Abstract
Endogenous circadian rhythms prepare the cardiovascular (CV) system for optimal function to match the daily anticipated behavioral and environmental cycles, including variable activities when awake during the day and recuperation when sleeping at night. The overall day-night patterns in most CV variables result from the summation of predictable circadian effects with variable behavioral and environmental effects on the CV system. The circadian system has also been implicated in the morning peak in the incidence of adverse CV events, including myocardial infarction, stroke, and sudden cardiac death. We discuss the resting and stress-reactive circadian control of CV physiology in humans and suggest future research opportunities, including improving CV therapy by optimally timing therapy relative to a person’s internal body clock time.
2. Introduction
Using rigorous experimental protocols in humans1 endogenous circadian rhythms have been discovered in numerous variables that can influence overall CV function. For instance, rhythms in cortisol, BP, heart rate, and autonomic function persist independent of the effects of daily environmental and behavioral cycles, such as the daily light/dark, wake/sleep, activity/rest, and eating/fasting cycles[*1–3]. These endogenous circadian rhythms have evolved to optimize function based on anticipated daily environmental and behavioral cycles, and based on the assumption that being physiologically prepared to respond to an anticipated stressor is advantageous compared to waiting for the stressor to occur and then reacting. For instance, cortisol increases are very sluggish in response to an acute stress, whereas the circadian system (the individual or summated functional effects of the central and peripheral clocks) increases cortisol well before the anticipated wakening in the morning, which ‘primes the pump’ for rapid CV adaptation to maintain homeostasis in the face of rapid changes in posture and increased physical activity immediately upon awakening [4]. In addition to preparing us for the anticipated exposure to variable mental and physical stresses during the day, the circadian system also serves to optimize our CV physiology for the anticipated habitual schedule of resting, recuperation, repair, and preparation during the night. In animal models, the peripheral circadian clock in the cardiomyocyte has been shown to facilitate myocardial processes of contractility, gene expression and metabolism, and tolerance to ischemia reperfusion injury [5–7]. On a continuous basis, the endogenous rhythms of resting CV physiology and CV recuperation summate with the CV responses to common behaviors and environmental changes across the day and night and lead to the characteristic day-night patterns in CV physiology (Figure 1, for BP).
Figure 1. Day-night pattern in BP.
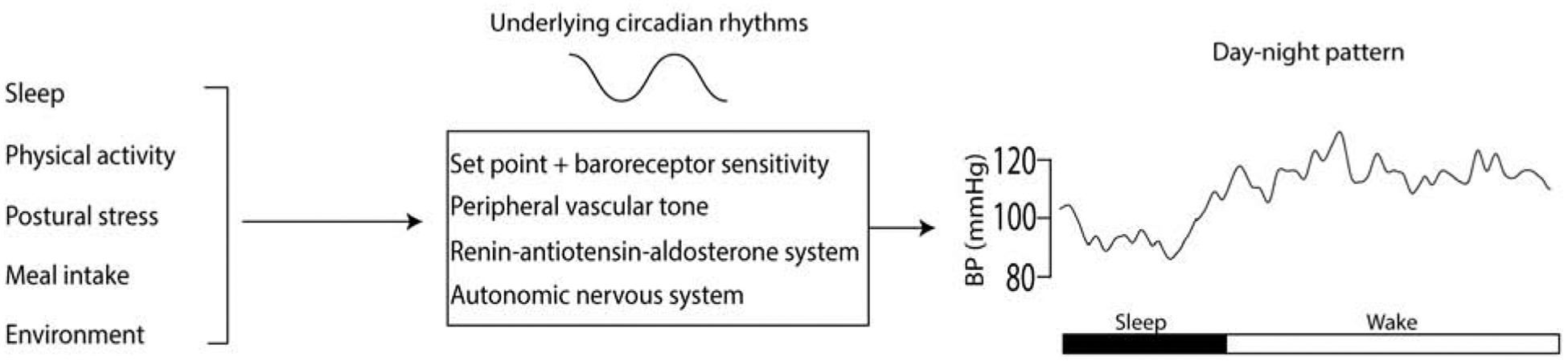
In healthy individuals, BP has a characteristic day night pattern with an increase in BP upon awakening, and higher levels during the wake period compared to sleep. This day night pattern is a result of the composite effects of different behaviors and the environment, interacting with the underlying circadian rhythms in BP, peripheral vascular tone, renin-angiotensin-aldosterone system and the autonomic nervous system.
There are substantial day/night patterns of adverse cardiovascular (CV) events, such as myocardial infarction and stroke, with most epidemiological studies in the general population detecting peak incidence in the morning [8–11]. The role of circadian system has been studied as a potential factor playing a role in these day/night patterns primarily because many CV variables such as blood pressure (BP) and markers of hemostasis abruptly increase in the morning hours when vascular endothelial function (VEF) is attenuated [12–17]. As explained in section 5, there is now proof that the morning rise in some of these markers is driven by the circadian system, whereas some markers are more affected by behaviors. Theoretically, the same endogenous rhythms that are advantageous in healthy humans, may however, be perilous in vulnerable individuals, e.g., people with underlying CV disease, and can lead to an increased risk for adverse CV events at specific times of day (Figure 2).
Figure 2. Interaction between individual susceptibility, circadian rhythms, behavioral triggers and environmental factors can increase the risk for adverse cardiovascular events.
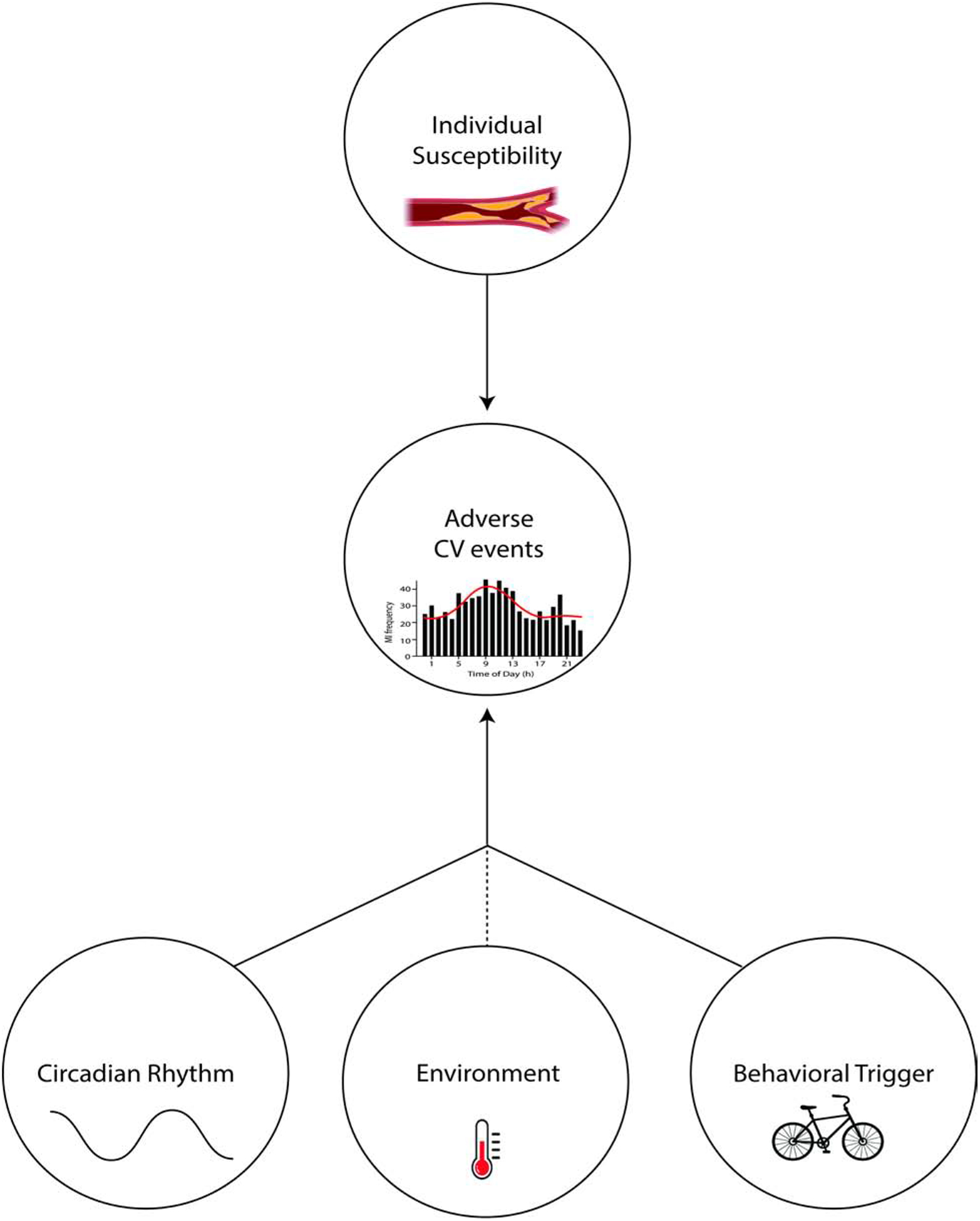
In vulnerable individuals (e.g. people with atherosclerosis), a behavioral trigger (e.g. exercise) can interact with underlying circadian rhythms in resting CV variables (e.g. hemostatic system) and lead to an adverse CV event. This interaction can also be affected by environmental factors (e.g. exercising in extreme temperatures).
Here we briefly review: 1) the hierarchy of circadian control, and 2) endogenous circadian rhythms in CV variables under both resting and stressed conditions. We then discuss the role of the circadian system in CV disease and highlight areas for future work, including studying people with existing CV disease and the potential to improve clinical outcomes by timing therapies to specific circadian phases.
3. Hierarchy of circadian control
In mammals, the central circadian clock is located in the suprachiasmatic nucleus in the anterior portion of the hypothalamus and is primarily entrained to the 24-hour environmental cycles (e.g., as seasons change) by light cues via the retino-hypothalamic tract. Peripheral circadian clocks also exist throughout the organism from the cell to the organ level. These peripheral clocks work on a transcription-translation negative feedback network wherein clock genes facilitate the translation of proteins, which in turn repress the clock genes, a cycle that takes ~24 hours. While all peripheral clocks can cycle even in the absence of inputs from the suprachiasmatic nucleus [18,19], this central clock helps to synchronize all peripheral circadian clocks via neural, hormonal, and systemic pathways [20] [21] and body temperature [22]. Circadian clocks across the CV system, including the heart, the vasculature, the kidney and the autonomic nervous system [20] [23–25] affect resting CV variables, their responsivity to stresses during the day and CV system’s ability to rest and recuperate at night.
4. Protocols to study the endogenous circadian system in humans
Separating the effects of behaviors from those of the endogenous circadian system, and ultimately studying the interaction between the two require rigorous multi-day laboratory protocols that schedule all behaviors (including sleep and wake) and control all environmental factors, including living in dim-light and time-isolation to avoid resetting the central circadian clock (please see Thosar et al. [26] for detailed illustrations of these protocols). One protocol is the ‘constant routine’ (CR) [27], in which participants live under conditions of constant wakefulness in a temperature-controlled suite in a semi-recumbent position for >24 hours. In an attempt to provide continuous nutrition while maintaining caloric balance, participants consume small identical iso-caloric meals, typically every hour, as opposed to three large meals per day. Circadian rhythms in CV function are then uncovered by making repeated measurements across >24 h [3,28,29]. One limitation of this constant routine protocol is that due to continued wakefulness, there is an accumulation of sleep loss, which could affect variables of interest. Nonetheless, in many instances, this effect is minimal compared to circadian variation and can be statistically accounted for [3,28,29]. A more sophisticated protocol, that can avoid sleep loss is called the ‘forced desynchrony’(FD) [30], wherein behaviors and circadian rhythms are ‘desynchronized’ by scheduling participants to live on a non-24-hour ‘day’. Standardized behaviors and physiological tests can then be evenly distributed across all phases of the circadian cycle. Using this method, it is possible to statistically separate the effects of all three components: behaviors, the circadian system, and their interactions. An underlying assumption of the FD is that in the dim light conditions, the circadian pacemaker ‘free-runs’ and synchronization between central and peripheral circadian clocks is preserved, as has been demonstrated for numerous physiological markers including melatonin, cortisol, core body temperature, and plasminogen activator-inhibitor 1 (PAI-1) [2,31,32].
5. Circadian rhythms in resting cardiovascular variables
5.1. Hemodynamic system
Blood pressure is higher during the day compared to the night [14,15]. Furthermore, BP immediately upon awakening is a predictor of adverse CV events [33,34] (also see [35] for race differences). Changes in common behaviors, including upright posture and physical activity, do not fully explain the day/night patterns in BP [36]. To test whether the circadian system contributes to the diurnal variation in BP, early studies using modified CR protocols and reported an absence of a circadian rhythm in BP [37,38]. However, the lack of strict light and physical activity control confounded the interpretation of results. In contrast, Shea et al. studied healthy young adults (mean age 26y) in three separate circadian protocols (CR, 20-hour FD, and 28-hour FD) [3]. They discovered that the circadian rhythm in BP exhibited a trough in the early morning and a peak in the evening corresponding to ~8 PM suggesting that the circadian rhythm in BP is unlikely to play a role in morning peak in adverse CV events [39,40]. More recently, using a 5-hour 20-minute FD, Thosar et al. confirmed these results in midlife adults (mean age 51y) [1], although the circadian phase for the peak in BP was advanced by ~2 hours compared to the previous report [1]. These authors also failed to find a circadian rhythm in heart rate [1]. Aging can alter the circadian rhythms in melatonin, body temperature, and cortisol [41–43], and it is possible that aging alters circadian CV rhythms in midlife participants, and this ought to be studied further due to the potential clinical significance.
5.2. Autonomic nervous system
The autonomic nervous system is an important regulator of a multitude of CV processes. Using modified CR protocols, some studies have demonstrated that cardiac vagal activity is driven by the homeostatic sleep pressure [44], whereas others have concluded that it is driven by the circadian system [45–47]. In contrast, recent CR and FD protocols with tighter control of lighting, activity, and meal intake reported that both sympathetic and cardiac vagal tone at rest were both under circadian control and that basal sympathetic activity peaked around mid-day, whereas cardiac vagal activity peaked in the morning ~8 AM [2,48,49]. The circadian peak in the stress hormone cortisol was also around the time of awakening [2]. Together, these results suggest that the circadian trough in BP in the morning may be related to cardiac vagal tone, yet the circadian peak in BP appears to be unrelated to the circadian rhythms in autonomic nervous system activity. The aforementioned studies included different indirect indices of the autonomic nervous system including, cardiac inter-beat interval, pNN50, high-frequency power, and plasma levels of epinephrine and norepinephrine. Therefore, data must be interpreted while considering the strengths and weaknesses of each measurement. Use of the direct measurement of sympathetic neural activity (e.g., muscle sympathetic nerve activity) remains an area of opportunity in this field.
5.3. Renin-Angiotensin-Aldosterone system
Similar to the autonomic nervous system, the renin-angiotensin-aldosterone system is an important regulator of BP and systemic vascular resistance [50,51]. In humans, plasma renin activity and aldosterone have a day/night pattern with high levels early in the morning compared to the evening, and a longstanding observation has been that these morning increases in humans are due to prior nocturnal sleep [52,53]. More recently, these findings for aldosterone were revisited using two separate protocols: one to isolate the effects of nocturnal sleep while controlling the effects of the circadian system (e.g., dim light environment), and the second, a 5-hour 20-minute FD to identify any circadian rhythmicity. It was discovered that nocturnal sleep was not responsible for the morning rise in aldosterone, but rather, there is a robust circadian rhythm in aldosterone with a peak at the circadian phase corresponding to ~7 AM [*54]. Unfortunately, this study did not include measurement of plasma renin activity or high frequency measurements of sodium and potassium levels to aid clinical interpretation of these aldosterone data. Furthermore, standard clinical assessment of aldosterone in hypertensive patients is performed in the morning, thus since the morning peak is driven by the circadian system, clinicians need to aware of any history of shift work or jet-lag in their patients before interpretation of their results [55].
5.4. Vascular endothelial function (VEF)
The vascular endothelium is a single layer of cells that lines the entire vascular network and performs functions that are critical to maintaining blood flow to vital organs [56,57]. In humans, VEF is relatively impaired in the morning [17,58] compared to other times of the day. Using two separate multi-day inpatient studies, which controlled for the effects of the circadian system, Thosar et al. [1,59] recently discovered that the morning impairment in VEF is due to the effects of the circadian system and not due to the effects of prior nocturnal sleep or the inactivity that typically accompanies sleep. In addition, mechanistic variables that affect VEF, namely endothelin-1 [60], and oxidative stress [61], both exhibited circadian rhythms with an increase across the morning hours and a peak around noon [1]. The morning increase in these biomarkers constitutes an increase in CV risk even though these markers’ circadian peaks did not match the trough in VEF. These findings have important clinical implications in relation to adverse CV events, especially in people with existing CV disease who may have a concomitant surge in BP during the morning hours [62–64]. The next logical steps in this area are to study people with CV disease with a day night pattern of adverse symptoms (e.g., angina[65]) and to extend these findings to the coronary vasculature.
5.5. Hemostatic system
Blood clotting is a vital function to prevent excessive blood loss during injury, whereas fibrinolytic activity is antithrombotic and keeps blood vessels patent to ensure perfusion to organ systems. In humans, these factors display a day-night pattern with the greatest hemostatic activity occurring in the morning [13,66]. Using an FD protocol in healthy young adults, the increase in plasminogen activator inhibitor-1 (PAI-1) in the morning was found to be due to the effects of the circadian system [*67]. The magnitude of this circadian increase was several times larger than the increase in PAI-1 during exercise. In a similar FD study, the circadian system was found to cause a morning peak in platelet surface-activated GPIIb-IIIa, the final part of the platelet aggregation pathway [68]. These results demonstrate that the circadian system creates a pro-thrombotic milieu in the morning in healthy humans, which perhaps conferred some evolutionary advantage to prevent blood loss during morning activities [69]. However, in vulnerable individuals, this same circadian increase in hemostasis in the morning in the presence of other factors such as impaired VEF and the morning surge in BP could potentially trigger an adverse CV event (Figure 2).
6. Interacting effects on cv function of behaviors and the circadian system
The CV responses to all of the common behaviors (e.g., sleep and exercise) that we typically encounter across the day and night have been well-established. These CV responses to behaviors are superimposed on the basal circadian rhythms in CV physiology described above (Figure 1). Importantly, these behaviors could occur at any circadian phase; for instance, emergency workers may be exposed to stresses at any time of day or night. Thus, it is important to determine the nature of these interacting effects, namely, are the behavioral and circadian CV effects simply additive, or are there more complex interactions (e.g., multiplicative). Moreover, the daily pattern of adverse CV events may be caused by these interactions. For instance, common behaviors such as arousal from sleep, a sudden change in posture, and an abrupt increase in physical activity during the morning could trigger adverse events at specific circadian phases in vulnerable individuals (Figure 2). Below, we consider how mild behavioral stresses interact with the circadian system when studied in constant environmental conditions. However, we must acknowledge that more robust stresses can occur in real life, and indeed that similar interactions may also occur between the circadian system and environmental stresses, such as heat and cold exposure. Moreover, all three may interact, which could be critically important, for instance, in susceptibility to heat exhaustion at specific times of the day. This is an area that needs to be studied in humans.
6.1. Circadian interactions with physical and mental stresses
Habitual physical activity is beneficial for CV and overall health, but in sedentary adults, physical exertion performed in the morning can increase the risk for adverse CV events [70]. Using a 20-hour FD in healthy humans to study the circadian reactivity of CV variables to exercise (60% of maximum heart rate for 15 minutes), it was discovered that the reactivity of BP and other CV markers to exercise is non-uniform across the circadian cycle [2,*71]. The increase in BP during exercise and BP recovery after exercise was the highest in the biological evening [2]. Conversely, the slowest recovery in BP occurred in the morning hours (~9 AM [71]), which could be related to the increased vasoconstrictor tone around that time [72]. Increases in plasma epinephrine and norepinephrine during exercise exhibited two large peaks at circadian phases corresponding to 07:00–10:30 and 20:00–22:00 [2]. Such ultradian rhythms have been demonstrated in cardiac β-adrenergic receptor density and function in animal models [73], and assessment of circadian changes in such receptor density would be valuable in humans. Moreover, the increase in plasma epinephrine from rest to exercise was twice as large at ~9 AM compared to ~5 AM [2]. Along with increased reactivity of the sympathetic nervous system, parasympathetic withdrawal during exercise was also the highest in the morning hours [2]. These same authors also discovered that the reactivity of epinephrine and norepinephrine to standardized mild mental stress peaked around noon. There was no statistical interaction between circadian phase and mental stress battery, but similar to exercise, sympathovagal balance in reaction to mental stress was significantly higher at ~9 AM [*74]. If such circadian effects of the autonomic nervous system to mental or physical stress persists in vulnerable people, it could explain the increased frequency of morning adverse CV events.
6.2. Circadian interaction with postural change
Most people get out of bed and stand up in the morning, yet this simple change in posture, after a prolonged period of recumbence across the night, is a large CV challenge that causes pooling of blood in the legs and significant compensatory changes in multiple CV variables [75]. To isolate the effects of posture alone, Hu et al. studied the circadian reactivity to passive postural change performed on a tilt table (moving from supine to 60° head-up for 15 min) [76]. Presyncope occurred in half of the healthy participants but almost exclusively during the circadian phases corresponding to the biological night (22:30–10:30) [76]. This study uncovered a period of relative susceptibility to presyncope during the night when most people sleep, which could also explain why in the general population, the greatest likelihood of syncope occurs in the morning [77]. However, overnight vulnerability to syncope finding has implications for people who get out of bed frequently at night (e.g., parents of young children, elderly with nocturia) or people who are active at night (e.g., shift workers).
7. Clinical implications, future directions and conclusions
7.1. Circadian rhythms in people with existing cardiovascular disease
To begin to understand the clinical significance of circadian rhythms in CV function, Butler et al. have studied people with obstructive sleep apnea as these patients commonly experience adverse CV events in the middle of the night, unlike the morning risk in the general population [78] [79]. These authors discovered that central circadian clock markers such as cortisol and melatonin were not disrupted, but circadian rhythms in BP peaked much later in people with obstructive sleep apnea compared to healthy controls [*79]. Although the peak in the BP rhythm did not correspond to the commonly observed time of adverse CV events, this finding suggests that certain diseases could result in a shift in peripheral CV rhythms, with as yet unknown clinical consequences. In the area of CV surgery, it was recently discovered that aortic valve replacement surgeries scheduled in the morning carry a higher rate of mortality compared to afternoon surgeries [*80]. Furthermore, these authors performed an ex-vivo analysis of human myocardium revealing an endogenous time of day variation in ischemia-reperfusion tolerance which was attributed to increased expression of the nuclear receptor Rev-Erbα in the morning. Indeed, recent animal work suggests that Rev-Erbα is a mediator of clock-controlled processes such as mitochondrial function, metabolism, signaling, and contractile function in the heart [81]. A major research opportunity is to study people with existing CV disease who have either stable, or disrupted day-night patterns in CV physiology (e.g., people with angina [82] or non-dipping hypertension [83]).
7.2. Chronotherapy
Chronotherapy or time-based “circadian medicine” has been considered “Medicine in the fourth dimension”[84]. In the CV area, most large chronotherapy trials have targeted not the internal, but the external clock time, and most trials have focused on hypertension. For instance, the MAPEC trial of nearly 2,000 people with resistant hypertension showed that at least one anti-hypertensive medication consumed at bedtime, compared to all hypertensive medications in the morning, reduced the relative risk of all CV events and CV mortality by >60% during 5.6 years of follow-up [85]. The same authors conducted an even larger multi-center trial (HYGIA) of over 19,000 hypertensive patients [**86] and reproduced the MAPEC trial results. They discovered that evening dosing rather than upon awake dosing improved BP control and significantly diminished the occurrence of almost all adverse CV disease outcomes (relative risk 0.55, 95% CI[0.50–0.61]), including myocardial infarction and stroke. At the moment, there is a debate on the generalizability of the results in the HYGIA trial [87,88], along with a concern for inducing silent ischemia with nighttime medications [89]. A better understanding of sleep physiology and circadian rhythms in people with hypertension will undoubtedly help to better interpret the results of future clinical trials. One hundred nineteen formulations on the World Health Organization’s essential medicine list target products of rhythmic genes [90]. Furthermore, >50% of the top best-selling drugs in the US have short half-lives (<6 h required for the drug concentration to decrease to half of its initial dose) [90]. The next step is to conduct randomized controlled clinical trials to target medications, especially those with short half-lives, to match the peaks in endogenous circadian rhythms of physiology to maximize drug potency and avoid overmedication [91]. Targeting drug delivery based on internal circadian time will however, require the development of a more convenient circadian phase marker that can be used in the clinic. Commonly used circadian phase markers such as the dim-light melatonin onset, or cortisol measurements, which require numerous serial assays from samples taken while the patient remains in dim light (DLMO) in the laboratory or at home [*92,93], or continuous measurement of core body temperature are cumbersome and perhaps not suited for personalized medicine. Overall, circadian medicine has only been sporadically used in CV medicine across many decades (see [94] for a review). Yet, based on all the recent findings in circadian CV physiology, this area is now ripe for exploration to improve clinical outcomes and reduce side effects of drugs.
Highlights.
The suprachiasmatic nuclei are the seat of the central circadian clock.
Peripheral circadian clocks exist throughout the cardiovascular (CV) system.
Together these clocks orchestrate resting and responsive rhythms in CV physiology.
Day-night CV patterns result from the summation of circadian and behavioral effects.
Timing drugs to specific circadian phases is a new opportunity to optimize therapy.
8. Acknowledgements:
Funding: This work is supported by NIH grants R01HL140577, HL142064, and KL2 TR002370, and by the Oregon Institute of Occupational Health Sciences at Oregon Health & Science University via funds from the Division of Consumer and Business Services of the State of Oregon (ORS 656.630).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9 Declaration of interest
Nothing declared
Throughout this review we refer to the “circadian” as being driven by the internal body clock (i.e. endogenous), independent of the effects of the environment or behaviors. Until researchers begin to use this term consistently it is advisable to read the protocols of all studies in this area for appropriate data interpretation. For example, many studies report differences in CV variables and CV pathophysiology across the day and the night without controlling for variable behaviors and environmental changes across the 24-hours, such as temperature, meal intake, physical activity, and sleep. Such 24-hour CV patterns have often been described as ‘circadian’ variations even though these studies have generally not assessed the endogenous nature of this variability. We use the term ‘circadian clock’ to infer the molecular clocks centrally in the suprachiasmatic nuclei and in the peripheral cells and tissues.
10 References
- 1.Thosar S, Berman A, Herzig M, McHill A, Bowles N, Swanson C, Clemons N, Butler M, Clemons A, Emens J: Circadian rhythm of vascular function in midlife adults. Arteriosclerosis, Thrombosis, and Vascular Biology 2019, 39:1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper shows that the attenuation in vascular endothelial function is due to the effects of the circadian system. Blood biomarkers of the vascular function are also rhythmic with increases in the morning. Finally, these authors failed to find a circadian rhythm in heart rate in midlife adults.
- 2.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA: Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proceedings of the National Academy of Sciences 2010, 107:20541–20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shea SA, Hilton MF, Hu K, Scheer FA: Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circulation Research 2011, 108:980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. : Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 1999, 284:2177–2181. [DOI] [PubMed] [Google Scholar]
- 5.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai J-Y, Bugger H, Zhang D: Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. American Journal of Physiology-Heart and Circulatory Physiology 2008, 294:H1036–H1047. [DOI] [PubMed] [Google Scholar]
- 6.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME: Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res 2010, 106:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, et al. : Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms 2014, 29:257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T: Circadian variation in the frequency of onset of acute myocardial infarction. New England Journal of Medicine 1985, 313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 9.Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, Maria Malagoni A, Haus E, Manfredini F: Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagic events. Chronobiology International 2005, 22:417–453. [DOI] [PubMed] [Google Scholar]
- 10.Twidale N, Taylor S, Heddle WF, Ayres BF, Tonkin AM: Morning increase in the time of onset of sustained ventricular tachycardia. The American journal of cardiology 1989, 64:1204–1206. [DOI] [PubMed] [Google Scholar]
- 11.Manfredini R, Portaluppi F, Zamboni P, Salmi R, Gallerani M: Circadian variation in spontaneous rupture of abdominal aorta. The Lancet 1999, 353:643–644. [DOI] [PubMed] [Google Scholar]
- 12.Angleton P, Chandler WL, Schmer G: Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation 1989, 79:101–106. [DOI] [PubMed] [Google Scholar]
- 13.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE: Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. New England Journal of Medicine 1987, 316:1514–1518. [DOI] [PubMed] [Google Scholar]
- 14.Millar-Craig M, Bishop C, Raftery E: Circadian variation of blood-pressure. The Lancet 1978, 311:795–797. [DOI] [PubMed] [Google Scholar]
- 15.Degaute J-P, Van De Borne P, Linkowski P, Van Cauter E: Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 1991, 18:199–210. [DOI] [PubMed] [Google Scholar]
- 16.Bau PFD, Bau CHD, Naujorks AA, Rosito G, Fuchs FD: Diurnal variation of vascular diameter and reactivity in healthy young men. Brazilian Journal of Medical and Biological Research 2008, 41:500–503. [DOI] [PubMed] [Google Scholar]
- 17.Otto ME, Svatikova A, de Mattos Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V: Early morning attenuation of endothelial function in healthy humans. Circulation 2004, 109:2507–2510. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H: Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000, 288:682–685. [DOI] [PubMed] [Google Scholar]
- 19.Balsalobre A, Damiola F, Schibler U: A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998, 93:929–937. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi JS: Molecular components of the circadian clock in mammals. Diabetes Obes Metab 2015, 17 Suppl 1:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD: Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci 1999, 2:1051–1053. [DOI] [PubMed] [Google Scholar]
- 22.Buhr ED, Yoo SH, Takahashi JS: Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson A, London B, Block G, Menaker M: Cardiovascular tissues contain independent circadian clocks. Clinical and experimental hypertension 2005, 27:307–311. [PubMed] [Google Scholar]
- 24.Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME: The intrinsic circadian clock within the cardiomyocyte. American Journal of Physiology-Heart and Circulatory Physiology 2005, 289:H1530–H1541. [DOI] [PubMed] [Google Scholar]
- 25.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D: Molecular clock is involved in predictive circadian adjustment of renal function. Proceedings of the National Academy of Sciences 2009, 106:16523–16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thosar SS, Butler MP, Shea SA: Role of the circadian system in cardiovascular disease. The Journal of clinical investigation 2018, 128:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minors DS, Waterhouse JM: The use of constant routines in unmasking the endogenous component of human circadian rhythms. Chronobiology international 1984, 1:205–216. [DOI] [PubMed] [Google Scholar]
- 28.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS: Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab 2005, 90:2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avery DH, Dahl K, Savage MV, Brengelmann GL, Larsen LH, Kenny MA, Eder DN, Vitiello MV, Prinz PN: Circadian temperature and cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression. Biological psychiatry 1997, 41:1109–1123. [DOI] [PubMed] [Google Scholar]
- 30.Kleitman N: Sleep and wakefulness as alternating phases in the cycle of existence. JAMA 1939, 113:2086. [Google Scholar]
- 31.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk D-J: Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 1999, 277:R1152–R1163. [DOI] [PubMed] [Google Scholar]
- 32.Scheer FA, Shea SA: Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014, 123:590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kario K, Shimada K, Pickering TG: Clinical implication of morning blood pressure surge in hypertension. Journal of cardiovascular pharmacology 2003, 42:S87–S91. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Thijs L, Hansen TW, Kikuya M, Boggia J, Richart T, Metoki H, Ohkubo T, Torp-Pedersen C, Kuznetsova T: Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010, 55:1040–1048. [DOI] [PubMed] [Google Scholar]
- 35.Booth III JN, Jaeger BC, Huang L, Abdalla M, Sims M, Butler M, Muntner P, Shimbo D: Morning Blood Pressure Surge and Cardiovascular Disease Events and All-Cause Mortality in Blacks: The Jackson Heart Study. Hypertension 2020, 75:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris CJ, Hastings JA, Boyd K, Krainski F, Perhonen MA, Scheer FA, Levine BD: Day/night variability in blood pressure: influence of posture and physical activity. American journal of hypertension 2013, 26:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Dongen HP, Maislin G, Kerkhof GA: Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiology international 2001, 18:85–98. [DOI] [PubMed] [Google Scholar]
- 38.Kerkhof GA, Dongen HPV, Bobbert AC: Absence of endogenous circadian rhythmicity in blood pressure? American journal of hypertension 1998, 11:373–377. [DOI] [PubMed] [Google Scholar]
- 39.Peters RW, Zoble RG, Liebson PR, Pawitan Y, Brooks MM, Proschan M: Identification of a secondary peak in myocardial infarction onset 11 to 12 hours after awakening: the Cardiac Arrhythmia Suppression Trial (CAST) experience. Journal of the American College of Cardiology 1993, 22:998–1003. [DOI] [PubMed] [Google Scholar]
- 40.Kono T, Morita H, Nishina T, Fujita M, Hirota Y, Kawamura K, Fujiwara A: Circadian variations of onset of acute myocardial infarction and efficacy of thrombolytic therapy. Journal of the American College of Cardiology 1996, 27:774–778. [DOI] [PubMed] [Google Scholar]
- 41.Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair N: Circadian rhythms of melatonin and cortisol in aging. Biological psychiatry 1989, 25:305–319. [DOI] [PubMed] [Google Scholar]
- 42.Vitiello MV, Smallwood RG, Avery DH, Pascualy RA, Martin DC, Prinz PN: Circadian temperature rhythms in young adult and aged men. Neurobiology of aging 1986, 7:97–100. [DOI] [PubMed] [Google Scholar]
- 43.Van Cauter E, Leproult R, Kupfer DJ: Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. The Journal of Clinical Endocrinology & Metabolism 1996, 81:2468–2473. [DOI] [PubMed] [Google Scholar]
- 44.Anders D, Vollenweider S, Cann J, Hofstetter M, Flammer J, Orgül S, Kräuchi K: Heart-rate variability in women during 40-hour prolonged wakefulness. Chronobiology international 2010, 27:1609–1628. [DOI] [PubMed] [Google Scholar]
- 45.van Eekelen AP, Houtveen JH, Kerkhof GA: Circadian variation in cardiac autonomic activity: reactivity measurements to different types of stressors. Chronobiology International 2004, 21:107–129. [DOI] [PubMed] [Google Scholar]
- 46.Burgess HJ, Trinder J, Kim Y, Luke D: Sleep and circadian influences on cardiac autonomic nervous system activity. American Journal of Physiology-Heart and Circulatory Physiology 1997, 273:H1761–H1768. [DOI] [PubMed] [Google Scholar]
- 47.Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, DIJK DJ: Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. Journal of sleep research 2007, 16:148–155. [DOI] [PubMed] [Google Scholar]
- 48.Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA: Endogenous circadian control of the human autonomic nervous system. Comput Cardiol 2000, 27:197–200. [PubMed] [Google Scholar]
- 49.Hu K, Ivanov P, Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA: Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci U S A 2004, 101:18223–18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattsson C, Young WF Jr: Primary aldosteronism: diagnostic and treatment strategies. Nature Reviews Nephrology 2006, 2:198. [DOI] [PubMed] [Google Scholar]
- 51.Funder JW: Minireview: aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology 2010, 151:5098–5102. [DOI] [PubMed] [Google Scholar]
- 52.Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G: Aldosterone release during the sleep-wake cycle in humans. American Journal of Physiology-Endocrinology And Metabolism 1999, 276:E43–E49. [DOI] [PubMed] [Google Scholar]
- 53.Charloux A, Gronfier C, Chapotot F, Ehrhart J, Piquard F, Brandenberger G: Sleep deprivation blunts the night time increase in aldosterone release in humans. Journal of sleep research 2001, 10:27–33. [DOI] [PubMed] [Google Scholar]
- 54.Thosar SS, Rueda JF, Berman AM, Lasarev MR, Herzig MX, Clemons NA, Roberts SA, Bowles NP, Emens JS, Ellison DH: Separate and interacting effects of the endogenous circadian system and behaviors on plasma aldosterone in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2019, 316:R157–R164. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper is important because it shows that the morning peak in aldosterone is due to the effects of the circadian system and not prior nocturnal sleep.
- 55.Weibel L, Brandenberger G: Disturbances in hormonal profiles of night workers during their usual sleep and work times. Journal of Biological Rhythms 1998, 13:202–208. [DOI] [PubMed] [Google Scholar]
- 56.Vita JA, Keaney JF Jr: Endothelial function: a barometer for cardiovascular risk? Edited by: Am Heart Assoc; 2002. [DOI] [PubMed] [Google Scholar]
- 57.Bonetti PO, Lerman LO, Lerman A: Endothelial dysfunction a marker of atherosclerotic risk. Arteriosclerosis, Thrombosis, and Vascular Biology 2003, 23:168–175. [DOI] [PubMed] [Google Scholar]
- 58.Gaenzer H, Sturm W, Kirchmair R, Neumayr G, Ritsch A, Patsch J: Circadian variation of endothelium-dependent vasodilatation of the brachial artery as a confounding factor in the evaluation of endothelial function. Atherosclerosis 2000, 149:227. [DOI] [PubMed] [Google Scholar]
- 59.Thosar SS, Berman AM, Herzig MX, Roberts SA, Lasarev MR, Shea SA: Morning impairment in vascular function is unrelated to overnight sleep or the inactivity that accompanies sleep. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2018, 315:R986–R993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurihara H, Yamaoki K, Nagai R, Yoshizumi M, Takaku F, Satoh H, Inui J, Yazaki Y: Endothelin: a potent vasoconstrictor associated with coronary vasospasm. Life sciences 1989, 44:1937–1943. [DOI] [PubMed] [Google Scholar]
- 61.Forstermann U, Munzel T: Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006, 113:1708–1714. [DOI] [PubMed] [Google Scholar]
- 62.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS: Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. International journal of cardiology 2009, 134:52–58. [DOI] [PubMed] [Google Scholar]
- 63.Hirsch L, Shechter A, Feinberg MS, Koren-Morag N, Shechter M: The impact of early compared to late morning hours on brachial endothelial function and long-term cardiovascular events in healthy subjects with no apparent coronary heart disease. International journal of cardiology 2011, 151:342–347. [DOI] [PubMed] [Google Scholar]
- 64.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K: Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives a prospective study. Circulation 2003, 107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 65.Taylor C, Hodge E, White D: The circadian rhythm of angina pectoris. Journal of Cardiovascular Pharmacology 1991, 17:S43–45. [DOI] [PubMed] [Google Scholar]
- 66.Andreotti F, Davies GJ, Hackett DR, Khan MI, De Bart AC, Aber VR, Maseri A, Kluft C: Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. The American journal of cardiology 1988, 62:635–637. [DOI] [PubMed] [Google Scholar]
- 67.Scheer FA, Shea SA: Human circadian system causes morning peak in pro-thrombotic plasminogen activator inhibitor-1 (PAI-1) independent of sleep/wake cycle. Blood 2013:blood-2013-2007-517060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheer FA, Michelson AD, Frelinger AL 3rd, Evoniuk H, Kelly EE, McCarthy M, Doamekpor LA, Barnard MR, Shea SA: The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One 2011, 6:e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young ME: PAI at breakfast (whether you like it or not). Blood, The Journal of the American Society of Hematology 2014, 123:466–468. [DOI] [PubMed] [Google Scholar]
- 70.Willich SN, Lewis M, Lowel H, Arntz H-R, Schubert F, Schroder R: Physical exertion as a trigger of acute myocardial infarction. New England Journal of Medicine 1993, 329:1684–1690. [DOI] [PubMed] [Google Scholar]
- 71.Qian J, Scheer FA, Hu K, Shea SA: The circadian system modulates the rate of recovery of systolic blood pressure after exercise in humans. Sleep 2020, 43:zsz253. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The paper shows that the circadian system modulates the rate of recovery of BP after exercise with fastest recovery in the biological afternoon. This has important implications for exercise prescription and interpretation of clinical tests of stress recovery.
- 72.Panza JA, Epstein SE, Quyyumi AA: Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med 1991, 325:986–990. [DOI] [PubMed] [Google Scholar]
- 73.Witte K, Parsa-Parsi R, Vobig M, Lemmer B: Mechanisms of the circadian regulation of β-adrenoceptor density and adenylyl cyclase activity in cardiac tissue from normotensive and spontaneously hypertensive rats. Journal of molecular and cellular cardiology 1995, 27:1195–1202. [DOI] [PubMed] [Google Scholar]
- 74.Scheer FA, Chellappa SL, Hu K, Shea SA: Impact of mental stress, the circadian system and their interaction on human cardiovascular function. Psychoneuroendocrinology 2019, 103:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper shows that the effects of mental stress and the endogenous circadian system on cardiovascular function occurred in conjunction, such that mental stress in the circadian morning caused greatest sympathovagal balance. This is clinically meaningful because summation of effects could contribute to the increased morning cardiovascular vulnerability.
- 75.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D: Effect of standing on neurohumoral responses and plasma volume in healthy subjects. Journal of Applied Physiology 1998, 84:914–921. [DOI] [PubMed] [Google Scholar]
- 76.Hu K, Scheer FA, Laker M, Smales C, Shea SA: Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 2011, 123:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du Fay De Lavallaz J, Badertscher P, Nestelberger T, Flores D, Miró Ò, Salgado E, Geigy N, Christ M, Cullen L, Than M: Circadian, weekly, seasonal, and temperature-dependent patterns of syncope aetiology in patients at increased risk of cardiac syncope. Ep Europace 2019, 21:511–521. [DOI] [PubMed] [Google Scholar]
- 78.Kuniyoshi FHS, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC: Day–night variation of acute myocardial infarction in obstructive sleep apnea. Journal of the American College of Cardiology 2008, 52:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butler MP TS, Smales C, et al. : Effects of obstructive sleep apnea on endogenous circadian rhythms assessed during relaxed wakefulness; an exploratory analysis. Chronobiology International 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper is important because these authors it shows a shift in the circadian rhythms of blood pressure, but not central circadian markers such as melatonin, in people with obstructive sleep apnea compared to healthy controls.
- 80.Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C: Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. The Lancet 2017. [DOI] [PubMed] [Google Scholar]; *This paper shows that aortic valve replacement surgeries scheduled in the morning carry a higher rate of mortality compared to afternoon surgeries. This could be attributed to increased expression of the nuclear receptor Rev-Erbα in the morning.
- 81.Mia S, Kane MS, Latimer MN, Reitz CJ, Sonkar R, Benavides GA, Smith SR, Frank SJ, Martino TA, Zhang J: Differential effects of REV-ERBα/β agonism on cardiac gene expression, metabolism, and contractile function in a mouse model of circadian disruption. American Journal of Physiology-Heart and Circulatory Physiology 2020, 318:H1487–H1508. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper highlights the role of REV-ERBα/β as mediating the circadian clock controlled processes in the myocardium. Specifically, these authors discovered that REV-ERBα/β was a mediator of glycogen synthesis, cardiomyocyte size, interstitial fibrosis, and contractile function abnormalities in the hearts of cardiomyocyte-specific-BMAL1-knockout mice.
- 82.El-Tamimi H, Mansour M, Pepine CJ, Wargovich TJ, Chen H: Circadian variation in coronary tone in patients with stable angina. Circulation 1995, 92:3201–3205. [DOI] [PubMed] [Google Scholar]
- 83.Zweiker R, Eber B, Schumacher M, Toplak H, Klein W: “Non-dipping” related to cardiovascular events in essential hypertensive patients. Acta Medica Austriaca 1993, 21:86–89. [PubMed] [Google Scholar]
- 84.Cederroth CR, Albrecht U, Bass J, Brown SA, Dyhrfjeld-Johnsen J, Gachon F, Green CB, Hastings MH, Helfrich-Förster C, Hogenesch JB: Medicine in the fourth dimension. Cell metabolism 2019, 30:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hermida RC, Ayala DE, Mojón A, Fernández JR: Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiology international 2010, 27:1629–1651. [DOI] [PubMed] [Google Scholar]
- 86.Hermida RC, Crespo JJ, Domínguez-Sardiña M, Otero A, Moyá A, Ríos MT, Sineiro E, Castiñeira MC, Callejas PA, Pousa L: Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. European heart journal 2019. [DOI] [PubMed] [Google Scholar]; **This study is important because it has direct relevance for patients with hypertension. These authors found that consuming an entire dose of anti-hypertensive medication at bedtime, compared to all doses upon awakening, led to significantly improved cardiovascular outcomes during the >6year median patient follow up. We also recommend reading the discussions in response to this article.
- 87.Guthrie G, Poulter, Macdonald T, Ford I, Mackenzie I, Findlay E, Williams B, Brown M, Lang C, Webb D: Chronotherapy in hypertension: the devil is in the details. European Heart Journal 2020, 41:1606–1607. [DOI] [PubMed] [Google Scholar]
- 88.Hermida RC, Mojón A, Fernández JR, Investigators HP: Comparing the design of the primary-care based Hygia Chronotherapy Trial and the Internet-Based TIME Study. European Heart Journal 2020, 41:1608–1608. [DOI] [PubMed] [Google Scholar]
- 89.Lemmer B, Middeke M: A commentary on the Spanish hypertension studies MAPEC and HYGIA. Chronobiology International 2020:1–3. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB: A circadian gene expression atlas in mammals: Implications for biology and medicine. Proceedings of the National Academy of Sciences 2014, 111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu G, Ruben MD, Francey LJ, Smith DF, Sherrill JD, Oblong JE, Mills KJ, Hogenesch JB: A single-sample circadian biomarker that performs across populations and platforms. bioRxiv 2019:820811. [Google Scholar]
- 92.Dijk D-J, Duffy JF: Novel approaches for assessing circadian rhythmicity in humans: a review. Journal of Biological Rhythms 2020, 35:421–438. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper reviews the novel approaches for assessing circadian phases of the central and peripheral and compares them to the gold standard methods of circadian phase assessment.
- 93.Burgess HJ, Wyatt JK, Park M, Fogg LF: Home Circadian Phase Assessments with Measures of Compliance Yield Accurate Dim Light Melatonin Onsets. Sleep 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Litinski M, Scheer FA, Shea SA: Influence of the Circadian System on Disease Severity. Sleep Med Clin 2009, 4:143–163. [DOI] [PMC free article] [PubMed] [Google Scholar]


