Abstract
In this review, we provide a comprehensive overview of the utility of steroid profiling for diagnosis of management of overt Cushing syndrome and mild autonomous cortisol secretion.
A diagnosis of Cushing syndrome is made through a multistep process that includes confirmation of endogenous hypercortisolism, followed by determination of its cause. Steroid metabolomic testing applied to serum or urine steroids and their metabolites can provide additional and novel insights into alterations of steroid biosynthesis and metabolism and its causes. In particular, increased availability and advances in mass spectrometry-based steroid analysis, coupled with machine learning-based algorithms, have facilitated the development of tailored diagnostic and subtyping approaches for autonomous cortisol secretion and might be useful for detecting low grade autonomous glucocorticoid secretion and in predicting and monitoring of disease severity and associated comorbidities.
Keywords: diagnosis, Cushing syndrome, mass spectrometry, mild autonomous cortisol secretion, metabolomics, steroid profiling
INTRODUCTION
Overt Cushing syndrome (CS) is mostly caused by increased stimulation of the adrenals by adrenocorticotropin (ACTH), due to a pituitary tumor (Cushing disease; 70 to 75%) or ectopic ACTH secretion (10 to 15%). In the remaining 10 to 15% cases, autonomous cortisol secretion from an adrenocortical adenoma, or, less frequently, adrenocortical carcinoma, are the most common causes(1). Rarely, autonomous adrenal cortisol excess is caused by primary bilateral macronodular adrenal hyperplasia(2), or primary pigmented nodular adrenocortical disease(1).
The incidence rate of adrenal tumors has increased 10-fold over the last 20 years(3). Adrenal incidentalomas occur in 5–7% of adults undergoing cross-sectional abdominal imaging, and the majority of these are benign adrenocortical adenomas(3, 4). When evaluated with an overnight dexamethasone suppression test, 30–50% of patients with adrenal adenomas demonstrate mild autonomous cortisol secretion (MACS)(3, 4). Patients with MACS lack clinical signs of overt CS, but suffer a higher rates of cardiovascular comorbidities, abnormal bone health, increased frailty, and mortality(5–10).
Diagnosis of both CS and MACS can be challenging. The diagnosis of CS is based on clinical evaluation and measurement of a combination of biochemical parameters(11), while the diagnosis of MACS has more recently been based on dexamethasone suppression testing(12, 13). In this review, we will discuss the role of steroid profiling in making a diagnosis of CS or MACS.
DIAGNOSTIC CHALLENGES OF CUSHING SYNDROME
CS presents with a wide range of clinical features that include obesity, abdominal fat redistribution, dorsocervical and supraclavicular fat pads, striae, thinning of the skin, easy bruising, and proximal myopathy. Patients may present with recent onset, or worsening of hypertension, diabetes mellitus type 2, dyslipidemia, osteoporosis, depression, or anxiety(11). However, no single clinical feature is 100% predictive of CS and the clinical diagnosis might be difficult in mild CS cases.
The Endocrine Society Practice guidelines recommend using at least three tests to diagnose hypercortisolism. These include evaluation for the loss of normal diurnal variation in cortisol secretion (late-night salivary cortisol), assessment of cortisol secretion in a 24-hour period (urine free cortisol), and documentation of a loss of feedback inhibition of cortisol on hypothalamic pituitary adrenal axis (overnight dexamethasone suppression testing)(11, 14).
It is important to recognize potential pitfalls of these standards of care diagnostic tests used for CS. All these tests might be false negative if optimal sampling conditions are not carefully adhered to. However, false positive results are also observed. All tests potentially perform sub optimally in individuals engaged in heavy physical activity, alcohol or drug consumption, and those who are stressed, very obese, or suffer from mental illness, such as depression or anxiety (15, 16). Usually the result is a false positive test in these scenarios.
The loss of the normal diurnal cortisol rhythm in CS forms the basis of using late-night salivary cortisol level sampling as a diagnostic test(17, 18). In patients without CS, salivary cortisol is low during the night, while patients with CS frequently present with elevated late-night salivary cortisol levels. False positive result might be observed when the sampling of saliva is done too early, as maximum suppression of cortisol occurs around midnight, while insufficient duration of contact with the absorptive sampling device might cause false low/negative results. Likewise, false negative results occur when spit or sputum are sampled, rather than saliva. Late night salivary cortisol is inaccurate and should not be performed in shift workers. Contamination of the sample with blood might cause false positive results since cortisol concentrations in blood are substantially higher than in saliva. Gum lesions or over-zealous tooth brushing are the usual culprits. Contamination with topical hydrocortisone preparations can also cause false positive results(19). Finally, it should be noted that late night salivary cortisol testing is frequently normal in patients with MACS.
Collection of 24h urine for measurements of free cortisol depends critically on the accuracy of the urine collection. Published rates of inaccurate 24h urine collections are 6–68% (with most studies showing rates of 30%), which can lead to either false low or false high cortisol measurements when normalized to the 24h collection period(20–23). Since free urinary cortisol represents only around 1% of a person’s glucocorticoid metabolome, even small collection errors can be significant. Again, urine cortisol might be normal in mild CS and is almost always normal in patients with MACS. In addition, for all the above-mentioned tests, results and interpretation may differ based on assays used and cutoffs applied (24–28). Low dose dexamethasone (1 mg) overnight suppression testing lacks specificity as the result is dependent on dexamethasone absorption and metabolism as well as on fluctuations in cortisol binding proteins. Certain medications can influence dexamethasone metabolism through induction, or inhibition, of CYP3A4. Measurement of serum dexamethasone concentrations to complement serum cortisol measurements might be helpful when the results are equivocal or incongruent with the clinical picture(29). Changes in cortisol binding globulin concentrations, or less frequently, albumin, might also affect the interpretation of total cortisol concentrations. A high estrogen state, such as late follicular or early ovulatory phase, use of oral contraceptive therapy, especially with higher estrogen concentrations (>20μg), or pregnancy, increase cortisol binding globulin, and as a result total cortisol concentrations, thus affecting the interpretation of both baseline and post-dexamethasone cortisol concentrations(30, 31).
Once hypercortisolism is confirmed, it is important to determine whether it is ACTH-dependent. This usually involves additional dynamic testing and pituitary imaging. Notably, up to 50% of patients with pituitary CS do not have a clearly visible pituitary adenoma (32, 33), necessitating additional testing, such as inferior petrosal sinus sampling, which is associated with a small, but serious risk of complications(34–36) When no pituitary source is found, sometimes further imaging is performed to locate peripheral neuroendocrine tumor(s) that might be secreting ACTH or corticotropin releasing hormone.
Patients with ACTH-independent hypercortisolism usually present with unilateral adrenal adenoma, bilateral macronodular hyperplasia or adenomas, micronodular hyperplasia, or adrenal cortical carcinoma. Once ACTH-independent hypercortisolism is confirmed, abdominal cross-sectional imaging is the next step.
At this time, published literature demonstrates that steroid profiling accurately distinguished between ACTH-independent and ACTH-dependent CS(37–40), however, very limited data exist on the value of steroid profiling in distinguishing ectopic from pituitary CS(37, 38).
CHALLENGES OF DIAGNOSIS OF MILD AUTONOMOUS CORTISOL SECRETION
MACS is diagnosed in up to 50% of patients with incidentally discovered adrenal adenomas(4). MACS is more common in postmenopausal women, patients with large, or bilateral tumors(6, 41). Unlike patients with CS, patients with MACS do not present with typical physical features, such as striae, supraclavicular pads, dorsocervical pads, or proximal myopathy. Moreover, most patients with MACS do not progress towards overt CS(42). The diagnosis is based on biochemical parameters only(12, 13).
Over the years, multiple biochemical definitions have been used to diagnose MACS(41, 42). In a systematic review and meta-analysis of cardiovascular outcomes in patients with MACS, at least 13 different definitions of MACS were used in 26 studies(41). Diagnosis was based on a combination of various tests evaluating the hypothalamic-pituitary-adrenal axis: 1 or 2 mg overnight or 2 day dexamethasone suppression tests, measurement of cortisol in 24-hour urine, late-night salivary- or serum cortisol, measurement of ACTH, assessment of circadian cortisol variation with morning and afternoon cortisol measurements, and other tests(41). In addition, the diagnostic cutoffs for tests varied considerably. For example, serum morning cortisol cut-offs of 1.8, 3, and 5 mcg/dL following the overnight 1 mg dexamethasone suppression have been used(41). This significant heterogeneity in MACS diagnosis across the studies makes it difficult to interpret the results. 2016 European guidelines for the management of adrenal incidentalomas(13), and a more recent White Paper from the American Association for Clinical Endocrinologists(12) suggested a simplified approach to the diagnosis of MACS, based on serum cortisol >1.8 mcg/dL after 1 mg overnight dexamethasone suppression. Possible MACS is diagnosed when post-dexamethasone cortisol is between 1.9 and 5 mcg/dL, and definite MACS is diagnosed when cortisol is > 5 mcg/dL. Measurement of ACTH and dehydroepiandrosterone sulfate (DHEAS) is further suggested to help the diagnosis of MACS(12, 13).
Several small studies investigated the accuracy of serum DHEAS in MACS. When a DHEAS cutoff of 40 mcg/dL was used, the area under the curve for MACS diagnosis varied between 0.76 and 0.79(43, 44). One study demonstrated a higher diagnostic accuracy using the ratio of the DHEAS concentration to the DHEAS lower reference range limit (area under the curve of 0.95), but included patients with higher degrees of cortisol autonomy (all patients had ACTH <10 pg/mL) (45).
At present, in order to make a diagnosis of MACS, clinicians usually request additional confirmatory tests after an initially abnormal dexamethasone suppression test(12, 13). These include repeating dexamethasone suppression test or obtaining baseline test to measure ACTH and DHEAS. Performing a 24h urine collection for free cortisol or late-night salivary cortisol measurements are also occasionally obtained but are not usually helpful as these are frequently normal in patients with MACS. Notably, only a minority of patients with adrenal incidentalomas undergo dexamethasone suppression test(3), possibly because of the burden of additional testing in the context of newly discovered adrenal mass. Steroid profiling has the potential to simply the diagnosis of MACS by potentially replacing some of these tests.
Patients with MACS present with a high prevalence and incidence of cardiovascular risk factors, such as hypertension, diabetes mellitus type 2, dyslipidemia, and obesity, as well as cardiovascular events(41, 42). Patients with MACS and post-dexamethasone cortisol between 1.9 and 5 mcg/dL present with higher frailty when compared to patients with nonfunctioning adrenal adenomas(8). In addition, patients with MACS have a higher prevalence and incidence of fractures(5, 6). However, while as a group, patients with MACS have a higher burden of comorbidities and have a higher mortality, individual risk of MACS-related comorbidities is difficult to determine. The 2016 European guidelines on management of adrenal incidentaloma suggest adrenalectomy for patients with a higher degree of cortisol autonomy (post-dexamethasone cortisol >5.0mcg/dL) and the presence of comorbidities related to cortisol excess such at type 2 diabetes mellitus, obesity, hypertension, or low bone mass(13). In patients with a lower degree of cortisol autonomy, or patients without comorbidities, the decision on adrenalectomy is individualized, or not generally recommended. In a systematic review and meta-analysis of patients with MACS, adrenalectomy led to improvement of cardiovascular risk factors(41), while a conservative approach in general led to worsening of cardiovascular risk factors(41, 42). A more accurate method of diagnosing clinically relevant MACS will help clinicians be more confident in recognizing MACS-related comorbidities in patients with established cardiovascular risk factors and selecting patients who will benefit from adrenalectomy early prior to development of comorbidities. Steroid profiling is potentially a valuable tool in establishing the diagnosis of MACS(46)
PRINCIPLES OF STEROID METABOLOMICS
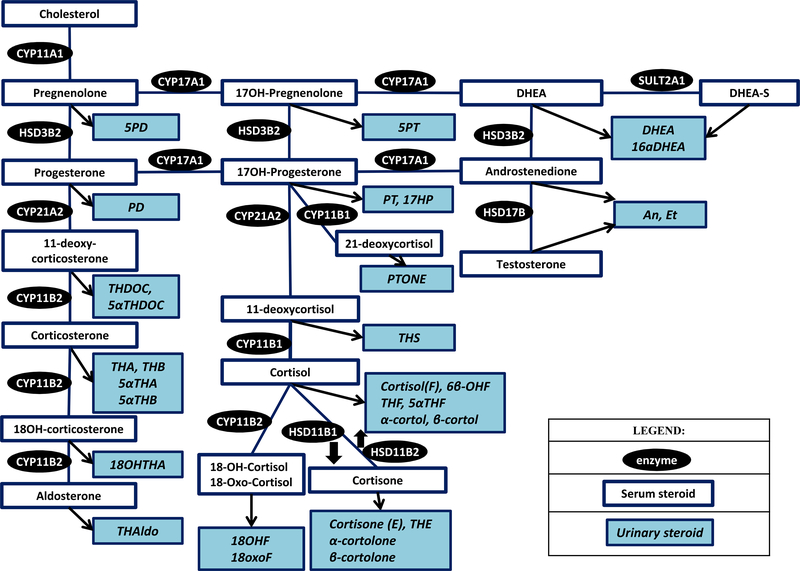
The adrenal cortex is the major site of steroidogenesis. Circulating steroids are available for action through binding to the cellular steroid receptors and can be measured in serum or plasma (serum/plasma steroid profiling), Table 1, Figure 1. Notably, steroid production follows a diurnal rhythm, with most measurements performed in the morning. Steroids can undergo metabolism in the liver, or can be directly excretes in the urine(47, 48). Measurements of steroids in a 24h urine sample represent a more comprehensive view of steroidogenesis, as it reflects both steroid production and metabolism, Table 1, Figure 1.
Table 1.
Steroid hormones, precursor and metabolites in serum/plasma and urine
| Adrenal Steroid | Serum Steroid | Urinary Steroid | |
|---|---|---|---|
| Full Name | Full Name | Abbreviated | |
| Glucocorticoid metabolites | Cortisol | Cortisol | F |
| α-cortol | α-cortol | ||
| β-cortol | β-cortol | ||
| 6β-Hydroxycortisol | 6β-OH-F | ||
| 5α-Tetrahydrocortisol | 5α-THF | ||
| Tetrahydrocortisol | THF | ||
| 11-β-Hydroxyetiocholanolone | 11β-OH-ET | ||
| Cortisone |
Cortisone | E | |
| α-cortolone | α-cortolone | ||
| β-cortolone | β-cortolone | ||
| Tetrahydrocortisone | THE | ||
| 11β-Oxoetiocholanolone | 11-oxo-ET | ||
| Glucocorticoid precursors | 21-deoxycortisol | Pregnanetriolone | PTONE |
| 17-hydroxyprogesterone | Pregnanetriol | PT | |
| 17-Hydroxy-pregnanolone | 17HP | ||
| 3α,5α-17-Hydroxy-pregnanolone | 3α5α-17HP | ||
| Progesterone | Pregnanediol | PD | |
| 11-deoxycortisol | Tetrahydro-11-deoxycortisol | THS | |
| Mineralocorticoid precursors and metabolites | 11-deoxycorticosterone | Tetrahydro-11-deoxycorticosterone | THDOC |
| 5α-Tetrahydro-11-deoxycorticosterone | 5α-THDOC | ||
| Corticosterone | Tetrahydro-11-dehydrocorticosterone | THA | |
| Tetrahydrocorticosterone | THB | ||
| 5α-Tetrahydro-11-dehydrocorticosterone | 5α-THA | ||
| 5α-Tetrahydrocorticosterone | 5α-THB | ||
| 18-hydroxycorticosterone | 18-hydroxytetrahydro-11-dehydrocorticosterone | 18OH-THA | |
| Aldosterone | 3α,5β-tetrahydroaldosterone | 3α5β-THAldo | |
| 18 - hydroxycortisol | 18 -hydroxycortisol | 18OHF | |
| 18 - oxocortisol | 18-oxocortisol | 18oxoF 18oxoTHF |
|
| Androgen precursors and metabolites | 17-Hydroxypregnenolone | Pregnenetriol | 5PT |
| Pregnenolone | Pregnenediol | 5PD | |
| Dehydroepiandrosterone and dehydroepiandrosterone sulfate | Dehydroepiandrosterone | DHEA | |
| 16α-Hydroxydehydroepiandrosterone | 16α-OH-DHEA | ||
| Androstenedione, testosterone | 11β-Hydroxyandrosterone | 11β-OH-AN | |
| 11β-Oxoetiocholanolone | 11-oxo-ET | ||
| Androsterone | An | ||
| Etiocholanolone | Etio | ||
Fig. 1.
Steroid pathway diagram indicating the serum and urine steroid metabolites. (Abbreviations: Refer Table 1).
In the blood, single steroids have been traditionally measured by immunoassays, and more recently with liquid chromatography-tandem mass spectrometry. Multi-steroid panels measuring serum/plasma steroids or 24h urine steroids have been developed and studied in various adrenal disorders such as adrenal cortical carcinomas, CS, MACS, primary aldosteronism, and congenital adrenal hyperplasia(37, 38, 40, 49, 50).
STEROID MEASUREMENTS
Steroid measurements have historically been performed with competitive immunoassays since steroids and their metabolites are of insufficient size to allow binding by two separate antibodies, as is done in immunometric assays (also known as “sandwich assays”). Unfortunately, this reduces the analyte specificity of steroid immune assays. The problem is exacerbated by the fact that different steroids are very similar to each other and their respective metabolites. Moreover, steroid hormone concentrations can vary widely during a day, through longer time frames, between sexes and with age. Hence, even if a competitive steroid assay has only 1% cross reactivity with a different steroid, substantial analytical errors might occur. For example, an estradiol measurement in an adult male, with testosterone concentrations several orders of magnitude higher than estradiol concentrations, would have a substantial false high bias. These cross-reactivity problems have been compounded by the advent of automated immunoassays. The principle advantage of automated immunoassays are their rapid turnaround time, high throughput, and excellent precision; however, these assays no longer use radioactive tritium – which is a near perfect hydrogen mimic – as a label, but bulky color-, fluorescence-, or chemiluminescent reporters, thus often further reducing specificity. Finally, the automated assays also do not separate unconjugated hormones from conjugated hormones and hormone metabolites(51, 52)
Another limitation of competitive immunoassays is their limited dynamic range of only 1–2 log. Consequently, these assays are only accurate and precise over a narrow range and might require dilution of samples that contain too much analyte(53)
Finally, different competitive immunoassays for the same steroid frequently give significantly different results. This leads to difficulties in interpreting results, particularly if fixed diagnostic cut-offs are used, rather than cut-offs that are based on an individual assay(54, 55).
Due to these limitations of steroid immunoassays there has been a trend to replace immunoassays with mass spectrometry (MS) assays(56, 57). MS overcomes most of the specificity and dynamic range challenges of immunoassays and offers improved between-assay comparability(58). For some MS based steroid assays, e.g. testosterone, estradiol and 25-OH Vitamin D, the USA Center of disease control offers an accuracy-based proficiency testing scheme to which most commercial laboratories that use MS for measurement subscribe (https://www.cdc.gov/labstandards/hs_certified_participants.html). This has resulted in very good measurement agreement across the country for these analytes.
The superior performance of MS is due to its multiple levels of analyte identification using well understood physico-chemical methods. The most common method is liquid-chromatography, tandem mass spectrometry (LC-MS/MS)(56). There are at least two dimensions of chemical selection: (i) sample extraction/clean up, followed enriches the sample in the desired target analyte(s) while discarding many other compounds, which is followed by (ii) 1 (or 2) dimensional chromatography which further separates the analyte of interest from similar substances. The actual MS will then ionize the sample, and focus on the analyte containing fraction by measuring its mass over charge ratio (m/z), and then fragmenting the measured substance(s) and measuring the fragment(s)’ m/z. The chance that an interference can pass all these hurdles is very low (although it can still sometimes happen. Based on this setup, it is also much easier to obtain comparable results in for the same analyte in different laboratories. The measurement precision/reproducibility of MS is comparable to manual extracted competitive immunoassays; however, MS is usually somewhat worse than what is seen with automated competitive immunoassays. The latter also have the advantage of quicker analytical turnaround time (and are still favored for applications were this is deemed essential).
There are several variations on the MS approach, which include gas chromatography combined with MS (usually single stage MS – no fragmentation), and high-resolution, accurate-mass MS (HRAM-MS). GC-MS is nearly equivalent in terms of analytical performance to LS-MS/MS but has a significantly slower turn-around time.
By contrast, HRAM-MS offers 10–100x the resolution of standard resolution LC-MS/MS by using different types of MS instruments (principally Time of Flight instruments or Electrostatic Orbital Traps)(59). This increases specificity and measurement accuracy further and allows quite extensive multiplexing. While LC-MS/MS is for simultaneous measurement of more than a handful of analytes is very challenging, HRAM-MS allows multiplexing of 20 to over 100om different analytes with excellent accuracy and acceptable analytical turn-around time. This has enabled the impressive metabolomic advances, including for steroid metabolomics. Coupled with artificial intelligence or machine learning algorithms(49), this creates the foundation for much deeper insights into CS and MACS.
STEROID METABOLOME IN OVERT CUSHING SYNDROME AND MACS
Steroid profiling in CS depends on the etiology (ACTH-dependent vs ACTH independent), and the severity of CS. In all patients with CS, excessive amounts of glucocorticoids are observed in either blood or urine measurements. Androgen production/excretion is very low in ACTH-independent CS, while elevated in the ACTH-dependent CS. In patients with MACS, steroid profiling results are is similar to ACTH-independent CS, though milder abnormalities are seen.
ACTH-dependent CS
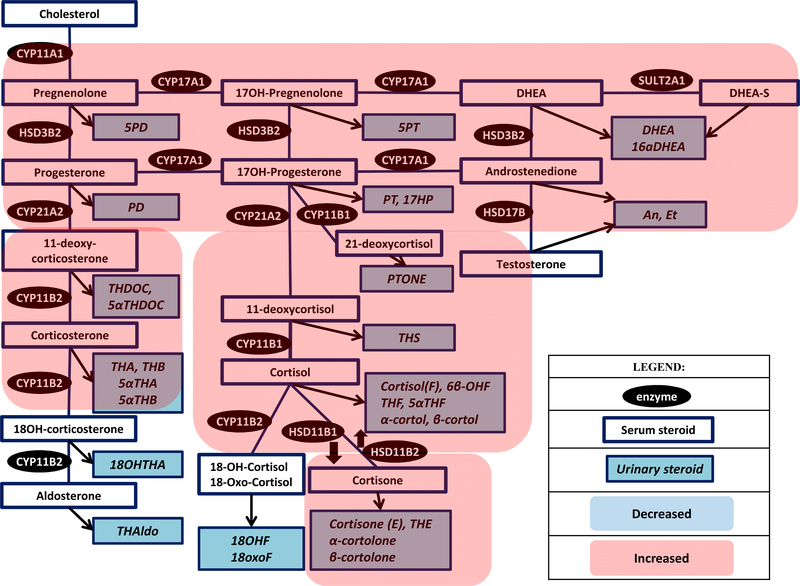
As expected in CS, cortisol and cortisol metabolites are significantly increased in proportion to the severity of CS. Excessive cortisol production in CS overwhelms the capacity of the enzyme that converts cortisol to cortisone, HSD11B2(38, 60). This leads to reduced inactivation of cortisol with increased ratio of cortisol to cortisone metabolites, measured by the (Tetrahydrocortisol + 5α-Tetrahydrocortisol) / Tetrahydrocortisone (THF+5αTHF/THE) ratio, and a higher urinary cortisol metabolites excretion with an intact A ring(60, 61). In a study of 22 patients with CS, THF+5αTHF/THE ratio was twice higher (median of 1.8 (range, 1.1–10), as compared to 0.8 (range, 0.5–1.5) in patients without CS). The degree of THF+5αTHF/THE ratio elevation was similar in patients with pituitary and adrenal CS, but was much higher in ectopic CS (median of 4.1)(60).
Interestingly, a significant correlation between THF+5αTHF/THE and urinary cortisol excretion was observed, as well as hypokalemia(60). Excessive ACTH stimulation also leads to increase in mineralocorticoids, with elevated urinary metabolite of the 11-deoxycorticosterone(61). Mineralocorticoid excess may contribute to clinical manifestations, such as hypertension, but plays a relatively minor role when compared to cortisol. Cortisol is more available to act at the mineralocorticoid receptor and contributes to hypertension, hypokalemia, and alkalosis frequently seen in severe CS. In addition, 5α-reductase action is decreased in CS, reflected by a high THF to 5αTHF ratio. Patients with CS were reported to have a more than 3 times higher THF/5αTHF ratio when compared to patients without CS (median of 6.2 (range, 1.1–36.5) vs 1.7 (range, 0.4–2.8)). The degree of THF/5αTHF ratio elevation was similar in patients with pituitary, ectopic, and adrenal CS(60).
Over the last several years, several studies have reported steroid profiling in patients with ACTH-dependent CS(37, 38, 40), Table 2, Figure 2. In one study that included 51 patients with pituitary CS and 12 patients with ectopic CS and utilized a LC-MS/MS 15 steroid plasma steroid panel(38), all patients with CS demonstrated increase in 11-deoxycortisol, 21-deoxycortisol, and cortisol, but also 11-deoxycorticosterone and corticosterone. Androgens (DHEA, DHEAS, and androstenedione) were elevated in ACTH-dependent CS. Patients with ACTH dependent disease demonstrated the lowest concentration of aldosterone, in comparison to adrenal disease and plasma 18-oxocortisol was particularly low in ectopic CS, suggestive of a decreased CYP11B2 activity. Notably, use of 10 selected steroids (11-deoxycortisol, 11-deoxycorticosterone, cortisol, cortisone, corticosterone, 18-oxocortisol, aldosterone, DHEA, DHEAS, androstenedione) correctly classified all patients with ectopic CS, and 88% of patients with pituitary CS(38). In another study that used a GC-MS-based 94 serum steroid panel in 67 patients with pituitary CS and 6 patients with ectopic CS (37), similar findings were demonstrated, with increase in androgens, mineralocorticoid precursors, and glucocorticoids. Notably, a metabolite of androstenedione, 11β-hydroxyepiandrosterone distinguished pituitary from ectopic CS with 100% sensitivity and 93.6% specificity, though the results were not corrected for the degree of hypercortisolism, and the sample size of patients with ectopic CS was small (37). Hines et al utilized a 26-steroid quantitative high resolution accurate mass spectrometry in a very small study of 4 patients with pituitary CS had increased urinary androgen metabolites (androsterone, etiocholanolone, DHEA, 16α-OH-DHEA, pregnanetriol, and pregnenediol) and glucocorticoid metabolites (cortisol, 6β-OH-cortisol, tetrahydrocortisol, 5α-tetrahydrocortisol, β-cortol, 11β-hydroxyandrosterone, 11β-oxoetiocholanolone, cortisone, tetrahydrocortisone, α-cortolone, and β-cortolone)(40).
Table 2.
Studies investigating serum steroid profiling in diagnosing MACS and CS*
| Author, type of study | Patients | Steroid profile | Results | Comments |
|---|---|---|---|---|
| Di Dalmazi et al1, 2015 Retrospective cross-sectional study |
28 MACS 66 nonfunctioning adrenal adenomas 188 age- and sex- matched volunteers |
10 serum steroid panel (liquid chromatography-tandem mass spectrometry, sera) Glucocorticoid precursors and glucocorticoids: cortisol, 11-deoxycortisol, 21-deoxycortisol Mineralocorticoid precursors and mineralocorticoids: corticosterone, 11-deoxycorticosterone Androgen precursors and androgens: progesterone, 17-hydroxyprogesterone, androstenedione, DHEA, testosterone |
MACS ↑ACTH simulated levels of 21-deoxycortisol and 11-deoxycorticosterone ↓basal and ACTH stimulated DHEA and androstenedione |
Accuracy for MACS diagnosis: - DHEA: AUC of 0.760 - Androstenedione: AUC of 0.705 |
| Eisenhofer et al, 20182 Retrospective cross-sectional study |
51 pituitary CS 21 adrenal CS 12 ectopic CS 138 excluded CS 227 age- and sex- matched volunteers |
15 plasma steroid panel (liquid chromatography-tandem mass spectrometry, plasma) Glucocorticoid precursors, metabolites, and glucocorticoids: cortisol, 11-deoxycortisol, 21-deoxycortisol, 18-oxocortisol, 18-hydrocortisol, cortisone Mineralocorticoid precursors and mineralocorticoids: corticosterone, 11-deoxycorticosterone, aldosterone Androgen precursors and androgens: progesterone, pregnenolone, 17-hydroxyprogesterone, androstenedione, DHEA, DHEA-sulfate, testosterone |
All CS ↑ 11-deoxycortisol (289%) ↑ 21-deoxycortisol (150%) ↑ 11-deoxycorticosterone (133%) ↑ Corticosterone (124%) ↑ Cortisol (122%) ACTH dependent CS ↑ all steroids except: ↓aldosterone ↓18-oxocortisol Adrenal CS ↓↓ androgens (DHEA, DHEA-sulfate, androstenedione |
Use of 7 selected steroids (11-deoxycortisol, 11-deoxycorticosterone, cortisol, aldosterone, 21-deoxycortisol, DHEA, DHEA-sulfate) →accuracy of CS diagnosis: AUC=0.930 Use of 10 selected steroids (11-deoxycortisol, 11-deoxycorticosterone, cortisol, cortisone, corticosterone, 18-oxocortisol, aldosterone, DHEA, DHEA-sulfate, androstenedione): - 0% misclassification of ectopic CS - 5% misclassification of adrenal CS (1/21) - 12% misclassification of pituitary CS (6/51) |
| Masjkur et al, 20193 Retrospective cross sectional study |
21 adrenal CS 35 MACS 152 excluded CS 277 age- and sex- matched volunteers (some overlap in populations with Eisenhofer et al) |
Adrenal CS and MACS ↑ 11-deoxycortisol and 11-deoxycorticosterone ↓ DHEA and DHEA-sulfate Adrenal CS ↓ androstenedione MACS ↑ corticosterone |
Use of 14 steroids (all except 18-oxocortisol): - AUC of 0.9777 for diagnosing MACS - Overall misclassification of 4.8% - Adrenal CS: misclassification of 5% - MACS: misclassification of 3% |
|
| Hana et al, 20194 | 67 Pituitary CS 6 ectopic CS 16 adrenal adenoma 7 bilateral adrenal hyperplasia with overt CS 42 volunteers |
94 serum steroid panel (gas chromatography tandem mass spectrometry) Conjugated an unconjugated steroids within the androgen, glucocorticoid and mineralocorticoid pathways (see figure 1 and table 1) |
Pituitary CS and ectopic CS ↑ androgens Ectopic CS ↑ mineralocorticoid precursors Adrenal adenomas and BMAH ↓ androgens |
Discrimination of pituitary CS from ectopic CS: 100% sensitivity and 93.6% specificity for 11β-hydroxyepiandrosterone sulfate (metabolite of androstenedione) |
| Studies investigating urine steroid profiling in diagnosing MACS and CS* | ||||
| Hines et al, 20175 Prospective cross-sectional study |
4 pituitary CS 4 adrenal CS 57 nonfunctioning adrenal adenomas |
26 urine steroid panel (liquidchromatography, high-resolution, accurate mass spectrometry, 24h urine) Steroids within the androgen, glucocorticoid and mineralocorticoid pathways (see figure 1 and table 1) |
Pituitary CS ↑ androgens ↑ glucocorticoids Adrenal CS ↓androgens ↑ glucocorticoids (lower degree than pituitary CS) |
3 analytes (Etiocholanolone, 11β-Hydroxyandrosterone and α-cortolone) showed statistical significance differences in differentiating pituitary vs adrenal CS. |
| Kotlowska et al, 20176 Retrospective cross-sectional study |
25 adrenal incidentaloma with “possible” MACS 16 CS 37 volunteers |
19 urine steroid panel (gas-chromatography, mass spectrometry, 24h urine) Steroids within the androgen, glucocorticoid and mineralocorticoid pathways (see figure 1 and table 1) |
CS (subtype unknown), compared to “possible” MACS ↑ androsterone, etiocholanolone, pregnenetriol, tetrahydrocortisone, tetrahydrocortisol, allo-tetrahydrocortiol, and α-cortol Adrenal incidentaloma, possible MACS (compared to volunteers) ↑ tetrahydrocorticosterone, tetrahydrocortisol, allo-tetrahydrocortiol, and α-cortol |
Urine steroid profiling: - CS: 0% misclassification - Adrenal incidentaloma with possible MACS: 20% misclassification |
Abbreviations: DHEA: dehydroepiandrosterone, CS: Cushing syndrome; MACS: mild autonomous cortisol secretion; AUC: area under the curve
Only studies investigating multi-steroid assays published since 2010 and including at least 5 patients are included
Di Dalmazi G, Fanelli F, Mezzullo M, et al. Steroid Profiling by LC-MS/MS in Nonsecreting and Subclinical Cortisol-Secreting Adrenocortical Adenomas. J Clin Endocrinol Metab. 2015;100(9):3529-3538.
Eisenhofer G, Masjkur J, Peitzsch M, et al. Plasma Steroid Metabolome Profiling for Diagnosis and Subtyping Patients with Cushing Syndrome. Clin Chem. 2018;64(3):586-596.
Masjkur J, Gruber M, Peitzsch M, et al. Plasma Steroid Profiles in Subclinical Compared With Overt Adrenal Cushing Syndrome. J Clin Endocrinol Metab. 2019;104(10):4331-4340.
Hana V, Jr., Jezkova J, Kosak M, Krsek M, Hana V, Hill M. Serum steroid profiling in Cushing's syndrome patients. J Steroid Biochem Mol Biol. 2019;192:105410.
Hines JM, Bancos I, Bancos C, et al. High-Resolution, Accurate-Mass (HRAM) Mass Spectrometry Urine Steroid Profiling in the Diagnosis of Adrenal Disorders. Clin Chem. 2017;63(12):1824-1835.
Kotlowska A, Puzyn T, Sworczak K, Stepnowski P, Szefer P. Metabolomic Biomarkers in Urine of Cushing's Syndrome Patients. Int J Mol Sci. 2017;18(2).
Fig. 2.
Steroid pathway diagram indicating the observed changes in serum and urine steroid metabolite concentrations in a patient with ACTH-dependent Cushing syndrome (Abbreviations: Refer Table 1).
Adrenal CS and MACS
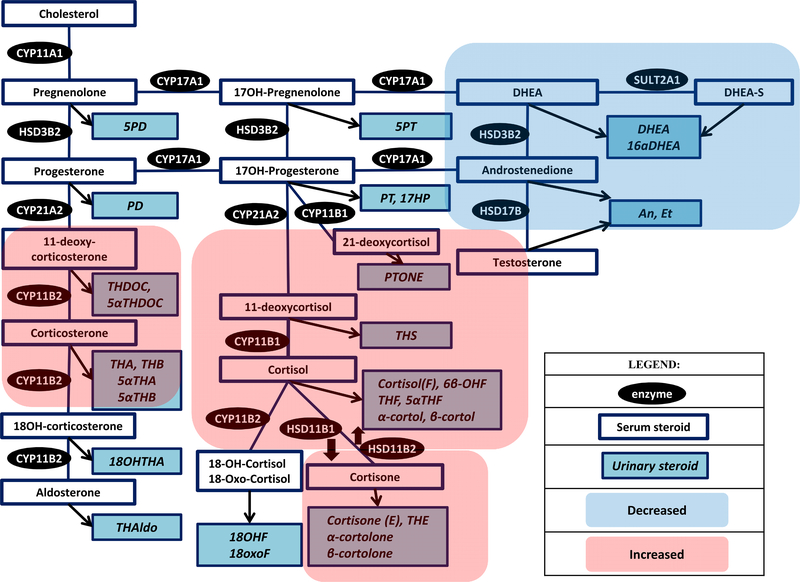
In patients with ACTH-independent CS, abnormalities in glucocorticoids and glucocorticoid metabolites are similar to ACTH-dependent CS, though of usually of a lesser degree than ectopic CS(60). Patients with MACS demonstrate much less pronounced, but similar, steroid profiling findings to patients with adrenal CS. Patients with ACTH-independent CS and MACS demonstrate decreased levels of androgens, Table 2, Figure 3.
Fig. 3.
Steroid pathway diagram indicating the observed changes in serum and urine steroid metabolite concentrations in a patient with adrenal CS and MACS. (Abbreviations: Refer Table 1).
One of the earliest reports on application of LC-MS/MS based multi-steroid profiling in patients was published in 2015. In this study of 28 patients with MACS, 66 patients with nonfunctioning adrenal adenomas, and 188 age and sex matched volunteers, the authors used a LCMS-based 10 serum steroid panel with measurements performed at baseline and after ACTH stimulation(50). Patients with MACS had lower basal and ACTH stimulated levels of serum DHEA and androstenedione, and higher ACTH-stimulated levels of 21-deoxycortisol and 11-deoxycorticosterone when compared to patients without MACS (50). Receiver-operating characteristic curves demonstrated an area under the curve of 0.760 for DHEA and 0.705 for androstenedione for the diagnosis of MACS (50). In two studies with overlapping populations that included both patients with MACS and adrenal CS and used a LC-MS/MS-based 15 plasma steroid profiling assay (38, 39), patients demonstrated increased 11-deoxycortisol and 11-deoxycorticosterone, and decreased DHEAS and DHEA-sulfate. In addition, patients with adrenal CS had decreased androstenedione, while patients with MACS also demonstrated increased corticosterone. Using 14 steroids, the area under the curve was 0.977 for diagnosing MACS(39). Overall misclassification of adrenal CS was 5%, and misclassification of MACS was 3%(39).
In a small study of patients with adrenal CS that utilized a high resolution accurate mass spectrometry 24h urine steroid profiling, etiocholanolone, 11β-hydroxyandrosterone and α-cortolone distinguished pituitary CS from adrenal CS(40). In another study that used a GCMS based 19 urine steroid panel, patients with adrenal incidentalomas and possible MACS, demonstrated increased concentrations of tetrahydrocortisol, tetrahydrocorticosterone, etiocholanolone, pregnenetriol, allo-tetrahydrocortisol, and α-cortol and a decrease of tetrahydrocortisone, when compared to patients with adrenal tumors, indicating hormonal activity that may have been present(62).
Clinical implications
Steroid profiling is a promising tool and has the potential to aid clinicians to make a more definitive diagnosis of overt CS or MACS. Though most studies discussed above had a small sample size, their results have been concordant in distinguishing ACTH dependent and ACTH independent causes of endogenous cortisol excess. These preliminary results need further validation in a prospective study of patients with CS.
There are limitations that prevent the wide use of steroid profiling in clinical practice. At present, these tests are available mainly in tertiary care settings. Multi-steroid analysis is not straight-forward and an understanding of the chemical and analytical processes is essential for implementation in an institution. Accuracy of the assay is completely dependent on accurate construction of the standard curve and pre-analytical processing, before the sample is even analyzed. Regular quality assurance and diagnostic test accreditation is required to ensure accuracy and reproducibility of measurements. Finally for most clinicians, steroid profiling might be difficult to interpret and the result will likely require a built in algorithm that is easy to integrate into practice.
Summary
The diagnostic potential of steroid profiling in disorders of endogenous cortisol excess is promising. Multianalyte assays by LC-MS/MS are facilitating measurements of large panel of steroids overcoming traditional interpretation based on a single hormone value. In the future, steroid profiling combined with customized computational approaches and machine-based learning may provide improvement in diagnosis of steroidogenic disorders, in particular – simplifying the hormonal workup and disease subtyping of CS, and possibly providing a more accurate diagnosis of MACS.
Practice points
Diagnosis of mild autonomous cortisol secretion frequently requires multiple biochemical tests and clinical evaluation for cortisol-related morbidity
Steroid profiling may supplement or improve current standard of care tests in the diagnosis of Cushing syndrome and mild autonomous cortisol secretion
Research agenda
Steroid profiling combined with customized computational or machine-based learning needs further validation in prospective studies of patients with cortisol excess prior to introduction to clinical practice
Acknowledgments
Role of funding source
This work was partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) USA under award K23DK121888 (to IB). The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health USA.
Footnotes
Conflict of interest
IB reports consulting with Corcept, Strongbridge, HRA Pharma, and Sparrow Pharmaceutics outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913–27. [DOI] [PubMed] [Google Scholar]
- 2.Assie G, Libe R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med. 2013;369(22):2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(11):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reimondo G, Castellano E, Grosso M, Priotto R, Puglisi S, Pia A, et al. Adrenal Incidentalomas are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. J Clin Endocrinol Metab. 2020;105(4). [DOI] [PubMed] [Google Scholar]
- 5.Athimulam S, Bancos I. Evaluation of bone health in patients with adrenal tumors. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):125–32. [DOI] [PubMed] [Google Scholar]
- 6.Athimulam S, Delivanis D, Thomas M, Young WF, Khosla S, Drake MT, et al. The Impact of Mild Autonomous Cortisol Secretion on Bone Turnover Markers. J Clin Endocrinol Metab. 2020;105(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delivanis DA, Athimulam S, Bancos I. Modern Management of Mild Autonomous Cortisol Secretion. Clin Pharmacol Ther. 2019;106(6):1209–21. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Atkinson EJ, Achenbach SJ, LeBrasseur N, Bancos I. Frailty in Patients With Mild Autonomous Cortisol Secretion is Higher Than in Patients with Nonfunctioning Adrenal Tumors. J Clin Endocrinol Metab. 2020;105(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405. [DOI] [PubMed] [Google Scholar]
- 11.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaidya A, Hamrahian A, Bancos I, Fleseriu M, Ghayee HK. The Evaluation of Incidentally Discovered Adrenal Masses. Endocr Pract. 2019;25(2):178–92.*
- 13.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Chen K, Wang S, Zhu H, Lu L, Zhang X, et al. The Optimal Cut-off of BIPSS in Differential Diagnosis of ACTH-dependent Cushing’s Syndrome: Is Stimulation Necessary? J Clin Endocrinol Metab. 2020;105(4). [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf). 2005;63(6):642–9. [DOI] [PubMed] [Google Scholar]
- 16.Butler PW, Besser GM. Pituitary-adrenal function in severe depressive illness. Lancet. 1968;1(7554):1234–6. [DOI] [PubMed] [Google Scholar]
- 17.Elias PC, Martinez EZ, Barone BF, Mermejo LM, Castro M, Moreira AC. Late-night salivary cortisol has a better performance than urinary free cortisol in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2014;99(6):2045–51. [DOI] [PubMed] [Google Scholar]
- 18.Raff H Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(10):3647–55. [DOI] [PubMed] [Google Scholar]
- 19.Kidambi S, Raff H, Findling JW. Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing’s syndrome. Eur J Endocrinol. 2007;157(6):725–31. [DOI] [PubMed] [Google Scholar]
- 20.Mericq MV, Cutler GB Jr. High fluid intake increases urine free cortisol excretion in normal subjects. J Clin Endocrinol Metab. 1998;83(2):682–4. [DOI] [PubMed] [Google Scholar]
- 21.Boyd C, Wood K, Whitaker D, Ashorobi O, Harvey L, Oster R, et al. Accuracy in 24-hour Urine Collection at a Tertiary Center. Rev Urol. 2018;20(3):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cote AM, Firoz T, Mattman A, Lam EM, von Dadelszen P, Magee LA. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol. 2008;199(6):625 e1–6. [DOI] [PubMed] [Google Scholar]
- 23.John KA, Cogswell ME, Campbell NR, Nowson CA, Legetic B, Hennis AJ, et al. Accuracy and Usefulness of Select Methods for Assessing Complete Collection of 24-Hour Urine: A Systematic Review. J Clin Hypertens (Greenwich). 2016;18(5):456–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galeandro L, Sieber-Ruckstuhl NS, Riond B, Hartnack S, Hofmann-Lehmann R, Reusch CE, et al. Urinary corticoid concentrations measured by 5 different immunoassays and gas chromatography-mass spectrometry in healthy dogs and dogs with hypercortisolism at home and in the hospital. J Vet Intern Med. 2014;28(5):1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecori Giraldi F, Saccani A, Cavagnini F, Study Group on the Hypothalamo-Pituitary-Adrenal Axis of the Italian Society of E. Assessment of ACTH assay variability: a multicenter study. Eur J Endocrinol. 2011;164(4):505–12. [DOI] [PubMed] [Google Scholar]
- 26.Briegel J, Sprung CL, Annane D, Singer M, Keh D, Moreno R, et al. Multicenter comparison of cortisol as measured by different methods in samples of patients with septic shock. Intensive Care Med. 2009;35(12):2151–6. [DOI] [PubMed] [Google Scholar]
- 27.Miller R, Plessow F. Transformation techniques for cross-sectional and longitudinal endocrine data: application to salivary cortisol concentrations. Psychoneuroendocrinology. 2013;38(6):941–6. [DOI] [PubMed] [Google Scholar]
- 28.Turpeinen U, Hamalainen E. Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab. 2013;27(6):795–801. [DOI] [PubMed] [Google Scholar]
- 29.Ueland GA, Methlie P, Kellmann R, Bjorgaas M, Asvold BO, Thorstensen K, et al. Simultaneous assay of cortisol and dexamethasone improved diagnostic accuracy of the dexamethasone suppression test. Eur J Endocrinol. 2017;176(6):705–13. [DOI] [PubMed] [Google Scholar]
- 30.Coe CL, Murai JT, Wiener SG, Levine S, Siiteri PK. Rapid cortisol and corticosteroid-binding globulin responses during pregnancy and after estrogen administration in the squirrel monkey. Endocrinology. 1986;118(1):435–40. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi AC, Bahri A, Breen LA, Barnes SC, Powrie JK, Thomas SM, et al. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin Endocrinol (Oxf). 2007;66(5):632–5. [DOI] [PubMed] [Google Scholar]
- 32.Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F. Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab. 1999;84(2):440–8. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury IN, Sinaii N, Oldfield EH, Patronas N, Nieman LK. A change in pituitary magnetic resonance imaging protocol detects ACTH-secreting tumours in patients with previously negative results. Clin Endocrinol (Oxf). 2010;72(4):502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller DL, Doppman JL, Peterman SB, Nieman LK, Oldfield EH, Chang R. Neurologic complications of petrosal sinus sampling. Radiology. 1992;185(1):143–7. [DOI] [PubMed] [Google Scholar]
- 35.Bonelli FS, Huston J 3rd, Carpenter PC, Erickson D, Young WF Jr., Meyer FB. Adrenocorticotropic hormone-dependent Cushing’s syndrome: sensitivity and specificity of inferior petrosal sinus sampling. AJNR Am J Neuroradiol. 2000;21(4):690–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Lefournier V, Martinie M, Vasdev A, Bessou P, Passagia JG, Labat-Moleur F, et al. Accuracy of bilateral inferior petrosal or cavernous sinuses sampling in predicting the lateralization of Cushing’s disease pituitary microadenoma: influence of catheter position and anatomy of venous drainage. J Clin Endocrinol Metab. 2003;88(1):196–203. [DOI] [PubMed] [Google Scholar]
- 37.Hana V Jr., Jezkova J, Kosak M, Krsek M, Hana V, Hill M. Serum steroid profiling in Cushing’s syndrome patients. J Steroid Biochem Mol Biol. 2019;192:105410.*
- 38.Eisenhofer G, Masjkur J, Peitzsch M, Di Dalmazi G, Bidlingmaier M, Gruber M, et al. Plasma Steroid Metabolome Profiling for Diagnosis and Subtyping Patients with Cushing Syndrome. Clin Chem. 2018;64(3):586–96.*
- 39.Masjkur J, Gruber M, Peitzsch M, Kaden D, Di Dalmazi G, Bidlingmaier M, et al. Plasma Steroid Profiles in Subclinical Compared With Overt Adrenal Cushing Syndrome. J Clin Endocrinol Metab. 2019;104(10):4331–40.*
- 40.Hines JM, Bancos I, Bancos C, Singh RD, Avula AV, Young WF, et al. High-Resolution, Accurate-Mass (HRAM) Mass Spectrometry Urine Steroid Profiling in the Diagnosis of Adrenal Disorders. Clin Chem. 2017;63(12):1824–35.*
- 41.Bancos I, Alahdab F, Crowley RK, Chortis V, Delivanis DA, Erickson D, et al. THERAPY OF ENDOCRINE DISEASE: Improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(6):R283–R95. [DOI] [PubMed] [Google Scholar]
- 42.Elhassan YS, Alahdab F, Prete A, Delivanis DA, Khanna A, Prokop L, et al. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess: A Systematic Review and Meta-analysis. Ann Intern Med. 2019;171(2):107–16. [DOI] [PubMed] [Google Scholar]
- 43.Yener S, Yilmaz H, Demir T, Secil M, Comlekci A. DHEAS for the prediction of subclinical Cushing’s syndrome: perplexing or advantageous? Endocrine. 2015;48(2):669–76. [DOI] [PubMed] [Google Scholar]
- 44.Ueland GA, Grinde T, Methlie P, Kelp O, Lovas K, Husebye ES. Diagnostic testing of autonomous cortisol secretion in adrenal incidentalomas. Endocr Connect. 2020;9(10):963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dennedy MC, Annamalai AK, Prankerd-Smith O, Freeman N, Vengopal K, Graggaber J, et al. Low DHEAS: A Sensitive and Specific Test for the Detection of Subclinical Hypercortisolism in Adrenal Incidentalomas. J Clin Endocrinol Metab. 2017;102(3):786–92. [DOI] [PubMed] [Google Scholar]
- 46.Fanelli F, Di Dalmazi G. Serum steroid profiling by mass spectrometry in adrenocortical tumors: diagnostic implications. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):160–5. [DOI] [PubMed] [Google Scholar]
- 47.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78(3):C113–8. [DOI] [PubMed] [Google Scholar]
- 48.Storbeck KH, Schiffer L, Baranowski ES, Chortis V, Prete A, Barnard L, et al. Steroid Metabolome Analysis in Disorders of Adrenal Steroid Biosynthesis and Metabolism. Endocr Rev. 2019;40(6):1605–25.*
- 49.Bancos I, Taylor AE, Chortis V, Sitch AJ, Jenkinson C, Davidge-Pitts CJ, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773–81.*
- 50.Di Dalmazi G, Fanelli F, Mezzullo M, Casadio E, Rinaldi E, Garelli S, et al. Steroid Profiling by LC-MS/MS in Nonsecreting and Subclinical Cortisol-Secreting Adrenocortical Adenomas. J Clin Endocrinol Metab. 2015;100(9):3529–38.*
- 51.Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol. 2014;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol. 2015;173(2):D1–12. [DOI] [PubMed] [Google Scholar]
- 53.Grebe SK. Laboratory testing in thyroid disorders. Luster M, Duntas L, Wartofsky L, editors. Cham, Switzerland: Springer International Publishing; 2019. [Google Scholar]
- 54.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16(9):1713–9. [DOI] [PubMed] [Google Scholar]
- 55.Gaudl A, Kratzsch J, Ceglarek U. Advancement in steroid hormone analysis by LC-MS/MS in clinical routine diagnostics - A three year recap from serum cortisol to dried blood 17alpha-hydroxyprogesterone. J Steroid Biochem Mol Biol. 2019;192:105389. [DOI] [PubMed] [Google Scholar]
- 56.Grebe SK, Singh RJ. LC-MS/MS in the Clinical Laboratory - Where to From Here? Clin Biochem Rev. 2011;32(1):5–31. [PMC free article] [PubMed] [Google Scholar]
- 57.Ketha H, Kaur S, Grebe SK, Singh RJ. Clinical applications of LC-MS sex steroid assays: evolution of methodologies in the 21st century. Curr Opin Endocrinol Diabetes Obes. 2014;21(3):217–26. [DOI] [PubMed] [Google Scholar]
- 58.Vesper HW, Bhasin S, Wang C, Tai SS, Dodge LA, Singh RJ, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids. 2009;74(6):498–503. [DOI] [PubMed] [Google Scholar]
- 59.Grebe SKG, Singh RJ. Clinical peptide and protein quantification by mass spectrometry (MS). Trac-Trend Anal Chem. 2016;84:131–43. [Google Scholar]
- 60.Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617–20.*
- 61.Ulick S, Wang JZ, Blumenfeld JD, Pickering TG. Cortisol inactivation overload: a mechanism of mineralocorticoid hypertension in the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1992;74(5):963–7. [DOI] [PubMed] [Google Scholar]
- 62.Kotlowska A, Puzyn T, Sworczak K, Stepnowski P, Szefer P. Metabolomic Biomarkers in Urine of Cushing’s Syndrome Patients. Int J Mol Sci. 2017;18(2).*