects of EA on the Protein Expression of p-PI3K, p-Akt, p-JNK1/2, and β-Arrestin2 in the L4-6 Spinal Dorsal Horn of Rats with Bone Cancer Pain-Morphine Tolerance
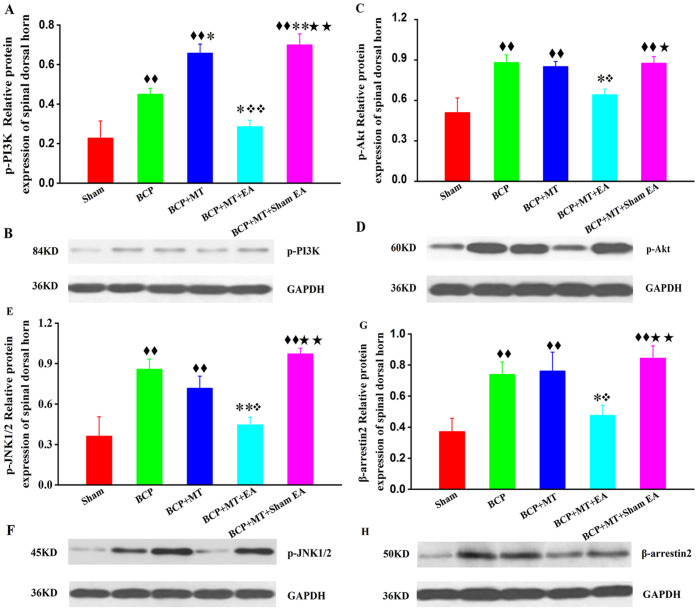
Compared with those in the sham group, the protein expression levels of p-PI3K (phosphorylated phosphatidylinositol 3-kinase), p-Akt (phosphorylated protein kinase B), p-JNK1/2 (phosphorylated c-Jun NH2-terminal kinase 1/2), and β-arrestin2 in the BCP, BCP + MT, and BCP + MT + sham EA groups were significantly increased (P < .01, Figure 3A-H). The protein expression levels of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the BCP + MT + EA group were higher than those in the sham group, but the difference was not statistically significant (P > .05, Figure 3A-H). Compared with that in the BCP group, the protein expression of p-PI3K in the BCP + MT and BCP + MT + sham EA groups was significantly increased (P < .01 or P < .05, Figure 3A and B). The protein expression levels of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the BCP + MT + EA group were significantly lower than those in the BCP group (P < .01 or P < .05, Figure 3A-H). Compared with those in the BCP + MT group, the protein expression levels of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the BCP + MT + EA group were significantly decreased (P < .01 or P < .05, Figure 3A-H), and there was no significant difference between the BCP + MT group and the BCP + MT + sham EA group (P > .05). The protein expression levels of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the BCP + MT + EA group were significantly lower than those of the BCP + MT + sham EA group (P < .01 or P < .05, Figure 3A-H).
Figure 3.
The quantitative data and the representative bands for the protein expression levels of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the spinal dorsal horn of rats with bone cancer pain-morphine tolerance.
The protein expression levels of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the BCP, BCP + MT, and BCP + MT + sham EA groups but not the BCP + MT + EA group were significantly increased (A, C, E, and G). Representative band of p-PI3K, P-Akt, p-JNK1/2, and β-arrestin2(B, D, F, and H). The data are presented as the mean ± SEM (n = 5/group). Compared with the sham group, P < .01; compared with the BCP group, P < .01, P < .05; compared with the BCP + MT group, P < .01, P < .05; compared with the BCP + MT + EA group, P < .01, P < .05.
A PI3K Inhibitor Delayed the Occurrence of Bone Cancer Pain-Morphine Tolerance
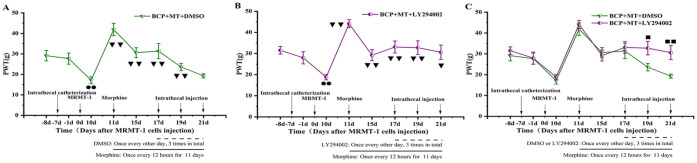
There was no significant difference in the basal (−8 days) PWT between groups before intrathecal catheterization (P > .05). Compared with the basal PWT, the PWT 1 day before MRMT-1 cell implantation was not decreased, but the PWT on the 10th day after MRMT-1 cell implantation was lower (P < .01, Figure 4A and B). This indicated that the model of bone cancer pain was successfully established. On the 11th day (the first day after morphine injection), the PWT was significantly higher than that on the 10th day (P < .01, Figure 4A and B). On the 15th day, the PWT was also higher than that on the 10th day but was lower than that on the 11th day, indicating that the analgesic effect of morphine was weakened after repeated morphine injection. There was no significant difference between the PWT of the BCP + MT + DMSO group on the 10th day and the 21st day (P > .05). This showed that morphine tolerance developed on the 11th day after the injection of morphine (Figure 4A). The PWT of the BCP + MT + LY294002 group was higher on the 17th, 19th, and 21st days than on the 10th day (P < .01 or P < .05, Figure 4B). The PWT of the BCP + MT + LY294002 group was markedly higher than that of the BCP + MT + DMSO group on the 19th and 21st days (P < .01 or P < .05, Figure 4C).
Figure 4.
Effect of a PI3K inhibitor on the PWT of rats with bone cancer pain-morphine tolerance.
A bone cancer pain model was established successfully on the 10th day after MRMT-1 cell implantation. The PWT of the BCP + MT + DMSO and BCP +MT + LY294002 groups increased significantly on the first day after morphine injection (A and B). On the 17th, 19th, and 21st days after MRMT-1 cell implantation, rats were intrathecally injected with 20 μl of LY294002 (dissolved in 5% DMSO, 0.25 μg/μl) or PBS 30 minutes before morphine injection. After 11 days of daily morphine injection, morphine tolerance developed in the BCP + MT + DMSO group but not in the BCP + MT + LY294002 group (A-C). The data are presented as the mean ± SEM (n = 10/group). Compared with the basal (−8 days) PWT, P < .01; compared with the 10th day after MRMT-1 cell implantation, P < .01, P < .05; compared with the BCP + MT + DMSO group, P < .01, P < .05.
Effects of a PI3K Inhibitor on the Protein Expression of p-Akt, p-JNK1/2, and β-Arrestin2 in the L4-6 Spinal Dorsal Horn of Rats with Bone Cancer Pain-Morphine Tolerance
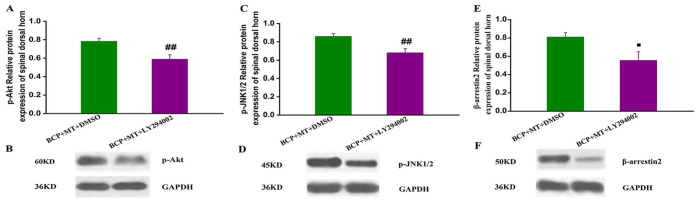
Compared with those in the BCP + MT + DMSO group, the protein expression levels of p-Akt, p-JNK1/2, and β-arrestin2 in the BCP + MT + LY294002 group were significantly reduced (P < .01 or P < .05, Figure 5A-F).
Figure 5.
The quantitative data and the representative bands for the protein expression levels of p-Akt, p-JNK1/2, and β-arrestin2 in the spinal dorsal horn of rats with bone cancer pain-morphine tolerance.
The protein expression levels of p-Akt, p-JNK1/2, and β-arrestin2 were significantly reduced in the BCP + MT + LY294002 group A, C, and E. Representative band of p-Akt, p-JNK1/2 and β-arrestin2(B, D, and F). The data are presented as the mean ± SEM (n = 8/group). Compared with the BCP + MT + DMSO group, ##P < .01, #P < .05.
A PI3K agonist antagonized the effect of EA on the development of bone cancer pain-morphine tolerance
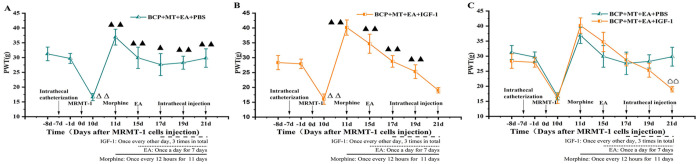
There was no significant difference in the basal (−8 days) PWT between groups before intrathecal catheterization (P > .05). Compared with the basal PWT, the PWT 1 day before MRMT-1 cell implantation was not decreased, but the PWT 10 days after MRMT-1 cell implantation was lower (P < .01). This indicated that the model of bone cancer pain was successfully established (Figure 6A and B). On the 11th day (the first day after morphine injection), the PWT was significantly higher than that on the 10th day (P < .01) (Figure 6A and B). On the 15th day, the PWT of the 2 groups was also higher than that on the 10th day (P < .05) but lower than that on the 11th day, indicating that the analgesic effect of morphine was weakened after repeated morphine injection (Figure 6A and B). There was no significant difference between the PWT of the BCP + MT + EA + IGF-1 group on the 10th day and the 21st day (P > .05). This showed that morphine tolerance developed on the 11th day after morphine injection (Figure 6B). The PWT of the BCP + MT + EA + PBS group was higher on the 21st day than on the 10th day (P < .01) (Figure 6A). Furthermore, the PWT of the BCP + MT + EA + IGF-1 group was markedly lower than that of the BCP + MT + EA + PBS group on the 21st day (P < .01) (Figure 6C).
Figure 6.
Effect of a PI3K agonist on the PWT of rats with bone cancer pain-morphine tolerance.
A bone cancer pain model was established successfully on the 10th day after MRMT-1 cell implantation. The PWT of the BCP + MT + EA + PBS group and BCP + MT + EA + IGF-1 group increased significantly on the first day after morphine injection (A and B). EA intervention was performed once a day for 7 days beginning on the 15th day after cell implantation. On the 17th, 19th, and 21st days after MRMT-1 cell implantation, the rats were intrathecally injected with 20 μl of IGF-1 (dissolved in PBS, 0.36 μg/μl) or PBS 30 minutes before morphine injection. After 11 days of daily morphine injection, morphine tolerance developed in the BCP + MT + EA + IGF-1 group but not in the BCP + MT + EA + PBS group (A-C). The data are presented as the mean ± SEM (n = 9/group). Compared with the basal (−8 days) PWT, P < .01; compared with the 10th day after MRMT-1 cell implantation, P < .01, P < .05; compared with the BCP + MT + EA + PBS group, >P < .01.
Discussion
In this study, a bone cancer pain model was established by injecting MRMT-1 mammary gland carcinoma cells into the marrow cavity of the tibia, and the PWT decreased gradually after tumor cell implantation. Compared with that of the sham group (Figure 2) and the rats before modeling (Figures 4 and 6), the PWT was decreased significantly on the 10th day after cell implantation, suggesting that the bone cancer pain model was established successfully. This was consistent with a previous report.13 When morphine was injected into rats with bone cancer pain on the first day, the PWT significantly increased, indicating that there was an obvious analgesic effect of morphine. After repeated injection of morphine, the PWT decreased gradually until the PWT was similar to that of the BCP group (Figure 2) and that on the 10th day after modeling (Figures 4 and 6), indicating the development of morphine tolerance. In experiment 1, compared with that of the BCP group, the PWT of the BCP + MT group and BCP + MT + sham EA group was decreased significantly on the 12th day of repeated morphine injection, which indicated that morphine tolerance had developed at this point; in contrast, the PWT of the BCP + EA group remained at a high level (P < .01), indicating that EA attenuated the development of morphine tolerance (Figure 2). The above results were similar to those of previous studies.13 In experiment 2, the PWT of the BCP + MT + DMSO group was decreased significantly on the 11th day of morphine injection, and morphine tolerance had developed. However, the PWT of the BCP + MT + LY294002 group was maintained at a high level and was higher than that of the BCP + MT + DMSO group (P < .01), which indicated that LY294002 inhibited the development of morphine tolerance (Figure 4). A previous study showed that the PI3K inhibitor LY294002 resisted morphine tolerance by inhibiting the PI3K/Akt signaling pathway.29 In experiment 3, on the 11th day of morphine injection, the PWT of the BCP + MT + EA + IGF-1 group was decreased significantly, and the difference was not statistically significant compared with the PWT on the 10th day after cell implantation, suggesting that morphine tolerance had developed at this point. However, the PWT of the BCP + MT + EA + PBS group was maintained at a high level and was higher than that of the BCP + MT + EA + IGF-1 group, and this difference was statistically significant (P < .01), which indicated that the effect of EA on morphine tolerance was blocked by IGF-1 (Figure 6).
Morphine is an opioid receptor agonist that produces analgesic effects mainly by acting on MOR. Morphine tolerance is thought to be caused by neuronal adaptation to repeated drug administration. Some signaling proteins and neuropeptides are involved in morphine tolerance.30 Long-term morphine therapy can cause opioid receptor-mediated changes in the nervous system, including internalization, desensitization, downregulation, and phosphorylation of the opioid receptor or heterodimerization with other receptors.31 In addition, changes in glutamate receptor function, protein kinase C (PKC) activation, and G-protein uncoupling are related to morphine tolerance.4
PI3K/Akt signaling is activated as a result of the ligand-dependent activation of G protein-coupled receptors (GPCRs). Morphine binds to MOR (a GPCR) to activate PI3K.32 As a lipid kinase, activated PI3K phosphorylates the D-3 position of PI lipids to produce phosphatidylinositol-3,4,5-triphosphate (PIP3), which can serve as a membrane-embedded second messenger to activate the downstream protein kinase AKT.33 The MOR-triggered activation of PI3K/Akt is involved in the development of morphine tolerance and further activates MAPK to promote the development of morphine tolerance.14 Chen34,35 proposed that chronic administration of morphine, which acts on MOR, may activate MAPKs, including ERK, P38 and JNK, through PKA, PKC and PI3K, and ultimately leads to morphine tolerance. In addition, Shi found that the activity of JNK was reduced after PI3K/AKT was inhibited.36 According to the current research, there is an upstream and downstream relationship between the PI3K/Akt and JNK signaling pathways. LY294002 is a specific inhibitor of PI3K that competes with the binding of adenosine 5′-triphosphate to the catalytic subunit of PI3K and inhibits the phosphorylation of Akt.37 DAMGO is a selective μ-opioid receptor peptide that induces Akt phosphorylation and antinociceptive tolerance.38 However, intrathecal injection of the PI3K inhibitor LY294002 can reduce DAMGO-induced morphine tolerance and reduce the protein levels of p-JNK, p-ERK1/2, and p-p38.14 In this study, the protein expression of p-PI3K, p-Akt, and p-JNK1/2 was upregulated in the spinal dorsal horn of rats with bone cancer pain-morphine tolerance. Spinal administration of LY294002 significantly downregulated the protein expression of p-Akt and p-JNK1/2, and EA significantly downregulated the protein expression of p-PI3K, p-Akt, and p-JNK1/2. The present study shows that the effect of EA intervention is similar to that of LY294002 administration, which can be blocked by IGF-1 (a PI3K agonist). We speculate that the mechanism involved in the effect of EA on the attenuation of morphine tolerance is associated with inhibition of the PI3K/Akt/JNK1/2 signaling pathway.
β-Arrestin2 is involved in morphine tolerance and plays an important role. β-Arrestin2, as a scaffolding protein, regulates the signaling cascade of commonly used analgesics, including the JNK pathway; JNK binds to β-arrestin2 and modulates the analgesic effects of morphine.39 Morphine can activate and bind to Gαi-coupled μ receptors, and its binding to these receptors leads to the dissociation of Gαi and Gβγ and induces β-arrestin2 recruitment.40 MOR is phosphorylated by the action of G protein-coupled receptor kinases (GRKs) after binding with morphine. Phosphorylated MOR can bind to the regulatory protein β-arrestin2 and become uncoupled from G proteins, resulting in MOR desensitization.41 The deletion of β-arrestin2 accelerates the resensitization of MOR, and the disruption of β-arrestin2-dependent receptor trafficking promotes the resensitization of MOR, thus reducing morphine tolerance.14 It has been found that mice lacking β-arrestin2 do not exhibit μ-opioid receptor desensitization after chronic morphine treatment and that these animals fail to develop antinociceptive tolerance.42 When MOR agonists induce antinociceptive tolerance, the expression of β-arrestin2 in the locus coeruleus, cortex, and striatum increases significantly, while the intrathecal administration of β-arrestin2 antisense oligonucleotides slows the progression of morphine tolerance.43 Yang et al44 found that the antinociceptive effects of intrathecal morphine are increased and maintained in rats that receive β-arrestin2 siRNA. miR-365 participates in morphine tolerance by regulating the expression of β-arrestin2. The overexpression of miR-365 induced by lentivirus-miR-365 leads to decreased expression of the target gene β-arrestin2 and reduced morphine tolerance.4 In this study, the protein expression of β-arrestin2 was upregulated in the spinal dorsal horn of rats with bone cancer pain-morphine tolerance. However, EA significantly downregulated the protein expression of β-arrestin2 in the spinal dorsal horn. These results suggest that EA attenuates morphine tolerance and may be involved in inhibiting the expression of β-arrestin2 to increase the resensitization of MOR.
EA can effectively relieve acute and chronic pain33 and is widely used in clinical treatment and scientific research. The NCCN cancer treatment guidelines also recommend acupuncture for the treatment of cancer pain. In addition, some studies have shown that EA can effectively delay the development of morphine tolerance in rats.13,26,45,46 EA can increase the mRNA expression of MOR and KOR in the DRG of bone cancer pain rats.47 EA may inhibit MOR desensitization, promote MOR endocytosis or resensitivity, and increase the expression of MOR in the locus ceruleus to alleviate morphine tolerance.13,26 In this report, EA was shown to downregulate the protein expression of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the spinal dorsal horn of rats with bone cancer pain-morphine tolerance. We presume that the effect of EA in attenuating morphine tolerance may be related to the inhibition of the PI3K/Akt/JNK1/2 signaling pathway and β-arrestin2 and may inhibit MOR desensitization or promote the endocytosis or resensitization of MOR to improve the expression of MOR mRNA in the spinal cord.
In this study, it was observed that (1) morphine tolerance occurred after repeated intraperitoneal injection of morphine into rats with bone cancer pain; (2) the protein expression levels of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the L4-6 spinal dorsal horn were upregulated in the BCP, BCP + MT, and BCP + MT + sham EA groups; (3) the EA intervention attenuated the development of morphine tolerance and downregulated the protein expression of p-PI3K, p-Akt, p-JNK1/2, and β-arrestin2 in the L4-6 spinal dorsal horn during intraperitoneal injection of morphine; (4) LY294002 (a PI3K inhibitor) attenuated the development of morphine tolerance and downregulated the protein expression of p-Akt, p-JNK1/2, and β-arrestin2 in the L4-6 spinal dorsal horn; (5) IGF-1 (a PI3K agonist) antagonized the ability of EA to attenuate morphine tolerance.
Conclusion
The mechanism underlying the ability of EA to attenuate morphine tolerance may be associated with inhibition of the PI3K/Akt/JNK1/2 signaling pathway.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_1534735421995237 for Electroacupuncture Attenuates Morphine Tolerance in Rats with Bone Cancer Pain by Inhibiting PI3K/Akt/JNK1/2 Signaling Pathway in the Spinal Dorsal Horn by Bin Jiang, Xuemei Zhong, Junfan Fang, Aijun Zhang, Wen WangD, Yi Liang, Jianqiao Fang, Feng Chen and Junying Du in Integrative Cancer Therapies
Supplemental material, sj-pdf-1-ict-10.1177_1534735421995237 for Electroacupuncture Attenuates Morphine Tolerance in Rats with Bone Cancer Pain by Inhibiting PI3K/Akt/JNK1/2 Signaling Pathway in the Spinal Dorsal Horn by Bin Jiang, Xuemei Zhong, Junfan Fang, Aijun Zhang, Wen WangD, Yi Liang, Jianqiao Fang, Feng Chen and Junying Du in Integrative Cancer Therapies
Acknowledgments
Thanks to Department of Neurobiology and Acupuncture Research of the Third Clinical Medical College of Zhejiang Chinese Medical University for providing the experimental facilities and Director of the department Xiaomei Shao for her careful guidance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Zhejiang Provincial Natural Science Fund of China (LY16H270017) and the Jiaxing Key Discipline of Chinese and Western Medicine-Acupuncture (Derived) (2019XK-B01).
ORCID iDs: Bin Jiang  https://orcid.org/0000-0001-5777-9842
https://orcid.org/0000-0001-5777-9842
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Cheng YC, Tsai RY, Sung YT, et al. Melatonin regulation of transcription in the reversal of morphine tolerance: microarray analysis of differential gene expression. Int J Mol Med. 2019;43:791-806. doi: 10.3892/ijmm.2018.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Günther T, Dasgupta P, Mann A, et al. Targeting multiple opioid receptors – improved analgesics with reduced side effects? Br J Pharmacol. 2018;175:2857-2868. doi: 10.1111/bph.13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bu H, Liu X, Tian X, Yang H, Gao F. Enhancement of morphine analgesia and prevention of morphine tolerance by downregulation of β-arrestin 2 with antigene RNAs in mice. Int J Neurosci. 2015;125:56-65. doi: 10.3109/00207454.2014.896913 [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Xu W, Zhong T, et al. miR-365 targets β-arrestin 2 to reverse morphine tolerance in rats. Sci Rep. 2016;6: 1-11. doi: 10.1038/srep38285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Shu Y, Ji Q, Liu J, He X, Li W. Attenuation of morphine analgesic tolerance by rosuvastatin in naïve and morphine tolerance rats. Inflammation. 2015;38:134-141. doi: 10.1007/s10753-014-0015-y [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Xu W, Shao J, et al. miR-219-5p targets CaMKIIγ to attenuate morphine tolerance in rats. Oncotarget. 2017;8:28203-28214. doi: 10.18632/oncotarget.15997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eidson LN, Murphy AZ. Persistent peripheral inflammation attenuates morphine-induced periaqueductal gray glial cell activation and analgesic tolerance in the male rat. J Pain. 2013;14:393-404. doi: 10.1016/j.jpain.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan AY, Miller DW, Bolash B, et al. Acupuncture’s role in solving the opioid epidemic: evidence, cost-effectiveness, and care availability for acupuncture as a primary, non-pharmacologic method for pain relief and management-white paper 2017. J Integr Med. 2017;15:411-425. doi: 10.1016/S2095-4964(17)60378-9 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Li Z, Han F. Electroacupuncture for patients with irritable bowel syndrome: a systematic review and meta-analysis protocol. Medicine (Baltimore). 2018;97:e11627. doi: 10.1097/MD.0000000000011627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355-375. doi: 10.1016/j.pneurobio.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 11. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258-261. doi: 10.1016/j.neulet.2003.12.019 [DOI] [PubMed] [Google Scholar]
- 12. Sima L, Fan B, Yan L, Shui Y. Effects of electroacupuncture treatment on bone cancer pain model with morphine tolerance. Evid Based Complement Alternat Med. doi: 10.1155/2016/8028474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi RY, Fu TF, Cai YQ, et al. Electroacupuncture intervention relieves pain possibly by promoting MOR endocytosis in locus coeruleus in bone cancer pain rats with morphine tolerance. Zhen Ci Yan Jiu. 2019;44:161-169. doi: 10.13702/j.1000-0607.180635 [DOI] [PubMed] [Google Scholar]
- 14. Dai WL, Liu XT, Bao YN, et al. Selective blockade of spinal D2DR by levo-corydalmine attenuates morphine tolerance via suppressing PI3K/Akt-MAPK signaling in a MOR-dependent manner. Exp Mol Med. 2018;50:1-12. doi: 10.1038/s12276-018-0175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcus DJ, Zee M, Hughes A, et al. Tolerance to the antinociceptive effects of chronic morphine requires c-Jun N-terminal kinase. Mol Pain. 2015;11:34. doi: 10.1186/s12990-015-0031-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ducourneau VR, Dolique T, Hachem-Delaunay S, et al. Cancer pain is not necessarily correlated with spinal overexpression of reactive glia markers. Pain. 2014;155:275-291. doi: 10.1016/j.pain.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 17. Bian J, Zhu S, Ma W, Li C, Ashraf MA. Analgesic effect and possible mechanism of SCH772984 intrathecal injection on rats with bone cancer pain. Saudi Pharm J. 2016;24:354-362. doi: 10.1016/j.jsps.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding X, Yang W, Liu XD, Yang X, Wang HM, Tai J. Spinal SHP2 contributes to exaggerated incisional pain in adult rats subjected to neonatal and adult incisions via PI3K. Neuroscience. 2018;385:102-120. doi: 10.1016/j.neuroscience.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 19. Yan Y, Liang Y, Ding T, Chu H. PI3K/Akt signaling pathway may be involved in MCP-1-induced P2X4R expression in cultured microglia and cancer-induced bone pain rats. Neurosci Lett. 2019;701:100-105. doi: 10.1016/j.neulet.2019.02.024 [DOI] [PubMed] [Google Scholar]
- 20. Han Y, Li Y, Xiao X, et al. Formaldehyde up-regulates TRPV1 through MAPK and PI3K signaling pathways in a rat model of bone cancer pain. Neurosci Bull. 2012;28:165-172. doi: 10.1007/s12264-012-1211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medhurst SJ, Walker K, Bowes M, et al. A rat model of bone cancer pain. Pain. 2002;96:129-140. doi: 10.1016/s0304-3959(01)00437-7 [DOI] [PubMed] [Google Scholar]
- 22. Mei HX, Zhou MH, Zhang XW, et al. Effects of miR-338 on morphine tolerance by targeting CXCR4 in a rat model of bone cancer pain. Biosci Rep. 2017;37:BSR20160517. doi: 10.1042/BSR20160517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qing P, Chai TQ, Ding HM, Zhao CH, Hu J. Effect of electroacupuncture combined with rehabilitation training on neurological function and expression of neuronal growth associated protein 43 and synaptophysin in rats with focal cerebral ischemia/reperfusion injury. Zhen Ci Yan Jiu. 2016;41:314-320. [PubMed] [Google Scholar]
- 24. Xu JT, Tu HY, Xin WJ, Liu XG, Zhang GH, Zhai CH. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol. 2007;206:269-279. doi: 10.1016/j.expneurol.2007.05.029 [DOI] [PubMed] [Google Scholar]
- 25. Haninec P, Houst’ava L, Stejskal L, Dubový P. Rescue of rat spinal motoneurons from avulsion-induced cell death by intrathecal administration of IGF-I and Cerebrolysin. Ann Anat. 2003;185:233-238. doi: 10.1016/S0940-9602(03)80030-4 [DOI] [PubMed] [Google Scholar]
- 26. Fu T, Wang L, Du J, et al. Effects of electroacupuncture on expression of μ-opioid receptor in nucleus ceruleus in rats with bone cancer pain-morphine tolerance. Zhongguo Zhen Jiu. 2017;37:513-520. doi: 10.13703/j.0255-2930.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 27. Sun RQ, Tu YJ, Yan JY, Willis WD. Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain. 2006;120:86-96. doi: 10.1016/j.pain.2005.10.017 [DOI] [PubMed] [Google Scholar]
- 28. Liu HT, Zhang HF, Si R, et al. Insulin protects isolated hearts from ischemia/reperfusion injury: cross-talk between PI3-K/Akt and JNKs. Sheng Li Xue Bao. 2007;59:651-659. [PubMed] [Google Scholar]
- 29. Li Z, Peng X, Jia X, et al. Spinal heat shock protein 27 participates in PDGFRβ-mediated morphine tolerance through PI3K/Akt and p38 MAPK signalling pathways. Br J Pharmacol. 2020;177:5046-5062. doi: 10.1111/bph.15169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin WY, Yu LC. Involvement of protein kinase C in morphine tolerance at spinal levels of rats. ACS Chem Neurosci. 2010;1:122-128. doi: 10.1021/cn900005d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu JT, Zhao JY, Zhao X, et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest. 2014;124:592-603. doi: 10.1172/JCI70236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667-676. doi: 10.1023/B:APPT.0000045801.15585.dd [DOI] [PubMed] [Google Scholar]
- 33. Kim HN, Kim YR, Jang JY, Shin HK, Choi BT. Electroacupuncture inhibits phosphorylation of spinal phosphatidylinositol 3-kinase/Akt in a carrageenan-induced inflammatory rat model. Brain Res Bull. 2012;87:199-204. doi: 10.1016/j.brainresbull.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 34. Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40:101-107. doi: 10.1007/s12035-009-8074-z [DOI] [PubMed] [Google Scholar]
- 35. Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci. 2008;28:5836-5845. doi: 10.1523/JNEUROSCI.4170-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi H, Lin B, Huang Y, et al. Basic fibroblast growth factor promotes melanocyte migration via activating PI3K/Akt-Rac1-FAK-JNK and ERK signaling pathways. IUBMB Life. 2016;68:735-747. doi: 10.1002/iub.1531 [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Gao J, Wang M, et al. Effects of scalp electroacupuncture on the PI3K/Akt signalling pathway and apoptosis of hippocampal neurons in a rat model of cerebral palsy. Acupunct Med. 2018;36:96-102. doi: 10.1136/acupmed-2016-011335 [DOI] [PubMed] [Google Scholar]
- 38. Tian Y, Liu M, Mao-Ying QL, et al. Early single Aspirin-triggered Lipoxin blocked morphine anti-nociception tolerance through inhibiting NALP1 inflammasome: Involvement of PI3k/Akt signaling pathway. Brain Behav Immun. 2015;50:63-77. doi: 10.1016/j.bbi.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 39. Mittal N, Tan M, Egbuta O, et al. Evidence that behavioral phenotypes of morphine in β-arr2-/- mice are due to the unmasking of JNK signaling. Neuropsychopharmacology. 2012;37:1953-1962. doi: 10.1038/npp.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yudin Y, Rohacs T. The G-protein-biased agents PZM21 and TRV130 are partial agonists of μ-opioid receptor-mediated signalling to ion channels. Br J Pharmacol. 2019;176:3110-3125. doi: 10.1111/bph.14702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiang R, Hu Y, Cao B. The role of β-arrestins in mediating G protein-coupled receptors (GPCRs)-related signal pathways. Chin J Biochem Molec Biol. 2013;29:122-127. doi: 10.13865/j.cnki.cjbmb.2013.02.001 [DOI] [Google Scholar]
- 42. Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720-723. doi: 10.1038/35047086 [DOI] [PubMed] [Google Scholar]
- 43. Li Y, Liu X, Liu C, et al. Improvement of morphine-mediated analgesia by inhibition of β-arrestin2 expression in mice periaqueductal gray matter. Int J Mol Sci. 2009;10:954-963. doi: 10.3390/ijms10030954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang CH, Huang HW, Chen KH, Chen YS, Sheen-Chen SM, Lin CR. Antinociceptive potentiation and attenuation of tolerance by intrathecal β-arrestin 2 small interfering RNA in rats. Br J Anaesth. 2011;107:774-781. doi: 10.1093/bja/aer291 [DOI] [PubMed] [Google Scholar]
- 45. Zheng YX, Yu YH, Wang GL. Analysis of the effects and the possible mechanism of electroacupuncture on the formation of morphine tolerance in rats with inflammatory pain. China J Mod Med. 2009;19:1315-1318. [Google Scholar]
- 46. Huang MN, Zhang YX, Yu YH, Wang GL. The effect of electroacupuncture on changes of the phosphorylation of capsaicin receptor in chronic morphine tolerance rats. Tianjin Med J. 2011;39:542-545. [Google Scholar]
- 47. Liang Y, Du JY, Fang JF, et al. Analgesic effect of electro-acupuncture and its intervention on peripheral opioid receptor expression in rats with bone cancer pain. CJTCMP. 2018;33:4877-4881. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_1534735421995237 for Electroacupuncture Attenuates Morphine Tolerance in Rats with Bone Cancer Pain by Inhibiting PI3K/Akt/JNK1/2 Signaling Pathway in the Spinal Dorsal Horn by Bin Jiang, Xuemei Zhong, Junfan Fang, Aijun Zhang, Wen WangD, Yi Liang, Jianqiao Fang, Feng Chen and Junying Du in Integrative Cancer Therapies
Supplemental material, sj-pdf-1-ict-10.1177_1534735421995237 for Electroacupuncture Attenuates Morphine Tolerance in Rats with Bone Cancer Pain by Inhibiting PI3K/Akt/JNK1/2 Signaling Pathway in the Spinal Dorsal Horn by Bin Jiang, Xuemei Zhong, Junfan Fang, Aijun Zhang, Wen WangD, Yi Liang, Jianqiao Fang, Feng Chen and Junying Du in Integrative Cancer Therapies