Abstract
Traumatic brain injury (TBI) is a leading cause of death and disability. Mounting evidence indicates that the immune system is critically involved in TBI pathogenesis, where it is deployed to dispose of neurotoxic material generated from head trauma and to instruct the wound healing process. However, the immune response to brain damage must be carefully held in check as aberrant regulation of immune signaling can lead to deleterious neuroinflammation, brain pathology, and neurological dysfunction. Efficient clearance of neurotoxic material by microglia (the brain’s resident phagocytes) and the glymphatic-meningeal lymphatic drainage system are paramount to keeping the immune system in balance following head trauma. In this review, we highlight emerging evidence that defines pivotal roles for microglia and the recently discovered glymphatic-meningeal lymphatic system in TBI pathogenesis.
Neuroimmune aspects of traumatic brain injury (TBI)
Traumatic brain injury (TBI) caused by an impact or sudden jolt to the head can result in brain damage and cellular stress, subsequently triggering activation and mobilization of the immune system (Figure 1). While the immune system is deployed as a beneficial strategy to coordinate the clearance of neurotoxic debris and to promote tissue repair, a dysregulated immune response following head trauma can also lead to secondary tissue damage, brain atrophy, and neurological dysfunction in vertebrates [1-9]. Balancing the beneficial and detrimental arms of the immune response is required for the development of improved strategies to effectively manage recovery after TBIs.
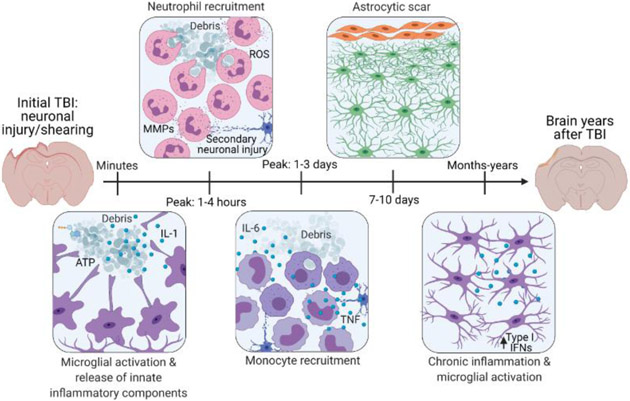
Figure 1. Traumatic brain injury (TBI) in humans and mice is characterized by an inflammatory response mediated by brain-resident cells and infiltrating immune cells.
The initial injury, while of differing severity depending on the injury mechanism, results in the death of impacted brain cells. These dying cells trigger the activation of nearby cells and the recruitment of immune cells from the periphery [27]. Microglia are the first to respond by extending their processes towards the site of injury to sample debris and wall off damage [37]. They respond to ATP and cytokines such as IL-1 released from dying cells [37]. Within hours of injury, neutrophils are recruited to the injury site and secrete other inflammatory modulators such as reactive oxygen species (ROS) and matrix metalloproteinases (MMPs) which continue to establish the immune response and remodel the extracellular matrix [4, 5]. Monocytes are present at the injury site at peak numbers from 1-4 days post-injury. They continue to engulf debris and secrete cytokines such as IL-1, IL-6, and TNF that are important for the immune response [3]. While this immune response is critical for clearing debris and promoting wound healing, it can also result in less desirable outcomes, including secondary neuronal injury and the formation of an astrocytic scar. Moreover, microglia can remain activated and express elevated concentrations of type I IFNs for months to years following TBI in mammals [43, 55, 56]. This figure was created using BioRender (https://biorender.com/).
Efficient clearance of damage/danger-associated molecular patterns (DAMPs) that are generated during TBI is a prerequisite to keep the immune system in check. For instance, DAMPs that are not swiftly eliminated from tissues can trigger local activation of pattern recognition receptors (PRRs) leading to proinflammatory cytokine production, cell death, and secondary tissue damage in vertebrates [10-13]. The two major ways in which potentially damaging substances are removed from organs in vertebrates are through engulfment and disposal by tissue-resident phagocytes or via lymphatic drainage to peripheral lymph nodes where large numbers of professional phagocytes reside [10, 11, 14]. In this review, we highlight exciting recent evidence suggesting that targeting the mammalian brain’s professional phagocytic cells, microglia, and the recently discovered glymphatic-meningeal lymphatic drainage system might offer much-needed strategies to alleviating TBI (Figure 2).
Figure 2. Key roles for neuroimmune cleanup crews following TBI.
Removal of neurotoxic material and excessive inflammatory products following brain injury is critical for preventing prolonged inflammation and secondary tissue damage following TBI. Both microglia, the brain-resident immune cell type with phagocytic capacities, and the glymphatic and meningeal lymphatic systems play pivotal roles in ensuring efficient disposal of neurotoxic debris from the brain [10-13, 83, 93-95]. Deficits in either microglial phagocytic function or glymphatic/lymphatic system drainage can result in increased buildup of neurotoxic material in the brain [10-13, 83, 99]. Recognition of neurotoxic material by pattern recognition receptors (PRRs) can trigger proinflammatory cytokine production (e.g. IL-1β, IFN-α/β, and TNF-α) and secondary tissue damage [3, 47-52]. dCLN, deep cervical lymph nodes; PRR, pattern recognition receptor. Black arrow indicates release of cytokines. This figure was created using BioRender (https://biorender.com/).
Microglia: the brain’s cellular cleanup crew
Microglia are central nervous system (CNS)-resident macrophages that serve as the professional phagocytes of the mammalian brain. Unlike most other macrophage populations that originate from the hematopoietic compartment, microglia are instead yolk-sac-derived, and uniquely express the surface markers TMEM119 and P2RY12 under steady-state conditions in mice [15-18] (Figure 3). The differentiation of microglia from yolk-sac derived progenitor cells is dependent on transcription factors PU.1 and IRF8, as well as on colony stimulating factor 1 receptor (CSF1R), TGFβ, and IL-34 signaling [18, 19]. Compared to circulating monocytes/macrophages in the periphery, which typically turn over every few days, microglia are extremely long-lived and have been reported to survive without local replenishment for upwards of a year in mice, and an estimated 4.2 years in humans [20, 21]. The long-lived nature of microglia in comparison to most other peripheral myeloid cell populations has been exploited in the field to target genetic manipulations of microglia [22, 23]. Microglia require tonic colony CSF1R signaling for their survival, and their reliance on continuous CSF1R signaling has also been leveraged to develop tools to target microglial depletion and manipulation [24] (Figure 3). Indeed, both pharmacological and genetic approaches that inhibit CSF1R signaling are routinely used to deplete microglia from the CNS in mammals [24]. Following the cessation of CSF1R inhibition, any remaining microglia that were spared from the depletion approach can rapidly repopulate the brain parenchyma in a manner of a few days [24]. These microglia depleting approaches have helped to illuminate roles for microglia in various homeostatic neurological processes, as well as in disease states in mice. For instance, sustained microglia depletion with the CSF1R inhibitor (Plexxikon (PLX) 5622) treatment in the 5xFAD mouse model of Alzheimer’s disease (AD) was shown to restrain amyloid beta (Aβ) plaque accumulation throughout the brain parenchyma in comparison to 5xFAD mice that received vehicle treatment [25]. Moreover, as described further on, microglia depletion strategies followed by recolonization of the brain with spared microglia can ameliorate neuroinflammation, brain pathology, and cognitive dysfunction in mouse models of TBI.
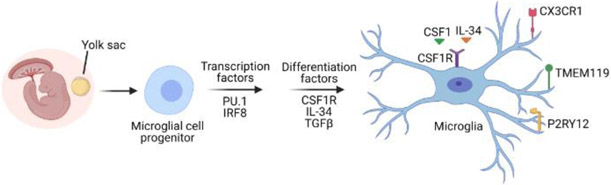
Figure 3. Microglial development and maturation in mice.
Microglia arise from the yolk sac and inhabit the brain at early time points in neurodevelopment before the blood-brain barrier (BBB) is established [15]. Their maintenance in the brain is strictly dependent on tonic signaling through the colony stimulating factor 1 receptor (CSF1R). The reliance of microglia on continuous CSF1R activation for survival in the brain has provided opportunities to deplete microglia genetically and pharmacologically by targeting this receptor [24]. Homeostatic microglia commonly express TMEM119, P2RY12 and CX3CR1; however, TMEM119 and P2RY12 are markers thought to be unique to microglia and not shared with monocytes that can infiltrate the brain in disease contexts [16, 17]. This figure was created using BioRender (https://biorender.com/).
Over the last few years, we have witnessed an explosion of findings on the roles of microglia in the CNS. Like other tissue-resident macrophage populations, microglia serve instrumental roles in the first line of defense against pathogens and are also necessary for the phagocytic containment and disposal of cellular debris and irritants in the CNS [26-28]. For example, knockdown of microglia with PLX5622 treatment in mice before infection with neurotropic West Nile virus (WNV) has resulted in greater morbidity and mortality than observed in WNV-infected mice that received control pretreatment [29, 30]. In addition to these canonical macrophage functions, microglia also take on specialized, tissue-specific roles, including coordinating synaptic reorganization in development and adulthood [23, 31-33], sculpting neural circuits [34], and regulating adult neurogenesis in mice [35, 36].
Microglia are activated following TBI as a protective strategy to dispose of neurotoxic material and to coordinate wound healing [4]. Their highly dynamic nature, consistent tiling throughout the brain, and phagocytic capacities all establish microglia as key first-responders to brain injury [5, 37]. Despite these initial beneficial responses, emerging evidence also suggests that dysregulated microglial responses can negatively impact neuronal health and ultimately contribute to various forms of neurodegenerative and psychiatric disease in mice [38-44]. For example, defects in microglial phagocytosis caused by loss-of-function mutations in the microglial scavenger receptor TREM2 (triggering receptor expressed on myeloid cells 2) have been shown to lead to greater Aβ pathology and neuronal cell loss in various different familial mouse models of AD [45, 46].
Microglia are potent producers of various proinflammatory cytokines that possess neurotoxic properties such as IL-1, TNF-α, and type I interferons (IFNs), and the unrestrained release of these mediators post-TBI can create inhospitable environments for neurons [3, 47-52]. Indeed, targeted ablation of IL-1, TNF-α, or type I IFN signaling with both genetic approaches and blocking antibodies in mice has been shown to ameliorate proinflammatory cytokine production, neuronal loss, and neurological dysfunction following TBI, compared with wild-type (WT) and vehicle treatment controls [3, 47-50, 52]. Moreover, persistent proinflammatory cytokine signaling can also lead to phagocytic compromise in microglia, which can incite a perpetuating inflammatory cascade leading to secondary brain damage in mice [53, 54]. This has been most extensively explored in the APP/PS1 mouse model of AD in which ablation of inflammasome-driven inflammation with NLRP3 deletion (Nlrp3−/−) boosts Aβ uptake by microglia relative to APP/PS1 controls [53, 54].
For reasons that remain poorly understood, microglia can remain activated for years to even decades post-TBI in humans [43, 55, 56], and this has spurred great interest in exploring whether microglial depletion strategies might be leveraged to treat head trauma tissue injury. Using various controlled cortical impact (CCI) injury mouse models of TBI, recent studies have shown that depletion and subsequent repopulation of the microglial compartment can offer an effective strategy to resolve deleterious neuroinflammation and limit brain damage; however, this still remains a somewhat contentious approach given that CSF1R inhibition-based microglial depletion may lead to depletion of other myeloid cell populations in the periphery and can also cause immunosuppression and increased susceptibility to CNS infection [29, 30, 57, 58].
Nevertheless, the potential therapeutic value of microglial replacement was first shown in studies that used an inducible diphtheria toxin A (DTA)-driven transgenic mouse model. This mouse model takes advantage of a tetracycline-off inducible transgenic CaM/Tet-DTA where DTA is expressed in forebrain excitatory neurons, and upon withdrawal of doxycycline from the diet and introduction of diphtheria toxin, neuronal death in the CA1 region of the hippocampus and cortex is selectively induced [59]. This approach was undertaken in this study to localize brain injury and neuronal loss to areas of the brain that are commonly disrupted in various forms of TBI and neurodegenerative disease in mammals [59]. Following the induction of neurodegeneration with diphtheria toxin treatment, mice were rested for 1 week and then received PLX5622 in their food for 2 weeks to transiently deplete microglia in lesioned brains. Microglial elimination and subsequent repopulation in this model of neuronal injury dampened neuroinflammation -- as evidenced from decreased expression of genes involved in proinflammatory signaling (e.g. Il1b, Ccl3, C4a, and Cxcl10), increased dendritic spine density, and improved cognitive performance in the Morris water maze (MWM), relative to untreated lesioned mice [59] (Figure 4). These findings provided some of the earliest evidence that microglial elimination followed by recolonization might provide a potential therapeutic strategy to ameliorate neuroinflammation and promote brain recovery following head injury.
Figure 4. Depletion and repopulation of microglia can lead to beneficial outcomes following brain injury in mice.
Microglia are initially deployed following TBI as a protective strategy to phagocytose neurotoxic material and coordinate wound healing responses. However, unchecked and/or chronic activation of microglia in TBI is thought to cause deleterious neuroinflammation and secondary tissue damage [59-61]. Microglia depletion followed by subsequent recolonization of the brain has been shown to dampen proinflammatory responses, limit neurodegeneration, and promote adult neurogenesis in an IL-6-dependent fashion [59-61]. Moreover, microglia ablation followed by repopulation can ultimately result in improved cognitive and motor functions. Without this microglial depletion/repopulation intervention, injured brains exhibit increased inflammasome activation, more neuronal degeneration, and increased production of harmful cytokines, including type I IFNs [59-61]. Inflammasome activation can result in the release of ASC specks which have been shown to seed amyloid beta (Aβ) aggregation and spread [66]. Whether amyloid beta deposition and spread can be prevented through microglial depletion and subsequent repopulation approaches remains to be investigated. IFN, interferon; IL, interleukin. Upward arrows indicate increases and downward arrows denote decreases. This figure was created using BioRender (https://biorender.com/).
In agreement with this initial report, recent studies using more traditional models of TBI. such as CCI injury models, have also confirmed that microglial elimination and subsequent recolonization can lead to improved clinical outcomes following head trauma [60, 61]. In one study, TBI mice received a week-long treatment with PLX5622 to deplete chronically activated microglia at one month post CCI injury [60]. Repopulated microglia in the injured brain displayed a ramified morphology reminiscent of what is typically displayed in homeostatic microglia from an uninjured brain. This is in contrast to the protracted, hyperactivated (i.e. ameboid) microglia morphology traditionally seen without depletion and repopulation intervention in TBI [44]. Moreover, TBI mice that underwent microglial recolonization also exhibited improved recovery of long-term motor and cognitive function at 3 months post CCI, and this was associated with dampened production of proinflammatory cytokines (e.g. IL-1β), smaller cortical lesions, and reduced neuronal cell death in the hippocampus, compared to vehicle-treated TBI mice (Figure 4) [60].
To gain insights into the potential mechanisms underlying the apparent neuroprotective effects of microglial depletion and recolonization in TBI, the role of dysregulated NLRP3 inflammasome signaling was examined in this model, as genes in this pathway (e.g. Nlrp3, Casp1, and Il1b) were downregulated in microglia that repopulated the injured brain relative to TBI mice that received vehicle [60]. Inflammasomes are multiprotein innate immune signaling complexes that orchestrate activation-induced cleavage of caspase-1 in response to a wide range of microbes and DAMPs. The activated cleavage form of caspase-1 can then coordinate the production of the proinflammatory cytokines IL-1β and IL-18, as well as execute a gasdermin-D-mediated form of cell death, commonly referred to as pyroptosis [62]. In this study, microglia expressed high amounts of IL-1β and active caspase-1 at 2 months post-TBI, and microglia repopulation was effective in recalibrating the expression of the inflammasome machinery (i.e. IL-1β and active caspase-1) to levels observed in uninjured mice (Figure 4). These findings are notable given the mounting evidence suggesting that inflammasomes are pivotal drivers of neuroinflammation and brain pathology following head trauma in mice [2, 63-65]. Of note, recent studies have also demonstrated that NLRP3 inflammasome activation-induced release of ASC specks can seed Aβ aggregation and spread [66]. In these studies, ASC specks enhanced the aggregation of both human and mouse Aβ in cell culture assays [66]. Moreover, blocking ASC speck formation in vivo with intrahippocampal anti-ASC antibody injection was effective in limiting the spread of Aβ pathology in Aβ-overexpressing transgenic mice (APP/PS1 mice) compared with APP/PS1 mice that received control antibody [66]. While TBI is recognized as a major risk factor for developing AD later in life in both humans and mice [67-69], what mechanistically accounts for this remains poorly understood. It is possible that microglia-coordinated release of ASC specks might be one factor that contributes to an increased risk of AD post-TBI. If so, microglia depletion and repopulation might offer a strategy to potentially limit this, although this possibility remains to be rigorously tested.
In a separate study, repopulation of microglia immediately following TBI in mice was also reported to attenuate learning and memory deficits, and these benefits lasted for at least 9 months post-injury [61] (Figure 4). Here, two independent and complementary approaches to explore the effects of microglial repopulation on TBI pathogenesis were leveraged. One approach involved depleting microglia by feeding mice with chow containing PLX5622 for 21 days prior to CCI induction, subsequently returning mice to normal chow at the time of CCI induction [61]. As a secondary strategy to repopulate the brain with new microglia immediately after TBI, this group also used CX3CR1creERT2xiDTR mice in their studies [61]. Both of these acute phase microglia repopulation strategies resulted in improved spatial learning and memory in the active place avoidance (APA), MWM, and Y-maze behavioral tests when compared to TBI mice that did not undergo microglial depletion and repopulation [61].
They also observed that microglia depletion and replenishment in this CCI model of head trauma resulted in elevated adult neurogenesis in the hippocampus relative to TBI mice that did not undergo microglia depletion and reconstitution [61]. These improvements in neurogenesis were seen when genetically targeting microglia through intra-peritoneal administration of diphtheria toxin, as well as through local administration of diphtheria toxin to the hippocampus in CX3CR1creERT2xiDTR mice [61]. Repopulation of microglia led to an increase in both hippocampal doublecortin (DCX)-positive immature neurons and TBR2-positive neural precursor cells [61]. Deficits in adult neurogenesis are commonly seen in many mouse models of TBI and this is thought to contribute to hippocampal neuronal loss and subsequent cognitive decline following head trauma [70, 71]. This might also be the case in the previously described experimental systems [61]; however, additional studies are needed to formally prove that in this TBI mouse model, the improved cognitive function observed with microglia repopulation is due to increased adult neurogenesis. Of note, IL-6 was shown to drive both the improved neurogenesis and behavioral outcomes seen after injury with repopulating microglia [61]. This was surprising as IL-6 is traditionally thought to be part of the detrimental inflammatory response after brain injury [72, 73]; thus, IL-6 production by hippocampal granule cells might actually be necessary for improved hippocampal neurogenesis after TBI, but this warrants further investigation [61]. In this study, microglia were involved in stimulating IL-6 production by hippocampal granule cells [61] (Figure 4); however, how microglia accomplish this at the mechanistic level remains to be defined. The identification of microglia-derived factors that stimulate IL-6 production by hippocampal granule cells post-TBI in mice might help uncover potential new therapeutic strategies to boost adult neurogenesis and ideally treat neurological conditions such as TBI and AD, which are driven by neuronal loss.
In addition to the induction of neurogenesis and the blunting of inflammasome activation, there are likely other mechanisms through which microglia depletion and repopulation can contribute to improved clinical outcomes in TBI. In these recent microglia recolonization studies, transcriptomic analyses provided a rich list of other molecular pathways that can be explored to gain a deeper understanding of how microglia might contribute to TBI pathogenesis at a mechanistic level [59-61]. Specifically, following TBI in mice, microglia displayed a proinflammatory signature characterized by the upregulation of genes involved in type I IFN, complement, and toll-like receptor (TLR) signaling that lasted for at least 2 months [60, 74]. In contrast, the repopulating microglia inhabiting the brain post-TBI exhibited a considerably different gene signature, including the substantial downregulation of proinflammatory genes involved in the type I IFN pathway (e.g. Irf7, Ifit3, and Mx1) and upregulation of genes involved in wound repair (e.g. Fn1, Alcam, and Cspg4) [61]. Moreover, microglial repopulation at one-month post-injury was associated with reduced expression of genes associated with oxidative stress (e.g. Cybb and Ncf1), proinflammatory responses (e.g. Il1r1 and Nlrp3), and apoptotic cell death (e.g. Bax, Casp3, and Casp7) [60]. Together, these findings suggest that repopulating microglia might potentially serve to “reset” the environment in the brain following injury by reducing inflammatory and stress responses, and this possibility merits further attention.
While these three recent studies have sparked considerable excitement about the therapeutic potential of targeting microglia to treat TBI in humans, it should be noted that microglial manipulation does not appear to be universally beneficial in all contexts of head trauma. Of note, in one of these recent studies [61], repopulation by new microglia was required to achieve the beneficial effects associated with microglial depletion and the neuroprotective effects were not achieved with persistent microglial elimination alone [61]. Specifically, continuous microglia depletion with PLX5622 treatment following CCI injury did not rescue cognitive performance of mice in the MWM, Y-maze, or the APA tests relative to CCI injury mice that received vehicle [61]. These findings are consistent with previous optic nerve injury studies demonstrating that long-term microglial depletion without repopulation does not offer any neuroprotective benefits in mice [75]. However, of note, a recent study showed that sustained depletion of microglia with chronic PLX5622 treatment following midline fluid percussion injury (mFPI) in mice could lead to improved cognitive performance in the novel objection location and recognition tests at 30 days post-injury compared to mFPI mice that received vehicle treatment [76]. This suggests that the effects of sustained microglia depletion on TBI disease progression might be influenced by the type and/or severity of injury, and thus, a nuanced approach must be taken when considering the timing and length of microglial depletion used to treat TBI.
Taken together, these findings suggest that the beneficial effects of microglia depletion treatment in TBI are likely due to the neuroprotective functions provided by the microglial population that repopulates this depleted niche, instead of stemming from the eliminated chronically activated microglia present in the CNS at early stages post-injury. Nevertheless, it is still possible that long-term depletion of microglia without replacement might help to treat other aspects of TBI-associated disease sequelae, such as limiting the long-term risk of developing neurodegenerative disease later in life, and this warrants further testing. The pursuit of such future long-term microglia depletion modalities will, however, have to weigh the risk of potential side-effects. For instance, as described earlier, the depletion of microglia with CSF1R inhibitor treatments in mice can limit the generation of protective immunity to CNS infections, such as with WNV, and lead to greater incidence of morbidity and mortality if infection does occur [29, 30, 57, 58].
Of note, a handful of older studies using less sophisticated microglia depletion approaches also concluded that microglia ablation does not always offer neuroprotective benefits following TBI, and in some cases can actually lead to worsened clinical outcomes in mice [77, 78]. However, there were a number of technical caveats with the microglia manipulation techniques used in these studies (i.e. depletion of bulk CD11b-expressing cells via DT treatment of CD11b-DTR (diphtheria toxin receptor) mice or valganciclovir treatment of CD11b-TK (thymidine kinase) mice) [77, 78]. For example, these approaches were only found to promote partial depletion of microglia from the brain. Moreover, many of these approaches were also known to profoundly affect various aspects of the peripheral immune system, including potently depleting multiple myeloid cell populations. Lastly, some of these knockdown approaches were also reported to incite collateral neuroinflammation independent of head trauma, which introduced other confounding variables [77, 78].
On the one hand, these findings collectively suggest a working model whereby long-term activation and inflammation propagated by microglia might facilitate ongoing neuronal death and lead to increased risk for the development of other neurodegenerative diseases. On the other hand, a complete lack of microglia in the CNS does not appear to be beneficial and can be harmful in some contexts. By contrast, depletion and repopulation of less-activated microglia following brain injury might serve as a ‘middle-ground’ in terms of resetting the inflammatory response, while still allowing microglia to perform their necessary healing functions. Despite these exciting recent advances, further research is needed to determine the most ideal timepoint(s) for further developing this depletion/repopulation microglia strategy, and to also uncover additional molecular players mechanistically orchestrating beneficial or detrimental microglia functions following head trauma. Moreover, given the prominent role that microglia play in shaping neurodevelopment [31, 32, 34, 79], it will also be important to carefully evaluate how microglia depletion approaches affect TBI in different age groups, especially in the young.
The glymphatic-meningeal lymphatic system: drainage conduits of the brain
Clearance of neurotoxic material from the brain following TBI can also be coordinated at the systems level by the glymphatics and the meningeal lymphatics (Figure 5). Recent studies have begun to reveal pivotal roles for these interconnected drainage systems in head trauma. The glymphatic system is a macroscopic paravascular system that promotes the movement of waste and other solutes through the brain parenchyma [80]. In the glymphatic system, forces generated by pulsating arterial walls propel cerebrospinal fluid (CSF) deep into the brain along perivascular spaces. Aquaporin-4 (AQP4) water channels located along the vascular astrocytic endfeet also facilitate bulk CSF flow and intermixing with interstitial fluid (ISF) in mice [80, 81]. The intermixed CSF/ISF and any parenchymal contents can then efflux along veins out of the parenchyma and return to the subarachnoid space surrounding the brain. This collective process removes waste from the brain parenchyma, which is then collected by the meningeal lymphatics and is subsequently transported to the deep cervical lymph nodes (dCLNs) in the periphery (Figure 5) [82].
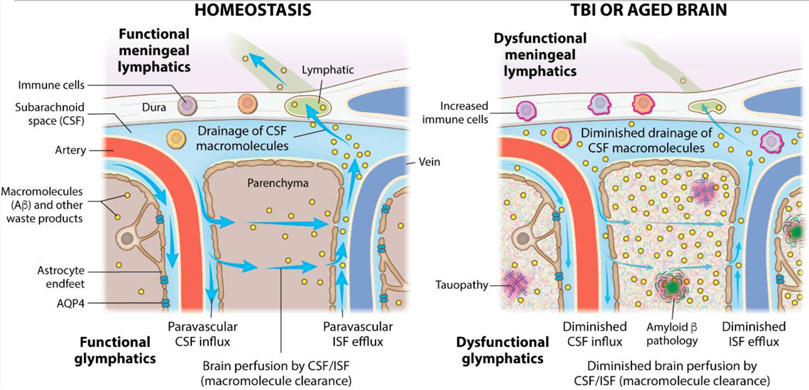
Key Figure, Figure 5. TBI and aging disrupt glymphatic-meningeal lymphatic system function in mice.
In homeostatic conditions, cerebrospinal fluid (CSF) moves along arterial vasculature into the brain where it intermixes with interstitial fluid (ISF) and other macromolecules such as amyloid beta (Aβ) in the brain parenchyma [80]. Aquaporin-4 (AQP4) water channels positioned along astrocytic endfeet are involved in coordinating bulk CSF flow and intermixing with ISF in mice [80, 81]. This fluid diffuses out along venous vasculature and returns to the CSF that surrounds the brain in the subarachnoid space. This makes up the glymphatic system. This CSF in the subarachnoid space can then be taken up by meningeal lymphatics in the dural meninges and taken to peripheral lymph nodes [88, 89]. After brain injury, flow in the lymphatic and glymphatic systems is slowed down, resulting in decreased movement of ISF through the brain, decreased outflow of CSF through the meningeal lymphatics, and a corresponding increase in protein accumulation in the brain parenchyma [83, 99]. Defects in the glymphatic-meningeal lymphatic system in mice can hinder the clearance of tau and Aβ aggregates from the brain [83, 93-95]. Arrows indicate the flow of CSF, ISF, and macromolecules through the brain parenchyma via the glymphatic system and leaving the brain through uptake by the meningeal lymphatics.
Moderate-to-severe TBI in mice can reduce glymphatic function by nearly 60% and these impairments can last for at least one month post-head trauma [83]. To further explore a functional role for this waste perfusion system in TBI, partial reduction of glymphatic capacity upon AQP4 deletion (Aqp4−/− mice) was investigated, as well as the disease outcomes following head trauma [83, 84]. While no effect of glymphatic dysfunction on lesion size was noted 28 days post-TBI in mice, moderate-to-severe TBI in AQP4-deficient mice resulted in exacerbated tauopathy, as evidenced from the increased accumulation of phospho-Tau seen in the cortices of Aqp4−/− TBI mice relative WT TBI mice [83]. Moreover, head trauma in AQP4-deficient mice led to greater axonal degeneration, neuroinflammation, and cognitive impairment than was observed in WT TBI mice [83]. Collectively, these findings established pivotal roles for the glymphatic system in head trauma, suggesting that efforts made to restore glymphatic function post-injury might offer novel putative strategies for treating TBI.
While the lymphatic vasculature in the meninges was first crudely described in the 1780s [85], it was not until the early 1990s when others reported that CSF could traffic to the dCLNs [86, 87]; only recently have we begun to uncover the unique anatomical and physiological features of the meningeal lymphatics [88, 89]. These are a series of draining vessels located in the meninges -- a tri-layer tissue residing between the brain parenchyma and skull. The meningeal lymphatic vessels drain CSF, ISF, CNS-derived molecules, and immune cells from the brain and meninges to the dCLNs [88-91]. Exciting recent work has revealed that the meningeal lymphatic pathway is crucially involved in draining various forms of neurotoxic material from the brain, including Aβ aggregates, extracellular tau, and alpha-synuclein aggregates in mice [92-95]. Moreover, emerging studies have also unraveled prominent roles for the meningeal lymphatic system in various mouse models of neurological disease, including AD, Parkinson’s disease, and glioblastoma [93, 96, 97]. In the case of AD, photoablation of the meningeal lymphatics in Aβ-overexpressing transgenic mice (5xFAD mice) resulted in increased Aβ pathology in the hippocampus and meninges relative to 5xFAD mice that received control treatment [93].
Great strides have also been recently made to define pivotal roles for the meningeal lymphatic system in brain injury pathogenesis [98, 99]. For instance, recent work revealed that sub-arachnoid hemorrhage (SAH)-induced brain injury disrupted meningeal lymphatic drainage function in mice [98, 100]. Mouse studies from our group also indicate that mild head trauma can lead to prominent defects in the drainage of fluorescent beads via the meningeal lymphatic system, and these deficits can last for at least one-month post-injury [99]. However, what contributes to meningeal lymphatic disruptions following head trauma currently remains poorly understood. One possibility is that the increased intracranial pressure (ICP) that typically accompanies TBI [101] may impinge on the meningeal lymphatic system. In support of this idea, our group recently showed that transient elevation of ICP with jugular vein ligation in mice caused impaired meningeal lymphatic drainage function compared to mice that underwent sham surgery [99]. In addition to increased ICP, there are likely other mechanistic factors contributing to the functional disruption of the meningeal lymphatic system. For instance, a combination of increased ICP, damage to the lymphatic vasculature from the injury, and the buildup of inflammatory cells, erythrocytes, and debris all likely conspire to cause lymphatic dysfunction in the context of brain injury, but these facets remain to be robustly tested.
Brain injury can have particularly grave consequences in individuals who have experienced multiple TBIs and in the elderly [102-106]. For instance, injuries of similar magnitude result in more severe neuronal cell loss, gliosis, and neurological impairment in aged mice than in younger controls [44, 99, 102, 107]. Mounting evidence also indicates that repetitive head trauma in mice can have debilitating consequences that include aggravated tauopathy, proinflammatory cytokine production, and cognitive dysfunction in the MWM test [104, 108-110]. The mechanisms behind the worsened outcomes in the elderly and in individuals with repetitive head injuries remains unclear. Of note, recent findings in mice have revealed a significant decrease in meningeal lymphatic functioning with aging (Figure 5) [93, 111, 112]. Moreover, a single head injury in mice can induce pronounced disruptions in both glymphatic and lymphatic function [83, 99]. This has led us and others to question whether pre-existing meningeal lymphatic dysfunction might help to explain some of the more severe outcomes often seen in repetitive TBI, or following brain injury in the elderly.
Consistent with this idea, recent findings show that pre-existing impairment in meningeal lymphatic drainage can result in more severe neuroinflammatory responses and neurological dysfunction than in the case of injury alone [98, 99]. For instance in a SAH mouse model, disruption of meningeal lymphatic function with either visudyne-induced photoablation, or treatment with the VEGFR3 tyrosine kinase inhibitor MAZ51 (intraperitoneal) before injury led to increased proportions of CD16+/CD32+ proinflammatory microglia when compared to anti-inflammatory CD206+ microglia in the injured brain [98] (Figure 6). Moreover, compared to injured mice that received control treatment prior to SAH, meningeal lymphatic disruption with either visudyne-induced photoablation or MAZ51 treatment before SAH-induction exacerbated anxiety-related behaviors and cognitive deficits in injured mice [98]. Recent findings from our laboratory have also shown than disruption of the meningeal lymphatics with visudyne-induced photoablation one week before mild TBI induction resulted in hyperactivation of astrocytes and microglia, enhanced expression of complement- and proinflammatory cytokine-related genes, and more severe cognitive dysfunction relative to mice receiving control pre-treatment before TBI induction [99]. Consistent with the idea that pre-existing meningeal lymphatic defects can lead to exacerbated TBI pathogenesis, recuperating meningeal lymphatic function in aged mice (20-22 months of age) with adeno-associated virus serotype 1(AAV1)-based delivery of the lymphatic growth factor VEGF-C before TBI induction was effective in mitigating the exacerbated gliosis seen in aged TBI mice relative to aged TBI mice that received control AAV1 expressing GFP [99] (Figure 6). Collectively, these findings suggest that pre-existing meningeal lymphatic dysfunction might contribute to explain the more aggressive clinical disease often seen in repetitive head injury, or following TBI in the elderly.
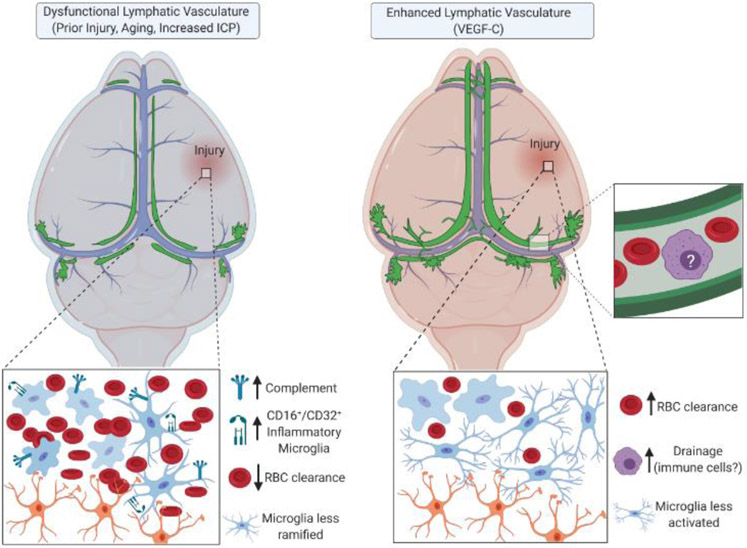
Figure 6. Meningeal lymphatic involvement in rodent brain injury.
Baseline disruption or dysfunction of the meningeal lymphatic system leads to increased inflammation following brain injury, including elevated complement signaling, activated microglia, and decreased red blood cell clearance [98, 99]. Enhancement of the meningeal lymphatic system with VEGF-C treatment can help to limit disease pathogenesis in TBI [99]. ICP, intracranial pressure; RBC, red blood cell; VEGF-C, vascular endothelial growth factor-C. Arrows indicate increased or decreased cells or proteins. This figure was created using BioRender (https://biorender.com/).
Our understanding of the meningeal lymphatic system is constantly evolving and recent work has begun to reveal additional pathways and players thought to influence this brain drainage conduit. For instance, the cribriform plate near the nasal cavities offers a potential exit point for drainage, yet this portion of the lymphatic system has been understudied in disease contexts [89, 113]. However, emerging evidence does indeed suggest that the cribriform drainage pathway can modulate the infiltration of autoimmune antigen-specific CD4+ T cells in experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis (as evidenced from adoptive transfer tracing experiments and MAZ51 treated EAE mice) [113]. In this model, EAE neuroinflammation also promoted the expansion of lymphatic vessel volume near the cribriform plate, and CD11c-expressing myeloid cells were found trafficking these lymphatic vessels (as evidenced in CD11c-eYFP transgenic reporter mice)[113]. Furthermore, while the majority of studies to date have focused on the dorsal region of the meningeal lymphatics, recent studies have also uncovered the presence of meningeal lymphatic vasculature at the base of the skull in mice [112]. Similar to the previously identified dorsal meningeal lymphatics [88, 89], these newly described basal meningeal lymphatics in mice can also contribute to the clearance of macromolecules injected into the CSF [112]. Thus, the interplay between the different anatomical portions of the meningeal lymphatic vasculature is currently a matter of great debate in the field. Indeed, further studies are still needed to rigorously investigate how TBI impacts the cribriform plate and the basal meningeal lymphatics.
Concluding Remarks
Recent findings suggest that depletion and subsequent repopulation of the microglial compartment may offer a promising strategy to reset a portion of the immune response following TBI [59-61]. These findings showed that recolonization of microglia following head trauma might help limit proinflammatory responses and neurodegeneration, while also promoting neurogenesis and improved cognitive function in TBI mice [59-61]. Similar microglial depletion and/or repopulation approaches have also had beneficial effects in a spectrum of neurological disease models, including those used to study autism and AD in mice [25, 41, 113-115]. This has sparked significant pharmaceutical interest in the design of putatively effective strategies to specifically and safely deplete microglia in humans. Hopefully, many of these emergent microglia-modulating therapies might make their way into clinical trials for TBI in the near future. However, as mentioned earlier, great care must be taken in the design of these studies, given that prolonged microglia depletion has been shown to derail protective immunity to CNS infections in mice [29, 30, 57, 58].
The glymphatic and meningeal lymphatic systems have also emerged as instrumental players in TBI [83, 98-100, 116]. However, additional research is needed to determine how the glymphatic-meningeal lymphatic system regulates immune responses in TBI and how this drainage pathway is affected in TBI patients (see Outstanding Questions). Of relevance, in vivo magnetic resonance (MRI) studies have confirmed the presence of meningeal lymphatic vessels in humans and nonhuman primates [97, 117, 118]. Moreover, recent studies employing contrast-enhanced MRI in Parkinson’s disease (PD) patients have also shown that meningeal lymphatic drainage function can be non-invasively evaluated in humans and that drainage defects are a hallmark of idiopathic PD [97]. These findings in humans open the door to the exciting possibility that we may be able to leverage similar imaging approaches in TBI patients to evaluate meningeal lymphatic dysfunction. Such an approach might potentially offer a much-needed strategy to assess recovery following head trauma.
Outstanding Questions.
What mechanistically accounts for the increased risk of developing neurodegenerative and psychiatric disease following head trauma? Studies in both humans and rodents have linked TBI with an increased risk of Alzheimer’s disease, multiple sclerosis, chronic traumatic encephalopathy, and depression, yet the connection between brain injury and these diseases is not fully understood. The possibility exists that TBI induces disruptions to the brain’s waste clearance systems (i.e. glymphatic-meningeal lymphatic system and microglia), which might help to explain this link; however, future studies are needed to formally test this.
What are the molecular players that underlie the protective effects associated with microglia repopulation seen in rodent models after TBI? Identification of the mechanistic factors that underlie the neuroprotective effects of microglial repopulation may help identify novel putative therapeutic targets to treat TBI.
What is the origin of the neuroprotective macrophages that repopulate the brain following microglia depletion? Understanding whether the repopulation is due to proliferation of the remaining few microglia or infiltration of monocytes that adopt microglial-like phenotypes will be essential for knowing if, when, and how to promote this beneficial effect.
What is the impact of repetitive TBI on microglia and meningeal lymphatic function? Does this contribute to the exacerbated neurological disease symptoms commonly seen following repetitive TBI in both humans and rodents?
What are the effects of glymphatic and meningeal lymphatic dysfunction on microglial biology? Do microglia and/or meningeal macrophages affect the functioning of these two interconnected drainage systems? The understanding of the meningeal lymphatic system is in its infancy; understanding how cells in the brain can affect this system and vice versa may be important for understanding both components in disease states.
What pathways contribute to the functional modulation of meningeal lymphatic drainage following head trauma? We still lack a complete understanding of the cellular and molecular mechanisms capable of boosting or impeding meningeal lymphatic function. Identifying such factors might aid in the design of meningeal lymphatic rejuvenation strategies to treat TBI and other neurological disorders that are affected by meningeal lymphatics such as Alzheimer’s and Parkinson’s diseases.
Are the glymphatic and meningeal lymphatic systems affected in human TBI patients? Imaging techniques to assess meningeal lymphatic functionality in humans are just being developed. Leveraging these emerging technologies to study human TBI can offer a deeper understanding of these systems in humans and may help clinicians to more precisely evaluate head trauma severity and recovery.
The complex interactions between the brain and the immune system that occur after brain injury are still not fully understood. However, it is becoming increasingly clear that proper disposal of neurotoxic material generated following head trauma is required to prevent chronic inflammatory responses and secondary brain damage from unfolding. In particular, recent findings have begun to define pivotal roles for microglia and the glymphatic and meningeal lymphatic systems in protecting the injured brain from spurious immune responses. Collectively, recent advances in microglia and glymphatic-meningeal lymphatic biology summarized in this review suggest that targeting these neuroimmune waste cleanup crews might offer novel strategies to alleviating TBI.
Highlights.
Traumatic brain injury (TBI), caused by blows or jolts to the head due to falls, vehicular accidents, sports-related collisions, and military combat, among other accidents, affects millions of people worldwide each year. Current trends project that TBI will become the third leading cause of death and disability in coming decades.
TBI has been shown to cause debilitating impairments in motor function, cognition, sensory function, and mental health in animal models and humans. Additionally, TBI has been associated with an increased risk for developing other neurological and psychiatric disorders, including chronic traumatic encephalopathy (CTE), Alzheimer’s disease, amyotrophic lateral sclerosis, anxiety, depression, aggression, and personality disorders.
Microglia, as professional phagocytic immune cells in the brain, can remain activated for years and even decades post-TBI in humans. And while they are centrally involved in the disposal of neurotoxic material and instructing tissue repair, microglia can also become dysregulated and cause pathological neuroinflammatory responses and secondary neurodegeneration.
Recent studies in mice suggest that microglia replacement strategies might be effective in limiting neuroinflammation, secondary brain damage, and cognitive decline in TBI. At the mechanistic level, recolonization of the microglial compartment following head trauma can dampen NLRP3 inflammasome activation, as well as promote neuroprotection and neurogenesis in an IL-6-dependent fashion.
The recently discovered glymphatic and meningeal lymphatic systems are also crucially involved in neurotoxic waste disposal and can impact TBI disease pathogenesis in mice. Even mild head trauma can lead to long-lasting defects in meningeal lymphatic drainage and lymphatic rejuvenation can mitigate TBI-driven neuroinflammation in mice.
ACKNOWLEDGMENTS
We apologize to authors whose work could not be referenced in this review due to space limitations. We thank members of the Lukens lab and the Center for Brain Immunology and Glia (BIG) for valuable discussions. We thank Anita Impagliazzo for producing the artwork that appears in Figure 5. The illustrations featured in Figures 1-4, 6 were made by the authors using BioRender (https://biorender.com/). This work was supported by The National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01NS106383; awarded to J.R.L.), The Alzheimer’s Association (AARG-18-566113; awarded to J.R.L.), The Owens Family Foundation (Awarded to J.R.L.), and The University of Virginia Research and Development Award (Awarded to J.R.L.). A.C.B. was supported by a Medical Scientist Training Program Grant (5T32GM007267-38), an Immunology Training Grant (5T32AI007496-25), a Wagner Fellowship, and an F30 grant from the National Institutes on Aging (NIA, F30AG069396-01).
Glossary
- Active place avoidance test
Behavioral test; assesses spatial navigation and learning. Rodents on a rotating turntable are motivated to regularly change their location using spatial cues to avoid being shocked in defined regions of the turntable.
- Adult neurogenesis
process of generating new neurons in the adult brain from adult neural precursor cells.
- Alpha-synuclein
Abundant protein in neuronal tissue; can become misfolded and aggregated in Parkinson’s disease; may spread disease by being passed from neuron to neuron.
- Amyloid beta
protein derived from the cleavage of amyloid precursor protein that aggregates into oligomers and plaques in Alzheimer’s disease.
- Anti-inflammatory CD206+ microglia
Subset that responds to brain injury by dampening inflammation.
- Aquaporin-4
water channel protein in astrocytes; implicated in the proper functioning of the glymphatic system
- ASC specks
multiprotein complex comprising the inflammasome adaptor protein ASC. ASC specks form during inflammasome activation and can be secreted into the extracellular space.
- Astrocytic endfeet
distal processes of astrocytes lining the blood vessels to create the blood brain barrier
- Brain parenchyma
functional tissue that includes neurons and glia
- CD16+/CD32+ proinflammatory microglia
in a pro-inflammatory response following brain injury, secrete cytokines and chemokines
- Cerebrospinal fluid
Fluid produced by the cells lining the brain ventricles; traffics throughout the ventricles and surrounds the brain in the sub-arachnoid space; intermixes with the interstitial fluid and can be taken up by the meningeal lymphatic vasculature into the periphery
- Complement
component of the innate immune system comprising soluble, circulating proteins
- Controlled cortical impact
relies on mechanical impact of exposed brain tissue at a fixed velocity, depth, and angle
- Cribriform plate
Portion of the ethmoid bone located at the base of the skull and the roof of the nasal cavities. Lymphatic vasculature in the cribriform plate region may contribute to drainage of material from the brain
- CSF1R
CSF1R signaling is required for microglial development and survival. Binds CSF1 and IL-34. CSF1R inhibitors are experimentally used to deplete microglia from the CNS
- CX3CR1
Chemokine receptor (binds CX3CL1); regulates cell adhesion and migration; selectively expressed by microglia in the CNS and also by specific myeloid cell populations in the periphery
- DAMPS
Molecules released from damaged, stressed, or dying cells that potently activate innate immune signaling pathways
- Deep cervical lymph nodes
located in the neck; collect CSF from the meningeal lymphatics
- Doublecortin (DCX) positive immature neurons
found in neurodevelopment and adult neurogenesis. Doublecortin is a marker for neurogenesis
- Gliosis
activation of glial cells with injury or disease; can refer to both activation-induced proliferation of glia as well as activation-associated changes in glial cell morphology
- Glymphatic system
paravascular pathway; facilitates interstitial fluid and solute movement along veins and arteries within the brain parenchyma
- Hippocampal granule cells
Neurons found in the dentate gyrus of the hippocampus; involved in memory formation
- Inflammasomes
Multiprotein innate immune signaling complexes; orchestrate cytokine release (IL-1, IL-18) and a form of cell death known as pyroptosis
- Interstitial fluid
present throughout the brain parenchyma; circulates along parenchymal arteries and veins; comprises cerebrospinal fluid, blood plasma, and cellular waste
- Meningeal lymphatics
vessels in the meningeal space between the exterior of the brain and the skull; centrally involved in the drainage of material from the brain and meninges to the dCLNs in the periphery
- Meninges
tri-layered tissue residing between the skull and brain parenchyma. The dura (outer, thick layer): houses the meningeal lymphatic vessels. The middle layer: arachnoid mater. The pia (thin): tightly adherent to the brain parenchyma. The sub-arachnoid space (with CSF), resides between the pia and the arachnoid
- Midline fluid percussion injury
TBI model; a fluid pressure pulse is generated at the midline of the brain
- Morris water maze
Behavioral test to measure spatial learning and memory in rodents. Rodents use spatial cues to locate a hidden platform submerged in a pool of water
- Neurotoxic debris/material
includes protein aggregates (e.g. amyloid beta and alpha-synuclein), myelin debris, damaged organelles, inflammatory cytokines, and cell debris released as a part of necrosis or pyroptosis
- NLRP3
Intracellular Nod-like receptor protein that coordinates inflammasome formation and activation in response to a wide-range of pathogen-derived triggers, environmental irritants, cellular perturbations, and damage/danger-associated molecular patterns
- Novel objection location and recognition test
behavioral test; evaluates learning and memory; assesses the ability of the rodent to identify the presence of a novel object as well as changes in the location of familiar objects
- Pattern recognition receptors
Immune receptors activated in response to molecules expressed by microbes as well as damage/danger-associated molecular patterns
- Pyroptosis
form of cell death triggered by inflammasome activation
- Ramified morphology
characteristic of homeostatic microglia; contrasts the ameboid morphology of activated microglia
- Sub-arachnoid hemorrhage
potentially life-threatening condition triggered by a bleeding vessel on the surface of the brain and causing neuropathology
- Synaptic reorganization
Changes in neuronal synapse numbers, size, and/or morphology in the adult brain. Microglia can contribute to this process by either engulfing synaptic material or promoting synaptic growth
- Tau
protein in neurons that stabilizes microtubules. In AD, tau can become aggregated (neurofibrillary tangles) and contribute to disease progression
- Tauopathy
Neurodegenerative pathology characterized by tau misfolding and accumulation
- TBR2 positive neural precursor cells
TBR2 (T-box protein TBR2/EOMES); protein preferentially enriched in intermediate progenitors ; marker of neurogenesis
- Toll like receptors
A class of pattern recognition receptor; orchestrates innate immune activation in response to pathogen-derived molecules and damage/danger-associated molecular patterns
- Traumatic brain injury (TBI)
results e.g. from a fall, blow, or jolt to the body. There is a wide range of severities of TBI including mild, moderate, and severe injuries
- TREM2
Receptor expressed on microglia; involved in the phagocytosis of amyloid beta and apoptotic neurons
- Y-maze test
behavioral test; evaluates spatial learning and memory. Testing occurs in a Y-shaped maze with three opaque arms at 120° angles from each other. Once in the center of the maze, the rodent can freely explore the three arms. After multiple arm entries, the subject should show a tendency to enter a less recently visited arm, indicative of healthy spatial memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Woodcock T and Morganti-Kossmann MC (2013) The role of markers of inflammation in traumatic brain injury. Front Neurol 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortezaee K et al. (2018) Inflammasome: Its role in traumatic brain and spinal cord injury. J Cell Physiol 233 (7), 5160–5169. [DOI] [PubMed] [Google Scholar]
- 3.McKee CA and Lukens JR (2016) Emerging Roles for the Immune System in Traumatic Brain Injury. Front Immunol 7, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corps KN et al. (2015) Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72 (3), 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth TL et al. (2014) Transcranial amelioration of inflammation and cell death after brain injury. Nature 505 (7482), 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon DW et al. (2017) The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol 13 (9), 572. [DOI] [PubMed] [Google Scholar]
- 7.Morganti-Kossmann MC et al. (2019) The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathol 137 (5), 731–755. [DOI] [PubMed] [Google Scholar]
- 8.Webster KM et al. (2017) Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation 14 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verboon LN et al. (2021) The Immune System's Role in the Consequences of Mild Traumatic Brain Injury (Concussion). Front Immunol 12, 620698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon IK et al. (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14 (3), 166–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morioka S et al. (2019) Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity 50 (5), 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etchegaray JI et al. (2016) Defective Phagocytic Corpse Processing Results in Neurodegeneration and Can Be Rescued by TORC1 Activation. J Neurosci 36 (11), 3170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pluvinage JV et al. (2019) CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568 (7751), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliver G et al. (2020) The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 182 (2), 270–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginhoux F et al. (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330 (6005), 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki Y et al. (2003) Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 44 (3), 242–50. [DOI] [PubMed] [Google Scholar]
- 17.Bennett ML et al. (2016) New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 113 (12), E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prinz M et al. (2019) Microglia Biology: One Century of Evolving Concepts. Cell 179 (2), 292–311. [DOI] [PubMed] [Google Scholar]
- 19.Kierdorf K et al. (2013) Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16 (3), 273–80. [DOI] [PubMed] [Google Scholar]
- 20.Reu P et al. (2017) The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep 20 (4), 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuger P et al. (2017) Microglia turnover with aging and in an Alzheimer's model via long-term in vivo single-cell imaging. Nat Neurosci 20 (10), 1371–1376. [DOI] [PubMed] [Google Scholar]
- 22.Goldmann T et al. (2013) A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci 16 (11), 1618–26. [DOI] [PubMed] [Google Scholar]
- 23.Parkhurst CN et al. (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155 (7), 1596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elmore MR et al. (2014) Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82 (2), 380–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangenberg E et al. (2019) Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer's disease model. Nat Commun 10 (1), 3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sierra A et al. (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7 (4), 483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marquez-Ropero M et al. (2020) Microglial Corpse Clearance: Lessons From Macrophages. Front Immunol 11, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damisah EC et al. (2020) Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv 6 (26), eaba3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funk KE and Klein RS (2019) CSF1R antagonism limits local restimulation of antiviral CD8(+) T cells during viral encephalitis. J Neuroinflammation 16 (1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seitz S et al. (2018) Pharmacologic Depletion of Microglia Increases Viral Load in the Brain and Enhances Mortality in Murine Models of Flavivirus-Induced Encephalitis. J Virol 92 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens B et al. (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131 (6), 1164–78. [DOI] [PubMed] [Google Scholar]
- 32.Stephan AH et al. (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35, 369–89. [DOI] [PubMed] [Google Scholar]
- 33.Wang C et al. (2020) Microglia mediate forgetting via complement-dependent synaptic elimination. Science 367 (6478), 688–694. [DOI] [PubMed] [Google Scholar]
- 34.Schafer DP et al. (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74 (4), 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Aparicio I et al. (2020) Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J Neurosci 40 (7), 1453–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato K (2015) Effects of Microglia on Neurogenesis. Glia 63 (8), 1394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davalos D et al. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8 (6), 752–8. [DOI] [PubMed] [Google Scholar]
- 38.Butovsky O and Weiner HL (2018) Microglial signatures and their role in health and disease. Nat Rev Neurosci 19 (10), 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickman S et al. (2018) Microglia in neurodegeneration. Nat Neurosci 21 (10), 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Priller J and Prinz M (2019) Targeting microglia in brain disorders. Science 365 (6448), 32–33. [DOI] [PubMed] [Google Scholar]
- 41.Ennerfelt HE and Lukens JR (2020) The role of innate immunity in Alzheimer's disease. Immunol Rev 297 (1), 225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witcher KG et al. (2021) Traumatic brain injury causes chronic cortical inflammation and neuronal dysfunction mediated by microglia. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witcher KG et al. (2018) Traumatic brain injury-induced neuronal damage in the somatosensory cortex causes formation of rod-shaped microglia that promote astrogliosis and persistent neuroinflammation. Glia 66 (12), 2719–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krukowski K et al. (2018) Traumatic Brain Injury in Aged Mice Induces Chronic Microglia Activation, Synapse Loss, and Complement-Dependent Memory Deficits. Int J Mol Sci 19 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y et al. (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell 160 (6), 1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jay TR et al. (2017) Disease Progression-Dependent Effects of TREM2 Deficiency in a Mouse Model of Alzheimer's Disease. J Neurosci 37 (3), 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clausen F et al. (2009) Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 30 (3), 385–96. [DOI] [PubMed] [Google Scholar]
- 48.Clausen F et al. (2011) Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 34 (1), 110–23. [DOI] [PubMed] [Google Scholar]
- 49.Karve IP et al. (2016) Ablation of Type-1 IFN Signaling in Hematopoietic Cells Confers Protection Following Traumatic Brain Injury. eNeuro 3 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scherbel U et al. (1999) Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci U S A 96 (15), 8721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrett JP et al. (2020) Interferon-beta Plays a Detrimental Role in Experimental Traumatic Brain Injury by Enhancing Neuroinflammation That Drives Chronic Neurodegeneration. J Neurosci 40 (11), 2357–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdullah A et al. (2018) STING-mediated type-I interferons contribute to the neuroinflammatory process and detrimental effects following traumatic brain injury. J Neuroinflammation 15 (1), 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tejera D et al. (2019) Systemic inflammation impairs microglial Abeta clearance through NLRP3 inflammasome. EMBO J 38 (17), e101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heneka MT et al. (2013) NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493 (7434), 674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramlackhansingh AF et al. (2011) Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 70 (3), 374–83. [DOI] [PubMed] [Google Scholar]
- 56.Johnson VE et al. (2013) Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136 (Pt 1), 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangale V et al. (2020) Microglia influence host defense, disease, and repair following murine coronavirus infection of the central nervous system. Glia 68 (11), 2345–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheeler DL et al. (2018) Microglia are required for protection against lethal coronavirus encephalitis in mice. J Clin Invest 128 (3), 931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rice RA et al. (2017) Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 65 (6), 931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henry RJ et al. (2020) Microglial Depletion with CSF1R Inhibitor During Chronic Phase of Experimental Traumatic Brain Injury Reduces Neurodegeneration and Neurological Deficits. J Neurosci 40 (14), 2960–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willis EF et al. (2020) Repopulating Microglia Promote Brain Repair in an IL-6-Dependent Manner. Cell 180 (5), 833–846 e16. [DOI] [PubMed] [Google Scholar]
- 62.Lamkanfi M and Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157 (5), 1013–22. [DOI] [PubMed] [Google Scholar]
- 63.Kerr N et al. (2018) Inflammasome proteins as biomarkers of traumatic brain injury. PLoS One 13 (12), e0210128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuwar R et al. (2019) A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J Neuroinflammation 16 (1), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Brien WT et al. (2020) The NLRP3 inflammasome in traumatic brain injury: potential as a biomarker and therapeutic target. J Neuroinflammation 17 (1), 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Venegas C et al. (2017) Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer's disease. Nature 552 (7685), 355–361. [DOI] [PubMed] [Google Scholar]
- 67.Plassman BL et al. (2000) Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55 (8), 1158–66. [DOI] [PubMed] [Google Scholar]
- 68.Nemetz PN et al. (1999) Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am J Epidemiol 149 (1), 32–40. [DOI] [PubMed] [Google Scholar]
- 69.Johnson VE et al. (2012) Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol 22 (2), 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X et al. (2016) Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. J Neurotrauma 33 (8), 721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ngwenya LB and Danzer SC (2018) Impact of Traumatic Brain Injury on Neurogenesis. Front Neurosci 12, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hergenroeder GW et al. (2010) Serum IL-6: a candidate biomarker for intracranial pressure elevation following isolated traumatic brain injury. J Neuroinflammation 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minambres E et al. (2003) Correlation between transcranial interleukin-6 gradient and outcome in patients with acute brain injury. Crit Care Med 31 (3), 933–8. [DOI] [PubMed] [Google Scholar]
- 74.Makinde HM et al. (2020) Microglia Adopt Longitudinal Transcriptional Changes After Traumatic Brain Injury. J Surg Res 246, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilla AM et al. (2017) Microglia Are Irrelevant for Neuronal Degeneration and Axon Regeneration after Acute Injury. J Neurosci 37 (25), 6113–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Witcher KG et al. (2021) Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia. J Neurosci 41 (7), 1597–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bennett RE and Brody DL (2014) Acute reduction of microglia does not alter axonal injury in a mouse model of repetitive concussive traumatic brain injury. J Neurotrauma 31 (19), 1647–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frieler RA et al. (2015) Depletion of macrophages in CD11b diphtheria toxin receptor mice induces brain inflammation and enhances inflammatory signaling during traumatic brain injury. Brain Res 1624, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zengeler KE and Lukens JR (2021) Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat Rev Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iliff JJ et al. (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33 (46), 18190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iliff JJ et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4 (147), 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louveau A et al. (2017) Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127 (9), 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iliff JJ et al. (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34 (49), 16180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren Z et al. (2013) 'Hit & Run' model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab 33 (6), 834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daniel Moreno-Zambrano DS, Avila David, Santibáñez Rocío (2018) Lymphatics of the Central Nervous System: Forgotten first descriptions. Neurology. [Google Scholar]
- 86.Cserr HF et al. (1992) Drainage of Brain Extracellular Fluid into Blood and Deep Cervical Lymph and its Immunological Significance. Brain Pathology 2 (4), 269–276. [DOI] [PubMed] [Google Scholar]
- 87.Kida S et al. (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathology and Applied Neurobiology 19 (6), 480–488. [DOI] [PubMed] [Google Scholar]
- 88.Louveau A et al. (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523 (7560), 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aspelund A et al. (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212 (7), 991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Antila S et al. (2017) Development and plasticity of meningeal lymphatic vessels. J Exp Med 214 (12), 3645–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Esposito E et al. (2019) Brain-to-cervical lymph node signaling after stroke. Nat Commun 10 (1), 5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zou W et al. (2019) Blocking meningeal lymphatic drainage aggravates Parkinson's disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl Neurodegener 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Da Mesquita S et al. (2018) Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature 560 (7717), 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wen YR et al. (2018) Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer's disease. Neural Regen Res 13 (4), 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel TK et al. (2019) Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener 14 (1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song E et al. (2020) VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577 (7792), 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ding XB et al. (2021) Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson's disease. Nat Med 27 (3), 411–418. [DOI] [PubMed] [Google Scholar]
- 98.Chen J et al. (2020) Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat Commun 11 (1), 3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bolte AC et al. (2020) Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun 11 (1), 4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pu T et al. (2019) Persistent Malfunction of Glymphatic and Meningeal Lymphatic Drainage in a Mouse Model of Subarachnoid Hemorrhage. Exp Neurobiol 28 (1), 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haider MN et al. (2018) Intracranial pressure changes after mild traumatic brain injury: a systematic review. Brain Inj 32 (7), 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Susman M et al. (2002) Traumatic Brain Injury in the Elderly: Increased Mortality and Worse Functional Outcomes at Discharge Despite Lower Injury Severity. J Trauma 53 (2), 219–223. [DOI] [PubMed] [Google Scholar]
- 103.McKee AC et al. (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68 (7), 709–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winston CN et al. (2016) Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma. Am J Pathol 186 (3), 552–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stern RA et al. (2019) Tau Positron-Emission Tomography in Former National Football League Players. N Engl J Med 380 (18), 1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alosco ML et al. (2020) Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology 95 (7), e793–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morganti JM et al. (2016) Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury. J Neuroinflammation 13 (1), 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shitaka Y et al. (2011) Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 70 (7), 551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng WH et al. (2019) CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice. Alzheimers Res Ther 11 (1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mannix R et al. (2014) Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J Neurosurg 121 (6), 1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma Q et al. (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8 (1), 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ahn JH et al. (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572 (7767), 62–66. [DOI] [PubMed] [Google Scholar]
- 113.Hsu M et al. (2019) Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun 10 (1), 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ikezu S et al. (2020) Inhibition of colony stimulating factor 1 receptor corrects maternal inflammation-induced microglial and synaptic dysfunction and behavioral abnormalities. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spangenberg EE and Green KN (2017) Inflammation in Alzheimer's disease: Lessons learned from microglia-depletion models. Brain Behav Immun 61, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plog BA et al. (2015) Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 35 (2), 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Absinta M et al. (2017) Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuo PH et al. (2018) Meningeal Lymphatic Vessel Flow Runs Countercurrent to Venous Flow in the Superior Sagittal Sinus of the Human Brain. Tomography 4 (3), 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]