Abstract
Objective
Nearly 5% of patients with breast cancer carry germline BRCA mutations, which are more common in triple-negative breast cancer (TNBC). Previous clinical trials demonstrated the therapeutic efficacy of poly (ADP-ribose) polymerase inhibitors (PARPis) against BRCA-mutated metastatic breast cancer. The current study conducted a systemic review and meta-analysis of the clinical efficiency and safety of PARPis, either alone or combined with chemotherapy, in patients with TNBC.
Methods
We searched PubMed, EMBASE, and ClinicalTrials.gov to identify randomized controlled trials comparing PARPi therapy with chemotherapy, and comparisons of chemotherapy plus PARPis with chemotherapy alone were included. The study endpoints included the clinical response, progression-free survival, and adverse event rates.
Results
PARPi therapy was revealed to improve progression-free survival in patients with advanced breast cancer, either alone or in combination with chemotherapy. Subgroup analysis illustrated that patients with mutant BRCA1 and mutant BRCA2 and those who had not been treated with platinum-based agents could specifically benefit from PARPis.
Conclusion
PARPi monotherapy can significantly improve clinical outcomes in patients with advanced breast cancer, especially those with TNBC, those who had not previously received platinum therapy, and those with mutant BRCA1/2. PARPis combined with chemotherapy represent new treatment options for patients with advanced cancer.
Keywords: poly (ADP-ribose) polymerase, breast cancer, BRCA, chemotherapy, platinum, clinical trial, HER2, homologous recombination, DNA repair
Introduction
Breast cancer is one of the most common malignant tumors in women. According to National Cancer Institute statistics, nearly 252,710 women were diagnosed with breast cancer in 2018, and 40,610 women died of the disease.1 Advanced metastasis is an important factor threatening the lives of patients. Chemotherapy, endocrine therapy, radiotherapy, and targeted therapy are the primary treatments for patients with advanced breast cancer. Currently, a widely used targeted therapy in clinical practice is anti-HER2 therapy, including HER2 antibodies and tyrosine kinase inhibitors (TKIs).2 However, triple-negative breast cancer (TNBC), which accounts for approximately 15% of breast cancers, lacks therapeutic targets.3 Chemotherapy has always been the main systemic treatment for TNBC. Because of the lack of targets for the three causes of breast cancer and the lack of targeted drugs, patient prognosis is poor, prompting clinicians to develop significant efforts to discovering treatable molecular targets.3 Interestingly, patients with TNBC often carry germline BRCA (gBRCA) mutations.4,5 Research data from Chinese patients with breast cancer revealed that the frequency of BRCA gene mutation in TNBC was approximately 11%.6
BRCA1 and BRCA2 are key tumor suppressor genes for homologous recombination (HR) repair. The proteins encoded by these genes are involved in the repair of DNA double-strand breaks, cell growth, and prevention of the abnormal cell division that leads to the occurrence of tumors. Poly (ADP-ribose) polymerase (PARP), as a DNA break sensor, is activated after DNA damage, and it recognizes and binds to the DNA break site and participates in the repair of DNA single-strand damage in tumor cells. For tumors with abnormal HR repair function, PARP inhibitors (PARPis) suppress PARP enzymatic activity and increase the formation of PARP–DNA complexes, leading to the repair of DNA damage in tumor cells and promoting apoptosis.7 In prior research, PARPis enhanced the efficacy of radiotherapy, alkylating agents, and platinum-based chemotherapy by inhibiting the repair of DNA damage in tumor cells and promoting apoptosis.8 Since 2003, clinical studies on the utilization of PARPis in solid malignancies have been increasingly reported. Breast and ovarian cancers, which are most frequently associated with BRCA mutations, were demonstrated to respond to PARPis.9,10 Several PARPis, such as olaparib, rucaparib, and niraparib, have been approved by the US Food and Drug Administration (FDA) as maintenance therapies for recurrent ovarian cancer.11–14 A meta-analysis of the efficacy of PARPis as maintenance treatments for platinum-sensitive recurrent ovarian cancer suggested that these drugs were effective regardless of BRCA mutation status, and substantial improvements of progression-free survival (PFS) were observed for patients with germline mutations.15 Currently, several clinical studies on PARPis for advanced breast cancer are underway. The randomized phase III trials OlympiAD16 and EMBRACA17 compared the effects of olaparib and talazoparib with doctor-selected chemotherapy in patients with gBRCA-mutant, HER2-negative breast cancer. PFS, as the primary endpoint, was significantly prolonged in the PARPi group. The FDA approved the two drugs for the clinical treatment of patients with gBRCA1/2-mutant, HER2-negative metastatic breast cancer who had previously received chemotherapy. BRCA mutation is an important therapeutic target for TNBC. Preclinical studies confirmed that PARPis could induce synergistic lethal effects in BRCA-mutant tumors. The results of OlympiAD further confirmed the role of PARPi therapy in patients with gBRCA-mutant, HER2-negative metastatic breast cancer from a clinical perspective. However, the results from phase II and III randomized controlled trials (RCTs) have been inconsistent.
In this study, we assessed the efficacy of PARPi therapy in patients with advanced breast cancer through a meta-analysis, including subgroup analyses.
Methods
Study search strategy
We first initiated a systemic review of the literature according to the Cochrane and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.18 We used several methods to screen the final studies for our research. PubMed, Embase, and ClinicalTrials.gov were searched according to the keywords represented in the titles and abstracts, including “breast cancer,” “PARP,” “chemotherapy,” and “BRCA.” This study was not registered with PROSPERO.
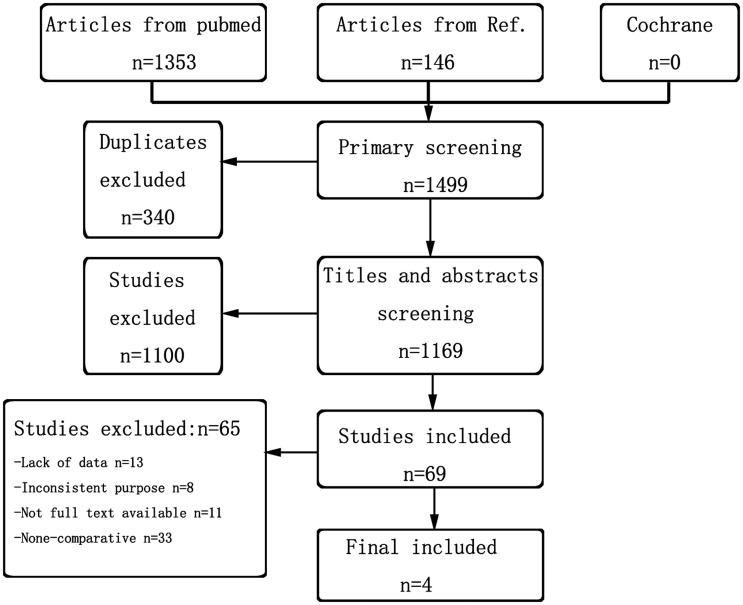
In total, 1499 articles were screened using the online databases. Among them, 146 were identified via a manual search of article references. Figure 1 presents the details of the search results. Of the searched studies, 340 were duplicates, and 1100 did not match the study aim based on a comprehensive reading of the titles and abstracts. After removing these articles, we further screened the remaining 69 studies by reading the full text intensively. Consequently, four clinical trials providing sufficient data were included in the meta-analyses.16,17,19,20 The characteristics of the included studies are summarized in Table 1.
Figure 1.
Flow chart of study selection.
Table 1.
Characteristics of the included studies.
| Clinical trials | Recruited patients | No. of patients | Design | Studied PARPi | Treatment/arms |
|---|---|---|---|---|---|
| Kummar et al., 2016 | Adult patients with refractory/metastatic TNBC | 45 | Open-label, multicenter, randomized phase II study | Veliparib (ABT-888) | A: Cyclophosphamide 50 mg once daily (n = 18)B: Cyclophosphamide 50 mg once daily + veliparib 60 mg daily in 21-day cycles (n = 21) |
| O’Shaughnessy et al., 2014 | Adult patients with refractory/metastatic TNBC | 519 | Open-label, multicenter, randomized phase III study | Iniparib | A: GC alone (n = 258) B: GC + iniparib (at a dose of 5.6 mg/kg body weight) on days 1, 4, 8, and 11 of each 21-day cycle (n = 261) |
| Litton et al., 2018 | Patients with advanced breast cancer and a germline BRCA1/2 mutation | 431 | Open-label, multicenter, randomized phase III study | Talazoparib | A: Standard single-agent therapy* (n = 287) B: Talazoparib (1 mg once daily) (n = 144) |
| Robson et al., 2017 | Patients with advanced breast cancer and a germline BRCA1/2 mutation | 302 | Open-label, multicenter, randomized phase III study | Olaparib | A: Standard single-agent therapy* (n = 97) B: Olaparib tablets (300 mg twice daily, n = 205) |
TNBC, triple-negative breast cancer; GC, gemcitabine (1000 mg/m2 body surface area) and carboplatin (at a dose equivalent to an area under the concentration–time curve of 2) on days 1 and 8.
*Standard single-agent therapy indicates the physician’s choice, including capecitabine, eribulin, gemcitabine, or vinorelbine, in continuous 21-day cycles.
Study criteria
Studies were eligible for inclusion if they were multicenter phase II or phase III RCTs. The included patients were diagnosed with advanced breast cancer, and they were randomly assigned to treatment with chemotherapy, PARPis, or both. The included studies reported at least one of the following clinical outcomes: response rate, PFS, overall survival (OS), and toxicity. Studies that had only one arm, those designed for neoadjuvant therapy, and those using other targeted therapies were excluded.
Study evaluation and data extraction
The Jadad score was used to assess the quality of each included study. The scoring criteria include the generation of random sequences, blinding procedure, and adscription of withdrawals and dropouts.21 We extracted the following data from the included articles: number of patients enrolled, chemotherapy regimen, treatment group, BRCA1 and BRCA2 status, hormone receptor and HER2 status, toxicity, and efficacy.
Statistical analysis
The clinical data, including the number of patients, clinical efficacy, and toxicity, were extracted from the included articles. PFS was defined as the time from randomization to objective radiologic disease progression. According to the modified Response Evaluation Criteria in Solid Tumors, version 1.1, the clinical response (CR) rate was defined as the sum of the stable disease, complete response, and partial response rates. Quantitative statistical combinations were calculated using Review Manager (version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) using fixed-effects or random-effects modeling considering the existing study variations. Heterogeneity was quantified using the I2 statistic. The fixed-effects or random-effects model selection principles were based on the value of I2. For I2 < 40%, which indicated low heterogeneity, the fixed-effects model was chosen. For I2 ≥ 40%, the random-effects model was chosen. The integrative results were represented as the odds ratio (OR) between groups and the 95% confidence interval (CI). In all analyses, P < 0.05 indicated statistical significance.
Results
All four included trials were open-label, multicenter phase II or III RCTs. All recruited patients were diagnosed with advanced breast cancer. Two studies compared PARPis with standard chemotherapy,16,17 and the other two studies primarily evaluated PARPis combined with chemotherapy.19,20 The methodological quality of the trials was assessed using the Jadad score (Table 2). The quality scores ranged from 4 to 5, indicating good quality despite the lack of double-blind studies.
Table 2.
Jadad scale.
| Clinical trials | Kummar et al., 2016 | O’Shaughnessy et al., 2014 | Litton et al., 2018 | Robson et al., 2017 |
|---|---|---|---|---|
| Randomization | 2 | 2 | 2 | 2 |
| Concealment of allocation | 2 | 2 | 2 | 2 |
| Double blinding | 0 | 0 | 0 | 0 |
| Withdrawals and dropouts | 0 | 1 | 1 | 1 |
| Jadad scorea | 4 | 5 | 5 | 5 |
aMethodological quality of meditative movement studies reviewed using Jadad scoring criteria. The maximum score is 7. Scores of 1 to 3 indicated low quality, whereas scores of 4 to 7 indicated high quality.
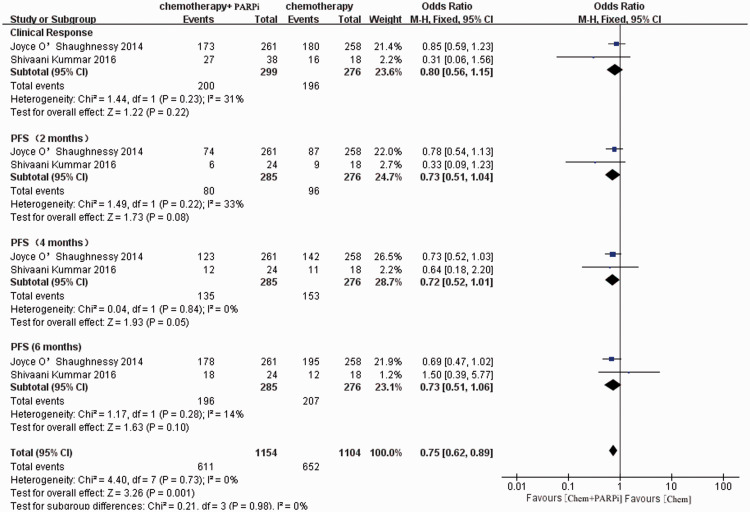
Clinical efficacy of PARPis combined with chemotherapy
Two earlier reports evaluated the clinical efficacy of PARPis plus chemotherapy in patients with advanced breast cancer.19,20 We combined the results of these studies and chose PFS and CR rates as the endpoints.
The meta-analysis illustrated that the addition of PARPis to chemotherapy did not increase the CR rate as expected (OR = 0.80, 95% CI = 0.56–1.15, P = 0.22). However, the combination regimens were linked to significantly improved PFS rates (OR = 0.72, 95% CI = 0.62–0.89, P = 0.001). The forest plot illustrated that the addition of PARPis to chemotherapy improved the long-term survival of patients (Figure 2).
Figure 2.
Forest plot of the pooled relative risk of clinical efficacy from the included studies reporting the clinical outcome associated with the combination of PARPis and chemotherapy. Horizontal lines represent 95% CIs.
M-H, Mantel–Haenszel; df, degrees of freedom; chem., chemotherapy; PARPi, poly ADP-ribose polymerase inhibitor; CI, confidence interval.
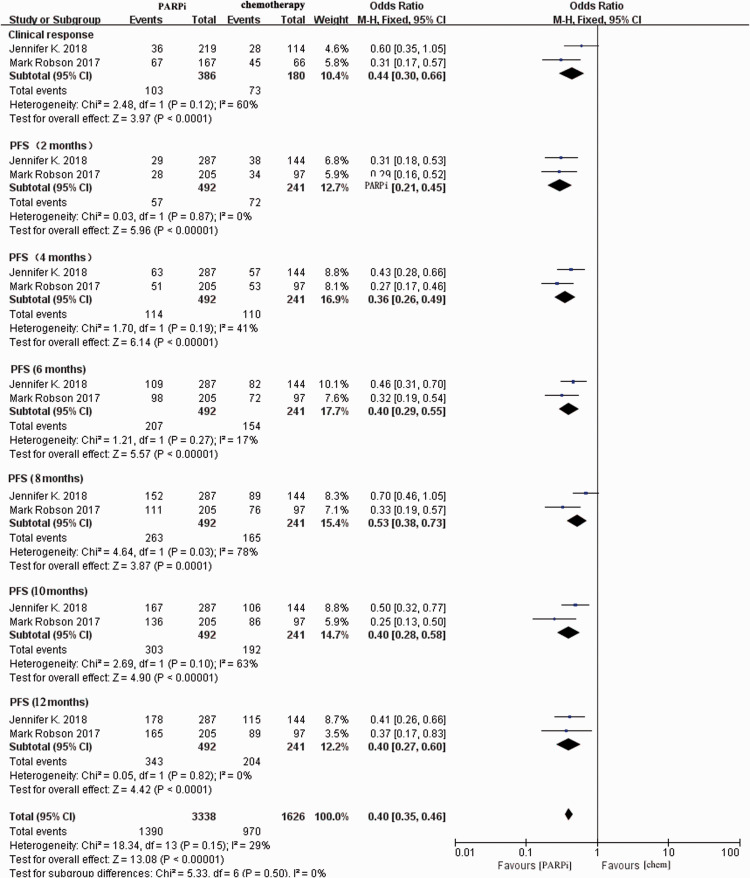
Clinical efficacy of PARPi versus chemotherapy
Two included trials reported the result of single-agent PARPi therapy versus standard therapy in patients with advanced breast cancer and gBRCA1/2 mutations.16,17
The results demonstrated that PARPi treatment (olaparib16 or talazoparib17) was statistically associated with better CR rates (OR = 0.44, 95% CI = 0.30–0.66, P < 0.0001) and increased PFS rates (OR = 0.40, 95% CI = 0.35–0.46, P < 0.0001, Figure 3). The analysis of PFS rates from 2 to 12 months in the forest plot illustrated the continuous effects of PARPis. Statistical heterogeneity was not obvious (Cochran’s Q test, P = 0.16, I2 = 30%).
Figure 3.
Forest plot of pooled relative risk of clinical efficacy from the included studies reporting clinical outcomes associated with PARPi monotherapy compared with chemotherapy. Horizontal lines represent 95% CIs.
M-H, Mantel–Haenszel; df, degrees of freedom; chem., chemotherapy; PARPi, poly ADP-ribose polymerase inhibitor; CI, confidence interval.
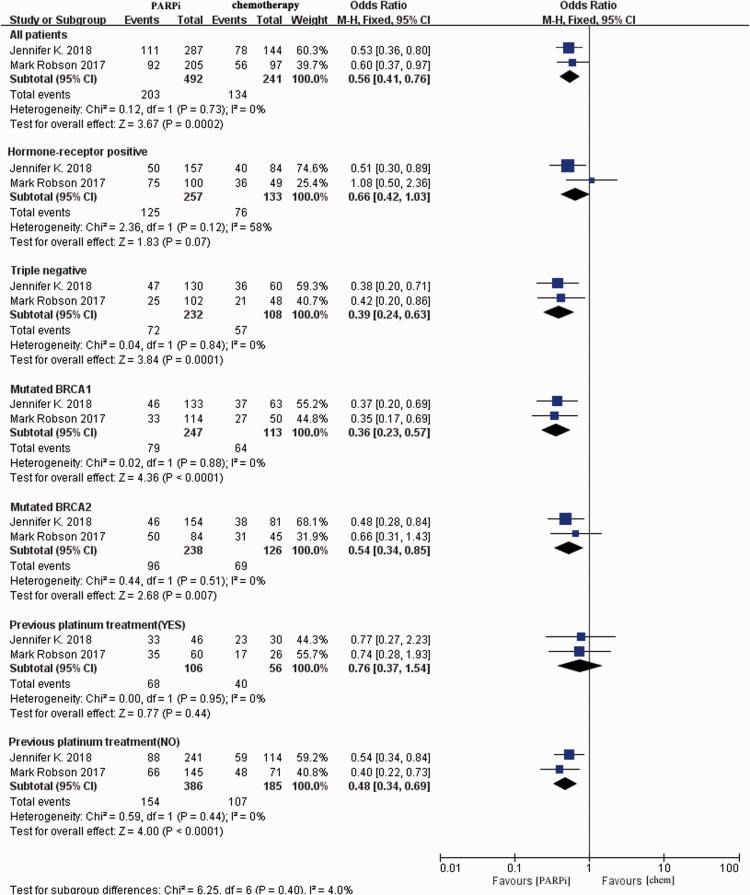
We conducted subgroup analyses based on the BRCA status, receptor status (hormone receptor-positive or triple-negative), and receipt of platinum treatment. Among all subgroups analyzed, the PARPi arm was significantly preferred in terms of PFS in four subgroups: TNBC (OR = 0.39, 95% CI = 0.24–0.63, P = 0.0001), mutant BRCA1 (OR = 0.36, 95% CI = 0.23–0.57, P < 0.0001), mutant BRCA2 (OR = 0.54, 95% CI = 0.34–0.85, P = 0.007), and no prior platinum treatment (OR = 0.48, 95% CI = 0.34–0.69, P < 0.0001, Figure 4). Patients with hormone receptor-positive cancer and those who previously received platinum treatment did not significantly benefit from PARPis.
Figure 4.
Pooled subgroup analysis of the relative risk of survival from the included studies reporting the disease-free survival of specific patients (e.g., triple-negative breast cancer, BRCA1/2 mutation, receipt of previous platinum therapy). Horizontal lines represent 95% CIs.
M-H, Mantel–Haenszel; df, degrees of freedom; chem., chemotherapy; PARPi, poly ADP-ribose polymerase inhibitor; CI, confidence interval.
Assessment of serious adverse events
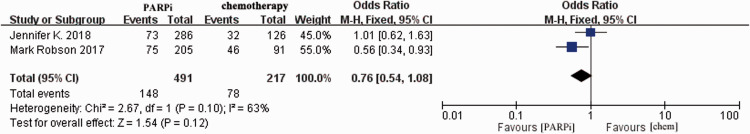
Adverse events related to the treatment were recorded in all of the included clinical trials. The main toxic effects were reflected in the blood and digestive systems. First, we compared the risks of grade 3 and 4 side effects between PARPi therapy and chemotherapy (Figure 5). Robson et al.16 reported fewer adverse effects in the single-agent PARPi arm. The current study revealed no differences in terms of serious side effects (≥Grade 3) between the PARPi and standard chemotherapy arms (OR = 0.76, 95% CI = 0.54–1.08, P = 0.12).
Figure 5.
Pooled analysis of side effects comparing PARPis with chemotherapy. Horizontal lines represent 95% CIs.
M-H, Mantel–Haenszel; df, degrees of freedom; chem., chemotherapy; PARPi, poly ADP-ribose polymerase inhibitor; CI, confidence interval.
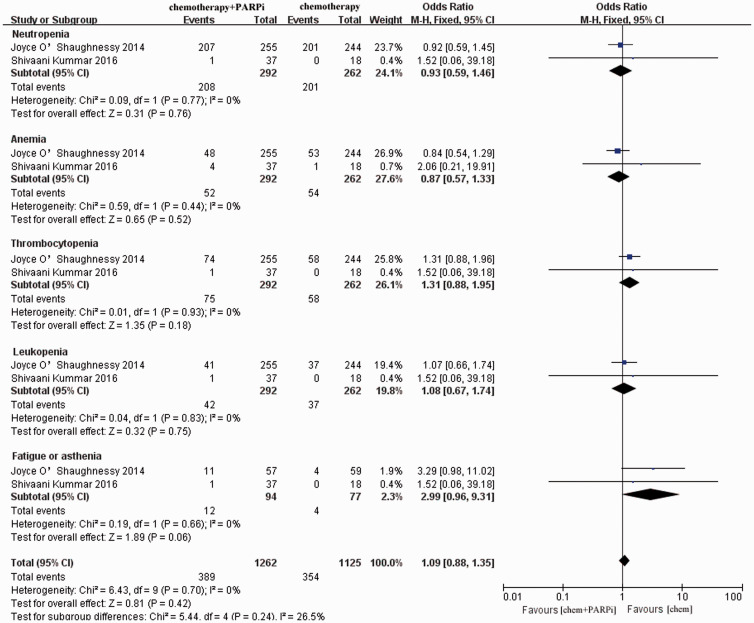
The other two studies reported data for the specific adverse reactions of PARPis in combination with chemotherapy.19,20 The most common side effects were neutropenia, anemia, thrombocytopenia, leucopenia, and fatigue or asthenia. From the forest graph (Figure 6), the incidence of all of the aforementioned adverse events were similar between the PARPi monotherapy and combination treatment groups (OR = 1.09, 95% CI = 0.88–1.35).
Figure 6.
Pooled analysis of specific side effects of the combination of PARPis with chemotherapy. Horizontal lines represent 95% CIs.
M-H, Mantel–Haenszel; df, degrees of freedom; chem., chemotherapy; PARPi, poly ADP-ribose polymerase inhibitor; CI, confidence interval.
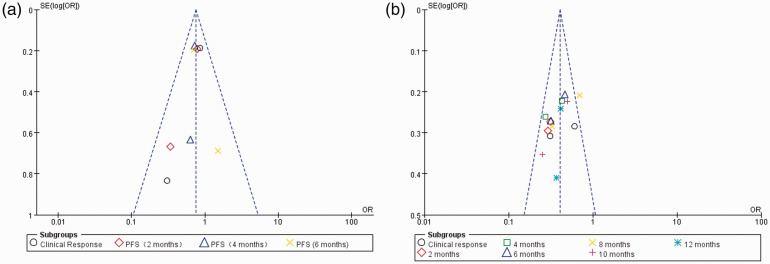
Publication bias
All meta-analyses in our study were divided into two parts: PARPi alone or combined with chemotherapy versus chemotherapy alone. Therefore, the publication bias assessments were divided into two parts. The funnel plot (Figure 7) presented no evidence of remarkable asymmetry in the monotherapy and combination arms (P = 0.5 and P = 0.98, respectively).
Figure 7.
Funnel plot for publication bias. (a) Funnel plot of the relationship between PARPi combination therapy and clinical efficacy. (b) Funnel plot of the relationship between PARPi monotherapy and clinical efficacy.
PARPi, poly ADP-ribose polymerase inhibitor.
Discussion
The current meta-analysis assessed the efficiency, safety, and benefits of PARPis. Relative to standard chemotherapy, PARPi monotherapy appeared to be effective and safe for patients with advanced breast cancer. Subgroup analysis illustrated that patients with TNBC, BRCA1 mutation, and no prior history of platinum therapy more strongly benefited from PARPi treatment. In addition, the combination of PARPis and chemotherapy significantly improved the survival of patients with TNBC.
Chemotherapy remains the primary treatment for patients with metastatic breast cancer at present. In recent years, several studies demonstrated the efficacy of platinum drugs against TNBC. Platinum drugs cause DNA cross-linking, hinder DNA synthesis, and inhibit tumor growth.22 However, resistance to chemotherapy, which is the main cause of treatment failure in patients advanced breast cancer and poor prognosis, is extremely common. Therefore, preclinical and clinical trials have been devoted to identifying new therapeutic targets.
Studies have revealed that 5% to 10% of patients with breast cancer have a clear genetic mutation, called hereditary breast cancer,23,24 in which BRCA1/2 gene mutation accounts for 15% of such lesions.25,26 Most BRCA-associated breast cancers are triple-negative.27 Interestingly, BRCA1 is the most studied gene associated with platinum resistance, and BRCA1-deficient tumor cells are more sensitive to cisplatin and other platinum drugs.28 A previous study also revealed that breast cancer cells with BRCA1/2 mutations are more sensitive to DNA cross-linking agents, such as cisplatin, carboplatin, and mitomycin.29
PARPis induce DNA single-strand breaks by blocking the repair of single-stranded DNA breakpoints, whereas BRCA mutants cannot initiate HR to repair DNA duplexes. PARP and BRCA are genes with synergistic lethal effects against tumor cells. Therefore BRCA mutants are sensitive to PARPis, leading to satisfactory clinical effects.16,30 The 2017 American Society of Clinical Oncology (ASCO) meeting reported the results of Phase III clinical trials of olaparib for patients with metastatic breast cancer. In January 2018, the FDA approved olaparib for the treatment of HER2-negative metastatic breast cancer carrying the BRCA gene mutation. In 2020, ASCO, the American Society of Radiation Oncology, and the Society of Surgical Oncology convened expert teams to formulate recommendations for the treatment of patients with breast cancer and susceptible germline mutations based on systematic reviews of the literature. The teams proposed that for HER2-negative breast cancer with BRCA1/2 mutations, olaparib or talazoparib should be used instead of chemotherapy in the first three lines of treatment. For BRCA1/2 mutation carriers with metastatic HER2-negative breast cancer, there are no data directly comparing the efficacy of PARPis and platinum-based chemotherapy.31 The current meta-analysis integrated data for olaparib and talazoparib, and the clinical effects were consistent with the reported phase III studies.16,17 Furthermore, patients with BRCA1/2 mutations and those who have not received platinum therapy can significantly benefit from PARPis. The results from the subgroup analysis were not completely consistent with the previously reported results, which revealed that the clinical outcomes of BRCA1 mutation carriers were worse than those of BRCA2 mutation carriers.32 The correlation between BRCA1/2 gene mutation and the prognosis of breast cancer is unclear.33,34 We speculated that mutant BRCA1 and BRCA2 played different roles in the response to PARPis. It is possible that the previously reported worse survival of BRCA2-mutant cancer was related to the different response to PARPis analyzed in our study. However, additional prospective studies are needed for confirmation.
Although PARPis are extremely effective against BRCA-mutant and platinum-resistant ovarian cancer, we found that patients with breast cancer who had previously received platinum-based treatment did not benefit from PARPis compared with the effects of chemotherapy. The specific mechanism is unclear at present. PARPis are currently approved for HER2-negative metastatic breast cancer carrying BRCA germline mutations. According to the latest guideline, platinum-based chemotherapy is recommended preferentially.35 However, our study suggested that patients who did not receive platinum treatment could benefit from PARPis, which means that PARPis would have greater utility in the adjuvant treatment stage. Clinical trials using PARPis in the adjuvant and neoadjuvant phases are underway. BrighTNess (NCT02032277) was a phase III trial assessing the combination of veliparib and chemotherapy in the neoadjuvant setting;36 BRE09-146 (NCT01074970) was a phase II trial that evaluated rucaparib combined with cisplatin in the adjuvant phase.37 Results from BrighTNess illustrated that a grossly subtherapeutic dose of PARPis in combination with standard doses of chemotherapy did not significantly improve clinical outcomes. We must await additional clinical trials and other research.
Although the original purpose of PARPis was to increase the sensitivity of tumor cells to chemotherapy by causing DNA damage, the clinical outcomes of combination studies of chemotherapy and PARPis were heterogeneous. The main reason is that the side effects of chemotherapy on normal healthy cells tend to limit the drug dosage, and combined usage with PARPis will increase side effects in healthy cells. Preclinical studies illustrated that high doses of PARPis combined with relatively low doses of chemotherapy could inhibit the proliferation of tumor cells.38,39 The safety and efficacy of this combination therapy are being tested in clinical trials. A phase III clinical trial reported the results of iniparib plus chemotherapy compared with chemotherapy alone in metastatic TNBC. Unfortunately, the trial did not meet the expected primary endpoints of PFS and OS.20 In our meta-analyses, the combination of PARPis with chemotherapy provided survival benefits for patients.
Clinical scientists are also working to further improve the clinical remission rate of TNBC and overcome the occurrence of drug resistance. PARPi combination treatments are worthy of further study. Clinical trials of PARPis combined with immune checkpoint inhibitors are also undergoing. The rationale for these combinations is that tumors with HR defects usually carry more genetic mutations, which may lead to the production of more new antigens and induction of stronger anti-tumor immune responses.40 Several studies, including the TOPACIO (NCT02657889), MEDIOLA (NCT02734004), and NCT02849496 trials, combined PARPis with immune checkpoint inhibitors.41–43 In the setting of ovarian cancer, the results of one Phase I study (TOPACIO/Keynote-162)44 demonstrated that niraparib combined with pembrolizumab was feasible and safe, with no expected toxicity observed. Strategies using immune checkpoint inhibitors are generally not hindered by additive toxicities in breast cancer, but the utility of combining PARPis with immunotherapy has not been particularly effective to date.
Conclusion
PARPi monotherapy could obviously improve the clinical outcomes of patients with advanced breast cancer, especially those with TNBC, BRCA1/2 mutations, and no prior history of platinum therapy. The combination of PARPis with chemotherapy represents a novel option for such patients. In patients with advanced TNBC who responded to previous platinum therapy, PARPis can be considered. Future PARPi studies should cover the following points: the selection of the most suitable patients for PARPi therapy, the development of drug resistance, and the optimum combination therapy.
Footnotes
Author contributions: ZC and KC contributed to study conception and design. ZC, XW, and YZ reviewed the literature and designed the article structure. ZC, KC, and YZ contributed to the acquisition and analysis of data. ZC and XW participated in data interpretation. ZC and KC were major contributors to writing the manuscript. XW, XL, and YZ revised and edited the manuscript critically for important intellectual content. ZC, KC, YZ, and XL gave final approval of the version to be published.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by National Natural Science Foundation of China (grant number, 81802623).
ORCID iDs: Zheling Chen https://orcid.org/0000-0002-3073-1811
References
- 1.Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018; 124: 2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponde N, Brandao M, El-Hachem G, et al. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev 2018; 67: 10–20. [DOI] [PubMed] [Google Scholar]
- 3.Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016; 13: 674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 2015; 33: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Klemp JR, Kimler BF, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat 2014; 145: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Meng H, Yao L, et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin Cancer Res 2017; 23: 6113–6119. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenblick A, De Azambuja E, Azim HA, Jr, et al. An update on PARP inhibitors–moving to the adjuvant setting. Nat Rev Clin Oncol 2015; 12: 27–41. [DOI] [PubMed] [Google Scholar]
- 8.De Vos M, Schreiber V, Dantzer F.The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol 2012; 84: 137–146. [DOI] [PubMed] [Google Scholar]
- 9.Geenen JJJ, Linn SC, Beijnen JH, et al. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin Pharmacokinet 2018; 57: 427–437. [DOI] [PubMed] [Google Scholar]
- 10.Dizon DS.PARP inhibitors for targeted treatment in ovarian cancer. Lancet 2017; 390: 1929–1930. [DOI] [PubMed] [Google Scholar]
- 11.Tucker H, Charles Z, Robertson J, et al. NICE guidance on olaparib for maintenance treatment of patients with relapsed, platinum-sensitive, BRCA mutation-positive ovarian cancer. Lancet Oncol 2016; 17: 277–278. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott LJ.Niraparib: First Global Approval. Drugs 2017; 77: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 14.Musella A, Bardhi E, Marchetti C, et al. Rucaparib: An emerging parp inhibitor for treatment of recurrent ovarian cancer. Cancer Treat Rev 2018; 66: 7–14. [DOI] [PubMed] [Google Scholar]
- 15.Tomao F, Bardhi E, Di Pinto A, et al. Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat Rev 2019; 80: 101909. [DOI] [PubMed] [Google Scholar]
- 16.Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017; 377523–533. [DOI] [PubMed] [Google Scholar]
- 17.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018; 379753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65–W94. [DOI] [PubMed] [Google Scholar]
- 19.Kummar S, Wade JL, Oza AM, et al. Randomized phase II trial of cyclophosphamide and the oral poly (ADP-ribose) polymerase inhibitor veliparib in patients with recurrent, advanced triple-negative breast cancer. Invest New Drugs 2016; 34: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Shaughnessy J, Schwartzberg L, Danso MA, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol 2014; 32: 3840–3847. [DOI] [PubMed] [Google Scholar]
- 21.Daouacher G, Walden M.A simple reconstruction of the posterior aspect of rhabdosphincter and sparing of puboprostatic collar reduces the time to early continence after laparoscopic radical prostatectomy. J Endourol 2014; 28: 481–486. [DOI] [PubMed] [Google Scholar]
- 22.Baselga J, Gomez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 2013; 31: 2586–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellisen LW, Haber DA.Hereditary breast cancer. Annu Rev Med 1998; 49: 425–436. [DOI] [PubMed] [Google Scholar]
- 24.Castera L, Krieger S, Rousselin A, et al. Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. Eur J Hum Genet 2014; 22: 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen FC, Van Overeem Hansen T, Sorensen CS.Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer 2016; 16: 599–612. [DOI] [PubMed] [Google Scholar]
- 26.Stratton MR, Rahman N.The emerging landscape of breast cancer susceptibility. Nat Genet 2008; 40: 17–22. [DOI] [PubMed] [Google Scholar]
- 27.De Summa S, Pinto R, Sambiasi D, et al. BRCAness: a deeper insight into basal-like breast tumors. Ann Oncol 2013; 24: viii13–viii21. [DOI] [PubMed] [Google Scholar]
- 28.Tassone P, Di Martino MT, Ventura M, et al. Loss of BRCA1 function increases the antitumor activity of cisplatin against human breast cancer xenografts in vivo. Cancer Biol Ther 2009; 8: 648–653. [DOI] [PubMed] [Google Scholar]
- 29.Lord CJ, Ashworth A.BRCAness revisited. Nat Rev Cancer 2016; 16: 110–120. [DOI] [PubMed] [Google Scholar]
- 30.Wang YQ, Wang PY, Wang YT, et al. An Update on Poly(ADP-ribose)polymerase-1 (PARP-1) Inhibitors: Opportunities and Challenges in Cancer Therapy. J Med Chem 2016; 59: 9575–9598. [DOI] [PubMed] [Google Scholar]
- 31.Tung NM, Boughey JC, Pierce LJ, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol 2020; 38: 2080–2106. [DOI] [PubMed] [Google Scholar]
- 32.Rijnsburger AJ, Obdeijn IM, Kaas R, et al. BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC Screening Study. J Clin Oncol 2010; 28: 5265–5273. [DOI] [PubMed] [Google Scholar]
- 33.Zhong Q, Peng HL, Zhao X, et al. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res 2015; 21: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine (Baltimore) 2016; 95: e4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017; 28: 3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geyer CE, O'Shaughnessy J, Untch M, et al. Phase 3 study evaluating efficacy and safety of veliparib (V) plus carboplatin (Cb) or Cb in combination with standard neoadjuvant chemotherapy (NAC) in patients (pts) with early stage triple-negative breast cancer (TNBC). J Clin Oncol 2017; 35: 520. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.520 [Google Scholar]
- 37.PARP inhibition for triple negative breast cancer (ER-/PR-/HER2-)With BRCA1/2 mutations. https://ClinicalTrials.gov/show/NCT01074970.
- 38.Li M, Yu X.The role of poly(ADP-ribosyl)ation in DNA damage response and cancer chemotherapy. Oncogene 2015; 34: 3349–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owonikoko TK, Zhang G, Deng X, et al. Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Med 2014; 3: 1579–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao B, Li CW, Lim SO, et al. Deglycosylation of PD-L1 by 2-deoxyglucose reverses PARP inhibitor-induced immunosuppression in triple-negative breast cancer. Am J Cancer Res 2018; 8: 1837–1846. [PMC free article] [PubMed] [Google Scholar]
- 41.Niraparib in combination with pembrolizumab in patients with triple-negative breast cancer or ovarian cancer. https://ClinicalTrials.gov/show/NCT02657889.
- 42.Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination With Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1-3 Inhibitor Cediranib in Women's Cancers: A Dose-Escalation, Phase I Study. J Clin Oncol 2017; 35: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Javelin parp medley: avelumab plus talazoparib in locally advanced or metastatic solid tumors. https://ClinicalTrials.gov/show/NCT03330405. [DOI] [PMC free article] [PubMed]
- 44.Konstantinopoulos PA, Waggoner S, Vidal GAet al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination With Pembrolizumab in Patients With Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol 2019; 5(8): 1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed]