Abstract
Background
Several papers have shown contradictory evidence about the relationship between smoking and COVID-19-related deaths. There is little evidence about smoking and risk of infection. We aim to examine association between smoking and COVID-19 infection and subsequent mortality.
Methods
This was a prospective study with participants from the UK Biobank cohort. Participants who lived in England were followed up from 01/02/2020 to 28/06/2020 with data linked to hospital episode statistics, Office for National Statistics and Public Health England PCR tests. We compared current-smokers, previous-smokers with never-smokers and estimated risk ratio (RR) of COVID-19 infection and subsequent mortality using Poisson regression adjusting for age, sex, ethnicity, body mass index and socio-economic status. Interactions between smoking status and age and sex were tested for using multiplicative interactions, and analyses were stratified by median age (49–68 years, 69–86 years) and sex.
Results
In total, 402,978 participants were included in the analyses. The majority were never smokers, 226,294 (56.2%), 140,090 (34.8%) were previous smokers, and 39,974 (9.9%) current smokers. COVID-19 infection was identified in 1591 (0.39%) people, and 372/1591 (23.4%) died. Amongst the younger participants, smokers were nearly twice as likely to become infected with COVID-19 than never smokers (RR 1.88 [1.49–2.38]) whereas there was no difference for those aged 69+ (RR 1.05 [0.82–1.34]). In contrast, amongst the older participants, smokers were twice as likely to die from COVID-19 compared to non-smokers (RR 2.15 [1.11–4.16]) whereas there was no difference for those under the age of 69 (RR 1.22[0.83–1.79]). Similar patterns were observed for previous smokers. The impact of smoking was similar in men and women.
Conclusion
The association between smoking and COVID-19 infection and subsequent death is modified by age. Smokers and previous smokers aged under 69 were at higher risk of COVID-19 infection, suggesting the risk is associated with increased exposure to SARS-COV-2 virus. Once infected, older smokers were twice as likely to die from COVID-19 than never smokers, possibly mediated by increased risk of chronic conditions/illnesses.
Keywords: smoking, COVID-19, UK Biobank
Introduction
There has been some debate as to whether smoking increases the risk of SARS-CoV-2 infection and subsequent disease (COVID-19) and related mortality. Available evidence regarding the impact of smoking on disease progression and death amongst COVID-19 patients is conflicting. A large study based on electronic health records from the United Kingdom identified a counter-intuitive lower risk of COVID-19 mortality amongst smokers than ex-smokers.1 A smaller French study also suggests there were fewer smokers among COVID-19 patients than the general French population.2 These findings are in contrast with two systematic reviews of 19 and 47 studies of COVID-19 patients which came to the conclusion that there is a significant association between smoking and progression of COVID-19 amongst infected patients.3,4 Further studies of a cohort of 2.4 million app users have also shown how smoking increases risk of hospitalization, and severity.5 This highlights that the study of the association between smoking and COVID-19 morbidity and mortality is complex and requires further investigation.6
There are currently several pressing issues related to smoking and COVID-19 that need to be resolved. First, there is a need to disentangle the risks of smoking and COVID-19 morbidity and mortality. Smokers may be more or less likely to become infected than never smokers or previous smokers. Once infected the chance of survival may also differ between smokers, never smokers and previous smokers. Also, we do not know whether the impact of smoking differs in men and women or in younger versus elderly people.
In this study, we used data from the UK biobank cohort which is one of the largest study samples including reliable information on smoking status, COVID-19 infection, and mortality in the Spring of 2020. Our aim was to examine the association between smoking and COVID-19 infection, and the association between smoking and COVID-19 mortality among those infected.
Materials and Methods
Data Sources
We used data from the UK Biobank study and include all England participants who were alive on 1 February 2020 and had given permission to use their data by 7 February 2020. UK Biobank includes data from all four countries, Wales, Scotland, England, and Northern Ireland, but COVID-19 test data were only available for England. We used information on participants which were collected via questionnaires, physical measurements and interviews in baseline assessments (2006–10).7,8
Study Population and Follow-Up
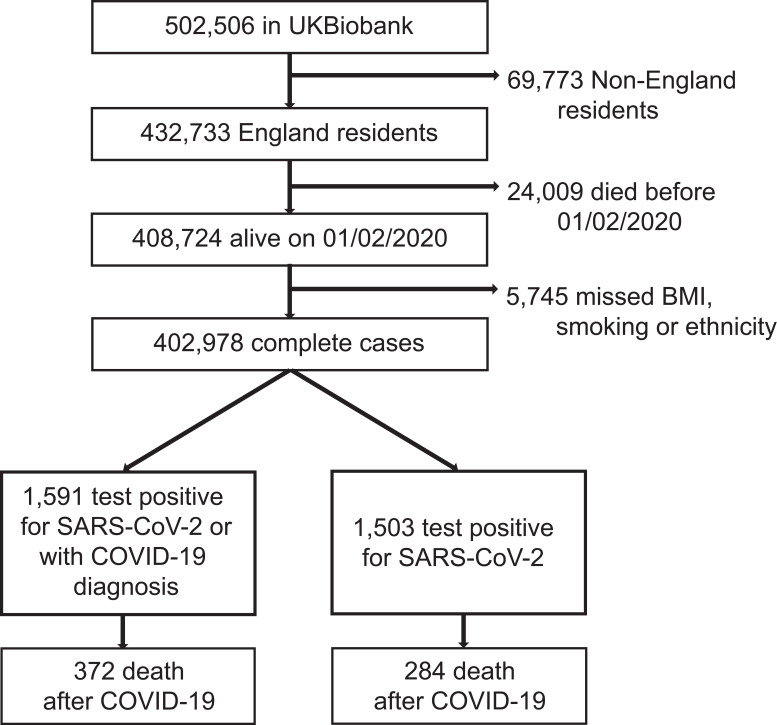
Of the 502,655 UK Biobank participants, 408,724 (81.31%) were eligible for inclusion in the study. However, 5746 (1.40%) participants were excluded due to missing information on body mass index, smoking or ethnicity and 402,978 were included in the regression analyses. The population selection process is shown in Figure 1.
Figure 1.
Selection process of eligible participants in UK.
Participants were followed-up until death or the end of the study (28/06/2020). The analysis of COVID-19 morbidity was based on the full cohort. The analysis of mortality was based on the cohort of people who had confirmed or suspected COVID-19 infection (n=1591).
Study Outcomes
This study included two separate outcomes: 1) COVID-19 infection, 2) death with COVID-19. COVID-19 infection was defined based on a SARS-CoV-2 positive PCR test or having COVID codes on death registry. PCR test information was retrieved from UK Biobank linkage to Public Health England COVID-19 test data.9 We obtained data on who was tested and test results. Patients were considered positive if one or more of the tests performed were positive for SARS-CoV-2. Death data was provided to UK Biobank by NHS Digital from linkage with NHS Central Register (NHSCR). Patients were considered to have died with COVID-19 if they died after a positive test or had a codified COVID cause of death.
Exposures and Measurements
The main exposure variable was smoking status. This information was collected during the UK Biobank recruitment visits (2006–2010). Smoking status is based on two questions: “Do you smoke tobacco now?” and “In the past, how often have you smoked tobacco?”. These questions were recoded into a single variable with the following categories: Current, previous, never and prefer not to answer. Sex and year of birth were acquired from the National Health Service Central Register (NHSCR) at recruitment. Information on ethnicity was classified using Office of National Statistics groups into Black/Black British, White, and other groups. Socioeconomic status was based on the index of multiple deprivation (IMD) and derived from the place of residency. IMD England 2010 index, rank, and deciles were used to stratify participants into IMD quintiles.
We identified data from linked Hospital Episodes Statistics (HES) on a number of chronic illnesses and other conditions which have previously been considered to be associated with COVID-19 morbidity and mortality, hypertensive disease, diabetes mellitus, ischemic heart diseases, other forms of heart disease including heart failure, chronic lower respiratory diseases (COPD or asthma), and renal failure (see Supplemental Table 1).
Permission to Use of the Data
This research was conducted using the UK Biobank Resource under Application Number 46,228. Although the original application was unrelated to COVID-19 work, an exception was made to allow these linked data to be used for COVID-19 research without further applications, to maximize the speed of the proposed study.
Statistical Analysis
We calculated the proportion of never smokers, previous smokers and current smokers for each category of baseline characteristics for the full cohort and for the cohort who became infected with COVID-19. Patients with missing data or a “Prefer not to Answer” response were subsequently excluded in the regression analyses.
We fitted multivariable Poisson models. The first model to estimate the incidence risk ratios (IRR) of COVID-19 infection according to smoking status and the second to estimate the IRR of death amongst those infected. We produced non-adjusted models as well as models adjusting for confounding including sex, age, deprivation, ethnicity, body mass index (BMI) and all of them. To assess the modification effect of age and sex on the association between smoking exposure and COVID-19 outcomes, we added multiplicative interaction terms to the unadjusted models. We stratified the models by age (below and above the median age 69) and sex where the likelihood ratio test comparing the model with and without the multiplicative interaction terms was statistically significant (2-sided P <.05).
In these analyses, we contrasted: 1) current smokers against never smokers and 2) previous smokers against never smokers. Thus, we used the analyses of previous smokers as a “negative control” analysis. This allowed us to evaluate whether smoking per se increases the risk of COVID-19 infection and subsequent mortality or other factors may be “at play”.
We considered whether to include chronic illnesses/conditions in our regression analyses, but decided against it as many of these are likely to be on the causal pathway between smoking and the outcome rather than actual confounding the associations between smoking and COVID-19 infection and COVID-19 mortality.
Finally, we conducted a sensitivity analysis with only those who tested positive. The results of this analysis are reported in Supplemental Table 2.
Data management was performed in Python 3.7.6. All analyses were performed in STATA version 15.1.
Results
In the full cohort (COVID-19 naïve cohort), 224,451 (55.7%), 139,056 (34.5%), and 39,471 (9.8%) reported that they were never smoking, previous smoking and current smoking, respectively (Table 1). The proportion of current smokers declined with age. It was highest among the 49–54 years old (14.5%) and lowest among the 80–86 years old (5.4%) (Table 1). Among the men 11.6% were current smokers while 8.4% of the women were current smokers. In general, many chronic illnesses/conditions were higher among current and previous smokers than never smokers. For example, of those infected with COVID-19 - a 1/3 of the smokers had hypertensive diseases while ¼ of the never smokers had hypertensive diseases (Table 1).
Table 1.
Baseline Characteristics in COVID-19 Naïve and Infection Cohorts Stratified by Smoking Status
| COVID-19 Naïve Cohort | COVID-19 Infection Cohorta | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Never Smoking | Previous Smoking | Current Smoking | Total | Never Smoking | Previous Smoking | Current Smoking | |
| N | 402,978 | 224,451 | 139,056 | 39,471 | 1591 | 771 | 628 | 192 |
| Socio-demographics | ||||||||
| Age | ||||||||
| 49–54 | 24,674 (100.0) | 14,978 (60.7) | 6111 (24.8) | 3585 (14.5) | 121 (100.0) | 77 (63.6) | 32 (26.4) | 12 (9.9) |
| 55–59 | 52,153 (100.0) | 32,310 (62.0) | 12,803 (24.5) | 7040 (13.5) | 266 (100.0) | 158 (59.4) | 73 (27.4) | 35 (13.2) |
| 60–64 | 59,535 (100.0) | 36,085 (60.6) | 16,319 (27.4) | 7131 (12.0) | 193 (100.0) | 110 (57.0) | 59 (30.6) | 24 (12.4) |
| 65–69 | 68,395 (100.0) | 38,599 (56.4) | 22,794 (33.3) | 7002 (10.2) | 182 (100.0) | 91 (50.0) | 65 (35.7) | 26 (14.3) |
| 70–74 | 94,777 (100.0) | 50,476 (53.3) | 36,462 (38.5) | 7839 (8.3) | 262 (100.0) | 107 (40.8) | 111 (42.4) | 44 (16.8) |
| 75–79 | 79,756 (100.0) | 39,646 (49.7) | 34,514 (43.3) | 5596 (7.0) | 398 (100.0) | 150 (37.7) | 208 (52.3) | 40 (10.1) |
| 80–86 | 23,688 (100.0) | 12,357 (52.2) | 10,053 (42.4) | 1278 (5.4) | 166 (100.0) | 75 (45.2) | 80 (48.2) | 11 (6.6) |
| Sex | ||||||||
| Female | 222,790 (100.0) | 134,105 (60.2) | 70,053 (31.4) | 18,632 (8.4) | 756 (100.0) | 439 (58.1) | 231 (30.6) | 86 (11.4) |
| Male | 180,188 (100.0) | 90,346 (50.1) | 69,003 (38.3) | 20,839 (11.6) | 832 (100.0) | 329 (39.5) | 397 (47.7) | 106 (12.7) |
| Ethnicity | ||||||||
| White | 379,697 (100.0) | 208,186 (54.8) | 134,724 (35.5) | 36,787 (9.7) | 1387 (100.0) | 634 (45.7) | 587 (42.3) | 166 (12.0) |
| Mixed | 2505 (100.0) | 1229 (49.1) | 824 (32.9) | 452 (18.0) | 12 (100.0) | 4 (33.3) | 7 (58.3) | 1 (8.3) |
| Asian or Asian British | 8492 (100.0) | 6585 (77.5) | 1109 (13.1) | 798 (9.4) | 68 (100.0) | 48 (70.6) | 10 (14.7) | 10 (14.7) |
| Black or Black British | 7108 (100.0) | 5045 (71.0) | 1233 (17.3) | 830 (11.7) | 86 (100.0) | 62 (72.1) | 14 (16.3) | 10 (11.6) |
| Chinese | 1311 (100.0) | 1045 (79.7) | 170 (13.0) | 96 (7.3) | 7 (100.0) | 5 (71.4) | 2 (28.6) | 0 (0.0) |
| Other ethnic group | 3865 (100.0) | 2361 (61.1) | 996 (25.8) | 508 (13.1) | 28 (100.0) | 15 (53.6) | 8 (28.6) | 5 (17.9) |
| IMD quintiles | ||||||||
| 1 [least deprived] | 120,042 (100.0) | 72,141 (60.1) | 40,530 (33.8) | 7371 (6.1) | 338 (100.0) | 173 (51.2) | 132 (39.1) | 33 (9.8) |
| 2 | 96,479 (100.0) | 55,439 (57.5) | 33,559 (34.8) | 7481 (7.8) | 294 (100.0) | 151 (51.4) | 115 (39.1) | 28 (9.5) |
| 3 | 72,196 (100.0) | 39,995 (55.4) | 25,257 (35.0) | 6944 (9.6) | 285 (100.0) | 132 (46.3) | 118 (41.4) | 35 (12.3) |
| 4 | 62,865 (100.0) | 32,502 (51.7) | 22,215 (35.3) | 8148 (13.0) | 313 (100.0) | 145 (46.3) | 129 (41.2) | 39 (12.5) |
| 5 [most deprived] | 51,396 (100.0) | 24,374 (47.4) | 17,495 (34.0) | 9527 (18.5) | 358 (100.0) | 167 (46.6) | 134 (37.4) | 57 (15.9) |
| BMI | 27.4 (4.7) | 27.1 (4.8) | 27.8 (4.7) | 27.0 (4.7) | 28.8 (5.4) | 28.5 (5.4) | 29.5 (5.3) | 28.0 (5.5) |
| Medical conditions | ||||||||
| Hypertensive diseases | 101,644 (25.2) | 50,863 (22.7) | 41,054 (29.5) | 9727 (24.6) | 459 (28.9) | 193 (25.1) | 200 (31.8) | 66 (34.4) |
| Diabetes mellitus | 26,578 (6.6) | 12,514 (5.6) | 10,946 (7.9) | 3118 (7.9) | 179 (11.3) | 81 (10.5) | 71 (11.3) | 27 (14.1) |
| Ischemic heart diseases | 35,155 (8.7) | 15,681 (7.0) | 15,424 (11.1) | 4050 (10.3) | 182 (11.5) | 69 (9.0) | 87 (13.9) | 26 (13.5) |
| Other heart disease | 38,993 (9.7) | 18,788 (8.4) | 16,403 (11.8) | 3802 (9.6) | 240 (15.1) | 102 (13.3) | 112 (17.8) | 26 (13.5) |
| Chronic respiratory diseases | 42,581 (10.6) | 20,074 (8.9) | 16,391 (11.8) | 6116 (15.5) | 187 (11.8) | 82 (10.7) | 74 (11.8) | 31 (16.1) |
| Renal failure | 15,617 (3.9) | 7362 (3.3) | 6472 (4.7) | 1783 (4.5) | 165 (10.4) | 71 (9.2) | 70 (11.1) | 24 (12.5) |
Note: aSARS-CoV-2 tested positive or COVID-19 hospitalization.
Abbreviations: BMI, body mass index; IMD, the index of multiple deprivation.
During the study period, 1591/402,978 (0.39%) tested positive for COVID-19 or had a death certificate with a COVID-19 cause. Table 2 shows the incidence risk ratios (IRR) for COVID-19 infection and related mortality according to smoking status. In total, 192 (0.49%) current smokers, 628 (0.45%) previous smokers and 771 (0.34%) never smokers were infected with COVID-19. Current smoking was associated with greater risk of COVID-19 infection compared to never smoking across unadjusted [IRR: 1.42 (95% CI, 1.21–1.66)], age-sex adjusted [IRR: 1.38 (1.18–1.1.62)] and socio-demographics adjusted [IRR: 1.27 (1.08–1.50)] models. Previous smoking was similarly associated with an increased risk of COVID-19 infection (Table 2).
Table 2.
Associations of Smoking Status with Covid-19 Infection and Subsequent Death
| Model | Covariates Adjustment | Never[Ref] | Covid-19 Infection a | Never[Ref] | Covid-19 Death b | ||
|---|---|---|---|---|---|---|---|
| Current | Previous | Current | Previous | ||||
| Model 1 | None | 1 | 1.42 [1.21,1.66] | 1.31 [1.18,1.46] | 1 | 1.59 [1.17,2.17] | 1.52 [1.22,1.90] |
| Model 1 + Age | 1 | 1.43 [1.22,1.67] | 1.30 [1.17,1.45] | 1 | 1.50 [1.10,2.05] | 1.16 [0.93,1.45] | |
| Model 1 + Sex | 1 | 1.37 [1.17,1.60] | 1.28 [1.15,1.42] | 1 | 1.52 [1.11,2.07] | 1.41 [1.12,1.76] | |
| Model 1 + Ethnicity | 1 | 1.43 [1.22,1.68] | 1.40 [1.26,1.56] | 1 | 1.60 [1.17,2.18] | 1.51 [1.21,1.89] | |
| Model 1 + IMD | 1 | 1.21 [1.03,1.42] | 1.28 [1.15,1.42] | 1 | 1.59 [1.17,2.17] | 1.53 [1.22,1.91] | |
| Model 1 + BMI | 1 | 1.42 [1.22,1.67] | 1.27 [1.14,1.41] | 1 | 1.61 [1.18,2.20] | 1.50 [1.20,1.87] | |
| Model 1 + Age + Sex | 1 | 1.38 [1.18,1.62] | 1.27 [1.14,1.41] | 1 | 1.46 [1.07,2.00] | 1.10 [0.88,1.39] | |
| Model 1 + Age + Sex + Ethnicity | 1 | 1.40 [1.19,1.64] | 1.34 [1.20,1.49] | 1 | 1.46 [1.07,2.00] | 1.13 [0.90,1.42] | |
| Model 1 + Age + Sex + IMD | 1 | 1.19 [1.01,1.40] | 1.23 [1.10,1.37] | 1 | 1.44 [1.06,1.98] | 1.11 [0.88,1.40] | |
| Model 1 + Age + Sex + BMI | 1 | 1.39 [1.18,1.63] | 1.22 [1.10,1.36] | 1 | 1.49 [1.09,2.03] | 1.08 [0.86,1.36] | |
| Model 2 | Model 1 + all socio-demographics | 1 | 1.27 [1.08,1.50] | 1.26 [1.13,1.40] | 1 | 1.49 [1.09,2.05] | 1.11 [0.88,1.40] |
Notes: aSARS-CoV-2 tested positive or with COVID-19 death cause. bDeath among participants with SARS-CoV-2 tested positive or with COVID-19 death cause. The statistical analysis was based on complete cases. Ethnicity was adjusted with four categories (white, Asian or Asian British, black or black British, others).
Abbreviations: IMD, the index of multiple deprivation; COPD, chronic obstructive pulmonary disease.
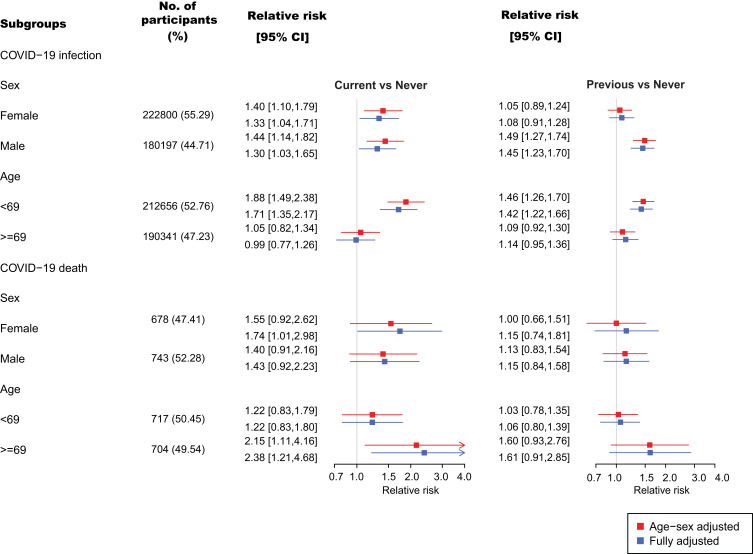
TCRM_A_310463There was an interaction effect of sex (P < 0.001) and age (P < 0.001) with smoking status on the risk of COVID-19 infection. Smokers under the age of 69 years were nearly twice as likely to become infected with COVID-19 than never smokers [adjusted IRR 1.88 (1.49–2.38)] whereas there was no differential risk associated with smoking for participants aged 69+ [age-sex adjusted IRR. 1.05 (0.82–1.34)]. Similar patterns were observed for previous smokers (Figure 2). Among previous smokers, the risk of COVID-19 infection was higher among men than women (Figure 2), but there was no sex difference for current smokers.
Figure 2.
Relative risks of COVID-19 infection and subsequent death by sex and age.
In the 1591 individuals who tested positive for COVID-19 or had a death certificate with a COVID-19 cause, the proportion of smokers was slightly different from the COVID-19 naïve cohort, 771 (48.5%), 628 (39.5%) and 192 (12%) reported that they were never, previous smoking or current smoking, respectively (Table 1). The smoking pattern also presented a reverse “U” shape with age, with fewer individuals smoking in the youngest and oldest group (Table 1). In total 372/1591 (23.4%) of the individuals who were infected with COVID-19 died subsequently. This amounted to 56 (29%) of the current smokers, 175 (27.9%) of previous smokers and 141 (18%) never smokers. Current smoking was associated with greater risk of death compared to never smoking across unadjusted [IRR 1.59 (1.17–2.17)], age-sex adjusted [IRR 1.46 (1.07–2.00)] and socio-demographics adjusted [IRR 1.49 (1.09–2.05)] models (Table 2).
Interaction effect of age (P = 0.031) was also detected for COVID-19 death. Older smokers aged 69 and above were more than twice as likely to die from COVID-19 compared to non-smokers [age-sex adjusted IRR 2.15 (1.11–4.16)]] while the association was less pronounced in those younger than 69 years old [age-sex adjusted IRR 1.22 (0.83–1.79)]. Similar patterns were observed for previous smokers (Figure 2).
A sensitivity analysis of mortality restricted to those with confirmed (test+) COVID obtained similar results for outcomes of COVID-19 infection and related death (Supplementary Table 2).
Discussion
To our knowledge, this is the first study to date investigating the association between smoking and risk of COVID-19 infection. We found that both current and previous smoking were associated with increased risk of COVID-19 infection in those aged below 69 whereas there was no difference between current smokers, previous smokers and never smokers for those aged 69 and above. In contrast, the COVID-19 mortality of smokers aged 69+ were twice as high compared to non-smokers (IRR 2.15 [1.11–4.16]) while there was little or no difference between current smokers and never smokers under the age of 69 (IRR 1.22 [0.83–1.79]). The patterns were similar for previous smokers.
Interpretation and Comparison with Previous Studies
It is well established that smoking can cause a plethora of respiratory diseases including lung cancer,10 asthma,11 pneumothorax,12 and chronic obstructive pulmonary disease.13 There are also some evidence to suggest smoking may contribute to a decline in immunity against infections, although it plays dual roles in both innate and adaptive immune response.14 Li et al found that smoking is associated with a low number and impaired function of regulatory B cells among patients with Helicobacter pylori (H. pylori) infection.15 Also, evidence from an experimental animal model has demonstrated that smoke exposure could reduce effector and memory T cells in mice infected with Mycobacterium tuberculosis.16 Most recently, Alraddadi et al analysed 535 patients with laboratory-confirmed Middle East respiratory syndrome coronavirus (MERS-CoV) infection and identified smoking as an independent risk factor associated with MERS-CoV illness.17 Smoking could also lead to a prolonged viral RNA shedding of SARS-CoV-2, as it happens with other respiratory infections.18 It also seems to promote the expression of genes, such as ACE 2 and TMPRSS2, that facilitate epithelial entry of SARS-CoV-2.19
Social determinants of health and deprivation, often related with smoking, have a big impact on respiratory disease and confound this association. In tuberculosis, for example, socioeconomic factors are associated with therapy failure and drug resistance, and lead to worse outcomes overall.20,21
Our overall findings suggest that there was an association between current smoking and COVID-19 infection. Yet, our stratified analyses suggest that the relationship between smoking and COVID-19 infection is complex. We only found an association between smoking and COVID-19 infection in those aged under 69 and similarly for previous smokers, but not for those aged 69 and above. It, therefore, seems plausible that the increased risk of COVID-19 infection in current and previous smokers was associated with increased risk of exposure to SARS-CoV-2 virus eg via increased occupational exposure rather than increased susceptibility to the virus among smokers.
Previous evidence on the impact of smoking on disease progression and death amongst COVID-19 patients is mixed and based on studies from many different settings.1,3,6
We found that current smoking was associated with a substantial risk of COVID-19 death in those aged 69 years and above (IRR 2.15 1.11 to 4.16). Yet, the risk of COVID-19 death was not much higher in current smokers than never smokers under 69 years (IRR 1.22 0.83 to 1.79). Similar patterns with age were observed for previous smokers. This suggests that the association between smoking and COVID-19 death may be multifaceted. The adverse impact of smoking on COVID-19 death may be due to a direct weakening of the immune system. However, the elevated risk of dying from COVID-19 among older current smokers and previous smokers, but not among those aged below 69 suggest other factors may be at play. For example, we observed that the prevalence of chronic illnesses/conditions such as diabetes, ischemic heart diseases, renal failure, and chronic lower respiratory diseases (COPD and asthma) was higher among current smokers and previous smokers than never smokers. Thus, it is likely that current smoking and previous smoking impact the risk of COVID-19 death indirectly via these chronic illnesses/conditions.
Strengths and Limitations of the Study
Unlike most of the published studies that retrospectively reviewed smoking history amongst hospitalized patients with COVID-19, this is the first population-based study which prospectively examined association between smoking status and risk of being infected by SARS-CoV-2. Despite not fully representative of the whole UK population, participants from UK Biobank are much less prone to significant sampling bias inevitable in hospital-based studies and enables our findings more generalizable to other settings.
Our study has some limitations. First, the identification of COVID-19 infection might be underestimated by using the laboratory-confirmed cases as suggested by the most recent Office for National Statistics.22 However, such underestimation is likely to be non-differential between exposure groups and would bias the genuine association towards the null. Second, the smoking information was collected at baseline between 2006 and 2010 and may have changed by 2020 when participants entered this study. However, it is unlikely that people will start smoking after 40 years old, and therefore misclassification exposure would limit within current and previous smoking groups, such as switching between current smokers and previous smokers. Third, this study was conducted among participants aged 49 years or older. Thus, these findings may not be generalizable to younger people whose immune response may modify the effect of smoking on COVID-19 outcomes, especially given that a noticeable interaction effect of age has been detected.
Clinical Implications
We found that the risk of COVID-19 infection was elevated for both current and previous smokers under the age of 69, but not for those 69 and above. The clinical implication of these findings is that change in smoking habits is unlikely to have major impact on the risk of COVID-19 infection.
Factors such as age, male sex, obesity and chronic conditions/illnesses have been identified as independent risk factors for severe COVID-19 infection and death,1,23 enabling the health professionals more effectively and precisely locate limited medical resources for the treatment of COVID-19. Our study suggests that current and past smoking history should also be taken into consideration when assessing the risk of COVID-19 death in those aged 69 and above. Although smoking on its own may have a limited direct impact on the risk of COVID-19 death both smoking and previous smoking appears be indirect indicators of the elevated risk of dying from COVID-19 via their associations with chronic conditions/illnesses.
Conclusion
The association between smoking and COVID-19 infection and subsequent death is modified by age. Smokers and previous smokers aged under 69 were at higher risk of COVID-19 infection, suggesting the risk is associated with increased exposure to SARS-COV-2 virus. Once infected, older smokers were twice as likely to die from COVID-19 than never smokers, possibly mediated by increased risk of chronic conditions/illnesses.
Funding Statement
The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). DPA is funded through a NIHR Senior Research Fellowship (Grant number SRF-2018-11-ST2-004). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. APU is supported by Fundación Alfonso Martin Escudero and the Medical Research Council (grant numbers MR/K501256/1, MR/N013468/1). The Funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Ethical Approval
The North West Multi-center Research Ethics Committee (MREC) approved the collection and use of UK Biobank data (16/NW/0274). UKBiobank approved the application used to access the data (Application 46,228) and its access to COVID-19 data on 17/04/2020.
Disclosure
Professor Daniel Prieto-Alhambra reports fees for speakers services and advisory board membership from Amgen and fees for consultancy services from UCB Biopharma and Les Laboratoires Servier, outside the submitted work. He reports HTA Funding Committee membership; and Janssen, on behalf of IMI-funded EHDEN and EMIF consortiums, and Synapse Management Partners have supported training programmes organised by DPA’s department and open for external participants. The authors report no other conflicts of interest in this work.
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyara M, Tubach F, Pourcher V, et al. Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. 2020. doi: 10.32388/WPP19W.3 [DOI] [Google Scholar]
- 3.Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;ntaa082. doi: 10.1093/ntr/ntaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy RK, Charles WN, Sklavounos A, et al. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2021;93(2):1045–1056. doi: 10.1002/jmv.26389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkinson NS, Rossi N, El-Sayed_Moustafa J, et al. Current smoking and COVID-19 risk: results from a population symptom app in over 2.4 million people. Thorax. 2021:thoraxjnl-2020-216422. doi: 10.1136/thoraxjnl-2020-216422 [DOI] [PubMed] [Google Scholar]
- 6.Smoking and COVID-19. Available from: https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19. Accessed May 8, 2021.
- 7.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins R. What makes UK biobank special? Lancet. 2012;379(9822):1173–1174. doi: 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- 9.Armstrong J, Rudkin JK, Allen N, et al. Dynamic linkage of COVID-19 test results between public health England’s second generation surveillance system and UK biobank. Microb Genom. 2020;6(7). doi: 10.1099/mgen.0.000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peto R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329. doi: 10.1136/bmj.321.7257.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson NC. Asthma and cigarette smoking. Eur Respir J. 2004;24(5):822–833. doi: 10.1183/09031936.04.00039004 [DOI] [PubMed] [Google Scholar]
- 12.Sahu KK, Mishra AK, Goldman Y. A rare case of pneumopericardium secondary to COVID-19. Heart Lung. 2020;49:679–680. doi: 10.1016/j.hrtlng.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonnesen P, Marott J, Nordestgaard B, Bojesen S, Lange P. Secular trends in smoking in relation to prevalent and incident smoking-related disease: a prospective population-basedstudy. Tob Induc Dis. 2019;17. doi: 10.18332/tid/112459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu F, Liang C-L, Liu H, et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. 2017;8(1):268–284. doi: 10.18632/oncotarget.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Wulan H, Song Z, et al. Regulatory B cell function is suppressed by smoking and obesity in H. pylori-infected subjects and is correlated with elevated risk of gastric cancer. PLoS One. 2015;10(7):e0134591. doi: 10.1371/journal.pone.0134591 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Shang S, Ordway D, Henao-Tamayo M, et al. Cigarette smoke increases susceptibility to tuberculosis—evidence from in vivo and in vitro models. J Infect Dis. 2011;203(9):1240–1248. doi: 10.1093/infdis/jir009 [DOI] [PubMed] [Google Scholar]
- 17.Alraddadi BM, Watson JT, Almarashi A, et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(1):49–55. doi: 10.3201/eid2201.151340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondi A, Castilletti C, Gagliardini R, et al. Risk and predictive factors of prolonged viral RNA shedding in upper respiratory specimens in a large cohort of COVID-19 patients admitted to an Italian reference hospital. Int J Infect Dis. 2021;105:532–539. doi: 10.1016/j.ijid.2021.02.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J, Kasper B, Petersen F, Yu X. Association of cigarette smoking, COPD, and lung cancer with expression of SARS-CoV-2 entry genes in human airway epithelial cells. Front Med. 2020;7:619453. doi: 10.3389/fmed.2020.619453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Gennaro F, Pizzol D, Cebola B, et al. Social determinants of therapy failure and multi drug resistance among people with tuberculosis: a review. Tuberculosis. 2017;103:44–51. [DOI] [PubMed] [Google Scholar]
- 21.Pizzol D, Veronese N, Marotta C, et al. Predictors of therapy failure in newly diagnosed pulmonary tuberculosis cases in Beira, Mozambique. BMC Res Notes. 2018;11:99. doi: 10.1186/s13104-018-3209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coronavirus (COVID-19) infection survey - office for national statistics. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/coronaviruscovid19infectionsurveydata. Accessed May 8, 2021.
- 23.Age, male sex, obesity, and underlying illness emerge as risk factors for severe covid-19 or death BMJ. Available from: https://www.bmj.com/company/newsroom/age-male-sex-obesity-and-underlying-illness-emerge-as-risk-factors-for-severe-covid-19-or-death-finds-largest-cohort-study-to-date/. Accessed May 8, 2021.