Abstract
Secreted frizzled-related protein-4 (SFRP4) belongs to a family of soluble ovarian-expressed proteins that participate in female reproduction, particularly in rodents. In humans, SFRP4 is highly expressed in cumulus cells (CCs). However, the mechanisms that stimulate SFRP4 in CCs have not been examined. We hypothesise that oocyte-secreted factors such as growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are involved in the regulation of SFRP4. Human CCs were collected from patients undergoing fertility treatments and treated with GDF9 or BMP15 or their combination in the presence of FSH or vehicle. FSH treatment significantly decreased SFRP4 mRNA levels when compared with nontreated cells. However, SFRP4 mRNA levels were increased significantly by GDF9 plus BMP15 in a concentration-dependent manner in the presence or absence of FSH. The combination of GDF9 plus BMP15 also increased SFRP4 protein levels and decreased the activity of the β-catenin/T cell factor-responsive promoter significantly. GDF9 plus BMP15 inhibited steroidogenic acute regulatory protein and LH/hCG receptor stimulation by FSH, while treatment with SFRP4 blocked the stimulatory effect of FSH on these genes. The evidence demonstrates that GDF9 and BMP15 act in coordination to stimulate SFRP4 expression and suggests that SFRP4 mediates the anti-luteinising effects of the oocyte in human CCs.
Keywords: oocyte-secreted factors, secreted frizzled-related protein-4, growth differentiation factor 9, bone morphogenetic protein 15, human cumulus cells, female reproduction
Introduction
Wnt is a large family of secreted glycolipoproteins, of which some members are essential for normal ovarian follicle maturation and steroid production (Bovolenta et al., 2008). A key and most studied Wnt signalling factor is the transcriptional co-activator β-catenin (Ctnnb1). In the ovary, Wnts and β-catenin modulate the response of granulosa cells (GCs) to gonadotropins and are essential regulators of ovarian function and fertility (Hsieh et al., 2005; Boyer et al., 2010; Fan et al., 2010; Stapp et al., 2014). Recent studies involving loss- and gain-of-function of Wnt members or their signalling partners established the importance of Wnt4, Fzd4 (a Wnt receptor) and Ctnnb1 in normal folliculogenesis, luteal function and steroidogenesis. Conditional deletion of Wnt4 in ovarian GCs or genomic knockout of Fzd4 in mice causes subfertility or complete infertility, respectively (Hsieh et al., 2005; Boyer et al., 2010), whereas conditional deletion of Ctnnb1 in GCs shows that β-catenin facilitates FSH-induced follicular growth, decreases follicle atresia and represses oocyte maturation, ovulation and luteinisation (Fan et al., 2010). However, the function and mechanisms controlled by the Wnt signalling pathway in humans remain to be determined.
Secreted frizzled-related protein-4 (SFRP4) belongs to a family of soluble proteins containing a frizzled-like cysteine-rich domain, which allows these proteins to bind Wnt ligands. SFRPs antagonise Wnt signalling by preventing their interaction with Wnt receptors (Bovolenta et al., 2008). SFRPs are expressed in the ovary and appear to play a role in female reproduction. In particular, in rodents, SFRP4 expression increases in GCs and cumulus cells (CCs) during luteinisation and remains expressed in the corpus luteum (Hsieh et al., 2003). SFRP4 knockout female mice produce larger litters than wild-type littermates (Zamberlam et al., 2019). These findings suggest that SFRP4 attenuates the response of ovarian cells to gonadotropins and that SFRP4 may decrease follicle survival, ovulatory rates and fertility. Indeed, in the rat ovary, insitu hybridisation studies demonstrated a correlation between SFRP4 expression and apoptosis, suggesting an association between SFRP4 and apoptosis (Drake et al., 2003).
Findings in human GCs suggest that the regulation of SFRP4 differs from what is known in rodents. For instance, SFRP4 transcript levels are significantly higher in human CCs than in mural GCs, whereas in rodents, SFRP4 is equally expressed in both cell types (Hernandez-Gonzalez et al., 2006; Maman et al., 2011). In addition, in human CCs, FSH decreases SFRP4 expression (Maman et al., 2011); in contrast, in rats GCs, SFRP4 expression increases 60-fold after FSH stimulation (Hsieh et al., 2003). Moreover, whereas in rodents, LH/hCG stimulates SFRP4 (Hsieh et al., 2003), in human mural GCs from IVF patients, hCG inhibits SFRP4 expression but stimulates progesterone production (Maman et al., 2011). Moreover, SFRP4 expression is lower in mural GCs from preovulatory luteinised follicles than in those from less mature large follicles (Maman et al., 2011). Using human luteinised GCs, we recently showed that hCG strongly inhibits the expression of SFRP4 but stimulates markers of luteinisation such as steroidogenic acute regulatory protein (StAR) and cytochrome side-chain cleavage (P450scc) (Convissar et al., 2019), in agreement with an inverse correlation between luteinisation and SFRP4 expression in humans. Finally, the maturation of human oocytes from germinal vesicle to Metaphase II, a process stimulated by LH, is accompanied by a reduction in SFRP4 expression in the CCs (Huang et al., 2013), further supporting an inhibitory role of LH on SFRP4 in humans. Thus, the evidence suggests species-specific molecular and cellular differences in the regulation of SFRP4 in ovarian cells.
This evidence reveals that SFRP4 is differentially regulated in rodents and humans, and while human SFRP4 is highly expressed in CCs, the mechanisms that stimulate SFRP4 in CCs have not been examined. Here, we hypothesised that oocyte-secreted factors (OSF) such as growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are involved in the regulation of SFRP4 and demonstrate that these factors strongly stimulate SFPR4 in human CCs.
Materials and methods
Study population
This study was conducted on CCs from women undergoing IVF treatment referred to the University of Illinois Fertility Center under an Institutional Review Board-exempt protocol. The patients underwent a GnRH antagonist procedure. Briefly, recombinant FSH (rFSH) (150–225 IU, Gonal-F®, Merck Serono SA, Aubonne, Switzerland) was administered on Day 3 from the beginning of the menstrual cycle and sustained until at least two follicles achieve the size of 14–15 mm. Next, a GnRH antagonist (0.25 mg, Cetrotide®, Merck Serono SA) was administered and continued until the dominant follicles achieved the size of 18 mm. Then, hCG (10 000 IU, Choriomon®, IBSA, Lugano, Switzerland) was administered to trigger oocytes maturation. After 36 h of hCG administration, transvaginal ultrasound-guided follicular aspiration was performed for oocyte retrieval. The follicles with a size above 18 mm were used for the isolation of cumulus–oocyte complexes (COCs). Each patient produces an average of 6–10 COCs and 50 000–250 000 CCs. Cells from each patient were cultured separately. A total of 49 patients were included in the study and distributed into each experiment as follows: Fig. 1A, 9 patients; Fig. 1B, 9 patients; Fig. 2, 4 patients; Fig. 3, 8 patients; Fig. 4A, 4 patients; Fig. 4B, 4 patients; Fig. 5, 4 patients; Fig. 6, 7 patients. Each patient represents a biological replicate. No patient information was collected for reporting.
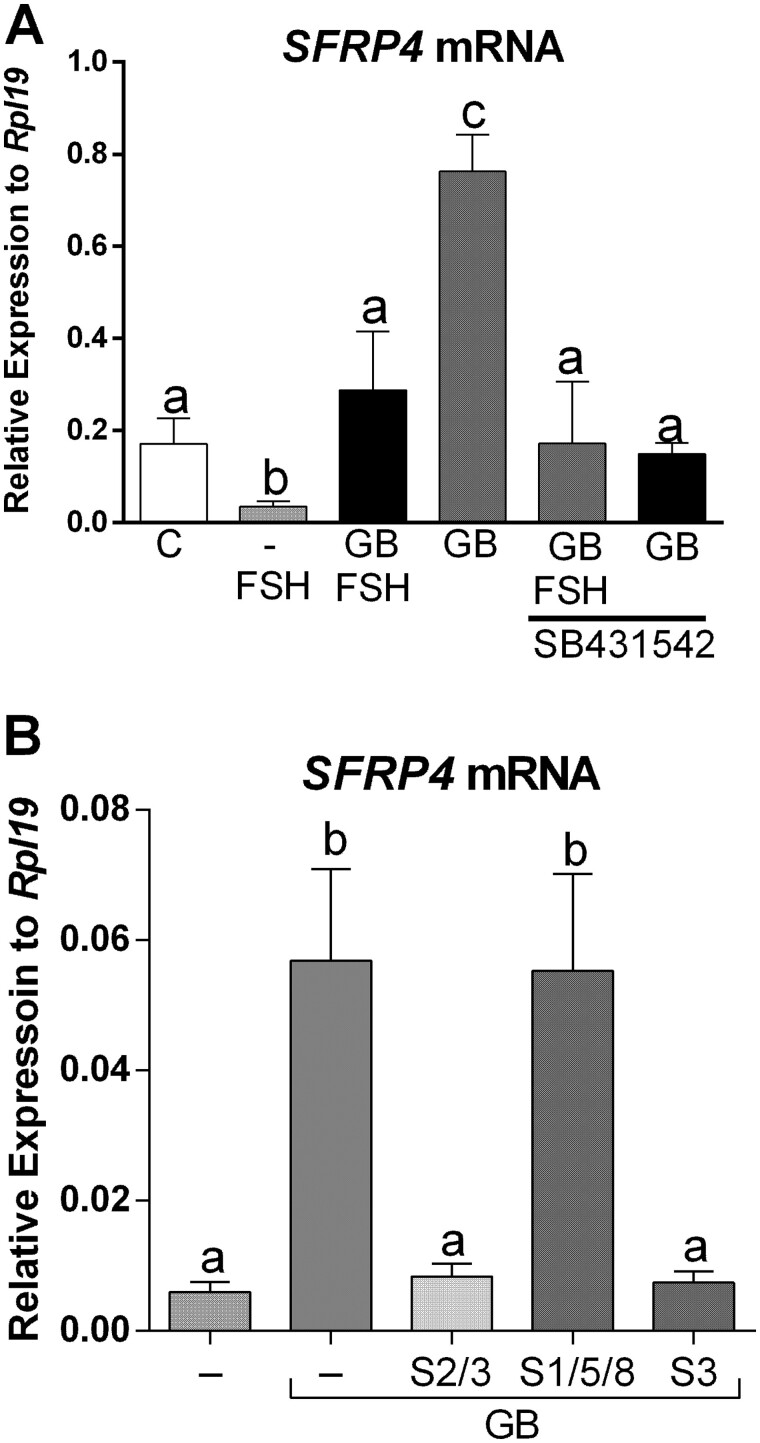
Figure 1.
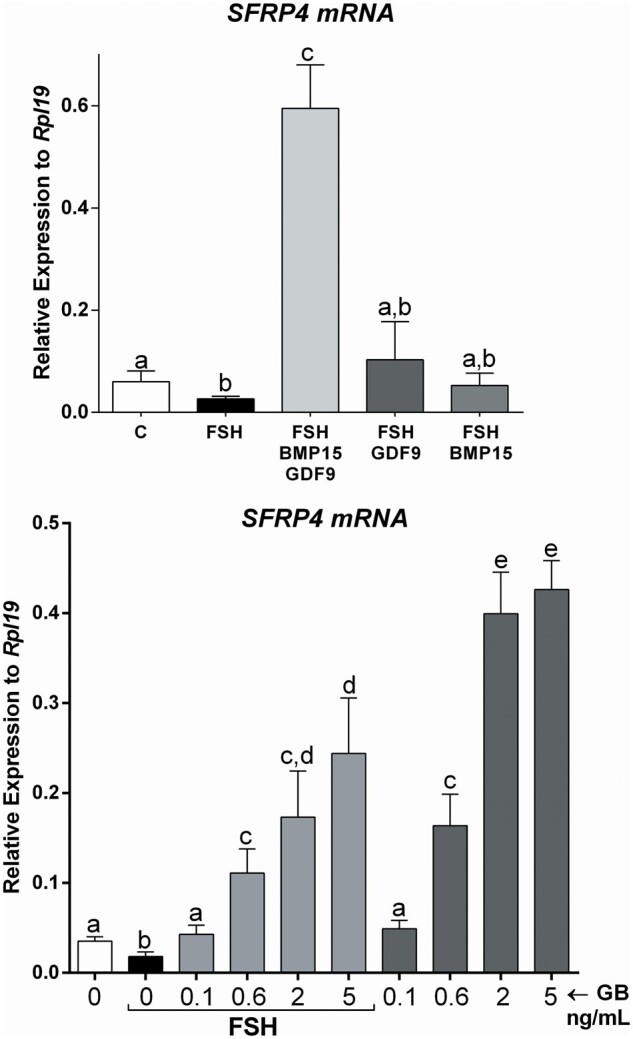
The combination of GDF9 and BMP15 strongly stimulates SFRP4 expression in primary human cumulus cells. (A) Primary human cumulus cells (CCs) were treated for 48 h with vehicle (C), GDF9 (G: 5 ng/ml), BMP15 (B: 5 ng/ml), GDF9 plus BMP15 combined (GB: 5 ng/ml each) in the presence or absence of FSH (50 ng/ml). SFRP4 mRNA levels were determined by qPCR and expressed relative to Rpl19. Columns represent the mean ± SEM. Columns with different letters differ significantly by one-way ANOVA analysis with repeated measures and Bonferroni correction, a–b and b–c P < 0.05, a-c P < 0.01 (n = 9). (B) Primary human CCs were treated for 48 h with vehicle (C), GB: 0.1, 0.6, 2 or 5 ng/ml G and B each, in the presence or absence of FSH (50 ng/ml). SFRP4 mRNA levels were determined by qPCR and expressed relative to Rpl19. Columns represent the mean ± SEM. Columns with different letters differ significantly by one-way ANOVA analysis with Bonferroni correction, a–b, d–e, c–e, a–c P < 0.05, and a–e P < 0.01 (n = 9).
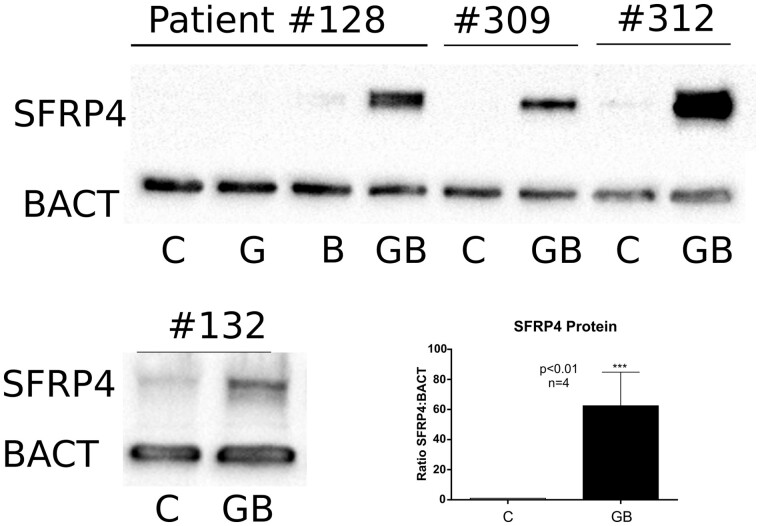
Figure 2.
The combination of GDF9 and BMP15 stimulates SFRP4 protein synthesis. Cumulus cells were obtained from four patients. For Patient no. 128, cells were treated with GDF9 (G: 5 ng/ml), BMP15 (B: 5 ng/ml) or GDF9 and BMP15 combined (GB: 5 ng/ml each). The cells from the other patients (#309, #312, #132) were treated with vehicle or GB (5 ng/ml each). SFRP4 and β-actin protein levels were quantified by the western blot. The bands corresponding to the vehicle or GDF9 plus BMP15-treated cells were quantified for all patients, plotted in the inserted graph where columns represent the mean ± SEM, and analysed using paired t-test, (***) P < 0.01 (n = 4). Full blot images are presented in Supplementary figure S1.
Figure 3.
GDF9 and BMP15 regulate SFRP4 via SMAD3. (A) Primary human cumulus cell (CCs) were treated with the SMAD2/3 inhibitor SB431542 (1µM) for 1 h before the addition of GDF9 and BMP15 (GB: 5 ng/ml each) and FSH (50 ng/ml). Cells were harvested 48 h later. (B) Cells were treated with the SMAD3 inhibitor SIS3 (S3: 5 µM), the SMAD1/5/8 inhibitor LDN-193189 (S1/5/8, 100 nM) or SB431542 (S2/3, 1µM) for 1 h before the addition of GDF9 plus BMP15 (GB: 5 ng/ml each). For both graphs, SFRP4 mRNA levels were determined by qPCR and expressed relative to Rpl19. Columns represent the mean ± SEM, columns with different letters differ significantly by one-way ANOVA analysis with Bonferroni correction, a–b P < 0.05, a–c, b–c P < 0.01 (n = 8).
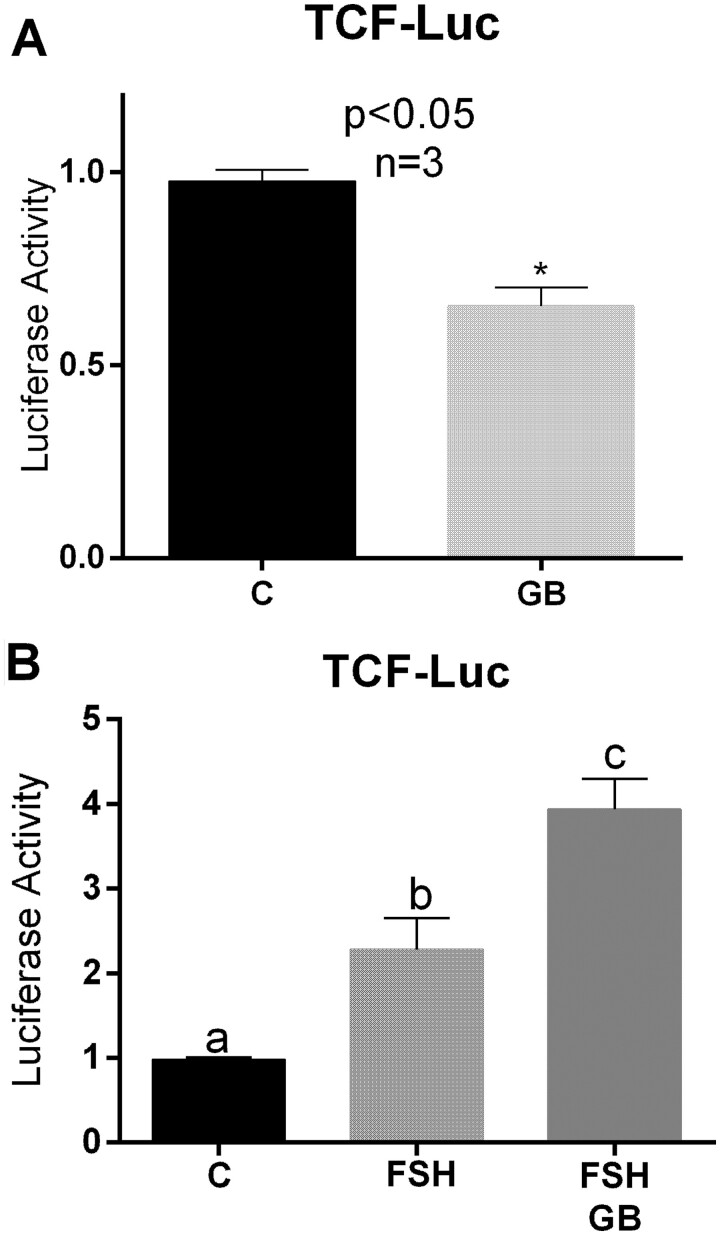
Figure 4.
Treatment with GDF9 and BMP15 decreases β-catenin/TCF activity. (A) Cells were infected with lentivirus carrying the β-catenin/TCF-Luc reporter and incubated overnight prior to 48 hours treatment with vehicle (C) or GDF9 and BMP15 (GB: 5 ng/ml each). Luciferase activity was quantified and expressed relative to the control. Columns represent the mean ± SEM and analysed using paired t-test, P < 0.05 (n = 4). (B) Cells were processed as in A but treated with FSH (50ng/ml) or FSH plus GDF9 plus BMP15 (GB). Columns represent the mean ± SEM, columns with different letters differ significantly by one-way ANOVA with Bonferroni correction, a–b P < 0.05, a–c P < 0.01, b–c P < 0.05 (n = 4).
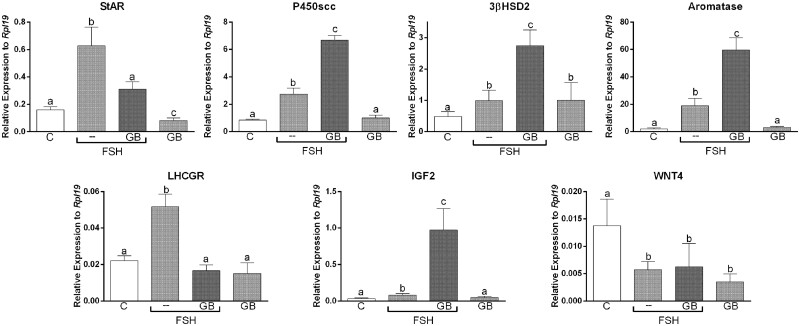
Figure 5.
Treatment with GDF9 plus BMP15 inhibits expression of luteinisation markers but potentiates granulosa cells preovulatory differentiation markers. Primary human cumulus cell (CCs) were treated for 48 h with vehicle (C) or GDF and BMP15 combined (GB: 5 ng/ml each) in the presence or absence of FSH (50 ng/ml). RNA levels were determined by qPCR and expressed relative to Rpl19. Columns represent the mean ± SEM. Columns with different letters differ significantly by one-way ANOVA analysis with repeated measures and Bonferroni correction, a–b P < 0.05, a–c P < 0.01, b–c P < 0.05 P < 0.01 (n = 4).
Figure 6.
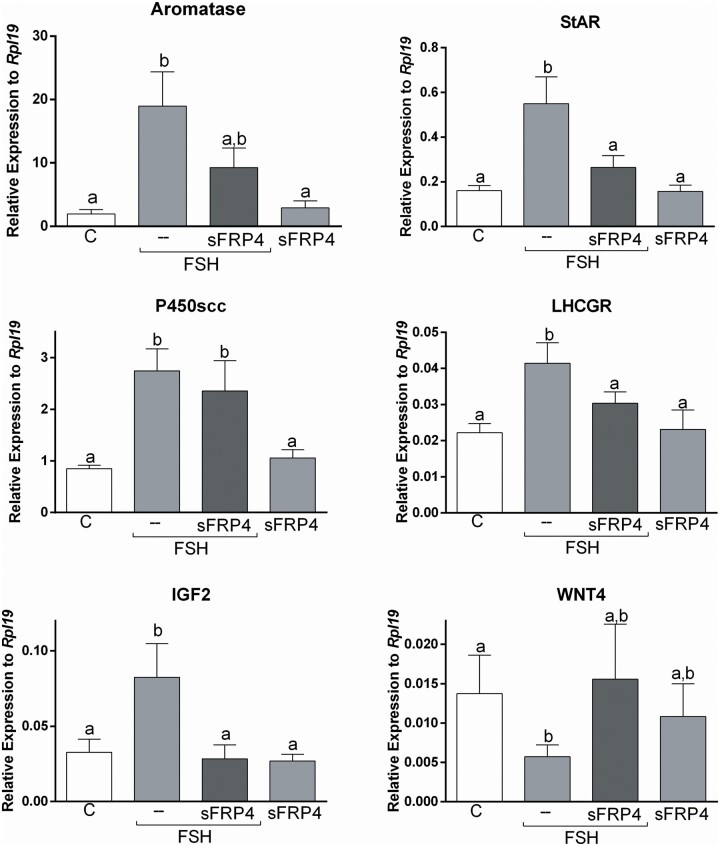
SFRP4 and FSH actions in human cumulus cells. Primary human cumulus cell (CCs) were treated for 48 h with vehicle (C) or SFRP4 (30 ng/ml) in the presence or absence of FSH (50 ng/ml). RNA levels were determined by qPCR and expressed relative to Rpl19. Columns represent the mean ± SEM. Columns with different letters differ significantly by one-way ANOVA analysis with repeated measures and Bonferroni correction, a–b P < 0.05, a–c P < 0.01, b–c P < 0.05 P < 0.01 (n = 7).
Human CC culture
Human CCs were collected from patients on the day of oocyte retrieval after ovarian stimulation; follicles were aspirated, COCs from MII oocytes were identified, and CCs were separated manually. CCs from all aspirated follicles were pooled for each patient, centrifuged at 2000g for 2 min, resuspended in phenol-red free DMEM/F12 medium (Sigma-Aldrich, St. Louis, MO, USA), and then dispersed by gentle pipetting to produce a single-cell suspension. We have used this methodology in several previous studies and have shown that CCs from these patients are healthy and respond to FSH and oocyte-secreted factors (Zamah et al., 2015; Convissar et al., 2017; Stocco et al., 2017; Hobeika et al., 2019; Armouti et al., 2020; Hobeika et al., 2020).
Cells were cultured on plates precoated with BD Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at a density of 6 × 104/ml in serum- and phenol-red free DMEM/F12 supplemented with penicillin (50 IU/ml), streptomycin (50 g/ml), sodium bicarbonate (1.2 g/l; Sigma-Aldrich), and bovine serum albumin (BSA: 0.25 w/v; Sigma-Aldrich). Cells were cultured for at least 48 h prior to treatment with various combinations of hormones and signalling inhibitors. Treatments included human recombinant FSH (Serono), GDF9 (R&B, Minneapolis, MN, USA), BMP15 (R&B), inhibitors of SMAD2/3 (SB431542, Tocris, Minneapolis, MN, USA), SMAD3 (SIS3; Cayman Chemical Company, Ann Arbor, Michigan, USA) or SMAD1/5/8 (LDN-193189, Selleck Chemicals, Pittsburgh, PA, USA).
Total RNA isolation and gene expression quantification
Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) as recommended in the manufacturer's protocol. Total RNA (1 µg) was reverse-transcribed using anchored oligo-dT primers (Integrated DNA Technologies, Coralville, IA, USA), and Moloney murine leukaemia virus reverse transcriptase (Invitrogen) at 37 °C for 2 h. The resulting cDNA was diluted to a final concentration of 10 ng/µl. Quantitative real-time PCR (qPCR) was performed using intron-spanning primers specific for the detection of ribosomal protein L19 (RPL19), SFRP4, StAR, P450scc, WNT4, aromatase (CYP19A1) or insulin-like growth factor 2 (IGF2) as previously described (Baumgarten et al., 2015, Stocco et al., 2017). All determinations were performed in duplicate, and the number of copies for each gene was calculated using a standard curve made with a serial dilution of the respective cDNA (Stocco, 2004). Once the number of copies of the gene of interest was obtained, the relative expression was calculated as the ratio between the copy number of the gene of interest and the copy number of the housekeeping gene Rpl19. Primer sequences are available upon request. Target gene expression was adjusted to the expression of RPL19, an internal control, for each sample.
Western blotting
Cultured CCs were harvested in ice-cold radioimmunoprecipitation assay lysis buffer, and whole-cell lysates were used in western blotting as previously described (Zhou et al., 2013; Baumgarten et al., 2015). The primary antibodies used were SFRP4 (1:1000; Abcam, Cambridge, UK) and β-actin (ACTB) (1:1000; Proteintech, Chicago, IL, USA) as a loading control. Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA) was used to capture blot images and to quantify band intensities, which were adjusted relative to ACTB.
Promoter activity assay
Lentiviruses containing the β-catenin/T cell factor (TCF)-Luc reporter construct were generated as previously described (Zhou et al., 2013). Empty plasmids were used as controls. Cells were infected with lentivirus and incubated overnight prior to treatment with FSH, GDF9, BMP15 or their combination. Luciferase activity was determined in 50 μl of lysates after 48 h of treatment, as previously described (Zhou et al., 2013).
Statistical analysis
The number of patients used in each experiment is indicated in the corresponding figure legends. Data for continuous variables are presented as mean values ± SEM. Statistical comparisons of mean values between groups were performed with paired t-tests, and multiple comparisons were performed with one-way ANOVA with repeated measures followed by Bonferroni adjustment where appropriate. Differences were considered to be statistically significant if the P values were less than 0.05.
Results
The combination of GDF9 and BMP15 stimulates SFRP4 mRNA expression
Because a previous report showed that SFRP4 is highly expressed in human CCs when compared to mural cells (Maman et al., 2011) and since CCs are the primary target of GDF9 and BMP15, we first examined whether treatment with these factors affects SFRP4 expression in CCs. Primary human CCs were treated with GDF9 or BMP15 alone or in combination (5 ng/ml each) in the presence of 50 ng/ml of FSH. This concentration of FSH was selected based on concentration-dependent studies previously reported (Convissar et al., 2017; Hobeika et al., 2019). FSH treatment of human CCs significantly decreased SFRP4 mRNA levels when compared with nontreated cells (one-way ANOVA; P < 0.05; Fig. 1A). Conversely, FSH treatment with the addition of GDF9 and BMP15 in combination strongly stimulated (6-fold) SFRP4 mRNA levels (P < 0.01). Strikingly, treatment with either GDF9 or BMP15 alone did not stimulate SFRP4 (Fig. 1A).
Since GDF9 and BMP15 cotreatment strongly stimulated SFRP4 expression, we conducted concentration-dependent experiments in the presence or absence of FSH. CCs were treated with increasing concentrations of GDF9 and BMP15 in the presence of 50 ng/ml of FSH or vehicle (C: control). As shown in Fig. 1B, SFRP4 mRNA levels increased significantly in human GCs treated with GDF9 and BMP15 (GB) at concentrations of 0.1, 0.6, 2 and 5 ng/ml in the presence or absence of FSH. Cotreatment with FSH blunted SFRP4 stimulation by GDF9 and BMP15.
GDF9 plus BMP15 stimulate SFRP4 protein levels
Next, we examined whether the effect of GDF9 plus BMP15 on SFRP4 mRNA levels translates into an increase in SFRP4 protein. SFRP4 protein levels were measured in total cell lysates using the western blot. Cells from four patients were used to test the effect of GDF9 and BMP15 alone or in combination on SFRP4 protein expression. While neither GDF9 nor BMP15 alone could induce SFRP4, cotreatment with GDF9 and BMP15 strongly stimulated SFRP4 protein expression (Fig. 2). Full western blots for SFRP4 and β-actin (BACT) without cropping are provided in Supplementary Fig. S1. We quantified SFRP4 protein levels in all patients and expressed them as a ratio to BACT. A paired t-test comparing the GDF9 plus the BMP15-treated group with the untreated control group (C) showed that GDF9 plus BMP15 induced a statistically significant increase in SFRP4 protein levels (Fig. 2; P < 0.01).
GDF9 and BMP15 regulate SFRP4 via SMAD signalling
It is known that GDF9 and BMP15 activate SMAD2/3 and SMAD1/5/8 signalling pathways, respectively (Moore et al., 2003; Mazerbourg et al., 2004; Liu et al., 2018). To investigate the mechanisms by which GDF9 plus BMP15 induces SFRP4 expression, specific SMAD signalling inhibitors were added to the culture media 1 h before commencing FSH or FSH plus GDF9 plus BMP15 treatments. The concentration of SMAD inhibitors used was based on previous publications in mouse and human GCs (Peng et al., 2013b; Liu et al., 2018). After 48 h, cells were harvested for SFRP4 mRNA determination.
Initial experiments were conducted using the inhibitor SB431542, which targets TGFβR1/ALK5, ALK4 and ALK7, preventing activation of both Smad2 and Smad3. As shown in Fig. 3A, treatment with SB431542 prevented the stimulation of SFRP4 mRNA levels by GDF9 and BMP15 either in the presence or absence of FSH. To confirm the role of SMAD2/3 in the regulation of SFRP4 by GDF9 and BMP15, we tested the effects of SIS3 that specifically targets SMAD3 or LDN-193189 that inhibits ALK2 and ALK3 preventing the activation of SMAD1/5/8. The results show that inhibition of SMAD1/5/8 had no significant impact on the induction of SFRP4 mRNA levels by GDF9 plus BMP15 (Fig. 3B). However, the induction of SFRP4 by GDF9 plus BMP15 was inhibited by SIS3 to the same extent as by SB431542.
SFRP4 represses Wnt signalling in human CCs
By sequestering canonical Wnts, SFRP4 decreases the activity of the Wnt signalling effector CTNNB1 (β-catenin). β-Catenin is a transcriptional coregulator of several FSH targeted genes, including aromatase and LHCGR (Fan et al., 2010). Therefore, we next tested whether treatment with GDF9 plus BMP15 affects the activity of a β-catenin responsive reporter. For this purpose, human CCs were transfected with a luciferase reporter construct in which luciferase expression is under the control of a β-catenin/TCF response element. Cells were then treated with or without GDF9 plus BMP15. A basal level of β-catenin/TCF activity was detected in transfected cells cultured in control medium (C: Fig. 4A). The addition of GDF9 and BMP15 decreased the activity of the β-catenin/TCF-responsive promoter significantly (GB: Fig. 4A).
To examine the response in human cells and to determine whether GDF9 and BMP15 interact with FSH to regulate β-catenin/TCF activity, we transfected human CCs with the β-catenin/TCF-LUC reporter. We then treated the cells with FSH in the presence or absence of GDF9 plus BMP15 for 48 h. FSH treatment alone stimulated β-catenin/TCF activity strongly compared to untreated cells (P < 0.05) (Fig. 4B). Cotreatment with GDF9 and BMP15 potentiated the stimulatory effect of FSH (P < 0.01) (F+GB: Fig. 4B).
Treatment with GDF9 and BMP15 inhibits StAR and LHCGR stimulation by FSH
Our findings suggest that treatment with GDF9 plus BMP15 inhibits Wnt/β-catenin actions in human CCs. β-catenin has been shown to regulate FSH action in rodent GCs (Fan et al., 2010). Next, we examined whether FSH targets are affected by GDF9 plus BMP15 treatment. For this experiment, we treated cells with FSH in the presence or absence of GDF9 plus BMP15. The findings demonstrated that GDF9 plus BMP15 enhances the effect of FSH on aromatase, 3βHSD2 and P450scc expression (Fig. 5). We also quantified IGF2 mRNA levels, which are regulated by FSH in CCs (Baumgarten et al., 2015). We observed that treatment with GDF9 plus BMP15 potentiates the stimulation of IGF2 expression by FSH. In contrast, GDF9 plus BMP15 blocked the induction of StAR and LHCGR expression by FSH (Fig. 5). GDF9 plus BMP15 treatment alone also decreased StAR mRNA levels significantly when compared to controls.
Since Wnt4 is expressed in GCs and corpora lutea in mice (Hsieh et al., 2002) and is essential for follicle development (Boyer et al., 2010), we examined the effect of GDF9 plus BMP15 on the steady-state levels of Wnt4 in human CCs. As shown in Fig. 5, treatment with FSH decreased Wnt4 mRNA levels, but this effect was not affected by cotreatment with GDF9 plus BMP15. Treatment with GDF9 and BMP15 alone also led to a significant decrease in the expression of Wnt4.
Treatment with SFRP4 mimics the inhibition of StAR and LHCGR by GDF9 and BMP15
Our findings demonstrate that GDF9 and BMP15 strongly stimulate SFRP4 expression in human CCs. Since SFRP4 is a secreted protein, we next tested if the addition of SFRP4 to the media mimics GDF9 and BMP15 effects. As shown in Fig. 6, the addition of SFRP4 to the media blocked the stimulatory effect of FSH on StAR and LHCGR expression (P < 0.05), mimicking the effect of GDF9 and BMP15. However, treatment with SFRP4 also blocked FSH stimulation of aromatase and IGF2 (P < 0.05). SFRP4 did not impact the induction of P450scc by FSH. Finally, treatment with SFRP4 partially blocked the inhibitory effect of FSH on the expression of Wnt4, although this effect was not statistically significant.
Discussion
Cumulus cells play an essential role in the reproduction by integrating endocrine and local signals to coordinate oocyte maturation, follicle growth and ovulation. The close interaction of CCs with the oocyte suggests that oocyte-secreted factors control their function. Here, we report that the two main oocyte-secreted factors found in humans, GDF9 and BMP15, work in concert to stimulate the expression of SFRP4 in human CCs. Insights on the mechanisms regulating SFRP4 expression in the human ovary could help our understanding of how the oocyte controls its environment. Moreover, since SFRP4 is a secreted factor, monitoring SFRP4 levels in the follicular fluid may be of value to estimate oocyte health.
Human CCs collected from COCs isolated from women undergoing IVF have been adopted to investigate human GC biology (Zhou et al., 2013; Baumgarten et al., 2015; Convissar et al., 2017; Pogrmic-Majkic et al., 2018; Convissar et al., 2019; Hobeika et al., 2019, 2020). We have demonstrated that CCs respond to FSH and express low levels of luteinisation markers compared to mural cells (Zhou et al., 2013; Baumgarten et al., 2015; Convissar et al., 2017; Pogrmic-Majkic et al., 2018; Convissar et al., 2019; Hobeika et al., 2019, 2020). Here, we also show that CCs from IVF patients are a well-suited experimental approach to study the role of oocyte-secreted factors in human GC function.
GDF9 and BMP15 work together to stimulate SFRP4 in human CCs. However, treatment of CCs with GDF9 or BMP15 alone does not affect SFRP4 expression. In agreement with these findings, we recently showed that only cotreatment with GDF9 and BMP15 effectively stimulates anti-Müllerian hormone expression in primary human CCs (Convissar et al., 2017). This strong synergism between GDF9 and BMP15 has been proposed to be mediated by a GDF9: BMP15 heterodimer (Peng et al., 2013a; Mottershead et al., 2015). However, GDF9: BMP15 heterodimers have not been detected in either the mouse ovary or in human follicular fluid (Peng et al., 2013a) and whether these factors form heterodimers in vivo remains to be determined. A recent report suggested that monomeric forms of BMP15 and GDF9 may interact with their cell-surface receptors to initiate synergistic actions without needing to form heterodimers in solution (Heath et al., 2017). Additional experiments are required to determine the mechanism of the collaborative effects between GDF9 and BMP15 on the regulation of human CCs and the stimulation of SFRP4.
A leading question is ‘What is the function of SRFP4 stimulation by GDF9 and BMP15?’ From our findings, it is clear that the GDF9 and BMP15 act in concert to inhibit Wnt signalling in CCs. We demonstrated that treatment with GDF9 plus BMP15 decreases the activity of a β-catenin/TCF-LUC reporter, diminishes the expression of Wnt4 and blocks the stimulation of genes known to be stimulated by Wnt signalling, including StAR and LHCGR. Moreover, treatment with SFRP4 decreases the stimulatory effect of FSH on StAR, aromatase, IGF2 and LHCGR. However, this effect does not seem to apply to every gene stimulated by FSH. For instance, we observed that neither GDF9 plus BMP15 nor SFRP4 impacted the stimulation of P450scc or 3βHSD by FSH. Based on these findings, we hypothesise that GDF9 plus BMP15 predominantly inhibits genes involved in luteinisation, such as StAR and LHCGR, a function that is supported by the potent inhibition of SFRP4 expression by hCG in human cells. Therefore, we propose that GDF9 and BMP15 mediate the anti-luteinising effects of oocytes in humans. However, the effects are gene-specific, targeting luteinising genes but not genes involved in oestradiol production. How the GDF9 plus BMP15 combination differentially modulates these two pathways remains to be investigated.
In rodents, SFRP4 increases during luteinisation (Hsieh et al., 2003); thus, it is expected that SFRP4 plays a crucial role in luteal function. However, recent evidence does not support this concept. In SFRP4 knockout mice, luteinisation, progesterone secretion and cumulus expansion proceed normally (Zamberlam et al., 2019). In contrast, that report demonstrated that the responsiveness of GCs to FSH increases in the absence of SFRP4. GCs of SFRP4 knockout mice respond to FSH expressing higher levels of aromatase compared to wild-type animals (Zamberlam et al., 2019), supporting the inhibitory effect of SFRP4 on aromatase expression that we observed in human CCs. However, we detected opposite effects on the expression of LHCGR in human CCs in which LHCGR expression was potently inhibited by GDF9 plus BMP15 and SFRP4 treatments, whereas in the GCs of SFRP4 knockout mice, LHCGR expression increases significantly compared to controls (Zamberlam et al., 2019). Thus, while some effects of SFRP4 are conserved between humans and rodents, significant differences also exist. Therefore, the use of experimental models, such as human CCs, is essential to better understand the role of SFRP4 in human ovaries.
How SFRP4 may differentially target Wnt-regulated genes is not known. Differential effects of Wnt signalling in GCs have been reported. For instance, in the mouse, Wnt3A dampens the ability of FSH to upregulate steroidogenic enzymes such as aromatase, StAR and P450scc (Stapp et al., 2014). However, other reports show that overexpression of β-catenin contributes to FSH induction of aromatase expression in rodents (Parakh et al., 2006; Stapp et al., 2014) and bovine (Castanon et al., 2012) GCs. Moreover, Wnt4 and FSH synergistically promote GC proliferation and oestrogen biosynthesis, whereas β-catenin prevents luteinisation of granulosa cells and the expression of LH target genes (Fan et al., 2010). We propose that the induction of SFRP4 by GDF9 and BMP15 in human CCs prevents luteinisation by inhibiting the expression of LHCGR. It is unclear how GDF9 plus BMP15 can potentiate the stimulation of aromatase expression by FSH, which has been shown to need β-catenin and, at the same time, stimulate the expression of an inhibitor of Wnt signalling such as SFRP4. It is possible that β-catenin regulates aromatase expression in a Wnt-independent manner or that different pathways are involved in Wnt stimulation of aromatase and luteinisation inhibition. Multiple members of the Wnt signalling pathway are involved in the regulation of ovarian function; thus, further research is needed to determine the role of each Wnt member, especially in humans.
Previous studies using bovine granulosa cells showed that GDF9 and BMP15 have synergistic effects on the activation of the SMAD signalling pathway and mural GC proliferation (Liu et al., 2018). Our findings show that inhibition of SMAD3, but not SMAD 1/5/8 activation, abolishes SFRP4 stimulation by GDF9 plus BMP15; thus, these oocyte-secreted factors act through SMAD3 in human CCs.
A limitation of this study is the under-representation of samples from women with diminished ovarian reserve or low oocyte yield at the time of oocyte retrieval as these would have yielded a suboptimal number of cells. Thus, the possibility of variable responses of CCs from patients with different infertility aetiologies cannot be ruled out. However, CCs from different patients were studied separately, suggesting that the mechanisms activated by oocyte-secreted factors regulating SFRP4 are conserved.
The biological mechanisms controlled by GDF9 and BMP15 remain unexplored in humans. Even in rodents, there are few genes known to be regulated by GDF9 and BMP15. The evidence reported here suggests that GDF9 and BMP15 act in coordination to stimulate SFRP4 expression in human CCs. As human oocytes produce both GDF9 and BMP15, our findings may represent the physiological effects of the combination of both factors as compared to experiments using only GDF9 or BMP15. Our findings also suggest that GDF9 and BMP15 mediate the anti-luteinising effects of the oocyte in human CCs.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
Study conceptualisation, Methodology and Formal analysis and investigation: S.E. and C.S. Supply of human samples: N.J.W., M.A.F. and H.S. Writing—original draft preparation: S.E. Writing—review and editing: S.E., N.J.W., M.A.F., H.S. and C.S. Project administration and funding acquisition: C.S.
Funding
This work was supported by NIH Grant no. R01HD097202 (C.S.).
Conflict of interest
The authors declare that no conflict of interest could be perceived as prejudicing the impartiality of the research reported.
Supplementary Material
References
- Armouti M, Winston N, Hatano O, Hobeika E, Hirshfeld-Cytron J, Liebermann J, Takemori H, Stocco C.. Salt inducible kinases are critical determinants of female fertility. Endocrinology 2020;161:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten SC, Convissar SM, Zamah AM, Fierro MA, Winston NJ, Scoccia B, Stocco C.. FSH regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J Clin Endocrinol Metab 2015;100:E1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J.. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 2008;121:737–746. [DOI] [PubMed] [Google Scholar]
- Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, DeMayo FJ, Richards JS, Boerboom D.. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J 2010;24:3010–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon BI, Stapp AD, Gifford CA, Spicer LJ, Hallford DM, Hernandez Gifford JA.. Follicle-stimulating hormone regulation of estradiol production: possible involvement of WNT2 and beta-catenin in bovine granulosa cells. J Anim Sci 2012;90:3789–3797. [DOI] [PubMed] [Google Scholar]
- Convissar S, Armouti M, Fierro MA, Winston NJ, Scoccia H, Zamah AM, Stocco C.. Regulation of AMH by oocyte-specific growth factors in human primary cumulus cells. Reproduction 2017;154:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convissar S, Winston NJ, Fierro MA, Scoccia H, Zamah AM, Stocco C.. Sp1 regulates steroidogenic genes and LHCGR expression in primary human luteinized granulosa cells. J Steroid Biochem Mol Biol 2019;190:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JM, Friis RR, Dharmarajan AM.. The role of sFRP4, a secreted frizzled-related protein, in ovulation. Apoptosis 2003;8:389–397. [DOI] [PubMed] [Google Scholar]
- Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS.. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol 2010;24:1529–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath DA, Pitman JL, McNatty KP.. Molecular forms of ruminant BMP15 and GDF9 and putative interactions with receptors. Reproduction 2017;154:521–534. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS.. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 2006;20:1300–1321. [DOI] [PubMed] [Google Scholar]
- Hobeika E, Armouti M, Fierro MA, Winston N, Scoccia H, Zamah AM, Stocco C.. Regulation of insulin-like growth factor 2 by oocyte-secreted factors in primary human granulosa cells. J Clin Endocrinol Metab 2020;105:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobeika E, Armouti M, Kala H, Fierro MA, Winston NJ, Scoccia B, Zamah AM, Stocco C.. Oocyte-secreted factors synergize with FSH to promote aromatase expression in primary human cumulus cells. J Clin Endocrinol Metab 2019;104:1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS.. Mice null for Frizzled4 (Fzd4-/-) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod 2005;73:1135–1146. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Johnson MA, Greenberg NM, Richards JS.. Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology 2002;143:898–908. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Mulders SM, Friis RR, Dharmarajan A, Richards JS.. Expression and localization of secreted frizzled-related protein-4 in the rodent ovary: evidence for selective up-regulation in luteinized granulosa cells. Endocrinology 2003;144:4597–4606. [DOI] [PubMed] [Google Scholar]
- Huang X, Hao C, Shen X, Liu X, Shan Y, Zhang Y, Chen L.. Differences in the transcriptional profiles of human cumulus cells isolated from MI and MII oocytes of patients with polycystic ovary syndrome. Reproduction 2013;145:597–608. [DOI] [PubMed] [Google Scholar]
- Liu C, Yuan B, Chen H, Xu M, Sun X, Xu J, Gao Y, Chen C, Jiang H, Zhang J.. Effects of MiR-375-BMPR2 as a key factor downstream of BMP15/GDF9 on the Smad1/5/8 and Smad2/3 signaling pathways. Cell Physiol Biochem 2018;46:213–225. [DOI] [PubMed] [Google Scholar]
- Maman E, Yung Y, Cohen B, Konopnicki S, Dal Canto M, Fadini R, Kanety H, Kedem A, Dor J, Hourvitz A.. Expression and regulation of sFRP family members in human granulosa cells. Mol Hum Reprod 2011;17:399–404. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, Ritvos O, Hsueh AJ.. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol 2004;18:653–665. [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S.. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem 2003;278:304–310. [DOI] [PubMed] [Google Scholar]
- Mottershead DG, Sugimura S, Al-Musawi SL, Li JJ, Richani D, White MA, Martin GA, Trotta AP, Ritter LJ, Shi J. et al. Cumulin, an oocyte-secreted heterodimer of the transforming growth factor-beta family, is a potent activator of granulosa cells and improves oocyte quality. J Biol Chem 2015;290:24007–24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, Nilson JH.. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc Natl Acad Sci USA 2006;103:12435–12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM.. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA 2013a;110:E776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM.. Reply to Mottershead et al.: GDF9:BMP15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA 2013b;110:E2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogrmic-Majkic K, Samardzija D, Stojkov-Mimic N, Vukosavljevic J, Trninic-Pjevic A, Kopitovic V, Andric N.. Atrazine suppresses FSH-induced steroidogenesis and LH-dependent expression of ovulatory genes through PDE-cAMP signaling pathway in human cumulus granulosa cells. Mol Cell Endocrinol 2018;461:79–88. [DOI] [PubMed] [Google Scholar]
- Stapp AD, Gomez BI, Gifford CA, Hallford DM, Hernandez Gifford JA.. Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PLoS One 2014;9:e86432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco C. In vivo and in vitro inhibition of cyp19 gene expression by prostaglandin F2a in murine luteal cells: implication of GATA-4. Endocrinology 2004;145:4957–4966. [DOI] [PubMed] [Google Scholar]
- Stocco C, Baumgarten SC, Armouti M, Fierro MA, Winston NJ, Scoccia B, Zamah AM.. Genome-wide interactions between FSH and insulin-like growth factors in the regulation of human granulosa cell differentiation. Hum Reprod 2017;32:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamah AM, Baumgarten SC, Convissar SM, Fierro MA, Winston N, Stocco C, Scoccia B.. FSH regulates IGF2 expression in human granulosa cells in an akt-dependent manner. American Society for Reproductive Medicine, 71st Annual Meeting. Baltimore, MD, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamberlam G, Lapointe E, Abedini A, Rico C, Godin P, Paquet M, DeMayo FJ, Boerboom D.. SFRP4 is a negative regulator of ovarian follicle development and female fertility. Endocrinology 2019;160:1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, Stocco C.. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol 2013;27:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.