Abstract
Objective
Monochorionic, diamniotic (MCDA) twin pairs are predisposed to various pregnancy complications due to the unique placental angioarchitecture of monochorionicity. Few studies have evaluated the outcomes of weight-discordant MCDA pairs without selective fetal growth restriction (SFGR) or the risk factors for development of SFGR. This study aims to describe the natural history of expectant, noninvasive management of weight-discordant MCDA twins and to evaluate risk factors associated with progression to SFGR.
Study Design
This was a retrospective cohort study at a single, tertiary care center in the United States. All MCDA twins with isolated intertwin weight discordance (ITWD)≥20% diagnosed before 26 weeks’ gestational age (GA) were included. The primary outcome of descriptive analyses was overall pregnancy outcome, incorporating both survival to delivery and GA at delivery, as defined by the North American Fetal Therapy Network. The secondary outcome was SFGR in one twin (defined as estimated fetal weight < 10% for GA) and factors associated with this progression. Only those with fetal ultrasound (US) within 4 weeks of delivery were included in this secondary analysis.
Results
Among 73 MCDA pairs with ITWD, 73% had a good pregnancy outcome, with dual live delivery at a median GA of 33 weeks. Among the 34 pairs with adequate US follow-up, 56% developed SFGR. There were no differences in GA at delivery or discordance at birth between those who did and those who did not develop SFGR. There was a nonsignificant association between increasing ITWD at diagnosis and subsequent development of SFGR.
Conclusion
Expectant, noninvasive management can be considered in MCDA twin pregnancies with ITWD≥20% diagnosed before 26 weeks. This approach is associated with a good pregnancy outcome in the majority of cases, even after the development of SFGR in the smaller twin.
Keywords: monochorionic, unequal placental sharing, growth discordance, discordant MCDA twins
The unique placental angioarchitecture of monochorionicity predisposes monochorionic, diamniotic (MCDA) twin pregnancies to important complications, including twin–twin transfusion syndrome (TTTS), twin anemia-polycythemia sequence, polyhydramnios affecting a recipient-like twin (PART), and unequal placental sharing.1 Although TTTS is familiar to providers and patients, it occurs in only 8 to 10% of MCDA twin pregnancies.2,3 Unequal placental sharing, however, is twice as common, affecting up to 22% of MCDA twin pregnancies.4 Unequal placental sharing can manifest as intertwin weight discordance (ITWD) or frank selective fetal growth restriction (SFGR). While numerous studies have described outcomes for SFGR in MCDA twins, few have evaluated outcomes of ITWD without SFGR or risk factors for development of SFGR.5–7
This retrospective cohort study aims to describe the natural history of expectant, noninvasive management for weight-discordant MCDA twin pregnancies and to evaluate risk factors associated with progression from ITWD to SFGR. We hypothesized that the majority of weight-discordant MCDA pairs have good pregnancy outcome without fetal intervention, and that the risk of progression to SFGR is associated with the gestational age (GA) and degree of discordance at the time of initial ITWD diagnosis.
Materials and Methods
This was a retrospective cohort study of all MCDA twin pregnancies with weight discordance identified prior to 26 weeks’ GA at the University of California San Francisco (UCSF) Fetal Treatment Center from January 2008 to July 2019. Inclusion criteria were MCDA twin pairs diagnosed with ITWD greater than or equal to 20%, based on sonographic estimated fetal weight (EFW)8 and the standard formula as recommended by the American College of Obstetricians and Gynecologists and the Royal College of Obstetricians and Gynaecologists9,10:
| (1) |
Exclusion criteria were the presence of TTTS or PART, demise of one twin prior to first evaluation, presence of SFGR at first evaluation, major structural abnormalities, known genetic anomaly, and absence of birth outcome data. TTTS and PART were diagnosed according to previously defined criteria,11,12 and SFGR was diagnosed if EFWsmaller twin was less than the 10th percentile for GA by ultrasound (US). We excluded pregnancies > 26 weeks’ GA as this represents the typical GA cutoff for fetal intervention (e.g., selective reduction or selective fetoscopic laser photocoagulation).
The primary outcome of this study was good, mixed, or poor pregnancy outcome of weight-discordant MCDA twins. These categories of pregnancy outcomes were as defined by the North American Fetal Therapy Network.13 A good outcome was defined as dual live delivery at or beyond 30.0 weeks’ GA; a mixed outcome was defined as loss of one twin or dual live delivery between26.0 and 29.9 weeks’ GA; and a poor outcome was defined as loss of both twins or dual live delivery before26.0 weeks’ GA. Those who experienced spontaneous loss of one twin and elected to terminate the remaining pregnancy were categorized as having a mixed outcome. The secondary outcome of this study was SFGR in one twin and factors associated with this progression, including maternal age, GA at the time of ITWD diagnosis, degree of ITWD at initial diagnosis, presence of oligohydramnios at initial diagnosis (defined as deepest vertical pocket < 2 cm), presence of absent or reversed end-diastolic flow (AREDF) in the umbilical artery (UA) at initial diagnosis, presence of arterioarterial (AA) anastomosis by Doppler US, and discordant cord insertion sites. Defined as one normal cord insertion and one marginal or velamentous cord insertion, discordance in cord insertion sites has previously been reported as a proxy for unequal placental sharing and has been shown to correlate with weight discordance in twin pairs.4
All MCDA twin pregnancies referred to the UCSF Fetal Treatment Center underwent detailed sonographic evaluation, including complete anatomic survey, calculation of EFW, identification of placental cord insertion site, UA Doppler evaluation, and Doppler interrogation for presence of AA anastomosis. Using targeted color and spectral Doppler interrogation, sonographers identified an AA anastomosis as a vessel coursing along the fetal surface of the placenta, between cord insertion sites, with pulsatile bidirectional flow.14 This procedure is routinely performed for all MCDA twin pairs evaluated at the UCSF Fetal Treatment Center, based on the understanding that this intertwin connection has important implications for MCDA complications.15,16 Patients seen at this site typically have serial sonograms every 1 to 4 weeks, with exact frequency determined based on the presence of pathology, maternal comorbidities, and other factors. If serial sonograms demonstrated clinical stability, the patient returned to the referring provider, with decisions regarding continue surveillance, re-referral, admission, and delivery at the discretion of the referring provider.
The UCSF Fetal Treatment Center database was queried and the UCSF electronic medical system reviewed by a single Maternal-Fetal Medicine provider (N.C.S.) to extract the variables for this study, including maternal demographics (age, parity, tobacco use, and use of assisted reproductive technology [ART]), US findings (EFW, placental cord insertion site, AREDF in the UA, and presence of AA anastomosis), and delivery details (GA at delivery and neonatal birth weight [BW]). Many patients referred to the UCSF Fetal Treatment Center for consultation and sonographic evaluation ultimately returned to their primary providers for delivery. For those patients who delivered elsewhere, the Fetal Treatment Center staff (including K.A.G.) directly contacted patients and their primary providers to obtain information regarding delivery details and neonatal outcomes.
Fisher’s exact test compared proportions for categorical variables, and Kruskal–Wallis’ test compared median values for nonparametric continuous variables. For analyses of the secondary outcome (SFGR and risk factors for progression), we included only those MCDA pairs with adequate US information to serially assess EFW until delivery, defined as last UCSF US within 4 weeks of delivery since the greatest time point between serial growth US in the UCSF Fetal Treatment Center was 4 weeks. Those with an interval of more than 4 weeks between last growth US and date of delivery were deemed as having inadequate US to assess for development of SFGR and were subsequently excluded from this analysis. Multivariable logistic regression generated odds ratios (ORs) for development of SFGR, adjusting for GA at the time of ITWD diagnosis, degree of ITWD at initial diagnosis, presence of AA anastomosis, discordant cord insertion sites, presence of oligohydramnios at initial diagnosis, presence of AREDF at initial diagnosis, and maternal age. Statistical significance was set at a p-value of < 0.05 or 95% confidence interval (CI) not overlapping 1.0 for multivariable analyses. All statistical analyses were performed using STATA v13.0 (StataCorp, College one normally grown twin). At presentation, 26% had evidence of AREDF in the UA, and 3% had oligohydramnios. The vast majority of the 73 weight-discordant MCDA pairs had a good outcome with expectant management, with dual live twin delivery at or beyond 30 weeks occurring in 53 cases (74%, Fig. 2). Only three pregnancies (4%) ended in a poor outcome of delivery before 26 weeks. Seventeen (23%) experienced a mixed outcome of delivery between 26 and 30 weeks (14) or loss of one twin (3). All three cases with the loss of one twin involved spontaneous intrauterine fetal demise (IUFD) of one twin followed by termination of the surviving twin. In the first of these cases, IUFD was diagnosed at 21.0 weeks in the normally grown twin, with no clear etiology for the demise. In the second case, IUFD was diagnosed at 25.9 weeks in the smaller twin with SFGR and AEDF. In the third case, IUFD was diagnosed at 23.0 weeks in the smaller twin with anhydramnios, SFGR with ITWD of 60%, and abnormal Doppler indices including AEDF, deepening of the a-wave in the ductus venosus, and notching in the umbilical vein. Among the 70 with ongoing pregnancies, the median GA at delivery was 33 weeks (IQR: 30.4–35.0), with a median BW discordance of 28% (IQR: 20.1–35.1). The median latency from diagnosis of ITWD to delivery was 81 days (IQR: 60–102).
Fig. 2.
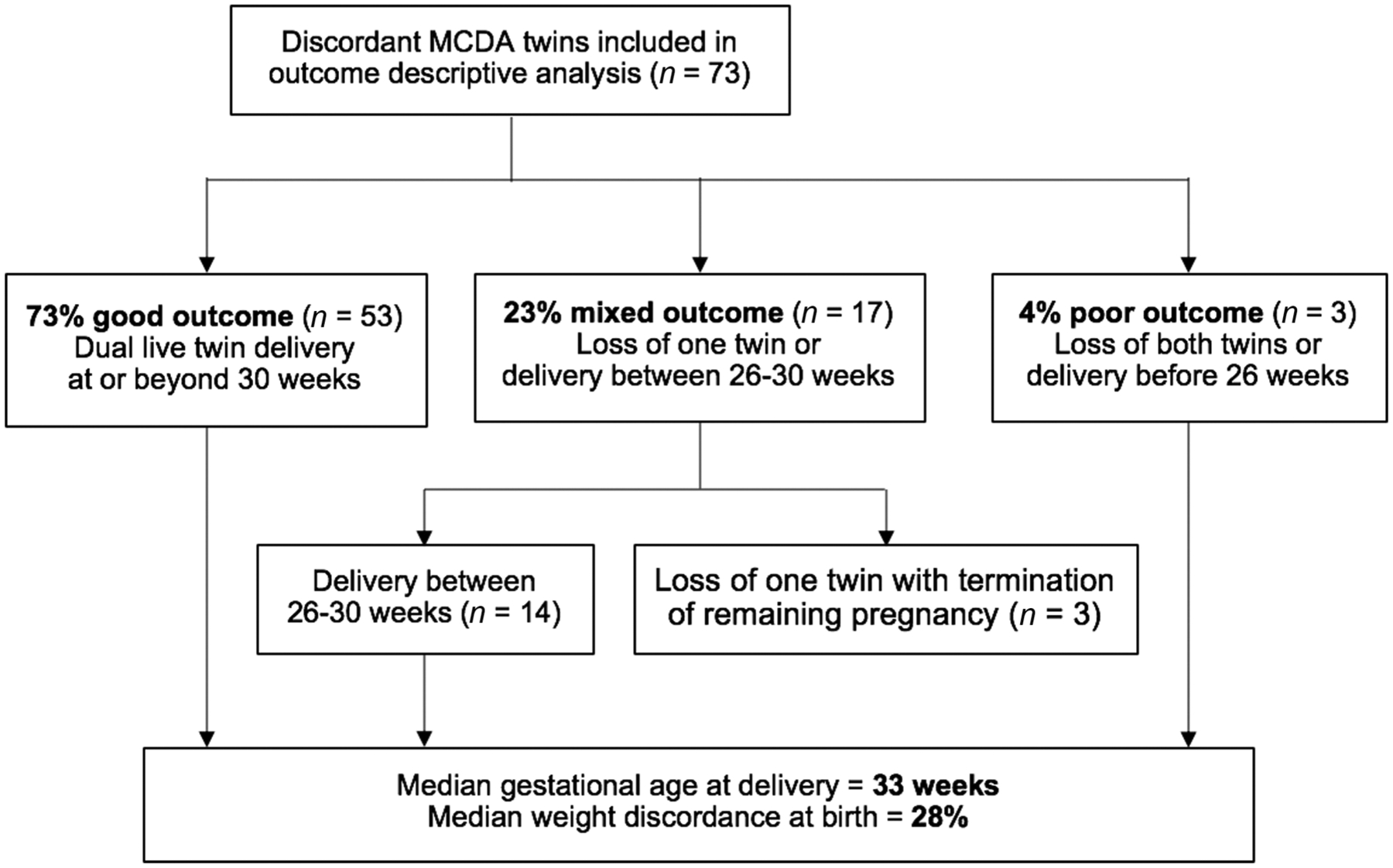
Overall pregnancy outcomes among monochorionic, diamniotic (MCDA) twin pregnancies diagnosed with intertwin weight discordance≥20% before 26 weeks of gestation.
Of the 70 continuing pregnancies, 36 had insufficient US data to assess for development of SFGR. Nineteen of the remaining 34 cases (56%) developed SFGR, with a median GA of 27 weeks (IQR: 23.7–33.1) at the time of SFGR diagnosis. The median latency from diagnosis of SFGR to delivery was Station, TX). This study was approved by the UCSF Committee on Human Research (study number 17–23011, approval date September 14, 2017).
Results
Between January 2008 and July 2019, 607 MCDA twin pregnancies were evaluated at the UCSF Fetal Treatment Center with at least one detailed US (Fig. 1). A total of 518 were excluded, due to TTTS on initial evaluation (282), PART on initial evaluation (106), absence of weight discordance (58), major structural abnormality (35), absence of birth outcome data(17), demise of one twin prior to first UCSF evaluation (6), presence of SFGR on first UCSF evaluation (10), or diagnosis of weight discordance after 26 weeks of gestation (4). Among the 89 remaining weight-discordant MCDA twin pairs, 4 MCDA pairs went on to develop TTTS, and 9 pairs went on to develop PART. These pregnancies were excluded from subsequent analyses. Three patients, who elected for termination of pregnancy after the pregnancy was diagnosed with ITWD≥20%, were also excluded from subsequent analyses.
Fig. 1.
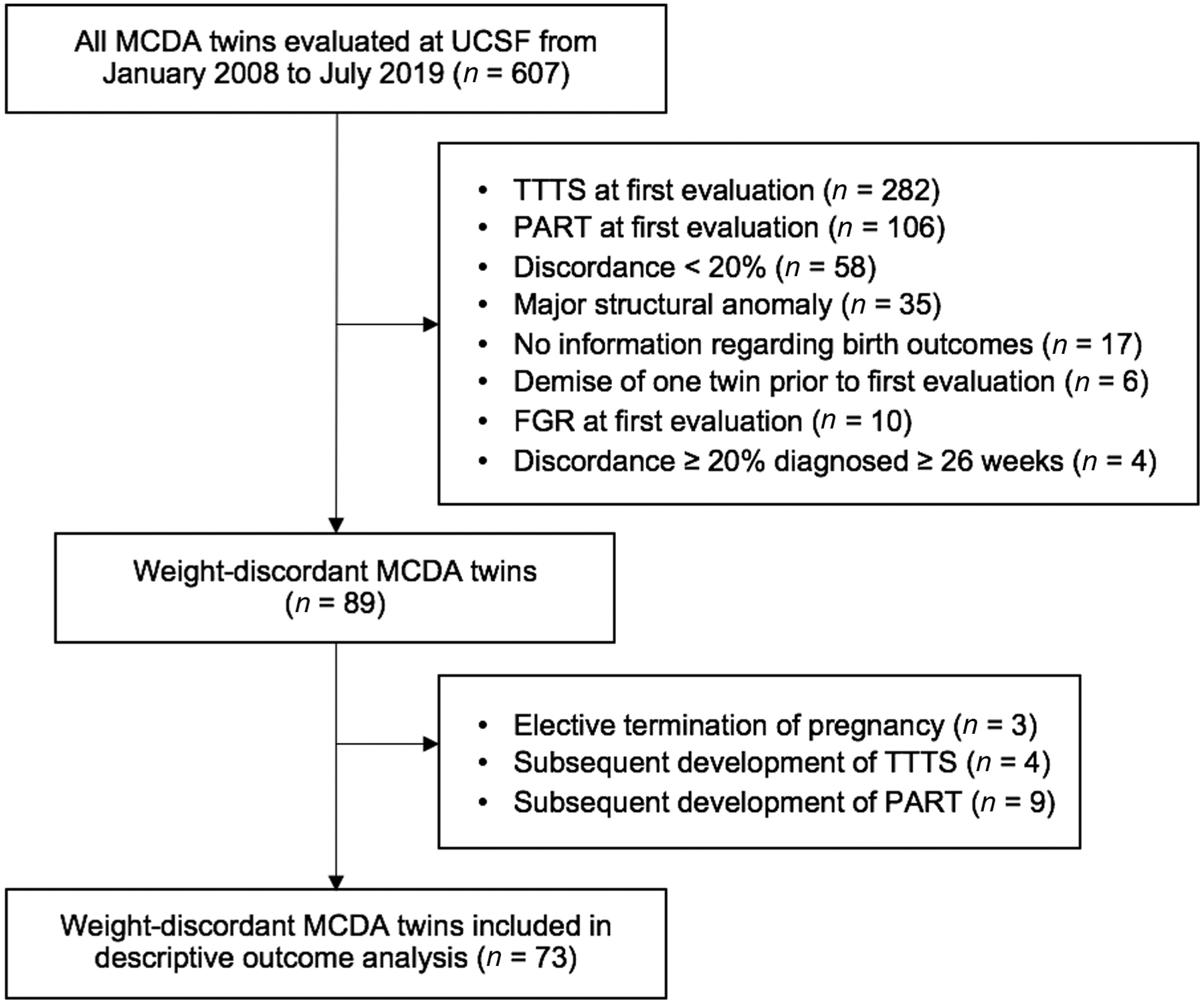
Flow diagram of MCDA twin pregnancies included in this study. FGR, fetal growth restriction; MCDA, monochorionic, diamniotic; PART, polyhydramnios affecting a recipient-like twin; TTTS, twin–twin transfusion syndrome; UCSF, University of California San Francisco.
Among the remaining 73 weight-discordant MCDA pairs, the median GA at the time of initial ITWD diagnosis was 21 weeks (interquartile range [IQR]: 18.4–22.1) and the median degree of ITWD was 28% (IQR: 24.4–33.0). In all cases, ITWD was due to one normally grown twin and one smaller twin (rather than one excessively grown twin and 38 days (IQR: 7–65). There were no statistically significant differences in maternal age, nulliparity, tobacco use, ART, presence of AA anastomosis, presence of oligohydramnios at diagnosis, or discordant cord insertion sites between those who developed SFGR and those who did not (Tables 1 and 2). There was a higher rate of AREDF at initial diagnosis in those who did not develop SFGR (5% with SFGR vs. 33% without SFGR, p = 0.03), although this finding was no longer statistically significant on multivariate analysis. The majority of cases in both groups developed AREDF in the UA of the smaller twin at some point in pregnancy, but there was no significant difference in the rate of AREDF between the groups (53% with SFGR vs. 67% without SFGR, p= 0.50).
Table 1.
Characteristics of overall cohort and groups by development of SFGR
| All discordant MCDA twins (n = 73) | By development of SFGRa | |||
|---|---|---|---|---|
| SFGR present (n = 19) | SFGR absent (n = 15) | p-Value | ||
| Maternal age, y | 32 (27.5–35.2) | 33 (27.9–40.0) | 31 (25.8–35.3) | 0.32 |
| Nulliparity | 38 (52%) | 10 (53%) | 8 (53%) | > 0.99 |
| Tobacco useb | 1 (2%) | 0 | 0 | - |
| Spontaneous twin pregnancyc | 13 (50%) | 2 (22%) | 3 (50%) | 0.33 |
| GA at diagnosis of ITWD, wk | 21 (18.4–22.1) | 21 (18.4–23.0) | 21 (19.3–23.1) | 0.40 |
| GA at deliveryd, wk | 33 (30.4–35.0) | 34 (32.3–35.3) | 32 (27.6–34.9) | 0.19 |
Abbreviations: GA, gestational age; ITWD, intertwin weight discordance; MCDA, monochorionic diamniotic; SFGR, selective fetal growth restriction. Note: Continuous variables presented as median (interquartile range) and categorical variables as n (%).
n = 34 for this analysis due to inclusion of only those with adequate UCSF ultrasound follow-up to assess for development of SFGR (defined as last UCSF ultrasound within 4 weeks of delivery).
n=41 for all and n=25 for SFGR comparison due to missing data regarding tobacco use.
n = 26 for all and n = 15 for SFGR comparison due to missing data regarding use of assisted reproductive technology.
n = 70 for all due to exclusion of three pregnancies with spontaneous intrauterine fetal demise followed by elective termination of pregnancy.
Table 2.
Sonographic findings of overall cohort and groups by development of SFGR
| All discordant MCDA twins (n = 73) | By development of SFGRa | |||
|---|---|---|---|---|
| SFGR present (n = 19) | SFGR absent (n = 15) | p-Value | ||
| AA anastomosis presentb | 51 (76%) | 14 (82%) | 12 (80%) | > 0.99 |
| Normal twin with central cord insertionc | 69 (97%) | 18 (100%) | 14 (93%) | 0.46 |
| Small twin with marginal/velamentous cord insertiond | 56 (78%) | 15 (79%) | 12 (80%) | > 0.99 |
| Discordant cord insertion sitese | 55 (77%) | 14 (78%) | 13 (87%) | 0.67 |
| ITWD at presentation, % | 28 (24.4–33.0) | 31 (24.0–39.0) | 28 (24.4–31.8) | 0.11 |
| Oligohydramnios at presentation | 2 (3%) | 1 (5%) | 0 | 0.37 |
| AREDF in umbilical artery at presentation | 19 (26%) | 1 (5%) | 5 (33%) | 0.03 |
| AREDF in umbilical artery at any timef | 34 (67%) | 10 (53%) | 10 (67%) | 0.50 |
Abbreviations: AA, arterioarterial; AREDF, absent or reversed end-diastolic flow; ITWD, intertwin weight discordance; MCDA, monochorionic diamniotic; SFGR, selective fetal growth restriction.
Note: Continuous variables presented as median (interquartile range) and categorical variables as n (%).
n = 34 for this analysis due to inclusion of only those with adequate UCSF ultrasound follow-up to assess for development of SFGR (defined as last UCSF ultrasound within 4 weeks of delivery).
n = 67 for all and n= 32 for SFGR comparison due to missing data regarding AA anastomoses.
n = 71 for all and n = 33 for SFGR comparison due to missing data regarding cord insertion.
n = 72 for all due to missing data regarding cord insertion.
n = 71 for all and n = 33 for SFGR comparison due to missing data regarding cord insertion.
n = 51 for all due to inclusion of only those with adequate UCSF ultrasound follow-up to assess for this finding (defined as last UCSF ultrasound within 4 weeks of delivery).
Similarly, there were no significant differences between the groups in GA at initial diagnosis of ITWD or degree of discordance at initial diagnosis. With regard to outcomes, there were no significant differences in overall pregnancy outcome, GA at delivery, or degree of BW discordance between those who did and those who did not develop SFGR (Table 3). On logistic regression, each additional 10% of ITWD was associated with a nearly threefold increase in the risk of developing SFGR, a finding that approached but did not reach statistical significance (OR: 2.9, 95% CI: 0.87–9.81, p = 0.08). This finding remained similar when adjusting for GA at ITWD diagnosis, degree of ITWD at initial diagnosis, presence of AA anastomosis, discordant cord insertion sites, presence of oligohydramnios at initial diagnosis, presence of AREDF at initial diagnosis, and maternal age (adjusted OR:3.4, 95% CI: 0.74–15.63, p = 0.12).
Table 3.
Outcomes of overall cohort and groups by development of SFGR
| All discordant MCDA twins (n = 70) | By development of SFGRa | |||
|---|---|---|---|---|
| SFGR present (n = 19) | SFGR absent (n = 15) | p-Value | ||
| Pregnancy outcomeb | ||||
| Good (dual delivery ≥ 30.0 wk) | 53 (73%) | 17 (89%) | 10 (67%) | 0.10 |
| Mixed (single loss or delivery 26.0–29.9 wk) | 17 (23%) | 2 (11%) | 5 (33%) | |
| Poor (dual loss or delivery < 26.0 wk) | 3 (4%) | 0 | 0 | |
| GA at delivery, wk | 33 (30.4–35.0) | 34 (32.3–35.3) | 32 (27.6–34.9) | 0.19 |
| BW discordance, % | 28 (20.1–35.1) | 29 (22.2–43.8) | 23 (13.4–30.2) | 0.12 |
| Latency from ITWD to delivery, d | 81 (60–102) | 88 (67–109) | 78 (47–101) | 0.18 |
| Latency from SFGR to deliveryc, d | 38 (7–65) | - | - | - |
| Latency from AREDF to deliveryd, d | 51 (40–78) | - | - | - |
Abbreviations: AREDF, absent or reversed end-diastolic flow; BW, birth weight; GA, gestational age; ITWD, intertwin weight discordance; MCDA, monochorionic, diamniotic; SFGR, selective fetal growth restriction.
Note: Continuous variables presented as median (interquartile range) and categorical variables as n (%).
n = 34 for this analysis due to inclusion of only those with adequate UCSF ultrasound follow-up to assess for development of SFGR (defined as last UCSF ultrasound within 4 weeks of delivery).
For this primary outcome, all n = 73 discordant MCDA twins were included. The remaining outcomes included n = 70, excluding the 3 who opted for termination of pregnancy following spontaneous intrauterine fetal demise of one twin.
Latency among the n = 19 who developed SFGR.
Latency among the n = 32 who developed AREDF
Discussion
At a single referral center, the majority of MCDA twins with ITWD≥20% before 26 weeks had a good pregnancy outcome with noninvasive, expectant management, with dual live delivery at a median GA of 33 weeks. Although > 50% of those with ITWD progressed to SFGR, there were no differences in GA at delivery or BW discordance between those who did and those who did not develop SFGR. There was a nonsignificant association between increasing ITWD at diagnosis and subsequent development of SFGR.
Prior reports have demonstrated increased morbidity and mortality among MCDA twins with ITWD, although it is unclear whether these risks are limited to those with concomitant SFGR or whether the risks extend to those with ITWD alone.5–7,17 We are reassured by our finding of a good outcome in the majorityofcases with ITWD. The process underlying this positive outcome is likely related to the complex angioarchitecture of the monochorionic placenta. In-depth placental studies demonstrate that ITWD is characterized by multiple arteriovenous (AV) anastomoses, with a paucity of superficial AA and venovenous anastamoses.18 These connections may serve to enhance blood flow toward the smaller twin, thus offsetting the effects of its smaller placental share. Using placental injection studies, Denbow et al found that BW discordance correlated with placental territory discordance and with degree of balance in AV anastomoses. They also reported a phenomenon of “rescue transfusion” in cases of ITWD, whereby the smaller twin (with its smaller placental territory) increases its functional placental share by recruiting blood flow from its cotwin via AV anastomoses.15 Given the suspected importance of these intertwinvascularconnections, caution should be taken when considering selective fetoscopic laser photocoagulation for ITWD, an intervention that has been utilized by some groups.19,20 The dichorionization effect of selective fetoscopic laser photocoagulation aims to reduce or eliminate intertwin vascular anastomoses, potentially disrupting connections that the smaller twin has “recruited” for support, thus leaving thesmaller twin with an extremely small portion of the monochorionic placenta and with subsequent risk of FGR or even IUFD. We similarly advise caution when considering selective reduction of the smaller twin with the intention to protect the normally grown twin from the risk of neurologic morbidity or even mortality that accompanies spontaneous IUFD of its cotwin.21,22 Selective reduction for this indication is not benign and can be associated with a dual twin loss rate of up to 56%,23 which is considerably higher than the rate of dual twin loss reported in the present series. In fact, we report zero cases of spontaneous dual twin loss; any dual twin loss was the result of a single IUFD followed by elective termination of the remaining pregnancy. Furthermore, spontaneous IUFD of the smaller twin occurred in only 2 of the 73 cases of ITWD in our series, corresponding to a rate of less than 3%. These rates should be incorporated into counseling of those who are considering selective reduction for ITWD.
Increasing degree of ITWD at initialdiagnosis appeared to be associated with development of SFGR, but this finding did not reach statistical significance, likely due to small sample size. We suspect, however, that this finding is real, since the vast majorityof the associated CI lies above 1 (for every 10% increase in ITWD, SFGR OR: 2.9, 95% CI: 0.87–9.81 and adjusted OR: 3.4, 95% CI: 0.74–15.63). We were otherwise unable to identify predictors for development of SFGR among these MCDA twin pairs and thus are unable to explain why some ITWD pairs develop SFGR while others do not. However, there were no differences in pregnancy outcome between those who did and those who did not develop SFGR. The development of SFGR in a MCDA pair with ITWD may be due to differential placental growth across gestation. Baumann et al demonstrated that monochorionic placentas reach maximal growth potential in the third trimester, with limited growth of the placenta beyond 26 weeks.24 This timing coincides with our finding that SFGR developed at a median GA of 27 weeks. It is possible that the plasticity and growth of the monochorionic placenta reaches a “tipping point” beyond which it is no longer able to sustain adequate growth of the smaller twin in the cases that develop SFGR, although further studies are needed to support this theory.
This study is limited by its observational, retrospective design and by its small sample size. The latter, however, is to be expected for a rare complication (ITWD) of a rare condition (MCDA twin pregnancy), and the present sample size is considerably larger than other similar studies.17 Although the single-center nature of this study may limit generalizability to other centers, this design ensured standardized diagnosis, monitoring, and management of a particular patient population and thus minimized inconsistencies in practice patterns that could have confounded the outcomes of interest. As a study conducted at a tertiary referral center, there is the potential for referral bias, and cases with more mild presentations and/or more stable courses may have been missed. In this situation, our referred cohort would represent the most severe cases with the highest likelihood of adverse outcome. Despite this, 73% had a good pregnancy outcome with dual live delivery≥30 weeks’ GA. We would thus expect the nonreferred cases to have an even higher rate of good pregnancy outcome, although further studies are needed to confirm this. The use of a referral center also subjects this study to limitations related to loss to follow-up, as many patients delivered at other institutions. However, our center has a systematic method of collecting delivery and immediate neonatal outcomes for patients who deliver elsewhere, and we found that this follow-up was missing for only 2% of all MCDA twin referrals during the study period. Nevertheless, this follow-up does not include detailed outcomes regarding specific neonatal complications such as respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and longer term neonatal events. Our status as a referral center also limited our sample size in the secondary analysis of development of SFGR. Many patients initially evaluated at our institution ultimately returned to their local, referring providers for care in the later part of pregnancy, and decisions regarding continued US surveillance during that time were at the discretion of those providers. Without access to the medical records at our many referring sites, we were unable to include MCDA pregnancies that did not undergo US at our site within 4 weeks of delivery, even though they may have received an US with a local provider closer to the time of delivery. Another limitation of this study is the absence of postnatal placental angioarchitecture evaluation.
Affecting nearly one in four MCDA pregnancies, unequal placental sharing is a relativelycommon complication of monochorionicity that manifests prenatally as ITWD.4 In our cohort of MCDA pairs with ITWD, the majority had a good immediate pregnancyoutcome, with dual livetwin deliveryat a median GA of 33 weeks, even in the setting of SFGR and/or AREDF in the UA of the smaller twin. Importantly, this outcome occurred in the setting of noninvasive, expectant management, which avoids the risks inherent to fetal intervention. To our knowledge, this is the largest reported cohort of noninvasive, expectant management of ITWD in MCDA twin pairs without concomitant complications. Providers caring for pregnancies complicated by isolated ITWD can incorporate our findings into clinical counseling and provide reassurance to expectant parents that most cases experience a good immediate pregnancy outcome, as defined by survival to delivery and GA at delivery.
Key Points.
Nearly 75% of weight-discordant mo/di twins have a good pregnancy outcome.
Weight-discordant mo/di twins deliver at a mean gestational age of 33 weeks without invasive therapy.
Noninvasive management should be considered for weight-discordant mo/di twins.
Acknowledgments
A portion of these data was presented in poster format at the 39th Annual Meeting of the Society for Maternal-Fetal Medicine (SMFM) in Las Vegas, NV, February 16, 2019.
Funding
T.N.S. is supported by grant 5K12HD001262-18 from the National Institutes of Health (NIH). The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
This study was approved by the University of California San Francisco (UCSF) Committee on Human Research (study number 17–23011, approval date September 14, 2017).
Conflict of Interest
None declared.
References
- 1.Rand L, Lee H. Complicated monochorionic twin pregnancies: updates in fetal diagnosis and treatment. Clin Perinatol 2009;36(02):417–430, x–xi [DOI] [PubMed] [Google Scholar]
- 2.Acosta-Rojas R, Becker J, Munoz-Abellana B, Ruiz C, Carreras E, Gratacos ECatalunya and Balears Monochorionic Network. Twin chorionicity and the risk of adverse perinatal outcome. Int J Gynaecol Obstet 2007;96(02):98–102 [DOI] [PubMed] [Google Scholar]
- 3.Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008;199(05):514. e1–514.e8 [DOI] [PubMed] [Google Scholar]
- 4.Fick AL, Feldstein VA, Norton ME, Wassel Fyr C, Caughey AB, Machin GA. Unequal placental sharing and birth weight discordance in monochorionic diamniotic twins. Am J Obstet Gynecol 2006;195(01):178–183 [DOI] [PubMed] [Google Scholar]
- 5.Breathnach FM, McAuliffe FM, Geary M, et al. ;Perinatal Ireland Research Consortium. Definition of intertwin birth weight discordance. Obstet Gynecol 2011;118(01):94–103 [DOI] [PubMed] [Google Scholar]
- 6.Khalil AA, Khan N, Bowe S, et al. Discordance in fetal biometry and Doppler are independent predictors of the risk of perinatal loss in twin pregnancies. Am J Obstet Gynecol 2015;213(02):222. e1–222.e10 [DOI] [PubMed] [Google Scholar]
- 7.D’Antonio F, Odibo AO, Prefumo F, et al. Weight discordance and perinatal mortality in twin pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;52(01):11–23 [DOI] [PubMed] [Google Scholar]
- 8.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 1991;181(01): 129–133 [DOI] [PubMed] [Google Scholar]
- 9.Hayes EJCommittee on Practice Bulletins—Obstetrics Society for Maternal–Fetal Medicine. Practice bulletin 169: multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol 2016;128(04):e131–e146 [DOI] [PubMed] [Google Scholar]
- 10.Kilby MD, Bricker Lon behalf of the Royal College of Obstetricians and Gynaecologists. Management of monochorionic twin pregnancy. Br J Obstet Gynaecol 2016;124:e1–e45 [Google Scholar]
- 11.Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin-twin transfusion syndrome. J Perinatol 1999;19(8 Pt 1):550–555 [DOI] [PubMed] [Google Scholar]
- 12.Washburn EE, Sparks TN, Gosnell KA, Rand L, Gonzalez JM, Feldstein VA. Polyhydramnios affecting a recipient-like twin: risk of progression to twin-twin transfusion syndrome and outcomes. Am J Perinatol 2018;35(04):317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery SP, Hasley SK, Catov JM, et al. ;North American Fetal Therapy Network. North American Fetal Therapy Network: intervention vs expectant management for stage I twin-twin transfusion syndrome. Am J Obstet Gynecol 2016;215(03):346.e1–346.e7 [DOI] [PubMed] [Google Scholar]
- 14.Taylor MJ, Denbow ML, Tanawattanacharoen S, Gannon C, Cox PM, Fisk NM. Doppler detection of arterio-arterial anastomoses in monochorionic twins: feasibility and clinical application. Hum Reprod 2000;15(07):1632–1636 [DOI] [PubMed] [Google Scholar]
- 15.Denbow ML, Cox P, Taylor M, Hammal DM, Fisk NM. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol 2000;182(02):417–426 [DOI] [PubMed] [Google Scholar]
- 16.Zhao DP, de Villiers SF, Slaghekke F, et al. Prevalence, size, number and localization of vascular anastomoses in monochorionic placentas. Placenta 2013;34(07):589–593 [DOI] [PubMed] [Google Scholar]
- 17.Harper LM, Weis MA, Odibo AO, Roehl KA, Macones GA, Cahill AG. Significance of growth discordance in appropriately grown twins. Am J Obstet Gynecol 2013;208(05):393.e1–393.e5 [DOI] [PubMed] [Google Scholar]
- 18.Bajoria R Vascular anatomy of monochorionic placenta in relation to discordant growth and amniotic fluid volume. Hum Reprod 1998;13(1O):2933–2940 [DOI] [PubMed] [Google Scholar]
- 19.Chmait RH, Korst LM, Bornick PW, Allen MH, Quintero RA. Fetal growth after laser therapy for twin-twin transfusion syndrome. Am J Obstet Gynecol 2008;199(01):47.e1–47.e6 [DOI] [PubMed] [Google Scholar]
- 20.Maschke C, Franz AR, Ellenrieder B, Hecher K, Diemert A, Bartmann P. Growth after intrauterine laser coagulation for twin-twin transfusion syndrome. Arch Dis Child Fetal Neonatal Ed 2010;95(02):F115–F117 [DOI] [PubMed] [Google Scholar]
- 21.Ong SSC, Zamora J, Khan KS, Kilby MD. Prognosis for the co-twin following single-twin death: a systematic review. BJOG 2006;113(09):992–998 [DOI] [PubMed] [Google Scholar]
- 22.Shek NWM, Hillman SC, Kilby MD. Single-twin demise: pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2014;28(02): 249–263 [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Zou G, Yang Y, Zhou F, Tao D. Risk factors for fetal death after radiofrequency ablation for complicated monochorionic twin pregnancies. Prenat Diagn 2018;38(07):499–503 [DOI] [PubMed] [Google Scholar]
- 24.Baumann MU, Marti M, Durrer L, et al. Placental plasticity in monochorionic twins: Impact on birth weight and placental weight. Placenta 2015;36(09):1018–1023 [DOI] [PubMed] [Google Scholar]


