Abstract
Objective
Alterations in eating behaviour are one of the diagnostic features of behavioural variant frontotemporal dementia (bvFTD). It is hypothesised that underlying brain network disturbances and atrophy to key structures may affect macronutrient preference in bvFTD. We aimed to establish whether a preference for dietary fat exists in bvFTD, its association with cognitive symptoms and the underlying neural mechanisms driving these changes.
Methods
Using a test meal paradigm, adapted from the obesity literature, with variable fat content (low 20%, medium 40% and high 60%), preference for fat in 20 bvFTD was compared to 16 Alzheimer’s disease (AD) and 13 control participants. MRI brain scans were analysed to determine the neural correlates of fat preference.
Results
Behavioural variant FTD patients preferred the high‐fat meal compared to both AD (U = 61.5; p = 0.001) and controls (U = 41.5; p = 0.001), with 85% of bvFTD participants consistently rating the high‐fat content meal as their preferred option. This increased preference for the high‐fat meal was associated with total behavioural change (Cambridge Behavioural Inventory: rs = 0.462; p = 0.001), as well as overall functional decline (Frontotemporal Dementia Rating Scale: rs = −0.420; p = 0.03). A preference for high‐fat content in bvFTD was associated with atrophy in an extended brain network including frontopolar, anterior cingulate, insular cortices, putamen and amygdala extending into lateral temporal, posteromedial parietal and occipital cortices.
Conclusions
Increased preference for fat content is associated with many of the canonical features of bvFTD. These findings offer new insights into markers of disease progression and pathogenesis, providing potential treatment targets.
Introduction
Increasing evidence indicates that changes in metabolism and eating behaviour play a key role in the pathogenesis and disease progression of the neurodegenerative conditions of frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). 1 These two disorders are often linked by a common pathology and shared genetic features with a repeat expansion of the C9orf72 gene being the most common genetic abnormality across both disorders. 2
Changes in eating behaviour are one of the core diagnostic criteria for the diagnosis of behavioural‐variant FTD (bvFTD), 3 with increased total caloric intake and sucrose preference highly indicative of a diagnosis of bvFTD. 4 Eating abnormalities have been linked to alterations in complex neural networks involving the hypothalamus, 5 interacting with reward and autonomic pathways 4 and have been linked to a range of complex metabolic changes including an increase in body mass index (BMI), dyslipidaemia and insulin resistance. 6 Recent evidence has shown that lipid changes and changes in eating behaviour are associated with improved survival along the FTD‐ALS spectrum. 7 , 8 Changes in eating behaviour in bvFTD have also been associated with hypothalamic atrophy and disturbances in key hypothalamic peptides including agouti‐related protein (AgRP). AgRP plays a central role in the melanocortin pathway with increased AgRP level in bvFTD hypothesised as a possible cause of increased caloric intake in this syndrome. 5
The melanocortin system is a critical central nervous system pathway involving the hypothalamus that plays a foundational role in eating and metabolism. Recent obesity research has shown that preferences in macronutrient intake may be associated with changes in melanocortin signalling with melanocortin MC4R pathway deficiency patients displaying a strong preference for high‐fat foods. 9 Reduced melanocortin pathway function due to reduced proopiomelanocortin (POMC) mRNA levels, but increased AgRP mRNA levels have been found in both TDP‐43 and FUS ALS mouse models and following starvation associated with increased overall food intake. 10 Increased AgRP levels are found in both bvFTD and in obese patients. In the obese population, this increased level has been hypothesised to result in increased caloric intake and fat preference following starvation. 9 While mounting evidence points to some commonalities between bvFTD and obesity, no study to our knowledge has empirically documented whether patients with bvFTD also display an increased preference for high‐fat content foods.
Here, we aimed to address this gap in the literature by investigating fat preference in bvFTD using a novel ecologically valid experimental paradigm adapted from the obesity literature, and to delineate the neural mechanisms associated with this change in fat preference. We hypothesised that patients with bvFTD would show an increased preference for high‐fat content food and this would be associated with degeneration of key frontal and temporal brain regions known to support reward and autonomic processing.
Methods
Participants
Twenty individuals diagnosed with bvFTD (n = 20) were compared to disease‐matched cases of Alzheimer’s disease (AD) (n = 16) and 13 control subjects. Participants were recruited from the FRONTIER clinic at the Brain and Mind Centre, the University of Sydney, Australia. Diagnostic assessment comprised a comprehensive neuropsychological assessment and neurological examination, and a structural brain MRI. Diagnosis was determined by multidisciplinary consensus by a neurologist and neuropsychologist in accordance with current clinical diagnostic criteria. 3 , 11 Disease severity was assessed by the Frontotemporal Dementia Rating Scale. 12 Healthy control participants were matched for age, education and BMI. All controls scored above the cut‐off score (88/100) on the Addenbrooke’s Cognitive Examination (ACE‐III). 13 , 14 Additional cognitive measures included the Trail Making Test (TMT) as an index of executive function, 15 and the Facial Affect Selection Task (FAST) to assess emotion processing. 16 Height and weight were measured (shoes removed) to derive the BMI. All carers of participants completed the revised Cambridge Behaviour Inventory (CBI‐R 17 ) to determine severity of behavioural symptoms, comprising a total score, as well as 10 subdomain scores including eating habits.
Ethics statement
This study was approved by the South Eastern Sydney Local Health District and the University of New South Wales ethics committees. All research was conducted in accordance with the relevant guidelines (STROBE) and regulations and informed written consent was obtained from all participants.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
Eating behaviour and fat preference
Preference for fatty food was measured through an ad libitum lunch test meal, adapted from obesity research, 9 where fat content was covertly manipulated by varying the amount of canola oil added to the meal. Participants were offered three options of a chicken korma meal varying only in fat content (low: 20%; medium: 40%; high: 60%). All participants were first offered a 10‐g tasting pot of each meal and asked to rank on a visual analogue scale (VAS) from 0 to 10 (5 = neutral) how much they liked each meal (i.e. liking rating of meal), how fatty it was (i.e. perceived fattiness of the meal) and which meal was their favourite (i.e. meal choice). Following the ratings, participants were encouraged to eat any of the three meals until they were comfortably full. Following the completion of the meal, amount (in grams) of each version of the meal eaten was recorded. Patients were not allowed to add any condiments to change the taste or nutritional content of the meals and each meal had comparable appearance and palatability.
To capture changes in everyday eating behaviour, the eating habits subdomain score was extracted from the CBI‐R. In addition, carers were asked to complete the Appetite and Eating Habits Questionnaire (APEHQ). 18
Statistical analyses
Data were analysed using SPSS Statistical software, version 24.0. Demographic variables (i.e. age, education, BMI and ACE‐III total score) and eating behaviour results (i.e. the amount of meal consumed, and the CBI‐R eating habits subdomain score and total score) were compared across groups (AD, bvFTD and controls) using one‐way analysis of variance (ANOVA) followed by Tukey HSD or Games‐Howell post hoc tests in case of violation of homogeneity of variances assumption. Categorical variables (i.e. sex and meal choice) were analysed using Chi‐squared tests. Other clinical (i.e. disease duration, FRS) and eating behaviour (i.e. APEHQ total score) variables specific to patient groups were analysed using independent sample t‐tests.
Comparisons of meal choice across groups were examined using Chi‐square tests followed by post‐hoc comparisons. Similarly, comparisons of meal ratings across groups (liking ratings and perceived fattiness) were examined using nonparametric Kruskal–Wallis tests followed by post‐hoc Mann–Whitney tests. Wilcoxon‐signed ranks tests were used to explore within‐group differences in the perceived fattiness of each meal. Associations between liking ratings and relevant clinical variables (e.g. ACE scores, APEHQ total score) were examined using Spearman rank correlations. Finally, linear regression analyses were run to explore whether fattiness perception (i.e. the rating of perceived fattiness of the meal) predicted meal liking (i.e. liking rating of the meal) in each participant group separately.
Imaging
Brain imaging acquisition
The majority of participants (bvFTD = 17; AD = 13; controls = 13) underwent whole‐brain structural MRI (3T GE Discovery MR750 scanner), fitted with a standard 8‐channel head coil. High‐resolution T1‐weighted images were acquired using the following protocol: matrix 256 × 256, 200 slices, 1 mm2 in‐plane resolution, slice thickness = 1 mm, echo time = 2.6ms, repetition time=5.8ms and flip angle=8°.
Voxel‐based morphometry analysis
VBM analysis was conducted on the T1‐weighted images, using the FMRIB Software Library (FSL) package, version 6.0.0 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM). In the first instance, brain extraction was conducted using the BET algorithm in FSL. 19 Each extracted scan was visually checked to ensure that no brain matter was excluded and no non‐brain matter (e.g. dura mater, skull) remained. Brain extracted images were then segmented into cerebrospinal fluid, grey matter and white matter using the FMRIB Automatic Segmentation Tool 20 (FAST). Following which, the grey matter partial volumes were non‐linearly registered to the Montreal Neurological Institute Standard space (MNI152) using FNIRT with a b‐spline representation of the registration warp field. An equal number of the registered grey matter images from each group (a total of 39 scans) was selected and concatenated into a grey matter template specific to this study with non‐linear (non‐affine) registration to ensure equal representation and minimise potential bias. Each voxel of each registered grey matter image was divided by the Jacobian of the warp field to correct for any contraction/enlargement caused by the non‐linear component of the transformation. Smoothing of the segmented and modulated normalised grey matter images was then conducted using a Gaussian kernel of 3 mm.
Whole‐brain general linear models were performed to explore neural correlates of fatty food preference. First, differences in the pattern of cerebral grey matter intensity between each patient group and controls (i.e. AD vs. controls; and bvFTD vs. controls) were examined using independent t tests to identify the pattern of whole‐brain atrophy within each patient group. Next, separate analyses were conducted on each patient group (combined with controls to increase power) 21 to determine disease‐specific neural correlates of fatty food preference using the Liking Rating for the high‐fat content meal. A negative t‐contrast was run exploring associations between higher preference ratings for the high‐fat content meal and lower grey matter intensity across the entire brain. For all analyses, significance was set at p < 0.001 uncorrected for multiple comparisons, with a cluster extent threshold of 100 contiguous voxels.
Results
Demographics
No significant group differences were found for age, level of education and BMI (Table 1). Sex distribution differed across groups, with more males than females in the bvFTD group (p = 0.004) and the reverse distribution in the control group (p = 0.007; see Table 1). Importantly, the patient groups did not differ in disease duration or disease severity as measured by the FRS (both p values > 0.05). Compared with controls, both bvFTD (p < 0.001) and AD (p < 0.001) groups demonstrated significantly poorer overall cognitive performance (ACE‐III total score), with the AD group also scoring lower than the bvFTD group (p = 0.006). Characteristic impairments in emotion processing were also observed in bvFTD (p = 0.001) and AD (p = 0.002) relative to controls. Both bvFTD and AD showed behavioural disturbances with disproportionate impairments in bvFTD relative to AD (CBI Total: p = 0.005).
Table 1.
Demographic and clinical characteristics of patient groups and healthy controls.
| Controls (n = 13) | bvFTD (n = 20) | AD (n = 16) | F | p | Post‐hoc | |
|---|---|---|---|---|---|---|
| Gender (Male/Female) | 3/10 | 16/4 | 8/8 | 10.569 1 | 0.005 | – |
| Male | 3 | 16 | 8 | – | 0.004 | bvFTD > AD, Controls |
| Female | 10 | 4 | 8 | – | 0.007 | Controls > AD, bvFTD |
| Age (years) | 66.5 ± 6.8 | 62.7 ± 7.9 | 65.1 ± 9.1 | 0.998 | 0.376 | – |
| Education (years) | 15.2 ± 4.3 | 12.3 ± 3.9 | 13.7 ± 2.7 | 2.243 | 0.118 | – |
| BMI | 25.6 ± 4.5 | 30.3 ± 8.6 | 25.5 ± 3.9 | 2.953 | 0.064 | – |
| ACE Total (/100) | 95.7 ± 3.5 | 76.4 ± 14.6 | 61 ± 17.9 | 40.608 | <0.001 | Controls > bvFTD and AD; bvFTD > AD |
| TMT B‐A Time (seconds) | 36.9 ± 38.5 | 97.7 ± 76.4 | 87.6 ± 91.9 | 4.619 | 0.022 | Controls < bvFTD, AD |
| FAST (/42) | 40.4 ± 1.3 | 30.4 ± 7.9 | 28.4 ± 7.8 | 17.275 | <0.001 | Controls > bvFTD, AD |
| Disease duration (months) | – | 5.2 ± 3.6 | 3.5 ± 1.5 | −1.933 2 | 0.064 | – |
| FRS (Rasch) | – | 0.3 ± 2.1 | 1.2 ± 1.3 | 1.085 2 | 0.293 | – |
| CBI‐R | ||||||
| Total score | 3.5 ± 3.7 | 41.3 ± 14.5 | 26.8 ± 10.7 | 79.077 | <0.001 | bvFTD > AD > Controls |
| Eating habits score | 1.7 ± 2.9 | 44.7 ± 20.9 | 11.3 ± 11.9 | 41.473 | <0.001 | bvFTD > Controls, AD |
| APEHQ total | – | 63.3 ± 41.7 | 15.3 ± 13 | −3.820 2 | 0.002 | bvFTD > AD |
Means ± standard deviation.
ACE, Addenbrooke’s Cognitive Examination; AD, Alzheimer’s disease; APEHQ, Appetite and Eating Habits Questionnaire; BMI, Body Mass Index; bvFTD, behavioural‐variant frontotemporal dementia; CBI‐R, Cambridge Behavioural Inventory–Revised; FAST, Facial Affect Selection Task; FRS, Frontal Rating Scale; TMT, Trail Making Test.
Chi‐square value.
Independent sample t‐test value.
In terms of eating changes, bvFTD patients were rated as displaying greater alterations in eating habits (CBI‐R eating habits subdomain: p < 0.001), as well as significant changes in everyday eating behaviours (APEHQ total scores: p = 0.002) compared with the AD group.
Lunch test meal and fat preference
A Chi‐square test revealed a significant group difference in terms of meal choice (p = 0.001; χ2 = 21.851). Post‐hoc comparisons revealed that a significantly higher proportion of bvFTD patients (85%) selected the high‐fat content meal (60% fat content), and least commonly the low‐fat content meal (20% fat content), as their preferred meal (both p values <0.001). In contrast, AD patients (p = 0.009) and controls (p = 0.036) were more likely to choose the low‐fat content meal, with only 19% of AD and 23% of controls choosing the high‐fat content option.
Within‐group Wilcoxon‐signed ranks tests revealed that bvFTD, AD and controls demonstrated the expected significantly higher perceived fattiness rating in the high‐fat meal compared to the medium‐ and low‐fat meal; and in the medium‐fat meal compared to low‐fat meal (all p values ≤0.014), with an effect approaching statistical significance in the AD group for high‐ versus medium‐fat meal (p = 0.053). This suggests that all groups could successfully differentiate among the low‐, medium‐ and high‐fat content meals in terms of their perceived fattiness (Fig. 1). Furthermore, between‐group Kruskal‐Wallis tests revealed that control participants labelled all meals as significantly more fatty than the AD or bvFTD groups (all values p < 0.05), whereas bvFTD and AD groups did not differ for their perceived fattiness ratings of each meal (all p values > 0.05), supporting the fact that the AD and bvFTD group had a similar perception of fattiness.
Figure 1.
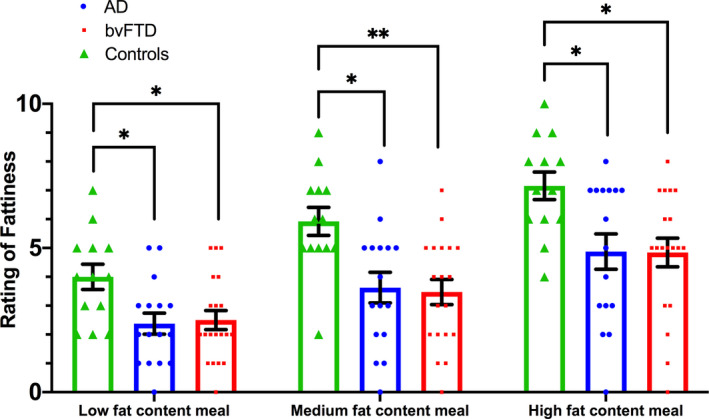
Bar plots displaying perceived fattiness rating of each meal. Each dot represents each individual data point; error bars represent standard error of the mean (SEM). AD, Alzheimer’s disease; bvFTD, behavioural‐variant frontotemporal dementia. *p < 0.05, **p ≤ 0.001.
Finally, we explored the liking ratings assigned by participants to each of the test meal options. BvFTD patients rated the high‐fat content meal significantly more favourably compared to both AD (U = 61.5; p = 0.001) and controls (U = 41.5; p = 0.001; Fig. 2 and Table 2), with no significant differences observed between AD and controls (p > 0.05). Liking ratings for the low‐ and medium‐fat content meals were not found to differ across groups (all p values >0.05), suggesting the preference for the high‐fat content meal was specific to the bvFTD group. In light of the statistical differences in sex distribution driven by the bvFTD group, an additional one‐way analysis of covariance (ANCOVA) was performed to compare the differences in liking rating of the high‐fat meal between groups with sex included as a covariate. After controlling for the confounding effect of sex, differences in the liking rating between diagnosis groups remained highly significant (p = 0.003) with a large effect size (partial eta2 = 0.225). Subsequent post‐hoc comparisons revealed that bvFTD continued to demonstrate significantly higher liking ratings for the high‐fat content meal compared to both AD (p = 0.013) and control (p = 0.007) groups after accounting for the effect of sex. The groups did not differ in the total amount of the chosen meal that they consumed for lunch.
Figure 2.
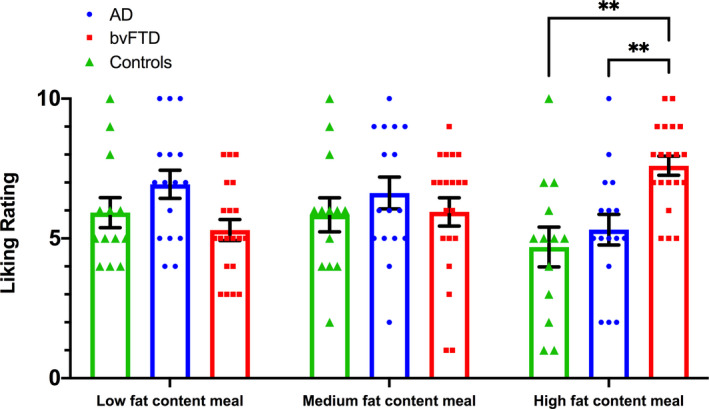
Bar plots depicting liking rating of each meal. Each dot represents each individual data point; error bars represent standard error of the mean (SEM). AD, Alzheimer’s disease; bvFTD, behavioural‐variant frontotemporal dementia. **p ≤ 0.001 compared to both AD and controls.
Table 2.
Fat preference results in patient groups and healthy controls.
| Controls (n = 13) | bvFTD (n = 20) | AD (n = 16) | H | p | Post‐hoc | |
|---|---|---|---|---|---|---|
| Meal choice as most liked (low/medium/high) | 7/3/3 | 1/2/17 | 8/4/3 | 21.851 1 | <0.001 | – |
| Low‐fat content | 7 | 1 | 8 | – | 0.046 | More likely: AD |
| <0.001 | Less likely: bvFTD | |||||
| Medium‐fat content | 3 | 2 | 4 | – | – | |
| High‐fat content | 3 | 17 | 3 | – | 0.009 | Less likely: AD |
| <0.001 | More likely: bvFTD | |||||
| 0.036 | Less likely: Control | |||||
| Liking rating of low‐fat content meal (/10) | 5.92 ± 1.94 | 5.30 ± 1.69 | 6.94 ± 2.02 | 5.347 | 0.069 | – |
| Liking rating of medium‐fat content meal (/10) | 5.85 ± 2.19 | 5.95 ± 2.26 | 6.63 ± 2.28 | 1.038 | 0.595 | – |
| Liking rating of high‐fat content meal (/10) | 4.69 ± 2.56 | 7.60 ± 1.54 | 5.31 ± 2.18 | 15.203 | <0.001 | bvFTD > AD, Controls |
| Perceived fattiness rating of low‐fat content meal (/10) | 4.0 ± 1.58 | 2.50 ± 1.47 | 2.38 ± 1.46 | 8.056 | 0.018 | Controls > AD, bvFTD |
| Perceived fattiness rating of medium‐fat content meal (/10) | 5.92 ± 1.75 | 3.47 ± 1.90 | 3.63 ± 2.13 | 11.885 | 0.003 | Controls > AD, bvFTD |
| Perceived fattiness rating of high‐fat content meal (/10) | 7.15 ± 1.73 | 4.84 ± 2.17 | 4.88 ± 2.45 | 9.058 | 0.011 | Controls > AD, bvFTD |
| Amount of the chosen meal consumed (gram) | 225.2 ± 58.5 | 256.4 ± 98.6 | 209.2 ± 78.4 | 10.225 | 0.305 | – |
Means ± standard deviation. H, Kruskal‐Wallis test statistic; Post‐hoc = Mann‐Whitney U post‐hoc comparison results.
AD, Alzheimer disease; bvFTD, behavioural‐variant Frontotemporal Dementia.
Chi‐square value.
A linear regression using perceived fattiness as the predictor and liking rating for high‐fat meal as the dependent variable was performed to further examine whether perceived level of fattiness predicted preference for high‐fat food. The level of perceived fattiness rating for the high‐fat content meal was not found to be a significant predictor of the liking rating within the AD (p = 0.769), bvFTD (p = 0.069) or control (p = 0.336) groups. This supports our assertion that the fatty food preference observed in the bvFTD group was not primarily driven by an effect of fattiness perception.
Associations between eating behaviour and clinical measures
Across the entire participant sample (n = 49), liking ratings for the high‐fat content meal were significantly and positively correlated with the CBI‐R total (rs = 0.462; p = 0.001) and CBI‐R eating habits subdomain (rs = 0.493; p = <0.001) scores. Liking of the high‐fat content meal was also correlated with greater functional impairment (FRS: rs = −0.420; p = 0.037), as well as emotion processing disturbances (total FAST; rs = −0.394; p = 0.013) and executive dysfunction (TMT B‐A time; rs = 0.355; p = 0.011), confirming that the increased fat preference correlated with core cognitive features of bvFTD.
Additional exploratory correlational analyses were conducted exclusively within the bvFTD group. Significant and positive correlations emerged between liking ratings for the high‐fat content meal and executive dysfunction (TMT B‐A time; rs = 0.657; p = 0.004). However, no significant associations were found between liking ratings and the CBI‐R, functional impairment (FRS scores) or emotion processing disturbances (total FAST; all p values >0.05). However, it is important to take into account the fact that power to detect statistical significance may be limited in light of the smaller sample size (20 bvFTD patients), when considered together with the small variance in behavioural abnormalities and clinical severity in the current sample of bvFTD patients. In line with this, the association between FAST scores and liking ratings for the high‐fat content meal was approaching significance (p = 0.058) with a high rs value of −0.439.
VBM results
Patterns of whole‐brain atrophy
Compared with controls, bvFTD showed characteristic cortical and subcortical grey matter intensity reduction predominantly in bilateral frontal poles extending into frontal orbital cortex, insular cortex, putamen and amygdala, as well as bilateral temporal pole, lateral occipital cortex and cerebellum, and right anterior cingulate and paracingulate gyrus (see Table S1). The AD group, in contrast, demonstrated the typical cortical and subcortical grey matter intensity reduction reported previously, largely concentrated in the left temporal lobe and hippocampus, and bilateral lateral occipital cortex, putamen and amygdala.
Neural correlates of high‐fat preference
Associations between grey matter intensity and liking rating of the high‐fat content meal were carried out separately for each patient group combined with controls (i.e. bvFTD with controls and AD with controls) in order to identify the associations between grey matter reduction and fatty food preference specific to each dementia syndrome.
In bvFTD combined with controls (Fig. 3, Table 3), significant associations were identified predominantly in bilateral frontopolar and insular cortex, right paracingulate gyrus, anterior and posterior cingulate gyrus, middle temporal gyrus and lateral occipital cortex. Subcortical regions including the bilateral putamen and left amygdala were further implicated. In AD combined with controls, no significant results were detected at the cluster threshold of 100 contiguous voxels.
Figure 3.
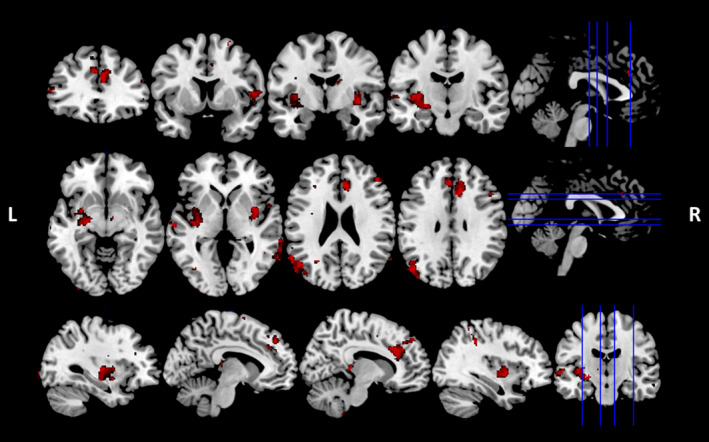
Voxel‐based morphometry analyses showing grey matter intensity reduction that correlates significantly with higher liking rating of the high‐fat content meal in bvFTD group combined with controls. Coloured voxels show significant grey matter intensity reduction at the threshold of p < 0.001 (uncorrected) with a cluster threshold of 100 contiguous voxels. L, Left hemisphere; R, Right hemisphere.
Table 3.
Voxel‐based morphometry results of significant grey matter intensity reduction that correlates significantly with liking rating of the high‐fat content meal in each patient group combined with controls.
| Group | Cluster size, voxels | MNI coordinates | Brain regions | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| bvFTD | 558 | −50 | −82 | 18 | Left lateral occipital cortex extending into angular gyrus |
| 487 | −20 | −10 | −10 | Left amygdala extending into putamen and insular cortex | |
| 465 | 10 | 28 | 20 | Right paracingulate gyrus extending into anterior cingulate gyrus | |
| 225 | 38 | −8 | −4 | Right insular cortex extending into right putamen | |
| 146 | 52 | 42 | 4 | Right frontal pole | |
| 144 | 64 | −58 | −4 | Right middle temporal gyrus | |
| 110 | 58 | −70 | 4 | Right lateral occipital cortex, inferior division extending into middle temporal gyrus | |
| 104 | 22 | 58 | 12 | Right frontal pole | |
| 101 | 12 | −38 | 2 | Right posterior cingulate gyrus extending into right thalamus | |
| AD | NS | – | – | – | – |
All t values reported ≥4.454.
Significant grey matter intensity reduction in each patient group combined with controls voxel‐wise at the threshold of p < 0.001 (uncorrected) with a cluster threshold of 100 contiguous voxels.
AD, Alzheimer’s disease; bvFTD, behavioural‐variant Frontotemporal Dementia; MNI, Montreal Neurological Institute; NS, No significant results.
Further exploratory analyses were run with age included as a covariate variable (see Table S1) and focusing on the bvFTD group only (see Table S2) using a more liberal threshold at p < 0.01 (uncorrected) with a cluster threshold of 50 contiguous voxels was used to compensate for the impact of an additional covariate variable and a smaller sample size on statistical power. Within the bvFTD group only, bilateral superior occipital cortex extending into angular gyrus and middle temporal cortex, bilateral frontal pole as well as left insular cortex, frontal medial and orbital cortex, posterior cingulate gyrus and right anterior cingulate and paracingulate gyrus were implicated in higher liking rating of the high‐fat content meal. After controlling for the effect of age, left putamen, right inferior lateral occipital cortex and adjacent inferior and middle temporal gyrus, and right paracingulate gyrus and anterior cingulate gyrus were found to be associated with liking rating of the high‐fat content meal in bvFTD group combined with controls. Overall, these analyses revealed largely similar regions of anterior and posterior cingulate, middle temporal gyrus and occipital cortices were significantly associated with liking rating of the high‐fat content meal.
Discussion
It is increasingly recognised that changes in eating behaviour and metabolism are associated with neurodegeneration 1 , 22 ; however, it has not been known whether these changes are specific to certain macronutrients and whether these changes are related to central brain neurodegeneration. For the first time in bvFTD, we have quantified the degree of fat preference using an ecologically valid ad libitum test meal paradigm. Using voxel‐based morphometry analyses we identified the potential neural drivers of this increased preference for fat content. In line with our original hypotheses, we found a strong preference for fat exclusively in the bvFTD group, with over 85% of cases selecting the meal option with the highest fat content. This preference for fat content was not evident in either the AD or healthy control groups, suggesting a fundamental change that is specific to the pathogenesis of bvFTD.
Looking at potential associations between the increased preference for fat content and clinical variables in bvFTD revealed important clues regarding the potential mechanisms underlying this effect. Liking ratings for the high‐fat option were correlated with overall disease staging on the FRS along with key discriminatory cognitive features of bvFTD, including eating changes, executive dysfunction and facial emotion processing, 23 Collectively, these findings suggest that an increased preference for fat content is associated with a number of the canonical features of bvFTD and may form an indicator for disease progression and pathogenesis. Importantly all participants were able to detect changes in perceived fattiness across all the meal options and there was no difference in perceived fattiness between the AD and bvFTD groups. Our linear regression modelling demonstrated that perceived perception of fat content did not predict liking ratings in either the patient or control groups, suggesting that changes in fat preference are likely due to factors other than the perception or taste of fat. A candidate mechanism in this regard is the direct neural response to fat; however, this proposal requires validation in further empirical studies. While there was no statistical significance difference between the total amount of the meal consumed between the groups, as expected the bvFTD group tended to consume a greater total amount. Increased intake of foods by patients with bvFTD has been well documented and forms one of the major diagnostic features.
Increased liking for high‐fat content on the test meal in bvFTD was associated with grey matter intensity decrease in a distributed neural network involving the frontoinsular cortices, middle temporal gyrus, anterior and posterior cingulate gyrus, bilateral occipital cortex as well as subcortical regions including the left amygdala, and bilateral putamen. Frontoinsular cortices are one of the earliest regions targeted by the bvFTD pathological process 24 and are typically implicated in the many socioemotional disturbances characteristic of this syndrome. 25 Frontopolar regions are commonly implicated in decision‐making processes, including evaluating the outcomes of decisions, 26 and may reflect the cognitive evaluation of different choices in the test meal paradigm. Increased fat preference also correlated with atrophy in the middle temporal gyrus, a region known to be associated with emotion processing and semantic processing, suggesting that knowledge of foods may play a role in changes in fat preference. 4 , 27
We further found evidence for anterior and posterior cingulate gyrus involvement in changes in fat preference in bvFTD potentially reflecting the integrative roles of these regions in supporting anticipation of reward, task reinforcement 28 , 29 and in controlling visceromotor, endocrine and skeletomotor outputs, 30 potentially via integration of cognitive with autonomic information. 31 In healthy individuals, activity of the cingulate cortex has been associated with increased BMI suggesting a role for this structure in regulating eating. 32 These cognitive aspects likely interact with reward processes via connections between the anterior cingulate cortex and insula which are implicated in the integration of taste via connections with the gustatory cortex in the insula, 33 and reward, acting as a relay centre between the basal ganglia and frontal structures. 34 The insula has been shown to regulate the processing of external sensory information linked to reward processing 35 and together with the anterior cingulate cortex forms the salience network. 24 The insula has been implicated extensively in food intake, 36 and benefits from extensive connections with orbitofrontal cortex and anterior cingulate cortex regions that undergo changes between obese and lean individuals. 37 , 38 The insula is also involved early in the course of bvFTD 39 and is associated with disrupted pain and temperature symptoms in bvFTD, 40 indicating it as a strong candidate as a hub in a sensory homeostatic signalling network. As such, our findings suggest that degeneration of the insula and its connections may modulate many aspects of eating changes in bvFTD, of which increased preference for high‐fat content appears to be a central feature.
We further found evidence for involvement of subcortical regions including the amygdala and putamen, in the origin of increased fat preference in bvFTD. The amygdala has a long‐established role in emotion processing, yet has been widely implicated in reward processing particularly in relation to food consumption and eating behaviour, with studies suggesting activity in the amygdala may positively reinforce food consumption. 41 The putamen is also potentially ideally located to support complex eating behaviours, situated within the brain’s frontostriatal reward pathways. 42 Indeed, previous functional MRI studies have demonstrated that increased fat intake is associated with lower putamen activity in healthy individuals, 43 suggesting an important role in reward evaluation during food consumption. To our knowledge, no study to date has employed functional MRI to study changes in eating behaviour in FTD, however, we suggest that such studies will be invaluable to determine how large‐scale network dysfunction relates to reward processing and positive/negative feedback.
Finally, we found evidence for occipital contributions to increased fat preference in bvFTD, with volume loss in the lateral occipital cortex correlating with liking ratings for the high‐fat option. This association likely reflects the highly visual way in which we approach eating, providing pleasurable or aversive feedback via reward pathways, and influencing the subsequent decision‐making phase. Interestingly, the contribution of such visual associations has been shown in other diagnoses with abnormal eating behaviour such as Prader‐Willi syndrome, 44 offering a novel platform for future investigations in bvFTD.
Previously, we have demonstrated that bvFTD is characterised by a strong sucrose preference. 4 While some of the regions appear to overlap between fat and sucrose preference, it is likely that different neural pathways control macronutrient intake in bvFTD. The brain regions commonly implicated in fat and sucrose preference in bvFTD include the frontal cortices, amygdala, putamen and insula and occipital regions. Other regions such as the anterior and posterior cingulate cortices appear to be specifically associated with fat preference. The unique contribution of the cingulate cortex to the increase in fat content in bvFTD remains unclear and future studies will be required to tease apart the precise role of the anterior and posterior subdivisions of the cingulate to these changes in eating behaviour.
Behavioural‐variant FTD patients appear to have a unique preference for both sucrose and fat intake. In genetically obese individuals, changes in the hypothalamus specifically involving disruption of melanocortin function lead to an increased preference for high‐fat food, but a decreased preference for sucrose‐rich food. 9 This stands in contrast with the global increased preference of high‐fat and high‐sucrose food in bvFTD. Changes in melanocortin function and hypothalamic volume have been related to eating behaviour and BMI change in bvFTD. 5 , 45 To understand the differences between bvFTD and genetically obese cohorts, it will be important to determine via pathological examination and serum analyses if changes in fat preference are also related to changes in melanocortin function in bvFTD. It will also be important to determine the interaction between cortical atrophy and hypothalamic neuroendocrine function more broadly.
It has been hypothesised that increased fat intake in obese patients with disruption of melanocortin dysfunction secondary to mutations in melanocortin receptors is an adaptive response to a starvation state, whereby patients increase their fat intake in order to maintain energy balance. In both FTD and ALS, an increase in fat intake has been hypothesised to be secondary to a state of increased energy metabolism. 1 Previously increased fat intake via carer survey has been shown in ALS (both pure and those with cognitive changes) 46 and presymptomatic ALS cohorts. 47 Further research is required to understand the role that fat intake may play in the setting of increased energy metabolism in the neurodegenerative process, and whether it is an adaptive mechanism to counteract a hypermetabolic state or is driven by the neurodegenerative process.
As the first study to explore the neurocognitive mechanisms modulating increased fat preference in bvFTD, our findings provide an important platform for future research on this topic. Such studies could focus on the staging at which increased fat preference emerges and whether such food preferences may be an early prodromal marker of disease and its ability to be used as a marker of disease progression. Future studies using larger population sizes and longitudinal approaches will be particularly interesting. It will also be important to examine the differences and similarities between the eating and metabolic profiles of patients with neurodegeneration and genetically obese cohorts. In turn, this could offer an attractive means of monitoring at‐risk genetic cohorts, for early harbingers of disease. Moreover, this approach could identify the contribution of obesity to disease pathogenesis and progression, improving our capacity to intervene effectively.
Author Contribution
Rebekah Ahmed: Study design, conducting experiments, analyses, drafting of manuscript. Nga Yan Tse: conducting experiments, analyses, drafting of manuscript. Yu Chen: analyses, drafting of manuscript. Elana Henning: analyses, drafting of manuscript. John Hodges: analyses, drafting of manuscript. Matthew C Kiernan: analyses, drafting of manuscript. Muireann Irish: analyses, drafting of manuscript. Sadaf Farooqi: Study design, analyses, drafting of manuscript. Olivier Piguet: analyses, drafting of manuscript.
Conflict of Interest
The authors declare no competing financial interests. Rebekah Ahmed: Reports no disclosures. Nga Yan Tse: Reports no disclosures. Yu Chen Reports no disclosures. Elana Henning: Reports no disclosures. John Hodges: Reports no disclosures. Matthew C Kiernan: Reports no disclosures. Muireann Irish: Reports no disclosures. Sadaf Farooqi: Reports no disclosures. Olivier Piguet: Reports no disclosures.
Supporting information
Table S1. Voxel‐based morphometry results showing significant associations between grey matter intensity reduction and liking rating of the high‐fat content meal in bvFTD combined with controls.
Table S2. Voxel‐based morphometry results showing significant associations between grey matter intensity reduction and liking rating of high‐fat content meal in the bvFTD group only.
Funding Information
This work was supported in part by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neurone disease, from the National Health and Medical Research Council of Australia (NHMRC) program grant (#1037746 to OP, MK and JRH) and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program (#CE110001021 to OP and JRH) and other grants/sources (NHMRC project grant #1003139 to OP) and Royal Australasian College of Physicians, MND Research Institute of Australia. We are grateful to the research participants involved with the ForeFront research studies. RA is a NHMRC Early Career Fellow (#1120770). MCK was supported by an NHMRC Practitioner Fellowship (#1156093), and MI is supported by an Australian Research Council Future Fellowship (FT160100096). OP is an NHMRC Senior Research Fellow (#1103258).
Funding Statement
This work was funded by National Health and Medical Research Council of Australia grants (#1037746, #1003139, #1120770, #1156093, and #1103258; Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program grants #CE110001021 and FT160100096; Royal Australasian College of Physicians grant ; MND Research Institute of Australia grant ; Australian Research Council Future Fellowship grant .
References
- 1. Ahmed RM, Irish M, Piguet O, et al. Amyotrophic lateral sclerosis and frontotemporal dementia: distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol 2016;15:332–342. [DOI] [PubMed] [Google Scholar]
- 2. Burrell JR, Halliday GM, Kril JJ, et al. The frontotemporal dementia‐motor neuron disease continuum. Lancet 2016;388:919–931. [DOI] [PubMed] [Google Scholar]
- 3. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed RM, Irish M, Henning E, et al. Assessment of eating behavior disturbance and associated neural networks in frontotemporal dementia. JAMA Neurol 2016;73:282–290. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed RM, Latheef S, Bartley L, et al. Eating behavior in frontotemporal dementia: peripheral hormones vs hypothalamic pathology. Neurology 2015;85:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed RM, MacMillan M, Bartley L, et al. Systemic metabolism in frontotemporal dementia. Neurology 2014;83:1812–1818. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed RM, Dupuis L, Kiernan MC. Paradox of amyotrophic lateral sclerosis and energy metabolism. J Neurol Neurosurg Psychiatry 2018;89(10):1013–1014. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed RM, Highton‐Williamson E, Caga J, et al. Lipid metabolism and survival across the frontotemporal dementia‐amyotrophic lateral sclerosis spectrum: relationships to eating behavior and cognition. J Alzheimers Dis 2018;61:773–783. [DOI] [PubMed] [Google Scholar]
- 9. van der Klaauw AA, Keogh JM, Henning E, et al. Divergent effects of central melanocortin signalling on fat and sucrose preference in humans. Nat Commun 2016;7:13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vercruysse P, Sinniger J, El Oussini H, et al. Alterations in the hypothalamic melanocortin pathway in amyotrophic lateral sclerosis. Brain 2016;139(Pt 4):1106–1122. [DOI] [PubMed] [Google Scholar]
- 11. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mioshi E, Hsieh S, Savage S, et al. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010;74:1591–1597. [DOI] [PubMed] [Google Scholar]
- 13. Hsieh S, Schubert S, Hoon C, et al. Validation of the Addenbrooke's Cognitive Examination III in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord 2013;36:242–250. [DOI] [PubMed] [Google Scholar]
- 14. So M, Foxe D, Kumfor F, et al. Addenbrooke's Cognitive Examination III: psychometric characteristics and relations to functional ability in dementia. J Int Neuropsychol Soc 2018;24:854–863. [DOI] [PubMed] [Google Scholar]
- 15. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–214. [DOI] [PubMed] [Google Scholar]
- 16. Miller LA, Hsieh S, Lah S, et al. One size does not fit all: face emotion processing impairments in semantic dementia, behavioural‐variant frontotemporal dementia and Alzheimer's disease are mediated by distinct cognitive deficits. Behav Neurol 2012;25:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wedderburn C, Wear H, Brown J, et al. The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatry 2008;79:500–503. [DOI] [PubMed] [Google Scholar]
- 18. Ahmed RM, Irish M, Kam J, et al. Quantifying the eating abnormalities in frontotemporal dementia. JAMA Neurol 2014;71:1540–1546. [DOI] [PubMed] [Google Scholar]
- 19. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 21. Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain 2012;135(Pt 7):2178–2191. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed RM, Ke YD, Vucic S, et al. Physiological changes in neurodegeneration ‐ mechanistic insights and clinical utility. Nat Rev Neurol 2018;14:259–271. [DOI] [PubMed] [Google Scholar]
- 23. Kamminga J, Kumfor F, Burrell JR, et al. Differentiating between right‐lateralised semantic dementia and behavioural‐variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry 2015;86:1082–1088. [DOI] [PubMed] [Google Scholar]
- 24. Seeley WW. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct 2010;214:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dermody N, Wong S, Ahmed R, et al. Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer's disease and the behavioral‐variant of frontotemporal dementia. J Alzheimers Dis 2016;53:801–816. [DOI] [PubMed] [Google Scholar]
- 26. Tsujimoto S, Genovesio A, Wise SP. Evaluating self‐generated decisions in frontal pole cortex of monkeys. Nat Neurosci 2010;13:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piwnica‐Worms KE, Omar R, Hailstone JC, Warren JD. Flavour processing in semantic dementia. Cortex 2010;46:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex 2006;16:1040–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 2007;11:168–176. [DOI] [PubMed] [Google Scholar]
- 30. Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992;2:435–443. [DOI] [PubMed] [Google Scholar]
- 31. Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 2001;29:537–545. [DOI] [PubMed] [Google Scholar]
- 32. Volkow ND, Wang G‐J, Telang F, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samuelsen CL, Gardner MP, Fontanini A. Thalamic contribution to cortical processing of taste and expectation. J Neurosci 2013;33:1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol 2011;300:R1266–R1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heni M, Kullmann S, Ketterer C, et al. Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Hum Brain Mapp 2014;35:918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kullmann S, Pape A‐A, Heni M, et al. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cereb Cortex 2013;23:1247–1256. [DOI] [PubMed] [Google Scholar]
- 38. Kullmann S, Heni M, Veit R, et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012;33:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 2008;65:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fletcher PD, Downey LE, Golden HL, et al. Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain 2015;138(Pt 11):3360–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Douglass AM, Kucukdereli H, Ponserre M, et al. Central amygdala circuits modulate food consumption through a positive‐valence mechanism. Nat Neurosci 2017;20:1384–1394. [DOI] [PubMed] [Google Scholar]
- 42. Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci 2018;19:470–484. [DOI] [PubMed] [Google Scholar]
- 43. Doornweerd S, van Duinkerken E, de Geus EJ, et al. Overweight is associated with lower resting state functional connectivity in females after eliminating genetic effects: a twin study. Hum Brain Mapp 2017;38:5069–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogura K, Fujii T, Abe N, et al. Regional cerebral blood flow and abnormal eating behavior in Prader‐Willi syndrome. Brain Dev 2013;35:427–434. [DOI] [PubMed] [Google Scholar]
- 45. Piguet O, Petersén Å, Yin Ka Lam B, et al. Eating and hypothalamus changes in behavioral‐variant frontotemporal dementia. Ann Neurol 2011;69:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahmed RM, Caga J, Devenney E, et al. Cognition and eating behavior in amyotrophic lateral sclerosis: effect on survival. J Neurol 2016;263:1593–1603. [DOI] [PubMed] [Google Scholar]
- 47. Huisman MHB, Seelen M, van Doormaal PTC, et al. Effect of presymptomatic body mass index and consumption of fat and alcohol on amyotrophic lateral sclerosis. JAMA Neurol 2015;72:1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Voxel‐based morphometry results showing significant associations between grey matter intensity reduction and liking rating of the high‐fat content meal in bvFTD combined with controls.
Table S2. Voxel‐based morphometry results showing significant associations between grey matter intensity reduction and liking rating of high‐fat content meal in the bvFTD group only.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.


