To the Editor
In the context of food oral immunotherapy (OIT) the identification of biomarkers that can replace current standard burdensome and risky double-blind placebo-controlled food challenges (DBPCFC), which are used pre- and post-therapy1, would be of great value in clinical settings. With this goal in mind, we undertook a comprehensive immune response analysis on blood cells obtained from a sub-group of multi-allergic participants in a clinical multi-OIT study (ClinicalTrials.gov number, NCT02626611) 2 to evaluate potential biomarker candidates. In the open-label phase of the multi-OIT study (n = 70, age 5–22 years), participants received omalizumab (weeks 1–16) and multi-OIT (2–5 allergens; 1 g each; weeks 8–30), after which they were tested by food challenge (week 30). Subsequently, 60 eligible participants (excluding 10 drop-outs) were randomized 1:1:1 to receive in a blind manner either 0 mg, 300 mg, or 1 g of food allergens (weeks 30–36). These participants were then tested again by food challenge at week 36.
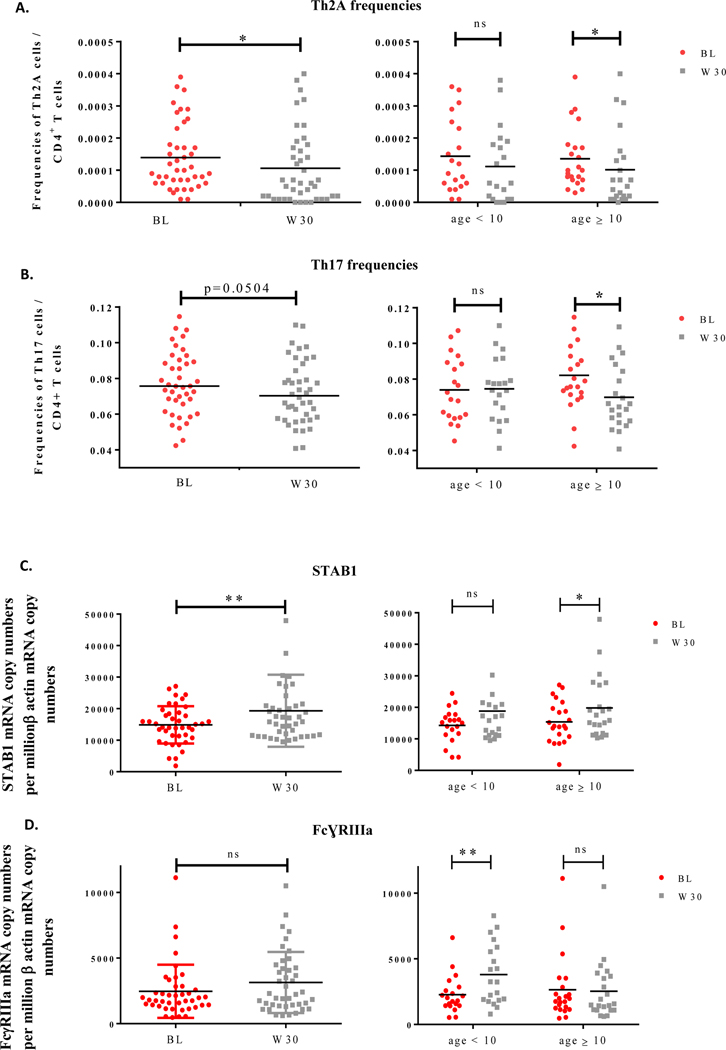
We analysed systemic changes in immune parameters, such as T helper (Th) cell and innate lymphoid cell (ILC) frequencies as well as dendritic cell (DC)-associated markers, occurring after 30-week multi-OIT, in a subset of 42 out of the 60 eligible patients. The demographics and baseline characteristics of patients are provided in Supplementary Table S1. Peripheral blood mononuclear cells (PBMCs) were stained with fluorescently labeled antibodies as described in Supplementary Methods according to the gating strategy shown in Supplementary Figure S1. We observed lower frequencies of both Th2A (p<0.05) and Th17 (p=0.0504) subsets in PBMCs at week 30 when compared to baseline (Figure 1.A and B, left panels). For the sake of simplicity, we divided ages in two groups instead of three groups since only 3 participants were over 15-year-old. Noticeably, the decrease of Th2A cell frequencies was statistically significant (p<0.05) only for patients aged 10 and over, although a positive trend was also detected in younger patients (Figure 1.A, right panel). Additionally, multi-OIT markedly (p<0.05) decreased Th17 cell frequencies in patient aged 10 and over, but no differences were seen for patients under 10-year-old. (Figure 1.B, right panel). To further explore cellular immune responses, we performed a detailed analysis of changes in innate immune cells by flow cytometry. However, no alterations were observed between groups in terms of cell frequencies for both type 1, 2 and 3 innate lymphoid as well as for DC subsets (data not shown). In addition, we monitored PBMCs for expression of markers associated with type 1 (i.e. MX1), type 2 (i.e. GATA3 and CD141), or regulatory (i.e. C1q, STAB1 and FcγRIIIa) DCs previously identified to correlate with clinical efficacy of allergen immunotherapy (AIT) 3, 4 (See Supplementary Methods). We did not observe any changes in DC1 or DC2-associated markers. In contrast, 2 out of 3 DCreg markers exhibited statistical differences before and after multi-OIT. The expression of STAB1 was significantly (p<0.05) upregulated in PBMCs from multi-OIT patients at week 30 when compared to baseline (Figure 1.C, left panel) particularly for patients aged 10 and over (Figure 1.C, right panel). Multi-OIT did not alter the expression of FcγRIIIa in patients’ blood when considering the whole subset (Figure 1.D, left panel). However, FcγRIIIa expression was significantly (p<0.05) increased in PBMCs from patients under 10 and correlated with a decrease of Th17 cell frequencies (Supplementary Figure S2; center panel) but was unchanged in patients older than 10 years of age (Figure 1.D, right panel).
Figure 1: Th2A and Th17 cell frequencies decreased while the regulatory marker STAB1 and FcγRIIIa increased after multi OIT.
PBMCs from patients receiving multi OIT (n=42) were analysed at baseline (BL) and week 30 (W30) for Th2A (A) and Th17 (B) cell frequencies by flow cytometry and for STAB1 (C) and FcɣRIIIa (D) gene expression by quantitative PCR. Age-related factors were analysed for patients under 10 and aged 10 and over. Wilcoxon tests were used for statistical analysis. *P<0.05; **p<0.01; ns=non-significant.
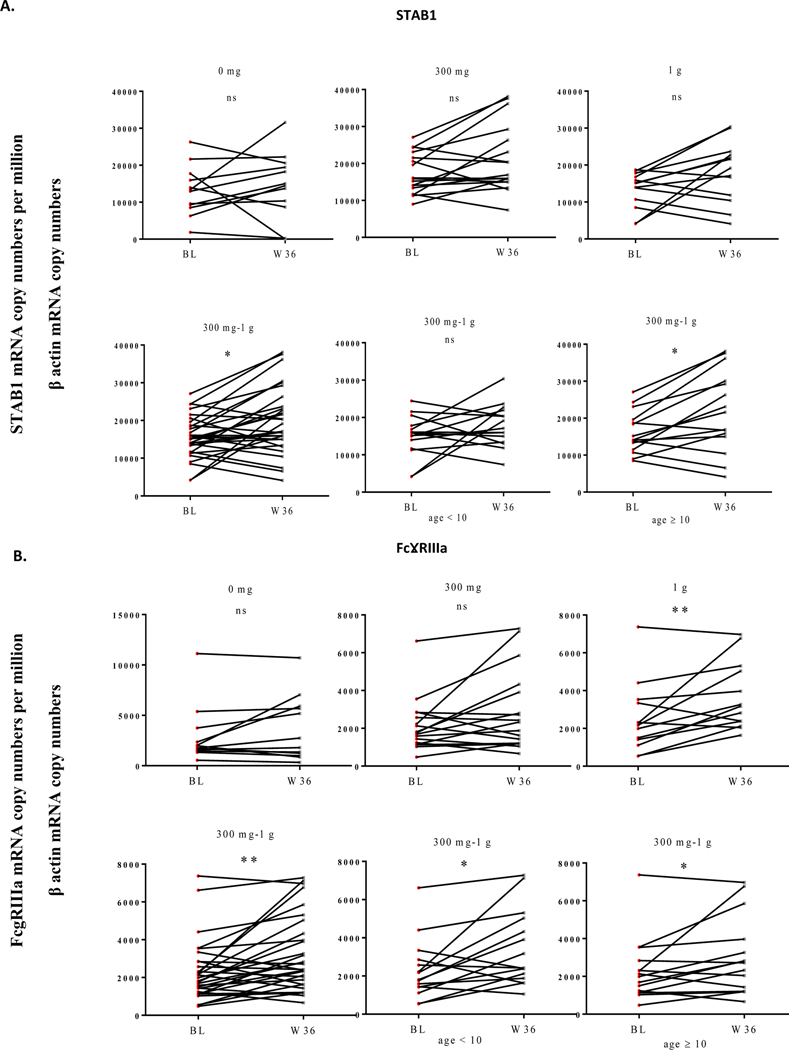
At week 36, immune changes were also analysed in sub-groups of patients receiving either 0 mg (n=12), 300 mg (n=17), or 1 g (n=13) as aforementioned. No differences in terms of Th2A or Th17 cell frequencies (Supplementary Figure S3) or STAB1 expression (Figure 2.A, upper panel) were observed between baseline and week 36 within the 3 groups (0 mg, 300 mg, 1 g), likely due to a limited number of patients. It is thus difficult to conclude on the value of following such markers after cessation of OIT. Only on pooling data from the 300 mg and 1 g treated groups, STAB1 was observed to be significantly (p<0.05) upregulated following a 36-week multi OIT, particularly in PBMCs from patients aged 10 and over (Figure 2.A, lower panel). Strikingly, FcγRIIIa expression was significantly (p<0.01) increased in PBMCs from patients receiving 1 g of food allergens up to week 36 (Figure 2.B, upper panel). On pooling data from the 300 mg and 1 g treated groups, we confirmed that this marker is significantly increased in PBMCs irrespective of age groups at W36 (but not at W30), suggesting that this DCreg marker is regulated in a time- and age-dependent fashion (Figure 2.B, lower panel).
Figure 2: Regulatory markers STAB1 and FcγRIIIa are confirmed to be upregulated in PBMCs from patients after extension of multi OIT.
At week 30 (W30), patients receiving OIT were randomised in 3 groups: patients interrupting the treatment (0 g; n=12), patients continuing OIT with 300 mg (n=17) or 1 g (n=13) until week 36 (W36). PBMCs from patients were analysed at baseline (BL) and W36 for STAB1 (A) and FcɣRIIIa (B) gene expression by quantitative PCR. Age related factor were analysed for patients under 10 and aged 10 and over. Wilcoxon tests were used for statistical analysis. *P<0.05; **p<0.01; ns=non-significant.
These results establish for the first time that omalizumab-facilitated multi-OIT induces changes in immune polarization-based readouts. Multi-OIT promoted a decrease in Th2A and Th17 cell frequencies while increasing regulatory markers in blood, particularly evidenced for patients aged 10 and over for which desensitization has been successful. Such results will need to be confirmed with a larger cohort of patients in DBPC clinical trials since we acknowledge the limits of our study due to a low number of participants. Additionally, long-term therapy should be considered as it may enable sustained effect and allow cessation of immunotherapy 2. Our findings are fully aligned with previous reports monitoring follow-up candidate biomarkers in AIT studies for respiratory allergies3, 4. Although a surrogate biomarker of AIT efficacy has not been validated so far, immune changes documented in peripheral blood or mucosal tissues of patients include the downregulation of allergen-specific Th2 cells 5, 6. On the other hand, a limited number of studies have analyzed a change in Th17 polarization with mixed conclusions 7, 8. We also confirmed the interest of both STAB1 and FcγRIIIa as hallmark DCreg markers in the mechanism of action of immunotherapy 3, 4. This is consistent with observations that STAB1 is expressed by tolerogenic DCs/macrophages and that it contributes to fetal implantation in the human decidua 4, 9. In contrast, the identification of FcγRIIIA as a DCreg marker was less expected since this receptor is more involved in inflammatory mechanisms 4.
Altogether, our findings pave the way for further understanding of the mechanisms involved in food immunotherapy. Positive outcomes on systemic immune parameters, as observed in this study, confirm the value of monitoring (particularly in PBMCs from pediatric patients), Th2/Th17 cell frequencies and DCreg markers as potential replacements for oral food challenges. We also suggest that successful multi-OIT involve different immune pathways according to the maturation level of the immune systems during infancy.
Supplementary Material
Acknowledgment
Funding sources include the National Institute of Health Grant #5R01AI140134
Footnotes
Conflict of interest
Co-authors (SL and LM) are employees at Stallergenes Greer.
Dr. Sharon Chinthrajah: Receives grant support from CoFAR NIAID, Aimmune, DBV Technologies, Astellas, AnaptysBio, Novartis, Regeneron, Stallergenes-Greer, and Boehringer Ingelheim, and is a scientific advisory board member for Alladapt Immunotherapeutics.
Shu Chen Lyu: Nothing to disclose
Dr. Kari Nadeau: Dr. Nadeau receives grant support from NIAID, Food Allergy Research & Education (FARE), End Allergies Together, Allergenis, Ukko; personal fees from Regeneron, AstraZeneca, ImmuneWorks, Cour; sponsored research from Novartis, Sanofi, Astellas, Nestle; Sponsored research for clinical trials from Genentech, Aimmune Therapeutics, DBV Technologies, AnaptysBio, Stallergenes-Greer, Regeneron, and Adare Pharmaceuticals; data and Safety Monitoring Board member at Novartis and NHLBI; co-founded Before Brands, Alladapt Immunotherapeutics, and ForTra; grant awardee for NIAID, NHLBI, NIEHS, and EPA; Director for FARE and World Allergy Organization (WAO) Center of Excellence.
References
- 1.Sindher SB, Long A, Acharya S, Sampath V, Nadeau KC. The Use of Biomarkers to Predict Aero-Allergen and Food Immunotherapy Responses. Clin Rev Allergy Immunol. 2018;55:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andorf S, Purington N, Kumar D, et al. A Phase 2 Randomized Controlled Multisite Study Using Omalizumab-facilitated Rapid Desensitization to Test Continued vs Discontinued Dosing in Multifood Allergic Individuals. EClinicalMedicine. 2019;7:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmer A, Bouley J, Le Mignon M, et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. The Journal of allergy and clinical immunology. 2012;129:1020–1030. [DOI] [PubMed] [Google Scholar]
- 4.Gueguen C, Bouley J, Moussu H, et al. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J Allergy Clin Immunol. 2016;137:545–558. [DOI] [PubMed] [Google Scholar]
- 5.Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72:1156–1173. [DOI] [PubMed] [Google Scholar]
- 6.Berings M, Karaaslan C, Altunbulakli C, et al. Advances and highlights in allergen immunotherapy: On the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol. 2017;140:1250–1267. [DOI] [PubMed] [Google Scholar]
- 7.Bonvalet M, Moussu H, Wambre E, et al. Allergen-specific CD4+ T cell responses in peripheral blood do not predict the early onset of clinical efficacy during grass pollen sublingual immunotherapy. Clin Exp Allergy. 2012;42:1745–1755. [DOI] [PubMed] [Google Scholar]
- 8.Gomez E, Fernandez TD, Dona I, et al. Initial immunological changes as predictors for house dust mite immunotherapy response. Clin Exp Allergy. 2015;45:1542–1553. [DOI] [PubMed] [Google Scholar]
- 9.Palani S, Maksimow M, Miiluniemi M, Auvinen K, Jalkanen S, Salmi M. Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages. European Journal of Immunology. 2011;41:2052–2063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.