Abstract
For treatment and diagnosis of cancer, antibodies have proven their value and now serve as a first line of therapy for certain cancers. A unique class of antibody fragments called nanobodies, derived from camelid heavy chain-only antibodies, are gaining increasing acceptance as diagnostic tools and are considered also as building blocks for chimeric antigen receptors as well as for targeted drug delivery. The small size of nanobodies (~15 kDa), their stability, ease of manufacture and modification for diverse formats, short circulatory half-life, and high tissue penetration, coupled with excellent specificity and affinity, account for their attractiveness. Here we review applications of nanobodies in the sphere of tumor biology.
Introduction
In this review we capture developments in the application of antibody fragments, called nanobodies, to tumor biology, covering both diagnostics and therapeutics. Spontaneous or engineered, immune responses against cancers are seen as a powerful adjunct to other forms of treatment. The ensemble of antigen presenting cells (APCs), CD4+ T cells, CD8+ T cells and B cells regulate adaptive immunity. CD4+ T cells (helper T cells) respond when they recognize antigen presented on class II major histocompatibility complex (MHC-II) molecules on the surface of APCs. Activated helper T cells and their products enhance the adaptive immune response through activation of B cells, NK cells and macrophages. B cells present antigen via MHC-II, which is recognized by helper T cells. Helper T cells then secrete signals to differentiate B cells into immunoglobulin (Ig)-secreting plasma cells. Secreted Ig serves various purposes, from neutralization of infectious agents to enhancement of phagocytosis or complement-assisted destruction of pathogens. These effector functions are attributable mostly to crosslinking of fragment crystallizable (Fc) receptors.
In most mammals, Igs are composed of a heavy chain and a light chain, each containing a variable and a constant region. A unique type of Igs, devoid of light chains, was discovered in sharks [1] and in camelid species in 1989 [2]. Engineering of the heavy chains of the camelid heavy-chain only antibodies (hcAbs) yields single-domain antibody (sdAb) fragments, also known as nanobodies (Nb) or VHHs (figure 1A). In select cases, it has been possible to generate sdAbs from the heavy chain variable segments of human and mouse (conventional) Igs [3–7]. While such human or mouse VH segments can be expressed in the absence of a light chain and retain proper solubility and antigen binding properties [8,9], this is not always the case. Therein lies the importance of the discovery and development of the camelid hcAbs.
Figure 1. Nanobodies and their targets in relation to the tumor (microenvironment).
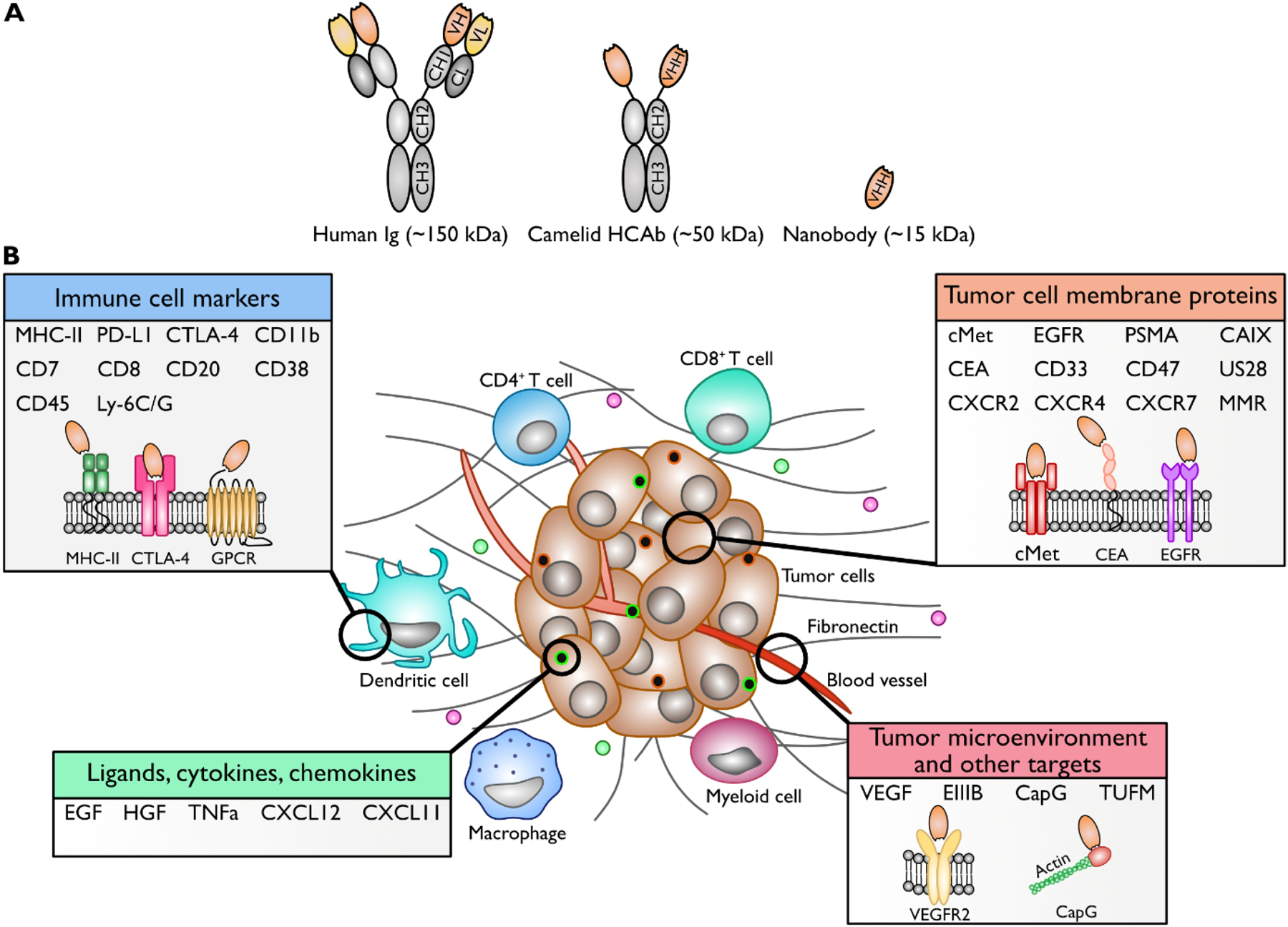
A. Schematic representation of a conventional human Ig, camelid HCab, and a nanobody. B. Schematic overview of the tumor-associated targets for which nanobodies have currently been established. Important targets are immune cell markers, tumor cell (membrane) proteins, receptor ligands, and proteins associated with the tumor microenvironment.
Of late, sdAbs are having a major impact on how Igs and their derivatives are used in research and in practical applications. Despite being only ~1/10th the size of their full-sized counterparts, nanobodies retain the characteristics of antigen specificity and binding affinity. Other favorable attributes of nanobodies are their solubility [10] and stability [11], as well as ease of production in bacteria, thus enabling large-scale production [12]. Their small size (~15 kDa) endows nanobodies with excellent tissue penetration [13] and rapid clearance from the circulation (t1/2 < 30 min) [14]. Because of their unique characteristics and relative ease of production, nanobodies are increasingly used in a variety of applications, such as delivery of drugs or radioisotopes, as well as imaging of tumors and other tissue types. The half-life of nanobodies can be extended at will, for instance by chemical modification with polyethylene glycol (PEG) [15], through fusion of the nanobody to serum albumin nanoparticles [16] or to a serum albumin-binding nanobody [14]. The field of nanobodies continues to advance rapidly. Several excellent reviews on the generation, properties and application of nanobodies across broad areas of biomedical interest have appeared [12,17–28]. The purpose of this review is to focus on recent applications of nanobodies in tumor immunology, primarily in the context of diagnostics, imaging, and therapeutics. We provide an overview of available nanobodies and the (tumor) targets they recognize, as well as their applications. While in many cases nanobodies are used in lieu of conventional antibodies, possibly to avoid intellectual property conflicts, it is helpful to think of nanobodies as immunological tools with unique properties.
Tumor-targeting nanobodies
Nanobodies have similar antigen-binding properties as conventional antibodies. However, because nanobodies employ a single Ig variable domain for antigen recognition, they can access epitopes that are beyond the reach of conventional antibodies or antibody derivatives such as single chain Fv fragments (scFvs). For example, nanobodies can penetrate into a cleft on a protein’s surface or at a domain-domain interface. Currently available nanobodies for tumor-relevant targets are listed in Table 1. Figure 1B shows an overview of nanobody targets in relation to the tumor (microenvironment). In some cases, the nanobodies cross-react with homologous targets from other species. This may facilitate the transition from pre-clinical to clinical applications. Examples include cross-reactivity with human and murine antigen for the anti-EGFR nanobody 8B6 [29], the anti-HER2 nanobody 2Rs15d [30] and the nanobody directed against the EIIIB splice variant of fibronectin [31].
Table 1.
Currently available nanobodies for tumor-relevant targets.
| Target | Disease examples | Origin | Model system tested | Nanobody name | References |
|---|---|---|---|---|---|
| ARTC2 | Murine (ART2.2 in Llama matahari) | CD38 KO mice | S+16a | [98] | |
| CAIX | Breast Cancer (ductal carcinoma) | rCAIX in Camelus dromedarius | PC3 and HeLa cell lines | K24 | [93] |
| Human (HeLa cells in Llama glama) | DCIS and CAIX xenograft-bearing SCID/beige mice | B9 | [94] | ||
| CapG | Breast Cancer TNBC, melanoma, PDAC | Human (Recombinant CapG in Llama glama) | MDA-MB-231 cells, MDA-MB-231 cells in nude mice | CAPNb2 | [92] |
| CD11b | Innate immune cell marker | Murine (BMDC in Llama glama) | BMDC and macrophage cell lines | V36, 76, 51, 81, B10 and 42 | [84] |
| HPV E7 xenograft bearing mice | VHHCD11b (also known as VHHDC13) | [19] | |||
| CD20 | B16 melanoma Melanoma, lung cancer, breast cancer | Human (hCD20-encoding plasmid and hCD20pos cells in Llama glama) | hCD20pos B16 xenograft-bearing mice | 9077, 9079 | [86] |
| CD33 | AML | rCD33 in Llama glama | THP-1 tumor xenograft-bearing mice | Nb_7, Nb_21, Nb_22 | [95] |
| CD38 | Multiple myeloma | Human (rCD38 ectodomain, C-terminal domain, or cDNA expression vector for full-length CD38 in Llama glama) | LP-1, OPM2 and RPMI8226 myeloma cell lines, Primary malignant plasma cells | MU375, MU1053, MU551 | [182] |
| Human CD38-expressing DC27.10 cells in nude mice | WF211, MU1067, JK36, JK2, MU523, WF14 and MU738 | [87] | |||
| CD45 | Mouse (Mouse BDMC cells in Llama glama) | In vitro assays | G7 and 32b | [84] | |
| CD47 | AML, NHL, gastric, ovarian, colon and hepatocellular cancer | Mouse (Ig-like V-type domain (ECD) of mouse CD47 in alpaca) | Tubo-EGFR mouse breast cancer cell line, BALB/c BMDMs, B16F10 cells | A4 | [97] |
| BMDMs and B16F10 xenograft-bearing C57BL/6 mice | A4 fusion to IgG2a Fc (A4Fc) | [135] | |||
| Human (hCD47(ECD)-Fc in Camelus bactrianus) | Raji cell lymphoma NOG mice, cynomolgus monkeys | HuNb1-IgG4 | [96] | ||
| CD7 | Leukemia | Human (CD7+ Jurkat cells in Llama glama) | Leukemia cell lines, CEM xenograft-bearing nude mice | VHH6 | [74] |
| T-ALL PDX model for humanized VHH6 | Humanized VHH6 | [75] | |||
| CD8 | B16 melanoma, pancreatic cancer | Human and mouse (recombinant mouse CD8αβ heterodimer in alpacas) | C57BL/6 mice with B16 and B16 GVAX, MMTV-PyMT transgenic mouse model, human biopsy tumor sections | VHH-X118 | [83] |
| CEA | Epithelial cancers (lung, thyroid, pancreas, uterus, breast, ovary, colorectal) | Human and murine (CEA in Camelus dromedarius) | LS174T cells and LS174T xenograft-bearing mice | cAb-CEA5 | [64] |
| Human (CEA in Vicugna pacos) | LS174T cells and MC38(CEA) mouse colon cancer cells | JJB-B2 | [66] | ||
| H460 xenograft-bearing nude mice | 99mTc-nanobody | [65] | |||
| c-Met | Brain, liver, pancreatic and gastric cancer, multiple myeloma | Human (c-MET-Fc in Llama glama) | hMSCs | Anti-c-Met nanobody, bispecific | [52] Nb patent by Beste et al., WO 2012/042026 A1 |
| Human (A431 cells in Llama glama) | A549 cells, MKN-45 cells | G2 | [51] | ||
| CTLA-4 | B16 melanoma | Human (CTLA-4 protein in Camelus dromedarius) | B16/B6 melanoma cell injected C57BL/6 mice | Nb16 | [76] |
| Murine (CTLA-4 ECD fused to Fc domain in alpaca) | H11 | [77] | |||
| CXCL11 | Pre-B lymphoma | Human (Chemokine mixture in Llama glama) | HEK293T cells | 11B1, 11B7 | [60] |
| CXCL12 | 12A4 | ||||
| CXCR2 | Acute and chronic inflammatory diseases, cancer metastases | Human (CXCR2-expressing cells or pVAX1-hCXCR2 DNA in Llama glama) | CHO-CXCR2 cells | 127D1, 163E3 | [55] |
| CXCR4 | HIV-1, tumor growth and metastasis, WHIM syndrome | Human (CXCR4-expressing HEK293T cells in Llama glama) 90% sequence identity with murine ortholog | Cynomolgus monkeys | 238D2and 238D4 (mono- and biparatopic) | [56] |
| HEK293T and CXCR4-R334X overexpressing K652 cell lines | 10A10 | [57] | |||
| Human (CXCR4-expressing lipoparticles in Llama glama) | SUP-T1 and Jurkat cells | VUN400, VUN401, VUN402 | [58] | ||
| CXCR7 | Head and neck cancer | Human (CXCR7-expressing HEK293 cells or pVAX1-CSCR7DNA in Llama glama) | 22A xenograft-bearing nude mice | NB1, NB2, NB3, NB4, NB5 (mono- and biparatopic) | [59] |
| EGFR | Epithelial cancers | Human (EGFRvIII peptide in Camelus bactrianus) | Ascites fluid of NSCLC | OR1–83, OR2–83 | [183] |
| Human (A431 cells in Llama glama) | Murine xenograft models | Ia1, IIIa3, L2–3.40, 9G8 | [34] | ||
| EGa1 | [184] | ||||
| 8B6 | [29] | ||||
| aEGFR-aEGFR-aAlb | [14] | ||||
| 7C12, 7D12 | [35] | ||||
| CONAN-1 (7D12-9G8-Alb1) | [36] | ||||
| OA-cb6 | [37] | ||||
| OR1–83, OR2–83 | [183] | ||||
| Fibronectin (EIIIB) | Mammary carcinoma | Mixture of ECM proteins, domains and peptides in alpaca | LM2 xenografts in NSG mice | NJB2 | [31] |
| HER2 | Breast cancer | Human (HER2-Fc recombinant fusion protein in Camelus dromedarius) | HER2+ SKOV3 tumor bearing mice | 2Rs15d, 1R136d | [30,154] |
| Human (MCF7 or BT474 cells in Llama glama) | SKBR3 xenograft-bearing mice | 11A4 | [38] | ||
| Human (SKBR3 cells in Llama glama) | BT474M1 xenograft-bearing mice | 5F7GGC | [39] | ||
| HGF | Glioma | Human (HGF in Llama glama) | U87 MG xenograft-bearing mice | 1E2-Alb8, 6E10-Alb8 | [53] |
| Ly-6C/Ly-6G | Myeloid cells in immune diseases and cancer | Mouse (mouse splenocytes in alpaca) | NUP98/HOXB4 cells and C57BL/6j mice | VHH16, VHH21 | [88] |
| MHC-II | Pancreatic cancer | Murine (murine splenocytes in alpaca) | panc02-tumors in C57/BL6 mice | VHH7, VHHDC8, and VHHDC15 | [90] |
| Graft versus Host Disease | Human (Purified HLA antigen in Vicugna pacos) | Xenograft model of GvHD | VHH4 | [89] | |
| MMR | TAMs infiltrating tumors | Human (MMR EC in Vicugna pacos) | TS/A and 3LL-R tumor-bearing mice | Nb cl1 | [72] |
| Human and murine (recomb. Monomeric fusion proteins in Vicugna pacos) | 3.49 | [73] | |||
| PD-L1 | NSCLC, colon, thyroid, uterus, pancreas, and ovary cancer | Human (PD-L1 Fc fusion protein in Camelus bactrianus) | PD-L1+ A375 cells + hPBMCs xenograft-bearing nude mice | KN035 | [118] |
| Murine (RAW264.7 cells in Camelus dromedarius) | TC-1 (WT and PD-L1 KO) in WT or PD-L1 KO mice | C3, E2 | [80] | ||
| Human (PD-L1-Fc protein in alpaca) | PD-L1+ MCF7 and 624-MEL xenograft-bearing nude mice | K2 | [79] | ||
| Human clinical trial | Human NSCLC patients | NM-01 | [81] | ||
| PSMA | Prostate cancer | Human (Purified PSMA antigen in Camelus dromedarius) | In vitro binding predictions | C9, C24, N14, N50 | [71] |
| Human (rPSMA in Camelus bactrianus) | LNcaP and PC3 cells | C3 | [69] | ||
| Human (LNCaP cells, PSMA peptide, rPSMA EC in Camelus dromedarius) | PC-3 and LNCaP xenograft-bearing nude mice | PSMA30 | [68] | ||
| Human (4 different PCa cell lines in Llama glama) | PC-310 and PC-3 xenograft-bearing NMRI mice | JVZ-007 | [67] | ||
| LNCaP, C4-2 or MKN45 xenograft bearing BALB/c-nu nude mice | [70] | ||||
| TNFα | Sarcomas, melanomas, carcinomas | DNA sequences encoding the camelidae antihuman TNFa single-domain) | MCF-7, T-47D and MDA-MB-231 cell lines, 4T-1 breast cancer mouse model | anti-TNF-VHH | [99] |
| TUFM | Glioblastoma | Human (GBM stemlike cells in Alpaca) | Several GBM cell lines and tissues | Nb206 | [91] |
| VEGF(R-2) | Angiogenesis in solid tumors | Human (293KDR cells in Camelus dromedarius) | HUVEC cells | 3VGR19 | [42] |
| Human (VEGF121 in Camelus dromedarius) | Nb22, Nb23, Nb35, Nb42; Humanized Nb42 | [44,45] | |||
| Human sdAb from HuSdl™ | NTV1 | [43] through HuSdl™ | |||
| Chorioallantoic membrane | VA12 | [46] | |||
| Viral GPCR US28 | Glioblastoma | pVAX1-US28 DNA boosted with HEK293T-US28 expressing cells in Llama glama | U251 cells, intracranial GBM mouse model | (bivalent) US28 nanobody | [62] |
| pcDEF3 vector encoding for VHL/E US28 in Llama glama | U251 cells | VUN100 | [63] | ||
| In silico | Nb7 | [61] |
EGFR family
Members of the epidermal growth factor receptor (EGFR) family are often over-expressed on the surface of tumor cells of epithelial origin and play a role in their proliferation, survival, and in angiogenesis [32]. Antibodies that target the EGF receptor have been proven successful in cancer treatment. An example is cetuximab, a full-size chimeric mouse/human monoclonal antibody specific for the EGFR [33]. Therefore, EGFR family members have been among the first tumor markers targeted by nanobodies. EGFR1-targeting nanobodies were identified by phage display, using competitive elution with the ligand EGF to identify specific binders [34]. Using the same EGFR phage nanobody repertoire and selecting for the EGFR extracellular domain, the nanobodies 7C12 and 7D12 [35] and 9G8 [34] were identified. The former competes with cetuximab, the latter does not. Multivalent nanobody molecules can be built by fusion of individual nanobody gene segments or through chemical conjugation methods. EGFR-specific nanobodies were formatted into bivalent molecules in different combinations, all of which inhibited tumor cell proliferation in an in vitro epidermoid cancer model. Specifically, the combination of 7D12–9G8 anti-EGFR nanobodies performed best in inhibiting EGFR signaling and reduced the growth of human epidermoid carcinoma A431 cells. When linked to Alb1, a serum albumin-binding nanobody, the construct was called CONAN-1, which strongly inhibited EGF-induced signaling, leading to tumor regression in A431 xenograft-bearing mice [36].
Using similar methods, the anti-EGFR nanobodies 8B6 and OA-cb6 were obtained [29,37]. Nanobodies that recognize HER2, another member of the EGFR family, specifically target HER2+ SKOV3 ovarian cancer cell-derived tumors in vivo [30]. HER2-targeting nanobodies 11A4 [38] and 5F7GGC [39] have been used for a variety of (clinical) applications, described elsehwere in this review.
VEGFR2 and VEGF
Vascular epithelial growth factor receptor 2 (VEGFR2) is part of the human VEGFR family of receptors and is present on vascular endothelial cells. Its ligand, VEGF, is secreted by cell types such as macrophages and tumor cells, thereby inducing downstream signaling pathways involved in cell proliferation, angiogenesis and metastasis [40,41]. This makes VEGF and VEGFR2 appealing targets for nanobody-based therapies, for example to prevent the formation of new blood vessels on which tumors rely for nutrient and oxygen supply. The anti-VEGFR2 nanobody 3VGR19 was obtained by phage display on recombinant extracellular domains of the VEGFR2 receptor. It inhibits VEGFR2 signaling, thereby inhibiting the formation of capillary-like structures, as shown in an in vitro study on human umbilical vein endothelial cells (HUVEC) [42]. Ma et al. isolated an anti-angiogenic VEGFR2-D3 specific nanobody NTV1 from HuSdl™, a human single domain antibody library of ‘camelized’ human antibodies [43]. In similar fashion, nanobodies specific for VEGF were obtained. These inhibit endothelial cell proliferation in an in vitro angiogenesis assay using HUVECs [44]. A humanized version of one of these nanobodies, Nb42, has also been generated [45]. Lastly, the nanobody VA12, which specifically targets the binding domain of VEGF-A, showed anti-angiogenic potential in a chorioallantoic membrane assay [46].
c-Met and HGF
Hepatocyte growth factor (HGF) binds to the c-Met receptor [47], which activates pathways responsible for cancer progression, angiogenesis and metastasis [48]. For several different epithelial and nonepithelial cancers, overexpression of HGF and the c-Met receptor are associated with a poor prognostic outcome [49,50]. Nanobodies against c-Met and HGF have been produced. The anti-cMet nanobody G2 competes with HGF for binding to the c-Met receptor [51]. Schmidt Slørdahl et al. used a bispecific nanobody, with one nanobody to target c-Met and the other nanobody to enable binding to human serum albumin for half-life extension. This bispecific anti-c-Met nanobody inhibited the interaction of c-Met with HGF and led to a reduction in cell migration and adhesion in multiple myeloma cells. This bispecific nanobody was even more efficient at inhibiting tumor growth than a conventional bivalent monoclonal anti-c-Met antibody [52].
The bispecific albumin- and HGF-specific 1E2-Alb8 and 6E10-Alb8 nanobodies showed a dose-dependent inhibition of HGF-induced proliferation of Bx-PC3 human pancreatic cancer cells. Nude mice bearing human glioma U-87 MG xenografts were treated with an anti-HGF nanobody, resulting in significant inhibition in tumor growth compared to the control group. Both 1E2-Alb8 and 6E10-Alb8 nanobodies show potential as a treatment option for multiple myeloma and other HGF-c-Met driven cancer types [53].
Other targets
In addition to the molecules described above, many other tumor-associated antigens have served as targets for nanobody development. Chemokine receptors, which are G-protein coupled receptors (GPCR), are overexpressed in a wide variety of malignancies [54]. Chemokines and their receptors drive migration and activation of a variety of cell types relevant for both innate and adaptive immune responses. If the goal is to interfere with cell migration, these molecules would appear to be ideal targets in view of the superior tissue penetration of nanobodies. Such nanobodies might neutralize the inhibition of chemorepellent signals, which would otherwise prohibit access of therapeutically efficacious immune cells to the tumor microenvironment. Conversely, immunosuppressive cells require chemoattractants to arrive at the site of the tumor. Nanobodies that target GPCRs and its ligands include reagents specific for human CXCR2 [55], CXCR4 [56–58], CXCR7 [59], CXCL11 and CXCL12 [60], and the viral GPCR US28 [61–63].
Furthermore, nanobodies have been identified that target human tumor-associated (trans)membrane proteins such as carcinoembryonic antigen (CEA) [64–66], prostate-specific membrane antigen (PSMA) [67–71], and human and murine macrophage mannose receptor (MMR) [72,73].
Other important targets are immune cell markers such as human CD7 [74,75], human and murine CTLA-4 [76,77], human and murine PDL-1 [78–82], murine CD8 [83] murine CD11b [19,84,85], human CD20 [86], human CD38 [87], mouse CD45 [84], mouse Ly-6C/Ly-6G [88], human and murine MHC-II [89,90]. Other targets include fibronectin [31], TUFM [91], CapG [92], CAIX [93,94], CD33 [95], human and murine CD47 [96,97], murine ARTC2 [98], and TNFα [99] (table 1).
Nanobodies for diagnosis through imaging
Molecular imaging has become an important tool in cancer research, both for understanding the underlying biology of a disease, as well as for diagnosis and therapy [100]. Molecular imaging requires a targeting moiety labeled with a diagnostic radioisotope [101] or a suitable fluorophore. Radiolabeled monoclonal antibodies have been used extensively as targeting moieties, but their effectiveness is limited by the large size of full-sized Igs and their comparatively long circulatory half-life [102]. Notwithstanding their large size, conventional fully human monoclonal antibodies used for therapy have been converted into imaging agents. This strategy has the obvious advantage that agents approved for clinical use can be used with only slight modification for imaging purposes, and with minimal risk of immunogenicity and unexpected adverse outcomes, especially given the modest amounts of imaging agent administered. Only recently have nanobodies been used in first human trials [28]. Aside from the kidneys, uptake of radiolabeled nanobodies in non-targeted organs is usually low, resulting in a high target-to-background ratio shortly after administration. This allows same-day imaging and the use of shorter-lived radioisotopes, in contrast to the low target-to-background ratio found shortly after administration of 89Zr-labeled full-sized monoclonal antibodies used for the same purpose [102,103].
These characteristics explain why nanobodies have been used in molecular imaging techniques such as positron emission tomography (PET) [104], single photon emission computed tomography (SPECT) [29], near-infrared fluorescence imaging (NIR) [105], and ultrasound-based molecular imaging [106] (figure 2A).
Figure 2. Overview of the applications of nanobodies in cancer diagnosis and therapy.
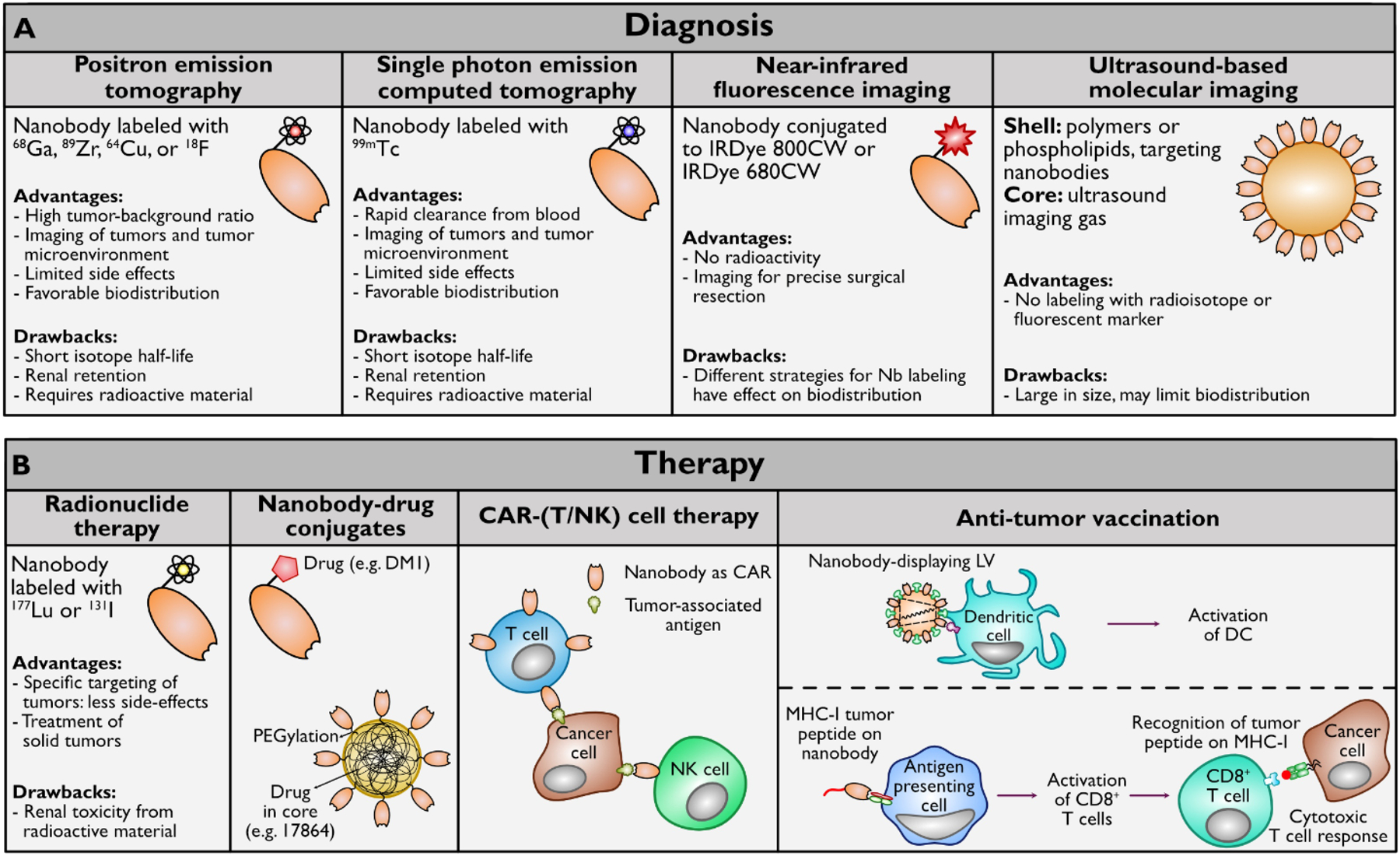
A. Nanobodies have been successful in diagnosis through molecular imaging techniques such as PET, SPECT, NIR, and ultrasound-based molecular imaging. B. Nanobodies can be used in a variety of tumor therapies, such as targeted radionuclide therapy, nanobody-drug conjugates, adoptive cell transfer, and vaccination.
PET imaging
PET imaging uses positron-emitting radiotracers. Positrons collide with electrons in the tissue. This produces energy in the form of photons, which can be detected with a PET scanner [107]. Isotopically labeled Igs and Ig fragments used as PET imaging agents show exquisite specificity for select targets in vivo [108,109]. The EGFR-targeting 7D12 nanobody, radiolabeled with 68/67Ga or 89Zr, was among the first nanobodies to be used for PET imaging. The PET images of A341 tumor-bearing mice show clearly visible tumors with good tumor-background contrast [104]. Some anti-HER2 nanobodies have also been used for imaging purposes, and the lead compound 2Rs15d has been studied in some detail. Coupled to 68Ga-NOTA, the nanobody yielded high-contrast images of tumors in SKOV3 tumor-bearing rats [110]. The use of this nanobody has also successfully been translated to the clinic, with the first in-human phase I study of 68Ga-NOTA-2Rs15d used in PET/CT scans of HER2-overexpressing cancer patients. The nanobody-based imaging agent showed favorable biodistribution and high accumulation in the primary lesions and/or metastases of the patients without side effects, indicating its safety and clinical potential [111]. Two phase II studies with this tracer have since been initiated, evaluating its potential to detect local and distant metastases in breast cancer patients (clinicaltrials.gov, NCT03331601 and NCT03924466). A similar approach with the anti-MMR nanobody 3.49 in 3LL-R tumor-bearing mice gave equally encouraging results, with promise for use in a phase I and II clinical trial (clinicaltrials.gov, NCT04168528) [112].
Labeling of biomolecules with 68Ga requires a specific 68Ge68/Ga generator. The relatively short half-life of 68Ga (T1/2 < 68 min) [113] can result in low resolution PET images. These challenges can perhaps be overcome using 18F for radiolabeling of nanobodies. 18F has a half-life of ~109.8 min [114] and radiolabeling with 18F provides better biodistribution and tumor targeting, as has been shown in vivo in PET/CT images of HER2+ SKOV3-tumor bearing mice when compared to labeling with 68Ga [115]. 18F labeling has also been performed on the anti-MMR 3.49 nanobody and resulted in specific visualization of the tumors of 3LL-R tumor-bearing mice [73].
Imaging of the myeloid compartment within the tumor microenvironment (TME) via PET is considered a desirable goal, as tumors are often infiltrated with myeloid-derived suppressor cells (MDSCs) [15]. Treatment with checkpoint blocking antibodies such as anti-PD-1 and anti-CTLA4 has changed the landscape of tumor therapy [116,117], and can likewise affect the distribution of myeloid cells within the tumor [118–120]. Thus, imaging the myeloid compartment within tumors can aid in understanding responses to cancer immunotherapies [15]. Nanobodies modified for use as PET imaging agents have now been applied to a variety of targets in pre-clinical models, directed against class II MHC (VHH7, VHH4), PD-L1, CTLA-4, fibronectin EIIIB (NJB2), CD8 (X118), CD11b (DC13), CD36 (DC20), and CD45 [15,31,82,89,90,121,122] labeled with 18F, 64Cu, or 89Zr. Several tumor models have thus been examined, including the mouse B16 melanoma, PANC02 pancreatic adenocarcinoma, MC38 colorectal adenocarcinoma, and C3.43 human papillomavirus-induced cancer models. All of these agents visualize tumors by virtue of the fact that myeloid cells and lymphocytes are present in the TME [19].
SPECT with Micro-CT imaging
Single photon emission computed tomography (SPECT) imaging uses gamma-emitting radioisotopes. EGFR-targeting nanobodies 7D12 and 7C12, labeled with 99mTc, have been used in SPECT and micro-CT applications. Both nanobodies showed clear localization to the tumors of A431 xenograft-bearing mice [35]. SPECT imaging with the 99mTc-labeled anti-EGFR nanobody 8B6 also showed good tumor localization in mice bearing DU145 and A431 tumor xenografts [29]. When 99mTc-2Rs15d was evaluated for tumor accumulation by SPECT and Micro-CT, it showed clear accumulation at the tumor site of HER2+ SKOV3 or LS174T xenograft-bearing mice, whereas no tumor localization of 99mTc-2Rs15d was observed in tumors of HER2− xenografted mice [30]. 99mTc-labeled NbCEA5, evaluated by total pinhole SPECT and Micro-CT, showed rapid clearance from the blood and efficient tumor targeting in LS174T xenografted mice [123]. The same held true for the 99mTc-labeled anti-MMR nanobody cl1 evaluated for tumortargeting potential in TS/A and 3LL-R tumor-bearing mice, imaged using pinhole SPECT and Micro-CT [72]. For diagnostic purposes, visualization of PD-L1 expression levels in patients can be valuable. SPECT imaging with 99mTc-labeled anti-PD-L1 nanobodies showed intense and specific uptake in PD-L1-overexpressing tumor models of melanoma and breast cancer in mice [79]. Moreover, these results were translated for human application in a phase I clinical trial on sixteen patients with non-small cell lung cancer (NSCLC), where an 99mTc labeled anti-PD-L1 nanobody showed clear visualization of the primary NSCLC tumors and metastases, while presenting favorable biodistribution and limited side-effects [81].
NIR fluorescence
The use of isotopically labeled imaging agents has as an obvious drawback the risk of radiation exposure for both patient and physician. Shorter lived isotopes with a high positron yield such as 18F in principle allow imaging shortly after administration of the 18F-labeled agent, but this requires that tissue penetration and clearance from the circulation are compatible with visualization of the target of interest. Methods that do not rely on the use of radioisotopes therefore remain attractive alternatives, although these, too, have their limitations. Fluorescence-based methods suffer from absorption of light of the excitation and emission wavelengths by tissue and bodily fluids. Nonetheless, suitably labeled nanobodies have been used in these optical applications. The HER2-targeting nanobody 11A4 conjugated to a near-infrared fluorophore IRDye 800CW, localized specifically to the tumor site of HER2+ SKBR3 xenograft-bearing mice, while maintaining good biodistribution. Near-infrared fluorescence imaging (NIR) has been exploited to enable image-guided surgery for the precise resection of HER2+ tumors. In a clinical setting, this NIR-conjugated anti-HER2 nanobody should allow specific non-invasive classification of HER2-postive tumors and more precise surgical tumor resection [38]. A similar approach was used to label the EGFR-targeting nanobody 7D12. NIR fluorescence identified OSC-19 tongue tumors .Ex vivo fluorescence imaging of histology sections showed localization of the nanobody to cervical lymph node metastases [124]. The anti-carbonic anhydrase IX (CAIX) nanobody B9 has been exploited for the same purpose and yielded acceptable images in an orthotopic xenograft mouse model [94]. Because the tumor microenvironment is often hypoxic and CAIX is a marker enzyme of hypoxia, this approach should allow its non-invasive visualization. Kijanka et al. conjugated the 11A4 and B9 nanobodies to either IRDye 800CW or IRDye 680RD and injected both simultaneously into MCF10DCIS breast cancer xenograft-bearing mice. The results indicate the possibility of imaging and surgical resection of heterogeneous tumors at improved tumor-to-background ratios [38]. Using the 2Rs15d nanobody labeled with IRDye 800CW, NIR fluorescence image-guided surgery aided the precise debulking of ovarian tumors in SKOV3 xenograft-bearing mice [125]. The anti-ARTC2 nanobody S+16a has been conjugated to the fluorescent dye AlexaFluor-680 and was used for in vivo NIR imaging and ex vivo dissection of ARTC2-positive tumors in mice [126]. Combined, these examples show that fluorescence-based methods that exploit nanobodies as the targeting moieties have considerable potential, not only in the characterization of the tumor microenvironment, but also as an adjunct to surgery aimed at physical elimination of a tumor. Nevertheless, a study comparing the biodistribution of random and site-specific labeled 2Rs15d nanobodies shows the effect of different conjugation strategies on nanobodies’ properties, which should be considered when developing nanobody-based fluorescent imaging agents [127].
Ultrasound-based molecular imaging
A wide branch of molecular imaging is ultrasound-based. Microbubbles or nanobubbles can be used as ultrasound contrast agents (Zhang et al. 2019). Nanobubbles can have various types of shells (polymers or phospholipids) and cores (gas, liquid, or solid) [129,130]. They can carry antibodies specific for tumor-associated antigens, aiding in the early diagnosis of different malignancies. The large molecular weight of full-sized antibody-particle complexes results in a limited number of nanobubbles that actually reach the intended target site. Therefore, the use of nanobodies may improve nanobubble performance [106] as tested with nanobubbles filled with C3F8 ultrasound imaging gas and carrying an anti-PSMA nanobody. The modified nanobubble specifically adhered to prostate cancer cells and displayed high specificity in prostate cancer xenograft imaging in vivo [70].
Several issues must be addressed before nanobodies can be fully implemented for imaging in a clinical setting. Importantly, nanobodies show high renal retention due to reabsorption in the proximal tubules, caused by megalin receptors [131]. Kidney retention can lead to renal damage, especially when the nanobody is labeled with a radioisotope or equipped with a cytotoxic drug. Kidney retention also produces a strong signal in several imaging applications, possibly overshadowing the signal of the desired molecular targets when physically close to the kidneys. Several strategies have been pursued to address these issues, such as coadministration of gelofusin or positively charged amino acids, which interact with megalin receptors and thereby reduce kidney retention [131]. Modification of nanobody imaging agents with PEG can also mitigate this problem, as observed with the anti-CD8 nanobody X118, used to image T cell infiltration into mouse B16 and Panc02 tumors in vivo via PET [83]. Lastly, incorporation of a brush border enzyme-cleavable linker, a glycine-lysine dipeptide, between the 18F-containing moiety and the 2Rs15d nanobody reduced renal activity levels as seen in micro-PET/CT images of SKOV-3 xenograft bearing mice [132].
Nanobodies for therapy
Nanobodies as checkpoint blockade therapies
Conventional checkpoint blockade therapies use monoclonal antibodies to bind to immune checkpoints such as PD-1 or CTLA-4 to improve the anti-tumor immune response [116,117,133,134]. The anti-PD-L1 nanobody KN035 fused to Fc (KN035-Fc) induced strong T cell responses and inhibited tumor growth of A375-PD-L1 cells in NOD-SCID mice in vivo (Zhang et al. 2017). The anti-CTLA-4 nanobody H11 alone failed to control B16 tumor growth in mice treated with the GVAX immunotherapy, but when linked to a murine Fc region, H11 resulted in better overall survival than an anti-mouse CTLA-4 monoclonal antibody [77]. CD47 is an antiphagocytic ligand (the “don’t eat me” signal) exploited by tumors. It does so by blunting antibody-mediated phagocytosis through binding to signal regulatory protein alpha (SIRPα) on phagocytes. The anti-CD47 nanobody A4 alone or in combination with a tumor-specific antibody fails to generate antitumor immunity against syngeneic B16 tumors, but CD47 antagonism substantially improved response rates against B16 tumors when used in combination with PD-L1 blockade [97]. Interestingly, administration of the A4 nanobody synergized with PD-L1, but not CTLA4 blockade [135].
Nanobody-drug conjugates
Specific tumor-targeted therapies include the use of antibody-drug conjugates (ADCs). ADCs exploit the targeting efficiency of antibodies combined with the action of the cytotoxic payload conjugated to it [136,137]. This ought to result in specific targeting of the cancer cells, thus alleviating off-target side-effects. The appeal of this approach is reflected by the large number of clinical trials that use ADCs (registered on clinicaltrials.gov), with almost 40 being completed and over 80 in progress. Popular targets for ADCs are HER2, c-MET, CD30, and PSMA.
Despite evidence for the effectiveness of ADCs, there are drawbacks to the use of monoclonal antibodies in cancer therapy. These include a limited capacity of antibodies to penetrate the tumor due to their relatively large size. Smaller antigen-binding fragments such as Fabs, scFVs, minibodies, and diabodies have therefore attracted attention as a platform for ADCs. Nonetheless, the efficiency of these smaller formats is often limited because of decreased stability, lower affinity, or difficulties in production [12]. Nanobodies can overcome most of these challenges, due to their shorter circulatory half-life, increased tissue penetration, stability and ease of production [136]. Figure 2B shows an overview of the described uses for nanobodies in cancer therapy.
Nanobody-drug conjugates under investigation include a nanobody-albumin nanoparticle (NANAP), which has an albumin core modified on its surface with EGFR-targeting nanobodies conjugated to PEG (EGa1-PEG). The NANAP is loaded with the multikinase inhibitor 1786. When internalized and digested in lysosomes, it causes the intracellular release of the kinase inhibitor and inhibition of proliferation of EGFR-positive 14C squamous head and neck cancer cells [16]. Furthermore, conjugation of the drug Mertansine (DM1) to an MHC-II targeting nanobody, VHH7, resulted in a reduction in liver metastases in mice engrafted with the A20 lymphoma [138]. The central role of MDSCs in driving cancer progression has raised interest in their depletion via ADCs for therapeutic benefit. In mice, CD11b is expressed on several myeloid cell types including monocytes, macrophages, and granulocytes, whereas Ly-6C is highly expressed on monocytes with lower levels on granulocytes, while Ly-6G is expressed on granulocytes [139,140]. Thus, the anti-CD11b nanobody DC13 and Ly-6C/Ly-6G-specific nanobodies (VHH16 and VHH21, respectively) were conjugated to Pseudomonas exotoxin A to deplete myeloid cells in vitro and in vivo [88]. All conjugates showed cytotoxicity in vitro. However, granulocytes were more sensitive than monocytes to Ly-6C/Ly6-G-specific immunotoxins in vivo despite similar binding of the nanobody-immunotoxins to each cell type, indicating the need to thoroughly characterize myeloid-specific ADC candidates.
Targeted radionuclide therapy (TRNT)
TRNT is an increasingly prevalent anti-cancer therapy, designed to deliver cytotoxic radiation to cancer cells, with delivery vehicles such as monoclonal antibodies, antibody fragments, or other small molecules equipped with a suitable radioisotope. Targeted delivery should limit exposure of healthy tissue to radiation. TRNT using antibodies has been approved by the FDA for Ibritumomab tiuxetan, a 90Y-labeled CD20-targeting monoclonal antibody for radioimmunotherapy of non-Hodgkin’s lymphoma [141–143], and the similar 131I-tositumomab [144]. Furthermore, promising results in early clinical trials have been obtained for antibodies specific for CD33 [145,146], or preclinical results for a combination of CD20 and CD22 targeting antibodies [147,148]. Nevertheless, the targeting of (large) solid tumors remains a challenge, as shown in trials with antibodies specific for MUC1 [149], CEA [150–152], and CEA [153]. Because the poor penetration of labeled antibodies into solid tumor tissue is to a large extent due to their size, smaller labeled molecules such as peptides and nanobodies, have been explored as alternatives for TRNT, especially for the treatment of solid tumors.
D’Huyvetter et al. were the first to use a nanobody for TRNT, in a study with mice bearing HER2+ SKOV3 xenografts treated with the 177Lu-DTPA-2Rs15d nanobody. The treated mice showed an almost complete arrest in tumor growth and significantly longer disease-free survival compared to the control group, while no evidence of renal inflammation or necrosis was observed [154]. The same nanobody, labeled with 131I, has been used in a phase I clinical trial with breast cancer patients (NCT02683083) [155]. The 5F7GGC nanobody, labeled with the residualizing agent N-succinimidyl 4-guanidinomethyl 3-125/131-I-iodobenzoate (*I-SGMIB), designed to trap radioiodine inside a tumor cell [156], showed promising results in targeting HER2+ cancers with different radioisotopes useful for TRNT [157].
The promising results with Ibritumomab tiuxetan prompted researchers to repeat this strategy with CD20-specific nanobodies, which should limit the toxicity seen with mAbs in non-targeted tissues. The nanobody 9079, radiolabeled with 177Lu, showed better disease-free survival when used for treating mice with B16 melanoma compared to controls. More importantly, minimal renal toxicity was seen when mice were treated with 177Lu-DTPA-sdAb 9079 [86]. The results of these preclinical studies underscore how the unique characteristics of nanobodies could be leveraged perhaps also in a clinical setting. Further optimization to decrease renal retention is necessary to further reduce any possible adverse effects.
Nanobody-based carrier delivery systems
To increase tumor efficacy and decrease toxicity in non-targeted tissues, it is important to target the delivery of a drug or compound to the tumor. Nanoparticles used as carriers for targeted drug delivery include liposomes, polymeric nanoparticles, micelles, and albumin nanoparticles [158]. Despite their differences in structure and mechanism of action, they all depend on a targeting ligand at the surface of the nanocarrier to achieve adequate specificity.
Conjugation of the anti-EGFR nanobody EGa1 to PEGylated liposomes induced internalization and downregulation of EGFR in 14C cells, both in vitro and in vivo [159]. When formulated as a polymeric PEGylated micelle, similar receptor binding and internalization were observed, making micelles promising systems for active drug targeting [160]. To this end, EGa1-decorated micelles were loaded with temoporfin (mTHPC), a photosensitizer compound used in the clinic for photodynamic therapy (PDT) of head and neck squamous cell carcinoma (HNSCC). These micelles show prolonged circulation in vivo compared to free mTHPC, indicating a potential of these micelles to improve the selectivity and efficacy of PDT in EGFR+ tumors [161]. Extracellular vesicles (EV) are also being explored as nanoparticles for therapeutic purposes [162]. To be tumor specific, such EVs must be equipped with a targeting moiety. By anchoring EVs through a glycosylphosphatidylinositol (GPI) anchor to the EGa1 nanobody, the engineered EVs showed localization to and internalization in EGFR-expressing cells, but the conditions will require further improvement for pre-clinical use [163].
Tumor vaccination, lentiviral vector-based cancer therapy, and CAR-T cells
Vaccination against cancer would be a valuable prophylactic or therapeutic strategy and would benefit from specifically delivering tumor antigens to APCs. To this end, lentiviral vectors (LVs) have been used to deliver cancer autoimmune antigens to APCs [164]. Antibodies [165], and more importantly nanobodies, can be used to specifically deliver these LVs to APCs. LVs displaying the dendritic cell-targeting nanobody DC2.1 exclusively transduce only DCs and macrophages in vitro and in vivo [166]. Tropism of human adenovirus serotype 5 (Ad5), which can efficiently transduce human cells, can be altered by capsid modifications that incorporate a nanobody against human CEA (hCEA). These CEA nanobody-expressing Ad5 vectors successfully transduced murine MC38 cells that express hCEA [66]. In a similar manner, nanobodies can be used to improve the targeting and transduction of adeno-associated viral vectors, as shown by the successful transduction of myeloma cells with AAV1P5 displaying an anti-CD38 nanobody [167].
Another vaccination strategy focuses on activating cytotoxic CD8+ T cells through targeted delivery of cancer antigens to APCs by anti-CD11b nanobodies [168]. This has been explored for HPV+ tumors driven by the E6 and E7 genes of the oncogenic HPV type 16 strain. Vaccination based on anti-cd11b nanobodies conjugated to E7-peptide antigens elicited a strong CD8+ T cell response in vivo and showed slower tumor growth and longer overall survival in an in vivo C3.43 cancer model [19]. These results highlight a new role for nanobodies in tumor vaccination strategies. In a similar approach, a strong Th1 immune response against the tumor-specific antigen MUC1 was generated by attaching a site-specifically glycosylated MUC1 peptide to the class II MHC-targeting nanobody VHH7 [122]. The enhanced production of antibodies in response to immunization with the nanobody-peptide adduct implied the induction of an adequate CD4 T helper response in vivo.
Adoptive cell transfer (ACT) employs a patient’s own immune cells to target cancer cells. The T cells are engineered to express a cloned T cell receptor (TCR) or chimeric antigen receptor (CAR) that targets a tumor antigen of interest, the latter allowing for recognition of non-MHC restricted antigens. An ACT strategy using T cells engineered with a CAR comprised of an scFv against mouse VEGFR2 was effective in eliminating several different vascularized syngeneic tumors in mice [169]. Multiple CAR-T cells derived from antibodies or ScFvs are currently under investigation in a clinical setting. Some clinical trials show an immune response directed against the CAR-T cells [170–172], presumably due to immunogenicity to the non-human scFv component in the CAR constructs [173]. This problem might be solved by using humanized nanobody-based CARs. Albert et al. used their UniCAR system, a unique type of CAR T cell that can be redirected via simultaneously infused target modules (TM), allowing the UniCAR to be switched off in the absence of target modules. The UniCAR decorated with anti-EGFR nanobodies effectively target A431 cells in vivo [174], and showed an even better anti-tumor responses when formulated as a bivalent α-EGFR-EGFR nanobody-based UniCAR [175]. A VEGFR2-nanobody specific CAR showed promising results in vitro, with high concentrations of secreted IL-2 and IFN-ƴ by the CAR T-cells, as well as a cytotoxic activity measured by an LDH release assay in response to the VEGFR2 antigen on target cells [176]. Bispecific CAR-T cells that target two antigens simultaneously might be effective to counteract potential antigen-escape in tumor cells. In vitro experiments show the great potential of a bispecific anti-CD20 and anti-HER2 nanobody-based CAR, which targets and kills Jurkat cells expressing either one or both antigens [177]. Targeting the TME rather than the tumor directly can be beneficial for targeting multiple tumor types. Anti-PD-L1-nanobody based CAR-T cells slow tumor growth rates in vivo in B16 and MC38 models. CAR-T cells based on a nanobody against the fibronectin splice variant EIIIB, which is exclusively expressed on tumor stroma and in the neovasculature, as found around tumors, significantly slowed B16 melanoma growth in vivo [178]. The anti-tumor efficacy of the EIIIB-nanobody CAR-T cells was improved in cells that simultaneously secreted nanobodies against PD-L1 or CTLA4, and their systemic cytotoxicity was reduced by secretion of a CD47 nanobody by the CAR T cells [179]. Because the sequence of the EIIIB splice variant is identical for mouse and man, there may be a future for the clinical use of human CAR T cells equipped with this nanobody as a recognition module.
These examples primarily focus on engineering the patient’s autologous T cells. However, selecting non-malignant T cells is difficult for patients with T cell-specific cancer such as T-ALL. To overcome this problem, CAR-NK cells can be used. An anti-CD7 nanobody-based CAR on NK cells showed an inhibitory effect on tumor cells in a PDX mouse model [180]. Bispecific anti-CD38 nanobody-based CAR-NK cells effectively deplete CD38+ cells from patient-derived multiple myeloma bone marrow cells in vitro [181]. Nanobody-based CAR-T cell therapy is now being pursued in clinical trials for CD19/CD20 bispecific targeting in patients with B Cell lymphoma (NCT03881761) and BCMA targeting in multiple myeloma (NCT03664661).
Conclusions
Research has illuminated a valuable role for nanobodies in cancer diagnostics and therapy. Their biophysical properties are fundamentally distinct from those of their conventional two-chain counterparts. The small size, antigen specificity, binding affinity, and stability of nanobodies allows successful targeting of antigens in the tumor, the tumor microenvironment and of the immune cells that are recruited there. Nanobodies are increasingly being used as a diagnostic tool in molecular imaging techniques such as PET, SPECT and NIR fluorescence imaging, as evidenced also by successful early clinical trials. As therapeutic agents, nanobodies can aid delivery of drugs or radioisotopes and can be used for tumor vaccination strategies and CAR-T cell therapy. The full range of possible applications of nanobodies has yet to be explored, but as a complement or an alternative to conventional immunoglobulins: nanobodies are here to stay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, Flajnik MF, A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks, Nature. 374 (1995). [DOI] [PubMed] [Google Scholar]
- [2].Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hammers C, Bajyana Songa E, Bendahman N, Hammers R, Naturally occurring antibodies devoid of light chains, Nature1. 363 (1993) 446–448. [DOI] [PubMed] [Google Scholar]
- [3].Davies J, Riechmann L, Antibody VH domains as small recognition units, Biotechnology. 13 (1995) 475–479. 10.1038/nbt0595-475. [DOI] [PubMed] [Google Scholar]
- [4].Reiter Y, Schuck P, Boyd LF, Plaksin D, An antibody single-domain phage display library of a native heavy chain variable region: Isolation of functional single-domain VH molecules with a unique interface, J. Mol. Biol 290 (1999) 685–698. 10.1006/jmbi.1999.2923. [DOI] [PubMed] [Google Scholar]
- [5].Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, Wang XW, Dimitrov DS, Ho M, Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma, PNAS. 110 (2013) 1–9. 10.1073/pnas.1217868110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li N, Fu H, Hewitt SM, Dimitrov DS, Ho M, Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma, PNAS. (2017) E6623–E6631. 10.1073/pnas.1706055114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen W, Zhu Z, Feng Y, Xiao X, Dimitrov DS, Construction of a large phage–displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain, J. Mol. Biol 382 (2008) 779–789. 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ward ES, Guusow D, Griffiths AD, Jones PT, Winter G, Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli., Nature. 341 (1989) 544–546. [DOI] [PubMed] [Google Scholar]
- [9].Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM, Domain antibodies: proteins for therapy, Trends Biotechnol. 21 (2003) 484–490. 10.1016/j.tibtech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- [10].Tanha J, Xu P, Chen Z, Ni F, Kaplan H, Narang SA, MacKenzie CR, Optimal Design Features of Camelized Human Single-domain Antibody Libraries, J. Biol. Chem 276 (2001) 24774–24780. 10.1074/jbc.M100770200. [DOI] [PubMed] [Google Scholar]
- [11].Van Der Linden RHJ, Frenken LGJ, De Geus B, Harmsen MM, Ruuls RC, Stok W, De Ron L, Wilson S, Davis P, Verrips CT, Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies, Biochim. Biophys. Acta 1431 (1999) 37–46. 10.1016/S0167-4838(99)00030-8. [DOI] [PubMed] [Google Scholar]
- [12].Kijanka M, Dorresteijn B, Oliveira S, Van Bergen En Henegouwen PMP, Nanobody-based cancer therapy of solid tumors, Nanomedicine. 10 (2015) 161–174. 10.2217/nnm.14.178. [DOI] [PubMed] [Google Scholar]
- [13].Fang T, Lu X, Berger D, Gmeiner C, Cho J, Schalek R, Ploegh H, Lichtman J, Nanobody immunostaining for correlated light and electron microscopy with preservation of ultrastructure, Nat. Methods 15 (2018) 1029–1032. 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tijink BM, Laeremans T, Budde M, Stigter-Van Walsum M, Dreier T, De Haard HJ, Leemans CR, Van Dongen GAMS, Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: Taking advantage of modular Nanobody technology, Mol. Cancer Ther 7 (2008) 2288–2297. 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- [15].Rashidian M, LaFleur MW, Verschoor VL, Dongre A, Zhang Y, Nguyen TH, Kolifrath S, Aref AR, Lau CJ, Paweletz CP, Bu X, Freeman GJ, Inmaculada Barrasa M, Weinberg RA, Sharpe AH, Ploegh HL, Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade, PNAS. 116 (2019) 16971–16980. 10.1073/pnas.1905005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Altintas I, Heukers R, Van Der Meel R, Lacombe M, Amidi M, Van Bergen En Henegouwen PMP, Hennink WE, Schiffelers RM, Kok RJ, Nanobody-albumin nanoparticles (NANAPs) for the delivery of a multikinase inhibitor 17864 to EGFR overexpressing tumor cells, J. Control. Release 165 (2013) 110–118. 10.1016/j.jconrel.2012.11.007. [DOI] [PubMed] [Google Scholar]
- [17].Iezzi ME, Policasro L, Werbajh S, Podhajcer O, Canziani GA, Single-Domain Antibodies and the Promise of Modular Targeting in Cancer imaging and Treatment, Front. Immunol 9 (2018) 1–11. 10.3389/fimmu.2018.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ingram JR, Schmidt FI, Ploegh HL, Exploiting Nanobodies’ Singular Traits, Annu. Rev. Immunol 36 (2018). 10.1146/annurev-immunol-042617-053327. [DOI] [PubMed] [Google Scholar]
- [19].Woodham AW, Cheloha RW, Ling J, Rashidian M, Kolifrath SC, Mesyngier M, Duarte JN, Bader JM, Skeate JG, Da Silva DM, Kast WM, Ploegh HL, Nanobody–antigen conjugates elicit HPV-specific antitumor immune responses, Cancer Immunol. Res 6 (2018) 870–880. 10.1158/2326-6066.CIR-17-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheloha RW, Fischer FA, Woodham AW, Daley E, Suminski N, Gardella TJ, Ploegh HL, Improved GPCR ligands from nanobody tethering, Nat. Commun 11 (2020) 1–11. 10.1038/s41467-020-15884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bannas P, Hambach J, Koch-Nolte F, Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics, Front. Immunol 8 (2017) 1–13. 10.3389/fimmu.2017.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Muyldermans S, Nanobodies: Natural Single-Domain Antibodies, Annu. Rev. Biochem 82 (2013) 775–797. 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- [23].Rahbarizadeh F, Ahmadvand D, Sharifzadeh Z, Nanobody; an Old Concept and New Vehicle for Immunotargeting, Immunol. Invest 40 (2011) 299–338. 10.3109/08820139.2010.542228. [DOI] [PubMed] [Google Scholar]
- [24].Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, Cauerh A, Danquah VW, Rissiek B, Scheuplein F, Schwarz N, Adriouch S, Boyer O, Seman M, Licea A, V Serreze D, Goldbaum FA, Haag F, Koch-nolte F, Single domain antibodies: promising experimental and therapeutic tools in infection and immunity, Med. Microbiol. Immunol 198 (2009) 157–174. 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hu Y, Liu C, Muyldermans S, Nanobody-Based Delivery Systems for Diagnosis and Targeted Tumor Therapy, Front. Immunol 8 (2017). 10.3389/fimmu.2017.01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].De Meyer T, Muyldermans S, Depicker A, Nanobody-based products as research and diagnostic tools, Trends Biotechnol. 32 (2014) 263–270. 10.1016/j.tibtech.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [27].Chanier T, Chames P, Nanobody Engineering: Toward Next Generation Immunotherapies and Immunoimaging of Cancer, Antibodies. 8 (2019). 10.3390/antib8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lecocq Q, De Vlaeminck Y, Hanssens H, Huyvetter MD, Raes G, Keyaerts M, Devoogdt N, Breckpot K, Theranostics Theranostics in immuno-oncology using nanobody derivatives, Theranostics. 9 (2019) 7772–7791. 10.7150/thno.34941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang L, Gainkam LOT, Caveliers V, Vanhove C, Keyaerts M, Baetselier P, Bossuyt A, Revets H, Lahoutte T, SPECT imaging with 99mTc-labeled EGFR-specific nanobody for in vivo monitoring of EGFR expression, Mol. Imaging Biol 10 (2008) 167–175. 10.1007/s11307-008-0133-8. [DOI] [PubMed] [Google Scholar]
- [30].Vaneycken I, Devoogdt N, Van Gassen N, Vincke C, Xavier C, Wernery U, Muyldermans S, Lahoutte T, Caveliers V, Preclinical screening of anti‐HER2 nanobodies for molecular imaging of breast cancer, FASEB J. 25 (2011) 2433–2446. 10.1096/fj.10-180331. [DOI] [PubMed] [Google Scholar]
- [31].Jailkhani N, Ingram JR, Rashidian M, Rickelt S, Tian C, Mak H, Jiang Z, Ploegh HL, Hynes RO, Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix, PNAS. 116 (2019) 14181–14190. 10.1073/pnas.1817442116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Oda K, Matsuoka Y, Funahashi A, Kitano H, A comprehensive pathway map of epidermal growth factor receptor signaling, Mol. Syst. Biol (2005) 1–17. 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gibson TB, Ranganathan A, Grothey A, Randomized Phase III Trial Results of Panitumumab, a Fully Human Anti—Epidermal Growth Factor Receptor Monoclonal Antibody, in Metastatic Colorectal Cancer, Clin. Colorectal Cancer 6 (2006) 29–31. [DOI] [PubMed] [Google Scholar]
- [34].Roovers RC, Laeremans T, Huang L, De Taeye S, Verkleij AJ, Revets H, De Haard HJ, Van Bergen En Henegouwen PMP, Efficient inhibition of EGFR signalling and of tumour growth by antagonistic anti-EGFR Nanobodies, Cancer Immunol. Immunother 56 (2007) 303–317. 10.1007/s00262-006-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gainkam LOT, Huang L, Caveliers V, Keyaerts M, Hernot S, Vaneycken I, Vanhove C, Revets H, De Baetselier P, Lahoutte T, Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT, J. Nucl. Med 49 (2008) 788–795. 10.2967/jnumed.107.048538. [DOI] [PubMed] [Google Scholar]
- [36].Roovers RC, Vosjan MJWD, Laeremans T, El Khoulati R, De Bruin RCG, Ferguson KM, Verkleij AJ, Van Dongen GAMS, Van Bergen En Henegouwen PMP, A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth, Int. J. Cancer 129 (2011) 2013–2024. 10.1002/ijc.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Omidfar K, Amjad Zanjani FS, Hagh AG, Azizi MD, Rasouli SJ, Kashanian S, Efficient growth inhibition of EGFR over-expressing tumor cells by an anti-EGFR nanobody, Mol. Biol. Rep 40 (2013) 6737–6745. 10.1007/s11033-013-2790-1. [DOI] [PubMed] [Google Scholar]
- [38].Kijanka M, Warnders F-J, El Khattabi M, Lub-De Hooge M, Van Dam GM, Ntziachristos V, De Vries L, Oliveira S, Van Bergen En Henegouwen PMP, Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery, Eur. J. Nucl. Med. Mol. Imaging 40 (2013) 1718–1729. 10.1007/s00259-013-2471-2. [DOI] [PubMed] [Google Scholar]
- [39].Pruszynski M, Koumarianou E, Vaidyanathan G, Revets H, Devoogdt N, Lahoutte T, Zalutsky MR, Targeting breast carcinoma with radioiodinated anti-HER2 Nanobody, Nucl. Med. Biol 40 (2013) 52–59. 10.1016/j.nucmedbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L, VEGF receptor signalling in control of vasular function, Nat. Rev. Mol. Cell Biol 7 (2006) 359–371. [DOI] [PubMed] [Google Scholar]
- [41].Holmes K, Roberts OL, Thomas AM, Cross MJ, Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition, Cell. Signal 19 (2007) 2003–2012. 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- [42].Behdani M, Zeinali S, Khanahmad H, Karimipour M, Asadzadeh N, Azadmanesh K, Khabiri A, Schoonooghe S, Habibi Anbouhi M, Hassanzadeh-Ghassabeh G, Muyldermans S, Generation and characterization of a functional Nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor, Mol. Immunol 50 (2012) 35–41. 10.1016/j.molimm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- [43].Ma L, Gu K, Zhang C-H, Chen XT, Jiang Y, Melcher K, Zhang J, Wang M, Xu HE, Generation and characterization of a human nanobody against VEGFR-2, Acta Pharmacol. Sin 37 (2016) 857–864. 10.1038/aps.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kazemi-Lomedasht F, Behdani M, Bagheri KP, Habibi-Anbouhi M, Abolhassani M, Arezumand R, Shahbazzadeh D, Mirzahoseini H, Inhibition of angiogenesis in human endothelial cell using VEGF specific nanobody, Mol. Immunol 65 (2015) 58–67. 10.1016/j.molimm.2015.01.010. [DOI] [PubMed] [Google Scholar]
- [45].Kazemi-Lomedasht F, Muyldermans S, Habibi-Anbouhi M, Behdani M, Design of a humanized anti vascular endothelial growth factor nanobody and evaluation of its in vitro function, Iran. J. Basic Med. Sci 21 (2018) 260–266. 10.22038/ijbms.2018.24898.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ebrahimizadeh W, Mousavi Gargari SL, Javidan Z, Rajabibazl M, Production of Novel VHH Nanobody Inhibiting Angiogenesis by Targeting Binding Site of VEGF, Appl. Biochem. Biotechnol 176 (2015) 1985–1995. 10.1007/s12010-015-1695-y. [DOI] [PubMed] [Google Scholar]
- [47].Bottaro DP, Rubin JS, Faletto DL, Chan AML, Kmiecik TE, Vande Woude GF, Aaronson SA, Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product, Science (80-.). 251 (1991) 802–804. 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- [48].Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G, Targeting MET in cancer: rationale and progress, Nat. Rev. Cancer 12 (2012) 89–103. [DOI] [PubMed] [Google Scholar]
- [49].Boccaccio C, Comoglio PM, Invasive growth: a MET-driven genetic programme for cancer and stem cells, Nat. Rev. Cancer 6 (2006) 637–645. [DOI] [PubMed] [Google Scholar]
- [50].Wallenius V, Hisaoka M, Helou K, Levan G, Mandahl N, Meis-Kindblom JM, Kindblom L-G, Jansson JO, Overexpression of the hepatocyte growth factor (HGF) receptor (Met) and presence of a truncated and activated intracellular HGF receptor fragment in locally aggressive/malignant human musculoskeletal tumors, Am. J. Pathol 156 (2000) 821–829. 10.1016/S0002-9440(10)64950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Heukers R, Altintas I, Raghoenath S, De Zan E, Pepermans R, Roovers RC, Haselberg R, Hennink WE, Schiffelers RM, Kok RJ, Van Bergen en Henegouwen PMP, Targeting hepatocyte growth factor receptor (Met) positive tumor cells using internalizing nanobody-decorated albumin nanoparticles, Biomaterials. 35 (2014) 601–610. 10.1016/j.biomaterials.2013.10.001. [DOI] [PubMed] [Google Scholar]
- [52].Schmidt Slørdahl T, Denayer T, Helen Moen S, Standal T, Børset M, Ververken C, Baade Rø T, Anti-c-MET Nanobody® - A new potential drug in multiple myeloma treatment, Eur. J. Haematol 91 (2013) 399–410. 10.1111/ejh.12185. [DOI] [PubMed] [Google Scholar]
- [53].Vosjan MJWD, Vercammen J, Kolkman JA, Stigter-Van Walsum M, Revets H, Van Dongen GAMS, Nanobodies targeting the hepatocyte growth factor: Potential new drugs for molecular cancer therapy, Mol. Cancer Ther 11 (2012) 1017–1025. 10.1158/1535-7163.MCT-11-0891. [DOI] [PubMed] [Google Scholar]
- [54].Balkwill F, Cancer and the chemokine network, Nat. Rev. Cancer 4 (2004) 540–550. 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- [55].Bradley ME, Dombrecht B, Manini J, Willis J, Vlerick D, De Taeye S, Van Den Heede K, Roobrouck A, Grot E, Kent TC, Laeremans T, Steffensen S, Van Heeke G, Brown Z, Charlton SJ, Cromie KD, Potent and efficacious inhibition of CXCR2 signaling by biparatopic nanobodies combining two distinct modes of action, Mol. Pharmacol 87 (2015) 251–262. 10.1124/mol.114.094821. [DOI] [PubMed] [Google Scholar]
- [56].Jähnichen S, Blanchetot C, Maussang D, Gonzalez-Pajuelo M, Chow KY, Bosch L, De Vrieze S, Serruys B, Ulrichts H, Vandevelde W, Saunders M, De Haard HJ, Schols D, Leurs R, Vanlandschoot P, Verrips T, Smit MJ, CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells, PNAS. 107 (2010) 20565–20570. 10.1073/pnas.1012865107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].De Wit RH, Heukers R, Brink HJ, Arsova A, Maussang D, Cutolo P, Strubbe B, Vischer HF, Bachelerie F, Smit MJ, CXCR4-specific nanobodies as potential therapeutics for WHIM syndrome, J. Pharmacol. Exp. Ther 363 (2017) 35–44. 10.1124/jpet.117.242735. [DOI] [PubMed] [Google Scholar]
- [58].Van Hout A, Klarenbeek A, Bobkov V, Doijen J, Arimont M, Zhao C, Heukers R, Rimkunas R, de Graaf C, Verrips T, van der Woning B, de Haard H, Rucker JB, Vermeire K, Handel T, Van Loy T, Smit MJ, Schols D, CXCR4-targeting nanobodies differentially inhibit CXCR4 function and HIV entry, Biochem. Pharmacol 158 (2018) 402–412. 10.1016/j.bcp.2018.10.015. [DOI] [PubMed] [Google Scholar]
- [59].Maussang D, Mujić-Delić A, Descamps FJ, Stortelers C, Vanlandschoot P, Stigter-Van Walsum M, Vischer HF, Van Roy M, Vosjan M, Gonzalez-Pajuelo M, Van Dongen GAMS, Merchiers P, Van Rompaey P, Smit MJ, Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo, J. Biol. Chem 288 (2013) 29562–29572. 10.1074/jbc.M113.498436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Blanchetot C, Verzijl D, Mujić-Delić A, Bosch L, Rem L, Leurs R, Verrips CT, Saunders M, De Haard H, Smit MJ, Neutralizing nanobodies targeting diverse chemokines effectively inhibit chemokine function, J. Biol. Chem 288 (2013) 25173–25182. 10.1074/jbc.M113.467969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Burg JS, Ingram JR, Venkatakrishnan AJ, Jude KM, Dukkipati A, Feinberg EN, Angelini A, Waghray D, Dror RO, Ploegh HL, Garcia KC, Structural basis for chemokine recognition and activation of a viral G protein–coupled receptor John, Science (80-.). 347 (2015) 1113–1117. 10.1126/science.aaa5026.Structural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Heukers R, Fan TS, de Wit RH, van Senten JR, De Groof TWM, Bebelman MP, Lagerweij T, Vieira J, de Munnik SM, Smits-de Vries L, van Offenbeek J, Rahbar A, van Hoorick D, Söderberg-Naucler C, Würdinger T, Leurs R, Siderius M, Vischer HF, Smit MJ, The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth, Oncogene. 37 (2018) 4110–4121. 10.1038/s41388-018-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].De Groof TWM, Mashayekhi V, Fan TS, Bergkamp ND, Toraño JS, Van Senten JR, Heukers R, Smit MJ, Oliveira S, Nanobody-Targeted Photodynamic Therapy Selectively Kills Viral GPCR-Expressing Glioblastoma Cells, Mol. Pharm 16 (2019) 3145–3156. 10.1021/acs.molpharmaceut.9b00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cortez-Retamozo V, Backmann N, Senter PD, Wernery U, De Baetselier P, Muyldermans S, Revets H, Efficient Cancer Therapy with a Nanobody-Based Conjugate, Cancer Res. 64 (2004) 2853–2857. 10.1158/0008-5472.CAN-03-3935. [DOI] [PubMed] [Google Scholar]
- [65].Wang H, Meng A-M, Li S-H, Zhou X-L, A nanobody targeting carcinoembryonic antigen as a promising molecular probe for non-small cell lung cancer, Mol. Med. Rep 16 (2017) 625–630. 10.3892/mmr.2017.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kaliberov SA, Kaliberova LN, Buggio M, Tremblay JM, Shoemaker CB, Curiel DT, Adenoviral targeting using genetically incorporated camelid single variable domains, Lab. Investig 94 (2014) 893–905. 10.1038/labinvest.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chatalic KLS, Veldhoven-Zweistra J, Bolkestein M, Hoeben S, Koning GA, Boerman OC, De Jong M, Van Weerden WM, A novel 111In-labeled anti-prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer, J. Nucl. Med 56 (2015) 1094–1099. 10.2967/jnumed.115.156729. [DOI] [PubMed] [Google Scholar]
- [68].Evazalipour M, D’Huyvetter M, Tehrani BS, Abolhassani M, Omidfar K, Abdoli S, Arezumand R, Morovvati H, Lahoutte T, Muyldermans S, Devoogdt N, Generation and characterization of nanobodies targeting PSMA for molecular imaging of prostate cancer, Contrast Media Mol. Imaging 9 (2014) 211–220. 10.1002/cmmi.1558. [DOI] [PubMed] [Google Scholar]
- [69].Zare H, Rajabibazl M, Rasooli I, Ebrahimizadeh W, Bakherad H, Ardakani LS, Gargari SLM, Production of nanobodies against prostate-specific membrane antigen (PSMA) recognizing LnCaP cells, Int. J. Biol. Markers 29 (2014) 169–179. 10.5301/jbm.5000063. [DOI] [PubMed] [Google Scholar]
- [70].Fan X, Wang L, Guo Y, Tu Z, Li L, Tong H, Xu Y, Li R, Fang K, Ultrasonic nanobubbles carrying anti-PSMA nanobody: Construction and application in prostate cancer-targeted imaging, PLoS One. 10 (2015) 1–13. 10.1371/journal.pone.0127419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Saerens D, Kinne J, Bosmans E, Wernery U, Muyldermans S, Conrath K, Single domain antibodies derived from dromedary lymph node and peripheral blood lymphocytes sensing conformational variants of prostate-specific antigen, J. Biol. Chem 279 (2004) 51965–51972. 10.1074/jbc.M409292200. [DOI] [PubMed] [Google Scholar]
- [72].Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K, Bouwens L, Lahoutte T, De Baetselier P, Raes G, Devoogdt N, Van Ginderachter JA, Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages, Cancer Res. 72 (2012) 4165–4177. 10.1158/0008-5472.CAN-11-2994. [DOI] [PubMed] [Google Scholar]
- [73].Blykers A, Schoonooghe S, Xavier C, D’Hoe K, Laoui D, D’Huyvetter M, Vaneycken I, Cleeren F, Bormans G, Heemskerk J, Raes G, De Baetselier P, Lahoutte T, Devoogdt N, Van Ginderachter JA, Caveliers V, PET imaging of macrophage mannose receptor-expressing macrophages in tumor stroma using 18F-radiolabeled camelid single-domain antibody fragments, J. Nucl. Med 56 (2015) 1265–1271. 10.2967/jnumed.115.156828. [DOI] [PubMed] [Google Scholar]
- [74].Tang J, Li J, Zhu X, Yu Y, Chen D, Yuan L, Gu Z, Zhang X, Qi L, Gong Z, Jiang P, Yu J, Meng H, An G, Zheng H, Yang L, Novel CD7-specific nanobody-based immunotoxins potently enhanced apoptosis of CD7-positive malignant cells, Oncotarget. 7 (2016) 34070–34083. 10.18632/oncotarget.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yu Y, Li J, Zhu X, Tang X, Bao Y, Sun X, Huang Y, Tian F, Liu X, Yang L, Humanized CD7 nanobody-based immunotoxins exhibit promising anti-T-cell acute lymphoblastic leukemia potential, Int. J. Nanomedicine 12 (2017) 1969–1983. 10.2147/IJN.S127575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wan R, Liu A, Hou X, Lai Z, Li J, Yang N, Tan J, Mo F, Hu Z, Yang X, Zhao Y, Lu X, Screening and antitumor effect of an anti-CTLA-4 nanobody, Oncol. Rep 39 (2018) 511–518. 10.3892/or.2017.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ingram JR, Blomberg OS, Rashidian M, Ali L, Garforth S, Fedorov E, Fedorov AA, Bonanno JB, Le Gall C, Crowley S, Espinosa C, Biary T, Keliher EJ, Weissleder R, Almo SC, Dougan SK, Ploegh HL, Dougan M, Anti-CTLA-4 therapy requires an Fc domain for efficacy, PNAS. (2018) 3912–3917. 10.1073/pnas.1801524115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhang F, Wei H, Wang X, Bai Y, Wang P, Wu J, Jiang X, Wang Y, Cai H, Xu T, Zhou A, Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade, Cell Discov. 3 (2017) 1–12. 10.1038/celldisc.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Broos K, Lecocq Q, Xavier C, Bridoux J, Nguyen TT, Corthals J, Schoonooghe S, Lion E, Raes G, Keyaerts M, Devoogdt N, Breckpot K, Evaluating a single domain antibody targeting human PD-L1 as a nuclear imaging and therapeutic agent, Cancers (Basel). 11 (2019) 1–19. 10.3390/cancers11060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Broos K, Keyaerts M, Lecocq Q, Renmans D, Nguyen T, Escors D, Liston A, Raes G, Breckpot K, Devoogdt N, Non-invasive assessment of murine PD-L1 levels in syngeneic tumor models by nuclear imaging with nanobody tracers, Oncotarget. 8 (2017) 41932–41946. 10.18632/oncotarget.16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Xing Y, Chand G, Liu C, Cook GJR, O’Doherty J, Zhao L, Wong NCL, Meszaros LK, Ting HH, Zhao J, Early phase I study of a 99mTc-labeled anti-programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non-small cell lung cancer, J. Nucl. Med 60 (2019) 1213–1220. 10.2967/jnumed.118.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ingram JR, Dougan M, Rashidian M, Knoll M, Keliher EJ, Garrett S, Garforth S, Blomberg OS, Espinosa C, Bhan A, Almo SC, Weissleder R, Lodish H, Dougan SK, Ploegh HL, PD-L1 is an activation-independent marker of brown adipocytes, Nat. Commun 8 (2017). 10.1038/s41467-017-00799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rashidian M, Ingram JR, Dougan M, Dongre A, Whang KA, LeGall C, Cragnolini JJ, Bierie B, Gostissa M, Gorman J, Grotenbreg GM, Bhan A, Weinberg RA, Ploegh HL, Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells, J. Exp. Med 214 (2017) 2243–2255. 10.1084/jem.20161950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rossotti M, Tabares S, Alfaya L, Leizagoyen C, Moron G, González-Sapienza G, Streamlined method for parallel identification of single domain antibodies to membrane receptors on whole cells, Biochim. Biophys. Acta 1850 (2015) 1397–1404. 10.1016/j.bbagen.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Rashidian M, Keliher EJ, Bilate AM, Duarte JN, Wojtkiewicz GR, Jacobsen JT, Cragnolini J, Swee LK, Victora GD, Weissleder R, Ploegh HL, Noninvasive imaging of immune responses, PNAS. 112 (2015) 6146–6151. 10.1073/pnas.1502609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Krasniqi A, D’Huyvetter M, Xavier C, Van der Jeught K, Muyldermans S, Van Der Heyden J, Lahoutte T, Tavernier J, Devoogdt N, Theranostic radiolabeled anti-CD20 sdAb for targeted radionuclide therapy of non-hodgkin lymphoma, Mol. Cancer Ther 16 (2017) 2828–2839. 10.1158/1535-7163.MCT-17-0554. [DOI] [PubMed] [Google Scholar]
- [87].Fumey W, Koenigsdorf J, Kunick V, Menzel S, Schütze K, Unger M, Schriewer L, Haag F, Adam G, Oberle A, Binder M, Fliegert R, Guse A, Zhao YJ, Lee HC, Malavasi F, Goldbaum F, Van Hegelsom R, Stortelers C, Bannas P, Koch-Nolte F, Nanobodies effectively modulate the enzymatic activity of CD38 and allow specific imaging of CD38+ tumors in mouse models in vivo, Sci. Rep 7 (2017) 1–13. 10.1038/s41598-017-14112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bachran C, Schröder M, Conrad L, Cragnolini JJ, Tafesse FG, Helming L, Ploegh HL, Swee LK, The activity of myeloid cell-specific VHH immunotoxins is target-, epitope-, subset- and organ dependent, Sci. Rep 7 (2017) 2–11. 10.1038/s41598-017-17948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Van Elssen CHMJ, Rashidian M, Vrbanac V, Wucherpfennig KW, El Habre Z, Sticht J, Freund C, Jacobsen JT, Cragnolini J, Ingram J, Plaisier L, Spierings E, Tager AM, Ploegh HL, Noninvasive imaging of human immune responses in a human xenograft model of graft-versus-host disease, J. Nucl. Med 58 (2017) 1003–1008. 10.2967/jnumed.116.186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rashidian M, Keliher EJ, Dougan M, Juras PK, Cavallari M, Wojtkiewicz GR, Jacobsen JT, Edens JG, Tas JMJ, Victora G, Weissleder R, Ploegh H, Use of 18F-2-fluorodeoxyglucose to label antibody fragments for immuno-positron emission tomography of pancreatic cancer, ACS Cent. Sci 1 (2015) 142–147. 10.1021/acscentsci.5b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Samec N, Jovcevska I, Stojan J, Zottel A, Liovic M, Myers MP, Muyldermans S, Šribar J, Križaj I, Komel R, Glioblastoma-specific anti-TUFM nanobody for in-vitro immunoimaging and cancer stem cell targeting, Oncotarget. 9 (2018) 17282–17299. 10.18632/oncotarget.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Van Impe K, Bethuyne J, Cool S, Impens F, Ruano-Gallego D, De Wever O, Vanloo B, Van Troys M, Lambein K, Boucherie C, Martens E, Zwaenepoel O, Hassanzadeh-Ghassabeh G, Vandekerckhove J, Gevaert K, Fernández LÁ, Sanders NN, Gettemans J, A nanobody targeting the F-actin capping protein CapG restrains breast cancer metastasis, Breast Cancer Res. 15 (2013) 1–15. 10.1186/bcr3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Araste F, Ebrahimizadeh W, Rasooli I, Rajabibazl M, Mousavi Gargari SL, A novel VHH nanobody against the active site (the CA domain) of tumor-associated, carbonic anhydrase isoform IX and its usefulness for cancer diagnosis, Biotechnol. Lett 36 (2014) 21–28. 10.1007/s10529-013-1340-1. [DOI] [PubMed] [Google Scholar]
- [94].van Brussel ASA, Adams A, Oliveira S, Dorresteijn B, El Khattabi M, Vermeulen JF, van der Wall E, Mali WPTM, Derksen PWB, van Diest PJ, van Bergen en Henegouwen PMP, Hypoxia-Targeting Fluorescent Nanobodies for Optical Molecular Imaging of Pre-Invasive Breast Cancer, Mol. Imaging Biol 18 (2016) 535–544. 10.1007/s11307-015-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Romão E, Krasniqi A, Maes L, Vandenbrande C, Sterckx YG-J, Stijlemans B, Vincke C, Devoogdt N, Muyldermans S, Identification of nanobodies against the acute myeloid leukemia marker CD33, Int. J. Mol. Sci 21 (2020). 10.3390/ijms21010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ma L, Zhu M, Gai J, Li G, Chang Q, Qiao P, Cao L, Chen W, Zhang S, Wan Y, Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential, J. Nanobiotechnology 18 (2020) 1–15. 10.1186/s12951-020-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sockolosky JT, Dougan M, Ingram JR, Ho CCM, Kauke MJ, Almo SC, Ploegh HL, Garcia KC, Durable antitumor responses to CD47 blockade require adaptive immune stimulation, PNAS. 113 (2016) E2646–54. 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Koch-Nolte F, Reyelt J, Schößow B, Schwarz N, Scheuplein F, Rothenburg S, Haag F, Alzogaray V, Cauerhff A, Goldbaum FA, Single domain antibodies from llama effectively and specifically block T cell ecto‐ADP‐ribosyltransferase ART2.2 in vivo, FASEB J. 21 (2007) 3490–3498. 10.1096/fj.07-8661com. [DOI] [PubMed] [Google Scholar]
- [99].Ji X, Peng Z, Li X, Yan Z, Yang Y, Qiao Z, Liu Y, Neutralization of TNFα in tumor with a novel nanobody potentiates paclitaxel-therapy and inhibits metastasis in breast cancer, Cancer Lett. 386 (2017) 24–34. 10.1016/j.canlet.2016.10.031. [DOI] [PubMed] [Google Scholar]
- [100].Weissleder R, Molecular imaging in cancer, Science (80-.). 312 (2006) 1168–1171. 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- [101].Hong H, Zhang Y, Sun J, Cai W, Molecular imaging and therapy of cancer with radiolabeled nanoparticles, Nano Today. 4 (2009) 399–413. 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].de Vos J, Devoogdt N, Lahoutte T, Muyldermans S, Camelid single-domain antibody-fragment engineering for (pre)clinical in vivo molecular imaging applications: adjusting the bullet to its target, Expert Opin. Biol. Ther 13 (2013) 1149–1160. 10.1517/14712598.2013.800478. [DOI] [PubMed] [Google Scholar]
- [103].Knowles SM, Wu AM, Advances in immuno-positron emission tomography: Antibodies for molecular imaging in oncology, J. Clin. Oncol 30 (2012) 3884–3892. 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Vosjan MJWD, Perk LR, Roovers RC, Visser GWM, Stigter-Van Walsum M, Van Bergen En Henegouwen PMP, Van Dongen GAMS, Facile labelling of an anti-epidermal growth factor receptor Nanobody with 68Ga via a novel bifunctional desferal chelate for immuno-PET, Eur. J. Nucl. Med. Mol. Imaging 38 (2011) 753–763. 10.1007/s00259-010-1700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Oliveira S, Van Dongen GAMS, Stigter-Van Walsum M, Roovers RC, Stam JC, Mali W, Van Diest PJ, Van Bergen En Henegouwen PMP, Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody, Mol. Imaging 11 (2012) 33–46. 10.2310/7290.2011.00025. [DOI] [PubMed] [Google Scholar]
- [106].Hernot S, Unnikrishnan S, Du Z, Shevchenko T, Cosyns B, Broisat A, Toczek J, Caveliers V, Muyldermans S, Lahoutte T, Klibanov AL, Devoogdt N, Nanobody-coupled microbubbles as novel molecular tracer, J. Control. Release 158 (2012) 346–353. 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Berger A, Positron emission tomography, Br. Med. J (2003). [Google Scholar]
- [108].Vaidyanathan G, Bigner DD, Zalutsky MR, Fluorine-18-labeled monoclonal antibody fragments: A potential approach for combining radioimmunoscintigraphy and positron emission tomography, J. Nucl. Med 33 (1992) 1535–1541. [PubMed] [Google Scholar]
- [109].Cai W, Olafsen T, Zhang X, Cao Q, Gambhir SS, Williams LE, Wu AM, Chen X, PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anticarcinoembryonic antigen diabody, J. Nucl. Med 48 (2007) 304–310. [PubMed] [Google Scholar]
- [110].Xavier C, Vaneycken I, D’Huyvetter M, Heemskerk J, Keyaerts M, Vincke C, Devoogdt N, Muyldermans S, Lahoutte T, Caveliers V, Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer, J. Nucl. Med 54 (2013) 776–784. 10.2967/jnumed.112.111021. [DOI] [PubMed] [Google Scholar]
- [111].Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, Vanhoeij M, Duhoux FP, Gevaert T, Simon P, Schallier D, Fontaine C, Vaneycken I, Vanhove C, De Greve J, Lamote J, Caveliers V, Lahoutte T, Phase I study of 68Ga-HER2-Nanobody for PET/CT assessment of HER2 expression in breast carcinoma, J. Nucl. Med 57 (2016) 27–33. 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- [112].Xavier C, Blykers A, Laoui D, Bolli E, Vaneyken I, Bridoux J, Baudhuin H, Raes G, Everaert H, Movahedi K, Van Ginderachter JA, Devoogdt N, Caveliers V, Lahoutte T, Keyaerts M, Clinical Translation of [68Ga]Ga-NOTA-anti-MMR-sdAb for PET/CT Imaging of Protumorigenic Macrophages, Mol. Imaging Biol 21 (2019) 898–906. 10.1007/s11307-0180-1302-5. [DOI] [PubMed] [Google Scholar]
- [113].Banerjee SR, Pomper MG, Clinical applications of Gallium-68, Appl. Radiat. Isot 76 (2013) 2–13. 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Garcia-Torano E, Ibarra MR, The half-life of 18F, Appl. Radiat. Isot 68 (2010) 1561–1565. [DOI] [PubMed] [Google Scholar]
- [115].Xavier C, Blykers A, Vaneycken I, D’Huyvetter M, Heemskerk J, Lahoutte T, Devoogdt N, Caveliers V, 18F-nanobody for PET imaging of HER2 overexpressing tumors, Nucl. Med. Biol 43 (2016) 247–252. 10.1016/j.nucmedbio.2016.01.002. [DOI] [PubMed] [Google Scholar]
- [116].Leach DR, Krummel MF, Allison JP, Enhancement of Antitumor Immunity by CTLA-4 Blockade, Science (80-.). 271 (1996) 1734–1736. [DOI] [PubMed] [Google Scholar]
- [117].Lipson EJ, Forde PM, Hammers H, Emens A, Taube JM, Topalian SL, Antagonists of PD-1 and PD-L1 in Cancer Treatment, Semin. Oncol 42 (2015) 587–600. 10.1053/j.seminoncol.2015.05.013.Antagonists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang Y, Velez-Delgado A, Mathew E, Li D, Mendez FM, Flannagan K, Rhim AD, Simeone DM, Beatty GL, Pasca Di Magliano M, Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer, Gut. 66 (2017) 124–136. 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bocanegra A, Fernandez-Hinojal G, Zuazo-Ibarra M, Arasanz H, Garcia-Granda MJ, Hernandez C, Ibañez M, Hernandez-Marin B, Martinez-Aguillo M, Lecumberri MJ, de Lascoiti AF, Teijeira L, Morilla I, Vera R, Escors D, Kochan G, PD-L1 expression in systemic immune cell populations as a potential predictive biomarker of responses to PD-L1/PD-1 blockade therapy in lung cancer, Int. J. Mol. Sci 20 (2019) 1–13. 10.3390/ijms20071631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Pico De Coaña Y, Masucci G, Hansson J, Kiessling R, Myeloid-derived suppressor cells and their role in CTLA-4 blockade therapy, Cancer Immunol. Immunother 63 (2014) 977–983. 10.1007/s00262-014-1570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Dedman JR, Gracy RW, Harris BG, A method for estimating sequence homology from amino acid compositions. The evolution of Ascaris employing aldolase and glyceraldehyde-3-phosphate dehydrogenase, Comp. Biochem. Physiol. B, Basic Heal 49 (1974) 715–731. [DOI] [PubMed] [Google Scholar]
- [122].Fang T, Van Elssen CHMJ, Duarte JN, Guzman JS, Chahal JS, Ling J, Ploegh HL, Targeted antigen delivery by an anti-class II MHC VHH elicits focused αmUC1(Tn) immunity, Chem. Sci 8 (2017) 5591–5597. 10.1039/c7sc00446j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Vaneycken I, Govaert J, Vincke C, Caveliers V, Lahoutte T, De Baetselier P, Raes G, Bossuyt A, Muyldermans S, Devoogdt N, In vitro analysis and in vivo tumor targeting of a humanized, grafted Nanobody in mice using pinhole SPECT/micro-CT, J. Nucl. Med 51 (2010) 1099–1106. 10.2967/jnumed.109.069823. [DOI] [PubMed] [Google Scholar]
- [124].Van Driel PBAA, Van Der Vorst JR, Verbeek FPR, Oliveira S, Snoeks TJA, Keereweer S, Chan B, Boonstra MC, Frangioni JV, Van Bergen En Henegouwen PMP, Vahrmeijer AL, Lowik CWGM, Intraoperative fluorescence delineation of head and neck cancer with a fluorescent Anti-epidermal growth factor receptor nanobody, Int. J. Cancer 134 (2014) 2663–2673. 10.1002/ijc.28601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Debie P, Vanhoeij M, Poortmans N, Puttemans J, Gillis K, Devoogdt N, Lahoutte T, Hernot S, Improved Debulking of Peritoneal Tumor Implants by Near-Infrared Fluorescent Nanobody Image Guidance in an Experimental Mouse Model, Mol. Imaging Biol 20 (2018) 361–367. 10.1007/s11307-017-1134-2. [DOI] [PubMed] [Google Scholar]
- [126].Bannas P, Lenz A, Kunick V, Well L, Fumey W, Rissiek B, Haag F, Schmid J, Schütze K, Eichhoff A, Trepel M, Adam G, Ittrich H, Koch-Nolte F, Molecular imaging of tumors with nanobodies and antibodies: Timing and dosage are crucial factors for improved in vivo detection, Contrast Media Mol. Imaging 10 (2015) 367–378. 10.1002/cmmi.1637. [DOI] [PubMed] [Google Scholar]
- [127].Debie P, Van Quathem J, Hansen I, Bala G, Massa S, Devoogdt N, Xavier C, Hernot S, Effect of dye and conjugation chemistry on the biodistribution profile of near-infrared-labeled nanobodies as tracers for image-guided surgery, Mol. Pharm 14 (2017) 1145–1153. 10.1021/acs.molpharmaceut.6b01053. [DOI] [PubMed] [Google Scholar]
- [128].Zhang J, Chen Y, Deng C, Zhang L, Sun Z, Wang J, Yang Y, Lv Q, Han W, Xie M, The optimized fabrication of a novel nanobubble for tumor imaging, Front. Pharmacol 10 (2019) 1–15. 10.3389/fphar.2019.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kogan P, Gessner RC, Dayton PA, Microbubbles in imaging: Applications beyond ultrasound, Bubble Sci. Eng. Technol 2 (2010) 3–8. 10.1179/175889610X12730566149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yin T, Wang P, Zheng R, Zheng B, Cheng D, Zhang X, Shuai X, Nanobubbles for enhanced ultrasound imaging of tumors, Int. J. Nanomedicine 7 (2012) 895–904. 10.2147/IJN.S28830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Gainkam LOT, Caveliers V, Devoogdt N, Vanhove C, Xavier C, Boerman O, Muyldermans S, Bossuyt T. Lahoutte, Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice, Contrast Media Mol. Imaging 6 (2011) 85–92. 10.1002/cmmi.408. [DOI] [PubMed] [Google Scholar]
- [132].Zhou Z, Devoogdt N, Zalutsky MR, Vaidyanathan G, An Efficient Method for Labeling Single Domain Antibody Fragments with 18 F Using Tetrazine-Trans-Cyclooctene Ligation and a Renal Brush Border Enzyme-Cleavable Linker, Bioconjug. Chem 29 (2018) 4090–4103. 10.1021/acs.bioconjchem.8b00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH, Coinhibitory Pathways in Immunotherapy for Cancer, Annu. Rev. Immunol 34 (2016) 539–573. 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- [134].Curran MA, Montalvo W, Yagita H, Allison JP, PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors, PNAS. 107 (2010) 4275–4280. 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ingram JR, Blomberg OS, Sockolosky JT, Ali L, Schmidt FI, Pishesha N, Espinosa C, Dougan SK, Garcia KC, Ploegh HL, Dougan M, Localized CD47 blockade enhances immunotherapy for murine melanoma, PNAS. 114 (2017) 2–7. 10.1073/pnas.1710776114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Peters C, Brown S, Antibody-drug conjugates as novel anti-cancer chemotherapeutics, Biosci. Rep 35 (2015). 10.1042/BSR20150089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Thomas A, Teicher BA, Hassan R, Antibody-drug conjugates for cancer therapy, Lancet Oncol. 17 (2016) e254–e262. 10.22088/jbums.19.7.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Fang T, Duarte JN, Ling J, Li Z, Guzman JS, Ploegh HL, Structurally-defined αMHC-II nanobody-drug conjugates: Therapeutic and imaging platforms for B-cell lymphoma, Angew. Chemie Int. Ed 55 (2016) 2416–2420. 10.1016/j.pep.2015.11.007.Simple. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, Goubau D, Reis e Sousa C, GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c+MHCII+ Macrophages and Dendritic Cells, Immunity. 42 (2015) 1197–1211. [DOI] [PubMed] [Google Scholar]
- [140].Becher B, Schlitzer A, Chen J, Mair F, Sumatoh HR, Wei K, Teng W, Low D, Ruedl C, Riccardi-castagnoli P, Poidinger M, Greter M, Ginhoux F, Newell EW, High-dimensional analysis of the murine myeloid cell system, Nat. Immunol 15 (2014) 1181–1189. 10.1038/ni.3006.nature. [DOI] [PubMed] [Google Scholar]
- [141].Knox SJ, Goris ML, Trisler K, Negrin R, Davis T, Liles T-M, Grillo-Lopez A, Chinn P, Varns C, Ning S-C, Fowler S, Deb N, Becker M, Marquez C, Levy R, Yttrium-90-labeled Anti-CD20 Monoclonal Antibody Therapy of Recurrent B-Cell Lymphoma, Clin. Cancer Res 2 (1996) 457–470. [PubMed] [Google Scholar]
- [142].Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-López AJ, Multani P, White CA, Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma, J. Clin. Oncol 20 (2002) 2453–2463. 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- [143].Witzig TE, White CA, Wiseman GA, Gordon LI, Emmanouilides C, Raubitschek A, Janakiraman N, Gutheil J, Schilder RJ, Spies S, Silverman DHS, Parker E, Grillo-López AJ, Phase I/II Trial of IDEC-Y2B8 Radioimmunotherapy for Treatment of Relapsed or Refractory CD20+ B-Cell Non-Hodgkin’s Lymphoma, J. Clin. Oncol 17 (1999) 3793–3803. [DOI] [PubMed] [Google Scholar]
- [144].Vose JM, Bierman PJ, Loberiza FR, Enke C, Hankins J, Bociek RG, Chan WC, Weisenburger DD, Armitage JO, Phase II Trial of 131-Iodine Tositumomab with High-Dose Chemotherapy and Autologous Stem Cell Transplantation for Relapsed Diffuse Large B Cell Lymphoma, Biol. Blood Marrow Transplant 19 (2013) 123–128. 10.1016/j.bbmt.2012.08.013. [DOI] [PubMed] [Google Scholar]
- [145].Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, Ballangrud ÅM, Hamacher KA, Ma D, Humm JL, Brechbiel MW, Molinet R, Scheinberg DA, Targeted α particle immunotherapy for myeloid leukemia, Blood. 100 (2002) 1233–1239. 10.1182/blood.v100.4.1233.h81602001233_1233_1239. [DOI] [PubMed] [Google Scholar]
- [146].Sgouros G, Ballangrud ÅM, Jurcic JG, McDevitt MR, Humm JL, Erdi YE, Mehta BM, Finn RD, Larson SM, Scheinberg DA, Pharmacokinetics and dosimetry of an α-particle emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia, J. Nucl. Med 40 (1999) 1935–1946. [PubMed] [Google Scholar]
- [147].Mattes MJ, Sharkey RM, Karacay H, Czuczman MS, Goldenberg DM, Therapy of advanced B-lymphoma xenografts with a combination of 90Y-anti-CD22 IgG (epratuzumab) and unlabeled anti-CD20 IgG (veltuzumab), Clin. Cancer Res 14 (2008) 6154–6160. 10.1158/1078-0432.CCR-08-0404. [DOI] [PubMed] [Google Scholar]
- [148].Sharkey RM, Press OW, Goldenberg DM, A re-examination of radioimmunotherapy in the treatment of non-Hodgkin lymphoma: prospects for dual-targeted antibody/radioantibody therapy, Blood. 113 (2009) 3891–3895. 10.1182/blood-2008-11-188896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Verheijen RH, Massuger LF, Benigno BB, Epenetos AA, A AL, Soper JT, Markowska J, R RV, T TJ, Stamp G, Spiegel G, Thurston D, Falke T, Lambert J, Seiden MV, Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission., J. Clin. Oncol 24 (2006) 571–578. [DOI] [PubMed] [Google Scholar]
- [150].Wong JYC, Chu DZ, Yamauchi DM, Williams LE, Liu A, Wilczynski S, Wu AM, Shively JE, Doroshow JH, Raubitschek AA, A Phase I Radioimmunotherapy Trial Evaluating 90 Yttrium-labeled Anti-Carcinoembryonic Antigen (CEA) Chimeric T84.66 in Patients with Metastatic CEA-producing Malignancies, Clin. Cancer Res 6 (2000) 3855–3863. [PubMed] [Google Scholar]
- [151].Liersch T, Meller J, Kulle B, Behr TM, Markus P, Langer C, Ghadimi BM, Wegener WA, Horak JKID, Becker H, Goldenberg DM, Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: five-year safety and efficacy results., J. Clin. Oncol 23 (2005) 6763–6770. [DOI] [PubMed] [Google Scholar]
- [152].Myers R, Harvey M, Kaufmann TJ, Greiner SM, Krempski JW, Raffel C, Shelton SE, Soeffker D, Zollman P, Federspiel MJ, Blanco M, Galanis E, Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas, Hum. Gene Ther 19 (2008) 690–698. 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Meredith RF, Khazaeli MB, Plott WE, Grizzle WE, Liu T, Schlom J, Russell CD, Wheeler RH, LoBuglio AF, Phase II Study with Interferon of Dual 131I-labeled Monoclonal Antibody Therapy in Patients with Metastatic Colorectal, Clin. Cancer Res 2 (1996) 1811–1818. [PubMed] [Google Scholar]
- [154].D’Huyvetter M, Vincke C, Xavier C, Aerts A, Impens N, Baatout S, De Raeve H, Muyldermans S, Caveliers V, Devoogdt N, Lahoutte T, Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody, Theranostics. 4 (2014) 708–720. 10.7150/thno.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].D’Huyvetter M, De Vos J, Xavier C, Pruszynski M, Sterckx YGJ, Massa S, Raes G, Caveliers V, Zalutsky MR, Lahoutte T, Devoogdt N, 131I-labeled anti-HER2 camelid sdAb as a theranostic tool in cancer treatment, Clin. Cancer Res 23 (2017) 6616–6628. 10.1158/10780-432.CCR-17-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Stein R, Govindan SV, Hayes M, Griffiths GL, Hansen HJ, Horak ID, Goldenberg DM, Advantage of a residualizing iodine radiolabel in the therapy of a colon cancer xenograft targeted with an anticarcinoembryonic antigen monoclonal antibody, Clin. Cancer Res 11 (2005) 2727–2734. 10.1158/1078-0432.CCR-04-2100. [DOI] [PubMed] [Google Scholar]
- [157].Pruszynski M, Koumarianou E, Vaidyanathan G, Revets H, Devoogdt N, Lahoutte T, Lyerly HK, Zalutsky MR, Improved tumor targeting of anti-her2 nanobody through n-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling, J. Nucl. Med 55 (2014) 650–656. 10.2967/jnumed.113.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Torchilin VP, Multifunctional nanocarriers, Adv. Drug Deliv. Rev 58 (2006) 1532–1555. 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- [159].Oliveira S, Schiffelers RM, van der Veeken J, van der Meel R, Vongpromek R, van Bergen en Henegouwen PMP, Storm G, Roovers RC, Downregulation of EGFR by a novel multivalent nanobody-liposome platform, J. Control. Release 145 (2010) 165–175. 10.1016/j.jconrel.2010.03.020. [DOI] [PubMed] [Google Scholar]
- [160].Talelli M, Rijcken CJF, Oliveira S, Van Der Meel R, Van Bergen En Henegouwen PMP, Lammers T, Van Nostrum CF, Storm G, Hennink WE, Nanobody - Shell functionalized thermosensitive core-crosslinked polymeric micelles for active drug targeting, J. Control. Release 151 (2011) 183–192. 10.1016/j.jconrel.2011.01.015. [DOI] [PubMed] [Google Scholar]
- [161].Liu Y, Scrivano L, Peterson JD, Fens MHAM, Beltrán Hernández I, Mesquita B, Toraño JS, Hennink WE, van Nostrum CF, Oliveira S, EGFR-Targeted Nanobody Functionalized Polymeric Micelles Loaded with mTHPC for Selective Photodynamic Therapy, Mol. Pharm 17 (2020) 1276–1292. 10.1021/acs.molpharmaceut.9b01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ, Extracellular vesicles in cancer — implications for future improvements in cancer care, Nat. Rev. Clin. Oncol 15 (2018) 617–638. 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- [163].Kooijmans SAA, Gómez Aleza C, Roffler SR, van Solinge WW, Vader P, Schiffelers RM, Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting, J. Extracell. Vesicles 5 (2016) 1–11. 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Breckpot K, Aerts J, Thielemans K, Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics, Gene Ther. 14 (2007) 847–862. 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- [165].Gennari F, Lopes L, Verhoeyen E, Marasco W, Collins MK, Single-Chain Antibodies That Target Lentiviral Vectors to MHC Class II on Antigen-Presenting Cells, Hum. Gene Ther 20 (2009) 554–562. [DOI] [PubMed] [Google Scholar]
- [166].Goyvaerts C, De Groeve K, Dingemans J, Van Lint S, Robays L, Heirman C, Reiser J, Zhang X-Y, Thielemans K, De Baetselier P, Raes G, Breckpot K, Development of the Nanobody display technology to target lentiviral vectors to antigen-presenting cells, Gene Ther. 19 (2012) 1133–1140. 10.1038/gt.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Eichhoff AM, Börner K, Albrecht B, Schäfer W, Baum N, Haag F, Körbelin J, Trepel M, Braren I, Grimm D, Adriouch S, Koch-Nolte F, Nanobody-Enhanced Targeting of AAV Gene Therapy Vectors, Mol. Ther. - Methods Clin. Dev 15 (2019) 211–220. 10.1016/j.omtm.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Duarte JN, Cragnolini JJ, Swee LK, Bilate AM, Bader J, Ingram JR, Rashidfarrokhi A, Fan T, Schiepers A, Hanke L, Ploegh HL, Generation of Immunity against Pathogens via Single-Domain Antibody–Antigen Constructs Joao, J. Immunol 197 (2016) 4838–4847. 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, Feldman SA, Restifo NP, Rosenberg SA, Immunotherapies based on adoptive cell transfer are highly effective in the treatment of metastatic melanoma, but the use of this approach in other cancer histologies has been hampered by the identification of appropriate target molecules. Immunologic a, 120 (2010) 3953–3968. 10.1172/JCI43490DS1. [DOI] [Google Scholar]
- [170].Davila ML, Riviere I, Wang X, Bartido S, Park J, Chung SS, Stefanski J, Borquez-ojeda O, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DCG, Arcila ME, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R, Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy, Sci. Transl. Med 6 (2014). 10.1126/scitranslmed.3008226.Efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA, Chimeric antigen receptor T cells for sustained remissions in leukemia, N. Engl. J. Med 371 (2014) 1507–1517. 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Lee DW, Kochenderfer JN, Stetler-stevenson M, Cui YK, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL, T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial, Lancet Oncol. 385 (2015) 517–528. 10.1016/S0140-6736(14)61403-3.T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gorovits B, Koren E, Immunogenicity of Chimeric Antigen Receptor T-Cell Therapeutics, BioDrugs. 33 (2019) 275–284. 10.1007/s40259-019-00354-5. [DOI] [PubMed] [Google Scholar]
- [174].Albert S, Arndt C, Feldmann A, Bergmann R, Bachmann D, Koristka S, Ludwig F, Ziller-Walter P, Kegler A, Gärtner S, Schmitz M, Ehninger A, Cartellieri M, Ehninger G, Pietzsch HJ, Pietzsch J, Steinbach J, Bachmann M, A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform, Oncoimmunology. 6 (2017) 1–17. 10.1080/2162402X.2017.1287246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Albert S, Arndt C, Koristka S, Berndt N, Bergmann R, Feldmann A, Schmitz M, Pietzsch J, Steinbach J, Bachmann M, From mono- to bivalent: Improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo, Oncotarget. 9 (2018) 25597–25616. 10.18632/oncotarget.25390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hajari Taheri F, Hassani M, Sharifzadeh Z, Behdani M, Arashkia A, Abolhassani M, T cell engineered with a novel nanobody-based chimeric antigen receptor against VEGFR2 as a candidate for tumor immunotherapy, IUBMB Life. 71 (2019) 1259–1267. 10.1002/iub.2019. [DOI] [PubMed] [Google Scholar]
- [177].De Munter S, Ingels J, Goetgeluk G, Bonte S, Pille M, Weening K, Kerre T, Abken H, Vandekerckhove B, Nanobody based dual specific CARs, Int. J. Mol. Sci 19 (2018) 1–11. 10.3390/ijms19020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Xie YJ, Dougan M, Jailkhani N, Ingram J, Fang T, Kummer L, Momin N, Pishesha N, Rickelt S, Hynes RO, Ploegh H, Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice, PNAS. 116 (2019) 7624–7631. 10.1073/pnas.1817147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Xie YJ, Dougan M, Ingram JR, Pishesha N, Fang T, Momin N, Ploegh HL, Improved Antitumor Efficacy of Chimeric Antigen Receptor T Cells that Secrete Single-Domain Antibody Fragments, Cancer Immunol. Res 8 (2020) 518–530. 10.1158/2326-6066.CIR-19-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].You F, Wang Y, Jiang L, Zhu X, Chen D, Yuan L, An G, Meng H, Yang L, A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia., Am. J. Cancer Res 9 (2019) 64–78. [PMC free article] [PubMed] [Google Scholar]
- [181].Hambach J, Riecken K, Cichutek S, Schütze K, Albrecht B, Petry K, Röckendorf JL, Baum N, Kröger N, Hansen T, Schuch G, Haag F, Adam G, Fehse B, Bannas P, Koch-nolte F, Targeting CD38-Expressing Multiple Myeloma and Burkitt Lymphoma Cells In Vitro with Nanobody-Based Chimeric Antigen Receptors (Nb-CARs), Cells. 9 (2020) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Li T, Qi S, Unger M, Hou YN, Deng QW, Liu J, Lam CMC, Wang XW, Xin D, Zhang P, Koch-nolte F, Hao Q, Zhang H, Cheung Lee H, Juan Zhao Y, Immuno-targeting the multifunctional CD38 using nanobody, Nat. Publ. Gr 6 (2016) 1–11. 10.1038/srep27055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Omidfar K, Rasaee MJ, Modjtahedi H, Forouzandeh M, Taghikhani M, Golmakani N, Production of a novel camel single-domain antibody specific for the type III mutant EGFR, Tumor Biol. 25 (2004) 296–305. 10.1159/000081395. [DOI] [PubMed] [Google Scholar]
- [184].Hofman EG, Ruonala MO, Bader AN, van den Heuvel D, Voortman J, Roovers RC, Verkleij AJ, Gerritsen HC, van Bergen en Henegouwen PMP, EGF induces coalescence of different lipid rafts, J. Cell Sci 121 (2008) 2519–2528. 10.1242/jcs.028753. [DOI] [PubMed] [Google Scholar]


