Abstract
Objective:
Duloxetine is an FDA-approved treatment for both osteoarthritis (OA) pain and depression, but uptake of duloxetine in knee OA management varies. We examined the cost-effectiveness of adding duloxetine to knee OA care with or without depression screening.
Methods:
We used the Osteoarthritis Policy Model, a validated computer microsimulation of knee OA, to examine the value of duloxetine for knee OA patients with moderate pain by comparing three strategies: 1) usual care (UC); 2) duloxetine for those who screen positive for depression on the Patient Health Questionnaire 9 (PHQ-9) + UC; and 3) universal duloxetine + UC. Outcomes included quality-adjusted life years (QALYs), lifetime direct medical costs, and incremental cost-effectiveness ratios (ICERs), discounted at 3% annually. Model inputs, drawn from published literature and national databases, included: annual cost of duloxetine, $721-$937; average pain reduction for duloxetine, 17.5 points on the WOMAC pain scale (0–100); likelihood of depression remission with duloxetine, 27.4%. We considered two willingness-to-pay (WTP) thresholds of $50,000/QALY and $100,000/QALY. We varied parameters related to the PHQ-9 and duloxetine’s cost, efficacy, and toxicities to address uncertainty in model inputs.
Results:
The screening strategy led to an additional 17 QALYs per 1,000 subjects and increased costs by $289/subject (ICER=$17,000/QALY). Universal duloxetine led to an additional 31 QALYs per 1,000 subjects and $1,205/subject (ICER=$39,300/QALY). Under the majority of sensitivity analyses, universal duloxetine was cost-effective at the $100,000/QALY threshold.
Conclusion:
Adding duloxetine to usual care for knee OA patients with moderate pain, regardless of depressive symptoms, is cost-effective at frequently-used WTP thresholds.
Knee osteoarthritis (OA) is a prevalent, disabling and costly condition that affects over 14 million Americans and over 263 million individuals worldwide.(1, 2) Co-morbid anxiety and depression are common among individuals with OA, with up to 41% experiencing either one or both of these conditions,(3) and place additional burdens on the healthcare system.(4, 5) Depression is associated with worse clinical outcomes, including more severe OA pain,(6–8) lower quality of life,(4, 6, 9) worse total knee replacement (TKR) outcomes,(10) and increased opioid utilization.(11) Despite evidence of its detrimental impact on OA outcomes, depression is not well-managed in this population; only 33% of adults with OA and depression receive adequate depression treatment.(12)
Duloxetine, a serotonin-norepinephrine reuptake inhibitor that is FDA-approved for major depressive disorder and knee OA, is effective in treating depression and OA pain independent of depressive symptoms.(13) Given the negative impact depression has on OA management, incorporating a treatment that could affect both conditions simultaneously could improve outcomes. Duloxetine may also alleviate some of the economic burden posed by medical costs associated with depression by leading to remission. However, while depression screening is recommended for all adults and may be especially important in this population,(14) some studies raise questions about the feasibility and efficacy of incorporating screening into routine care.(15) Additionally, rheumatologists and orthopedists may be reluctant to screen for depression due to time constraints or feeling that other providers would be better suited to depression management.(16, 17)
In this analysis, we aimed to examine whether screening OA patients for depression and treating only those who screen positive with duloxetine offers better value than offering duloxetine to knee OA patients regardless of depression assessment.
Materials and Methods
Analytic Overview
The Osteoarthritis Policy (OAPol) Model is a validated microsimulation model of the progressive natural history and management of knee OA.(18–20) We used the OAPol Model to examine whether adding duloxetine to usual care (UC) in knee OA patients with moderate pain who no longer receive adequate pain relief from NSAIDs could be cost-effective and whether depression screening offers a way to optimize benefits and reduce adverse effects from duloxetine.
We simulated a cohort of knee OA patients with demographic and clinical characteristics similar to subjects in the Osteoarthritis Initiative (OAI). The primary outcomes were quality-adjusted life expectancy (QALE), lifetime medical costs, and incremental cost-effectiveness ratios (ICERs). We calculated ICERs as the difference in lifetime medical costs divided by the difference in QALE between two strategies. All costs were reported in 2018 USD, and analyses were conducted from a healthcare sector perspective, which includes costs paid by all payers, including public, private, and individuals.(21) Costs and quality-adjusted life years (QALYs) were discounted 3% annually.(21) We considered two well-established willingness-to-pay (WTP) thresholds of $50,000 and $100,000/QALY.(22) Treatment strategies were considered cost-effective if they improved QALE and produced an ICER below the WTP threshold. Any strategy that increased cost and decreased QALE was called “dominated.”
To address uncertainty in our inputs, we performed sensitivity analyses varying parameters of the Patient Health Questionnaire 9 (PHQ-9), the prevalence of depressive symptoms, and the cost, efficacy, and adverse effects of duloxetine. Model inputs and sensitivity analyses are described in Table 1.
Table 1.
Cohort and treatment characteristics and model input parameters varied in sensitivity analyses (costs in 2018 USD).
|
Cohort Characteristics
| ||||||||||||||||||||||
| Parameter | Cohort with depressive symptoms - Mean (SD) | Cohort without depressive symptoms – Mean (SD) | Data Source | |||||||||||||||||||
|
| ||||||||||||||||||||||
| Mean age (years) | 60 (9) | 60 (9) | Average age of those with depressive symptoms in OAI(23) | |||||||||||||||||||
| Percent Female | 74.2% | 60.9% | OAI(23) | |||||||||||||||||||
| Percent White Non-Hispanic | 62.1% | 80.1% | ||||||||||||||||||||
| WOMAC Pain | 40.1 (22.8) | 24.7 (19.4) | ||||||||||||||||||||
| Body Mass Index | 31.4 (4.8) | 29.6 (4.1) | ||||||||||||||||||||
| Prevalence of depressive symptoms | 23% | MCBS(27) | ||||||||||||||||||||
|
| ||||||||||||||||||||||
|
Quality of life (QoL) weights by age, obesity, and WOMAC Pain for cohort without depressive symptoms, with no comorbidities (scale of 0–1)
| ||||||||||||||||||||||
| WOMAC Pain | Obese (BMI > 30) | Source | ||||||||||||||||||||
|
| ||||||||||||||||||||||
| 45–54 | 55–64 | 65–74 | 75+ | Derivations using a model proposed by Brazier et al., 2004 and data from the Osteoarthritis Initiative(23) | ||||||||||||||||||
| Values for subjects with no comorbid conditions; additional comorbid conditions carry QoL decrement | 1–16 | 0.814 | 0.820 | 0.844 | 0.827 | |||||||||||||||||
| 16–40 | 0.778 | 0.784 | 0.808 | 0.791 | ||||||||||||||||||
| 40–70 | 0.712 | 0.718 | 0.742 | 0.725 | ||||||||||||||||||
| 71–100 | 0.654 | 0.660 | 0.683 | 0.666 | ||||||||||||||||||
| Non-obese | ||||||||||||||||||||||
| 1–16 | 0.825 | 0.831 | 0.855 | 0.838 | ||||||||||||||||||
| 16–40 | 0.789 | 0.795 | 0.819 | 0.802 | ||||||||||||||||||
| 40–70 | 0.723 | 0.729 | 0.753 | 0.736 | ||||||||||||||||||
| 71–100 | 0.664 | 0.670 | 0.694 | 0.677 | ||||||||||||||||||
|
| ||||||||||||||||||||||
| QoL decrement due to depression: 0.0625 | Sullivan et al., 2006(29) | |||||||||||||||||||||
|
| ||||||||||||||||||||||
| Background medical costs by age for non-depressed subjects with 0–1 comorbidity | ||||||||||||||||||||||
| Age Group | Data Source | |||||||||||||||||||||
|
| ||||||||||||||||||||||
| 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80+ | Pope et al., 2004, MCBS, NHANES 2015–2016, Red Book Online(26–28, 38) | ||||||||||||||||
| $5,199 | $5,741 | $5,587 | $6,244 | $7,138 | $10,342 | |||||||||||||||||
|
| ||||||||||||||||||||||
| Additional cost for subjects with depressive symptoms | $1,081 | Derived from MCBS, 2015–2016(27) | ||||||||||||||||||||
|
| ||||||||||||||||||||||
|
Strong opioid utilization among subjects with and without depressive symptoms
| ||||||||||||||||||||||
| Depressive symptoms | 5.95% | Derived from MCBS, 2015–2016(27) | ||||||||||||||||||||
| No depressive symptoms | 3.61% | |||||||||||||||||||||
|
| ||||||||||||||||||||||
| Duloxetine Treatment Characteristics | ||||||||||||||||||||||
| Parameter | Model Input Value | Data Source | ||||||||||||||||||||
| Annual Cost (First Year) | $721 | Red Book Online, Medicare Physician Fee Schedule, Annual Cost of Dispensing Study(38–40) | ||||||||||||||||||||
| Annual Cost (Subsequent Years) | $937 | |||||||||||||||||||||
| Treatment-Related Toxicity Probability | 46% | Nelson et al., 2006(37) | ||||||||||||||||||||
| Likelihood of Discontinuation in First Year | 23% | Chappell et al., 2011(13) | ||||||||||||||||||||
|
| ||||||||||||||||||||||
| Duloxetine Pain Efficacy | ||||||||||||||||||||||
| Pain Group (WOMAC Pain Scale) | Average First Year Pain Decrement (SD)(13) | Probability of Pain Failure in Subsequent Years(34) | ||||||||||||||||||||
|
| ||||||||||||||||||||||
| 1–15 | 8 (4) | 24% | ||||||||||||||||||||
| 16–40 | 14 (5) | 24% | ||||||||||||||||||||
| 41–70 | 18 (6) | 50% | ||||||||||||||||||||
| 70–100 | 21 (6) | 75% | ||||||||||||||||||||
| Duloxetine Depression Efficacy | ||||||||||||||||||||||
| Parameter | Model Input Value | Data source | ||||||||||||||||||||
|
| ||||||||||||||||||||||
| Likelihood of depressive symptom remission | 27% | Raskin et al., 2007(35) | ||||||||||||||||||||
| Likelihood of depression relapse in subsequent years | 44% | IMPACT Trial(36) | ||||||||||||||||||||
|
| ||||||||||||||||||||||
| Sensitivity Analyses | ||||||||||||||||||||||
| Parameter Varied | Values or Range of Change | |||||||||||||||||||||
|
| ||||||||||||||||||||||
| Duloxetine depression efficacy | 21%−33%; 0% | |||||||||||||||||||||
| Duloxetine pain efficacy | 6–17 points on the WOMAC scale, stratified by pain group, and 9–23 points (confidence intervals of data reported by Chappell et al., 2011(13)) | |||||||||||||||||||||
| Cost of duloxetine | $444 - $1555 | |||||||||||||||||||||
| Treatment-related toxicity probability | 56.4% | |||||||||||||||||||||
| Increased complexity of physician visit | $152 for first visit (HCPS code: 99214),(40) compared to $120 (base case, HCPS code: 99213) | |||||||||||||||||||||
| PHQ-9 sensitivity | 40.7% - 81.3%(42) | |||||||||||||||||||||
| PHQ-9 specificity | 42.7% - 85.3%(42) | |||||||||||||||||||||
| Prevalence of depressive symptoms | 0% - 23%(27) | |||||||||||||||||||||
The OAPol Model
The OAPol Model simulates cohorts of knee OA patients (model subjects) using prespecified distributions of demographic and clinical characteristics, including age, sex, race/ethnicity, body mass index (BMI), comorbidities (cardiovascular disease, cancer, diabetes, and inflammatory arthritis), and structural and symptomatic severity of knee OA. Using Monte Carlo simulation, subjects transition through different health states that include changes to knee OA structural and symptomatic severity, BMI classes, and comorbidities. Each annual model cycle, each subject accrues QALYs, and the sum of these QALYs (between the subject’s initialization and death) averaged across all subjects is the QALE. Symptomatic severity is defined by pain ratings on the 100-point Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scale, and ratings are split into five pain groups (<1, 1–15, 16–40, 41–70, and 71–100). Changes in these health states are associated with quality of life (QoL) changes, and subjects incur costs related to OA management and background (non-OA) medical costs each year. Further details about the OAPol Model have been published.(18–20)
For each treatment regimen, a pain decrement is determined from distributions reported in published literature. Once a subject fails to get pain relief from that regimen, they are evaluated for the subsequent regimen (Figure 1). A subject may also move to the next regimen due to voluntary discontinuation or experiencing a toxicity that would cause their physician to discontinue that treatment. The cost of each regimen includes medication (if relevant), procedures, physician visits, and medical care for toxicities.
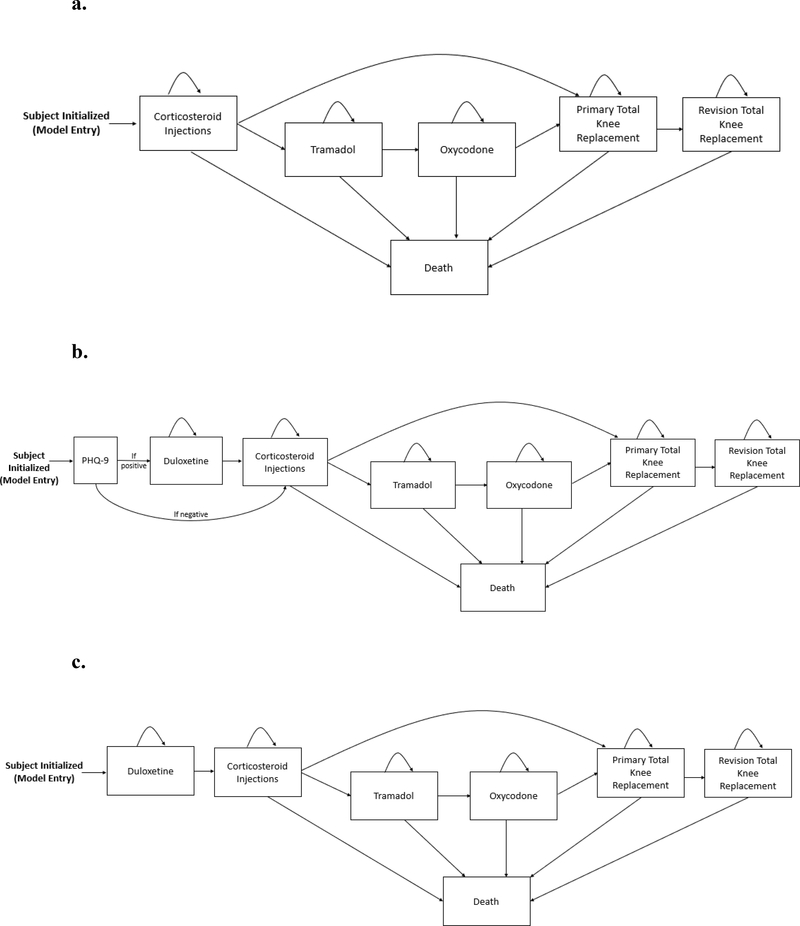
Figure 1.
Panel A. The usual care (UC) treatment sequence for knee OA in the OAPol Model. Subjects are initialized based on specific cohort characteristics and progress through the regimens outlined, including corticosteroids, tramadol, oxycodone (for some subjects, others skip opioid regimens), total knee replacement, and revision total knee replacement. Subjects remain on each treatment until it is no longer effective. Death can occur at any point in the sequence.
Panel B. The depression screening treatment sequence for knee OA in the OAPol Model. Subjects are initialized based on specific cohort characteristics and are screened for depression using the Patient Health Questionnaire 9 (PHQ-9) at initialization. If they screen positive, they receive duloxetine before UC, consisting of corticosteroids, tramadol, oxycodone (for some subjects, others skip opioid regimens), total knee replacement, and revision total knee replacement. If they screen negative, they proceed directly to UC. Subjects remain on each treatment until it is no longer effective. Death can occur at any point in the sequence.
Panel C. The universal duloxetine treatment sequence for knee OA in the OAPol model. Subjects are initialized based on specific cohort characteristics and receive duloxetine before progressing through the rest of the UC treatments, including corticosteroids, tramadol, oxycodone (for some subjects, others skip opioid regimens), total knee replacement, and revision total knee replacement. Subjects remain on each treatment until it is no longer effective. Death can occur at any point in the sequence.
Cohort Characteristics
The cohort characteristics were based on those with knee OA enrolled in the OAI.(23) Using OAI subjects’ responses to the Center for Epidemiological Studies Depression Scale, we modeled the demographic characteristics of two cohorts: with and without depressive symptoms. We chose to simulate a cohort not currently receiving mental health care, as the population represented in this analysis are those for whom duloxetine would not replace ongoing depression treatments. We considered knee OA patients that had failed to achieve adequate pain relief from NSAIDs, physical therapy, and lifestyle modifications.(24, 25)
Both cohorts (with and without depressive symptoms) had a mean starting age of 60 (SD: 9) and a mean BMI of 31 kg/m2 and 30 kg/m2, respectively. The average starting pain on the WOMAC scale was 40 (SD: 23) for those with depressive symptoms and 25 (SD: 19) for those without.(23) These OAI pain data are consistent with studies demonstrating that individuals with depression experience greater pain.(6, 8) The prevalence and incidence of the comorbidities were derived from the 2014–2016 National Health and Nutrition Examination Survey (NHANES) and were stratified by age, race, and sex.(26) Background medical costs were derived from NHANES(26) and the Medicare Current Beneficiary Survey (MCBS),(27) and we used a risk-adjustment model developed by Pope et al. to stratify costs by age and number of comorbidities.(28) QoL values were derived from OAI and were stratified by age and number of comorbidities.(23)
Depression-Related Inputs
We used data from two depression screening questions in MCBS to estimate the prevalence of individuals with depressive symptoms not receiving depression treatment. 23% of the MCBS cohort responded positively to a question about feeling sad, blue or depressed or having two or more weeks when they lost interest or pleasure in valued activities over the past year and were not receiving depression treatment;(27) note that we use the term “depressive symptoms” to refer to these subjects, as the screening questionnaire does not provide enough information to determine whether they meet Diagnostic and Statistical Manual 5 criteria for major depressive disorder.
We applied a QoL decrement of 0.0625 to those with depressive symptoms.(29) Depressed individuals utilize the healthcare system at higher rates,(5) and those who responded positively to MCBS depression screening questions and were not receiving treatment incurred an additional $1,081 in medical costs compared to those who responded negatively (adjusted for number of comorbidities), which we added to the background medical costs for subjects with depressive symptoms.(27) Mortality rates were estimated using Centers for Disease Control and Prevention 2014 US Life Tables.(30) We estimated a 58% increased risk of all-cause mortality in those with depressive symptoms.(31)
Treatment Strategies
We considered three treatment strategies (Figure 1):
Usual care (UC)–subjects are treated with a sequence of corticosteroid injections, tramadol, oxycodone, TKR, and revision TKR.
Screening–subjects are screened for depressive symptoms using the PHQ-9 and receive duloxetine if positive; if negative, or after failure of duloxetine, subjects receive the UC treatment sequence.
Universal duloxetine–all subjects receive duloxetine; after failure of duloxetine, subjects receive the UC treatment sequence.
We calibrated the proportion of subjects taking strong opioids to reflect data from MCBS, stratified by depressive symptom status (Table 1).(27) The pain reduction for TKR was derived from the AViKA cohort and stratified by depressive symptom status.(32) Further details on the UC regimens have been previously published.(19, 33)
Duloxetine Characteristics
Efficacy
We derived duloxetine’s pain efficacy from a 13-week randomized controlled trial that reported a mean WOMAC decrease of 17.5 points (100-point scale) among those taking duloxetine.(13) We stratified this pain decrement by subjects’ initial pain group (Table 1). Since this trial did not report the durability of pain relief after 12 months of treatment, we assumed that the sustainability was similar to that reported in NSAID trials (24%−75% experiencing pain failure, stratified by pain at regimen initiation).(34)
To derive duloxetine’s efficacy in reducing depressive symptoms (“depression efficacy”), we used a randomized trial of duloxetine for depression treatment in adults aged 65 or older.(35) 27.4% achieved depression remission, measured on the Hamilton Depression Rating Scale. This trial similarly did not report long-term efficacy, so we used durability data from the Improving Mood Promoting Access to Collaborative Care Treatment (IMPACT) trial, a collaborative care intervention for elderly patients with depression in which 73% percent took anti-depressant medication.(36) Of those who achieved depression remission at 12 months, 44% experienced depression relapse at 24 months. Those who achieved depressive symptom remission received a QoL increase of 0.0625.(29)
Toxicity
We derived toxicities from a pooled analysis of four clinical trials.(37) Only adverse effects that occurred at rates significantly different from placebo were included, which were nausea, constipation, insomnia, dry mouth, somnolence, sweating, and fatigue. Toxicities were associated with QoL decreases, and in some cases, additional treatment costs (Appendix 1). The likelihood of experiencing one of these toxicities was 46%. Of those who experienced a toxicity, 37.5% discontinued the duloxetine regimen.(13) Overall, 23% of those taking duloxetine discontinued; 17.4% discontinued due to a toxicity, and 5.6% discontinued for other reasons.(13)
Cost
We derived the annual cost of a 60 mg daily dose of generic duloxetine from Red Book Online in October 2018. We used the average wholesale acquisition cost for 30-unit packages, which was $444/year.(38) In addition to the pharmaceutical cost, we included a monthly dispensing fee (as not all insurance plans cover three-month prescriptions),(39) as well as the cost of three physician visits, derived from the 2018 Medicare Physician Fee Schedule.(40) We assumed that those who discontinued did so in the first year of taking duloxetine and reduced the first-year cost of the regimen accordingly. The annual cost of the regimen was $721 in the first year and $937 in subsequent years (Table 1).
Depression Screening Characteristics
For the depression screening strategy, we used characteristics of the PHQ-9, a validated, self-administered, 9-question form designed to screen for depression in primary care and similar settings.(41) The sensitivity and specificity of the PHQ-9 have been estimated at 81.3% and 85.3%, respectively.(42)
Sensitivity Analyses
We varied input parameters in one-way and scenario-based deterministic sensitivity analyses and a probabilistic sensitivity analysis to examine the robustness of the results.
Deterministic Sensitivity Analyses
We varied parameters related to the cost, toxicities, and efficacy of duloxetine, as well as the parameters of the PHQ-9 and depressive symptom prevalence. We conducted a “tipping point” analysis for the cost of duloxetine, in which we increased the pharmaceutical cost and determined the prices at which the two strategies crossed WTP thresholds. We also conducted a scenario-based sensitivity analysis in which we classified the first physician visit as more complex, and thus more expensive. Additionally, we performed a sensitivity analysis in which subjects who achieved depressive symptom remission continued to incur the increased background medical costs associated with depressive symptoms.
Additional studies of duloxetine have found that sexual dysfunction and falls may occur at higher rates among those taking duloxetine. Nelson et al. (2013) report that treatment-emergent sexual dysfunction occurred in 46.4% of those taking duloxetine compared to 28.8% of those on placebo.(37) Sullivan et al. (2004) modeled treatment for this toxicity as two physician visits and a month’s prescription of sildenafil for 25% of those who experience it;(43) we derived these costs from the Medicare Physician Fee Schedule and Red Book Online.(38, 40) Nelson et al. (2013) report that falls occurred in 17.3% of those on duloxetine compared to 11.6% of those on placebo.(44) We modeled the risk of fracture due to falls. Soh et al. (2020) found that the proportion of those who self-reported a fracture was 23% of the number that self-reported a fall.(45) We applied this probability to the increased fall risk among those taking duloxetine. With these additional toxicities, the overall toxicity probability was 56.4%, and 25.8% of those taking duloxetine discontinued in the first year. Data on costs and QoL decrements for these toxicities are presented in Appendix 1.
To examine combinations of some of the least favorable variations, we conducted scenario-based sensitivity analyses of selected reductions in the pain and depression efficacy of duloxetine. We used the lower 95% confidence intervals from the respective trials and included analyses of duloxetine having no depression efficacy or no pain efficacy. We also examined scenarios in which the PHQ-9 sensitivity and specificity were 50% of base case values and the prevalence of depressive symptoms was 25% of base case.
Probabilistic Sensitivity Analysis
We performed a probabilistic sensitivity analysis (PSA) drawing from distributions of pain efficacy, depression efficacy, toxicity rates, PHQ-9 sensitivity, and depressive symptom prevalence. 500 iterations of probabilistic inputs were drawn and run through the model. A normal distribution was used for the pain decrements, and beta distributions were used for the toxicities, depression efficacy, prevalence of depressive symptoms, and PHQ-9 sensitivity. We constructed a cost-effectiveness acceptability curve to depict the proportion of time each strategy was the preferred strategy, or that which produced the greatest QALE while maintaining the ICER below a certain WTP threshold, under several WTP thresholds.
Results
Base Case
The average duration on duloxetine was 2.4 years for subjects with depressive symptoms and 3.2 years for those without. The screening strategy resulted in a cost increase of $289 per subject over usual care and an increase of 17 QALYs per 1,000 subjects, which produced an ICER of $16,961/QALY (Table 2). Compared to the screening strategy, the universal duloxetine strategy led to a cost increase of $1,205 per subject and an increase of 31 QALYs per 1,000 subjects, resulting in an ICER of $39,288/QALY.
Table 2.
Results of base case and selected scenario sensitivity analyses.
| Treatment Sequence | Quality-Adjusted Life Expectancy (QALE) | Lifetime Medical Costs | Incremental Cost-Effectiveness Ratio |
|---|---|---|---|
| Base Case | |||
| Usual care | 11.0458 | $186,914 | - |
| Depression screening | 11.0628 | $187,203 | $16,961/QALY |
| Universal duloxetine | 11.0935 | $188,408 | $39,288/QALY |
| Sensitivity Analyses | |||
| No remission of increased background medical costs for subjects with depressive symptom remission | |||
| Usual care | 11.0458 | $186,914 | - |
| Depression screening | 11.0632 | $187,325 | $23,528/QALY |
| Universal duloxetine | 11.0940 | $188,558 | $40,076/QALY |
| Increased visit complexity for first physician visit | |||
| Usual care | 11.0458 | $186,914 | - |
| Depression screening | 11.0628 | $187,213 | $17,515/QALY |
| Universal duloxetine | 11.0935 | $188,440 | $40,007/QALY |
| Increased toxicity rate | |||
| Usual care | 11.0458 | $186,914 | - |
| Depression screening | 11.0588 | $187,202 | $22,210/QALY |
| Universal duloxetine | 11.0782 | $188,373 | $60,271/QALY |
| No pain efficacy and decreased depression efficacy of duloxetine | |||
| Usual care | 11.0458 | $186,914 | - |
| Depression screening | 11.0448 | $186,959 | Dominated |
| Universal duloxetine | 11.0382 | $187,155 | Dominated |
| PHQ-9 sensitivity and specificity at 50% of base case; 5.75% depressive symptom prevalence | |||
| Usual care | 11.5714 | $191,060 | - |
| Depression screening | 11.5958 | $192,046 | $40,330/QALY |
| Universal duloxetine | 11.6151 | $192,787 | $38,430/QALY |
Sensitivity Analyses
One-way Sensitivity Analyses
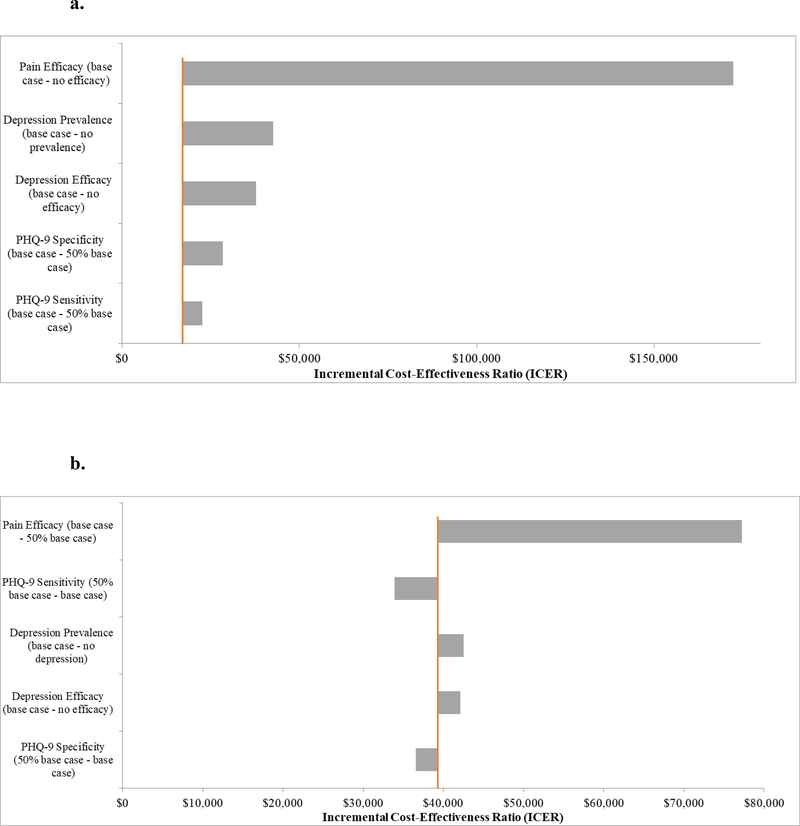
The results of the one-way sensitivity analyses are presented in Figure 2. Pain efficacy had the greatest impact on the results; for the screening strategy, duloxetine providing no pain efficacy resulted in an ICER of $172,455/QALY, and universal duloxetine was dominated by the screening strategy. At 50% pain efficacy, the screening strategy and universal duloxetine had ICERs of $23,417/QALY and $77,224/QALY, respectively. For the other parameters varied (depression efficacy, prevalence of depressive symptoms, and the sensitivity and specificity of the PHQ-9), ICERs for both the screening strategy and universal duloxetine remained below $50,000/QALY for the most conservative values tested, including when the depressive symptom prevalence was zero.
Figure 2.
Panel A. Univariate sensitivity analyses of parameters related to duloxetine, prevalence of depressive symptoms, and the Patient Health Questionnaire 9 (PHQ-9) for the screening strategy. Incremental cost-effectiveness ratios (ICERs) for the screening strategy are presented in relation to variations in the pain and depression efficacy of duloxetine, the prevalence of depressive symptoms, and the sensitivity and specificity of the PHQ-9. All parameters were held at base case values except for the parameter listed on the vertical axis, which was varied according to the values listed. The orange line represents the base case.
Panel B. Univariate sensitivity analyses of parameters related to duloxetine, prevalence of depressive symptoms, and the PHQ-9 for the universal duloxetine strategy. ICERs for universal duloxetine compared to the screening strategy are presented in relation to variations in the pain and depression efficacy of duloxetine, the prevalence of depressive symptoms, and the sensitivity and specificity of the PHQ-9. All parameters were held at base case values except for the parameter listed on the vertical axis, which was varied according to the values listed. Parameters presented are the same as those presented for the screening strategy, with the exception that the lowest value of pain efficacy presented is 50% of base case, as the no pain efficacy scenario was dominated. The orange line represents the base case.
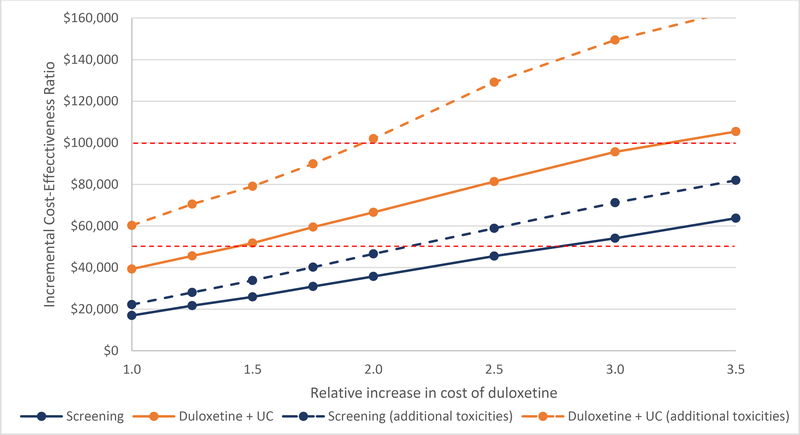
Figure 3 presents the ICERs for the tipping point sensitivity analysis of the annual cost of duloxetine. We varied the cost under base case parameters and with additional toxicities. The screening strategy with base case toxicities resulted in ICERs that crossed the $50,000/QALY WTP threshold between 2.5 and 3 times the base case cost of duloxetine. The universal duloxetine strategy crossed this threshold between 1.25 and 1.5 times the base case cost. Considering the $100,000/QALY WTP threshold, the screening strategy was cost-effective at all values tested, and the universal duloxetine strategy crossed this threshold between 3 and 3.5 times base case cost. Under the increased toxicity scenario, which included fracture and sexual dysfunction, the screening strategy crossed the $50,000/QALY WTP threshold between 2 and 2.5 times the base case cost. The universal duloxetine strategy crossed the $100,000/QALY threshold between 1.75 and 2 times the base case cost of duloxetine.
Figure 3.
Tipping point sensitivity analysis of the cost of duloxetine. The incremental cost-effectiveness ratios (ICERs) for depression screening and universal duloxetine are presented in relation to relative increases in the cost of an annual prescription of 60 mg of generic duloxetine. Solid lines represent base case toxicity values; dotted lines represent the increased toxicity parameters, which includes an increased risk of falls and sexual dysfunction. Red lines represent the $50,000 and $100,000/QALY willingness-to-pay thresholds.
Scenario-based Sensitivity Analyses
Maintaining increased background medical costs during depressive symptom remission resulted in ICERs of $23,528/QALY and $40,076/QALY for the screening and universal duloxetine strategies, respectively. Additional toxicities increased the ICERs to $22,210/QALY and $60,271/QALY for the two strategies. Increasing the cost of the first physician visit, during which duloxetine would be prescribed, had minimal impact (Table 2).
We varied the pain and depression efficacy of duloxetine together using the lower 95% confidence intervals reported by the trials.(13, 35) We also considered extreme scenarios in which duloxetine had no pain or depression efficacy. Under a conservative scenario (decreased pain efficacy and no depression efficacy), the screening strategy ICER was $51,204/QALY, and the universal duloxetine ICER was $53,980/QALY. When pain efficacy was assumed to be zero and depression efficacy was reduced, both strategies were dominated by usual care.
To examine less favorable variations of screening parameters, we reduced the sensitivity and specificity of the PHQ-9 to 50% of base case values and the prevalence of depressive symptoms to 5.75% (25% of base case). Under this unfavorable scenario, the screening strategy ICER was $40,330/QALY, and the universal duloxetine ICER was $38,430/QALY (Table 2).
Probabilistic Sensitivity Analyses
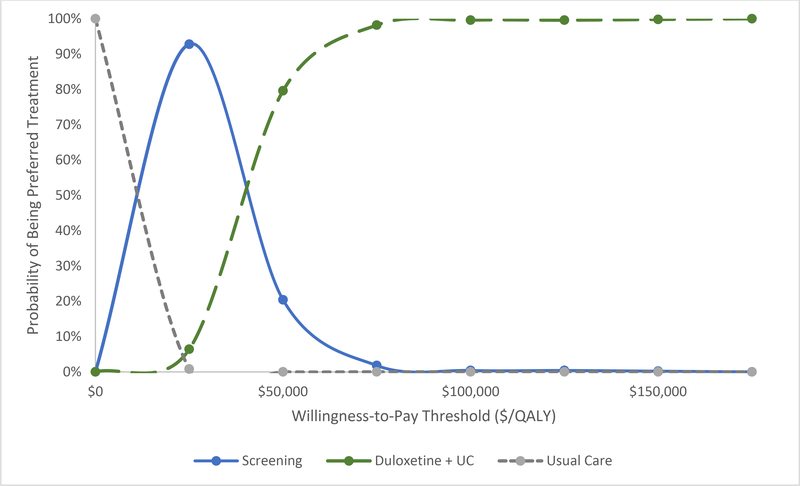
Figure 4 depicts the results of the PSA. The cost-effectiveness acceptability curve depicts the proportion of times each strategy was the preferred option at willingness-to-pay thresholds from $0 to $150,000/QALY. At a WTP threshold of $25,000/QALY, the screening strategy was the preferred strategy in 93% of cases, and universal duloxetine was the preferred treatment in 6% of cases. Universal duloxetine was the preferred strategy in 80% of cases at the $50,000/QALY WTP threshold. At the $100,000/QALY threshold, universal duloxetine was the preferred option 100% of the time.
Figure 4.
Probabilistic sensitivity analyses were conducted varying the toxicity rates, pain and depression efficacy of duloxetine, the sensitivity of the PHQ-9, and prevalence of depressive symptoms. 500 iterations of probabilistic inputs were run. Incremental cost-effectiveness ratios (ICERs) were calculated for each iteration comparing UC (grey dashed line), the depression screening strategy (blue), and universal duloxetine (green). The ICERs for these strategies were compared to a range of willingness-to-pay (WTP) thresholds, and that which produced the greatest quality-adjusted life expectancy while remaining below the WTP threshold was termed the preferred strategy. The probability of being the preferred strategy is plotted against various WTP thresholds.
Discussion
We used a widely-published, validated computer simulation model to evaluate the cost-effectiveness of incorporating duloxetine into knee OA care for patients with moderate pain under two strategies, including adding duloxetine to usual care for only those with depressive symptoms or incorporating duloxetine without depression screening. Base case results suggest that adding duloxetine to usual care without depression screening would maximize QALE while keeping ICERs under $50,000/QALY. Results from the PSA reinforced the value of the universal duloxetine strategy; it was the preferred strategy in 80% of iterations at the $50,000/QALY threshold.
As pharmaceutical costs vary across health systems, pharmacies, and payers, we conducted a tipping point analysis to determine at what cost the strategies crossed WTP thresholds. According to data reported on the prices that Medicare plans pay for duloxetine, 75% of plans pay less than $537/year,(46) which falls between our base case ($444/year) and 1.25x base case, indicating that the universal duloxetine strategy is likely to be cost-effective at prices paid by federal insurers. Additionally, the base case cost was greater than that reported in the Federal Supply Schedule, which is the source the Second Panel on Cost-Effectiveness recommends using for cost-effectiveness analyses.(21) We chose a more conservative base case cost estimate and found universal duloxetine to be cost-effective even above this cost.
Even under a variety of conservative scenarios surrounding duloxetine’s efficacy and toxicities, the ICERs for universal duloxetine did not exceed $77,300/QALY, except for the scenario in which duloxetine had no pain efficacy. We added fracture and sexual dysfunction as additional toxicities, and while they resulted in less favorable ICERs (>$50,000/QALY), they remained below the $100,000/QALY threshold. Similarly, decreasing the pain and depression efficacy of duloxetine led to somewhat less favorable results, though even with decreased pain efficacy and no depression efficacy, the ICER was $53,980/QALY. The only scenario under which universal duloxetine was not cost-effective was when it provided no pain efficacy. The results were robust to a variety of conservative analyses, with the greatest ICER of the sensitivity analyses—except for the scenario in which duloxetine did not affect pain—being $77,224.
The universal duloxetine strategy has an advantage in being simple to implement. While improving depressive symptoms may increase the efficacy of OA treatments, rheumatologists and orthopedists may be hesitant to make OA-related clinical decisions based on a patient’s depressive symptoms.(16, 17) The dual benefits of duloxetine may improve outcomes for knee OA patients; providing it to broader populations rather than targeting those with depressive symptoms can add value to knee OA care. The cost-effectiveness of universal duloxetine was driven more by its pain-relieving properties than its effect on depressive symptoms, as it was cost-effective even in a population without depressive symptoms (Figure 2), underscoring the benefit of not restricting its use to individuals with depressive symptoms.
To the best of our knowledge, this is the first study to examine the cost-effectiveness of duloxetine for knee OA that accounts for duloxetine’s pain and depression efficacy. Wielage et al. (2013) found that duloxetine was a cost-effective treatment for knee OA,(47) though this analysis did not account for any effect on depressive symptoms and derived QoL coefficients using a dose-response relationship between pain and QoL, which may overstate treatment effects. We addressed these two limitations in this analysis. Duloxetine has shown potential for being cost-effective for other chronic conditions, including fibromyalgia and chronic low back pain,(48, 49) though these analyses similarly did not account for depression efficacy. Our results build on Wielage et al.’s findings to indicate that duloxetine may provide good value where other treatments, such as opioids, have not been shown to be cost-effective.(33) Duloxetine may add to a limited range of cost-effective oral analgesics, including NSAIDs,(50) that can be used before surgical intervention.
There are several limitations to this analysis. The trials used to derive efficacy and toxicities associated with duloxetine were relatively short-term, and we had to make assumptions about the likelihood of treatment failure after one year. The Chappell et al. (2011) trial reports the WOMAC decrease in terms of the total WOMAC score, while we model the WOMAC pain subscale. These scales are correlated, and we assumed them to be comparable, though they are not equivalent. We also used studies of diagnosed depression to derive some data for our cohort with depressive symptoms. Additionally, model subjects can only be on one regimen at a time and move sequentially from one to another, when in practice, individuals may combine or cycle through treatments. We modeled depressive symptoms as a binary state and were unable to account for partial improvement. We conducted sensitivity analyses to address these uncertainties, but further clinical studies could clarify these characteristics of duloxetine and provide longer-term data to model cost-effectiveness with more certainty. Lastly, prior studies highlight the influence of subjects’ pain at regimen initiation on a pharmaceutical’s cost-effectiveness,(19) thus these findings may have limited generalizability to populations with different characteristics, such as pain.
Incorporating duloxetine’s dual efficacy for pain and depressive symptoms offers a better understanding of duloxetine’s potential value. Given the economic burden that depression and knee OA place on the healthcare system and the prevalence of inadequately treated depression in this population,(12) identifying treatments that can address these issues together is valuable. This analysis provides evidence that, even without screening for depressive symptoms, introducing duloxetine after NSAIDs fail to provide relief in knee OA patients with moderate pain offers good value as a pain management option.
Supplementary Material
Significance and Innovation.
Depression is poorly managed in knee osteoarthritis (OA) populations and is associated with more severe pain and lower quality of life.
Duloxetine is effective at reducing both OA pain and depressive symptoms, but there have been no studies evaluating the cost-effectiveness of incorporating duloxetine into knee OA care that account for duloxetine’s effect on depression.
We used a validated microsimulation model of knee OA to evaluate the cost-effectiveness of adding duloxetine to usual knee OA care under two strategies: adding duloxetine only for subjects who screen positive for depressive symptoms on the Patient Health Questionnaire 9, or for all subjects, regardless of depressive symptoms.
The depression screening strategy had an incremental cost-effectiveness ratio (ICER) of $16,961/QALY, and the universal duloxetine strategy had an ICER of $39,288/QALY, indicating that incorporating duloxetine into usual knee OA care without depression assessment provides good value.
Acknowledgments
Support: NIH/NIAMS K24AR057827, R01AR074290, P30AR72577, NIMH R25MH094612, K24 AR070892
Financial disclosures: EL has received research support from Samumed, Flexion Therapeutics, and Pfizer and has received consulting fees from Pfizer. JNK has received research support from Samumed and Flexion Therapeutics. DJH is a consultant for Pfizer, Merck Serono, and Lilly. TN is a consultant for Pfizer, Lilly, EMD Serono, and Novartis. NL, JS, ER, SS, and RE have no disclosures.
References
- 1.Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res (Hoboken). 2016;68(12):1743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;398(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axford J, Butt A, Heron C, Hammond J, Morgan J, Alavi A, et al. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clinical rheumatology. 2010;29(11):1277–83. [DOI] [PubMed] [Google Scholar]
- 4.Rosemann T, Gensichen J, Sauer N, Laux G, Szecsenyi J. The impact of concomitant depression on quality of life and health service utilisation in patients with osteoarthritis. Rheumatology international. 2007;27(9):859–63. [DOI] [PubMed] [Google Scholar]
- 5.Simon GE, VonKorff M, Barlow W. Health care costs of primary care patients with recognized depression. Archives of general psychiatry. 1995;52(10):850–6. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Kudesia P, Shi Q, Gandhi R. Anxiety and depression in patients with osteoarthritis: impact and management challenges. Open Access Rheumatol 2016;8:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KW, Han JW, Cho HJ, Chang CB, Park JH, Lee JJ, et al. Association between comorbid depression and osteoarthritis symptom severity in patients with knee osteoarthritis. The Journal of bone and joint surgery American volume. 2011;93(6):556–63. [DOI] [PubMed] [Google Scholar]
- 8.Rosemann T, Laux G, Szecsenyi J, Wensing M, Grol R. Pain and osteoarthritis in primary care: factors associated with pain perception in a sample of 1,021 patients. Pain medicine (Malden, Mass). 2008;9(7):903–10. [DOI] [PubMed] [Google Scholar]
- 9.Sambamoorthi U, Shah D, Zhao X. Healthcare burden of depression in adults with arthritis. Expert review of pharmacoeconomics & outcomes research. 2017;17(1):53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Olivo MA, Landon GC, Siff SJ, Edelstein D, Pak C, Kallen MA, et al. Psychosocial determinants of outcomes in knee replacement. Annals of the rheumatic diseases. 2011;70(10):1775–81. [DOI] [PubMed] [Google Scholar]
- 11.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014;66(10):1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harman JS, Edlund MJ, Fortney JC, Kallas H. The influence of comorbid chronic medical conditions on the adequacy of depression care for older Americans. Journal of the American Geriatrics Society. 2005;53(12):2178–83. [DOI] [PubMed] [Google Scholar]
- 13.Chappell AS, Desaiah D, Liu-Seifert H, Zhang S, Skljarevski V, Belenkov Y, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain practice : the official journal of World Institute of Pain. 2011;11(1):33–41. [DOI] [PubMed] [Google Scholar]
- 14.Guglielmo D, Hootman JM, Boring MA, Murphy LB, Theis KA, Croft JB, et al. Symptoms of Anxiety and Depression Among Adults with Arthritis - United States, 2015–2017. MMWR Morbidity and mortality weekly report. 2018;67(39):1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kigozi J, Jowett S, Nicholl BI, Lewis M, Bartlam B, Green D, et al. Cost-Utility Analysis of Routine Anxiety and Depression Screening in Patients Consulting for Osteoarthritis: Results From a Clinical, Randomized Controlled Trial. Arthritis Care Res (Hoboken). 2018;70(12):1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heiman E, Kravitz RL, Wise BL. Rheumatologists’ Approaches to Diagnosis and Treatment of Depression. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2016;22(6):307–11. [DOI] [PubMed] [Google Scholar]
- 17.Sheehy C, Murphy E, Barry M. Depression in rheumatoid arthritis—underscoring the problem. Rheumatology. 2006;45(11):1325–7. [DOI] [PubMed] [Google Scholar]
- 18.Losina E, Silva GS, Smith KC, Collins JE, Hunter DJ, Shrestha S, et al. Quality-Adjusted Life-Years Lost Due to Physical Inactivity in the United States Osteoarthritis Population. Arthritis Care Res (Hoboken). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Losina E, Usiskin IM, Smith SR, Sullivan JK, Smith KC, Hunter DJ, et al. Cost-effectiveness of generic celecoxib in knee osteoarthritis for average-risk patients: a model-based evaluation. Osteoarthritis and cartilage. 2018;26(5):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Annals of internal medicine. 2011;154(4):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama. 2016;316(10):1093–103. [DOI] [PubMed] [Google Scholar]
- 22.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. The New England journal of medicine. 2014;371(9):796–7. [DOI] [PubMed] [Google Scholar]
- 23.Osteoarthritis Initiative [Internet]. 2013. [Google Scholar]
- 24.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis and cartilage. 2019;27(11):1578–89. [DOI] [PubMed] [Google Scholar]
- 25.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis & Rheumatology. 2020;72(2):220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survery (NHANES), 2014–2016. 2016. [Google Scholar]
- 27.Medicare Current Beneficiary Survey (MCBS) [Internet]. 2009–2013. [Google Scholar]
- 28.Pope GKJ, Ingber M, Freeman S, Sekar R, Newhart C. Evaluation of the CMS-HCC Risk Adjustment Model RTI International, 2011. [Google Scholar]
- 29.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Medical decision making : an international journal of the Society for Medical Decision Making. 2006;26(4):410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Life Tables, 2014 [Internet]. 2014. [Google Scholar]
- 31.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. The American journal of psychiatry. 2014;171(4):453–62. [DOI] [PubMed] [Google Scholar]
- 32.Losina E, Collins JE, Wright J, Daigle ME, Donnell-Fink LA, Strnad D, et al. Postoperative Care Navigation for Total Knee Arthroplasty Patients: A Randomized Controlled Trial. Arthritis Care Res (Hoboken). 2016;68(9):1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SR, Katz JN, Collins JE, Solomon DH, Jordan JM, Suter LG, et al. Cost-Effectiveness of Tramadol and Oxycodone in the Treatment of Knee Osteoarthritis. Arthritis Care Res (Hoboken). 2017;69(2):234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott DL, Berry H, Capell H, Coppock J, Daymond T, Doyle DV, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford, England). 2000;39(10):1095–101. [DOI] [PubMed] [Google Scholar]
- 35.Raskin J, Wiltse CG, Siegal A, Sheikh J, Xu J, Dinkel JJ, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. The American journal of psychiatry. 2007;164(6):900–9. [DOI] [PubMed] [Google Scholar]
- 36.Hunkeler EM, Katon W, Tang L, Williams JW Jr., Kroenke K, Lin EH, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ (Clinical research ed). 2006;332(7536):259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson JC, Lu Pritchett Y, Martynov O, Yu JY, Mallinckrodt CH, Detke MJ. The safety and tolerability of duloxetine compared with paroxetine and placebo: a pooled analysis of 4 clinical trials. Primary care companion to the Journal of clinical psychiatry. 2006;8(4):212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Red Book Online [Internet]. Micromedex. 2018. [cited October 2018]. Available from: micromedexsolutions.com. [Google Scholar]
- 39.Coalition for Community Pharmacy Action: National Community Pharmacists Association. The cost of dispensing study: an independent comparative analysis of U.S. prescription dispensing costs. 2015. [Google Scholar]
- 40.Centers for Medicare and Medicaid Services. Medicare Fee Schedules 2018. Baltimore, MD: 2018. [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell AJ, Yadegarfar M, Gill J, Stubbs B. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych open. 2016;2(2):127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan PW, Valuck R, Saseen J, MacFall HM. A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS drugs. 2004;18(13):911–32. [DOI] [PubMed] [Google Scholar]
- 44.Nelson JC, Oakes TM, Liu P, Ahl J, Bangs ME, Raskin J, et al. Assessment of falls in older patients treated with duloxetine: a secondary analysis of a 24-week randomized, placebo-controlled trial. The primary care companion for CNS disorders. 2013;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soh SE, Barker AL, Morello RT, Ackerman IN. Applying the International Classification of Functioning, Disability and Health framework to determine the predictors of falls and fractures in people with osteoarthritis or at high risk of developing osteoarthritis: data from the Osteoarthritis Initiative. BMC musculoskeletal disorders. 2020;21(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.New Medicare Part D “Ski Slope” Shows Seniors’ Wild Drug Pricing Ride: 46brooklyn; 2019. [updated July 2019; cited 2020 April 24]. Available from: https://www.46brooklyn.com/research/2019/7/14/new-viz-shows-part-d-pricing-distortions.
- 47.Wielage RC, Bansal M, Andrews JS, Klein RW, Happich M. Cost-utility analysis of duloxetine in osteoarthritis: a US private payer perspective. Applied health economics and health policy. 2013;11(3):219–36. [DOI] [PubMed] [Google Scholar]
- 48.Beard SM, Roskell N, Le TK, Zhao Y, Coleman A, Ang D, et al. Cost effectiveness of duloxetine in the treatment of fibromyalgia in the United States. Journal of medical economics. 2011;14(4):463–76. [DOI] [PubMed] [Google Scholar]
- 49.Wielage RC, Bansal M, Andrews JS, Wohlreich MM, Klein RW, Happich M. The cost-effectiveness of duloxetine in chronic low back pain: a US private payer perspective. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16(2):334–44. [DOI] [PubMed] [Google Scholar]
- 50.Katz JN, Smith SR, Collins JE, Solomon DH, Jordan JM, Hunter DJ, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis and cartilage. 2016;24(3):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.