Abstract
According to the Centers for Disease Control and Prevention, chronic liver disease and cirrhosis is the 11th leading cause of death in the United States. Common causes of chronic liver disease include alcohol, viral hepatitis, and non-alcoholic steatohepatitis (NASH). Inflammation is a critical driver in the progression of liver disease to liver fibrosis and ultimately cirrhosis. While the severity of chronic liver disease extends over a continuum, the management is more easily differentiated between compensated and decompensated cirrhosis. In this review, we discuss pathophysiology, clinical features and management of common complications of liver cirrhosis based on literature review and the current clinical practice guidelines of the American Association for the Study of Liver Diseases (AASLD).
Keywords: cirrhosis, chronic liver disease, end-stage liver disease, decompensated cirrhosis, compensated cirrhosis, hepatorenal syndrome
Introduction
The spectrum of chronic liver disease involves liver inflammation, fibrosis and cirrhosis. While fibrosis was previously viewed as an irreversible disease process, further understanding of pathophysiology and risk factors shows that liver regeneration and regression of fibrosis is possible. Once the diagnosis of liver cirrhosis is established, it is important to distinguish between compensated and decompensated disease. Patients with compensated liver cirrhosis are often asymptomatic and possess indolent disease. Decompensated liver cirrhosis is characterized by complications of portal hypertension including ascites, spontaneous bacterial peritonitis, hepatic encephalopathy and variceal bleeding. The transition from compensated to decompensated cirrhosis occurs at a rate of 5% per year and can be accompanied by a reduction in median survival time from 12 years to 2 years.1 In this review, we discuss the pathophysiology, clinical features and management of common complications of liver cirrhosis based on literature review and the current clinical practice guidelines of the AASLD.
Pathophysiology of Liver Fibrosis
The process of liver fibrosis begins with hepatocytes undergoing cell death in response to liver injury.2 This leads to activation of peri-sinusoidal hepatic stellate cells, which transform into myofibroblasts that begin depositing an excess of fibrous extracellular matrix into the space of Disse and portal tracts.3–5 This abnormal healing process is further driven by inflammatory cytokines and reactive oxygen species.4,5 The new thickened and congested space of Disse separates hepatocytes from sinusoidal blood flow.3 This results in islands of regenerative hepatocytes surrounded by fibrous tissue. Initially, liver fibrosis remains limited to periportal or perivenular regions. As chronic injury promotes persistent fibrous formation, fibrosis can expand to bridge across lobules, between portal regions, and/or between portal regions and central veins.6 As cirrhosis progresses, hepatic vasculature becomes distorted leading to hepatic congestion, poor venous flow, and increased portal pressure. Of note, histologic patterns of regenerative nodules, parenchymal loss, and patterns of scarring may differ based on the underlying disease.2,3
Assessment and Monitoring of Chronic Liver Disease
From a laboratory perspective, there are multiple scoring methods that utilize specific serum markers to screen for liver fibrosis and cirrhosis. APRI (AST-Platelet Ratio Index) and FIB-4 (Fibrosis-4) are most commonly used in Hepatitis B, Hepatitis C, and HIV/Hepatitis C. An APRI score >1 has 76% sensitivity and 72% specificity for cirrhosis.7,8 Similarly, a FIB-4 score >3.25 has a positive predictive value of 82% for advanced fibrosis and a specificity of 98% for cirrhosis.8 (Table 1) Overall, these scores are useful for physicians to evaluate disease progression and identify patients with liver cirrhosis.9,10 Imaging in the form of ultrasound elastography (Fibroscan) has also been utilized as a non-invasive method to assess the amount of liver stiffness. (Table 2) Ultrasound elastography has been most validated in chronic hepatitis C, chronic hepatitis B, and alcoholic liver disease with respective recommended cut-off values for detecting cirrhosis of 12.5kPa, 11kPa, and 12.5kPa.11 In NASH, a score of 14.9 is correlated with 90% specificity for ruling-in cirrhosis.12 Table 2 shows the general ranges correlating to fibrosis that have been accepted irrespective of underlying chronic liver disease.13 Furthermore, two predictive models are used to determine the prognosis of patients with liver cirrhosis, the Child-Pugh and MELD (model for end-stage liver disease) score. The Child-Pugh score is based on the degree of ascites, concentration of bilirubin and albumin, prothrombin time and degree of encephalopathy. It is associated with the grade of liver dysfunction and the likelihood of developing complications of liver cirrhosis. Patients with a score of 5 or 6 have Child-Pugh A cirrhosis (well compensated), those with a score of 7 to 9 have Child-Pugh B cirrhosis (significant hepatic dysfunction), and those with a score of 10 to 15 have Child-Pugh C cirrhosis (decompensated cirrhosis). The MELD score includes creatinine, bilirubin and prothrombin time. It is predominantly used to predict 30-day mortality rates in patients with liver cirrhosis and prioritize patients for liver transplantation.
Table 1.
Non-Invasive Evaluation of Liver Fibrosis/Cirrhosis
| Scoring System | Formula | Cirrhosis |
|---|---|---|
| APRI | 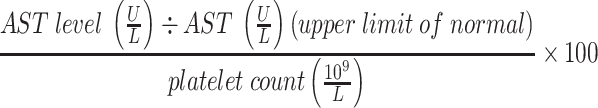 |
Score >1 |
| FIB-4 | 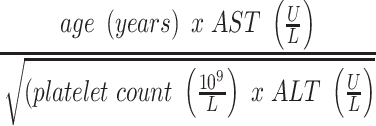 |
Score >3.25 |
Table 2.
Liver Stiffness Scores in Ultrasound Elastography
| Metavir Score119 | F0-F1 (Normal-Mild) | F2 (Mild-Moderate) | F3 (Moderate) | F4 (Severe) |
|---|---|---|---|---|
| Liver stiffness (kPa) | 2.5–7 | 7–9.5 | 9.5–12.5 | 12.5–75 |
Clinical Features of Cirrhosis
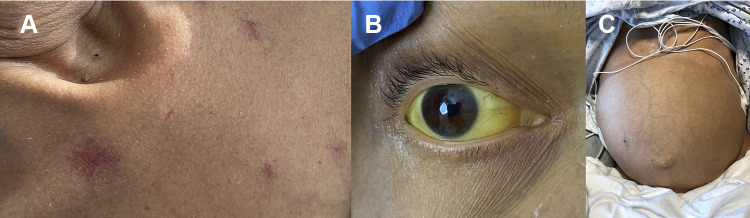
There are several signs of advanced liver disease that can be visualized on physical examination including palmar erythema, spider nevi (Figure 1A), gynecomastia, scleral icterus (Figure 1B), decreased body hair, and testicular atrophy. Palmar erythema is characterized by reddening of the thenar and hypothenar eminences. Spider nevi and gynecomastia are related to increased plasma levels of estrogen.14 Jaundice results from biliary pigmentation within tissue and is observed in the sclera with bilirubin levels as low as 2.5 mg/dL. Additional findings such as caput medusae, hemorrhoids, and splenomegaly can be observed in the setting of increased portal pressure.2,14
Figure 1.
Clinical features of cirrhosis. (A) Spider nevi. (B) Severe scleral icterus. (C) Ascites.
Bleeding Disorders and Coagulopathy in Cirrhosis
Patients with cirrhosis possess a hemostatic imbalance with an increased risk of bleeding and a concomitant risk of thrombosis. Patients are at risk of bleeding due to coagulation factor deficiencies, thrombocytopenia, platelet dysfunction, and an altered fibrinolytic system. The deficiency in coagulation factors predominantly affects the vitamin K dependent factors: II, VII, IX, and X.15,16 Splenic sequestration and decreased levels of thrombopoietin production results in thrombocytopenia. Moreover, fibrinolysis occurs due to accelerated intravascular coagulation and fibrinolysis (AICF), which causes premature clot dissolution.17 Conversely, deficiencies in Protein C and S with elevations in the von Willebrand factor can lead to a hypercoagulable state. Elevated levels of endothelial-derived von Willebrand factor and endothelial-derived factor VIII also promote hypercoagulability and may contribute to the progression of liver disease by thrombosis of small vessels within the liver. This can result in ischemia and atrophy in a process known as parenchymal extinction.15,18,19
Assessment of bleeding risk in patients with cirrhosis prior to high-risk procedures is complex and requires collaboration between different specialists.20 Newer guidelines are emerging for the best practice in coagulation management for cirrhosis patients.21 Currently, prior to high-risk procedures, platelets should be between 30 and 50 × 109/L.21 Pending discussions with specialists, the INR may need to be corrected with vitamin K. Fresh frozen plasma for correction of an elevated INR is not recommended.21 Low fibrinogen levels can indicate the severity of liver disease, and the role of cryoprecipitate in reducing bleeding outcomes is being studied.22 For elective procedures, thrombopoietin agonists have been approved.15 Second-generation agents, avatrombopag and lusutrombopag, result in a more modest increase in platelets when compared to first-generation agents and avoid thrombotic complications.23–26 Lastly, anti-fibrinolytic therapy should be considered in cases where there is bleeding from mucosal surfaces or puncture wound sites that are exposed to saliva and ascites as these substances accelerate fibrinolysis.
Esophageal Varices
Progression of liver fibrosis can lead to worsening portal hypertension and formation of portosystemic venous shunts including esophageal varices, gastric varices and hemorrhoids. Varices develop at a rate of 5% per year with significantly higher rates in patients with decompensated cirrhosis.27 They are present in roughly 85% of patients with decompensated cirrhosis and a hepatic venous pressure gradient (HVPG) of more than 10 mmHg.28,29 The risk of variceal hemorrhage is dependent on variceal size (small varices less than 5 mm versus large varices greater than 5 mm), the presence of red wale marks or cherry-red spots on varices and the severity of liver dysfunction as indicated by the Child-Pugh Score.30–32 Cirrhotic patients should undergo diagnostic endoscopy to document the presence or absence of varices and to determine their risk for variceal hemorrhage. Of note, the BAVENO VI Consensus Workshop defined criteria to identify patients with compensated cirrhosis in whom endoscopy can be avoided. These include liver stiffness <20 kPa by transient elastography and a platelet count >150,000/mm.3,33 Based on the individual risk of variceal hemorrhage, primary prophylaxis with non-selective beta-blockers and/or endoscopic band ligation may be indicated.30 (Figure 2) The use of non-selective beta-blockers reduces portal pressure and the risk of variceal hemorrhage. Carvedilol is commonly preferred due to intrinsic anti-alpha-1 receptor activity and greater portal pressure reduction, but other non-selective beta blockers such as propranolol and nadolol can also be used.34
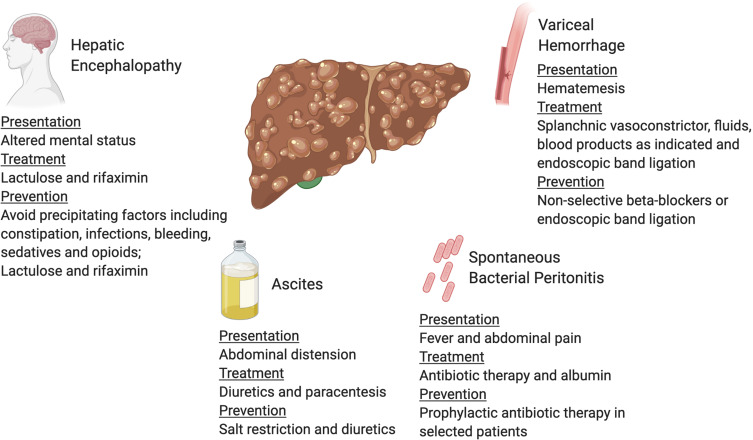
Figure 2.
Complications of decompensated cirrhosis. Image created with the BioRender software.
Esophageal Variceal Hemorrhage
A serious and life-threatening complication of worsening portal hypertension is esophageal variceal hemorrhage. (Figure 3A) Active variceal bleeding is associated with a 6-week mortality rate greater than 15%.28,33,35,36 Patients at greatest risk for hemorrhage have large esophageal varices (>5 mm) with high wall tension. Additional sources of bleeding in portal hypertension include gastric varices and portal hypertensive gastropathy.35 Patients with esophageal variceal hemorrhage require escalation of care to an intensive care unit for closer monitoring of hemodynamics and consideration of intubation for airway protection. The initial steps in the management include establishing large bore intravenous access, fluid resuscitation with crystalloid fluids and checking blood type for transfusions to achieve a hemoglobin level of 8 g/dL. (Table 3) Higher hemoglobin levels are counterproductive as over transfusion may worsen portal hypertension and outcomes.33,37 Patients with severe coagulopathy and thrombocytopenia may benefit from platelet transfusions. Vasoactive therapy with octreotide, a splanchnic vasoconstrictor, should be initiated before endoscopic evaluation to decrease the incidence of active bleeding during endoscopy. Splanchnic vasoconstrictors should be continued for 5 days to reduce the risk of re-bleeding.38,39 The final goal is to control the bleeding with endoscopic band ligation.28,36,40 (Figure 3B) Salvage therapy for uncontrolled bleeding includes balloon tamponade if the creation of a transjugular intrahepatic portosystemic shunt (TIPS) is intended within the next 24 hours.41,42 Patients with a HVPG greater than 20 mmHg are at greatest risk of uncontrolled bleeding, early re-bleeding and death.28 For secondary prophylaxis of variceal hemorrhage, combined therapy with non-selective beta-blockers and endoscopic band ligation is recommended.30,43
Figure 3.
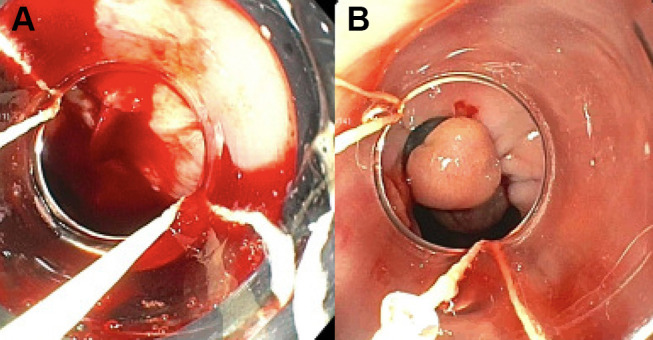
Esophageal varices. (A) Bleeding varix. (B) Bleeding varix status post banding.
Table 3.
General Recommendations for Common Complications of Decompensated Cirrhosis
| Complication | Treatment |
|---|---|
| Esophageal variceal hemorrhage |
|
| Ascites |
|
| Spontaneous bacterial peritonitis (SBP) |
|
| Hepatic encephalopathy |
|
| Hypervolemic hyponatremia |
|
| Hepatorenal syndrome |
|
Patients with variceal hemorrhage are also at high risk for infection, such as pneumonia, spontaneous bacterial peritonitis and urinary tract infection.44 A meta-analysis has shown that antibiotic prophylaxis with ceftriaxone for 7 days decreases the risk of infection, helps control bleeding and improves overall survival45
Portal and Deep Vein Thrombosis
Portal vein thrombosis (PVT) is commonly seen in patients with advanced cirrhosis due to increased venous stasis in the portal vein.46 A hypercoagulable workup is typically not required in patients with no prior history of clotting.15 Anticoagulation should be provided to symptomatic patients and liver transplant candidates to promote recanalization and prevent progression.15 Intravenous heparin and low-molecular weight heparin are options for acute portal vein thrombosis, and direct anticoagulation (DOAC) agents are considered safe and effective options for chronic PVT in early stages of cirrhosis. In those patients with advanced end-stage liver disease, the risk to benefit comparison must be considered prior to initiating anticoagulation therapy. Prior to the initiation of anticoagulation, patients should be screened and treated for esophageal varices if necessary.47 A recent systematic review and meta-analysis found that up to 50% of patients treated for 6 months with anticoagulation had complete recanalization of the portal vein.48 Moreover, patients with cirrhosis are also at increased risk of venous thromboembolism.49,50 Based on clinical evidence, DVT prophylaxis should be provided to patients with liver cirrhosis who are hospitalized and not actively bleeding.15,51,52
Fluid Complications in Cirrhosis
Ascites
Ascites is defined as overt abdominal distention caused by the accumulation of fluid in the peritoneal cavity. (Figure 1C) It is the most common complication of cirrhosis with approximately 50% of patients developing ascites within 10 years of diagnosis.53,54 Mild ascites is only detectable by ultrasound, while moderate to severe ascites can cause overt abdominal distension. First-line treatment is sodium restriction to less than 2 g per day, while fluid restriction can additionally be recommended if a patient has concurrent hyponatremia. (Table 3) Next steps in management include the initiation of diuretics that usually consist of furosemide and spironolactone. Compared to other fluid overload syndromes, the use of spironolactone is essential as it will block hyperaldosteronism in cirrhosis. Diuretics are often started at 40 mg furosemide and 100 mg spironolactone daily doses and titrated using a 1:2.5 ratio.55,56 Patients who become intolerant or refractory to diuretics due to renal dysfunction may require serial large volume paracentesis (LVP) and/or consideration of a transjugular intrahepatic portosystemic shunt (TIPS).54 Once a patient receives a LVP it is important to re-expand intravascular volume with albumin (recommended dose is 6–8 g of albumin per liter of fluid removed if ≥ 5 liters are removed) to prevent paracentesis induced circulatory dysfunction (PICD), a complication that arises due to decreased vascular resistance and hypovolemia with subsequent activation of the renin-angiotensin-aldosterone and sympathetic system.57
Spontaneous Bacterial Peritonitis
Cirrhosis patients with fever, abdominal pain, and acute kidney injury should be evaluated for spontaneous bacterial peritonitis (SBP), an infection in the ascites fluid.58 (Figure 2) There are specific cirrhosis-associated alterations of the immune system that make these patients highly susceptible to infection.59,60 SBP is diagnosed by performing paracentesis and examining the fluid for a neutrophil cell count and bacterial culture. The criterion for diagnosis is a neutrophil count ≥ 250 cells/mm3 and/or a positive bacterial culture.61,62 Initial treatment for SBP includes empiric broad-spectrum antibiotics, preferably an intravenous third-generation cephalosporin until there is speciation of culture studies and antibiotic susceptibilities available.63 (Table 3) Albumin is recommended on day 1 (1.5 g/kg) and 3 (1 g/kg) based on a landmark trial showing a significant decrease in mortality from 29% to 10% with albumin administration.64 Albumin also decreases the risk of renal failure as studies have found that albumin infusion is particularly effective in patients with a serum creatinine ≥ 1 mg/dl and a serum bilirubin ≥ 4 mg/dl.65 Following an episode of SBP, patients should be on secondary prophylaxis with options including ciprofloxacin, norfloxacin, and bactrim to decrease risk of recurrence and mortality.66,67 (Table 3) Primary prophylaxis of SBP is indicated in patients with an ascites fluid protein lower than 1.5 g/dL and at least one of the following: Child-Pugh score ≥ 9 and serum bilirubin level ≥3 mg/dl, creatinine >1.2 mg/dL, blood urea nitrogen ≥ 25 mg/dL or serum sodium ≤ 130.62,68,69 It is also noteworthy that proton pump inhibitors, frequently used in patients with cirrhosis, may increase the risk of SBP and should be restricted to those with a clear indication.70
Hepatic Hydrothorax
Respiratory function can become compromised in about 5% of patients with decompensated cirrhosis by a condition known as hepatic hydrothorax.62 This condition is associated with a large pleural effusion, typically right sided in 85% of patients, due to passage of peritoneal fluid through small defects in the diaphragm.62,71 (Figure 4) Management is similar to ascites which includes sodium restriction and diuretics. Thoracentesis is indicated for symptomatic patients and if spontaneous bacterial empyema is suspected. Insertion of a chest tube is contraindicated due to infectious complications and poor wound healing.62
Figure 4.
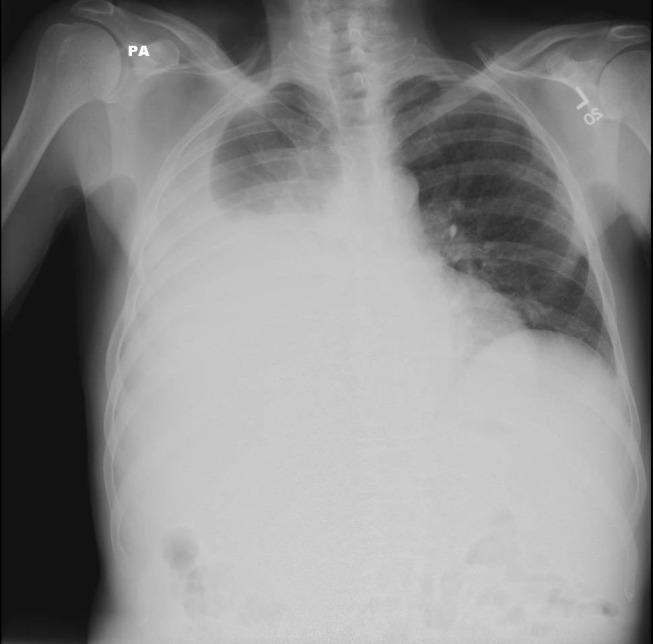
Chest Radiograph Demonstrating Hepatic Hydrothorax.
Hyponatremia
Portal hypertension and splanchnic vasodilation, secondary to the release of nitrous oxide from endothelial cells, results in decreased effective circulatory volume. This decreased volume mimics a hypovolemia setting which activates the renin-angiotensin-aldosterone pathway, activates the sympathetic nervous system, and releases anti-diuretic hormone.72 Symptomatic hyponatremia with confusion, nausea, vomiting, gait disturbance and muscle cramping may occur if sodium levels fall below 125 mEq/L.73 Severe hyponatremia has been associated with worse outcomes and often coincides with refractory ascites, frequent large volume paracentesis, hepatic encephalopathy, SBP, hepatorenal syndrome (HRS) and acute on chronic liver failure.74–78 Initial therapies include fluid restriction (1.5–2 liters per day), discontinuation of diuretics, administration of albumin, and the use of hypertonic saline in severe and symptomatic cases.72 (Table 3) Vaptans are not recommended for correction of hyponatremia due to reports of drug induced liver injury.76
Hepatic Encephalopathy
Hepatic encephalopathy (HE) is a clinical diagnosis associated with brain dysfunction due to liver insufficiency, portal hypertension, portosystemic shunting, and astrocyte swelling.79 Almost a quarter of patients develop HE within 5 years after the diagnosis of liver cirrhosis.80,81 HE presents within a spectrum of non-specific neurologic and psychiatric manifestations.82 According to the severity of manifestations, HE can be classified as follows:79
Minimal: Detected only during neuropsychiatric testing.
Grade 1: Patient is oriented to person, place, time and situation but shows psychomotor retardation (slowed speech, decreased movement, impaired cognitive function).
Grade 2: Patient is disoriented to time and presents with asterixis (characterized by flapping hand tremor).
Grade 3: Patient is somnolent with disorientation to person, time, place and situation.
Grade 4: Patient is comatose with lack of response to painful stimuli.
It is important to note that while ammonia is a surrogate marker of HE in patients with acute liver failure, blood ammonia levels poorly correlate with the degree of HE in patients with chronic liver disease and cirrhosis.83 Thus, testing for blood ammonia levels has no diagnostic value and is not recommended.
HE can occur spontaneously or by precipitating conditions including infection, bleeding, diuretic overdose, electrolyte disorders and constipation.84 (Figure 2) It is critical to identify the existence of precipitating factors because almost 90% of patients can be treated successfully by solely correcting the underlying cause.84,85 Treatment and prevention of HE typically involves lactulose, a non-absorbed disaccharide laxative, that promotes the predominance of non-urease-producing bacteria in the gut microbiome, leading to decreased colonic ammonia production and absorption.86 (Table 3) Patients are advised to aim for three to four soft stools per day.79,87 An adjunct medication for secondary prevention is rifaximin, a non-absorbed antibiotic used to decrease the enteric bacterial flora.79,88,89 Combined treatment with lactulose and rifaximin has been shown to reduce the frequency of hospitalization.89
Hepatorenal Syndrome
Hepatorenal syndrome (HRS) is a serious complication of acute or chronic liver disease. Patients with decompensated cirrhosis have a 18% and 40% probability of developing HRS at 1 and 5 years, respectively.90 HRS is a diagnosis of exclusion fulfilling the following major criteria: cirrhosis with ascites, serum creatinine greater than 1.5 mg/dL, no improvement of serum creatinine (≤ 1.5 mg/dL) after discontinuing diuretics and providing a 3 day albumin challenge (1g/kg, max daily is 100g), absence of shock or exposure to nephrotoxic drugs, and absence of parenchymal kidney disease.91 Based on practice guidelines, the recommended management of HRS includes albumin, midodrine and octreotide. (Table 3) This regimen has shown significant reduction in mortality.92 For patients in the intensive care unit, norepinephrine or vasopressin can be used instead of midodrine. The goal of these therapies is to improve kidney function by promoting renal blood flow.
Hepatocellular Carcinoma
The incidence of hepatocellular carcinoma (HCC) in cirrhosis is between 1 and 8% per year.93 According to the 2018 AASLD guidelines, patients with Child-Pugh A or B cirrhosis should be routinely screened with an ultrasound every 6 months with or without obtaining an alpha fetoprotein level (AFP).93 Lesions ≥ 10 mm and an AFP >20 ng/mL should prompt further evaluation with non-invasive diagnostic imaging such as a multiphase CT or MRI. The Liver Imaging Reporting and Data System (LI-RADS), a standardized reporting and classification system created by the American College of Radiology, is integrated into the AASLD non-invasive diagnostic criteria for HCC. Imaging features diagnostic for definitive HCC are those consistent with a LI-RADS 5 lesion. A lesion ≥ 20 mm is classified as LI-RADS 5 if there is arterial-phase hyperenhancement and one or more of the following: size increase in a mass by ≥ 50% in ≤ 6 months (threshold growth), enhancing capsule or non-peripheral washout. A lesion between 10 and 19 mm meets criteria for a LI-RADS 5 lesion if at least two of the former criteria are met, or there is arterial-phase hyperenhancement with either non-peripheral washout or threshold growth.
Treatment options for HCC include local ablation with ethanol or acetic acid, radiofrequency ablation, microwave ablation, laser, and cryotherapy. Surgical resection and orthotopic liver transplantation represent additional treatment options. According to the Barcelona Clinic Liver Cancer staging system, treatment options for very early HCC (Stage 0) characterized by one nodule < 2 cm include local resection, radiofrequency (RFA), and microwave ablation (MWA). For early HCC (Stage A), classified as one nodule or up to three nodules less than 3 cm, treatment with all modalities can be considered. However, local resection is favored for patients with intact liver function and lack of clinically significant portal hypertension. Frequent surveillance imaging following resection should be conducted in the first year, as the risk of HCC recurrence is highest in the first year and then at least every 3–6 months thereafter.93 For patients who are not surgical candidates, RFA is preferred over ethanol ablation based on randomized control studies showing improved survival outcomes.93–95 In terms of thermal ablation therapies, a recent systematic review and meta-analysis showed that MWA is associated with a greater reduction in local tumor progression and is non-inferior in regard to safety when compared to RFA.96 Surveillance imaging following ablation should be obtained at least every 3 months during the first year due to the high risk of recurrence and then every 6 months.93
Liver transplantation is another option for patients within the T1 and T2 Milan criteria classified as having one lesion of less than 5 cm or up to three lesions each between 1 and 3 cm with no evidence of vascular invasion or extra-hepatic metastases. The Milan criteria have been widely accepted for liver transplantation in patients with HCC given the markedly improved recurrence-free and overall survival rates at 4-5 years of close to 75%.97 AASLD currently recommends bridging transplantation with locoregional therapy, such as ablation, transarterial chemoembolization (TACE), transarterial radioembolization (TARE), for patients within the T2 Milan criteria to delay progression and subsequent delisting from the transplant list, as well as for patients with lesions beyond the Milan criteria.97
For a patient with a multinodular liver, intermediate HCC (Stage B), treatment options are locoregional therapy with TACE or TARE. To date, there are conflicting data and a paucity of studies to establish superiority between TACE and TARE methods.13,98,99 Moreover, studies investigating the benefit of systemic therapy with TACE have shown inconsistent results in regard to improved survival and is currently not recommended for intermediate HCC.100–106 For advanced HCC (Stage C), associated with malignant portal vein invasion and/or extrahepatic metastasis, treatment with systemic therapy is available and includes multi-kinase inhibitors, sorafenib or lenvatinib, that are approved by the US Food and Drug Administration (FDA) as first-line agents.107–110 Second line agents include alternative multi-kinase inhibitors, such as regorafenib and cabozantinib, and immunotherapeutic agents such as nivolumab and pembrolizumab that target the programmed cell death-1 receptor.93,111
End-Stage Liver Disease Management
Transjugular Intrahepatic Portosystemic Shunt (TIPS)
TIPS has been used for the past two decades to mitigate the complications of portal hypertension. This shunt provides an alternative path for blood flow through the portal system into the inferior vena cava. The indications for TIPS include refractory ascites, refractory hepatic hydrothorax, uncontrolled esophageal or gastric variceal hemorrhage and uncontrolled bleeding from portal hypertensive gastropathy while on non-selective beta blockers.112 (Table 3) The absolute contraindications for TIPS include congestive heart failure, systemic infection, severe pulmonary hypertension, multiple hepatic cysts, and unrelieved biliary obstruction.112 The MELD score can be used to predict the 3 month mortality risk following TIPS creation, which was its original purpose prior to organ allocation.113 As a clinician, it is important to be mindful of complications that can arise from TIPS. The most common complication is new onset or worsening hepatic encephalopathy (incidence of 20–31%). Another complication is TIPS dysfunction, defined as stenosis or occlusion.112 TIPS patency can be evaluated by Doppler ultrasound and this imaging study should be considered when a cirrhosis patient decompensates. The expanded use of polytetrafluoroethylene (ePTFE)-covered stents has reduced the complications of occlusion and stenosis by maintaining patency of the shunt.112,114–116
Liver Transplant
Liver transplantation should be considered in patients with complications from end-stage liver disease refractory to medical therapy. These include refractory ascites, recurrent blood loss due to portal hypertensive gastropathy, refractory variceal hemorrhage, hepatorenal syndrome, hepatocellular dysfunction with a MELD ≥ 15, refractory hepatic encephalopathy, and non-metastatic HCC.117 Allocation of transplants is regulated by the United Network for Organ Sharing (UNOS) and prioritization of patients is based on a modified version of the MELD score, MELD-Na, as studies have shown that hyponatremia correlates with higher mortality rates on the waiting list.117,118 Identifying and referring patients to transplant centers early is essential for a complete evaluation of their candidacy.
Conclusion
This article reviews the management of patients with liver cirrhosis based on practice guidelines from the American Association for the Study of Liver Diseases and literature review. Patients with compensated cirrhosis are usually asymptomatic and have a greater likelihood of preserving liver function if risk factors are removed and the underlying condition is treated. Progression of fibrosis to cirrhosis may lead to worsening of portal hypertension and complications including variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome, as well as hepatocellular carcinoma. Given the high morbidity and mortality of these complications, it is important to identify and refer these patients early to transplant centers for evaluation of liver transplantation.
Disclosure
The authors have no conflicts of interest.
References
- 1.Liberal R, Grant CR. Cirrhosis and autoimmune liver disease: current understanding. World J Hepatol. 2016;8(28):1157–1168. doi: 10.4254/wjh.v8.i28.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V, Aster A. Robbins Basic Pathology. 10th ed. Elsevier; 2018. [Google Scholar]
- 3.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371(9615):838–851. doi: 10.1016/S0140-6736(08)60383-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J Hepatol. 2015;62(1 Suppl):S15–24. doi: 10.1016/j.jhep.2015.02.039 [DOI] [PubMed] [Google Scholar]
- 5.Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127(1):55–64. doi: 10.1172/JCI88881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Axley P, Mudumbi S, Sarker S, Kuo YF, Singal AK. Patients with stage 3 compared to stage 4 liver fibrosis have lower frequency of and longer time to liver disease complications. PLoS One. 2018;13(5):e0197117. doi: 10.1371/journal.pone.0197117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–736. doi: 10.1002/hep.24105 [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Ann Intern Med. 2013;159(5):372. doi: 10.7326/0003-4819-159-5-201309030-00021 [DOI] [PubMed] [Google Scholar]
- 9.Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21(41):11567–11583. doi: 10.3748/wjg.v21.i41.11567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapper EB, Lok AS. Use of liver imaging and biopsy in clinical practice. N Engl J Med. 2017;377(8):756–768. doi: 10.1056/NEJMra1610570 [DOI] [PubMed] [Google Scholar]
- 11.Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Association CGCotAG. American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology. 2017;152(6):1536–1543. doi: 10.1053/j.gastro.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156–163.e2. doi: 10.1016/j.cgh.2018.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig JM, Zhang D, Xing M, Kim HS. Meta-analysis: adjusted indirect comparison of drug-eluting bead transarterial chemoembolization versus 90Y-radioembolization for hepatocellular carcinoma. Eur Radiol. 2017;27(5):2031–2041. doi: 10.1007/s00330-016-4548-3 [DOI] [PubMed] [Google Scholar]
- 14.Udell JA, Wang CS, Tinmouth J, et al. Does this patient with liver disease have cirrhosis? JAMA. 2012;307(8):832–842. doi: 10.1001/jama.2012.186 [DOI] [PubMed] [Google Scholar]
- 15.O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34–43.e1. doi: 10.1053/j.gastro.2019.03.070 [DOI] [PubMed] [Google Scholar]
- 16.Intagliata NM, Davis JPE, Caldwell SH. Coagulation pathways, hemostasis, and thrombosis in liver failure. Semin Respir Crit Care Med. 2018;39(5):598–608. doi: 10.1055/s-0038-1673658 [DOI] [PubMed] [Google Scholar]
- 17.Joist JH. AICF and DIC in liver cirrhosis: expressions of a hypercoagulable state. Am J Gastroenterol. 1999;94(10):2801–2803. doi: 10.1111/j.1572-0241.1999.02801.x [DOI] [PubMed] [Google Scholar]
- 18.Sinegre T, Duron C, Lecompte T, et al. Increased factor VIII plays a significant role in plasma hypercoagulability phenotype of patients with cirrhosis. J Thromb Haemost. 2018;16(6):1132–1140. doi: 10.1111/jth.14011 [DOI] [PubMed] [Google Scholar]
- 19.Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21(5):1238–1247. [PubMed] [Google Scholar]
- 20.Intagliata NM, Argo CK, Stine JG, et al. Concepts and controversies in haemostasis and thrombosis associated with liver disease: proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost. 2018;118(8):1491–1506. doi: 10.1055/s-0038-1666861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2020. doi: 10.1002/hep.31646 [DOI] [PubMed] [Google Scholar]
- 22.Budnick I, Davis J, Sundararaghavan A, et al. Transfusion with cryoprecipitate for very low fibrinogen levels does not affect bleeding or survival in critically Ill cirrhosis patients. Thromb Haemost. 2021. doi: 10.1055/a-1355-3716 [DOI] [PubMed] [Google Scholar]
- 23.Afdhal NH, Giannini EG, Tayyab G, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367(8):716–724. doi: 10.1056/NEJMoa1110709 [DOI] [PubMed] [Google Scholar]
- 24.Tateishi R, Seike M, Kudo M, et al. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol. 2019;54(2):171–181. doi: 10.1007/s00535-018-1499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terrault NA, Hassanein T, Howell CD, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol. 2014;61(6):1253–1259. doi: 10.1016/j.jhep.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 26.Terrault N, Chen YC, Izumi N, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155(3):705–718. doi: 10.1053/j.gastro.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 27.Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38(3):266–272. doi: 10.1016/s0168-8278(02)00420-8 [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Diseases PGCotAAftSoL, Gastroenterology PPCotACo. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922–938. doi: 10.1002/hep.21907 [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5(3):419–424. doi: 10.1002/hep.1840050313 [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. doi: 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 31.Beppu K, Inokuchi K, Koyanagi N, et al. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27(4):213–218. doi: 10.1016/s0016-5107(81)73224-3 [DOI] [PubMed] [Google Scholar]
- 32.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983–989. doi: 10.1056/NEJM198810133191505 [DOI] [PubMed] [Google Scholar]
- 33.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43(1):167–176. doi: 10.1016/j.jhep.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 34.Li T, Ke W, Sun P, et al. Carvedilol for portal hypertension in cirrhosis: systematic review with meta-analysis. BMJ Open. 2016;6(5):e010902. doi: 10.1136/bmjopen-2015-010902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bendtsen F, Krag A, Møller S. Treatment of acute variceal bleeding. Dig Liver Dis. 2008;40(5):328–336. doi: 10.1016/j.dld.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 36.D’Amico G, De Franchis R, Group CS. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38(3):599–612. doi: 10.1053/jhep.2003.50385 [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Pagán JC, Bosch J. Endoscopic band ligation in the treatment of portal hypertension. Nat Clin Pract Gastroenterol Hepatol. 2005;2(11):526–535. doi: 10.1038/ncpgasthep0323 [DOI] [PubMed] [Google Scholar]
- 38.Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet. 1995;346(8979):865–868. doi: 10.1016/s0140-6736(95)92708-5 [DOI] [PubMed] [Google Scholar]
- 39.Seo YS, Park SY, Kim MY, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60(3):954–963. doi: 10.1002/hep.27006 [DOI] [PubMed] [Google Scholar]
- 40.Bañares R, Albillos A, Rincón D, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology. 2002;35(3):609–615. doi: 10.1053/jhep.2002.31354 [DOI] [PubMed] [Google Scholar]
- 41.Chau TN, Patch D, Chan YW, Nagral A, Dick R, Burroughs AK. “Salvage” transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998;114(5):981–987. doi: 10.1016/s0016-5085(98)00640-4 [DOI] [PubMed] [Google Scholar]
- 42.García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379. doi: 10.1056/NEJMoa0910102 [DOI] [PubMed] [Google Scholar]
- 43.Puente A, Hernández-Gea V, Graupera I, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int. 2014;34(6):823–833. doi: 10.1111/liv.12452 [DOI] [PubMed] [Google Scholar]
- 44.Villanueva C, Escorsell À. Optimizing general management of acute variceal bleeding in cirrhosis. Curr Hepatol Rep. 2014;13(3):198–207. doi: 10.1007/s11901-014-0241-7 [DOI] [Google Scholar]
- 45.Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29(6):1655–1661. doi: 10.1002/hep.510290608 [DOI] [PubMed] [Google Scholar]
- 46.Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31(3):366–374. doi: 10.1111/j.1365-2036.2009.04182.x [DOI] [PubMed] [Google Scholar]
- 47.Ageno W, Beyer-Westendorf J, Garcia DA, Lazo-Langner A, McBane RD, Paciaroni M. Guidance for the management of venous thrombosis in unusual sites. J Thromb Thrombolysis. 2016;41(1):129–143. doi: 10.1007/s11239-015-1308-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology. 2017;153(2):480–487.e1. doi: 10.1053/j.gastro.2017.04.042 [DOI] [PubMed] [Google Scholar]
- 49.Gulley D, Teal E, Suvannasankha A, Chalasani N, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53(11):3012–3017. doi: 10.1007/s10620-008-0265-3 [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8(9):800–805. doi: 10.1016/j.cgh.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 51.Intagliata NM, Henry ZH, Shah N, Lisman T, Caldwell SH, Northup PG. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014;34(1):26–32. doi: 10.1111/liv.12211 [DOI] [PubMed] [Google Scholar]
- 52.Turco L, de Raucourt E, Valla DC, Villa E. Anticoagulation in the cirrhotic patient. JHEP Rep. 2019;1(3):227–239. doi: 10.1016/j.jhepr.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Planas R, Montoliu S, Ballesté B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4(11):1385–1394. doi: 10.1016/j.cgh.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 54.Ginés P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93(2):234–241. doi: 10.1016/0016-5085(87)91007-9 [DOI] [PubMed] [Google Scholar]
- 55.Runyon BA. Care of patients with ascites. N Engl J Med. 1994;330(5):337–342. doi: 10.1056/NEJM199402033300508 [DOI] [PubMed] [Google Scholar]
- 56.Pérez-Ayuso RM, Arroyo V, Planas R, et al. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84(5 Pt 1):961–968. doi: 10.1016/0016-5085(83)90198-1 [DOI] [PubMed] [Google Scholar]
- 57.Kim JH. What we know about paracentesis induced circulatory dysfunction? Clin Mol Hepatol. 2015;21(4):349–351. doi: 10.3350/cmh.2015.21.4.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45(1):223–229. doi: 10.1002/hep.21443 [DOI] [PubMed] [Google Scholar]
- 59.Bolte FJ, Rehermann B. Mucosal-associated invariant T cells in chronic inflammatory liver disease. Semin Liver Dis. 2018;38(1):60–65. doi: 10.1055/s-0037-1621709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60(6):1310–1324. doi: 10.1016/j.jhep.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 61.Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology. 1984;4(6):1209–1211. doi: 10.1002/hep.1840040619 [DOI] [PubMed] [Google Scholar]
- 62.Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–1653. doi: 10.1002/hep.26359 [DOI] [PubMed] [Google Scholar]
- 63.Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5(3):457–462. doi: 10.1002/hep.1840050319 [DOI] [PubMed] [Google Scholar]
- 64.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403–409. doi: 10.1056/NEJM199908053410603 [DOI] [PubMed] [Google Scholar]
- 65.Sigal SH, Stanca CM, Fernandez J, Arroyo V, Navasa M. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56(4):597–599. doi: 10.1136/gut.2006.113050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988;8(1):27–31. doi: 10.1002/hep.1840080107 [DOI] [PubMed] [Google Scholar]
- 67.Ginés P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12(4 Pt 1):716–724. doi: 10.1002/hep.1840120416 [DOI] [PubMed] [Google Scholar]
- 68.Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133(3):818–824. doi: 10.1053/j.gastro.2007.06.065 [DOI] [PubMed] [Google Scholar]
- 69.Saab S, Hernandez JC, Chi AC, Tong MJ. Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short-term survival in cirrhosis: a meta-analysis. Am J Gastroenterol. 2009;104(4):993–1001;quiz 1002. doi: 10.1038/ajg.2009.3 [DOI] [PubMed] [Google Scholar]
- 70.Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64(4):1265–1272. doi: 10.1002/hep.28737 [DOI] [PubMed] [Google Scholar]
- 71.Garbuzenko DV, Arefyev NO. Hepatic hydrothorax: an update and review of the literature. World J Hepatol. 2017;9(31):1197–1204. doi: 10.4254/wjh.v9.i31.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol. 2015;21(11):3197–3205. doi: 10.3748/wjg.v21.i11.3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581–1589. doi: 10.1056/NEJM200005253422107 [DOI] [PubMed] [Google Scholar]
- 74.Angeli P, Wong F, Watson H, Ginès P, Investigators C. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44(6):1535–1542. doi: 10.1002/hep.21412 [DOI] [PubMed] [Google Scholar]
- 75.Jenq CC, Tsai MH, Tian YC, et al. Serum sodium predicts prognosis in critically ill cirrhotic patients. J Clin Gastroenterol. 2010;44(3):220–226. doi: 10.1097/MCG.0b013e3181aabbcd [DOI] [PubMed] [Google Scholar]
- 76.Alukal JJ, John S, Thuluvath PJ. Hyponatremia in cirrhosis: an update. Am J Gastroenterol. 2020;115(11):1775–1785. doi: 10.14309/ajg.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 77.Pereira G, Baldin C, Piedade J, et al. Combination and sequential evaluation of acute-on-chronic liver failure (ACLF) and hyponatremia and prognosis in cirrhotic patients. Dig Liver Dis. 2020;52(1):91–97. doi: 10.1016/j.dld.2019.08.013 [DOI] [PubMed] [Google Scholar]
- 78.Lopes-Secundo TM, Sevá-Pereira T, Correa BR, et al. Serum sodium, model for end-stage liver disease, and a recent invasive procedure are risk factors for severe acute-on-chronic liver failure and death in cirrhotic patients hospitalized with bacterial infection. Eur J Gastroenterol Hepatol. 2018;30(9):1055–1059. doi: 10.1097/MEG.0000000000001184 [DOI] [PubMed] [Google Scholar]
- 79.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210 [DOI] [PubMed] [Google Scholar]
- 80.Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895. doi: 10.1016/s0168-8278(99)80144-5 [DOI] [PubMed] [Google Scholar]
- 81.Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53(5):744–749. doi: 10.1136/gut.2003.020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–721. doi: 10.1053/jhep.2002.31250 [DOI] [PubMed] [Google Scholar]
- 83.Kundra A, Jain A, Banga A, Bajaj G, Kar P. Evaluation of plasma ammonia levels in patients with acute liver failure and chronic liver disease and its correlation with the severity of hepatic encephalopathy and clinical features of raised intracranial tension. Clin Biochem. 2005;38(8):696–699. doi: 10.1016/j.clinbiochem.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 84.Strauss E, da Costa MF. The importance of bacterial infections as precipitating factors of chronic hepatic encephalopathy in cirrhosis. Hepatogastroenterology. 1998;45(21):900–904. [PubMed] [Google Scholar]
- 85.Strauss E, Tramote R, Silva EP, et al. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39(6):542–545. [PubMed] [Google Scholar]
- 86.Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375(17):1660–1670. doi: 10.1056/NEJMra1600561 [DOI] [PubMed] [Google Scholar]
- 87.Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328(7447):1046. doi: 10.1136/bmj.38048.506134.EE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shah NL, Banaei YP, Hojnowski KL, Cornella SL. Management options in decompensated cirrhosis. Hepat Med. 2015;7:43–50. doi: 10.2147/HMER.S62463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081. doi: 10.1056/NEJMoa0907893 [DOI] [PubMed] [Google Scholar]
- 90.Ginès A, Escorsell A, Ginès P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105(1):229–236. doi: 10.1016/0016-5085(93)90031-7 [DOI] [PubMed] [Google Scholar]
- 91.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–1318. doi: 10.1136/gut.2006.107789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52(3):742–748. doi: 10.1007/s10620-006-9312-0 [DOI] [PubMed] [Google Scholar]
- 93.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 94.Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocellular carcinoma: a meta-analysis. J Hepatol. 2010;52(3):380–388. doi: 10.1016/j.jhep.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 95.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49(2):453–459. doi: 10.1002/hep.22648 [DOI] [PubMed] [Google Scholar]
- 96.Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:6407–6438. doi: 10.2147/OTT.S204340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lingiah VA, Niazi M, Olivo R, Paterno F, Guarrera JV, Pyrsopoulos NT. Liver transplantation beyond Milan criteria. J Clin Transl Hepatol. 2020;8(1):69–75. doi: 10.14218/JCTH.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Facciorusso A, Serviddio G, Muscatiello N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: a systematic review and meta-analysis. World J Hepatol. 2016;8(18):770–778. doi: 10.4254/wjh.v8.i18.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–1163.e2. doi: 10.1053/j.gastro.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized Phase III trial. Hepatology. 2014;60(5):1697–1707. doi: 10.1002/hep.27290 [DOI] [PubMed] [Google Scholar]
- 101.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi: 10.1016/j.jhep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 102.Wu FX, Chen J, Bai T, et al. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer. 2017;17(1):645. doi: 10.1186/s12885-017-3545-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9(6):e100305. doi: 10.1371/journal.pone.0100305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Y, Chen B, Liu N, et al. Overall survival in response to sorafenib with transarterial chemoembolization for BCLC stage B hepatocellular carcinoma: propensity score analysis. Int J Clin Pharmacol Ther. 2017;55(6):498–508. doi: 10.5414/CP202787 [DOI] [PubMed] [Google Scholar]
- 105.Zhang X, Wang K, Wang M, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8(17):29416–29427. doi: 10.18632/oncotarget.15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L, Zhao W, Wang M, et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18(1):138. doi: 10.1186/s12876-018-0849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 108.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 109.Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial. Expert Rev Anticancer Ther. 2009;9(6):739–745. doi: 10.1586/era.09.41 [DOI] [PubMed] [Google Scholar]
- 110.Kane RC, Farrell AT, Madabushi R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14(1):95–100. doi: 10.1634/theoncologist.2008-0185 [DOI] [PubMed] [Google Scholar]
- 111.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 112.Boyer TD, Haskal ZJ; Diseases AAftSoL. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2010;51(1):306. doi: 10.1002/hep.23383 [DOI] [PubMed] [Google Scholar]
- 113.Kamath PS, Kim WR; Group ALDS. The model for end-stage liver disease (MELD). Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563 [DOI] [PubMed] [Google Scholar]
- 114.Bureau C, Garcia Pagan JC, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27(6):742–747. doi: 10.1111/j.1478-3231.2007.01522.x [DOI] [PubMed] [Google Scholar]
- 115.Angermayr B, Cejna M, Koenig F, et al. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stent grafts versus bare stents. Hepatology. 2003;38(4):1043–1050. doi: 10.1053/jhep.2003.50423 [DOI] [PubMed] [Google Scholar]
- 116.Rössle M, Siegerstetter V, Huber M, Ochs A. The first decade of the transjugular intrahepatic portosystemic shunt (TIPS): state of the art. Liver. 1998;18(2):73–89. doi: 10.1111/j.1600-0676.1998.tb00132.x [DOI] [PubMed] [Google Scholar]
- 117.Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–1165. doi: 10.1002/hep.26972 [DOI] [PubMed] [Google Scholar]
- 118.Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(6):1652–1660. doi: 10.1053/j.gastro.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 119.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–847. doi: 10.1016/j.jhep.2008.02.008 [DOI] [PubMed] [Google Scholar]