Abstract
Air pollution is associated with preterm birth (PTB), potentially via inflammation. We recently showed the mixture benzene, toluene, ethylbenzene, and xylene (BTEX) is associated with PTB. We examined if ambient BTEX exposure is associated with mid-pregnancy inflammation in a sample of 140 African-American women residing in Detroit, Michigan. The Geospatial Determinants of Health Outcomes Consortium study collected outdoor air pollution measurements in Detroit; these data were coupled with Michigan Air Sampling Network measurements to develop monthly BTEX concentration estimates at a spatial density of 300m2. First trimester and mid-pregnancy BTEX exposure estimates were assigned to maternal address. Mid-pregnancy (mean 21.3 ± 3.7 weeks gestation) inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin [IL]-6, IL-10, IL-1β, and tumor necrosis factor-α) were measured with enzyme immunoassays. After covariate adjustment, for every 1-unit increase in first trimester BTEX, there was an expected mean increase in log-transformed IL-1β of 0.05 ± 0.02 units (P = 0.014) and an expected mean increase in log-transformed tumor necrosis factor-α of 0.07 ± 0.02 units (P = 0.006). Similarly, for every 1-unit increase in mid-pregnancy BTEX, there was a mean increase in log IL-1β of 0.06 ± 0.03 units (P = 0.027). There was no association of either first trimester or mid-pregnancy BTEX with high-sensitivity C-reactive protein, IL-10, or IL-6 (all P > 0.05) Ambient BTEX exposure is associated with inflammation in mid-pregnancy in African-American women. Future studies examining if inflammation mediates associations between BTEX exposure and PTB are needed.
Keywords: air pollution, BTEX, inflammation, cytokines, racial disparity
1. Introduction1
Air pollutants impact birth outcomes (Nieuwenhuijsen et al. 2013, Guo et al. 2019) including preterm birth (PTB). We (Cassidy-Bushrow et al. 2020) and others (Llop et al. 2010, Santos and Nascimento 2019, Serrano-Lomelin et al. 2019) found exposure to the mixture benzene, toluene, ethylbenzene, and xylene (BTEX) or to individual BTEX components in ambient air is associated with PTB. BTEX compounds are volatile organics in the atmosphere originating primarily from motor vehicle exhaust and local stationary sources (Brunekreef and Holgate 2002, Hinwood et al. 2007). While a small body of evidence links benzene with measures of immune function and inflammation (Baiz et al. 2011, Minciullo et al. 2014), to our knowledge no studies have specifically examined if ambient BTEX exposure is associated with altered inflammation in pregnancy.
Successful pregnancy requires tight control of both the maternal and fetal immune system (Mor et al. 2011, Mor et al. 2017). Dysregulation of maternal and fetal immune systems is linked to adverse pregnancy outcomes (Christiaens et al. 2008, Chatterjee et al. 2014, Ragsdale et al. 2019). For example, inflammation is a critical mediator of parturition; however, when triggered earlier, such as in cases of infection, the resulting inflammatory cascade can induce PTB (Nadeau et al. 2016). Inflammatory processes may impact associations between air pollutants, such as BTEX, and PTB (Vadillo-Ortega et al. 2014). Adverse health effects from benzene may be due to chronic inflammation and an immune-mediated response (Gross and Paustenbach 2018). Minciullo et al. (2014) summarized evidence of altered cytokine expression due to benzene, which included tumor necrosis factor-α (TNF-α), Interleukin (IL)-1β, IL-2, and IL-10. However, there is limited information on how prenatal BTEX exposure impacts the maternal immune system.
Detroit, Michigan has one of the highest PTB rates in the United States, with African-American women in Michigan having a 54% higher PTB rate than non-African American women (March of Dimes 2018, Martin et al. 2019). African-American women are also more likely to be exposed to environmental toxicants (Burris et al. 2011, Burris and Hacker 2017). Thus, the goal of this study was to examine if ambient BTEX exposure estimated through high resolution spatial monitoring (Miller et al. 2010, Lemke et al. 2014, O’Leary and Lemke 2014, Miller et al. 2019) was associated with inflammatory biomarkers in the second trimester of pregnancy in African-American women in Detroit (Cassidy-Bushrow et al. 2012, Bobbitt et al. 2015).
2. Materials and Methods
2.1. Study Population
The study population has been described previously (Cassidy-Bushrow et al. 2012, Bobbitt et al. 2015). Pregnant African-American women who were patients in the Henry Ford Health System (HFHS), ages 18-44 years, and in the second trimester of pregnancy (~13-28 weeks gestation) were eligible. Recruitment spanned February 2009 to June 2010. Written informed consent was obtained. Study protocols were approved by Institutional Review Boards at HFHS and Wayne State University.
A total of 203 women completed the study; 142 resided in the boundaries of air pollution measurement (i.e., within Detroit as described below). Two women without inflammatory biomarker measurements were excluded. Our final analytic sample size was 140 women.
2.2. BTEX Estimation and Exposure Assignment
The Geospatial Determinants of Health Outcomes Consortium (GeoDHOC) performed high resolution spatial monitoring of air pollution in Detroit at 68 sites during September 5-20, 2008 and 86 sites during May 29-June 13, 2009 (Miller et al. 2010, Lemke et al. 2014, O’Leary and Lemke 2014, Miller et al. 2019). During the sampling period, passive samplers measured BTEX concentrations at a spatial sampling density of ~5 km2 per sample. Ordinary kriging was used to model total BTEX concentration at a geospatial resolution approximating a city block scale (i.e., 300 m x 300 m); these models were combined with temporally detailed 3-year air contaminant time series data from the Michigan Air Sampling Network to generate monthly BTEX concentration maps for Detroit covering January 2008 through December 2010 (O’Leary and Lemke 2014).
Maternal residential address at the time of inflammatory biomarker measurement was self-reported and used to estimate ambient BTEX exposures. We defined the bounds of exposure by last menstrual period (LMP) date (lower bound) and date of inflammatory biomarker measure (upper bound). Exposures for pregnancies with a LMP date on or before the 15th of each month were considered to begin in that month while exposures for pregnancies with a LMP date after the 15th of a month were considered to begin the following month (Cassidy-Bushrow et al. 2020). We calculated mid-pregnancy BTEX exposure by averaging the monthly BTEX exposure estimates for each month of the pregnancy through the time of inflammatory biomarker measurement. A similar approach was used to estimate average first trimester BTEX exposure. For visualization purposes, first trimester and mid-pregnancy BTEX exposure was dichotomized at the median (7.30 μg/m3 and 7.98 μg/m3 for first trimester and mid-pregnancy BTEX, respectively). The relative fraction of individual BTEX components observed in the GeoDHOC and Michigan Air Sampling Network measurements are presented in Supplementary Table S1.
2.3. Mid-Pregnancy Inflammatory Biomarkers
All assays were performed with serum obtained in the second trimester (stored at −80°) (Cassidy-Bushrow et al. 2012). High-sensitivity C-reactive protein (hs-CRP; mg/L) was measured by enzyme immunoassay (BioCheck, Inc., Foster City, CA). The limit of detection (LOD) was 0.1 mg/L. IL-1β, IL-6, IL-10 and TNF-α (pg/ml) were assayed on a Bio-Plex 200 System using Bio-Plex Pro Cytokine Assay custom 4-plex kits (Bio-Rad, Hercules, CA); the LOD was 0.6, 2.6, 0.3, and 6.0 pg/ml, respectively. Women with inflammatory biomarkers < LOD were set to the mid-point between 0 and LOD (Alper et al. 2010). We calculated the IL-6 to IL-10 ratio as a measure of the pro-inflammatory to anti-inflammatory milieu and the IL-10 to TNF-α ratio as a measure of the anti-inflammatory to pro-inflammatory milieu (Taniguchi et al. 1999, Kaislasuo et al. 2020).
2.4. Pilot Analysis: BTEX Metabolite Measurement
Our ambient BTEX exposure assignments reflect estimated exposures, which may be subject to misclassification bias (Bove et al. 2002). We measured two BTEX metabolites in maternal plasma: the benzene metabolite N-acetyl-S-phenyl-L-cysteine (PMA) and the toluene metabolite N-acetyl-S-benzyl-L-cysteine (BMA) in a subset of 25 women. An online concentration high-performance liquid chromatography/electrospray ionization tandem mass spectrometry method was utilized; online concentration provides a high throughput workflow that provides economic and quick response time advantages compared to solid phase extraction (SPE) performed by manual or automated technologies. This concentrating step provides two orders of magnitude of method sensitivity as compared to SPE methods. The metabolites are separated by reversed phase chromatography and quantified by multiple reaction monitoring. For both metabolites, the LOD was 3.0 parts per trillion; data were reported as (1) a quantitative level, (2) an assignment of being detectable but under the limit of quantification, or (3) as undetectable. For every 10 samples that were analyzed, a duplicate and spiked (plasma fortified with a known concentration of the standard) sample were run. The percent recovery for spiked samples was between 73%-124% for PMA and 97%-119% for BMA. The percent relative standard deviation for duplicate samples run was 12%-28% for BMA (none of the duplicate samples contained PMA). Because of the large number that were undetectable (68% PMA and 16% BMA), for analysis women were categorized as having detectable vs. undetectable metabolite levels for each metabolite.
2.5. Covariate Assessment
Participants self-reported date of birth, marital status, education, employment status, cigarette smoking, alcohol use, and pre-pregnancy height and weight. Pre-pregnancy body mass index (BMI) was calculated as weight (kg)/height (m2). Parity was abstracted from the medical record.
2.6. Statistical Analysis
SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses. Each inflammatory biomarker, after log-transformation to reduce non-normality, was examined separately. We compared women included and excluded from the analysis by demographic characteristics and inflammatory biomarker variables using an independent t-test for continous variables and a chi-square test for categorical variables.
Linear regression models were used to examine the association of BTEX exposure (first trimester and mid-pregnancy, separately) with each inflammatory biomarker/ratio. The 140 women resided in 127 unique census tracts, thus there was minimal clustering and models did not account for spatial clustering. Models were fit unadjusted, and then adjusted for potential confounding variables previously identified as being associated with ≥2 of the inflammatory biomarkers (maternal age, pre-pregnancy BMI, and education) (Cassidy-Bushrow et al. 2012). Although all inflammatory biomarkers were measured in the second trimester, the gestational age at the time of measurement varied from 13.1 to 27.9 weeks; inflammatory biomarker levels change over pregnancy (Ferguson et al. 2014), thus we refit models additionally adjusted for gestational age at time of measurement.
Benzene (PMA) and toluene (BMA) metabolites were available for 25 women. A Wilcoxon rank sum test was used to compare ambient BTEX in both the first trimester and mid-pregnancy and each inflammatory biomarker by whether or not each metabolite was detectable.
3. Results
Table 1 presents the comparison of the 140 women included in the analysis to the 58 women excluded from the analysis. While there was no difference in any inflammatory biomarker (all P > 0.160), women excluded from the analysis were statistically significantly more likely to be married, to have at least a high school education, to be currently employed, and to have a lower mean parity. The mean gestational age at the time of inflammatory biomarker measurement was 21.3 ±3.7 weeks. The mean BTEX exposure in the first trimester was 7.9 ± 2.3 μg/m3 and in mid-pregnancy was 8.0 ±1.8 μg/m3 (Table 1); these were statistically significantly correlated (r = 0.77, P < 0.001). Only 14 women (10%) experienced PTB; neither BTEX estimate was associated with PTB (all P > 0.501).
Table 1.
Descriptive characteristics of the study population and a comparison of women included and excluded from the analytic sample.
| Variable | Included in Analytic Sample | Excluded from Analytic Sample | P-value |
|---|---|---|---|
| N | 140 | 58a | |
| Maternal age (years) | 26.3±5.8 | 27.2±6.3 | 0.366 |
| Married | 29 (20.7%) | 21 (36.2%) | 0.022 |
| ≥ High school | 116 (82.9%) | 55 (94.8%) | 0.026 |
| Currently employed | 62 (44.3%) | 40 (69.0%) | 0.002 |
| Pre-pregnancy BMI (kg/m2) | 29.5±7.7 | 28.5±7.3 | 0.395 |
| Parity | 1.1±1.2 | 0.7±0.9 | 0.012 |
| Gestational age at time of study visit (weeks) | 21.3±3.7 | 20.7±3.8 | 0.289 |
| Smoked during pregnancy | 12 (8.6%) | 2 (3.5%) | 0.241 |
| Alcohol consumption in last month | 6 (4.3%) | 1 (1.7%) | 0.676 |
| Log hs-CRPb | 1.19±1.08 | 1.12±0.76 | 0.635 |
| Log IL-6c | 1.28±0.56 | 1.14±0.62 | 0.160 |
| Log IL-10c | 0.62±0.37 | 0.59±0.29 | 0.564 |
| Log IL-1βcc | −0.45±0.57 | −0.33±0.58 | 0.194 |
| Log TNF-αc | 1.71±0.65 | 1.78±0.68 | 0.484 |
| IL-6/IL-10 ratio | 2.22±1.12 | 2.04±1.29 | 0.368 |
| IL-10/TNF-α ratio | 0.43±0.32 | 0.38±0.25 | 0.248 |
| First trimester BTEX exposure (μg/m3)d | 7.9±2.3 | ||
| Mid-pregnancy BTEX exposure (μg/m3)e | 8.0±1.8 | ||
BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α; BTEX, benzene, toluene, ethylbenzene, xylene.
Data are mean ± standard deviation or N (%).
N = 56 for inflammatory biomarkers
On raw scale, in units of mg/L.
On raw scale, in units of pg/ml.
First trimester BTEX exposure is the average exposure during the first trimester of pregnancy.
Mid-pregnancy BTEX exposure is the average exposure from the start of pregnancy through the time of inflammatory biomarker measurement.
Bold font indicates variable with P < 0.05.
The distribution of each mid-pregnancy inflammatory biomarker and ratio by dichotomized first trimester BTEX exposure is presented in Figure 1. Women with higher first trimester BTEX exposure had statistically significant lower hs-CRP (P = 0.022) and IL-10 to TNF-α ratio (P = 0.001) and higher TNF-α (P < 0.001) and IL-1β (P = 0.028) compared to women with lower first trimester BTEX exposure; there were no differences in IL-6, IL-10, or the IL-6 to IL-10 ratio (Figure 1; all P > 0.411). After adjusting for maternal age, pre-pregnancy BMI, high school education, and gestational age at inflammatory biomarker measurement, first trimester BTEX was statistically significantly positively associated with log-transformed IL-1β (P = 0.014) and log-transformed TNF-α (P = 0.006); for every 1-unit increase in first trimester BTEX, there was an expected mean increase in log-transformed IL-1β of 0.05 ± 0.02 units and in log-transformed TNF-α of 0.07 ± 0.02 units in mid-pregnancy (Table 2). There was no association of first trimester BTEX and hs-CRP, IL-6, IL-10, the IL-6 to IL-10 ratio, or the IL-10 to TNF-α ratio (Table 2).
Figure 1.
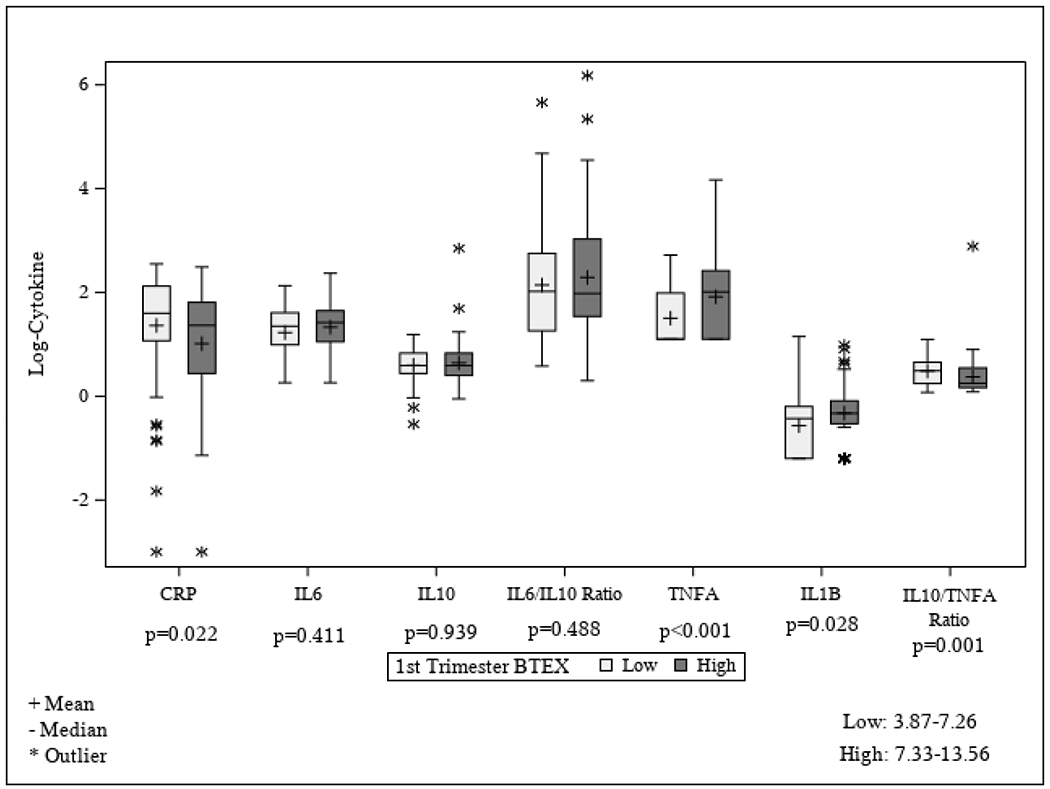
Distribution of each log-transformed inflammatory biomarker (or biomarker ratio) by first trimester BTEX exposure (defined as high exposure if above the median and low exposure if below the median; median = 7.30 μg/m3). First trimester BTEX exposure is the average exposure during the first trimester of pregnancy. BTEX, benzene, toluene, ethylbenzene, xylene; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α.
Table 2:
Association of first trimester BTEX exposure and mid-pregnancy BTEX exposure with mid-pregnancy inflammatory biomarkers
| Variable | Log hs-CRP | Log IL-6 | Log IL-10 | Log IL-1β | Log TNF-α | IL-6/IL-10 | IL-10/TNF-α |
|---|---|---|---|---|---|---|---|
| β±se | β±se | β±se | β±se | β±se | β±se | β±se | |
| (P-value) | (P-value) | (P-value) | (P-value) | (P-value) | (P-value) | (P-value) | |
| First trimester BTEX exposure (μg/m3)a | |||||||
| Unadjusted | −0.04±0.04 | 0.02±0.02 | 0.02±0.01 | 0.05±0.02 | 0.06±0.02 | 0.02±0.04 | −0.0±0.01 |
| (p=0.302) | (p=0.295) | (p=0.197) | (p=0.020) | (p=0.009) | (p=0.667) | (p=0.719) | |
| Adjusted for maternal age, pre-pregnancy | −0.02±0.03 | 0.03±0.02 | 0.02±0.01 | 0.05±0.02 | 0.06±0.02 | 0.03±0.04 | −0.00±0.01 |
| BMI, and high school education | (p=0.494) | (p=0.154) | (p=0.184) | (p=0.020) | (p=0.008) | (p=0.458) | (p=0.737) |
| Adjusted for maternal age, pre-pregnancy | −0.02±0.03 | 0.03±0.02 | 0.02±0.01 | 0.05±0.02 | 0.07±0.02 | 0.04±0.04 | −0.01±0.01 |
| BMI, high school education, and GA at inflammatory biomarker measurement | (p=0.609) | (p=0.135) | (p=0.206) | (p=0.014) | (p=0.006) | (p=0.372) | (p=0.656) |
| Mid-pregnancy BTEX exposure (μg/m3)b | |||||||
| Unadjusted | −0.11±0.05 | −0.0±0.03) | 0.04±0.02 | 0.05±0.03 | 0.04±0.03 | −0.06±0.05 | 0.01±0.02 |
| (p=0.033) | (p=0.900) | (p=0.044) | (p=0.065) | (p=0.190) | (p=0.285) | (p=0.552) | |
| Adjusted for maternal age, pre-pregnancy | −0.07±0.04 | 0.01±0.03 | 0.04±0.02 | 0.05±0.03 | 0.04±0.03 | −0.03±0.05 | 0.01±0.02 |
| BMI, and high school education | (p=0.110) | (p=0.787) | (p=0.043) | (p=0.049) | (0.166) | (p=0.496) | (p=0.525) |
| Adjusted for maternal age, pre-pregnancy | −0.06±0.04 | 0.01±0.03 | 0.04±0.02 | 0.06±0.03 | 0.05±0.03 | −0.02±0.05 | 0.01±0.02 |
| BMI, high school education, and GA at inflammatory biomarker measurement | (p=0.189) | (p=0.700) | (p=0.052) | (p=0.027) | (p=0.115) | (p=0.661) | (p=0.647) |
β, parameter estimate; se, standard error; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α; BTEX, benzene, toluene, ethylbenzene, xylene; GA, gestational age
First trimester BTEX exposure is the average exposure during the first trimester of pregnancy
Mid-pregnancy BTEX exposure is the average exposure from the start of pregnancy through the time of inflammatory biomarker measurement
Bold font indicates variable with P < 0.05
The distribution of each inflammatory biomarker and ratio by dichotomized mid-pregnancy BTEX exposure is presented in Figure 2; there were no statistically significant differences in any inflammatory biomarker or ratio between women in the higher vs. lower mid-pregnancy BTEX exposure category (all P > 0.054). After adjusting for maternal age, pre-pregnancy BMI, high school education, and gestational age at inflammatory biomarker measurement, for every 1-unit increase in mid-pregnancy BTEX, there was a mean increase in log IL-1β of 0.06 ± 0.03 units (P = 0.027). There was no association of mid-pregnancy BTEX exposure and hs-CRP, IL-6, IL-10, TNF-α, the IL-6 to IL-10 ratio, and the IL-10 to TNF-α ratio after covariate adjustment (Table 2).
Figure 2.
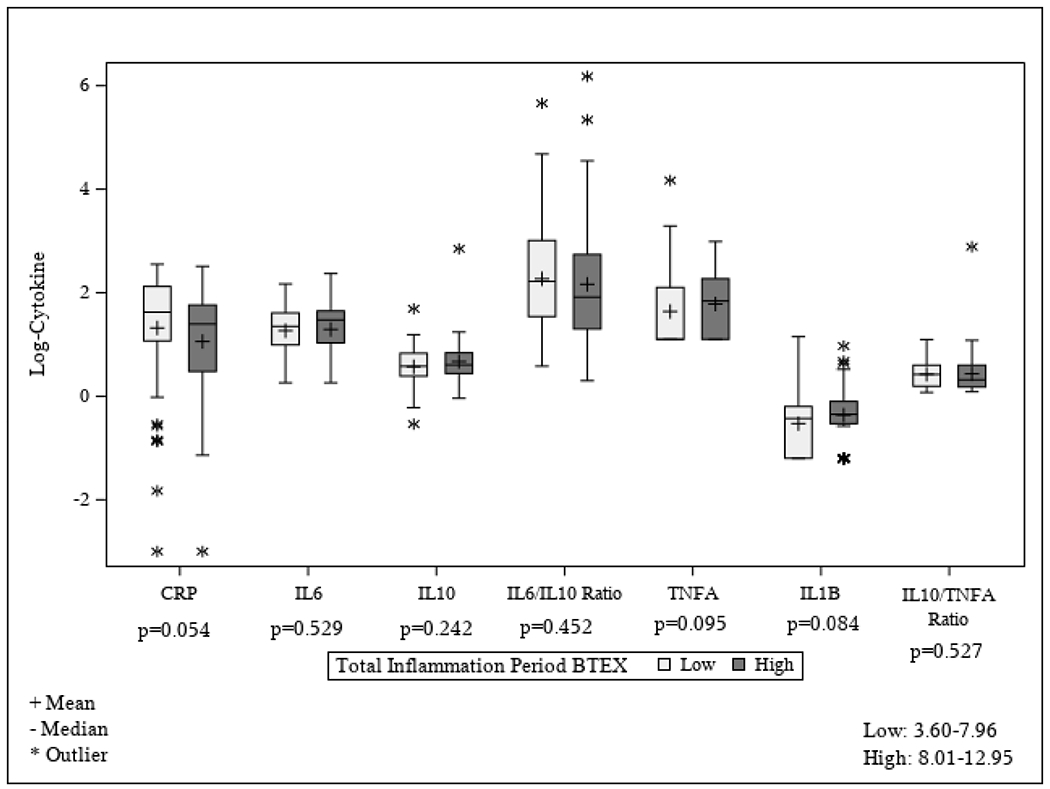
Distribution of each log-transformed inflammatory biomarker (or biomarker ratio) by mid-pregnancy BTEX exposure (defined as high exposure if above the median and low exposure if below the median; median = 7.98 μg/m3). Mid-pregnancy BTEX exposure is the average exposure from the start of pregnancy through the time of inflammatory biomarker measurement. BTEX, benzene, toluene, ethylbenzene, xylene; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α.
3. Benzene (PMA) and Toluene (BMA) Metabolites
In the subset of 25 women with metabolite data, 8 (32%) women had detectable PMA and 21 (84%) had detectable BMA. Although not statistically significant (Table 3; all P > 0.51), women with detectable BMA had higher first trimester BTEX exposure levels compared to women without detectable BMA and women with detectable PMA had higher first trimester and mid-pregnancy BTEX exposures compared to women without detectable PMA. Women with detectable BMA had statistically significant higher mean log IL-6 and log IL-1β levels compared to women without detectable BMA (Table 3; both P = 0.04). There was no other association of BMA with any inflammatory biomarker or with PMA and any inflammatory biomarker (Table 3).
Table 3.
Pilot analysis comparing ambient BTEX levels (first trimester and mid-pregnancy) and inflammatory biomarkers by detectable vs. non-detectable benzene (PMA) and toluene (BMA) metabolites.
| PMA (benzene) | BMA (toluene) | |||||
|---|---|---|---|---|---|---|
| Measure | Detectable | Non-detectable | P-value | Detectable | Non-detectable | P-value |
| N | 8 | 17 | 21 | 4 | ||
| First trimester BTEX (μg/m3)a | 7.44±1.19 | 7.12±1.33 | 0.67 | 7.31±1.30 | 6.79±1.16 | 0.51 |
| Mid-pregnancy BTEX (μg/m3)b | 7.44±1.44 | 6.93±1.49 | 0.58 | 7.01±1.30 | 7.44±2.28 | 0.67 |
| Log hs-CRP | 1.14±0.80 | 0.91±0.98 | 0.76 | 1.05±0.82 | 0.62±1.41 | 0.48 |
| Log IL-6 | 1.41±0.33 | 1.27±0.72 | 0.87 | 1.43±0.58 | 0.71±0.57 | 0.04 |
| Log IL-10 | 0.55±0.27 | 0.37±0.31 | 0.13 | 0.50±0.18 | 0.08±0.56 | 0.11 |
| Log IL-1β | −0.58±0.49 | −0.35±0.49 | 0.28 | −0.33±0.47 | −0.84±0.42 | 0.04 |
| Log TNF-α | 1.84±0.74 | 1.85±0.79 | 0.87 | 1.95±0.78 | 1.35±0.51 | 0.15 |
| IL-6/IL-10 | 2.44±0.79 | 2.92±1.85 | 0.63 | 2.92±1.67 | 2.11±1.27 | 0.31 |
| IL-10/TNF-α | 0.34±0.24 | 0.30±0.21 | 0.63 | 0.30±0.20 | 0.38±0.39 | 0.61 |
BTEX, benzene, toluene, ethylbenzene, xylene; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor-α.
First trimester BTEX exposure is the average exposure during the first trimester of pregnancy
Mid-pregnancy BTEX exposure is the average exposure from the start of pregnancy through the time of inflammatory biomarker measurement
Data are mean ± standard deviation.
Bold font indicates variable with P < 0.05
4. Discussion
We found higher estimated BTEX exposure was associated with higher levels of the inflammatory biomarkers IL-1β and TNF-α in mid-pregnancy in a sample of pregnant African-American women. Overall, the most consistent evidence was for an association of BTEX exposure with higher levels of mid-pregnancy IL-1β. This finding is supported by evidence both from estimated ambient BTEX exposure and from the toluene metabolite BMA in maternal plasma. We recently showed that higher ambient BTEX exposure in pregnancy is associated with elevated risk of PTB in Detroit (Cassidy-Bushrow et al. 2020), thus the current study suggests that inflammatory mechanisms may be involved in the association between BTEX exposure and PTB.
Exposure to BTEX (or its components) is associated with alterations in inflammatory biomarkers; however, most evidence comes from studies in men and non-pregnant women. In the Gulf Long-Term Follow-up study (GuLF), benzene and toluene, measured in blood, were positively associated with IL-1β levels in obese men but inversely associated with IL-1β levels in non-obese men (Werder et al. 2020). In our cohort, where the majority of women were overweight (26.4%) or obese (41.6%) pre-pregnancy (Cassidy-Bushrow et al. 2012), we also found evidence that higher ambient BTEX exposure, as well as having detectable levels of a toluene metabolite, was associated with higher IL-1β levels. Evidence supporting a role of BTEX or its constituents with TNF-α is mixed. In a study of adults in Iran, residing in areas with higher levels of the mixture benzene, toluene, xylene and styrene was associated with higher TNF-α levels (Samadi et al. 2019). In contrast, in men in the GulF study, neither benzene, toluene or ethylbenzene were associated with TNF-α (Werder et al. 2020).
Normal pregnancy is characterized by changes in the maternal immune system (Mor et al. 2011, Mor et al. 2017). Atypical alterations in inflammatory biomarkers are associated with poorer birth outcomes. In a study of 495 women in Malaysia, plasma IL-1β at the time of labor was higher in women who delivered PTB (Langmia et al. 2016). In the Pregnancy Outcomes and Community Health study, conducted in Michigan, higher mid-pregnancy IL-1β but not TNF-α was associated with greater odds of PTB (Gargano et al. 2008). In data from the Perinatal Research Center in Nashville, TN, in African-American women both IL-1β and TNF-α in plasma at the time of labor were statistically significantly higher in those with PTB, whereas in Caucasian women, there was no statistically significant difference by PTB status in these biomarkers (Brou et al. 2012). In women with a history of previous PTB, higher second trimester serum TNF-α is associated with increased risk of recurrent PTB (Vogel et al. 2007). In a review by Pandey et al. (2017), TNF-α is described as a pro-inflammatory cytokine that promotes production of metalloproteinases, which in turn can weaken fetal membrane strength and block progesterone release, each of which are associated with PTB. IL-1β, which is the inflammatory biomarker most consistently associated with BTEX in the current study, is also a pro-inflammatory cytokine; higher levels of IL-1β may stimulate prostaglandin secretion and myometrium contraction, which can lead to PTB (Equils et al. 2020). BTEX may influence the risk of PTB by altering inflammatory cytokines that have a potential biologic role in PTB. Importantly, the biomarkers in the current study were measured during mid-pregnancy, prior to onset of labor. Many of the aforementioned studies measured biomarkers at parturition and differences in inflammatory biomarkers during labor may relate more strongly to differences in parturition of a preterm versus term infant and could account for discrepancies in findings.
Mechanistically, BTEX may lead to altered systemic inflammation via activation of innate immune system cells (including Natural Killer [NK] cells) (Guo et al. 2020); these cells help maintain a normal tissue microenvironment by protecting against pathogens and irritants. Once innate immune system cells are activated, they differentiate and produce a wide range of inflammatory cytokines to recruit and activate additional immune cells, thereby amplifying the inflammatory response until the foreign stimuli is removed. However, previous studies have largely focused on the toxic effects of benzene, rather than BTEX as a whole. As described in the review by Bahadar et al (2014), benzene exposure can negatively impact numerous organ systems, including the hematopoietic, immune and reproductive systems, with the toxicity occurring via mechanisms including formation of reactive oxygen species, oxidative DNA damage, DNA methylation changes, and chromosomal abnormalities. Reactive oxygen species, for example, are produced by NK cells in response to foreign stimuli and can further enhance the inflammatory process by promoting cell death of infected or damaged cells (Hensley et al. 2000). A better understanding of the molecular mechanisms by which BTEX exposure may lead to increased inflammation is needed.
We found some differences in associations comparing first trimester BTEX exposure to mid-pregnancy BTEX exposure with the inflammatory biomarkers. After adjusting for selected risk factors, ambient BTEX exposure level in the first trimester was significantly associated with IL-1β and TNF-α while mid-pregnancy BTEX exposure was only associated with IL-1β. In the current study, data on inflammation was available only at a single time point obtained during the second trimester; inflammatory biomarker levels change over pregnancy (Ferguson et al. 2014), thus we may be missing important time points of measurement and interactions of these biomarkers. Future studies with additional timed measurements of BTEX exposure and inflammatory biomarkers are needed to better understand temporal relationships.
We used maternal address reported at the time of inflammatory biomarker measurement to estimate BTEX exposure. Change in residence and lack of accounting for mother’s work or other addresses may result in exposure misclassification. Further, indoor BTEX levels can be greater than outdoor levels (Minciullo et al. 2014), thus estimates based on outdoor levels may underestimate true exposure. We conducted a pilot study measuring two BTEX metabolites in maternal plasma, PMA and BMA, to capture a measure of direct, rather than estimated, BTEX exposure. While urine is the preferred matrix for measuring BTEX metabolites, we did not have available urine samples. Although not statistically significant, women with detectable PMA had higher estimated BTEX exposure levels (both in the first trimester and mid-pregnancy) than women without detectable PMA; women with detectable BMA also had higher estimated first trimester BTEX exposure compared to women without detectable BMA. This is in contrast to a study of 61 women in Cape Town, South Africa, where personal BTEX exposure (and the individual BTEX constituents) was measured over a 7-day period using compact passive diffusion samplers and PMA and BMA measured in urine; higher personal toluene exposure was not associated with BMA (r = 0.12, P > 0.05) but was statistically significantly and inversely associated with PMA (r = −0.22, P < 0.05) and personal benzene exposure was not associated with PMA (r = 0.01, P > 0.05) or BMA (r = −0.07, P > 0.05) (Everson et al. 2019). Given our small sample size (n = 25 with metabolite and ambient BTEX exposure data), we may be underpowered. Women with detectable BMA also had higher levels of IL-6 and IL-1β. Due to the small sample size, we did not adjust for potential confounders; however, these results, together with the consistent evidence that both first trimester and mid-pregnancy estimated BTEX exposure were also associated with IL-1β, provide further support that maternal BTEX exposure is associated with inflammation. Future studies that capture additional address information and measure indoor exposure levels and direct exposure to environmental contaminants (e.g., additional metabolites of BTEX) are needed to better understand the association of BTEX and inflammation in pregnancy.
4.1. Strengths and Limitations
Our pollution models were only applicable to women residing within Detroit; thus only 69.0% of the original cohort was included in the analysis. Although there were some expected socioeconomic differences in women included vs. excluded from the analytic sample, there was no difference in inflammatory biomarker levels. Our results may not be generalizable to women of other races or to women living outside of major urban cities. While the current study only measured circulating/systemic markers of inflammation, it is important to note that the placenta, a key player in preterm labor, is a source of inflammatory markers in maternal circulation.
Nonetheless, measures of in utero inflammation, such as in cervicovaginal or amniotic fluid may be a more specific marker of inflammation relevant to PTB (Buxton et al. 2019), but, these require more invasive approaches. Future studies should consider measurements of systemic inflammation with confirmation of in utero or histologic inflammation in a subset of participants. As only 14 women in the analytic sample had a PTB, we were unable to conduct a mediation analysis; future studies examining the potential mediating effect of inflammation between BTEX exposure and PTB are needed.
Strengths of the current study include that our estimates of ambient air pollution exposure have uniquely high spatial and temporal resolution capable of representing exposure variability during pregnancy. Such data may be critical to more accurately quantify neighborhood scale and seasonally varying exposure levels (Ross et al. 2013). We were able to explore potential confounders, including maternal education and pre-pregnancy BMI. Importantly, this study focuses on African-American pregnant women, a group at highest risk for PTB (Martin et al. 2019).
4.2. Conclusion
African-American women are disproportionately more likely to experience adverse birth outcomes (Martin et al. 2019) and be exposed to environmental toxicants (Burris et al. 2011, Burris and Hacker 2017). Our study showing ambient BTEX exposure is associated with mid-pregnancy inflammation may lead to future studies of environmental factors and potential mechanisms driving racial disparities in women and children’s health.
Supplementary Material
Highlights.
Exposure to BTEX is associated with PTB
BTEX is associated with inflammation in the non-pregnant state
BTEX exposure is positively associated with mid-pregnancy IL-1β and TNF-α
BTEX may influence PTB via inflammation, but this requires further study
Acknowledgements
Funding:
This work was supported by the Institute for Population Sciences, Health Assessment, Administration, Services, and Economics (INPHAASE), the Kellogg Foundation and the Center for Urban Response to Environmental Stressors NIH P30 ES020957. The study sponsors were not involved in the design of the study, in the collection, analysis or interpretation of data, in the writing of the manuscript or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: BMA, N-acetyl-S-benzyl-L-cysteine; BMI, body mass index; BTEX, benzene, toluene, ethylbenzene, and xylene; GeoDHOC, Geospatial Determinants of Health Outcomes Consortium; GulF, Gulf Long-Term Follow-up study; HFHS, Henry Ford Health System; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; LMP, last menstrual period; LOD, limit of detection; NK, Natural Killer; PMA, N-acetyl-S-phenyl-L-cysteine; PTB, preterm birth; SPE, solid phase extraction; TNF, tumor necrosis factor.
Conflicts of Interest
All authors state that there are no conflicts to disclose.
References
- Alper CM, et al. , 2010. Nasal secretion concentrations of IL-5, IL-6, and IL-10 in children with and without upper respiratory tract viruses. Arch. Otolaryngol. Head Neck Surg 136, 281–6 [DOI] [PubMed] [Google Scholar]
- Bahadar H, Mostafalou S, Abdollahi M 2014. Current understandings and perspectives on non-cancer health effects of benzene: a global concern. Toxicol. Appl. Pharmacol 276, 83–94 [DOI] [PubMed] [Google Scholar]
- Baiz N, et al. , 2011. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth. 11, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbitt KR, et al. , 2015. Early pregnancy vitamin D and patterns of antenatal inflammation in african-american women. J. Reprod. Immunol 107, 52–8 [DOI] [PubMed] [Google Scholar]
- Bove F, et al. , 2002. Drinking water contaminants and adverse pregnancy outcomes: A review. Environ. Health Perspect 110, 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou L, et al. , 2012. Dysregulated biomarkers induce distinct pathways in preterm birth. BJOG. 119, 458–73 [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST, 2002. Air pollution and health. Lancet. 360, 1233–42 [DOI] [PubMed] [Google Scholar]
- Burris HH, et al. , 2011. Racial/ethnic disparities in preterm birth: Clues from environmental exposures. Curr. Opin. Pediatr 23, 227–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Hacker MR, 2017. Birth outcome racial disparities: A result of intersecting social and environmental factors. Semin. Perinatol 41, 360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton MA, et al. , 2019. Air pollution and inflammation: Findings from concurrent repeated measures of systemic and reproductive tract cytokines during term pregnancy in Mexico City. Sci. Total Environ 681, 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, et al. , 2020. Prenatal airshed pollutants and preterm birth in an observational birth cohort study in Detroit, Michigan, USA. Environ. Res 189, 109845. [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, et al. , 2012. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J. Reprod. Immunol 94, 202–9 [DOI] [PubMed] [Google Scholar]
- Chatterjee P, et al. , 2014. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 5, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens I, et al. , 2008. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol 79, 50–7 [DOI] [PubMed] [Google Scholar]
- Equils O, et al. , 2020. The role of the IL-1 system in pregnancy and the use of IL-1 system markers to identify women at risk for pregnancy complications. Biol. Reprod 103, 684–694 [DOI] [PubMed] [Google Scholar]
- Everson F, et al. , 2019. Personal NO2 and volatile organic compounds exposure levels are associated with markers of cardiovascular risk in women in the Cape Town region of South Africa. Int. J. Environ. Res. Public Health. 16, 2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2014. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am. J. Reprod. Immunol 72, 326–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano JW, et al. , 2008. Mid-pregnancy circulating cytokine levels, histologic chorioamnionitis and spontaneous preterm birth. J. Reprod. Immunol 79, 100–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SA, Paustenbach DJ, 2018. Shanghai health study (2001-2009): What was learned about benzene health effects? Crit. Rev. Toxicol 48, 217–251 [DOI] [PubMed] [Google Scholar]
- Guo H, Ahn S, Zhang L 2020. Benzene-associated immunosuppression and chronic inflammation in humans: a systematic review. Occup. Environ. Med doi: 10.1136/oemed-2020-106517. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LQ, et al. , 2019. Ambient air pollution and adverse birth outcomes: A systematic review and meta-analysis. Journal of Zhejiang University. Science. B. 20, 238–252 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hensley K, et al. , 2000. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med 28, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Hinwood AL, et al. , 2007. Risk factors for increased BTEX exposure in four Australian cities. Chemosphere. 66, 533–41 [DOI] [PubMed] [Google Scholar]
- Kaislasuo J, et al. , 2020. Il-10 to Tnf-alpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am. J. Reprod. Immunol 83, e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmia IM, et al. , 2016. Impact of IL1b gene polymorphisms and interleukin 1b levels on susceptibility to spontaneous preterm birth. Pharmacogenet. Genomics 26, 505–509 [DOI] [PubMed] [Google Scholar]
- Lemke LD, et al. , 2014. Geospatial relationships of air pollution and acute asthma events across the Detroit-Windsor international border: Study design and preliminary results. J. Expo. Sci. Environ. Epidemiol 24, 346–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop S, et al. , 2010. Preterm birth and exposure to air pollutants during pregnancy. Environ Res. 110, 778–85 [DOI] [PubMed] [Google Scholar]
- March of Dimes, 2018. 2018 premature birth report card United States. Available at: https://www.marchofdimes.org/materials/PrematureBirthReportCard-United%20States-2018.pdf
- Martin JA, et al. , 2019. Births: Final data for 2018. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 68, 1–47 [PubMed] [Google Scholar]
- Miller L, et al. , 2010. Intra-urban correlation and spatial variability of air toxics across an international airshed in Detroit, Michigan (USA) and Windsor, Ontario (Canada). Atmos. Environ 44, 1162–1174 [Google Scholar]
- Miller L, et al. , 2019. Interannual variation of air quality across an international airshed in Detroit (USA) and Windsor (Canada): A comparison of two sampling campaigns in both cities. Atmos. Environ 198, 417–426 [Google Scholar]
- Minciullo PL, et al. , 2014. Cytokine network involvement in subjects exposed to benzene. J. Immunol. Res 2014, 937987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, et al. , 2017. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol 17, 469–482 [DOI] [PubMed] [Google Scholar]
- Mor G, et al. , 2011. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci 1221, 80–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau HC, et al. , 2016. Infection and preterm birth. Semin. Fetal Neonatal Med 21, 100–5 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, et al. , 2013. Environmental risk factors of pregnancy outcomes: A summary of recent meta-analyses of epidemiological studies. Environ. Health. 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary BF, Lemke LD, 2014. Modeling spatiotemporal variability of intra-urban air pollutants in Detroit: A pragmatic approach. Atmos. Environ 94, 417–427 [Google Scholar]
- Pandey M, et al. , 2017. Interplay of cytokines in preterm birth. Indian J. Med. Res 146, 316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale HB, et al. , 2019. Regulation of inflammation during gestation and birth outcomes: Inflammatory cytokine balance predicts birth weight and length. Am J Hum Biol. 31, e23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross Z, et al. , 2013. Spatial and temporal estimation of air pollutants in New York City: Exposure assignment for use in a birth outcomes study. Environ Health. 12, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi MT, et al. , 2019. Association of long term exposure to outdoor volatile organic compounds (BTXS) with pro-inflammatory biomarkers and hematologic parameters in urban adults: A cross-sectional study in Tabriz, Iran. Ecotoxicol. Environ. Saf 180, 152–159 [DOI] [PubMed] [Google Scholar]
- Santos D, Nascimento LFC, 2019. Maternal exposure to benzene and toluene and preterm birth. A longitudinal study. Sao Paulo Medical J. 137, 486–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Lomelin J, et al. , 2019. Interdisciplinary-driven hypotheses on spatial associations of mixtures of industrial air pollutants with adverse birth outcomes. Environ Int. 131, 104972. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, et al. , 1999. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit. Care Med 27, 1262–4 [DOI] [PubMed] [Google Scholar]
- Vadillo-Ortega F, et al. , 2014. Air pollution, inflammation and preterm birth: A potential mechanistic link. Med Hypotheses. 82, 219–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel I, et al. , 2007. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol. 75, 133–40 [DOI] [PubMed] [Google Scholar]
- Werder EJ, et al. , 2020. Blood BTEXS and heavy metal levels are associated with liver injury and systemic inflammation in gulf states residents. Food Chem. Toxicol 139, 111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


