Abstract
Objectives:
To determine whether the Memorial Sloan Kettering Frailty Index (MSK-FI) is associated with decision-making in older women surgically treated for advanced-stage ovarian cancer.
Methods:
We retrospectively applied the MSK-FI to women ≥70 years with newly diagnosed advanced-stage ovarian cancer surgically treated at our institution from 01/2001–05/2017. MSK-FI components, including 10 comorbidities and functional assessment, were extracted from medical records. The MSK-FI ranges from 0–11, with higher scores indicating greater frailty. The primary outcome was the association between frailty and rate of primary debulking surgery (PDS), for which a multivariable logistic regression was used, adjusted for stage and histology.
Results:
We identified 430 women treated with PDS (n=231, 54%) or neoadjuvant chemotherapy/interval debulking (n=199, 46%) with complete data. MSK-FI score distribution was: “0”, 95 patients (22%); “1”, 172 (40%); “2”, 89 (21%); and “3+”, 74 (17%). More-frail patients were less likely to have undergone PDS (OR for a unit increase of MSK-FI: 0.64; 95%CI, 0.53–0.77; p<0.0001). Grade 3+ complications and unintended intensive care admission occurred in 40 (9%) and 38 (9%) women, respectively, but were not associated with frailty (OR 1.21; 95%CI, 0.96–1.52; p=0.11). More-frail patients were more likely to delay postoperative chemotherapy (non-linear association p=0.009) and less likely to enroll in research (OR 0.84; 95%CI, 0.70–1.00; p=0.049). Greater frailty was associated with poorer overall survival (HR 1.16; 95%CI, 1.05–1.30; p=0.005).
Conclusions:
Frailty, as calculated by the MSK-FI, is strongly associated with treatment approach in older women with advanced ovarian cancer, suggesting objective or subjective correlates of the MSK-FI influence decision-making.
Keywords: MSK-FI, frailty index, frailty, ovarian cancer, surgery, neoadjuvant chemotherapy
Introduction
An estimated 75% of patients with newly diagnosed ovarian cancer will present with advanced-stage disease. These women are treated with either upfront primary debulking surgery (PDS) followed by postoperative platinum-based chemotherapy or with neoadjuvant platinum-based chemotherapy (NACT) followed by interval debulking surgery (IDS) and further platinum-based postoperative chemotherapy. The goal of either PDS or IDS is complete gross resection of all visible disease, which in some cases requires extensive surgical effort.1,2 There is evidence to suggest that IDS compared with PDS requires less surgical effort, leading some surgeons to prefer this approach.3,4 Several prospective randomized trials have not found a difference in outcomes between women treated with PDS and NACT/IDS5–7; however, numerous retrospective studies and one randomized trial have shown improved survival in women who undergo PDS,8–11 albeit none of these studies are without flaws and biases.
The choice between PDS and NACT/IDS relies heavily on two factors – (1) the extent of disease spread and whether a complete gross resection can be achieved, and (2) the fitness of the patient to withstand an extensive surgery as determined by the treating physician. An increasing number of tools have been developed to help gynecologic oncologists assess resectability of disease,12–14 yet there is a paucity of data on how to effectively evaluate surgical fitness, especially in the elderly population. Despite this triage gap, data have shown improved survival in appropriately selected older women treated with PDS, even when extensive resection is required, albeit at increased risk of morbidity and mortality.14,15 Understanding surgical parameters for older patients with ovarian cancer is imperative, as the median age at diagnosis is 65 years and the overall population continues to age.16,17
We believe it is time to shift away from the debate of PDS vs. NACT/IDS and focus on appropriate patient selection for PDS with complete gross resection in order to maximize the number of women who can undergo PDS safely. In order to accomplish this, however, we must develop and use reliable and objective tools to quantify fitness and frailty, something that has been lacking from the majority of both randomized and retrospective studies thus far. We need tools that can be used by surgeons to objectively evaluate fitness in everyday practice.
We recently published a paper that described the development of the Memorial Sloan Kettering Frailty Index (MSK-FI), which takes into account 10 areas of comorbidity and includes a functional assessment.18 The objective of this study was to evaluate the association between the MSK-FI and treatment decision-making in older women with advanced ovarian cancer treated with surgery. Our primary aim was to determine the association between frailty, as determined by the MSK-FI, and treatment with PDS. We hypothesized that patients considered more frail would be less likely to undergo PDS and would be offered NACT/IDS instead. As a secondary aim, we sought to assess whether frailty was associated with enrollment in any research study or treatment-based trial.
METHODS
After obtaining Institutional Review Board approval, we identified all women 70 years of age or older with newly diagnosed advanced-stage epithelial ovarian cancer surgically treated with either PDS or IDS at our institution between January 2001 and May 2017, when a change in data collection methods occurred. The age bracket of 70 and older was chosen because of institutional guidelines for enhanced screening for frailty in this population. Women treated with chemotherapy alone were excluded. Variables extracted from the medical record included date of diagnosis, age at diagnosis, date of recurrence, date of last follow-up or death, stage, grade, histology, treatment approach (PDS vs. NACT/IDS), date of surgery, residual disease after surgery, postoperative admission to the intensive care unit (ICU), discharge location (home, home with services, rehabilitation), 30-day postoperative complications, date of postoperative chemotherapy initiation, and clinical trial participation.
To retrospectively apply the MSK-FI to the cohort, components of the MSK-FI were extracted from the medical record; these included the following 10 areas of comorbidity, which were identified using International Classification of Disease codes: (1) chronic obstructive pulmonary disease or pneumonia within 30 days of surgery, (2) diabetes mellitus, (3) congestive heart failure, (4) myocardial infarction, (5) coronary artery disease, (6) hypertension, (7) peripheral vascular disease, (8) impaired sensorium including Alzheimer disease, delirium, dementia, Lewy body disease, mild cognitive impairment, and memory loss, (9) cerebrovascular accident, and (10) transient ischemic attack. In addition, we performed a functional assessment based on four patient-reported activities of daily living (bathing, dressing, grooming, and walking outside the home) and one instrumental activity of daily living (preparing meals), which are collected for all patients on the institutional adult intake form. The MSK-FI score ranges from 0 to 11, with a higher score indicating a higher level of frailty. There was no cut-off value to delineate fit vs. frail patients, as the MSK-FI is treated as a continuous variable.
To test our primary aim, we used a multivariable logistic regression with PDS as the outcome and MSK-FI as the predictor as a continuous variable and adjusted for pathology stage (III vs. IV) and high-grade serous histology (yes vs. no). For the secondary aim, our institution has various research studies for patients with advanced ovarian cancer, ranging from surveys or tissue banking for research to interventional clinical trials, such as those testing novel chemotherapy agents or surgical techniques. As such, we utilized separate multivariable logistic regression models for each of the outcomes, with continuous MSK-FI as the predictor, and stage, high-grade serous histology, whether patients underwent NACT (yes vs. no), and age at diagnosis as covariates.
Regardless of whether patients undergo PDS or IDS, they are treated with platinum-based postoperative chemotherapy. We tested whether frailty is associated with time to initiation of postoperative chemotherapy using a multivariable linear regression with days to adjuvant chemotherapy as the outcome, continuous MSK-FI as the predictor, and covariates of stage, high-grade serous histology, use of NACT, and residual disease after surgery, which was defined as complete gross resection (no visible residual tumor) vs. residual between 1 and 10 mm vs. residual >10 mm. Patients who did not receive postoperative chemotherapy at our institution, either due to declining treatment or treatment at another hospital, were excluded from this analysis.
To determine the association between the MSK-FI and overall survival (OS), we used a multivariable Cox regression model, with continuous MSK-FI as the predictor. We then explored ways in which frailty may impact OS by ascertaining whether frailty is associated with postoperative complications or discharge location. We used a multivariable logistic regression model, with major complication within 30 days of surgery as the outcome. We also looked at an alternative definition of complications, where the outcome included either major complications within 30 days of surgery or unintended ICU admission. Due to the limited number of patients who were discharged to rehabilitation, we did not have enough events to look at discharge locations as three levels (home vs. home with services vs. rehabilitation). Instead, we combined patients who were discharged to home with services and patients discharged to rehabilitation and used a multivariable logistic regression with discharge to home with services or rehabilitation as the outcome and continuous MSK-FI as the predictor. In the models for OS and complications, we adjusted for NACT use, stage, and high-grade serous histology.
For each of our outcomes, we tested the MSK-FI score for nonlinearity using restricted cubic splines with knots at the tertiles. We found no evidence of a nonlinear relationship between the MSK-FI score and PDS, enrollment in any trial, enrollment in treatment-based trial, OS, 30-day postoperative complications, or discharge location; therefore MSK-FI was included in these models as a linear variable. However, we did observe evidence of a nonlinear relationship between MSK-FI and time to postoperative chemotherapy (p=0.025), and therefore, for this model, MSK-FI was included as a nonlinear term. All statistical analyses were conducted using STATA 15.0 (StataCorp, College Station, TX).
RESULTS
Of 551 initially eligible patients, 5 were excluded due to missing stage or outcomes data. Functional status, which is necessary to calculate MSK-FI score, could not be determined in 116 patients due to missing data, resulting in a final cohort of 430 women. We compared patients and disease characteristics between patients with and without missing variables. Patients with available data had a lower rate of chronic obstructive pulmonary disease and pneumonia 30 days prior to surgery (4.4% vs. 10%; p=0.022); however, there were no other characteristics differences between the two groups (Supplementary Table 1).
Patient and disease characteristics are shown in Table 1. Half of our patients were 70–75 years of age, and approximately one-quarter were older than 80 years of age. Approximately equal numbers of patients had undergone PDS or NACT/IDS. Approximately 4 of 5 patients had an MSK-FI score of 1 or higher.
Table 1.
Patient and disease characteristics for the entire cohort (N=430). Data presented as medians (interquartile range) and frequency, in percent (%)
| Age at Surgery, years | 75 (72–79) |
| IV | 152 (35%) |
| Neoadjuvant Chemotherapy | 199 (46%) |
| Number of NACT Cycles | 5 (3, 6) |
| High-Grade Serous | 359 (83%) |
| Residual >10 mm | 76 (18%) |
| Grade 3+ Postoperative Complication | 40 (9.3%) |
| Unintended ICU Admission | 38 (8.8%) |
| Major Complication or ICU Admission | 67 (16%) |
| Rehabilitation | 33 (7.7%) |
| Participation in Treatment-Based Trial | 39 (9.1%) |
| 4+ | 20 (4.7%) |
NACT, neoadjuvant chemotherapy; NOS, not otherwise specified; ICU, intensive care unit
Table 2 shows the results of multivariable regression analyses assessing the association between MSK-FI and various outcomes. We saw evidence of an association between MSK-FI and undergoing PDS (odds ratio [OR] for unit increase in MSK-FI: 0.64; 95% confidence interval [CI], 0.53–0.77; p<0.0001); in other words, patients who were more frail were less likely to have undergone PDS. Figure 1 represents the probability of PDS by MSK-FI score. This shows, for example, that for patients with stage III disease and high-grade serous histology, a patient with an MSK-FI score of 2 would have a 63% predicted probability of undergoing PDS, compared to a predicted probability of 41% for a patient with an MSK-FI score of 4.
Table 2.
Association between MSK-FI and outcome on multivariable analysis (N=430).
| Outcome | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Primary debulking surgery | 0.64 | 0.53, 0.77 | <0.0001 |
| Participation in any trial | 0.84 | 0.70, 1.00 | 0.049 |
| Participation in a treatment-based trial | 0.91 | 0.66, 1.25 | 0.6 |
| Grade 3+ 30-day postoperative complication | 1.25 | 0.95, 1.65 | 0.11 |
| Grade 3+ 30-day postoperative complication or unintended ICU admission | 1.21 | 0.96, 1.52 | 0.11 |
| Discharge home with services or rehabilitation | 1.11 | 0.92, 1.35 | 0.3 |
| Overall survival | 1.16* | 1.05, 1.30 | 0.005 |
hazard ratio
ICU, intensive care unit
Figure 1.
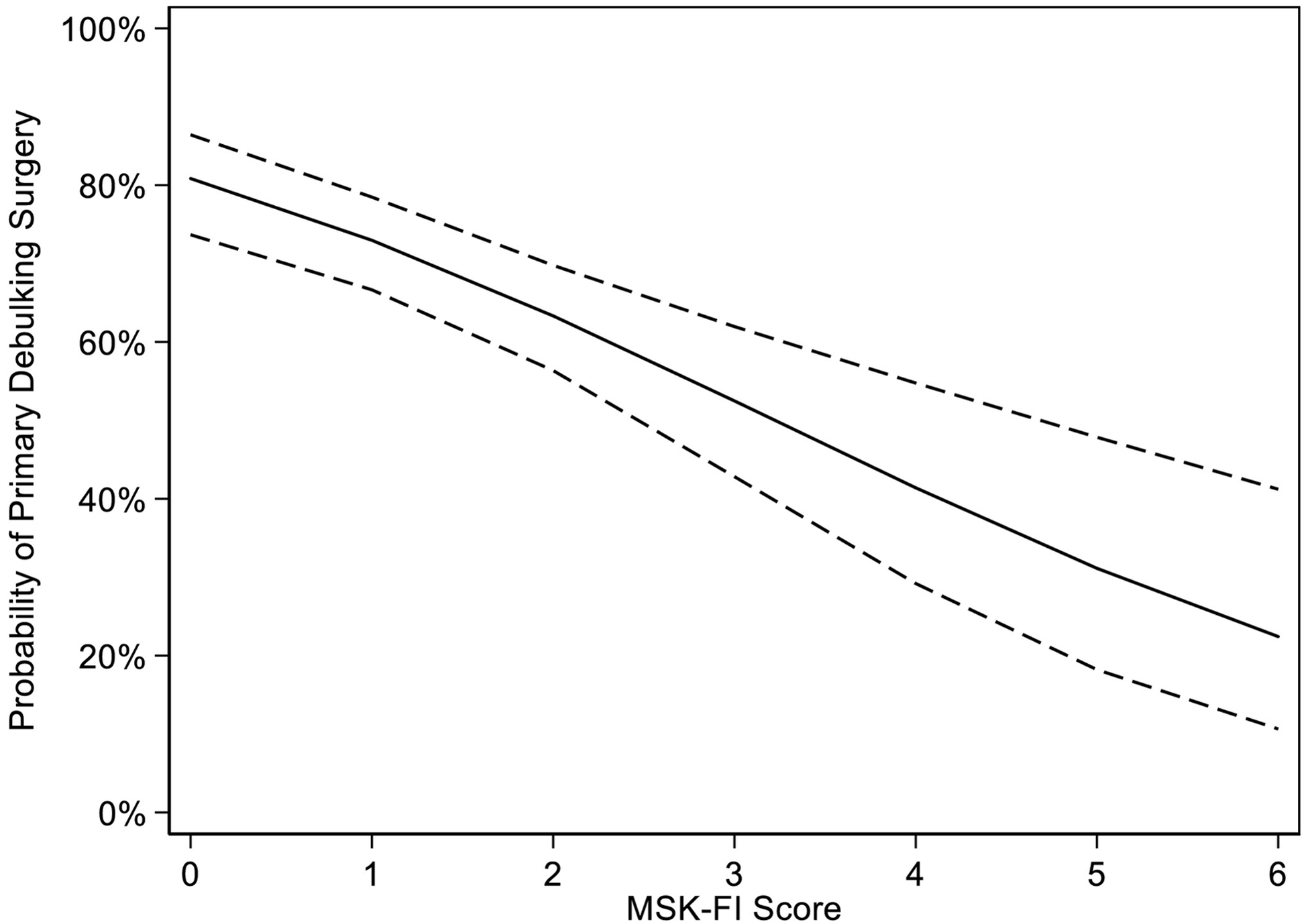
Predicted probability of primary debulking surgery based on MSK-FI score, and corresponding 95% CIs (odds ratio per unit increase in MSK-FI: 0.64; 95% CI, 0.53–0.77; p<0.0001).
Figure 2 depicts the association between MSK-FI and initiation of postoperative chemotherapy for patients with stage III, high-grade serous disease treated with PDS that led to residual disease of 10 mm or less. Greater frailty was associated with later initiation of adjuvant chemotherapy (non-linear association p=0.009). In particular, among the least-frail patients, on average, those with an MSK-FI score of 1 had nearly a week delay in beginning adjuvant chemotherapy compared to patients with an MSK-FI score of 0.
Figure 2.
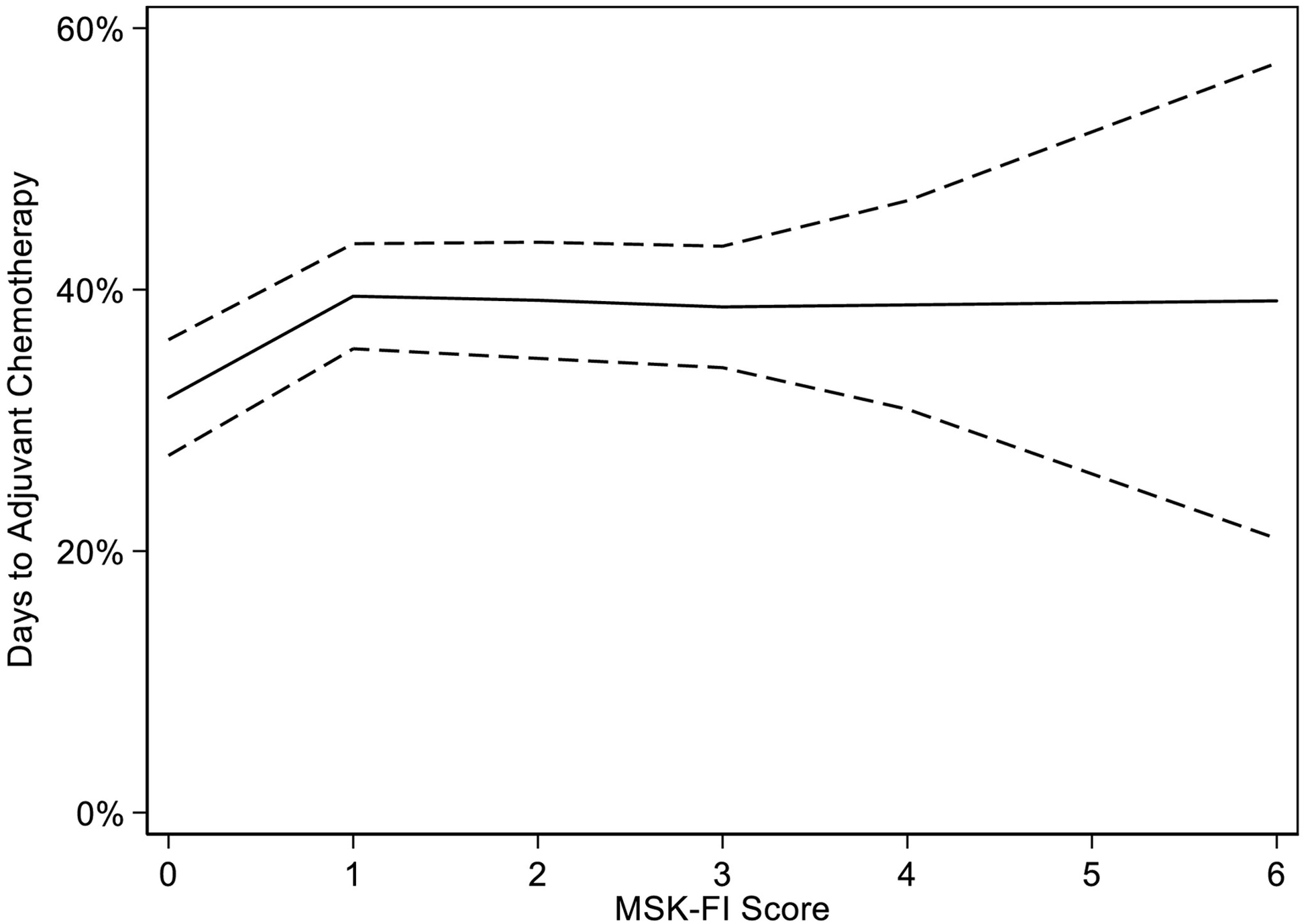
Predicted time to initiation of adjuvant chemotherapy based on MSK-FI score, and corresponding 95% CIs (non-linear association, p=0.009).
Patients with greater frailty were less likely to enroll in a research study (OR for a unit increase in MSK-FI: 0.84; 95% CI, 0.70–1.00; p=0.049). There was no association between enrollment and MSK-FI for treatment-based trials (Figure 3), although overall accrual rates were low.
Figure 3.
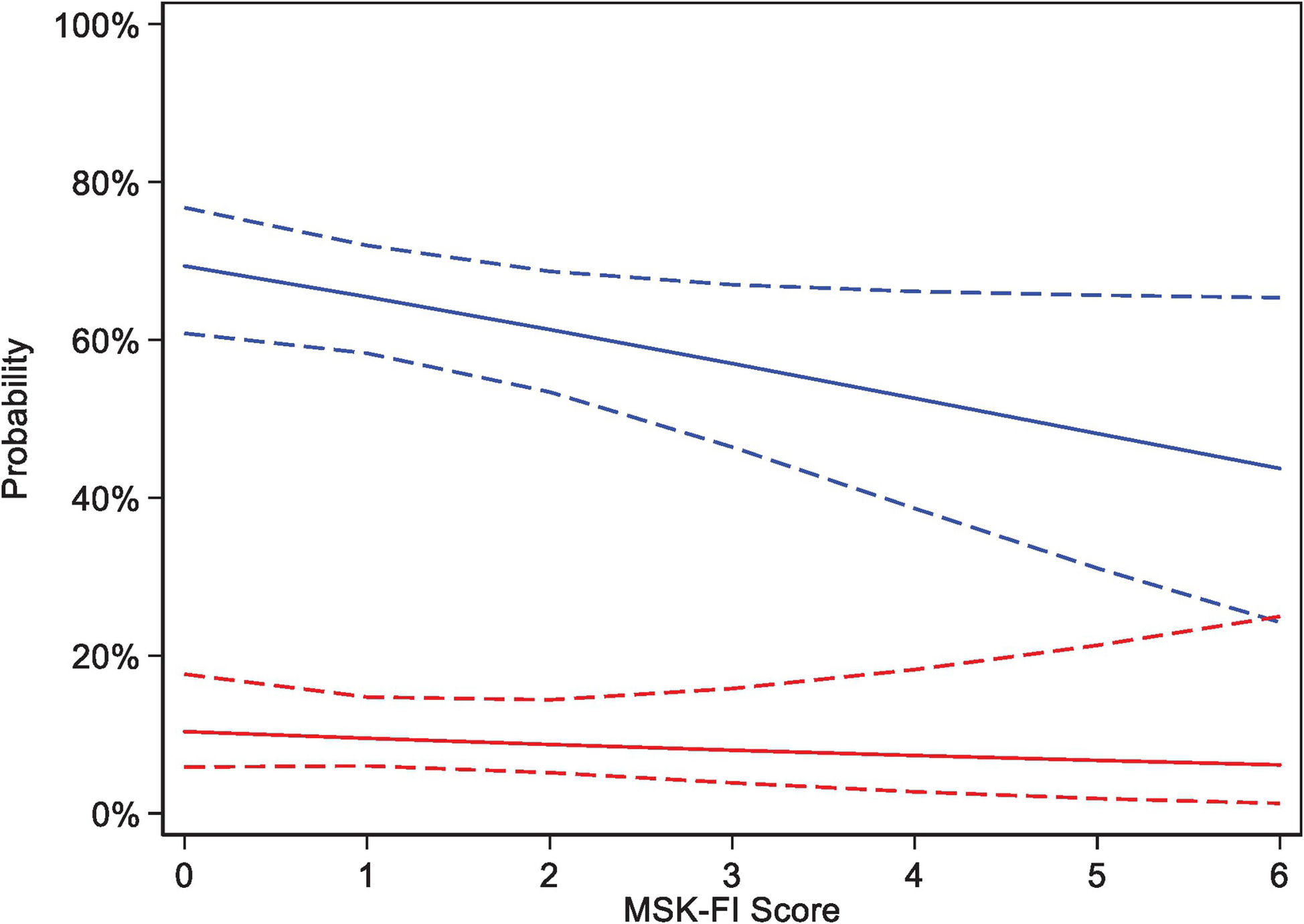
Predicted probability of participation in any research study (blue, odds ratio per unit increase in MSK-FI: 0.84; 95% CI, 0.70–1.00; p=0.049) and participation in a treatment-based trial (red, odds ratio per unit increase in MSK-FI: 0.91; 95% CI, 0.66–1.25; p=0.6) based on MSK-FI score, and corresponding 95% CIs.
Among our cohort, 258 patients died. The median follow-up was 30 months (IQR, 15–54) after surgery. As expected, greater frailty was associated with worse OS (hazard ratio [HR] for a unit increase on the MSK-FI: 1.16; 95% CI, 1.05–1.30; p=0.005). For patients with stage III, high-grade serous disease who had undergone PDS, the predicted 2-year survival probability was 71% for a patient with an MSK-FI score of 2 compared to 63% for a patient with an MSK-FI score of 4 (Figure 4). We saw non-significant higher rates of complications and discharge to home with services or rehabilitation in patients with greater frailty (Table 2), suggesting that the mechanism in which frailty is associated with OS is not only through higher rates of grade 3+ complications or location of discharge.
Figure 4.
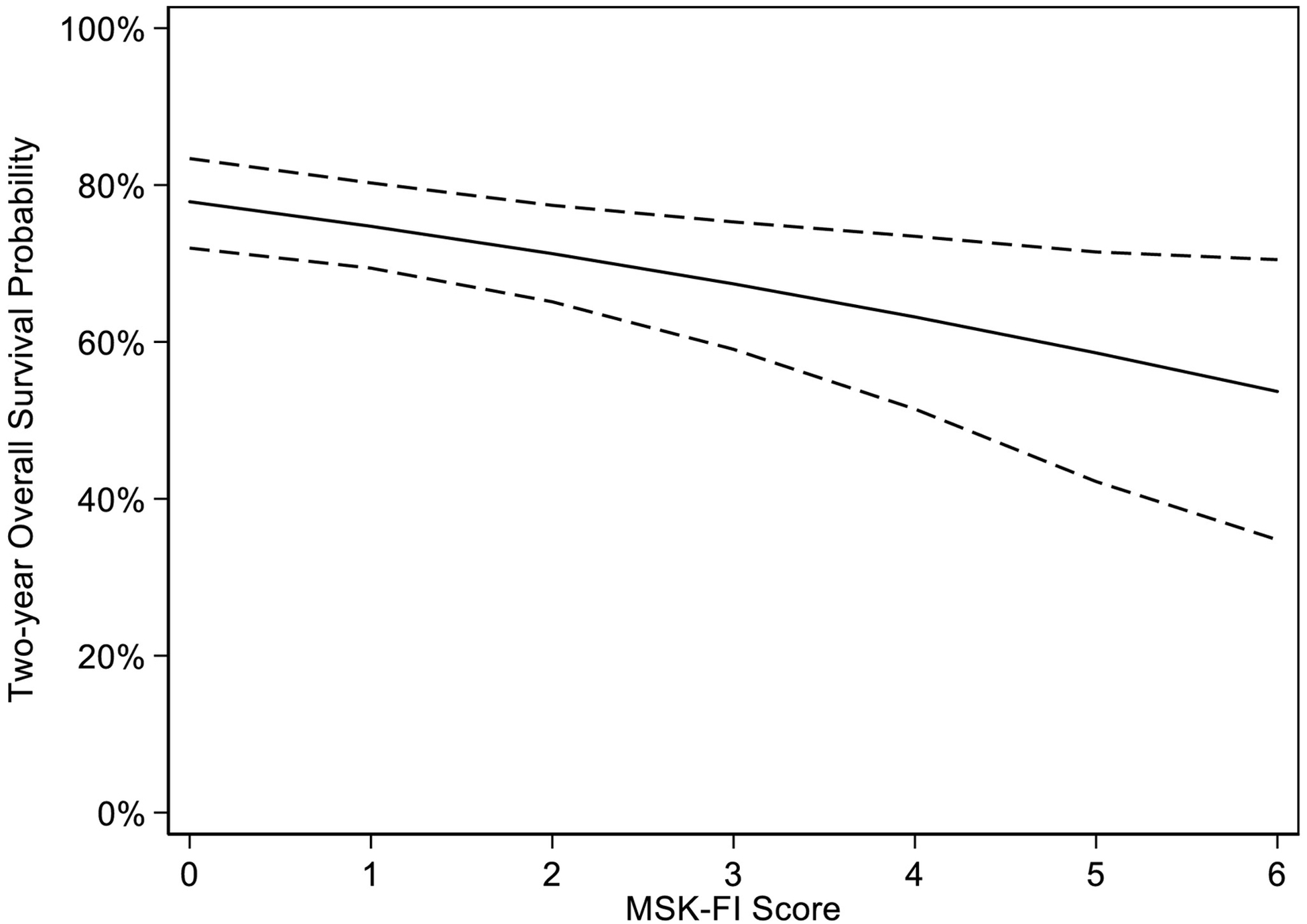
Estimated 2-year overall survival probability based on MSK-FI score and bootstrapped 95% CIs (hazard ratio per unit increase in MSK-FI: 1.16; 95% CI, 1.05–1.30; p=0.005).
DISCUSSION
The care of older woman with newly diagnosed advanced-stage ovarian cancer is highly complex, with an intricate interplay between disease, treatment, survival, and underlying fitness and comorbidity. While geriatrics is a rapidly growing field, the application of geriatric principles to the care of older women with ovarian cancer is still in its early stages. We demonstrated that a retrospectively calculated MSK-FI is strongly associated with the decision between PDS and NACT/IDS. We were able to show that women with higher frailty scores on the MSK-FI were less likely to be treated with PDS and had worse OS. More importantly, we were able to show that greater frailty was not associated with grade 3+ postoperative complications and unintended ICU admission; however, both outcomes were uncommon. Our findings suggest that oncologists are using subjective or objective correlates of the MSK-FI in decision-making. The propensity of an oncologist to recommend PDS vs. NACT/IDS would likely be influenced by a qualitative judgment of the likely impact of the comorbidities documented in the medical history. Decision-making may also be influenced by a clinical impression of the patient as frail. Although such impressions are correlated with frailty (a patient with congestive heart failure naturally presents as more frail than a patient without congestive heart failure), the correlation is imperfect; a patient with diabetes and hypertension who has fully recovered from a transient ischemic attack, for example, may give a false impression of high physiologic reserve. This raises the question of whether formal assessment of frailty using the MSK-FI could improve the decision between PDS and NACT/IDS, and ultimately, improve patient outcome. There would be two mechanisms for such an effect. First, formal quantitative estimation of frailty with the MSK-FI may have better prognostic characteristics than informal qualitative estimation based on medical history. Second, subjective assessment of frailty is prone to a number of cognitive biases and may therefore be inferior to an objective assessment of comorbidity. Our goal is to test and validate our findings prospectively in a clinical trial. We would calculate the MSK-FI score for all women who present to clinic with newly diagnosed ovarian cancer and then randomize providers to knowing or not knowing the MSK-FI result. This way, we hope to assess whether knowing the MKS-FI score influences treatment decisions. Such a design has already been used to try and predict severe chemotherapy toxicity in the field of medical oncology.19,20 We also hope to validate these findings externally.
The choice between PDS and NACT/IDS is critical for the management of advanced ovarian cancer. So far, three randomized trials have not shown a survival benefit with PDS,5–7 yet many retrospective studies as well as the most recent randomized trial have shown a survival advantage for women treated with PDS, although these studies are not without faults.8–11 Further, it seems that the most survival benefit is gained when complete gross resection is achieved at the time of PDS.2 The optimal approach in older women is still not known. This is in part due to a preconceived idea that older women cannot withstand PDS, and indeed, two of the randomized trials excluded women older than 75 years of age.7,11 The result of this bias can also be seen on a population scale, as the rate of NACT use in women 65 and older has increased from 16% in 2000 to 35.4% in 2013.21 It is thus paramount to develop tools that will help surgeons determine patient fitness and choose between PDS and NACT/IDS in an objective fashion. Frailty is defined as a decrease in the physiologic reserve beyond what is expected for the normal aging process.22 Fitness is the opposite of frailty. Data suggest that fitness, instead of chronologic age, should be used to in the treatment decision-making process. Frailty has been shown to be associated with both postoperative complications23 and chemotherapy toxicity.24 There are many reported tools for evaluating fitness, but geriatric assessment remains the gold standard. Geriatric assessment has been shown to be expedient (15 minutes on average) and can produce specific recommendations, although some suggest it is lengthy and difficult to interpret.25 Other quicker tools that are commonly used unfortunately still carry a component of subjectivity. On the other hand, it is clear that the MSK-FI is objective, short, easy to use, and produces readily interpretable results.
Pre- and postoperative interventions can lessen the impact of frailty on outcome. Prior data from our institution demonstrated successful completion of cytoreductive surgery in frail women with advanced ovarian cancer by using a co-management approach that included surgical and geriatric services.26 Not only would we be able to perform PDS more frequently in older women found to be fit on an objective assessment, but we could potentially also perform PDS on women with modifiable frailty factors if appropriate support services and multi-specialty management were used.
We found that patients with a higher MSK-FI score were less likely to participate in research studies, although we were not able to demonstrate that women with a higher MSK-FI score were less likely to participate in clinical trials specifically, likely because of overall low enrollment, as enrollment in a treatment-based trial was <10% in our cohort of older women. Even through approximately two-thirds of all cancers are diagnosed in adults 65 years of age and older, only approximately 25% of clinical trial participants are 65 or older.27 This data gap on the efficacy and safety of a particular treatment for older adults limits their access to new treatments.28 The many obstacles that prevent these patients from participating in clinical trials include comorbidity, immobility and communication issues, ageism and economic constraints, among others.28 We must work toward overcoming such obstacles in a systematic fashion, including policy changes, in order to include more older women in clinical trials for gynecologic malignancies, as without this, the “data free zone” will never be closed.
Either upfront treatment approach, PDS or NACT, imposes stress on the patient; however, unlike NACT, the stress imposed by PDS cannot be easily taken away. The MSK-FI serves as a screening tool to identify patients who would benefit from the NACT “stress test”, so they can be treated first with chemotherapy that can be dose-reduced or discontinued if the patient develops significant morbidity. The time over the course of NACT may allow for the mitigation of comorbidities, optimizing a patient’s ability to tolerate the stress of surgery. During this time, nutritional intervention, pre-habilitation, and the optimal management of chronic medical conditions may allow the patient to regain some fitness, as demonstrated by the growing volume of literature dedicated to pre-habilitation specifically within gynecologic oncology.29,30 Women identified as frail by the MSK-FI would be afforded the benefits of pre-habilitation prior to surgery. It is also likely that some women identified as frail by the MSK-FI and treated with NACT will experience continued deterioration of functional status and will never undergo surgery due to the unacceptable risk of mortality.
Our study is not without its limitations. Since it only used data from a single institution, it is difficult to know if the association between the MSK-FI and PDS is generalizable across the United States and internationally. It is also extremely challenging to capture and account for all of the factors that are involved in choosing between PDS and NACT/IDS. Given the systematic approach to these patients at our institution, with the use of departmental algorithms, the bias toward older patients would be homogeneous across all included patients. Additionally, we excluded patients in our cohort with missing data necessary to calculate the MSK-FI. While it is possible that including an additional 116 women into the cohort could affect the trends seen, as it represents approximately 25% of the cohort, we do not expect this to be the case, as comparison between patients with and without complete data yielded minimal differences.
In conclusion, we showed that, when applied retrospectively, the MSK-FI is strongly associated with the clinical decision to proceed with PDS vs. NACT/IDS in older women with advanced-stage epithelial ovarian cancer. We believe its use as a formal decision-making aid should be evaluated in a prospective fashion.
Supplementary Material
Highlights.
Higher MSK-FI score was associated with forgoing primary debulking surgery
Higher MSK-FI score was associated with delays in chemotherapy and worse overall survival
Major complications or unintended intensive care unit admission were not associated with frailty
Funding
Funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Outside the submitted work, Dr. Chi reports personal fees from Bovie Medical Co., Verthermia Inc. (now Apyx Medical Corp.), C Surgeries, and Biom ‘Up. He also reports previous stock ownership in Intuitive Surgical, Inc. and TransEnterix, Inc. The other authors have nothing to disclose.
References
- 1.Sioulas VD, Shiavone MB, Kadouri D, Zivanovic O, Long Rocher K, O’Cearbhaill R, Abu-Rustum NR, Levine DA, Sonoda Y, Gardner GJ, Leitao MM Jr, Chi DS. Optimal primary management of bulky stage IIIC ovaraina, fallopian tube and peritoneal carcinoma: are the only options complete gross resection at primary debulking surgery of neoadjuvant chemotherapy? Gynecol Oncol, 2017. 145(1); 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng JH, Cowan RA, Zhou Q, Iasonos A, Byrne M, Polcino T, Polen-De C, Gardner GJ, Sonoda Y, Zivanovic O, Abu-Rustum NR, Long Rocher K, and Chi DS. Continuous improvement in primary debulking surgery for advanced ovarian cancer: do increased complete gross resection rates independently lead to increased progression-free and overall survival? Gynecol Oncol, 2018. 151(1), 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markauskas A, Mogensen O, dePont Christense R, and Tine Jensen P. Primary surgery or interval debulking for advanced epithelial ovarian cancer: does it matter? Int J of Gynecol Cancer, 2014. 24(8); 1420–1428. [DOI] [PubMed] [Google Scholar]
- 4.Rafii A, Stoeckle E, Jean-Laurent M, Ferron G, Morice P, Houvenaeghel G, Lecuru F, Leblanc E, and Querleu D. Multi-center evaluation of post-operative morbidity and mortality after optimal cytoreductive surgery for advanced ovarian cancer. PLoS ONE, 2012. 7(7); e39415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomized, controlled non-inferiority trial. The Lancet, 2015. 386(9990); 249–257. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine, 2010. 363(10); 943–953. [DOI] [PubMed] [Google Scholar]
- 7.Fagotti A, Vizzielle G, Ferrandina G, Fanfani F, Gallotta V, et al. Survival analyses form a randomized trial of primary debulking surgery versus neoaduvant chemotherapy for advanced epithelial ovarian cancer with high tumor load (SCORPION trial). JCO, 2018. 36(15, supplement); 5516. [Google Scholar]
- 8.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol, 2012. 124(1); 10–14. [DOI] [PubMed] [Google Scholar]
- 9.Mueller JJ, Zhou QC, Iasonoa A, O’Cearbhaill RE, Alvi FA, et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol, 2016. 140(3), 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May T, Comeau R, Sun P, Kotsopoulos J, Narod S, et al. A comparison of survival outcomes in advanced serous ovarian cancer patients treated with primary debulking surgery versus neoadjuvant chemotherapy. Int J of Gynecol Cancer, 2017. 27(4); 669–674. [DOI] [PubMed] [Google Scholar]
- 11.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, et al. Comaprison of survival between upfront primary debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in pahse III randomized trial: JCOG0602. JSO, 2018. 36; 5500. [Google Scholar]
- 12.Fagotti A, Ferrandina G, Fanfani F, Ercoli A, Lorusso D, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Annals of Surgical Oncology, 2006. 13(8); 1156–1161. [DOI] [PubMed] [Google Scholar]
- 13.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Mironov S, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol, 2014. 134(4), 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippova OT, Chi DS, Long Rocher K, Sonoda Y, Zivanovic O, et al. Geriatric co-management leads to safely performed cytoreductive surgery in older women with advanced stage ovarian cancer treated at a tertiary care cancer center. Gynecol Oncol, 2019. 154(1), 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langstraat C, Aletti GD, and Cliby WA. Morbidity, mortality and overall survival in elderly women undergoing primary surgical debulking for ovaria cancer: a delicate balance requiring individualization. Gynecol Oncol, 2011. 123(2); 187–191. [DOI] [PubMed] [Google Scholar]
- 16.Tew WP. Ovarian cancer in the older woman. J of Geriatric Oncology, 2016. 7(5); 354–361. [DOI] [PubMed] [Google Scholar]
- 17.Korc-Grodzicki B, Downey RJ, Shahrokni A, Kingham TP, Patel SG, and Audisio RA. Surgical consideration in older adults with cancer. JCO, 2014. 32(24), 2647–2653. [DOI] [PubMed] [Google Scholar]
- 18.Shahrokni A, Tin A, Alexander K, Sarraf S, Afonso A, et al. Development and evaluation of a new frailty index for older surgical patients with cancer. JAMA Netw Open, 2019. 2(5); e193545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moth EB, Kiely BE, Stefanic N, Naganathan V, Martin A, et al. Predicting chemotherapy toxicity in older adults: comparing the predictive value of the CARG Toxicity Score with oncologists’ estimates of toxicity based on clinical judgement. Journal of Geriatric Oncology, 2019. 10; 202–9. [DOI] [PubMed] [Google Scholar]
- 20.Ortland I, Ott MM, Kowar M, Sippel C, Jaehde U, et al. Comapring the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. Journal of Geriatric Oncology, 2020. 11; 997–1005. [DOI] [PubMed] [Google Scholar]
- 21.Meyer LA, He W, Sun CC, Zhao H, Wright AA, Suidan RS, et al. Neoadjuvant chemotherapy in elderly women with ovarian cancer: rate of use and effectiveness. Gynecol Oncol, 2018. 150; 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uppal S, Igwe E, Rice LW, Spencer RJ, Rose SI. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol, 2015. 137; 98–101. [DOI] [PubMed] [Google Scholar]
- 23.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg, 2013. 206; 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freyer G, Feay JF, Touzel S, Provencal J, Weber B, Lacquin JP, et al. Comperehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol, 2005. 16; 1795–1800. [DOI] [PubMed] [Google Scholar]
- 25.Shahrokni A, Tin A, Downey RJ, Strong V, Mahmoudzadeh S, et al. Electronic rapid fitness assessment: a novel tool for preoperative evaluation of the geriatric oncology patients. J Natl Compr Canc Netw, 2017. 15(2); 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippova OT, Chi DS, Long Roche K, Sonoda Y, Zivanovic O et al. Geriatric co-management leads to safely performed cytoreductive surgery in older women with advanced stage ovarian cancer treated at a tertiary care cancer center. Gynecol Oncol, 2019. 154; 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis JH, Kilgore ML, Goldman DP, Trimple EL, Kaplan R, et al. Participation of patients 65 years of age or older in cancer clinical trials. JCO, 2003. 21(7)l 1383–1389. [DOI] [PubMed] [Google Scholar]
- 28.Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, and Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. American Journal of Public Health, 2010. 100(S1); S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miralpeix E, Mancebo G, Gayete S, Corcoy M, Sole-Sedeno JM. Role and impact of multimodal prehabilitation for gynecologic oncology patient in an Enhanced Recovery After Surgery (ERAS) program. Int J Gynecol Cancer, 2019. 29; 1235–43. [DOI] [PubMed] [Google Scholar]
- 30.Schneider S, Armbrust R, Spies C, du Bois A, Sehouli J. Prehabilitation programs and ERAS protocols in gynecologic incology: a comprehensive review. Archives of Gynecology and Obstetrics, 2020. 301; 315–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


