Abstract
The trigeminal blink reflex plays an important role in protecting the corneal surface from damage and preserving visual function in an unpredictable environment. The closing phase of the reflex, produced by activation of the orbicularis oculi muscles, consists of an initial, small, ipsilateral R1 component, followed by a larger, bilateral R2 component. We investigated the circuitry that underlies this reflex in macaque (Macaca fascicularis and mulatta) monkeys by use of single and dual tracer methods. Injection of retrograde tracer into the facial nucleus labeled neurons in the principal trigeminal nucleus, and in the spinal nucleus pars oralis and interpolaris, bilaterally, and in pars caudalis, ipsilaterally. Injection of anterograde tracer into the principal trigeminal nucleus labeled axons that directly terminated on orbicularis oculi motoneurons, with an ipsilateral predominance. Injection of anterograde tracer into pars caudalis of the spinal trigeminal nucleus labeled axons that directly terminated on ipsilateral orbicularis oculi motoneurons. The observed pattern of labeling indicates that the reticular formation ventromedial to the principal and spinal nuclei also contributes extensive bilateral input to orbicularis oculi motoneurons. Thus, much of the trigeminal sensory complex is in a position to supply monosynaptic drive for lid closure, and the adjacent reticular formation can supply a disynaptic drive. These findings indicate that assignment of the R1 and R2 components of the blink reflex to different parts of trigeminal sensory complex cannot be exclusively based on subdivision connectional relationships with facial motoneurons. The characteristics of the R2 component may be due, instead, to other circuit properties.
Keywords: facial nucleus, trigeminal nucleus, primate, eyelid, somatosensory, classical conditioning, associative learning
Graphical Abstract

INTRODUCTION
We are able to view the world because light passes through and is refracted by the cornea. This transparent, avascular organ is made up of a specialized connective tissue covered by a delicate, noncornified, stratified squamous epithelium on its external surface. It is bathed in a tear film that supplies it with oxygen and creates a smooth refractive interface with the air. Corneal integrity is critical for proper vision; consequently, it is protected by the eyelids. These spread the tear film over the surface and can swiftly close to protect the eye through the action of the orbicularis oculi (ObOc) muscle. This muscle lies within the eyelid and extends up over the supra- and infraorbital ridges (Porter et al., 1989; Evinger, 1995). Information from a number of different sensory systems can trigger reflex activation of this muscle to produce closure of the eye in a blink (Tackmann et al., 1982; Gruart et al., 1995). One of these is the trigeminal sensory system. Indeed, the eyelids themselves boast sensitive mechanoreceptors attached to a lever system in the form of the eyelashes. These activate trigeminal primary afferents that will result in a reflex blink. Moreover, the cornea is itself densely innervated with sensory fibers, the vast majority of which are nociceptors. Their activation can produce a blink, or, if the cornea is damaged, prolonged activation of the reflex circuitry to produce continuous activation of the ObOc muscle and long term closure of the eye. In addition, information on the condition of the tear film is relayed by corneal afferents through the trigeminal sensory nuclei and influences regulation of the spontaneous blink rate that spreads the film (Cruz et al., 2011; Kaminer et al., 2011; Rahman et al., 2015; Nosch et al., 2016). Hyperactivity in the blink circuitry leads to involuntary closure of the eyes, a syndrome termed blepharospasm, for which there is currently limited etiological understanding and only symptomatic treatment (Hallett, 2002; Evinger, 2015).
Testing of the trigeminal blink reflex is part of a neurological exam (Fine et al., 1992), and this reflex has been extensively studied in both humans (Ongerboer de Visser, 1983; Evinger et al., 1991; Aramideh et al., 1997; Bour et al., 2000) and experimental animals (guinea pig: Evinger et al., 1984; Pellegrini et al., 1995; cat: Gruart et al., 1995; rabbit: Gruart et al., 2000; rat: Powers et al., 1997). In particular, this reflex has been used as a model system in a variety of studies examining associative learning using either an air puff delivered to the cornea or electrical stimulation of the supraorbital nerve as the unconditioned stimulus (rabbit: Kelly et al., 1990; Thompson, 1990; Welsh & Harvey, 1991; cat: Gruart et al., 1994; mouse: Boele et al., 2010). It has also been employed in other learning paradigms, such as habituation and prepulse inhibition (rat: Powers et al., 1997; Schicatano et al., 2000). A blink is initiated by activation of the ObOc muscle and concurrent inactivation of the levator palpebrae superioris muscle (Evinger et al., 1984; Triggo et al., 1999). Some animals also protect their eye with a nictitating membrane, and the eye can also be pulled into the orbit through the action of retractor bulbi muscles (cat: Delgado-Garcia et al., 1990). However, macaques and humans have small or vestigial nictitating membranes and lack retractor bulbi muscles. They may co-contract their rectus muscles to withdraw the eye, to supplement the regular blink lid movements. This produces blink associated eye movements (human: Bour et al., 2000; monkey: Schultz et al., 2010). The present study concentrated on the trigeminal circuits targeting ObOc motoneurons, as these represent the main controllers of the closing phase of the primate trigeminal blink reflex. These motoneurons are located in the dorsal division of the facial nucleus (VII) of macaque monkeys (Porter et al., 1989; Welt & Abbs, 1990; termed the intermediate subdivision by VanderWerf et al., 1998).
Following elicitation of the reflex by electrical activation of the supraorbital nerve, the ensuing blink is actually a complex multiphasic phenomenon. Electromyographic recordings from the orbicularis oculi muscles reveal that there is an initial, short latency response, termed the R1, followed, after a pause, by a longer latency response termed the R2 (human: Kugelberg, 1952; Aramideh & Ongerboer de Visser, 2002; guinea pig: Pellegrini et al., 1995; cat: Gruart et al., 1995; LeDoux et al., 1997). Some authorities have described an even longer latency R3 response when pain fibers are activated (human: Ellrich & Hopf, 1996). The R1 is smaller and shorter in duration than the R2 in humans, but the R1 is more substantial in most tested species. Moreover, the R1 response does not show nearly the degree of plasticity that the R2 response does (guinea pig: Evinger & Manning, 1988; Pellegrini et al., 1995). At threshold, the R1 in primates is generally unilaterally elicited, while the R2 is bilaterally evoked (Tackmann et al., 1982; Porter et al., 1993; Bour et al., 2000). However, the R2 is unilateral at threshold in the lateral-eyed animals that have been tested, with bilateral responses elicited only by stronger stimuli (rat: Basso et al., 1993; guinea pig: Pelligrini et al., 1995). Stimulation of the cornea by an air puff, in contrast, only results in the single, bilateral wave of reflex activity, with a time course similar to that of the R2 (rabbit: McCormick et al., 1982).
Several different pathways have been suggested as the source of the R1 and R2 components of the supraorbitally elicited reflex. Based on work in guinea pigs, Pelligrini and colleagues (1995) suggested that the R1 pathway is provided by a monosynaptic connection from the medullary portion of the spinal trigeminal nucleus (sV) to ObOc motoneurons, while the R2 pathway originates in pars caudalis of the spinal trigeminal nucleus (sVc) at the level of C1, and is relayed to ObOc motoneurons by interneurons in the reticular formation. Based on work in rabbits, van Ham and Yeo (1996B) suggested that the R1 pathway to ObOc motoneurons utilizes monosynaptic connections from all levels of sV, as well as the principal trigeminal nucleus (pV), while the R2 pathway utilizes polysynaptic ascending trigeminotrigeminal and trigeminoreticular connections. Based on reflex testing in humans with brainstem lesions, Aramideh and colleagues (1997; see also Ongerboer de Visser, 1983) suggested that the R1 pathway is due to an ipsilateral monosynaptic projection by neurons in pV to ObOc motoneurons, while the R2 pathway derives from sVc, and relays via interneurons in the medullary reticular formation that project bilaterally to ObOc motoneurons.
In view of the variety of different opinions concerning trigeminal reflex pathways, and the general dearth of data on trigeminal brainstem connections in primates obtained using modern tracing techniques (baboon and squirrel monkey: Tiwari & King, 1974), we sought to examine the connections from pV and sVc to ObOc motoneurons. We undertook these studies using both conventional tracer techniques to label facial nucleus afferents, and dual tracer techniques in which a retrograde tracer was used to label ObOc motoneurons and an anterograde tracer was used to label trigeminal nucleus afferent input to blink-related motoneurons. Connections between the labeled elements were examined at both the light microscopic (LM) and electron microscopic (EM) level.
METHODS
These experiments utilized Macaca fascicularis (n=15) and Macaca mulatta (n=3) monkeys. Both male (n=8) and female (n=10) monkeys were employed. No obvious differences were seen between the two species or the two sexes with respect to terminal distribution. Data from these animals was also used in other non-conflicting studies. The procedures used were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center. Animals were initially sedated with ketamine HCl (10 mg/kg, IM). Atropine sulfate (0.05 mg/kg, IM) was given to suppress production of mucus and dexamethasone (2.5 mg/kg, IV) was given to preclude edema. Animals were anesthetized with isoflurane gas (1-3%) and their heads were stabilized in a stereotaxic apparatus. An IV line allowed infusion of supplemental fluids. Body temperature and heart rate were monitored and kept within normal limits. Following surgery, the incision area was infused with Sensorcaine, and animals were provided with postoperative analgesia in the form of buprenorphine (0.01 mg/kg, IM).
Surgical Procedures
Injections of pV (n=6) and VII (n=1) were made as follows. A craniotomy was placed near the midline over the occipital-parietal border. The medial bank of cortex lying over the pontomesencephalic junction was then aspirated to reveal the tentorium cerebelli, which was incised to reveal the junction of the anterior cerebellar cortex and midbrain. These were then gently separated to reveal the middle cerebellar peduncle and the exiting trochlear nerve. This nerve was used as a landmark for the surface point where a 1.0 μl Hamilton syringe needle was introduced into the brainstem at its juncture with the peduncle. The needle was angled 1-2° tip medial, and was advanced 4.0-4.5 mm to target pV and 5.5 mm to include VII in the injection. In the retrograde tracer injection cases, we injected 0.01-0.02 μl of a 2.0 % wheatgerm agglutinin conjugated to horseradish peroxidase (WGA-HRP) solution into either pV (n= 1) or VII (n= 1). In the anterograde tracer experiments, we injected 0.1-0.5 μl of 10% biotinylated dextran amine (BDA) into pV (n=5). We also made control injections with this approach where the BDA was injected lateral to (n=1) or medial to (n=1) pV. Following the injection, hydrated Gelfoam was placed in the aspiration defect and the incision was closed with suture.
Injections of sV were directed at the medullary portion of pars caudalis (sVc) (n=4), as this region receives a dense projection from both the eyelid and the cornea in the monkey (Marfurt & Echtenkamp, 1988; May & Porter, 1998). The animal’s head was tipped nose down in the stereotaxic apparatus by ~30° and the body was supported so that there was little traction on the neck. A midline incision was made between obelion and C2 of the vertebral column, and the skin was retracted laterally. The muscles at the back of the neck were separated at the midline and retracted laterally to reveal the atlanto-occipital membrane. This membrane was incised to allow visualization of the dorsal surface of the brainstem. The head was then further flexed to ~40° nose down. The tuberculum cinereum overlying the spinal trigeminal nucleus could then be visualized. In additional experiments, we targeting the cervical portion of the sVc by also removing a portion of the neural arch of C1 (n=2). The tracer, 10% BDA, was held in a 5.0 μl Hamilton syringe that was angled in the sagittal plane, as necessary to allow targeting of various parts of sVc. We injected 0.2 μl at each injection site. In some cases we made 2-3 injections along the rostrocaudal course of the part of the nucleus being targeted. We also made control injections with this approach where the medullary reticular formation (n=1), the lateral funiculus (n=1), or the spinal cord below C3 were targeted (n=1). Following the injections, a piece of Gelfilm was placed in the defect caused by cutting the atlanto-occipital membrane so that the animal could re-establish normal intracranial pressure. Then, suture was used to close the muscles in layers, and to stabilize the reapproximated skin.
For the dual tracer cases, a second injection was made 19 days after the initial BDA injection of pV (n=4), or sVc (n=3) or control injections (n=2). The orbicularis oculi muscle was injected with 10.0 μl of a cocktail that contained 2 % WGA-HRP and 5 % horseradish peroxidase (HRP). The solution was held in a 25 μl Hamilton syringe and injected beneath the skin through a 25 G needle, spreading the bolus among multiple sites on the upper and lower eye lid. It should be noted that in some of the BDA injection cases, retrograde tracers were place in other muscles or other parts of the brain where the pattern of labeling would not confound the results presented here.
Animals survived for 2 days following the WGA-HRP injections (21 days following the BDA injections). They were once again sedated with ketamine HCl (10 mg/kg, IM), then deeply anesthetized with sodium pentobarbital (50 mg/kg, IP). They were perfused through the heart with buffered saline (0.1 M, pH 7.2 phosphate buffer (PB) and 0.85% NaCl) followed by a mixed aldehyde fixative (1.0% paraformaldehyde and 1.25-1.5% glutaraldehyde in 0.1 M, pH 7.2 PB). The brains were blocked in the frontal plane, removed, and postfixed in the same fixative for 2 hours at 4° C. They were then stored in 0.1 M, pH 7.2 PB at 4° until processed.
Histology and Analysis
The brainstems were cut into 100 μm frontal sections using a vibratome (Lancer Series 1000) and collected in 0.1 M, pH 7.2 PB. The cervical spinal cord was cut into 100 μm horizontal or frontal sections. A minimum of 1 in 3 sections were reacted to visualize the tracers. To reveal presence of HRP, sections were incubated in a solution containing 0.005 % tetramethylbenzidine (TMB), 0.25% ethanol and 0.24% ammonium molybdate in 0.1 M, pH 6.0 PB. The reaction was then initiated by the addition of 0.1125% H2O2 and the sections were gently agitated at room temperature for 1-2 hrs and then overnight at 4°C. The blue reaction product was stabilized by incubation in a 5.0% ammonium molybdate 0.1 M, pH 6.0 PB solution.
For dual tracer experiments, we followed the procedure described above, and then further stabilized the TMB reaction product by incubation in 0.05% diaminobenzidine HCl (DAB) in a solution of 0.1 M, pH. 7.2 PB. The reaction was initiated by addition of 0.05% H2O2 and run until the reaction product was brown. Next, sections were reacted to reveal the BDA. Following treatment with 0.3% Triton X-100 in 0.1 M, pH 7.2 PB, they were incubated in a solution containing 0.2% avidin-HRP in 0.3% Triton X-100, 0.1 M, pH 7.2 PB. Incubation started with gentle agitation at room temperature for 4 hours, followed by overnight incubation at 4°C. After rinsing, the sections were placed in a solution of 0.05% DAB in 0.1 M, pH. 7.2 PB to which 0.01% cobalt chloride and nickel ammonium sulfate was added to produce a black reaction product. The reaction was then initiated through the addition of 0.05% H2O2. In all cases, sections were mounted onto gelatin coated slides, counterstained with cresyl violet, dehydrated, cleared and coverslipped.
Individual sections were drawn using a Wild M8 stereoscope with a drawing tube. Labeled elements were charted onto them with an Olympus BH-2 microscope. This microscope and its attached drawing tube were used to illustrate other features of the labeling pattern, as well. Subdivision boundaries in the trigeminal sensory nucleus were established with reference to Noriega and Wall (1991) and an atlas (Paxinos et al., 2000). Examples of labeled cells and axonal arbors were photographed using a Nikon Eclipse E600 microscope equipped with a Nikon DS-Ri1 digital camera. Images were imported using Nikon Elements software, which could be used to combine multiple Z-axis focal planes. Image contrast, brightness and tone were adjusted in Adobe Photoshop to match the view seen through the microscope.
For EM (n=4), a second 1 in 3 series of sections was reacted using the dual tracer technique. However, for the EM sections, 0.1% Triton X-100 was used in the avidin-HRP steps. Following the final reaction, VII was located in free-floating sections and the area containing labeled ObOc motoneurons was cut from the section. These samples were processed using conventional EM methods (Barnerssoi & May, 2016). They were osmicated and en bloc stained with 0.5 % uranyl acetate, before being dehydrated in a graded series of ethanols, followed by 100 % propylene oxide. Next, they were flat embedded in TAAB epoxy 812 resin and attached to resin blocks. Semithin sections (1-2 μm) were taken and stained with toluidine blue to aid trimming of the sample to include just the labeled area. Ultrathin sections (70-90 nm) were cut on an ultramicrotome (Reichert-Jung Ultracut E) and collected onto copper grids. The sections were stained with lead citrate, and analyzed using a Zeiss EM 10C electron microscope. Negatives were digitized and then assembled into figures with Adobe Photoshop.
RESULTS
WGA-HRP Injections
The distribution of retrogradely labeled neurons seen following an injection of WGA-HRP that included VII is shown in figure 1 (Case 1). The tracer spread along the injection track to include pV, the pontine reticular formation adjacent to pV, pars oralis of the spinal trigeminal nucleus (sVo), and the lateral corner of the parabrachial nuclei. (Fig. 1a-d). On the ipsilateral side, retrogradely labeled cells (dots) were observed at all levels of sV. They were particularly numerous in sVc (Fig. 1e-l), including the portion of the nucleus found in the upper two levels of the cervical spinal cord (C1 & C2) (Fig. 1k-l). Labeled cells were also common in the reticular formation medial and ventromedial to sV (Fig. 1e-j). On the contralateral side, retrogradely labeled neurons were observed in pV (Fig. 1a-c), sVo (Fig. 1c-e) and sVi (Fig. 1). Contralateral neurons were still present in sV at the junction between sVi and sVc (Fig. 1h), but were not observed more caudally in contralateral sVc (Fig. 1i-l). Labeled neurons were also observed in the contralateral pontine reticular formation, just medial to pV and ventral to the exiting facial nerve (Fig. 1a-c). More caudally, they were located in the core of the medullary reticular formation (Fig. 1d-j).
Figure 1.
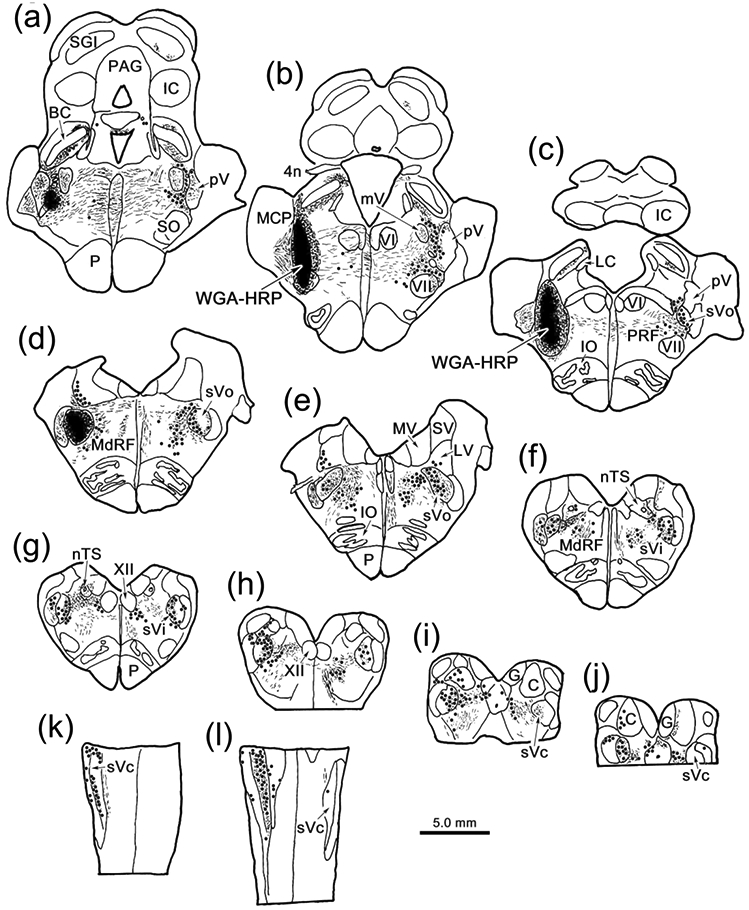
Brainstem distribution of cells supplying input to VII. An injection of WGA-HRP that included VII (b-c), as well as pV (a-b) and sVo (c-d), retrogradely labeled neurons (dots), anterogradely labeled terminals (stipple), and labeled axons (lines) throughout the brainstem (a-j) and rostral levels of the cervical spinal cord (k-l). Examples a-j are frontal sections arranged in rostral to caudal order, as are the chartings in other figures. The rostral spinal cord (k-l) was cut horizontally.
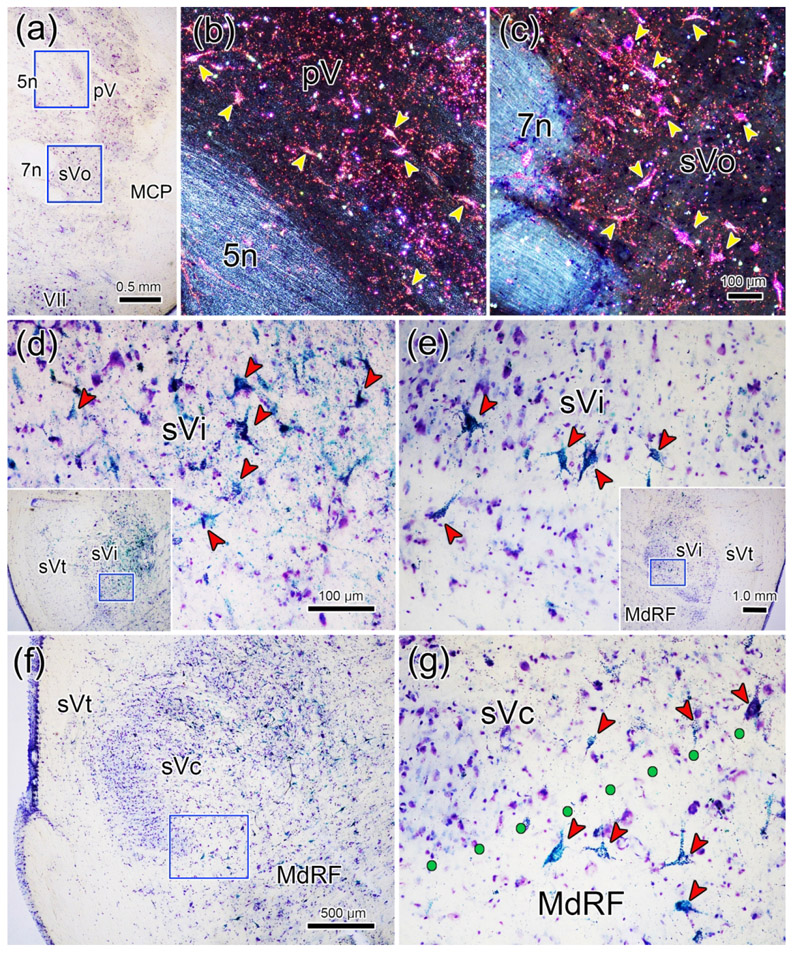
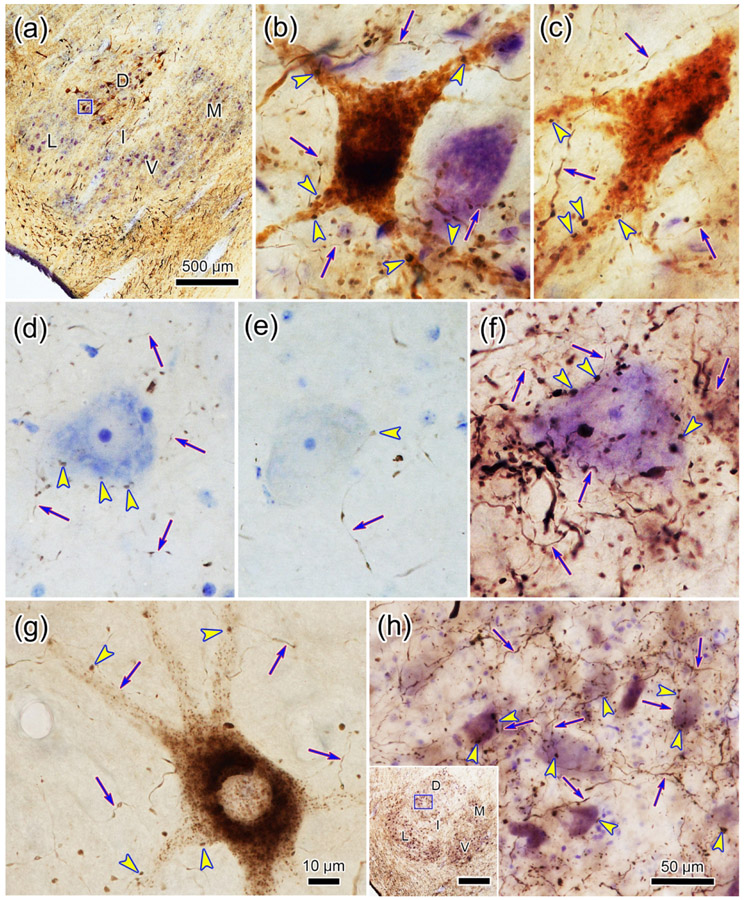
Examples of the retrograde labeling are shown in figure 2. In the pons, numerous retrogradely labeled neurons (arrowheads) are present contralaterally. These were located lateral to the motor root of the trigeminal nerve in pV (Fig. 2b) and the facial nerve in rostral sVo (Fig. 2c). A large number of retrogradely labeled cells (arrowheads) were also observed within ipsilateral sVi (Fig. 2d), including cells found ventrally, where the ophthalmic division is located (Kerr et al. 1968; Price et al., 1976). Fewer cells were labeled contralaterally in sVi, but many of these were also found ventrally (Fig, 2e). At the level of sVc (Fig 2f), labeled cells were primarily found ipsilaterally. Labeled neurons were present within the ophthalmic division (Fig. 2g), but were even more prominent in the medullary reticular formation, immediately ventral to sVc.
Figure 2.
Retrogradely labeled neurons (arrowheads) from the injection shown in figure 1.a-c: Numerous retrogradely labeled neurons are present contralateral to the injection in pV (b) and sVo (c) of the pons, as demonstrated with crossed polarizers. The upper and lower boxes in a indicate the regions shown in b and c, respectively. d-e: The ventral portion of sVi, the location of the ophthalmic subdivision, contains numerous retrogradely labeled neurons on the ipsilateral (d) and contralateral (e) sides. Boxes in the inserts indicate the location of samples d and e. f-g: Retrogradely labeled neurons were also present in the ophthalmic portion of sVc, ipsilaterally. However, even more retrogradely labeled neurons were present in the adjacent medullary reticular formation (MdRF). Box in f indicates region shown ingG. Dots indicate the border between sVc and MdRF in g. Scale in c = b; in d = e&g; and in insert e = insert d.
As this injection site included pV, and we have documented the presence of extensive intratrigeminal connection in the macaque (Warren & May, 2013), we tested whether the cells labeled in Case 1 might be trigeminotrigeminal neurons by injecting WGA-HRP into pV (Case 2). Figure 3 shows the pattern of brainstem labeling in a case where the WGA-HRP injection site involved pV, but did not involve VII (Fig. 3a-c). Once again, retrogradely labeled neurons were located ipsilaterally in all subdivisions of sV (Fig. 3e-k). Contralaterally, retrogradely labeled neurons were present in pV (Fig. 3c-d), sVo (Fig. 3e) and sVi (Fig. 3f-g), but not sVc (Fig 3i-k). A few contralateral cells were present at the junction between sVi and sVc (Fig. 3h). There was also considerable bilateral retrograde labeling in the reticular formation. This WGA-HRP injection also produce anterograde labeling. Of particular interest here is a terminal field (stippling) present in the dorsal subdivision of VII, which contains ObOc motoneurons (Fig. 3c-d). As shown in figure 4a, while labeled terminals were present throughout VII, ipsilaterally, they were distinctly denser in the dorsal, lateral and intermediate subdivisions. On the contralateral side, very sparse terminal labeling was present in the contralateral dorsal subdivision of VII (Fig. 4b).
Figure 3.
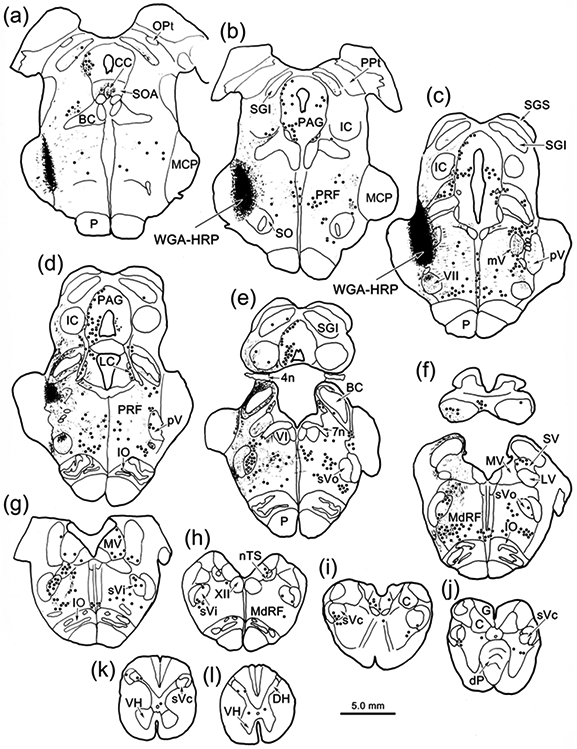
Brainstem distribution of pV connections. An injection of WGA-HRP that included pV (c), retrogradely labeled neurons (dots), anterogradely labeled terminals (stipple), and labeled axons (lines) throughout the brainstem (a-j) and in rostral levels of the cervical spinal cord (k-l). Note the presence of dense terminal labeling in ipsilateral VII (c-d).
Figure 4.
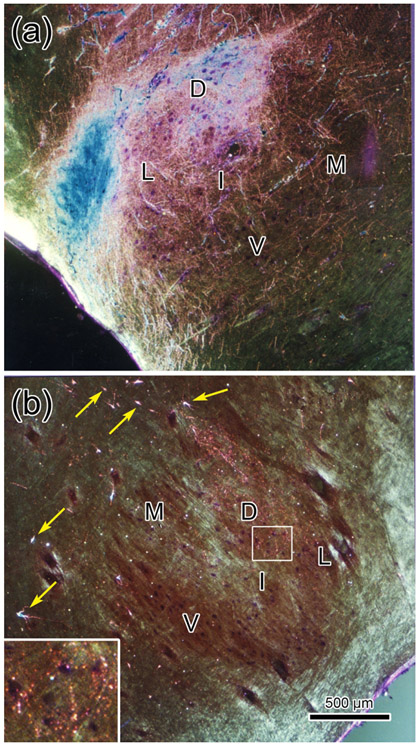
Trigeminal terminal fields in VII under crossed polarizer illumination. Following a WGA-HRP injection that included pV and rostral sVo (see Fig. 3), dense anterogradely labeled terminal fields were present in the dorsal (D), lateral (L) and intermediate (I) subdivisions of ipsilateral VII (a), with far fewer labeled terminals present in the ventral (V) and medial (M) subdivisions. On the contralateral side (b) only a very sparse projection to the dorsal division was present. The portion of the contralateral terminal field in the boxed region is shown at higher magnification in the insert. Retrogradely labeled neurons (arrows) were present dorsal and medial to the facial nucleus. Scale in b = a.
BDA Injections of the Principal Nucleus
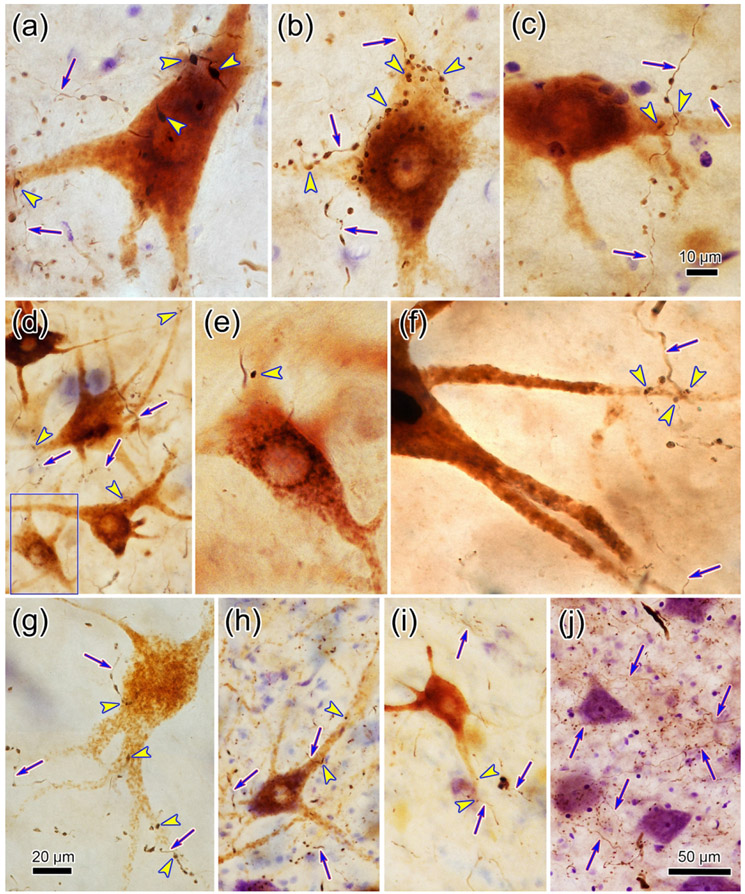
In light of the similarity between the labeling patterns of pV injections that included or did not include VII, we further documented trigeminofacial projections with the anterograde tracer experiments by injecting BDA into pV. In the illustrated case (Case 3), the BDA injection is centered in pV (Fig. 5a-b), with some tracer invading the lateral aspect of the parabrachial nuclei and extending just below pV. Labeled terminals (stipple) were present throughout ipsilateral VII, but were densest in the dorsal subdivision (Fig. 5b-c). On the contralateral side, they were mainly found in the dorsal subdivision of VII (Fig. 5b-c). They were also observed bilaterally in sVo (Fig. 5d). Retrograde tracers were placed in the ObOc muscles in this case. The relationship of these pV terminals to ObOc motoneurons in Case 3 is shown in figure 6. Examples of labeling in VII ipsilateral to (Fig. 6a) and contralateral to (Fig. 6b) the BDA injection site in pV are illustrated. The WGA-HRP reaction product was visible well out into the primary dendrites of the large, multipolar facial motoneurons (shading). The BDA labeled trigeminal axons (arrows) displayed numerous en passant boutons, and occasional terminal boutons. Numerous examples of close associations (arrowheads) between the boutons and the somata and dendrites of the labeled motoneurons were observed on both sides of VII in this case. Examples of axonal relationships from this case are further illustrated in figure 7a-c. The darker, BDA labeled trigeminal axonal arbors displayed boutons which made close associations (arrowheads) with the primary dendrites and somata of the brown, retrogradely labeled, ObOc motoneurons located ipsilateral (Fig. 7a-b) to the pV injection. Contralateral to the pV injection (Fig. 7c), close associations were also observed. However, these were fewer in number and tended to be on dendrites of the motoneurons.
Figure 5.
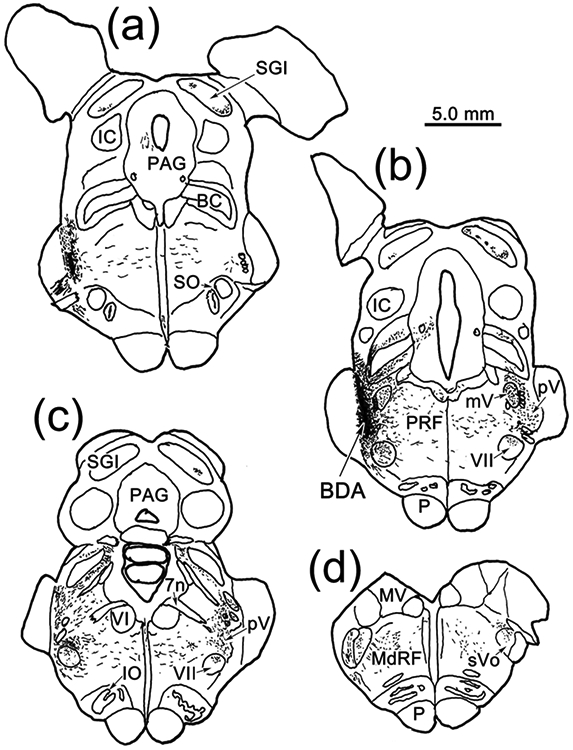
Distribution of rostral trigeminal nucleus projections anterogradely labeled with BDA. The injection, which included pV (a-b) and sVo (c-d), labeled axons (lines) and their terminals (stipple) in the midbrain (a-c), pons (a-c) and rostral medulla (c-d). BDA labeled terminal fields were present in both ipsilateral and contralateral VII (b-c).
Figure 6.
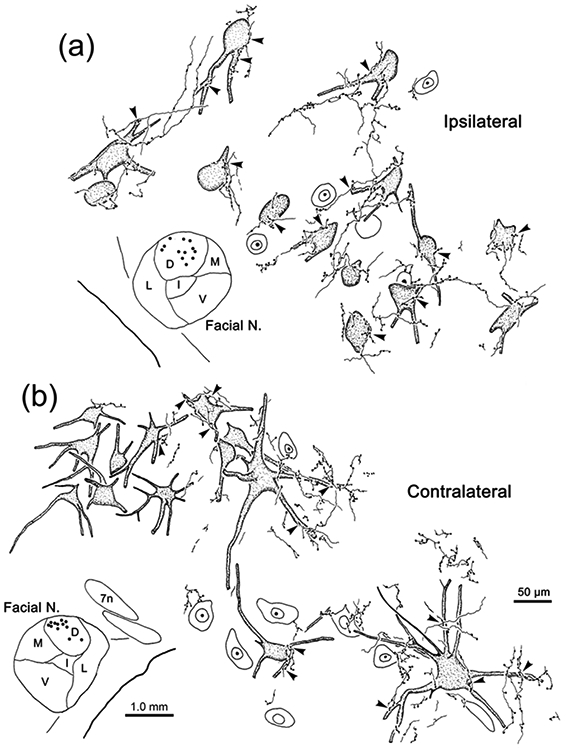
Bilateral projections to ObOc motoneurons. Axonal arbors were labeled with BDA following a pV injection (see Fig. 5). Facial motoneurons (dots in insets) in the dorsal subdivision of ipsilateral VII (a) and contralateral VII (b) were labeled due to an injection of retrograde tracers into the ObOc muscle. Close associations (arrowheads) were observed between the somata and dendrites of retrogradely labeled ObOc motoneurons (shading) and the boutons of anterogradely labeled axonal arbors. Scales in b = a.
Figure 7.
Examples of close associations (arrowheads) between the boutons of BDA labeled axonal arbors (arrows) and ObOc motoneurons following pontine injections. a-c: Retrogradely labeled ObOc motoneurons from the facial nucleus ipsilateral (a-b) and contralateral (c) to the BDA injection (Fig. 5) display numerous close associations with labeled boutons. d-j: Patterns of labeling observed in VII for other rostral injection cases illustrated in figure 8. d-f: In Case 4 (Fig. 8), which had a small pV injection, numerous close associations were observed on ObOc motoneurons ipsilateral to the BDA injection (d-e), but only a very few were seen on contralateral ObOc motoneurons (f). g: In Case 5 (Fig. 8), where the BDA injection also involved the adjacent pontine reticular formation, many more labeled boutons were associated with ObOc motoneurons contralaterally. h-i: In Case 7 (Fig. 8), which primarily involved sVo, a considerable number of close associations were observed on ObOc motoneurons ipsilateral to the BDA injection (h), and far fewer were seen in association with contralateral ObOc motoneurons (i). j: Case 6 (Fig. 8), which had the largest BDA injection, the dorsal subdivision of contralateral VII displayed numerous BDA labeled arbors among the counterstained somata. Scale in c = a-b, e-f and in j = d, h-i. Boxed region in d is shown in e.
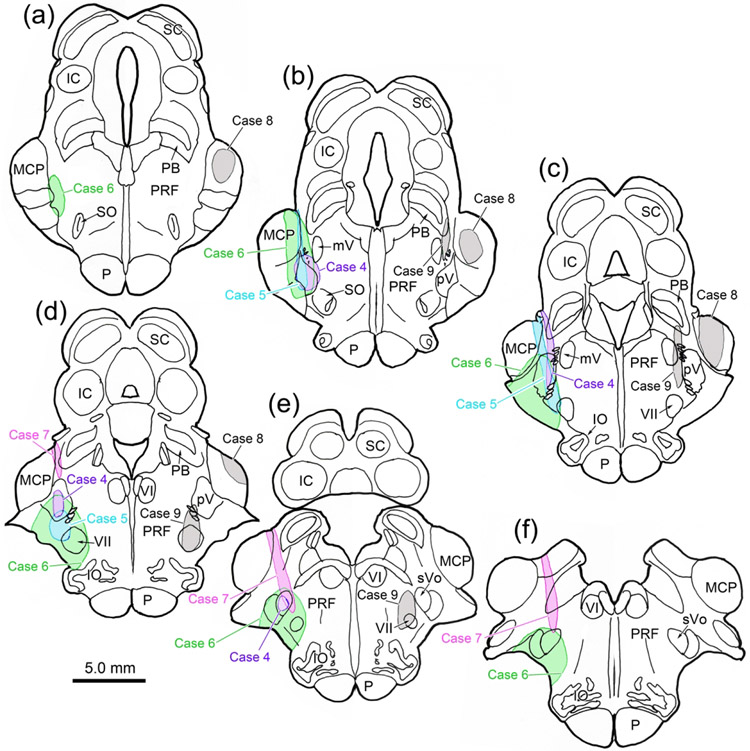
Other cases (Cases 4&5) with pV injections of BDA (Fig. 8) produced similar patterns of termination. Dense terminations were seen in the ipsilateral dorsal division of VII and quite sparse terminations were seen in the contralateral dorsal division. In both cases, labeled terminals were associated with ObOc motoneurons. The smallest injection of pV (Case 4; Fig. 8b-e), did not involve adjacent reticular formation and was centered laterally in pV, although it did involve the lateral parabrachial region and extended slightly into sVo. It produced a considerable number of labeled terminal arbors in ipsilateral VII (Fig. 7d), many of which displayed boutons that had close associations (arrowhead) with ObOc motoneurons (Fig. 7d-e). The most impressive labeled arbor we observed contralaterally is shown in figure 7f, but this is one of just a handful that were present in a nucleus nearly devoid of labeled axons. Case 5 (Fig. 8b-d) was centered in medial pV and extended ventrally to include part of VII. It produced much heavier projections and more boutons in close association with ObOc motoneurons in contralateral VII (Fig. 7g). The largest BDA injection (Case 6) included both Vp and sVo, as well as VII (Fig. 8a-f). It produced the most extensive labeling within the dorsal division of contralateral VII (Fig. 7j). Case 7 primarily involved sVo (Fig. 8e-f). It also produced terminal fields in the dorsal subdivision of VII, ipsilaterally (Fig. 7h) and contralaterally (Fig. 7i). The ipsilateral projection was more modest than that seen with the pV injections.
Figure 8.
Rostral pontine BDA injections for Cases 4-9. a-f: Injection sites from each case are charted on a rostral to caudal series of sections. Injections involving pV and sVo are shown on the left. Control injections are shown on the right to ease visualization.
No labeled axon arbors were observed in VII either ipsilaterally or contralaterally from a control injection placed in the middle cerebellar peduncle (Case 8, Fig. 8a-d). The medial control injection (Case 9, Fig. 8b-e) extended through the lateral corner of the parabrachial nuclei, included the pontine reticular formation immediately medial to pV, and terminated in VII. Examination of contralateral VII did not reveal any terminal label, although labeled terminal arbors were present in the contralateral pontine reticular formation.
EM evidence indicates that the close associations seen at the LM level represent synaptic contacts between the labeled elements. In samples taken from both the ipsilateral (Fig. 9) and contralateral (Fig. 10) facial nucleus, flocculent electron dense material was present in the cytoplasm of the WGA-HRP labeled ObOc motoneuron dendrites (Den*; Fig. 9c-f; 10d-e) and somata (Soma*; Fig. 10f-g). The BDA reaction product sometimes filled labeled axon terminals (At*) so densely that their internal structure was obscured (Fig. 9c; 10a). However, in other labeled examples, the terminals were less densely labeled, but still could be differentiated from unlabeled terminals (At; Fig. 9a,e,f; 10a,g). In these cases, the presence of clear, spherical vesicles was apparent in the labeled terminals (Fig. 9a-b; 10b-c). The extent of the observable postsynaptic density (arrowheads) varied, but examples in which the synapse was clearly asymmetric were observed (Fig. 9a-c; 10a). Examples of both axodendritic (Fig. 9c-f; 10d-e) and axosomatic (Fig. 10f-g) synaptic contacts between the labeled terminals and labeled cells were present, indicating a monosynaptic drive.
Figure 9.
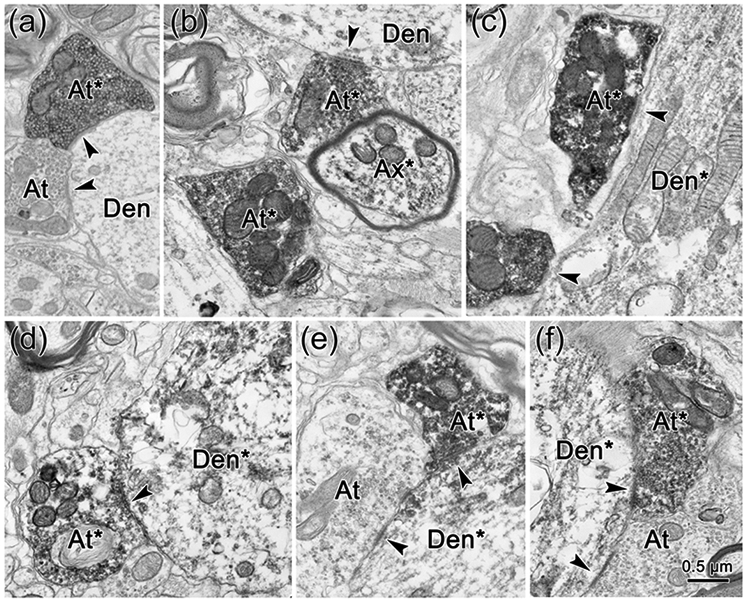
Direct synaptic contacts on ObOc motoneurons ipsilateral to the trigeminal injection. a-f: BDA labeled axon terminals (At*) and axons (Ax*) are much more electron dense than unlabeled terminals (At). They synaptically contact (arrowhead) unlabeled dendrites (Den)(a-b), and retrogradely labeled dendrites (Den*) (c-f), which have flocculent reaction product in their cytoplasm.
Figure 10.
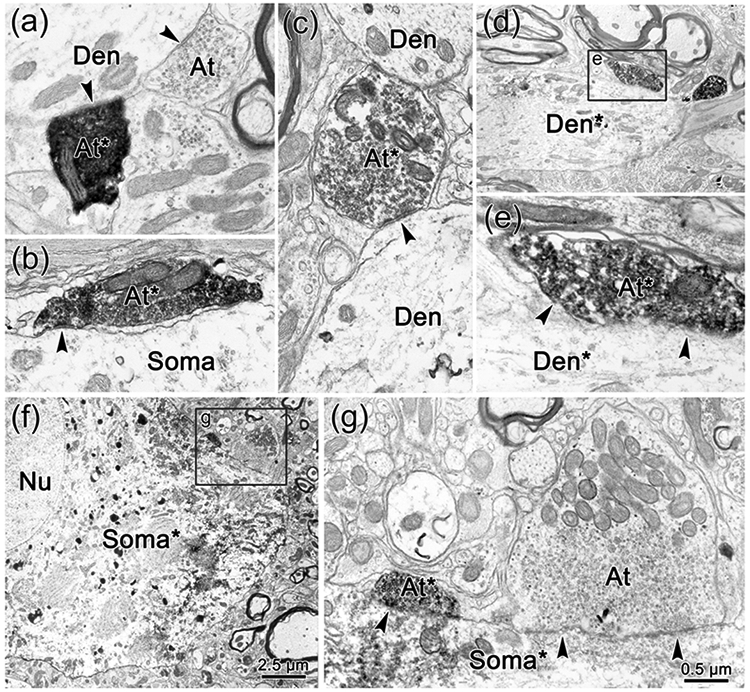
Direct synaptic contacts on ObOc motoneurons contralateral to the trigeminal injection. a-g: BDA labeled axon terminals (At*) and axons (Ax*) are much more electron dense than unlabeled terminals (At). They synaptically contact (arrowhead) unlabeled dendrites (Den)(a,c), and somata (Soma)(b), as well as retrogradely labeled dendrites (Den*) (d-e) and somata (Soma*)(f-g), which have flocculent reaction product in their cytoplasm. Boxed areas in d and f are shown at higher magnification in e and g, respectively.
BDA Injections of the Spinal Trigeminal Nucleus
To discriminate between ascending intratrigeminal connections (Warren & May, 2013; Fig. 3) and ascending trigeminofacial projections (Fig. 1), we confirmed the presence of a trigeminal projection to ObOc motoneurons by injecting BDA into sVc. An example (Case 10) is shown in figure 11. Since the sVc projection shown in figure 1 was ipsilateral, we used bilateral injections in this case. The injection sites were centered in sVc near the spinomedullary junction (Fig. 11f-g). They both extended into the medullary reticular formation ventral to the targeted nuclei (Fig. 11d-e). Both these injections produced terminal label (stipple) within VII that was densest in the dorsal division, which contains the ObOc motoneurons (Fig. 11a-c). Terminal fields were also present ventrally in sVo (Fig. 11a-c) and pV (not illustrated), as well as in the adjacent reticular formation (Fig. 11a-c). In this case, we had also injected WGA-HRP into the ObOc muscles. Figure 12 shows an example of the labeling in VII on the right side of the case shown in figure 11. The retrogradely labeled motoneurons (shaded) in the dorsal subdivision of VII showed reaction product well out into their primary dendrites. BDA labeled axons arborized throughout the region. These axonal arbors were studded with numerous, mostly en passant boutons. Many of these displayed close associations (arrowheads) with the somata and dendrites of the ObOc motoneurons. Images from this case show the ObOc labeling pattern in the dorsal subdivision (Fig. 13a). The brown WGA-HRP reaction product can be seen in the somata and dendrites of the ObOc motoneurons (Fig. 13b-c). Darker, brown-black reaction product is present in the numerous BDA labeled axons (arrows). Close associations (arrowheads), suggestive of synaptic contact, are present between the BDA labeled boutons and the ObOc cells.
Figure 11.
Brainstem distribution of spinal trigeminal pars caudalis (sVc) projections. Bilateral injections of BDA centered in sVc (e-g) at the spinomedullary junction, that included the adjacent medullary reticular formation, anterogradely labeled axons (lines), as well as terminals (stipple), throughout the brainstem (a-g) and in rostral levels of the cervical spinal cord (h). Note the presence of dense terminal labeling in VII (a-c).
Figure 12.
Projection of sVc onto ObOc motoneurons. BDA labeled axonal arbors from the case illustrated in figure 11 are shown. b: Facial motoneurons in the dorsal subdivision of VIII (dots) on the left side were labeled due to an injection of retrograde tracers into the left ObOc muscle. a: Close associations (arrowheads) were observed between the somata and dendrites of retrogradely labeled ObOc motoneurons (shading) and the boutons of axonal arbors anterogradely labeled with BDA. Unlabeled, counterstained neurons are not shaded.
Figure 13.
Examples of close associations (arrowheads) between the boutons of BDA labeled axonal arbors (arrows) and ObOc motoneurons following medullary and spinal cord injections. a-c: Labeling from the case illustrated in figure 12. Numerous motoneurons were labeled in the dorsal subdivision of VII following an injection of the ObOc muscle (a). Boxed area indicates region shown in b. A second example is shown in c. Numerous close associations between the labeled elements are present in both. d-h: Examples of terminal arbor labeling from cases illustrated in figure 14. A small injection, largely confined to sVc (Case 12, Fig. 14) still produced numerous BDA labeled boutons in close association with counterstained somata in the ipsilateral dorsal subdivision of VII (d), and scattered arbors in the contralateral subdivision (e). A more rostral injection that substantially involved the medullary reticular formation (Case 13, Fig. 14) produced very dense terminal label in the ipsilateral dorsal subdivision of VII (f), and more modest terminal labeling in the contralateral subdivision, where close associations with labeled ObOc motoneurons were observed (g). An injection of sVc at upper cervical levels (Case 15, Fig. 14) also produced extensive anterograde labeling in the dorsal subdivision of ipsilateral VII. Boxed region in inset is shown at higher magnification in h. Scale in g = b-f. Scale in insert h = 500 μm.
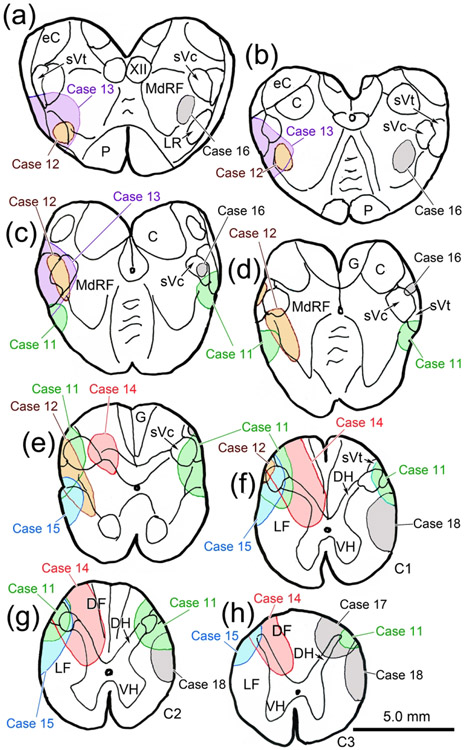
The other cases confirmed and extended the findings revealed in Case 10 (Fig. 14). Case 11 had bilateral injections at the spinomedullary border (Fig. 14c-h), similar to those of Case 10, and a similar level of terminal labeling was observed in VII, with a similar abundance of close associations with labeled ObOc motoneurons (not illustrated). Case 12 was a much smaller, unilateral injection that involved a small part of sVc (Fig, 14c-f) and extended into the adjacent medullary reticular formation ventral to sVc (Fig.14d-e). It produced a relatively modest BDA labeled terminal field in the dorsal subdivision of ipsilateral VII (Fig. 13d) where labeled boutons were observed in close association (arrowheads) with counterstained somata. Scattered labeled axons were also encountered in the dorsal subdivision of the contralateral nucleus (Fig. 13e). Case 13 was a larger injection that extended further rostral than the others (Fig. 14a-c).
Figure 14.
Caudal medulla and spinal cord BDA injections for Cases 11-18. Injection sites from each case are charted on a rostral to caudal series of sections of the medulla (a-e) and spinal cord (f-h). Injections involving sVc are shown on the left or bilaterally (Case 11). Control injections are shown on the right to ease visualization.
It also involved the medullary reticular formation at the levels illustrated and further rostrally in the brainstem. It produced very dense labeling of terminal arbors within the dorsal subdivision of ipsilateral VII (Fig. 13f). It also produced the most anterograde labeling on the contralateral side of the sVc injections. While the number of contralateral labeled axons was modest, compared to ipsilateral projections, a number of close associations (arrowheads) with retrogradely labeled ObOc motoneurons were still observed (Fig. 13g).
Two cases had injections centered in the spinal levels of sVc located at C1 and C2 (Cases 14 & 15) (Fig. 14f-h). In both cases, the injection site also included portions of the dorsal horn, lateral and dorsal funiculi, and extended to C3. They produced similar labeling patterns. An example of the extensive terminal labeling they produced in the dorsal subdivision of ipsilateral VII is shown in figure 13h.
We also made control injections at and below the spinomedullary junction. Case 16 primarily involved the medullary reticular formation ventral to sVc (Fig. 14a-d), although a small piece of sVc itself was injected (Fig. 14c). This injection produced a relatively dense terminal field in the dorsal subdivision of ipsilateral VII, and a few labeled axons that displayed boutons in close association with ObOc motoneurons on the contralateral side (not illustrated). Thus, its terminal distribution pattern looked similar to that of Case 12 (Fig. 13d-e). Case 17, which was a dorsolateral injection constrained to C3 (Fig. 14h) and areas caudal to C3, produced no terminal labeling VII. Similarly, Case 18, which occupied the lateral funiculus, but did not involve sVc (Fig. 14f-h), produced no terminal labeling in VII. The results in these two controls (Cases 17&18) suggest that the labeling we observed in the sVc injection cases was not due fiber-of-passage uptake by previously described spinofacial projections (cat: Tanaka et al., 1971; 1978).
Close associations between labeled boutons and labeled cells indicate the likely presence of a synaptic contact, but do not prove that a synapse is present. So we extended our analysis of the ipsilateral trigeminofacial sVc projection to the EM level. The WGA-HRP reaction product produced flocculent electron dense crystals (arrows) in the labeled somata (Soma*) (Fig. 15c) and labeled dendrites (Fig. 15b,c,f) of ObOc motoneurons. Electron dense reaction product filled the BDA labeled terminals (At*), in some cases making it difficult to characterize their constituent elements (Fig. 15b,d). In other cases, it just outlined the clear, spherical vesicles with a dark fuzz (Fig. 15a,d-f). Numerous examples of labeled terminal profiles (At*) contacting labeled dendrites (Den*) were observed indicating a monosynaptic connection. The appearance of the synaptic densities (arrowheads) varied. Some were clearly asymmetric and even exhibited postsynaptic bars (Fig. 15a). In others, the postsynaptic portion of the density was quite modest (Fig. 15e-f).
Figure 15.
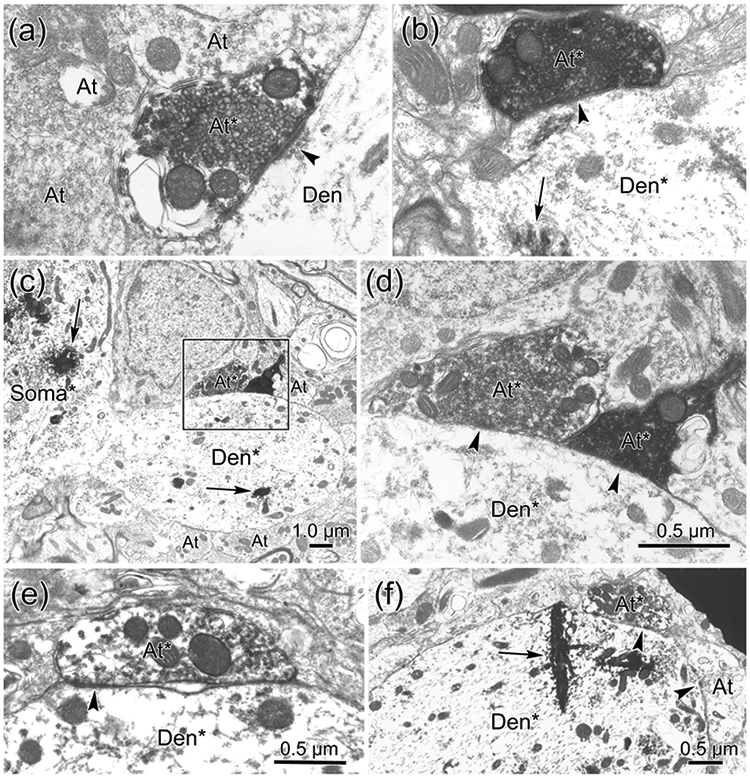
Direct synaptic contacts on ObOc motoneurons ipsilateral to a sVc injection of BDA. a-f: BDA labeled axon terminals (At*) are more electron dense than unlabeled terminals (At). They synaptically contact (arrowhead) unlabeled dendrites (Den)(a), as well as retrogradely labeled dendrites (Den*) (b-f), which have flocculent reaction product in their cytoplasm. Boxed region in c is shown at higher magnification in d. Scale in d = a-b.
DISCUSSION
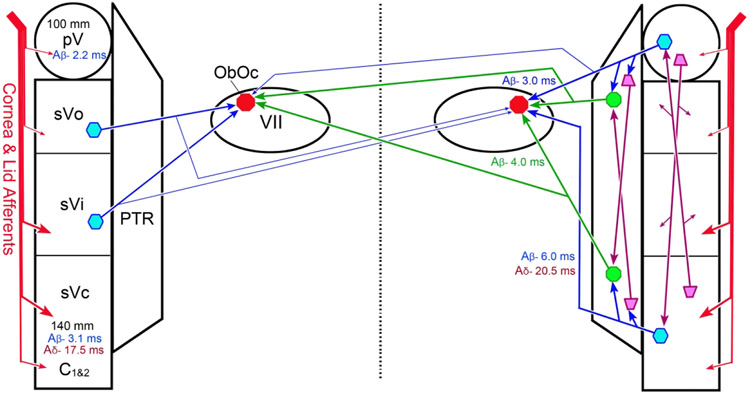
The results of the experiments described here suggest that ObOc motoneurons in the facial nucleus receive direct inputs from trigeminal sensory neurons that are likely to initiate the trigeminal blink reflex in the macaque (Fig. 16, blue arrows). We have primarily demonstrated projections from pV and sVc in this study (Fig. 16, right), but we have also observed evidence for likely projections from sVo and sVi (Fig. 16 left). The ipsilateral trigeminofacial projection onto ObOc motoneurons is quite extensive, but a contralateral projection is also present. However, the number of cells providing the contralateral projection decreases dramatically at caudal levels of sVc. In addition to the pV and sV projections, the results of the present experiments also support a reticulofacial projection to ObOc motoneurons by cells in the pontine and medullary reticular formation located immediately ventromedial to pV and sVc (Fig. 16, green arrows); an area that has been termed the paratrigeminal reticular formation (PTR) (van Ham & Yeo, 1996B). Projections by this reticular area project more densely to contralateral VII than trigeminal nuclei efferents. Thus, it is likely that the reticulofacial projection represents part of a disynaptic circuit that supports the trigeminal blink reflex. The connections we have observed may also function in other circuits that produce eyelid closure following trigeminal sensory activation, including those that regulate spontaneous blink rate to maintain the tear film (Cruz et al., 2011; Kaminer et al., 2011; Rahman et al., 2015; Nosch et al., 2016).
Figure 16.
Schematic of the connections underlying the trigeminal blink reflex. On the left, the afferent input to the trigeminal sensory nuclei is indicated, along with distances between the supraorbital notch and pV and sVc, and the estimated times for activation by Aβ and Aδ fibers. On the right, the patterns of connections between pV and sVc and both ipsilateral and contralateral ObOc motoneurons in VII that were primarily examined in this study are shown (blue arrows). These include connections via the paratrigeminal reticular formation (PTR) (green arrows). On the left, additional projections from sVo and sVi that are likely are shown. The times for monosynaptic activation of ObOc motoneurons by Aβ pathways are indicated in blue, and disynaptic activation in green. Aδ monosynaptic activation is indicated in magenta. Interconnections between rostral and caudal trigeminal subdivisions and medullary and pontine PTR are also included (magenta arrows), but the interconnection between other levels of the trigeminal sensory nucleus are not included, for simplicity. Thinner lines indicates a smaller projection.
Technical Considerations
Spread of the tracer outside of the injection targets limits the strength of the conclusions we can make from the results. The injections of pV included the lateral aspect of the parabrachial nuclei. Bilateral projections by these nuclei to the facial nucleus have been reported (rat: Hinrichsen & Watson, 1983; Li et al., 1997; cat: Takeuchi et al., 1979; Fort et al., 1989). Our control injection that included the lateral part of the parabrachial nuclei did not produce terminals in the dorsal subdivision of contralateral VII (Case 9, Fig. 8), in agreement with the pattern observed using retrograde trans-synaptic transport of rabies virus from the orbicularis oculi muscle in the rat (Morcuende et al., 2002). However, we can not rule out the possibility that a portion of the terminals observed contacting ipsilateral ObOc motoneurons following pV injections may be due to a parabrachial nuclei projection. All but one of the pV injections involved the immediately adjacent pontine reticular formation. However, the case that did not (Case 4, Fig. 8), still produced substantial terminations on ObOc motoneurons in ipsilateral VII (Fig. 7e), although it produced very few labeled axonal arbors in contralateral VII. This suggests the crossed trigeminofacial projection from pV is modest. The contribution of sVo by itself (Case 7, Fig. 8) is also relatively modest (Fig. 7i). On the other hand, the very dense contralateral projection labeled when pV, sVo and the surrounding reticular formation were injected (Case 6, Fig. 8), indicates this general region is responsible for an extensive crossed projection (Fig. 7j).
Similarly, our medullary injections of sVc all included the adjacent reticular formation. Thus, we cannot discriminate which of these two sources are responsible for the terminals we observed in VII. However, in one case with a cervical sVc injection that did not involve medullary reticular formation (Case 14, Fig. 14), we still saw extensive terminal labeling in the dorsal subdivision of ipsilateral VII (Fig. 13h). Conversely, we also saw terminals labeled in this division with a medullary reticular formation injection that largely avoided sVc (Case 16, Fig. 14) supporting the presence of a reticulofacial projection. Furthermore, both regions contained retrogradely labeled neurons following an injection of ipsilateral VII. Taken together, our results suggest that both sources contribute input to ObOc motoneurons. Indeed, retrograde tracer studies in other species where injections were confined to VII support an ipsilateral projection of both sVc and the adjacent medullary reticular formation (rat: Hinrichsen & Watson, 1983; Li et al., 1997; cat: Fort et al., 1989), as does retrograde trans-synaptic transport from the ObOc muscle (rat: Morcuende et al., 2002) and an anterograde transport study (rabbit: van Ham & Yeo, 1996B).
Comparison to Previous Studies
A bilateral projection from pV to ObOc motoneurons has not, to the best of our knowledge, been described previously in a monkey. In rats, conventional retrograde tracers only label cells in ipsilateral pV following injections confined to VII (Li et al., 1997), as do trans-synaptic tracers injected into the ObOc muscle (Morcuende et al., 2002). However, the latter study revealed cells lying ventrally in contralateral sVo. Only ipsilateral projections to the subdivision of VII containing ObOc motoneurons have been described with anterograde methods (cat: Holstege et al., 1986A; 1988; rabbit: van Ham & Yeo, 1996B; rat: Pinganaud et al., 1999). On the other hand, retrograde tracers label a few cells in contralateral pV in cats (Takeuchi et al., 1979; Fort et al., 1989).Thus, while an ipsilateral projection may be a common mammalian feature, the presence of this crossed trigeminofacial projection may be related to frontally placed eyes. Similarly, motor area corticofacial projections for the upper face are also bilateral in the monkey (Morecraft et al., 2001)
Our macaque data point to projections from the pontine reticular formation, particularly the region near the exiting facial nerve, as being an import source of the contralateral projection from pV to the dorsal subdivision of VII. One previous study in cats observed retrogradely labeled cells in this region on both sides of the pons following VII injections (Fort et al., 1989), but another only observed them ipsilaterally (Takeuchi et al., 1979), and those in rats did not observe them at all (Li et al., 1997, Morcuende et al., 2002). Based on anterograde transport of tritiated amino acids in cats, Holstege and colleagues (1986A; 1986B; 1988) have argued strongly that the reticular formation ventromedial to pV and sVo projects densely and selectively to the portion of ipsilateral VII that contains ObOc motoneurons, as well as to retractor bulbi motoneurons in the accessory abducens nucleus, making it an important center for initiating the blink reflex. Support for this contention comes from a variety of sources. Afferents from pV terminate in the area ventromedial to the nucleus (rat: Zerari-Mailly et al., 2001), and there are monosynaptic projections from this region that terminate on VII motoneurons (Mogoseanu et al., 1994). Furthermore, c-Fos activity is increased in this population following electrical stimulation of the supraorbital nerve (rat: Zerari-Mailly et al., 2003). Physiological recordings in the reticular formation medial to pV and sVo reveal neurons whose activity correlates with reflex blinking in cats (Siegel et al., 1983; Tamai et al., 1986). Our findings indicate that this region likely plays an important role in blinking in primates, and particularly contributes to bilateral blinking.
The ipsilateral direct projection from sVc that targets ObOc motoneurons shown here has not, to the best of our knowledge, been described previously in a monkey. However, a projection from sVc to ipsilateral VII in general was first demonstrated by use of axonal degeneration techniques (squirrel monkey and baboon: Tiwari & King, 1974; rat: Erzurumlu & Killackey, 1979). There is also evidence for such a trigeminofacial projection from retrograde studies in other species (cat: Takeuchi et al., 1979; Fort et al., 1989; guinea pig: Pellegrini et al., 1995), and neurons in rat sVc were labeled trans-synaptically following rabies injections of the ipsilateral ObOc muscle (Morcuende et al., 2002). Similarly, injections of anterograde tracers into medullary portion of sVc produced labeled terminals in the subdivision of VII that contains ObOc motoneurons (rabbit: van Ham & Yeo, 1996B; rat: Pinganaud et al., 1999). None of these studies reported the presence of labeled facial nucleus afferent cells at cervical levels of sV, with the exception of the trans-synaptic retrograde study of ObOc circuits in rat (Morcuende et al., 2002). Furthermore, in contradistinction to our results, no projection to VII from an injection of tritiated amino acid into sVc at cervical levels was observed in the cat (Holstege et al., 1986A). Taken together, these studies and our own strongly suggest that sVc provides direct input to ipsilateral ObOc motoneurons, but also indicates that there are species variations in the levels of sVc projecting to VII, with all sVc levels contributing in monkeys.
As our injections generally included the medullary reticular formation ventromedial to sVc, and retrogradely labeled cells in this region were numerous follow the WGA-HRP injection of VII (Fig. 1h-j), we believe it is likely that a medullary reticulofacial projection targeting ObOc motoneurons is also present in the macaque. It appears to be denser projection than that of sVc (Fig. 2g). Evidence for such a reticulofacial projection is found in retrograde studies of other species (cat: Takeuchi et al., 1979; Fort et al., 1989; guinea pig: Pellegrini et al., 1995; rat: Li et al., 1997; Morcuende et al., 2002). Interestingly, the cat studies revealed bilateral projections, while the lateral-eye rat and guinea pig only showed ipsilateral labeling. The pattern of our cases suggest that cells with crossed projections are much more common at more rostral levels of the medulla. There is other evidence that the region of medullary reticular formation ventromedial to sVc is involved in the blink reflex. Injections of sVc and sVi produce labeled terminals in this reticular region in rats (Zerari-Mailly et al., 2001), and these reticular cells show c-Fos activity in response to supraorbital nerve stimulation (Zerari-Mailly et al., 2003). Injection of tritiated amino acids into this region in cats labeled terminals in the ObOc territory of VII (Holstege et al., 1986A). Finally, reticulofacial neurons located ventromedial to sVc could be antidromically driven from VII and were synaptically driven by electrical stimulation of the supraorbital nerve (Inagaki et al., 1989).
Functional Implications
The data we have described in the monkey and the studies described above indicate that pV and sV are strongly and monosynaptically connected to ObOc motoneurons in ipsilateral VII (Fig. 16, right side, blue arrows). They are also connected disynaptically via strong connections between the PTR, which lies immediately ventromedial to the trigeminal complex, and ObOc motoneurons (Fig. 16, right side, green arrows). These pathways are capable of transmitting activity due to both painful and non-nociceptive sensory activation to VII. However, our discussion will mainly center on the non-nociceptive activation of ObOc motoneurons following supraorbital stimulation. Studies of the trigeminal blink reflex have commonly suggested that the R1 component of the blink reflex is supported by a monosynaptic connection between neurons in the trigeminal nuclei and ObOc motoneurons. We have provided anatomical evidence for such a projection in the monkey that agrees with physiological findings indicating direct projections to VII in other species (cat: Fanardjian et al., 1983; rat: Hirata et al., 2000; Henriquez & Evinger, 2007). We also observed evidence for monosynaptic crossed trigeminofacial projections. Normally, the R1 in humans is ipsilateral (Aramideh et al., 2002), although it is bilateral in other species, albeit sometimes with a different threshold (guinea pig: Pellegrini et al., 1995; cat: LeDoux et al., 1997). In addition, a contralateral R1 can be elicited in humans when the reflex is facilitated, suggesting that the connections are present, but their ability to elicit the reflex is suppressed under normal conditions (Willer et al., 1984).
A number of studies have argued that only discrete portions of the trigeminal sensory complex provide the source of the R1 component. It has been suggested that this component originates in pV in humans, based on the fact lesions in this region disrupt the R1, but leave the R2 component intact (Ongerboer de Visser, 1983; Aramideh et al., 1997). The data presented here support the idea that the pV and the adjacent pontine reticular formation, which would have been involved in pontine lesions, supply short latency mono- and disynaptic pathways to ObOc motoneurons. It should be noted that since this region of reticular formation is immediately adjacent to pV, the disynaptic pathway is likely to only be about 1 ms slower than the monosynaptic pathway. The relatively small afferent terminal projection from the eyelid and cornea within the macaque pV (Marfurt & Echtenkamp, 1988; May & Porter, 1998) correlates with the very small R1 observed in humans at around 10-11 ms (Evinger et al., 1991; Powers et al., 1997; Hammond et al., 1996). However, it is not clear why the mono- and disynaptic pathways from sVc, which we observed in the macaque, and which are presumably spared in these human pontine lesions, are not capable of producing an R1 reflex. The ascending ipsilateral projections certainly appear dense enough to elicit activity in ObOc motoneurons.
The distance to sVc could be a factor. We measured the approximate distance from the supraorbital notch to pV (<100 mm) and sVc (<140 mm) in the macaque (Fig. 16). Assuming a 45 m/s conduction velocity for Aβ fibers, which are believed to initiate the R1 reflex (Pelligrini et al., 1995), the monosynaptic pathway through pV would activate ObOc motoneurons at around 3 ms and the disynaptic pathway would activate them around 4 ms. (The pV would be activated at about 2.2 ms.) This agrees with recordings from cat ObOc motoneurons that show a mean activation time of 3.7 ms (Trigo et al., 1999). This same study indicated it takes another 2 ms for activity to reach the ObOc muscle. So we would estimate that the fastest R1 in the macaque would be on the order of 6 ms. Unfortunately, the R1 and R2 latencies have not, to the best of our knowledge, been determined in a monkey. In cats, the R1 ranges from 6-8 ms (Gruart et al., 1995; LeDoux et al., 1997). So the pathway through the pV of a macaque, whose head is larger than that of a cat, would easily accomplish activation of the ObOc muscle in the appropriate time. These same Aβ fibers would activate sVc at around 3.1 ms. Based on a conduction time of 2 ms (Fanardjian & Manvelyan, 1987) and a 1 ms synaptic delay, between sVc and VII, the longer pathway would result in ObOc activity around 6 ms after supraorbital stimulation (Fig. 16). Thus, activity produced by this longer pathway still would lie within the duration (6-10 ms) of the R1. It appears then that either the blink circuitry in macaques and humans is dramatically different, or the presence of a pontine lesion must suppress the R1 outflow from the medulla.
Based on work in the guinea pig, an alternative model proposes that the R1 originates from an area stretching from caudal sVi to rostral sVc, where supraorbital afferents terminate relatively densely (Pelligrini et al., 1995). This study demonstrated that the large R1 characteristic of this species is suppressed by lesions or pharmacological inactivation of this region. The presence of a substantial mono- and, via the adjacent medullary reticular formation, disynaptic projection from sVc in the current report, certainly supports the possibility that this area can drive the R1 component of the reflex. Also, in one animal (not described in this study), we saw labeled terminals closely associated with ObOc motoneurons ipsilateral to a sVi BDA injection. Similar to the guinea pig, the macaque sVi and sVc also receive a major primary afferent projection from the cornea and eyelids (Marfurt & Echtenkamp, 1988; May & Porter, 1998). Pelligrini and colleagues (1995) further argued that the sVc at and below the spinomedullary junction, does not contribute to the R1 component of the reflex, even though it receives extensive supraorbital afferent input, since lesions at the spinomedullary junction did not seem to affect R1. Furthermore, physiological and pharmacological studies indicate that the caudal and rostral portions of sVc have rather different response properties (Meng et al., 1997; Hirata et al., 2000; 2003). Our macaque data, on the other hand, show a considerable ipsilateral projection to the dorsal division of VII from C1&2. Furthermore, both species receive a substantial input to C1&2 from the eyelid and the supraorbital nerve (Marfurt & Echtenkamp, 1988; Pelligrini et al., 1995; May & Porter, 1998). So there is no connectional reason why the caudal end of sVc cannot contribute to the R1 component of the reflex in the macaque.
The source of the R2 component is even more problematic. Lesion studies in humans indicate that it requires pathways passing through the medulla (Ongerboer de Visser, 1983; Aramideh et al., 1997). Work in the guinea pig suggests that the levels of sVc at and below the spinomedullary junction play a critical role in producing the R2 (Pelligrini et al., 1995). Others have argued that the longer latency of the R2 is due to the involvement of slower afferent fibers, not the use of different trigeminal circuits (Ellrich et al., 1997). We estimated the latency of Aδ activation of the macaque trigeminal circuits assuming a conduction velocity of 8 m/s (Fig. 16). Neurons in sVc would be activated at around 17.5 ms after supraorbital stimulation, leading to activity in ObOc motoneurons around 20.5 ms and EMG activity around 22.5 ms. This is similar to the 23 ms latency observed for the cat R2 (LeDoux et al., 1997). However, it leaves unexplained how R2 activation could be evoked in guinea pigs using electrical stimulation amplitudes that did not activate Aδ fibers (Pelligrini et al., 1995). On the other hand, if Aβ fibers are the source of both components, it becomes necessary to hypothesize a polysynaptic brainstem chain involving a series of several cells in order to explain the 10-20 ms delay (depending on species) between the initiation of the R1 and R2 components or possibly a circuit involving motor cortex (Morecraft et al., 2001).
A related question is the source of the silent period of approximately 5 ms between the two components of the reflex. It appears that suspension of activity is due to inhibition of the reflex. Facial muscles, and the orbicularis oculi in particular, move very quickly and are directed by motoneurons firing at high rates (McCormick et al., 1984; Trigo et al., 1999). As a result, they are prone to hyperactivity disorders, such as blepharospasm. So the silent period after the R1 may represent an inhibitory response that modulates the initial activity until the more nuanced R2 is initiated. There is ample evidence for such inhibition (Pellegrini and Evinger 1995; Powers et al., 1997). On the other hand, the latency argument would posit that this interval is due, in part, to a gap between the slow end of Aβ fiber conduction velocities (19 m/s) and the fast end of the Aδ fibers (9 m/s), which we estimate in monkey, would be around 8 ms. However, this interval is still present if one initiates the reflex via just Aβ afferent activity (Pelligrini et al., 1995). So one is left with the question of what brain region is able to maintain the blink-related activity during this silent period without activating ObOc motoneurons, and still be able to eventually trigger the R2 component.
A third model for producing the trigeminal blink reflex has been proposed based on findings in the rabbit (van Ham & Yeo, 1996B). It suggests that the R2 is the product of a network consisting of trigeminotrigeminal, trigeminoreticular and reticulculoreticular connections that feed into VII. The connectional data on trigeminal and reticular projections to ObOc motoneurons described here in monkeys are in agreement with this model, as is our previous study that demonstrated extensive trigeminotrigeminal projections (monkey: Warren & May, 2013)(Fig. 16, right side, magenta cells). These connections help explain why physiological examinations have shown a complete representation of the face and a variety of physiological cell types at all levels of the trigeminal sensory complex (monkey: Kerr et al., 1968; Price et al., 1976), even though the density and type of primary afferent input seems to vary considerably between levels (monkey: Marfurt & Echtenkap, 1988; May & Porter, 1998; cat: Panneton & Burton, 1981; rat: Marfurt & Del Torro, 1987; rabbit: van Ham & Yeo, 1996A; guinea pig, Pelligrini et al., 1995).
Considering the fact that none of the extant theories explains all the data concerning the R2 response, we would like to suggest another possibility related to the normal blink activation, as opposed to the artificial stimulation of blinks. Perhaps the initial Aβ input to this intrinsic circuitry sets up a reverberating pattern of activity within the trigeminal sensory nuclei and/or PTR that eventually results in production of the R2 component, particularly if it is reinforced by Aδ input, as would probably normally occur. The fact that stimulation of both Aβ and Aδ fibers produces a much more substantial R2 response supports this idea (guinea pig: Pelligrini et al., 1995). Furthermore, activity that corresponds to each of the reflex components has been observed in PTR cells (cat: Tamai et al., 1986; Inagaki et al., 1989). The delay between the responses would also allow other systems a chance to reinforce or suppress the R2, which is known to be the more plastic of the responses, by up or down regulating the trigeminotrigeminal and PTR circuits (Evinger & Manning, 1988; Powers et al., 1997; Schicatano et al., 2000). The more extensive crossed reticulofacial projections we observed in the present study, might also explain why bilateral blink R2 responses generally occur at lower stimulus levels than the R1 in humans (human: Tackmann et al. 1982; Aramideh & Ongerboer de Visser, 2002). Testing this hypothesis will require recording from the PTR, while activating the blink reflex in an awake behaving animal.
Acknowledgements:
We would like to thank the technicians who have assisted us in surgeries, undertaken the histological processing and provided assistance with illustrations for this work: Jayne Bernanke, Olga Golanov, Jennifer Cotton and Jinrong Wei. We are also beholden to Glen Hoskins for his preparation of the EM samples.
Grant support: This work was supported by NEI grant EY09762 to PJM, EY014263 to PJM & SW, and a grant from the Benign Essential Blepharospasm Research Foundation to SW & PJM.
List of Abbreviations
- 4n
trochlear nerve
- 5n
trigeminal nerve
- 7n
facial nerve
- At
axon terminal
- At*
labeled axon terminal
- Ax*
labeled axon
- BDA
biotinylated dextran amine
- BC
brachium conjunctivum
- C
cuneate nucleus
- C1-3
cervical cord levels 1-3
- CC
caudal central subdivision
- D
dorsal division of the facial nucleus
- Den
dendrite
- Den*
labeled dendrite
- DF
dorsal funiculus
- DH
dorsal horn
- dP
decussation of the pyramids
- eC
external cuneate nucleus
- G
gracile nucleus
- I
intermediate division of facial nucleus
- IC
inferior colliculus
- IO
inferior olive
- L
lateral division of facial nucleus
- LC
locus coeruleus
- LF
lateral funiculus
- LR
lateral reticular nucleus
- LV
lateral vestibular nucleus
- M
medial division of facial nucleus
- MdRF
medullary reticular formation
- MCP
middle cerebellar peduncle
- mV
trigeminal motor nucleus
- MV
medial vestibular nucleus
- nTS
nucleus tractus solitarius
- Nu
nucleus
- ObOc
orbicularis oculi
- OPt
olivary pretectal nucleus
- P
pyramid
- PAG
periaqueductal gray
- PB
parabrachial nuclei
- PRF
pontine reticular formation
- PPt
posterior pretectal nucleus
- PTR
paratigeminal reticular formation
- pV
principal trigeminal nucleus
- SC
superior colliculus
- SGI
intermediate gray layer
- SGS
superficial gray layer
- SO
superior olive
- SOA
supraoculomotor area
- Soma*
labeled soma
- SV
superior vestibular nucleus
- sVc
spinal trigeminal nucleus, pars caudalis
- sVi
spinal trigeminal nucleus, pars intermedius
- sVo
spinal trigeminal nucleus, pars oralis
- sVt
spinal trigeminal tract
- V
ventral division of facial nucleus
- VH
ventral horn
- VI
abducens nucleus
- VII
facial nucleus
- WGA-HRP
wheat germ agglutinin conjugated horseradish peroxidase
- XII
hypoglossal nucleus
Footnotes
Conflict of Interest: Neither author has any conflicts of interest, financial or otherwise, with respect to the work described in this manuscript.
Data Sharing: The data (essentially the boxes of slides from the cases on which this work has been based) are available for borrowing upon reasonable request to the authors.
The blink reflex is critical for protecting the eyes and spreading the tear film, and is a commonly used learning model. We demonstrate monosynaptic trigeminal (shown here) and disynaptic reticular formation pathways connecting the principal and the spinal trigeminal nucleus (pars caudalis) with orbicularis oculi motoneurons in the macaque monkey.
References
- Aramideh M, Ongerboer de Visser BW, Koelman JH, Majoie CB, Holstege G (1997) The late blink reflex response abnormality due to lesion of the lateral tegmental field. Brain 120:1685–92. doi: 10.1093/brain/120.9.1685. [DOI] [PubMed] [Google Scholar]
- Aramideh M, Ongerboer de Visser BW (2002) Brainstem reflexes: electrodiagnostic techniques, physiology, normative data, and clinical applications, Muscle Nerve 26:14–30. doi: 10.1002/mus.10120. [DOI] [PubMed] [Google Scholar]
- Barnerssoi M, May PJ (2016) Postembedding immunohistochemistry for inhibitory neurotransmitters in conjunction with neuroanatomical tracers. In: Transmission Electron Microscopy Methods for Understanding the Brain; Ed: Van Bockstaele EJ; Neuromethods; vol 115, pp 181–203. [Google Scholar]
- Basso MA, Strecker RE, Evinger C (1993) Midbrain 6-hydroxydopamine lesions modulate blink reflex excitability. Exp Brain Res 94:88–96. doi: 10.1007/BF00230472. [DOI] [PubMed] [Google Scholar]
- Boele H-J, Koekkoek SKE, De Zeeuw CI (2010) Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model. Front Cell Neurosci 3:19. doi: 10.3389/neuro.03.019.2009. eCollection 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour LJ, Aramideh M, Ongerboer de Visser BW (2000) Neurophysiological aspects of eye and eyelid movements during blinking in humans. J Neurophysiol 83:166–76. doi: 10.1152/jn.2000.83.1.166. [DOI] [PubMed] [Google Scholar]
- Cruz AA, Garcia DM, Pinto CT, Cechetti SP (2011) Spontaneous eyeblink activity. Ocul Surf 9(1):29–41. doi: 10.1016/s1542-0124(11)70007-6. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia JM, Evinger C, Escudero M, Baker R (1990) Behavior of accessory abducens and abducens motoneurons during eye retraction and rotation in the alert cat. J Neurophysiol 64:413–22. doi: 10.1152/jn.1990.64.2.413. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Hopf HC (1996) The R3 component of the blink reflex: normative data and application in spinal lesions. Electroencephalogr Clin Neurophysiol 101:349–54. doi: 10.1016/0924-980x(96)95684-2. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Bromm B, Hopf H C (1997) Pain-evoked blink reflex. Muscle Nerve 20:265–70. doi: . [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Killackey HP (1979) Efferent connections of the brainstem trigeminal complex with the facial nucleus of the rat. J Comp Neurol 188:75–86. doi: 10.1002/cne.901880107. [DOI] [PubMed] [Google Scholar]
- Evinger C, Shaw MD, Peck CK, Manning KA, Baker R (1984) Blinking and associated eye movements in humans, guinea pigs, and rabbits. J Neurophysiol 52:323–39. doi: 10.1152/jn.1984.52.2.323. [DOI] [PubMed] [Google Scholar]
- Evinger C, Manning KA (1988) A model system for motor learning: adaptive gain control of the blink reflex. Exp Brain Res 70:527–38. doi: 10.1007/BF00247600. [DOI] [PubMed] [Google Scholar]
- Evinger C, Manning KA, Sibony PA (1991) Eyelid movements. Mechanisms and normal data. Invest Ophthalmol Vis Sci 32:387–400. [PubMed] [Google Scholar]
- Evinger C (1995) A brain stem reflex in the blink of an eye. Physiology 10:147–153. [Google Scholar]
- Evinger C (2015) Benign essential blepharospasm is a disorder of neuroplasticity: Lessons from animal models. J Neuroophthalmol 35:374–9. doi: 10.1097/WNO.0000000000000317. [DOI] [PubMed] [Google Scholar]
- Fanardjian VV, Kasabyan SA, Manvelyan LR (1983) Mechanisms regulating the activity of facial nucleus motoneurones- 2. Synaptic activation from the caudal trigeminal nucleus. Neuroscience 9:823–35. doi: 10.1016/0306-4522(83)90271-3. [DOI] [PubMed] [Google Scholar]
- Fanardjian VV, Manvelyan LR (1987) Mechanisms regulating the activity of facial nucleus motoneurons- IV. Influences from the brainstem structures. Neuroscience 20:845–53. doi: 10.1016/0306-4522(87)90245-4. [DOI] [PubMed] [Google Scholar]
- Fine EJ, Sentz L, Soria E (1992) The history of the blink reflex. Neurology 42(2):450–4.doi: 10.1212/wnl.42.2.450. [DOI] [PubMed] [Google Scholar]
- Fort P, Sakai K, Luppi PH, Salvert D, Jouvet M (1989) Monoaminergic, peptidergic, and cholinergic afferents to the cat facial nucleus as evidenced by a double immunostaining method with unconjugated cholera toxin as a retrograde tracer. J Comp Neurol 283:285–302. doi: 10.1002/cne.902830209. [DOI] [PubMed] [Google Scholar]
- Gruart A, Blázquez P, Delgado-García JM (1994) Kinematic analyses of classically-conditioned eyelid movements in the cat suggest a brain stem site for motor learning. Neurosci Lett 175:81–4. doi: 10.1016/0304-3940(94)91083-9. [DOI] [PubMed] [Google Scholar]
- Gruart A, Blázquez P, Delgado-García JM (1995) Kinematics of spontaneous, reflex, and conditioned eyelid movements in the alert cat. J Neurophysiol 74:226–48. doi: 10.1152/jn.1995.74.1.226. [DOI] [PubMed] [Google Scholar]
- Gruart A, Schreurs BG, del Toro ED, Delgado-García JM. (2000) Kinetic and frequency-domain properties of reflex and conditioned eyelid responses in the rabbit. J Neurophysiol 83:836–52. doi: 10.1152/jn.2000.83.2.836. [DOI] [PubMed] [Google Scholar]
- Hallett M (2002) Blepharospasm: recent advances. Neurology 59:1306–12. doi: 10.1212/01.wnl.0000027361.73814.0e. [DOI] [PubMed] [Google Scholar]
- Hammond G, Thompson T, Proffitt T, Driscoll P (1996) Functional significance of the early component of the human blink reflex. Behav Neurosci 110:7–12. doi: 10.1037/0735-7044.110.1.7. [DOI] [PubMed] [Google Scholar]
- Henriquez VM, Evinger C (2007) The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res 179:691–702. doi: 10.1007/s00221-006-0826-7. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Watson CD (1983) Brain stem projections to the facial nucleus of the rat, Brain Behav Evol 22:153–63. doi: 10.1159/000121514. [DOI] [PubMed] [Google Scholar]
- Hirata H, Takeshita S, Hu JW, Bereiter DA (2000) Cornea-responsive medullary dorsal horn neurons: modulation by local opioids and projections to thalamus and brain stem. J Neurophysiol 84:1050–61. doi: 10.1152/jn.2000.84.2.1050. [DOI] [PubMed] [Google Scholar]
- Hirata H, Okamoto K, Bereiter DA (2003) GABA(A) receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis. J Neurophysiol 90:2837–49. doi: 10.1152/jn.00544.2003. Epub 2003 Jul 30. [DOI] [PubMed] [Google Scholar]
- Holstege G, Tan J, van Ham JJ, (1986A) Afferent projections to the orbicularis oculi motoneuronal cell group. An autoradiographical tracing study in the cat. Brain Res 374:306–20. doi: 10.1016/0006-8993(86)90425-7. [DOI] [PubMed] [Google Scholar]
- Holstege G, van Ham JJ, Tan J, Graveland GA (1986B) Anatomical observations on the afferent projections to the retractor bulbi motoneuronal cell group and other pathways possibly related to the blink reflex in the cat. Brain Res 374:321–34. doi: 10.1016/0006-8993(86)90426-9. [DOI] [PubMed] [Google Scholar]
- Holstege G, Tan J, van Ham JJ, (1988) Anatomic observations on afferent projections of orbicularis oculi and retractor bulbi motoneuronal cell groups and other pathways possibly related to the blink reflex in the cat. In: Cellular Mechanisms of Conditioning and Behavioral Plasticity Ed: Woody CD, Plenum Publishing Corp., pp 273–286. [Google Scholar]
- Inagaki M, Takeshita K, Nakao S, Shiraishi Y, Oikawa T (1989) An electrophysiologically defined trigemino-reticulo-facial pathway related to the blink reflex in the cat. Neurosci Lett 96:64–9. doi: 10.1016/0304-3940(89)90244-9. [DOI] [PubMed] [Google Scholar]
- Kaminer J, Powers AS, Horn KG, Hui C, Evinger C (2011) Characterizing the spontaneous blink generator: an animal model. J Neurosci 31(31):11256–67. doi: 10.1523/JNEUROSCI.6218-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Zuo CC, Bloedel JR (1990) Classical conditioning of the eyeblink reflex in the decerebrate-decerebellate rabbit. Behav Brain Res 38:7–18. doi: 10.1016/0166-4328(90)90019-b. [DOI] [PubMed] [Google Scholar]
- Kerr FW, Kruger L, Schwassmann HO, Stern R (1968) Somatotopic organization of mechanoreceptor units in the trigeminal nuclear complex of the macaque. J Comp Neurol 134:127–44. doi: 10.1002/cne.901340202. [DOI] [PubMed] [Google Scholar]
- Kugelberg E (1952) Facial reflexes. Brain 75:385–96. doi: 10.1093/brain/75.3.385. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Lorden JF, Weir AD, Smith JM (1997) Blink reflex to supraorbital nerve stimulation in the cat. Exp Brain Res 116:104–12. doi: 10.1007/pl00005730. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizun N (1997) Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J Comp Neurol 378:283–94. doi: . [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Del Toro DR (1987) Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol 261:450–9. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Echtenkamp SF (1988) Central projections and trigeminal ganglion location of corneal afferent neurons in the monkey, Macaca fascicularis. J Comp Neurol 272:370–82. doi: 10.1002/cne.902720307. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD (1998) The distribution of primary afferent terminals from the eyelids of macaque monkeys. Exp Brain Res 123:368–81. doi: 10.1007/s002210050582. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Benetti AP, Bereiter DA (1997) Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. J Neurophysiol 77:43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- Mogoseanu D, Smith AD, Bolam JP (1994) Monosynaptic innervation of facial motoneurones by neurones of the parvicellular reticular formation. Exp Brain Res 101:427–38. doi: 10.1007/BF00227336. [DOI] [PubMed] [Google Scholar]
- Morcuende S, Delgado-Garcia J-M, Ugolini G (2002) Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. J Neurosci 22:8808–18. doi: 10.1523/JNEUROSCI.22-20-08808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS (2001) Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain 124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Noriega AL, Wall JT (1991) Parcellated organization in the trigeminal and dorsal column nuclei of primates. Brain Res 565:188–94. doi: 10.1016/0006-8993(91)91649-l. [DOI] [PubMed] [Google Scholar]
- Nosch DS, Pult H, Albon J, Purslow C, Murphy JP (2016) Relationship between corneal sensation, blinking, and tear film quality. Optom Vis Sci 93:471–81. doi: 10.1097/OPX.0000000000000827. [DOI] [PubMed] [Google Scholar]
- Ongerboer de Visser BW (1983) Anatomical and functional organization of reflexes involving the trigeminal system in man: jaw reflex, blink reflex, corneal reflex, and exteroceptive suppression Adv Neurol 39:727–38. [PubMed] [Google Scholar]
- Panneton WM, Burton H (1981) Corneal and periocular representation within the trigeminal sensory complex in the cat studied with transganglionic transport of horseradish peroxidase. J Comp Neurol 199:327–44. doi: 10.1002/cne.901990303. [DOI] [PubMed] [Google Scholar]
- Paxinos GF, Huang X-F, Toga AW (2000) The Rhesus Monkey Brain in Stereotaxic Coordinates. Scademic Press, San Diego. [Google Scholar]
- Pellegrini JJ, Horn AK, Evinger C (1995) The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res 107:166–80. doi: 10.1007/BF00230039. [DOI] [PubMed] [Google Scholar]
- Pinganaud G, Bernat I, Buisseret P, Buisseret-Delmas C (1999) Trigeminal projections to hypoglossal and facial motor nuclei in the rat. J Comp Neurol 415:91–104. doi: . [DOI] [PubMed] [Google Scholar]
- Porter JD, Burns LA, May PJ (1989) Morphological substrate for eyelid movements: innervation and structure of primate levator palpebrae superioris and orbicularis oculi muscles. J Comp Neurol 287:64–81. doi: 10.1002/cne.902870106. [DOI] [PubMed] [Google Scholar]
- Porter JD, Stava MW, Gaddie IB, Baker RS (1993) Quantitative analysis of eyelid movement metrics reveals the highly stereotyped nature of monkey blinks. Brain Res 609:159–66. doi: 10.1016/0006-8993(93)90869-o. [DOI] [PubMed] [Google Scholar]
- Powers AS, Schicatano EJ, Basso MA, Evinger C (1997) To blink or not to blink: inhibition and facilitation of reflex blinks. Exp Brain Res 113:283–90. doi: 10.1007/BF02450326. [DOI] [PubMed] [Google Scholar]
- Price DD, Dubner R, Hu JW (1976) Trigeminothalamic neurons in nucleus caudalis responsive to tactile, thermal, and nociceptive stimulation of monkey's face. J Neurophysiol 39:936–53. doi: 10.1152/jn.1976.39.5.936. [DOI] [PubMed] [Google Scholar]
- Rahman M, Okamoto K, Thompson R, Katagiri A, Bereiter DA (2015) Sensitization of trigeminal brainstem pathways in a model for tear deficient dry eye. Pain 156:942–950. doi: 10.1097/j.pain.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicatano EJ, Peshori KR, Gopalaswamy R, Sahay E, Evinger C (2000) Reflex excitability regulates prepulse inhibition. J Neurosci 20:4240–7. doi: 10.1523/JNEUROSCI.20-11-04240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KP, Williams CR, Busettini C (2010) Macaque pontine omnipause neurons play no direct role in the generation of eye blinks. J Neurophysiol 103:2255–74. doi: 10.1152/jn.01150.2009. Epub 2010 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS, Wheeler RL (1983) Behavioral organization of reticular formation: studies in the unrestrained cat. II. Cells related to facial movements. J Neurophysiol 50:717–23. doi: 10.1152/jn.1983.50.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackmann W, Ettlin T, Barth R (1982) Blink reflexes elicited by electrical, acoustic and visual stimuli. I. Normal values and possible anatomical pathways. Eur Neurol 21:210–6. doi: 10.1159/000115483. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Nakano K, Uemura M, Matsuda K, Matsushima R, Mizuno N (1979) Mesencephalic and pontine afferent fiber system to the facial neucleus in the cat: a study using the horseradish peroxidase and silver impregnation techniques. Exp Neurol 66:330–42. doi: 10.1016/0014-4886(79)90084-0. [DOI] [PubMed] [Google Scholar]
- Tamai Y, Iwamoto M, Tsujimoto T (1986) Pathway of the blink reflex in the brainstem of the cat: interneurons between the trigeminal nuclei and the facial nucleus. Brain Res 380:19–25. doi: 10.1016/0006-8993(86)91424-1. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yu H, Kitai ST (1971) Trigeminal and spinal inputs to the facial nucleus. Brain Res 33:504–8. doi: 10.1016/0006-8993(71)90126-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Takeuchi Y, Nakano K (1978) Cells of origin of the spino-facial pathway in the cat: a horseradish peroxidase study. Brain Res 142:580–5. doi: 10.1016/0006-8993(78)90922-8. [DOI] [PubMed] [Google Scholar]
- Thompson RF (1990) Neural mechanisms of classical conditioning in mammals Philosophical Transactions: Biological Sciences 329: 161–170. [DOI] [PubMed] [Google Scholar]
- Tiwari RK, King RB (1974) Fiber projections from trigeminal nucleus caudalis in primate (squirrel monkey and baboon). J Comp Neurol 158:191–205. doi: 10.1002/cne.901580206. [DOI] [PubMed] [Google Scholar]
- Trigo JA, Gruart A, Delgado-García JM (1999) Discharge profiles of abducens, accessory abducens, and orbicularis oculi motoneurons during reflex and conditioned blinks in alert cats. J Neurophysiol 81:1666–84. doi: 10.1152/jn.1999.81.4.1666. [DOI] [PubMed] [Google Scholar]
- van Ham JJ, Yeo CH (1996A) The central distribution of primary afferents from the external eyelids, conjunctiva, and cornea in the rabbit, studied using WGA-HRP and B-HRP as transganglionic tracers. Exp Neurol 142:217–25. doi: 10.1006/exnr.1996.0193. [DOI] [PubMed] [Google Scholar]
- van Ham JJ, Yeo CH (1996B) Trigeminal inputs to eyeblink motoneurons in the rabbit. Exp Neurol 142:244–57. doi: 10.1006/exnr.1996.0195. [DOI] [PubMed] [Google Scholar]
- VanderWerf F, Aramideh M, Otto JA, Ongerboer de Visser BW (1998) Retrograde tracing studies of subdivisions of the orbicularis oculi muscle in the rhesus monkey. Exp Brain Res 121:433–41. doi: 10.1007/s002210050478. [DOI] [PubMed] [Google Scholar]
- Warren S, May PJ (2013) Morphology and connections of intratrigeminal cells and axons in the macaque monkey. Front Neuroanat 29; 7:11. doi: 10.3389/fnana.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Harvey JA (1991) Pavlovian conditioning in the rabbit during inactivation of the interpositus nucleus. J Physiol 444:459–80. doi: 10.1113/jphysiol.1991.sp018888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt C, Abbs JH (1990) Musculotopic organization of the facial motor nucleus in Macaca fascicularis: a morphometric and retrograde tracing study with cholera toxin B-HRP. J Comp Neurol 291:621–36. doi: 10.1002/cne.902910409. [DOI] [PubMed] [Google Scholar]
- Willer JC, Boulu P, Bratzlavsky M (1984) Electrophysiological evidence for crossed oligosynaptic trigemino-facial connections in normal man. J Neurol Neurosurg Psychiatry 47:87–90. doi: 10.1136/jnnp.47.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerari-Mailly F, Pinganaud G, Dauvergne C, Buisseret P, Buisseret-Delmas C (2001) Trigemino-reticulo-facial and trigemino-reticulo-hypoglossal pathways in the rat. J Comp Neurol 429:80–93. doi: . [DOI] [PubMed] [Google Scholar]
- Zerari-Mailly F, Dauvergne C, Buisseret P, Buisseret-Delmas C (2003) Localization of trigeminal, spinal, and reticular neurons involved in the rat blink reflex. J Comp Neurol 467:173–84. doi: 10.1002/cne.10917. [DOI] [PubMed] [Google Scholar]