Abstract
Background
Tick-borne pathogens other than Borrelia burgdorferi sensu lato – the causative agent of Lyme borreliosis – are common in Ixodes ricinus ticks. How often these pathogens cause human disease is unknown. In addition, diagnostic tools to identify such diseases are lacking or reserved to research laboratories. To elucidate their prevalence and disease burden, the study ‘Ticking on Pandora’s Box’ has been initiated, a collaborative effort between Amsterdam University Medical Center and the National Institute for Public Health and the Environment.
Methods
The study investigates how often the tick-borne pathogens Anaplasma phagocytophilum, Babesia species, Borrelia miyamotoi, Neoehrlichia mikurensis, spotted fever group Rickettsia species and/or tick-borne encephalitis virus cause an acute febrile illness after tick-bite. We aim to determine the impact and severity of these tick-borne diseases in the Netherlands by measuring their prevalence and describing their clinical picture and course of disease.
The study is designed as a prospective case-control study. We aim to include 150 cases – individuals clinically suspected of a tick-borne disease – and 3 matched healthy control groups of 200 persons each. The controls consist respectively of a group of individuals with either a tick-bite without complaints, the general population and of healthy blood donors. During a one-year follow-up we will acquire blood, urine and skin biopsy samples and ticks at baseline, 4 and 12 weeks. Additionally, participants answer modified versions of validated questionnaires to assess self-reported symptoms, among which the SF-36, on a 3 monthly basis.
Discussion
This article describes the background and design of the study protocol of ‘Ticking on Pandora’s Box’. With our study we hope to provide insight into the prevalence, clinical presentation and disease burden of the tick-borne diseases anaplasmosis, babesiosis, B. miyamotoi disease, neoehrlichiosis, rickettsiosis and tick-borne encephalitis and to assist in test development as well as provide recommendations for national guidelines.
Trial registration
NL9258 (retrospectively registered at Netherlands Trial Register, trialregister.nl in in February 2021).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06190-9.
Keywords: Prospective case-control study, Study protocol, Ixodes ricinus ticks, Tick-borne pathogens, Tick-borne diseases, Hard tick-borne fever, Fever after tick-bite
Background
Tick-borne diseases (TBDs) have been predicted to increase in Europe due to a combination of human behaviour, climatic and environmental changes [1]. Annually tens of thousands of European patients acquire Lyme borreliosis (LB) and tick-borne encephalitis (TBE) [2–5]. In contrast, the prevalence and impact of other TBDs, in Europe in general and in the Netherlands in particular, are currently unknown [6–11]. These TBDs are caused by several tick-borne pathogens (TBPs) – including Anaplasma phagocytophilum, Babesia divergens, Babesia microti, Babesia venatorum, Borrelia miyamotoi, Neoehrlichila mikurensis, Rickettsia helvetica and Rickettsia monacensis – which are all detected in Ixodes ricinus in the Netherlands [7, 12–19].
With annually approximately one and a half million tick-bites in the Netherlands alone [11] and roughly one third of the ticks being infected with at least one potential TBP other than Borrelia burgdorferi sensu lato [19], numerous individuals are exposed to other TBPs each year. Recent molecular detection data indicate that the probability of infection with another TBP after a tick-bite in the Netherlands is 2–3%, which is comparable to the risk of contracting LB [20]. It should be noted that – in analogy to an infection with B. burgdorferi s.l. – not everyone who gets infected with another TBP will develop symptomatic disease. Nevertheless there is mounting published and unpublished data showing molecular and serological evidence of infection with various TBPs in humans in Europe, including the Netherlands, in both asymptomatic and symptomatic patients [21–32]. These are the main reasons why the Minister of Health and the National Health Council [33], requested more insight in the public health relevance of other TBPs.
The clinical course of TBDs other than LB varies widely and can range from a typical febrile illness several weeks after a tick-bite [30, 34] to a less frequent severe illness and even death in immunocompromised patients, depending on the TBP [28, 34]. Moreover, co-infections with multiple TBPs appear to be able to alter the course of acute LB [35–41]. However, even in endemic regions in the USA and Europe, co-infections seem to only occur occasionally [21, 24, 42–44].
Lack of awareness, case definitions, laboratory diagnostics, as well as a non-characteristic clinical presentation are among the reasons why these other TBDs often go undiagnosed [19, 45]. With the current study, we aim to determine the impact and severity of other TBDs in the Netherlands by measuring their prevalence and describing their clinical picture and the course of disease.
Methods/design
Study design
This is a prospective case-control study with a one-year follow-up to assess the disease prevalence and impact of the TBDs anaplasmosis, babesiosis, B. miyamotoi disease (BMD), neoehrlichiosis, rickettsiosis and TBE after a tick-bite in the Netherlands. For this purpose, cases – individuals who develop fever after tick-bite – are compared with several healthy control groups. This study represents a collaborative effort between Amsterdam University Medical Center (former AMC, Amsterdam, the Netherlands) and the National Institute for Public Health and the Environment (RIVM, Bilthoven, the Netherlands). The study has been approved by the Medical Ethics Committee of the Amsterdam UMC (registration no. NL61446.094.17), and is conducted according to the principles of the Declaration of Helsinki.
Study population
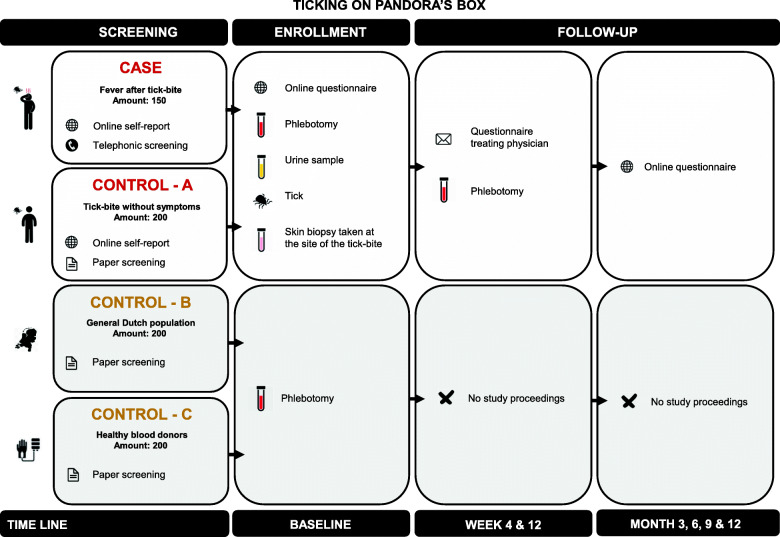
We aim to enroll four groups in the case-control study (Fig. 1). Cases are 150 adults from the Netherlands who report a febrile episode within 4 weeks after tick-bite (Table 1). Strict case descriptions are used for the selection, which includes an objectified fever (measured temperature ≥ 38.0 °C) within the last 4 weeks, developed in the course of 4 weeks after tick-bite. Cases who have not seen the tick attached or who describe signs and symptoms that have another reasonable cause besides a TBD, are excluded. We will also include three type of healthy control participants, consisting of 200 tick-bite controls (A), 200 randomly sampled general Dutch population controls (B) and 200 blood donor controls (C). These groups are frequency matched with the cases by age and gender. Furthermore, both the tick-bite (A) and blood donor controls (C) frequency match to the cases by geographical residence. The tick-bite controls (A) also match by month of tick-bite. All individuals aged ≥16 years reporting on the online inclusion platform or visiting the outpatient department of infectious diseases of the Amsterdam UMC are eligible.
Fig. 1.
Flowchart of study design
Table 1.
Inclusion and exclusion criteria
| Cases | |
| Inclusion criteria: | |
| • Subjects are ≥16 years old; | |
| • Subjects report a tick-bite acquired within the last 2 months; | |
| • Subjects report an objectified (measured rectally, axillary, orally or tympanic) fever (defined as ≥38.0 °C) within the last 4 weeks, developed in the course of 4 weeks after tick-bite; | |
| • Subjects live or stay in the Netherlands during the course of the study. | |
| Exclusion criteria: | |
| • Subjects with evident signs or symptoms of another cause of the fever besides a TBD; | |
| • Subjects unable to provide informed consent or do not have sufficient proficiency of the Dutch language. | |
| Tick-bite controls (A) | |
| Inclusion criteria: | |
| • Subjects are ≥16 years old; | |
| • Subjects report a tick-bite acquired within the last 2 months; | |
| • Subjects frequency match to cases by gender, age, province of residence and month of tick-bite acquirement; | |
| • Subjects live or stay in the Netherlands during the course of the study. | |
| Exclusion criteria: | |
| • Subjects develop an objectified (measured rectally, axillary, orally or tympanic) temperature > 37.3 °C within 4 weeks after the tick-bite; | |
| • Subjects with evident signs or symptoms of a currant infectious disease; | |
| • Subjects unable to provide informed consent or do not have sufficient proficiency of the Dutch language. | |
| Population background controls (B) | |
| Inclusion criteria: | |
| • Subjects are ≥16 years old; | |
| • Subjects participating in the national serosurvey PIENTER-3 of the National Institute for Public Health and Environment (RIVM) of the Netherlands; | |
| • Subjects frequency match to cases by gender and age. | |
| Exclusion criterium: | |
| • Subjects unable to give informed consent or do not have sufficient proficiency of the Dutch, English, French, Spanish, Arabian, Turkish or Papiamentu language. | |
| Healthy blood donors (C) | |
| Inclusion criteria: | |
| • Subjects are ≥18 years old; | |
| • Subjects presenting as a healthy blood donor at the national blood bank Sanquin; | |
| • Subjects frequency match to cases by gender, age and province of residence. | |
| Exclusion criterium: | |
| • Subjects unable to give informed consent. |
Recruitment, inclusion and follow-up of participants
Recruitment started in May 2018. The cases and tick-bite controls (A) are prospectively recruited in a similar manner as described in the study protocols of LymeProspect [46] and VICTORY [47]. The majority of the cases are recruited online, however some participants are additionally included through the outpatient department of infectious diseases of the Amsterdam UMC after evaluation by one of the investigators. The recruitment procedures, and in- and exclusion criteria for the cases are identical in both routes. The tick-bite controls (A) are solely recruited online.
Recruitment, inclusion and follow-up of both the cases and tick-bite controls (A) occur online at www.tekenradar.nl, a secured online platform operated by the RIVM and Wageningen University. People can visit the website after referral by their treating physician or on their own initiative. Cases thus recruited primarily have fever after a tick-bite, whereas the tick-bite controls (A) exclusively report a tick-bite without meeting any criteria of a possible infectious disease diagnosis. Written informed consent is obtained from all eligible participants. Importantly, to verify the complaints or diagnosis after online enrollment the participant’s treating physician is consulted. Blood collection can be done locally or in the participating hospital and is performed at baseline, after 4 weeks and on an optional basis after 12 weeks (Table 2). In addition to the first phlebotomy at baseline, the participants are asked to send in a urine sample and the tick that has bitten them. Cases with skin manifestation have the option of giving additional informed consent for skin biopsy.
Table 2.
Data collection and measurements for study participants. Cases and tick-bite controls
| Baseline | 4 weeks | 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|---|---|
| Written information and informed consent | X | |||||
| Baseline characteristics | X | |||||
| Physical examination | Xa | Xa | ||||
| Recording of clinical manifestation, treatment and concomitant medication | X | X | X | X | X | |
| Recording of adverse events | X | X | ||||
| Laboratory measurements | ||||||
| General laboratory measurements | X | Xb | Xb, c | |||
| Cultures - TBPs | Xd | |||||
| Multiplex qPCR - TBPs | X | X | Xc | |||
| C6 EIA and confirmatory immunoblot – Borrelia burgdorferi s.l. | X | X | Xc | |||
| ELISA and confirmatory VNT - TBEV | X | X | Xc | |||
| IFA – Anaplasma phagocytophilum, Babesia microti, Rickettsia SFG | X | X | Xc | |||
| Immunofluorescence protein microarray – Borrelia miyamotoi | X | X | Xc | |||
| Questionnaires | ||||||
| Comorbidities and pre-existent symptoms | X | X | X | X | X | |
| Somatic symptoms | X | X | X | X | X | |
| Pain, physical and social functioning: SF-36 | X | X | X | X | X | |
| Relevant medical information from the treating physician | X | |||||
For explanation of abbreviations, see the main text
aParticipants included through the outpatient department of infectious diseases of the Amsterdam UMC
bGeneral laboratory measurements are repeated only at a consecutive time-point when deviating at baseline
cLaboratory measurements are only performed when the third optional phlebotomy is performed
dCultures are performed for participants meeting case criteria only
The two other healthy control groups (B and C), are recruited in a separate way to match the cases. Firstly, the general Dutch population controls (B) are retrospectively selected from the existing national serosurvey PIENTER-3, which was carried out throughout 2016–2017 [48]. Additionally, we prospectively recruited blood donor controls (C) from the National blood bank Sanquin in 2018–2019, for which an independent local protocol was approved. In both study arms, written informed consent was obtained and a singular phlebotomy performed at baseline (Table 3). Details on these two study arms are provided in the supplemental material.
Table 3.
Data collection and measurements for study participants. General Dutch population and healthy blood donors
| Baseline | 4 weeks | |
|---|---|---|
| Written information and informed consent | X | |
| Baseline characteristics | X | |
| Recording of adverse events | X | X |
| Laboratory measurements | ||
| C6 EIA and confirmatory immunoblot – Borrelia burgdorferi s.l. | X | |
| ELISA and confirmatory VNT - TBEV | X | |
| IFA – Anaplasma phagocytophilum, Babesia microti, Rickettsia SFG | X | |
| Immunofluorescence protein microarray – Borrelia miyamotoi | X | |
For explanation of abbreviations, see the main text
Epidemiological and clinical measurements
For all participants in the four study arms standard demographical characteristics are available, including age, gender and place of residence. Both cases and tick-bite control (A) participants also provide information on comorbidities and details on previous tick exposure and previous or current episodes of TBDs at baseline and throughout follow-up. Photographs of skin manifestations after tick-bite are obtained for blinded evaluation by independent experts (Table 2). For the general population control group (B) comorbidities and details on tick exposure and any previous episodes of LB are reported (Table 3).
Laboratory measurements
At baseline general laboratory measurements are performed in a two-step model on the blood samples to gain insight in the general health status of the participant. Thereafter different study materials are used to provide evidence of a current TBD. For that purpose, specific cultures, molecular assays and serology are performed in an attempt to find evidence of laboratory confirmed LB, anaplasmosis, babesiosis, BMD, neoehrlichiosis, rickettsiosis and/or TBE. Details on the various tests are provided in the supplemental material. All samples are processed blinded, i.e. without any markings related to the identity of the participant, type of participant or time-point.
Questionnaires
Cases and tick-bite control (A) participants will be asked to fill out online questionnaires in both the screening phase as throughout the follow-up phase. Primarily, some screening questions will be displayed, checking for inclusion criteria. In addition, a study physician will screen for other causes of the fever in the medical history of the participant by telephonic interview. Participants with other probable causes of fever are excluded. During a one-year follow-up participants will fill out 3-monthly questionnaires on determinants of health, assessment of symptoms, co-morbid disorders and pre-existing symptoms. These questionnaires consist of modified versions of validated questionnaires, among which the physical and social functioning and the severity and impact of pain through the SF-36 [49, 50], and are specified in the supplementary material.
Outcome measures and data analysis
The primary outcome measures the prevalence of the different TBPs tested in blood, urine and skin biopsy in a group of participants who develop fever within 4 weeks after tick-bite in the Netherlands, in whom other causes of the fever are excluded. This can either be through direct laboratory (molecular or culture) or indirect (serology) evidence. This measurement will be compared to the prevalence of infection with the same TBPs in the different control groups.
The secondary outcomes measure the long term sequelae and the clinical manifestations as well as the course of disease of participants with evidence of an infection with the different TBPs. These data will be obtained from the questionnaires and information from the treating physicians and measured by laboratory tests, culture, molecular and serological analyses in both cases and control groups.
Sample size
Based on our previous research [20] we postulate that in circa 3% (0,03) of the tick-bite controls and in 12% (0,12) of the cases evidence of TBD will be found. Assuming to include 200 tick-bite controls and 150 cases this would yield a power of 89% (alpha < 0,05) to detect a significant difference of disease prevalence between the cases and controls. Additionally, we will include 200 general Dutch population and 200 healthy blood donor controls to enhance this outcome. Details on the power calculations are provided in the supplementary material.
Discussion
The Pandora study aims to evaluate the prevalence of the different TBDs in cases compared to several control groups, together with the determination of clinical manifestations and long term sequelae of the different TBDs. Additionally, acquired study materials – such as bodily materials from well-defined patients and clinical TBP isolates – will serve to validate, improve and develop diagnostic tests.
In general, not much is known about the prevalence and disease burden of the TBDs Anaplasma phagocytophilum, Babesia divergens, Babesia microti, Babesia venatorum, B. miyamotoi, Neoehrlichila mikurensis, Rickettsia helvetica, Rickettsia monacensis and tick-borne encephalitis virus (TBEV) in the Netherlands [20, 30]. Nevertheless they have been observed repetitively in a vast amount of eco-epidemiological studies on Ixodes ricinus [11, 15, 51, 52]. Altogether, with the increasing risk of exposure to and infection with these TBPs, it is crucial to obtain better insight into the risk of disease development.
The main explanation for the presumed underdiagnosis of these other TBDs, is that current diagnostic tools for the TBDs – with exception of TBE – are virtually non-existent, of questionable quality, or poorly validated in a European setting [19]. As a consequence, the awareness of other TBDs among physicians and the public is low. Therefore, to gain more knowledge on the prevalence and nature of TBDs in the Netherlands it is imperative to improve laboratory diagnostic tests.
Our study is unprecedented compared to other studies on other TBDs in Europe, as the patient populations are strictly defined according to consensus criteria, the study includes both tick-bite and healthy controls from the general population as well as blood donors, and is appropriately powered. Furthermore, a wide variety of supportive laboratory tests will be performed which could aid to provide evidence of disease and thus TBP pathogenicity.
In conclusion, the Pandora study has a translational approach consisting of a prospective case-control study to estimate the prevalence and impact of TBDs in the Netherlands and to acquire materials, which directs further improvement and development of diagnostic tests. The results of this study aims to make substantial contributions to and insights into the clinical manifestation, diagnostics and possible treatment of TBDs in the Netherlands. Such information could be used for the modification of existing national guidelines and could be extrapolated to other Western European countries.
Supplementary Information
Additional file 1. (1) Specifics of laboratory measurements. (2) Questionnaires. (3) Sample size calculation.
Acknowledgements
The authors acknowledge the following colleagues for their valuable assistance in setting up and performing this study: G.A. (Anneke) Oei, BASc; J.I. (Jasmin) Ersöz, BASc; Nienke Verhaar, BASc; Jeanine Ursinus, MD; M.E. (Ewoud) Baarsma, MD; Amber Vrijlandt, MD; Joris Koetsveld, MD-PhD; J.J.A. (Jos) Trentelman, MASc; Abhijeet Nayak, PhD; A.P. (Alje) van Dam, MD-PhD; C.W. (Wim) Ang, MD-PhD (all Amsterdam UMC); Arieke Docters-van Leeuwen, BASc; Manoj Fonville, BASc; Ankje de Vries, BASc; Lola Tulen, BASc; (all RIVM).
Trial status
Protocol version 1: 02-01-2018
Protocol version 2: 06-02-2018
Protocol version 3: 13-02-2018
Recruitment started: 16-04-2018
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AMC
Academic Medical Center
- Amsterdam UMC
Amsterdam University Medical Centers
- AST
Aspartate aminotransferase
- B. burgdorferi s.l.
Borrelia burgdorferi sensu lato
- B. miyamotoi
Borrelia miyamotoi
- BMD
Borrelia miyamotoi disease
- CCMO
Central Committee on Research Involving Human Subjects
- CRP
C-reactive protein
- DFM
Dark field microscopy
- EDTA
Ethylenediaminetetraacetic acid
- EIA
Enzyme immunoassay
- ELISA
Enzyme-linked immunosorbent assay
- GGT
Gamma-glutamyl transferase
- IFA
Immunofluorescence assay
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- LB
Lyme borreliosis
- LDH
Lacate dehydrogenase
- MCV
Mean corpuscular volume
- MKP
Modified Kelly-Pettenkofer
- MLST
Multilocus sequence typing
- PHQ-15
Patient Health Questionnaire
- RBC
Red blood cell
- RIVM
Dutch National Institute for Public Health and the Environment
- (RT-)PCR
(Real-time) polymerase chain reaction
- SF-36: SFG
Spotted fever group; Health Status Inventory
- TBD
Tick-borne disease
- TBE
Tick-borne encephalitis
- TBEV
Tick-borne encephalitis virus
- TBP
Tick-borne pathogen
- TiC-P
Trimbos/iMTA questionnaire for Costs associated with Psychiatric Illness
- VNT
Virus neutralization test
- WBC
White blood cell
Authors’ contributions
DH was primarily responsible for drafting this protocol and for the protocol implementation. SAG assists in protocol implementation. MGH, TA, AW, HS, CCvdW and KK held the position of consultations throughout the set-up and implementation of the protocol. HS, CCvdW and JWH are the principal investigators for this project and as such collaborated on the design of this protocol and the supervision of the first author. JWH conceived of the study and functions as the project leader. All authors contributed to the refinement of this manuscript and approved the final version.
Funding
This study is funded by the Netherlands Organization for Health Research and Development (ZonMw, project number 52200–30-07), which has a peer-reviewed the grant application. It was additionally made possible by a small donation through the AMC Foundation (amcfoundation.nl). JWH and HS are supported by the NorthTick, European Union, European Regional Development Fund, in the North Sea Region Programme. None of the funding organizations had or will have any role in the design or the data analysis and interpretation of the study.
Availability of data and materials
We intend to share our datasets either in a publicly available repository or present it in the main manuscript or additional supporting files in a readable format.
Declarations
Ethics approval and consent to participate
This protocol and all associated documents (e.g., participant information form & informed consent form) have been reviewed and approved by the Medical Ethics Committee of the Amsterdam UMC/AMC. This study is registered with the Central Committee on Research Involving Human Subjects (CCMO) of the Netherlands under number NL61446.094.17. Should additional amendments arise, then these will be submitted to the aforementioned Medical Ethics Committee. Participants are all 16 years or older of age and have given written informed consent before enrolment. All study data are processed under a code, which is only accessible to researchers directly involved with the project or persons performing monitoring functions (e.g., the Medical Ethics Committee). Personal information is processed according to the provisions of the European Union’s General Data Protection Regulation. This research is conducted according to the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lindgren E, Andersson Y, Suk JE, Sudre B, Semenza JC. Public health. Monitoring EU emerging infectious disease risk due to climate change. Science. 2012;336(6080):418–419. doi: 10.1126/science.1215735. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro Surveill. 2011;16(27):19906. [PubMed]
- 3.Kunze U, Isw TBE. Tick-borne encephalitis-still on the map: report of the 18th annual meeting of the international scientific working group on tick-borne encephalitis (ISW-TBE) Ticks Tick Borne Dis. 2016;7(5):911–914. doi: 10.1016/j.ttbdis.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Hofhuis A, Harms M, Bennema S, van den Wijngaard CC, van Pelt W. Physician reported incidence of early and late Lyme borreliosis. Parasit Vectors. 2015;8(1):161. doi: 10.1186/s13071-015-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofhuis A, Bennema S, Harms M, van Vliet AJ, Takken W, van den Wijngaard CC, van Pelt W. Decrease in tick bite consultations and stabilization of early Lyme borreliosis in the Netherlands in 2014 after 15 years of continuous increase. BMC Public Health. 2016;16(1):425. doi: 10.1186/s12889-016-3105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 7.Heyman P, Cochez C, Hofhuis A, van der Giessen J, Sprong H, Porter SR, Losson B, Saegerman C, Donoso-Mantke O, Niedrig M, Papa A. A clear and present danger: tick-borne diseases in Europe. Expert Rev Anti-Infect Ther. 2010;8(1):33–50. doi: 10.1586/eri.09.118. [DOI] [PubMed] [Google Scholar]
- 8.Kirby CS, 3rd, Williams SC, Magnarelli LA, Bharadwaj A, Ertel SH, Nelson RS. Expansion of zoonotic babesiosis and reported human cases, Connecticut, 2001-2010. J Med Entomol. 2014;51(1):245–252. doi: 10.1603/ME13154. [DOI] [PubMed] [Google Scholar]
- 9.Molloy PJ, Telford SR, 3rd, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. 2015;163(2):91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 10.Dahlgren FS, Heitman KN, Drexler NA, Massung RF, Behravesh CB. Human granulocytic anaplasmosis in the United States from 2008 to 2012: a summary of national surveillance data. Am J Trop Med Hyg. 2015;93(1):66–72. doi: 10.4269/ajtmh.15-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofhuis A, Harms M, van den Wijngaard C, Sprong H, van Pelt W. Continuing increase of tick bites and Lyme disease between 1994 and 2009. Ticks Tick Borne Dis. 2015;6(1):69–74. doi: 10.1016/j.ttbdis.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Sprong H, Wielinga PR, Fonville M, Reusken C, Brandenburg AH, Borgsteede F, Gaasenbeek C, van der Giessen JW. Ixodes ricinus ticks are reservoir hosts for rickettsia helvetica and potentially carry flea-borne rickettsia species. Parasit Vectors. 2009;2(1):41. doi: 10.1186/1756-3305-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wielinga PR, Fonville M, Sprong H, Gaasenbeek C, Borgsteede F, van der Giessen JW. Persistent detection of Babesia EU1 and Babesia microti in Ixodes ricinus in the Netherlands during a 5-year surveillance: 2003-2007. Vector Borne Zoonotic Dis. 2009;9(1):119–122. doi: 10.1089/vbz.2008.0047. [DOI] [PubMed] [Google Scholar]
- 14.Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte EJ, Takken W, Heyman P, Medlock JM, Heylen D, Kleve J, Sprong H. Prevalence of Neoehrlichia mikurensis in ticks and rodents from north-West Europe. Parasit Vectors. 2012;5(1):74. doi: 10.1186/1756-3305-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coipan EC, Jahfari S, Fonville M, Maassen CB, van der Giessen J, Takken W, Takumi K, Sprong H. Spatiotemporal dynamics of emerging pathogens in questing Ixodes ricinus. Front Cell Infect Microbiol. 2013;3:36. doi: 10.3389/fcimb.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Heylen D, Heyman P, van Maanen C, Butler CM, Foldvari G, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014;7(1):365. doi: 10.1186/1756-3305-7-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tijsse-Klasen E, Fonville M, van Overbeek L, Reimerink JH, Sprong H. Exotic Rickettsiae in Ixodes ricinus: fact or artifact? Parasit Vectors. 2010;3(1):54. doi: 10.1186/1756-3305-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Duijvendijk G, Coipan C, Wagemakers A, Fonville M, Ersoz J, Oei A, Foldvari G, Hovius J, Takken W, Sprong H. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasit Vectors. 2016;9:97. doi: 10.1186/s13071-016-1389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprong H, Azagi T, Hoornstra D, Nijhof AM, Knorr S, Baarsma ME, Hovius JW. Control of Lyme borreliosis and other Ixodes ricinus-borne diseases. Parasit Vectors. 2018;11(1):145. doi: 10.1186/s13071-018-2744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, Sprong H. Molecular detection of tick-borne pathogens in humans with tick bites and erythema Migrans, in the Netherlands. PLoS Negl Trop Dis. 2016;10(10):e0005042. doi: 10.1371/journal.pntd.0005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tijsse-Klasen E, Jacobs JJ, Swart A, Fonville M, Reimerink JH, Brandenburg AH, van der Giessen JW, Hofhuis A, Sprong H. Small risk of developing symptomatic tick-borne diseases following a tick bite in the Netherlands. Parasit Vectors. 2011;4(1):17. doi: 10.1186/1756-3305-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382(9892):658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofhuis A, Herremans T, Notermans DW, Sprong H, Fonville M, van der Giessen JW, van Pelt W. A prospective study among patients presenting at the general practitioner with a tick bite or erythema migrans in the Netherlands. PLoS One. 2013;8(5):e64361. doi: 10.1371/journal.pone.0064361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tijsse-Klasen E, Sprong H, Pandak N. Co-infection of Borrelia burgdorferi sensu lato and rickettsia species in ticks and in an erythema migrans patient. Parasit Vectors. 2013;6(1):347. doi: 10.1186/1756-3305-6-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hing M, Woestyn S, Van Bosterhaut B, Desbonnet Y, Heyman P, Cochez C, Silaghi C, Sprong H, Fournier PE, Raoult D, et al. Diagnosis of human granulocytic anaplasmosis in Belgium by combining molecular and serological methods. New Microbes New Infect. 2014;2(6):177–178. doi: 10.1002/nmi2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahfari S, Herremans T, Platonov AE, Kuiper H, Karan LS, Vasilieva O, Koopmans MP, Hovius JW, Sprong H. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes New Infect. 2014;2(5):144–149. doi: 10.1002/nmi2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonville M, Friesema IH, Hengeveld PD, Docters van Leeuwen A, Jahfari S, Harms MG, van Vliet AJ, Hofhuis A, van Pelt W, Sprong H, et al. Human exposure to tickborne relapsing fever spirochete Borrelia miyamotoi, the Netherlands. Emerg Infect Dis. 2014;20(7):1244–1245. doi: 10.3201/eid2007.131525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silaghi C, Beck R, Oteo JA, Pfeffer M, Sprong H. Neoehrlichiosis: an emerging tick-borne zoonosis caused by Candidatus Neoehrlichia mikurensis. Exp Appl Acarol. 2016;68(3):279–297. doi: 10.1007/s10493-015-9935-y. [DOI] [PubMed] [Google Scholar]
- 29.Koetsveld J, Tijsse-Klasen E, Herremans T, Hovius JW, Sprong H. Serological and molecular evidence for spotted fever group rickettsia and Borrelia burgdorferi sensu lato co-infections in The Netherlands. Ticks Tick Borne Dis. 2016;7(2):371–377. doi: 10.1016/j.ttbdis.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Azagi THD, Kremer K, Hovius JW, Sprong H. Evaluation of disease causality of rare Ixodes ricinus-borne infections in Europe. Pathogens. 2020;9(150):1–24. doi: 10.3390/pathogens9020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoornstra D, Koetsveld J, Sprong H, Platonov AE, Hovius JW. Borrelia miyamotoi disease in an immunocompetent patient, Western Europe. Emerg Infect Dis. 2018;24(9):1770–1772. doi: 10.3201/eid2409.180806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henningsson AJ, Asgeirsson H, Hammas B, Karlsson E, Parke A, Hoornstra D, Wilhelmsson P, Hovius JW. Two cases of Borrelia miyamotoi meningitis, Sweden, 2018. Emerg Infect Dis. 2019;25(10):1965–1968. doi: 10.3201/eid2510.190416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netherlands HCot . A closer look at Lyme disease. In. The Hague: Health Council of the Netherlands; 2013. [Google Scholar]
- 34.Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31(6):260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Krause PJ, Telford SR, 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996;275(21):1657–1660. doi: 10.1001/jama.1996.03530450047031. [DOI] [PubMed] [Google Scholar]
- 36.Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, Closter L, Christianson D, Telford SR, Persing D, Radolf JD, Spielman A, the Deer‐Associated Infection Study Group Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34(9):1184–1191. doi: 10.1086/339813. [DOI] [PubMed] [Google Scholar]
- 37.Belongia EA. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002;2(4):265–273. doi: 10.1089/153036602321653851. [DOI] [PubMed] [Google Scholar]
- 38.Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from ixodes ticks. Clin Microbiol Rev. 2006;19(4):708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knapp KL, Rice NA. Human Coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res. 2015;2015:587131. doi: 10.1155/2015/587131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32(1):30–42. doi: 10.1016/j.pt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Homer MJ, Aguilar-Delfin I, Telford SR, 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13(3):451–469. doi: 10.1128/CMR.13.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steere AC, McHugh G, Suarez C, Hoitt J, Damle N, Sikand VK. Prospective study of coinfection in patients with erythema migrans. Clin Infect Dis. 2003;36(8):1078–1081. doi: 10.1086/368187. [DOI] [PubMed] [Google Scholar]
- 43.Moniuszko A, Dunaj J, Swiecicka I, Zambrowski G, Chmielewska-Badora J, Zukiewicz-Sobczak W, Zajkowska J, Czupryna P, Kondrusik M, Grygorczuk S, et al. Co-infections with Borrelia species, Anaplasma phagocytophilum and Babesia spp. in patients with tick-borne encephalitis. Eur J Clin Microbiol Infect Dis. 2014;33(10):1835–1841. doi: 10.1007/s10096-014-2134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strle F, Bogovic P, Cimperman J, Maraspin V, Ogrinc K, Rojko T, Stupica D, Lusa L, Avsic-Zupanc T, Smrdel KS, et al. Are patients with erythema migrans who have leukopenia and/or thrombocytopenia coinfected with Anaplasma phagocytophilum or tick-borne encephalitis virus? PLoS One. 2014;9(7):e103188. doi: 10.1371/journal.pone.0103188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jahfari S, de Vries A, Rijks JM, Van Gucht S, Vennema H, Sprong H, Rockx B. Tick-borne encephalitis virus in ticks and roe deer, the Netherlands. Emerg Infect Dis. 2017;23(6):1028–1030. doi: 10.3201/eid2306.161247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vrijmoeth HD, Ursinus J, Harms MG, Zomer TP, Gauw SA, Tulen AD, Kremer K, Sprong H, Knoop H, Vermeeren YM, van Kooten B, Joosten LAB, Kullberg BJ, Hovius JWR, van den Wijngaard CC. Prevalence and determinants of persistent symptoms after treatment for Lyme borreliosis: study protocol for an observational, prospective cohort study (LymeProspect) BMC Infect Dis. 2019;19(1):324. doi: 10.1186/s12879-019-3949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Schoor FR, Baarsma ME, Gauw SA, Joosten LAB, Kullberg BJ, van den Wijngaard CC, Hovius JW. Validation of cellular tests for Lyme borreliosis (VICTORY) study. BMC Infect Dis. 2019;19(1):732. doi: 10.1186/s12879-019-4323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verberk JDM, Vos RA, Mollema L, van Vliet J, van Weert JWM, de Melker HE, van der Klis FRM. Third national biobank for population-based seroprevalence studies in the Netherlands, including the Caribbean Netherlands. BMC Infect Dis. 2019;19(1):470. doi: 10.1186/s12879-019-4019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart JW, Quitkin FM, McGrath PJ, Rabkin JG, Markowitz JS, Tricamo E, Klein DF. Social functioning in chronic depression: effect of 6 weeks of antidepressant treatment. Psychiatry Res. 1988;25(2):213–222. doi: 10.1016/0165-1781(88)90053-4. [DOI] [PubMed] [Google Scholar]
- 50.Aaronson N, Muller M, Cohen P, Essink-Bot M, Fekkes M, Sanderman R, Sprangers M, te Velde A, E V Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–1068. doi: 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 51.Estrada-Pena A, Pfaffle M, Baneth G, Kleinerman G, Petney TN. Ixodoidea of the Western Palaearctic: a review of available literature for identification of species. Ticks Tick Borne Dis. 2017;8(4):512–525. doi: 10.1016/j.ttbdis.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Takumi K, Sprong H, Hofmeester TR. Impact of vertebrate communities on Ixodes ricinus-borne disease risk in forest areas. Parasit Vectors. 2019;12(1):434. doi: 10.1186/s13071-019-3700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. (1) Specifics of laboratory measurements. (2) Questionnaires. (3) Sample size calculation.
Data Availability Statement
We intend to share our datasets either in a publicly available repository or present it in the main manuscript or additional supporting files in a readable format.