Abstract
Background:
Germline CDH1 mutation carriers are at risk for early-onset diffuse gastric cancer (DGC) and female carriers have an additional risk of lobular breast cancer. The reported literature GC risk of 70% has led to the recommendation for germline mutation carriers to undergo prophylactic total gastrectomy (PTG). The objective of this research was to examine post-surgical clinical outcomes and to identify which of the domains/symptoms from the European Organisation for Research and Treatment of Cancer QOL Questionnaire (EORTC QLQ-C30) were determinants of overall quality of life (QOL) in individuals undergoing PTG.
Methods:
Participants were recruited through multiple sources. Postsurgical clinical outcomes were obtained from hospital records. Participants completed validated questionnaires measuring generic and condition specific QOL (PROMIS, EORTC and SF 36v.II) at a single point in time.
Results:
The mean QOL in this cohort was 70.6 (SD = 25.6), which is comparable to reference values from the general populations in Norway and Sweden. Role and social function plus the symptoms anxiety, pain, taste, dyspnea and diarrhea were significant predictor variables for QOL (p<0.05).
Conclusions:
Although this study reveals good overall QOL for individuals after PTG, attention should be given to managing symptoms as part of long term care to further enhance QOL. The function/symptom scores were associated with worse overall health and global health status and thus may mark a real need for more attentive post-surgical care.
Keywords: Diffuse gastric cancer, total prophylactic gastrectomy, clinical outcomes, quality of life, predictor variables
Introduction
Clinically defined hereditary diffuse gastric cancer (c-HDGC) [OMIM #137215] is characterized by early-onset, multi-generational diffuse gastric cancer (DGC) and lobular breast cancer (LBC).
Approximately 40% of HDGC families have germline mutations in the CDH1 gene (E-cadherin) [ENSG00000039068; OMIM *192090]. Since its implication in HDGC, there have been over 100 different pathogenic germline mutations reported across multiple ethnicities [1]. A recent study of 75 families with pathogenic CDH1 mutations predicted the cumulative incidence of gastric cancer as 70% (95% confidence interval [CI], 40%−94%) for males and 56% (95% CI, 27%−90%) for females by age 80 years. The risk of breast cancer for females was 42% (95% CI, 23%−68%) by 80 years [1].
Identifying individuals who have an increased risk of developing GC allows for cancer prevention strategies to be put into place. Given that DGC is difficult to detect by current screening modalities, prophylactic total gastrectomy (PTG) is considered the treatment of choice for patients with a germline mutation of the CDH1 gene [2]. This surgery is effective in preventing DGC but is associated with long-term complications [3–15] (Table 1). Improving our understanding of patient outcomes through assessment of both functional status and health related QOL (HRQOL) will provide important clues to improve post-surgical management. There are currently only two smaller (32 and 18 participants) studies that document the QOL in HDGC [14,15]. Given that recommendations are being made for carriers to undergo PTG to decrease their mortality from the increased cancer risk, it is imperative that further evidence-based clinical management of these individuals post-surgery.
Table 1.
A literature summary of cases/families and their post-surgical outcomes after PTG.
| Report (year) | No. of cases (# of kindred) | Age at surgery (years) | Sex | Postoperative complications (# affected) | # PTG specimens with signet ring cells | Follow-up (months) |
|---|---|---|---|---|---|---|
| Huntsman et.al. (2001) [3] | 5 (2) | 22–40 | 4 F 2 M | None | 5/5 (100%) | Not provided |
| Lewis et.al. (2001) [4] | 6 (2) | 22–40 | 4 F 2 M | Anastomic stricture (1) Septic phlebitis (1) | Not provided | 18 |
| Chun et. al. (2001) [5] | 5 (1) | 37 – 47 | 3 F 2 M | None | 5/5 (100%) | Not provided |
| Newman & Mulholland (2006) [6] | 2 (1) | 46/35 | Diarrhea (2) Ileus (1) Wound infection (1) | 0 | 6 months | |
| Norton et.al. (2007) [7] | 6 (1) | 51–57 | 4 F 2 M | None | 6/6 (100%) | 12 |
| Hebbard et.al. (2009) [8] | 23 (3) | 26–63 | 14 F 9 M | Subclinical leak (4) Wound infection (4) Venous thromboembolism (3) Anastomotic leak with abscess (2) Pneumonia (2) Urinary tract infection (2) Ileus (1) Intra-abdominal abscess (1) Small bowel obstruction (1) | Not provided | Retrospective medical chart review (surgeries done approx. 12–36 months prior) |
| Hackenson et.al. (2010) [9] | 6 (1) | 21 – 51 | 3 F 3 M | Esophageal stricture (1) Small bowel obstruction (1) | 100% | Not provided |
| Pandalai et.al. (2011) [10] | 10 (6) | 27–51 | 4 F 6 M | SBO (2) Anastomotic stricture (1); Intussusception after 2 years (1) Pulmonary embolism (1) | 9/10 (90%) | > 12 |
| Chen et.al. (2011) [11] | 13 (6) | 18–70 | 9 F 4 M | None reported | 12/13 (92%) | 1–55months (Median 37.2) |
| Li et.al. (2013) [12] | 2 (1) | 32,38 | 2 F | Tachycardia (1) | 0/2 | 35 |
| Bardram (2014) [13] | 6 (2) | 26–45 | 3F 3 M | None reported | 6/6 (100%) | 5–10 |
| Worster et.al, (2014) [14] | 32 (17) | 16–64 | 17 F 15 M | Dysphagia (3) Seizures (2) Adhesions (1) Anastomic leak (1) Dumping/Diarrhea (1) Nausea, vomiting, dumping (1) Postop bleed (1) | 27/28 (96%) | 0 – 24 |
| Muir et.al (2016) [15] | 13 (7) | 23–63 | 11 F 2 M | Anastomotic stricture (3) Incisional hernia (1) Severe dumping (1) | 9/13 (69%) | Median 24 |
| Strong et.al (2017) [16] | 41 (?) | 20–71 | 27 F 14 M | Pulmonary complications (6) Anastomic Leak, esophagus (6) Wound infection (3) Duodenal stump leak (2) Gastrointestinal bleeding ((2) Multiple organ system failure (1) Pulmonary embolus (1) Supraventricular arrhythmia (1) Urinary tract infection (1) | 35/41 (85%) | 1 to 96 months (Median 16 months) |
This study has endeavored to augment the knowledge base and in so doing contribute to the evidence base used to guide decision-making for patients contemplating PTG and their clinical management after surgery. Understanding the characteristics or conditions that predict subsequent QOL may help clinicians identify patients who may be at increased risk of adverse psychosocial problems.
Methods
Study Design and Patient Accrual
This retrospective, multicentre study in which data were collected on one occasion from CDH1 mutation carriers who had undergone PTG was approved by the Behavioural Research Ethics Board of the University of British Columbia (Certificate number H11–00956) and local IRBs in Newfoundland. All participants provided written informed consent. Participants from BC were invited directly by one of the authors (PK). Participants outside BC were recruited through their genetics provider or surgeon. The study was advertised on the National Society for Genetic Counsellors and No Stomach for Cancer websites. Patient accrual was done between 2013 and 2016.
Data Collection Tools
Medical records were reviewed for family history of cancer, CDH1 mutation, postsurgical complications and pathological evaluation of resected stomach. All participants completed four validated questionnaires at one time point. Functional status/condition specific QOL was assessed using the EORTC QOLQ-C30 version 3 [17] and the EORTC STO22 [18] questionnaires. The EORTC QOLQ-C30, a 30-item cancer-specific questionnaire, assesses the QOL of cancer patients within four domains including functional scales, symptom scales, global QOL and single items. There are five functional scales (physical, role, cognitive, emotional and social), three symptom scales (pain, fatigue, nausea/ vomiting), a global health/QOL scale and single items for the evaluation of symptoms (dyspnea, appetite loss, constipation, diarrhea and sleep disturbance) commonly reported by patients with cancer. No item in the instrument occurs more than once so that each of the scales with multi-items has a different set. The QOLQ-STO22 GC module is a 22-item form used together with the EORTC QLQ-C30 to assess QOL in individuals with GC. It includes five multi-scale items: dysphagia, pain, reflux, eating and anxiety and four single items (dry mouth, tasting, body image and hair loss) covering disease and treatment related symptoms and emotional consequences of GC. Both questionnaires demonstrate clinical validity and reliability in GC patients.
Anxiety and depression were measured using instruments from the Patient Reported Outcomes Measurement Information System (PROMIS) bank. The Adult PROMIS Emotional Distress – Anxiety v.1 has 29 items and measures anxiety symptoms over the past seven days using five Likert response categories for each question. The Adult PROMIS Emotional Distress—Depression v.1, a 28-item instrument, measures depressive symptoms and focuses on assessing self-reported negative mood, views of self, social cognition, and a decrease in the positive affect and engagement over the past seven days. The PROMIS-Depression item bank does not measure behavioural and somatic symptoms such as changes in appetite or sleeping patterns which can have confounding effects when assessing patients with comorbid physical conditions [19]. There are five Likert response categories for each question. A question about satisfaction with surgery which was rated on five-point Likert-type scale was also asked.
Data Analyses
Descriptive statistics were used to characterize the sample. Time elapsed between surgery and survey was divided into three groups: (≤12 months), (13–60 months) and (> 60 months) to see if symptoms and functional status improved with time after surgery.
All surveys were examined for completeness, coded in a standardized format and entered into IBM SPSS version 22. All instruments required calculation of scores and were done according to scale authors. Responses from the EORTC QOLQ-C30 and QOLQ-STO22 were linearly transformed to the 0 to 100 scale. A higher score on the functional scales indicates a better QOL, with 100 being a perfect score. The lower a symptom score, the less the symptom burden with 0 being a score indicating no reported symptoms. Missing values for both EORTC QOLQ-C30 and QOLQ-STO22 were handled according to the recommendations in the EORTC-QOQ-C30 scoring manual [20].
The computations for the PROMIS instruments were done within the NIH PROMIS Assessment Center (http://www.assessmentcenter.net/). Each item on the Anxiety and Depression instruments has a range in total score from 8 to 40; higher scores indicate greater severity of the emotional distress for both. A score was approximated if a participant skipped a question. The final score was represented by the T-score, a standardized score with a mean of 50 and a standard deviation (SD) of 10. A score of 50 (SD = 50) is the average for the United States general population.
To examine if any of the demographic variables and symptoms affected the overall QOL of the participants, a linear multiple regression model was fit with QOL as a response variable all variables were entered as predictor variables into R. A full linear model was first ran involving all predictor variables and QOL as the response variable. Model reduction was also performed using a stepwise procedure that optimizes the AIC criterion. A restriction was placed on the parameters within the reduced model.
All analyses, apart from stepwise regression, were performed using SPSS. Stepwise regression was performed in R. The two-sided significance level for all statistical tests was p < 0.05. As this was an exploratory study, no adjustment was made for multiple comparisons.
Results
Participants
The demographic data of the combined sample of 53 individuals, who had undergone PTG between 2004 and 2013, is displayed in Table 2.
Table 2.
Demographic Characteristics of Study Participants (N=53)
| Variable | Number (%) |
|---|---|
| Sex | |
| Male | 14 (26%) |
| Female | 39 (74%) |
| Age at time of surgery | |
| <50 years | 34 (64%) |
| >50 years | 19 (36%) |
| Age at time of survey | |
| <50 years | 31 (58%) |
| >50 years | 22 (42%) |
| Ethnicity | |
| White | 52 (98%) |
| Asian | 1 (2%) |
| Marital status | |
| Married (including common-law) | 41 (77%) |
| Widowed | 0 (0%) |
| Divorced/Separated | 5 (10%) |
| Never Married | 7 (13%) |
| Education | |
| Less than a university bachelor’s degree | 21 (40%) |
| Bachelor’s Degree and above | 32 (60%) |
| Employment | |
| Working | 35 (66%) |
| Homemaker | 6 (11%) |
| Retired | 7 (13%) |
| Student | 2 (4%) |
| Unable to work | 3 (6%) |
| Surgical Type | |
| Open surgery | 47 (89%) |
| Laparoscopic surgery | 6 (11%) |
| Time between surgery and survey (months) | |
| ≤ 12 | 8 (15%) |
| 13 – 60 | 24 (45%) |
| >60 | 21(40%) |
Post-surgical Clinical Outcomes and Complications after PTG
Medical records were received and reviewed for 52 participants; one participant did not provide consent. At least eight kindred were involved, but kindred relationships were not available for most participants. All participants were asymptomatic at the time of surgery. The surgeries were performed in 11 different hospitals by 14 surgeons. All participants had a PTG with stapled esophagojejunal anastomosis (50 – 60cm) and Roux-en-Y reconstruction. Six (12%) of participants underwent laparoscopic total gastrectomy. The mean length of hospital stay was 8.9 days with a median of 7 days (range 4 – 18 days). Length of stay for three of the individuals undergoing laparoscopic TG was 4 – 7 days.
Twenty-three participants experienced post-surgical complications either during the hospital stay or within six months of surgery (Table 3). One participant experienced seven oesophageal strictures, of which four were managed with dilation within the first 12 months. Another participant experienced pleural effusion eight days after surgery and required pleurocentesis. This same individual then developed a clot in the right lung on day 10 and a small wound infection (on day 18). At 12 months post-surgery, this individual needed post-oesophageal dilation for distal and proximal dysphagia. The patient with bile reflux and two of the patients with strictures between 1 −12 months after a laparoscopic procedure.
Table 3. A summary of the postsurgical complications within the first year following prophylactic gastrectomy among the study participants (N=52).
Numbers in parentheses indicate the number of participants who suffered the complication.
| Complications experienced in hospital/within 1 month of surgery | Complications experienced between 1–12 months post-surgery |
|---|---|
| Anastomic leak (2) | Anastomic strictures (12) a |
| Urinary tract infection (1) | Bile reflux (1)b |
| Wound infection (3) | Duodenal obstruction (1) |
| Pulmonary embolism (1) | Partial obstruction at jejunal anastomosis site (1) |
| Pulmonary effusion (1) | |
| Shortness of breath (1) | Moderate iron deficiency (1) |
| Small bowel obstruction (1) | Ileus obstruction (1) |
Pathology reports were received for only 41 of the participants despite this information being specifically requested on the Authority to Divulge form. Thirty two (78%) had signet ring cells, indicative of DGC, in their post-gastrectomy specimens. Foci were distributed throughout the entire stomach and ranged from 1 to 52 per stomach. All foci were staged at T1N0M0.
The median weight of participants before and after surgery was 73 kilograms (range 46 – 140 kg) and 60 kilograms (range 40 – 104 kg) respectively. The median loss of weight overall was 11 kilograms (mean was 15kg) and ranged between 0– 46 kilograms. At the time of the study, the majority (69%) of participants were maintaining a healthy weight. Sixteen percent were underweight at the time of survey and 14% were obese. Of the eight participants who were underweight at the time of the survey, three had already been underweight pre-surgery.
Functional scales and symptoms (EORTC C30 and STO22)
The overall QOL score was 70.6 (SD = 25.6) on the EORTC-QLQ C30. Figure 1 shows comparisons between the three time-elapsed groups of the function and symptoms scales from the EORTC C30 and STO 22 instruments.
Figure 1.
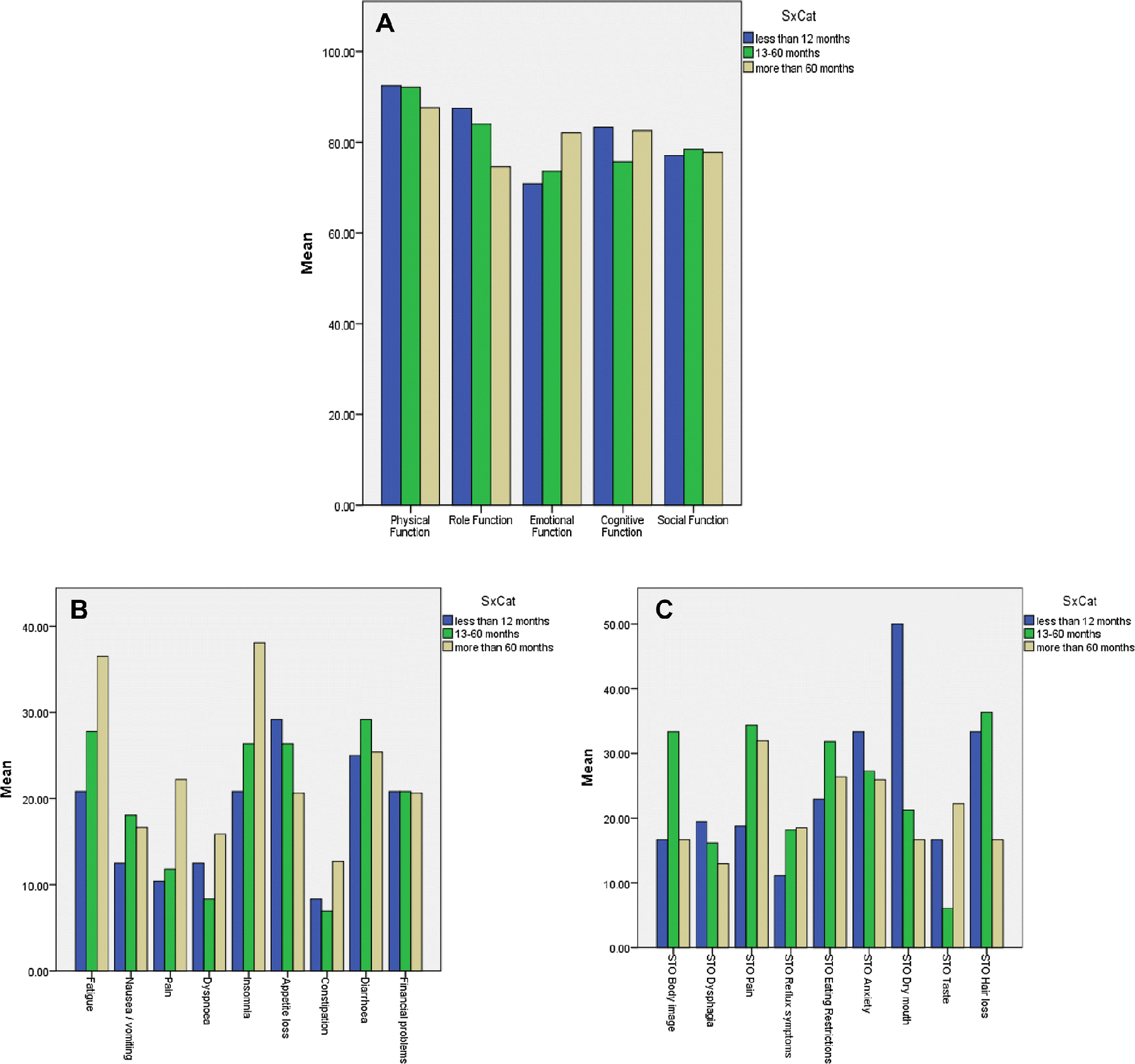
a. Bar chart comparing EORTC C30 functional scales between the three time-elapsed groups. b. Bar chart comparing EORTC C30 symptoms between the three time-elapsed groups. c. Bar chart comparing means of STO 22 symptoms between the three time-elapsed groups.
Anxiety:
Fifty participants completed this instrument. The mean score was 48 (SD 11) with a range of 31.6 to 72.2. Twenty-seven (54%) of the study participants scored less than 50, which is below the average score of the normal population. Thirty-seven (74%) of the study participants scored at the “none to slight” anxiety level.
Depression:
Fifty-two participants completed the questionnaire. The mean score for the depression instrument was 46 (SD=12) with a range between 33.5 and 81.6. Thirty-five (67%) participants scored below 50 points. Three participants (6%) scored in the severe depression range.
Patient Satisfaction with Surgery:
Forty-seven (88%) of the participants were either very satisfied (79%) or satisfied (9%) with their decision to have surgery. Two (4%) participants were neither satisfied nor dissatisfied. Three (6%) participants were not satisfied with the decision to have surgery; one of these indicated dissatisfaction because of the symptoms being experienced but also was extremely satisfied with the surgery because s/he was still alive. The individual with 2 answers was left out of all the analyses involving satisfaction.
Predictors of QOL after PTG
While the full model (including all possible predictors) statistically predicted QOL (R2 = .84, F (17,32) = 9, p < .001) none of the variables were statistically significant individually. This could be attributed to a small sample size and lack of power. A reduced model was obtained using a stepwise procedure with the AIC criterion, shown in Table 4. All the predictor variables in this model, except for the symptoms depression, body image and eating restrictions were statistically significant.
Table 4.
The reduced model analysis results
| Estimate | Std Error | P | |
|---|---|---|---|
| (Intercept) | 54.3 | 12.2 | 8.5e-05 |
| Cognitive Function | 0.1 | 0.06 | 0.03 * |
| Role Function | 0.3 | 0.08 | 0.001 ** |
| Social Function | 0.3 | 0.1 | 0.004 ** |
| Depression | 0.2 | 0.1 | 0.2 |
| Anxiety | −0.7 | 0.1 | 0.0004 *** |
| Body Image | 0.07 | 0.04 | 0.1 |
| Pain | −0.2 | 0.07 | 0.008 ** |
| Eating | 0.1 | 0.08 | 0.1 |
| Taste | 0.2 | 0.07 | 0.001 ** |
| Dyspnea | −0.1 | 0.06 | 0.013 * |
| Diarhhea | −0.1 | 0.05 | 0.01 * |
The coefficient of variation,R2, was 0.94 and adjusted R2 is 0.92. These predictor variables explain 92% of the variability of the data which was a significant improvement over the full model.
The reduced model to predict QOL, with nine significant variables, statistically predicted QOL R2 = .92, F(14,35) = 40, p < .001. As can be seen in Table 4, these predictors had significant positive regression weights, indicating participants with worse scores on these scales were expected to have lower QOL after controlling for the other variables in the model.
Discussion and Conclusions
This study data sheds further light on post-surgical complications, symptoms and their impact on the individual after PTG. In this cohort, the mean global health status/QOL was 70.6 (SD = 25.6) was comparable to reference values from the general population in Norway and Sweden (71.2 ± 22.4), (p=0. 51) (20). As expected, the mean score for global health status/QOL for this cohort is much higher than the reference values for gastric cancer patients (53.1±26.5) [21].
The findings suggest that this cohort of CDH1 carriers overall appear to enjoy a satisfactory QOL after PTG despite their symptom burden. Ferrans and Powers (1993), after studying a group of haemodialysis patients, suggested that individuals tend to re-define or amend their life goals to conserve their well-being despite adverse changes in their lives [22]. It is possible that a redefinition of life goals may also explain relatively favourable self-reported QOL in this study sample. In addition, these individuals may have developed various coping strategies that enabled them to manage their symptoms in the time since PTG.
Post-surgical complications including major complications (anastomic leak, pulmonary embolism and effusion) were seen in 46% of participants and were within the range of 31–56% of the previously reported series [14–16] but much lower than the large Newfoundland cohort [8]. There was no difference in any of the scales between participants who had post-operative complications and those who did not. Two other studies have shown this lack of influence of post-operative complications on QOL [23,24] in individuals who had undergone gastrectomy.
Participants in this study had an average of 19% weight loss after the surgery, similar to the weight loss seen in the other studies [3–16] thus cementing the observation that most individuals do struggle to maintain their weight after the surgery. Given that micronutrient deficiencies, which can lead to severe clinical complications, can occur even years after a TG, a dietician should be involved pre-and post-surgery so that continuing assessment and management can prevent clinical complications due to potential micronutrient deficiencies.
Of the 41 pathology reports in this study, only 32 (78%) were shown to have foci of signet ring cells. This cohort has a much smaller percentage of gastrectomy specimens with signet ring cell foci than the > 90% seen in previously reported series possibly because many of the specimens from this cohort of participants were not examined according to the investigations recommended by the IGCLC [2]. Recently, a histopathological review of published and unpublished data on TG specimens underlined the importance of using the IGCLC protocol to detect the microscopic foci [25]; in specimens where the protocol was implemented, there was a 95.3% detection rate compared to the 62.5% rate when no protocol was utilized. Given that many mutation carriers have signet ring cell foci has led to the idea that this surgery is curative rather than prophylactic [9].
In this study, there was no significant difference seen in any of the individual symptoms in either the EORTC QLQ-C30 or the STO22 instruments when looking at the three groups that were stratified according to time elapsed since surgery and survey. It may be that the numbers of participants in each group was too small to pick up any potential difference. This needs to be repeated with a larger number of patients within each group.
In the regression model, some of the “meal-related” symptoms from the STO22 questionnaire proved to be significant. Abdominal pain revealed high (56.2±16.3) overall scores, pain on both the EORTC QLQ-C30 and STO22 were high (56.2±16.3 and 49.1±23.7). Pain scores reported by Worster’s study [15] were much lower in at 2 years after (mean score of 1.99 (SD = 0.74). The scores for this study are also higher than those reported for gastric cancer patients (31.4±31.7).
Pain (STO22 symptom) was a significant predictor variable in the overall regression model revealing the clinical influence of this symptom in an individual’s overall QOL (Table 4). Dyspnea and taste were also significant predictor variables in the regression model (Table 4). Previous studies have reported that patients who have undergone gastric surgery have reported changes in their taste of food and drink after surgery, including aversions to certain foods [26–28]. In one study [27], 45% of patients also reported loss of taste and/or smell after gastrectomy; this was transient in most cases but in some patients the changes were long term. Although there is no known mechanism for why this occurs, it has been speculated that vagal influences might be responsible [27], that is, after the surgery, there could be an initial disruption in the homeostasis in olfaction and gestation followed by vacillating hypersensity and hyposensitivity before a static level of sensation is finally achieved. It would be important to discover how and to what extent, these symptoms affect QOL and the longevity of these effects in individuals after PTG.
Anxiety and depression, which are prevalent in patients with cancer or other chronic conditions [29,30], scored in the “none to slight” range for both anxiety and depression (71% and 76% respectively). All four of the individuals who scored in the severe range for anxiety and depression had been diagnosed with anxiety disorder, depression or associative identity disorder prior to the PTG. Anxiety has been shown to be independently associated with a poorer overall QOL in cancer patients [31]. Anxiety was a significant predictor in the reduced model in this study. Given that anxiety is a common response in cancer patients [32] and that anecdotal evidence (personal communications) has indicated that several individuals suffer from anxiety after PTG, this result is not a surprise. Anxiety is a common response to threats of mortality and suffering and anxiety is believed to help patients to cope with the fluctuating situations in their lives. Anxiety is dynamic and for the majority of cancer patients, it peaks at different times during the course of their disease [33]. Therefore, it would be important for healthcare professionals to elicit the specific concerns of individuals after PTG and provide appropriate intervention if needed.
A comparison of the five functioning scales in the EORTC QLQ-C30 between this study sample, the Worster [14] PTG study cohort and the general population reference values [21] is shown in Table 5. The participants in this cohort did not appear to fare as well as the Worster study participants in the cognitive scales (p=0.003). They also did much worse than the general population reference population in the cognitive functioning scale (p<0.001). Further investigations are needed to understand why this difference in the cognitive scales exists.
Table 5.
Group means of this study population, Worster study cohort and general population reference values.
| This Study | Worster et. al (2014) [15] | EORTC reference values for general population [286] | ||
|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | p-value* |
| Physical | 89.5 (15.9) | 86.7 (1.6) | 89.8 (16.2) | 0.29 |
| Role | 79.3 (25.6) | 77.1 (24.3) | 84.7 (25.4) | 0.40 |
| Emotional | 74.9 (24.6) | 84.4 (16.9) | 76.3 (22.8) | 0.01 |
| Cognitive | 78.2 (27.1) | 91.7 (16.1) | 86.1 (20) | 0.003** |
| Social | 76.6 (26.5) | 76.0 (25.8) | 87.5 (22.9) | 0.44 |
SD = Standard deviation
p-value is for comparison between the means of this study sample and the Worster study cohort.
Cognitive functioning was significant in the reduced model in this study. Worster’s [14] cohort revealed a significant decrease in cognitive scores within the first month after surgery, but scores recovered to baseline within two years. In several studies of patients with GC who have undergone gastrectomy, cognitive functioning is largely unaffected by surgery and tends to stay near baseline levels throughout follow-up [34, 35] or are actually back to baseline after three months [36, 37]. This study measured patient outcomes at one time frame; given that approximately half of the participants in this cohort were within three years of their surgery when completing this survey, it is possible that group may have skewed the scores on this variable. Further work on larger numbers of participants will help tease this effect out. In the meantime, in those with impaired cognitive functioning, a complete medical checkup and consult with a neurologist or a psychiatrist should be provided. In addition, the patient ought to be informed of the positive impact of maintaining their social activity.
The importance of the role functioning scale is reflected in its significant contribution to the regression model. This scale did not return to baseline in the Worster study [14] and has also been shown to recover either in the short (one year) term or long term (five years or more) [34, 38,39]. Poor role functioning may be a result of weakness and fatigue, diarrhea and even with age [34]. Anxiety was a significant predictor variable that can affect one’s ability to cope and thus one’s QOL. In individuals who are not able to adjust and make changes to their lifestyle after PTG, it would be important to provide interventions based on the level of anxiety. Intervention is important in individuals who have moderate to severe anxiety as it may manifest itself in one’s role functioning, as well as presenting physical symptoms such as fatigue, nausea/vomiting and even pain.
Social function was significant in the regression model. Social support is an important predictor of coping with difficult life issues and predicts the well-being of everyone from young children to the elderly in many contexts [40]. Studies have shown that social support can improve the several different aspects of QOL in cancer patients [30, 41]. Assessing an individual’s social support after PTG could help identify those whose QOL might benefit from improved social support such as seeking out peer support, being a part of different networks and participating socially.
This research provides further information on clinical outcomes, post-surgical symptoms and QOL in individuals after PTG. These individuals are usually asymptomatic prior to their surgery and generally in good health. Although the worry of gastric cancer is generally eliminated for those undergoing PTG, the surgery comes with a price – inevitable post-surgical symptoms may affect QOL. This re-enforces the recommendation of having a multidisciplinary team involved in the pre- and post-surgical care of these individuals.
Psychosocial issues in patients who have had PTG are also important to assess. This study has shown that social function is an important predictor of QOL in this cohort. This is not surprising as the social environment of an individual has strong impact on one’s well-being and also provides protection from the harmful effects of potentially stressful life events that include major surgery [39]. It would be unreasonable to suggest such a drastic surgery if QOL were seriously impaired, and it is reassuring that we found that QOL scores were high in this study sample.
This study suggests that PTG does not usually have long-term negative implications for a person’s QOL. Most of the individuals in this study did not exhibit anxiety or depression either on the PROMIS or EORTC instruments but were functioning at psychosocial levels that are comparable to the general population. It is hoped that these study outcomes will reassure individuals who are contemplating having the surgery.
Routine measurement of QOL before and after PTG might afford a number of benefits, including identification and prioritization of patients’ problems, screening for hidden concerns and improving the efficacy of the outpatient consultation. Recognition of the potential negative side effects of PTG may also spur the development of more efficient screening of DGC to avert the need for this surgery in the first place.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, Santos TA, Claro I, Carvalho J, Nielsen C, Padilla S, Lum A, Talhouk A, Baker-Lange K, Richardson S, Lewis I, Lindor NM, Pennell E, MacMillan A, Fernandez B, Keller G, Lynch H, Shah SP, Guilford P, Gallinger S, Corso G, Roviello F, Caldas C, Oliveira C, Pharoah PD, Huntsman DGD. G. (2015). Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncology, 1(1), 23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 2.van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE, Hardwick RH, Ausems MG, Bardram L, Benusiglio PR, Bisseling TM, Blair V, Bleiker E, Boussioutas A, Cats A, Coit D, DeGregorio L, Figueiredo J, Ford JM, Heijkoop E, Hermens R, Humar B, Kaurah P, Keller G, Lai J, Ligtenberg MJ, O’Donovan M, Oliveira C, Pinheiro H, Ragunath K, Rasenberg E, Richardson S, Roviello F, Schackert H, Seruca R, Taylor A, Ter Huurne A, Tischkowitz M, Joe ST, van Dijck B, van Grieken NC, van Hillegersberg R, van Sandick JW, Vehof R, van Krieken JH, Fitzgerald RC (2015). Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. Journal of medical genetics, 52(6), 361–374. doi: 10.1136/jmedgenet-2015-103094. Epub 2015 May 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huntsman DG, Carneiro F, Lewis FR, MacLeod PM, Hayashi A, Monaghan KG, Maung R, Seruca R, Jackson CE, Caldas C (2001). Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. New England Journal of Medicine, 344(25), 1904–1909. [DOI] [PubMed] [Google Scholar]
- 4.Lewis FR, Mellinger JD, Hayashi A, Lorelli D, Monaghan KG, Carneiro F, Huntsman DG, Jackson CE, Caldas C. (2001). Prophylactic total gastrectomy for familial gastric cancer. Surgery 130(4): 612–7; discussion 617–9. [DOI] [PubMed] [Google Scholar]
- 5.Chun YS, Lindor NM, Smyrk TC, Petersen BT, Burgart LJ, Guilford PJ, Donohue JH. (2001). Germline E-cadherin gene mutations: is prophylactic total gastrectomy indicated? Cancer. 92(1):181–7. [DOI] [PubMed] [Google Scholar]
- 6.Newman EA and Mulholland MW. (2006). Prophylactic gastrectomy for hereditary diffuse gastric cancer syndrome. J Am Coll Surg, 202(4):612–617. [DOI] [PubMed] [Google Scholar]
- 7.Norton JA, Ham CM, Van Dam J, Jeffrey RB, Longacre TA, Huntsman DG, Chun N, Kurian AW, Ford JM (2007). CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Annals of surgery, 245(6): 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebbard PC, PC, Macmillan A, Huntsman D, Kaurah P, Carneiro F, Wen X, Kwan A, Boone D, Bursey F, Green J, Fernandez B, Fontaine D, Wirtzfeld DA. (2009). Prophylactic total gastrectomy (PTG) for Hereditary Diffuse Gastric Cancer (HDGC): the Newfoundland experience with 23 patients.Ann Surg. Onc 16(7):1890–5. [DOI] [PubMed] [Google Scholar]
- 9.Hackenson D, Edelman DA., McGuire T, Weaver DW, & Webber JD (2010). Prophylactic laparoscopic gastrectomy for hereditary diffuse gastric cancer: a case series in a single family. JSLS: Journal of the Society of Laparoendoscopic Surgeons, 14(3), 348. doi: 10.4293/108680810X12924466007449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandalai PK, Lauwers GY, Chung DC, Patel D, Yoon SS. (2011). Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery 149(3):347–55. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Kingham K, Ford JM, Rosing J, Van Dam J, Jeffrey RB, Longacre TA, Chun N, Kurian A, Norton JA. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol 2011. September;18(9):2594–8. doi: 10.1245/s10434-011-1648-9. Epub 2011 Mar 18. [DOI] [PubMed] [Google Scholar]
- 12.Li J, McBean E, Li X, Berho M, Szomstein S, & Rosenthal RJ (2013). Laparoscopic prophylactic total gastrectomy with linear stapler side-to-side esophagojejunal anastomosis for hereditary diffuse gastric cancer syndrome in 2 siblings. Surgical Laparoscopy Endoscopy & Percutaneous Techniques, 23(3), e124–e126. doi: 10.1097/SLE.0b013e3182773e38. . [DOI] [PubMed] [Google Scholar]
- 13.Bardram L, Hansen TV, Gerdes AM, Timshel S, Friis-Hansen L, & Federspiel B (2014). Prophylactic total gastrectomy in hereditary diffuse gastric cancer: identification of two novel CDH1 gene mutations—a clinical observational study. Familial cancer, 13(2), 231–242. doi: 10.1007/s10689-013-9698-8. [DOI] [PubMed] [Google Scholar]
- 14.Worster E, Liu X, Richardson S, Hardwick RH, Dwerryhouse S, Caldas C, Fitzgerald RC. (2014). The impact of prophylactic total gastrectomy on health-related quality of life: a prospective cohort study. Ann Surg 260(1):87–93. [DOI] [PubMed] [Google Scholar]
- 15.Muir J, Aronson M, Esplen MJ, Pollett A, Swallow CJ. Gastrointest Surg J. (2016). Prophylactic Total Gastrectomy: a Prospective Cohort Study of Long-Term Impact on Quality of Life December;20(12):1950–1958. Epub 2016 Oct 17. [DOI] [PubMed] [Google Scholar]
- 16.Strong VE, Gholami S, Shah MA, Tang LH, Janjigian YY, Schattner M, Selby LV, Yoon SS, Salo-Mullen E, Stadler ZK, Kelsen D, Brennan MF, Coit DG. Total Gastrectomy for Hereditary Diffuse Gastric Cancer at a Single Center: Postsurgical Outcomes in 41 Patients. Ann Surg 2017. December;266(6):1006–1012. doi: 10.1097/SLA.0000000000002030. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, … & Takeda F (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Journal of the national cancer institute, 85(5), 365–376. [DOI] [PubMed] [Google Scholar]
- 18.Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O., … & Coens C (2004). Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. European Journal of Cancer; 40(15), 2260–2268. [DOI] [PubMed] [Google Scholar]
- 19.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D; PROMIS Cooperative Group. (2011). Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger, Assessment, 18(3): 263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayers PM, Aaronson NK, Bjordal K., Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. (2001). EORTC QLQ-C30 Scoring Manual
- 21.Scott N, Fayers P, Aaronson N, Bottomley A, de Graeff A, Groenvold M, … & AG, M. (2008). EORTC QLQ-C30. Reference values. Brussels: EORTC. [Online]. Available at: http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf.
- 22.Ferrans CE and Powers MJ. (1993). Quality of life of hemodialysis patients. ANNA J; . 20(5):575–81. discussion 582. [PubMed] [Google Scholar]
- 23.Jakstaite G, Samalavicius NE, Smailyte G, & Lunevicius R (2012). The quality of life after a total gastrectomy with extended lymphadenectomy and omega type oesophagojejunostomy for gastric adenocarcinoma without distant metastases. BMC surgery, 12(1), 11. doi: 10.1186/1471-2482-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz De Liano A, Oteiza Martinez F, Ciga MA, Aizcorbe M, Cobo F, & Trujillo R (2003). Impact of surgical procedure for gastric cancer on quality of life. British Journal of Surgery, 90(1), 91–94. [DOI] [PubMed] [Google Scholar]
- 25.Rocha JP, Gullo I, Wen X, Devezas V, Baptista M, Oliveira C, Carneiro F. Pathological features of total gastrectomy specimens from asymptomatic hereditary diffuse gastric cancer patients and implications for clinical management. Histopathology 2018. December;73(6):878–886. Epub 2018 Oct 9. Review. [DOI] [PubMed] [Google Scholar]
- 26.Tichansky DS, Boughter JD Jr, Madan AK. (2006). Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric band. Surg Obes Relat Dis;2:440–4. [DOI] [PubMed] [Google Scholar]
- 27.Harris AM and Griffin SM. (2003). Postoperative taste and smell deficit after upper gastrointestinal cancer surgery- an unreported complication. J Surg Oncol; 82:147–50. [DOI] [PubMed] [Google Scholar]
- 28.Carey S, Laws R, Ferrie S, Young J, Allman-Farinelli M. (2013). Struggling with food and eating – life after major upper gastrointestinal surgery. Support Care Cancer; 21: 2749–2757. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Yu Z, Wu J, Kean J, & Monahan PO. (2014). Operating Characteristics of PROMIS Four‐Item Depression and Anxiety Scales in Primary Care Patients with Chronic Pain. Pain Medicine, 15(11), 1892–1901. doi: 10.1111/pme.12537. Epub 2014 Aug 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda T, Onuoha FN, & Munakata T (2006). The effect of postoperative symptom experience, and personality and psychosocial factors on depression among postgastrectomy patients in Japan. Gastroenterology nursing, 29(6), 437–444. [DOI] [PubMed] [Google Scholar]
- 31.Brown LF, Kroenke K, Theobald DE, Wu J, & Tu W. (2010). The association of depression and anxiety with health‐related quality of life in cancer patients with depression and/or pain. Psycho‐Oncology, 19(7), 734–741. doi: 10.1002/pon.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, Zabora JR. (2009). Mixed anxiety/depression symptoms in a large cancer cohort: Prevalence by cancer type. Psychosomatics; 50:383–391. [DOI] [PubMed] [Google Scholar]
- 33.Stark D, Kiely M, Smith A, Velikova G, House A, Selby P. (2002). Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. J Clin Oncol;20(14):3137–48. [DOI] [PubMed] [Google Scholar]
- 34.Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, … & Kim S (2012). Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Annals of surgery, 256(6), 1008–1013. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, & Nakao A (2011). Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World journal of surgery, 35(2), 357–364. [DOI] [PubMed] [Google Scholar]
- 36.Huang JL, Wei HB, Zheng ZH, Wei B, Chen TF, Huang Y, Guo WP, Hu B. (2010). Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer. Digestive surgery, 27(4), 291–296. doi: 10.1159/000281818. Epub 2010 Jul 31 [DOI] [PubMed] [Google Scholar]
- 37.Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, & Whang-Peng J. (2008). Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. British journal of cancer, 98(1), 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong H, Kwon OK, & Yu W (2012). Changes of quality of life after gastric cancer surgery. Journal of gastric cancer, 12(3), 194–200. doi: 10.5230/jgc.2012.12.3.194. Epub 2012 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, & Whang-Peng J. (2008). Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. British journal of cancer, 98(1), 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen S & Wills TA. (1985). Stress, social support, and the buffering hypothesis. Psychological bulletin, 98(2), 310. [PubMed] [Google Scholar]
- 41.Tian J & Hong JS. (2014). Assessment of the relationship between resilience and quality of life in patients with digestive cancer. World journal of gastroenterology: WJG, 20(48), 18439. doi: 10.3748/wjg.v20.i48.18439. [DOI] [PMC free article] [PubMed] [Google Scholar]


