Abstract
Objective
The goal of this exploratory study was to evaluate the effects of an exercise intervention – progressive resistance training (PRT) on the metabolome of people with MS (pwMS) and to link these to changes in clinical outcomes.
Methods
14 pwMS with EDSS <4.0 and 13 age‐ and sex‐matched healthy controls completed a 12‐week in‐person PRT exercise intervention. Outcome measures included: plasma metabolomics analysis, cardiovascular fitness tests, EDSS, timed 25‐foot walk (T25FW), six‐minute walk test (6MWT), hip strength, and modified fatigue impact scale (MFIS). We identified changes in the metabolome with PRT intervention in both groups using individual metabolite abundance and weighted correlation network defined metabolite module eigenvalues and then examined correlations in changes in metabolite modules with changes in various clinical outcomes.
Results
In both groups PRT intervention improved hip strength, distance walked in 6WMT, speed of walking, while fatigue (MFIS) was improved in pwMS. Fatty acid, phospholipid, and sex steroid metabolism were significantly altered by PRT in pwMS but not in controls. Changes in fatigue (MFIS score) were strongly inversely correlated and hip strength was moderately correlated with change in sex steroid metabolite module in pwMS. A similar relationship was noted between change in dehydroepiandrosterone sulfate abundance (sex steroid metabolite) and fatigue in pwMS. We also noted an inverse correlation between changes in fatty acid metabolism and cardiovascular fitness in pwMS.
Interpretation
PRT‐induced metabolic changes may underlie improved clinical parameters in pwMS and may warrant investigation as potential therapeutic targets in future studies.
Introduction
Multiple sclerosis is a chronic autoimmune neurological disorder that has both inflammatory and degenerative components. 1 Genetic and environmental factors play a role in conferring risk for MS. The circulating metabolome provides unique insights into the pathophysiology of disease and can identify prognostic and diagnostic biomarkers. 2 , 3 Multiple studies have demonstrated that the metabolome is altered in MS patients and may be linked to disease severity and course. 4 , 5 The precise mechanism by which various altered metabolic pathways affect the disease is unknown.
Exercise has beneficial effects on various aspects of MS disease. Exercise training improves strength and mobility in healthy controls as well as individuals with MS. 6 , 7 For example, walking ability in pwMS is affected by hip weakness, 8 which we have shown can be improved through targeted progressive resistance training (PRT). 9 Exercise studies also show functional benefits in mood, 10 cardiovascular fitness, 6 fatigue 11 , 12 and cognition. 13 However, the benefits of exercise extend beyond symptomatic improvements, positively affecting brain volume in the hippocampus, 14 reducing oxidative stress, 15 and potentially increasing the production of neurotrophic factors such as brain‐derived neurotrophic factor (BDNF), 16 which offer the potential for immunomodulation and neuroprotection. However, the degree to which an individual with MS responds to exercise can vary widely 9 and the precise mechanisms by which interventions such as PRT produce these beneficial effects is not known.
Exercise has effects on the metabolome both acutely and in a more chronic fashion. 17 , 18 , 19 Changes, however, vary depending on the population studied and the exercise paradigm utilized. The effects of exercise on metabolism in people with MS (pwMS) are not known. We hypothesized that some of the beneficial effects of exercise in pwMS may be mediated by changes in the metabolome and hence in this exploratory study we sought to determine the effects of a progressive resistance training (PRT) exercise program on the metabolome of pwMS and to determine whether these changes were related to improvement in strength, mobility and fatigue.
Methods
Study participants
We recruited participants with relapsing‐remitting MS (RRMS) who satisfied the inclusion and exclusion criteria as follows. Participants were adults with a confirmed diagnosis of RRMS, not on a disease‐modifying therapy (DMT) or on the same DMT for the past 6 months, EDSS score <4.0, and no co‐morbid medical conditions that would prevent exercise. Exclusion criteria included other neurological diseases, MS exacerbation in prior 8 weeks, pregnancy, congestive cardiac failure, peripheral artery disease, cancer, pulmonary or renal failure, unstable angina, uncontrolled hypertension, orthopedic or pain conditions limiting exercise or use of medications that could increase the risk for cardiovascular fitness testing. We also recruited age‐, race and sex‐matched healthy control (HC) participants. All participants provided informed consent prior to participation in the study and the study was approved by the Johns Hopkins University Institutional Review Board.
Study intervention
All participants underwent a 12‐week intervention targeting hip strengthening by a progressive resistance training (PRT) program. 9 This involved three sessions a week in‐person and participants were asked to attend a minimum of 33 sessions. The rehabilitation program selectively isolates and targets large muscle groups acting on the hip. This intervention was chosen as we have previously shown hip weakness is a major contributor to walking dysfunction in MS. Details of the exercise intervention have been previously published. 9 For the analysis, compliance with the intervention was defined as a completion of a minimum of 20 sessions over 10−14 weeks. The minimum compliance was established based on our prior study showing a significant change in 8 weeks of strength training and given the allowance to miss two sessions / month. The range of total weeks duration was provided to participants to allow for missed visits due to vacation or illness and because the demand is high for participants to come in person three times a week who are working full time.
Study outcome measures
This study was a pretest–posttest controlled design. Outcome measures were obtained within 2 weeks of starting or stopping the exercise intervention. Demographic information including age, sex, height, and weight were also collected.
Metabolomics assays
Blood draw
All participants underwent phlebotomy at baseline and end‐of‐study visits. Time of day and time of last meal were recorded at sample collection. While no uniform protocol was enforced across participants, individual participant circumstances at the baseline visit were repeated at the end‐of‐study (same time of day and time from last meal). Blood was processed using a standardized protocol and plasma was separated, aliquoted, and stored at –80°C until it was utilized for metabolomics analyses at the end of the study.
Metabolomics analysis
Global metabolomics analyses were performed at Metabolon Inc. as previously described. 20 In brief, recovery standards were added before the extraction process for quality control. To remove protein and recover chemically diverse metabolites, proteins were precipitated with methanol under vigorous shaking for 2 min, followed by centrifugation. The resulting extract was divided into five fractions: analysis by ultra‐high‐performance liquid chromatography‐tandem mass spectrometry (UPLC‐MS/MS) (positive ionization); UPLC‐MS/MS (negative ionization); UPLC‐MS/MS polar platform (negative ionization); gas chromatography‐mass spectrometry; and one aliquot reserved for backup. Metabolite identification was performed by automated comparison of the ion features in the study samples with a reference library of standard metabolites. Quantification of peaks was performed using the AUC. Raw values for the area counts for each metabolite were normalized (correcting for variation resulting from instrument inter‐day tuning differences) by the median value for each run day.
Overall impairment
An expanded disability status scale (EDSS) was conducted at baseline and end of study visits on each participant to assess overall impairment related to MS. These were performed by the study neurologist ‐ PB.
Participant reported measures
Fatigue
The Modified Fatigue Impact Scale (MFIS) was used to assess the impact of fatigue on everyday life and hence provide a measure of severity of MS‐related fatigue. 21
Physical activity
The self‐report of the Godin Shephard Leisure‐Time Physical Activity Exercise Questionnaire was used to assess average weekly physical activity. 22
Quantitative performance measures
Strength
Per protocol in [9], hip flexor strength was measured by hand‐held dynamometry using a Microfet2 (Hoggan Health Industries). An average of two trials of maximal effort for each hip was calculated and the sum of both sides was used in the analysis. Changes in summed strength were used to identify responders (≥5.1 lbs) and non‐responders (<5.1 lbs) based on a calculation of the 95% minimal detectable change from our previous strength training study. 9
Walking
Walking speed was measured by the Timed 25 Foot Walk Test (T25FW). Participants are asked to walk 25’ feet as quickly and safely as possible. The time in seconds is reported. 23
Physical fitness
Two measures were used to assess physical fitness. The distance obtained from the six‐minute walk test (6MWT) during which participants walked back and forth on a 20‐m track attempting to cover the maximum distance possible. 24 The second measure was the estimated VO2 max, following NHANES protocol, 25 participants walked on a treadmill at fixed exercise workloads and heart rate response was assessed. VO2 max was estimated by validated formulae. Two participants (1 MS 60 year and 1 HC 66 years) did not perform the VO2 max estimate test as resting vital signs at the pretest excluded their participation (SBP > 180 mmHg, DBP > 100 mmHg; HR > 100 bpm): one female with MS, 60 years (resting BP 144/97; HR 94); one female HC, 66 years (resting BP 145/101; HR 95). Both subjects were cleared by their physicians for participation in the exercise intervention and vital signs were monitored daily.
Statistical analysis
Metabolomics data analysis
The metabolite abundance data generated by global metabolomics analysis were subjected to quality control and pre‐processing as follows. We first removed metabolites that were missing in more than 30% of the samples, followed by the imputation of missing values using a k‐nearest neighbors (kNN) method. We then normalized metabolite concentrations by log transformation followed by autoscaling of these values. This processed data was then used to run Sign‐rank (non‐parametric) tests to determine which metabolites changed over the course of the study. We set a False‐discovery rate (FDR) adjusted p value of 0.2 as the threshold to determine significance. We also utilized an alternative strategy of using weighted correlation network analysis (WGCNA) for dimension reduction, 4 , 26 by generating 17 modules of highly correlated metabolites and used the eigen‐metabolite values for each module in downstream analyses. We utilized Wilcoxon signed‐rank test to compare WGCNA derived eigen‐metabolite values to determine which modules were altered with PRT intervention. We adjusted for multiple comparisons using a Benjamini–Hochberg FDR correction with a Q value of 0.2 to generate FDR‐adjusted p values.
Self‐reported and quantitative performance measures analyses
Non‐parametric tests were used for all measures due to the small sample size. Differences between the groups were assessed using the Kruskal–Wallis Test. To assess within‐group changes pre‐post training the Wilcoxon matched‐pair signed‐rank test was used.
Responders/non‐responders
Assignment of participants as responder or non‐responder to the intervention was determined by changes in the summed strength after training. A participant was labeled a responder if the change exceeded the minimal detectable change (MDC) of 5.1 pounds. The Minimal Detectable Change based on a 95% confidence interval using the formal MDC = 1.96 × Standard Error of Measurement (SEM) × (Shirley Ryan Ability Lab; https://www.sralab.org/statistical‐terms‐use). The SEM used was assessed from a combination of the MS participants in this study and those with an EDSS <4.0 from our prior PRT study 9 for a total group of pwMS with n = 28, mean change in summed hip strength = 8.6 ± 1.94 (SEM).
Relationships between training‐induced changes and changes in the metabolome
To assess the relationship between changes in the metabolome and changes in performance measures we used Spearman’s Rank‐Order correlations. Significance was set at p < 0.05. Given the exploratory nature of the study and these analyses, we did not perform corrections for multiple hypothesis testing for these analyses.
Data availability
Deidentified data are available from the corresponding author on reasonable request.
Results
Cohort characteristics
We enrolled 20 MS participants of which 14 completed the exercise intervention and 18 healthy controls of which 13 completed the exercise intervention. Compliance was high with only three participants having less than 33 sessions. The demographic characteristics of participants that completed the PRT intervention are shown in Table 1. Baseline differences between the two groups were not significant (p > 0.05) for any demographic or outcome measure except for walking speed (T25FW, p < 0.01) with the pwMS walking slower than the age‐matched controls.
Table 1.
Demographic characteristics of study participants.
| Characteristic | Multiple sclerosis (n = 14) | Healthy control (n = 13) |
|---|---|---|
| Age (mean ± SD), years | 42 ± 13 | 39 ± 14 |
| Sex (M:F) | 2:12 | 2:11 |
| BMI (mean ± SD), kg/m2 | 26.5 ± 5.9 | 25.6 ± 4.3 |
| Godin‐Shephard exercise activity scale (mean ± SD) | 29 ± 17 | 37 ± 30 |
| EDSS [median (range)] | 1.5 (0–3) | – |
| Days of exercise completed (mean ± SD), days | 31 ± 5 | 33.4 ± 1 |
| Exercise intervention duration (mean ± SD), weeks | 12.4 ± 2.2 | 11.7 ± 0.9 |
Effects of exercise program on the metabolome in pwMS and HCs
We identified 51 metabolites that changed in pwMS (using FDR‐adjusted p value cut‐off <0.2) and no metabolites that changed significantly in HCs (using the same cutoff) following the PRT intervention. The top 25 metabolites are shown in Table 2 for the MS group. The majority of metabolites that changed belong to fatty acid and phospholipid metabolic pathways.
Table 2.
Metabolites altered by PRT intervention in pwMS.
| Metabolite 1 | p value | FDR adjusted p value |
|---|---|---|
| 1‐palmitoleoyl‐GPC | 0.000122 | 0.059001 |
| Myristate | 0.000244 | 0.059001 |
| 3‐hydroxyisobutyrate | 0.000366 | 0.059001 |
| Linolenate | 0.000366 | 0.059001 |
| 1‐palmitoyl‐GPC | 0.00061 | 0.059001 |
| 1‐stearoyl‐GPC | 0.00061 | 0.059001 |
| Hydantoin‐5‐propionate | 0.00061 | 0.059001 |
| Margarate | 0.00061 | 0.059001 |
| Palmitate | 0.00061 | 0.059001 |
| 1‐1‐enyl‐stearoyl‐GPE | 0.000854 | 0.067583 |
| 10‐nonadecenoate | 0.000854 | 0.067583 |
| 1‐1‐enyl‐palmitoyl‐GPC | 0.001221 | 0.075858 |
| Arachidonate | 0.001221 | 0.075858 |
| Oleate/vaccinate | 0.001221 | 0.075858 |
| Dihomolinoleate | 0.001709 | 0.08746 |
| Linoleate | 0.001709 | 0.08746 |
| Stearidonate | 0.001709 | 0.08746 |
| Tetradecanedioate | 0.002319 | 0.1121 |
| 5‐dodecenoate | 0.003052 | 0.13275 |
| Glycerol | 0.003052 | 0.13275 |
| 3‐3‐hydroxyphenylpropionate sulfate | 0.004028 | 0.14603 |
| 5,6‐dihydrouridine | 0.004028 | 0.14603 |
| indole‐3‐carboxylate | 0.004028 | 0.14603 |
| Stearate | 0.004028 | 0.14603 |
| 3‐aminoisobutyrate | 0.005249 | 0.15222 |
Top 25 metabolites are shown.
Of the 17 metabolite modules identified by WGCNA, six modules changed significantly with PRT in the MS group (Table 3). The top metabolites in the modules, derived from the WGCNA analysis that changed in the MS group with PRT, are also shown in Table 3. Consistent with the analysis of individual metabolites, the modules that changed were primarily related to fatty acid, phospholipid, and sex steroid metabolism.
Table 3.
Modules altered by PRT in pwMS with primary metabolites included in the modules
| Module | Metabolites | p value | FDR adjusted p value |
|---|---|---|---|
| Black (Acylcarnitine metabolism) | Laurylcarnitine | 0.018 | 0.10 |
| 5‐dodecenoylcarnitine | |||
| Myristoleoylcarnitine | |||
| Decanoylcarnitine | |||
| Octanoylcarnitine | |||
| Hexanoylcarnitine | |||
| 3‐hydroxydecanoylcarnitine | |||
| Nonanoylcarnitine | |||
| 3‐hydroxyoleoylcarnitine | |||
| Pink (Fatty acid metabolism) | 3‐hydroxysebacate | 0.013 | 0.11 |
| Dodecanedioate | |||
| Tetradecanedioate | |||
| Hexanoylglutamine | |||
| 3‐hydroxyoctanoate | |||
| 3‐hydroxyhexanoate | |||
| Yellow (Fatty acid metabolism) | Palmitate | 0.0076 | 0.13 |
| 10‐nonadecenoate | |||
| Dihomolinoleate | |||
| Myristate | |||
| Linoleate | |||
| Oleate | |||
| Margarate | |||
| Docosapentaenoate (DPA) | |||
| Brown (Steroid metabolism) | Dehydroepiandrosterone sulfate (DHEAS) | 0.03 | 0.13 |
| Androstenediol‐3beta,17beta‐monosulfate | |||
| Pregnenetriol sulfate | |||
| Pregnenediol sulfate | |||
| Pregnenetriol disulfate | |||
| Androstenediol‐3alpha,17alpha‐monosulfate | |||
| Androstenediol‐3beta,17beta‐disulfate | |||
| Tan (Ether lipid metabolism) | 1,1‐enyl palmitoyl GPE | 0.03 | 0.13 |
| 1,1‐enyl stearoyl GPE | |||
| 1,1‐enyl oleoyl GPE | |||
| 1,1‐enyl stearoyl‐2‐arachidonoyl GPE | |||
| 1,1‐enyl stearoyl‐2‐oleoyl GPE | |||
| 1,1‐enyl palmitoyl‐2‐linoleoyl GPE | |||
| 1,1‐enyl stearoyl‐2‐linoleoyl GPE | |||
| Salmon (Phospholipid metabolism) | 1‐stearoyl GP | 0.03 | 0.13 |
| Oleoylcholine | |||
| Palmitoylcholine | |||
| Linoleoylcholine | |||
| 1‐palmitoyl GPI | |||
| Arachidonoylcholine |
Exercise program leads to changes in fitness and disability metrics in pwMS
We examined the effect of the PRT on measures of strength, cognition, fatigue, and overall disability. We noted that in MS participants the PRT intervention led to significant increases in hip strength, distance walked during the 6MWT, faster walking speed (on timed 25‐foot walk [T25FW]), and reduced fatigue (based on MFIS scores) (Table 4). Healthy controls also had significant changes in hip strength, distance walked during the 6MWT and walking speed (Table 4). The effects of PRT, however, were not uniform within all pwMS and utilizing the MDC for change in hip strength of 5.1 lbs, we noted that 8/14 pwMS demonstrated a response to the PRT intervention in terms of a meaningful increase in hip strength. In comparison, 12/13 healthy controls demonstrated strength improvement above the MDC reference.
Table 4.
Effect of PRT intervention on clinical outcomes in pwMS and HCs.
| Outcome | Multiple sclerosis | p value | Healthy control | p value | ||
|---|---|---|---|---|---|---|
| Pre‐PRT | Post‐PRT | Pre‐PRT | Post‐PRT | |||
| Hip strength (pounds) | 88.5 ± 18 | 96 ± 24 | 0.008 | 93.6 ± 20 | 107 ± 23 | 0.002 |
| 6MWT (meters) | 589 ± 81 | 616 ± 85 | 0.01 | 613 ± 59 | 632 ± 71 | 0.03 |
| T25FW (seconds) | 3.76 ± 0.39 | 3.46 ± 0.5 | 0.008 | 3.4 ± 0.4 | 3.2 ± 0.5 | 0.02 |
| EDSS | 1.5 ± 0.15 | 1.2 ± 0.3 | 0.4 | – | – | – |
| VO2 max estimated 1 (ml/kg/min) | 34.15 ± 11.8 | 35.3 ± 11.8 | 0.72 | 35.4 ± 6.8 | 35.4 ± 6.8 | 1.0 |
| MFIS total | 23 ± 5 | 14.6 ± 4.5 | 0.0056 | – | – | – |
| MFIS physical subscale | 10.6 ± 2.3 | 6.4 ± 1.9 | 0.005 | – | – | – |
| MFIS cognitive subscale | 10.3 ± 2.6 | 7 ± 2.6 | 0.012 | – | – | – |
| Godin Leisure scale | 36 ± 29 | 44 ± 17 | 0.096 | 29 ± 17 | 42 ± 21 | 0.38 |
n = 12 for each group since one participant from each group was unable to complete this measurement.
In the pwMS group, there was no difference in baseline characteristics or training intensity between responders and non‐responders. Compared with non‐responders, the responders also showed significantly greater changes in other measures (non‐responder vs. responder mean (SD)): 6MWT (20 m ± 32 vs. 31 m ± 35), T25FW (−0.17 s ± 0.29 vs. −0.41 s ± 0.4), MFIS total (−2 ± 5 vs. −13 ± 5), and MFIS physical, (−0.5 ± 1 vs. −7 ± 4).
Association between the change in the metabolome and effects on fitness and disability metrics in MS
We then studied the relationship between change in metabolite levels and alterations in the various clinical outcomes. To do this we first calculated Spearman correlation coefficients between change in various clinical outcomes and the change in eigen‐metabolite values for the various WGCNA derived metabolite modules. We noted strong inverse correlations between change in the sex steroid metabolite module scores (brown module) and change in fatigue scores (both MFIS – r = −0.65, p = 0.01 [Fig. 1A] and MFIS physical subscale scores – r = −0.64, p = 0.01 [Fig. 1B]). We also noted a moderate correlation between the change in the brown metabolite module and change in hip strength (r = −0.54, p = 0.03, Fig. 1E). The eigenmetabolite in this module was dehydroepiandrosterone sulfate (DHEAS). As expected, change in DHEAS abundance over the course of the study was inversely correlated with change in fatigue scores (Fig. 1C and D).
Figure 1.
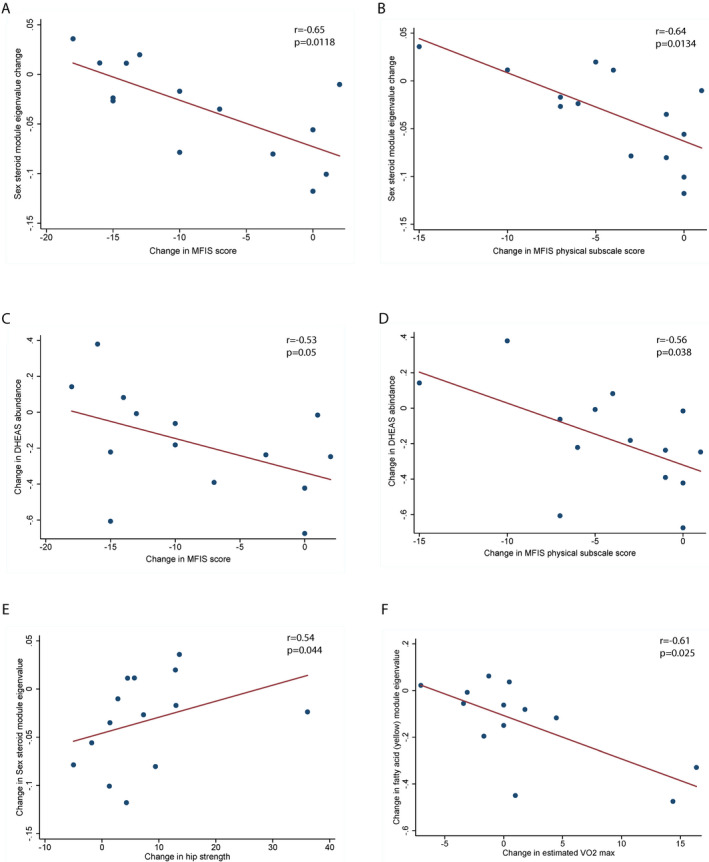
Relationship between change in the metabolome and clinical measures in pwMS. (A) Scatter plot depicting a strong inverse relationship between change in sex steroid metabolite module eigenvalue and fatigue (MFIS) scores. (B) Scatter plot depicting a strong inverse relationship between change in sex steroid metabolite module eigenvalue and score on the physical subscale of MFIS. (C) Scatter plot depicting a strong inverse relationship between change in DHEAS abundance and fatigue (MFIS) scores. (D) Scatter plot depicting a strong inverse relationship between change in DHEAS abundance and score on the physical subscale of MFIS. (E) Scatter plot depicting the moderate correlation between change in sex steroid metabolite module eigenvalue and hip strength. (F) Scatter plot depicting a strong inverse relationship between change in fatty acid metabolism module eigenvalue and cardiovascular fitness (measured by estimated VO2 max). p and r values for A–F are derived from Spearman’s correlations.
Additionally, we noted a significant inverse correlation between the change in yellow module (fatty acid metabolism) scores and change in estimated VO2 max which is a measure of overall cardiovascular fitness (r = −0.61, p = 0.025 [Fig. 1F]).
We also analyzed whether changes in the metabolome differed by status as a responder or non‐responder to the PRT intervention (as defined above). We noted significant differences in the change in sex steroid module (brown) eigenmetabolite values between the responder and non‐responder groups (p = 0.028). Similarly, changes in other metabolite modules (black [acylcarnitine metabolism] and pink [fatty acid metabolism]) were also noted to be significantly different between the responder and non‐responder groups (p = 0.038 and p = 0.029, respectively).
Discussion
We demonstrate that a 12‐week PRT intervention led to significant changes in the metabolome in pwMS. These changes primarily involved fatty acid, phospholipid, and sex steroid metabolism and were related to changes in strength, fatigue, and cardiovascular endurance.
Consistent with prior studies, we demonstrate numerous beneficial effects of an exercise intervention in pwMS. 9 , 27 , 28 We noted improved hip strength, faster walking speed, and improved fatigue following PRT. The results of this study provide additional evidence for the pleiotropic benefits of exercise in pwMS.
We noted significant changes in the metabolome of pwMS primarily involving fatty acid, phospholipid and sex steroid metabolism. We noted similar results utilizing both individual metabolite abundances and metabolite modules obtained using WGCNA. Previous studies of exercise interventions in healthy controls have shown great variability in the changes in the resting metabolome following exercise 17 , 19 , 29 , 30 with some showing minimal change attributed to the presence of effects of a variety of additional variables on the metabolome. A recent study examining effects of exercise in army recruits showed that the resting metabolome was altered with changes in some classes of metabolites that overlapped with our findings—including certain lipids and products of fatty acid oxidation. 29 Several changes, we noted in the metabolome of pwMS, are compatible with those seen in prior studies of exercise interventions in other populations. Interestingly, in our cohort, we did not note similar changes in the metabolome of healthy controls following PRT. Most recent studies of exercise modalities used to study perturbations in the metabolome of healthy controls rely on much higher intensity levels and shorter intervals (hours not weeks) of exercise with results showing relatively short decay (24 h) in the changes observed. 31 Our results suggest that pwMS may require a less intense intervention to see beneficial lasting changes in the metabolome than health controls. This could potentially point to a specific effect of the PRT intervention in pwMS or perhaps that the intervention did not represent a significant/sufficient metabolic stressor for the healthy control group.
We noted associations between the change in the metabolome with PRT and effects on measures of strength, endurance, and fatigue in pwMS, suggesting the possibility that alterations in the metabolome may be mediating some of the beneficial effects noted on clinical outcomes. Specifically, changes in sex steroid metabolism were correlated with improvement in fatigue and increase in hip strength. This effect appeared to be driven by metabolites like DHEAS—a neurosteroid that has previously been associated with a multitude of beneficial biological effects. 32 , 33 Multiple studies have examined the relationship between exercise and levels of sex steroids in healthy controls, especially DHEA and DHEAS, with the majority of studies appearing to demonstrate increased levels with exercise though some studies of resistance exercise showed discordant results between the two metabolites. 34
In a previous study, low DHEAS levels were noted in pwMS compared to controls, especially in fatigued pwMS. 35 Similar relationships have also been noted in other autoimmune diseases like Sjogren’s and Lupus. Studies have also examined the benefit of supplementation of DHEA on symptoms in Sjogren’s syndrome 36 ; however, similar studies have not been conducted in MS. Side effects associated with DHEA supplementation in trials in other conditions have generally been mild and included hirsutism, acne, and greasy skin. 36 , 37 Further studies to assess the effects of interventions such as DHEA supplementation in fatigued pwMS may be warranted based on our observations.
Another interesting observation in our study was the inhomogeneity of the response to PRT intervention in pwMS. Using an MDC cut‐off we noted that just over half of the participants were classified as responders to PRT based on a change in hip strength. While there was no difference in training intensity or baseline characteristics between the two groups, the responders also showed greater improvements in several other outcomes and in particular, in their reduction in fatigue (MFIS total and physical sub‐score). The fact that some metabolic changes (such as sex steroid metabolism) differed between responders and non‐responders raises the possibility that metabolic changes may explain the varied responsiveness of pwMS for beneficial effects of exercise on clinical outcomes. DHEA supplementation has been tested as a means to augment the beneficial effects of exercise on a variety of end points. 38 , 39 Our results also suggest that further research is required to determine whether supplementation of sex steroids like DHEA may help augment the benefit derived by pwMS through an exercise intervention.
The strengths of our study include the fact that the exercise intervention targeted hip weakness that is a specific impairment limiting function in pwMS, and that the PRT was conducted in‐person under direct physical therapist supervision thus ensuring an adequate dose of exercise for all participants. We also enrolled a fairly homogenous cohort of participants with low disability levels to allow for unrestricted participation in the exercise program. Another strength of the study was that we utilized quantitative outcome measures to assess exercise responsiveness and fitness (i.e., dynamometry, estimated VO2 max) to assess associations with changes in the metabolome. These measures are less subjective compared to typical rating scales (manual muscle testing, self‐reported activity levels) and require minimal equipment and training to quantify clinical outcomes making the results of exercise intervention studies more clinically applicable.
The main limitations are the small sample size of the study, partly due to early termination due to the COVID‐19 pandemic and the dropout rate (due to inclusion of low disability pwMS who work full time and are hence more likely to dropout due to the time commitment) which may have reduced our ability to identify additional changes in the metabolome produced by the PRT intervention and may reduce the generalizability of findings by biasing towards those more likely to be compliant with intensive intervention. The small sample size may also increase the risk of identifying false‐positive associations between the various outcomes. An additional limitation that may reduce the generalizability of the findings is that we selected participants with RRMS and low levels of disability to allow for optimal participation in the exercise intervention.
In conclusion, we demonstrated the pleiotropic beneficial effects of a resistance training intervention in pwMS and identified changes in sex steroid metabolism (such as DHEAS) that were associated with changes in hip strength and fatigue. Given the exploratory nature of the current study, future studies to confirm the effects of PRT on the metabolome are warranted. This will also provide rationale to investigate sex steroid supplementation to treat MS‐related fatigue and augment response to exercise interventions.
Conflict of Interest
J Keller reports no disclosures relevant to the manuscript; K Zackowski reports no disclosures relevant to the manuscript; S Kim reports no disclosures relevant to the manuscript; IJ Chidobem reports no disclosures relevant to the manuscript; MD Smith reports no disclosures relevant to the manuscript; F Farhadi reports no disclosures relevant to the manuscript; P Bhargava reports no disclosures relevant to the manuscript.
Funding Information
This study was funded by a Hypothesis‐Discovery Award from the Department of Defense to KZ and a Career Transition Award from the National Multiple Sclerosis Society to PB.
Funding Statement
This work was funded by Department of Defense grant ; National Multiple Sclerosis Society grant .
Contributor Information
Kathleen Zackowski, Email: kathleen.zackowski@nmss.org.
Pavan Bhargava, Email: pbharga2@jhmi.edu.
References
- 1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018;378:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhargava P, Calabresi PA. Metabolomics in multiple sclerosis. Mult Scler 2016;22(4):451–460. [DOI] [PubMed] [Google Scholar]
- 3. Bhargava P, Anthony D. Metabolomics in multiple sclerosis disease course and progression. Mult Scler 2020;26:591–598. [DOI] [PubMed] [Google Scholar]
- 4. Bhargava P, Fitzgerald KC, Calabresi PA, Mowry EM. Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation. JCI Insight 2017;2(19):e95302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villoslada P, Alonso C, Agirrezabal I, et al. Metabolomic signatures associated with disease severity in multiple sclerosis. Neurol ‐ Neuroimmunol Neuroinflamm 2017;4:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Platta ME, Ensari I, Motl RW, Pilutti LA. Effect of exercise training on fitness in multiple sclerosis: a meta‐analysis. Arch Phys Med Rehabil 2016;97:1564–1572. [DOI] [PubMed] [Google Scholar]
- 7. Robinson AG, Dennett AM, Snowdon DA. Treadmill training may be an effective form of task‐specific training for improving mobility in people with Parkinson’s disease and multiple sclerosis: a systematic review and meta‐analysis. Physiotherapy 2019;105:174–186. [DOI] [PubMed] [Google Scholar]
- 8. Fritz NE, Marasigan RER, Calabresi PA, et al. The impact of dynamic balance measures on walking performance in multiple sclerosis. Neurorehabil Neural Repair 2015;29:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keller JL, Fritz N, Chiang CC, et al. Adapted resistance training improves strength in eight weeks in individuals with multiple sclerosis. J Vis Exp 2016;2016:53449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamson BC, Ensari I, Motl RW. Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta‐analysis. Arch Phys Med Rehabil 2015;96:1329–1338. [DOI] [PubMed] [Google Scholar]
- 11. Pilutti LA, Greenlee TA, Motl RW, et al. Effects of exercise training on fatigue in multiple sclerosis: a meta‐analysis. Psychosom Med. 2013;75:575–580. [DOI] [PubMed] [Google Scholar]
- 12. Razazian N, Kazeminia M, Moayedi H, et al. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: a systematic review and meta‐analysis. BMC Neurol 2020;20(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Felippe LA, Salgado PR, De Souza SD, et al. A controlled clinical trial on the effects of exercise on cognition and mobility in adults with multiple sclerosis. Am J Phys Med Rehabil 2019;98:97–102. [DOI] [PubMed] [Google Scholar]
- 14. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Sousa CV, Sales MM, Rosa TS, et al. The antioxidant effect of exercise: a systematic review and meta‐analysis. Sports Med 2017;47:277–293. [DOI] [PubMed] [Google Scholar]
- 16. Mackay CP, Kuys SS, Brauer SG. The effect of aerobic exercise on brain‐derived neurotrophic factor in people with neurological disorders: a systematic review and meta‐analysis. Neural Plast 2017;2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brennan AM, Benson M, Morningstar J, et al. Plasma metabolite profiles in response to chronic exercise. Med Sci Sports Exerc 2018;50:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med 2010;2:33ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karl JP, Margolis LM, Murphy NE, et al. Military training elicits marked increases in plasma metabolomic signatures of energy metabolism, lipolysis, fatty acid oxidation, and ketogenesis. Physiol Rep 2017;5:e13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhargava P, Fitzgerald KC, Venkata SLV, et al. Dimethyl fumarate treatment induces lipid metabolism alterations that are linked to immunological changes. Ann Clin Transl Neurol 2019;6:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kos D, Kerckhofs E, Carrea I, et al. Evaluation of the Modified Fatigue Impact Scale in four different European countries. Mult Scler 2005;11:76–80. [DOI] [PubMed] [Google Scholar]
- 22. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985;10:141–146. [PubMed] [Google Scholar]
- 23. Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology 2010;74(Suppl 3):S8–S15. [DOI] [PubMed] [Google Scholar]
- 24. Goldman MD, Marrie RA, Cohen JA. Evaluation of the six‐minute walk in multiple sclerosis subjects and healthy controls. Mult Scler 2008;14:383–390. [DOI] [PubMed] [Google Scholar]
- 25. Center for Health Statistics N . National Health and Nutrition Examination Survey CARDIOVASCULAR FITNESS PROCEDURES MANUAL.
- 26. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mokhtarzade M, Ranjbar R, Majdinasab N, et al. Effect of aerobic interval training on serum IL‐10, TNFα, and adipokines levels in women with multiple sclerosis: possible relations with fatigue and quality of life. Endocrine 2017;57:262–271. [DOI] [PubMed] [Google Scholar]
- 28. Grazioli E, Tranchita E, Borriello G, et al. The effects of concurrent resistance and aerobic exercise training on functional status in patients with multiple sclerosis. Curr Sports Med Rep 2019;18:452–457. [DOI] [PubMed] [Google Scholar]
- 29. Koay YC, Stanton K, Kienzle V, et al. Effect of chronic exercise in healthy young male adults: a metabolomic analysis. Cardiovasc Res 2020;117(2):613–622. [DOI] [PubMed] [Google Scholar]
- 30. Ali AM, Burleigh M, Daskalaki E, et al. Metabolomic profiling of submaximal exercise at a standardised relative intensity in healthy adults. Metabolites 2016;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakaguchi CA, Nieman DC, Signini EF, et al. Metabolomics‐based studies assessing exercise‐induced alterations of the human metabolome: a systematic review. Metabolites 2019;9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klinge CM, Clark BJ, Prough RA. Dehydroepiandrosterone research: past, current, and future. Vitam Horm 2018;108:1–28. [DOI] [PubMed] [Google Scholar]
- 33. Ohlsson C, Vandenput L, Tivesten Å. DHEA and mortality: what is the nature of the association? J Steroid Biochem Mol Biol 2015;145:248–253. [DOI] [PubMed] [Google Scholar]
- 34. Collomp K, Buisson C, Lasne F, Collomp R. DHEA, physical exercise and doping. J Steroid Biochem Mol Biol 2015;145;206–212. [DOI] [PubMed] [Google Scholar]
- 35. Téllez N, Comabella M, Julià EV, et al. Fatigue in progressive multiple sclerosis is associated with low levels of dehydroepiandrosterone. Mult Scler 2006;12:487–494. [DOI] [PubMed] [Google Scholar]
- 36. Virkki LM, Porola P, Forsblad‐D’Elia H, et al. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA‐deficient patients with primary Sjögren’s syndrome. Arthritis Care Res 2010;62:118–124. [DOI] [PubMed] [Google Scholar]
- 37. Papierska L, Rabijewski M, Kasperlik‐Załuska A, Zgliczyński W. Effect of DHEA supplementation on serum IGF‐1, osteocalcin, and bone mineral density in postmenopausal, glucocorticoid‐treated women. Adv Med Sci 2012;57:51–57. [DOI] [PubMed] [Google Scholar]
- 38. Kenny AM, Boxer RS, Kleppinger A, et al. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc 2010;58:1707–1714. [DOI] [PubMed] [Google Scholar]
- 39. Sato K, Iemitsu M, Aizawa K, et al. DHEA administration and exercise training improves insulin resistance in obese rats. Nutr Metab 2012;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data are available from the corresponding author on reasonable request.


