Summary
The discovery of hepcidin has provided a solid foundation for understanding the mechanisms of systemic iron homeostasis and the etiologies of iron disorders. Hepcidin assures the balance of circulating and stored iron levels for multiple physiological processes including oxygen transport and erythropoiesis, while limiting the toxicity of excess iron. The liver is the major site where regulatory signals from iron, erythropoietic drive and inflammation are integrated to control hepcidin production. Pathologically, hepcidin dysregulation by genetic inactivation, ineffective erythropoiesis, or inflammation leads to diseases of iron deficiency or overload such as iron refractory iron deficiency anemia, anemia of inflammation, iron loading anemias and hereditary hemochromatosis. In this review, we discuss recent insights into the molecular mechanisms governing hepcidin regulation, how these pathways are disrupted in iron disorders, and how this knowledge is being used to develop novel diagnostic and therapeutic strategies.
Keywords: iron, anemia, hepcidin, thalassemia, hereditary hemochromatosis, bone morphogenetic protein
1. Iron biology
Iron is essential for nearly all organisms including humans, animals, plants and microbes.1 Many enzymes require iron to function in biological processes including electron transfer, oxidative metabolism and energy production.2 However, excess iron can participate in the Fenton reaction to generate reactive oxygen species leading to toxicity. The adult human body contains a total of approximately 3–5 g of iron.3 Among this, 2–3 g of iron is incorporated into hemoglobin and myoglobin in red blood cells (RBCs) and muscles respectively. About 1 g is stored as ferritin and hemosiderin, mainly in macrophages and hepatocytes. Another 150 mg of iron is incorporated into iron-sulfur cluster (Fe-S) proteins. Only 1–3 mg of iron is present in the circulation, mainly bound to plasma iron carrier protein transferrin (TF). Under iron overload conditions, circulating iron also exists in the form of non-transferrin bound iron (NTBI) and loosely binds to carriers such as albumin and citrate.1
The daily fluxes of iron in the human body occur mainly in three ways.1 First, a stream of approximately 20–25 mg of iron generated from the degradation of senescent RBCs in macrophages is released into the circulation and transported to the bone marrow for erythropoiesis. Second, a loss of about 1–2 mg of iron occurs through sloughing of skin and minor bleeding, and is replenished by dietary absorption in the duodenum. Lastly, stored iron is released from hepatocytes to supply iron needs, and excess iron is taken up by hepatocytes for storage. Recent studies have also demonstrated a functional role for iron export directly from RBCs and iron reclamation by the kidney in the systemic iron economy.4,5
2. The hepcidin-ferroportin axis
Hepcidin was originally discovered as an antimicrobial peptide hormone two decades ago.6–8 Genetic knockout of the Hamp gene (encoding hepcidin) in mice and HAMP variants in humans result in iron overload and severe hemochromatosis,9–11 whereas hepcidin overexpression in transgenic mice leads to severe iron-deficiency anemia.12 Since then, numerous in vitro and in vivo studies have demonstrated that hepcidin functions as the major iron-regulatory hormone controlling systemic iron homeostasis.
The human HAMP gene encodes the 84-amino-acid pre-pro-peptide form of hepcidin, consisting of an N-terminal 24-amino-acid endoplasmic reticulum targeting signal sequence, a 35-amino-acid pro-region piece with a furin cleavage site, and a C-terminal mature 25-amino-acid peptide.13 Posttranslational cleavage occurs in the cells and mature hepcidin is secreted into circulation. Hepcidin is an amphipathic peptide with eight cysteine residues and is highly conserved across species. Mutagenesis studies showed that the N-terminal 5 amino acids are essential for hepcidin activity, whereas the cysteine residues are not.13 This has led to the development of minihepcidins and other hepcidin mimetics for therapeutics.14
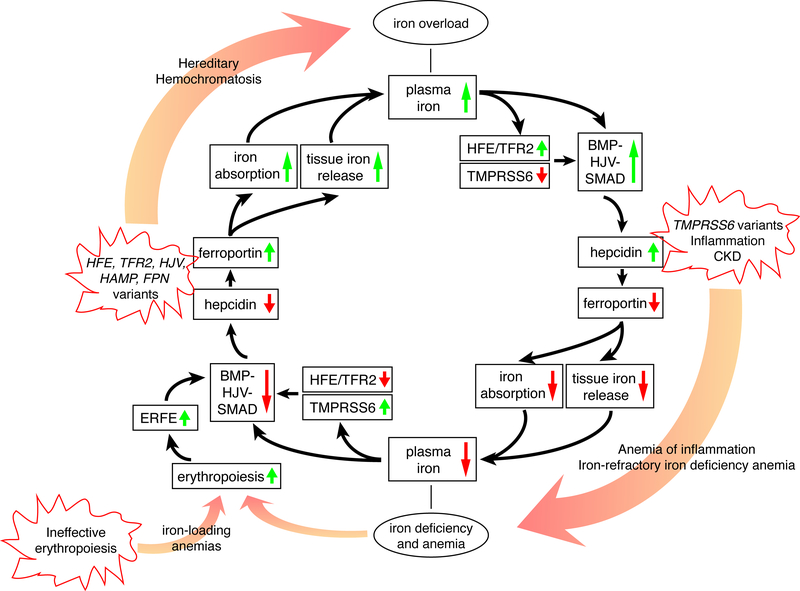
Hepcidin regulates the systemic iron economy by binding to ferroportin (FPN), a 12 transmembrane domain protein that functions as the only iron exporter in vertebrates. Hepcidin binding causes FPN to be ubiquitinated, internalized and degraded, thereby inhibiting iron export.15,16 Hepcidin binding also occludes FPN to interfere with iron export directly.17 Because FPN is highly expressed in duodenal enterocytes, iron-recycling macrophages, and hepatocytes, hepcidin-mediated FPN occlusion and degradation impairs dietary iron absorption and limits release of stored iron, thereby lowering circulating iron levels. Loss of Fpn in mice leads to severe anemia with iron sequestration in reticuloendothelial macrophages and duodenal enterocytes, phenotypically similar to hepcidin overexpression.18 In humans, heterozygous variants in FPN that disrupt iron export activity also lead to iron accumulation in reticuloendothelial macrophages, with some patients displaying marginal iron-restricted erythropoiesis and a tendency toward anemia in the setting of high iron demand, such as menarche and aggressive phlebotomy.19–22 Missense variants of FPN that disrupt hepcidin-FPN binding or the conformational change required for endocytosis and degradation lead to severe iron overload in mice and humans, resembling the phenotype of Hamp knockout mice.17,23,24 Together, these data highlight a critical role of the hepcidin-ferroportin axis in regulating systemic iron homeostasis (Figure 1).
Figure 1. The iron regulatory cycle.
The inner circle with black arrows illustrates how iron homeostasis is regulated by the hepcidin-ferroportin axis. Starting from the top, high plasma and/or tissue iron leads to the activation of the HFE/TFR2 system and the BMP/HJV/SMAD signaling pathway that upregulate hepcidin expression. Hepcidin degrades ferroportin which limits iron absorption and tissue iron release, thereby lowering plasma iron concentration (bottom). Starting from the bottom, iron deficiency activates TMPRSS6 and suppresses the HFE/TFR2 and BMP/HJV/SMAD signaling pathway, thereby lowering hepcidin expression, increasing ferroportin activity, and increasing iron absorption from the diet and mobilization from body stores to increase plasma iron. Anemia (bottom) stimulates erythropoiesis. Proliferating red blood cell precursors secrete ERFE that inhibits BMP/SMAD signaling to lower hepcidin and increase iron availability. The outer circle of this figure displays how genetic variants or diseases lead to iron deficiency or overload. In hereditary hemochromatosis (HH), genetic variants in HFE, TFR2, HJV, HAMP or FPN (gain of function) downregulate hepcidin and/or activate ferroportin, which eventually lead to iron overload. TMPRSS6 inactivation, inflammation or CKD upregulate hepcidin, leading to iron refractory iron deficiency anemia (IRIDA) or anemia of inflammation (AI). Ineffective erythropoiesis in iron-loading anemias such as β-thalassemia leads to excessive ERFE production, hepcidin downregulation and iron overload.
3. Regulation of hepcidin
Hepcidin is predominantly expressed in the liver, which is the main source of circulating hepcidin to control systemic iron homeostasis.25 This is in accordance with the anatomical fact that the hepatic portal vein collects blood from both superior the mesenteric vein and splenic vein, which contain iron absorbed by the intestine and iron released from degraded senescent RBCs in splenic macrophages. The liver is therefore poised to sense changes in circulating iron levels, which are a key signal governing hepcidin production. In addition to circulating iron levels, three other signals comprise the major contributors to hepcidin regulation: (1) tissue iron stores, (2) demand for erythropoiesis, and (3) infection and inflammation.1 Although hepcidin is predominantly expressed in the liver, there are many other organs and cells that also express hepcidin, including the heart, brain, kidney, pulmonary vasculature, macrophages, and dendritic cells, where hepcidin may participate in local iron regulation.26–28
The major signaling pathways that sense changes in circulating or tissue iron levels to control hepcidin synthesis are the homeostatic iron regulator (HFE)/transferrin receptor 2 (TFR2) system29–31 and the bone morphogenetic protein (BMP)/hemojuvelin (HJV)/mothers against decapentaplegic homolog (SMAD) signaling pathway (Figure 2).32–44 The importance of these proteins in mediating hepcidin regulation by iron is demonstrated by the findings that genetic variants or inactivation in HFE, TFR2, or components of the BMP/HJV/SMAD signaling pathway in the liver each lead to hepcidin deficiency and iron overload in both humans and mice.33,36–39,43–49
Figure 2. Molecular pathways governing hepcidin regulation in the liver.
As tissue iron levels increase, iron is captured by unknown receptor(s) on the surface of liver sinusoidal endothelial cells (LSECs), leading to the stabilization and translocation of NRF2 into the nucleus to induce BMP6 expression. BMP2 expression is also increased by unknown mechanisms. BMP6 and BMP2, most likely in the form of heterodimers (BMP2/6), bind to BMP type I (ALK2 and ALK3) and type II receptors (ACTR2A and BMPR2) and the co-receptor HJV to phosphorylate SMAD1/5/8, which together with SMAD4 activates the transcription of hepcidin. As serum iron levels increase, holo-transferrin (TF-2Fe) is captured by TFR1 and TFR2 on the surface of hepatocytes. It is postulated that HFE dissociates from TFR1 and forms a complex with TFR2 and HJV, which activates hepcidin via an interaction with the BMP/SMAD signaling pathway. Neogenin (NEO) stabilizes HJV and the BMP receptor complex. In the setting of iron deficiency, TMPRSS6 cleaves HJV from the membrane surface to inhibit hepcidin production. Soluble HJV may also contribute to hepcidin suppression by sequestering BMP2/6 ligands. Under erythropoietic conditions, ERFE is secreted by red blood cell precursors and blocks BMP2/6 from binding to the BMP receptor complex, resulting in hepcidin downregulation. Under inflammatory conditions, macrophages release IL-6 and increase hepcidin transcription via the JAK-STAT3 pathway.
The BMP/HJV/SMAD signaling pathway is the major transcriptional regulator of hepcidin expression.50 In response to increases in tissue iron levels, liver endothelial cells activate nuclear factor erythroid 2-related factor 2 (NRF2) and potentially other transcription factors51 to increase the production of BMP6 ligand. Secreted BMP6, together with endothelial cell-derived BMP2, binds to a complex of BMP type I (ALK2 and ALK3) and type II (ACTR2A and BMPR2) receptors and the co-receptor HJV on the hepatocyte membrane to induce phosphorylation of intracellular SMAD1, SMAD5, and SMAD8 transcription factors, which function together with SMAD4 to induce hepcidin transcription (Figure 2). The relative roles of these BMP-SMAD signaling components in hepcidin regulation has recently been reviewed.50 Transmembrane protease serine 6 (TMPRSS6) works as a negative regulator of hepcidin expression in response to iron deficiency through cleavage of HJV (see section 4.2).52 Neogenin (NEO) is a binding partner of HJV that is thought to promote hepcidin expression by functioning as a scaffold protein, although the molecular details are still uncertain.53–56
Circulating iron levels, in the form of iron-bound transferrin, are sensed by TFR2 and HFE to regulate hepcidin production. TFR2 binds to iron-bound transferrin directly and is stabilized by the interaction.57 HFE is displaced from transferrin receptor 1 (TFR1) upon transferrin binding, thereby freeing HFE to signal to hepcidin.58 HFE may also participate in sensing tissue iron levels in hepatocytes since TFR1 levels are decreased by hepatocyte iron loading via the iron regulatory protein system, which would contribute to increased HFE-mediated hepcidin signaling in this context.59 Studies from genetic mouse models suggest that the HFE/TFR2 system regulates hepcidin via a functional interaction with BMP/HJV/SMAD signaling pathway,60,61 possibly through protein-protein interactions between HFE, TFR2, and HJV and/or between HFE and the BMP type I receptor ALK3 (Figure 2).62–64
Erythropoiesis requires iron for the generation of hemoglobin. Anemia, hypoxia, and other inducers of erythropoietic drive stimulate the kidney to produce erythropoietin (EPO), which stimulates erythroferrone (ERFE) production by red blood cell precursors in the bone marrow.65 Circulating ERFE binds to BMP ligands to prevent their interaction with BMP receptors, thereby lowering hepcidin expression and promoting the release of stored iron to support erythropoiesis (Figure 2).66–68 Additional ERFE-independent pathways also contribute to hepcidin suppression by erythropoietic drive. In particular, erythropoiesis leads to a rapid utilization and transient reduction in circulating diferric transferrin, which is sensed by the liver via the iron sensing pathway discussed above.69,70 Growth and differentiation factor 15 (GDF15), platelet-derived growth factor-BB, and GDF11 have also been proposed to play a role, but their functional contribution is less certain.71–73
Limiting iron is an evolutionary host defense mechanism to protect against microbes, which require iron to grow and proliferate. This is achieved, at least in part, by the upregulation of hepcidin.74 The major signaling pathway for infectious organisms to induce hepcidin is by stimulating production of interleukin 6 (IL-6), which upregulates hepcidin transcription via the JAK-STAT3 pathway (Figure 2).75,76 Other cytokines such as IL-22, IL-1β, and activin B may also contribute,77 although the role of activin B as a hepcidin inducer by inflammation in vivo has been challenged.78 The BMP-SMAD signaling pathway and the HFE/TFR2/HJV system influence hepcidin expression in the context of inflammation mainly by influencing the basal hepcidin setpoint.44,79–83
In addition to the major hepcidin regulators described above, a number of other factors have been described to contribute to hepcidin regulation. Growth factors, such as epidermal growth factor (EGF) and hepatocyte growth factor (HGF), and sex hormones, such as testosterone and estrogen, suppress hepcidin expression to boost iron need during growth and development.84–86 The molecular mechanisms by which growth factors and sex hormones regulate hepcidin are still not fully understood, but most functionally intersect with the BMP-SMAD signaling pathway.50 Protein misfolding-induced endoplasmic reticulum (ER) stress serves as an intracellular signal to transcriptionally activate hepcidin via cyclic AMP response element-binding protein H (CREBH) and the SMAD signaling pathway.87,88 Post-transcriptional hepcidin regulation by the hepcidin RNA-binding protein HuR may also contribute.89 The immunophilin FKBP12 suppresses hepcidin by binding and inhibiting the BMP type I receptor ALK2 to avoid uncontrolled activation of the pathway, effects that can be reversed by the FKBP12 sequestering immunosuppressive medications rapamycin and tacroliums.90
4. Clinical implications of hepcidin regulation
Given the central role of hepcidin in systemic iron homeostasis, it is not surprising that hepcidin dysregulation has a major role in the pathogenesis of iron-related disorders, including iron-refractory iron deficiency anemia (IRIDA), anemia of inflammation (AI), iron loading anemias such as β-thalassemia, and hereditary hemochromatosis (HH) (Figure 1). The hepcidin-ferroportin axis is therefore an attractive therapeutic target to ameliorate or even reverse the symptoms of such diseases. Measurement of hepcidin levels has also been explored as diagnostic tool.
4.1. Hepcidin assays
A number of assays have been developed to measure circulating hepcidin levels.91 The two major assay methods are mass spectrometry (MS)-based assays and immunoassays. MS-based assays can distinguish the bioactive hepcidin-25 isoform from other isoforms, but the clinical utility of this distinction is uncertain, and these assays require specialized equipment. Although relative hepcidin levels are largely consistent among many MS and immunoassays tested, absolute hepcidin levels are widely variable, thereby precluding universal reference ranges.92 Harmonization initiatives are underway to address this challenge.93 Although hepcidin assays are not currently available for routine clinical use, there are certain clinical contexts where hepcidin assays have shown some promise, including the diagnosis of IRIDA, distinguishing iron deficiency anemia (IDA) from AI, and guiding iron supplementation strategies,91 as discussed below.
4.2. Iron-deficiency anemia (IDA) and iron-refractory iron-deficiency anemia (IRIDA)
IDA results from insufficient body iron stores and remains one of the most common types of anemia worldwide.94 Iron deficiency typically results from insufficient dietary intake due to poverty, malnutrition and iron-poor diets. Blood loss is another big cause of IDA ranging from worm infection, menstruation, chronic blood loss from the gastrointestinal tract, and blood donation. Other causes include pregnancy, poor intestinal iron absorption and urinary loss of iron due to kidney diseases. IDA is manifest by a microcytic, hypochromic anemia with low hemoglobin (Hb, <13 g/dl for men, <12 g/dl for women), low MCV, low MCH, low serum transferrin saturation (TF sat, <16%), and low serum ferritin (SF, <10–30 μg/L).94 Higher cutoffs are suggested in patients with underlying inflammatory disorders or other co-morbidities as well as the elderly. In addition to addressing the underlying etiology, common treatments for IDA include oral iron therapy, although caution may be warranted in malaria endemic settings.95 For patients with severe IDA, chronic blood loss, or those intolerant or unresponsive to oral iron compounds, intravenous (IV) iron therapy or RBC transfusions may be required.
IRIDA represents a rare inherited type of IDA where patients do not respond to oral iron and are only partially responsive to IV iron. IRIDA is caused by variants of the gene encoding TMPRSS6, also known as Matriptase 2.96–98 Contrary to IDA, where iron deficiency is the primary process and hepcidin levels are maximally suppressed to optimize iron availability, IRIDA is caused by inappropriately high production of hepcidin, leading to impaired iron absorption from the diet and impaired iron release from body stores (Figure 1).96–98 IRIDA patients therefore differ from IDA patients by exhibiting higher ferritin levels, reflecting increased macrophage iron, and normal/high hepcidin in the absence of inflammation.94,99 Measurement of hepcidin levels may be helpful to confirm the diagnosis of IRIDA in the appropriate clinical context.91
Mechanistically, TMPRSS6 is an endogenous suppressor of hepcidin production in the context of iron deficiency. TMPRSS6 expression is increased by iron deficiency100 and functions to cleave HJV from the membrane surface,52 thereby decreasing BMP/SMAD signaling and hepcidin production (Figure 2). TMPRSS6 deleterious variants associated with IRIDA impair cleavage of HJV, thereby leading to inappropriately high levels of BMP/HJV/SMAD signaling and hepcidin production.52 Additionally, TMPRSS6 gene variants are also linked to altered iron parameters and hemoglobin levels in population studies.101,102
Current management for IRIDA includes repeated IV iron infusions.103 Preclinical studies have demonstrated that antagonizing hepcidin or BMP signaling may also be an effective way to treat patients with IRIDA. As proof of principle, Tmprss6/Bmp6 double knockout mice are neither anemic nor iron overloaded.104 Moreover, BMP type I receptor inhibitors and hemojuvelin antibodies were effective to lower hepcidin and improve hemoglobin levels in Tmprss6 knockout mice.105,106
4.3. Anemia of inflammation
Anemia of inflammation (AI, also known as anemia of chronic disease) is an immune-driven disorder that displays reduced erythropoietic activity and dysregulation of iron homeostasis.77,107 Whereas IDA is characterized by absolute iron deficiency, AI represents a state of functional iron deficiency where insufficient iron is available for erythropoiesis despite normal or elevated body iron stores. AI is associated with a number of underlying diseases from chronic rheumatologic disorders and infections to chronic kidney disease (CKD), malignancy, congestive heart failure, chronic obstructive pulmonary disease, obesity, and critical illness.77,107 Observational data indicate that anemia and iron deficiency in these disease states is associated with adverse outcomes, including an increased risk of hospitalizations, cardiovascular disease, cognitive and neuromuscular impairment, and mortality.108–112 However, the truly independent risk of anemia and/or iron deficiency remain uncertain given the association of anemia and iron deficiency with numerous comorbidities. Mechanistically, AI is caused by inflammatory cytokines such as IL-6 increasing the transcription of hepcidin, thereby sequestering iron in macrophage stores, decreasing dietary iron absorption, and decreasing serum iron levels (Figure 1). In parallel, toll-like receptor signaling in macrophages directly inhibit ferroportin expression independent of hepcidin to contribute to the hypoferremia. Ferritin is also upregulated by cytokines such as TNF-α to sequester iron. In addition to restricting iron availability, inflammatory cytokines also inhibit erythropoietic activity by suppressing the production and activity of erythropoietin and by directly targeting erythropoietic cells. Meanwhile, cytokine-activated macrophages reduce the lifespan of existing erythrocytes. These events together lead to the anemic state.
AI is characterized by a normochromic, normocytic anemia with low levels of hemoglobin, low to normal levels transferrin saturation, and serum ferritin >100 μg/L occurring in the presence of clinical or laboratory evidence of inflammation.77,113 Higher serum ferritin levels reflecting preserved body iron stores is the biggest distinguishing factor between AI and IDA. A particularly challenging scenario is identifying patients in whom IDA coexists with AI, which can occur in up to 20–85% of AI patients.77 Several studies have suggested that measurement of hepcidin levels may be helpful in this context since hepcidin levels are lower in patients with superimposed IDA compared to patients with pure AI; however, hepcidin levels alone are not diagnostic.114–119 Lower hepcidin levels may also be helpful to identify patients who would be responsive to oral iron or in whom iron supplementation strategies would be safe in settings with a high prevalence of infection.114,118,120 However, there are still several limitations that preclude the use of hepcidin assays in clinical practice at the present time.91
Direct treatment of inflammation would be an ideal way to ameliorate anemia in AI. Indeed, IL-6 receptor antibodies and IL-6 antibodies have been demonstrated to lower hepcidin levels and improve anemia in patients with rheumatoid arthritis and multicentric Castleman’s disease.121–123 However, such treatment is not always feasible.107 More specific anemia management with erythropoietin stimulating agents (ESAs) and iron has been studied most extensively in the treatment of anemia of chronic kidney disease (CKD), where an impairment in kidney EPO production also contributes to the development of anemia. Although ESAs are effective in improving hemoglobin levels, reducing transfusion requirements and improving quality of life, they have not been demonstrated to improve harder clinical outcomes in prospective randomized controlled trials (RCTs) in CKD patients.108 Moreover, targeting normal hemoglobin levels with ESAs in CKD patients has been associated with increased adverse outcomes including stroke, cardiovascular events, and death.124,125 Regarding iron supplementation, there is RCT evidence in CKD patients on hemodialysis that high-dose IV iron administered to patients with TF sat <40% and ferritin <700 μg/L improves hard clinical outcomes (a composite of death, myocardial infarction, heart failure hospitalization, and stroke) without increasing infectious complications, compared to low-dose IV iron administered only to patients with overt IDA (TF sat <20%, ferritin <200 μg/L).126, 127 However, management of patients with higher ferritin levels is less certain. Additionally, for other AI populations that are less well-studied, there remains the theoretical concern of adverse consequences from iron supplementation due to iron overload, oxidative stress, and a permissive environment for infectious organisms.128
Novel hepcidin-lowering or antagonizing therapeutics are an attractive approach in patients with AI because this would target the underlying pathophysiologic mechanisms of iron restriction to facilitate iron mobilization from body stores and improve dietary iron absorption. Hepcidin monoclonal antibodies were shown to enhance red cell hemoglobinization in a mouse model of AI129 and were evaluated in a phase 1 trial in patients with cancer-associated anemia.130 Likewise, hepcidin anticalin and spiegelmer have been shown to elevate serum iron concentration in both cynomolgus monkey and humans.131–133 Ferroportin antibodies blocking access to hepcidin were also shown to have efficacy in increasing serum iron levels in cynomolgus monkeys and humans without and with CKD.134 In addition to agents antagonizing hepcidin, agents targeting the BMP-SMAD pathway to inhibit hepcidin production have also been evaluated for the treatment of AI in rodent models and early stage human clinical trials. These include soluble HJV fragment that sequesters BMP ligands,135 HJV monoclonal antibodies,105 BMP6 monoclonal antibodies134,136 and small molecule inhibitors of BMP type I receptors.135,137–139 However, as with traditional iron supplementation strategies, the potential risks of increasing iron availability on inducing oxidative stress and creatinine a permissive environment for infections128 will need to be evaluated, as well as potential off-target effects of inhibiting BMP-SMAD signaling.
Another interesting class of emerging anemia therapies are the HIF prolyl-hydroxylase (PHD) inhibitors. HIF transcription factors are heterodimers composed of an α subunit (such as HIF1α and HIF2α) and a β subunit (such as HIF1β). Under normoxia and iron sufficient conditions, HIFα subunits are hydroxylated by PHDs and degraded. Inhibition of PHDs stabilizes HIFs to increase expression of HIF regulated genes with multifactorial effects to ameliorate anemia including increased EPO production, hepcidin downregulation, and increased intestinal iron absorption. Notably, although EPO is a direct HIF target, hepcidin is an indirect target that is suppressed via EPO-mediated ERFE activation,140 an effect shared with other ESAs. However, HIF-PHIs may also have the added benefit of increasing dietary iron absorption by inducing FPN and other proteins involved in intestinal iron uptake that are direct HIF targets.141,142 HIF-PHIs have shown efficacy in improving hemoglobin levels in phase 3 trials in CKD patients,143–146 and are already approved for clinical use in Asia. The clinical impact of HIF-PHIs on iron homeostasis remains to be clarified. Larger, longer term clinical trials in more diverse patient populations will also be needed to better understand the potential safety concerns of HIF-PHIs given the large number of other genes that are also HIF targets, with particular concerns related to tumor promoting effects, vascular retinopathies, pulmonary arterial hypertension, and cyst progression in polycystic kidney disease among others.
4.4. β-thalassemia
β-thalassemia is an inherited hemoglobin disorder characterized by ineffective erythropoiesis, anemia, hematopoietic expansion and organomegaly, hypercoagulability and increased intestinal iron absorption.147 Depending on the severity of these conditions and the extent of α-globin to β-globin chain imbalance, β-thalassemia is classified into major, intermedia, or minor. In recent years, β-thalassemia and other thalassemias are clinically categorized into transfusion-dependent thalassemia (TDT) or non–transfusion-dependent thalassemia (NTDT). Patients with β-thalassemia major often fall under TDT while patients with β-thalassemia intermedia fall under NTDT.147
Iron overload is an important cause of morbidity and mortality in thalassemia patients leading to end organ damage in the liver, heart, and endocrine glands.147 In addition to blood transfusions, which provide 200–250 mg of iron per unit, hyperabsorption of dietary iron as a consequence of hepcidin-FPN axis dysregulation is an important contributing factor to iron overload in thalassemia patients.147 Mechanistically, ineffective erythropoiesis leads to an increase in ERFE production that suppresses hepcidin expression (Figure 1).148,149 Other erythroid suppressors of hepcidin may also contribute, but their functional role is less certain (see section 3). As iron overload progresses iron may stimulate the production of hepcidin, but hepcidin levels are still relatively low compared to tissue iron levels.150,151 Anemia and hypoxia may also contribute to dietary iron hyperabsorption by stabilizing intestinal HIF2 expression leading to an increase in FPN and other HIF target proteins involved in intestinal iron uptake (see section 4.3).152
Conventional management of β-thalassemia includes transfusion and iron chelation.147 In patients with TDT, blood transfusion can successfully bring Hb concentrations back to 9–10.5 g/dl. However, patients may receive an average of 10–30 mg of iron per day. Iron chelation is then used as a complementary treatment in patients with regular transfusions or patients with NTDT who develop iron overload. Currently available iron chelation therapy includes desferrioxamine (also known as deferoxamine), deferiprone and deferasirox. Although these drugs have improved patient outcomes, they do not target the underlying pathophysiologic mechanisms of disease and do not prevent ongoing dietary iron hyperabsorption. Moreover, iron chelators can be cumbersome to administer (particularly desferrioxamine, which is given parenterally) and are associated with adverse effects.14
Novel therapeutics targeting the hepcidin-FPN pathway could represent an advantage of better targeting the pathophysiology of iron overload in thalassemia. Proof of principle studies indicate that hepcidin overexpression improves tissue iron loading in the Hbbth3/+ β-thalassemia intermedia mouse model.153 Notably, hepcidin overexpression also ameliorated anemia and reversed ineffective erythropoiesis and splenomegaly in the thalassemic mice.153 This is thought to be a consequence of limiting iron availability to erythroid precursors, thereby decreasing heme production, reducing insoluble hemichrome aggregates, limiting reactive oxygen species, and potentially lowering free α-globin chain accumulation, since similar findings have been reported with many other strategies that cause iron restriction.151 Subsequent studies have shown that treatment with minihepcidins (stable truncated hepcidin peptides that retain the biological function of hepcidin) similarly improved iron overload and anemia in Hbbth3/+ and Hbbth1/th2 (resembling human TDT) mice.154,155 Hepcidin mimetics have also shown efficacy in preclinical models and are currently in phase 2 clinical trials in β-thalassemia patients (ClinicalTrials.gov Identifier: NCT03802201, NCT03381833).156 Alternatively, inhibiting ERFE to block its hepcidin suppressive effects has also been shown to be effective in attenuating the phenotype of β-thalassemia in mice.67,148 Inhibiting the hepcidin suppressor TMPRSS6 has also been efficacious in pre-clinical studies,157–160 and a TMPRSS6 antisense oligonucleotide is in early stage clinical trials (ClinicalTrials.gov Identifier: NCT04059406). In addition to agents targeting the hepcidin-ferroportin axis, a number of other novel therapeutics are emerging for β-thalassemia, including hematopoietic stem cell transplantation, gene therapy, gene editing, TGF-β superfamily ligand traps, and JAK2 inhibitors.147
4.5. Hereditary hemochromatosis (HH)
HH is a genetic disorder of iron overload characterized by dietary iron hyperabsorption with excessive tissue iron accumulation eventually leading to diverse pathologies including hepatic cirrhosis, diabetes mellitus, cardiomyopathy, arthropathy and skin pigmentation.161 The common underlying pathophysiology of HH is hepcidin deficiency and/or FPN overactivity due to pathogenic variants in the genes encoding hepcidin itself (HAMP), FPN, or other key mediators of hepcidin regulation by iron (HFE, TFR2, or HJV) (Figure 1). HH types are categorized by the age of onset and the underlying genetic variants, including type 1 (HFE variants, adulthood onset), type 2 (HJV or HAMP, juvenile), type 3 (TFR2, before age 30), and type 4 (FPN gain-of-function variants, adulthood).162
Biochemical evidence of HH includes high levels of transferrin saturation (TSAT, ≥45–50% for men, >40–45% for women) and serum ferritin (SF, >200–300 μg/L for men, >150–200 μg/L for women).163,164 Genetic testing for common HFE variants is typically performed in patients in whom HH is suspected based on clinical grounds, and HFE C282Y homozygosity confirms a diagnosis of HH.165 With the advent of genetic testing, liver biopsy is mainly recommended to stage fibrosis and rule out concurrent liver disease in HFE C282Y homozygotes with SF >1000 μg/L and/or elevated liver enzymes.163,165 For patients who are not HFE C282Y homozygotes, evaluation for other liver or hematologic disorders should be performed, and MRI or liver biopsy can be used to measure tissue iron concentration, evaluate for alternative liver diseases, and stage fibrosis.163,165 Although serum hepcidin measurement is not routinely used, it could have some clinical utility in diagnosing non-HFE HH patients and guiding follow-up genetic testing, with reduced hepcidin levels relative to iron overload seen in type I-3 HH (most markedly reduced in type 2) and increased hepcidin levels in type 4.91
Phlebotomy is the most common treatment considering its accessibility and effectiveness, removing approximately 200–250 mg of iron from the patient per blood unit. This treatment improves many HH clinical features, including some degree of hepatic fibrosis reversal, diabetes control, cardiac function, and skin pigmentation, and significantly improves mortality when initiated before the development of cirrhosis and/or diabetes. However, other clinical manifestations are less responsive or unresponsive, including arthropathy, hypogonadism, and advanced cirrhosis.163 Iron chelators may be used if the patient does not tolerate phlebotomy, is refractory, or if phlebotomy is contraindicated.163 Targeting hepcidin deficiency is also a promising new therapeutic approach to treat HH patients. Pre-clinical studies have shown efficacy for mini-hepcidins, recombinant BMP6, and TMRPSS6 antisense oligonucleotides and RNAi166,167,158,159 in rodent models of hemochromatosis. Hepcidin mimetics are currently under evaluation for HH treatment in phase 2 clinical trials (ClinicalTrials.gov Identifier: NCT04202965, ClinicalTrials.gov Identifier: NCT03395704).
5. Conclusion
The past two decades have witnessed an explosion of new knowledge regarding the molecular underpinnings of the physiology of systemic iron homeostasis and the pathophysiology of iron disorders. This has identified a key role for the hepcidin-ferroportin axis and the molecular mediators of hepcidin regulation by iron, inflammation, and erythropoietic drive. Future advances in this area would benefit from better model systems to study the molecular mechanisms of iron sensing and provide more precise structural and functional knowledge regarding the liver membrane proteins that contribute to hepcidin regulation. Novel therapeutic strategies targeting these molecular pathways are currently in clinical development for the treatment of iron disorders.
Grant support and acknowledgements
This work was supported by National Institutes of Health grant RO1-DK087727 and the Patricia and Scott Eston Massachusetts General Hospital Research Scholar Award to JLB. VMAM is supported by Programa Propio I+D+I grant provided by Universidad Politécnica de Madrid.
Footnotes
Conflict of interest disclosures
JLB has ownership interest in Ferrumax Pharmaceuticals and has been a consultant for Disc Medicine, Incyte Corporation, and Alnylam Pharmaceuticals. All other authors declare no conflict of interest.
References
- 1.van Swelm RPL, Wetzels JFM, Swinkels DW. The multifaceted role of iron in renal health and disease. Nat Rev Nephrol. 2020;16:77–98. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan J, Ward DM. The essential nature of iron usage and regulation. Curr Biol. 2013;23:R642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta Mol Cell Res. 2012;1823:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D-L, Ghosh MC, Ollivierre H, Li Y, Rouault TA. Ferroportin deficiency in erythroid cells causes serum iron deficiency and promotes hemolysis due to oxidative stress. Blood. 2018;132:2078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Zheng X, Zhang J, Zhao S, Wang Z, Wang F, et al. Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury. Am J Physiol Renal Physiol. 2018;315:F1042–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause A, Neitz S, Mägert H-J, Schulz A, Forssmann W-G, Schulz-Knappe P, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–50. [DOI] [PubMed] [Google Scholar]
- 7.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. J Biol Chem. 2001;276:7806–10. [DOI] [PubMed] [Google Scholar]
- 8.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–9. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–2. [DOI] [PubMed] [Google Scholar]
- 11.Lesbordes-Brion J-C, Viatte L, Bennoun M, Lou D-Q, Ramey G, Houbron C, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108:1402–5. [DOI] [PubMed] [Google Scholar]
- 12.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth E, Preza GC, Jung C-L, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsarou A, Pantopoulos K. Hepcidin Therapeutics. Pharmaceuticals (Basel). 2018;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemeth E Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science. 2004;306:2090–3. [DOI] [PubMed] [Google Scholar]
- 16.Qiao B, Sugianto P, Fung E, del-Castillo-Rueda A, Moran-Jimenez M-J, Ganz T, et al. Hepcidin-Induced Endocytosis of Ferroportin Is Dependent on Ferroportin Ubiquitination. Cell Metab. 2012;15:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aschemeyer S, Qiao B, Stefanova D, Valore EV, Sek AC, Ruwe TA, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 19.Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, Cassanelli S, et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest. 2001;108:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrangelo A The ferroportin disease. Blood Cells Mol Dis. 2004;32:131–8. [DOI] [PubMed] [Google Scholar]
- 21.Pietrangelo A Ferroportin disease: pathogenesis, diagnosis and treatment. Haematologica. 2017;102:1972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corradini E, Buzzetti E, Pietrangelo A. Genetic iron overload disorders. Mol Aspects Med. 2020;75:100896. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes A, Preza GC, Phung Y, De Domenico I, Kaplan J, Ganz T, et al. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood. 2009;114:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altamura S, Kessler R, Gröne H-J, Gretz N, Hentze MW, Galy B, et al. Resistance of Ferroportin to Hepcidin Binding causes Exocrine Pancreatic Failure and Fatal Iron Overload. Cell Metab. 2014;20:359–67. [DOI] [PubMed] [Google Scholar]
- 25.Zumerle S, Mathieu JRR, Delga S, Heinis M, Viatte L, Vaulont S, et al. Targeted disruption of hepcidin in the liver recapitulates the hemochromatotic phenotype. Blood. 2014;123:3646–50. [DOI] [PubMed] [Google Scholar]
- 26.Lakhal-Littleton S, Wolna M, Chung YJ, Christian HC, Heather LC, Brescia M, et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. eLife. 2016;5:e19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakhal-Littleton S, Crosby A, Frise MC, Mohammad G, Carr CA, Loick PAM, et al. Intracellular iron deficiency in pulmonary arterial smooth muscle cells induces pulmonary arterial hypertension in mice. Proc Natl Acad Sci U S A. 2019;116:13122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bessman NJ, Mathieu JRR, Renassia C, Zhou L, Fung TC, Fernandez KC, et al. Dendritic cell–derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science. 2020;368:186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, et al. Decreased Liver Hepcidin Expression in the Hfe Knockout Mouse. Blood Cells Mol Dis. 2002;29:361–6. [DOI] [PubMed] [Google Scholar]
- 30.Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O’Kelly J, et al. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105:376–81. [DOI] [PubMed] [Google Scholar]
- 31.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105:1803–6. [DOI] [PubMed] [Google Scholar]
- 32.Niederkofler V Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang FW. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R-H, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. [DOI] [PubMed] [Google Scholar]
- 35.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–9. [DOI] [PubMed] [Google Scholar]
- 36.Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth M-P. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–81. [DOI] [PubMed] [Google Scholar]
- 38.Steinbicker AU, Bartnikas TB, Lohmeyer LK, Leyton P, Mayeur C, Kao SM, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118:4224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayeur C, Leyton PA, Kolodziej SA, Yu B, Bloch KD. BMP type II receptors have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism. Blood. 2014;124:2116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canali S, Zumbrennen-Bullough KB, Core AB, Wang C-Y, Nairz M, Bouley R, et al. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood. 2017;129:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koch P-S, Olsavszky V, Ulbrich F, Sticht C, Demory A, Leibing T, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. 2017;129:415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canali S, Wang C-Y, Zumbrennen-Bullough KB, Bayer A, Babitt JL. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am J Hematol. 2017;92:1204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C-Y, Core AB, Canali S, Zumbrennen-Bullough KB, Ozer S, Umans L, et al. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood. 2017;130:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Xiao X, Bayer A, Xu Y, Dev S, Canali S, et al. Ablation of Hepatocyte Smad1, Smad5, and Smad8 Causes Severe Tissue Iron Loading and Liver Fibrosis in Mice. Hepatology. 2019;70:1986–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I–like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. [DOI] [PubMed] [Google Scholar]
- 46.Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, et al. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci U S A. 1998;95:2492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camaschella C, Roetto A, Calì A, De Gobbi M, Garozzo G, Carella M, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–5. [DOI] [PubMed] [Google Scholar]
- 48.Papanikolaou G, Samuels ME, Ludwig EH, MacDonald MLE, Franchini PL, Dubé M-P, et al. Mutations in HFE2 cause iron overload in chromosome 1q–linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. [DOI] [PubMed] [Google Scholar]
- 49.Roetto A, Di Cunto F, Pellegrino RM, Hirsch E, Azzolino O, Bondi A, et al. Comparison of 3 Tfr2-deficient murine models suggests distinct functions for Tfr2-alpha and Tfr2-beta isoforms in different tissues. Blood. 2010;115:3382–9. [DOI] [PubMed] [Google Scholar]
- 50.Xiao X, Alfaro-Magallanes VM, Babitt JL. Bone morphogenic proteins in iron homeostasis. Bone. 2020;138:115495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim PJ, Duarte TL, Arezes J, Garcia-Santos D, Hamdi A, Pasricha S-R, et al. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat Metab. 2019;1:519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The Serine Protease Matriptase-2 (TMPRSS6) Inhibits Hepcidin Activation by Cleaving Membrane Hemojuvelin. Cell Metab. 2008;8:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee D-H, Zhou L-J, Zhou Z, Xie J-X, Jung J-U, Liu Y, et al. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood. 2010;115:3136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagihara M, Endo M, Hata K, Higuchi C, Takaoka K, Yoshikawa H, et al. Neogenin, a receptor for bone morphogenetic proteins. J Biol Chem. 2011;286:5157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Healey EG, Bishop B, Elegheert J, Bell CH, Padilla-Parra S, Siebold C. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat Struct Mol Biol. 2015;22:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao N, Maxson JE, Zhang RH, Wahedi M, Enns CA, Zhang A-S. Neogenin Facilitates the Induction of Hepcidin Expression by Hemojuvelin in the Liver. J Biol Chem. 2016;291:12322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104:4287–93. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The Transferrin Receptor Modulates Hfe-Dependent Regulation of Hepcidin Expression. Cell Metab. 2008;7:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fillebeen C, Charlebois E, Wagner J, Katsarou A, Mui J, Vali H, et al. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood. 2019;133:344–55. [DOI] [PubMed] [Google Scholar]
- 60.Corradini E, Garuti C, Montosi G, Ventura P, Andriopoulos B, Lin HY, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137:1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corradini E, Rozier M, Meynard D, Odhiambo A, Lin HY, Feng Q, et al. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology. 2011;141:1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Besson-Fournier C, Latour C, Gourbeyre O, Aguilar-Martinez P, Silvestri L, Roth M-P, et al. Transferrin Receptor 2 and HFE Regulate Hepcidin Levels Independently of Bmp6 and Hemojuvelin. Blood. 2014;124:746–746. [Google Scholar]
- 63.Latour C, Besson-Fournier C, Meynard D, Silvestri L, Gourbeyre O, Aguilar-Martinez P, et al. Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin. Hepatology. 2016;63:126–37. [DOI] [PubMed] [Google Scholar]
- 64.Xiao X, Dev S, Canali S, Bayer A, Xu Y, Agarwal A, et al. Endothelial Bone Morphogenetic Protein 2 (Bmp2) Knockout Exacerbates Hemochromatosis in Homeostatic Iron Regulator (Hfe) Knockout Mice but not Bmp6 Knockout Mice. Hepatology. 2020;72:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132:1473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arezes J, Foy N, McHugh K, Quinkert D, Benard S, Sawant A, et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood. 2020;135:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C-Y, Xu Y, Traeger L, Dogan DY, Xiao X, Steinbicker AU, et al. Erythroferrone lowers hepcidin by sequestering BMP2/6 heterodimer from binding to the BMP type I receptor ALK3. Blood. 2020;135:453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirciov CSG, Wilkins SJ, Hung GCC, Helman SL, Anderson GJ, Frazer DM. Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis. Haematologica. 2018;103:1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Artuso I, Pettinato M, Nai A, Pagani A, Sardo U, Billoré B, et al. Transient decrease of serum iron after acute erythropoietin treatment contributes to hepcidin inhibition by ERFE in mice. Haematologica. 2019;104:e87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanno T, Bhanu NV, Oneal PA, Goh S-H, Staker P, Lee YT, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–101. [DOI] [PubMed] [Google Scholar]
- 72.Sonnweber T, Nachbaur D, Schroll A, Nairz M, Seifert M, Demetz E, et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut. 2014;63:1951–9. [DOI] [PubMed] [Google Scholar]
- 73.Fang Z, Zhu Z, Zhang H, Peng Y, Liu J, Lu H, et al. GDF11 contributes to hepatic hepcidin (HAMP) inhibition through SMURF1-mediated BMP-SMAD signalling suppression. Br J Haematol. 2020;188:321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–3. [DOI] [PubMed] [Google Scholar]
- 75.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Besson-Fournier C, Gineste A, Latour C, Gourbeyre O, Meynard D, Martin P, et al. Hepcidin upregulation by inflammation is independent of Smad1/5/8 signaling by activin B. Blood. 2017;129:533–6. [DOI] [PubMed] [Google Scholar]
- 79.Wallace DF, McDonald CJ, Ostini L, Subramaniam VN. Blunted hepcidin response to inflammation in the absence of Hfe and transferrin receptor 2. Blood. 2011;117:2960–6. [DOI] [PubMed] [Google Scholar]
- 80.Latour C, Besson-Fournier C, Gourbeyre O, Meynard D, Roth M-P, Coppin H. Deletion of BMP6 worsens the phenotype of HJV-deficient mice and attenuates hepcidin levels reached after LPS challenge. Blood. 2017;130:2339–43. [DOI] [PubMed] [Google Scholar]
- 81.Fillebeen C, Wilkinson N, Charlebois E, Katsarou A, Wagner J, Pantopoulos K. Hepcidin-mediated hypoferremic response to acute inflammation requires a threshold of Bmp6/Hjv/Smad signaling. Blood. 2018;132:1829–41. [DOI] [PubMed] [Google Scholar]
- 82.Gallitz I, Lofruthe N, Traeger L, Bäumer N, Hoerr V, Faber C, et al. Deficiency of the BMP Type I receptor ALK3 partly protects mice from anemia of inflammation. BMC Physiol. 2018;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang C-Y, Canali S, Bayer A, Dev S, Agarwal A, Babitt JL. Iron, erythropoietin, and inflammation regulate hepcidin in Bmp2-deficient mice, but serum iron fails to induce hepcidin in Bmp6-deficient mice. Am J Hematol. 2019;94:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goodnough JB, Ramos E, Nemeth E, Ganz T. Inhibition of hepcidin transcription by growth factors. Hepatology. 2012;56:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou Y, Zhang S, Wang L, Li J, Qu G, He J, et al. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511:398–403. [DOI] [PubMed] [Google Scholar]
- 86.Latour C, Kautz L, Besson-Fournier C, Island M-L, Canonne-Hergaux F, Loréal O, et al. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology. 2014;59:683–94. [DOI] [PubMed] [Google Scholar]
- 87.Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, et al. ER Stress Controls Iron Metabolism Through Induction of Hepcidin. Science. 2009;325:877–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Canali S, Vecchi C, Garuti C, Montosi G, Babitt JL, Pietrangelo A. The SMAD Pathway Is Required for Hepcidin Response During Endoplasmic Reticulum Stress. Endocrinology. 2016;157:3935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belot A, Gourbeyre O, Palin A, Rubio A, Largounez A, Besson-Fournier C, et al. Endoplasmic reticulum stress controls iron metabolism through TMPRSS6 repression and hepcidin mRNA stabilization by RNA-binding protein HuR. Haematologica. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colucci S, Pagani A, Pettinato M, Artuso I, Nai A, Camaschella C, et al. The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood. 2017;130:2111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127:2809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kroot JJC, Kemna EHJM, Bansal SS, Busbridge M, Campostrini N, Girelli D, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94:1748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diepeveen LE, Laarakkers CMM, Martos G, Pawlak ME, Uğuz FF, Verberne KESA, et al. Provisional standardization of hepcidin assays: creating a traceability chain with a primary reference material, candidate reference method and a commutable secondary reference material. Clin Chem Lab Med. 2019;57:864–72. [DOI] [PubMed] [Google Scholar]
- 94.Camaschella C Iron-Deficiency Anemia. Longo DL, editor. N Engl J Med. 2015;372:1832–43. [DOI] [PubMed] [Google Scholar]
- 95.Spottiswoode N, Fried M, Drakesmith H, Duffy PE. Implications of Malaria On Iron Deficiency Control Strategies. Adv Nutr. 2012;3:570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, et al. The Serine Protease TMPRSS6 Is Required to Sense Iron Deficiency. Science. 2008;320:1088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finberg KE, Heeney MM, Campagna DR, Aydınok Y, Pearson HA, Hartman KR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Folgueras AR, de Lara FM, Pendás AM, Garabaya C, Rodríguez F, Astudillo A, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112:2539–45. [DOI] [PubMed] [Google Scholar]
- 99.Camaschella C Iron deficiency. Blood. 2019;133:30–9. [DOI] [PubMed] [Google Scholar]
- 100.Zhao N, Nizzi CP, Anderson SA, Wang J, Ueno A, Tsukamoto H, et al. Low intracellular iron increases the stability of matriptase-2. J Biol Chem. 2015;290:4432–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benyamin B, Ferreira MAR, Willemsen G, Gordon S, Middelberg RPS, McEvoy BP, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. 2009;41:1173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. 2009;41:1170–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Falco L, Sanchez M, Silvestri L, Kannengiesser C, Muckenthaler MU, Iolascon A, et al. Iron refractory iron deficiency anemia. Haematologica. 2013;98:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nai A, Rubio A, Campanella A, Gourbeyre O, Artuso I, Bordini J, et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood. 2016;127:2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kovac S, Boser P, Cui Y, Ferring-Appel D, Casarrubea D, Huang L, et al. Anti-hemojuvelin antibody corrects anemia caused by inappropriately high hepcidin levels. Haematologica. 2016;101:e173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belot A, Gourbeyre O, Fay A, Palin A, Besson-Fournier C, Latour C, et al. LJ000328, a novel ALK2/3 kinase inhibitor, represses hepcidin and significantly improves the phenotype of IRIDA. Haematologica. 2020;105:e385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ganz T Anemia of Inflammation. N Engl J Med. 2019;381:1148–57. [DOI] [PubMed] [Google Scholar]
- 108.KDOQI, National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47:S11–145. [DOI] [PubMed] [Google Scholar]
- 109.Awan AA, Walther CP, Richardson PA, Shah M, Winkelmayer WC, Navaneethan SD. Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol Dial Transplant. 2019; [DOI] [PubMed] [Google Scholar]
- 110.Lam CSP, Doehner W, Comin-Colet J, IRON CORE Group. Iron deficiency in chronic heart failure: case-based practical guidance. ESC Heart Fail. 2018;5:764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho ME, Hansen JL, Peters CB, Cheung AK, Greene T, Sauer BC. An increased mortality risk is associated with abnormal iron status in diabetic and non-diabetic Veterans with predialysis chronic kidney disease. Kidney Int. 2019;96:750–60. [DOI] [PubMed] [Google Scholar]
- 112.Litton E, Lim J. Iron Metabolism: An Emerging Therapeutic Target in Critical Illness. Crit Care. 2019;23:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weiss G, Goodnough LT. Anemia of Chronic Disease. N Engl J Med. 2005;352:1011–23. [DOI] [PubMed] [Google Scholar]
- 114.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–86. [DOI] [PubMed] [Google Scholar]
- 115.Lasocki S, Baron G, Driss F, Westerman M, Puy H, Boutron I, et al. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med. 2010;36:1044–8. [DOI] [PubMed] [Google Scholar]
- 116.van Santen S, van Dongen-Lases EC, de Vegt F, Laarakkers CMM, van Riel PLCM, van Ede AE, et al. Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2011;63:3672–80. [DOI] [PubMed] [Google Scholar]
- 117.Bergamaschi G, Di Sabatino A, Albertini R, Costanzo F, Guerci M, Masotti M, et al. Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis. 2013;19:2166–72. [DOI] [PubMed] [Google Scholar]
- 118.Pasricha S-R, Atkinson SH, Armitage AE, Khandwala S, Veenemans J, Cox SE, et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci Transl Med. 2014;6:235re3. [DOI] [PubMed] [Google Scholar]
- 119.Shu T, Jing C, Lv Z, Xie Y, Xu J, Wu J. Hepcidin in tumor-related iron deficiency anemia and tumor-related anemia of chronic disease: pathogenic mechanisms and diagnosis. Eur J Haematol. 2015;94:67–73. [DOI] [PubMed] [Google Scholar]
- 120.Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, et al. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;119:1922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song S-NJ, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013;15:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Casper C, Chaturvedi S, Munshi N, Wong R, Qi M, Schaffer M, et al. Analysis of Inflammatory and Anemia-Related Biomarkers in a Randomized, Double-Blind, Placebo-Controlled Study of Siltuximab (Anti-IL6 Monoclonal Antibody) in Patients With Multicentric Castleman Disease. Clin Cancer Res. 2015;21:4294–304. [DOI] [PubMed] [Google Scholar]
- 124.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–98. [DOI] [PubMed] [Google Scholar]
- 125.Pfeffer MA, Burdmann EA, Chen C-Y, Cooper ME, de Zeeuw D, Eckardt K-U, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–32. [DOI] [PubMed] [Google Scholar]
- 126.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N Engl J Med. 2019;380:447–58. [DOI] [PubMed] [Google Scholar]
- 127.Macdougall IC, Bhandari S, White C, Anker SD, Farrington K, Kalra PA, et al. Intravenous Iron Dosing and Infection Risk in Patients on Hemodialysis: A Prespecified Secondary Analysis of the PIVOTAL Trial. J Am Soc Nephrol. 2020;31:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Macdougall IC, Bircher AJ, Eckardt K-U, Obrador GT, Pollock CA, Stenvinkel P, et al. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;89:28–39. [DOI] [PubMed] [Google Scholar]
- 129.Cooke KS, Hinkle B, Salimi-Moosavi H, Foltz I, King C, Rathanaswami P, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122:3054–61. [DOI] [PubMed] [Google Scholar]
- 130.Vadhan-Raj S, Abonour R, Goldman JW, Smith DA, Slapak CA, Ilaria RL, et al. A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. J Hematol Oncol. 2017;10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schwoebel F, van Eijk LT, Zboralski D, Sell S, Buchner K, Maasch C, et al. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013;121:2311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hohlbaum AM, Gille H, Trentmann S, Kolodziejczyk M, Rattenstetter B, Laarakkers CM, et al. Sustained plasma hepcidin suppression and iron elevation by Anticalin-derived hepcidin antagonist in cynomolgus monkey: Sustained hepcidin suppression by antagonist. Br J Pharmacol. 2018;175:1054–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Renders L, Budde K, Rosenberger C, van Swelm R, Swinkels D, Dellanna F, et al. First-in-human Phase I studies of PRS-080#22, a hepcidin antagonist, in healthy volunteers and patients with chronic kidney disease undergoing hemodialysis. Eller K, editor. PLoS ONE. 2019;14:e0212023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sheetz M, Barrington P, Callies S, Berg PH, McColm J, Marbury T, et al. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br J Clin Pharmacol. 2019;85:935–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118:4977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petzer V, Tymoszuk P, Asshoff M, Carvalho J, Papworth J, Deantonio C, et al. A fully human anti-BMP6 antibody reduces the need for erythropoietin in rodent models of the anemia of chronic disease. Blood. 2020;136:1080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Steinbicker AU, Sachidanandan C, Vonner AJ, Yusuf RZ, Deng DY, Lai CS, et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117:4915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun CC, Vaja V, Chen S, Theurl I, Stepanek A, Brown DE, et al. A hepcidin lowering agent mobilizes iron for incorporation into red blood cells in an adenine-induced kidney disease model of anemia in rats. Nephrol Dial Transplant. 2013;28:1733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood. 2017;129:1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012;122:4635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shah YM, Matsubara T, Ito S, Yim S-H, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, et al. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140:2044–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen N, Hao C, Liu B-C, Lin H, Wang C, Xing C, et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381:1011–22. [DOI] [PubMed] [Google Scholar]
- 144.Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med. 2019;381:1001–10. [DOI] [PubMed] [Google Scholar]
- 145.Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Reusch M. Phase 3, Randomized, Double-Blind, Active-Comparator (Darbepoetin Alfa) Study of Oral Roxadustat in CKD Patients with Anemia on Hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Akizawa T, Nangaku M, Yonekawa T, Okuda N, Kawamatsu S, Onoue T, et al. Efficacy and Safety of Daprodustat Compared with Darbepoetin Alfa in Japanese Hemodialysis Patients with Anemia: A Randomized, Double-Blind, Phase 3 Trial. Clin J Am Soc Nephrol. 2020;15:1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391:155–67. [DOI] [PubMed] [Google Scholar]
- 148.Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015;126:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ganz T, Jung G, Naeim A, Ginzburg Y, Pakbaz Z, Walter PB, et al. Immunoassay for human serum erythroferrone. Blood. 2017;130:1243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Porter JB, Cappellini MD, Kattamis A, Viprakasit V, Musallam KM, Zhu Z, et al. Iron overload across the spectrum of non-transfusion-dependent thalassaemias: role of erythropoiesis, splenectomy and transfusions. Br J Haematol. 2017;176:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rivella S Iron metabolism under conditions of ineffective erythropoiesis in β-thalassemia. Blood. 2019;133:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, Xie L, et al. Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc Natl Acad Sci U S A. 2013;110:E4922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest. 2010;120:4466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Casu C, Goldberg A, Nemeth E, Ganz T, Gardenghi S, MacDonald B, et al. Treatment With Minihepcidin Peptide Improves Anemia and Iron Overload In a Mouse Model Of Thalassemia Intermedia. Blood. 2013;122:431–431. [Google Scholar]
- 155.Casu C, Chessa R, Liu A, Gupta R, Drakesmith H, Fleming R, et al. Minihepcidins improve ineffective erythropoiesis and splenomegaly in a new mouse model of adult β-thalassemia major. Haematologica. 2020;105:1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taranath R, Bourne G, Zhao L, Frederick B, King C, Liu D. Regulation of Iron Homeostasis By PTG-300 Improves Disease Parameters in Mouse Models for Beta-Thalassemia and Hereditary Hemochromatosis. Blood. 2019;134:3540–3540. [Google Scholar]
- 157.Nai A, Pagani A, Mandelli G, Lidonnici MR, Silvestri L, Ferrari G, et al. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of β-thalassemia. Blood. 2012;119:5021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo S, Casu C, Gardenghi S, Booten S, Aghajan M, Peralta R, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J Clin Invest. 2013;123:1531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schmidt PJ, Toudjarska I, Sendamarai AK, Racie T, Milstein S, Bettencourt BR, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(−/−) mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood. 2013;121:1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Casu C, Aghajan M, Oikonomidou PR, Guo S, Monia BP, Rivella S. Combination of Tmprss6- ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica. 2016;101:e8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pantopoulos K Inherited Disorders of Iron Overload. Front Nutr. 2018;5:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fleming RE, Ponka P. Iron Overload in Human Disease. N Engl J Med. 2012;366:348–59. [DOI] [PubMed] [Google Scholar]
- 163.Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. Diagnosis and management of hemochromatosis: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fitzsimons EJ, Cullis JO, Thomas DW, Tsochatzis E, Griffiths WJH, British Society for Haematology. Diagnosis and therapy of genetic haemochromatosis (review and 2017 update). Br J Haematol. 2018;181:293–303. [DOI] [PubMed] [Google Scholar]
- 165.Kowdley KV, Brown KE, Ahn J, Sundaram V. ACG Clinical Guideline: Hereditary Hemochromatosis. Am J Gastroenterol. 2019;114:1202–18. [DOI] [PubMed] [Google Scholar]
- 166.Ramos E, Ruchala P, Goodnough JB, Kautz L, Preza GC, Nemeth E, et al. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. 2012;120:3829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Corradini E, Schmidt PJ, Meynard D, Garuti C, Montosi G, Chen S, et al. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. 2010;139:1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]