Abstract
Acute pulmonary thromboembolism is associated with high mortality, similar to that of myocardial infarction and stroke. We studied the clinical presentation and management of pulmonary thromboembolism in the Indian population. An analysis of 140 patients who presented with acute pulmonary thromboembolism at a large volume center in India from June 2015 through December 2018 was performed. The mean age of our study population was 50 years with 59% being male. Comorbidities including deep vein thrombosis, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease were present in 52.9%, 40%, 35.7% and 7.14% of patients, respectively. Out of 140 patients, 40 (28.6%) patients had massive pulmonary thromboembolism, 36 (25.7%) sub-massive pulmonary thromboembolism, and 64 (45.7%) had low-risk pulmonary thromboembolism. Overall, in-hospital mortality was 25.7%. Multivariate regression analysis found chronic kidney disease and pulmonary thromboembolism severity to be the only independent risk factors. Thrombolysis was performed in 62.5% of patients with a massive pulmonary thromboembolism and 63.9% of patients with a sub-massive pulmonary thromboembolism. In the massive pulmonary thromboembolism group, patients receiving thrombolytic therapy had lower mortality compared with patients who did not receive therapy (p=0.022), whereas this difference was not observed in patients in the sub-massive pulmonary thromboembolism group. We conclude that patients with acute pulmonary thromboembolism in India presented more than a decade earlier than our western counterparts, and it was associated with poor clinical outcomes. Thrombolysis was associated with significantly reduced in-hospital mortality in patients with massive pulmonary thromboembolism.
Keywords: acute pulmonary embolism, thrombolysis, shock, acute cor-pulmonale
Introduction
Venous thromboembolism (VTE) constitutes a disease spectrum ranging from deep venous thromboembolism (DVT) of the extremities to massive pulmonary thromboembolism (PE), which is associated with high morbidity and mortality if left untreated. Incidence of VTE varies from 0.5 to 2 per 1000 inhabitants.1,2 The exact incidence of PE remains unknown, as it may go undetected in 40–50% of patients with DVT.6 An autopsy-based study showed that approximately one-third of patients with a fatal PE could be identified before death.4 The International Cooperative Pulmonary Embolism Registry (ICOPER) demonstrated that the mortality rates for massive and sub-massive PE were 58.3% and 15.1%, respectively.5
There are limited data with regard to PE, its management and associated complications in India, with the majority of the clinical evidence being derived largely from case reports and a select few small-sized studies.6 An autopsy-based study from Northern India showed PE to be the cause of death in 15.9% of all hospitalized patients.7 A recent epidemiological study including 2420 Asian patients reported that the rate of symptomatic VTE or sudden death due to VTE to be 2.3% higher than their western counterparts.8 According to the guidelines set out by the American College of Cardiology (ACC) 9 and the European Society of Cardiology (ESC),10 therapeutic management of patients with PE included anti-coagulation, intravenous and catheter-directed thrombolysis (CBT), and surgical embolectomy. We sought to study the clinical characteristics, treatment strategy, in-hospital outcomes, and prognostic factors of patients with pulmonary embolism in India.
Methods
Study design
Our study was a non-randomized, retrospective, single-center, observational study, and was an investigator-initiated non-funded research project. Patients >18 years of age who were diagnosed and treated at our Institute from June 2015 through December 2018 were enrolled in the study. The study was approved by the Institute’s Ethics Committee. Informed consent was not obtained from the patients, as this study was a retrospective study which was based on a database. Patients with acute PE that was diagnosed by computer tomography-pulmonary angiography (CT-PA), patients with acute PE superimposed on a background of chronic PE, and patients in shock with screening echocardiogram showing evidence of PE were included in the study. Patients with chronic PE or diagnosed with PE in the remote past who were admitted for other medical reasons, patients with no demonstrable PE by CT-PA, and no echocardiographic evidence of PE were excluded from the study.
Data collection
Two physicians (MK and AVR) collected the patients’ data from the electronic database information system of our institution. If there was any discrepancy in the data collected, a third physician (NBS) served as an arbitrator to resolve any issues. Information about presenting symptoms, co-morbidities, laboratory results, and findings of imaging studies which included venous Doppler, trans-thoracic echocardiogram (TTE), and CT-PA was collected. Data pertaining to treatment administered to individual patients including anticoagulation therapy, thrombolytic therapy, and surgical thrombectomy were also collected. Patients were categorized into massive, sub-massive, and low-risk PE groups based on the 2011 American Heart Association (AHA) guidelines9 (Supplementary document) with the demographics, treatment, and outcomes being analyzed separately for each group. The primary outcome of the study was in-hospital mortality. Bleeding outcomes were evaluated and graded based on the Bleeding Academic Research Consortium (BARC), with patients who had a BARC grade of ≥3 being considered significant.11,12
Statistical analysis
We expressed categorical data as ratio or proportion or percentage, and continuous data as mean ± standard deviation or median (Interquartile range), as appropriate. Continuous variables were analyzed by t-test or ANOVA when appropriate. We used the Chi-square test to assess the significance of categorical variables. Multiple logistic regression analysis was performed to identify the risk factors for in-hospital mortality. A two-tailed p-value <0.05 was considered significant. All analyses were performed using SPSS 26 (IBM, New York).
Results
Baseline characteristics
One hundred and forty patients were included in the study, with a mean age of 50 ± 15 years (41% female) (Table 1). The most common presenting symptom was NYHA class III and NYHA class IV dyspnea (61.5%), followed by chest pain (35%) and cough (32%). The mean duration from symptom onset to hospital presentation was 3.2 days with 75% of patients presenting within five days. DVT, diabetes mellitus, hypertension, recent surgery, recent trauma, malignancy, coronary artery disease, and COPD were present in 52.9%, 40%, 35.7%, 25.7%, 17.1%, 16.4%, 10.3%, and 7.14% of patients, respectively. The mean pulse rate was 109 beats per minute, and the mean respiratory rate was 25 breaths per minute. The in-hospital mortality was 25.7%. Right ventricular dysfunction and severe pulmonary hypertension (PH)were noticed in 50% and 5.7% of patients, respectively.
Table 1.
Baseline characteristics of study population
| Baseline characteristics of the study population (n = 140) | Count (No.) | Percentage of patients/±1 standard deviation |
|---|---|---|
| Age (year) | 50.3 | ±14.87 |
| Sex | ||
| Female | 57 | 40.7% |
| Male | 83 | 59.3% |
| Symptoms | ||
| Dyspnea | ||
| NYHA I | 23 | 16.4% |
| NYHA II | 31 | 22.1% |
| NYHA III | 53 | 37.9% |
| NYHA IV | 33 | 23.6% |
| Syncope | 14 | 10.0% |
| Chest Pain | 49 | 35.0% |
| Cough | 45 | 32.1% |
| Hemoptysis | 14 | 10.0% |
| Palpitations | 19 | 13.6% |
| Risk factors | ||
| Deep venous thrombosis | 74 | 52.9% |
| Diabetes | 56 | 40.0% |
| Malignancy | 23 | 16.4% |
| Hypertension | 50 | 35.7% |
| Chronic kidney disease | 13 | 9.2% |
| Recent surgery | 36 | 25.7% |
| Pregnancy | 3 | 2.1% |
| Chronic obstructive pulmonary disease | 10 | 7.1% |
| Trauma | 24 | 17.1% |
| Travel | 1 | 0.7% |
| Pulse (per minute) | 108.89 | ±19.19 |
| Respiratory rate (per minute) | 25.62 | ±6.984 |
| Pulmonary embolism severity index | ||
| <65 | 38 | 27.1% |
| 66–85 | 36 | 25.7% |
| 86–105 | 20 | 14.2% |
| 106–125 | 16 | 11.4% |
| >125 | 30 | 21.4% |
| Right ventricular dysfunction | 70 | 50% |
| Pulmonary hypertension (PH)/right ventricular systolic pressure (RVSP) | ||
| No PH (PH<30mmHG) | 59 | 42.1% |
| Mild PH ( PH-30-49mmHg) | 41 | 29.2% |
| Moderate PH (PH- 50-69mmHg) | 32 | 22.8% |
| Severe PH (PH->70mmHg) | 8 | 5.7% |
| Thrombolysis done | 50 | 35.7% |
| Bleeding Academic Research Consortium (BARC) Bleeding ≥3 | 13 | 9.2% |
| Mortality | 36 | 25.7% |
Univariate and multivariate analysis
It was found that chronic kidney diseases (CKDs), chronic obstructive pulmonary disease (COPD), PESI score, pulmonary embolism severity index (PESI class), PE severity, and PH were significantly different between patients who survived and patients who died (Table 2). Patients with massive PE had PESI scores ranging between 124 and 144. Patients with sub-massive PE had PESI scores ranging between 81 and 99, while those with low-risk PE had PESI scores ranging between 59 and 69. Correlation between PESI and death was highly significant (p<0.001). Patients who survived had PESI scores between 72.5 and 83.95, whereas patients who died had PESI scores between 113.73 and 141.33, thereby emphasizing the importance of the PESI score as a prognostic tool. The receiver operating curve (ROC) comparing PESI score with in-hospital mortality showed a significant positive relationship between the two variables. The area under the curve for the ROC curve was 0.852 (CI: 0.772–0.932) with a p-value <0.001. Using a PESI score of 129 as a cutoff for predicting mortality, a strong positive correlation was seen between the two variables (p<0.001). Patients with a PESI score of ≥129 were found to have a mortality rate of 78.3%. However, multivariate regression analysis found massive PE to be an independent risk factor for in-hospital mortality (Table 3). A negative association of mortality with CKD status was also noted.
Table 2.
Univariate analysis for in-hospital mortality in patients with pulmonary embolism
| Characteristics | Live | Death | p value |
|---|---|---|---|
| Age (year) | 49.65 ± 15.35 | 52.25 ± 13.424 | 0.369 |
| Sex | |||
| Female | 41 (39.4%) | 16 (44.40%) | 0.597 |
| Male | 63 (60.6%) | 20 (55.60%) | |
| Acute worsening of symptoms (days) | 3.34 ± 3.818 | 2.89 ± 2.435 | 0.512 |
| Dyspnea NYHA I | 20 (19.2%) | 3 (8.3%) | 0.077 |
| NYHA II | 26 (25.1%) | 5 (13.90%) | |
| NYHA III | 38 (36.5%) | 13 (41.70)% | |
| NYHA IV | 20 (19.2%) | 9 (36.1%) | |
| Syncope | 9 (8.7%) | 5 (13.90%) | 0.367 |
| Chest pain | 39 (37.5%) | 10 (27.80%) | 0.292 |
| Cough | 32 (30.80%) | 13 (36.10%) | 0.554 |
| Hemoptysis | 12 (11.50%) | 2 (5.60%) | 0.302 |
| Palpitations | 14 (13.5%) | 5 (13.90%) | 0.949 |
| DVT | 55 (52.9%) | 19 (52.80%) | 0.991 |
| DM | 38 (36.50%) | 18 (50.00% | 0.155 |
| Malignancy | 17 (16.3%) | 6 (16.70%) | 0.964 |
| Systemic hypertension | 34 (32.7%) | 16 (44.40%) | 0.205 |
| Recent surgery | 29 (27.90%) | 7 (19.40%) | 0.318 |
| Pregnancy | 2 (1.90%) | 1 (2.80%) | 0.760 |
| CKD | 5 (4.8%) | 8 (22.2%) | 0.002 |
| COPD | 4 (3.80%) | 6 (16.70%) | 0.010 |
| Trauma | 19 (18.30%) | 5 (13.90%) | 0.548 |
| Travel | 1 (1.00%) | 0 (0%) | 0.555 |
| PESI | |||
| ≤65 | 36 (34.6%) | 2 (5.6%) | 0.001 |
| 66–85 | 33 (31.7%) | 3 (8.3%) | |
| 86–105 | 16 (15.4%) | 4 (11.1%) | |
| 106–125 | 13 (12.3%) | 3 (8.3%) | |
| >125 | 6 (5.8%) | 24 (66.7%) | |
| Pulmonary embolism severity index | 78.26 ± 29.273 | 127.53 ± 40.790 | <0.001 |
| PTE severity | |||
| Massive | 11 (10.6%) | 29 (80.6%) | <0.001 |
| Sub massive | 33 (31.7%) | 3 (8.3%) | |
| Low risk | 60 (57.7%) | 4 (11.1%) | |
| RV Dysfunction | 43 (41.3%) | 27 (75.0%) | 0.001 |
| Pulmonary hypertension(PH)/ Right ventricular systolic pressure (RVSP) | |||
| No PH (PAH<30mmHG) | 47 (45.20% | 12 (33.30%) | 0.001 |
| Mild PH ( PAH-30–49mmHg) | 36 (34.60%) | 5 (13.90%) | |
| Moderate PH (PAH- 50–69mmHg) | 15 (14.4%) | 17 (47.2%) | |
| Severe PH (PAH->70mmHg) | 06 (5.8%) | 02 (5.6%) | |
| Thrombolysis-done | 34 (32.7%) | 16 (44.4%) | 0.205 |
Table 3.
Multivariate analysis for in-hospital mortality in patients with pulmonary embolism.
| Parameters | Significance (p) | Odds ratio |
95% Confidence interval |
|
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Chronic kidney disease | 0.012 | 0.105 | 0.018 | 0.612 |
| Chronic obstructive pulmonary disease | 0.36 | 0.41 | 0.061 | 2.78 |
| Massive PE | <0.0001 | 67.27 | 8.89 | 508.74 |
| Sub massive PE | 0.548 | 1.95 | 0.21 | 18.64 |
| Right ventricular dysfunction | 0.29 | 2.80 | 0.41 | 19.17 |
| Mild pulmonary hypertension | 0.71 | 1.55 | 0.16 | 15.4 |
| Moderate pulmonary hypertension | 0.80 | 0.74 | 0.70 | 7.72 |
| Severe pulmonary hypertension | 0.25 | 3.72 | 0.39 | 35.41 |
Subgroup analysis
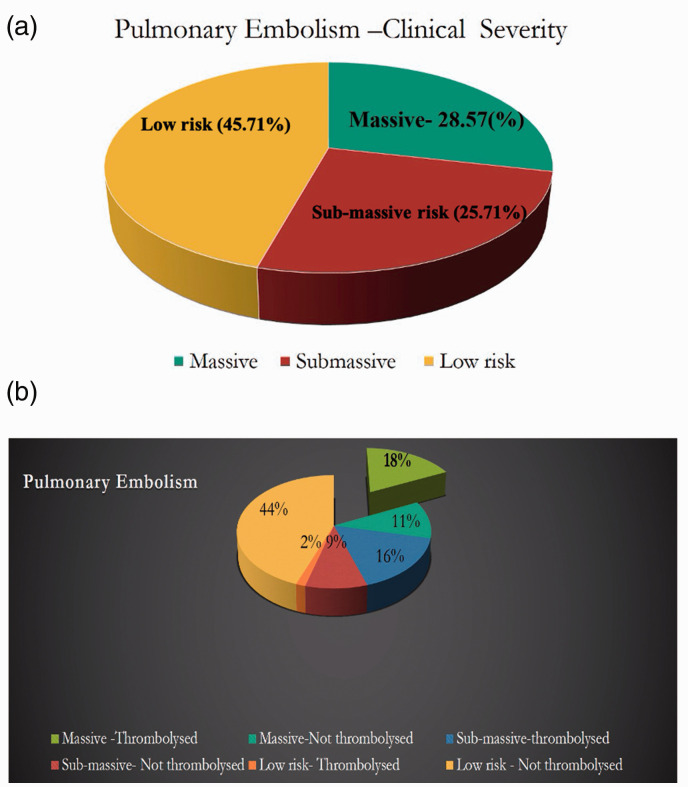
Out of 140 patients, 40 (28.6%) had a massive PE, 36 (25.7%) had a sub-massive PE, and 64 (45.7%) had a low-risk PE (Fig. 1a). Age (p=0.003), COPD (p=0.008), and PESI score (p=0.001) were found to be significantly different in the sub-group analysis. Patients within the massive PE group were found to be significantly younger as compared to the rest (Table 4). Sub-group analysis showed that patients with a massive PE had an in-hospital mortality rate of 72.5% as opposed to 8.3% and 6.3% in patients with a sub-massive PE and a low-risk PE, respectively (Table 4). Further, subgroup analysis of patients with massive PE based on their thrombolytic status showed no difference between those who received thrombolytic therapy and those who had not received thrombolytic therapy except in hypertension and PESI class. Patients who had not received thrombolytic therapy had a history of hypertension in 57.1% as compared with 16.7% in the other group (p=0.01). In the non-thrombolytic therapy group, 92.9% belonged to PESI class 5 status as compared with 50% in the thrombolytic group (p=0.02).
Fig. 1.
(a) The classification of patients with pulmonary embolism based on clinical severity. (b) The percentage of patients who received thrombolysis in each category.
Table 4.
Pulmonary embolism and clinical outcomes based on clinical severity.
| Characteristics |
Pulmonary thromboembolism Severity |
|||
|---|---|---|---|---|
| Massive(n=40) | Sub massive(n=36) | Low risk(n=64) | p value | |
| Age (year) | 47 ± 14 | 50 ± 16 | 52 ± 15 | 0.003 |
| Sex | ||||
| Female | 16 (40%) | 11 (30.60%) | 30 (46.90%) | 0.279 |
| Male | 24 (60%) | 25 (69.40%) | 34 (53.10%) | |
| Dyspnea | ||||
| NYHA I | 4 (10%) | 0 (0%) | 19 (29.70%) | Stat test could not be performed |
| NYHA II | 3 (7.50%) | 11 (30.60%) | 17 (26.60%) | |
| NYHA III | 16 (40%) | 16 (44.40)% | 21 (32.80%) | |
| NYHA IV | 17 (42.50%) | 9 (25%) | 7 (10.90%) | |
| Syncope | 6 (15%) | 4 (11.10%) | 4 (6.30%) | 0.340 |
| Chest pain | 12 (30%) | 16 (44.40%) | 21 (32.80%) | 0.371 |
| Cough | 11 (27.50%) | 12 (33.30%) | 22 (34.40%) | 0.754 |
| Hemoptysis | 3 (7.50%) | 4 (11.10%) | 7 (10.90%) | 0.823 |
| Palpitations | 6 (15%) | 7 (19.40%) | 6 (9.40% | 0.352 |
| Deep venous thrombosis | 20 (50%) | 19 (52.80%) | 35 (54.70%) | 0.897 |
| Diabetes | 15 (37.50%) | 15 (41.70% | 26 (40.60%) | 0.925 |
| Malignancy | 6 (15%) | 7 (19.40%) | 10 (15.60%) | 0.849 |
| Hypertension | 14 (35%) | 15 (41.70%) | 21 (32.80%) | 0.671 |
| Recent surgery | 9 (22.50%) | 8 (22.20%) | 19 (29.70%) | 0.614 |
| Pregnancy | 1 (2.50%) | 1 (2.80%) | 1 (1.60%) | 0.906 |
| Chronic kidney disease | 6 (15%) | 3 (3.80%) | 4 (6.30%) | 0.318 |
| Chronic obstructive pulmonary disease | 7 (17.50%) | 2 (5.60%) | 1 (1.60%) | 0.008 |
| Trauma | 7 (17.50%) | 6 (16.70%) | 11 (17.20%) | 0.995 |
| Travel | 1 (2.50%) | 0 (0%) | 0 (0%) | 0.284 |
| Duration (days) | 4 ± 5 | 6 ± 4 | 6 ± 7 | 0.287 |
| Acute worsening (days) | 3 ± 2 | 4 ± 2 | 3 ± 5 | 0.154 |
| Pulmonary embolism severity index | 134 ± 32 | 90 ± 27 | 64 ± 20 | <0.001 |
| Outcome | ||||
| Live | 11 (27.50%) | 33 (91.70%) | 60 (93.80%) | <0.001 |
| Death | 29 (72.50%) | 3 (8.30%) | 4 (6.30%) | |
| Pulmonary embolism severity index | ||||
| Live | 119.91 ± 21.585 | 89.64 ± 27.26 | 64.37 ± 20.54 | Not significant |
| Death | 139.83 ± 33.37 | 97.33 ± 25.15 | 61 ± 17.91 | |
| Lysed | ||||
| Thrombolyzed | 25 (62.50%) | 23 (63.90%) | 2 (3.10%) | <0.001 |
| Not thrombolyzed | 15 (37.50%) | 13 (36.10%) | 62 (96.90%) | |
| Bleeding | ||||
| Yes | 5 (12.50% | 5 (13.90%) | 3 (4.70%) | 0.223 |
| No | 35 (87.50%) | 31 (86.10%) | 61 (95.30%) | |
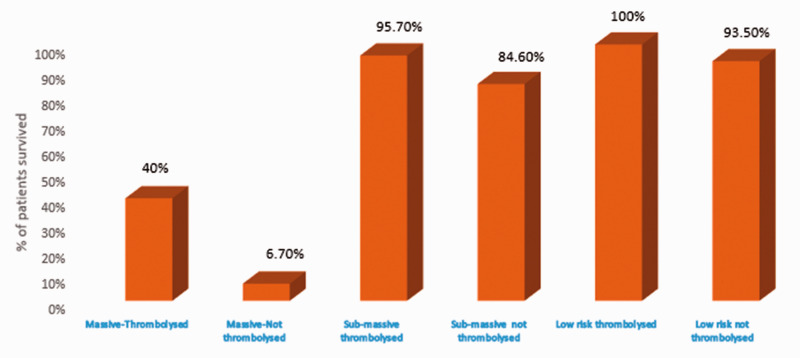
| Thrombolyzed | ||||
| Live | 10 (40%)a | 22 (95.7%)b | 2 (100 %)c | |
| Death | 15 (60%)a | 1 (4.3%)b | 0 (0.1%)c | |
| Not thrombolyzed | ||||
| Live | 1 (6.7%)a | 11 (84.6%)b | 58 (58.1 %)c | |
| Death | 14 (93.3%)a | 2 (15.4%)b | 4 (3.9%)c | |
ap=0.022.
b,cp=not significant.
Use of thrombolytic therapy and its clinical effects
Thrombolytic therapy was given to 62.5% of patients who had massive PE and 63.9% of patients who had sub-massive PE (Fig. 1b). In-hospital mortality was significantly lower in patients who had a massive PE and received thrombolytic therapy compared with those who did not receive thrombolytic therapy (p=0.02) (Fig. 2 and Table 4). In contrast, in-hospital mortality was not significantly different between those patients who had sub-massive PE and received thrombolytic therapy compared with those who did not. In the low-risk PE group, two patients received intravenous thrombolytic therapy. One patient received thrombolytic therapy to prevent complications of post-thrombotic syndrome due to a large thrombus burden of DVT involving the left common iliac vein and inferior vena cava. The second patient had protein S deficiency and a history of recurrent PE with a large thrombus burden of DVT. The second patient underwent thrombolysis with tenecteplase, and during the post-thrombolysis phase, an IVC filter was placed. In total, 9.2% had significant bleeding, while 14% of patients with PE treated with thrombolytic therapy developed BARC grade ≥3 bleeding requiring a blood transfusion. In patients who were not administered thrombolytic therapy, 5.6% of them required a blood transfusion due to anemia or malignancy-related coagulopathy.
Fig. 2.
The survival of patients with different clinical presentation based on their status of thrombolysis.
Use of catheter-directed therapy and other medications
Three patients with acute massive PE received catheter-directed therapy (CDT). One patient had a history of a hemorrhagic cerebro-vascular accident (CVA) and survived, whereas the two other patients died from their disease. One patient with low-risk PE and significant DVT involving proximal deep veins was thrombolyzed peripherally using CDT. All survived patients were initially anticoagulated using unfractionated heparin or low molecular weight heparin and subsequently transitioned to one of the following oral anticoagulants warfarin, nicoumalone, apixaban, rivaroxaban, or dabigatran.
Discussion
To the best of our knowledge, this is the largest study from India evaluating the presenting symptoms and clinical outcomes in patients with PE. In our study, 25.7% of patients with PE died, while 72.5% of patients with massive PE died. Nearly two-thirds of patients with massive PE received thrombolytic therapy. The in-hospital mortality for the patients with massive PE who received thrombolytic therapy was 60%, while for those who did not receive thrombolytic therapy was 93.3%, with an absolute risk reduction of 33.3% (NNT=3).
Indians appear to have a greater propensity towards developing PE at an earlier age, especially massive PE, and are associated with higher mortality. In this study, we found that the mean age of the Indian population having PE was 50 years as opposed to above 65 years as seen in the Western population.13,14 Our finding is in accordance with the findings observed in the Arrive registry which is one of the largest studies on VTE from India.15 A recent trial in South India also found that the mean age of presentation was 52 years.16 PE should therefore be suspected in any patient with unexplained or new-onset dyspnea, chest pain, palpitations, syncope, or unexplained hypotension.17 Early detection and prompt treatment are vital to the management of patients with PE .18
Previously published studies have found that a PESI score >125 was associated with a higher risk of mortality.19 We also found that a PESI score >129 was highly predictive of mortality in our univariate analysis alone. Of patients with a PESI score >129, 78.4% died. Multiple factors were found to be associated with in-hospital mortality in patients with PE in the univariate analysis. However, massive PE was an independent predictors of in-hospital mortality in the multivariate analysis. Nearly two-thirds of patients with massive PE received thrombolytic therapy. Patients who had a massive PE and received thrombolytic therapy had a lower mortality rate (NNT=3), thereby emphasizing the importance of initiating therapy promptly, specifically in critically ill patients with acute cor-pulmonale due to PE.
Thrombolytic therapy is associated with a higher rate of bleeding complications. It was observed that 12.5% of patients with massive PE who received thrombolytics developed bleeding BARC grade ≥3, requiring a blood transfusion. For patients who were deemed to be of high bleeding risk for systemic thrombolytics, catheter-directed therapy (CDT) is a potential alternative. 20,21 CDT can be in the form of catheter-assisted embolectomy or catheter-directed intra-pulmonary thrombolysis and are proposed to have increased efficacy with better safety outcomes. Since we can directly instill the thrombolytic agent at a lower dosage into the thrombus via a side hole catheter, the rate of major systemic bleeding is reported to be lower as compared to systemic thrombolytic therapy20,21 though occasional pulmonary hemorrhage can occur.22 These interventions need more research and standardization before they are widely used. At present, CDT can be used in patients with massive PE who have a high bleeding risk or failed systemic thrombolysis, provided appropriate expertise is available.23 In our study, three patients with acute massive PE received catheter-directed therapy. One patient survived, while the other two succumbed to their illness.
Of the patients who had sub-massive PE, 64% of them received thrombolytic therapy. Unlike patients with massive PE treated with thrombolytic therapy, in-hospital mortality was not affected by the administration of thrombolytic therapy in the sub-massive group. There is a clear discordance between the major societal guidelines in the management of sub-massive PE.10,23 A large, randomized trial, PEITHO – Pulmonary Embolism International Thrombolysis Trial has shown that fibrinolytic therapy decreased hemodynamic decompensation, while increasing the risk of major hemorrhage and stroke when compared with anticoagulation alone in patients with high-risk sub-massive PE 24 without any effect on mortality. Therefore, bleeding risk associated with thrombolytic therapy is an important factor that warrants consideration in patients with PE.
Higher mortality in patients with massive PE especially in those who had not received the thrombolytic therapy may be inherently biased by the critical status of the patients which were shown by a higher PESI score compared with those who had received thrombolytic therapy. Also, a delay in clinical presentation, failure to administer thrombolytic agents, and associated co-morbid illnesses may add to the increased mortality observed in the overall population.
VTE-associated pulmonary embolism should be given equal importance as myocardial infarction and acute stroke considering the poor outcomes noticed in less-industrialized countries like India. Institution of regional centers which serve patients with PE, and an active pulmonary embolism response team (PERT) which consists of a multi-pronged, collaborative approach among various subspecialties in order to effectively coordinate the care of patients with massive PE has been associated with improved outcomes.25–27
Conclusion
In our study, acute PE presented more than a decade earlier in Indian patients compared with their western counterparts and was associated with a very high mortality if left undetected or untreated. Thrombolytic therapy was associated with significantly reduced in-hospital mortality in patients with massive PE. Public education of this illness, promptly recognizing acute pulmonary thromboembolism and the concept of Pulmonary Embolism Response Team (PERT)with the creation of regional centers of excellence serving such patients will likely be instrumental in achieving improved patient outcomes.
Limitations
This was a single-center study done at a teaching hospital in Chennai, India. Therefore, the results of this study may not apply to other types of practice and other regions. Also, this was a retrospective, observational study dependent on the medical records and a computer-based patient database system; therefore, all possible limitations of a retrospective study hold true.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894021992678 for Clinical profile and management of patients with acute pulmonary thromboembolism – a single centre, large observational study from India by Thoddi Ramamurthy Muralidharan, Sankaran Ramesh, Balakrishnan Vinod Kumar, Aditya V. Ruia, Mohan Kumar, Akshaya Gopalakrishnan, Gurpreet S. Johal, Amit Hooda, Rohit Malhotra, Reza Masoomi, Mahalakshmi Ramadoss, Vinodhini Subramanian, Maria J. Kalsingh, Panchanatham Manokar, Jebaraj Rathinasamy, Shanmugasundram Sadhanandham, Jayanthy V. Balasubramaniyan, Preetam Krishnamurthy, Jayanthy S. Murthy, Sadagopan Thanikachalam and Nagendra Boopathy Senguttuvan in Pulmonary Circulation
Acknowledgements
We thank SRIHER for the facilities and support provided to conduct this study. We would like to thank all the staffs and fellows of the department of Cardiology and the members of the central research facility, SRIHER.
Footnotes
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval: The study was approved by the Institute’s Ethics Committee. Informed consent was not obtained from the patients, as this study was a retrospective study which was based on a database
Guarantor: T.R Muralidharan.
Authors’ contributions: ▪
ORCID iDs: Balakrishnan V. Kumar https://orcid.org/0000-0001-9949-4271
Aditya V. Ruia https://orcid.org/0000-0002-3169-1989
Nagendra B. Senguttuvan https://orcid.org/0000-0003-4979-596X
References
- 1.Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology [ESC]. Eur Heart J 2008; 29: 2276–2315. [DOI] [PubMed] [Google Scholar]
- 2.Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med 2010; 38(4 Suppl): S495–501. [DOI] [PubMed] [Google Scholar]
- 3.Meignan M, Rosso J, Gauthier H, et al. Systematic lung scans reveal a high frequency of silent pulmonary embolism in patients with proximal deep venous thrombosis. Arch Intern Med 2000; 160: 159–164. [DOI] [PubMed] [Google Scholar]
- 4.Pineda LA, Hathwar VS, Grant BJ. Clinical suspicion of fatal pulmonary embolism. Chest 2001; 120: 791–795. [DOI] [PubMed] [Google Scholar]
- 5.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry [ICOPER]. Lancet 1999; 353: 1386–1389. [DOI] [PubMed] [Google Scholar]
- 6.Mohan B, Tandon R, Bansal R, et al. , Determinants of in-hospital clinical outcome in patients with sub-massive pulmonary embolism. Indian Heart J 2018; 70(Suppl 3): S90–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakkar N, Vasishta RK. Pulmonary embolism in medical patients: an autopsy-based study. Clin Appl Thromb Hemost 2008; 14: 159–167. [DOI] [PubMed] [Google Scholar]
- 8.Leizorovicz A, Turpie AGG, Cohen AT, et al. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J Thromb Haemost 2005; 3: 28–34. [DOI] [PubMed] [Google Scholar]
- 9.Jaff Michael R, McMurtry MS, Archer Stephen L, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123: 1788–1830. [DOI] [PubMed] [Google Scholar]
- 10.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society [ERS]The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology [ESC]. Eur Heart J 2020; 41: 543–603. [DOI] [PubMed] [Google Scholar]
- 11.Vranckx P, White HD, Huang Z, et al. Validation of BARC bleeding criteria in patients with acute coronary syndromes: the TRACER trial. J Am Coll Cardiol 2016; 67: 2135–2144. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Baber U, Kini A, et al. Comparison of bleeding definitions, Barc, Gusto, Timi and Varc in patients undergoing balloon aortic valvuloplasty: results from a two-center registry. J Am Coll Cardiol 2019; 59: E65. [Google Scholar]
- 13.Tsymbalyuk N, Mostovoy Y, Slepchenko N. Study of pulmonary embolism prevalence depending on age and sex by autopsy data. Eur Respir J 2012 40: 3985. [Google Scholar]
- 14.Stein PD, Hull RD, Kayali F, et al. Venous thromboembolism according to age: the impact of an aging population. Arch Intern Med 2004; 164: 2260–2265. [DOI] [PubMed] [Google Scholar]
- 15.Kamerkar DR, John MJ, Desai SC, et al. Arrive: a retrospective registry of Indian patients with venous thromboembolism. Indian J Crit Care Med 2016; 20: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calwin Davidsingh S, Srinivasan N, Balaji P, et al. Study of clinical profile and management of patients with pulmonary embolism – single center study. Indian Heart J 2014; 66: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendelboe AM, McCumber M, Hylek EM, et al. Global public awareness of venous thromboembolism. J Thromb Haemost 2015; 13: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 18.Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primer 2018; 4: 18028. [DOI] [PubMed] [Google Scholar]
- 19.Dentali F, Riva N, Turato S, et al. Pulmonary embolism severity index accurately predicts long-term mortality rate in patients hospitalized for acute pulmonary embolism. J Thromb Haemost 2013; 11: 2103–2110. [DOI] [PubMed] [Google Scholar]
- 20.Giri J, Sista AK, Weinberg I, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation 2019; 140: e774–e801. [DOI] [PubMed] [Google Scholar]
- 21.Barco S, Konstantinides SV. Catheter-directed thrombolysis for acute pulmonary embolism: where do we stand? Lung India Off Organ Indian Chest Soc 2017; 34: 221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown KN, Devarapally SR, Lee L, et al. Catheter directed thrombolysis of pulmonary embolism. In: StatPearls. Treasure Island [FL]: StatPearls Publishing, www.ncbi.nlm.nih.gov/books/NBK536918/ (accessed 30 March 2020). [PubMed]
- 23.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–352. [DOI] [PubMed] [Google Scholar]
- 24.Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370: 1402–1411. [DOI] [PubMed] [Google Scholar]
- 25.Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133: 98–103. [DOI] [PubMed] [Google Scholar]
- 26.Kabrhel C, Jaff MR, Channick RN, et al. A multidisciplinary pulmonary embolism response team. Chest 2013; 144: 1738–1739. [DOI] [PubMed] [Google Scholar]
- 27.Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team [MGH PERT]: creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract 2014; 42: 31–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894021992678 for Clinical profile and management of patients with acute pulmonary thromboembolism – a single centre, large observational study from India by Thoddi Ramamurthy Muralidharan, Sankaran Ramesh, Balakrishnan Vinod Kumar, Aditya V. Ruia, Mohan Kumar, Akshaya Gopalakrishnan, Gurpreet S. Johal, Amit Hooda, Rohit Malhotra, Reza Masoomi, Mahalakshmi Ramadoss, Vinodhini Subramanian, Maria J. Kalsingh, Panchanatham Manokar, Jebaraj Rathinasamy, Shanmugasundram Sadhanandham, Jayanthy V. Balasubramaniyan, Preetam Krishnamurthy, Jayanthy S. Murthy, Sadagopan Thanikachalam and Nagendra Boopathy Senguttuvan in Pulmonary Circulation