Abstract
Background
Many dental procedures produce aerosols (droplets, droplet nuclei and splatter) that harbour various pathogenic micro‐organisms and may pose a risk for the spread of infections between dentist and patient. The COVID‐19 pandemic has led to greater concern about this risk.
Objectives
To assess the effectiveness of methods used during dental treatment procedures to minimize aerosol production and reduce or neutralize contamination in aerosols.
Search methods
Cochrane Oral Health’s Information Specialist searched the following databases on 17 September 2020: Cochrane Oral Health’s Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library, 2020, Issue 8), MEDLINE Ovid (from 1946); Embase Ovid (from 1980); the WHO COVID‐19 Global literature on coronavirus disease; the US National Institutes of Health Trials Registry (ClinicalTrials.gov); and the Cochrane COVID‐19 Study Register. We placed no restrictions on the language or date of publication.
Selection criteria
We included randomized controlled trials (RCTs) and controlled clinical trials (CCTs) on aerosol‐generating procedures (AGPs) performed by dental healthcare providers that evaluated methods to reduce contaminated aerosols in dental clinics (excluding preprocedural mouthrinses). The primary outcomes were incidence of infection in dental staff or patients, and reduction in volume and level of contaminated aerosols in the operative environment. The secondary outcomes were cost, accessibility and feasibility.
Data collection and analysis
Two review authors screened search results, extracted data from the included studies, assessed the risk of bias in the studies, and judged the certainty of the available evidence. We used mean differences (MDs) and 95% confidence intervals (CIs) as the effect estimate for continuous outcomes, and random‐effects meta‐analysis to combine data. We assessed heterogeneity.
Main results
We included 16 studies with 425 participants aged 5 to 69 years. Eight studies had high risk of bias; eight had unclear risk of bias. No studies measured infection. All studies measured bacterial contamination using the surrogate outcome of colony‐forming units (CFU). Two studies measured contamination per volume of air sampled at different distances from the patient's mouth, and 14 studies sampled particles on agar plates at specific distances from the patient's mouth.
The results presented below should be interpreted with caution as the evidence is very low certainty due to heterogeneity, risk of bias, small sample sizes and wide confidence intervals. Moreover, we do not know the 'minimal clinically important difference' in CFU.
High‐volume evacuator
Use of a high‐volume evacuator (HVE) may reduce bacterial contamination in aerosols less than one foot (~ 30 cm) from a patient's mouth (MD −47.41, 95% CI −92.76 to −2.06; 3 split‐mouth RCTs, 122 participants; very high heterogeneity I² = 95%), but not at longer distances (MD −1.00, −2.56 to 0.56; 1 RCT, 80 participants).
One split‐mouth RCT (six participants) found that HVE may not be more effective than conventional dental suction (saliva ejector or low‐volume evacuator) at 40 cm (MD CFU −2.30, 95% CI −5.32 to 0.72) or 150 cm (MD −2.20, 95% CI −14.01 to 9.61).
Dental isolation combination system
One RCT (50 participants) found that there may be no difference in CFU between a combination system (Isolite) and a saliva ejector (low‐volume evacuator) during AGPs (MD −0.31, 95% CI −0.82 to 0.20) or after AGPs (MD −0.35, −0.99 to 0.29). However, an 'n of 1' design study showed that the combination system may reduce CFU compared with rubber dam plus HVE (MD −125.20, 95% CI −174.02 to −76.38) or HVE (MD −109.30, 95% CI −153.01 to −65.59).
Rubber dam
One split‐mouth RCT (10 participants) receiving dental treatment, found that there may be a reduction in CFU with rubber dam at one‐metre (MD −16.20, 95% CI −19.36 to −13.04) and two‐metre distance (MD −11.70, 95% CI −15.82 to −7.58). One RCT of 47 dental students found use of rubber dam may make no difference in CFU at the forehead (MD 0.98, 95% CI −0.73 to 2.70) and occipital region of the operator (MD 0.77, 95% CI −0.46 to 2.00).
One split‐mouth RCT (21 participants) found that rubber dam plus HVE may reduce CFU more than cotton roll plus HVE on the patient's chest (MD −251.00, 95% CI −267.95 to −234.05) and dental unit light (MD −12.70, 95% CI −12.85 to −12.55).
Air cleaning systems
One split‐mouth CCT (two participants) used a local stand‐alone air cleaning system (ACS), which may reduce aerosol contamination during cavity preparation (MD −66.70 CFU, 95% CI −120.15 to −13.25 per cubic metre) or ultrasonic scaling (MD −32.40, 95% CI ‐ 51.55 to −13.25).
Another CCT (50 participants) found that laminar flow in the dental clinic combined with a HEPA filter may reduce contamination approximately 76 cm from the floor (MD −483.56 CFU, 95% CI −550.02 to −417.10 per cubic feet per minute per patient) and 20 cm to 30 cm from the patient's mouth (MD −319.14 CFU, 95% CI ‐ 385.60 to −252.68).
Disinfectants ‒ antimicrobial coolants
Two RCTs evaluated use of antimicrobial coolants during ultrasonic scaling. Compared with distilled water, coolant containing chlorhexidine (CHX), cinnamon extract coolant or povidone iodine may reduce CFU: CHX (MD −124.00, 95% CI −135.78 to −112.22; 20 participants), povidone iodine (MD −656.45, 95% CI −672.74 to −640.16; 40 participants), cinnamon (MD −644.55, 95% CI −668.70 to −620.40; 40 participants). CHX coolant may reduce CFU more than povidone iodine (MD −59.30, 95% CI −64.16 to −54.44; 20 participants), but not more than cinnamon extract (MD −11.90, 95% CI −35.88 to 12.08; 40 participants).
Authors' conclusions
We found no studies that evaluated disease transmission via aerosols in a dental setting; and no evidence about viral contamination in aerosols.
All of the included studies measured bacterial contamination using colony‐forming units. There appeared to be some benefit from the interventions evaluated but the available evidence is very low certainty so we are unable to draw reliable conclusions.
We did not find any studies on methods such as ventilation, ionization, ozonisation, UV light and fogging.
Studies are needed that measure contamination in aerosols, size distribution of aerosols and infection transmission risk for respiratory diseases such as COVID‐19 in dental patients and staff.
Keywords: Adolescent; Adult; Aged; Child; Child, Preschool; Humans; Middle Aged; Young Adult; Aerosols; Air Filters; Air Microbiology; Bacterial Infections; Bacterial Infections/prevention & control; Colony Count, Microbial; Colony Count, Microbial/methods; Dentistry; Disinfectants; Infection Control, Dental; Infection Control, Dental/economics; Infection Control, Dental/instrumentation; Infection Control, Dental/methods; Occupational Diseases; Occupational Diseases/prevention & control; Randomized Controlled Trials as Topic; Randomized Controlled Trials as Topic/statistics & numerical data; Rubber Dams; Suction; Virus Diseases; Virus Diseases/prevention & control
Plain language summary
Do measures that aim to reduce aerosol production during dental procedures prevent the transmission of infectious diseases?
Why is this question important?
Most dental care procedures create tiny drops of liquid that float in the air, called aerosols. For example, to remove the film of bacteria (plaque) that builds on teeth, dentists use scaling machines (scalers). Scalers vibrate at high speed and use a flow of water to wash away the plaque. This produces aerosols that are made of air, water, and the patient’s saliva, which may also contain micro‐organisms such as bacteria, fungi and viruses.
Aerosols that contain bacteria, fungi or viruses can spread infectious diseases. Limiting the production of these aerosols could help to prevent disease transmission in a dental setting.
A range of approaches can be used to reduce production of potentially infectious aerosols during dental procedures. These include:
‐ ways to decontaminate the mouth before aerosols are produced, for example by using anti‐microbial mouthwash;
‐ ways to prevent aerosols from leaving the mouth (for example, placing a rubber sheet – known as a ‘dam’ – around the tooth that is to be treated, to isolate the treatment zone from saliva; or using a straw‐like suction tube known as a saliva ejector);
‐ local ventilation using a suction device (known as a high‐volume evacuator) that draws up a large volume of air and evacuates aerosols from the treatment zone;
‐ general ventilation, to reduce the concentration of aerosols in the air, for example by keeping windows open;
‐ decontamination of air‐borne aerosols, for example using ultraviolet light to sterilize the air.
These can be used alone, or in combination.
We analysed the evidence from research studies to find out whether interventions that aim to reduce aerosol production during dental procedures can prevent the transmission of infectious diseases. We also wanted to find out about the cost of the interventions, whether patients and dentists found them acceptable, and whether the interventions were easy to implement.
How did we identify and evaluate the evidence?
First, we searched for all relevant studies in the medical literature that compared interventions to reduce aerosol production during dental procedures against other interventions or no intervention. We then compared the results, and summarized the evidence from all the studies. Finally, we assessed how certain the evidence was. To do this, we considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we categorized the evidence as being of very low, low, moderate or high certainty.
What did we find?
We found 16 studies that involved a total of 425 people. Studies involved between one and 80 participants, who were aged between 5 and 69 years. Six studies were conducted in the USA, five in India, two in the UK and one each in Egypt, the Netherlands and the United Arab Emirates.
The studies evaluated one or more of the following devices:
‐ high‐volume evacuator (7 studies);
‐ hands‐free suction device (2 studies);
‐ saliva ejector (1 study);
‐ rubber dam (3 studies);
‐ rubber dam with a high‐volume evacuator (1 study); or
‐ air cleaning system (1 study).
None of the studies evaluated the risk infectious disease transmission. Nor did they evaluate cost, acceptability or ease of implementation.
All 16 studies measured changes in the levels of bacterial contamination in aerosols, but we assessed the evidence as being of very low certainty. This means that we have very little confidence in the evidence, and that we expect further research to change the findings of our review. We therefore cannot deduce from this evidence whether there is an effect on levels of bacterial contamination. No studies investigated viral or fungal contamination.
What does this mean?
We do not know whether interventions that aim to reduce aerosol production during dental procedures prevent the transmission of infectious diseases. This review highlights the need for more and better‐quality studies in this area.
How up to date is this review?
The evidence in this Cochrane Review is current to September 2020.
Summary of findings
Summary of findings 1. Comparison 1. High‐volume evacuation (HVE) compared to no HVE for reducing the level of contamination in aerosols.
| High‐volume evacuation (HVE) compared to no HVE for reducing the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed dental operatory Intervention: high‐volume evacuation (HVE) Comparison: no HVE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no HVE | Risk with HVE | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols (CFU/mm³) during ultrasonic scaling and air polishing at less than 1 foot from oral cavity |
The mean CFU level ranged from 13.50 to 107.13 | MD 47.41 CFU lower (92.76 lower to 2.06 lower) | ‐ | 122 (3 RCTs)a b c | ⊕⊝⊝⊝ VERY LOW 1 | 2 CCTs found an imprecise result that crossed the line of no effect (−50.19, 95% CI −109.71 to 9.33). |
|
Reduction in level of contamination in aerosols (CFU/mm³) during ultrasonic scaling and air polishing at more than 1 foot from oral cavity |
The mean CFU level was 12.50 | MD 1 CFU lower (2.56 lower to 0.56 higher) | ‐ | 80 (1 RCT)c | ⊕⊝⊝⊝ VERY LOW 2 | 1 CCT found a reduction in contamination with HVE at the same distance (MD −13.56, 95% CI −23.18 to −3.94, 30 participants). |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aKing 1997, bMuzzin 1999cDesarda 2014
1. Downgraded 1 level for unclear risk of bias in at least 2 domains, 2 levels for inconsistency due to substantial heterogeneity and 2 levels for imprecision due to wide confidence intervals
2. Downgraded 1 level for unclear risk of selection bias and reporting bias and 2 levels for imprecision due to wide confidence intervals
Summary of findings 2. Comparison 2. HVE compared to conventional dental suction for reduction in the level of contamination in aerosols.
| HVE compared to conventional dental suction for reduction in the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed dental operatory Intervention: HVE Comparison: conventional dental suction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional dental suction | Risk with HVE | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols during ultrasonic scaling at 40 cm |
The mean CFU level was 4.30 | MD 2.30 CFU lower (5.32 lower to 0.72 higher) | ‐ | 6 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in level of contamination in aerosols during ultrasonic scaling at 150 cm |
The mean CFU level was 10.30 | MD 2.20 CFU lower (14.01 lower to 9.61 higher) | ‐ | 6 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 2 levels for imprecision due to small sample size reported in a single study and 1 level for unclear risk of selection, detection and reporting bias
Summary of findings 3. Comparison 6. Rubber dam compared to no rubber dam for reduction in the level of contamination in aerosols.
| Rubber dam compared to no rubber dam for reduction in the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed dental operatory Intervention: rubber dam Comparison: no rubber dam | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no rubber dam | Risk with rubber dam | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols during restorative procedures at 1 meter from mouth |
The mean CFU level was 25.10 | MD 16.20 CFU lower (19.36 lower to 13.04 lower) | ‐ | 10 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in the level of contamination in aerosols during restorative procedures at 2 meters from mouth |
The mean CFU level was 20.40 | MD 11.70 CFU lower (15.82 lower to 7.58 lower) | ‐ | 10 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in the level of contamination in aerosols during restorative procedures at forehead |
The mean CFU level was 1.72 | MD 0.98 CFU higher (0.73 lower to 2.70 higher) | ‐ | 47 (1 RCT)b | ⊕⊝⊝⊝ VERY LOW 2 | |
|
Reduction in the level of contamination in aerosols during restorative procedures at occiput |
The mean CFU level was 1.44 | MD 0.77 CFU higher (0.46 lower to 2.00 higher) | ‐ | 47 (1 RCT)b | ⊕⊝⊝⊝ VERY LOW 2 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 1 level for unclear risk of selection, detection and reporting bias and 2 levels for imprecision due to small sample size reported in a single study
2. Downgraded 2 levels for high risk of selection and attrition bias and 2 levels for imprecision due to small sample size reported in a single study
Summary of findings 4. Comparison 7. Rubber dam + HVE compared to HVE for reduction in the level of contamination in aerosols.
| Rubber dam + HVE compared to no rubber dam + HVE for reduction in the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed dental operatory Intervention: rubber dam + HVE Comparison: no rubber dam + HVE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no rubber dam + HVE | Risk with rubber dam + HVE | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols during restorative procedures at participant's chest |
The mean CFU level was 280.00 | MD 251 CFU lower (267.95 lower to 234.05 lower) | ‐ | 21 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in level of contamination in aerosols during restorative procedures at dental unit light |
The mean CFU level was 13.00 | MD 12.70 CFU lower (12.85 lower to 12.55 lower) | ‐ | 21 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 2 levels for unclear risk of selection and reporting bias and high risk of detection bias, and 2 levels for imprecision due to small sample size reported in a single study
Background
The production of aerosols and splatter in dentistry is a major health concern as aerosols generated during dental procedures are contaminated with micro‐organisms, which can lead to spread of infections among dental professionals and their patients. The oral cavity harbours over 700 species of bacteria and other infectious microbes (e.g. viruses, fungi), which can be expelled through aerosol‐generating procedures (AGPs). This may be able to cause respiratory health effects or transmit diseases bidirectionally. As procedures in a dental clinic generally involve close contact between patients and dentists, the risk of respiratory infection in this setting can be high (Meng 2020), though empirical evidence of respiratory infections in dental staff is scarce. Scannapieco 2004 did not find an increased risk of respiratory diseases among dental students. A recent systematic review of the risks of COVID‐19 among healthcare workers did not find studies on dental staff but it might have missed studies because dentists were not included in the search (Chou 2020).
The World Health Organization (WHO) has previously reported disease outbreaks of Ebola virus, Middle East respiratory syndrome (MERS‐CoV), severe acute respiratory syndrome (SARS‐CoV), swine flu, avian influenza (H5N1 flu), tuberculosis and measles across the world, and we are currently experiencing the COVID‐19 pandemic (WHO 2020a). Based on risk assessment, WHO has recommended airborne precautions for settings in which AGPs are performed (WHO 2020b), thus leading several countries to temporarily suspend all elective dental procedures. Dental professional organisations proposed infection control protocols (ADA 2020; ALOP 2020; BDA 2020a; CDC 2020; Dominiak 2020; NCUDSPH 2020); and recommendations to postpone elective procedures, surgeries and non‐urgent dental visits (ADA 2020; CDC 2020; NCUDSPH 2020). This new Rapid Review explores the evidence on the effectiveness of various methods that can be used to reduce contaminated aerosols and contamination in aerosols generated during dental procedures.
Description of the condition
Dental professionals have an important role in preventing the transmission of any infection. The possible routes for the spread of most viral, bacterial and fungal infections in a dental clinic are droplet, contact and airborne (Peng 2020). These routes can be bidirectional, meaning transmission may occur from patient to patient, patient to clinician or clinician to patient (Laheij 2012). It is unclear how much each form of transmission contributes to the risk of infection, but it is assumed that airborne transmission occurs only when a large volume of aerosol particles are generated (Harrel 2004).
In contrast to other health care workers, dentists mostly see patients who are healthy other than their dental condition. The risk of infection may thus especially occur with patients who are in the prodromal phase of an infection. The incubation period of common bacterial and viral infections ranges between two and 14 days during which the patient is asymptomatic but the chance of contamination and spread may still exist (Lessler 2009). The incubation period of the current pandemic due to COVID‐19 has been estimated at five to six days on an average, but it could be as long as 14 days (Meng 2020). The incubation period of SARS virus infection was reported to be 10 days, though with a low risk of transmission in the prodromal phase (Samaranayake 2004). This uncertainty makes it prudent to consider all patients to be potential sources of infection.
Transmission of respiratory infection in the dental clinic probably primarily occurs by direct contact with the respiratory droplets from the infected person on the mucous membranes of the dental staff. Also, indirect contact with surfaces in the immediate environment on which droplets or aerosols have settled can be a source of infection (WHO 2020b). Aerosol scientists have argued that COVID‐19 also spreads via aerosols in the air and that smaller particles can also be inhaled deep into the lungs and thus be a different cause of infection than droplets (Lewis 2020). Leung 2020 detected rhinovirus, influenza and human coronaviruses (excluding SARS‐CoV‐2) in respiratory droplets and aerosols. WHO states that airborne transmission may be possible during certain medical procedures such as intubation (WHO 2020b); and on 9 July 2020, issued a statement on the possible airborne transmission of COVID‐19 infection (WHO 2020c).
Differentiation of aerosols
Aerosols are differentiated based on particle size: splatter when they are greater than 50 µm; droplets when 11 µm to 50 µm; droplet nuclei when 10 µm or less. Most of the aerosols produced in the dental settings are extremely small (less than 5 µm) (Harrel 2004; James 2016). They vary in size depending on the procedures (Polednik 2014), and submicrometre particles have been demonstrated in various dental procedures in laboratory settings (Polednik 2014; Sotiriou 2008).
Splatter particles, being larger, are airborne only briefly. They fall to the ground or settle on surfaces in the dental operatory (Harrel 2004). Droplets remain suspended in the air until they evaporate, leaving droplet nuclei that may contain microbes related to respiratory infections. Droplet nuclei can contaminate surfaces to a range of three feet and may remain airborne for 30 minutes to two hours. If inhaled, the droplet nuclei can penetrate deep into the respiratory system and thus cause infection (Harrel 2004; James 2016; Kormuth 2018). Droplets cause infection by contaminating the mucous membranes but small airborne particles cause infection through inhalation. Prevention of contamination of the mucous membranes can simply be done with face shields or masks but inhalation prevention requires much better respiratory protection in the form of respirators or hoods with positive air pressure respirators. It is therefore useful to try to prevent both the production of splatter and smaller‐sized aerosols.
What is the composition of contaminated droplets or aerosols?
The oral cavity is a nidus for several bacteria and viruses. It also harbours bacteria and viruses from the nose, throat and respiratory tract. Hence, different strains of micro‐organisms and viruses are present in aerosols generated when dental AGPs are carried out, making them contaminated aerosols or bio‐aerosols or microbial aerosols (Zemouri 2017). In addition to micro‐organisms, the following are commonly present in dental aerosols: components of saliva, nasopharyngeal secretions, plaque, blood, tooth components and any material used in the dental procedures such as abrasives for air polishing and air abrasion. While multiple studies have been conducted to determine which dental procedure produces the most airborne bacterial contamination (Jain 2020; Monarca 2000; Polednik 2014; Rautemaa 2006), viral particles such as influenza, rhinoviruses, SARS coronavirus and bacteria such as Mycobacteria tuberculi and strict anaerobic bacteria could not be measured in these studies as the culture medium used was not suitable (Harrel 2004).
What are the sources of aerosols and splatter in the dental workplace?
A four‐fold increase of airborne bacteria has been observed in areas where dental aerosol‐producing equipment is used (Sawhney 2015). According to the General Dental Council in the UK, the following dental procedures are classified as AGPs: use of high‐speed handpieces for direct and indirect restorative procedures, ultrasonic scalers and high pressure 3:1 air syringe, polishing teeth, use of air‐driven surgical handpieces, air abrasion, slow‐speed polishing and opening teeth for drainage (FGDP 2020; GDC 2020). In addition to these procedures, WHO has added the following procedures in oral health care to the list of AGPs: definitive cementation of crown or bridge; surgical tooth extraction and implant placement (WHO 2020d). Moreover, some non‐AGPs, such as intraoral radiography, can evoke gag reflex leading to coughing or sneezing that results in aerosols (Mair 2020). The British Association of Oral Surgeons and British Association of Oral and Maxillofacial Surgeons advise that all urgent dental procedures, including oral examination, be treated as aerosol‐generating (FGDP 2020). Aerosol‐producing medical procedures are broadly classified as procedures that induce the patient to produce aerosols and those that mechanically create aerosols (Judson 2019). If we apply this classification for aerosol‐producing dental procedures, intraoral radiography and impression procedures that can induce gag reflex that leads to coughing would be categorized as the procedures that induce the patient to produce aerosols and the procedures listed above would be categorized as those that mechanically create aerosols. Dental handpieces, ultrasonic scalers, air polishers and air abrasion units produce the most visible aerosols. Each of these instruments removes material from the operative site thus generating aerosols by the action of rotary instruments, ultrasonic vibrations, or the combined action of water sprays and compressed air. Using the bacterial growth method, the ultrasonic scaler has been shown to produce the greatest amount of airborne contamination, followed by the air‐driven high‐speed handpiece, the air polisher and other instruments such as the air‐water syringe and prophylaxis angles (Barnes 1998; Gross 1992; Harrel 1996; Harrel 2004; Muzzin 1999). The particle size of these dental aerosols is less than 50 μm and their small size means they tend to be suspended in the air for longer periods of time (Cottone 1991).
One in vitro study reported that the position of the handpiece in the dental arch influences the amount of splatter. When the water spray is positioned closer to the oral aperture (e.g. near upper anterior teeth), it is more likely that there is escape of water from the mouth rather than its adhering to adjacent oral surfaces or the rubber dam (Dahlke 2012).
Description of the intervention
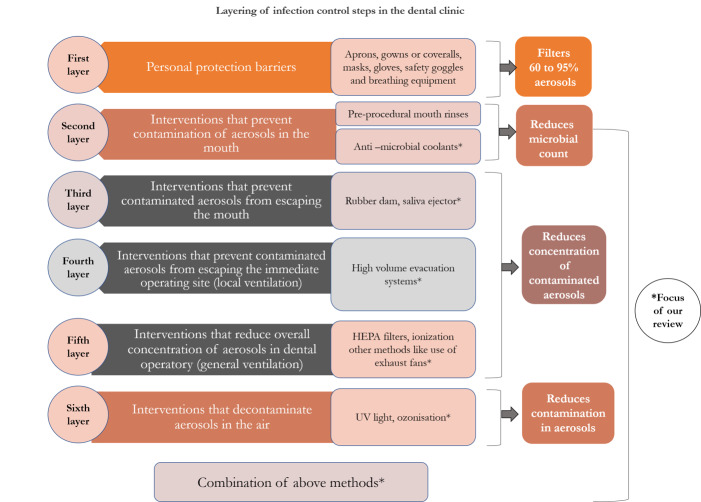
Harrel 2004 suggested layering infection control steps to reduce the potential danger from contaminated dental aerosols. These consisted of: 1. barrier protection – mask, gloves and eye protection; 2. preprocedural rinse with antiseptic mouthwash; 3. high‐volume evacuator; 4. high‐efficiency particulate air room filters and ultraviolet (UV) treatment of ventilation system. Many other techniques and devices have been introduced since the early 2000s. We devised an infographic based on Harrel 2004 to categorise interventions used to reduce contaminated aerosols produced during dental procedures (Figure 1).
1.
Key infection control methods (inspired by Harrel 2004). Copyright: Prashanti Eachempati.
HEPA: high efficiency particulate air; UV: ultraviolet.
Interventions that are not included in our review
Personal protective equipment (PPE)
PPE includes aprons, gowns or coveralls (a one‐piece suit), gloves, masks, breathing equipment (respirators) and goggles. PPE reduces operator (dentist, dental assistant or dental laboratory personnel) contact with aerosols thus protecting from exposure to microbial organisms in the aerosol. A Cochrane Review on this topic has recently been published (Verbeek 2020).
Preprocedural mouthrinses
Preprocedural mouthrinses (e.g. chlorhexidine, povidone iodine and hydrogen peroxide) have antimicrobial action; they help reduce the salivary concentration of microbial organisms thereby reducing the number of viable microbial organisms in the aerosols during AGPs (Eggers 2018; Harrel 2004). A limitation of the studies testing effectiveness of aerosol‐reducing interventions is the use of bacterial colony‐forming units (CFU) as a surrogate measurement tool to check for reduction in contaminated aerosol. Hence, in patients where the preprocedural rinses are used, the true efficacy of the other interventions may be obscured as the bacterial count in the saliva itself is controlled. The use of mouthrinses in the context of COVID‐19 specifically is currently being evaluated in reviews being undertaken jointly by Cochrane Oral Health and Cochrane Ear, Nose and Throat (Burton 2020a; Burton 2020b). We are writing a protocol for a review of preprocedural mouthrinses for prevention of any infectious disease and hope to publish a review before the end of 2020.
Interventions included in our review
-
Interventions that prevent contamination of aerosols in the mouth (Harrel 2004)
Anti‐microbial agents such as chlorhexidine and povidone iodine are used as coolants along with ultrasonic scalers to reduce the contamination of aerosols in the mouth (Sethi 2019).
-
Interventions that prevent contaminated aerosols from escaping the mouth (Harrel 2004)
Use of a rubber dam during AGPs prevents patient's saliva being mixed with the water spray generated from the drill or scaler.
Saliva ejectors (usually connected to low‐volume evacuators and hence known as low‐volume aspirators or conventional dental suction) reduce the aerosols escaping the mouth.
-
Interventions that prevent contaminated aerosols from escaping the immediate operating site (local ventilation)
Aerosols coming out of the mouth can be removed with local exhaust ventilation such as high‐volume evacuation systems (HVE).
-
Interventions that reduce overall concentration of aerosols in dental operatory (general ventilation)
Once the contaminated aerosols escape the immediate operating site and become airborne, air purification methods, such as high‐efficiency particulate air (HEPA) filters, can be used to tackle them. These aim to reduce the overall concentration of aerosols in the dental operatory (Harrel 2004; Yadav 2015).
Ionisation makes the aerosol particles unipolarly charged and thus they repel each other to deposit on surfaces (Yadav 2015).
Other methods, such as avoiding the use of fans that can recirculate the air (Warnakulasuriya 2020), and keeping windows open in the dental operatory room and using exhaust fans (Escombe 2019; Stockwell 2019), have been suggested.
-
Interventions that decontaminate aerosols in the air
UV light (Yadav 2015): UV has germicidal properties and short wavelength UV‐C (250 nm to 265 nm wavelength) is used for disinfection purposes.
Ozonisation (Yadav 2015): ozone, an allotrope of oxygen, owes its antimicrobial activity to its high oxidative potential.
Fumigation and fogging (Bali 2014 and McDonnell 2006, respectively): fumigation is a chemical method of decontaminating the air in an operating theatre or a clinic by spraying formaldehyde and potassium permanganate in liquid form; fogging uses a mixture of hydrogen peroxide and silver ion solution in the form of aerosols to control the contaminated aerosols (McDonnell 2006).
Combination of methods or other methods
Dentists can select different combinations of the above methods; for example, Cochran 1989 evaluated rubber dam together with HVE to reduce contamination in aerosols. Modifications of existing techniques or equipment may be used, or new devices: for example, Isolite illuminated dental isolation system (Zyris 2020).
How the intervention might work
Interventions that prevent contamination of aerosols in the mouth
The traditional use of water coolant during ultrasonic scaling or while using a high speed handpiece is to reduce the temperature on the tooth surface and surrounding tissues. However, anti‐microbial agents such as chlorhexidine gluconate and povidone iodine are used as ultrasonic coolants to prevent the contamination of aerosols in the mouth and biofilm formation (Sethi 2019). These agents are used in solution form and lesser concentrations than the agents used in preprocedural rinse or local irrigation. This reduces contamination of the waterlines; and penetration of the agent into the periodontal pocket increases and thus acts on the local microbia to prevent the contamination of aerosols produced (Jawade 2016).
Interventions that prevent contaminated aerosols from escaping the mouth
1. Rubber dam
This is a disposable rubber sheet that is stretched around the treated tooth or teeth, and works by isolating the treatment zone from saliva (Al‐amad 2017). Two studies observed a significant reduction in bacterial atmospheric contamination when rubber dams were used (Cochran 1989; Samaranayake 1989). However, contradictory results are reported by Al‐amad and colleagues, which showed an increase in the bacterial contamination on the headscarf of the students who used rubber dam (Al‐amad 2017). Rubber dam application in certain situations may not establish a perfect seal around the tooth and may even expose the gingiva due to reduced clinical crown height (when not using the split dam technique). This can lead to leakage of contaminated saliva which results in aerosols and thus reduces the efficiency of rubber dam isolation (Al‐amad 2017; Cochran 1989; Fors 1986). Rubber dam may not be of much use in prevention of contaminated aerosols when AGPs are performed on a carious tooth which not only harbours the caries‐causing microbial flora, but other microbial organisms including fungi and viruses.
2. Saliva ejectors (low‐volume evacuators or low‐volume aspirators)
A saliva ejector is a narrow, tubular device that provides suction to remove saliva, blood, tooth material and debris from the mouth during dental procedures to provide a clear operating field (Merriam‐Webster 2020). The use of saliva ejectors with low or high volume was shown to reduce the production of droplets and aerosols in one study (Yadav 2015); however, neither saliva ejectors nor HVE devices reduced the aerosols and splatter effectively in another study (Holloman 2015). Saliva ejectors in conjunction with HVE devices are more effective than saliva ejectors used alone (Graetz 2014). This is because of the smaller diameter of the tip, which is not capable of clearing the aerosols. Saliva ejectors are preferred in dental practices because of their usefulness in providing a clear operating field, convenient use and comfort as opposed to HVE devices (Graetz 2014; Jacks 2002).
Local ventilation (interventions that prevent contaminated aerosols from escaping the immediate operating site)
1. High‐volume suction evacuation (high‐volume evacuator devices or high‐volume aspirators)
HVE devices are suction devices fitted on an evacuation system that can draw a large volume of air within a short period of time (Avasthi 2018; Harrel 2004). The usual HVE device used in dentistry has a large opening (usually 8 mm or greater) and is attached to an evacuation system that will remove up to 2.8 cubic metres of air per minute (Harrel 2004). They have been tested in controlling aerosol production in dental settings and studies have shown varying results, with 90.8% reduction of aerosols (Jacks 2002) to no statistically significant difference between using and not using HVE devices (Desarda 2014). Proper distance should be maintained by clinicians while holding HVE devices. The device should be held approximately 6 mm to 15 mm away from the active ultrasonic tip or air polisher (Avasthi 2018).
General ventilation
1. High‐efficiency particulate air filters
A HEPA filter is composed of a mat of randomly arranged fibres and can remove 99.95% (European Standard) of particles measuring 0.3 μm in diameter, from the air that passes through (European Standard 2009 – EN 1822‐1:2009). In the USA, the Institute of Environmental Sciences and Technology (IEST) requires a certified HEPA filter to capture a minimum of 99.97% of contaminants 0.3 μm in size and larger, which means that for every 10,000 particles that pass through the filter, only three can be permitted to escape (Yadav 2015). Filtration involves physical removal of particulates from the air and is a vital aspect in achieving acceptable indoor air quality. Air purifiers utilise different types of filtration such as carbon, HEPA or a mixture such as a carbon/HEPA filtration unit. While a carbon filter is ideal for chemicals and odours in the air, HEPA is ideal for air particles. According to IEST, there are six types of filters used in HEPA (type A, B, C, D, E and F), dependent on performance (Veeck 2004). Portable HEPA filters are also available and are effective in particle reduction when tested in simulated hospital wards (Qian 2010).
2. Ionization
Ionizers or ionic air purifiers are devices that can either be wearable or stationary. They use charged electrodes to project negative ions into the air. These devices impart electrical charges of the same polarity on aerosol particles. These unipolarly charged particles then repel each other and move away from the breathing zone to be deposited on nearby surfaces (Grinshpun 2001). Another possible mechanism for how this works is that the micro‐organisms floating in the air attract these negatively charged ions and become heavier as a result and then precipitate onto surfaces. The micro‐organisms are not destroyed through this process, however. They remain viable and thus require further treatment through some more conventional form of disinfection (Yadav 2015).
3. Other methods
Other methods, such as avoiding the use of fans, keeping windows open at the dental operatory room and using exhaust fans may help by improving the air circulation (Escombe 2019; Meng 2020; Stockwell 2019).
Decontamination of aerosols in the air
1. Ultraviolet light
Air sterilization is done using UV irradiation. The DNA of all bacteria and viruses are ruptured, thus rendering them sterile and incapable of reproduction (Harrel 2004; Yadav 2015).
2. Ozonisation
Ozone attacks the cell membrane of bacteria, possibly through ozonolysis of carbon–carbon double bonds of membrane lipids leading to lysis of the cell (Gurley 1985). Laboratory studies have shown that ozone at a concentration of over 100 ppm with high humidity was highly virucidal against ribonucleic acid (RNA) viruses (Sato 1990). Ozone molecules are highly reactive and, when they come into contact with micro‐organisms, they react, rendering them harmless. Concerns are raised about the amount of ozone required to destroy pathogens in the air and whether that would present a health risk to dental personnel and patients (Yadav 2015); the half‐life of ozone is 20 minutes, however, and it decomposes to oxygen thus not posing a health hazard (Brown 1999).
3. Fumigation and fogging
Fumigation with formaldehyde was able to reduce Staphylococcus aureus, Streptococcus spp, Escherichia coli and Aspergillus spp in samples obtained in a maxillofacial operating theatre in India because of its bactericidal properties (Bali 2014).
Nowadays, this fumigation method is seldom used because of the carcinogenic effect of formaldehyde and fogging is preferred instead. Fogging uses a mixture of hydrogen peroxide and silver ion solution to control the contaminated aerosols through its bactericidal action (McDonnell 2006).
Why it is important to do this review
The recent COVID‐19 pandemic and similar communicable diseases pose a high risk to health professionals (Coulthard 2020; Laheij 2012; Peng 2020; Samaranayake 2004; Scannapieco 1999). AGPs such as dental drills and surgical drills used in oral surgery procedures form aerosols contaminated with bacteria, fungi and viruses (Al‐Eid 2018; Ishihama 2008; Szymańska 2007). Dentists who treat patients using such AGPs are at risk of contaminating and inoculating themselves if the patient is infected with infections such as COVID‐19 and SARS (Peng 2020; Samaranayake 2004). Dental assistants, other office staff members, and patients are also at risk of inoculation (Froum 2020). According to the US Department of Labor, dental hygienists, dental assistants and general dentists have the highest occupational risk for COVID‐19 with a risk score of 99.7% (hygienists), 92.5% (assistant) and 92.1% (general dentist) (Lu 2020). Similarly, the Occupational Safety and Health Act (OSHA) categorises occupations involved with aerosol production as very high risk (OSHA 2020). The first report on a dentist and two dental nurses contracting COVID‐19 infection was outlined by Wuhan Dental Hospital in the early weeks of the pandemic (Meng 2020).
Dental professionals in many countries have stopped routine care because of regulatory restrictions and fear of spreading COVID‐19 among their patients and beyond. This closure brings significant financial impact for dental professionals, especially for self‐employed practitioners (Coulthard 2020); or dental practices with a National Health Service contract (UK) that furloughed their staff. The Association of British Insurers warned that the majority of the dental clinics in the UK are not covered for business interruption claims due to the COVID‐19 pandemic (BDA 2020b). Moreover, the abrupt closure of dental services has left many patients midway through procedures such as root canal treatment, dentures, orthodontic treatment, fixed partial dentures and implant‐supported dentures. Patients may be in pain but in fear of attending for urgent treatment, and delayed treatment may exacerbate non‐urgent problems. This review will help dental professionals prepare themselves to adopt best practices during and after the COVID‐19 pandemic, by identifying the effective methods for reduction of contaminated aerosols in their dental clinics and thus reduction in the risk of infectious diseases spreading through aerosols.
Objectives
To assess the effectiveness of methods used during dental treatment procedures to minimize aerosol production and reduce or neutralize contamination in aerosols.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and controlled clinical trials (CCTs) conducted in a dental environment. We also included randomized and pseudo‐randomized (alternation) split‐mouth studies. When the authors mentioned that the order or the participants were randomly assigned, we classified the study as a RCT and in other cases as a CCT. We included studies where the unit of randomization is dental professionals, participants, quadrants (split‐mouth design), dental units or practices.
We excluded experimental studies conducted in a laboratory environment with mannequins not including real patients.
Types of participants
We included studies with dental healthcare providers (dentist, dental surgery assistant, dental hygienist, dental technologist, dental laboratory staff, dental aide or a dental trainee) and their patients undergoing a dental AGP.
Types of interventions
We included any method, procedure or policy that aimed to reduce contaminated aerosols in dental clinics compared to any other method including no treatment or combination of methods.
We categorised the interventions, primarily in the following categories.
Methods to prevent contaminated aerosols escaping from the mouth
Local ventilation
General ventilation
Decontamination of aerosols in the air
Combination of methods
Types of outcome measures
As this is a Rapid Review, we consider only the following key outcomes.
Primary outcomes
Incidence of infection of dental staff or patients
Reduction in volume of contaminated aerosols in the operative environment
The reduction of these aerosols can be measured directly as a decrease in the amount of particles, using optical particle counters, condensation nuclei counters, aerodynamic analyses, scanning mobility particle sizer spectrometers (Górny 2020), adenosine triphosphate (ATP) bioluminescence (Watanabe 2018), or use of fluorescent dye to count splatter (Veena 2015).
Reduction in level of contamination in aerosols in the operative environment
There is no generally accepted method for measuring contamination in bioaerosols (Ghosh 2015). Contamination in bioaerosols can be measured with various methods such as sampling volumes of air and collecting micro‐organisms by various physical methods such as impaction, impingement or filtration. For all methods, the amount of contamination is measured by the number of CFU on collection surfaces such as an agar plate per volume of air per minute. The CFU can further be identified and specified according to type of micro‐organism. The contamination can also be measured by having micro‐organisms settle on agar plates because of gravity. The findings can be expressed as a standard index of microbial air contamination (IMA) (Pasquarella 2000). We call this a surrogate outcome because gravitational settling is biased towards larger particles and does not inform about the size of the particles. The CFU can be the result of any contamination and not just from aerosols, thus making it a less reliable outcome measure.
Secondary outcomes
Costs for the interventions used (measured in local currency)
Acceptability and feasibility of the intervention to patients and dentists (measured using ordinal (e.g. Likert scale) or dichotomous (e.g. yes/no) data)
Search methods for identification of studies
Cochrane Oral Health's Information Specialist conducted systematic searches for RCTs and CCTs. There were no language, publication year or publication status restrictions. We contacted original authors for clarification and further data if trial reports had missing data or were unclear.
Electronic searches
Cochrane Oral Health's information specialist searched the following databases.
Cochrane Oral Health's Trials Register (to 17 September 2020) (see Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Register of Studies (to 17 September 2020) (see Appendix 2)
MEDLINE Ovid (1946 to 17 September 2020) (see Appendix 3)
Embase Ovid (1980 to 17 September 2020) (see Appendix 4)
WHO COVID‐19 Global literature on coronavirus disease database (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov; to 17 September 2020, see Appendix 5)
We modelled subject strategies on the search strategy designed for MEDLINE Ovid. Where appropriate, we combined them with subject strategy adaptations of the Highly Sensitive Search Strategies designed by Cochrane for identifying RCTs as described in the Cochrane Handbook for Systematic Reviews of Interventions, Technical Supplement to Chapter 4 (Lefebvre 2019).
Searching other resources
Cochrane Oral Health's information specialist searched the following databases to identify ongoing studies.
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov; to 17 September 2020; Appendix 6);
Cochrane COVID‐19 Study Register (covid-19.cochrane.org) (search via the Cochrane Register of Studies, to 17 September 2020; Appendix 7).
A search of the WHO's International Clinical Trials Registry Platform is mandatory for Cochrane Reviews; however, this database was not available at the time of the search due to the COVID‐19 pandemic. We will search this database for any updates of this review.
We also undertook a non‐systematic search of the internet using Google in May 2020.
We made efforts to identify full‐text papers regardless of date of publication; however, we did not delay the Rapid Review process. Any papers that we were unable to source quickly were listed as awaiting classification.
Data collection and analysis
Selection of studies
Two review authors (MP and GS) screened the titles and abstracts in duplicate. We initially tried to resolve any disagreements during the screening by discussion. If this was not successful, we consulted a third review author (arbiter ‐ MN) and reached consensus through further discussion. We used online Rayyan software to screen the titles and abstracts (Rayyan 2016).
Two review authors (MP and GS) screened the full‐text articles in duplicate and we entered the reasons for excluding full‐text articles in the Characteristics of excluded studies table. For included studies, we extracted useful information and data from the full‐text articles and completed the Characteristics of included studies table. We resolved any disagreements during the screening by discussion. If this was not successful, we consulted a third review author (arbiter ‐ MN) and reached consensus through further discussion.
Where studies with multiple publications were encountered, we planned to collate the reports of the same study so that each study, rather than each report, is the unit of interest for the review, and such studies have a single identifier with multiple references. We did not, however, encounter any such multiple publications.
Data extraction and management
One review author (PE) designed the data extraction form and another review author (JV) tested its suitability. One review author (PE) extracted the data using the data extraction form. One of the three review authors (SKN, MN and JV) verified the correctness and completeness of data extracted. We limited the data extraction to a minimal set of required data items.
Assessment of risk of bias in included studies
Two review authors (SKN and PE) assessed risk of bias, using the Cochrane 'Risk of bias' tool for RCTs, and reported the results in a table (Higgins 2019). We classified each domain at high, low or unclear risk of bias (Higgins 2019). For CCTs, we classified the randomization and allocation domain at high risk of bias, while the other domains were assessed in the same way as for RCTs. We attempted to contact the trial authors if information is not specified or is unclear. We tried to resolve any disagreements by discussion between the review authors. If we could not reach agreement, we consulted a third review author (arbiter ‐ MN).
Measures of treatment effect
We did not find any study describing the effect sizes as dichotomous outcomes. We reported continuous outcomes as mean differences (MD) and 95% CIs. If the included trials reported continuous outcomes obtained from different instruments, we planned to use the standardised mean difference (SMD) and 95% CI as the effect measure. We did not, however, encounter such studies in this review. We planned to qualitatively describe the costs for the interventions used; however, none of the included studies reported costs. For ordinal data, we planned to dichotomise the data and present the effect sizes as RR and 95% CIs; none of the studies used the ordinal data, however.
Unit of analysis issues
We did not anticipate that any cluster‐randomised studies would meet the inclusion criteria of this review. We identified multi‐arm trials and selected relevant arms for inclusion in our analyses. If more than two arms were relevant to this review, we split the control group between different comparisons so that participants were not double‐counted in meta‐analysis.
Dealing with missing data
If we encountered trials with missing data, we contacted the investigators of these studies wherever e‐mail addresses were available. We calculated the missing data from other data, such as standard deviations (SDs), from P values and graphs and from other studies, if needed. We planned to re‐analyse the data according to the intention‐to‐treat (ITT) principle whenever possible. However, none of the studies had given enough details to perform ITT analysis.
We did not include one trial in the meta‐analysis due to missing data. For trials reporting data in graphs, we derived the data using PlotDigitizer software (PlotDigitizer 2015). When mean and standard error (SE) were given, we calculated the standard deviation (SD) as given in the Cochrane Handbook for Systematic Reviews of Interventions Section 7.7.3.3 (Higgins 2011). In split‐mouth trials, mean difference (MD) and SE were calculated using the MD as described in the Handbook Section 16.4.6.3 (Higgins 2011). When mean and P value were given, we calculated SD according to the methods described in the Handbook Section 7.7.3.3 (Higgins 2011). When median and interquartile range were given, we used the data to calculate mean and SD according to the methods described in the Handbook Section 7.7.3.5 (Higgins 2011).
Assessment of heterogeneity
We assessed heterogeneity by visually inspecting the forest plots to determine closeness of point estimates with each other and overlap of CIs. We used the Chi² test with a P value of 0.1 to indicate statistical significance. We also used the I² statistic, following the interpretation recommended in the Cochrane Handbook for Systematic Reviews of Interventions Section 9.5 (0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity) (Higgins 2011).
Assessment of reporting biases
If we had included 10 or more studies, we would have constructed a funnel plot to investigate any potential reporting bias; we could not assess reporting bias, however, as none of the analyses had included 10 or more studies.
Data synthesis
We analysed the data using Review Manager 5 (Review Manager 2014). We analysed RCTs and CCTs separately. In the absence of substantial clinical or methodological heterogeneity, we performed a meta‐analysis using a random‐effects model. Where there was substantial or considerable heterogeneity identified by Chi² and I² tests, we investigated it using a subgroup analysis where possible. We used the generic inverse variance method when including split‐mouth studies.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity by performing the following subgroup analyses.
Type of AGP (e.g. ultrasonic and sonic scaling, tooth preparation using air turbine handpiece or air abrasion, three‐way syringe)
Type of clinical set‐up (e.g. single chair, polyclinic, operating theatre for minor oral surgery)
Types of filters used in HEPA (e.g. type A, B, C, D, E or F)
Procedure performed in anterior teeth or posterior teeth
Biological assessment used (CFU, fluorescent‐dye‐stained splatter)
We had insufficient data to conduct these analyses, however.
Sensitivity analysis
To explore the possible effect of losses to follow‐up on the effect estimates for the primary outcomes, we planned to perform sensitivity analyses. For dichotomous outcomes, we planned to vary the event rate within the missing participants from intervention and control groups within plausible limits. However, we did not find such data in the included studies.
For continuous outcomes, we performed sensitivity analyses for assumptions that we made in our analyses where we imputed SD or SE using P value or data obtained from graphs. We removed those studies at high risk of bias or CCTs and found no significant difference between the results of these analyses.
Summary of findings and assessment of the certainty of the evidence
We summarised the results of the analyses in 'Summary of findings' tables for the primary outcomes for all comparisons. We used the GRADE framework to evaluate the certainty of evidence for each outcome as high, moderate, low or very low (GRADEpro GDT), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We justified all decisions to downgrade the certainty of the evidence in footnotes.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies and Studies awaiting classification.
Results of the search
We retrieved 1134 references in total from the electronic database search. We also identified a further 11 studies via a non‐systematic search of Google Scholar, personal contacts, cross‐references of included studies and related systematic reviews. After removing duplicates, we screened 887 references by title and abstract, and excluded 860. Based on the full text, we excluded five studies. We could not get the required data of two studies and two were in pre‐print stage and hence await classification. We identified two ongoing trials. The remaining 16 studies met the inclusion criteria for this review (Figure 2).
2.
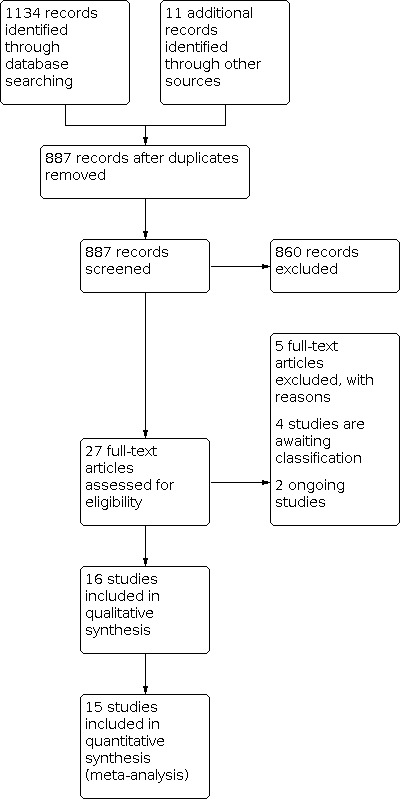
Study flow diagram.
Included studies
We included 16 trials in the review.
Characteristics of trial settings and investigators
All trials were in English. Fifteen trials were published in peer‐reviewed journals. One trial was published as a poster (Frere 2016).
Countries of origin
Six trials were from the USA (Cochran 1989; Frere 2016; Holloman 2015; King 1997; Muzzin 1999; Williams 1970); five trials from India (Desarda 2014; Devker 2012; Jawade 2016; Narayana 2016; Sethi 2019); two from the UK (Hallier 2010; Samaranayake 1989); one from Egypt (El‐Din 1997); one from the Netherlands (Timmerman 2004); and one from the United Arab Emirates (Al‐amad 2017).
Funding
Three trials were funded by private companies whose products were tested (Cochran 1989; Holloman 2015; Muzzin 1999). Williams 1970 was government funded. Six trials did not disclose any funding details or conflict of interest and the remaining six trials reported that they had not received any funding and had no conflicts of interest (Desarda 2014; El‐Din 1997; Hallier 2010; King 1997; Samaranayake 1989; Timmerman 2004).
Trial design
Eleven studies were RCTs (Al‐amad 2017; Cochran 1989; Desarda 2014; El‐Din 1997; Frere 2016; Holloman 2015; Jawade 2016; King 1997; Muzzin 1999; Sethi 2019; Timmerman 2004); and five were CCTs (Devker 2012; Hallier 2010; Narayana 2016; Samaranayake 1989; Williams 1970).
Five studies used parallel‐arm design (Al‐amad 2017; ; Holloman 2015; Jawade 2016; Samaranayake 1989; Sethi 2019), one of which was a CCT (Samaranayake 1989). Eleven studies used split‐mouth design (Cochran 1989; Desarda 2014; Devker 2012; El‐Din 1997; Frere 2016; Hallier 2010; King 1997; Muzzin 1999; Narayana 2016; Timmerman 2004; Williams 1970), four of which were CCTs (Devker 2012; Hallier 2010; Narayana 2016; Williams 1970).
Frere 2016 was a simulated trial and Cochran 1989 had both actual cavity preparation and simulation procedures. The remaining 14 were trials conducted on patients undergoing at least one of the AGPs.
Trial arms
Of the six studies using parallel‐arm design: Jawade 2016 and Sethi 2019 had three arms; Al‐amad 2017, Holloman 2015 and Samaranayake 1989 had two arms.
Of the 10 studies using a split‐mouth design: El‐Din 1997 and Hallier 2010 had four arms; Devker 2012, Frere 2016 and Narayana 2016 had three arms; Desarda 2014; King 1997, Muzzin 1999, Timmerman 2004 and Williams 1970 had two arms. Cochran 1989 had two phases and each phase had two arms.
Sample size
The minimum sample size was one (Frere 2016); and the maximum sample size was 80 (Desarda 2014). Though the sample size of Frere 2016 was one, the experiment was repeated 36 times on the same patient. None of the included studies mentioned the sample size calculation or power of the study.
AGP procedures tested
Nine studies tested interventions during ultrasonic scaling procedures (Desarda 2014; Devker 2012; Holloman 2015; Jawade 2016; King 1997; Narayana 2016; Sethi 2019; Timmerman 2004; Williams 1970). Three studies tested during restorative procedures (Al‐amad 2017; El‐Din 1997; Samaranayake 1989). One study tested during air polishing (Muzzin 1999); one study tested during high‐speed water spray (Frere 2016). One study tested during restorative and high‐speed water spray procedures (Cochran 1989); and another study during restorative procedures and ultrasonic scaling (Hallier 2010).
Clinical set‐up
Eleven studies used closed operatory separate from other clinical facilities for testing the interventions (Cochran 1989; Desarda 2014; Holloman 2015; Jawade 2016; King 1997; Muzzin 1999; Narayana 2016; Samaranayake 1989; Sethi 2019; Timmerman 2004; Williams 1970). One study used a partition measuring 2 m × 3 m in the pedodontics clinic (El‐Din 1997). One study used both large open multi‐chair clinical areas and a single‐chair closed operatory (Hallier 2010). Three studies did not mention any details of the clinical setup (Al‐amad 2017; Devker 2012; Frere 2016).
Fumigation of the operating room
Four studies fumigated the operatory used in the trial, before starting the procedure (Desarda 2014; Jawade 2016; Narayana 2016; Sethi 2019). Desarda 2014 used formalin and Narayana 2016 used formaldehyde and potassium permanganate crystals for fumigation. The other two studies did not report the details of the fumigation technique (Jawade 2016; Sethi 2019).
Dental unit waterlines
Three trials flushed water from the waterlines before starting the AGP, to reduce the biofilm present in the dental unit waterlines (Cochran 1989; Muzzin 1999; Sethi 2019). Other studies did not mention any details of flushing water from the waterlines before starting the AGPs.
In Cochran 1989, the handpiece and air‐water syringe lines were flushed for 30 seconds before each appointment, and then sprayed into sterile glass containers for 30 seconds. This water was subsequently quantitatively cultured for the presence of bacteria. In Sethi 2019, the ultrasonic unit was switched on and flushed for two minutes to get rid of contaminated water due to overnight stagnation in waterlines. In Muzzin 1999, the waterline of the air polisher was flushed for two minutes between each treatment.
Characteristics of participants
Age
The maximum participant age reported was 69 years (Timmerman 2004); the minimum was 5 years (El‐Din 1997). Two studies recruited only children (El‐Din 1997; Samaranayake 1989). Nine studies recruited adult participants only (Al‐amad 2017; Desarda 2014; Devker 2012; Holloman 2015; Jawade 2016; King 1997; Muzzin 1999; Sethi 2019; Timmerman 2004). The remaining four studies did not mention any details about the age group.
Sex
One trial recruited only female participants (Al‐amad 2017). Five studies recruited both male and female participants (Timmerman 2004; Muzzin 1999; King 1997; Jawade 2016; Devker 2012). The remaining trials did not mention if study participants were male or female. Frere 2016 had one male participant.
Inclusion/exclusion criteria
Most of the included studies recruited medically healthy people (Desarda 2014; Devker 2012; Frere 2016; Holloman 2015; Jawade 2016; King 1997; Muzzin 1999; Narayana 2016; Sethi 2019); or people who were not taking any antibiotic treatment or with a recent history of antibiotic treatment (Cochran 1989; Desarda 2014; Devker 2012; Holloman 2015; Jawade 2016; King 1997; Muzzin 1999; Sethi 2019; Timmerman 2004).
However, five studies did not mention any such inclusion/exclusion criteria (Al‐amad 2017; El‐Din 1997; Hallier 2010; Samaranayake 1989; Williams 1970).
Characteristics of intervention
1. HVE versus no HVE
Five split‐mouth trials tested HVE versus no HVE during AGPs for reduction in the contamination of aerosols. Three were RCTs (Desarda 2014; King 1997; Muzzin 1999) and two were CCTs (Devker 2012; Narayana 2016).
Desarda 2014 used HVE with a stainless‐steel tip of 12 mm diameter during ultrasonic scaling of maxillary incisors and canines. Reduction in contamination of aerosols was tested using nutrient agar plates for bacterial colonies at 12 and 20 inches (~ 30 and 50 cm) from the patient’s mouth.
King 1997 tested the effectiveness of HVE on reducing contamination of aerosols during ultrasonic scaling. They used an aerosol reduction device (a modified HVE) by attaching it to the ultrasonic scaling unit. One side (maxillary and mandibular) of the participant's mouth was scaled by using a magnetostrictive ultrasonic scaler without the aerosol reduction device (control), and the opposing side was scaled by using the ultrasonic scaler with the aerosol reduction device (intervention). The outcome measure of bacterial CFU was measured at six inches from the participant’s mouth and on the dentist's face shield.
Muzzin 1999 scaled one side (maxillary and mandibular) of the participant's mouth using an air polisher without the aerosol reduction device (control), and the opposing side an air polisher was used with the aerosol reduction device (intervention). Reduction in contamination of aerosols was tested using bacterial CFU in blood agar plates at 12 inches from the participant's mouth and on the operator's face mask.
In their multi‐arm split‐mouth CCT, Devker 2012 tested the use of HVE during ultrasonic scaling. Oral prophylaxis was done on a randomly selected side (control side) for a period of 10 minutes. After a gap of 30 minutes, a high‐volume suction tip was tied to the ultrasonic scaler. Oral prophylaxis was done on the other side (test side) of the same arch with high‐volume suction for a period of 10 minutes. Following the 10‐minute sampling period, blood agar plates were taken off. Reduction in contamination of aerosols was tested using blood agar plates for bacterial colonies at 6 inches from operator’s nose level, 6 inches from assistant’s nose level, 12 inches from participant’s chest level and 36 inches from participant’s right side. We did not consider other arms of this study as preprocedural rinse was used.
Narayana 2016 conducted a multi‐arm split‐mouth CCT to check the effectiveness of HVE and preprocedural rinse during supragingival ultrasonic scaling. Ultrasonic scaling was performed on first and fourth quadrants without using HVE and second and third quadrants with HVE to check for reduction in contamination of aerosols. The bacterial CFU was checked using blood agar plates placed on the left side of the participant at the dental assistant position.
2. HVE versus conventional dental suction (low‐volume evacuator (LVE))
One study (a split‐mouth RCT) evaluated this comparison (Timmerman 2004). Ultrasonic scaling was performed in patients with generalized adult periodontitis with HVE (intervention) or conventional dental suction (LVE). Two blood agar Petri dishes were placed at a distance of 40 cm and 150 cm from the participant's mouth to check for aerobic and anaerobic CFU.
3. Combination system versus saliva ejector (LVE)
One study (a parallel RCT) compared the effectiveness of a combination system (Isolite) and a traditional saliva ejector (Holloman 2015). It evaluated the reduction in the level of contamination in aerosols produced during simulated occlusal surface preparation with a high‐speed turbine handpiece. A combination system provides isolation of two quadrants simultaneously, illumination, continuous HVE, retraction of tissues, protection of airway and a comfortable way for the patient to keep their mouth open. The outcome was measured by bacterial CFU, which was collected by Dulbecco phosphate‐buffered saline (DPBS) solution during and after ultrasonic scaling and sent for aerobic and anaerobic bacterial culture. The DPBS Petri dish was placed centrally, six inches from the oral cavity.
4. Combination system versus rubber dam + HVE, and 5. Combination system versus HVE
One study compared the effectiveness of combination system (Isolite) with rubber dam plus HVE and HVE alone (Frere 2016). This was an 'n of 1' trial where a single male participant was recruited and 12 trials were conducted for each comparison (total of 36 trials). The sides of mouth were randomised to receive the intervention or control. Simulated occlusal preparation was the AGP and the outcome measured was the bacterial CFU obtained from five blood agar plates that were placed at standardized positions around the participant for each trial.
6. Rubber dam versus no rubber dam
Three studies assessed the efficacy of rubber dam to check the reduction in contaminated aerosols. Al‐amad 2017 evaluated the outcome from the head scarves of the female dental students who were performing restorative procedures. El‐Din 1997 and Samaranayake 1989 evaluated the outcome in children undergoing restorative procedures.
Al‐amad 2017 studied the effect of rubber dam on atmospheric bacterial aerosols during restorative procedures in a parallel‐arm RCT. The outcomes were measured during the dental cavity preparations on posterior teeth of patients by female dental students. The sampling for bacterial contamination was done from scarves of these students in forehead, left ear, submental triangle and occipital regions and bacterial CFU were counted in trypticase soy agar culture medium.
El‐Din 1997 conducted a four‐arm RCT where each arm had a split‐mouth design. The objective of this trial was to the check the effectiveness of preprocedural mouthrinse with chlorhexidine and rubber dam isolation for restorative procedures in children. Reduction in contamination of aerosols was measured equidistantly from the child's head—one each on the chest, on the left and right sides and behind the patient, one metre and two metres from the head‐rest of the dental chair—in blood agar culture medium. We have used data from 'rubber dam' and 'no rubber dam' groups only.
Samaranayake 1989 tested the efficacy of rubber dam in a clinical trial on children undergoing restorative procedures. The outcome measure was reduction in contamination of aerosols measured in bacterial CFU in blood agar medium placed at one metre, two metres and three metres from the head rest.
7. Rubber dam + HVE versus cotton roll + HVE
Cochran 1989 conducted an RCT with a split‐mouth design to check the effectiveness of rubber dam in reducing contaminated aerosols during restorative and high‐speed spraying procedures using handpiece and air‐water syringe. The outcome measured was bacterial CFU collected in Petri dishes containing agar (MM10), which was assessed at the dental unit light and patient’s chest area.
8. Air cleaning system versus no air cleaning system
One split‐mouth CCT evaluated the efficacy of an air cleaning system in reducing the contaminated aerosols (Hallier 2010). The study included dental‐aerosol‐generating and non‐aerosol‐generating procedures such as history and intraoral examination, ultrasonic scaling, cavity preparation using a high‐speed dental handpiece and tooth extraction under local anaesthesia performed in a closed operatory and multi‐dental chair clinic. The outcome was assessed in terms of bacterial CFU cultured in blood agar plates.
9. Laminar air on with HEPA versus laminar air off
One split‐mouth CCT evaluated the reduction in volume of contaminated aerosols (viable particles) and the reduction in level of contamination in aerosols during ultrasonic scaling procedures (Williams 1970).
10. Chlorhexidine coolant versus distilled water coolant, and 11. Chlorhexidine coolant versus cinnamon extract or povidone iodine coolant
Jawade 2016 evaluated two different ultrasonic liquid coolants on dental aerosols in a 3‐arm parallel RCT. One group underwent ultrasonic scaling with 2% povidone iodine in 0.1% solution as a coolant and the other group with 0.12% chlorhexidine in 0.06% dilution. The outcome assessed was reduction in bacterial contamination of aerosols measured as CFU in blood agar plates placed at 0.4 metres on right and left side and 2 metres behind the patient.
In a 3‐arm parallel‐design RCT conducted by Sethi 2019, chlorhexidine coolant was compared with cinnamon extract coolant during ultrasonic scaling procedures. Reduction in contamination of aerosols (measured at a distance of one foot (~ 30 cm) from mouth to patient’s chest, right side and left side) was measured as bacterial CFU in blood agar plates.
Both also compared chlorhexidine with distilled water as coolant during ultrasonic scaling procedures (Jawade 2016; Sethi 2019).
Methods of outcome measurement
None of the included studies evaluated our primary outcome 'Incidence of infection of dental staff or patients'. Nor did they evaluate our secondary outcomes of costs for the interventions used, and acceptability and feasibility of the intervention to patients and dentists.
All trials measured reduction in the contamination of aerosols using CFU.
One trial measured the reduction in contaminated aerosols per volume of air at about 1.5 metres from the floor and at about 20 cm to 30 cm from the patient's mouth and related to the size of the particles in the aerosol using impactors (Reyniers slit samplers and Andersen cascade samplers) and gravimetric settling plates and Rodac contact plates (Williams 1970). Another trial used an air suction pump connected to a Petri dish to measure the contamination per volume of air (Hallier 2010). All other trials used gravity sampling only to measure the level of aerosol contamination.
The type of culture medium used varied between the studies. Nine studies used blood agar medium for aerobic culture and measurement of CFU (Devker 2012; El‐Din 1997; Frere 2016; Hallier 2010; Jawade 2016; Muzzin 1999; Narayana 2016; Samaranayake 1989; Sethi 2019). Three studies used other culture media such as trypticase soy agar (Al‐amad 2017), agar (MM10) (Cochran 1989), and nutrient agar (Desarda 2014) for aerobic culture. Four studies used two culture media (Holloman 2015; King 1997; Timmerman 2004; Williams 1970), and two of these studies cultured both aerobic and anaerobic bacteria (Holloman 2015; Timmerman 2004). Holloman 2015 used Brucella agar (solid) and Petri dishes containing 20 mL of sterile Dulbecco phosphate‐buffered saline (DPBS) solution (liquid) for both aerobic and anaerobic culture. King 1997 used blood agar and Replicate organism detection and counting (RODAC) plates with trypticase soy agar, lecithin and polysorbate 80 for aerobic culture. Timmerman 2004 used brain heart infusion agar with 5% horse blood and one pair of plates was cultured aerobically, the other pair anaerobically. Williams 1970 used gravimetric agar settling plates and RODAC agar contact plates with blood agar growth medium incubated for aerobic culture.
Excluded studies
We excluded five studies based on the full‐text articles: Watanabe 2018 tested contamination pattern on PPE; Bentley 1994 tested which dental procedure produces more aerosols when an aerosol‐reducing device is used; Yamada 2011 did not have a control group; Muir 1978 used preprocedural rinse in all groups; and Larato 1967 used preprocedural antiseptic mouthrinse with no control group. See Characteristics of excluded studies.
Studies awaiting classification
Four studies are awaiting classification. We could not obtain required data of two studies (Worrall 1987; Klyn 2001); the other two studies were shared by the author (James Allison) and are still in pre‐print stage (Allison 2020; Llandro 2020). See Characteristics of studies awaiting classification.
Ongoing studies
We identified two ongoing studies that met the inclusion criteria for this review (ISRCTN10378358; NCT04430387).
Risk of bias in included studies
We assessed three RCTs at high risk of bias and eight RCTs as unclear because they each had at least two risk of bias domains that we judged to be unclear (Figure 3; Figure 4). We assessed the five CCTs at high risk of bias.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
RCTs
Only six RCTs gave the details of random sequence generation (Al‐amad 2017; Desarda 2014; Frere 2016; Holloman 2015; Jawade 2016; Sethi 2019) and only Sethi 2019 provided the details of allocation concealment (personal communication), which we assessed to have low risk of bias. The other five RCTs did not provide any information on allocation and thus we assessed them to have unclear risk of bias. Al‐amad 2017 mentioned that they had not concealed the allocation (personal communication) and was assessed as high risk of bias. The remaining five RCTs did not give any details on how random sequence was generated and thus we assessed them as unclear risk of bias.
CCTs
All CCTs are at high risk of selection bias. Four CCTs provided no information about confounding and selection bias (Devker 2012; Hallier 2010; Narayana 2016; Samaranayake 1989), while Williams 1970 stated that alternation was used to allocate participants to groups.
Blinding
None of the 16 included studies described if the participants and personnel were blinded or if blinding was possible. This could have not affected the performance, however, and thus we assessed these studies as low risk of bias. All 16 studies used bacterial CFU as the outcome measure; the studies that used assessor blinding or automated colony counters we assessed as having low risk of bias while we assessed the study using manual colony counting without assessor blinding as having high risk of bias. We assessed one study as having high risk of detection bias (Cochran 1989); and seven studies as having low risk of detection bias (Desarda 2014; Devker 2012; Frere 2016; Holloman 2015; Jawade 2016; King 1997; Muzzin 1999). The remaining studies had unclear risk of bias.
Incomplete outcome data
We assessed nine of the included studies at low risk of bias due to incomplete outcome data (Cochran 1989; El‐Din 1997; Frere 2016; Hallier 2010; Holloman 2015; King 1997; Samaranayake 1989; Timmerman 2004; Williams 1970). One study had excluded 16 readings because those readings were outliers and hence we assessed this as high risk of bias (Al‐amad 2017). However, the remaining six studies had no dropouts and thus we assessed them to have low risk of bias (Desarda 2014; Devker 2012; Jawade 2016; Muzzin 1999; Narayana 2016; Sethi 2019).
Selective reporting
Only one study had a registered study protocol and all the intended outcomes were reported (Frere 2016). We assessed the remaining 15 studies to have unclear risk of bias as no protocols were provided and we were not sure if all the planned outcomes were reported.
Other potential sources of bias
We assessed one study at high risk of bias because we were not sure if the quantity of bacterial colonisation would be the same after one hour (washout period) between each trial (Frere 2016), which could affect the CFU in the aerosols. We could not find any other relevant bias in any of the other 15 included studies and thus assessed them as being at low risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We present the effects of intervention under 11 comparisons for one outcome only.
1. High‐volume evacuation (HVE) versus no HVE
Five studies tested the reduction in contamination of aerosols under this comparison and the culture plates were placed at a distance of less than one foot (~ 30 cm) (Desarda 2014; Devker 2012; King 1997; Muzzin 1999; Narayana 2016). The trials were split‐mouth studies and hence we used the generic inverse variance (GIV) method to analyse the data, using mean differences and standard error. Two studies checked for any reduction in the contamination of aerosols on the operator’s face shield/mask (King 1997; Muzzin 1999). Another two studies evaluated the same outcome at more than one‐foot distance from the patient's oral cavity (Desarda 2014; Devker 2012). Narayana 2016 evaluated the contamination of aerosols during ultrasonic scaling procedures at a distance of less than one foot from patient's oral cavity. We separately analysed the data from RCTs and CCTs according to the guidance for combining studies (section 24.6.2.1) in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
We imputed the data from graph in Desarda 2014 and calculated SD using the mean and P value of 0.01 in Devker 2012 (Table 5).
1. Data imputed.
| Study ID | Outcome | Data imputed from | Mean/median (intervention arm) | SD | IQR (from graph) | Mean/median (control arm) | SD | IQR (from graph) | Statistical test used for comparison |
| Desarda 2014 (RCT ‐ split mouth) | Less than 1 ft | Graph | 12.5 | 3.5 | ‐‐ | 13.5 | 5.75 | ‐‐ | Student t test |
| More than 1 ft | 11.5 | 4.5 | ‐‐ | 12.5 | 5.5 | ‐‐ | |||
| Devker 2012 (RCT ‐ split mouth) | Less than 1 ft | SD calculated from mean and P value of 0.01 | 18.13 | 176.41 | ‐‐ | 107.13 | 176.41 | ‐‐ | Paired t test |
| More than 1 ft | 7.3 | 26.88 | ‐‐ | 20.86 | 26.88 | ‐‐ | |||
| Hallier 2010 (CCT ‐ split‐mouth design) | Cavity preparation | IQR is imputed from the graph. SD = IQR/1.35 (6.5.2.5, Higgins 2019) |
38.4 | 10.2 (imputed) | 34.5 48.3 |
105.1 | 37.19 (imputed) | 82.8 133 |
Wilcoxon signed rank test |
| Ultrasonic scaling | 38.5 | 6.15 (imputed) | 36 44.3 |
70.9 | 12.37 (imputed) | 62.1 78.8 |
|||
| Williams 1970 (CCT ‐ split mouth) | At 30 inches (approx 76 cm) from floor using Reynier slit sampler | Borrowed 80.67 and 2.6 from Larato 1967 and multiplied with the ratio of the outcomes in both studies to get an SD related to the size of the effect | 0.6* (*calculated from the data given in table 1 of the article) | 239.8 (imputed) | ‐‐ | 319.74* | 239.8 (imputed) | ‐‐ | ‐‐ |
| At 8 to 12 inches (20 to 30 cm) from participant's mouth using Andersen sampler | 2.04* | ‐‐ | 485.6* | ‐‐ | ‐‐ |
We summarised the key results in Table 1.
Reduction in contamination of aerosols at less than one‐foot distance (~ 30 cm) from patient’s oral cavity ‒ RCTs (Analysis 1.1): three studies reported this outcome for the comparison between HVE and no HVE. The meta‐analysis suggested a benefit from HVE (MD in CFU −47.41, 95% −92.76 to −2.06; 122 participants), but there was very high heterogeneity that we could not explain (I² = 95%).
Reduction in contamination of aerosols at less than one foot from patient’s oral cavity ‒ CCTs (Analysis 1.1): two split‐mouth studies reported this outcome. The effect estimates showed reduction in the level of contamination of aerosols in the HVE group but with wide confidence intervals crossing the line of no effect (MD −50.19, 95% CI −109.71 to 9.33; 2 studies, 45 participants).
Reduction in contamination of aerosols on operator’s face shield/mask (Analysis 1.1): two split‐mouth RCTs reported this outcome comparing HVE and no HVE. The meta‐analysis suggested a benefit from HVE (MD in CFU −15.71, 95% −46.37 to −14.95; 42 participants), but there was very high heterogeneity that we could not explain (I² = 95%).
Reduction in contamination of aerosols at more than one foot from patient’s oral cavity ‒ RCT (Analysis 1.1): one study reported this outcome comparing HVE and no HVE (Desarda 2014). The mean difference was small and the confidence interval crossed the line of no effect (MD −1.00, 95% CI −2.56 to 0.56; 80 participants).
Reduction in contamination of aerosols at more than one foot from patient’s oral cavity ‒ CCT (Analysis 1.1): one study reported this outcome comparing HVE and no HVE (Devker 2012). The mean difference showed reduction in contamination of aerosols in the HVE group (MD −13.56, 95% CI −23.18 to −3.94; 30 participants).
1.1. Analysis.

Comparison 1: High‐volume evacuation (HVE) versus no HVE, Outcome 1: Reduction in the level of contamination in aerosols
2. HVE versus conventional dental suction
One study of six participants evaluated reduction in contamination of aerosols by HVE compared to dental suction (Timmerman 2004). The effect estimates showed reduction in contamination of aerosols in the HVE group with wide confidence intervals crossing the line of no effect at 40 cm from the patient's mouth (MD −2.30, 95% CI −5.32 to 0.72) and 150 cm distance from the patient's mouth (MD −2.20, 95% CI −14.01 to 9.61) (Analysis 2.1). See Table 2.
2.1. Analysis.

Comparison 2: HVE versus conventional dental suction, Outcome 1: Reduction in the level of contamination in aerosols
3. Combination system versus saliva ejector
One study (50 participants) evaluated reduction in contamination of aerosols under this comparison (Holloman 2015). The study described the results as mean log10 values and we decided to use the log10 data in the analysis. The outcome was evaluated during and after AGP and hence we did subgroup analysis. The effect estimates showed reduction in contamination of aerosols in the combination system group with wide confidence intervals crossing the line of no effect in both the subgroups (MD −0.31, 95% CI −0.82 to 0.20 and MD −0.35, 95% CI −0.99 to 0.29, respectively) (Analysis 3.1). See Table 6.
3.1. Analysis.

Comparison 3: Combination system versus saliva ejector, Outcome 1: Reduction in the level of contamination in aerosols
2. Comparison 3. Combination system compared to saliva ejector for reduction in the level of contamination in aerosols.
| Combination system compared to saliva ejector for reduction in the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed dental operatory Intervention: combination system (Isolite) Comparison: saliva ejector | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with saliva ejector | Risk with combination system | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols during ultrasonic scaling |
The mean CFU level was 3.61 | MD 0.31 CFU lower (0.82 lower to 0.20 higher) | ‐ | 50 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | Mean data given in Log10 and the same was used in the analysis. |
|
Reduction in contamination of aerosols after ultrasonic scaling |
The mean CFU level was 2.00 | MD 0.35 CFU lower (0.99 lower to 0.29 higher) | ‐ | 50 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 2 levels for imprecision due to small sample size reported in a single study and 1 level for unclear risk of selection and reporting bias
4. Combination system versus rubber dam + HVE
One 'n of one' trial compared a combination system with the traditional rubber dam plus HVE (Frere 2016). It was a split‐mouth trial so we used the GIV method to analyse the data using mean differences and standard error. The effect estimates show better reduction in contamination of aerosols in the combination system group compared to the rubber dam plus HVE group (MD −125.20, 95% CI −174.02 to −76.38; 1 study (24 trials), 1 participant) (Analysis 4.1). See Table 7.
4.1. Analysis.

Comparison 4: Combination system versus rubber dam + HVE, Outcome 1: Reduction in the level of contamination in aerosols
3. Comparison 4. Combination system compared to rubber dam + HVE for reduction in the level of contamination in aerosols.
| Combination system compared to rubber dam + HVE for reduction in the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: dental clinic Intervention: combination system Comparison: rubber dam + HVE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of sites (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with rubber dam + HVE | Risk with combination system | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols during water spray using high speed handpiece |
The mean CFU level was 133.70 | MD 125.20 CFU lower (174.02 lower to 76.38 lower) | ‐ | 24 ('n of 1' design) (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 2 levels for unclear risk of selection bias and high risk of other bias, and 2 levels for imprecision due to small sample size reported in a single study
5. Combination system versus HVE
One 'n of one' trial compared a combination system with HVE. Since this was a split‐mouth trial, we used the GIV method to analyse the data using mean differences and standard error. The effect estimates show better reduction in contamination of aerosols in the combination system group than in the HVE group (MD −109.30, 95% CI −153.01 to −65.59; 1 study (24 trials), 1 participant) (Frere 2016) (Analysis 5.1). See Table 8.
5.1. Analysis.

Comparison 5: Combination system versus HVE, Outcome 1: Reduction in the level of contamination in aerosols
4. Comparison 5. Combination system compared to HVE for reduction in the level of contamination in aerosols.
| Combination system compared to HVE for reduction in the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: dental clinic Intervention: combination system Comparison: HVE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of sites (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with HVE | Risk with combination system | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols during water spray using high speed handpiece |
The mean CFU level was 117.80 | MD 109.30 CFU lower (153.01 lower to 65.59 lower) | ‐ | 24 ('n of 1' design) (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 2 levels for unclear risk of selection bias and high risk of other bias, and 2 levels for imprecision due to small sample size reported in a single study
6. Rubber dam versus no rubber dam
Three studies tested the efficacy of using rubber dam in reducing contaminated aerosols at different distances (Al‐amad 2017; El‐Din 1997; Samaranayake 1989). We analysed data based on the study design (RCT or CCT) and based on regions: namely, one metre from participant’s mouth, two metres from participant’s mouth, on operator’s forehead, left ear, submental triangle and occipital region (Analysis 6.1). See Table 3.
6.1. Analysis.

Comparison 6: Rubber dam versus no rubber dam, Outcome 1: Reduction in the level of contamination in aerosols
We used the data from personal communication in Al‐amad 2017 (Table 9).
5. Al‐amad 2017 data ‐ personal communication.
| Parallel‐arm trial | With rubber dam | Without rubber dam |
P value (student t test) |
| Mean CFU (SD) | Mean CFU (SD) | ||
| Point A (forehead) | 2.7014 (2.75) | 1.7165 (3.26) | 0.263 |
| Point B (left ear) | 2.1599 (2.02) | 1.200 (1.53) | 0.071 |
| Point C (submental triangle) | 1.2828 (1.36) | 0.7614 (0.72) | 0.110 |
| Point D (occiput) | 2.2064 (2.24) | 1.4400 (2.04) | 0.223 |
At one metre from participant’s mouth (RCT): one RCT investigated contaminated aerosols at one metre (El‐Din 1997). The effect estimates show fewer CFU in the rubber dam group compared to the no rubber dam (control) group (MD −16.20, 95% CI −19.36 to −13.04; 10 participants; Analysis 6.1).
At one metre from participant’s mouth (CCT): one CCT investigated contaminated aerosols at one metre (Samaranayake 1989). The effect estimates show fewer CFUs in the rubber dam group compared to the no rubber dam (control) group (MD −10.10, 95% CI −19.72 to −0.48; 20 participants; Analysis 6.1).
At two metres from participant’s mouth (RCT): one RCT investigated contaminated aerosols at two metres (El‐Din 1997). The effect estimates show fewer CFU in the rubber dam group compared to the no rubber dam (control) group (MD −11.70, 95% CI −15.82 to −7.58; 10 participants; Analysis 6.1).
At two metres from participant’s mouth (CCT): one CCT investigated contaminated aerosols at two metres (Samaranayake 1989). The effect estimates show fewer CFU in the rubber dam group compared to the no rubber dam (control) group (MD −2.80, 95% CI −4.65 to −0.95; 20 participants; Analysis 6.1).
At operator's forehead: one RCT investigated contaminated aerosols on the operator's forehead (Al‐amad 2017). The effect estimates show more CFU in the rubber dam group compared to no rubber dam (control) group with the confidence intervals crossing the line of no effect (MD 0.98, 95% CI −0.73 to 2.70; 47 participants; Analysis 6.1).
At operator's left ear: one RCT investigated contaminated aerosols on the operator's left ear (Al‐amad 2017). The effect estimates show more CFU in the rubber dam group compared to no rubber dam (control) group with the confidence intervals crossing the line of no effect (MD 0.96, 95% CI −0.08 to 2.00; 47 participants; Analysis 6.1).
At operator's submental region: one RCT investigated contaminated aerosols on the operator's submental region (Al‐amad 2017). The effect estimates show more CFU in the rubber dam group compared to no rubber dam (control) group with the confidence intervals crossing the line of no effect (MD 0.52, 95% CI −0.11 to 1.16; 47 participants; Analysis 6.1).
At operator's occipital region: one RCT investigated contaminated aerosols on the operator's occipital region (Al‐amad 2017). The effect estimates show more CFU in the rubber dam group compared to no rubber dam (control) group with the confidence intervals crossing the line of no effect (MD 0.77, 95% CI −0.46 to 2.00; 47 participants; Analysis 6.1).
7. Rubber dam + HVE versus cotton roll + HVE
One study compared the efficacy of rubber dam plus HVE with cotton roll and HVE in reducing the contamination of aerosols on the participant’s chest region and on the dental unit light (Cochran 1989). The effect estimates show better reduction in contamination of aerosols in rubber dam plus HVE intervention at either distance (MD −251.00, 95% CI −267.95 to −234.05; 1 study, 16 participants; and MD −12.70, 95% CI −12.85 to −12.55; 1 study, 16 participants, respectively; Analysis 7.1). See Table 4.
7.1. Analysis.

Comparison 7: Rubber dam + HVE versus cotton roll + HVE, Outcome 1: Reduction in the level of contamination in aerosols
8. Air cleaning system (ACS) versus no ACS
One CCT tested the efficacy of using ACS in reducing contaminated aerosols during cavity preparation and ultrasonic scaling (Hallier 2010). The study has described the results as mean in tables and IQR value in form of graphs. Hence we derived the interquartile range from the graph using PlotDigitizer software (PlotDigitizer 2015); and calculated the SD according to section 6.5.2.5 of the Handbook (Higgins 2019) (Table 5). We analysed the data under different subgroups for each procedure. The effect estimates show fewer CFU in ACS group for both the procedures with wide confidence intervals (MD −66.70, 95% CI −120.15 to −13.25; 2 participants; and MD −32.40, 95% CI −51.55 to −13.25; 2 participants, respectively; Analysis 8.1). See Table 10.
8.1. Analysis.

Comparison 8: Air‐cleaning system (ACS) versus no ACS, Outcome 1: Reduction in the level of contamination in aerosols
6. Comparison 8. Air cleaning system (ACS) compared to no ACS for reduction in the level of contamination in aerosols.
| Air cleaning system (ACS) compared to no ACS for reduction in the level of contamination in aerosols | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed dental operatory and open clinical area Intervention: air cleaning system (ACS) Comparison: no ACS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of sites (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no ACS | Risk with air cleaning system (ACS) | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols during cavity preparation |
The mean CFU level was 105.10 | MD 66.70 CFU lower (120.15 lower to 13.25 lower) | ‐ | 4 (1 CCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in level of contamination in aerosols during ultrasonic scaling |
The mean CFU level was 70.9 | MD 32.40 CFU lower (51.55 lower to 13.25 lower) | ‐ | 4 (1 CCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Begins at 'low' as non‐randomised evidence. Downgraded 1 level for unclear risk of detection bias and reporting bias and 2 levels for imprecision due to small sample size reported in a single study
9. Laminar air flow with HEPA filter versus without flow or filter
One CCT investigated the effectiveness of using laminar air flow with HEPA filter during ultrasonic scaling (Williams 1970). The effect estimates show reduction in the level of contamination in aerosols (fewer CFU) during the use of laminar air flow with HEPA filters compared to no laminar air flow or filter at less than 1 metre from the floor (MD −483.56, 95% CI −550.02 to −417.10; 50 participants; Analysis 9.1) and 20 cm to 30 cm from patient's mouth (MD −319.14, 95% CI −385.60 to −252.68; 50 participants; Analysis 9.1). Under this comparison, reduction in the volume of contaminated aerosols was also noted in the intervention group. We are unable to analyse the results, however, due to missing data. See Table 11.
9.1. Analysis.

Comparison 9: Laminar air flow with HEPA filter versus without flow or filter, Outcome 1: Reduction in the level of contamination in aerosols (CFU per cubic feet/minute/patient)
7. Comparison 9. Laminar air flow with HEPA filter compared to without flow or filter for preventing infectious diseases.
| Laminar air flow with HEPA filter compared to without flow or filter for preventing infectious diseases | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed operatory Intervention: Laminar air flow with HEPA filter Comparison: without flow or filter | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of particpants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with without flow or filter | Risk with Laminar air flow with HEPA filter | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols (CFU per cubic feet/minute/patient) using Reyniers slit samplers 30 inches (76 cm) from the floor |
The mean CFU level was 319.74 | MD 319.14 CFU lower (385.60 lower to 252.68 lower) | ‐ | 50 (1 CCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in level of contamination in aerosols (CFUs per cubic feet/minute/patient) using Andersen cascade sampler placed 8 to 12 inches (20 to 30 cm) from patient's mouth |
The mean CFU level was 485.60 | MD 483.56 CFU lower (550.02 lower to 417.10 lower) | ‐ | 50 (1 CCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Begins at 'low' as non‐randomised evidence. Downgraded 1 level for unclear risk of detection and reporting bias, and 2 levels for imprecision due to small sample size in single study
10. Antimicrobial coolant versus control coolant
Two RCTs investigated the effectiveness of using chlorhexidine, povidone iodine and cinnamon extract coolants compared with water coolant (control) during ultrasonic scaling on the right side of the patient (Jawade 2016; Sethi 2019). Hence we did the subgroup analysis based on the antimicrobial coolant. The effect estimates show fewer CFU in antimicrobial coolant group compared to the control group (MD −124.00, 95% CI −135.78 to −112.22; 20 participants; Analysis 10.1); (MD −656.45, 95% CI −672.74 to −640.16; 40 participants; Analysis 10.1); and MD −644.55, 95% CI −668.70 to −620.40; 40 participants; Analysis 10.1), respectively. See Table 12.
10.1. Analysis.

Comparison 10: Antimicrobial coolant versus control coolant, Outcome 1: Reduction in the level of contamination in aerosols
8. Comparison 10. Antimicrobial coolant compared to control coolant for preventing infectious diseases.
| Antimicrobial coolant compared to control coolant for preventing infectious diseases | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed operatory Intervention antimicrobial coolant Comparison: control coolant | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control coolant | Risk with antimicrobial coolant | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols in the operative environment | Not reported | |||||
|
Reduction in level of contamination in aerosols when chlorhexidine coolant was compared to distilled water during ultrasonic scaling at right side of patient |
The mean CFU level was 165.30 | MD 124 CFU lower (135.78 lower to 112.22 lower) | ‐ | 20 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in level of contamination in aerosols when povidone iodine coolant was compared to distilled water during ultrasonic scaling at side of patient |
The mean CFU level was 1064.05 | MD 656.45 CFU lower (672.74 lower to 640.16 lower) | ‐ | 40 (1 RCT)b | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in level of contamination in aerosols when cinnamon coolant was compared to distilled water during ultrasonic scaling at right side of patient |
The mean CFU level was 1064.05 | MD 644.55 CFU lower (668.70 lower to 620.40 lower) | ‐ | 40 (1 RCT)b | ⊕⊝⊝⊝ VERY LOW 1 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 1 level for unclear risk of selection bias and reporting bias and 2 levels for imprecision due to small sample size reported in a single study
11. Antimicrobial coolant A versus antimicrobial coolant B
Two RCTs investigated the effectiveness of using chlorhexidine with povidone iodine coolant or cinnamon coolant during ultrasonic scaling (Jawade 2016; Sethi 2019). The effect estimates show fewer CFU on the right side of the participants in chlorhexidine group compared to the povidone iodine group (MD −59.30, 95% CI −64.16 to −54.44; 20 participants; Analysis 11.1). The effect estimates show fewer CFU on the right side of the participants in the chlorhexidine group compared to cinnamon group (MD −11.90, 95% CI −35.88 to 12.08; 40 participants; Analysis 11.1); however, the confidence intervals are crossing the line of no effect and thus the conclusions may not be robust. See Table 13.
11.1. Analysis.

Comparison 11: Antimicrobial coolant A versus antimicrobial coolant B, Outcome 1: Reduction in the level of contamination in aerosols
9. Comparison 11. Antimicrobial coolant A compared to antimicrobial coolant B for preventing infectious diseases.
| Antimicrobial coolant A compared to antimicrobial coolant B for preventing infectious diseases | ||||||
| Population: people undergoing aerosol generating procedures Setting: closed operatory Intervention: antimicrobial coolant A Comparison: antimicrobial coolant B | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with antimicrobial coolant B | Risk with antimicrobial coolant A | |||||
| Rate of infection of dental staff or patients | Not reported | |||||
| Reduction in volume of contaminated aerosols | Not reported | |||||
|
Reduction in level of contamination in aerosols when chlorhexidine coolant was compared to cinnamon coolant during ultrasonic scaling at right side of patient |
The mean CFU level was 419.50 | MD 11.90 CFU lower (35.88 lower to 12.08 higher) | ‐ | 40 (1 RCT)a | ⊕⊝⊝⊝ VERY LOW 1 | |
|
Reduction in level of contamination in aerosols when chlorhexidine coolant was compared to povidone iodine coolant during ultrasonic scaling at right side of patient |
The mean CFU level was 100.60 | was MD 59.30 CFU lower (64.16 lower to 54.44 lower) | ‐ | 20 (1 RCT)b | ⊕⊝⊝⊝ VERY LOW 2 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony forming units; CI: confidence interval; MD: mean difference; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1. Downgraded 1 level for unclear risk of detection and reporting bias, and 2 levels for imprecision due to small sample size reported in a single study
2. Downgraded 1 level for unclear risk of selection and reporting bias, and 2 levels for imprecision due to small sample size reported in a single study
Discussion
Summary of main results
We found 16 studies that evaluated 11 comparisons in this Cochrane Review. The studies did not measure reduction in infection rates. All included trials presented the results for reduction in contamination of aerosols (two measured the reduction in contamination in a volume of air and the other 14 used gravity sampling only). None of the 16 studies provided any data on costs or acceptability and feasibility of the intervention to patients and dentists.
We produced 'Summary of findings' tables for all comparisons. We could not find any COMET recommendations for the most important outcome measures (COMET 2020). We assessed certainty of the evidence for the reduction in contamination of aerosols: we found very low certainty evidence for all the interventions included in this review and therefore we cannot draw any robust conclusions.
Overall completeness and applicability of evidence
It is understandable that there are no studies with infection rates as outcomes: infection rates can only be measured during an epidemic. In general, it is difficult to conduct studies during an epidemic because all resources are focused and used for prevention. It would also be very difficult to use a split‐mouth design with infection rates as an outcome because a two‐week wash‐out period is needed.
The only outcome measure reported was CFU. Different studies placed the culture plates or obtained the culture samples from different sites, however, such as patient's chest, right and left side of the patient, behind patient, at one, two and three metres' distance. A few studies collected the culture samples before and after the procedure while some studies collected the samples before, during and after the procedure. Use of CFU to measure the reduction in level of contamination in aerosols is a surrogate outcome as this measures only the bacterial component of the aerosols that was cultured. This may not represent the actual reduction of aerosols, and viral or fungal components of the aerosols are not considered. We are not sure how these results can be interpreted when considering their usefulness in reducing the risk of COVID‐19 due to aerosol‐generating procedures. In addition to this, we could not find any information on minimal clinically important difference in CFU in order to determine the success or failure of an intervention.
Logically, dental AGPs such as non‐carious tooth preparation will have lesser microbial load compared to dental AGPs such as cavity preparation of a carious tooth or ultrasonic scaling in a periodontitis patient. Each dental AGP poses a different level of risk and hence we have done subgroup analysis based on the type of dental AGPs, wherever applicable. Also, it is not possible to extrapolate the results of one AGP to another AGP as the level of risk in each AGP varies. We could not find any studies that tested the outcomes in procedures that could induce aerosols such as intraoral radiography, which could have more microbial load and travel larger distances, comparatively.
Four of the included studies tested the efficacy of rubber dam. One of these studies showed increase in CFU in the intervention group when compared to control (no rubber dam) (Al‐amad 2017). This is because the CFU were measured on the face of the operator unlike the other three studies where the CFU were measured away from the operator. The direction in which the aerosols can spread and the distance of the culture plates from the patient's mouth should be considered before interpreting these studies.
We could not find any studies on other methods such as ionisation, use of ventilation, ozonisation, UV light and fogging. There is a need to study these interventions. The recent (July 2020) statement released by WHO regarding the possible airborne transmission of COVID‐19 infection indicates the need for well‐designed research to study the efficacy of the above‐mentioned methods (WHO 2020c).
We had two studies that measured the volume of contaminated aerosols. There are a few laboratory studies on this aspect. Translation of the results of these studies is questionable, however, until we have more clinical trials on this outcome.
Although we had 16 trials included in this review, most of the comparisons were based on the results of single trials and could not be combined in meta‐analyses. The evidence is of very low certainty.
We encourage further high‐quality randomised controlled trials (RCTs) to be conducted that standardise the interventions and outcome measures evaluated.
Quality of the evidence
The certainty of the evidence for all comparisons was very low for the considered outcomes. Most comparisons were evaluated by single trials with a relatively small number of participants and low event rates. In analyses with RCTs, we downgraded the trials mainly for high/unclear risk of bias, imprecision and inconsistency. In analyses of CCTs, GRADE starts at 'low' and we downgraded further due to the small sample sizes and high or unclear risk of bias.
Potential biases in the review process
There may be unpublished data that we did not identify. We did not include other study designs such as in vitro experimental studies, observational studies, case series and case reports, which could have influenced the results of the review.
We planned this review because of the present COVID‐19 pandemic. However, we extended the scope of the review to all infections that could possibly affect dental staff due to dental AGPs as we considered the mechanism to be the same regardless of the causing mechanism. Even though there is a clear theoretical risk of infection given the close contact with patients and the exposure to patients' contaminated saliva and respiratory excretions, there is little empirical evidence of infection risks. Some authors have argued that dentists around the world have been doing AGPs for many years and yet the infections acquired by dentists due to these AGPs are not of a significant number (Fox 2010; Mair 2020; Porter 1991). After six months of the present COVID‐19 pandemic, it is estimated that at least 90,000 healthcare workers are infected with COVID‐19 (Mantovani 2020), but we have not been able to get an accurate number of dental healthcare workers infected with the SARS‐Cov‐2 virus due to dental AGPs. However, we consider that any potential source of infection in a dental clinic should be controlled or eradicated and thus there is a need for evidence‐based interventions to reduce the aerosols produced during dental procedures to prevent infections among dental staff.
We could have decided not to meta‐analyse the studies in analysis 1.1 and 1.3. These meta‐analyses had high heterogeneity that we were unable explain. In either case, the evidence is very low certainty and we need further research to draw any reliable conclusions.
Agreements and disagreements with other studies or reviews
We identified one systematic review (29 studies) with network meta‐analysis on this topic (Koletsi 2020). The review had very low to moderate certainty of evidence across all comparisons. Preprocedural mouthrinse with tempered chlorhexidine (CHX) 0.2% compared with non‐active control mouthrinse, prior to routine ultrasonic scaling was most effective toward reduced postprocedural bacterial load. Our review does not include preprocedural rinse interventions, but this will be assessed in a sister review to be published before the end of 2020.
Recently, the COVID‐19 Dental Services Evidence Review (CoDER) working group published a Rapid Review on AGPs and their mitigation in international dental guidance documents (Clarkson 2020). This review has compiled reports from national recommendations for AGPs from 58 countries and the majority of their recommendations lacked evidence. Forty‐eight per cent of these recommendations suggest a fallow period of 2 to 180 minutes after providing AGP treatment for non‐COVID patients. We did not come across any such fallow period in the included studies of our review.
Authors' conclusions
Implications for practice.
Infection transmission was not measured in the studies we identified for this review. The analyses suggested that the evaluated interventions may reduce bacterial contamination in aerosols, but it is not possible to draw any reliable conclusions based on the very low certainty evidence. We were unable to draw any conclusions regarding the superiority of any intervention over another. None of the included trials tested the reduction in level of viral contamination in aerosols.
Implications for research.
Further research should be undertaken to determine the most effective methods to reduce contaminated aerosols generated during dental procedures by conducting well‐planned randomised controlled trials (RCTs) including the outcomes developed through any of the core outcome consortiums such as COMET (COMET 2020). In designing such clinical trials, the following should be considered.
Evidence
The present evidence from controlled trials is insufficient to conclude that any of the interventions are effective for reducing contaminated aerosols. Trials should focus on testing similar methods of intervention. Trials should focus on direct outcome measurements such as viable particles in aerosols with small particle size. Studies should also measure patient‐related outcomes and cost effectiveness. Furthermore, reports on clinical trials would be improved by following CONSORT 2010 recommendations.
Population
Inclusion criteria for clinical trials should be well defined and different types of dental procedures should be included (such as cavity preparation, ultrasonic scaling, access cavity preparation, air polishing, minor oral surgery procedures and dental lab procedures). Trials should include both systemically healthy and compromised individuals as this would be a pragmatic approach.
Intervention
Intervention should focus on similar methods used in earlier studies. This will add on to the existing evidence pool allowing us to make more robust conclusions. In addition to these, we could not find any trials testing many of the interventions mentioned in the Background section of this review such as ionisation, UV light, ozonisation, fumigation and fogging and thus they should be tested for their usefulness.
Comparison
We found only single trials for most of the comparisons included in this review. RCTs need to be conducted keeping in mind already published studies so that the number of trials for a particular comparison increases.
Outcomes
Evidence is especially needed on viable small particles. This requires the measurement of particles' size and contamination, preferably based on air flow sampling. It would be helpful to have consensus on how and what to measure as the contamination outcome, for example through a COMET initiative. Cost effectiveness is important to consumers and should be added as an outcome in future RCTs.
We need a good systematic review on risk of respiratory infection in dental healthcare providers resulting from exposure to AGPs.
What's new
| Date | Event | Description |
|---|---|---|
| 14 October 2020 | Amended | Minor edit |
History
Protocol first published: Issue 7, 2020 Review first published: Issue 10, 2020
Acknowledgements
We are extremely grateful to Cochrane Oral Health: Anne Littlewood, Information Specialist; Laura MacDonald, Managing Editor; and Professor Anne Marie Glenny, Co‐ordinating Editor, and Professor Helen Worthington and Dr Phil Riley, Editors.
We are grateful to peer reviewers Julián Balanta Melo, Dr Nicola Innes and Derek Richards (with the team at Scottish Dental Clinical Effectiveness Programme) who gave us their valuable comments. We also thank Jennifer Hilgart and copy editor Jason Elliot‐Smith.
We thank Professor Dr Jaspal Singh Sahota, Chief Executive, Melaka‐Manipal Medical College, Melaka campus and Professor Dr Abdul Rashid Hj Ismail, Dean, Faculty of Dentistry, Melaka‐Manipal Medical College for constant support to undertake Cochrane Reviews.
We thank Professor Dr Prathap Tharyan, Jabez Paul Barnabas, Dr Mohan Kamath, Dr Thambu and Richard Kirubakaran, BVMC for Evidence‐Informed Healthcare and Health Policy, CMC Vellore, India for training us in Rapid Review methods.
We would like to thank Farhad Shokraneh, Information Specialist at Cochrane Schizophrenia, University of Nottingham, for peer reviewing the search strategy and Dr Tony Francis, Assistant Professor in Department of Conservative and Endodontics, Faculty of Dentistry, Melaka‐Manipal Medical College for the valuable input during the preparation of this protocol.
Review author GS would like to thank Dr Muneera Sabt Alsobaei, Chief of Dental Training Department, Ministry of Health, Bahrain, for the support given to be a part of this Cochrane Review.
We thank Ms Shazana Binti Mohd Selva, Chief Librarian, Melaka‐Manipal Medical College for timely supply of full‐text articles in the midst of COVID‐19 lockdown.
We acknowledge the prompt response of study authors Suhail Al‐Amad, Farhad Yeroshalmi and Alefiya Mamajiwala who helped us by providing the missing information and thank James Allison for mailing us links to their pre‐print publications.
Appendices
Appendix 1. Cochrane Oral Health’s Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see https://oralhealth.cochrane.org/trials.
1 MESH DESCRIPTOR Air microbiology AND INREGISTER 2 MESH DESCRIPTOR Air Pollution, Indoor AND INREGISTER 3 MESH DESCRIPTOR Aerosols AND INREGISTER 4 MESH DESCRIPTOR Inhalation Exposure AND INREGISTER 5 (aerosol* or bioaerosol*) AND INREGISTER 6 (droplet* or splatter* or spatter* or microbe* or bacillus or germ* or microorganism* or virus* or viral or coronavirus* or COVID* or "middle east? respiratory syndrome*" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome*" or "Wuhan virus*" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1) AND INREGISTER 7 (air near5 (pollut* or quality or impur*)) AND INREGISTER 8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 AND INREGISTER 9 #8 AND INREGISTER 10 MESH DESCRIPTOR Decontamination AND INREGISTER 11 MESH DESCRIPTOR Rubber Dams AND INREGISTER 12 MESH DESCRIPTOR Air Filters AND INREGISTER 13 MESH DESCRIPTOR Air ionization AND INREGISTER 14 MESH DESCRIPTOR Suction AND INREGISTER 15 MESH DESCRIPTOR Ozone AND INREGISTER 16 MESH DESCRIPTOR Ultraviolet Rays AND INREGISTER 17 MESH DESCRIPTOR Fumigation AND INREGISTER 18 ("high volume evacuat*" or HVE or "high volume aspirat*") AND INREGISTER 19 ((rubber near dam*) or (oral near dam*) or (dental near dam*) or (latex near dam*) or Kofferdam) AND INREGISTER 20 ("Optra Dam" or "OptraDam Plus" or OptiDam or FlexiDam or "Hygenic Fiesta") AND INREGISTER 21 ("saliva ejector" or "low volume aspirat*" or (suction near2 saliva)) AND INREGISTER 22 (air near5 (filter* or filtration or purif* or clean*)) AND INREGISTER 23 ((HEPA or "High Efficiency Particulate Air" or "High Efficiency Particulate Arrestance") near5 filter*) AND INREGISTER 24 (ionis* or ioniz*) AND INREGISTER 25 (ozonis* or ozoniz*) AND INREGISTER 26 (ultraviolet or UV or ultra‐violet or actinic) AND INREGISTER 27 ((aerosol* or bioaerosol* or droplet* or spatter or splatter) near2 reduc*) AND INREGISTER 28 (fog* or fumigat* or decontaminat* or "smoke out" or smokeout or depollut* or depurat*) AND INREGISTER 29 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 AND INREGISTER 30 #29 AND #9 AND INREGISTER
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
The search strategy below was executed in the Cochrane Register of Studies, limited to records in the CENTRAL register.
1 MESH DESCRIPTOR dentistry EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR dental facilities EXPLODE ALL AND CENTRAL:TARGET 3 MESH DESCRIPTOR Infection Control, Dental AND CENTRAL:TARGET 4 MESH DESCRIPTOR Dentists EXPLODE ALL AND CENTRAL:TARGET 5 MESH DESCRIPTOR Dental Staff EXPLODE ALL AND CENTRAL:TARGET 6 MESH DESCRIPTOR Dental Auxiliaries EXPLODE ALL AND CENTRAL:TARGET 7 MESH DESCRIPTOR Dental High‐Speed Equipment AND CENTRAL:TARGET 8 (dental or dentist* or hygienist*) AND CENTRAL:TARGET 9 ((oral or maxillofacial) near5 (care* or procedure* or surgery or surgical or medicine)) AND CENTRAL:TARGET 10 orthodonti* AND CENTRAL:TARGET 11 periodont* AND CENTRAL:TARGET 12 (tooth or teeth or gum* or endodont* or plaque* or pulpotom* or pulpectom* or "cavity prep*" or molar* or bicuspid* or premolar* or pre‐molar* or incisor* or canine* or eyetooth or eyeteeth or cuspid*) AND CENTRAL:TARGET 13 ((scal* near2 polish*) or "root canal" or (root near6 resect*) or (root* near3 planing) or apicectom* or apicoectom*) AND CENTRAL:TARGET 14 ((root* or periodont* or dental or subgingiv* or gingiv* or supragingiv*) near5 (scale or scaling or scaler* or curettage)) AND CENTRAL:TARGET 15 ("high speed air rotor*" or "low speed handpiece*" or "low speed hand piece*" or micromotor* or "turbine handpiece*" or "electrosurgery unit" or "air polisher*" or "prophy angle*" or "air‐water syringe*" or "high speed hand piece*" or "high speed handpiece*" or "three‐way air syringe*" or "threeway air syringe*" or "ultrasonic scaler*" or "hard‐tissue laser*" or "dental drill*" or "piezo unit*" or "piezo hand piece*" or "piezo handpiece*" or "rotary instrument*" or "air abrasion" or "water spray*") AND CENTRAL:TARGET 16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 AND CENTRAL:TARGET 17 MESH DESCRIPTOR Air microbiology AND CENTRAL:TARGET 18 MESH DESCRIPTOR Air Pollution, Indoor AND CENTRAL:TARGET 19 MESH DESCRIPTOR Aerosols AND CENTRAL:TARGET 20 MESH DESCRIPTOR Inhalation Exposure AND CENTRAL:TARGET 21 (aerosol* or bioaerosol*) AND CENTRAL:TARGET 22 (droplet* or splatter* or spatter* or microbe* or bacillus or germ* or microorganism* or virus* or viral or coronavirus* or COVID* or "middle east? respiratory syndrome*" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome*" or "Wuhan virus*" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1) AND CENTRAL:TARGET 120553 23 (air near5 (pollut* or quality or impur*)) AND CENTRAL:TARGET 24 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 AND CENTRAL:TARGET 25 #24 AND #16 AND CENTRAL:TARGET 26 MESH DESCRIPTOR Decontamination AND CENTRAL:TARGET 27 MESH DESCRIPTOR Rubber Dams AND CENTRAL:TARGET 28 MESH DESCRIPTOR Air Filters AND CENTRAL:TARGET 29 MESH DESCRIPTOR Air ionization AND CENTRAL:TARGET 30 MESH DESCRIPTOR Suction AND CENTRAL:TARGET 31 MESH DESCRIPTOR Ozone AND CENTRAL:TARGET 32 MESH DESCRIPTOR Ultraviolet Rays AND CENTRAL:TARGET 33 MESH DESCRIPTOR Fumigation AND CENTRAL:TARGET 34 ("high volume evacuat*" or HVE or "high volume aspirat*") AND CENTRAL:TARGET 35 ((rubber near dam*) or (oral near dam*) or (dental near dam*) or (latex near dam*) or Kofferdam) AND CENTRAL:TARGET 36 ("Optra Dam" or "OptraDam Plus" or OptiDam or FlexiDam or "Hygenic Fiesta") AND CENTRAL:TARGET 37 ("saliva ejector" or "low volume aspirat*" or (suction near2 saliva)) AND CENTRAL:TARGET 38 (air near5 (filter* or filtration or purif* or clean*)) AND CENTRAL:TARGET 39 ((HEPA or "High Efficiency Particulate Air" or "High Efficiency Particulate Arrestance") near5 filter*) AND CENTRAL:TARGET 40 (ionis* or ioniz*) AND CENTRAL:TARGET 41 (ozonis* or ozoniz*) AND CENTRAL:TARGET 42 (ultraviolet or UV or ultra‐violet or actinic) AND CENTRAL:TARGET 43 ((aerosol* or bioaerosol* or droplet* or spatter or splatter) near2 reduc*) AND CENTRAL:TARGET 44 (fog* or fumigat* or decontaminat* or "smoke out" or smokeout or depollut* or depurat*) AND CENTRAL:TARGET 45 #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 AND CENTRAL:TARGET 46 #45 AND #25 AND CENTRAL:TARGET
Appendix 3. MEDLINE Ovid search strategy
1. exp dentistry/ 2. exp dental facilities/ 3. infection control, dental/ 4. exp dentists/ 5. dental staff/ 6. exp dental auxiliaries/ 7. (dental or dentist$ or hygienist$).mp. 8. ((oral or maxillofacial) adj5 (care$ or procedure$ or surgery or surgical or medicine)).mp. 9. orthodonti$.mp. 10. periodont$.mp. 11. (tooth or teeth or gum$ or endodont$ or plaque$ or pulpotom$ or pulpectom$ or "cavity prep$" or molar$ or bicuspid$ or premolar$ or pre‐molar$ or incisor$ or canine$ or eyetooth or eyeteeth or cuspid$).mp. 12. ((scal$ adj2 polish$) or "root canal" or (root adj6 resect$) or (root$ adj3 planing) or apicectom$ or apicoectom$).mp. 13. ((root$ or periodont$ or dental or subgingiv$ or gingiv$ or supragingiv$) adj5 (scale or scaling or scaler$ or curettage)).mp. 14. Dental high speed equipment/ 15. ("high speed air rotor$" or "low speed handpiece$" or "low speed hand piece$" or micromotor$ or "turbine handpiece$" or "electrosurgery unit" or "air polisher$" or "prophy angle$" or "air‐water syringe$" or "high speed hand piece$" or "high speed handpiece$" or "three‐way air syringe$" or "threeway air syringe$" or "ultrasonic scaler$" or "hard‐tissue laser$" or "dental drill$" or "piezo unit$" or "piezo hand piece$" or "piezo handpiece$" or "rotary instrument$" or "air abrasion" or "water spray$").mp. 16. or/1‐15 17. Air microbiology/ 18. Air pollution, indoor/ 19. Aerosols/ 20. Inhalation exposure/ 21. (aerosol$ or bioaerosol$).mp. 22. (droplet$ or splatter$ or spatter$ or microbe$ or bacillus or germ$ or microorganism$ or virus$ or viral or coronavirus$ or COVID$ or "middle east? respiratory syndrome$" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome$" or "Wuhan virus$" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1).mp. 23. (air adj5 (pollut$ or quality or impur$)).mp. 24. or/17‐23 25. Decontamination/ 26. ("high volume evacuat$" or HVE or "high volume aspirat$").mp. 27. Rubber dams/ 28. ((rubber adj dam$) or (oral adj dam$) or (dental adj dam$) or (latex adj dam$) or Kofferdam).mp. 29. ("Optra Dam" or "OptraDam Plus" or OptiDam or FlexiDam or "Hygenic Fiesta").mp. 30. Suction/ 31. ("saliva ejector" or "low volume aspirat$" or (suction adj2 saliva)).mp. 32. Air filters/ 33. (air adj5 (filter$ or filtration or purif$ or clean$)).mp. 34. ((HEPA or "High Efficiency Particulate Air" or "High Efficiency Particulate Arrestance") adj5 filter$).mp. 35. Air ionization/ 36. (ionis$ or ioniz$).mp. 37. Ozone/ 38. (ozonis$ or ozoniz$).mp. 39. Ultraviolet rays/ 40. (ultraviolet or UV or ultra‐violet or actinic).mp. 41. ((aerosol$ or bioaerosol$ or droplet$ or spatter or splatter) adj2 reduc$).mp. 42. Fumigation/ 43. (fog$ or fumigat$ or decontaminat$ or "smoke out" or smokeout or depollut$ or depurat$).mp. 44. or/25‐43 45. 16 and 24 and 44
This subject search will be linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) (Lefebvre 2019).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. exp dentistry/ 2. dental facility/ 3. Exp dental personnel/ 4. (dental or dentist$ or hygienist$).mp. 5. ((oral or maxillofacial) adj5 (care$ or procedure$ or surgery or surgical or medicine)).mp. 6. orthodonti$.mp. 7. periodont$.mp. 8. (tooth or teeth or gum$ or endodont$ or plaque$ or pulpotom$ or pulpectom$ or "cavity prep$" or molar$ or bicuspid$ or premolar$ or pre‐molar$ or incisor$ or canine$ or eyetooth or eyeteeth or cuspid$).mp. 9. ((scal$ adj2 polish$) or "root canal" or (root adj6 resect$) or (root$ adj3 planing) or apicectom$ or apicoectom$).mp. 10. ((root$ or periodont$ or dental or subgingiv$ or gingiv$ or supragingiv$) adj5 (scale or scaling or scaler$ or curettage)).mp. 11. ("high speed air rotor$" or "low speed handpiece$" or "low speed hand piece$" or micromotor$ or "turbine handpiece$" or "electrosurgery unit" or "air polisher$" or "prophy angle$" or "air‐water syringe$" or "high speed hand piece$" or "high speed handpiece$" or "three‐way air syringe$" or "threeway air syringe$" or "ultrasonic scaler$" or "hard‐tissue laser$" or "dental drill$" or "piezo unit$" or "piezo hand piece$" or "piezo handpiece$" or "rotary instrument$" or "air abrasion" or "water spray$").mp. 12. or/1‐11 13. exp Air pollution/ 14. Aerosol/ 15. Environmental exposure/ 16. (aerosol$ or bioaerosol$).mp. 17. (droplet$ or splatter$ or spatter$ or microbe$ or bacillus or germ$ or microorganism$ or virus$ or viral or coronavirus$ or COVID$ or "middle east? respiratory syndrome$" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome$" or "Wuhan virus$" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1).mp. 18. (air adj5 (pollut$ or quality or impur$)).mp. 19. or/13‐18 20. Decontamination/ 21. ("high volume evacuat$" or HVE or "high volume aspirat$").mp. 22. Cofferdam/ 23. ((rubber adj dam$) or (oral adj dam$) or (dental adj dam$) or (latex adj dam$) or Kofferdam or cofferdam).mp. 24. ("Optra Dam" or "OptraDam Plus" or OptiDam or FlexiDam or "Hygenic Fiesta").mp. 25. Suction device/ 26. "saliva ejector" or "low volume aspirat$" or (suction adj2 saliva)).mp. 27. Air filter/ 28. (air adj5 (filter$ or filtration or purif$ or clean$)).mp. 29. ((HEPA or "High Efficiency Particulate Air" or "High Efficiency Particulate Arrestance") adj5 filter$).mp. 30. ionization/ 31. (ionis$ or ioniz$).mp. 32. Ozone/ 33. (ozonis$ or ozoniz$).mp. 34. Ultraviolet radiation/ 35. (ultraviolet or UV or ultra‐violet or actinic).mp. 36. ((aerosol$ or bioaerosol$ or droplet$ or spatter or splatter) adj2 reduc$).mp. 37. Fumigation/ 38. (fog$ or fumigat$ or decontaminat$ or "smoke out" or smokeout or depollut$ or depurat$).mp. 39. or/20‐38 40. 12 and 19 and 39
This subject search was linked to the Cochrane search filter for identifying randomised trials in Embase (2016 version) as referenced in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook.
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. human experiment/ 19. trial.ti. 20. or/1‐19 21. random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 22. Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 23. (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 24. (Systematic review not (trial or study)).ti. 25. (nonrandom$ not random$).ti,ab. 26. "Random field$".ti,ab. 27. (random cluster adj3 sampl$).ti,ab. 28. (review.ab. and review.pt.) not trial.ti. 29. "we searched".ab. and (review.ti. or review.pt.) 30. "update review".ab. 31. (databases adj4 searched).ab. 32. (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 33. Animal experiment/ not (human experiment/ or human/) 34. or/21‐33 35. 20 not 34
Appendix 5. WHO COVID‐19 Global literature on coronavirus disease database search strategy
(tw:((dental or dentist* or hygienist* or "oral health" or "oral care" or "oral medicine" or maxillofacial or "oral surgery" or orthodonti* or periodont*))) AND (tw:((aerosol or bioaerosol or droplet* or splatter* or spatter* or microbe* or bacillus or germ* or microorganism* or air*)))
Appendix 6. US National Institutes of Health Trials Registry (ClinicalTrials.gov) search strategy
Expert search interface:
( dentist OR dentistry OR dental OR EXPAND[Concept] "oral health" OR EXPAND[Concept] "oral care" ) AND ( EXPAND[Concept] "high volume evacuator" OR EXPAND[Concept] "high volume evacuation" OR HVE OR EXPAND[Concept] "high volume aspirator" OR EXPAND[Concept] "high volume aspiration" OR “rubber dam” OR “oral dam” OR “dental dam” OR “latex dam” OR EXPAND[Concept] "saliva ejector" OR EXPAND[Concept] "low volume aspiration" OR EXPAND[Concept] "low volume aspirator" OR suction OR filter or filtration OR purify OR HEPA OR EXPAND[Concept] "High Efficiency Particulate Air" OR EXPAND[Concept] "High Efficiency Particulate Arrestance" OR ionisation OR ionization OR ozone OR ozonisation OR ozonization OR ultraviolet OR UV OR ultra‐violet OR actinic OR fog OR fumigate OR fumigation OR decontaminate OR decontamination OR EXPAND[Concept] "smoke out" OR smokeout OR depollute OR depollution OR depurate OR depuration ) AND ( aerosol OR bioaerosol OR droplet OR splatter OR spatter* OR microbe OR bacillus OR germ OR microorganism OR virus OR viral OR coronavirus* OR COVID* OR EXPAND[Concept] "middle eastern respiratory syndrome" OR MERS OR MERS‐CoV OR EXPAND[Concept] "camel flu" OR SARS OR EXPAND[Concept] "sudden acute respiratory syndrome" OR EXPAND[Concept] "Wuhan virus" OR 2019‐nCoV OR SARS‐CoV‐2 OR SARS‐CoV OR SARS‐CoV‐1 OR SARS‐1 )
Appendix 7. Cochrane COVID‐19 Study Register search strategy
The search strategy below was executed in the Cochrane Register of Studies, limited to the COVID‐19 Study Register.
1 MESH DESCRIPTOR dentistry EXPLODE ALL AND INREGISTER 2 MESH DESCRIPTOR dental facilities EXPLODE ALL AND INREGISTER 3 MESH DESCRIPTOR Infection Control, Dental AND INREGISTER 4 MESH DESCRIPTOR Dentists EXPLODE ALL AND INREGISTER 5 MESH DESCRIPTOR Dental Staff EXPLODE ALL AND INREGISTER 6 MESH DESCRIPTOR Dental Auxiliaries EXPLODE ALL AND INREGISTER 7 MESH DESCRIPTOR Dental High‐Speed Equipment AND INREGISTER 8 (dental or dentist* or hygienist*) AND INREGISTER 9 ((oral or maxillofacial) near5 (care* or procedure* or surgery or surgical or medicine)) AND INREGISTER 10 orthodonti* AND INREGISTER 11 periodont* AND INREGISTER 12 (tooth or teeth or gum* or endodont* or plaque* or pulpotom* or pulpectom* or "cavity prep*" or molar* or bicuspid* or premolar* or pre‐molar* or incisor* or canine* or eyetooth or eyeteeth or cuspid*) AND INREGISTER 13 ((scal* near2 polish*) or "root canal" or (root near6 resect*) or (root* near3 planing) or apicectom* or apicoectom*) AND INREGISTER 14 ((root* or periodont* or dental or subgingiv* or gingiv* or supragingiv*) near5 (scale or scaling or scaler* or curettage)) AND INREGISTER 15 ("high speed air rotor*" or "low speed handpiece*" or "low speed hand piece*" or micromotor* or "turbine handpiece*" or "electrosurgery unit" or "air polisher*" or "prophy angle*" or "air‐water syringe*" or "high speed hand piece*" or "high speed handpiece*" or "three‐way air syringe*" or "threeway air syringe*" or "ultrasonic scaler*" or "hard‐tissue laser*" or "dental drill*" or "piezo unit*" or "piezo hand piece*" or "piezo handpiece*" or "rotary instrument*" or "air abrasion" or "water spray*") AND INREGISTER 16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 AND INREGISTER 17 MESH DESCRIPTOR Air microbiology AND INREGISTER 18 MESH DESCRIPTOR Air Pollution, Indoor AND INREGISTER 19 MESH DESCRIPTOR Aerosols AND INREGISTER 20 MESH DESCRIPTOR Inhalation Exposure AND INREGISTER 21 (aerosol* or bioaerosol*) AND INREGISTER 22 (droplet* or splatter* or spatter* or microbe* or bacillus or germ* or microorganism* or virus* or viral or coronavirus* or COVID* or "middle east? respiratory syndrome*" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome*" or "Wuhan virus*" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1) AND INREGISTER 23 (air near5 (pollut* or quality or impur*)) AND INREGISTER 24 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 AND INREGISTER 25 #24 AND #16 AND INREGISTER 26 MESH DESCRIPTOR Decontamination AND INREGISTER 27 MESH DESCRIPTOR Rubber Dams AND INREGISTER 28 MESH DESCRIPTOR Air Filters AND INREGISTER 29 MESH DESCRIPTOR Air ionization AND INREGISTER 30 MESH DESCRIPTOR Suction AND INREGISTER 31 MESH DESCRIPTOR Ozone AND INREGISTER 32 MESH DESCRIPTOR Ultraviolet Rays AND INREGISTER 33 MESH DESCRIPTOR Fumigation AND INREGISTER 34 ("high volume evacuat*" or HVE or "high volume aspirat*") AND INREGISTER 35 ((rubber near dam*) or (oral near dam*) or (dental near dam*) or (latex near dam*) or Kofferdam) AND INREGISTER 36 ("Optra Dam" or "OptraDam Plus" or OptiDam or FlexiDam or "Hygenic Fiesta") AND INREGISTER 37 ("saliva ejector" or "low volume aspirat*" or (suction near2 saliva)) AND INREGISTER 38 (air near5 (filter* or filtration or purif* or clean*)) AND INREGISTER 39 ((HEPA or "High Efficiency Particulate Air" or "High Efficiency Particulate Arrestance") near5 filter*) AND INREGISTER 40 (ionis* or ioniz*) AND INREGISTER 41 (ozonis* or ozoniz*) AND INREGISTER 42 (ultraviolet or UV or ultra‐violet or actinic) AND INREGISTER 43 ((aerosol* or bioaerosol* or droplet* or spatter or splatter) near2 reduc*) AND INREGISTER 44 (fog* or fumigat* or decontaminat* or "smoke out" or smokeout or depollut* or depurat*) AND INREGISTER 45 #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 AND INREGISTER 46 #45 AND #25 AND INREGISTER
Data and analyses
Comparison 1. High‐volume evacuation (HVE) versus no HVE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Reduction in the level of contamination in aerosols | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 At less than 1 foot from oral cavity ‐ RCT | 3 | Mean Difference (IV, Random, 95% CI) | ‐47.41 [‐92.76, ‐2.06] | |
| 1.1.2 At less than 1 foot from oral cavity ‐ CCT | 2 | Mean Difference (IV, Random, 95% CI) | ‐50.19 [‐109.71, 9.33] | |
| 1.1.3 On operator face shield/mask | 2 | Mean Difference (IV, Random, 95% CI) | ‐15.71 [‐46.37, 14.95] | |
| 1.1.4 At more than 1 foot from oral cavity ‐ RCT | 1 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.56, 0.56] | |
| 1.1.5 At more than 1 foot from oral cavity ‐ CCT | 1 | Mean Difference (IV, Random, 95% CI) | ‐13.56 [‐23.18, ‐3.94] |
Comparison 2. HVE versus conventional dental suction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Reduction in the level of contamination in aerosols | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.1 At 40 cm | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.2 At 150 cm | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Combination system versus saliva ejector.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Reduction in the level of contamination in aerosols | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.1 During AGP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1.2 After AGP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Combination system versus rubber dam + HVE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Reduction in the level of contamination in aerosols | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Combination system versus HVE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Reduction in the level of contamination in aerosols | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Rubber dam versus no rubber dam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Reduction in the level of contamination in aerosols | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.1 At 1 meter from mouth ‐ RCT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.2 At 1 meter from mouth ‐ CCT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.3 At 2 meters from mouth ‐ RCT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.4 At 2 meters from mouth ‐ CCT | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.5 At forehead | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.6 At left ear | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.7 At submental triangle | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1.8 At occiput | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Rubber dam + HVE versus cotton roll + HVE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Reduction in the level of contamination in aerosols | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1.1 At patient's chest | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1.2 At dental unit light | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Air‐cleaning system (ACS) versus no ACS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Reduction in the level of contamination in aerosols | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1.1 During cavity preparation | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1.2 During ultrasonic scaling | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 9. Laminar air flow with HEPA filter versus without flow or filter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Reduction in the level of contamination in aerosols (CFU per cubic feet/minute/patient) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.1 At about 30 inches (1.5 metres) from the floor | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1.2 At about 8 to 12 inches (20 to 30 cm) from participant's mouth | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 10. Antimicrobial coolant versus control coolant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Reduction in the level of contamination in aerosols | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1.1 Chlorhexidine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1.2 Povidone iodine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1.3 Cinnamon | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 11. Antimicrobial coolant A versus antimicrobial coolant B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Reduction in the level of contamination in aerosols | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1.1 Chlorhexidine vs cinnamon | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1.2 Chlorhexidine vs povidone iodine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐amad 2017.
| Study characteristics | ||
| Methods | Trial design: RCT ‒ parallel arm Location: College of Dental Medicine, University of Sharjah, The University City, Sharjah, United Arab Emirates Setting: dental clinic Language: English Number of centres: 1 Study period: 2013 to 2014 Funding source: no funding received Protocol: not available | |
| Participants | Age: not mentioned
Total number of participants: 52 female dental students
Inclusion criteria: female dental students in their 4th and 5th years, who would customarily wear headscarves To standardize the extent of the dental procedure, only dental cavity preparations on posterior teeth that were already planned for the patients were included. Exclusion criteria: not mentioned Number randomized: 52 (26 per group) Number evaluated (withdrawals/missing participants): 47 (5 dropouts: 4 from the rubber dam group and 1 from the non‐rubber dam group). 2 participants were excluded due to changes in the dental procedure type intraoperatively (from restorative cavity preparation to access opening and inlay preparation) and 3 students were excluded as they had to use a face shield. The final sample consisted of 47 students with 188 collection points (4 for each student). Of those collection points, 16 were outliers (more than 3 SDs from mean) and were excluded from statistical analysis. The majority of the outliers (13 collection points) belonged to the rubber dam group. The final number of collection points was 172 (188 minus 16 outliers). |
|
| Interventions |
Comparison: rubber dam versus no rubber dam Intervention: Group name: with rubber dam Number of intervention groups: 1 Number randomized to intervention group: 26 but evaluated 22 Description of intervention: students who consented to participate (n = 52) were randomly assigned into 2 equal groups using computer‐generated random numbers and then assigned to a dental clinic where they performed a routine restorative dental procedure. A colleague from the same group was assigned to assist each student by holding the surgical suction tube throughout the clinical procedure. All students wore similar PPE, consisting of a disposable apron, mask, gloves and plastic goggles. Half the sample was asked to perform this procedure while a rubber dam was placed over the tooth that was being treated, while the other half performed similar procedures without a rubber dam. Any co‐interventions: none Comparator: Group name: without rubber dam Number of control groups: 1 Number randomized to control group: 26 but evaluated 25 Description of control: same as above. Control group underwent procedures without a rubber dam. |
|
| Outcomes | Outcome tested: reduction in the level of contamination in aerosols (measured at 4 sampling areas: the area overlaying the forehead (designated as point A), the area overlaying the left ear (point B), the area overlaying the submental triangle (point C), and the area overlaying the occiput (point D))
Outcome measurement: CFU
Effect estimate: mean (SD not given) Key conclusions: "for each of the collection points, the average number of colony‐forming units (CFU) was higher in the rubber dam group than in the no rubber dam group. The difference between the two groups for each point was not statistically significant. However, when an adjustment was made for all collection points, the presence of a rubber dam was associated with significantly more bacteria‐containing aerosols based on the CFU counts (P = 0.009)" |
|
| Notes | Study author contacted for: allocation concealment, study protocol and SD. E‐mail sent on 26 July 2020. Received reply on 28 July 2020. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomization: computer‐generated random table Quote: “Students who consented to participate (n = 52) were randomly assigned into two equal groups using computer‐generated random numbers...” |
| Allocation concealment (selection bias) | High risk | No. No details given in the article; however in personal communication, the contact author reported that there was no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the CFU were manually counted or any automated colony counters were used ‐ there is subjectivity if manually counted. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “During the course of cavity preparation, 2 participants were excluded due to changes in the dental procedure type intra‐operatively (from restorative cavity preparation to access opening and inlay preparation) and 3 students had to use a face shield and were dropped out.” However, 16 observations were excluded because they were outliers. It is not clear what the effect estimate would be if those values were included. |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. SDs for observations are not reported. |
| Other bias | Low risk | None |
Cochran 1989.
| Study characteristics | ||
| Methods | Trial design: RCT ‐ split mouth Location: Indiana University School of Dentistry, Michigan, Indianapolis, USA Setting: closed operatory separate from other clinical facilities Language: English Number of centres: 1 Study period: not mentioned Funding source: grant from Hygiene corp. Akron, OH Study protocol: not available | |
| Participants | Age: not mentioned Total number of participants: part 1: n = 16; part 2: n = 10 Inclusion criteria: For part 1:
For part 2:
Exclusion criteria: not mentioned Number randomized: For part 1: 32 sites (16 in intervention and 16 in control) "Selection of isolation method, lesion and appointment was randomised". For part 2: 10 (5 in intervention and 5 in control) Number evaluated (withdrawals/missing participants): part 1: 16; part 2: 10 (no missing participants) |
|
| Interventions |
Comparision: rubber dam + HVE versus cotton roll (without rubber dam) + HVE Intervention: Group name: rubber dam + HVE Number of intervention groups: 1 (but study done in 2 parts using 2 different procedures) Number randomized to intervention group: 16 for part 1; 5 for part 2 Description of intervention: For part 1: rubber dam + HVE during restorative procedure (n = 16). 1 lesion of each pair (anterior and posterior) was restored using rubber dam isolation and HVE. Aerosol particle sampling was done during preparation, cleaning and restoration of all lesions and time required was recorded. Microbial samples were collected throughout the procedure time, which ranged from 11.8 to 23.8 minutes. For part 2: rubber dam + HVE during spraying procedure (n = 5). At appointments 1 week apart, the area from second molar to canine in each participant‐assigned arch was sprayed for 2 minutes with high‐speed handpiece spray followed by spray from air water syringe for 2 minutes. For the intervention group, the teeth were isolated during this procedure with rubber dam and HVE was also used. Microbial samples were collected throughout the procedure time, which was 8 minutes. Any co‐interventions: no Comparator: Group name: cotton roll (without rubber dam) + HVE Number of control groups: 1 (study done in 2 parts using 2 different procedures) Number randomized to control group: 16 for part 1; 5 for part 2 Description of control: For part 1: cotton roll (without rubber dam) + HVE during restorative procedure: (n = 16). 1 lesion of each pair (anterior and posterior) was restored using cotton roll isolation and high‐volume evacuation. Aerosol particle sampling was done during preparation, cleaning and restoration of all lesions and time required was recorded. For part 2: cotton roll (without rubber dam) + HVE during spraying procedure (n = 5). At appointments 1 week apart, the area from second molar to canine in each participant‐assigned arch was sprayed for 2 minutes with high‐speed handpiece spray followed by spray from air water syringe for 2 minutes. Teeth were isolated using cotton rolls and HVE was also used. |
|
| Outcomes | Outcome name: reduction in contamination of aerosols (in 2 areas – at dental unit light and 24 inches from mouth and particpant’s chest) (measured by reduction in CFU) Outcome measurement: CFU Effect estimate: mean + SE Key conclusions: "routine use of rubber dam combined with other accepted barrier techniques can contribute significantly to overall infection control program". | |
| Notes | We had hoped to contact the study author for the method of random sequence generation, allocation concealment and for the study protocol; however, no contact details were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not mentioned. Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes measured is CFU using stereomicroscopy (manual counting), which is a subjective method and thus may be affected by lack of assessor blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
Desarda 2014.
| Study characteristics | ||
| Methods | Trial design: RCT – split‐mouth design Location: Tatyasaheb Kore Dental College and Research Centre, New Pargaon, Kolhapur, Maharashtra, India Setting: fumigated closed operatory Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 30 to 60 years Total number of participants: 80 Inclusion criteria: patients aged 30 to 60 years with chronic generalized periodontitis were selected based on International Workshop for Classification of Periodontal Diseases, 1999 (AAP 1999) Exclusion criteria: patients with severely debilitating systemic diseases, with pacemakers, with a history of respiratory diseases, a history of previous periodontal treatment for 1‐year period, or on any antibiotics for 6‐month period Number randomized: 80 Number evaluated: 80 (no missing participants) | |
| Interventions |
Comparison: HVE versus no HVE Intervention: Group name: group Y – presence of high‐volume evacuator Number of intervention groups: 1 Number randomized to intervention group: not clear ‐ n = 80 assumed Description of intervention: maxillary incisors and canines were selected as an area for scaling. A piezoelectric scaler (BONARTTM) was used. Scaling was performed in the presence of the high‐volume evacuator in the above‐mentioned area. Power and water flow settings of the scaler were kept the same throughout the procedure. The high‐volume evacuator tip used in this study was stainless steel with a diameter of 12 mm. Nutrient agar plates were exposed for 20 minutes in each group and incubated at 37 °C for 24 hours. Any co‐interventions: no (but saliva ejector was used in both groups) Comparator: Group name: group X ‐ absence of high‐volume evacuator Number of control groups: 1 Number randomized: not clear ‐ n = 80 assumed Description of intervention: maxillary incisors and canines were selected as an area for scaling. A piezoelectric scaler (BONARTTM) was used. Scaling was performed in the absence of the high‐volume evacuator in the above‐mentioned area. Power and water flow settings of the scaler were kept the same throughout the procedure. |
|
| Outcomes | Outcome name: reduction in contamination of aerosols (nutrient agar plates were used to check CFU in 2 areas – 20 inches from participant's mouth and 12 inches from participant's mouth)
Outcome measurement: reduction in contamination of aerosols (difference in number of CFU)
Effect estimate: mean + SD Key conclusions: "within the limitations of the present study, the results showed no difference in reduction of aerosols with or without the use of a high‐volume evacuator when analyzed microbiologically. Thus, it was concluded that high‐volume evacuator when used as a separate unit without any modification is not effective in reducing aerosol count and environmental contamination". |
|
| Notes | Study authors contacted for: allocation concealment, blinding of participants and personnel, study protocol and details of statistical tests (authors used Student's t test; for a split‐mouth study, paired t‐test should be used). E‐mail sent on 18 June 2020 but no response received. Scaling was carried out for 10 minutes in both groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Coin toss was used to determine which procedure was to be performed first (i.e. with high‐volume evacuator or without high‐volume evacuator)” |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The next day, the nutrient agar plates were examined for colony forming units by a single microbiologist who was unaware of the procedure performed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
Devker 2012.
| Study characteristics | ||
| Methods | Trial design: CCT – split mouth (multi‐arm – but in our trial only 1 group comparing with and without HVE was used) Location: STES dental college and hospital, Pune, Maharashtra, India Setting: dental clinic Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 18 to 45 years Total number of participants: 30 (for HVE group, which is of interest for this review) Inclusion criteria:
Exclusion criteria:
Number randomized: 30. However, both the sides of the mouth and the order of the intervention were not randomised Number evaluated (withdrawals/missing participants): 30 |
|
| Interventions |
Comparison: HVE versus no HVE Intervention: •Group I: rinse with 0.2% chlorhexidine gluconate prior to scaling •Group II: use of HVE during ultrasonic scaling •Group III: rinse with 0.2% chlorhexidine gluconate prior to scaling and use of HVE during ultrasonic scaling Group name: group II ‐ test side ‐ presence of high‐volume evacuator alone Number of intervention groups: 1 Number randomized to intervention group: 30 Description of intervention: oral prophylaxis was done on a randomly selected side (control side) for a period of 10 minutes. After a gap of 30 minutes, high‐volume suction tip was tied to the ultrasonic scaler. Oral prophylaxis was done on the other side (test side) of the same arch with high‐volume suction for a period of 10 minutes. Following the 10‐minute sampling period, blood agar plates were taken off. Any co‐interventions: no (2 other groups were present: Group I ‐ with preprocedural rinse and Group III ‐ with preprocedural rinse + HVE, which are not relevant to our review) Comparator: Group name: group II – control side ‐ absence of high‐volume evacuator Number of control groups: 1 Number randomized to control group: 30 |
|
| Outcomes | Outcome name: reduction of microbial load in aerosols (blood agar plates were used to check CFU in 4 areas)
Reference point: mouth of the patient • At 6 inches (half a foot) from reference point (operator’s nose level) • At 6 inches (half a foot) from reference point (assistant’s nose level) • At 12 inches (1 foot) from reference point (participant’s chest level) • At 36 inches (3 foot) from reference point on participant’s right Outcome measurement: CFU Effect estimate: mean (SD not given) Key conclusions: the results of this study showed that preprocedural rinse and high‐volume suction were effective when used alone as well as together in reducing the microbial load of the aerosols produced during ultrasonic scaling. |
|
| Notes | Study authors contacted for: random sequence generation, allocation concealment, blinding of participants and personnel and study protocol. E‐mail sent on 26 July 2020 and we are yet to receive any response from the contact author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sides of the mouth and the order of the intervention were not randomised |
| Allocation concealment (selection bias) | High risk | Sides of the mouth and the order of the intervention were not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CFU is measured using digital colony counter, which is an objective method and will not be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
El‐Din 1997.
| Study characteristics | ||
| Methods | Trial design: RCT ‐ 4 arms and each arm had a split‐mouth design Location: Faculty of Dentistry, Tanta University, Egypt Setting: dental partition measuring 2 m × 3 m in the pedodontics clinic Language: English Number of centres: 1 Study period: not mentioned Funding source: none mentioned Study protocol: not available | |
| Participants | Age: 5 to 10 years Total number of participants: 20 (10 per group) Inclusion criteria:
Exclusion criteria: not mentioned Number randomized: 20 Number evaluated (withdrawals/missing participants): 20 (none) |
|
| Interventions |
Comparison: rubber dam versus no rubber dam Intervention: Group 1: conservative procedures performed under rubber dam isolation Group 2: CHX mouthrinse 30 minutes before starting the conservative procedure Group 3: CHX mouthrinse before application of the rubber dam Group 4 (control): conservative procedures performed without rubber dam isolation We used group 1 and group 4 data only. Number of intervention groups: 1 Number randomized to intervention group: 10 Description of intervention: 2 different methods of bacterial reduction were used for each child. Adjacent lesions were restored at appointments at least 1 week apart. The operative procedures were performed in the morning to minimize aerosol particle contamination of the environment. An air‐turbine‐driven handpiece was used, and the patient was seated in a reclining position. The length of the procedure varied from 5 to 15 minutes. The windows of the dental partition were opened prior to the procedure to ventilate the partition but were closed 30 minutes before recording background levels of atmospheric bacteria. The selection of the bacterial reduction method, the restoration of the caries tooth and the appointment were randomized and divided into 4 groups: 2 intervention and 2 control groups Any co‐interventions: no Comparator: Group name: conservative procedures performed without rubber dam isolation Number of control groups: 1 Number randomized to control group: 10 Description of control: same as above except that the control group was without rubber dam isolation |
|
| Outcomes | Outcome name: reduction in contamination of aerosols (measured equidistantly from the child's head, 1 each on the chest, on the left and right sides and behind the participant. Another 2 plates were placed 1 metre and 2 metres from the head‐rest of the dental chair) (measured by reduction in CFU) Outcome measurement: CFU Effect estimate: mean (SD) Key conclusions: during conservative procedure without rubber dam, which involved 5 to 15 minutes work on the patient, the airborne bacterial load increased from 8.8 to 25.1 CFU. The results of this study are comparable to those of other studies on the barrier efficiency of rubber dam. | |
| Notes | Study author to be contacted for: random sequence generation, allocation concealment and study protocol. We could not contact the authors as their e‐mail details were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the CFU were manually counted or any automated colony counters were used ‐ there is subjectivity if manually counted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
Frere 2016.
| Study characteristics | ||
| Methods | Trial design: RCT ‐ n of 1 trial, split‐mouth simulation trial Location: Jacobi Medical Center, Bronx, New York, USA Setting: dental clinic Language: English Number of centres: 1 Study period: 2015 to 2016 Funding source: not reported Study protocol: Albert Einstein College of Medicine IRB number 2015‐4827 | |
| Participants | Age: 18 years or older
Total number of participants: 1 (randomized for each procedure – 12 trials per group)
Inclusion criteria: no dental procedures completed in the preceding year, or antibiotic or steroid therapy in the preceding year
Exclusion criteria: respiratory infection; cardiovascular disease; received antibiotic or steroid therapy in the 12 months prior to the study; requiring antibiotic prophylaxis prior to dental procedures; and requiring medication for a medical condition
Number randomized: 36 trials were done (12 trials per group) Method of randomization: the order of the trials were randomized each day using computer‐generated randomization. Wash‐out period: each trial was performed 1 hour apart to allow the aerosols to clear the operatory and bacteria to recolonize the participant’s tooth surfaces. Number evaluated (withdrawals/missing participants): 36 (none) |
|
| Interventions |
Comparison: combination system (Isolite) versus rubber dam + HVE; combination system versus HVE Intervention: Group 1: combination system (Isolite) Group 2: rubber dam with HVE Group 3: HVE alone Number of intervention groups: 1 (group 1) Number randomized to intervention group: 12 Description of intervention: 36 trials were conducted. 3 trials were conducted 1 hour apart each day of the trials. Interventions and control procedures were done in randomized order. Simulated occlusal preparation with high‐speed handpiece ("with a bur blank") was used to generate aerosols. Any co‐interventions: no Comparator: Group name: rubber dam with HVE and HVE alone Number of control groups: 2 (group 2 and 3) Number randomized to control group: 12 Description of control: same as above. Control group underwent procedures with only HVE |
|
| Outcomes | Outcome name: reduction in contamination of aerosols (5 blood agar plates were placed at standardized positions around the participant for each trial. 1 plate was placed on the participant’s chest. 2 plates were attached to laboratory stands at the 9 o’clock and 3 o’clock position around the participant’s oral cavity. 1 plate was placed on the dental assistant’s cart at the 2 o’clock position. The last single plate was placed at the 7 o’clock position on a countertop away from the participant’s oral cavity.)
Outcome measurement: CFU
Effect estimate: mean (SD) Key conclusions: the results suggest that Isolite can be used for reduction of aerosol/spatter. There was no difference between HVE and HVE with rubber dam. |
|
| Notes | Study author (Farhad Yeroshalmi) provided random sequence generation details on personal communication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization was used (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CFU counting was done by blinded researcher who did hand counting of the colonies |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | We are not sure if the quantity of bacterial colonisation will be same after 1 hour (washout period) between each trial and whether it affects both the groups similarly. This can affect the CFU in the aerosols. |
Hallier 2010.
| Study characteristics | ||
| Methods | Trial design: CCT, split‐mouth design Location: School of Dentistry, Cardiff University, Heath Park, Cardiff, UK Setting: 3 dental clinics in the university ‐ clinic 1 and 2 ‐ large open multi‐chair clinical areas, clinic 3 ‐ single‐chair room Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: not mentioned Total number of participants: 8 (2 participants for each treatment episode, thereby allowing comparison of bioaerosols with and without the ACS in operation) Inclusion and exclusion criteria: not mentioned Number randomized: not available Number evaluated (withdrawals/missing participants): 8 (none) | |
| Interventions |
Comparison: air cleaning system versus no air cleaning system Intervention: Group name: with IQAir Flex Vac™ Air Cleaning System in operation. The air cleaning system is a general air filtering system consisting of High Efficiency Particulate Air (HEPA) pre‐filters, which retain particles less than 0.3 μm in size (which includes bacteria and many types of virus), a second filtration stage involves 4 cylinder gas filter cartridges, which remove mercury vapour, formaldehyde, glutaraldehyde and odours, and a final filtration stage comprises an electrostatically charged post‐filter. Number of intervention groups: 1 Number randomized to intervention group: not available Description of intervention: the study involved 8 participants (2 participants for each treatment episode, thereby allowing comparison of bioaerosols with and without the ACS in operation) treated by 8 dental students in an attempt to minimize participant and operator bias. Sampling was undertaken in the same dental units in each of the 3 clinics at baseline and during the 4 procedures. The ACS and sampling pump were placed in the same position throughout the study. The blood agar plates were replaced every 10 minutes in the sampling pump during the course of each treatment procedure. Each dental procedure tested was performed on different days. In the intervention group, the IQAir Air Cleaning System (ACS) was activated 1 hour before samples were taken and was operated continuously at 500 m³/hour. History and oral examination, using a standard probe and a mirror, were assessed in Clinic 1. The same clinic was used for the assessment of participants undergoing cavity preparation using a high‐speed dental handpiece. Ultrasonic scaling, in conjunction with high‐volume aspiration (HVA), was assessed in Clinic 2, while tooth extraction under local anaesthesia was assessed in Clinic 3. All baseline and procedure sampling were performed with the clinic windows closed and no air conditioning systems or fans on. The room temperature in all 3 clinical areas was between 21 °C and 24 °C. Any co‐interventions: no Comparator: Group name: without IQAir Flex Vac™ Air Cleaning System in operation Number of control groups: 1 Number randomized: not reported Description of control: same as above without the ACS in operation |
|
| Outcomes | Outcome name: mean bacterial count with and without ACS Outcome measurement: CFU/m³ with an air pump connected to a Petri dish (Buck Bio‐Culture™ (Model B30120; A. P. Buck, Inc) Effect estimate: mean (SD not given) Key conclusions: "the results of the present study also provide the first evidence that an ACS can significantly reduce the bioaerosol load during dental procedures. However, on no occasion was the level reduced to that encountered at baseline. Regardless of this, it can be concluded here that the IQ Air Flex Vac™ ACS was efficient at reducing the mean bacterial aerosols within a dental clinic." | |
| Notes | Study authors were contacted for: random sequence generation, allocation concealment, blinding, number randomised, age of participants, SD and study protocol. E‐mail sent on 26 July 2020 and we are yet to receive any response from the contact author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 1 participant was allocated to the group with the ventilation system on and another side of the same participant was treated with the system off. |
| Allocation concealment (selection bias) | High risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No details available for participant blinding. However, personnel blinding was not possible and neither of them will be able to alter their behaviour even if they knew the intervention received. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the CFU were manually counted or any automated colony counters were used ‐ there is subjectivity if manually counted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | Quote: "Each dental procedure tested was performed on different days." This ensured adequate washout period. |
Holloman 2015.
| Study characteristics | ||
| Methods | Trial design: RCT – parallel arm Location: General and Oral Health Center in the School of Dentistry at the University of North Carolina at Chapel Hill, USA Setting: single enclosed dental operatory Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 18 years or older Total number of participants: 50 (25 per group) Inclusion criteria:
Exclusion criteria:
Number randomized: not reported Number evaluated (withdrawals/missing participants): 50 (no dropouts) |
|
| Interventions |
Comparison: combination system versus saliva ejector Intervention: Group name: the test device was the Isolite illuminated dental isolation system (test device attaches to high‐volume suction, and is expected to behave similarly to the HVE) Number of intervention groups: 1 Number randomized to intervention group: 25 Description of intervention: study compared the reduction in aerosols and spatter when using the Isolite suction device in the intervention group during ultrasonic scaling in a clinical environment using a 30‐kHz Cavitron Select SPS Ultrasonic Scaler and a Dentsply 30K slimline scaling tip. Quote: "The airflow in the operatory was set to an exchange rate of 6 to 8 times per hour. Each participant was seated in a supine position during the cleaning and was treated by the same clinician. The clinician (J.L.H.) was a licensed dental hygienist with 5 years of clinical experience and 3 years of experience with the test device. Before ultrasonic scaling, a single Petri dish containing the 20 mL of sterile DPBS solution was placed centrally 6 inches from the oral cavity. At the onset of ultrasonic scaling, the lid to the Petri dish was removed for the duration of ultrasonic scaling, and the exposure time was recorded. Immediately after exposure to the ultrasonic scaler, the Petri dish was recapped and replaced with a second Petri dish containing 20 mL of fresh DPBS. The second Petri dish remained open for 35 minutes to collect postexposure aerosols; the operator then recapped it. The remainder of the participants’ prophylaxis proceeded without the use of any devices that would create aerosols or spatter, such as those used in coronal polishing or an air‐powder polisher. To prevent cross‐contamination of aerosols, we scheduled only 1 participant per day." Any co‐interventions: no Comparator: Group name: the positive control was the standard saliva ejector, a disposable attachment to the low‐volume suction hose. Number of control groups: 1 Number randomized to control group: 25 Description of control: same as above except that a standard saliva ejector, a disposable attachment to the low‐volume suction hose was used as the control. |
|
| Outcomes | Outcome name: reduction in the level of contamination in aerosols (trial authors call it reduction in aerosols and splatter. But the measurement is done using CFU)
Outcome measurement: CFU/ml
Effect estimate: mean log10 (SD) Key conclusions: practical implications of this study suggest that neither the Isolite device nor the saliva ejector effectively reduced aerosols and spatter during ultrasonic scaling |
|
| Notes | Study authors were contacted for: allocation concealment, blinding, number randomised and study protocol. E‐mail sent on 26 July 2020 and we are yet to receive any response from the contact author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomized with the flip of a coin into 1 of 2 treatment groups” |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded. Quote: "After incubation, the principal investigator counted the Brucella agar plates by hand and recorded the results. Each sample was marked with a number so that the investigator was masked as to the device used for each sample when recording CFUs." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Data for 2 participants were excluded from the analysis owing to incorrect dilution of the DPBS in their samples” |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
Jawade 2016.
| Study characteristics | ||
| Methods | Trial design: RCT – parallel, 3 arms Location: MIDSR Dental College, Latur, Maharashtra, India. Setting: closed dental operatory Language: English Number of centres: 1 Study period: July 2015 to October 2015 Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 22 to 55 years Total number of participants: 30 participants (10 per group) (17 male, 13 female) Inclusion criteria: minimum of 20 permanent functional teeth, and mean probing depth ≤ 5 mm and clinical attachment loss ≤ 3 mm measured with Williams Periodontal Probe (Hu‐Friedy) in at least 30% teeth sites Exclusion criteria: a history of systemic diseases like diabetes mellitus, hypertension, rheumatoid arthritis, etc, use of tobacco in any form, history of periodontal treatment in the preceding 6 months, pregnant and lactating females, thyroid dysfunction, use of antibiotic or other drugs that affect periodontal status in the preceding 6 months or allergic to chlorhexidine and povidone iodine Number randomized: 30 (10 per group) Number evaluated (withdrawals/missing participants): 30 (none) |
|
| Interventions |
Comparison: antimicrobial coolant A (povidone iodine) versus antimicrobial coolant B (chlorhexidine gluconate) Intervention: Group name: Group 2 (test group): ultrasonic scaling with 2% povidone iodine in 0.1% dilution (10 participants) 2% povidone iodine is diluted in 1:1 ratio in 1 litre water to prepare ultrasonic liquid coolant Group 3 (test group): ultrasonic scaling with 0.12% chlorhexidine in 0.06% dilution (10 participants) 0.12% chlorhexidine gluconate is diluted in 1:1 ratio in 1 litre water to prepare ultrasonic liquid coolant Number of intervention groups: 2 Number randomized to intervention group: 10 per group Description of intervention: ultrasonic scaling was carried out for 20 minutes by the clinician, with universal tip attached to the ultrasonic scaler. The normal rate of flow of water in ultrasonic scaler is 20 to 30 ml/min. The same rate of flow of water for each agent (2% povidone iodine in 0.1% dilution and 0.12% chlorhexidine in 0.06% dilution) while performing ultrasonic scaling was maintained. To assure that the room was free from aerosols, only 1 person was treated per day. For every scaling procedure, high‐vacuum suction was used. After the treatment, 3 coded blood agar plates were left uncovered for 20 minutes at the pre‐designated sites for gravitometric settling of airborne bacteria. After gravitometric settling of aerosols, blood agar plates were transferred to laboratory for incubation at 37 °C for 48 hours followed by colony counting procedure with the help of colony counter device by the microbiologist. Any co‐interventions: no Comparator: Group name: Group 1 (control group): ultrasonic scaling with distilled water (10 participants) Number of control groups: 1 Number randomized to control group: 10 Description of control: as described above except that the ultrasonic coolant was distilled water |
|
| Outcomes | Outcome name: reduction in contamination of aerosol (positions selected for agar plates was 0.4 metres on right, 0.4 metres on left and 2 metres behind the participant)
Outcome measurement: CFU (using blood agar plates)
Effect estimate: mean (SD) Key conclusions: this study indicates that chlorhexidine gluconate as an ultrasonic liquid coolant significantly reduces the microbial content of dental aerosols generated during scaling when compared with distilled water. Chlorhexidine gluconate showed better CFU reductions when compared with povidone iodine. Povidone iodine also showed better CFU reduction when compared with distilled water. |
|
| Notes | Study author were contacted for: allocation concealment, blinding and study protocol. E‐mail sent on 26 July 2020 and we are yet to receive any response from the contact author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “All subjects were assigned to one of the three groups by using randomization table...” Method of randomization: randomization table |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After gravitometric settling of aerosols, blood agar plates were transferred to laboratory for incubation at 37°C for 48 hours followed by colony counting procedure with the help of colony counter device by the microbiologist." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
King 1997.
| Study characteristics | ||
| Methods | Trial design: RCT – split‐mouth design Location: Baylor College of Dentistry, Dallas, USA Setting: single enclosed dental operatory Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 21 to 63 years; mean age 39 years Total number of participants: 12 (1 male, 11 female) Inclusion criteria: currently not taking antibiotics, not wearing a cardiac pacemaker, absence of respiratory infection Exclusion criteria: not mentioned Number randomized: 12 Number evaluated (withdrawals/missing participants): 12 (none) | |
| Interventions |
Comparison: HVE versus no HVE Intervention: Group name: with aerosol reduction device (ARD) Number of intervention groups: 1 Number randomized to intervention group: 12 Description of intervention: split mouth ‒ 1 side (maxillary and mandibular) of the mouth was scaled by using a magneto‐strictive ultrasonic scaler without the aerosol reduction device (control), and the opposing side was scaled by using the ultrasonic scaler with the aerosol reduction device (intervention). Any co‐interventions: nil Comparator: Group name: without ARD Number of control groups: 1 Number randomized to control group: 12 Description of control: as above. |
|
| Outcomes | Outcome name: reduction in contamination of aerosol (at 6 inches from the participant's mouth and on face shield)
Outcome measurement: CFU. "Three blood agar plates were then placed 6 inches in front of patient's mouth, right and left side. To ensure that the entire area of the face shield was sampled, 3× 21/i‐inch RODAC plates were used. The right, middle, and left sides of the face shield were lightly pressed by separate RODAC plates."
Effect estimate: mean (SD) Key conclusions: "these data suggest that an aerosol reduction device is effective in reducing the number of microorganisms generated during ultrasonic scaling, therefore decreasing the risk of disease transmission." |
|
| Notes | We wanted to contact the study author for method of randomisation, allocation concealment, blinding and study protocol, but their e‐mail details were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details about method of randomisation: "The right or left side of the subject's mouth was randomly assigned to one of the two treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quebec Colony Counter (Leica, Deerfield, IL) was used to count the colonies, which is an automated colony counter and is an objective finding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
Muzzin 1999.
| Study characteristics | ||
| Methods | Trial design: RCT – split‐mouth design Location: Baylor College of Dentistry, Dallas, USA Setting: 2 separate enclosed dental operatories Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 21 to 57 years – mean age 30 years Total number of participants: 30 (7 male, 23 female) Inclusion criteria: minimum of 20 permanent teeth (10 on each side of the mouth). Exclusion criteria:
Number randomized: 30 Number evaluated (withdrawals/missing participants): 30 |
|
| Interventions |
Comparison: HVE versus no HVE Intervention: Group name: with aerosol reduction device (ARD) Number of intervention groups: 1 Number randomized to intervention group: 30 Description of intervention: split mouth – 1 side (maxillary and mandibular) of the mouth was scaled by using an air polisher without the aerosol reduction device (control); and the opposing side, air polisher was used with the aerosol reduction device (intervention). Any co‐interventions: no Comparator: Group name: without ARD Number of control groups: 1 Number randomized to control group: 30 Description of control: as above |
|
| Outcomes | Outcome name: reduction in contamination of aerosol (at 2 areas – 12 inches from mouth and on face mask) Outcome measurement: CFU (using blood agar plates) Effect estimate: mean (SD) Key conclusions: the results of this investigation suggest that the aerosol reduction device attached to the air polisher is effective in reducing the amount of microbially contaminated aerosol and spatter that are generated during air polishing. | |
| Notes | We wanted to contact the study author for method of randomisation, allocation concealment, blinding of participants and study protocol, but their e‐mail details were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details about method of randomisation: "We randomly assigned the order of use of the air polisher with and without the aerosol reduction device as well as sampling of the right and left sides of the subject’s mouth." |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded. Quote: "Three microbiologists (C.B. and two others), blinded to each treatment group assignment, counted the number of CFUs on each plate using the Quebec Colony Counter and a hand‐tally counter." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
Narayana 2016.
| Study characteristics | ||
| Methods | Trial design: CCT – split mouth (multi‐arm) Location: G Pulla Reddy Dental College, Kurnool, Andhra Pradesh, Oxford Dental College, Bangalore, India Setting: good ventilated room measuring about 20 feet × 15 feet with single dental chair Language: English Number of centres: 2 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: not given
Total number of participants: 15 (for HVE group)
Inclusion criteria: patients undergoing supragingival scaling
Exclusion criteria: immunocompromised patients or patients with systemic diseases
Method of randomization: not mentioned
Method of allocation concealment: not mentioned Method of blinding: not mentioned Number evaluated (withdrawals/missing participants): 15 (no dropouts) |
|
| Interventions |
Comparison: HVE versus no HVE Intervention: Group A: with and without preprocedural rinse with 0.12% chlorhexidine rinse Group B: with and without HVE Group C: with and without preprocedural rinse with 0.12% chlorhexidine rinse and HVE Group name: Group B: 2nd and 3rd quadrant – with HVE Number of intervention groups: 1 Number of participants in the intervention group: 15 Description of intervention: Group B consists of 15 individuals with 1st and 4th quadrants undergoing supragingival scaling without HVE, 2nd and 3rd quadrants undergoing supragingival scaling with HVE. Any co‐interventions: no (2 other group was present Group A ‐ with and without preprocedural rinse and Group C – combination) Comparator: Group name: Group B: 1st and 4th quadrant ‐ without HVE Number of control groups: 1 Number of participants in the control group: 15 Description of control: Group B consists of 15 individuals with 1st and 4th quadrants undergoing supragingival scaling without HVE, 2nd and 3rd quadrants undergoing supragingival scaling with HVE. |
|
| Outcomes | Outcome name: reducing bioaerosol contamination during ultrasonic scaling procedure (blood agar plates were used to check CFU) Outcome measurement: CFU Effect estimate: mean (SD not given) Key conclusions: CFU were significantly reduced with the use of HVE in Group B individuals. | |
| Notes | We contacted the study authors for: SD and study protocol. E‐mail sent on 18 June 2020 and we are yet to receive any response from the contact author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled clinical trial |
| Allocation concealment (selection bias) | High risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the CFU were manually counted or any automated colony counters were used – there is subjectivity if manually counted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available. |
| Other bias | Low risk | None |
Samaranayake 1989.
| Study characteristics | ||
| Methods | Trial design: CCT Location: University of Glasgow, Dental Hospital and School, UK Setting: 12 feet by 15 feet in the pedodontic clinic Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 10 to 14 years Total number of participants: 20 Inclusion criteria: children who required restorative procedures Exclusion criteria: not mentioned Number of participants: 20 (10 per group) Confounding factors: not found Selection bias: not found Method of blinding: not available Number evaluated (withdrawals/missing participants): 20 (none) | |
| Interventions |
Comparison: rubber dam versus no rubber dam Intervention: Group name: with rubber dam Number of intervention groups: 1 Number in intervention group: 10 Description of intervention: 10 children in the intervention group underwent restorative procedures with rubber dam. Any co‐interventions: no Comparator: Group name: without rubber dam Number of control groups: 1 Number in control group: 10 Description of control: 10 children in the control group underwent restorative procedures without rubber dam. |
|
| Outcomes | Outcome name: reduction in contamination of aerosols (measured at 1 metre, 2 metres, 3 metres from head rest) Outcome measurement: CFU Effect estimate: mean + SE Key conclusions: results indicate that the use of a rubber dam perioperatively is associated with significantly higher bacterial aerosol levels and bacterial reduction was greatest 1 metre from head rest. | |
| Notes | Study author to be contacted for: method of randomisation, allocation concealment, blinding and study protocol. Study is 31 years old – missing data unlikely to be traceable. We could not contact the authors as their e‐mail details were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Details on confounding factors not found |
| Allocation concealment (selection bias) | High risk | Details of selection process not available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the CFU were manually counted or any automated colony counters were used – there is subjectivity if manually counted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available |
| Other bias | Low risk | None |
Sethi 2019.
| Study characteristics | ||
| Methods | Trial design: RCT – 3‐arm parallel design Location: MGV's K.B.H. Dental College and Hospital, Mumbai, India Setting: closed operatory Language: English Number of centres: 1 Study period: not mentioned Funding source: none mentioned Study protocol: not available | |
| Participants | Age: 18 to 55 years Total number of participants: 60 (20 per group) Inclusion criteria:
Exclusion criteria:
Number randomized: 60 (20 per group) Method of randomization: computer‐generated random sequence table Method of allocation concealment: allocated into 3 groups by 1 examiner while the treatment was performed by another examiner. Method of blinding: not mentioned Number evaluated (withdrawals/missing participants): 60 (none) |
|
| Interventions |
Comparison: chlorhexidine coolant versus distilled water or cinnamon extract coolant Intervention: Group name: Group I: chlorhexidine used as ultrasonic coolant (20 participants) Group II: cinnamon extract used as ultrasonic coolant (20 participants) Number of intervention groups: 2 Number randomized to intervention group: 20 per group Description of intervention: dental chairs with self‐contained water system were selected. The agents were added in the dental unit waterlines. Strict asepsis was observed. Participants were prepared to enter the operatory by wearing headcaps and autoclaved gowns. Participants were instructed to refrain from all actions that would generate aerosols e.g. conversation, sneezing, and coughing. Single‐sitting ultrasonic scaling was done for all patients for 20 minutes, using ultrasonic scaler. During each scaling procedure, saliva ejector was used. After the procedure, participants were asked about any discomfort noticed such as alteration in taste or burning sensation during debridement. Participants were asked to report to dental office if any adverse effects were experienced after treatment. Any co‐interventions: no Comparator: Group name: group III: distilled water used as ultrasonic coolant (20 participants). Number of control groups: 1 Number randomized to control group: 20 Description of control: same as above except that in the control group distilled water was used as the coolant. |
|
| Outcomes | Outcome name: reduction in contamination of aerosols (measured at distance of 1 foot from mouth to patient's chest, right side and left side) Outcome measurement: CFU Effect estimate: mean (SD) Key conclusions: no difference between the 2 intervention groups (chlorhexidine and cinnamon groups) but significant difference between both intervention groups and control (distilled water group) Within the limitations of this study, both cinnamon and chlorhexidine when used as an ultrasonic coolant effectively helped in the reduction of bacterial contamination in dental aerosols, which was seen by reduction in the CFU, after adding these agents in the DUWL. | |
| Notes | Study authors were contacted for: allocation concealment and study protocol. E‐mail sent on 26 July 2020 and reply received on 27 July 2020. The protocol was not published anywhere and was only submitted to ethics committee. Allocation concealment was carried out by an independent investigator who took care of the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence was used |
| Allocation concealment (selection bias) | Low risk | Quote: “..allocated into 3 groups by one examiner while the treatment was performed by another examiner” Quote from the personal communication: "Regarding allocation concealment: One of the authors was appointed for this work. The operator and the patient were blind regarding the groups. Only the author responsible for the concealment would take care of the groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the CFU were manually counted or any automated colony counters were used – there is subjectivity if manually counted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no published protocol available. |
| Other bias | Low risk | None |
Timmerman 2004.
| Study characteristics | ||
| Methods | Trial design: RCT – split‐mouth design Location: Centre for Dentistry, Amsterdam, the Netherlands Setting: closed operatory room Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: 43 to 69 years Total number of participants: 6 (3 male, 3 female) Inclusion criteria:
Exclusion criteria: use of antibiotics or topical antiseptics during a period of 30 days prior to the study Number randomized: 6 Number evaluated (withdrawals/missing participants): 6 |
|
| Interventions |
Comparison: HVE versus conventional dental suction (low‐volume evacuator) Intervention: Group name: with HVE (high‐volume evacuation) Number of intervention groups: 1 Number randomized to intervention group: 6 Description of intervention: the study included 17 treatment sessions, consisting of a 40‐minute episode of continuous plaque and calculus removal using an ultrasonic unit (EMS). The treatment sessions were carried out in 6 patients with generalized adult periodontitis and ranged from 2 to 4 sessions per patient according to their needs. The use of HVE and CDS was randomly assigned over the sessions within each patient. Before each treatment, the operating room was not used for 15 hours. To measure baseline microbial air pollution 2 Petri dishes containing blood agar were exposed for 10 minutes to the air. At the start of each treatment session, 2 Petri dishes were exposed for 5 minutes at a distance of 40 cm from the mouth of the patients. After 20 minutes, this procedure was repeated. At a distance of 150 cm, 2 Petri dishes were exposed for 20 minutes followed by exposure of 2 new Petri dishes for the rest of the session. The plates were cultured aerobically and anaerobically for 3 and 7 days, respectively Any co‐interventions: no Comparator: Group name: with conventional dental suction (CDS) Number of control groups: 1 Number randomized to control group: 6 Description of control: as described above |
|
| Outcomes | Outcome name: reduction in contamination of aerosols Outcome measurement: CFU (using blood agar plates), both aerobic and anerobic culture were done Effect estimate: mean (SD) Key conclusions: the results of the present study showed no differences when different methods of suction were used. This might indicate that the amount of aerosol with small particle size, able to carry bacteria over a larger distance, as produced by the present piezoelectric device, is relatively limited. The use of a high‐volume evacuator may, however, help to minimize risks of air microbial contamination. | |
| Notes | We contacted the study author for method of randomisation, allocation concealment, personnel blinding and study protocol. We sent an e‐mail on 26 July 2020 to the contact author and the e‐mail bounced back. We could not contact other authors as their e‐mail details are not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not possible. However, participants and personnel will not be able to alter their behaviour even if they know the received intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the CFU were manually counted or any automated colony counters were used – there is subjectivity if manually counted. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all 6 participants are reported |
| Selective reporting (reporting bias) | Unclear risk | We are not sure of reporting selective outcomes as there is no protocol available |
| Other bias | Low risk | None |
Williams 1970.
| Study characteristics | ||
| Methods | Trial design: CCT – split mouth (alternation) Location: University of Maryland School of Dentistry, Baltimore, Maryland, USA Setting: dental operatory Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available | |
| Participants | Age: not mentioned Total number of participants: 50 Inclusion criteria: patients who volunteered to receive prophylactic procedures, which involved cleaning and scaling of the maxillary and mandibular right or left sides of the dental arches on an alternating basis with an ultrasonic cleaning and scaling device Exclusion criteria: not mentioned Number alternated: 50 Method of randomization: alternation Method of allocation concealment: not mentioned Method of blinding: not mentioned Number evaluated (withdrawals/missing participants): 50 (nil) | |
| Interventions |
Comparison: laminar air flow and HEPA filter versus no air flow or filter Intervention: Group name: with laminar air flow and HEPA filter Number of intervention groups: 1 Number alternated to intervention site and control site: 50 Description of intervention: ceiling to floor laminar airflow that enters the room through a HEPA filter. In the dental procedure, the ultrasonic scaling device was used around all teeth supragingivally and subgingivally; dental tape was used interproximally and linen strips instituted where necessary. The teeth were polished with a mounted, webbed rubber cup and a flavoured prophylactic paste. At the completion of this procedure, the room was 'air washed' for 5 minutes, and then the other side of the mouth was given a dental prophylactic treatment while the environmental air in the room was washed with laminar air. Microbial samplings from dental aerosols were performed during the entire procedure. Any co‐interventions: no Comparator: Group name: without laminar air flow or filter Number of control groups: 1 Number alternated to control group: 50 Description of control: the other half of the mouth was given a complete prophylactic treatment without laminar air. |
|
| Outcomes | Outcome name: reduction in contaminated aerosols (measured as viable particle count = CFU)
Outcome measurement: microbial samplings from airborne dental aerosols were taken throughout the room by Reyniers slit samplers at about 1.5 metres from the floor, and the Andersen sampler collected oral aerosol samples approximately 20 cm to 30cm from the patient's mouth measured as CFU per cubic feet per minute. Surface contamination by dental aerosols was also assayed both by gravimetric settling plates and Rodac contact plates. Effect estimate: total (SD not given). We converted this to an average per patient and used the SD from a similar study (Larato 1967). Key conclusions: through the use of laminar airflow in a dental operatory, dental aerosols containing micro‐organisms disseminated into the environmental air by an ultrasonic scaling device can be significantly reduced (99.67%); the risks of exposure to airborne infection are considerably minimized; and surface contamination can be controlled to near‐sterile conditions. |
|
| Notes | Study is 50 years old – missing data unlikely to be traceable. We could not contact the authors as their e‐mail details were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation |
| Allocation concealment (selection bias) | High risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lack of blinding the participants and personnel will not have any effect on their behaviour |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available and hence not possible to judge |
| Other bias | Low risk | None |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bentley 1994 | Compared 2 different dental aerosol‐producing procedures after using an aerosol‐reducing device |
| Larato 1967 | Used preprocedural antiseptic mouthrinse and no control group |
| Muir 1978 | Evaluated preprocedural mouthrinse |
| Watanabe 2018 | Compared contamination of PPE before and after dental treatment |
| Yamada 2011 | No control group |
Characteristics of studies awaiting classification [ordered by study ID]
Allison 2020.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Pre‐print stage |
Klyn 2001.
| Methods | Trial design: RCT‐split mouth design Location: Keesler Air Force Base, United States Air Force, USA Setting: dental operatory Language: English Number of centres: 1 Study period: not mentioned Funding source: no financial interest in any products, equipment or companies cited in the manuscript Study protocol: not available |
| Participants | Age: 21 to 63 years Sex: not reported Total number of participants: 15 Inclusion criteria: volunteers whose treatment plan included complete mouth ultrasonic scaling without any history of cardiac or renal or hepatic or blood dyscrasia or immunosuppresive problems, antibiotic intake and not undergone dental treatment 3 months prior to the study period. Exclusion criteria: breastfeeding women Number randomised: not reported Randomisation of quadrant: done Random sequence generation: not reported Allocation concealment: not reported Method of blinding: the medical laboratory technician who recorded the CFU was blinded and reported based only on the culture plate number. Number evaluated (withdrawals/missing participants): none |
| Interventions | Comparison: ultrasonic scaling done on each quadrant. Each group was treated in a different room. Group 1: control (no aerosol reduction device (ARD) or preoperative CHX) Group 2: ARD only Group 3: preoperative CHX rinse only Group 4: use of both ARD and CHX rinse Washout period: not reported Number of intervention groups: 3 Intervention: Group name: ARD (Group 2) Description of intervention: SAFETY Suction ARD was attached to the high‐speed evacuation system and the cavitron handpiece Any co‐interventions: saliva ejector was used in all groups Comparator: Group name: control (Group 1) Group name: teeth were scaled using ultrasonic scaler for 5 minutes using a newly purchased 30,000 hz scaler and distilled water without ARD or CHX rinse. Number of control groups: 1 |
| Outcomes | Outcome name: reduction in level of contamination in aerosols Outcome measurement: CFU (using blood agar plates) aerobic culture were done. 3 culture plates were placed on a plexiglass mount 6 inches and 1 agar plate on left side 2 feet from patient's oral cavity. Effect estimate: mean (SD) Key conclusions: ARD or preoperative CHX rinse reduces bacterial contamination in aerosols during ultrasonic scaling. ARD reduces more contamination when compared to preoperative CHX rinse and combination of ARD and preoperative CHX rinse had no additional benefit when compared to the use of ARD alone. |
| Notes | Washout period not reported |
Llandro 2020.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Pre‐print stage |
Worrall 1987.
| Methods | Trial design: CCT Location: Periodontal clinic, Birmingham Dental Hospital, UK Setting: dental operatory Language: English Number of centres: 1 Study period: not mentioned Funding source: not mentioned Study protocol: not available |
| Participants | Age: not mentioned Sex: not mentioned Total number of participants: not reported Inclusion criteria: patients who require stain removal Exclusion criteria: not mentioned Method of blinding: not mentioned Number evaluated (withdrawals/missing participants): not reported |
| Interventions |
Comparison: Group 1: negative control (air polishing on a sterile stainless steel bowl) Group 2: positive control (air polishing on patient's teeth using conventional saliva ejector) Group 3: preoperative CHX mouthrinse Group 4: high‐volume aspiration with wide‐bore tip Number of intervention groups: 2 Intervention: Group name: high‐volume aspiration with wide‐bore tip Description of intervention: patients' teeth were air‐polished using Prophy Jet and a dental surgery assistant was holding the high‐volume aspiration apparatus Any co‐interventions: no Comparator: Group name: air polishing on a sterile stainless steel bowl Group name: air polishing on patient's teeth using conventional saliva ejector Number of control groups: 2 |
| Outcomes | Outcome name: reduction in level of contamination in aerosols Outcome measurement: CFU (using blood agar plates), aerobic culture were done. Culture plates placed at 1, 2 and 3 metres from the headrest of the chair. Culture was done 10 minutes before the procedure (resting), during the procedure and 20 minutes after the procedure. Effect estimate: mean (SE) Key conclusions: high‐volume aspirator is very effective in reducing airborne contamination produced during air‐polishing. If this is not available, 0.2% CHX pre‐rinsing is recommended prior to air‐polishing. |
| Notes | Number of participants not reported |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN10378358.
| Study name | Oral fluorescein as a biomarker for droplet and aerosol spread of COVID‐19 within a clinical environment |
| Methods | Multi‐centric randomised controlled trial. Saliva will be stained using Fluorescein 2%, 1 drop in the mouth. Aerosols will be imaged with hyperspectral, forensic photography and microscopy to detect minute droplet particles. |
| Participants | 9 groups of participants who will be undergoing 9 different AGPs including dental procedures involving high‐speed drilling/hygiene |
| Interventions | Rubber dam versus no rubber dam |
| Outcomes | Proportion of patients where the spread of airborne droplets during AGP is > 50 drops on a detection pad at 1 m |
| Starting date | 15 June 2020 |
| Contact information | Prof. Richard Newsom, Faculty of Science and Health, White Swan Road, Porstmouth, PO1 2DT, United Kingdom Phone: +44 (0)23 9284 2994 E‐mail: richard.newsom@port.ac.uk |
| Notes | Overall trial end date: 1 March 2022 |
NCT04430387.
| Study name | Evaluation of impact on environmental spatter using different isolation methods during hygiene appointment among pediatric patients |
| Methods | Randomised controlled trial ‐ single blinded "The image of the spots of fluorescence from the spatter collected will be captured using a digital camera with an amber‐colored lens cover. The image will be processed by a digital imaging software to get the number of the spots on each mask and film. The number of fluorescent spots is recorded to determine the amount of spatter produced." |
| Participants | Children from 4 to 15 years age who need dental prophylaxis or restorative procedure not requiring sedation or nitrous oxide |
| Interventions | Group 1 ‐ saliva ejector; Group 2 ‐ high‐volume evacuator (HVE); Group 3 ‐ DryShield |
| Outcomes | To collect, measure, and assess the environmental spatter produced during dental appointments under different isolation methods used in pediatric dentistry |
| Starting date | 11 June 2020 |
| Contact information | Contact: Di I Wu, The University of Texas Health Science Center, Houston, USA, Phone: 7135008220 E‐mail: di.wu@uth.tmc.edu |
| Notes | Overall trial end date: February 2022 |
Differences between protocol and review
We screened search records in duplicate and checked the eligibility of full texts in duplicate.
We did not specify in the protocol that we would analyse RCTs and CCTs separately. We also omitted to specify that we would use generic inverse variance for split‐mouth studies.
We intended to use the ROBINS‐I Risk of bias tool for non‐randomized studies (Sterne 2016). We finally decided that the studies did not contain enough information for a proper assessment of confounding and selection bias and used the Cochrane Risk of bias tool for all studies. For CCTs we rated the first two domain randomization and allocation concealment at high risk of bias. The other domains were applied in the same way for all studies.
We had intended to conduct a subgroup analysis for the position of the culture plates but decided that these were actually different outcomes that could not be combined. We reported the outcomes for a distance of less than one foot from the patient's mouth, more than one foot from the patient's mouth and on the dentist's head, separately. At a short distance, the culture plates catch bigger and heavier droplets; at a distance further away, the plates will catch smaller particles that are suspended in the air for a longer time.
Contributions of authors
SKN initiated this review and all authors contributed to drafting the protocol.
Responsibilities for the full review were as follows.
SKN: obtaining full‐text articles, data extraction, data analysis and final review draft preparation, and review update. PE: drafting protocol, data extraction, data analysis and final review draft preparation. MP: drafting protocol, screening of titles and abstracts, screening full texts and final review draft preparation. MN: drafting protocol, arbiter, data extraction, analysis and final review draft preparation. GS: drafting protocol, screening of titles and abstracts, screening full texts and final review draft preparation. JHV: drafting protocol, data extraction and analysis, writing results and discussion.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
-
Cochrane Oral Health Global Alliance, Other
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oral‐health.cochrane.org/partnerships‐alliances). Contributors over the past two years have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
Declarations of interest
SKN: none PE: none MP: none MN: none GS: none JHV: none
Edited (no change to conclusions)
References
References to studies included in this review
Al‐amad 2017 {published and unpublished data}
- *.Al-amad 2017 [pers comm]. Allocation concealment and standard deviations used in our trial. E-mail to Prashanti Eachempati 28 July 2020.
- *.Al-amad SH, Awad MA, Edher FM, Shahramian K, Omran TA. The effect of rubber dam on atmospheric bacterial aerosols during restorative dentistry. Journal of Infection and Public Health 2017;10(2):195-200. [DOI] [PubMed] [Google Scholar]
Cochran 1989 {published data only}
- Cochran MA, Miller CH, Sheldrake MA. The efficacy of the rubber dam as a barrier to the spread of microorganisms during dental treatment. Journal of American Dental Association 1989;119(1):141-4. [DOI] [PubMed] [Google Scholar]
Desarda 2014 {published data only}
- Desarda H, Gurav A, Dharmadhikari C, Shete A, Gaikwad S. Efficacy of high-volume evacuator in aerosol reduction: truth or myth? A clinical and microbiological study. Journal of Dental Research, Dental Clinics, Dental Prospects 2014;8(3):176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devker 2012 {published data only}
- Devker N, Mohitey J, Vibhute A, Chouhan VS, Chavan P, Malagi S, et al. A study to evaluate and compare the efficacy of preprocedural mouthrinsing and high volume evacuator attachment alone and in combination in reducing the amount of viable aerosols produced during ultrasonic scaling procedure. Journal of Contemporary Dental Practice 2012;13(5):681-9. [DOI] [PubMed] [Google Scholar]
El‐Din 1997 {published data only}
- El-Din ALT, Ghoname AE. Efficacy of rubber dam isolation as an infection control procedure in pediatric dentistry. Eastern Mediterranean Health Journal 1997;3(3):530-9. [Google Scholar]
Frere 2016 {published and unpublished data}
- *.Frere AD, Margulis KS, Yeroshalmi F, Badner V. Effectiveness of two isolation techniques in aerosol/spatter reduction. zyris.com/wp-content/uploads/2020/03/Effectiveness-of-Two-Isolation-Techniques-in-Aerosol-Spatter-Reduction.png?utm_campaign=Aerosol%20Emails&utm_medium=email&_hsmi=85208401&_hsenc=p2ANqtz-_iAoute0mH3PhRvvy8C2XbSjyi408OyHhHhHaTxSfqAHIYzkrb0ZPQBFNas7bLzAjzz3hAxlcbddIvK9VGg75xmiB0ug&utm_content=85208401&utm_source=hs_automation 2016.
- Yeroshalmi F [pers comm]. Random sequence generation details of our trial. E-mail to Sumanth Kumbargere Nagraj 24 June 2020.
Hallier 2010 {published data only}
- Hallier C, Williams DW, Potts AJC, Lewis MAO. A pilot study of bioaerosolreduction using an air cleaning system during dental procedures. British Dental Journal 2010;209(8):E14. [DOI: 10.1038/sj.bdj.2010.975] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holloman 2015 {published data only}
- Holloman JL, Mauriello SM, Pimenta L, Arnold RR. Comparison of suction device with saliva ejector for aerosol and spatter reduction during ultrasonic scaling. Journal of the American Dental Association (1939) 2015;146(1):27-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jawade 2016 {published data only}
- Jawade R, Bhandari V, Ugale G, Taru S, Khaparde S, Kulkarni A, et al. Comparative evaluation of two different ultrasonic liquid coolants on dental aerosols. Journal of Clinical and Diagnostic Research : JCDR 2016;10(7):ZC53-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 1997 {published data only}
- King TB, Muzzin KB, Berry CW, Anders LM. The effectiveness of an aerosol reduction device for ultrasonic sealers. Journal of Periodontology 1997;68:45-9. [DOI] [PubMed] [Google Scholar]
Muzzin 1999 {published data only}
- Muzzin KB, King TB, Berry CW. Assessing the clinical effectiveness of an aerosol reduction device for the air polisher. Journal of the American Dental Association 1999;130(9):1354-9. [DOI] [PubMed] [Google Scholar]
Narayana 2016 {published data only}
- Narayana TV, Mohanty L, Sreenath G, Vidhyadhari P. Role of preprocedural rinse and high volume evacuator in reducing bacterial contamination in bioaerosols. Journal of Oral and Maxillofacial Pathology 2016;20(1):59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samaranayake 1989 {published data only}
- Samaranayake LP, Reid J, Evans D. The efficacy of rubber dam isolation in reducing atmospheric bacterial contamination. ASDC Journal of Dentistry for Children 1989;56(6):442-4. [PMID: ] [PubMed] [Google Scholar]
Sethi 2019 {published data only}
- Mamajiwala A [pers comm]. Allocation concealment and protocol publication details of our trial. E-mail reply to Prashanti Eachempati 27 July 2020.
- *.Sethi KS, Mamajiwala A, Mahale S, Raut CP, Karde P. Comparative evaluation of the chlorhexidine and cinnamon extract as ultrasonic coolant for reduction of bacterial load in dental aerosols. Journal of Indian Society of Periodontology 2019;23(3):226-33. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Timmerman 2004 {published data only}
- Timmerman MF, Menso L, Steinfort J, Winkelhoff AJ, Weijden GA. Atmospheric contamination during ultrasonic scaling. Journal of Clinical Periodontology 2004;31:458-62. [DOI: 10.1111/j.1600-051X.2004.00511.x] [DOI] [PubMed] [Google Scholar]
Williams 1970 {published data only}
- Williams GH, Pollok NL, Shay DE, Barr CE. Laminar air purge of microorganisms in dental aerosols: prophylactic procedures with the ultrasonic scaler. Journal of Dental Research 1970;49(6):1498-504. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bentley 1994 {published data only}
- Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. Journal of American Dental Association 1994;125(May):579-84. [DOI] [PubMed] [Google Scholar]
Larato 1967 {published data only}
- Larato DC, Ruskin PF, Martin A. Effect of an ultrasonic scaler on bacterial counts in air. Journal of Periodontology 1967;38(6):550-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Muir 1978 {published data only}
- Muir KF, Ross PW, MacPhee IT, Holbrook WP, Kowolik MJ. Reduction of microbial contamination from ultrasonic scalers. British Dental Journal 1978;145:76-8. [DOI] [PubMed] [Google Scholar]
Watanabe 2018 {published data only}
- Watanabe A, Tamaki N, Yokota K, Matsuyama M, Kokeguchi S. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. Journal of Hospital Infection 2018;99:303-5. [DOI: 10.1016/j.jhin.2018.03.002] [DOI] [PubMed] [Google Scholar]
Yamada 2011 {published data only}
- Yamada H, Ishihama K, Yasuda K, Hasumi-Nakayama Y, Shimoji S, Furusawa K. Aerial dispersal of blood-contaminated aerosols during dental procedures. Quintessence International 2011;42(5):399-405. [PubMed] [Google Scholar]
References to studies awaiting assessment
Allison 2020 {unpublished data only}
- Allison JR, Currie CC, Edwards DC, et al. Evaluating aerosol and splatter following dental procedures: addressing new challenges for oral healthcare and rehabilitation. bioRxiv (pre-print server for Biology). [DOI: 10.1101/2020.06.25.154401] [DOI] [PMC free article] [PubMed]
Klyn 2001 {published data only}
- Klyn SL, Cummings DE, Richardson BW, Davis RD. Reduction of bacteria-containing spray produced during ultrasonic scaling. General dentistry 2001;49(6):648-52. [PubMed] [Google Scholar]
Llandro 2020 {unpublished data only}
- Llandro H, Allison J, Currie C, et al. Evaluating aerosol and splatter during orthodontic debonding: implications for the COVID-19 pandemic. medRxiv (pre-print server for Health Sciences). [DOI: 10.1101/2020.08.19.20178319] [DOI] [PMC free article] [PubMed]
Worrall 1987 {published data only}
- Worrall SF, Knibbs PJ, Glenwright HD. Methods of reducing bacterial contamination of the atmosphere arising from use of an air-polisher. British Dental Journal 1987;163(August):118-9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN10378358 {published data only}
- Oral fluorescein as a biomarker for droplet and aerosol spread of COVID-19 within a clinical environment. Ongoing study. 15 June 2020. Contact author for more information.
NCT04430387 {published data only}
- Evaluation of impact on environmental spatter using different isolation methods during hygiene appointment among pediatric patients. Ongoing study. 11 June 2020. Contact author for more information.
Additional references
ADA 2020
- American Dental Association. COVID-19 frequently asked questions. success.ada.org/en/practice-management/patients/coronavirus-frequently-asked-questions?utm_source=cpsorg&utm_medium=covid-nav&utm_content=nav-faq&utm_campaign=covid-19 (accessed 16 April 2020).
Al‐Eid 2018
- Al-Eid RA, Ramalingam S, Sundar C, Aldawsari M, Nooh N. Detection of visually imperceptible blood contamination in the oral surgical clinic using forensic luminol blood detection agent. Journal of International Society of Preventive & Community Dentistry 2018;8(4):327-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ALOP 2020
- The Latin American Association of Pediatric Dentistry (Asociación Latinoamericana de Odontopediatría ALOP). Recommendations for care in pediatric dentistry versus Covid-19. www.acop.com.co/2020/04/13/recomendaciones-de-atencion-en-odontopediatria-frente-al-covid-19/ (accessed 13 April 2020).
Avasthi 2018
- Avasthi A. High volume evacuator (HVE) in reducing aerosol – an exploration worth by clinicians. Journal of Dental Health, Oral Disorders & Therapy 2018;9(3):165-6. [Google Scholar]
Bali 2014
- Bali R, Sharma P, Nagrath S, Gupta P. Microbial isolations from maxillofacial operation theatre and its correlation to fumigation in a teaching hospital in India. Journal of Maxillofacial and Oral Surgery 2014;13(2):128-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 1998
- Barnes JB, Harrel SK, Rivera-Hidalgo F. Blood contamination of the aerosols produced by in vivo use of ultrasonic scalers. Journal of Periodontology 1998;69(4):434-8. [DOI] [PubMed] [Google Scholar]
BDA 2020a
- British Dental Association. Personal protective equipment (PPE). bda.org/advice/Coronavirus/Pages/face-mask-shortage.aspx (accessed 6 June 2020).
BDA 2020b
- British Dental Association. Coronavirus: the financial impact. bda.org/advice/Coronavirus/Pages/financial-impact.aspx (accessed 6 June 2020).
Brown 1999
- Brown K, Xu Y. Fogging for the disinfection of food processing factories and equipment. Trends in Food Science & Technology 1999;10(6-7):205-10. [Google Scholar]
Burton 2020a
- Burton MJ, Clarkson JE, Goulao B, Glenny A-M, McBain AJ, Schilder AG, et al. Antimicrobial mouthwashes (gargling) and nasal sprays to protect healthcare workers when undertaking aerosol-generating procedures (AGPs) on patients without suspected or confirmed COVID-19 infection. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013628. [DOI: 10.1002/14651858.CD013628] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2020b
- Burton MJ, Clarkson JE, Goulao B, Glenny A-M, McBain AJ, Schilder AG, et al. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD013626. [DOI: 10.1002/14651858.CD013626] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2020
- Centers for Disease Control and Prevention. Dental settings: interim infection prevention and control guidance for dental settings during the COVID-19 response. www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html (accessed 27 April 2020).
Chou 2020
- Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers. Annals of Internal Medicine 2020;173(2):120-36. [DOI: 10.7326/M20-1632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarkson 2020
- Clarkson J, Ramsay C, Richards D, Robertson C, & Aceves-Martins M, on behalf of the CoDER Working Group. Aerosol Generating Procedures and their Mitigation in International Dental Guidance Documents - A Rapid Review. oralhealth.cochrane.org/sites/oralhealth.cochrane.org/files/public/uploads/rapid_review_of_agps_in_international_dental_guidance_documents.pdf. 2020.
COMET 2020
- COMET. Core Outcome Measures in Effectiveness Trials. www.comet-initiative.org (accessed 19 May 2020).
CONSORT 2010
- CONSORT Group. CONSORT Statement. www.consort- statement.org/ (accessed 15 September 2019) 2010.
Cottone 1991
- Cottone JA, Terezhalmy GT, Molinari JA. Practical Infection Control in Dentistry. Baltimore (MD): Williams and Wilkins, 1991. [Google Scholar]
Coulthard 2020
- Coulthard P. Dentistry and coronavirus (COVID-19) – moral decision-making. British Dental Journal 2020;228(7):503-5. [DOI] [PubMed] [Google Scholar]
Dahlke 2012
- Dahlke WO, Cottam MR, Herring MC, Leavitt JM, Ditmyer MM, Walker RS. Evaluation of the spatter-reduction effectiveness of two dry-field isolation techniques. Journal of American Dental Association 2012;143:1199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dominiak 2020
- Dominiak M, Różyło-Kalinowska I, Gedrange T, Konopka T, Hadzik J, Bednarz W, et al. COVID-19 and professional dental practice. The Polish Dental Association Working Group recommendations for procedures in dental office during an increased epidemiological risk. Journal of Stomatology 2020;73(1):1-10. [Google Scholar]
Eggers 2018
- Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infectious Diseases and Therapy 2018;7(2):249-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Escombe 2019
- Escombe AR, Ticona E, Chávez-Pérez V, Espinoza M, Moore DA. Improving natural ventilation in hospital waiting and consulting rooms to reduce nosocomial tuberculosis transmission risk in a low resource setting. BMC Infectious Diseases 2019;19(1):88. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
European Standard 2009
- European Standard. High efficiency air filters (EPA, HEPA and ULPA). European Standard EN 1822-1:2009.
FGDP 2020
- Faculty of General Dental Practice (UK). COVID-19: updated guidance and resources as lockdown eases. fgdp.org.uk/news/covid-19-updated-guidance-and-resources-lockdown-eases 21 May 2020.
Fors 1986
- Fors UG, Berg JO, Sandberg H. Microbiological investigation of saliva leakage between the rubber dam and tooth during endodontic treatment. Journal of Endodontics 1986;12(9):396-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fox 2010
- Fox C. Evidence summary: what 'cost of illness' evidence is there about cross-infection related infections in dental practice? British dental journal 2010;209(2):87-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Froum 2020
- Froum S, Strange M. COVID-19 and the problem with dental aerosols. www.perioimplantadvisory.com/periodontics/oral-medicine-anesthetics-and-oral-systemic-connection/article/14173521/covid19-and-the-problem-with-dental-aerosols (accessed 6 June 2020).
GDC 2020
- GDC. Aerosol generating procedures. gdc-uk.org/docs/default-source/covid-19/use-of-aerosol-generating-procedures-(agps).pdf?sfvrsn=5a95b3b3_2 18 March 2020.
Ghosh 2015
- Ghosh B, Lal H, Srivastava A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environment International 2015;85:254-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 1 July 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Graetz 2014
- Graetz C, Bielfeldt J, Tillner A, Plaumann A, Dörfer CE. Spatter contamination in dental practices – how can it be prevented? Revista Medico-Chirurgicala a Societatii de Medici si Naturalisti din Lasi 2014;118:1122-34. [PMID: ] [PubMed] [Google Scholar]
Grinshpun 2001
- Grinshpun S, Mainelis G, Reponen T, Willeke K, Trunov MA, Adhikari A. Effect of wearable ionizers on the concentration of respirable airborne particles and microorganisms. Journal of Aerosol Science 2001;32(Suppl 1):S335-6. [Google Scholar]
Gross 1992
- Gross KB, Overman PR, Cobb C, Brockmann S. Aerosol generation by two ultrasonic scalers and one sonic scaler. A comparative study. Journal of Dental Hygiene 1992;66(7):314-8. [PubMed] [Google Scholar]
Gurley 1985
- Gurley B. Ozone: a pharmaceutical sterilant of the future? Journal of Parenteral Science and Technology 1985;39(6):256-61. [PubMed] [Google Scholar]
Górny 2020
- Górny RL. Microbial aerosols: sources, properties, health effects, exposure assessment – a review. KONA Powder and Particle Journal 2020;37:64-84. [DOI: 10.14356/kona.2020005] [DOI] [Google Scholar]
Harrel 1996
- Harrel SK. Clinical use of an aerosol-reduction device with an ultrasonic scaler. Compendium of Continuing Education in Dentistry 1996;17(12):1185-93. [PubMed] [Google Scholar]
Harrel 2004
- Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. Journal of the American Dental Association (1939) 2004;135(4):429-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011) edition. The Cochrane Collaboration, 2011. [www.handbook.cochrane.org] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Hoboken (NJ): Wiley-Blackwell, 2019. [Google Scholar]
Ishihama 2008
- Ishihama K, Iida S, Koizumi H, Wada T, Adachi T, Isomura-Tanaka E, et al. High incidence of blood exposure due to imperceptible contaminated splatters during oral surgery. Journal of Oral and Maxillofacial Surgery 2008;66(4):704-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacks 2002
- Jacks ME. A laboratory comparison of evacuation devices on aerosol reduction. Journal of Dental Hygiene 2002;76:202-6. [PubMed] [Google Scholar]
Jain 2020
- Jain M, Mathur A, Mathur A, Mukhi PU, Ahire M, Pingal C. Qualitative and quantitative analysis of bacterial aerosols in dental clinical settings: risk exposure towards dentist, auxiliary staff, and patients. Journal of Family Medicine and Primary Care 2020;9(2):1003-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2016
- James R, Mani A. Dental aerosols: a silent hazard in dentistry! International Journal of Scientific Research 2016;5:1761-3. [Google Scholar]
Judson 2019
- Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses 2019;11(10):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koletsi 2020
Kormuth 2018
- Kormuth KA, Lin K, Prussin AJ II, Vejerano EP, Tiwari AJ, Cox SS, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. Journal of Infectious Diseases 2018;218(5):739-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laheij 2012
- Laheij AM, Kistler JO, Belibasakis GN, Välimaa H, Soet JJ. Healthcare-associated viral and bacterial infections in dentistry. Journal of Oral Microbiology 2012;4:4. [DOI: 10.3402/jom.v4i0.17659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, Version 6 (updated July 2019). Cochrane, 2019. Available from: www.training.cochrane.org/handbook.
Lessler 2009
- Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infectious Diseases 2009;9(5):291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2020
- Leung NH, Chu DK, Shiu EY, Chan K-H, McDevitt JJ, Hau Benien JP, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Medicine 2020;26:676-80. [DOI: 10.1038/s41591-020-0843-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2020
Lu 2020
- Lu M. The front line: visualizing the occupations with the highest COVID-19 risk. Visual Capitalist 15 April 2020. [www.visualcapitalist.com/the-front-line-visualizing-the-occupations-with-the-highest-covid-19-risk/]
Mair 2020
- Mair AD, Korne PH. Decoding Dental Aerosols. https://secibonline.com/wp-content/uploads/2020/05/Decoding-Dental-Aerosols_May-212020.pdf [Accessed on 31 July 2020].
Mantovani 2020
- Mantovani C. Over 90,000 health workers infected with COVID-19 worldwide: nurses group. www.reuters.com/article/us-health-coronavirus-nurses/over-90000-health-workers-infected-with-covid-19-worldwide-nurses-group-idUSKBN22I1XH 6 May 2020.
McDonnell 2006
Meng 2020
- Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. Journal of Dental Research 2020;99(5):481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merriam‐Webster 2020
- Merriam-Webster Online Medical Dictionary. Saliva ejector. www.merriam-webster.com/medical/saliva%20ejector (accessed 5 May 2020).
Monarca 2000
- Monarca S, Grottolo M, Renzi D, Paganelli C, Sapelli P, Zerbini I, et al. Evaluation of environmental bacterial contamination and procedures to control cross infection in a sample of Italian dental surgeries. Occupational and Environmental Medicine 2000;57(11):721-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCUDSPH 2020
- Le Collège National des Chirurgiens-Dentistes Universitaires en Santé Publique. Recommendations to deal with the Covid19 epidemic. www.dentairesantepublique.fr/recommandations-face-a-lepidemie-covid19/ (accessed 18 March 2020).
OSHA 2020
- US Department of Labor: Occupational Safety and Health Administration. Guidance on preparing workplaces for COVID-19. www.osha.gov/Publications/OSHA3990.pdf (accessed prior to 16 June 2020).
Pasquarella 2000
- Pasquarella C, Pitzurra O, Savino A. The index of microbial air contamination. Journal of Hospital Infection 2000;46(4):241-56. [DOI] [PubMed] [Google Scholar]
Peng 2020
- Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. International Journal of Oral Science 2020;12(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
PlotDigitizer 2015 [Computer program]
- PlotDigitizer software. SourceForge, 3 June 2001 [revised 24 October 2015]. Available at sourceforge.net/projects/plotdigitizer.
Polednik 2014
- Polednik B. Aerosol and bioaerosol particles in a dental office. Environmental Research 2014;134:405-9. [DOI] [PubMed] [Google Scholar]
Porter 1991
- Porter SR. Infection control in dentistry. Current opinion in dentistry 1991;1(4):429-35. [PMID: ] [PubMed] [Google Scholar]
Qian 2010
- Qian H, Li Y, Sun H, Nielsen PV, Huang X, Zheng X. Particle removal efficiency of the portable HEPA air cleaner in a simulated hospital ward. Building Simulation 2010;3(3):215-24. [Google Scholar]
Rautemaa 2006
- Rautemaa R, Nordberg A, Wuolijoki-Saaristo K, Meurman J. Bacterial aerosols in dental practice – a potential hospital infection problem? Journal of Hospital Infection 2006;64(1):76-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayyan 2016 [Computer program]
- Rayyan QCRI, the Systematic Reviews web app. Doha, Qatar: Qatar Computing Research Institute, Hamad Bin Khalifa University, 2016.
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Samaranayake 2004
- Samaranayake LP, Peiris M. Severe acute respiratory syndrome and dentistry: a retrospective view. Journal of the American Dental Association (1939) 2004;135(9):1292-302. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 1990
- Sato H, Wananabe Y, Miyata H. Virucidal effect of ozone treatment of laboratory animal viruses. Jikken Dobutsu. Experimental Animals 1990;39(2):223-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sawhney 2015
- Sawhney A, Venugopal S, Babu GR, Garg A, Mathew M, Yadav M, et al. Aerosols how dangerous they are in clinical practice. Journal of Clinical and Diagnostic Research : JCDR 2015;9(4):ZC52-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scannapieco 1999
- Scannapieco F. Role of oral bacteria in respiratory infection. Journal of Periodontology 1999;70:793-801. [DOI] [PubMed] [Google Scholar]
Scannapieco 2004
- Scannapieco FA, Ho AW, DiTolla M, Chen C, Dentino AR. Exposure to the dental environment and prevalence of respiratory illness in dental student populations. Journal (Canadian Dental Association) 2004;70(3):170-4. [PMID: ] [PubMed] [Google Scholar]
Sotiriou 2008
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI: 10.1136/bmj.i4919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockwell 2019
- Stockwell RE, Ballard EL, O'Rourke P, Knibbs LD, Morawska L, Bell SC. Indoor hospital air and the impact of ventilation on bioaerosols: a systematic review. Journal of Hospital Infection 2019;103(2):175-84. [DOI] [PubMed] [Google Scholar]
Szymańska 2007
- Szymańska J. Dental bioaerosol as an occupational hazard in a dentist's workplace. Annals of Agricultural and Environmental Medicine : AAEM 2007;14(2):203-7. [PMID: ] [PubMed] [Google Scholar]
Veeck 2004
- Veeck AC. Types of HEPA filters. In: NAFA Guide to Air Filtration. 3 edition. Middleton (WI): NAFA, 2004. [Google Scholar]
Veena 2015
- Veena HR, Mahantesha S, Joseph Preethi A, Patil Sudhir R, Patil Suvarna H. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. Journal of Infection and Public Health 2015;8(3):260-5. [DOI] [PubMed] [Google Scholar]
Verbeek 2020
- Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD011621. [DOI: 10.1002/14651858.CD011621.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warnakulasuriya 2020
- Warnakulasuriya S. Protecting dental manpower from COVID-19 infection. Oral Diseases 2020 May 13 [Epub ahead of print]. [DOI: 10.1111/odi.13410] [DOI] [PMC free article] [PubMed]
WHO 2020a
- World Health Organization. WHO timeline – COVID-19. www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19 (accessed prior to 16 June 2020).
WHO 2020b
- World Health Organization. Maintaining essential health services: operational guidance for the COVID-19 context - Interim guide. WHO/2019-nCoV/essential_health_services/2020.2.1 June 2020:34 and 36.
WHO 2020c
- World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions 9 July 2020.
WHO 2020d
- World Health Organization. Considerations for the provision of essential oral health services in the context of COVID-19 - Interim guidance. https://apps.who.int/iris/handle/10665/333625 3 August 2020.
Yadav 2015
- Yadav N, Agrawal B, Maheshwari C. Role of high-efficiency particulate arrestor filters in control of air borne infections in dental clinics. SRM Journal of Research in Dental Sciences 2015;6(4):240-2. [Google Scholar]
Zemouri 2017
- Zemouri C, Soet H, Crielaard W, Laheij A. A scoping review on bio-aerosols in healthcare and the dental environment. PloS One 2017;12(5):e0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zyris 2020
- Zyris: dental isolation systems and mouthpieces (formerly Isolite). www.zyris.com/ 2020.
References to other published versions of this review
Kumbargere Nagraj 2020
- Kumbargere Nagraj S, Eachempati P, Paisi M, Nasser M, Sivaramakrishnan G, Verbeek JH. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013686. [DOI: 10.1002/14651858.CD013686] [DOI] [PMC free article] [PubMed] [Google Scholar]