Abstract
In this article, we will describe the properties of albumin and its biological functions, types of sources that can be used to produce albumin nanoparticles, methods of producing albumin nanoparticles, its therapeutic applications and the importance of albumin nanoparticles in the production of pharmaceutical formulations. In view of the increasing use of Abraxane and its approval for use in the treatment of several types of cancer and during the final stages of clinical trials for other cancers, to evaluate it and compare its effectiveness with conventional non formulations of chemotherapy Paclitaxel is paid. In this article, we will examine the role and importance of animal proteins in Nano medicine and the various benefits of these biomolecules for the preparation of drug delivery carriers and the characteristics of plant protein Nano carriers and protein Nano cages and their potentials in diagnosis and treatment. Finally, the advantages and disadvantages of protein nanoparticles are mentioned, as well as the methods of production of albumin nanoparticles, its therapeutic applications and the importance of albumin nanoparticles in the production of pharmaceutical formulations.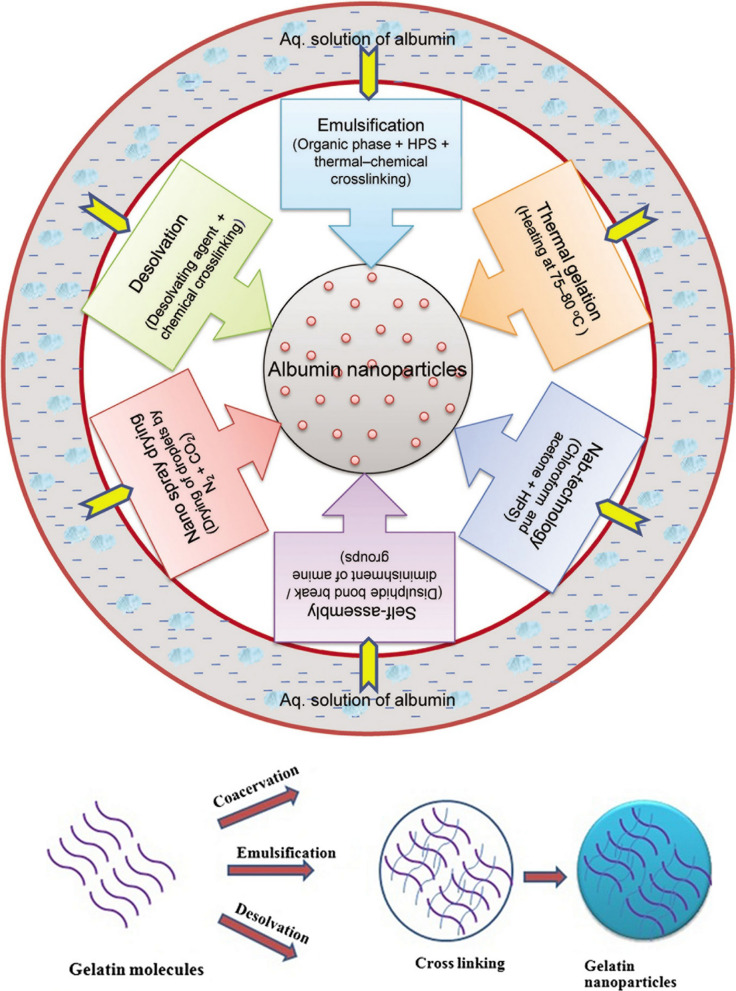
Keywords: Protein nanoparticles, Drug delivery, Animal protein, Plant proteins, Protein cages, Albumin nanoparticles
Introduction
Proteins and peptides are one of the most important and basic research fields in Nano medicine. At present, experts in various fields in Nano medicine, Nano biotechnology, pharmacy, toxicology, immunology and other medical sciences are studying the various dimensions of these vital biomolecules, including understanding the interaction of the resulting nanostructures with the body and their application in diagnostic fields and are engaged in therapy [1–8]. Plants have been continuously considered by researchers as safe and abundant, renewable and cheap resources in various pharmaceutical, medical and food industries. Nano carriers made from some plant proteins have the ability to control the release of their cargo over a long period of time [9, 10]. The possibility of transmitting the disease from plant sources to humans is rare, and for this reason, the use of plant proteins to produce nano carriers for therapeutic agents has been considered by experts in recent years [11–17]. On the other hand, some protein subunits, such as the capsid proteins of viruses, have the ability to self-assemble and create hollow nanometer structures with well-defined and reproducible geometric shapes [18, 19]. The empty space inside these nanostructures or their surface can be used as a reservoir to carry pharmaceutical and diagnostic agents. These nanostructures are called protein cages and have great potential in preparing pharmaceutical formulations. Albumin is one of the animal proteins that has been considered since the early twentieth century and has several therapeutic applications [20–22]. On the one hand, this protein has therapeutic applications and on the other hand, it is used in various formulations to carry pharmacological and diagnostic agents [23–25]. The many benefits and potentials of this protein have made it the focus of Nano medical researchers [17, 26, 27]. Abraxane is a drug formulation based on albumin nanoparticles that sold more than 2 $ billion in 2012 alone and is considered by experts to be one of the main approaches to treating all types of cancer in the near future [28, 29]. The following section covers the most recent uses of therapeutic nanoparticles as selective delivery mechanisms in a variety of diseases, and Table 1 summarizes the nano-drug formulations authorised by the Food and Drug Administration (FDA) and the Euro.
Table 1.
Food and Drug Administration and European Medicines Agency approved therapeutic nanoparticles
| Nanostructure | Production | Nanoparticle formulation | Drug | Indication(s) | Confirmed | Refs. |
|---|---|---|---|---|---|---|
| Liposomes | Marqibo® | Sphingomyelin and cholesterol | Vincristine sulfate | Acute lymphoid leukemia | FDA 2012 | [30] |
| Liposomes | Mepact® | 1-Palmitoyl-2-oleoyl-snglycero-3-phosphocholine and 1,2-Dioleoyl-sn-glycero-3-phospho-l-serine liposomes | Mifamurtide | Non-metastasizing osteosarcoma | Europe 2009 | [31] |
| Liposomes | Onivyde® | Nanoliposomes | Irinotecan | Pancreatic cancer, colorectal cancer |
FDA 2015 Europe 2016 |
[32] |
| Liposomes | Vyxeos® | Distearoylphosphatidylcholine, distearoylphosphatidylglycerol, cholesterol |
Daunorubicin Cytarabine |
Acute myeloid leukemia | FDA 2017 | [33] |
| Lipid-based (non-liposoma) | Onpattro® | Lipid nanoparticles | Transthyretin targeted siRNA | Transthyretin-mediated amyloidosis | FDA 2018 | [34] |
| Polymer-based | Glatopa® | l-glutamic acid polymer with l-alanine, l-lysine, and l-tyrosine (Glatiramer) | –- | Multiple sclerosis | FDA 2015 | [35] |
| Protein-drug conjugates | Kadcyla® | Maytansine derivative, DM1 | Trastuzumab | HER2 + breast cancer | FDA 2013 | [36] |
| Protein-drug conjugates | Abraxane® | Albumin | Paclitaxel | Non-small lung cancer, pancreatic cancer |
FDA 2012 Europe 2005, FDA 2013 Europe 2008 |
[37] |
| Protein-drug conjugates | Krystexxa® | PEGylated uricase | Pegloticase | Gout disease |
FDA 2010 Europe 2013 |
[38] |
| Protein-drug conjugates | Plegridy® | PEGylated interferon-1a | Interferon-1a | Multiple sclerosis |
FDA 2014 Europe 2014 |
[38] |
| Protein-drug conjugates | Adynovate® | PEGylated factor VIII | Factor VIII | Hemophilia | FDA 2015 | [39] |
| Protein-drug conjugates | Rebinyn® | Glycopegylated coagulation factor IX | Factor IX | Hemophilia | FDA 2015 | [40] |
Pean Medicines Agency (EMA) since 2009
Nano medicine and proteins
Nano medicine and proteins in the field of treatment
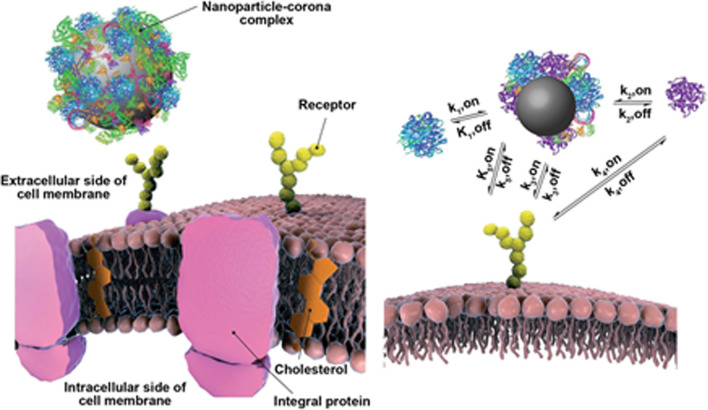
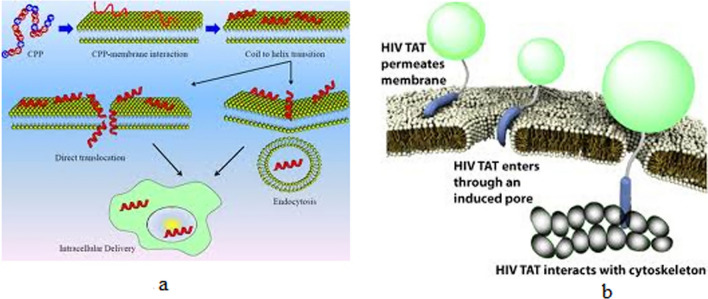
Many proteins and peptides such as insulin, vaccines, antibodies and various recombinant proteins have been used in medicine and therapy, and among the important research areas in Nano medicine is the development of new drug delivery systems to improve their function and properties [41–43]. On the other hand, one of the most important challenges facing Nano pharmaceutical formulations is the interaction of different blood proteins with them and the formation of a protein crown (Protein corona) around the nanoparticles, which plays an important role in the final performance of nanoparticles (Fig. 1). These include immune system proteins, including antibodies, and complement systems. Extensive efforts are being made to control the interaction of drug formulations with a variety of proteins, especially immune system proteins, to improve the performance of Nano drugs [44–47]. Various peptides and proteins have also been considered to target drug-containing nanoparticles to target tissue or tissues, such as tumor tissue, including cell-penetrating peptides (CPPs), antibodies, and phage peptides (Fig. 2). Finally, protein nanoparticles themselves, as drug carriers, are among the new drug delivery systems. Abraxane, which contains albumin nanoparticles containing the anti-cancer drug Paclitaxel, has been approved by the relevant international organizations in recent years, and the significant annual growth trend of this drug and its effectiveness in treating various cancers has attracted the attention of many researchers Has attracted drug delivery [48–51]. Mathematical modeling plays an important role in facilitating the design of drug delivery systems by identifying key factors and molecular mechanisms of release [52–54].
Fig. 1.
Nanoparticle protein crown: the formation of a protein crown around transferrin-targeted nanoparticles obscures the second transferrin binding and prevents it from binding to the surface of the target cell [48–51]
Fig. 2.
Cell penetrating peptides: a cell-penetrating peptides enter the cell by various mechanisms directly or through endocytosis pathways and can enter the cell-bound cargo. b AIDS TAT peptide is one of the cell-penetrating peptides that has been used in various studies to introduce nanoparticles into cells [48–51]
Nano medicine and proteins in the field of diagnosis
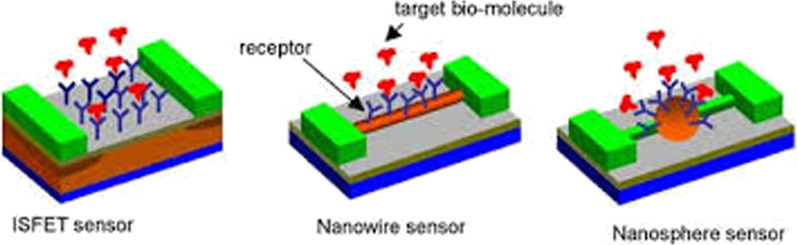
On the one hand, proteins are important factors in the diagnosis of diseases and on the other hand, they are used in the manufacture of sensors to diagnose other diseases [55–57]. Various Nano biosensors are being studied to detect a variety of proteins, antibodies, antigens and biomarkers, and factors such as antibodies and enzymes are being used to diagnose the disease [58–60]. For example, antibodies are used to detect a variety of viral diseases and the enzyme glucose oxidase is used in the manufacture of glucose Nano biosensors (Fig. 3).
Fig. 3.
Nano sensors in the detection of proteins: different types of Nano biosensors are made to detect antibodies, antigens and protein biomarkers [55–57]
Advantages and disadvantaged of the general protein-based nanoparticle fabrication methods
The appropriate properties of protein nanoparticles have made them one of the important options in drug delivery and tissue engineering. The advantages and disadvantages of these fabrication methods are summarized in Table 2. Some of the most important of these benefits are [61–66]:
-
Biocompatibility
Proteins are among the major biomolecules that make up the body of all living organisms and therefore have little toxicity, especially compared to synthetic polymers [67–69]. By absorbing water and creating a space repulsion, proteins can increase the stability of nanoparticles [70–72] and also reduce the recognition of Nano carriers by the immune system (Fig. 4).
-
Biodegradability
These molecules are broken down in the body and the amino acids they produce are used by surrounding tissues to make proteins or produce energy [73, 74].
-
Possibility of easy and cheap production
Usually proteins are abundant in nature and are renewable sources by plants, animals, humans and other organisms. It is also possible to mass-produce a variety of proteins by recombinant protein production methods [75, 76].
-
High drug binding capacity
Proteins generally have many types of functional groups and therefore have the ability to bind and carry significant amounts of drug by different mechanisms such as electrostatic interactions, hydrophobic interactions, and covalent bonds [77–79].
-
Proper uptake by cells
Usually, proteins and polymer nanoparticles are removed by the cell by different mechanisms, for example, one of the effective factors mentioned in the anticancer drug Abraxane is the uptake of albumin nanoparticles by vascular endothelial cells [80–82].
-
Targeting
The structure and sequence of the protein and the presence of numerous different functional groups allow the binding of the drug to specific sites in the protein and the binding of different targeting ligands to the protein Nano carrier [83, 84].
Table 2.
Advantages and disadvantaged of the general protein-based nanoparticle fabrication methods
| Method | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| pH Variation |
Control for particle size Control secondary structure of protein Control for zeta potential Produces chemically and physically stable particles Experimentally simple |
Post-fabrication drug loading is required Limited to small scale production |
[35] |
| Spray-drying |
Cost effective Experimentally simple Easily encapsulate hydrophilic drugs Useful for heat-sensitive samples Control for particle size |
Limited to small scale production Challenging to incorporate hydrophobic drugs |
[36] |
| Rapid Laminar Jet |
Control for particle size Production of uniform particles Production of strong, stable particles |
Possibility of coalescence Many parameters must be controlled for |
[37] |
| Phase Separation |
Specialized equipment is not required Particle size can be controlled by adjusting protein concentration Uniform particles are produced |
Particle sizes are limited to 50–500 nm in diameter Organic solvents are required Limited to small scale production |
[38] |
| Milling |
Cost effective Large scale production is possible Control of nanoparticle size Experimentally simple |
Heat is released during the process requiring chamber to be cooled Little control over nanoparticle shape Nanoparticles must be coarse |
[39] |
|
Polymer Chain Collapse |
Properties of the nanoparticle can be easily controlled by selection of the precursor chain Production of particles with high stability Particles with improved spherical shape are produced |
Particle size is limited to 5–20 nm in diameter Side reaction may be difficult to control |
[40] |
Fig. 4.
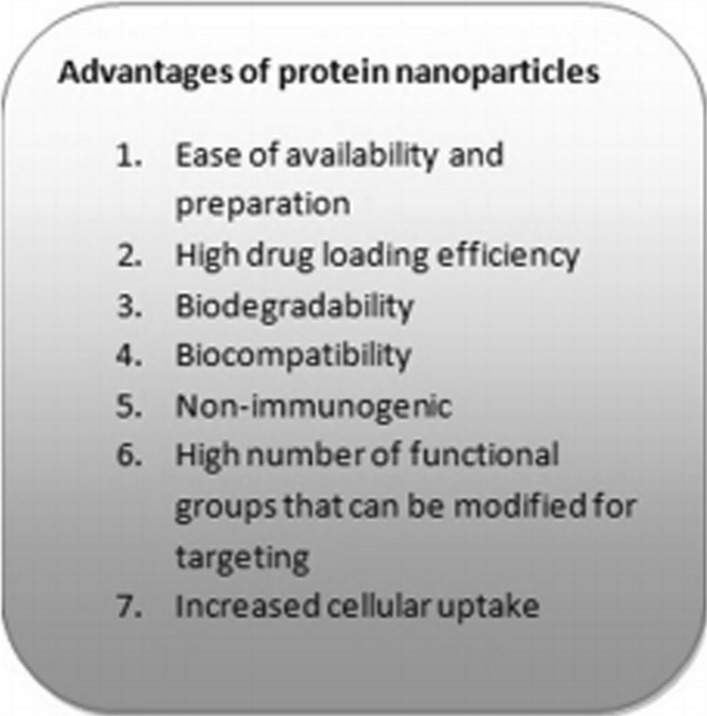
Advantages of protein nanoparticles in drug delivery [67]
Types of protein nanoparticles
Animal proteins
Gelatin nanoparticles
Gelatin is a denatured protein derived from the acidic hydrolysis or collagen base of animals. This biomolecule has been used for many years in the pharmaceutical, cosmetics and food industries. Gelatin stimulates the immune system due to denaturation. Gelatin is a Polyampholyte compound and has cationic and anionic active groups and hydrophobic groups in a ratio of 1:1:1, so that the gelatin molecule has 13% positive charge (amino acids lysine and arginine), 12% negative charge (Glutamic and aspartic amino acids) and 11% of hydrophobic amino acids (leucine, isoleucine, methionine and valine) [85–87]. The rest of the structure is made up of glycine, proline and hydroxyproline. Gelatin is commercially available as both cationic gelatin and anionic gelatin. Cationic gelatin is obtained from type 1 pig skin collagen under acidic hydrolysis and anionic type from bovine collagen under hydrolysis [88, 89]. Gelatin is used in various drug formulations in systemic use. It is used clinically as a plasma volume enhancer as well as a stabilizer in protein formulations, vaccines and gelatin sponges such as gel foam. Gelatin contains the arginine-lysine-glycine sequence in its structure. The above sequence is an important sequence in many extracellular matrix proteins and plays an important role in cell binding and cellular messaging by binding to the beta subunit of integrin receptors at the cell surface [90–92]. This property is one of the important advantages of gelatin over polymers that lack cell recognition and binding sites. The active groups of gelatin make it possible to make a variety of chemical changes on it directly or by using different linkers, this feature is very important, especially when producing targeted drug delivery carriers and the possibility of attaching significant amounts of drug to carriers. Gelatin nanoparticles have been used to deliver various types of hydrophilic and hydrophobic drugs, including various anti-cancer drugs, anti-AIDS drugs, anti-malarial drugs, and analgesics, treatment of infectious diseases, muscle relaxants, anti-inflammatory drugs, and treatment drugs. Diabetes is a topical ophthalmic drug, inhibitor of protein synthesis, activator of tissue plasminogen, gene delivery and delivery of protein drugs and vaccines [93–95]. SEM and TEM images of gelatin nanoparticles is shown in Fig. 5, which clearly indicates the smooth And spherical nanoparticles with an average diameter of about 100–300 nm.
Fig. 5.
Synthesis of gelatin nanoparticles
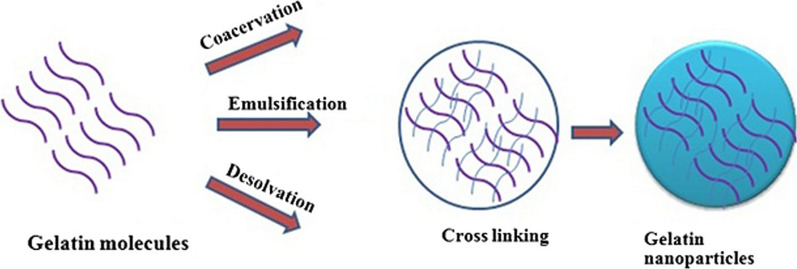
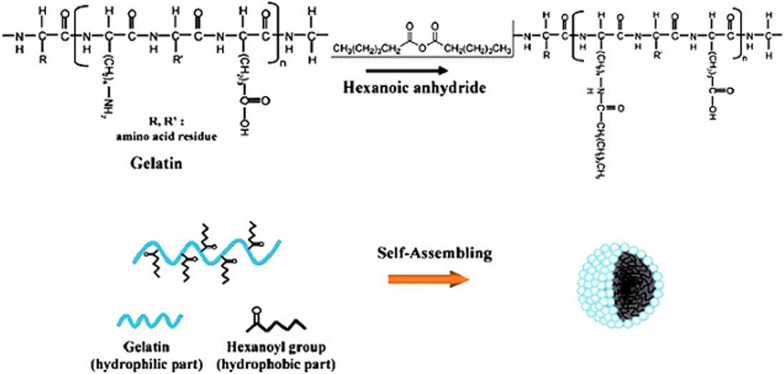
Several methods are used to make gelatinous nanoparticles (Fig. 6). These methods include precipitation, phase separation, emulsion-solvent evaporation, self-assembly of gelatin molecules (which have been deformed by the bonding of chemical groups) or self-assembly of drugs and gelatin molecules, micro emulsion, and so on. The following is an example of the self-assembly of gelatin molecules (Fig. 7) [96].
Fig. 6.
Synthesis of gelatin nanoparticles has been done in different ways, most of which in the final stages of synthesis, it is necessary to use a combination of linker (cross linking agent) to stabilize the nanoparticles
Fig. 7.
Synthesis of gelatin nanoparticles by self-assembly method: in this method, hexanoic anhydride molecules are first attached to it through the lysine roots in collagen, resulting in the formation of an amphipathic structure that spontaneously Nanoparticles accumulate [96]
Collagen nanoparticles
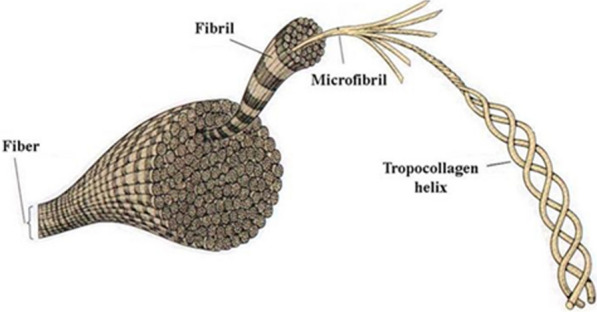
Collagen is a structural protein in the vertebrate body and is the most abundant protein in the mammalian body, accounting for 20–30% of the body's total proteins. The basic structure of collagen is tropocollagen three-stranded molecules that are twisted together in three spirals and connected by various nono covalent bonds [97–100]. Collagen is eventually produced by the formation of covalent crosslinks between tropocollagen molecules (Fig. 8). Due to its good biocompatibility and low stimulation of the immune system and biodegradability, this protein is widely used in medicine. Collagen nanoparticles are removed by the body's Reticuloendothelium system and can therefore increase the uptake of certain compounds, such as anti-AIDS drugs, into some cells, especially macrophages [101–104]. Collagen nanoparticles due to their small size with high contact surface, high absorption capacity and ability to disperse in water to form a stable and clear colloidal solution have been used as drug carriers for long-term release of antimicrobial and steroid drugs, especially in dermatology [105–107].
Fig. 8.
Milk proteins
Milk proteins are natural carriers of biologically active substances. Based on their structure, they can be classified into two categories [108, 109]:
The group of proteins with linear and flexible structure including caseins and proteins with spherical structure including whey proteins (whey) [110].
Beta-lactoglobin and alpha-lactoglobin are the main proteins in whey that have been studied to make drug Nano carriers. Among the characteristics of these proteins are their high resistance to breakdown by enzymes that break down proteins in the stomach [111, 112].
Casein
Casein is the main protein in milk. Its advantages as drug-carrying nanoparticles include low cost, easy access to its sources, high stability and nono Taxol [113, 114]. Many of the structural and physicochemical properties of caseins make it possible to use them as drug delivery systems. Some of these properties include the ability to bind to a variety of ions and molecules, exceptional stability and surface activity properties, excellent self-assembly and emulsification properties, and water-binding and gel-forming capacity. Caseins are not temperature sensitive, while whey globular proteins undergo denaturation and fundamental structural changes at temperatures above 70 °C [115–117]. The high tensile strength of casein films has made these proteins attractive in use as tablet coatings. Another important property of caseins is their protective effect, which is essential for the protection of sensitive cargoes. For example, casein with the ability to absorb strong light, especially in the wavelength range of 200–300 nm can protect its cargo against radiation, especially in the range of ultraviolet light [118–120]. The mentioned features suggest casein as a suitable candidate for building conventional and newer drug delivery systems such as nano-camels. However, limitations of caseins include immunosuppression and allergy concerns. It should be noted that casein in milk is absorbed as amino acids after decomposition in the gastrointestinal tract, but in cases such as direct intravenous injection of these proteins, the immune response to them should be considered [121–123].
Casein structure
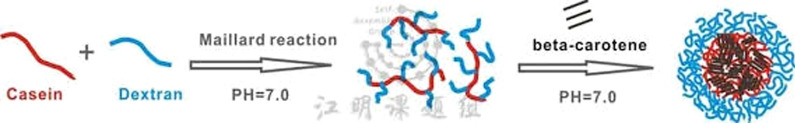
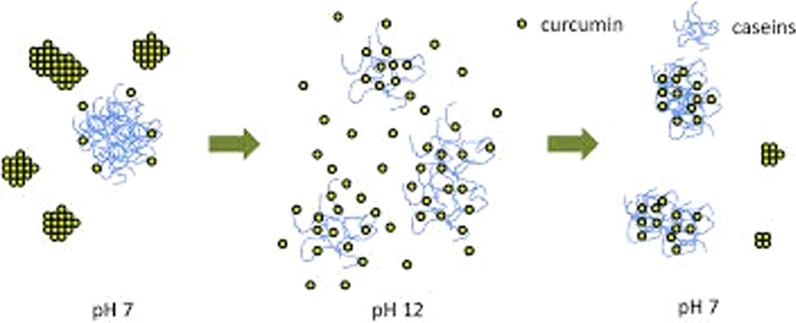
Milk casein contains about 94% protein and 6% low molecular weight compounds called colloidal calcium phosphate. These Phosphoprotein have a molecular weight of 19–25 kDa and an isoelectric pH of 4.6–4.8. Caseins contain multiple roots of hydrophilic and hydrophobic amino acids, and are therefore dual-protein proteins capable of producing block copolymers with a high tendency to self-regulate micelles in the range of 50–500 nm (average 250 nm) [124]. These spherical micelles have a hydrophobic inner part whose outer surface is surrounded by a layer of hydrophilic casein kappa (κ) that stabilizes the micelles by creating an electrostatic and spatial repulsion between the micelles (Fig. 9). In fact, casein micelles in milk are natural nano carriers that are responsible for transporting and supplying amino acids and calcium phosphate from mother to baby [125–127]. These micelles are very stable and maintain their structural stability while performing various processes on milk to prepare a variety of dairy products. More recently, casein or copolymer micelles have been used with other polymers to transport hydrophobic cargoes, effectively inhibiting vitamin D and omega-3 unsaturated fatty acids and beta-carotene (a precursor to vitamin A) against degradation and oxidation by Protect from ultraviolet light (Fig. 10). Casein nano micels have been used as carriers of various anticancer drugs such as curcumin, mitoxantrone, vinbelastin, docetaxel, and paclitaxel (Fig. 11). Beta casein is a candidate for targeting gastric tumors due to its degradability in the stomach. By degrading beta-casein nanoparticles in the stomach by the enzyme pepsin, paclitaxel is released from them and effectively reduces the growth of gastric cancer cells. Nano mysel protects the drug by protecting the drug inside it and preventing the drug from being released before it reaches the stomach, as well as preventing its toxic effect on higher areas of the gastrointestinal tract such as the mouth and esophagus. Gels from this protein are sensitive to pH changes and can be used in the construction of intelligent drug delivery systems. Another potential of casein nanoparticles is their ability to be lyophilized without the need for cryo-protectants when preparing drug formulations [128, 129].
Fig. 9.
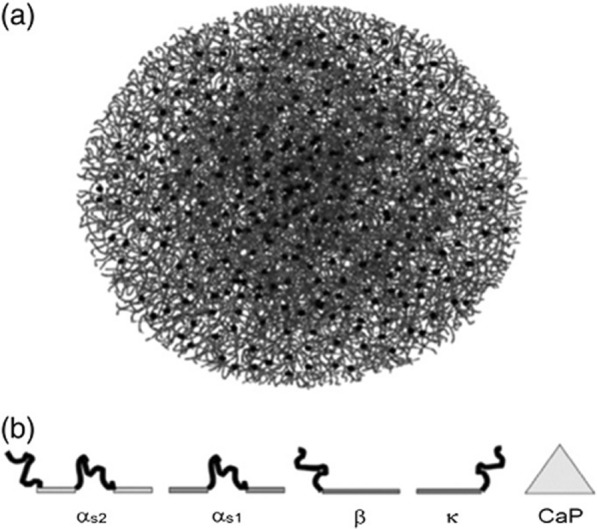
a Structure of casein micelles: filamentous casein monomers and black circles represent calcium phosphate Nano clusters. b Casein structural proteins: These proteins include hydrophobic regions (light linear parts) that react with each other and hydrophilic regions (dark ring parts) also interact with calcium phosphate Nano clusters [128, 129].
Fig. 10.
Cases of the casein and dextran copolymers have the ability to load and protect beta-carotene molecules [128, 129]
Fig. 11.
Production of casein nanoparticles containing curcumin: at pH 7 = curcumin molecules are complex (large particles) but with increasing to about 12 molecules of drug particles are dispersed and casein molecules are opened. By reducing the pH again to the range of 7, casein nano drates containing the drug curcumin are formed [128, 129]
Silk fibroin
Silk is a natural protein polymer produced by the larvae of some insects, such as silkworms and spiders. Due to their good biocompatibility, these proteins are being studied in drug delivery and tissue engineering. The main components of silk are the fibrin linear protein in the nucleus and the adhesive protein such as serein, which encapsulates the fibrin nucleus [130]. Among the potentials of these proteins in making nanoparticles and scaffolds with low decomposition rate is their self-assembly ability and mechanical properties. Fibroin has a lower inflammatory response at the site of degradation than widely used biocompatible synthetic polymers such as polylactic acid. Fibroin nanoparticles have the ability to protect proteins and peptides such as conjugated insulin and vascular endothelial growth factor in blood serum and solution containing the enzyme trypsin (enzyme that breaks down proteins in the human stomach) and increase the duration of active release of these compounds [131–133].
Elastin
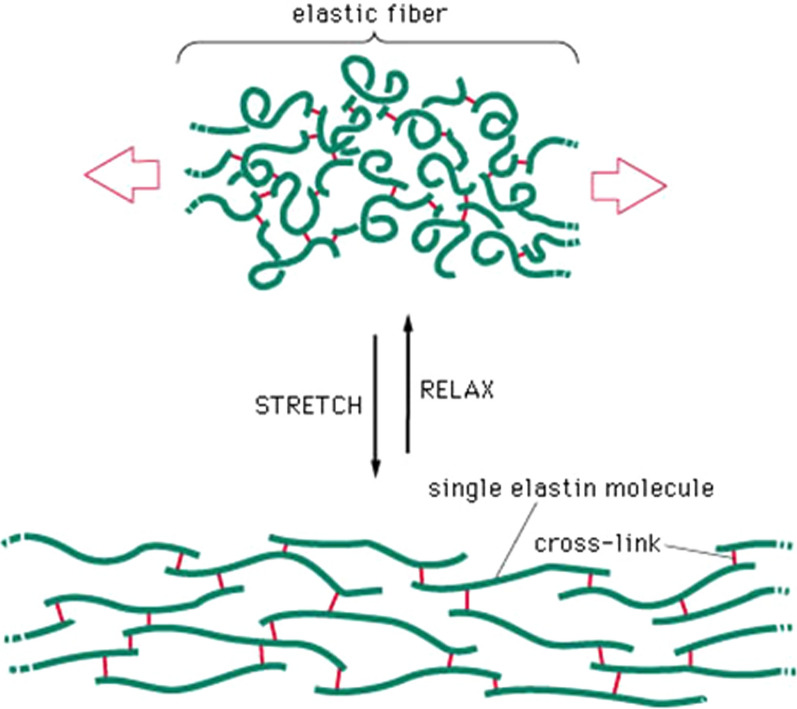
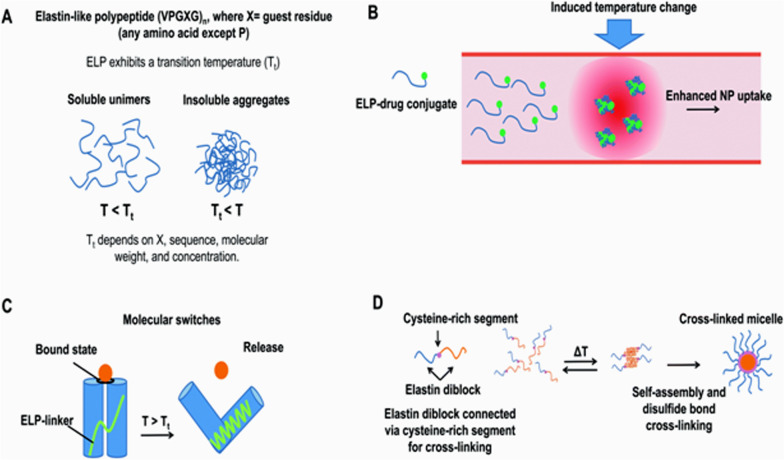
Elastin is the predominant protein in the extracellular matrix of arterial walls. This protein plays an important role in creating elastic properties and flexibility in the arteries when blood pressure changes, as well as in many other tissues of the body such as the lungs, skin and ligaments [134, 135]. In their natural environment, the components of elastin first converge in the form of a water-soluble precursor molecule called their tropoelastin, and then these precursors combine to form elastin fibers by forming covalent cross-links (Fig. 12). Genetic engineering techniques and the production of recombinant proteins have made it possible to make elastin-like polymers (ELPs) [136]. The basic structure of these proteins is similar to the repetitive sequences found in elastin, but genetic engineering methods have made it possible to add specific sequences and create the desired properties of researchers in these polymers [137]. Due to their similarity to natural elastin in the body, the immune system does not react to them and they have the ability to escape from the immune system [138]. The possibility of designing and manufacturing elastin-like proteins by genetic engineering techniques gives several benefits to these proteins and their nanoparticles, including the ability to achieve appropriate pharmacokinetic properties, the ability to precisely control molecular weight, and the production of single-size polymers. (monodisperese), the ability to attach multiple drug molecules to them and the ability to attach nanoparticle targeting agents to specific tissues or locations in the body [139–142]. Also, a variety of polymers designed with these methods have the ability to change the phase rapidly in response to temperature changes (Fig. 13).
Fig. 12.
Elastic fiber structure. Changing the pattern of bonds between elastin molecules causes elastic properties and flexibility in elastin fiber and tissue [139–142]
Fig. 13.
Temperature responsive ELP nanoparticles. ELP. A with n-repeating peptide sequence (Val-Pro-Gly-X-Gly) is a temperature-sensitive polypeptide that is insoluble in water above the transfer temperature (Tt) and soluble in water at the bottom. B Drug-ELP conjugates in heated tumor tissue can aggregate to produce drug-containing ELP nanoparticles that will increase in size depending on their uptake into the target tissue (EPR mechanism). C Molecular thermal switches based on ELP nanoparticles. D Production of temperature-sensitive micelles by ELP molecules containing cysteine-rich sequences [139–142]
Plant proteins
The use of plant protein nano carriers is a new approach in drug delivery. Unlike animal protein nano carriers, plant proteins such as zein and gliadin have a longer drug release ability due to their hydrophobic nature [143–145]. Also, due to high hydrophobicity, stable nanoparticles of plant proteins may be produced without the need for chemical and physical treatments and the use of chemical linker molecules, which are often used in the manufacture of animal protein Nano carriers [146–148]. Vegetable proteins are widely available and are much cheaper than animal proteins. They are also not at risk of transmitting animal diseases to humans, such as bovine insanity. The presence of different functional groups in these proteins makes it possible to change the surface of the resulting nanoparticles to regulate the physical and chemical properties and bind the targeting agent [149–152].
Zein
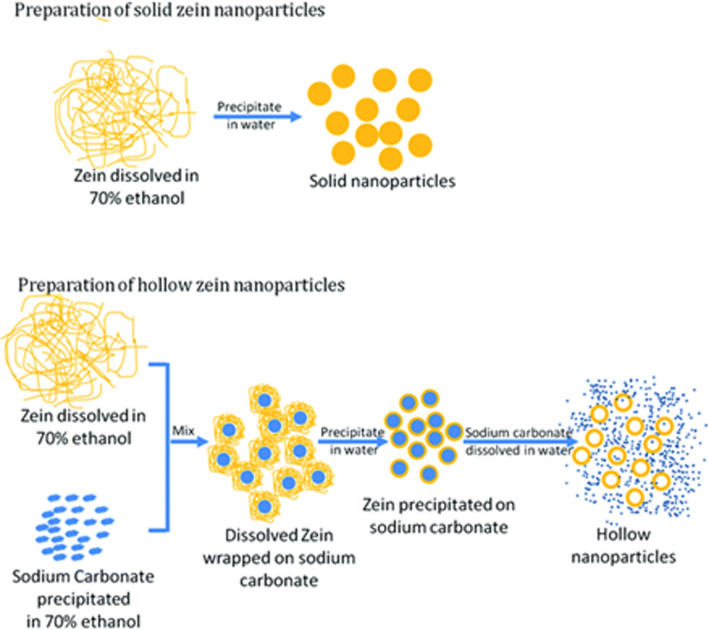
Saddle is a water-soluble, alcohol-soluble protein with a molecular weight of about 40 kDa, which is found mainly in cereals [153]. 75% of its amino acids are hydrophobic and 25% are hydrophilic. Because zein is a natural protein and has good biodegradability, it has been approved by international organizations for use in the food and pharmaceutical industries [154]. It is also used as a degradable coating in foods and drugs today due to its low water absorption, high temperature resistance and suitable mechanical properties. This protein forms nanoparticles in the alcoholic-aqueous solution in the range of 150–550 nm (Fig. 14) and due to high hydrophobicity is considered as a controlled drug delivery system for hydrophobic drugs. Also, the special shape of bricks like this protein enables the ability to carry hydrophilic compounds such as heparin, 5-fluorouracil, and doxorubicin and control their release and improve their effect [155–157].
Fig. 14.
Production methods of cup nanoparticles (nano sphere) and hollow nanoparticles (nano capsules) of zein [155–157]
Gliadin
Wheat gluten is a complex containing proteins and carbohydrates, of which proteins are the main components. These proteins include glutenin and gliadin. Isolation and detection of these proteins is done with 70% alcohol. Glutenin is an alcohol insoluble protein with a molecular weight of 106 kDa. Gliadin is a set of proteins that are separated from alcohol by 70% of gluten and have a molecular weight in the range of 25–100 kDa. The structure of these proteins also contains large amounts of the amino acid glutamine (about 40%) [158, 159]. Gliadin has little solubility in aqueous solutions [160–162]. Because they are similar to creatine, these proteins are rich in proline and have the ability to interact with the skin's creatine epidermis and have the potential to produce skin formulations. Various studies have shown the ability of Gliadin nanoparticles as drug release control systems for hydrophobic compounds and amphiphilic compounds such as vitamin A, vitamin E, amoxicillin [163, 164]. One of the useful properties of Gliadin is its high ability to bind to the body mucosa [165, 166]. Due to the presence of glutamine and hydrophobic amino acids in its structure, Gliadin on the one hand gives abundant hydrogen bonds with the mucous layer of the mucosa and on the other hand can interact with the cell membrane by hydrophobic interactions. For this reason, Gliadin nanoparticles have shown good potential in the preparation of oral formulations, especially for the treatment of gastric diseases such as gastric ulcers. Due to their interaction with gastric mucus, these nanoparticles increase bioavailability and increase the release time of the drug, resulting in the effective removal of Helicobacter pylori (the cause of gastric ulcer) from the mucosa of this organ [167, 168].
Lectin
Lectins are a diverse group of glycoproteins or proteins capable of binding to carbohydrates. Wheat germ agglutinin (WGA) is one of the most popular plant lectins that is of great interest. This protein has high stability, low toxicity and immunogenicity, resistance to proteolytic degradation as well as specific identification and binding site to glycosylated components of intestinal mucosa and therefore can improve the absorption of oral drug formulations [169–172]. In the last two decades, lectins in two the main area has been considered by the pharmaceutical industry. The first is to improve the absorption of existing drugs with low bioavailability and the second is to prepare targeted drug formulations in the treatment of cancers [173–176]. In addition, several types of lectins, including WGA, have shown significant antitumor effects by inducing apoptosis in cancer cells. Numerous proteins and phospholipids are present in cell membranes attached to different oligosaccharide roots and have the ability to bind specifically [177–179]. Lectins to these sugar roots at the cell surface are the basis for targeted drug delivery by lectins (Fig. 15). Because different cells produce and display different types of oligosaccharide chains on their surface, cancer cells also often show different oligosaccharide patterns than normal cells of the same type, so different lectins can be used as Carriers are used to deliver the drug to different tissues and cells (Fig. 16). Accordingly, many studies have been conducted in this field to cover different types of nanoparticles with lectins and to produce targeted drug delivery systems. Lectins can be introduced as the second generation of bioadhesive enhancers, because in addition to the release of nanoparticles from the mucosal layer of the mucosa by various mechanisms such as clathrin-dependent endocytosis and endocytosis by the Caveola pathway causes increased cell uptake of drug formulations [180]. Lectins with the ability to bind to carbohydrates on the surface of Helicobacter pylori can also increase the effectiveness of treatment [175, 176, 181]. Lectins are also useful in the development of oral vaccines. Nanoparticles containing pathogenic antigens and coated with lectins targeted to the surface of Peyer’s patches in the intestine enhance the immune response of the oral vaccine [182, 183]. Aged plaque cells are cells that display antigens and components of the immune system located in the gut [184, 185]. The ability to bind lectins to the mucosa is used not only for the gastrointestinal mucosa but also to improve drug delivery through non-oral pathways such as the nasal mucosa, vagina, lungs, eyes, as well as crossing the blood–brain barrier. odorranalectin, the smallest member of the lectin family, is less immunogenic than other lectins and has the ability to specifically detect and bind to L-fucose. This sugar is abundant on the surface of cells lining the nasal mucosa. Nanoparticles containing this lectin have increased nose-to-brain delivery to the brain [186–189].
Fig. 15.
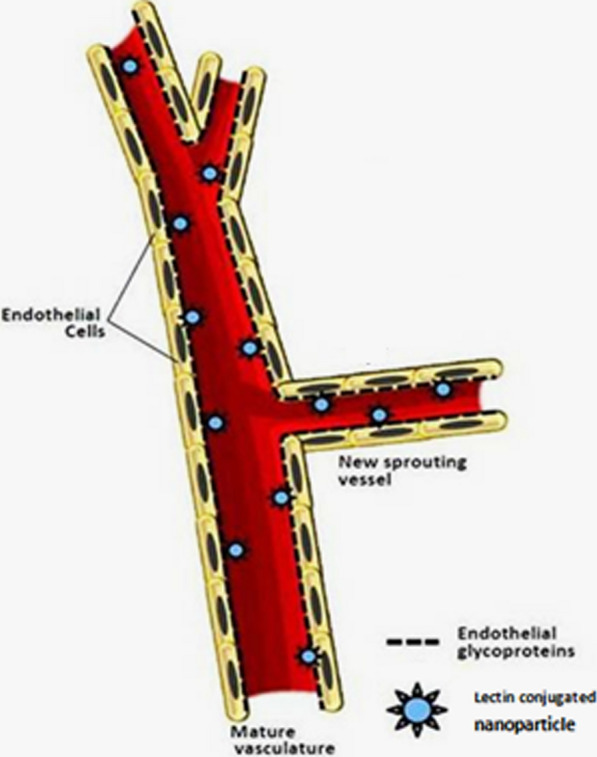
Nanoparticles coated with a variety of lectins have the ability to bind to glycoproteins of the luminal surface of vascular endothelial cell membranes and can be used in targeted drug delivery [186–189]
Fig. 16.
Some sugar sequences in tumors and viruses are different from those in normal cells. These sequences can be used as target lectin antigens in targeted drug delivery [186–189]
Soy proteins
Soybeans are currently one of the most abundant sources of plant protein. The fortified form of soy protein is called soy protein isolate (SPI). Soy protein extract is a balanced combination of polar, non-polar and pregnant amino acids that allows its use in a variety of drugs. The main components of SPI are glycinin with a molecular weight of 360 kDa and beta-kan glycinin with a molecular weight of 180 kDa. In aqueous solution, SPI proteins form a spherical structure consisting of a hydrophilic shell and a hydrophobic nucleus. Addition of precipitating agent (dissolvent = solvent) or linker molecules accumulate in different structures such as microsphere and hydrogel. By changing the amount of linker agents are added and as a result the percentage of binding in the resulting particles can be particle decomposition pattern to achieve the appropriate pattern Drug release altered Soy protein nanoparticles can be obtained from fresh SPI by desolvation or coacervation [190–193].
Protein cages
Viral protein cages
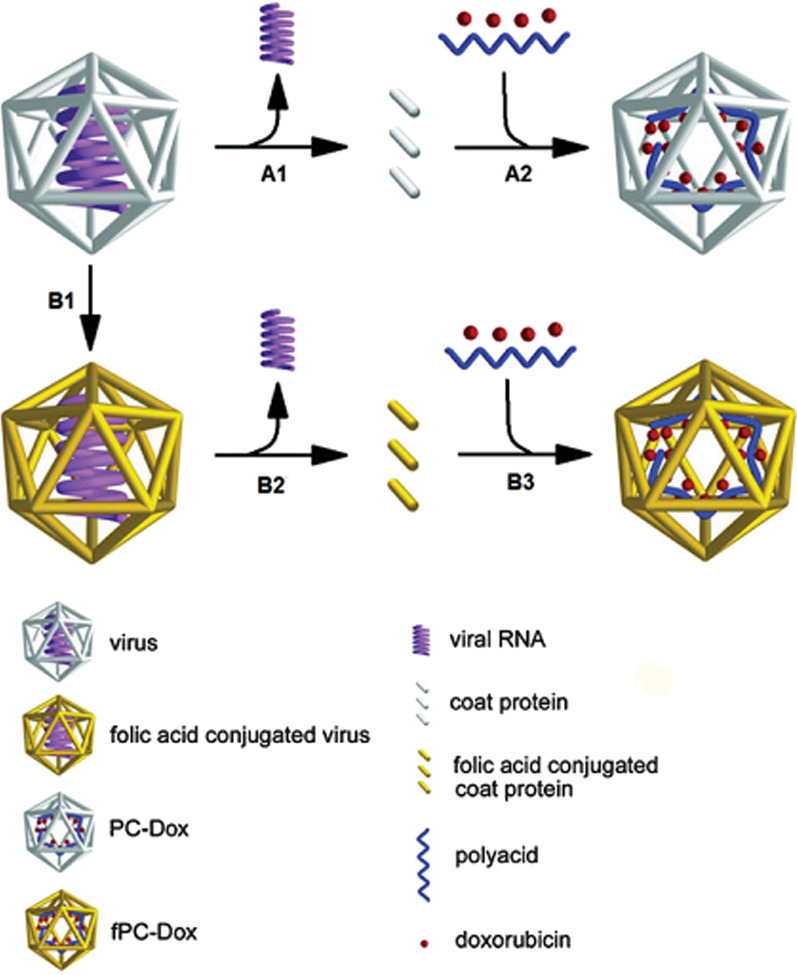
Protein cages are structures derived from viruses or virus-like particles. These particles often range in size from a few nanometers to a few tens of nanometers. Virus cages are actually the structural shell or capsid of viruses without their nucleic acid content. The shape, size and stability of virus cages depend on the type of virus. These cages consist of a limited number of subunits that accumulate in the form of porous nano spheres. In this structure, three distinct areas are significant, which are the inner and outer surface of the cage and the distance between the subunits. All three regions can be modified by chemical methods or genetic engineering methods (by changing the nucleotide sequence of subunits) without changing the structure of the cage, for use in applications in medical diagnosis and treatment [194]. Protein cages usually have good stability in different chemical environments (to cause chemical changes on them). In this way, a protein cage can be designed that has the ability to perform several operations simultaneously, such as drug loading, imaging agent, and cage targeting agent to a specific cell or tissue (Fig. 17). For example, by adding the amino acids cysteine and lysine to the cage by genetic engineering methods, it is possible to attach different drugs, imaging agents and fluorophores to the cage (Fig. 18). Another distinctive feature of protein cages compared to other protein structures is the uniform size of the cage. This property makes it possible to load relatively specific amounts of drug into these nanoparticles, which is an important pharmacokinetic feature of a drug formulation. The resulting protein cage is naturally stable in many physiological environments and protects drugs and therapeutic agents against chemical and enzymatic degradation. Cancer chemotherapy is another potential application of protein cages [195, 196]. Due to their size (tens of nanometers), nanometer cages do not pass through the endothelial layer of normal vessels, so they have a longer half-life in the bloodstream, but these cages are smaller than the pores of tumor tissue vessels (Fenestrate) and can enter and Adhesion to the surface of cells and tumor tissue can effectively inject significant amounts of chemotherapy drug into tumor tissue. The small size of the cages also helps these particles escape from the macrophage cells of the liver tissue [197, 198].
Fig. 17.
Mechanism of loading doxorubicin chemotherapy into the protein cage alone (A1, A2) or simultaneously with the binding of the targeting agent (folic acid) to the protein cage (B1, B2, B3) [195–198]
Fig. 18.
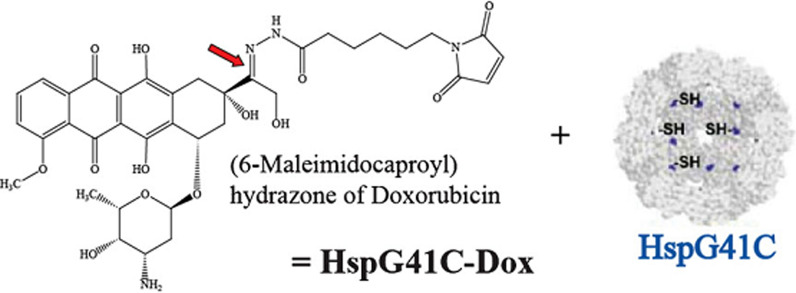
By genetic engineering methods, thiol (SH) cysteine roots are added to recombinant heat shock protein (HspG41C) nanocages. The resulting nano cages are able to bind to a significant number of doxorubicin drugs [195–198]
Non-viral protein cages
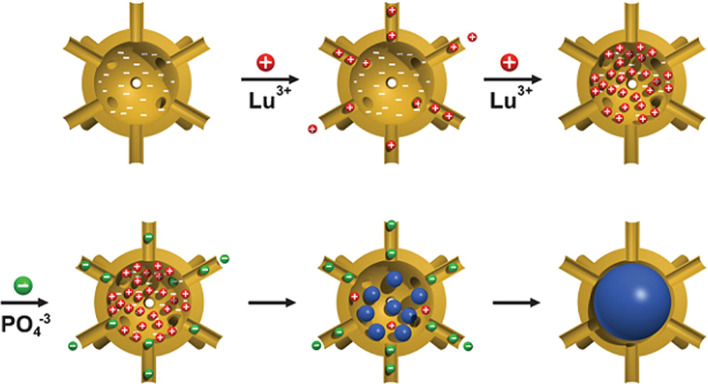
In addition to viruses, ferritin/apophytin protein cages and small heat shock shock protein are among the protein cages. Ferritin cages consist of 24 subunits that are arranged in the form of hollow structures with a diameter of 12 nm and an internal space of 8 nm. Normally in the body, this space is used to store about 4500 iron ions. In this structure, there are 14 channels for the exchange of materials between the cage and the external environment. These nano cages have been used to carry various molecules and ions [199]. Lutetium-177 (Lutetium-177) is a radioactive material that is highly regarded for imaging and radiotherapy applications in nuclear medicine due to its good half-life (6–7 days) and beta and gamma radiation. Numerous cancers, such as neuroendocrine, pancreatic, prostate, lung, bone marrow, and leukemia tumors, have been shown to be able to load large amounts of this radioactive substance into the body and increase its stability in the body. The higher dose of radiation to the tumor tissue will be during radiotherapy (Fig. 19).
Fig. 19.
Loading of luteum phosphate into ferritin nano cages by diffusion mechanism [199]
Disadvantages and limitations of protein nanoparticles
Despite the various advantages mentioned regarding the use of protein nanoparticles in drug delivery and tissue engineering, there are some limitations to these natural polymers in their use in the pharmaceutical and medical industries, some of the most important of which are [200–202]:
Proteins are natural polymers and most of them are heterogeneous mixtures of different sizes with different molecular weights. This feature reduces the possibility of reproducibility and the possibility of differences in product characteristics at different times (batch to batch variation) during mass production and industrial pharmaceutical uses. To overcome this problem, researchers are looking to produce recombinant proteins using genetic engineering techniques [203, 204]. The proteins produced by these methods are single in size and have a fixed and specific molecular weight. By designing their structure, it is possible to connect different groups to their surface, such as targeting factors, and also to adjust the rate of decomposition and release of the drug by them. In this regard, various types of proteins have been produced for use in drug delivery, including elastin proteins such as ELPs, recombinant human serum albumin (rHSA) and recombinant gelatin. It should be noted that the production of proteins by genetic engineering methods leads to an increase in the cost of products.
Immunogenicity: the human body has shown an immune response to foreign proteins and different degrees of immunogenicity are among the limitations of protein nanoparticles. However, when intravenous injection of albumin, gelatin, casein and zein nanoparticles [205–208], little immune response was observed [209].
Achieving a proper release pattern: since proteins are often hydrophilic molecules, most of them are not able to release the drug for a long time and their nanoparticles swell when they enter the body by absorbing water and the drug spreads rapidly outside. Therefore, chemical linker molecules such as formaldehyde and glutar aldehyde are usually used to stabilize their structure when preparing protein nanoparticles [210–214]. These interface molecules are often toxic, so one of the areas of active research in the field of protein nanoparticles is the achievement of suitable and non-toxic linkers. Also, plant proteins with hydrophobic nature have shown promising results in the production of protein nano carriers [215–219] with long-term release capacity.
The possibility of transmitting animal diseases such as bovine insanity to humans when using animal protein sources to produce nanoparticles [220–223].
Advantages of albumin nanoparticles as a drug delivery system
Formulations based on albumin nanoparticles have several advantages, some of which are due to the use of albumin as a structural unit of nanoparticles and others related to the properties of these nanoparticles, and Mathematical modeling plays an important role in facilitating the design of drug delivery systems by identifying key factors and molecular mechanisms of release [224–227]. Which are mentioned below [228, 229].
Albumin is one of the most important proteins in blood plasma and has many important physiological roles. The presence of high levels of albumin in the body makes the injection of significant amounts of it into the body without side effects or with low side effects. A history of albumin sensitivity is rare in individuals. One of the major advantages of albumin in mass production is its relatively easy access to the source and its price [230, 231].
Albumin nanoparticles are biocompatible, non- Taxol, immunogenic and biodegradable, and the residues from their degradation are amino acids that are used as a structural unit to make body proteins by the surrounding tissues.
Albumin has many different functional groups and therefore has the capacity to bind to significant amounts of the drug. Full understanding of the amino acid sequence and structure of albumin and multiple charged groups allows the binding of various drugs to albumin nanoparticles by various mechanisms including electrostatic attraction with negatively charged drugs (such as ganciclovir), positively charged ones (such as oligonucleotides), and dual compounds. Gives friend (like doxorubicin) and hydrophobic (like paclitaxel). Also, the presence of multiple functional groups on the surface of the resulting albumin nanoparticles, such as thiol, amine and carboxyl groups, makes it possible to easily change the surface and attach different ligand molecules to its surface to make nanoparticles with different activities and purposes [228, 229].
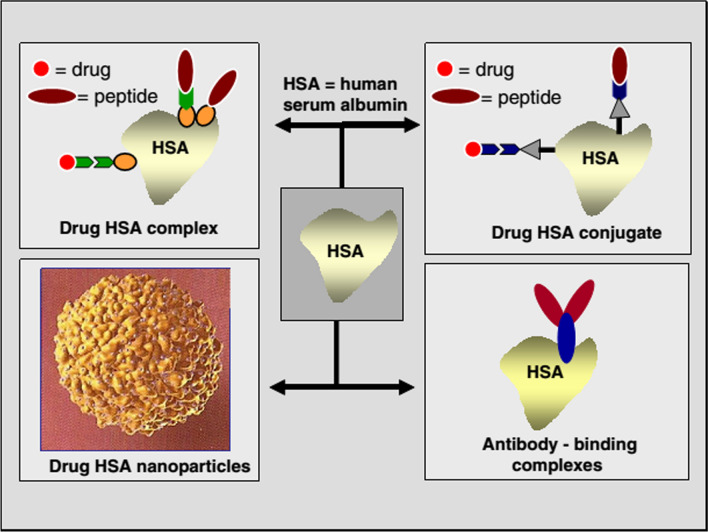
Various albumin-based drug delivery systems are commercially available and available in the market, including levmir and victoza in the treatment of diabetes, ozoralizumab in the treatment of rheumatoid arthritis, albuferon in the treatment of hepatitis C and the product. Albures 99mTc and Tc-Nanocoll 99 m can be mentioned in nuclear medicine (Figs. 20, 21), and albumin is also used as a carrier in the treatment of cancer and viral diseases. The four major methods that use albumin as a drug carrier are shown in Fig. 22. Drug precursors, and proteins and peptides can bind directly to this protein by non-covalent and covalent bonds or indirectly through an intermediate ligand having an albumin-binding group (top right and left). Alternatively, nano bodies are attached to albumin, or albumin replaces part of the immunoglobulin G antibody chain (fragment (Fc (bottom right)). Using albumin nanoparticles another important strategy has been to use albumin as a drug carrier (bottom left).
There are different methods for making albumin nanoparticles under mild environmental conditions.
Albumin as a structural unit of nanoparticles [232–234], an acidic protein, is very soluble and stable. Albumin is a flexible molecule and easily deforms depending on the environmental conditions in which it is located and also by changing the binding of ligands and returns to its original state at the first opportunity with the help of disulfide bonds, and this property is an advantage. It is important for it in the physiological environment and outside the body. This protein is able to regenerate its structure even though its numerous disulfide bridges are broken. Albumin does not have the conventional properties of most proteins, as it is a very stable and potent protein that, unlike many proteins, is in a wide range (pH = 4–6) and for a long time at high temperatures (more than 10 h at room temperature Above 60 °C) and also remains active in organic solvents. Its denaturation occurs only in non-physiological environments with severe changes in temperature, pH and ionic concentration of the environment. Introduces all the mentioned properties of albumin as a suitable subunit for making nanocarriers in drug delivery [199, 200, 228, 229].
As an anti-cancer drug formulation, albumin nanoparticles [235–237] accumulate in tumor tissue both through passive targeting and through active activation targeting, and therefore albumin nanoparticles have a high therapeutic ability. They have malignant solid tumors.
In general, albumin nanoparticles allow better control of drug release than liposomal formulations, which is effective in improving patient satisfaction and acceptance.
Fig. 20.
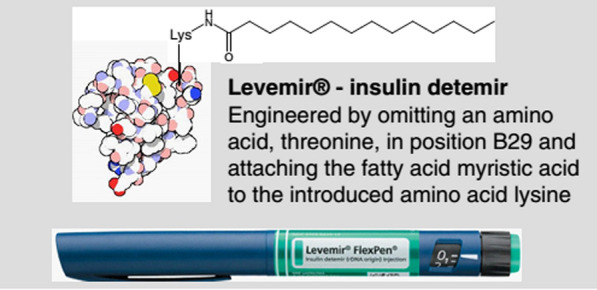
Structure of the drug Levemir used to treat diabetes [199, 200, 228, 229]
Fig. 21.
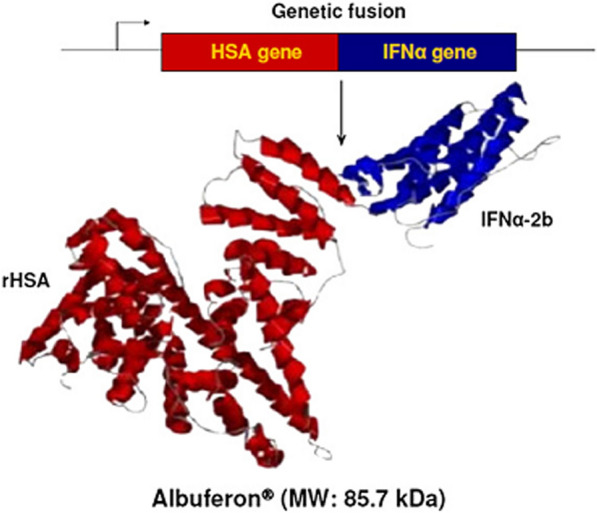
Alboferon is a genetic engineering product derived from the binding of the human albumin gene sequence to interferon alpha [199, 200, 228, 229]
Fig. 22.
Types of pharmaceutical formulations made using albumin as a carrier [199, 200, 228, 229]
Albumin
Over the past few decades, albumin has emerged as a powerful macromolecular carrier in medical therapeutic and diagnostic applications. This protein with a half-life of about 19 days in the bloodstream can play an important role in improving the pharmacokinetic properties as well as targeting drugs [238]. Abraxane Albumin Formulation Paclitaxel Anti-Cancer Drug Benefits the benefits of albumin in its antitumor function (Fig. 23).
Fig. 23.
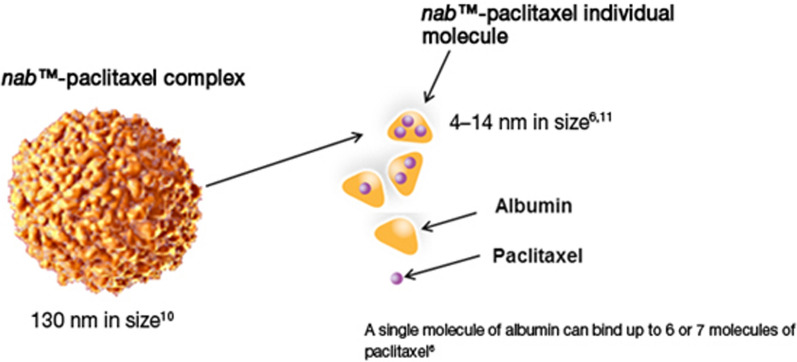
Structure of Abraxane (nabTM-paclitaxel)
Types of albumin
For commercial use, albumin is made from a variety of sources, such as egg white called ovalbumin, bovine serum albumin (BSA), and human serum albumin (HSA). Milk, soy and legumes are other sources of albumin [239].
Ovalbumin
Ovalbumin is one of the most widely used food proteins, which is widely used in the food industry. This molecule is a glycoprotein with a molecular weight of 47 kDa, contains 385 amino acid roots and has only one disulfide bond. The main reason for choosing and using this protein as a drug carrier is benefits such as easy access to its source and its cheap price. Other properties include the ability to form gels, suspensions and foams. Due to its pH-sensitive and temperature-sensitive properties, ovalbumin has the potential to be used as a drug release control agent [228].
Bovine serum albumin
This protein with a molecular weight of 69 kDa is widely used in drug delivery. The popularity of this protein is due to various advantages such as abundance of its source, cheap price, easy separation and purification from bovine serum, its high capacity to bind to ligands and also its wide acceptance in the pharmaceutical industry [240].
Human serum albumin
-
Albumin structure
Human serum albumin is a spherical soluble protein with a molecular weight of 66.5 kDa consisting of 585 Amino Acid consisting of a single polypeptide strand. This chain is a set of alpha-helix chains that form three separate second structures (Fig. 24). Albumin contains 35 cysteine roots, which play a fundamental role in the formation of the structure of this protein by forming 17 disulfide bonds. Also, the presence of a large number of charged amino acids such as lysine, arginine, glutamic acid and aspartic acid in its structure plays an important role in the various biological roles of albumin and also in the production of nanoparticles and the binding of various factors to it. The three-dimensional structure of human albumin is determined by X-ray Crystallography, according to which the albumin has a heart-like structure with dimensions of 80 by 30 angstroms [199, 241]. Of course, this structure is somewhat different in solution and all three of them are elliptical (Fig. 25).
-
Physiological functions of albumin
Albumin is one of the most important plasma proteins and has many important physiological roles. This protein makes up more than 60% of the mass of plasma proteins and its amount is about 35–50 g per liter of blood serum. Albumin alone is responsible for more than 80% of the plasma osmolality pressure, and also plays an important role in stabilizing blood pH by its buffering action. This protein, like many plasma proteins, is made in the liver and has a daily production rate of 10–15 g in the body, and its average half-life in human blood serum is 19 days. Albumin acts as a carrier of many molecules including fatty acids, eicosanoids, bile acids, steroid hormones, vitamins C, D, folate, copper, zinc, calcium, magnesium, as well as many drugs in the blood such as penicillin’s, sulfonamides, benzodiazepines And end lytic compounds are involved (Fig. 26). In its protective role, albumin binds to the toxic metabolite bilirubin and transports it to the liver for excretion. Albumin exerts its protective role by binding to toxic substances of external origin such as benzene and the carcinogenic compound Afflation and various other compounds. It is used as a therapeutic agent in human serum albumin in the treatment of various diseases such as shock, burns, albumin deficiency, trauma and cardiopulmonary surgery, acute respiratory problems and blood dialysis. Another important feature is its preferential absorption by inflamed tissues as well as tumor tissues, which is an important advantage for use as a drug carrier [200].
Fig. 24.

Albumin structure. Albumin is a polypeptide strand containing several alpha chains [199, 241]
Fig. 25.
Shows the schematic structure of albumin in solution [199, 241]
Fig. 26.
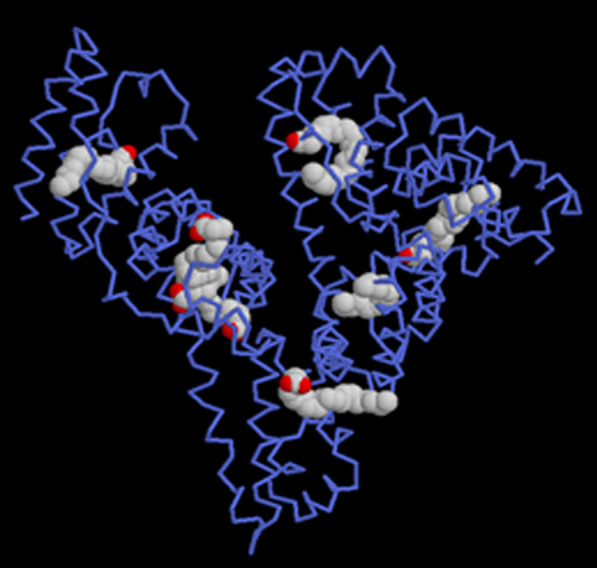
Albumin has multiple binding sites for a variety of biomolecules and drugs [200]
Mechanism of targeting by albumin nanoparticles
Passive targeting
Tumors have the ability to trap plasma proteins and use their amino acids as a source of energy and food during their proliferation. Tumor vascular endothelial wall is defective and has greater permeability than healthy vasculature, so the entry of some macromolecules into the interstitial fluid space that does not occur in small tissue vessels or occurs in small amounts increases in tumor tissue. On the other hand, lymphatic resection of interstitial fluid in tumor tissue is not performed well and the combination of the two leads to the accumulation of macromolecules in tumor tissue. This phenomenon is called Enhanced permeation and retention. Studies have also shown that the duration Long circulation time is one of the prerequisites for increasing tumor resection of proteins. Albumin with effective hydrodynamic diameter of 7.2 nm and long circulation is a suitable candidate for drug delivery to tumor tissues and EPR mechanism is one of the mechanisms to increase harvesting. It is a tumor (Fig. 27) [199, 200, 228, 229, 238–241].
Fig. 27.
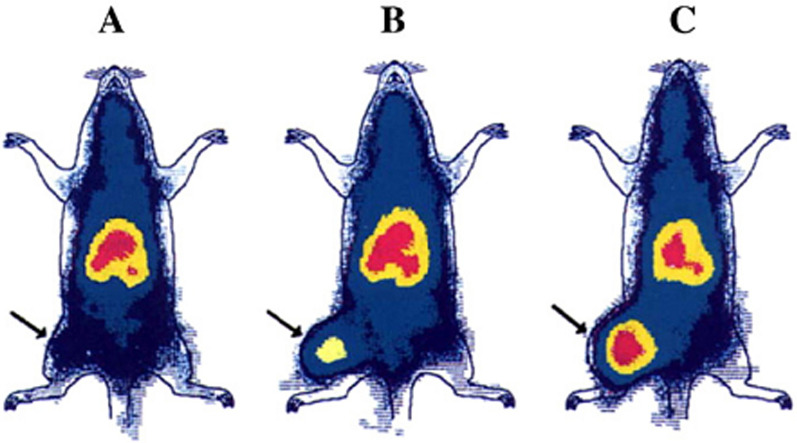
Accumulation of albumin-advance blue complex in a tumor formed in the left leg of a growing rat during (A) 24 h (B) 48 h, and (C) 72 h [199, 200, 228, 229, 238–241]
Targeted active
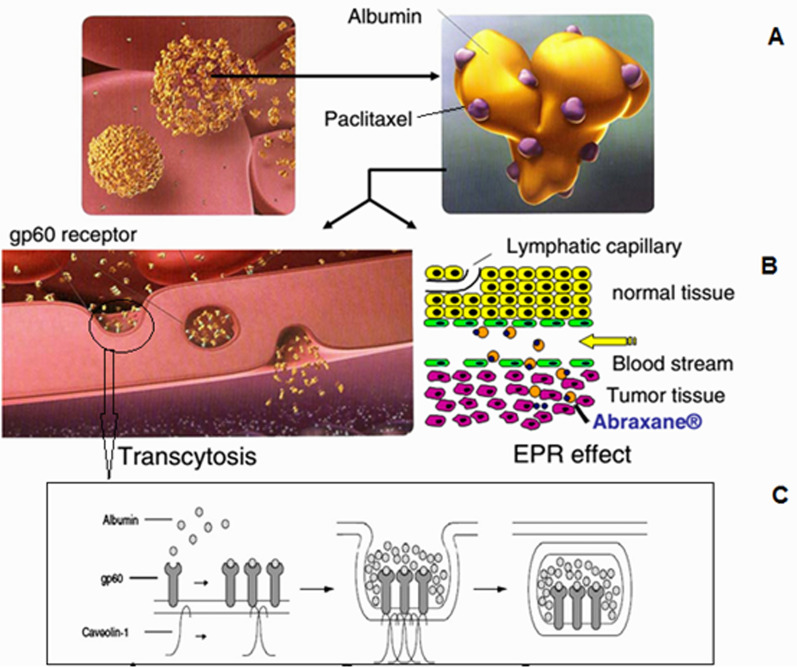
Albumin has a receptor called albobandine or glycoprotein 60 (with a molecular weight of 60 kDa) on the surface of endothelial luminal cells, the drug-albumin complex binds to these receptors. The affinity of the paclitaxel albumin complex for glycoprotein 60 is very high and in the nanomolar concentration range. This binding stimulates the accumulation of glycoprotein 60 molecules and then the accumulation of proteins called kaolin in place. These proteins are involved in the process of endocytosis. Thus, the drug-albumin complex enters from the luminal surface of endothelial cells and on the other hand it is released and enters the interstitial fluid space. In the interstitial space, this complex binds to an extracellular matrix protein called SPARK, increasing the shelf life of drug-containing albumin in the extracellular space and releasing paclitaxel over a long period of time in the vicinity of cancer cells. SPARK protein expression is increased in several cancers, and as a result, this property of albumin nanoparticles leads to active targeted drug delivery to tumor tissue (Fig. 28) [199, 200, 228, 229, 238–241].
Fig. 28.
A Structure of albumin nanoparticles in Abraham. B Two mechanisms that increase the accumulation of abraxas in tumor tissue. C Details of the mechanism by which albumin passes through endothelial cells into interstitial fluid space [199, 200, 228, 229, 238–241]
Abraxane commercial formulation of albumin nanoparticles containing paclitaxel
Taxol are a family of compounds with strong anti-cancer properties, and Paclitaxel is a member of this family. The most important limitation of these compounds for therapeutic use is their low solubility in physiological body fluids. For this reason, Taxol is used as a formulation with higher solubility of Paclitaxel for the treatment of cancer. Abraxane was developed to overcome the limitations of Taxol and has several advantages over Taxol in the treatment of various cancers, which are mentioned below (Fig. 29) [199, 200, 228, 229, 238–241].
In terms of CrEL-paclitaxel formulation with the brand name Taxol, it contains 50% ethanol and 50% chromophore to improve the solubility of Paclitaxel. Chromophore is a toxic compound that limits the use of Taxol, while nab-Paclitaxel under the brand name Abraxane (ABI 007) contains a formulation of Paclitaxel containing 3 to 4% albumin [199].
In terms of injection time, Taxol requires a long injection time of 3 to 24 h, while the duration of Abraxane injection is only 30 min.
Before injecting Taxol, it is necessary to prepare the patient by injecting various drugs such as corticosteroids and antihistamines to reduce the risk of hypersensitivity reactions. Including dexamethasone (12 and 6 h before injection), diphenhydramine (1 h before injection) and cimetidine (30 and 60 min before injection) while Abraxane does not require preparation. Also, unlike Abraxane, Taxol injection requires a special injection set that increases the cost of treatment [228, 229].
Due to the protection of paclitaxel in albumin nanoparticles in Abraxane, the maximum allowable dose of paclitaxel in this formulation is 260 mg / m2, which is significantly higher than Taxol (175 mg / m2). It should be noted that despite the possibility of using a higher dose of paclitaxel to treat cancer in Abraxane, the side effects of the drug, including neutropenia, are less than Taxol. Pharmacokinetically, due to linear release, Abraxane is superior to Taxol with non-linear release [228, 229].
Studies with radioactively labeled paclitaxel show that the drug passes more than 4 times the width of endothelial cells in the formulation of Abraxane relative to Taxol (Fig. 30) [199, 200, 228, 229, 238–241].
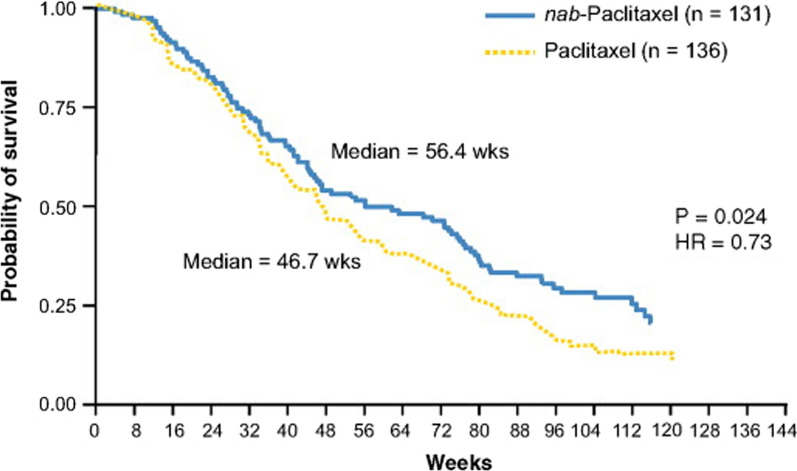
Intracorporeal studies show that at an injectable dose equal to paclitaxel in the two formulations of Taxol and Abraxane, the rate of drug accumulation in the tumor when using Abraxane is 33% higher than that of Taxol and Abraxane also causes a significant delay in tumor growth rate (Fig. 31) and also significantly increases the patient's lifetime (survival)[228, 229].
It is possible to actively target Abraxane using changes in their levels with antibodies and peptides [228, 229].
Fig. 29.
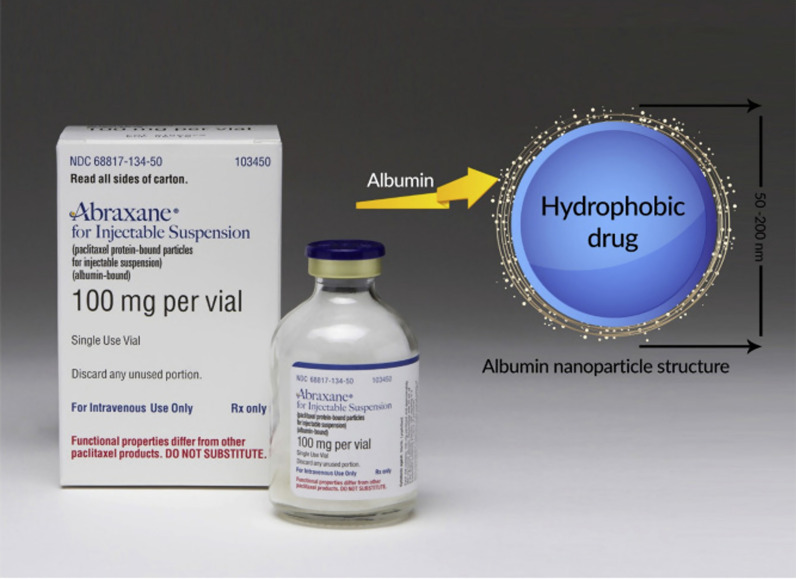
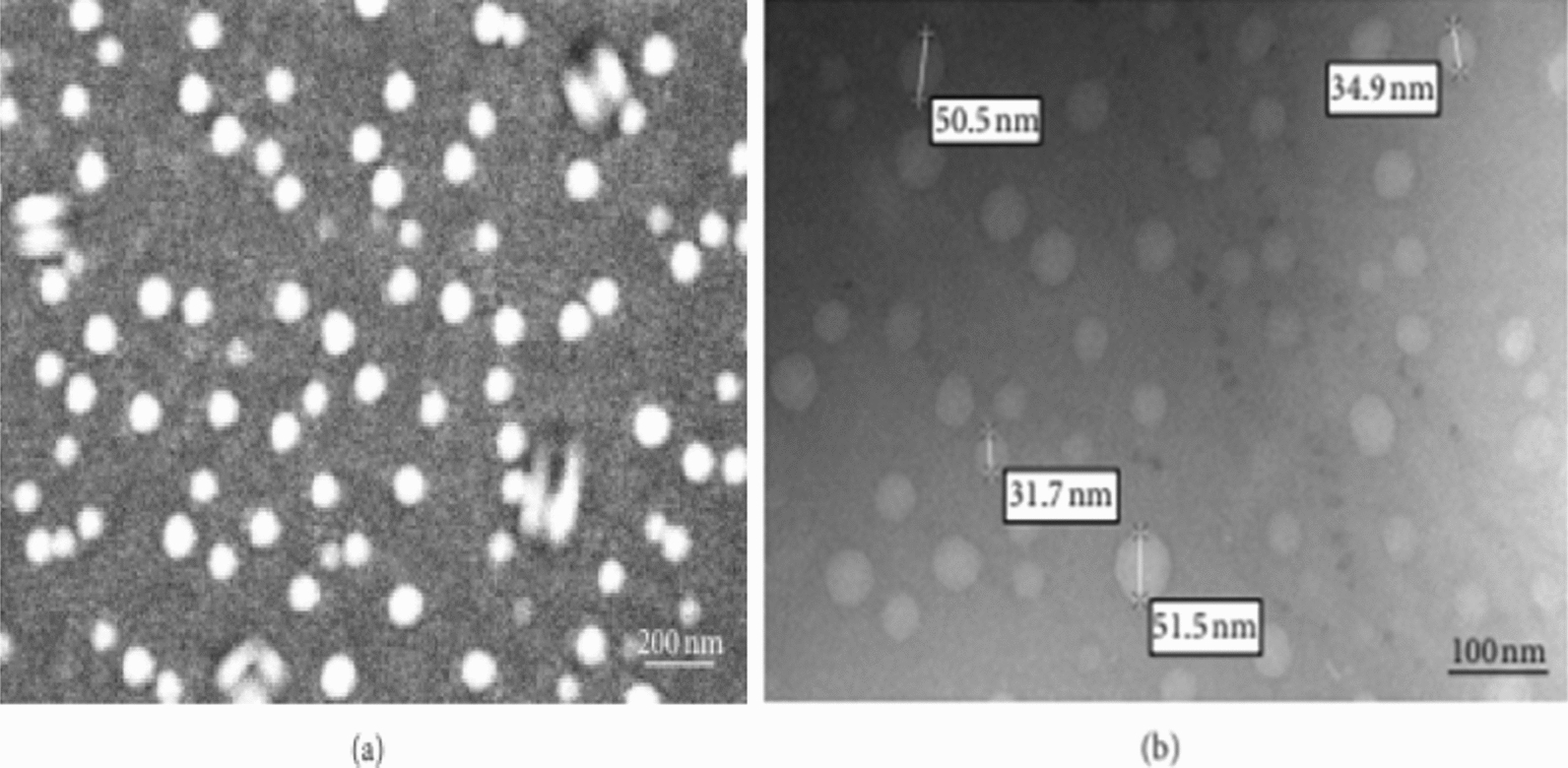
Abraxane formulation containing 135 nm particles of albumin nanoparticles containing paclitaxel [28, 29, 41–51, 55–67, 73–95, 97–159, 165–172, 177–180, 184–202, 228, 229, 238–242]
Fig. 30.
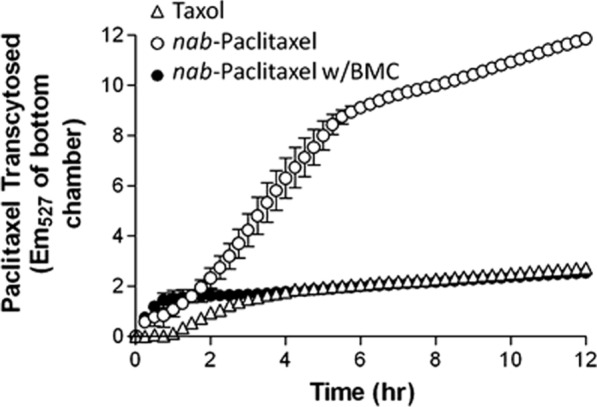
Comparison of paclitaxel entry into endothelial cells in three formulations of Taxol and Abraxane and Abraxane containing methyl beta-cyclodextrin: Abraxane increases the cellular uptake of paclitaxel by 4.4 times compared to Taxol. When cyclodextrin is used as an inhibitor of 60 gp glycoprotein (which, by binding to albumin, causes endocytosis and increases its cellular uptake), the rate of drug uptake is very similar to that of Taxol, indicating a direct role for albumin in increasing paclitaxel cellular uptake [228, 229]
Fig. 31.
Comparison of the effect of two formulations of Abraxane and Taxol in prolonging the tumor progression process in patients with metastatic breast cancer: Abraxane causes a significant delay in tumor progression compared to Taxol in equal time
Methods of producing albumin nanoparticles
There are several methods for making albumin nanoparticles [243–245], the most important of which is the emulsion-evaporation method used in the manufacture of Abraxane (Fig. 32). Some of these methods are [199–202, 228, 229, 238–241]:
Emulsion method—evaporation with the creation of cross connections (emulsion evaporation cross link method),
Phase separation method,
Simple coacervation method,
Self-assembly method (self-assembly),
-
5.
Nano spray drying method and thermal method (Thermal gelation).
-
6.
Production process of Abraxane formulation (nab-Technology): The basis of nab (nanoparicle albumin bound technology) is the use of evaporation emulsion method with the creation of crosslinks between albumin units for the stability of the resulting nanoparticles. The generalities of its production process are given (Fig. 33).
Fig. 32.
Methods of producing albumin nanoparticles [228]
Fig. 33.
Albumin nanoparticles by emulsion-evaporation method [199, 200, 228, 229]
Mathematical modelling of drug release kinetic
Equation (1) [246, 247, 248, 249] was used to quantify the normalized drug release from nanocarriers:
| 1 |
The total quantities of drug released at any time (t) and the final amounts of drug released, respectively, are Mt and Mf.
The stabilized drug release primarily for the early stage (0–8 h), which can be represented by Peppa’s model [250], a statistical and semi-empirical model:
| 2 |
where k is determined by the liposome's and drugs structural properties, and n is determined by the drug's release mechanism (Fickian diffusion or non-Fickian diffusion) and carrier geometry. (Times) is the dimension of k, and n is dimensionless.
In order to align the experimental drug release with models [251–254], nonlinear regression analysis was conducted using MATLAB software version 7.8 (Mathworks Inc., Natick, MA) [255–259].
The consistency of the fit was determined using root mean square error (RMSE) [260–265] and R-square (R2) [266–269]. A better fit is shown by higher R2 and lower RMSE values [238–239]. Expressions (3) and (4) can be used to quantify these:
| 3 |
| 4 |
where release (percent)exp,i is the experimental release used in every calculation, release (percent)pre,i is the expected release for this measurement, N is the number of observations, Z is the number of model constants [270–272], release (percent)exp is the total average data, and I is ith data, and N is the number of observations, Z is the number of model constants, release (percent)exp is the total average data, and release (percent)exp is the total It is assumed that there is no barrier to drug delivery in the buffer solution. Multiple regression methods were used to construct drug release model coefficients with different parameters such as buffer pH and temperature in order to generalize the drug release kinetic model [273, 274].
Conclusion
Nanoparticles are among the most promising carriers in modern drug delivery systems. Among these, protein nanoparticles due to numerous advantages such as easy access to their resources, renewable resources, reasonable price, biocompatibility and biodegradability, the existence of multiple functional groups to carry large amounts of drugs and the possibility of connecting targeting groups to them. To target nanoparticles to a specific target cell or tissue, they are considered. Various animal and plant proteins have been used to make nanodrates. Among the most important animal proteins used to make nanocarriers are gelatin, collagen, and elastin, milk proteins such as casein, albumin, and silk fibroids.
Due to their high hydrophobic nature, some plant proteins have the ability to produce nanocarriers that, unlike animal proteins, do not require chemical linkers to produce stable nanoparticles and can also retain their drug shipments for long periods of time. They have a long-term release of drug delivery systems. Abundant resources and easy and cheap access are other benefits of plant protein nanocarriers. Researchers in the pharmaceutical and medical industries are always looking for carriers whose repeatability is high during mass industrial production and it is possible to control their various properties, such as the connection of targeting agents. The properties of protein nanocages promise the production of ideal nanocarriers in the future.
Albumin is one of the most important proteins in blood plasma and has had several therapeutic applications in the past few decades. As a rich protein, blood has good biocompatibility and biodegradability. The existence of multiple functional groups makes it possible to carry significant amounts of therapeutic and diagnostic factors as well as targeting factors. It also has a high structural stability and can withstand a wide range of temperature and pH without adversely affecting their structure. The unique properties of albumin have led to the development of a variety of drug formulations based on this protein in the treatment of various diseases. Abraxane nanoparticle formulation the anti-cancer drug paclitaxel is currently used as an effective formulation in the treatment of several common cancers and is in the final stages of clinical trials for other cancers.
In the field of nanoparticle drug delivery, protein polymers and protein composite materials are gaining popularity. Their properties are suitable for drug delivery systems, and they have the potential to improve controlled release or targeting processes. Natural protein polymer is an appealing commodity from an economic standpoint because it is comparatively inexpensive, simple to produce, and reusable. Biodegradability and biocompatibility are the key benefits of protein-based nanoparticles over conventional materials. A key factor in deciding the effectiveness of a drug delivery operation is minimizing the host immune response. The aggregation of particle byproducts is reduced by the normal breakdown of these protein polymers, which is also safer for human wellbeing. The properties of protein materials such as silk fibroin, keratin, and elastin, as well as their use in nanoparticle drug delivery and biomedical applications, were the subject of this study. Protein-based nanoparticles can be processed in a number of ways, allowing their properties to be tailored for particular applications. Although there are still obstacles to conquer, there is a growing need in the medical sector for biocompatible protein nanoparticles. To address these obstacles, future research on protein-based nanoparticles must concentrate on the creation of large-scale manufacturing techniques that enable these particles to be produced in a commercially viable manner. To reduce off-target impact, functionalized particles capable of targeting particular areas of the body are likely to be produced. The fabrication and characteristics of protein nanoparticles must change as new pharmaceuticals are developed in order to provide suitable vehicles for drug delivery. The further new experiments are published and the functionality of these protein materials improves, the more people will be interested in them.
Acknowledgements
ERNAM-Erciyes University Nanotechnology Application and Research Center, Kayseri 38039, Turkey, Department of Analytical Chemistry, Faculty of Pharmacy, Erciyes University, Kayseri 38039, Turkey, Technology Research and Application Center (TAUM), Erciyes University, Kayseri 38039, Turkey, Chemicamed Chemical Inc. Erciyes University Technology Development Zone, 38039 Kayseri, Turkey.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
We considerate the submitted is original and unique work and it has not been submitted to another journal simultaneity. The work is single study and it’s not up into several parts and results are presented clearly, honestly, and without, falsification or inappropriate the data manipulation, the study submitted is part of my doctoral thesis. The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. This work does not contain any studies with human participants or animals performed by any of the authors. Finally, additional informed consent was obtained from all individual participants for whom identifying information is included in this paper.
Consent for publication
Not applicable.
Competing interests
The author declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cai R, Chen C. The crown and the scepter: roles of the protein corona in nanomedicine. Adv Mater. 2019;31(45):1805740. doi: 10.1002/adma.201805740. [DOI] [PubMed] [Google Scholar]
- 2.Ovais M, Guo M, Chen C. Tailoring nanomaterials for targeting tumor-associated macrophages. Adv Mater. 2019;31(19):1808303. doi: 10.1002/adma.201808303. [DOI] [PubMed] [Google Scholar]
- 3.Ovais M, Khalil AT, Raza A, et al. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl Microbiol Biotechnol. 2018;102(10):4393–4408. doi: 10.1007/s00253-018-8928-2. [DOI] [PubMed] [Google Scholar]
- 4.Kianfar E. Synthesis and characterization of AlPO4/ZSM-5 catalyst for methanol conversion to dimethyl ether. Russ J Appl Chem. 2018;91:1711–1720. doi: 10.1134/S1070427218100208. [DOI] [Google Scholar]
- 5.Kianfar E. Ethylene to propylene conversion over Ni-W/ZSM-5 catalyst. Russ J Appl Chem. 2019;92:1094–1101. doi: 10.1134/S1070427219080068. [DOI] [Google Scholar]
- 6.Kianfar E, Salimi M, Kianfar F, et al. CO2/N2 separation using polyvinyl chloride iso-phthalic acid/aluminium nitrate nanocomposite membrane. Macromol Res. 2019;27:83–89. doi: 10.1007/s13233-019-7009-4. [DOI] [Google Scholar]
- 7.Kianfar E. Ethylene to propylene over zeolite ZSM-5: improved catalyst performance by treatment with CuO. Russ J Appl Chem. 2019;92:933–939. doi: 10.1134/S1070427219070085. [DOI] [Google Scholar]
- 8.Kianfar E, Shirshahi M, Kianfar F, et al. Simultaneous prediction of the density, viscosity and electrical conductivity of pyridinium-based hydrophobic ionic liquids using artificial neural network. SILICON. 2018;10:2617–2625. doi: 10.1007/s12633-018-9798-z. [DOI] [Google Scholar]
- 9.Wan S, Kelly PM, Mahon E, et al. The “sweet” side of the protein corona: effects of glycosylation on nanoparticle–cell interactions. ACS Nano. 2015;9(2):2157–2166. doi: 10.1021/nn506060q. [DOI] [PubMed] [Google Scholar]
- 10.Hellstrand E, Lynch I, Andersson A, et al. Complete high-density lipoproteins in nanoparticle corona. FEBS J. 2009;276(12):3372–3381. doi: 10.1111/j.1742-4658.2009.07062.x. [DOI] [PubMed] [Google Scholar]
- 11.Ke PC, Lin S, Parak WJ, Davis TP, Caruso F. A decade of the protein corona. ACS Nano. 2017;11(12):11773–11776. doi: 10.1021/acsnano.7b08008. [DOI] [PubMed] [Google Scholar]
- 12.Setyawati MI, Tay CY, Docter D, Stauber RH, Leong DT. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem Soc Rev. 2015;44(22):8174–8199. doi: 10.1039/C5CS00499C. [DOI] [PubMed] [Google Scholar]
- 13.Salimi M, Pirouzfar V, Kianfar E. Novel nanocomposite membranes prepared with PVC/ABS and silica nanoparticles for C2H6/CH4 separation. Polym Sci Ser A. 2017;59:566–574. doi: 10.1134/S0965545X17040071. [DOI] [Google Scholar]
- 14.Kianfar F, Kianfar E. Synthesis of isophthalic acid/aluminum nitrate thin film nanocomposite membrane for hard water softening. J Inorg Organomet Polym. 2019;29:2176–2185. doi: 10.1007/s10904-019-01177-1. [DOI] [Google Scholar]
- 15.Kianfar E, Azimikia R, Faghih SM. Simple and strong dative attachment of α-diimine nickel (II) catalysts on supports for ethylene polymerization with controlled morphology. Catal Lett. 2020;150:2322–2330. doi: 10.1007/s10562-020-03116-z. [DOI] [Google Scholar]
- 16.Kianfar E. Nanozeolites: synthesized, properties, applications. J Sol-Gel Sci Technol. 2019;91:415–429. doi: 10.1007/s10971-019-05012-4. [DOI] [Google Scholar]
- 17.Liu H, Kianfar E. Investigation the synthesis of nano-SAPO-34 catalyst prepared by different templates for MTO process. Catal Lett. 2020 doi: 10.1007/s10562-020-03333-6. [DOI] [Google Scholar]
- 18.Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 19.Baimanov D, Cai R, Chen C. Understanding the chemical nature of nanoparticle–protein interactions. Bioconjug Chem. 2019;30(7):1923–1937. doi: 10.1021/acs.bioconjchem.9b00348. [DOI] [PubMed] [Google Scholar]
- 20.Jin J, Ovais M, Chen C. Stimulus-responsive gold nanotheranostic platforms for targeting the tumor microenvironment. Nano Today. 2018;22:83–99. doi: 10.1016/j.nantod.2018.08.007. [DOI] [Google Scholar]
- 21.Chen H, Gu Z, An H, et al. Precise nanomedicine for intelligent therapy of cancer. Sci China Chem. 2018;61(12):1503–1552. doi: 10.1007/s11426-018-9397-5. [DOI] [Google Scholar]
- 22.Shang L, Nienhaus GU. In situ characterization of protein adsorption onto nanoparticles by fluorescence correlation spectroscopy. Acc Chem Res. 2017;50(2):387–395. doi: 10.1021/acs.accounts.6b00579. [DOI] [PubMed] [Google Scholar]
- 23.Maffre P, Nienhaus K, Amin F, Parak WJ, Nienhaus GU. Characterization of protein adsorption onto FePt nanoparticles using dual-focus fluorescence correlation spectroscopy. Beilstein J Nanotechnol. 2011;2(1):374–383. doi: 10.3762/bjnano.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maffre P, Brandholt S, Nienhaus K, Shang L, Parak WJ, Nienhaus GU. Effects of surface functionalization on the adsorption of human serum albumin onto nanoparticles—a fluorescence correlation spectroscopy study. Beilstein J Nanotechnol. 2014;5(1):2036–2047. doi: 10.3762/bjnano.5.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treuel L, Brandholt S, Maffre P, Wiegele S, Shang L, Nienhaus GU. Impact of protein modification on the protein corona on nanoparticles and nanoparticle–cell interactions. ACS Nano. 2014;8(1):503–513. doi: 10.1021/nn405019v. [DOI] [PubMed] [Google Scholar]
- 26.Kianfar E, Salimi M, Hajimirzaee S, Koohestani B. Methanol to gasoline conversion over CuO/ZSM-5 catalyst synthesized using sonochemistry method. Int J Chem Reactor Eng. 2018;17.
- 27.Zhang X, et al. Pyramid Channel-based Feature Attention Network for image dehazing. Comput Vis Image Underst. 2020;197-198:103003. doi: 10.1016/j.cviu.2020.103003. [DOI] [Google Scholar]
- 28.Shang L, Yang L, Seiter J, et al. Nanoparticles interacting with proteins and cells: a systematic study of protein surface charge effects. ACS Appl Mater Interfaces. 2014;1(2):1300079. doi: 10.1002/admi.201300079. [DOI] [Google Scholar]
- 29.Dominguez-Medina S, Kisley L, Tauzin LJ, et al. Adsorption and unfolding of a single protein triggers nanoparticle aggregation. ACS Nano. 2016;10(2):2103–2112. doi: 10.1021/acsnano.5b06439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman JA, Deitcher SR. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharm. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatakrishnan K, Liu Y, Noe D, Mertz J, Bargfrede M, Marbury T, Farbakhsh K, Oliva C, Milton A. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate renal impairment. Br J Clin Pharm. 2014;77:986–997. doi: 10.1111/bcp.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets. 2016;9:3001–3007. doi: 10.2147/OTT.S105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 35.Komlosh A, Weinstein V, Loupe P, Hasson T, Timan B, Konya A, Alexander J, Melamed-Gal S, Nock S. Physicochemical and biological examination of two glatiramer acetate products. Biomedicines. 2019;7:49. doi: 10.3390/biomedicines7030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MT, Chen Y, Marhoul J, Jacobson F. Statistical modeling of the drug load distribution on trastuzumab emtansine (Kadcyla), a lysine-linked antibody drug conjugate. Bioconjug Chem. 2014;25:1223–1232. doi: 10.1021/bc5000109. [DOI] [PubMed] [Google Scholar]
- 37.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomed. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, et al. Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chelle P, Yeung CHT, Croteau SE, Lissick J, Balasa V, Ashburner C, Park YS, Bonanad S, Megías-Vericat JE, Nagao A, et al. Development and validation of a population-pharmacokinetic model for Rurioctacog Alfa Pegol (Adynovate®): a report on behalf of the WAPPS-Hemo Investigators Ad Hoc Subgroup. Clin Pharmacokinet. 2020;59:245–256. doi: 10.1007/s40262-019-00809-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Wang Di, Zhou Z, Ma Yi. Robust low-rank tensor recovery with rectification and alignment. IEEE Trans Pattern Anal Mach Intell. 2021;43(1):238–255. doi: 10.1109/TPAMI.2019.2929043. [DOI] [PubMed] [Google Scholar]
- 41.Fleischer CC, Payne CK. Nanoparticle–cell interactions: molecular structure of the protein corona and cellular outcomes. Acc Chem Res. 2014;47(8):2651–2659. doi: 10.1021/ar500190q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozboyaci M, Kokh DB, Corni S, Wade RC. Modeling and simulation of protein–surface interactions: achievements and challenges. Q Rev Biophys. 2016;49:e4. doi: 10.1017/S0033583515000256. [DOI] [PubMed] [Google Scholar]
- 43.Ozboyaci M, Kokh D, Wade R. Three steps to gold: mechanism of protein adsorption revealed by Brownian and molecular dynamics simulations. Phys Chem Chem Phys. 2016;18(15):10191–10200. doi: 10.1039/C6CP00201C. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Shang L, Maffre P, et al. The nature of a hard protein corona forming on quantum dots exposed to human blood serum. Small. 2016;12(42):5836–5844. doi: 10.1002/smll.201602283. [DOI] [PubMed] [Google Scholar]
- 45.Monopoli MP, Walczyk D, Campbell A, et al. Physical–chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133(8):2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 46.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 47.Nedelkov D, Kiernan UA, Niederkofler EE, Tubbs KA, Nelson RW. Investigating diversity in human plasma proteins. Proc Natl Acad Sci. 2005;102(31):10852–10857. doi: 10.1073/pnas.0500426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajipour MJ, Raheb J, Akhavan O, et al. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015;7(19):8978–8994. doi: 10.1039/C5NR00520E. [DOI] [PubMed] [Google Scholar]
- 49.Corbo C, Molinaro R, Tabatabaei M, Farokhzad OC, Mahmoudi M. Personalized protein corona on nanoparticles and its clinical implications. Biomater Sci. 2017;5(3):378–387. doi: 10.1039/C6BM00921B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahmoudi M, Abdelmonem AM, Behzadi S, et al. Temperature: the “ignored” factor at the nanobio interface. ACS Nano. 2013;7(8):6555–6562. doi: 10.1021/nn305337c. [DOI] [PubMed] [Google Scholar]
- 51.Laurent S, Burtea C, Thirifays C, Rezaee F, Mahmoudi M. Significance of cell “observer” and protein source in nanobiosciences. J Colloid Interface Sci. 2013;392:431–445. doi: 10.1016/j.jcis.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Fan M, Wang Di, Zhou P, Tao D. Top-k feature selection framework using robust 0–1 integer programming. IEEE Trans Neural Netw Learn Syst. 2020 doi: 10.1109/TNNLS.2020.3009209. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Wang T, Wang J, Tang G, Zhao L. Pyramid channel-based feature attention network for image Dehazing. Comput Vis Image Underst. 2020;197-198:103003. doi: 10.1016/j.cviu.2020.103003. [DOI] [Google Scholar]
- 54.Zhang X, Jiang R, Wang T, Wang J. Recursive neural network for video deblurring. IEEE Trans Circuits Syst Video Technol. 2020 doi: 10.1109/TCSVT.2020.3035722. [DOI] [Google Scholar]
- 55.Ghavami M, Saffar S, Emamy BA, et al. Plasma concentration gradient influences the protein corona decoration on nanoparticles. RSC Adv. 2013;3(4):1119–1126. doi: 10.1039/C2RA22093H. [DOI] [Google Scholar]
- 56.Viel KR, Ameri A, Abshire TC, et al. Inhibitors of factor VIII in black patients with hemophilia. N Engl J Med. 2009;360(16):1618–1627. doi: 10.1056/NEJMoa075760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walkey CD, Chan WC. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41(7):2780–2799. doi: 10.1039/C1CS15233E. [DOI] [PubMed] [Google Scholar]
- 58.Colapicchioni V, Tilio M, Digiacomo L, et al. Personalized liposome–protein corona in the blood of breast, gastric and pancreatic cancer patients. Int J Biochem Cell Biol. 2016;75:180–187. doi: 10.1016/j.biocel.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Caracciolo G, Farokhzad OC, Mahmoudi M. Biological identity of nanoparticles in vivo: clinical implications of the protein corona. Trends Biotechnol. 2017;35(3):257–264. doi: 10.1016/j.tibtech.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Monopoli MP, Åberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7(12):779. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 61.García-Álvarez R, Hadjidemetriou M, Sánchez-Iglesias A, Liz-Marzán LM, Kostarelos K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale. 2018;10(3):1256–1264. doi: 10.1039/C7NR08322J. [DOI] [PubMed] [Google Scholar]
- 62.Obst K, Yealland G, Balzus B, et al. Protein corona formation on colloidal polymeric nanoparticles and polymeric nanogels: impact on cellular uptake, toxicity, immunogenicity, and drug release properties. Biomacromol. 2017;18(6):1762–1771. doi: 10.1021/acs.biomac.7b00158. [DOI] [PubMed] [Google Scholar]
- 63.Chen D, Ganesh S, Wang W, Amiji M. The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles. Nanoscale. 2019;11(18):8760–8775. doi: 10.1039/C8NR09855G. [DOI] [PubMed] [Google Scholar]
- 64.Juling S, Niedzwiecka A, BöHmert L, et al. Protein corona analysis of silver nanoparticles links to their cellular effects. J Proteome Res. 2017;16(11):4020–4034. doi: 10.1021/acs.jproteome.7b00412. [DOI] [PubMed] [Google Scholar]
- 65.Müller J, Prozeller D, Ghazaryan A, et al. Beyond the protein corona-lipids matter for biological response of nanocarriers. Acta Biomater. 2018;71:420–431. doi: 10.1016/j.actbio.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 66.Wang M, Briggs MR. HDL: the metabolism, function, and therapeutic importance. Chem Rev. 2004;104(1):119–138. doi: 10.1021/cr020466v. [DOI] [PubMed] [Google Scholar]
- 67.Ritz S, SchöTtler S, Kotman N, et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromol. 2015;16(4):1311–1321. doi: 10.1021/acs.biomac.5b00108. [DOI] [PubMed] [Google Scholar]
- 68.Kianfar E. Investigation of the effect of crystallization temperature and time in synthesis of SAPO-34 catalyst for the production of light olefins. Pet Chem. 2021 doi: 10.1134/S0965544121050030. [DOI] [Google Scholar]
- 69.Kianfar E. Nano Biosensors: properties, applications and electrochemical techniques. J Mater Res Technol. 2021 doi: 10.1016/j.jmrt.2021.03.048. [DOI] [Google Scholar]
- 70.Kianfar F, Moghadam SRM, Kianfar E. Synthesis of spiro pyran by using silica-bonded N-propyldiethylenetriamine as recyclable basic catalyst. Indian J Sci Technol. 2015;8(11):68669. doi: 10.17485/ijst/2015/v8i11/71776. [DOI] [Google Scholar]
- 71.Kianfar E. Recent advances in synthesis, properties, and applications of vanadium oxide nanotube. Microchem J. 2019;145:966–978. doi: 10.1016/j.microc.2018.12.008. [DOI] [Google Scholar]
- 72.Wang M-R, Deng Li, Liu G-C, Wen L, Wang J-G, Huang K-B, Tang H-T, Pan Y-M. Porous organic polymer-derived nanopalladium catalysts for chemoselective synthesis of antitumor benzofuro[2,3-b]pyrazine from 2-bromophenol and isonitriles. Org Lett. 2019;21(13):4929–4932. doi: 10.1021/acs.orglett.9b01230. [DOI] [PubMed] [Google Scholar]
- 73.Lara S, Alnasser F, Polo E, et al. Identification of receptor binding to the biomolecular corona of nanoparticles. ACS Nano. 2017;11(2):1884–1893. doi: 10.1021/acsnano.6b07933. [DOI] [PubMed] [Google Scholar]
- 74.Nam NN, Han SY. Formation of high-density lipoprotein (HDL) coronas on silica nanoparticles occurs by adsorption of intact HDL particulates. Bull Korean Chem Soc. 2016;37(1):3–4. doi: 10.1002/bkcs.10622. [DOI] [Google Scholar]
- 75.Chen F, Wang G, Griffin JI, et al. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12(4):387. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bertrand N, Grenier P, Mahmoudi M, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat Commun. 2017;8(1):777. doi: 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao W, Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int J Pharm. 2018;552(1–2):328–339. doi: 10.1016/j.ijpharm.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Guo L, Wang T, Chen Z, He N, Chen Y, Yuan T. Light scattering based analyses of the effects of bovine serum proteins on interactions of magnetite spherical particles with cells. Chin Chem Lett. 2018;29(8):1291–1295. doi: 10.1016/j.cclet.2017.11.017. [DOI] [Google Scholar]
- 79.Schöttler S, Becker G, Winzen S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11(4):372. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 80.Ovais M, Chen C. Safety considerations for nanoparticles in tumor treatment. Nanomedicine. 2018;13(19):2373–2376. doi: 10.2217/nnm-2018-0267. [DOI] [PubMed] [Google Scholar]
- 81.Lesniak A, Fenaroli F, Monopoli MP, Åberg C, Dawson KA, Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano. 2012;6(7):5845–5857. doi: 10.1021/nn300223w. [DOI] [PubMed] [Google Scholar]
- 82.Ho YT, Kamm RD, Kah JCY. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale. 2018;10(26):12386–12397. doi: 10.1039/C8NR02393J. [DOI] [PubMed] [Google Scholar]
- 83.Guo L, Feng Z, Cai L, et al. Effects of a protein-corona on the cellular uptake of ferroferric oxide nanoparticles. J Nanosci Nanotechnol. 2016;16(7):7125–7128. doi: 10.1166/jnn.2016.11361. [DOI] [Google Scholar]
- 84.Mirshafiee V, Kim R, Park S, Mahmoudi M, Kraft ML. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials. 2016;75:295–304. doi: 10.1016/j.biomaterials.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Ding L, Yao C, Yin X, et al. Size, shape, and protein corona determine cellular uptake and removal mechanisms of gold nanoparticles. Small. 2018;14(42):1801451. doi: 10.1002/smll.201801451. [DOI] [PubMed] [Google Scholar]
- 86.Riviere JE, Jaberi-Douraki M, Lillich J, Azizi T, Joo H, Choi K, Thakkar R, Monteiro-Riviere NA. Modeling gold nanoparticle biodistribution after arterial infusion into perfused tissue: effects of surface coating, size and protein corona. Nanotoxicology. 2018;12(10):1093–1112. doi: 10.1080/17435390.2018.1476986. [DOI] [PubMed] [Google Scholar]
- 87.Baynes RE. Isolated perfused porcine skin flap. Curr Protoc Toxicol. 2001;8(1):541–549. doi: 10.1002/0471140856.tx0504s08. [DOI] [PubMed] [Google Scholar]
- 88.Leavens TL, Xia XR, Lee HA, Monteiro-Riviere NA, Brooks JD, Riviere JE. Evaluation of perfused porcine skin as a model system to quantitate tissue distribution of fullerene nanoparticles. Toxicol Lett. 2010;197(1):1–6. doi: 10.1016/j.toxlet.2010.03.1119. [DOI] [PubMed] [Google Scholar]
- 89.Leavens TL, Monteiro-Riviere NA, Inman AO, Brooks JD, Oldenburg SJ, Riviere JE. In vitro biodistribution of silver nanoparticles in isolated perfused porcine skin flaps. J Appl Toxicol. 2012;32(11):913–919. doi: 10.1002/jat.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee HA, Imran M, Monteiro-Riviere NA, Colvin VL, Yu WW, Riviere JE. Biodistribution of quantum dot nanoparticles in perfused skin: evidence of coating dependency and periodicity in arterial extraction. Nano Lett. 2007;7(9):2865–2870. doi: 10.1021/nl071563c. [DOI] [PubMed] [Google Scholar]
- 91.Stepien G, Moros M, PéRez-HernáNdez M, et al. Effect of surface chemistry and associated protein corona on the long-term biodegradation of iron oxide nanoparticles in vivo. ACS Appl Mater Interfaces. 2018;10(5):4548–4560. doi: 10.1021/acsami.7b18648. [DOI] [PubMed] [Google Scholar]
- 92.Shannahan JH, Podila R, Aldossari AA, et al. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol Sci. 2014;143(1):136–146. doi: 10.1093/toxsci/kfu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rahman M, Laurent S, Tawil N, Yahia LH, Mahmoudi M. Protein–nanoparticle interactions. Berlin: Springer; 2013. Nanoparticle and protein corona; pp. 21–44. [Google Scholar]
- 94.Salvati A, Pitek AS, Monopoli MP, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8(2):137. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 95.Varnamkhasti BS, Hosseinzadeh H, Azhdarzadeh M, et al. Protein corona hampers targeting potential of MUC1 aptamer functionalized SN-38 core–shell nanoparticles. Int J Pharm. 2015;494(1):430–444. doi: 10.1016/j.ijpharm.2015.08.060. [DOI] [PubMed] [Google Scholar]
- 96.Carvalho JA, Abreu AS, Ferreira VTP, Gonçalves EP, Tedesco AC, Pinto JG, Ferreira-Strixino J, Junior MB, Simioni AR. Preparation of gelatin nanoparticles by two step desolvation method for application in photodynamic therapy. J Biomater Sci Polym Ed. 2018;29(11):1287–1301. doi: 10.1080/09205063.2018.1456027. [DOI] [PubMed] [Google Scholar]
- 97.Dai Q, Yan Y, Ang C-S, et al. Monoclonal antibody-functionalized multilayered particles: targeting cancer cells in the presence of protein coronas. ACS Nano. 2015;9(3):2876–2885. doi: 10.1021/nn506929e. [DOI] [PubMed] [Google Scholar]
- 98.Dehouck B, Dehouck M-P, Fruchart J-C, Cecchelli R. Upregulation of the low density lipoprotein receptor at the blood–brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J Cell Biol. 1994;126(2):465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DalMagro R, Albertini B, Beretta S, et al. Artificial apolipoprotein corona enables nanoparticle brain targeting. Nanomed NBM. 2018;14(2):429–438. doi: 10.1016/j.nano.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Pareek V, Bhargava A, Bhanot V, Gupta R, Jain N, Panwar J. Formation and characterization of protein corona around nanoparticles: a review. J Nanosci Nanotechnol. 2018;18(10):6653–6670. doi: 10.1166/jnn.2018.15766. [DOI] [PubMed] [Google Scholar]
- 102.Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Protein–nanoparticle interactions: opportunities and challenges. Chem Rev. 2011;111(9):5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 103.Wang X, Wang M, Lei R, Zhu SF, Zhao Y, Chen C. Chiral surface of nanoparticles determines the orientation of adsorbed transferrin and its interaction with receptors. ACS Nano. 2017;11(5):4606–4616. doi: 10.1021/acsnano.7b00200. [DOI] [PubMed] [Google Scholar]
- 104.Ge C, Du J, Zhao L, et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci. 2011;108(41):16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Li J, Pan J, et al. Revealing the binding structure of the protein corona on gold nanorods using synchrotron radiation-based techniques: understanding the reduced damage in cell membranes. J Am Chem Soc. 2013;135(46):17359–17368. doi: 10.1021/ja406924v. [DOI] [PubMed] [Google Scholar]
- 106.Ding Z, Ma H, Chen Y. Interaction of graphene oxide with human serum albumin and its mechanism. RSC Adv. 2014;4(98):55290–55295. doi: 10.1039/C4RA09613D. [DOI] [Google Scholar]
- 107.Li S, Peng Z, Leblanc RM. Method to determine protein concentration in the protein–nanoparticle conjugates aqueous solution using circular dichroism spectroscopy. Anal Chem. 2015;87(13):6455–6459. doi: 10.1021/acs.analchem.5b01451. [DOI] [PubMed] [Google Scholar]
- 108.Pederzoli F, Tosi G, Vandelli MA, Belletti D, Forni F, Ruozi B. Protein corona and nanoparticles: how can we investigate on? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(6):e1467. doi: 10.1002/wnan.1467. [DOI] [PubMed] [Google Scholar]
- 109.Zhang J, Zhang X, Zhang F, Yu S. Solid-film sampling method for the determination of protein secondary structure by Fourier transform infrared spectroscopy. Anal Bioanal Chem. 2017;409(18):4459–4465. doi: 10.1007/s00216-017-0390-y. [DOI] [PubMed] [Google Scholar]
- 110.Yang H, Wang M, Zhang Y, et al. Detailed insight into the formation of protein corona: conformational change, stability and aggregation. Int J Biol Macromol. 2019;15(135):1114–1122. doi: 10.1016/j.ijbiomac.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 111.Wang M, Fu C, Liu X, Lin Z, Yang N, Yu S. Probing the mechanism of plasma protein adsorption on Au and Ag nanoparticles with FT-IR spectroscopy. Nanoscale. 2015;7(37):15191–15196. doi: 10.1039/C5NR04498G. [DOI] [PubMed] [Google Scholar]
- 112.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6(1):39. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 113.Amenabar I, Poly S, Nuansing W, et al. Structural analysis and mapping of individual protein complexes by infrared nanospectroscopy. Nat Commun. 2013;4:2890. doi: 10.1038/ncomms3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Assfalg M, Ragona L, Pagano K, et al. The study of transient protein–nanoparticle interactions by solution NMR spectroscopy. Biochim Biophys Acta Proteins Proteom. 2016;1864(1):102–114. doi: 10.1016/j.bbapap.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 115.Lundqvist M, Sethson I, Jonsson B-H. Protein adsorption onto silica nanoparticles: conformational changes depend on the particles’ curvature and the protein stability. Langmuir. 2004;20(24):10639–10647. doi: 10.1021/la0484725. [DOI] [PubMed] [Google Scholar]
- 116.Stayton PS, Drobny GP, Shaw WJ, Long JR, Gilbert M. Molecular recognition at the protein–hydroxyapatite interface. Crit Rev Oral Biol Med. 2003;14(5):370–376. doi: 10.1177/154411130301400507. [DOI] [PubMed] [Google Scholar]
- 117.Carril M, Padro D, Del Pino P, Carrillo-Carrion C, Gallego M, Parak WJ. In situ detection of the protein corona in complex environments. Nat Commun. 2017;8(1):1542. doi: 10.1038/s41467-017-01826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carrillo-Carrion C, Carril M, Parak WJ. Techniques for the experimental investigation of the protein corona. Curr Opin Biotechnol. 2017;46:106–113. doi: 10.1016/j.copbio.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 119.Wang X, Berger R, Ramos JI, et al. Nanopatterns of polymer brushes for understanding protein adsorption on the nanoscale. RSC Adv. 2014;4(85):45059–45064. doi: 10.1039/C4RA07623K. [DOI] [Google Scholar]
- 120.Guan G, Zhang S, Liu S, et al. Protein induces layer-by-layer exfoliation of transition metal dichalcogenides. J Am Chem Soc. 2015;137(19):6152–6155. doi: 10.1021/jacs.5b02780. [DOI] [PubMed] [Google Scholar]
- 121.Dobrovolskaia MA, Patri AK, Zheng J, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomed NBM. 2009;5(2):106–117. doi: 10.1016/j.nano.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schaefer J, Schulze C, Marxer EEJ, et al. Atomic force microscopy and analytical ultracentrifugation for probing nanomaterial protein interactions. ACS Nano. 2012;6(6):4603–4614. doi: 10.1021/nn202657q. [DOI] [PubMed] [Google Scholar]
- 123.Chong Y, Ge C, Yang Z, et al. Reduced cytotoxicity of graphene nanosheets mediated by blood-protein coating. ACS Nano. 2015;9(6):5713–5724. doi: 10.1021/nn5066606. [DOI] [PubMed] [Google Scholar]
- 124.Zong C, Xu M, Xu L-J, et al. Surface-enhanced Raman spectroscopy for bioanalysis: reliability and challenges. Chem Rev. 2018;118(10):4946–4980. doi: 10.1021/acs.chemrev.7b00668. [DOI] [PubMed] [Google Scholar]
- 125.Shashilov VA, Sikirzhytski V, Popova LA, Lednev IK. Quantitative methods for structural characterization of proteins based on deep UV resonance Raman spectroscopy. Methods. 2010;52(1):23–37. doi: 10.1016/j.ymeth.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 126.Zhang D, Neumann O, Wang H, et al. Gold nanoparticles can induce the formation of protein-based aggregates at physiological pH. Nano Lett. 2009;9(2):666–671. doi: 10.1021/nl803054h. [DOI] [PubMed] [Google Scholar]
- 127.Porcaro F, Roudeau S, Carmona A, Ortega R. Advances in element speciation analysis of biomedical samples using synchrotron-based techniques. Trends Analyt Chem. 2018;104:22–41. doi: 10.1016/j.trac.2017.09.016. [DOI] [Google Scholar]
- 128.Kumagai PS, Araujo AP, Lopes JL. Going deep into protein secondary structure with synchrotron radiation circular dichroism spectroscopy. Biophys Rev. 2017;9(5):517–527. doi: 10.1007/s12551-017-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Su H, Liu C, Hu S, et al. Progress in application of microbeam x-ray fluorescence spectroscopy in forensic science. Fa Yi Xue Za Zhi. 2013;29(1):43–48. [PubMed] [Google Scholar]
- 130.Ho YT, Poinard B, Yeo ELL, Kah JCY. An instantaneous colorimetric protein assay based on spontaneous formation of a protein corona on gold nanoparticles. Analyst. 2015;140(4):1026–1036. doi: 10.1039/C4AN01819B. [DOI] [PubMed] [Google Scholar]
- 131.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4(11):2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kambhampati S, Li J, Evans BS, Allen DK. Accurate and efficient amino acid analysis for protein quantification using hydrophilic interaction chromatography coupled tandem mass spectrometry. Plant Methods. 2019;15(1):46. doi: 10.1186/s13007-019-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahmoudi M, Shokrgozar MA, Sardari S, et al. Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale. 2011;3(3):1127–1138. doi: 10.1039/c0nr00733a. [DOI] [PubMed] [Google Scholar]
- 134.Timms JF, Cutillas PR. Overview of quantitative LC-MS techniques for proteomics and activitomics. In: Timms CPJ, editor. LC-MS/MS in proteomics. USA: Humana Press; 2010. pp. 19–45. [DOI] [PubMed] [Google Scholar]
- 135.Zhang H, Wu RA. Proteomic profiling of protein corona formed on the surface of nanomaterial. Sci China Chem. 2015;58(5):780–792. doi: 10.1007/s11426-015-5395-9. [DOI] [Google Scholar]
- 136.Hu Z, Zhao L, Zhang H, Zhang Y, Wu RA, Zou H. The on-bead digestion of protein corona on nanoparticles by trypsin immobilized on the magnetic nanoparticle. J Chromatogr A. 2014;1334:55–63. doi: 10.1016/j.chroma.2014.01.077. [DOI] [PubMed] [Google Scholar]
- 137.Fernández-Iglesias N, Bettmer J. Complementary mass spectrometric techniques for the quantification of the protein corona: a case study on gold nanoparticles and human serum proteins. Nanoscale. 2015;7(34):14324–14331. doi: 10.1039/C5NR02625C. [DOI] [PubMed] [Google Scholar]
- 138.Legat J, Matczuk M, Timerbaev A, Jarosz M. CE separation and ICP-MS detection of gold nanoparticles and their protein conjugates. Chromatographia. 2017;80(11):1695–1700. doi: 10.1007/s10337-017-3387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yin H, Chen R, Casey PS, Ke PC, Davis TP, Chen C. Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium. RSC Adv. 2015;5(90):73963–73973. doi: 10.1039/C5RA14870G. [DOI] [Google Scholar]
- 140.Kokkinopoulou M, Simon J, Landfester K, Mailänder V, Lieberwirth I. Visualization of the protein corona: towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale. 2017;9(25):8858–8870. doi: 10.1039/C7NR02977B. [DOI] [PubMed] [Google Scholar]
- 141.Stewart M, Mulenos M, Steele L, Sayes C. Differences among unique nanoparticle protein corona constructs: a case study using data analytics and multi-variant visualization to describe physicochemical characteristics. Appl Sci. 2018;8(12):2669. doi: 10.3390/app8122669. [DOI] [Google Scholar]
- 142.Tonigold M, Simon J, Estupiñán D, et al. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat Nanotechnol. 2018;13(9):862. doi: 10.1038/s41565-018-0171-6. [DOI] [PubMed] [Google Scholar]
- 143.Serpooshan V, Mahmoudi M, Zhao M, et al. Protein corona influences cell–biomaterial interactions in nanostructured tissue engineering scaffolds. Adv Funct Mater. 2015;25(28):4379–4389. doi: 10.1002/adfm.201500875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Durowoju IB, Bhandal KS, Hu J, Carpick B, Kirkitadze M. Differential scanning calorimetry—a method for assessing the thermal stability and conformation of protein antigen. J Vis Exp. 2017;121:e55262. doi: 10.3791/55262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.MüLler J, Simon J, Rohne P, et al. Denaturation via surfactants changes composition of protein corona. Bio macromolecules. 2018;19(7):2657–2664. doi: 10.1021/acs.biomac.8b00278. [DOI] [PubMed] [Google Scholar]
- 146.Nienhaus GU, Maffre P, Nienhaus K. Studying the protein corona on nanoparticles by FCS. In: Sergey YT, editor. Methods in enzymology. The Netherlands: Elsevier; 2013. pp. 115–137. [DOI] [PubMed] [Google Scholar]
- 147.Röcker C, Pötzl M, Zhang F, Parak WJ, Nienhaus GU. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat Nanotechnol. 2009;4(9):577. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 148.Huang R, Carney RP, Ikuma K, Stellacci F, Lau BL. Effects of surface compositional and structural heterogeneity on nanoparticle–protein interactions: different protein configurations. ACS Nano. 2014;8(6):5402–5412. doi: 10.1021/nn501203k. [DOI] [PubMed] [Google Scholar]
- 149.Zheng T, Bott S, Huo Q. Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl Mater Interfaces. 2016;8(33):21585–21594. doi: 10.1021/acsami.6b06903. [DOI] [PubMed] [Google Scholar]
- 150.Zheng T, Cherubin P, Cilenti L, Teter K, Huo Q. A simple and fast method to study the hydrodynamic size difference of protein disulfide isomerase in oxidized and reduced form using gold nanoparticles and dynamic light scattering. Analyst. 2016;141(3):934–938. doi: 10.1039/C5AN02248G. [DOI] [PubMed] [Google Scholar]
- 151.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V. Time evolution of the nanoparticle protein corona. ACS Nano. 2010;4(7):3623–3632. doi: 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- 152.Goy-LóPez S, JuáRez J, Alatorre-Meda M, et al. Physicochemical characteristics of protein–NP bioconjugates: the role of particle curvature and solution conditions on human serum albumin conformation and fibrillogenesis inhibition. Langmuir. 2012;28(24):9113–9126. doi: 10.1021/la300402w. [DOI] [PubMed] [Google Scholar]
- 153.Blundell EL, Healey MJ, Holton E, Sivakumaran M, Manstana S, Platt M. Characterisation of the protein corona using tunable resistive pulse sensing: determining the change and distribution of a particle’s surface charge. Anal Bioanal Chem. 2016;408(21):5757–5768. doi: 10.1007/s00216-016-9678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gessner A, Lieske A, Paulke BR, Müller RH. Influence of surface charge density on protein adsorption on polymeric nanoparticles: analysis by two-dimensional electrophoresis. Eur J Pharm Biopharm. 2002;54(2):165–170. doi: 10.1016/S0939-6411(02)00081-4. [DOI] [PubMed] [Google Scholar]
- 156.Lynch I, Dawson KA. Protein–nanoparticle interactions. Nano Today. 2008;3(1–2):40–47. doi: 10.1016/S1748-0132(08)70014-8. [DOI] [Google Scholar]
- 157.Ânia M, Emilio R, Angel M, Rafael G, Manuel F. Protein interactions and nanomaterials: a key role of the protein corona in nanobiocompatibility. In: Ansari MUR, editor. Protein–protein interaction assays. London: IntechOpen Limited; 2018. p. 29. [Google Scholar]
- 158.Sooväli L, Rõõm E-I, Kütt A, Kaljurand I, Leito I. Uncertainty sources in UV–Vis spectrophotometric measurement. Accred Qual Assur. 2006;11(5):246–255. doi: 10.1007/s00769-006-0124-x. [DOI] [Google Scholar]
- 159.Cedervall T, Lynch I, Lindman S, et al. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci. 2007;104(7):2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu L, Jiang S, Wu J, Zou Q. An in silico approach to identification, categorization and prediction of nucleic acid binding proteins. Brief Bioinform. 2020 doi: 10.1093/bib/bbaa171. [DOI] [PubMed] [Google Scholar]
- 161.Xian-Fang W, Peng G, Yi-Feng L, Hong-Fei L, Fan L. Predicting thermophilic proteins by machine learning. Curr Bioinformat. 2020;15:493. doi: 10.2174/1574893615666200207094357. [DOI] [Google Scholar]
- 162.Zou Q, Xing P, Wei L, Liu B. Gene2vec: gene subsequence embedding for prediction of mammalian N6-methyladenosine sites from mRNA. RNA. 2019;25(2):205–218. doi: 10.1261/rna.069112.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xue C, Bei J, Dengkui W, Guoli L, et al. Gate-tunable the interface properties of GaAs–WSe2 (1D–2D) vdWs heterojunction for high-responsivity, self-powered photodetector. Appl Phys Lett. 2021;118:041102. doi: 10.1063/5.0035275. [DOI] [Google Scholar]
- 164.Kianfar E. Production and identification of vanadium oxide nanotubes. Indian J Sci Technol. 2015;8(S9):455–464. doi: 10.17485/ijst/2015/v8iS9/68569. [DOI] [Google Scholar]
- 165.Kari OK, Rojalin T, Salmaso S, et al. Multi-parametric surface plasmon resonance platform for studying liposome–serum interactions and protein corona formation. Drug Deliv Transl Res. 2017;7(2):228–240. doi: 10.1007/s13346-016-0320-0. [DOI] [PubMed] [Google Scholar]
- 166.Sen R, Dasgupta D. Simple fluorescence assays probing conformational changes of Escherichia coli RNA polymerase during transcription initiation. In: Adhya S, Garges S, editors. Methods in enzymology. The Netherlands: Elsevier; 2003. pp. 598–605. [DOI] [PubMed] [Google Scholar]
- 167.Wang Y, Li M, Xu X, Tang W, Xiong L, Sun Q. Formation of protein corona on nanoparticles with digestive enzymes in simulated gastrointestinal fluids. J Agric Food Chem. 2019;67(8):2296–2306. doi: 10.1021/acs.jafc.8b05702. [DOI] [PubMed] [Google Scholar]
- 168.Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S, et al. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system) Theranostics. 2014;4(3):316. doi: 10.7150/thno.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clemments AM, Botella P, Landry CC. Protein adsorption from biofluids on silica nanoparticles: corona analysis as a function of particle diameter and porosity. ACS Appl Mater Interfaces. 2015;7(39):21682–21689. doi: 10.1021/acsami.5b07631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cedervall T, Lynch I, Foy M, et al. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed. 2007;46(30):5754–5756. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- 171.Docter D, Distler U, Storck W, et al. Quantitative profiling of the protein coronas that form around nanoparticles. Nat Protoc. 2014;9(9):2030. doi: 10.1038/nprot.2014.139. [DOI] [PubMed] [Google Scholar]
- 172.Mukherjee S, Dasari M, Priyamvada S, Kotcherlakota R, Bollu VS, Patra CR. A green chemistry approach for the synthesis of gold nanoconjugates that induce the inhibition of cancer cell proliferation through induction of oxidative stress and their in vivo toxicity study. J Mater Chem B. 2015;3(18):3820–3830. doi: 10.1039/C5TB00244C. [DOI] [PubMed] [Google Scholar]
- 173.Kianfar E. CO2 Capture with ionic liquids: a review. In: Advances in chemistry research. Vol. 67, USA: Nova Science Publishers; 2020.
- 174.Kianfar E. enhanced light olefins production via methanol dehydration over promoted SAPO-34. In: Chapter 4: Advances in chemistry research. Vol. 63, USA: Nova Science Publishers, Inc., 2020.
- 175.Kianfar E. Gas hydrate: applications, structure, formation, separation processes, Thermodynamics. In: Taylor JC, editor. Chapter 8: Advances in chemistry research. Vol. 62, USA: Nova Science Publishers, Inc., 2020.
- 176.Kianfar M, Kianfar F, Kianfar E. The effect of nano-composites on the mechanic and morphological characteristics of NBR/PA6 blends. Am J Oil Chem Technol. 2016;4(1):29–44. [Google Scholar]
- 177.Gorshkov V, Bubis JA, Solovyeva EM, Gorshkov MV, Kjeldsen F. Protein corona formed on silver nanoparticles in blood plasma is highly selective and resistant to physicochemical changes of the solution. Environ Sci Nano. 2019;6(4):1089–1098. doi: 10.1039/C8EN01054D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mbeh D, Javanbakht T, Tabet L, et al. Protein corona formation on magnetite nanoparticles: effects of culture medium composition, and its consequences on superparamagnetic nanoparticle cytotoxicity. J Biomed Nanotechnol. 2015;11(5):828–840. doi: 10.1166/jbn.2015.2000. [DOI] [PubMed] [Google Scholar]
- 179.Neunzehn J, Draude F, Golla-Schindler U, Arlinghaus HF, Wiesmann HP. Detection of protein coatings on nanoparticles surfaces by ToF-SIMS and advanced electron microscopy. Surf Interf Anal. 2013;45(9):1340–1346. doi: 10.1002/sia.5287. [DOI] [Google Scholar]
- 180.Pelaz B, Del Pino P, Maffre P, et al. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano. 2015;9(7):6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- 181.Kianfar E. The effect of nano-composites on the mechanic and morphological characteristics of NBR/PA6 blends. Am J Oil Chem Technol. 2016;4(1):27–42. [Google Scholar]
- 182.Kianfar F, Moghadam SRM, Kianfar E. Energy optimization of ilam gas refinery unit 100 by using HYSYS refinery software (2015) Indian J Sci Technol. 2015;8(S9):431–436. [Google Scholar]
- 183.Jietao H, Jing L, Yayu Z, Zekai L, Zhiwei Q, Zili L, Wei Y, et al. A new anti-biofilm strategy of enabling arbitrary surfaces of materials and devices with robust bacterial anti-adhesion via a spraying modified microsphere method. J Mater Chem A. 2019;7:26039–26052. doi: 10.1039/C9TA07236E. [DOI] [Google Scholar]
- 184.Blundell EL, Vogel R, Platt M. Determination of zeta potential via nanoparticle translocation velocities through a tunable nanopore: using DNA-modified particles as an example. J Vis Exp. 2016;116:e54577. doi: 10.3791/54577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chetwynd A, Guggenheim E, Briffa S, Thorn J, Lynch I, Valsami-Jones E. Current application of capillary electrophoresis in nanomaterial characterisation and its potential to characterise the protein and small molecule corona. Nanomaterials. 2018;8(2):99. doi: 10.3390/nano8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Forest V, Pourchez J. The nanoparticle protein corona: the myth of average. Nano Today. 2016;11(6):700–703. doi: 10.1016/j.nantod.2015.10.007. [DOI] [Google Scholar]
- 187.Santos-Martinez MJ, Inkielewicz-Stepniak I, Medina C, et al. The use of quartz crystal microbalance with dissipation (QCM-D) for studying nanoparticle-induced platelet aggregation. Int J Nanomedicine. 2012;7:243. doi: 10.2147/IJN.S26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang B, Anslyn EV. Chemosensors: principles, strategies, and applications. In: Wang B, Anslyn EV, editor. USA: Wiley; 2011.
- 189.Brewer SH, Glomm WR, Johnson MC, Knag MK, Franzen S. Probing BSA binding to citrate-coated gold nanoparticles and surfaces. Langmuir. 2005;21(20):9303–9307. doi: 10.1021/la050588t. [DOI] [PubMed] [Google Scholar]
- 190.Kaufman ED, Belyea J, Johnson MC, et al. Probing protein adsorption onto mercaptoundecanoic acid stabilized gold nanoparticles and surfaces by quartz crystal microbalance and ζ-potential measurements. Langmuir. 2007;23(11):6053–6062. doi: 10.1021/la063725a. [DOI] [PubMed] [Google Scholar]
- 191.Hospital A, Goñi JR, Orozco M, Gelpí JL. Molecular dynamics simulations: advances and applications. Adv Appl Bioinform Chem. 2015;8:37. doi: 10.2147/AABC.S70333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Puzyn T, Leszczynska D, Leszczynski J. Toward the development of “nano-QSARs”: advances and challenges. Small. 2009;5(22):2494–2509. doi: 10.1002/smll.200900179. [DOI] [PubMed] [Google Scholar]
- 193.Liu R, Rallo R, George S, et al. Classification NanoSAR development for cytotoxicity of metal oxide nanoparticles. Small. 2011;7(8):1118–1126. doi: 10.1002/smll.201002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Puzyn T, Rasulev B, Gajewicz A, et al. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat Nanotechnol. 2011;6(3):175. doi: 10.1038/nnano.2011.10. [DOI] [PubMed] [Google Scholar]
- 195.Walkey CD, Olsen JB, Song F, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8(3):2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 196.Eigenheer R, Castellanos ER, Nakamoto MY, Gerner KT, Lampe AM, Wheeler KE. Silver nanoparticle protein corona composition compared across engineered particle properties and environmentally relevant reaction conditions. Environ Sci Nano. 2014;1(3):238–247. doi: 10.1039/C4EN00002A. [DOI] [Google Scholar]
- 197.Tavanti F, Pedone A, Menziani MC. Multiscale molecular dynamics simulation of multiple protein adsorption on gold nanoparticles. Int J Mol Sci. 2019;20(14):3539. doi: 10.3390/ijms20143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zanganeh S, Spitler R, Erfanzadeh M, Alkilany AM, Mahmoudi M. Protein corona: opportunities and challenges. Int J Biochem Cell Biol. 2016;75:143–147. doi: 10.1016/j.biocel.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Control Rel. 2012;161:38–49. doi: 10.1016/j.jconrel.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 200.Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res Int. 2014;2014:1–12. doi: 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang W, Rollett A, Kaplan DL. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Exp Opin Drug Deliv. 2014;2014:1–13. doi: 10.1517/17425247.2015.989830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Elzoghby AO, El-Fotoh WSA, Elgindy NA. Casein-based formulations as promising controlled release drug delivery systems. J Control Rel. 2011;153:206–216. doi: 10.1016/j.jconrel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 203.Pang X, Gong K, Zhang X, Wu S, Cui Y, Qian BZ. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol Res. 2019;144:235–244. doi: 10.1016/j.phrs.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 204.Peijie W, Wei G, Miao S, Edouard NC, Zhang W, Lin J, Xie N. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front Cell Dev Biol. 2021 doi: 10.3389/fcell.2021.641469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kianfar E, Salimi M, Pirouzfar V, Koohestani B. Int J Chem Reactor Eng. 2018;16:1–7. doi: 10.1515/ijcre-2017-0229. [DOI] [Google Scholar]
- 206.Kianfar E. Comparison and assessment of zeolite catalysts performance dimethyl ether and light olefins production through methanol: a review. Rev Inorg Chem. 2019;39:157–177. doi: 10.1515/revic-2019-0001. [DOI] [Google Scholar]
- 207.Kianfar E, Salimi M. A review on the production of light olefins from hydrocarbons cracking and methanol conversion: In: Taylor JC, editor. Chapter 1: Advances in chemistry research. USA: Nova Science Publishers, Vol. 59; 2020.
- 208.Kianfar E, Razavi A. Zeolite catalyst based selective for the process MTG: A review: In Annett M, editor. Chapter 8: Zeolites: advances in research and applications, USA: Nova Science Publishers; 2020.
- 209.Wang B, Kong B, Li F, Liu Q, Zhang H, Xia X. Changes in the thermal stability and structure of protein from porcine longissimus dorsi induced by different thawing methods. Food Chem. 2020;316:126375. doi: 10.1016/j.foodchem.2020.126375. [DOI] [PubMed] [Google Scholar]
- 210.Kianfar E. Zeolites: properties, applications, modification and selectivity. In: Annett M, editor. Chapter 1: Zeolites: advances in research and applications. USA: Nova Science Publishers Inc.; 2020.
- 211.Kianfar E, Hajimirzaee S, Musavian SS, Mehr AS. Zeolite-based catalysts for methanol to gasoline process: a review. Microchem J. 2020:104822.
- 212.Kianfar E, Baghernejad M, Rahimdashti Y. Study synthesis of vanadium oxide nanotubes with two template hexadecylamin and hexylamine. Biol Forum. 2015;7:1671–1685. [Google Scholar]
- 213.Kkianfar E. Synthesizing of vanadium oxide nanotubes using hydrothermal and ultrasonic method. Lambert Academic Publishing. 2020:1–80. ISBN: 978-613-9-81541-8.
- 214.Kianfar E, Pirouzfar V, Sakhaeinia H. An experimental study on absorption/stripping CO2 using Mono-ethanol amine hollow fiber membrane contactor. J Taiwan Inst Chem Eng. 2017;80:954–962. doi: 10.1016/j.jtice.2017.08.017. [DOI] [Google Scholar]
- 215.Kianfar E, Viet C. Polymeric membranes on base of PolyMethyl methacrylate for air separation: a review. J Market Res. 2021;10:1437–1461. [Google Scholar]
- 216.Nnmousavian SS, Faravar P, Zarei Z, Zzimikia R, Monjezi MG, Kianfar E. Modeling and simulation absorption of CO2 using hollow fiber membranes (HFM) with mono-ethanol amine with computational fluid dynamics. J Environ Chem Eng. 2020;8(4):103946. doi: 10.1016/j.jece.2020.103946. [DOI] [Google Scholar]
- 217.Yang Z, Zhang L, Zhou Y, Wang H, Wen L, Kianfar E. Investigation of effective parameters on SAPO-34 Nano catalyst the methanol-to-olefin conversion process: a review. Rev Inorg Chem. 2020;40(3):91–105. doi: 10.1515/revic-2020-0003. [DOI] [Google Scholar]
- 218.Gao C, Liao J, Jingqiong Lu, Ma J, Kianfar E. The effect of nanoparticles on gas permeability with polyimide membranes and network hybrid membranes: a review. Rev Inorg Chem. 2020 doi: 10.1515/revic-2020-0007. [DOI] [Google Scholar]
- 219.Kianfar E, Salimi M, Koohestani B. Zeolite CATALYST: a review on the production of light olefins. Lambert Academic Publishing. 2020:1-116.ISBN:978-620-3-04259-7.
- 220.Kianfar E. Investigation on catalysts of “Methanol to light Olefins”. Lambert Academic Publishing. 2020:1–168. ISBN: 978-620-3-19402-9.
- 221.Kianfar E. Application of nanotechnology in enhanced recovery oil and gas importance & applications of nanotechnology. MedDocs Publishers. Vol. 5, Chapter 3, pp. 16–21; 2020
- 222.Kianfar E. Catalytic properties of nanomaterials and factors affecting it importance & applications of nanotechnology. MedDocs Publishers.Vol. 5, Chapter 4, pp. 22–25; 2020.
- 223.Wang M, et al. Toward an optimal kernel extreme learning machine using a chaotic moth-flame optimization strategy with applications in medical diagnoses. Neurocomputing. 2017;267:69–84. doi: 10.1016/j.neucom.2017.04.060. [DOI] [Google Scholar]
- 224.Zhang X, Wang T, Luo W, Huang P. Multi-level fusion and attention-guided CNN for image Dehazing. IEEE Trans Circuits Syst Video Technol. 2020 doi: 10.1109/TCSVT.2020.3046625. [DOI] [Google Scholar]
- 225.Zhang X, et al. Robust low-rank tensor recovery with rectification and alignment. IEEE Trans Pat Anal Mach Intell. 2019;2019:1. doi: 10.1109/TPAMI.2019.2929043. [DOI] [PubMed] [Google Scholar]
- 226.Zhang X, et al. Top-k feature selection framework using robust 0–1 integer programming. IEEE Trans Neural Netw Learn Syst. 2020;2020:1–15. doi: 10.1109/TNNLS.2020.3009209. [DOI] [PubMed] [Google Scholar]
- 227.Salimi M, Pirouzfar V, Kianfar E. Enhanced gas transport properties in silica nanoparticle filler-polystyrene nanocomposite membranes. Colloid Polym Sci. 2017;295:215–226. doi: 10.1007/s00396-016-3998-0. [DOI] [Google Scholar]
- 228.MaHam A, Tang Z, Hong W, Wang J, Lin Y. Protein-based nanomedicine platforms for drug delivery. Small. 2009;5:1706–1721. doi: 10.1002/smll.200801602. [DOI] [PubMed] [Google Scholar]
- 229.Jahanshahi M, Babaei Z. Protein nanoparticle: a unique system as drug delivery vehicles. Afr J Biotechnol. 2008;7.
- 230.Wang B, Li F, Pan N, Kong B, Xia X. Effect of ice structuring protein on the quality of quick-frozen patties subjected to multiple freeze-thaw cycles. Meat Sci. 2021;172:108335. doi: 10.1016/j.meatsci.2020.108335. [DOI] [PubMed] [Google Scholar]
- 231.Hajimirzaee S, Mehr AS, Kianfar E. Modified ZSM-5 Zeolite for conversion of LPG to aromatics. Polycyclic Aromat Compd. 2020 doi: 10.1080/10406638.2020.1833048. [DOI] [Google Scholar]
- 232.Kianfar E. Simultaneous prediction of the density and viscosity of the ternary system water-ethanol-ethylene glycol using support vector machine. Fine Chem Eng. 2020;1:69–74. [Google Scholar]
- 233.Kianfar E, Salimi M, Koohestani B. Methanol to gasoline conversion over CuO/ZSM-5 catalyst synthesized and influence of water on conversion. Fine Chem Eng. 2020;1:75–82. [Google Scholar]
- 234.Kianfar E. An experimental study PVDF and PSF hollow fiber membranes for chemical absorption carbon dioxide. Fine Chem Eng. 2020;1:92–103. [Google Scholar]
- 235.Kianfar E, Mafi S. Ionic liquids: properties, application, and synthesis. Fine Chem Eng. 2020;2:22. doi: 10.37256/fce.212021693. [DOI] [Google Scholar]
- 236.Faghih SM, Kianfar E. Modeling of fluid bed reactor of ethylene dichloride production in Abadan Petrochemical based on three-phase hydrodynamic model. Int J Chem React Eng. 2018;16:1–14. [Google Scholar]
- 237.Kianfar E, Mazaheri H. Methanol to gasoline: a sustainable transport fuel. In: Taylor JC, editor. Chapter 4: Advances in chemistry research. Vol. 66 USA: Nova Science Publishers, Inc.; 2020.
- 238.Ahmed AO. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J Control Rel. 2013;172:1075–1091. doi: 10.1016/j.jconrel.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 239.Copolovici DM, Langel K, Eriste E, Langel U. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano. 2014;8:1972–1994. doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- 240.Sripriyalakshmi S, Jose P, Ravindran A, et al. Recent trends in drug delivery system using protein nanoparticles. Cell Biochem Biophys. 2014;70:17–26. doi: 10.1007/s12013-014-9896-5. [DOI] [PubMed] [Google Scholar]
- 241.Jahanshahi M, Sanati MH, Babaei Z. Optimization of parameters for the fabrication of gelatin nanoparticles by the Taguchi robust design method. J Appl Stat. 2008;35(12):1345–1353. doi: 10.1080/02664760802382426. [DOI] [Google Scholar]
- 242.Tu C, Yin Z, Lin J, Bao F. A review of experimental techniques for measuring micro-to nano-particle-laden gas flows. Appl Sci. 2017;7(2):120. doi: 10.3390/app7020120. [DOI] [Google Scholar]
- 243.Kianfar E. A comparison and assessment on performance of zeolite catalyst based selective for theprocess methanol to gasoline: a review. In: Chapter 2: Advances in Chemistry Research. Vol. 63, NewYork: Nova Science Publishers, Inc.; 2020.
- 244.Kianfar E, Hajimirzaee S, Faghih SM et al. Polyvinyl chloride + nanoparticles titanium oxide Membrane for Separation of O2/N2. In: Advances in Nanotechnology. NY, USA: Nova Science Publishers, Inc. 2020.
- 245.Shan W, et al. Double adaptive weights for stabilization of moth flame optimizer: balance analysis, engineering cases, and medical diagnosis. Knowl Based Syst. 2020;214:106728. doi: 10.1016/j.knosys.2020.106728. [DOI] [Google Scholar]
- 246.Tu J, et al. Evolutionary biogeography-based whale optimization methods with communication structure: towards measuring the balance. Knowl Based Syst. 2021;212:106642. doi: 10.1016/j.knosys.2020.106642. [DOI] [Google Scholar]
- 247.Kianfar E. Introducing the application of nanotechnology in lithium-ion battery importance & applications of nanotechnology. MedDocs Publishers. Vol. 4, Chapter 4, pp. 1–7; 2020.
- 248.Kianfar E, Mazaheri H. Synthesis of nanocomposite (CAU-10-H) thin-film nanocomposite (TFN) membrane for removal of color from the water. Fine Chem Eng. 2020;1:83–91. [Google Scholar]
- 249.Jun Z, Bin L. A review on the recent developments of sequence-based protein feature extraction methods. Curr Bioinformat. 2019;14:190. doi: 10.2174/1574893614666181212102749. [DOI] [Google Scholar]
- 250.Korsmeyer RW, Gurny R, Doelker E, et al. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [Google Scholar]
- 251.Zhang X, et al. Recursive neural network for video deblurring. IEEE Trans Circ Syst Video Technol. 2020;2020:1. doi: 10.1109/TCSVT.2020.3035722. [DOI] [Google Scholar]
- 252.Zhang X, et al. Multi-level fusion and attention-guided CNN for image dehazing. IEEE Trans Circ Syst Video Technol. 2020;2020:1. doi: 10.1109/TCSVT.2020.3046625. [DOI] [Google Scholar]
- 253.Zhang X, et al. Robust feature learning for adversarial defense via hierarchical feature alignment. Inform Sci. 2020;560:256–270. doi: 10.1016/j.ins.2020.12.042. [DOI] [Google Scholar]
- 254.Chen H, et al. Multi-population differential evolution-assisted Harris hawks optimization: Framework and case studies. Futur Gener Comput Syst. 2020;111:175–198. doi: 10.1016/j.future.2020.04.008. [DOI] [Google Scholar]
- 255.Wang M, Chen HJASC. Chaotic multi-swarm whale optimizer boosted support vector machine for medical diagnosis. Appl Soft Comput. 2020;88:105946. doi: 10.1016/j.asoc.2019.105946. [DOI] [Google Scholar]
- 256.Xu Y, et al. Enhanced Moth-flame optimizer with mutation strategy for global optimization. Inf Sci. 2019;492:181–203. doi: 10.1016/j.ins.2019.04.022. [DOI] [Google Scholar]
- 257.Zhao X, et al. Chaos enhanced grey wolf optimization wrapped ELM for diagnosis of paraquat-poisoned patients. Comput Biol Chem. 2019;78:481–490. doi: 10.1016/j.compbiolchem.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 258.Li C, et al. Developing a new intelligent system for the diagnosis of tuberculous pleural effusion. Comput Methods Programs Biomed. 2018;153:211–225. doi: 10.1016/j.cmpb.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 259.Kianfar E, Salimi M, Pirouzfar V, Koohestani B. Int J Appl CeramTechnol. 2018;15:734–741. [Google Scholar]
- 260.Procopio MJ. An experimental analysis of classifier ensembles for learning drifting concepts over time in autonomous outdoor robot navigation, ProQuest, Boulder, 2007.
- 261.Xia J, et al. Ultrasound-based differentiation of malignant and benign thyroid Nodules: an extreme learning machine approach. Comput Methods Programs Biomed. 2017;147:37–49. doi: 10.1016/j.cmpb.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 262.Shen L, et al. Evolving support vector machines using fruit fly optimization for medical data classification. Knowl-Based Syst. 2016;96:61–75. doi: 10.1016/j.knosys.2016.01.002. [DOI] [Google Scholar]
- 263.Chen H-L, et al. An efficient hybrid kernel extreme learning machine approach for early diagnosis of Parkinson’s disease. Neurocomputing. 2016;184:131–144. doi: 10.1016/j.neucom.2015.07.138. [DOI] [Google Scholar]
- 264.Hu L, et al. An efficient machine learning approach for diagnosis of paraquat-poisoned patients. Comput Biol Med. 2015;59:116–124. doi: 10.1016/j.compbiomed.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 265.Xu X, Chen HLJSC. Adaptive computational chemotaxis based on field in bacterial foraging optimization. Soft Comput. 2014;18(4):797–807. doi: 10.1007/s00500-013-1089-4. [DOI] [Google Scholar]
- 266.Vidakovic B. Statistics for bioengineering sciences: with MATLAB and WinBUGS Support, 2nd edn. Springer Science & Business Media, 2011.
- 267.Zhang Y, et al. Boosted binary Harris hawks optimizer and feature selection. Eng Comput. 2020;25:26. [Google Scholar]
- 268.Zhang Y, et al. Towards augmented kernel extreme learning models for bankruptcy prediction: algorithmic behavior and comprehensive analysis. Neurocomputing. 2020;430:185–212. doi: 10.1016/j.neucom.2020.10.038. [DOI] [Google Scholar]
- 269.Zhao D, et al. Chaotic random spare ant colony optimization for multi-threshold image segmentation of 2D Kapur entropy. Knowl Based Syst. 2020;216:106510. doi: 10.1016/j.knosys.2020.106510. [DOI] [Google Scholar]
- 270.Yu C et al. SGOA: annealing-behaved grasshopper optimizer for global tasks. Eng Comput. 2021;1–28.
- 271.Hu J, et al. Orthogonal learning covariance matrix for defects of grey wolf optimizer: insights, balance, diversity, and feature selection. Knowl Based Syst. 2020;213:106684. doi: 10.1016/j.knosys.2020.106684. [DOI] [Google Scholar]
- 272.Zhao X, et al. Feature selection based on improved ant colony optimization for online detection of foreign fiber in cotton. Appl Soft Comput. 2014;24:585–596. doi: 10.1016/j.asoc.2014.07.024. [DOI] [Google Scholar]
- 273.Yu H et al. Dynamic Gaussian bare-bones fruit fly optimizers with abandonment mechanism: method and analysis. Eng Comput. 2020;1–29.
- 274.Kianfar E. Synthesis of characterization nanoparticles isophthalic acid/aluminum nitrate (CAU-10-H) using method hydrothermal. Advances in Chemistry Research. NY, USA: Nova Science Publishers, Inc. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.