Abstract
Background
Excessive delivery of free fatty acids (FFAs) to the liver promotes steatosis and insulin resistance (IR), with IR defined as reduced glucose uptake, glycogen synthesis and anti-lipolysis stimulated by normal insulin levels. Whether the associations between FFAs and diabetes development differ between patients with and without nonalcoholic fatty liver disease (NAFLD) remains unclear.
Methods
Consecutive subjects (2,220 NAFLD subjects and 1,790 non-NAFLD subjects according to ultrasound imaging) were enrolled from the First Affiliated Hospital of Sun Yat-sen University between 2009 and 2019. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.
Results
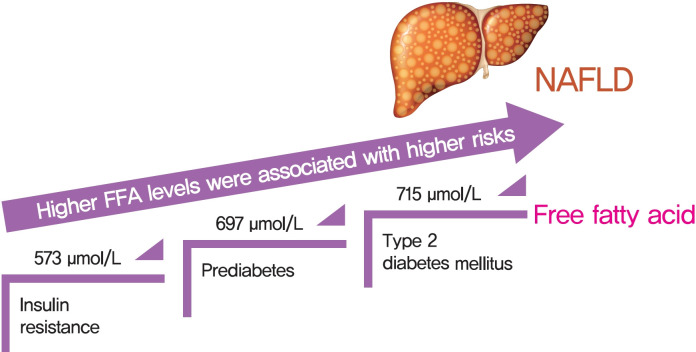
There was an approximate J-shaped relationship between FFA levels and HOMA-IR in the NAFLD group. Higher FFA concentration quartiles were associated with higher risks of IR (odds ratio [OR], 9.24; 95% confidence interval [CI], 6.43 to 13.36), prediabetes (OR, 10.48; 95% CI, 5.66 to 19.39), and type 2 diabetes mellitus (T2DM; OR, 19.43; 95% CI, 12.75 to 29.81) in the NAFLD group but not in the non-NAFLD group. The cut-off points for the FFA levels increased in a stepwise manner in discriminating IR, prediabetes and T2DM (573, 697, and 715 μmol/L) in the NAFLD group but not in non-NAFLD individuals.
Conclusion
A distinct dose-dependent relationship of FFA levels was found with IR, prediabetes and T2DM in NAFLD patients. Screening serum FFA levels in NAFLD patients would be valuable in preventing diabetes development.
Keywords: Diabetes mellitus, type 2; Fatty acids, nonesterifie; Non-alcoholic fatty liver disease
Graphical abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), characterized by an overaccumulation of intrahepatic lipids and subsequent lipotoxic injuries, is currently the leading form of chronic liver disease, with a prevalence of up to 26% worldwide [1]. In addition to the progression from simple steatosis to liver inflammation, fibrosis, and hepatocellular carcinoma (HCC), NAFLD also confers a substantial risk for the development of type 2 diabetes mellitus (T2DM), given the following shared established risk factors: obesity, metabolic syndrome, dyslipidemia, insulin resistance (IR), among others [2,3]. Previous studies have reported that NAFLD occurs in approximately 75% of patients with T2DM [4], whereas the incidences of T2DM in normal and overweight adults with NAFLD were 14.4% and 26.4% respectively, after 12.8 years of follow-up, with significantly higher adjusted hazard ratios (HRs) in overweight adults than in normal adults without NAFLD (HR, 3.59; 95% confidence interval [CI], 2.14 to 5.76 and HR, 6.77; 95% CI, 5.17 to 8.91) [5]. Moreover, the presence and metabolic traits of diabetes strongly predispose patients with NAFLD to a greater risk of developing HCC [6]. Therefore, the screening and prevention of diabetes in patients with early-stage NAFLD may be an important target for long-term management.
Free fatty acids (FFAs), also termed non-esterified fatty acids, are closely correlated with IR, NAFLD, and T2DM. FFAs in the blood originate from the hydrolysis of triacylglycerol, which is stored in the adipose tissue [7], and this process is inhibited by physiological insulin concentrations [8]. When the adipose tissue becomes desensitized to insulin, excessive circulating FFAs may enter visceral organs, resulting in lipid overload in the liver and pancreas [9]. High levels of intracellular FFA are converted to toxic levels of reactive oxygen species during lipid peroxidation in the mitochondria, which impairs insulin signaling and islet β-cell function, leading to the development of IR and T2DM [10]. Moreover, FFAs can prevent the inhibition of lipolysis via the action of insulin, which in turn promotes the secretion of FFAs into the plasma. Although serum FFA levels have been identified as an important mediator among IR, NAFLD, and T2DM [11], the association of FFAs with the progression from IR to T2DM in patients with and without NAFLD remains unclear. Identifying the associations among IR, NAFLD, and T2DM would be beneficial in the prevention of T2DM in patients with and without NAFLD through the establishment of individualized early detection strategies and prompt treatment deliveries.
Therefore, the purpose of this study was to characterize the relationship of serum FFA levels with the development of T2DM (including healthy, IR, prediabetes, and T2DM states) in a large population of subjects with and without NAFLD.
METHODS
Study population
This is a cross-sectional study of a single-center cohort in which subjects were enrolled consecutively from the NAFLD and Health Examination Center of the First Affiliated Hospital of Sun Yat-sen University, China. The subjects were enrolled from January 1, 2009, to December 30, 2019, with the following inclusion criteria: (1) subjects aged 18 to 80 years; (2) patients with complete serum FFA levels, glucose, lipid, and insulin measurement results; and (3) assessment of NAFLD, FFA levels, and oral glucose tolerance test (OGTT) performed within 2 weeks.
The exclusion criteria were as follows: (1) diagnosis or any evidence of viral hepatitis, autoimmune liver disease, HCC, or other end-stage liver diseases; (2) diagnosis of other types of diabetes; (3) pregnancy and breastfeeding; (4) a previous history of alcohol consumption of >30 g per day in men or >20 g per day in women; (5) the use of statins, fibrates, or anti-diabetic drugs in the past 6 months; and (6) trained athlete status.
The protocol was approved by the Independent Ethics Committee for Clinical Research and Animal Trials of the First Affiliated Hospital, Sun Yat-sen University (approval number: [2017]104), and informed consent was collected from all participants. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Clinical evaluation
Questionnaire interviews were conducted to collect patient histories, including patient demographics, past medical history, smoking status and alcohol consumption. All subjects underwent anthropometric measurements, including body weight, body height, waist circumference (WC) and blood pressure. Body mass index (BMI) was defined as the body weight in kilograms divided by the square of the body height in meters. WC was measured in centimeters at the midpoint between the lower margin of the rib cage and the top of the iliac crest using a nonelastic measuring tape. Sitting blood pressure was measured twice by physicians using an Omron (J710; Omron, Kyoto, Japan) electronic monitor, which was applied to the right upper arm after a 15-minute rest. Obesity was defined as having a BMI ≥25 kg/m2, and abdominal obesity was defined as having a WC ≥90 cm for men and WC ≥80 cm for women [12]. Hypertension was diagnosed in subjects with a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg or a previous diagnosis of hypertension [13].
Biochemical measurements
After fasting overnight for ≥8 hours, blood samples were taken, and the following biochemical parameters were assayed using the Abbott c8000 Automatic Biochemistry Analyzer (Abbott, Abbott Park, IL, USA): FFA (acyl CoA synthetase-acyl CoA oxidase [ACS-ACOD] method), lipid profiles, uric acid, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase, fasting plasma glucose (FPG), fasting insulin (FINS), and glycosylated hemoglobin (HbA1c). The 2-hour plasma glucose (2hPG) levels were measured during the 75 g OGTT. Serum lipid levels were determined directly using the enzymatic colorimetric method with the following Beckman Coulter reagent test kits: total cholesterol (OSR6216), triglycerides (OSR61118), low-density lipoprotein cholesterol (LDL-C; OSR6283), and high-density lipoprotein cholesterol (HDL-C; OSR6287). The HbA1c test was performed with the National Glycohemoglobin Standardization Program (NGSP) method in our study (Reagent test kit: VARIANT II TURBO HbA1c Kit 2.0; Bio-Rad Laboratories, Hercules, CA, USA). All laboratory measurements were performed within one day using the same fasting samples, with the exception of the OGTT.
IR, prediabetes, and T2DM assessment
Based on the homeostasis model assessment of insulin resistance (HOMA-IR) index, IR values were calculated using the equation FINS (µU/mL)×FPG (mmol/L)/22.5, and IR was confirmed if the HOMA-IR level was over 2.69 [14]. Additionally, the fasting adipose tissue insulin resistance (Adipo-IR) index was calculated with the equation FFA (mmol/L)×FINS (pmol/L) [15]. Prediabetes, defined as an impaired fasting glucose level, was determined by a fasting glucose value lower than 5.6 to 7.0 mmol/L and/or a 120-minute blood glucose level between 7.8 and 11.1 mmol/L observed in the OGTT (impaired glucose tolerance) or an HbA1c level between 5.7% and 6.4%. T2DM was defined by a fasting glucose value of ≥7 mmol/L, a 120-minute OGTT value of ≥11.1 mmol/L, and/or HbA1c ≥6.5% (48 mmol/mol) after a clinical physician excluded other types of diabetes [16].
Radiology examination
Because there can be discrepancies in the liver and kidney echo intensity, fatty liver was assessed in all subjects by abdominal ultrasonography based on the following criteria: the presence of posterior attenuation of the ultrasound beam, vessel blurring, difficult visualization of the gallbladder wall, and difficult visualization of the diaphragm [17]. A total of 979 subjects also underwent liver and pancreatic fat content (PFC) quantification via magnetic resonance imaging (MRI) of the upper abdomen with a 3.0-Tesla MRI scanner (Siemens 3.0T MAGNETOM Verio; Siemens, Munchen, Germany), which was performed within 2 weeks after the patient’s biochemical measurements were taken. The scanning protocol and imaging parameters, described in detail in our previous study [18], were as follows: TE1, 2.5 ms; TE2, 3.7 ms; repetition time, 5.47 ms; flip angle, 5°; receiver bandwidth, ±504.0 kHz per pixel; and slice thickness, 3.0 mm. The fat content was calculated for each patient using an irregularly shaped region of interest that covered the entire liver in 21 consecutive slices (maximum area centered). Based on the MRI proton density fat fraction (MRI-PDFF), the liver fat content (LFC) was classified as absent (<5%), mild (5% to 10%), moderate (10% to 25%), and severe (>25%) steatosis [19-21]. Pancreatic fat infiltration was defined as an average PFC ≥5%.
Statistical analysis
The software program R version 3.5.1 (http://www.Rproject.org), was used for statistical analyses. Continuous variables are shown as the mean±standard deviation or the median with interquartile range. Student’s t-test and the Mann-Whitney U test were used to compare continuous variables. The chi-square test was applied to compare categorical variables. Univariate and multivariate backward stepwise logistic regression was used to evaluate whether factors were independently associated with IR, prediabetes, and T2DM. We explored the nonlinear relationship between FFA levels and several parameters using smooth curve fitting analysis. Receiver operating characteristic (ROC) curve analysis was conducted for the predictive factors. A two-tailed P value less than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
In total, 4,010 subjects were enrolled in our study, including 2,220 subjects in the non-NAFLD group, 1,790 subjects in the NAFLD group and 941 subjects in the NAFLD subgroup defined by MRI-PDFF. As shown in Table 1, the ages and sex proportions were comparable among the groups. IR (40.2% vs. 10.2%), prediabetes (14.3% vs. 11.5%), and T2DM (31.1% vs. 16.6%) were more common in the NAFLD group than in the non-NAFLD group. Compared with the non-NAFLD subjects, the NAFLD subjects presented with higher BMIs (25.7 kg/m2 [23.7 to 28.0] vs. 21.9 kg/m2 [19.7 to 24.4], P<0.001) and WCs (89 cm [83 to 95] vs. 77 cm [71 to 85], P<0.001), as expected. Additionally, we found that compared with individuals without NAFLD, individuals with NAFLD tended to have higher blood pressure, ALT, AST, GGT, and uric acid levels and significant differences in their plasma lipid profiles, including triglycerides, total cholesterol, HDL-C, and LDL-C. Notably, there were no significant differences in the FFA levels between the non-NAFLD and NAFLD groups (561±234 μmol/L vs. 562±218 μmol/L). Of the parameters associated with diabetes, FPG, FINS, HbA1c, HOMA-IR, and Adipo-IR index were significantly higher in the patients with NAFLD than in the subjects without NAFLD (all P<0.05); however, there were no significant differences in the 2hPG levels. Additionally, NAFLD subjects defined by MRI-PDFF showed consistent results when compared to the non-NAFLD group and presented similar trends as those of the total NAFLD subjects.
Table 1.
Characteristic of enrolled subjects
| Characteristic | Non-NAFLD (n=2,220) | NAFLD (n=1,790) | NAFLD defined by MRI-PDFF (n=941) | P value |
|---|---|---|---|---|
| Age, yr | 46.3±15.8 | 45.6±20.6 | 45.8±19.9 | 0.455 |
| Male sex, % | 62.5 | 63.7 | 63.1 | 0.121 |
| Current smoker, % | 9.6 | 9.8 | 9.9 | 0.324 |
| Anthropometric parameters | ||||
| Body mass index, kg/m2 | 21.9 (19.7–24.4)b | 25.7 (23.7–28.0)a | 26.3 (23.8–28.7)a | <0.001 |
| Waist circumference, cm | 77 (71–85)b | 89 (83–95)a | 89 (83–95)a | <0.001 |
| Systolic blood pressure, mm Hg | 125 (117–138)b | 130 (120–142)a | 130 (120–141)a | <0.001 |
| Diastolic blood pressure, mm Hg | 81 (74–88)b | 85 (76–93)a | 86 (77–95)a | <0.001 |
| Liver enzyme | ||||
| Alanine aminotransferase, U/L | 25 (17–39)b | 34 (22–55)a | 37 (24–61)a | <0.001 |
| Aspartate aminotransferase, U/L | 25 (20–34)b | 28 (22–40)a | 29 (23–40)a | <0.001 |
| γ-Glutamyl transpeptidase, U/L | 23 (16–37)b | 38 (25–62)a | 39 (26–67)a | <0.001 |
| Alkaline phosphatase, U/L | 75 (65–90) | 76 (65–89) | 76 (65–89) | 0.276 |
| Metabolic parameters | ||||
| Uric acid, μmol/L | 339 (279–401)b | 399 (332–458)a | 408 (339–472)a | <0.001 |
| Cholesterol, mmol/L | 4.7 (4.0–5.5)b | 5.0 (4.3–5.7)a | 5.1 (4.4–5.8)a | <0.001 |
| Triglycerides, mmol/L | 1.0 (0.8–1.5)b | 1.5 (1.1–2.1)a | 1.5 (1.1–2.1)a | <0.001 |
| HDL-C, mmol/L | 1.2 (1.0–1.5)b | 1.1 (1.0–1.3)a | 1.1 (1.0–1.3)a | <0.001 |
| LDL-C, mmol/L | 2.8 (2.4–3.5)b | 3.2 (2.6–3.7)a | 3.2 (2.7–3.7)a | <0.001 |
| Free fatty acids, μmol/L | 561±234 | 562±218 | 567±206 | 0.405 |
| Fasting plasma glucose, mmol/L | 4.7 (4.3–5.3)b | 5.0 (4.6–5.7)a | 4.9 (4.5–5.4)a | <0.001 |
| Fasting insulin, μU/mL | 5.7 (4.0–8.2)b | 8.9 (6.5–12.5)a | 9.0 (6.5–12.8)a | <0.001 |
| HOMA-IR | 1.2 (0.8–1.8)b | 2.3 (1.4–4.0)a | 2.3 (1.4–4.1)a | <0.001 |
| Adipo-IR | 22.0 (14.3–34.2)b | 34.8 (21.6–53.1)a | 35.4 (21.9–56.1)a | <0.001 |
| HbA1c, % | 5.9 (5.4–7.2)b | 7.8 (6.2–10.0)a | 7.6 (6.0–9.4)a | <0.001 |
| 2-hr plasma glucose, mmol/L | 10.1 (7.0–13.4) | 9.2 (7.1–14.7) | 9.1 (7.2–14.5) | 0.909 |
| Insulin resistance, % | 10.2b | 40.2a | 40.5a | <0.001 |
| Prediabetes, % | 11.5b | 14.3a | 13.3a | 0.024 |
| Type 2 diabetes mellitus, % | 16.6b | 31.1a | 29.9a | <0.001 |
Values are presented as mean±standard deviation and median (interquartile range). NAFLD, nonalcoholic fatty liver disease; MRI-PDFF, magnetic resonance imaging proton density fat fraction; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; Adipo-IR, adipose tissue insulin resistance; HbA1c, glycosylated hemoglobin.
Significant difference compared to non-NAFLD group (P<0.05),
Significant difference compared to NAFLD group (P<0.05).
Associations among FFAs, lipid profiles, and IR
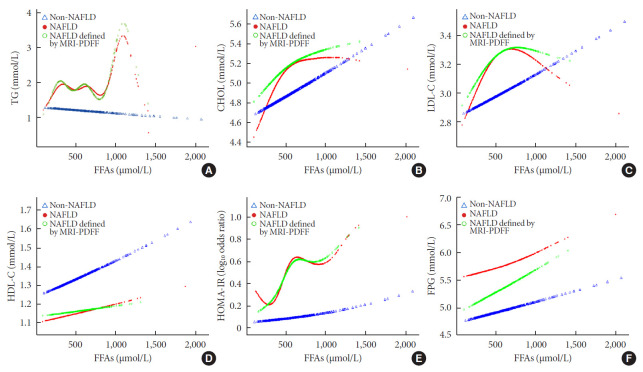
To explore the nonlinear association of FFA levels with traditional serum glucose and lipid metabolism markers in non-NAFLD and NAFLD subjects, we performed smooth curve fitting analyses after adjusting for age, sex, and BMI (Fig. 1). Among the NAFLD patients, plasma triglyceride concentrations fluctuated between 0 and 810 μmol/L as the FFA levels increased, increased sharply when the FFA levels reached 810 μmol/L, and then decreased when the FFA levels reached 1,121 μmol/L (Fig. 1A). In the non-NAFLD group, the triglyceride concentrations showed a stable, but not obvious, decreasing trend as the FFA levels increased. Total serum cholesterol levels in both groups displayed an increasing trend as the FFA levels increased; the curves in the NAFLD patients were sharper when the FFA levels were below 612 μmol/L but then became smooth (Fig. 1B). However, when the FFA levels increased to over 671 μmol/L in the NAFLD group, there was an obvious inverse U-shaped trend in the LDL-C concentration, whereas there was a positive linear correlation in the non-NAFLD group (Fig. 1C). For plasma HDL-C, both groups exhibited a similar and stable increasing trend, but the concentration was noticeably lower in the NAFLD patients (Fig. 1D). As shown in Fig. 1E, the HOMA-IR index rose more sharply as FFA levels increased in the NAFLD group and presented a J-shaped curve in general. However, there was an inverse J-shaped relationship when FFA levels increased between 500 and 1,000 μmol/L in the NAFLD group. As shown in Fig. 1F, compared to the FPG levels of the non-NAFLD subjects, which increased stably as FFA levels increased, the FPG levels of the NAFLD patients showed a decreasing trend as FFA levels increased; however, their levels increased sharply when FFA levels exceeded 748 μmol/L. The results of the NAFLD patients defined by the MRI-PDFF subgroup exhibited trends resembling those of the total NAFLD group in all of the above analyses.
Fig. 1.
Nonlinear relationships of lipid profiles, homeostasis model assessment of insulin resistance (HOMA-IR), and fasting plasma glucose levels with free fatty acids (FFAs) in non-nonalcoholic fatty liver disease (NAFLD), NAFLD and subgroup NAFLD defined by magnetic resonance imaging proton density fat fraction (MRI-PDFF) groups adjusted for age, sex, and body mass index. (A) Triglycerides (TG), (B) cholesterol (CHOL), (C) low-density lipoprotein cholesterol (LDL-C), (D) high-density lipoprotein cholesterol (HDL-C), (E) HOMA-IR, and (F) fasting plasma glucose (FPG).
Associations among FFAs, fat distribution and diabetes progression
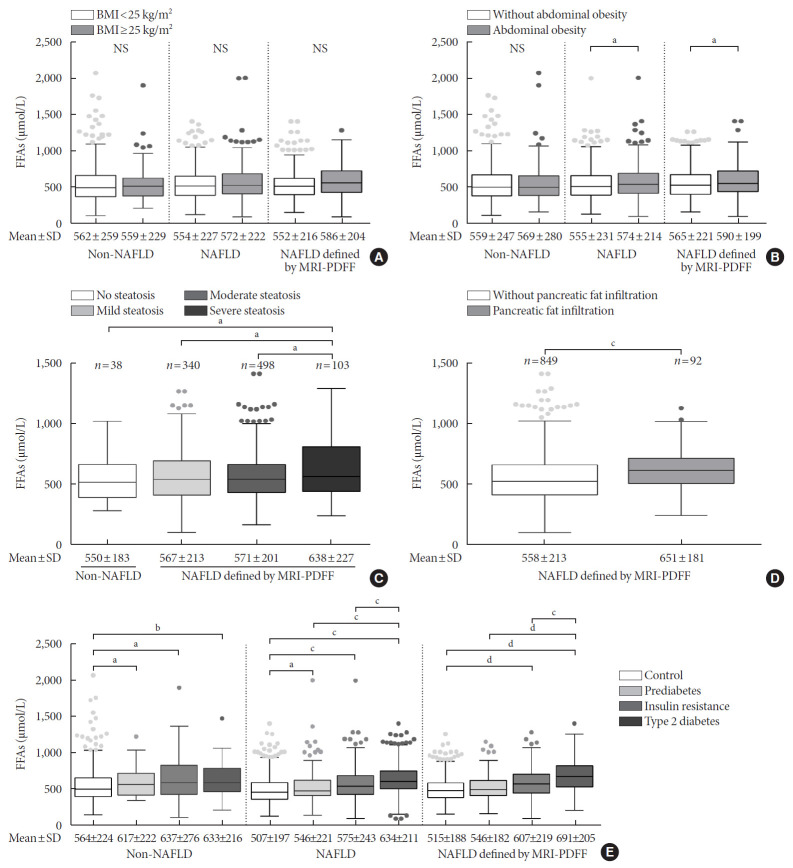
There were no significant differences in the FFA levels of subjects with or without obesity and subjects with or without NAFLD (Fig. 2A). The NAFLD patients with abdominal obesity presented higher levels of FFAs than those with no abdominal obesity (574±214 μmol/L vs. 555±231 μmol/L, P<0.05), and this difference was not apparent in the non-NAFLD subjects (Fig. 2B). In total, 979 subjects underwent an MRI scan to measure the fat content of their liver and pancreas. The subjects with LFC >25%, which indicates severe steatosis, showed higher FFA levels than the subjects with moderate, mild and no steatosis (638±227 μmol/L vs. 571±201 μmol/L vs. 567±213 μmol/L vs. 550±183 μmol/L, respectively; P<0.05) (Fig. 2C). Notably, higher levels of FFAs were detected in the NAFLD patients who exhibited pancreatic fat infiltration (PFC ≥5%; 651±181 μmol/L vs. 558±213 μmol/L, P<0.05) (Fig. 2D). Besides, FFA levels were significantly correlated with LFC (r=0.08, P=0.014) and PFC (r=0.18, P<0.001). In addition, a stepwise increase in FFA levels was found from a normal status to the IR, prediabetes, and T2DM stages within the NAFLD group and the NAFLD subgroup defined by MRI-PDFF, while a significant difference was observed between a normal status and the other three stages in the non-NAFLD group (Fig. 2E). The NAFLD subgroup defined by MRI-PDFF showed similar results as those of the total NAFLD group. With the purpose of exploring subtle differences in FFA levels in subjects with LFC data, a comparison of FFA levels among the 10 quantiles of LFC was conducted, as shown in Supplementary Fig. 1. Significant differences in FFA levels were found among quantile 7 (LFC, 11.2% to 14.1%), quantile 9 (LFC, 17.2% to 22.4%), and quantile 10 (LFC >22.4%). However, there was no significant correlation between FFA levels and quantiles of LFC in non-NAFLD and NAFLD subjects.
Fig. 2.
Comparison of free fatty acid levels among subgroups with different body fat distribution and diabetes development. Subgroups were divided by (A) obesity, (B) abdominal obesity, (C) liver steatosis, (D) pancreatic fat infiltration, and (E) diabetes development. Obesity was defined as a body mass index (BMI) ≥25 kg/m2; abdominal obesity was defined as a waist circumference >90 cm for men and >80 cm for women; subjects was classified as absent, mild, moderate and severe steatosis based on the average liver fat content assessed using the magnetic resonance imaging proton density fat fraction (MRI-PDFF); pancreatic fat infiltration was defined as an average pancreatic fat content ≥5%, as assessed by MRI-PDFF. NAFLD, nonalcoholic fatty liver disease; FFA, free fatty acid; NS, not significant; SD, standard deviation. aP<0.05, bP<0.01, cP<0.001, dP<0.0001.
Dose-dependent association of serum FFA levels with IR, prediabetes, and T2DM in NAFLD patients
Trend regression analyses were used to identify the role of FFA levels in predicting diabetes development. All the subjects were categorized based on the 25th (397 μmol/L), 50th (510 μmol/L), and 75th (647 μmol/L) FFA level percentiles (Table 2). In addition to the crude model, model 1 (adjusted for age and sex) and model 2 (adjusted for various biochemical parameters) were also built. In the NAFLD group, the subjects with the highest quartile of FFA levels had higher risk ratios for IR, prediabetes, and T2DM (odds ratios, 9.24 [6.43 to 13.36], 10.48 [5.66 to 19.39], and 19.43 [12.75 to 29.81], respectively; all P<0.05), even after adjusting for various confounders. Furthermore, significant differences were observed in the tests for trends. Analysis of the NAFLD subgroup defined by MRI-PDFF presented similar results. In contrast, in the non-NAFLD group, although there was a higher risk ratio in the subjects with a higher FFA level quartile, none of the analyses indicated significant trends after adjusting for confounders. Characteristic comparison among FFA levels quartiles is shown in Supplementary Table 1.
Table 2.
OR for quartiles of free fatty acids levels in predicting insulin resistance, prediabetes, and type 2 diabetes mellitus
| Variable | OR (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-NAFLD |
NAFLD |
NAFLD defined by MRI-PDFF |
||||||||
| Crude | Model 1a | Model 2b | Crude | Model 1a | Model 2b | Crude | Model 1a | Model 2b | ||
| Insulin resistance | ||||||||||
| FFA quartile 1 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | |
| FFA quartile 2 | 1.18 (0.70–1.92) | 1.19 (0.70–1.97) | 0.77 (0.42–1.37) | 1.41 (1.08–1.80) | 1.41 (1.11–1.83) | 1.24 (0.82–1.82) | 1.71 (1.23–2.35) | 1.66 (1.19–2.28) | 1.24 (0.85–1.85) | |
| FFA quartile 3 | 1.05 (0.60–1.75) | 1.08 (0.61–1.81) | 0.74 (0.38–1.35) | 2.86 (2.27–3.61) | 2.88 (2.27–3.63) | 1.95 (1.34–2.82) | 2.84 (2.10–3.81) | 2.75 (2.03–3.69) | 1.95 (1.34–2.83) | |
| FFA quartile 4 | 1.73 (1.10–2.71) | 1.62 (1.01–2.56) | 2.29 (1.35–3.85) | 9.77 (7.83–12.22) | 9.66 (7.75–12.11) | 9.24 (6.43–13.36) | 12.52 (9.32–16.81) | 12.13 (9.03–16.28) | 9.24 (6.44–13.32) | |
| P for trend | 0.179 | 0.267 | 0.335 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Prediabetes | ||||||||||
| FFA quartile 1 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | |
| FFA quartile 2 | 0.39 (0.25–0.60) | 0.34 (0.20–0.53) | 0.23 (0.08–0.56) | 1.08 (0.77–1.51) | 1.11 (0.78–1.55) | 2.03 (1.06–3.69) | 1.82 (1.18–2.81) | 1.82 (1.18–2.82) | 3 (1.75–5.16) | |
| FFA quartile 3 | 0.51 (0.33–0.77) | 0.4 (0.25–0.63) | 1.65 (0.79–3.36) | 2.08 (1.51–2.83) | 2.14 (1.55–2.91) | 2.81 (1.48–5.15) | 2.35 (1.56–3.53) | 2.39 (1.58–3.58) | 3.93 (3.07–4.43) | |
| FFA quartile 4 | 0.79 (0.54–1.15) | 0.7 (0.46–1.05) | 1.16 (0.59–2.24) | 2.87 (2.09–3.90) | 2.88 (2.11–3.95) | 10.48 (5.66–19.39) | 2.81 (2.19–3.70) | 2.77 (2.18–3.67) | 4.93 (3.70–6.05) | |
| P for trend | 0.817 | 0.59 | 0.255 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.014 | |
| Type 2 diabetes mellitus | ||||||||||
| FFA quartile 1 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | |
| FFA quartile 2 | 1 (0.7–1.38) | 1.06 (0.73–1.52) | 0.81 (0.38–1.58) | 1.1 (0.84–1.42) | 1.19 (0.91–1.55) | 3.68 (2.25–5.93) | 2.23 (1.85–4.15) | 2.08 (1.77–4.00) | 3.47 (2.12–5.65) | |
| FFA quartile 3 | 0.87 (0.61–1.23) | 0.83 (0.56–1.22) | 1.08 (0.52–2.11) | 1.7 (1.34–2.16) | 1.89 (1.46–2.40) | 6.5 (4.06–10.18) | 3.23 (2.46–5.85) | 3.23 (2.38–6.85) | 6.06 (3.82–9.53) | |
| FFA quartile 4 | 1.45 (1.06–1.97) | 1.4 (1.01–1.96) | 2.23 (1.25–3.98) | 6.02 (4.86–7.44) | 6.4 (5.12–8.02) | 19.43 (12.75–29.81) | 10.15 (8.23–14.31) | 10.31 (8.62–15.85) | 18.29 (11.94–27.94) | |
| P for trend | 0.153 | 0.258 | 0.071 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
Test for trend based on variable containing median value for each quartile. FFAs were categorized by 397, 510, and 647 mmol/L for the 25th, 50th and 75th percentiles, represented by FFA quartile 1, quartile 2, quartile 3, and quartile 4.
OR, odds ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; MRI-PDFF, magnetic resonance imaging proton density fat fraction; FFA, free fatty acid.
Adjusted for age and sex,
Adjusted for age, sex, smoking status, body mass index, waist circumference, triglycerides, cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, uric acid and homeostasis model assessment of insulin resistance index (except for insulin resistance analysis) in both groups. Liver fat content and pancreatic fat content were additionally adjusted in subgroup NAFLD defined by MRI-PDFF.
Predictive value of serum FFA levels for IR, prediabetes, and T2DM
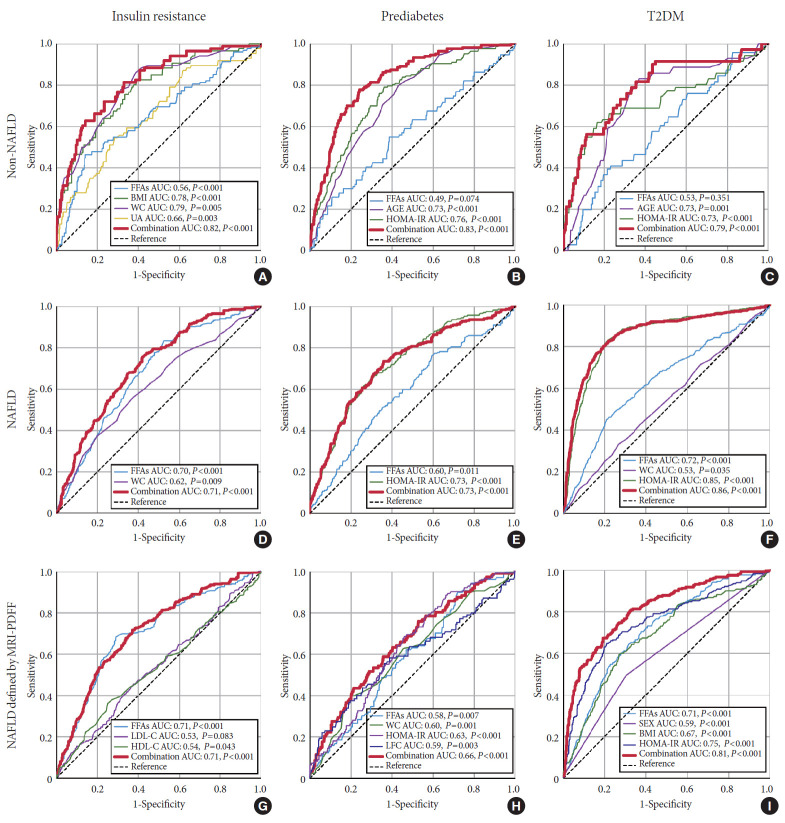
The independent risk factors for IR, prediabetes, and T2DM were identified via univariate and multivariate regression analysis (Supplementary Tables 2-4). For the purpose of evaluating the efficacy of FFA levels in predicting IR, prediabetes, and T2DM, ROC curves were drawn, as shown in Fig. 3. For predicting IR, prediabetes and T2DM, the areas under the curve (AUCs) for all of the independent risk factors combined, including FFA levels, were 0.82, 0.83, and 0.79 in non-NAFLD subjects, respectively (Fig. 3A, B, and C). While in NAFLD subjects, the AUCs for the combined independent risk factors were 0.71, 0.73, and 0.86, respectively (Fig. 3D, E, and F). For subgroup analysis in the NAFLD subgroup defined by MRI-PDFF, FFA levels were still an independent risk factor, although some other risk factors were identified. The AUCs for FFA levels and combined independent risk factors were similar to those for the total NAFLD group in predicting IR and T2DM. In predicting prediabetes, the AUC for the combined independent risk factors was lower than that of the total NAFLD group (Fig. 3G, H, and I). As shown in Table 3, the NAFLD subjects had lower cut-off values for FFA levels for predicting IR, prediabetes, and T2DM compared to the values for the non-NAFLD subjects, based on the ROC analysis (573 μmol/L vs. 815 μmol/L, 697 μmol/L vs. 938 μmol/L, and 715 μmol/L vs. 1,049 μmol/L, respectively). Additionally, cut-off values in the NAFLD subgroup defined by MRI-PDFF were found to be similar to those in the total NAFLD group and were still lower than those in the non-NAFLD group.
Fig. 3.
Receiver operator characteristic (ROC) curve of factors for predicting insulin resistance (IR), prediabetes and type 2 diabetes mellitus (T2DM) in non-nonalcoholic fatty liver disease (NAFLD), NAFLD and subgroup NAFLD defined by magnetic resonance imaging proton density fat fraction (MRI-PDFF) groups. (A, B, C) ROC curve for predicting IR, prediabetes and T2DM in patients with non-NAFLD, (D, E, F) ROC curve for predicting IR, prediabetes and T2DM in NAFLD individuals, (G, H, I) ROC curve for predicting IR, prediabetes and T2DM in the subgroup of NAFLD defined by MRI-PDFF. FFA, free fatty acid; AUC, area under curve; BMI, body mass index; WC, waist circumference; UA, uric acid; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; LFC, liver fat content.
Table 3.
Cut-off value of free fatty acids for prediction of insulin resistance, prediabetes, and type 2 diabetes mellitus
| Variable | Cut-off value, μmol/L | Sensitivity, % | Specificity, % | AUC |
|---|---|---|---|---|
| Insulin resistance | ||||
| Non-NAFLD | 815 | 72 | 75 | 0.82 |
| NAFLD | 573 | 72 | 62 | 0.71 |
| NAFLD defined by MRI-PDFF | 526 | 72 | 63 | 0.71 |
| Prediabetes | ||||
| Non-NAFLD | 938 | 81 | 77 | 0.83 |
| NAFLD | 697 | 71 | 69 | 0.73 |
| NAFLD defined by MRI-PDFF | 562 | 59 | 64 | 0.66 |
| Type 2 diabetes mellitus | ||||
| Non-NAFLD | 1,049 | 80 | 69 | 0.79 |
| NAFLD | 715 | 66 | 80 | 0.86 |
| NAFLD defined by MRI-PDFF | 582 | 80 | 68 | 0.81 |
AUC, area under curve; NAFLD, nonalcoholic fatty liver disease; MRI-PDFF, magnetic resonance imaging proton density fat fraction.
DISCUSSION
In the present study, we characterized the associations between circulating FFA levels and lipid profiles, IR, prediabetes and T2DM in NAFLD and non-NAFLD populations. Despite levels of FFA that were similar to those in patients without NAFLD, patients with NAFLD had many different metabolic profile features with high levels of plasma FFA. We determined that elevated FFA levels in blood were as an independent risk factor for T2DM, which could optimize the prediction of T2DM development, especially in large-scale NAFLD patient cohorts. Additionally, our study further indicated that lower FFA levels cut-off values significantly affect the sensitivity and specificity of the isokinetic test that is used as a tool for IR, prediabetes and T2DM detection.
Previous studies have reported higher levels of total plasma FFA in NAFLD patients compared with those in healthy controls, with most studies focused on obesity, metabolic syndromes, adipocyte cell size, hepatic inflammation, and advanced fibrosis [9,22-24]. Our study further suggests that a stepwise increase in the total FFAs concentration characterizes the progression from healthy to IR, prediabetes, and T2DM in NAFLD patients; different FFAs concentrations were observed for those with and without IR in the non-NAFLD controls. Interestingly, our study is the first to further stratify NAFLD patients based on their fat disposition in different organs, including whole body fat distribution via BMI, abdominal obesity via WC, steatosis severity, and pancreatic fat infiltration via MRI-PDFF. The analysis of the pancreatic fat infiltration subgroup showed that abnormal serum FFA levels may be linked to both abdominal obesity and pancreatic fat overaccumulation. Because fatty pancreas has an established association with β-cell dysfunction, and the latter may strongly mediate IR as anticipated, our study suggested that FFAs entrance and storage into the pancreas may be involved in the worsening of IR and the development of T2DM.
In our curve-fitting analysis for FFA levels and HOMA-IR index, a fluctuating J-shaped relationship was presented between FFA concentrations and the risks of HOMA-IR in NAFLD patients, while the corresponding relationship exhibited a more slowly growing linear curve in non-NAFLD subjects. These results suggest that the degree of IR in NAFLD patients was more subject to FFA changes. FFAs serve as the main energy substrate to physically generate adenosine triphosphate for many organs after β-oxidation. FFAs at extremely low levels may indicate insufficient energy status combined with severely impaired release of insulin and high serum glucose [23,25]; therefore, inverse effects of FFAs and HOMA-IR were observed in some NAFLD patients with lower glucose modulation capacity due to steatosis, liver inflammation, and fibrosis [26]. In contrast, elevated FFA levels in the blood primarily originate from excessive lipolysis in adipose tissue due to IR and obesity. This is because insulin functions as an important hormone to inhibit hydrolysis of triacylglycerol [8], and IR occurrence would attenuate the insulin-regulating effect, subsequently causing oversecretion of FFAs into circulation. Then, excessive FFAs can be delivered to muscle, liver and pancreas, leading to ectopic fat accumulation [27]. The overwhelming amount of intracellular lipids, including diacylglycerols and ceramides, would alter the activation of phosphatidylinositol 3-kinase, protein kinase θ, and protein kinase B (PKB), which accelerate IR progression [25].
Once FFAs are delivered to the liver, they undergo β-oxidation or reesterification into triacylglycerols, which in turn are transformed into very LDL-C and accelerate the secretion ratio, thereby enhancing the export of triglyceride and cholesterol into circulation [21]. Karjalainen et al. [28] reported that serum FFA levels were positively correlated with total triglyceride levels and very LDL-C levels in healthy individuals. However, our smooth curve fitting analysis observed that the positive association between triglyceride and FFAs was inverse in NAFLD states when FFA levels are greater than 1,121 μmol/L in the plasma, despite its persistence in non-NAFLD patients. This phenomenon might be owing to the impaired ability to incorporate plasma-derived fatty acids into triglycerides when there is an increased influx of FFAs into the liver in the presence of steatosis, which inhibits its metabolism [9]. In particular, the increased lipogenesis in NAFLD might modestly decrease during the progression to NASH [22]. Elevated levels of FFA, triglycerides, cholesterol, and LDL-C are all independent risk factors for IR and T2DM [29], and our study suggested that FFAs incorporation may amplify the predictive effects of other common risk factors for IR, prediabetes, and T2DM in NAFLD. When taken together with its pathophysiologic roles, these findings suggest that FFAs are an important target with therapeutic value for the management, progression, and detection of NAFLD.
We performed ROC analysis to address the issue of the optimal total FFA levels cut-off levels for predicting T2DM-related stages in NAFLD patients. Our main finding was that serum FFA levels may serve as the one of the combined indicators to discriminate IR, prediabetes and T2DM, with optimal cut-off values of 573, 697, and 715 μmol/L for individuals with NAFLD respectively, and 815, 938, and 1,049 μmol/L for non-NAFLD individuals, respectively. Thus, subjects with NAFLD might suffer a higher risk of diabetes than those without NAFLD when exposed to the same level of serum FFAs. NAFLD subjects had lower FFA levels cut-off values than non-NAFLD subjects for predicting IR, prediabetes and T2DM. This result was similar to that observed in another large population-based study in China that found that IR was enhanced at FFAs concentrations of 540 and 610 μmol/L in obese and nonobese groups, respectively, with obesity being much more prevalent among the NAFLD subjects [29].
Several limitations should be considered in the present study. First, the study followed a cross-sectional design carried out with hospital-based data, which lacked exercise information. The cut-off values for T2DM-related predictions require further validation in prospective cohorts. Second, the lack of IR assessment using the gold standard may reduce the predictive value of FFA levels among these participants. The indices of adipocyte IR are based on radionuclide tracing technology (i.e., isotope-labeled palmitate or glycerol or FFAs suppression during euglycemic-hyperinsulinemic clamp) or OGTT, and the relationship between adipose IR and FFAs could not be estimated due to the problems of multicollinearity or the costs of radionuclide tracing technology. Last, the use of total FFA levels instead of a specific type of FFA detected by gas chromatography with mass spectrometry can be considered a limitation, but the total FFA level was identified as a simple and useful marker that had been previously used in important studies on NAFLD.
In conclusion, a J-shaped relationship between FFA levels and IR risk in NAFLD patients was revealed in our study, which was different from the linear association that was observed in subjects without NAFLD. Our results suggested that the optimal serum FFA levels threshold required to monitor T2DM progression in Chinese patients with NAFLD was lower than that in non-NAFLD patients.
Acknowledgments
We are grateful to Professor Aihua Lin in School of Public Health, Sun Yat-sen University for her assistance in statistical analysis of this study. This work was supported by National Natural Science Foundation of China (81870404, 81670518, 8117-0392). Fuxi Li, Junzhao Ye and Yanhong Sun contributedequally to this work. Shiting Feng offered the imaging support with Science and Technology Program of Guangdong province, China (2013B021800290, 2014A020212118, 2017A020215015).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: J.Y., Y.S., B.Z.
Acquisition, analysis, or interpretation of data: F.L., J.Y., Y.L., T.W., C.S., Q.M., X.L., S.F.
Drafting the work or revising: F.L., J.Y.
Final approval of the manuscript: B.Z.
FUNDING
None
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0039.
Characteristic comparison among free fatty acid levels quartiles
Univariate logistic regression analysis for factors associated with IR, prediabetes, and T2DM in non-NAFLD and NAFLD groups
Multivariate logistic regression analysis for factors associated with IR, prediabetes, and T2DM in non-NAFLD and NAFLD groups
Univariate and multivariate logistic regression analysis for factors associated with IR, prediabetes, and T2DM in subgroup NAFLD defined by MRI-PDFF
Free fatty acid (FFA) levels among the 10 quantiles of liver fat content (LFC) in the subgroup with magnetic resonance imaging proton density fat fraction (MRI-PDFF) data. Dotted lines represent correlations based on individual data. Black dots represent the median (interquartile range) of LFC in each quantile group. Cut-off points discriminating the 10 groups were as follow: quantile 1 (<1.5%); quantile 2 (1.5% to 2.7%); quantile 3 (2.8% to 4.1%); quantile 4 (4.2% to 6.5%); quantile 5 (6.6% to 8.4%); quantile 6 (8.5% to 11.1%); quantile 7 (11.2% to 14.1%); quantile 8 (14.2% to 17.1%); quantile 9 (17.2% to 22.4%); quantile 10 (>22.4%).  represented as the mean value of liver fat contents in each FFA quantantile. NAFLD, nonalcoholic fatty liver disease. aP<0.05.
represented as the mean value of liver fat contents in each FFA quantantile. NAFLD, nonalcoholic fatty liver disease. aP<0.05.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–53. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda T, Hamaguchi M, Kojima T, Hashimoto Y, Ohbora A, Kato T, et al. The impact of non-alcoholic fatty liver disease on incident type 2 diabetes mellitus in non-overweight individuals. Liver Int. 2016;36:275–83. doi: 10.1111/liv.12912. [DOI] [PubMed] [Google Scholar]
- 6.Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71:808–19. doi: 10.1002/hep.31014. [DOI] [PubMed] [Google Scholar]
- 7.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology. 2018;68:1308–18. doi: 10.1002/hep.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia. 2013;56:949–64. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wree A, Schlattjan M, Bechmann LP, Claudel T, Sowa JP, Stojakovic T, et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metabolism. 2014;63:1542–52. doi: 10.1016/j.metabol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 11.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of nonalcoholic fatty liver disease. Prog Lipid Res. 2013;52:165–74. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–73. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Park S, Buranakitjaroen P, Chen CH, Chia YC, Divinagracia R, Hoshide S, et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: the Hope Asia Network. J Hum Hypertens. 2018;32:249–58. doi: 10.1038/s41371-017-0025-y. [DOI] [PubMed] [Google Scholar]
- 14.Xing X, Yang W, Yang Z. The diagnostic significance of homeostasis model assessment of insulin resistance in metabolic syndrome among subjects with different glucose tolerance. Chin J Diabetes. 2004;12:182–6. [Google Scholar]
- 15.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio metabolism study. Diabetes. 2017;66:815–22. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S14–31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 17.Ballestri S, Lonardo A, Romagnoli D, Carulli L, Losi L, Day CP, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012;32:1242–52. doi: 10.1111/j.1478-3231.2012.02804.x. [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, Luo Y, Zhang Z, Cai H, Li Y, Chan T, et al. MR quantification of total liver fat in patients with impaired glucose tolerance and healthy subjects. PLoS One. 2014;9:e111283. doi: 10.1371/journal.pone.0111283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67:1348–59. doi: 10.1002/hep.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, et al. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol Hepatol. 2015;13:1337–45. doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70:133–41. doi: 10.1016/j.jhep.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–38. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng R, Luo C, Li C, Du S, Okekunle AP, Li Y, et al. Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: a case-control study. Lipids Health Dis. 2017;16:165. doi: 10.1186/s12944-017-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:5832. doi: 10.1038/srep05832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SS, Seo YK. Excess accumulation of lipid impairs insulin sensitivity in skeletal muscle. Int J Mol Sci. 2020;21:1949. doi: 10.3390/ijms21061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanWagner LB, Ning H, Allen NB, Siddique J, Carson AP, Bancks MP, et al. Twenty-five-year trajectories of insulin resistance and pancreatic β-cell response and diabetes risk in nonalcoholic fatty liver disease. Liver Int. 2018;38:2069–81. doi: 10.1111/liv.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snel M, Jonker JT, Schoones J, Lamb H, de Roos A, Pijl H, et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. 2012;2012:983814. doi: 10.1155/2012/983814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karjalainen L, Pihlajamaki J, Karhapaa P, Laakso M. Impaired insulin-stimulated glucose oxidation and free fatty acid suppression in patients with familial combined hyperlipidemia: a precursor defect for dyslipidemia? Arterioscler Thromb Vasc Biol. 1998;18:1548–53. doi: 10.1161/01.atv.18.10.1548. [DOI] [PubMed] [Google Scholar]
- 29.Xin Y, Wang Y, Chi J, Zhu X, Zhao H, Zhao S, et al. Elevated free fatty acid level is associated with insulin-resistant state in nondiabetic Chinese people. Diabetes Metab Syndr Obes. 2019;12:139–47. doi: 10.2147/DMSO.S186505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristic comparison among free fatty acid levels quartiles
Univariate logistic regression analysis for factors associated with IR, prediabetes, and T2DM in non-NAFLD and NAFLD groups
Multivariate logistic regression analysis for factors associated with IR, prediabetes, and T2DM in non-NAFLD and NAFLD groups
Univariate and multivariate logistic regression analysis for factors associated with IR, prediabetes, and T2DM in subgroup NAFLD defined by MRI-PDFF
Free fatty acid (FFA) levels among the 10 quantiles of liver fat content (LFC) in the subgroup with magnetic resonance imaging proton density fat fraction (MRI-PDFF) data. Dotted lines represent correlations based on individual data. Black dots represent the median (interquartile range) of LFC in each quantile group. Cut-off points discriminating the 10 groups were as follow: quantile 1 (<1.5%); quantile 2 (1.5% to 2.7%); quantile 3 (2.8% to 4.1%); quantile 4 (4.2% to 6.5%); quantile 5 (6.6% to 8.4%); quantile 6 (8.5% to 11.1%); quantile 7 (11.2% to 14.1%); quantile 8 (14.2% to 17.1%); quantile 9 (17.2% to 22.4%); quantile 10 (>22.4%).  represented as the mean value of liver fat contents in each FFA quantantile. NAFLD, nonalcoholic fatty liver disease. aP<0.05.
represented as the mean value of liver fat contents in each FFA quantantile. NAFLD, nonalcoholic fatty liver disease. aP<0.05.