Abstract
Objective
This study aimed to investigate the colonization and prevalence of carbapenem-resistant Enterobacteriaceae (CRE) in pediatric liver transplant recipients and analyze the high-risk factors and prognosis of CRE infection.
Methods
A prospective study involving 152 pediatric patients undergoing liver transplantation was carried out. Anal swab bacteria cultures were collected when the patients entered the intensive care unit (ICU) and when they left in order to screen for intestinal CRE colonization. The results were grouped according to the occurrence of CRE infection following surgery, and the patients were divided into two groups: a CRE infection group and a non-CRE infection group. Univariate analysis and multiple logistic regression analysis were conducted to determine the independent risk factors of CRE infection and analyze the survival rate.
Results
Of the 152 pediatric liver transplant recipients enrolled in the study, there were 13 cases of postoperative CRE infection and 139 cases of non-CRE infection. The incidence of preoperative CRE infection, preoperative cytomegalovirus (CMV) infection, and preoperative sepsis in the CRE infection group was significantly higher than in the non-CRE infection group (P < 0.005). Intraoperative bleeding volume and operation times in the CRE infection group were also significantly higher than in the non-CRE infection group (P < 0.05). Furthermore, postoperative ICU treatment time, postoperative occurrence of unplanned surgery, postoperative mechanical ventilation of more than 24 hours, and the incidence of pre-ICU CRE colonization in the CRE infection group were significantly higher than in the non-CRE infection group (P < 0.05). Finally, the difference between the CRE infection group and the non-CRE infection group in six-month survival rate following surgery was significant (P < 0.001).
Conclusion
The independent risk factors of CRE infection following pediatric liver transplantation include preoperative CRE infection and pre-ICU CRE colonization. CRE infection progresses quickly, with a poor prognosis and a high mortality rate. The CRE screening of anal swabs is crucial for the early detection of CRE infection.
Keywords: carbapenem-resistant Enterobacteriaceae, CRE, liver transplantation, intestinal colonization, CT screening, anal swabs
Introduction
Over the past decade, carbapenem-resistant Enterobacteriaceae (CRE) infection has become a serious public health issue. CRE refers to any carbapenem-like antibiotics that are resistant to imipenem, meropenem, ertapenem, and doripenem, or to the Enterobacteriaceae that have been confirmed to produce carbapenemases. As one of the most dangerous infectious pathogens following organ transplantation in patients with low immunity, CRE seriously threatens not only the graft’s survival rate but also the recipient’s. In order to identify CRE colonization in asymptomatic patients, anal swabs or stool specimens should be cultured in accordance with the relevant criteria in the Chinese Expert Consensus on the Prevention and Control of Nosocomial Infections with Multidrug-Resistant Bacteria. CRE infection can be identified if patients experience any symptoms of clinical infection and their sputum, bronchoalveolar lavage fluid, blood, ascites, bile, wound secretion, or other specimen culture results indicate the presence of CRE alongside elevated procalcitonin (PCT), C-reactive protein (CRP), or other inflammatory indicators.
The present study aims to investigate CRE intestinal colonization and postoperative infection in pediatric liver transplant recipients, analyze the high-risk factors and prognosis of CRE infection, and discuss the prevention and treatment of the condition.
Data and Methods
Patient Data and Subgroups
Patient Data
The data of pediatric patients receiving liver transplantation in Tianjin First Central Hospital between August 1, 2017, and January 31, 2018, were retrospectively collected through the hospital’s electronic medical record system. Patients who had donor-derived CRE infection or who abandoned all treatment after operation were excluded, resulting in a total of 152 liver transplant recipients being included in the study. The group consisted of 65 males and 87 females, with an average age of seven months. Donor organs for pediatric liver transplantation were all from immediate family members or heart/brain dead donors, and all organs were donated voluntarily and signed informed consent, and that this was conducted in accordance with the Declaration of Istanbul (See Table 1 for other general preoperative conditions.)
Table 1.
Comparison of Basic Information of Two Groups of Liver Transplant Recipients
| Non-CRE Infection Group, n=139(91.4%) | CRE Infection Group, n=13(8.6%) | p | |
|---|---|---|---|
| Age(Mon) | 7(6–10) | 7(5–10.5) | 0.318 |
| Gender(M) | 59(42.4%) | 6(46.2%) | 0.96 |
| Preoperative hospitalization time (d) | 10(3–23) | 21(4–36) | 0.103 |
| Preoperative ICU stay | 0 | 0 | 0.543 |
| Primary disease(BA) | 126(90.6%) | 12(92.3%) | 0.810 |
| PELD score | 17(10–23) | 12(7–21) | 0.274 |
| Combined heart disease | 48(34.5%) | 5(38.5%) | 0.776 |
| Pre-operative Gelsemium | 65(46.8%) | 8(61.5%) | 0.308 |
| Preoperative antibiotic exposure | 57(41%) | 9(69.2%) | 0.05 |
| Cephalosporin III | 35(27.1%) | 6(46.2%) | 0.193 |
| Carbapenems | 35(27.1%) | 3(23.1%) | 0.867 |
| Anti-positive bacteria antimicrobial | 17(13.2%) | 3(23.1%) | 0.269 |
| Pre-operative pneumonia | 26(20.2%) | 4(30.8%) | 0.296 |
| Pre-operative cholangitis | 20(15.5%) | 2(15.4%) | 0.753 |
| Pre-operative sepsis | 10(7.8%) | 4(30.8%) | 0.0005 |
| Pre-operative peritonitis | 13(2.3%) | 0 | 0.249 |
| Pre-operative CMV infection | 5(3.9%) | 4(30.8%) | <0.0001 |
| Previous CRE infection before surgery | 1(0.7%) | 4(30.8%) | <0.001 |
| Preoperative internal indwelling central venous catheter | 20(14.4%) | 2(15.4%) | >0.05 |
Abbreviation: BA, biliary atresia.
Patient Grouping
Based on whether CRE infection was diagnosed within one month following liver transplantation, the patients were divided into two groups: a CRE infection group and a non-CRE infection group.
Microbiological Test Methods
Anal Swabs
Anal swab specimens were collected from the liver transplant recipients following surgery and were smeared on a CHROMagar CRE screening medium. If CRE growth was present, the strain was identified using an automatic microbial analysis system (Vitek-2 Compact, Biomerieux, France). If the swab separation and identification indicated the presence of CRE at the time of entering the intensive care unit (ICU), it was defined as pre-ICU CRE colonization; if the results indicated the presence of CRE at the time of leaving the ICU, it was defined as post-ICU CRE colonization.
Strain Identification and Drug Sensitivity Tests
Bacterial separation and identification were conducted using an automatic microbial analysis system (Vitek-2 Compact, Biomerieux, France) in accordance with the National Clinical Laboratory Procedures, and the sensitivity was carried out at the same time. The modified Hodge test was adopted in the carbapenemases phenotype test. CRE infection was determined according to the following criteria:
The patient’s sputum, bronchoalveolar lavage fluid, blood, ascites, bile, wound secretion, or other specimen culture results suggested the presence of CRE.
The patient also had pneumonia, septicemia, peritonitis, biliary tract infection, skin and soft-tissue infection, or other corresponding clinical symptoms.
These symptoms were accompanied by an increase in inflammatory indices, such as elevated PCT and CRP.
Preoperative Infection and Treatment
If pneumonia, peritonitis, cholangitis, septicemia, or other infections were present before surgery, corresponding treatment was carried out. In addition to the drug treatment for different pathogens, expectoration, atomization, and body position drainage were conducted to promote sputum excretion in patients with pneumonia; the infective ascites of patients with peritonitis were punctured and drained; the patients with cholangitis were treated with corresponding treatment. For patients with septicemia, infection foci were identified; if the infection source was the catheter, it was removed as soon as possible.
Nutritional support was strengthened before surgery and probiotics were given to increase intestinal immunity. Liver transplantation was then performed if the preoperative treatment achieved the corresponding standards, including normal body temperature, improved clinical symptoms, white blood cell count, PCT, CRP, and other indicators returning to normal, sputum, blood, ascites, and other culture results showing negative, and chest X-rays, CT scans, and other imaging examination showing normal results.
Surgical Conditions and Postoperative Management
The surgical conditions of the 152 patients are presented in Table 2. The postoperative management of the patients included the following measures.
Table 2.
Comparison of Surgical Data Between Two Groups of Liver Transplant Recipients
| Non-CRE Infection Group, n=139(91.4%) | CRE Infection Group, n=13(8.6%) | p | ||
|---|---|---|---|---|
| Surgical Procedure | DBD/DCD liver transplant | 32(23%) | 5(38.5%) | 0.0516 |
| Parental liver transplant | 106(76.3%) | 7(53.8%) | ||
| Assisted liver transplantation | 1(0.7%) | 1(7.7%) | ||
| Surgery time | 7.8±1.5 | 8.8±1.3 | 0.032 | |
| Intraoperative bleeding volume | 300(200–400) | 400(275–500) | 0.045 | |
| Intraoperative red blood cell transfusion volume | 2(2–3) | 3(2–4.5) | 0.255 | |
Abbreviations: DBD, brain-dead donor; DCD, cardiac-death donor.
Immunosuppressive Plans
An intraoperative administration of basiliximab in combination with methylprednisolone was used for immune induction. A triple anti-rejection regime using tacrolimus, methylprednisolone, and basiliximab was adopted following the operation.
Infection Prevention and Monitoring
A five-day postoperative routine intravenous administration of broad-spectrum antibiotics was adopted. Third-generation cephalosporins containing enzyme inhibitors, piperacillin, tazobactam, and ertapenem were generally selected for this. If the patient’s body temperature was normal after five days, and serum PCT < 0.5 μg/L, the antibiotics were stopped. In the case of fever, appropriate anti-infection treatments were selected according to clinical manifestations, serum PCT, CRP, sputum smear and culture, ascites routine and culture, blood culture, fungal glucan test, cytomegalovirus PCR, chest X-ray film, and chest and abdominal CT examination.
Routine Treatment After Liver Transplantation
Basiliximab 10 mg combined with methylprednisolone 10 mg/kg was used for immune induction. A triple anti-rejection regime using tacrolimus, methylprednisolone, and basiliximab was adopted following the operation. A second dose of basiliximab 10 mg was given on the fourth day after operation. Tacrolimus was generally administered orally or via gastric tube 24 hours after operation, with an initial dose of 0.15 mg/(kg·d), after which it was administered twice a day. The target concentration of tacrolimus in the blood in the first three months after operation was maintained at 6–10 μg/L.
Treatment of CRE Infection
If the patient was diagnosed with CRE infection after the operation, one of the following four treatments was administered based on the location of the infection, bacterial culture, and sensitivity results: (1) meropenem 40 mg/kg once/8 h + tigecycline 1.2 mg/kg once/12 h (double the first dose of tigecycline); (2) meropenem 40 mg/kg once/8 h + fosfomycin sodium 100 mg/kg once/8 h; (3) meropenem 40 mg/kg once/8 h + ertapenem 15 mg/kg once/12 h; (4) meropenem 40 mg/kg once/12 h + ceftazidime/averbatan (50 mg/kg once/8 h under 6 months of age, 5 mg/kg once/8 h, but no more than 2.5g once/8 h). The course of treatment lasted at least two weeks. At the same time, strict bedside isolation and aseptic operation was adhered to, and patients, bed units, and related equipment were decolonized with glucose chlorhexidine disinfectant every day.
Prevention of Vascular and Biliary Complications
Blood flow was monitored on a daily basis for one week after surgery. When vascular complications were suspected, an examination was carried out to obtain a clear diagnosis in order to determine the correct treatment.
Postoperative Liver Function Monitoring
Daily hemoglobin (Hb), alanine aminotransferase (ALT), total bilirubin (TB), and prealbumin (PA) levels were recorded five days prior to the operation, and the mean values were calculated. Liver function was re-examined at one month, three months, and six months after operation, and ALT and TB levels were recorded again.
Discharge and Follow-Up
If the patient’s condition was stable, they were discharged from the ICU two to four days after the operation. Patients who had a smooth recovery were generally discharged around one month after the operation. Routine bloods, liver function, blood tacrolimus concentration, and abdominal vascular color ultrasound were reviewed every one to two months for the first year following discharge.
Statistical Analysis
SPSS (version 18.0) was used for data processing. Quantitative data were described in terms of the median (Q1 and Q3), while qualitative data were described in terms of constitution ratio (%). For intergroup comparison, quantitative data were tested using the rank-sum test of independent samples, and qualitative data were tested using a t-test or Fisher’s exact test. Risk factors (χ2) were analyzed via univariate analysis and multivariate logistic regression analysis, while survival rates were compared using the Log rank test. P < 0.05 was considered statistically significant.
Results
Preoperative General Conditions and Operation Conditions
A total of 152 liver transplant patients were included in the study, of which 13 had a postoperative CRE infection and 139 had no CRE infection. Out of the 13 in the CRE infection group, five had CRE infection prior to surgery, four had a bloodstream infection, two had cases of carbapenem-resistant E. coli infection, and two had cases of carbapenem-resistant Klebsiella pneumoniae (CRKP) infection. One patient in the non-CRE infection group had a preoperative CRE abdominal infection, with the ascites culture suggesting CRKP. After treatment, five patients underwent liver transplantation once the preoperative blood culture or ascites culture were negative.
The incidence of preoperative CRE infection, preoperative cytomegalovirus (CMV) infection, and preoperative sepsis in the CRE infection group was significantly higher than in the non-CRE infection group (P < 0.005). However, the differences between the two groups in preoperative hospitalization time, internal indwelling central venous catheter, preoperative antibiotic exposure, and other infectious diseases were not statistically significant (P > 0.05) (Table 1). Intraoperative bleeding volume and operation time in the CRE infection group were significantly higher than in the non-CRE infection group (P < 0.05) (Table 2).
A comparison of general conditions, intraoperative conditions, and postoperative conditions between the CRE infection group and non-CRE infection group is shown in Table 3.
Table 3.
Comparison Between
| CRE Colonization Group n=44(28.9%) | Non-CRE Colonization Group n=108(71.1%) | p | |
|---|---|---|---|
| Age(Mon) | 7.4(4–19) | 8.1(5–36) | 0.318 |
| Gender(M) | 21(47.7%) | 44(40.7%) | 0.472 |
| Primary disease(BA) | 40(90.9%) | 98(90.7%) | 0.974 |
| PELD score | 17(10–23) | 12(7–21) | 0.274 |
| Preoperative hospitalization time (d) | 23(4–70) | 11(4–34) | 0.023 |
| Pre-operative Gelsemium | 28(63.9%) | 45(41.7%) | 0.02 |
| Preoperative antibiotic exposure | 27(61.4%) | 39(36.1%) | 0.007 |
| Pre-operative CRE infection | 4(9.1%) | 1(0.9%) | 0.025 |
| Preoperative internal indwelling central venous catheter | 7(15.9%) | 15(13.9%) | 0.801 |
| Surgery time (h) | 7.8±1.5 | 8.8±1.3 | 0.132 |
| Intraoperative bleeding volume (mL) | 320(200–400) | 370(275–500) | 0.055 |
| ICU treatment time (d) | 7.7(3–21) | 8(4–14) | 0.203 |
| Unplanned surgery | 9(20.5%) | 6(5.6%) | 0.013 |
| Postoperative blood purification | 2(4.5%) | 1(0.9%) | 0.201 |
| Postoperative mechanical ventilation >24h | 5(11.4%) | 5(4.6%) | 0.154 |
CRE Infection and CRE Colonization
In the 152 cases of liver transplantation, 44 cases of CRE intestinal colonization were screened out, and the colonization rate in this group was 28.9%. Of these, 26 (17.1%) were cases of pre-ICU intestinal colonization, and 18 (11.8%) were cases of post-ICU intestinal colonization. CRE infection occurred in 12 of the 44 patients with CRE colonization, with a CRE infection rate of 27.3%. CRE infection occurred in 1 of the 108 patients without CRE colonization, with a CRE infection rate of 0.9%.
Hospital stays in the CRE infection group were significantly longer than in the non-CRE infection group (P < 0.05). The preoperative gexi operation, exposure rates of third-generation cephalosporins or carbapenems, preoperative CRE infection rates, and postoperative occurrences of unplanned surgery were significantly higher in the CRE infection group than in the non-CRE infection group (all P < 0.05).
Postoperative Complications
The postoperative occurrence of unplanned surgery, postoperative mechanical ventilation of more than 24 hours, and the incidence of pre-ICU CRE colonization were significantly higher in the CRE infection group than in the non-CRE infection group (P < 0.05). A number of indicators, including the incidence of postoperative pneumonia and intestinal fistula and PCT levels one month after surgery (which were strongly correlated with CRE infection) were also significantly higher in the CRE infection group (P < 0.05). However, the differences in transplanted hepatic gall bladder, biliary tract complications, and various causes of liver dysfunction were not statistically significant between the two groups. CRE intestinal colonizers were found in both groups, including 12 patients in the CRE infection group and 32 in the non-CRE infection group. The incidence of pre-ICU CRE colonization in the CRE infection group was significantly higher than in the non-CRE infection group (69.2% vs 12.2%, P < 0.001; Table 4).
Table 4.
Postoperative Comparison of Two Groups of Liver Transplant Recipients
| Non-CRE Infection Group, n=139(91.4%) | CRE Infection Group, n=13(8.6%) | p | |
|---|---|---|---|
| Postoperative occurrence of unplanned surgery | 11(7.9%) | 4(30.7%) | 0.026 |
| Liver dysfunction | |||
| Primary non-functional | 1(0.7%) | 0 | >0.05 |
| Acute rejection | 6(4.3%) | 1(7.7%) | 0.472 |
| Drug-induced liver damage | 4(2.9%) | 0 | >0.05 |
| Liver dysfunction due to CMV infection | 3(2.2%) | 0 | >0.05 |
| Postoperative liver support therapy | 1(0.7%) | 1(7.7%) | 0.164 |
| Postoperative acute kidney injury | 35(25.2%) | 3(23.1%) | >0.05 |
| Postoperative renal replacement therapy | 1(0.7%) | 0 | >0.05 |
| Postoperative mechanical ventilation >24h | 7(5%) | 3(23.1%) | 0.042 |
| Pre-ICU CRE colonization | 17(12.2%) | 9(69.2%) | <0.001 |
| Post-ICU CRE colonization | 15(10.8%) | 3(23.1%) | 0.389 |
| Infectious complications | |||
| Pneumonia | 6(4.3%) | 3(23.1%) | 0.031 |
| Abdominal cavity infection | 32(23%) | 3(23.1%) | >0.05 |
| Sepsis | 5(3.6%) | 0 | >0.05 |
| CMV/EBV | 36(25.9%) | 4(30.8%) | 0.959 |
| Skin soft tissue infections | 1(0.7%) | 0 | >0.05 |
| Vascular complications | |||
| Stenosis of the portal anastomosis | 7(5%) | 1(7.7%) | 0.52 |
| Hepatic artery occlusion | 1(0.7%) | 1(7.7%) | 0.164 |
| Outflow tract obstruction | 2(1.4%) | 0 | >0.05 |
| Biliary complications | |||
| Gall bladder | 6(4.3%) | 0 | >0.05 |
| Biliary stenosis | 5(3.6%) | 0 | >0.05 |
| Intestinal fistula | 1(0.7%) | 2(15.4%) | 0.019 |
| Intestinal obstruction | 1(0.7%) | 0 | >0.05 |
| PCT level one month after surgery | 0.92(0.4–3.7) | 4.5(1.7–22) | 0.004 |
| FK506 concentration one month after surgery | 7.6±1.8 | 6.6±2.6 | 0.079 |
Note: EBV for human herpes virus type 4 (HIV-IV).
CRE Infection and Risk-Factor Analysis
Of the 152 liver transplant recipients, 13 had a CRE infection, all of which were CRKP. The median time of infection was five days after surgery (1–13 days). CRE was found in the immediate bacterial culture in five patients following the operation. Of the 13 infected patients, one had a CRE infection in three sites (abdominal cavity, blood flow, and lung), six had a CRE infection in two sites (abdominal cavity and blood flow), and six had a CRE infection in one site (four in the abdominal cavity, one in the blood flow, and one in the lung). The logistic regression analysis suggested that, in terms of children, the independent risk factors for contracting a CRE infection following liver transplantation include preoperative CRE infection and pre-ICU CRE colonization (Table 5).
Table 5.
Multifactorial Analysis of CRE Infection
| B | Standard Error | Wald | Degree of Freedom | p | Exp(B) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Pre-operative CRE infection | 11.703 | 4.758 | 6.050 | 1 | 0.014 | 61.333 | 6.194–607.3 |
| Pre-ICU CRE colonization | 3.833 | 1.163 | 10.865 | 1 | 0.001 | 46.192 | 4.729–451.182 |
Survival Rates
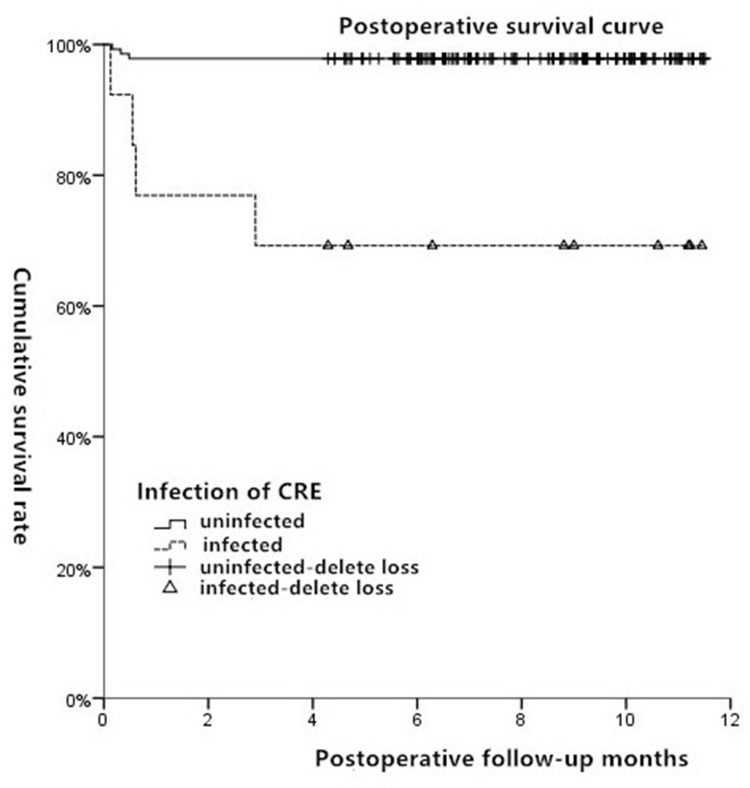
By August 1, 2018, the median follow-up time was nine months (6–12 months). Of the 152 recipients, seven died after surgery, with a median death time of 15 days (4–90 days). Four of these were in the CRE infection group, with their causes of death determined as postoperative abdominal infection and septic shock (day 4), pneumonia and liver failure (day 17), abdominal infection (day 19), and pneumonia and respiratory failure (day 90). The other three deaths occurred in the non-CRE infection group, with their causes of death determined as multiple organ failure secondary to primary graft nonfunction (day 5), Enterococcus faecium-induced abdominal infection and pneumonia (day 10), and Sphingomonas paucimobilis-induced abdominal infection, pneumonia, and respiratory failure (day 15). In the CRE infection group, the survival rates one month, three months, and six months after surgery were 76.9%, 69.2%, and 69.2%, respectively, while all survival rates in the non-CRE infection group were 97.8% (P < 0.001) (Figure 1).
Figure 1.
Survival curves of CRE infection group and non-CRE infection group after operation.
Discussion
According to statistics from the China Antimicrobial Resistance Surveillance System, the incidence of CRKP gradually increased from 3% to 24% between 2005 and 2007. The 2017 CRKP figures from 34 mainland China hospitals revealed an average resistance rate to imipenem of 20.9%.1,2 The number of isolated CRE strains is also increasing every year, with most occurring in transplantation departments. CRE infection seriously threatens survival rates of both the graft and the patient following organ transplantation. Therefore, the early identification and prevention of CRE infection has become a significant challenge in the field of anti-infection treatment following solid organ transplantation.
The human gut is a storage site and source of infection for various drug-resistant bacteria and genes. Feces can cause the diffusion of drug-resistant bacteria by contaminating the surrounding environment. The Center for Disease Control (CDC) recommends the use of fecal specimens to monitor carbapenem-producing bacteria. In view of this, Nanfang Hospital collected fecal samples on the day of admission from 5000 inpatients and found that the intestinal colonization rate of CRE was 1.16%.3 However, CRE has a higher intestinal colonization rate in liver transplant recipients: in a study of 237–386 overseas liver transplant recipients undergoing surgery within the last decade, the preoperative CRE colonization rate was found to be 1.97%–17.6%.4–7 However, few studies have been conducted in terms of the intestinal colonization and infection of CRE in Chinese liver transplant recipients, particularly in infants and young children. The current study included 152 children, who were found to have a 17.1% pre-ICU CRE colonization rate and a 11.8% post-ICU CRE colonization rate. These results were in line with those presented in the existing literature.
According to both domestic and foreign literature, the risk factors for contracting a multi-drug-resistant bacteria infection following liver transplantation include preoperative infection, long operation times, intraoperative bleeding volume of more than 1000 mL, prolonged mechanical ventilation time, and a Model for End-Stage Liver Disease (MELD) score of over 30 points.4–9 Based on the results of the current study, the risk factors for CRE infection can be divided into three categories: preoperative, intraoperative, and postoperative. Preoperative factors include CRE infection, CMV infection, and sepsis, all of which may be due to the application of postoperative immunosuppressants, with the original low immune function further decreasing and the risk of infection further increasing. Intraoperative factors include intraoperative bleeding volume and operation time: if the operation time is more than eight hours, if the bleeding volume is greater than 1500 mL, and if the transfusion volume is more than four units of red blood cells, the risk of postoperative infection is significantly increased.10 Liver transplantation surgery is highly traumatic, and the dramatic hemodynamic changes that occur during the operation lead to increased vascular permeability and an impaired intestinal mucosal barrier. Bacterial translocation from the colonized intestinal tract also causes systemic infection, including CRE infection. Postoperative factors include unplanned surgery, mechanical ventilation of more than 24 hours, and the incidence of pre-ICU CRE colonization: any unplanned surgery can mean that the resistant bacteria that were originally colonized in the gastrointestinal tract and skin mucosa cause an infection in other areas, and prolonged mechanical ventilation inevitably causes respiratory mucosal damage and a decline in the self-cleaning ability of the respiratory tract.
Among all the multi-drug-resistant bacteria infections, a unique risk factor for CRE infection is CRE intestinal colonization. Numerous studies have demonstrated that CRE intestinal colonization at any stage is a risk factor for CRE infection, and the probability of developing a CRE infection following preoperative CRE colonization surgery is between 18.2% and 83.3%, while the probability of developing CRE infection after surgery is between 46.7% and 50%.4–8 Although CRE infections can be controlled for a certain amount of time, CRE colonization can persist for longer periods. One previous study found that CRE colonization lasted for 2–3 months in 46% of patients, 6–12 months in 28% of patients, and more than a year in 14% of patients.11 In the present study, five patients had a preoperative CRE infection, with four of these having a new CRE infection or a recurrent infection following the operation and three having pre-ICU CRE intestinal colonization (75%). CRE was also found in five patients via bacterial culture immediately after operation, with four of these patients having pre-ICU CRE colonization. These results further suggest that CRE intestinal colonization can persist for a long period of time. Hence, the risk of long-term colonization and recurrent CRE infection is high.
The existing literature reports that the infection rate of CRKP following liver transplantation is between 2.5% and 15.7%4–8 and that the annual mortality rate of patients infected with CRE is between 45% and 50%, which is significantly higher than that of patients not infected with CRE (5–7%). The follow-up testing of the present study indicated that the mortality rate in the CRE infection group was 30.8% one year after surgery, while in the non-CRE infection group it was 2.3%, with the difference between the two groups being statistically significant (P < 0.001). As such, greater attention must be given to the prevention and treatment of CRE infection. Prior to surgery, the organ and liver function of the patient must be maintained, their nutritional support must be strengthened, and their normal intestinal flora and microecological balance must be protected. During the operation, precise hemostasis, more careful separation of the gastrointestinal adhesion sites, and more effective abdominal drainage must be conducted in order to avoid a recurrence of the laparotomy. In order to monitor any CRE colonization, routine anal swab screening should be carried out for all admitted patients, especially those with severe conditions, and the current screening frequency should be increased. When a patient tests positive and there are signs of infection, especially in terms of abdominal infection, medical personnel must be alert to the possible occurrence of CRE infection. The vital cluster strategies for controlling CRE colonization include strengthening the culture monitoring of rectal samples, strengthening the education of health-management personnel and the execution of contact isolation and hand hygiene, reducing the application of broad-spectrum antibiotics prior to surgery, monitoring any postoperative complications, preventing catheter-related infections, and choosing the intestinal decontamination.10,12–16
As an alternative therapeutic option for the decolonization of CRE, fecal microbiota transplantation (FMT), which involves gut microbiota with important roles in metabolic processes, immune modulation, and protection against pathogen colonization, can be considered. A previous study indicated that FMT is a potential option for CRE decolonization and that the gut microbiota of CRE carriers could be used to predict decolonization timing after FMT and determine the necessity of repeated FMT.17 FMT has also been reported to be very efficient for the eradication of Clostridium difficile.18 Dai et al studied the therapeutic role of FMT in patients with antibiotic-associated diarrhea, finding that 44.4% (8/18) of the patients recovered from abdominal symptoms without recurrence and survived for a minimum of 12 weeks after being discharged from ICU.19 A systematic review also found that FMT‐related adverse events (AEs) were mild or moderate and self‐limiting but that the methodology of FMT should be improved to reduce both delivery‐related AEs and microbiota‐related AEs.20 Therefore, FMT is a potential alternative therapeutic option for decolonizing CRE, but further investigation might be necessary to improve FMT-related safety during both the procedure and patient preparation.
Conclusion
This study conducted a preliminary analysis of the risk factors for CRE infection following liver transplantation. The results provide significant guidance for the early detection, prevention, and control of CRE infection. However, further randomized controlled studies are needed to gain a better understanding of the prevention of CRE infection in high-risk populations, the efficacy of preventive treatment compared with treatment following diagnosis, the treatment plan options for children, the screening of intestinal colonization, and methods for CRE decolonization.
Funding Statement
Chunfeng Project of Tianjin First Central Hospital (2019CF34). Chunfeng Project of Tianjin First Central Hospital (2020CF05). Key Project of Tianjin Health and Family Planning Commission (16KG107). State Natural Science Fund project (81670600). State Natural Science Fund project (81870444).
Ethics Approval
This study was conducted with approval from the Ethics Committee of Tianjin First Central Hospital. Written informed consent was obtained from all parents or legal guardians of participants. The guidelines outlined in the Declaration of Helsinki were followed.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang J, Yu W, Zhao LN, et al. Epidemiology and characteristics of antibacterial resistance in China. Chin J Clin Infect Dis. 2016;9(2):118–128. [Google Scholar]
- 2.Zhang Y, Zeng J, Liu W, et al. Emergence of a hypervirulent carbapenem—resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71(5):553–560. doi: 10.1016/j.jinf.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 3.Lu WT. The Prevalence and Resistance Mechanism of Carbapenem–Resistance Gram-Negative Bacilli in Stool Specimens from Patients. Guangzhou: SouthernMedical University; 2015. [Google Scholar]
- 4.Mazza E, Prosperi M, Panzeri MF, et al. Carbapenem-resistant Klebsiella pneumoniae infections early after liver transplantation: a single-center experience. Transplant Proc. 2017;49(4):677–681. doi: 10.1016/j.transproceed.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 5.Giannella M, Bartoletti M, Morelli MC, et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant. 2015;15:1708–1715. doi: 10.1111/ajt.13136 [DOI] [PubMed] [Google Scholar]
- 6.Pereira MR, Scully BF, Pouch SM, et al. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumonia infections in liver transplant recipients. Liver Transpl. 2015;21:1511–1519. doi: 10.1002/lt.24207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freire MP, Oshiro IC, Pierrotti LC, et al. Carbapenem-resistant Enterobacteriaceae acquired before liver transplantation: impact on recipient outcomes. Transplantation. 2017;101:811–820. doi: 10.1097/TP.0000000000001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen YH, Fan J, Zhou J, et al. Pulmonary infection and its risk factors after orthotopic liver transplantation. Chin J Liver Dis. 2007;15(11):833–836. [PubMed] [Google Scholar]
- 9.Li H, Gu Y, Xing TH, et al. Characterization and homology analysis of the multidrug-resistant gram-negative bacteria isolated from liver transplant recipients. Chin J Infect Chemother. 2011;11(6):457–462. [Google Scholar]
- 10.Huang X, Deng Z, Yuxin N, et al. Chinese experts’ consensus on prevention and control of multidrug resistance organism healthcare-associated infection. Chin J Infect Control. 2015;14(1):1–9. [Google Scholar]
- 11.Temkin E, Adler A, Lerner A, et al. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci. 2014;1323(1):22–42. doi: 10.1111/nyas.12537 [DOI] [PubMed] [Google Scholar]
- 12.Kritikos A, Manuel O. Bloodstream infections after solid-organ transplantation. Virulence. 2016;7:329–340. doi: 10.1080/21505594.2016.1139279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geladari A, Karampatakis T, Antachopoulos C, et al. Epidemiological surveillance of multidrug-resistant gram-negative bacteria in a solid organ transplantation department. Transpl Infect Dis. 2017;19:1–10. [DOI] [PubMed] [Google Scholar]
- 14.Freire MP, Villela soares oshiro IC, Bonazzi PR, et al. Surveillance culture for multidrug-resistant gram-negative bacteria: performance in liver transplant recipients. Am J Infect Control. 2017;45(3):e40–e44. doi: 10.1016/j.ajic.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Yang T, Liu WL. The clinical application of fecal microbiota transplantation. Electron Ed Chin Organ Transplant J. 2018;12(1):42–48. [Google Scholar]
- 16.Dinh A, Fessi H, Duran C, et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect. 2018;99:481–486. doi: 10.1016/j.jhin.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 17.Lee JJ, Yong D, Suk KT, et al. Alteration of gut microbiota in carbapenem-resistant Enterobacteriaceae carriers during fecal microbiota transplantation according to decolonization periods. Microorganisms. 2021;9(2):352. doi: 10.3390/microorganisms9020352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davido B, Batista R, Dinh A, et al. Fifty shades of graft: how to improve the efficacy of faecal microbiota transplantation for decolonization of antibiotic-resistant bacteria. Int J Antimicrob Agents. 2019;53(5):553–556. doi: 10.1016/j.ijantimicag.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 19.Dai M, Liu Y, Chen W, et al. Rescue fecal microbiota transplantation for antibiotic-associated diarrhea in critically ill patients. Crit Care. 2019;23(1):324. doi: 10.1186/s13054-019-2604-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcella C, Cui B, Kelly C, et al. In systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53(1):33–42. doi: 10.1111/apt.16148 [DOI] [PubMed] [Google Scholar]