Abstract
Background
To investigate age-related severity, patterns of retinal structural damage, and functional visual recovery in pediatric and adult cohorts of myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) optic neuritis (ON).
Methods
All MOGAD patients from the 5 participating centers were included. Patients with initial manifestation <18 years were included in the pediatric (MOGADped) cohort and patients with ≥18 years in the adult (MOGADadult) cohort. For patients with MOGAD ON, examinations at least ≥6 months after ON onset were included in the analyses. Using spectral domain optical coherence tomography (SD-OCT), we acquired peripapillary retinal nerve fiber layer thickness (pRNFL) and volumes of combined ganglion cell and inner plexiform layer (GCIPL). High- and 2.5% low-contrast visual acuity (HCVA, LCVA) and visual-evoked potentials (VEP) were obtained.
Results
Twenty MOGADped (10.3±3.7 years, 30 MOGAD ON eyes) and 39 MOGADadult (34.9±11.6 years, 42 MOGAD ON eyes) patients were included. The average number of ON episodes per ON eye was similar in both groups (1.8±1.3 and 2.0±1.7). In both pediatric and adult MOGAD, ON led to pronounced neuroaxonal retinal atrophy (pRNFL: 63.1±18.7 and 64.3±22.9 μm; GCIPL: 0.42±0.09 and 0.44±0.13 mm3, respectively) and moderate delay of the VEP latencies (117.9±10.7 and 118.0±14.5 ms). In contrast, visual acuity was substantially better in children (HCVA: 51.4±9.3 vs. 35.0±20.6 raw letters, p=0.001; LCVA: 22.8±14.6 vs. 13.5±16.4, p=0.028). Complete visual recovery (HCVA-logMAR 0.0) occurred in 73.3% of MOGADped and 31% MOGADadults ON eyes, while 3.3% and 31% demonstrated moderate to severe (logMAR > 0.5) visual impairment. Independent of retinal atrophy, age at ON onset significantly correlated with visual outcome.
Conclusion
Pediatric MOGAD ON showed better visual recovery than adult MOGAD ON despite profound and almost identical neuroaxonal retinal atrophy. Age-related cortical neuroplasticity may account for the substantial discrepancy between structural changes and functional outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-021-02160-9.
Keywords: Optical coherence tomography, Optic neuritis, Myelin oligodendrocyte glycoprotein IgG, MOGAD
Introduction
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is a newly defined autoimmune disorder of the central nervous system (CNS) [1]. While immune responses targeting myelin oligodendrocyte glycoprotein (MOG) have been extensively studied in experimental autoimmune encephalomyelitis [1], the clinical relevance of MOG-immunoglobulin (Ig)G was only recently appreciated following the identification of autoantibodies targeting conformational epitopes of full-length MOG in humans [2]. MOGAD patients may develop any combination of monophasic or relapsing acute disseminated encephalomyelitis (ADEM), non-ADEM encephalitis, neuromyelitis optica spectrum disorder (NMOSD), optic neuritis (ON), transverse myelitis (TM), brainstem encephalitis, or rarely multiple sclerosis (MS)-like demyelinating disease [3–5]. Clinical manifestations seem to be age-dependent: children < 10 years are more likely to develop an ADEM phenotype, while those ≥10 years are more likely to present with an NMOSD- or MS-like phenotype similar to adults [6]. In both pediatric and adult patients, isolated, bilateral, or recurrent ON are common clinical presentations [3, 5, 7, 8]. High levels of MOG expression and enhanced blood-brain barrier permeability in the optic nerve may explain its frequent involvement [9, 10]. Although the histopathology of cerebral lesions in MOGAD patients demonstrates relative preservation of axonal structures, severe visual impairment or functional blindness have been reported in >30% of MOGADadults ON patients, and OCT studies repeatedly demonstrated profound axonal degeneration with loss of retinal ganglion cells [11–14]. Although MOG-IgG antibodies have been frequently reported in children with ON, there are only a few studies investigating retinal changes and visual outcomes in this population [5, 15–17]. Moreover, there are no studies comparing ON course and outcome in children and adults with MOGAD. The latter is especially interesting as pediatric MS and MOGAD patients generally demonstrate better relapse recovery than adults [18–22]. Here, we compared the outcomes of MOGADped and MOGADadult ON including age-related (1) severity and patterns of retinal structural damage and (2) functional visual recovery.
Subjects and methods
Study population
We conducted an analysis of prospectively collected data from MOGADped and MOGADadult patients with or without ON. The inclusion criteria included the acute presentation of demyelinating disease, MOG-IgG seropositivity, availability of OCT, visual acuity data, and clinical information. If one or multiple ON events were known, visits could be considered at least 6 months after the last ON. During the study period (2018–2020), all patients tested positive for MOG-IgG were included who met the inclusion criteria and who were seen at five academic university centers specialized in neuroimmunological diseases (Department of Pediatric Neurology, Children’s Hospital Datteln, University Witten/Herdecke, Germany, N=9; Department of Neuropediatrics and Social Pediatrics University Hospital of Pediatrics and Adolescent Medicine, Ruhr-University Bochum, N=3; Neurology Department, St. Josef Hospital Bochum, Bochum, Germany, N=16; Institute of Clinical Neuroimmunology, LMU Hospital, Munich, Germany, N=29, and Institute of Pediatrics, University Hospital Vall d’Hebron, Barcelona, Spain, N=2) [total N=59]. Five patients were excluded due to incomplete examination data (n=2) or an acute ON at the time of examination (n=3) (see supplementary Figure 3). A part of the MOGAD cohort of the Institute of Clinical Neuroimmunology (13/29 patients) has already been published as a subset of other cohort analyses [12, 13]. Written informed consent was obtained from all patients participating in the study. The local ethics committees approved the study protocol in accordance with the Declaration of Helsinki (1964) in its currently applicable version. Two groups of patients were evaluated depending on the age at disease manifestation: group 1 with 20 MOG-IgG-positive children with initial manifestation < 18 years (MOGADped) and group 2 with 39 MOG-IgG-positive adult patients with initial manifestation ≥ 18 years (MOGADadult). Demographic (gender and age at initial manifestation) and clinical (disease duration, number and side of clinical ON episodes) data were collected for all patients. For the detection of MOG-IgG, serum samples were analyzed during the initial workup at least once by established cell-based assays at the discretion of each center using the laboratory’s cutoffs (MOG IFT, EUROIMMUN, Laboratory Stöcker, Germany; Reindl Lab, Medical University of Innsbruck, Innsbruck, Austria; Meinl Lab, LMU Hospital, Munich, Germany) [1, 2, 23].
Optical coherence tomography (OCT) and visual acuity
All centers used spectral-domain optical coherence tomography (SD-OCT, SPECTRALIS, Heidelberg Engineering, Heidelberg, Germany) with automatic real-time (ART) averaging. A scan around the optic nerve with an activated eye tracker (12°, 3.5 mm ring, 50≤ ART ≤100) and a macular volume scan (20° × 20°, 25 vertical B-scans, 20 ≤ ART ≤ 49) were performed as cylinders of 3 mm diameter around the fovea based on local protocols. The thickness of the peripapillary retinal nerve fiber layer (pRNFL) and the volumes of the macular retinal nerve fiber (mRNFL), the combined ganglion cell and inner plexiform layer (GCIPL), the inner nuclear layer (INL), the combined outer plexiform layer and outer nuclear layer (OPONL), and the total macular volume (TMV) were analyzed. The segmentation of all layers was performed semi-automatically using software from the SD-OCT manufacturer (Eye Explorer 1.9.10.0 with viewing module 6.3.4.0, Heidelberg Engineering, Heidelberg, Germany). Experienced evaluators carefully checked all scans for sufficient quality and segmentation errors and corrected them if necessary. The SD-OCT data in this study are analyzed and reported according to the recommendations of APOSTEL and OSCAR-IB [24, 25].
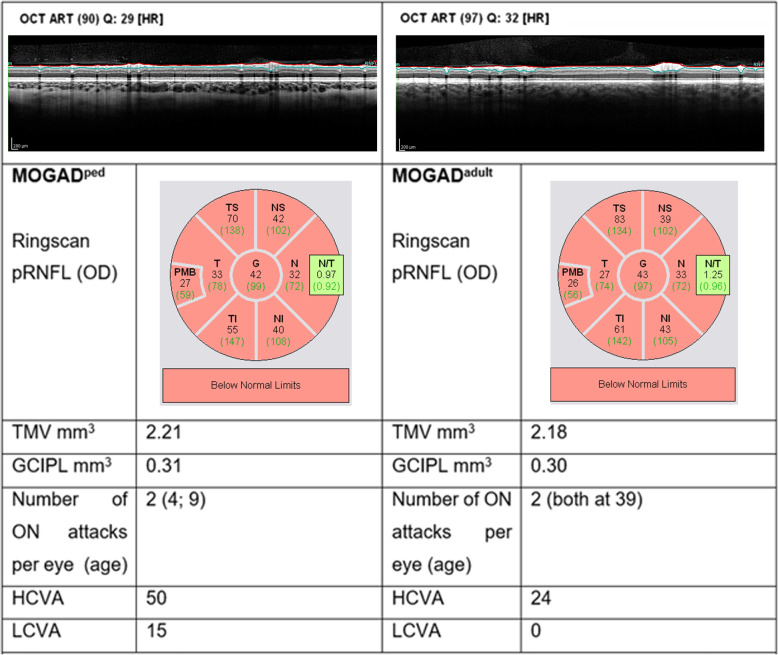
In addition, at the time of OCT examination, habitually corrected high-contrast and low-contrast monocular visual acuity (VA) was acquired using high contrast and 2.5% low-contrast Sloan letter charts placed in a retro-illuminated light box at 2 m distance. Each chart consists of 14 lines with 5 letters per line that are standardized with equal difficulty per line and equal spacing between the lines. The total number of correct letters identified on each chart was tested to determine high- and low-contrast VA (HCVA, LCVA; maximum, 70 letters). Characteristic pRNFL scans in a pediatric and an adult MOGAD patient are shown in Fig. 1.
Fig. 1.
Representative pediatric and adult retinal OCT scans after recurrent MOGAD-ON. Despite almost identical neuroaxonal retinal atrophy, functional vision is notably better in children than adults. Abbreviations: OD oculus dexter, G global pRNFL, N nasal pRNFL, I inferior pRNFL, T temporal pRNFL, PMB paillo-macular-bundle, TMV total macular volume, GCIPL combined ganglion cell and inner plexiform layer
Visual evoked potentials (VEP)
VEP data (Keypoint.net, Neurolite Software, Natus, Switzerland) were collected from occipital midline referred to a mid-frontal electrode according to the International Society for Clinical Electrophysiology of Vision standards. Pattern reversal VEP was produced by high-contrast, black and white checks. Each check has the size of 171.6 arc minute. The examination was performed in a dark room in a 1-m distance. P100 latency and the P100-P125 amplitude were collected. All VEP examinations were performed in Bochum (data available for 15 MOGADped and 12 MOGADadult patients).
Statistical methods
Clinical data, OCT, VEP, and VA results were compared between MOGADped and MOGADadult patients. The mean and standard deviation were calculated for continuous variables, frequency, and proportion for categorical variables. The non-parametric Mann-Whitney U test and chi-square test were used to compare two independent groups. Statistical significance was defined as p < 0.05.
SD-OCT data, VEP data, and HCVA/LCVA in the eyes with and without ON were analyzed within and between the MOGADped and MOGADadult cohorts using generalized estimating equation models (GEE) to account for within-patient inter-eye correlation. The correlation matrix parameter was set to “exchangeable.”
Further, we performed a Spearman correlation to identify the possible factors determining the visual outcome in MOGAD ON. Age at ON, number of ON episodes per eye, the extent of retinal degeneration (pRNFL and GCIPL thickness), and VEP P100 latency were included in the analysis. For cases of recurrent ON, we calculated an average age at ON onset. Due to a relatively small sample size, both groups were pooled. All factors that significantly correlated with visual outcome were included in GEE analysis. Data were analyzed with SPSS version 26 (IBM SPSS Statistics).
Results
Cohort description
We enrolled 20 MOG-IgG-positive children (MOGADped patients, female:male 13:7, mean age 10.3±3.7 years) and 39 MOG-IgG-positive adults (MOGADadult patients, female: male 20:19, mean age 34.9±11.6 years). From the medical history, 2 patients had no ON, 6 had unilateral ON, and 12 had bilateral ON in the MOGADped patient cohort (total 30 ON affected eyes). Accordingly, in the MOGADadult patient cohort, 15 had no ON, 6 had unilateral ON, and 18 had bilateral ON (total 42 ON affected eyes). The main clinical data of all patients are described in Table 1. Eight of 20 MOG-IgG-positive children were diagnosed with recurrent NMOSD, 6 children with recurrent ON (rON), 3 children with ADEM + rON, and 3 children with encephalomyelitis. Three of 39 MOG-IgG-positive adults were diagnosed with monophasic NMOSD, 12 with recurrent NMOSD, 14 with encephalomyelitis, 9 adults with rON, and 1 adult with ADEM. There were no differences in gender, disease duration, or number of previous ON episodes between the two groups. Eighteen (94.7%) of the MOGADped patients were on long-term immunotherapy at the time of SD-OCT (8 monotherapy with oral prednisone, 5 intravenous long-term immunoglobulin/subcutaneous immunoglobulin (IVIG/SCIG), 3 glatiramer acetate, 1 rituximab, 1 azathioprine, 1 oral prednisone as an add-on therapy) compared with 30 (76.9%) of MOGADadult patients (12 azathioprine, 7 rituximab, 1 oral prednisone as an add-on therapy, 3 methotrexate, 2 tocilizumab, 2 teriflunomide, 2 glatiramer acetate, 1 monotherapy with oral prednisone, and 1 long-term IVIG).
Table 1.
Demographic and main clinical characteristics of pediatric and adult cohorts
| MOGADped (n=20) | MOGADadult (n=39) | p | |
|---|---|---|---|
| Age at initial manifestation, median (range) | 10.5 (4–15) | 35.0 (19–64) | <0.001 |
| Females, n (%) | 13 (65.0%) | 20 (51.3%) | 0.157 |
| Ethnicity | 20 Caucasians |
36 Caucasians 3 Asians |
0.081 |
| Disease duration (years), median (range) | 4.5 (0–22) | 6.5 (0–24) | 0.084 |
| Patients with a clinical history of ON, n (%) | 18 (90.0%) | 24 (61.5%) | 0.001 |
| Patients with unilateral ON, n (%) | 6 (30.0%) | 6 (15.4%) | 0.062 |
| Patients with bilateral ON, n (%) | 12 (60.0%) | 18 (46.2%) | 0.154 |
| Patients with a simultaneous bilateral ON, n (%) | 8 (40.0%) | 14 (35.9%) | 0.663 |
| Total ON eyes, n (%) | 30 (75.0%) | 42 (53.8%) | 0.029 |
| Number of ON episodes per eye, mean (SD) | 1.8 (1.3) | 2.0 (1.7) | 0.864 |
| Time between ON and examination in months, median (range) | 7 (6–129) | 10 (6–155) | 0.2 |
Abbreviations: MOGAD myelin oligodendrocyte glycoprotein antibody-associated disease, ON optic neuritis, SD standard deviation
Pediatric and adult MOGAD ON cause profound neuroaxonal retinal atrophy
The main SD-OCT results are shown in Table 2 (for more detailed results, see supplementary Table 3 and supplementary Figure 4). The thickness of pRNFL was significantly reduced in MOGADped- and MOGADadult-ON eyes compared to non-ON eyes globally as well as in all segments (pRNFL S, pRNFL I, pRNFL T, pRNFL N, pRNFL PMB); the pRNFL N/T ratio remained unchanged (see Fig. 1 for illustrating OCT images). The total macular volume (TMV), as well as combined GCIPL volume, were also significantly decreased in ON eyes of both pediatric and adult patients compared to non-ON eyes. In contrast, the volume of the inner nuclear layer (INL) was increased in ON eyes of both groups.
Table 2.
Comparison of OCT and VEP measures as well as visual acuity between MOGAD-ON and MOGAD-NON eyes in pediatric and adult MOGAD patients
| Parameter | Pediatric patients | Adult patients | Pediatric patients | Adult patients | MOGAD-ON eyes | MOGAD-NON eyes | ||
|---|---|---|---|---|---|---|---|---|
| MOGAD-ON eyes (30 eyes, mean± SD) | MOGAD-NON eyes (10 eyes, mean± SD) | MOGAD-ON eyes (42 eyes, mean± SD) | MOGAD-NON eyes (36 eyes, mean± SD) | MOGAD-ON vs. MOGAD-NON eyes, p | MOGAD-ON vs. MOGAD-NON eyes, p | Pediatric vs. adult patients, p | Pediatric vs. adult patients, p | |
| pRNFL G | 63.12 ± 18.74 | 90.30 ± 13.40 | 64.26 ± 22.85 | 96.64 ± 20.67 | < 0.001 | 0.001 | 0.997 | 0.292 |
| TMV | 2.19 ± 0.11 | 2.30 ± 0.13 | 2.22 ± 0.12 | 2.31 ± 0.11 | 0.021 | 0.048 | 0.484 | 0.718 |
| mRNFL | 0.12 ± 0.02 | 0.14 ± 0.01 | 0.13 ± 0.02 | 0.14 ± 0.03 | < 0.001 | 0.537 | 0.012 | 0.278 |
| mGCIPL | 0.42 ± 0.09 | 0.57 ± 0.08 | 0.44 ± 0.13 | 0.57 ± 0.08 | < 0.001 | 0.012 | 0.555 | 0.853 |
| mINL | 0.28 ± 0.03 | 0.26 ± 0.02 | 0.29 ± 0.04 | 0.26 ± 0.02 | 0.001 | 0.017 | 0.793 | 0.994 |
| mOPONL | 0.77 ± 0.07 | 0.74 ± 0.08 | 0.77 ± 0.05 | 0.75 ± 0.05 | 0.307 | 0.949 | 0.779 | 0.589 |
| VEP P100 latency | 117.86 ± 10.67 | 112.44 ± 9.59 | 117.99 ± 14.51 | 118.15 ± 9.84 | 0.324 | 0.790 | 0.946 | 0.115 |
| VEP amplitude | 10.57 ± 6.12 | 8.52 ± 4.47 | 5.76 ± 2.79 | 7.61 ± 5.21 | 0.235 | 0.049 | – | – |
| HC VA | 51.36 ± 9.33 | 55.60 ± 8.88 | 34.97 ± 20.57 | 52.03 ± 8.67 | 0.245 | 0.002 | < 0.0001 | 0.325 |
| 2.5% LC VA | 22.83 ± 14.62 | 25.60 ± 13.94 | 13.54 ± 16.44 | 29.52 ± 13.89 | 0.963 | 0.017 | 0.028 | 0.451 |
GEE analysis: p value: significant results p < 0.05 are indicated in bold
Abbreviations: MOGAD-ON eyes with a history of ON, MOGAD-NON eyes without a history of ON, pRNFL peripapillary retinal nerve fiber layer (G global), TMV total macular volume, mRNFL macular RNFL, mGCIPL macular ganglion cell and inner plexiform layer, mINL macular inner nuclear layer, mOPONL macular outer plexiform and outer nuclear layer, HC high-contrast, LC low-contrast, VA visual acuity, pRNFL thickness in μm, macular volumes in mm3, VEP P100 latency in ms, VEP amplitude in uV, VA number of correctly stated letters
Comparing MOGADped-ON and MOGADadult-ON eyes, we found no differences in pRNFL thickness or volumes of the macular GCIPL, INL, and OPONL, while macular RNFL was significantly lower in MOGADped-ON eyes compared to MOGADadult-ON eyes. Macular microcysts were observed in 10% of MOGADped-ON and 7.7% of MOGADadult-ON eyes.
Peripapillary retinal atrophy patterns are similar independently of age
There was no difference in the pattern of neuroaxonal retinal atrophy between MOGADped and MOGADadult ON eyes (Table 3, supplementary results). MOGAD ON eyes showed no temporal predominance of pRNFL atrophy after 1.8±1.3 vs. 2.0±1.7 ON episodes per eye in children and adults respectively. The N/T ratio was comparable in affected eyes of pediatric and adult patients.
Children demonstrate significantly better visual outcome after MOGAD ON
VA testing revealed significantly worse HCVA and LCVA in affected MOGADadult-ON eyes (Table 2, supplementary Figure 4). This was not the case for affected MOGADped-ON eyes. Complete visual recovery (defined as HCVA logMAR 0.0) occurred in 73.3% of MOGADped-ON eyes vs. 31.0% MOGADadult-ON eyes. Only 3.3% of MOGADped-ON eyes vs. 31.0% of MOGADadult-ON eyes showed moderate to severe (HCVA logMAR > 0.5) visual impairment. HCVA and LCVA were significantly worse in affected eyes of MOGADadult versus MOGADped patients; there was no difference noted in unaffected non-ON eyes.
VEP are not different in affected eyes in pediatric and adult MOGAD patients
VEP latencies were only moderately prolonged and showed no significant differences between affected and unaffected eyes in both cohorts (Table 2). Interestingly, the VEP latencies were also almost identical in affected eyes in MOGADped and MOGADadult patients. Significant reduction of the VEP amplitudes was observed in the affected eyes of MOGADadult patients only. VEP amplitude varies with age and was not compared between MOGADped and MOGADadult patients.
Age at onset correlates with visual outcome in MOGAD ON
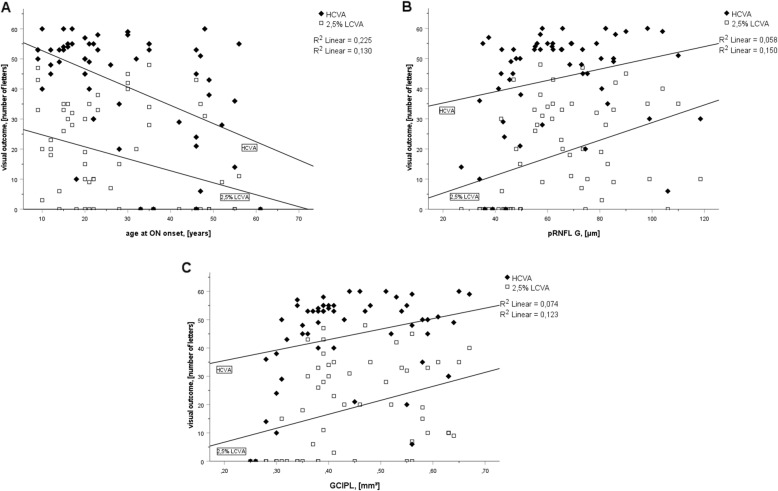
We next evaluated the effects of the number of previous ON attacks, age at ON onset, extent of neuroaxonal retinal atrophy and optic nerve signal transmission (P100 latency) on the HCVA, and LCVA in the entire study population (Fig. 2 and supplementary Figure 5). Neither the number of previous ON nor P100 latency correlated with HCVA or LCVA. Neuroaxonal retinal atrophy weakly correlated with HCVA (rho=0.311 CI95% 0.080–0.510, p=0.014 [pRNFL] and rho=0.282 CI95% 0.049–0.486, p=0.036 [GCIPL]) and more closely with a LCVA reduction (rho=0.498 CI95% 0.288 0.662, p<0.001 [pRNFL] and rho=0.448 CI95% 0.230 0.623, p=0.001 [GCIPL]) (Fig. 2). We observed a stronger correlation with the age at ON onset (rho=−0.565 CI95% −0.713–0.368, p<0.001 [HCVA] and rho=−0.460 CI95% −0.632–0.244, p<0.001 [LCVA]).
Fig. 2.
Scatterplots of age at ON onset (a), pRNFL G thickness (b), and GCIPL volume (c) against visual outcome (HCVA and 2.5% LCVA). HCVA correlated modestly with neuroaxonal retinal atrophy and more strongly with age at ON onset, whereas LCVA correlated moderately with both parameters
Discussion
In this study, we were able to demonstrate a significantly better visual recovery after ON in pediatric versus adult MOGAD patients. Interestingly, substantial differences in the functional outcome in these two groups were independent of structural neuroaxonal retinal atrophy and signal transmission in the optic nerve, so that other age-dependent mechanisms are likely to be responsible. Our results thus add nicely to the recently published work on clinical features and risk of relapse in children and adults with MOGAD [22]. In this study, overall better remission of general relapses was reported in MOGADped compared to MOGADadult patients, although the underlying mechanisms remained unclear [22].
On a structural level, we clearly observed both pRNFL and GCIPL atrophy in the affected eye compared with the unaffected eyes in MOGADped and MOGADadult patients, indicating combined neuroaxonal retinal atrophy. The extent of degeneration was comparable across both cohorts and in line with recent studies. Previously, we and others have shown that pRNFL parameters, TMV, mRNFL, mGCL, and mIPL are significantly reduced in MOGADadult-ON eyes compared to healthy controls [11–13]. There is little data on structural changes after MOGAD ON in children. Two other groups have reported significant neuroaxonal retinal atrophy following MOGADped-ON with an average and median pRNFL thickness of 68.7 ± 12.6 μm and 58 μm, respectively [26, 27]. These findings are comparable to our pRNFL measures (mean 63.1±18.7 μm). Data on GCIPL thickness are only available from one Chinese cohort. Similar to our study, the combined GCIPL volume in MOGADped-ON was significantly reduced [26].
The volume of the INL was increased in ON eyes of both MOGADped and MOGADadult patients. It has been suggested that the INL may serve as a biomarker for inflammatory processes, since an increase in INL volume is associated with MS-ON and risk for clinical relapse [28]. However, the meaning of the INL volume change in MOGAD remains uncertain and may occur in compensation to neuroaxonal retinal atrophy. We could not confirm an association between INL volume and relapse rate in our cohort (data not shown). Microcystic macular edema (MME) in the INL has been documented in MOGAD ON [12, 13]. We observed INL-MME in 10% vs. 7.7% of MOGADped- and MOGADadult-ON, but not in unaffected eyes. In contrast to MS, we did not observe predominant temporal atrophy or any other specific pattern of RNFL loss. We found a high rate of RNFL atrophy in all optic nerve head segments [29].
MOGAD ON has been reported, in general, to have a more favorable visual prognosis when compared to AQP-4 IgG-seropositive NMOSD ON [30–35]. Study results, however, varied significantly, probably due in part to MOGAD clinical heterogeneity [11, 12, 27, 30–32, 34, 36–38]. Our adult MOGAD cohort, being partly included in previous multicenter studies, showed significant visual impairment in a relatively high proportion of patients after MOGAD ON [12, 13]. Nevertheless, other studies also reported functional blindness in MOGAD ON patients, with a VA below logMAR 1.0 in 13% of MOGAD ON eyes [11, 13]. In contrast, we observed a very good functional recovery in the pediatric cohort despite similar levels of neuroaxonal retinal atrophy. Interestingly, another study also demonstrated a more favorable functional outcome in MOGAD ON compared to AQP4-IgG seropositive NMOSD ON despite similar structural pRNFL degeneration [37]. HCVA in our MOGADped-ON cohort was similar to that reported by Wan et al. (complete recovery in 85% of affected eyes [n=59, mean age 12.6, range 3.9–18.8]) [36]. In contrast, Eyre et al. showed that only 65% of children from the mixed cohort recovered completely; microstructural damage of the retina was the only factor correlating with visual recovery [38].
The association of better visual recovery with younger age at ON onset is a central finding of this study. The better visual recovery in younger patients was independent of the number of previous ON attacks per eye, microstructural retinal changes, or optic nerve signal transmission. This is generally consistent with previous observations of better visual recovery in younger patients with AQP4-abs-positive NMOSD-ON and MS-ON, although these studies did not control for the number of previous ON episodes, optic nerve signal transmission, and neuroaxonal retinal atrophy [21, 33, 34]. In a recently published analysis of the relapse recovery of children versus adults with MS, the probability of incomplete recovery increased 1.33-fold with each decade of life [39].
We propose that an active age-dependent neuroplasticity of the visual system, most likely at a cortical level, may explain our findings. First, there is evidence for the existence of immature neural visual circuits in children enabling further development of visual acuity in childhood and adolescence [40]. Indeed, the HCVA of healthy children up to the age of 8–10 years is inferior to that of healthy adults, and the age of 6–8 years is widely accepted as a “sensitive period” for the development of amblyopia [40–42]. Second, animal studies have shown that contrast sensitivity is less promoted by retinal integrity than by the maturation of visual circuits [43]. In children, contrast sensitivity matures between the age of 8 and 19 [41]. Accordingly, visual system maturation is likely to be ongoing in our MOGADped cohort (mean age 10.3±3.7 years). Indeed, we observed only minimal differences in HCVA and LCVA between affected and non-affected- MOGADped-ON eyes, regardless of the profound neuroaxonal retinal atrophy. Third, a large functional magnetic resonance imaging (fMRI) study demonstrated a clear association between fMRI activity in the lateral occipital cortex (as a marker of early adaptive neuroplasticity) and visual outcome in young adults (mean age 32 years) with acute ON. Interestingly, the fMRI activity was the strongest predictor of the visual outcome in this study and was also independent of other structural or electrophysiological parameters at baseline or 12 months follow-up [44].
Given the heterogeneity of MOGAD, we cannot exclude that age- or disease-dependent differences in the MOGAD autoimmunity contribute to the noted differences in ON recovery. Indeed, the role of MOG-IgG in disease pathogenesis is not clear, and the titer and epitope specificity of MOG-IgG may drive distinct effects during injury and recovery [34, 35]. Additionally, other cellular mechanisms, including T cell and microglial response, need to be further characterized [2, 14]. Nevertheless, in our cohorts, these immunologic variables did not impact structural or electrophysiologic metrics.
Our study has several limitations. Due to the limited dataset, the extent of visual impairment before the onset of ON and at nadir remains unknown and cannot be compared between the groups. Visual acuity was corrected habitually, and there is no visual field or color vision data. In addition, our study lacks MRI data on the extent and volume of optic nerve lesions and accompanying lesions in the post-geniculate visual pathways or occipital cortex. A prospective study combining longitudinal clinical data, OCT and neuro-imaging, visual field data, electrophysiology, and fMRI would be important to confirm our findings. Furthermore, studies in MS or aquaporin-4-IgG-positive NMOSD are needed to clarify if the demonstrated age-dependent VA improvement is universal or disease-specific.
Conclusion
In summary, a comparison of pediatric and adult MOGAD cohorts demonstrates age-dependent effects on visual recovery after ON. Despite almost identical neuroaxonal retinal atrophy, functional vision is notably better in children than in adults. Age-dependent cortical neuroplasticity seems to be the most plausible mechanism explaining this dissociation. Future studies, combining precise analysis of the anterior and posterior visual system, are needed to confirm the leading role of central neuroplasticity in determining visual recovery after MOGAD-ON. Identification of a functionally relevant cutoff age for the visual recovery in MOGAD-ON could be of high relevance for the prognosis and care.
Supplementary Information
Additional file 1: Supplementary Figure 3: Flow chart of patients included in the study. During the study period 64 MOGAD patients were identified in participating centers. Five patients were excluded due to incomplete examination data (n=2) or an acute ON at the time of examination (n=3). Depending on the age of manifestation patients were divided into 2 groups: group (1) 20 MOG-IgG-positive patients with initial manifestation < 18 years (MOGADped) and group (2) 39 MOG-IgG-positive patients with initial manifestation ≥ 18 years (MOGADadult). Supplementary Figure 4: Beeswarm plots showing the distribution of pRNFL G thickness (A), GCIPL volume (B), HCVA (C) and 2,5% LCVA (D) in MOGADped-ON and MOGADadult-ON. Despite profound neuroaxonal retinal atrophy in both groups, MOGADadult-ON eyes showed significantly worse visual outcome (HCVA and 2,5% LCVA) in comparison to MOGADped-ON. Supplementary Figure 5: Scatterplots of VEP P100 latency (A) and number of previous ON (B) against visual outcome (HCVA and 2,5% LCVA). Neither the number of previous ON nor P100-latency correlated with HCVA (rho=0.030 CI95% -0.288 -0.152, p=0.840 [number of previous ON] and rho=-0.028 CI95% -0.354 -0.290, p=0.851 [VEP P100 latency]) or LCVA (rho=-0.237 CI95% -0.422 -0.106, p=0.104 [number of previous ON] and rho=-0.263 CI95% -0.503 -0.078, p=0.071 [VEP P100 latency]). Supplementary Table 3: Demographic and main clinical characteristics of pediatric and adult cohorts with ON history (MOGAD-ON). Abbreviations: MOGAD myelin oligodendrocyte glycoprotein-antibody-associated disease, ON optic neuritis, SD standard deviation. Supplementary Table 4. Comparison of all OCT and VEP measures as well as visual acuity between in ON- and non-ON-eyes in pediatric and adult MOGAD patients. Abbreviations: MOGAD-ON eyes with a history of ON, MOGAD-NON eyes without history of ON, pRNFL peripapillary retinal nerve fibre layer (G global, S superior, l inferior, T temporal, N nasal, PMB papillomacular bundle, N/T nasal/temporal ratio), TMV total macular volume, mRNFL macular RNFL, mGCIPL macular ganglion cell and inner plexiform layer, mINL macular inner nuclear layer, mOPONL macular outer plexiform and outer nuclear layer, HCVA high-contrast, LCVA low-contrast visusal acuity. pRNFL thickness in μm and macular volumes in mm3, VEP P100 latency in ms, VEP amplitude in uV, VA in number of correctly stated letters. GEE analysis: B regression coefficient, p-value: significant results p < 0.05 are indicated in bold letters.
Acknowledgements
We would like to thank all patients and their families for participating in the study. We would like to thank Inga Sedleckiene, Yvonne Skrzypiec, Ute Rüther, Stefanie Stüwe (Bochum), Angelika Bamberger, and Luise Böhm (Munich) for their excellent technical support.
Authors’ contributions
JH, TP, KR, and IA: conception and design of the study, acquisition and analysis of the data, drafting of a significant portion of the manuscript or figures, and analysis or interpretation of the data. IK: conception and design of the study, acquisition and analysis of the data, and revision of the manuscript for content. CS, JLB, AKK, AFR, SCJ, ALH, TK, MK, EW, MR, CT, TL, KH, and RG: acquisition and analysis of the data, analysis or interpretation of the data, and revision of the manuscript for content. The authors read and approved the final manuscript.
Funding
JH is (partially) funded by the German Federal Ministry of Education and Research (Grant Numbers 01ZZ1603[A-D] and 01ZZ1804[A-H] (DIFUTURE)). Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all patients participating in the study. The local ethics committees approved the study protocol in accordance with the Declaration of Helsinki (1964) in its currently applicable version.
Consent for publication
Not applicable.
Competing interests
J. Havla reports grants for OCT research from the Friedrich-Baur-Stiftung and Merck; personal fees and non-financial support from Celgene, Merck, Alexion, Novartis, Roche, Santhera, Biogen, Heidelberg Engineering, and Sanofi Genzyme; and non-financial support of the Guthy-Jackson Charitable Foundation, all outside the submitted work.
T. Pakeerathan has no conflicts of interest.
C. Schwake has no conflicts of interest.
J.L. Bennett reports payment for study design/consultation from MedImmune/Viela Bio; personal fees from AbbVie, Alexion, Chugai, Clene Nanomedicine, Mitsubishi-Tanabe, Reistone Bio, Genentech, and Roche; grants and personal fees from Novartis, Mallinckrodt, and the National Institutes of Health; and has a patent for Aquaporumab issued.
I. Kleiter has received speaker honoraria or travel funding from Alexion, Biogen, Novartis, Merck, Mylan, Sanofi Genzyme, and Roche; travel funding from the Guthy-Jackson Charitable Foundation; and consulted for Alexion, Biogen, Celgene, Chugai, IQVIA, Novartis, Merck, and Roche.
A. Felipe-Rucián has no conflicts of interest.
S.C. Joachim received travel funding and/or speaker honoraria from Bayer, Novartis, and Roche and received research support from Bayer, Novartis, Roche, and Ursapharm.
A. S. Lotz-Havla has received travel expenses from BioMarin.
T. Kümpfel received speaker and/or consultation honoraria from Bayer Healthcare, Merck, Novartis Pharma, Roche Pharma, and Biogen as well as grant support from Novartis.
M. Krumbholz received travel funding and/or speaker honoraria from Merck, Novartis, and Roche and research support from Merck.
E. Wendel has nothing to declare.
M. Reindl (the University Hospital and Medical University of Innsbruck (Austria); employer of M. Reindl) receives payments for antibody assays (MOG, AQP4, and other autoantibodies) and for antibody validation experiments organized by Euroimmun and research grants from Euroimmun and Roche.
C. Thiels has no conflicts of interest.
T. Lücke has no conflicts of interest.
K. Hellwig has received travel grants from Biogen, Novartis, and Merck and received speaker and research honoraria from Biogen Idec Germany, Teva, Sanofi Genzyme, Novartis, Bayer Healthcare, Merck Serono, and Roche
R. Gold received speaker’s and board honoraria from Baxter, Bayer Schering, Biogen Idec, CLB Behring, Genzyme, Merck Serono, Novartis, Stendhal, Talecris, and Teva. His department received grant support from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, and Teva. All are not related to the content of this manuscript.
K. Rostasy has no conflict of interest related to this article.
I. Ayzenberg has received travel grants from Biogen Idec and Guthy-Jackson Charitable Foundation, served on scientific advisory boards for Roche and Alexion, and received research support from Diamed, not related to this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joachim Havla, Thivya Pakeerathan, Kevin Rostasy, and Ilya Ayzenberg contributed equally to the manuscript.
Contributor Information
Joachim Havla, Email: joachim.havla@med.lmu.de.
Ilya Ayzenberg, Email: ilya.ayzenberg@rub.de.
References
- 1.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, Franciotta D, Fujihara K, Jacob A, Kim HJ, Kleiter I, Kümpfel T, Levy M, Palace J, Ruprecht K, Saiz A, Trebst C, Weinshenker BG, Wildemann B. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spadaro M, Winklmeier S, Beltran E, Macrini C, Höftberger R, Schuh E, Thaler F, Gerdes LA, Laurent S, Gerhards R, et al. Pathogenic mechanisms of human autoantibodies against myelin oligodendrocyte glycoprotein. Mult Scler J. 2017;23:976–1023. doi: 10.1177/1352458517733228. [DOI] [Google Scholar]
- 3.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, Ciron J, Collongues N, Brassat D, Cotton F, Papeix C, Durand-Dubief F, Laplaud D, Deschamps R, Cohen M, Biotti D, Ayrignac X, Tilikete C, Thouvenot E, Brochet B, Dulau C, Moreau T, Tourbah A, Lebranchu P, Michel L, Lebrun-Frenay C, Montcuquet A, Mathey G, Debouverie M, Pelletier J, Labauge P, Derache N, Coustans M, Rollot F, de Seze J, Vukusic S, Marignier R, OFSEP and NOMADMUS Study Group Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90(21):e1858–e1869. doi: 10.1212/WNL.0000000000005560. [DOI] [PubMed] [Google Scholar]
- 4.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hummert MW, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wegener-Panzer A, Cleaveland R, Wendel EM, Baumann M, Bertolini A, Hausler M, Knierim E, Reiter-Fink E, Breu M, Sonmez O, et al. Clinical and imaging features of children with autoimmune encephalitis and MOG antibodies. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e731. doi: 10.1212/NXI.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacohen Y, Banwell B. Treatment approaches for MOG-Ab-associated demyelination in children. Curr Treat Options Neurol. 2019;21(1):2. doi: 10.1007/s11940-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, White OB, Broadley S, Lechner-Scott J, Vucic S, Henderson APD, Barnett MH, Reddel SW, Brilot F, Dale RC, Australasian and New Zealand MOG Study Group Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reindl M, Rostasy K. MOG antibody-associated diseases. Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e60. doi: 10.1212/NXI.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofman P, Hoyng P, vander Werf F, Vrensen GF, Schlingemann RO. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42(5):895–901. [PubMed] [Google Scholar]
- 10.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197(9):1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, Bellmann-Strobl J, Jarius S, Wildemann B, Reindl M, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13(1):282. doi: 10.1186/s12974-016-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havla J, Kumpfel T, Schinner R, Spadaro M, Schuh E, Meinl E, Hohlfeld R, Outteryck O. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol. 2017;264(1):139–151. doi: 10.1007/s00415-016-8333-7. [DOI] [PubMed] [Google Scholar]
- 13.Oertel FC, Outteryck O, Knier B, Zimmermann H, Borisow N, Bellmann-Strobl J, Blaschek A, Jarius S, Reindl M, Ruprecht K, Meinl E, Hohlfeld R, Paul F, Brandt AU, Kümpfel T, Havla J. Optical coherence tomography in myelin-oligodendrocyte-glycoprotein antibody-seropositive patients: a longitudinal study. J Neuroinflammation. 2019;16(1):154. doi: 10.1186/s12974-019-1521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, Joldic D, Pittock SJ, Tillema JM, Gorman M, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020;139(5):875–892. doi: 10.1007/s00401-020-02132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, Vucic S, Gorman MP, Benson LA, Alper G, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22(4):470–482. doi: 10.1177/1352458515593406. [DOI] [PubMed] [Google Scholar]
- 16.Wendel EM, Baumann M, Barisic N, Blaschek A, Coelho de Oliveira Koch E, Della Marina A, Diepold K, Hackenberg A, Hahn A, von Kalle T, et al. High association of MOG-IgG antibodies in children with bilateral optic neuritis. Eur J Pediatr Neurol. 2020;27:86–93. doi: 10.1016/j.ejpn.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Jonzzon S, Suleiman L, Yousef A, Young B, Hart J, Peschl P, Reindl M, Schaller KL, Bennett JL, Waubant E, Graves JS. Clinical features and outcomes of pediatric monophasic and recurrent idiopathic optic neuritis. J Child Neurol. 2020;35(1):77–83. doi: 10.1177/0883073819877334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocca MA, Absinta M, Ghezzi A, Moiola L, Comi G, Filippi M. Is a preserved functional reserve a mechanism limiting clinical impairment in pediatric MS patients? Hum Brain Mapp. 2009;30(9):2844–2851. doi: 10.1002/hbm.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, Tardieu M. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6(10):887–902. doi: 10.1016/S1474-4422(07)70242-9. [DOI] [PubMed] [Google Scholar]
- 20.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(4):770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 21.Lana-Peixoto MA, Andrade GC. The clinical profile of childhood optic neuritis. Arq Neuropsiquiatr. 2001;59(2B):311–317. doi: 10.1590/S0004-282X2001000300001. [DOI] [PubMed] [Google Scholar]
- 22.Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. Clinical Features and Risk of Relapse in Children and Adults with Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Ann Neurol. 2021;89(1):30–41.10.1002/ana.25909. Epub 2020 Oct 15. [DOI] [PubMed]
- 23.Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, Fryer JP, Mills J, Teegen B, Mindorf S, Ritter N, Krummrei U, Stöcker W, Eggert J, Flanagan EP, Ramberger M, Hegen H, Rostasy K, Berger T, Leite MI, Palace J, Irani SR, Dale RC, Probst C, Probst M, Brilot F, Pittock SJ, Waters P. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e674. doi: 10.1212/NXI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA, Schuman JS, Villoslada P, Calabresi P, Balcer L, Petzold A, Green AJ, Paul F, Brandt AU, Albrecht P, IMSVISUAL consortium The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303–2309. doi: 10.1212/WNL.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schippling S, Balk LJ, Costello F, Albrecht P, Balcer L, Calabresi PA, Frederiksen JL, Frohman E, Green AJ, Klistorner A, Outteryck O, Paul F, Plant GT, Traber G, Vermersch P, Villoslada P, Wolf S, Petzold A. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21(2):163–170. doi: 10.1177/1352458514538110. [DOI] [PubMed] [Google Scholar]
- 26.Song H, Zhou H, Yang M, Xu Q, Sun M, Wei S. Clinical characteristics and outcomes of myelin oligodendrocyte glycoprotein antibody-seropositive optic neuritis in varying age groups: a cohort study in China. J Neurol Sci. 2019;400:83–89. doi: 10.1016/j.jns.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Narayan RN, McCreary M, Conger D, Wang C, Greenberg BM. Unique characteristics of optical coherence tomography (OCT) results and visual acuity testing in myelin oligodendrocyte glycoprotein (MOG) antibody positive pediatric patients. Mult Scler Relat Disord. 2019;28:86–90. doi: 10.1016/j.msard.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Balk LJ, Coric D, Knier B, Zimmermann HG, Behbehani R, Alroughani R, Martinez-Lapiscina EH, Brandt AU, Sanchez-Dalmau B, Vidal-Jordana A, et al. Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study. Mult Scler J Exp Transl Clin. 2019;5:2055217319871582. doi: 10.1177/2055217319871582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Outteryck O, Majed B, Defoort-Dhellemmes S, Vermersch P, Zephir H. A comparative optical coherence tomography study in neuromyelitis optica spectrum disorder and multiple sclerosis. Mult Scler. 2015;21(14):1781–1793. doi: 10.1177/1352458515578888. [DOI] [PubMed] [Google Scholar]
- 30.Chen JJ, Flanagan EP, Jitprapaikulsan J, Lopez-Chiriboga ASS, Fryer JP, Leavitt JA, Weinshenker BG, McKeon A, Tillema JM, Lennon VA, et al. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2018;195:8–15. doi: 10.1016/j.ajo.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deschamps R, Pique J, Ayrignac X, Collongues N, Audoin B, Zephir H, Ciron J, Cohen M, Aboab J, Mathey G, et al. The long-term outcome of MOGAD: an observational national cohort study of 61 patients. Eur J Neurol. 2021;28(5):1659–1664. doi: 10.1111/ene.14746. [DOI] [PubMed] [Google Scholar]
- 32.Jitprapaikulsan J, Chen JJ, Flanagan EP, Tobin WO, Fryer JP, Weinshenker BG, McKeon A, Lennon VA, Leavitt JA, Tillema JM, Lucchinetti C, Keegan BM, Kantarci O, Khanna C, Jenkins SM, Spears GM, Sagan J, Pittock SJ. Aquaporin-4 and myelin oligodendrocyte glycoprotein autoantibody status predict outcome of recurrent optic neuritis. Ophthalmology. 2018;125(10):1628–1637. doi: 10.1016/j.ophtha.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 33.Akaishi T, Nakashima I, Takeshita T, Mugikura S, Sato DK, Takahashi T, Nishiyama S, Kurosawa K, Misu T, Nakazawa T, Aoki M, Fujihara K. Lesion length of optic neuritis impacts visual prognosis in neuromyelitis optica. J Neuroimmunol. 2016;293:28–33. doi: 10.1016/j.jneuroim.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Bruijstens AL, Breu M, Wendel EM, Wassmer E, Lim M, Neuteboom RF, Wickstrom R, consortium EUpM E.U. Paediatric MOG Consortium Consensus: Part 4 - Outcome of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:32–40. doi: 10.1016/j.ejpn.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Armangue T, Capobianco M, de Chalus A, Laetitia G, Deiva K, consortium EUpM E.U. Paediatric MOG Consortium Consensus: Part 3 - Biomarkers of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:22–31. doi: 10.1016/j.ejpn.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Wan MJ, Adebona O, Benson LA, Gorman MP, Heidary G. Visual outcomes in pediatric optic neuritis. Am J Ophthalmol. 2014;158(3):503–507. doi: 10.1016/j.ajo.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Zhao G, Chen Q, Huang Y, Li Z, Sun X, Lu P, Yan S, Wang M, Tian G. Clinical characteristics of myelin oligodendrocyte glycoprotein seropositive optic neuritis: a cohort study in Shanghai, China. J Neurol. 2018;265(1):33–40. doi: 10.1007/s00415-017-8651-4. [DOI] [PubMed] [Google Scholar]
- 38.Eyre M, Hameed A, Wright S, Brownlee W, Ciccarelli O, Bowman R, Lim M, Wassmer E, Thompson D, Hemingway C, Hacohen Y. Retinal nerve fibre layer thinning is associated with worse visual outcome after optic neuritis in children with a relapsing demyelinating syndrome. Dev Med Child Neurol. 2018;60(12):1244–1250. doi: 10.1111/dmcn.13757. [DOI] [PubMed] [Google Scholar]
- 39.Chitnis T, Aaen G, Belman A, Benson L, Gorman M, Goyal MS, Graves JS, Harris Y, Krupp L, Lotze T, Mar S, Ness J, Rensel M, Schreiner T, Tillema JM, Waubant E, Weinstock-Guttman B, Roalstad S, Rose J, Weiner HL, Casper TC, Rodriguez M, for the US Network of Paediatric Multiple Sclerosis Centers Improved relapse recovery in paediatric compared to adult multiple sclerosis. Brain. 2020;143(9):2733–2741. doi: 10.1093/brain/awaa199. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, Zhou J, Zhao H, Dai Y, Zhang Y, Tang Y, Zhou Y. Immature visual neural system in children reflected by contrast sensitivity with adaptive optics correction. Sci Rep. 2014;4:4687. doi: 10.1038/srep04687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leat SJ, Yadav NK, Irving EL. Development of visual acuity and contrast sensitivity in children. Journal of Optometry. 2009;2(1):19–26. doi: 10.3921/joptom.2009.19. [DOI] [Google Scholar]
- 42.Castaldi E, Lunghi C, Morrone MC. Neuroplasticity in adult human visual cortex. Neurosci Biobehav Rev. 2020;112:542–552. doi: 10.1016/j.neubiorev.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Kiorpes L, Tang C, Hawken MJ, Movshon JA. Ideal observer analysis of the development of spatial contrast sensitivity in macaque monkeys. J Vis. 2003;3(10):630–641. doi: 10.1167/3.10.6. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins TM, Toosy AT, Ciccarelli O, Miszkiel KA, Wheeler-Kingshott CA, Henderson AP, Kallis C, Mancini L, Plant GT, Miller DH, Thompson AJ. Neuroplasticity predicts outcome of optic neuritis independent of tissue damage. Ann Neurol. 2010;67(1):99–113. doi: 10.1002/ana.21823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 3: Flow chart of patients included in the study. During the study period 64 MOGAD patients were identified in participating centers. Five patients were excluded due to incomplete examination data (n=2) or an acute ON at the time of examination (n=3). Depending on the age of manifestation patients were divided into 2 groups: group (1) 20 MOG-IgG-positive patients with initial manifestation < 18 years (MOGADped) and group (2) 39 MOG-IgG-positive patients with initial manifestation ≥ 18 years (MOGADadult). Supplementary Figure 4: Beeswarm plots showing the distribution of pRNFL G thickness (A), GCIPL volume (B), HCVA (C) and 2,5% LCVA (D) in MOGADped-ON and MOGADadult-ON. Despite profound neuroaxonal retinal atrophy in both groups, MOGADadult-ON eyes showed significantly worse visual outcome (HCVA and 2,5% LCVA) in comparison to MOGADped-ON. Supplementary Figure 5: Scatterplots of VEP P100 latency (A) and number of previous ON (B) against visual outcome (HCVA and 2,5% LCVA). Neither the number of previous ON nor P100-latency correlated with HCVA (rho=0.030 CI95% -0.288 -0.152, p=0.840 [number of previous ON] and rho=-0.028 CI95% -0.354 -0.290, p=0.851 [VEP P100 latency]) or LCVA (rho=-0.237 CI95% -0.422 -0.106, p=0.104 [number of previous ON] and rho=-0.263 CI95% -0.503 -0.078, p=0.071 [VEP P100 latency]). Supplementary Table 3: Demographic and main clinical characteristics of pediatric and adult cohorts with ON history (MOGAD-ON). Abbreviations: MOGAD myelin oligodendrocyte glycoprotein-antibody-associated disease, ON optic neuritis, SD standard deviation. Supplementary Table 4. Comparison of all OCT and VEP measures as well as visual acuity between in ON- and non-ON-eyes in pediatric and adult MOGAD patients. Abbreviations: MOGAD-ON eyes with a history of ON, MOGAD-NON eyes without history of ON, pRNFL peripapillary retinal nerve fibre layer (G global, S superior, l inferior, T temporal, N nasal, PMB papillomacular bundle, N/T nasal/temporal ratio), TMV total macular volume, mRNFL macular RNFL, mGCIPL macular ganglion cell and inner plexiform layer, mINL macular inner nuclear layer, mOPONL macular outer plexiform and outer nuclear layer, HCVA high-contrast, LCVA low-contrast visusal acuity. pRNFL thickness in μm and macular volumes in mm3, VEP P100 latency in ms, VEP amplitude in uV, VA in number of correctly stated letters. GEE analysis: B regression coefficient, p-value: significant results p < 0.05 are indicated in bold letters.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.