Abstract
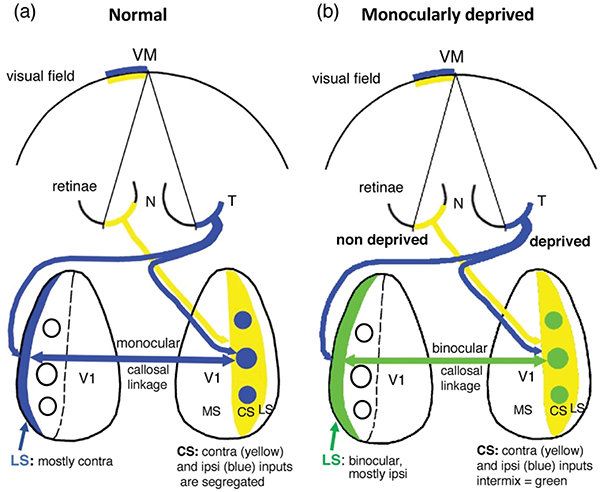
In Long Evans rats, ocular dominance columns (ODCs) in V1 overlap with patches of callosal connections. Using anatomical tracers, we found that ODCs and callosal patches are present at postnatal day 10 (P10), several days before eye opening, and about 10 days before the activation of the critical period for ocular dominance plasticity (~P20). In rats monocularly enucleated at P10 and perfused ~P20, ODCs ipsilateral to the remaining eye desegregated, indicating that rat ODCs are highly susceptible to monocular enucleation during a precritical period. Monocular enucleation during the critical period exerted significant, although smaller, effects. Monocular eye lid suture during the critical period led to a significant expansion of the ipsilateral projection from the non-deprived eye, whereas the contralateral projection invaded into, and intermixed with, ipsilateral ODCs innervated by the deprived eye. We propose that this intermixing allows callosal connections to contribute to the effects of monocular deprivation assessed in the hemisphere ipsilateral to the non deprived eye. The ipsilateral and contralateral projections from the deprived eye did not undergo significant shrinkage.
In contrast, we found that callosal patches are less susceptible to imbalance of eye input. In rats monocularly enucleated during either the precritical or critical periods, callosal patches were maintained in the hemisphere ipsilateral to the remaining eye, but desegregated in the hemisphere ipsilateral to the enucleated orbit. Callosal patches were maintained in rats binocularly enucleated at P10 or later. Similarly, monocular deprivation during the critical period had no significant effect on callosal patches in either hemisphere.
Keywords: Long Evans rats, Columnar organization, eye-specific domains, interhemispheric connections, primary visual cortex, monocular enucleation, monocular deprivation, desegregation, RRID:RGD 68073
Graphical Abstract

In normal rats, ocular dominance columns (ODCs) overlap with callosal patches in V1. ODCs (a) and callosal patches (c) are present by postnatal day 10 (P10). In rats monocularly enucleated (ME) at P10 and perfused before the onset of the critical period (~P20), ODCs desegregate in the hemisphere ipsilateral to the remaining eye (b), whereas callosal patches do not desegregate in this hemisphere (d).
1. INTRODUCTION
It is generally thought that the period during which ocular dominance columns (ODCs, cortical aggregates of neurons with the same eye preference) emerge and are susceptible to imbalance of visual experience between the eyes coincides with the physiologically defined critical period during which shifts in ocular dominance can be induced by imbalance of eye input (LeVay et al., 1978; Finney & Shatz, 1998; Issa et al., 1999; Ruthazer et al., 1999). However, studies in several species have provided evidence that ODCs develop before the beginning of the critical period for ocular dominance plasticity (ferrets: Crowley and Katz, 2000; cats: Crair et al., 2001). Whether ODCs are also plastic before the onset of the critical period, or whether they remain resistant to experience-dependent competitive interactions until the beginning of the critical period, is still highly debated. Crowley and Katz (2000) presented data supporting the second possibility. They reported that ODCs in ferrets were visible by postnatal day 16–18 (P16-P18), about 2 weeks before the onset of the critical period (P33, Issa et al., 1999), and that they were unaffected in ferrets monocularly enucleated between P7 and P14 and perfused between P17 and P21. They proposed that the establishment and plasticity of ODCs are temporally and mechanistically distinct phases of visual development.
Here we address this issue in the system of ODCs in Long Evans rats (Laing et al., 2015). In young and older rats, we examined the pattern of WGA-HRP labeling in tangential sections through V1 following intraocular injections of this transneuronal tracer. We found that ODCs similar to that in adults are visible by postnatal day P10, a few days before eye opening, and about 10 days before the beginning of the critical period (~P20, Fagiolini et al., 1994). In rats monocularly enucleated at P10 and perfused by P20 we found that ODCs ipsilateral to the remaining eye desegregate nearly completely. Contralaterally, the labeling was distributed throughout V1, such that regions devoid of labeling corresponding to ipsilateral eye territory were not observed. These results indicate that in rats, ODCs are highly susceptible to monocular enucleation during a precritical period, here defined as the period extending back from the beginning of the critical period to the time when ocular dominance columns form. We also examined the anatomical effects of either monocular enucleation or monocular deprivation by eyelid suture during the critical period. The effects of monocular deprivation were also assessed with physiological methods. In rats monocularly enucleated at the start of the critical period and perfused at adulthood, the anatomical effects were significant, but smaller than the effects observed during the precritical period. In rats monocularly deprived by eyelid suture, the ipsilateral projection from the non-deprived eye expanded significantly in V1. Again, the contralateral projections invaded into, and intermixed with, ipsilateral ODCs innervated by the deprived eye. The ipsilateral and contralateral projections from the deprived eye did not undergo significant shrinkage compared to controls. Our finding of significant differences between ODCs serving the deprived and non-deprived eye are consistent with studies of monocular deprivation during the critical period in other species (Hubel et al., 1970; Shatz and Stryker, 1978; LeVay et al., 1980; Horton and Hocking, 1997a).
Callosal connections in V1 of Long Evans rats form distinct patches that colocalize with ipsilateral ODCs in the central segment of V1 (Laing et al., 2015; Andelin et al., 2020). Moreover, Laing et al. (2015) found that the regions occupied by callosal patches responded preferentially to ipsilateral eye input. The fact that callosal patches are both spatially and physiologically coupled to ipsilateral ODCs prompted us to investigate whether callosal patches are plastic after they form, and if so, whether their plastic changes mirror those of ODCs. We therefore studied the effects that monocular enucleation and monocular deprivation during the precritical and critical periods have on callosal patches. The patterns of callosal connections in V1 of one hemisphere were analyzed in tangential sections of the flattened cortex following multiple intracortical injections of horseradish peroxidase (HRP) into the other hemisphere. We found that, as ODCs, callosal patches are visible at P10, but, unlike ODCs, they are resistant to monocular deprivation and to enucleation of one or both eyes, with the exception that they desegregate in the hemisphere ipsilateral to the enucleated orbit in monocularly enucleated rats. Our findings highlight that pathways that are closely correlated anatomically and physiologically during normal conditions can respond very differently to the same insults delivered during critical periods of development.
2. MATERIALS AND METHODS
Long Evans pigmented rats (RRID:RGD 68073) (Rattus norvegicus) were used and procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee at the University of Washington, and are in accordance with the animal care guidelines of the National Institutes of Health, USA.
2.1. Monocular enucleation/ deprivation
Rats were enucleated or monocularly deprived under isoflurane anesthesia (2.5% in air) administered first in an induction chamber and maintained with a vaporizer and nose cone. Local analgesics were applied and rats were returned to the animal colony after recovery from anesthesia. All monocularly deprived animals were checked daily to ensure that the lid suture remained intact.
2.2. Intraocular and intracortical injections of anatomical tracers, and histochemical processing
Intraocular and intracortical tracer injections were performed under anesthesia induced and maintained with isoflurane (5.0 and 2.0%, respectively) in air. A total volume of 4–6 μL of 3% horseradish peroxidase conjugated to wheat-germ agglutinin (WGA-HRP) in saline was pressure injected into one eye over 15 minutes through glass micropipettes (50–100 μm tip diameter). In rats, the diameter of the eyes ranges from ~50 mm at P10 and ~78 mm at adulthood (Pinazo-Duran et al., 2011). The injection site was 1.5–2 mm posterior to the corneal limbus, at a depth of 1.5–2 mm. Data on ipsilateral eye projections to V1 obtained similarly by Laing et al. (2015) in Long Evans rats were analyzed for comparison. Due to its transneuronal transport property, WGA-HRP has been widely used in studies of retino-thalamo-cortical projections and patterns of ODCs in a variety of species (see Laing et al., 2015, for references). To label the callosal connections, a craniotomy exposed an area over occipital cortex extending from ~2.0 to 7.0 mm lateral to the midline suture, and 0.0–6.0 mm anterior to lambda suture in rats P20 or older. In younger rats these distances were reduced by about 1.0 mm. The dura was kept intact and moist with saline. Throughout the exposed area, horseradish peroxidase (HRP, Sigma Co, 30% in saline) was pressure injected intracortically through glass micropipettes (50–100 μm tip diameter) in volumes varying from about 3 μL for ~P10 pups, to 4 μL for older rats. These volumes were delivered in 16 injections in young rats, or up to 20 injections in ~P20 or older rats. The cortical thickness varies from about 1.0 mm in ~P10 rats to 1.2 mm in adult rats. Injections were evenly spaced (0.2 μL each), at a depth of about 800 μm in young rats and 1.0 mm in adult rats, corresponding approximately to layers 5/6, from where the tracer diffuses throughout the cortical layers (Olavarria and Van Sluyters, 1985). After the injections, the bone chip was repositioned, and the skin was sutured by planes.
Following intraocular injections of WGA-HRP, the survival periods were 2–3 days for ~P10-P20 rats and 4 days for older rats, and 2 and 3 days, respectively, after intracortical injections of HRP. Animals were deeply anesthetized with pentobarbital sodium (100 mg/kg i.p.) and perfused through the heart with 0.9% saline followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4). Cortices were separated from the brains and flattened for sectioning in the tangential plane, whereas the remainder of the brain was left intact for coronal sectioning. The flattened cortices were left between glass slides for 24 hours in 0.1 M PB, after which time the tissue was transferred to a 3% PFA in 0.1 M PB, 20% sucrose solution for 1 additional hour. Tissue blocks to be sectioned in the coronal plane were kept in 3% PFA in 0.1 M PB and 20% sucrose for one or more days. All tissue was cut using a freezing microtome at 60 μm thickness and the sections were collected in 0.1 M PB. Sections were reacted for HRP with 3,3,5,5-tetramethylbenzidine as the chromogen (Mesulam, 1978).
2.3. Electrophysiology
Electrophysiological recordings were performed under urethane anesthesia (1200 mg/kg i.p.) in adult rats (~P40) that had been monocularly deprived by eyelid suture at P20. Atropine sulfate (0.1 mg/kg i.p.) was used to reduce tracheal secretions. Body temperature was kept at 37°C with a heating pad. Pupils were dilated with atropine sulfate ophthalmic solution (1%, Bausch and Lomb), artificial tears (polyethylene glycol eye drops) and silicone oil was used to protect the corneas and the cerebral cortex, respectively. The occipital cortex was exposed by drilling a window into the skull that extended from 2.0 to 7.0 mm in the mediolateral direction, and from the lambda suture to 5.0 mm in the anteroposterior direction. The dura mater was left intact. Multiunit activity was recorded with glass-insulated tungsten electrodes (1–2 MΩ; FHC) positioned perpendicularly to the cortical surface, at depths of 500–600 μm, and displayed on an oscilloscope and an audio monitor. Signals were filtered and amplified using a digital electrophysiology amplifier board (RHD 2132, Intan technologies, LLC) and USB interface board (RHD 2000 series, Intan Technologies, LLC), and stored on a computer for later analysis.
Full field visual stimuli were presented through custom made eye covers housing a white LED for each eye. The eye covers ensured that each eye was stimulated independently from the other eye. A custom-made remote control simultaneously sent a signal to one LED as well as the electrophysiology interface board, allowing visually evoked responses to be time-locked to stimulation. Each eye was stimulated separately while the non-stimulated eye was covered. Visual responses elicited from an eye ceased when the eye was covered, indicating that covering of the eyes was effective in preventing visual stimulation. Electrode penetrations, spaced at least 200 μm apart, were arranged in a grid over the lateral half of V1.
Action potentials (APs) were collected after filtering and thresholding recording traces of neural activity using a custom Matlab script (ver 2015a; Mathworks, Inc.). The spontaneous activity recorded during 300 milliseconds prior to stimulation was subtracted from responses recorded from each eye during the 300 milliseconds following stimulation onset. These time windows were chosen based on analysis of peak response times to visual stimulation. For each recording site, counts of the APs for each trial were summed and averaged for each eye. Using these values, a contralateral bias index (CBI) was calculated to evaluate the ocular preference of responses within each of the cortical regions analyzed, according to the formula:
R denotes responses, measured as the number of APs, following stimulations of either the contralateral or ipsilateral eye. This index ranges from −1 (purely ipsilateral response) to +1 (purely contralateral response). Values ~ 0 indicate balanced binocular responses (Andelin et al., 2020; Conde-Ocazionez et al., 2018). Rats were perfused immediately at the end of recording sessions, and the recorded hemispheres were flattened for tangential sectioning.
2.4. Data acquisition and analysis
Prior to histochemical processing, the tangential sections from flattened hemispheres were digitally scanned at 2400 dpi for identification of the V1 border, which was revealed by the abrupt transition in the density of myelination pattern upon passing from striate cortex to extrastriate cortex (Richter and Warner, 1974; Laing et al., 2012, 2015). The myelin pattern, optimally displayed in tangential sections passing through layer IV (∼500–650 μm deep), was confirmed in 2 or more sections from the same animal. When analyzing patterns of callosal connections, further information for identifying the border of V1 was provided by the relationship that is known to exist between this border with the 17/18a callosal band and other distinct features of the callosal pattern in V1 (Laing et al., 2012; Olavarria & van Sluyters, 1985). Following the histological processing for anatomical tracers, digital images of the labeling patterns were obtained by scanning the sections (Epson 4990). The patterns of retino-geniculo-cortical projections labeled with WGA-HRP were displayed either from one tangential section, or from reconstructions of two superimposed sections. The digitized images of myelin and anatomical labeling patterns were aligned with each other in Adobe Photoshop CS5 (Adobe Systems, Inc. CA), using the border of V1, the edges of sections, and radial blood vessels as fiducial markers. Since sections scanned to reveal the myelin pattern were subsequently reacted for HRP, aligned myelin and labeling images often came from the same sections. In all images, we used the level control in Photoshop to adjust the black and white brightness points so that they coincided with the lowest and highest points, respectively, of the image brightness histograms. We then applied similar values of enhancement filters to the entire images. These filters included noise and Gaussian filters to reduce sharp increases in density produced by blood vessels and other artifacts; high pass filters to remove gradual changes in labeling intensity; and brightness/contrast filters. ImageJ (ver 1.50e) was used to measure the area size of V1, analyze patchiness data in V1, and to obtain 3-D surface plots of the patterns of ipsilateral eye input in V1. The WGA-HRP and HRP labeled fields in V1 were thresholded in ImageJ to measure the area size of the labeled fields in regions corresponding to the central segment in V1. The thresholded images faithfully captured the patterns of densely labeled regions in all cases. The values were expressed as a percent of the area of V1 occupied by the WGA-HRP labeled fields or HRP labeled callosal connections. Measurements of the HRP labeled area did not include the band of callosal connections at the 17/18a border. In coronal sections, the border of the LGN was visualized by the contrast between the LGN and surrounding tissue produced by WGA-HRP labeled fibers traversing the nucleus. High-magnification images were obtained using a DMR Leica microscope coupled to a Leica DC 300F digital camera. To compare our results with those reported by Crowley and Katz (2000) in ferrets, we translated postnatal ages in rats and ferrets using the “translating time” regression model developed by Finlay and colleagues (Workman et al. 2013; see also, http://www.translatingtime.org).
2.4.1. Analysis of patchiness
The degree of patchiness (expressed as patch index) in the labeling distributions in V1 was evaluated by calculating the standard deviations (SD) of the grey value distributions in the region corresponding to the central segment in V1: images with large variations in labeling density (i.e., high patch index) contain a wide range of grey values and have large SD, whereas images with uniform distributions (i.e., low patch index) are more restricted in their range of grey values and have smaller SD. To examine whether increases in the tracer reaction times affect the grayscale values, we compared the mean grayscale values across the entire tissue sample across groups and found that the variance both within and between groups was not statistically significant (p > 0.05).
2.4.2. Electrophysiology.
On the basis of the distribution of afferent projections from the ipsilateral eye, V1 in rats has been subdivided into three main subdivisions or segments (Laing et al., 2015): (1) The central segment (CS), defined as the area receiving the bulk of ipsilateral retino-thalamo-cortical projections (outlined by the smooth line in Figure 1a). In this segment, the inputs from both eyes are normally segregated anatomically and functionally into ODCs. (2) The medial segment (MS), located medial to the CS, presumably representing the peripheral monocular visual field. (3) The lateral segment (LS), located between the CS and the lateral border of V1. These segments have their counterpart in the distribution of associated callosal connections in V1. In monocularly deprived rats (n = 7), the ocular preference of evoked response was assessed quantitatively at recording sites judged to be within the CS and LS in the hemisphere ipsilateral to the non-deprived eye. Data on ocular dominance preference in 10 normal adult Long Evans rats obtained similarly in a previous study from our laboratory (Andelin et al. (2020) were analyzed for comparison. The width of the LS in normal adult rats and in the hemisphere ipsilateral to the non-deprived eye was estimated to be 0.2–0.3 mm based on the patterns of ipsilateral retino-geniculate-V1projections. The locations of recording sites along with local blood vessels used as reference were marked on digital images of the intact cortical surface taken immediately before recordings began. The images were correlated with images of the most superficial tangential sections, and the recording sites were matched to the patterns made by electrode penetrations in these sections. The first tangential sections were aligned with deeper sections using the pattern of penetrating blood vessels, section borders, and other landmarks. The border of V1 was determined using the myelin pattern. Following histological processing, the binocularity at recording sites judged to be within regions corresponding to the central segment (CS) and lateral segment (LS) was calculated (see Figure 4 in Andelin et al., 2020). A total of 38 and 42 recoding sites were analyzed in normal and monocularly deprived rats, respectively, all of which were judged to be oriented perpendicularly to the cortical surface.
Figure 1.
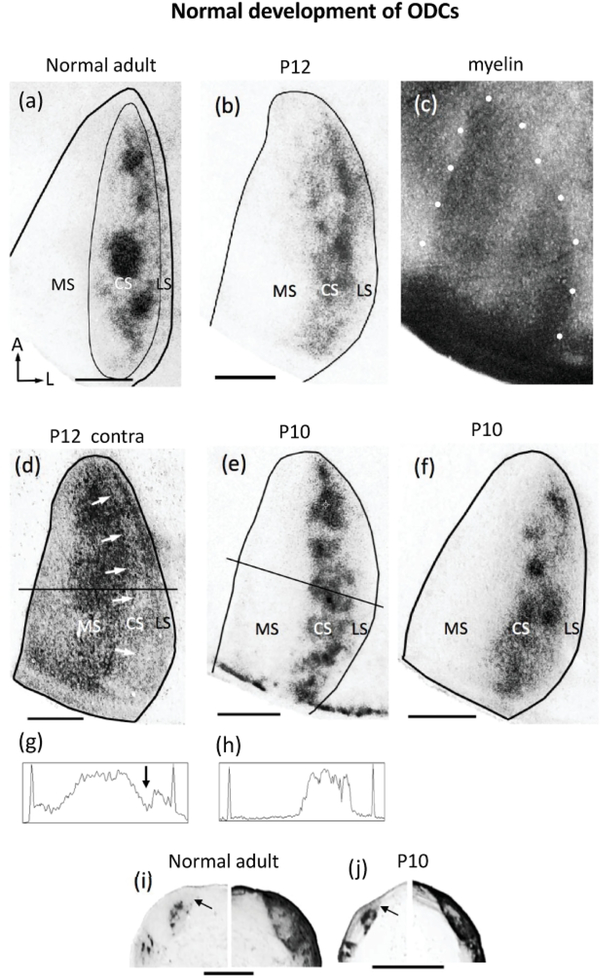
Development of ODCs in Long Evans rats revealed following WGA-HRP labeling of retino-geniculo-cortical projections. (a) Representative case of ipsilateral eye labeling in adult Long Evans rat (adapted from Laing et al., 2015). Thin line outlines the subdivision of V1 into medial (MS), central (CS) and lateral (LS) segments. Note the distinctly labeled ODCs in the central segment of V1 and the largely unlabeled LS, interposed between the ODCs and the lateral border of V1. Bottom, left arrows in (a) indicate anterior (A) and lateral (L). (b) Data from a P12 rat showing that the ipsilateral eye labeling in the CS is patchy, and that the lateral segment is present. The black line indicates the border of V1 determined based on the myelin pattern from the same case delineated by white dots in (c). (d) Labeling in the contralateral hemisphere in the case shown in (b). Arrows indicate reduced labeling in a region corresponding to the CS, which likely correspond to ipsilateral input from the eye not injected with WGA-HRP. The reduction in labeling at the level indicated by a horizontal line is indicated by an arrow in the density scan in (g). The reduction in the labeling density in medial V1 is likely due to the section passing out of layer 4 in this region. The peaks at both ends of the scan indicate the medial (left) and lateral (right) borders of V1. (e) Data from a P10 rat showing that patchiness of ipsilateral eye input is visible at this age. A density scan at the level indicated is shown in (h). (f) Data from another rat studied at P10, also showing patchy ipsilateral eye projections. (i) Labeling pattern in the LGN of a normal adult rat showing ipsilateral (left) and contralateral (right) eye projections. (j) Ipsilateral (left) and contralateral (right) labeling in the LGN of a P10 rat. Arrows in (i) and (j) indicate dorsomedial region that remains unlabeled after intraocular injections of WGA-HRP into the ipsilateral eye in normal young and adult rats. This LGN region innervates the LS in V1 (see Refs. in text). In the LGN contralateral to the injection in both adult and young rats, an area of reduced labeling is observed, likely corresponding to ipsilateral territory innervated by the non-injected eye. Cortical labeling in both hemispheres is represented as right hemispheres, lateral to the right. Scale bars = 1.0 mm.
2.4.3. Statistical analyses.
The Student t-test was used for comparing two groups (significance set at p< 0.05). ANOVA with Bonferroni posttest (significance set at p< 0.05) was used for multiple comparisons between groups.
3. Results
3.1. The tripartite subdivision of V1 and the pattern of ODCs are visible as early as postnatal day 10
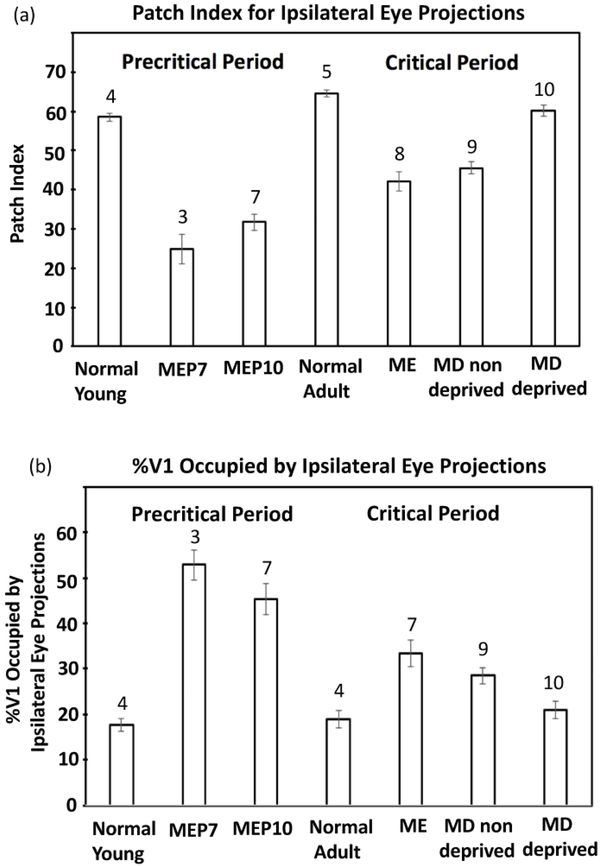
To investigate the distribution of retino-geniculo-cortical input to striate cortex (V1) in young rats, we injected the anatomical tracer WGA-HRP into one eye and analyzed the patterns of WGA labeling in tangential cortical sections. The transneuronal transport property of WGA-HRP has been demonstrated at both the optical and structural level (Itaya & van Hoesen, 1982), and the pattern of anterogradely labeled geniculo-cortical axon terminals in V1 was most distinct at the level of layer IV (500–650 μm) (Itaya & van Hoesen, 1982; Kageyama, Gallivan, Gallardo, & Robertson, 1990; Laing et al., 2015). On average, the patch index for normal adult rats was 64.51 (SEM = 0.974, n = 5), and ODCs occupied 18.96 % (SEM = 1.95, n = 4) of the area of V1. Contralateral WGA-HRP labeling was uniformly dense throughout V1, except for areas of reduced or absent labeling in the CS corresponding to ipsilateral eye recipient zones (Laing et al., 2015).
Kageyama & Robertson (1993) used the transneuronal WGA-HRP technique in rats to analyze the development of geniculo-cortical projections in coronal sections. Their work suggested that, if present, ODCs could be visualized during the second and third weeks of life. We were successful in rats perfused at P19, P12 and P10. However, in rats perfused at P7 (n = 2), we did not observe significant WGA-HRP labeling in V1 ipsilateral to the injected eye presumably because sparse LGN-V1 projections perpendicular to the cortical surface are difficult to detect in tangential sections. The outcome from our attempt at studying P7 rats (corresponding to P13 in the ferret) is consistent with the observation by Crowley and Katz (2000) that in ferrets perfused between P13-P15, the innervation density was too low to determine conclusively whether labeling fluctuations correspond to nascent ODCs.
In rats perfused at P10-P12 (n = 4), (corresponding to P21 and P26 in ferrets, respectively), we found that, as in adult rats (Figure 1a), labeling from the ipsilateral eye in young rats accumulates forming several patches arranged within a region corresponding to the CS (Figure 1b,e,f). On average, the fraction of V1 occupied by these patches (18.45%, SEM = 1.49, n = 4) is not significantly different (p > 0.05) from the fraction of V1 occupied by ODCs in adult normal rats (18.96, SEM = 1.95, n = 4), but the average patch index in young rats (58.35, SEM = 1.22, n = 4) was smaller (p < 0.05) than in normal adult rats (64.51, SEM = 0.97, n = 5). Medially, the MS is devoid of labeling, and laterally, between the CS and the border of V1, lies a region with little or no labeling corresponding to the LS (Figure 1b,e,f). The borders of V1 were determined from the myelin patterns (e.g., delineated by white dots in Figure 1c, see Material and Methods). Figure 1h shows a density scan across the anteroposterior level indicated in Figure 1e. The medial and lateral spikes in the trace correspond to the medial and lateral borders of V1. This figure illustrates that in these young rats the density of WGA-HRP labeling decreases laterally before reaching the lateral border of V1, leaving a gap of reduced or absent labeling that corresponds to the LS. Thus, as in normal adult rats, the LS in young rats receives little or no direct (i.e., retino-geniculo-cortical) input from the ipsilateral eye. In the hemisphere contralateral to the injected eye, labeling was observed in the MS and LS, whereas within the CS, an elongated area of reduced or absent labeling could be seen (arrows in Figure 1d, and arrow in the density scan in Figure 1g, taken at the level indicated in Figure 1d), likely corresponding to ipsilateral territory from the non-injected eye, consistent with the pattern in normal adult rats (Laing et al., 2015).
3.1.1. Retino-geniculate projections
In the LGN of normal rats perfused during the second or third week of life (Figure 1j) the location of the projection ipsilateral to the injected eye is similar to that in adult rats (cf. Figure 1i). In particular, a region located dorsomedially to the target of ipsilateral eye projections remains unlabeled in both normal adult rats (arrow in Figure 1i) and in normal young rats (arrow in Figure 1j ). This region provides direct input to lateral striate cortex (Lewis & Olavarria, 1995; Olavarria & Hiroi, 2003), and the fact that it remains unlabeled after an ipsilateral eye injection indicates that the LS in young rats, as in normal adult rats, does not receive significant direct ipsilateral eye input. Contralaterally, in both normal adult and young rats, the labeling was uniformly dense throughout the LGN, except for a small central region where the labeling was reduced or absent, presumably corresponding to the ipsilateral eye recipient zone.
In summary, our results show that a pattern of eye-specific segregation similar to that seen in adult rats can be readily recognized in V1 a few days before eye opening. Our finding that ODCs are clearly visible as early as P10 is in close correspondence with the report by Crowley and Katz (2000) that in ferrets perfused at P16-P18 (corresponding to rat P8-P9), injections into single LGN layers produced segregated patches of geniculo-cortical axons in layer
3.2. ODC plasticity during the precritical period
The critical period for ocular dominance plasticity starts around P20 in the rat (Fagiolini et al., 1994) and mice (Gordon and Stryker, 1996). Our results indicate that the pattern of ODCs in rats is present at least 10 days before the activation of this critical period. We were interested in determining whether rat ODCs are either susceptible to the effects of monocular enucleation before the onset of the critical period, or resistant to modifications, as reported for ODCs in ferrets (Crowley and Katz, 2000).
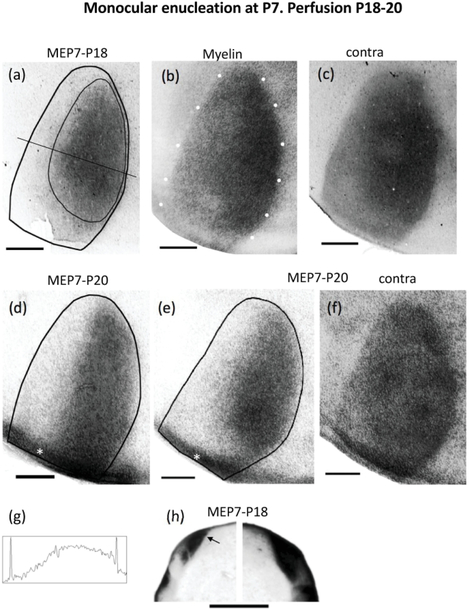
We analyzed rats monocularly enucleated at P7 and perfused at P18-P20 (n = 3). Figure 2 shows the patterns of V1 labeling following injection of WGA-HRP into the remaining eye. The borders of V1 were determined from the myelin patterns illustrated in Figure 2b (white dots) for case in a. In the hemispheres ipsilateral to the injection (Figure 2 a,d,e), distinct labeled patches were not observed in the CS. Instead, the labeling was uniformly distributed over a broad area that on average occupied 52.91% of the area of V1 (SEM = 3.30, n=3), significantly larger (p = 0.01) than the fraction of V1 occupied by the labeled area in normal young rats (18.45%, SEM = 1.495, n = 4). Correspondingly, the average patch index in rats monocularly enucleated at P7 (24.86, SEM = 3.65, n= 3) was significantly smaller (p <0.01) than in young normal rats (58.35, SEM = 1.22, n = 4). Figure 2 a,d,e shows that the labeled area extended medially in V1, and also laterally, essentially abolishing, or greatly reducing the width of presumptive LS, indicating that the ipsilateral eye provides strong input to this lateral region, which in normal rats is exclusively innervated by the contralateral eye (Laing et al., 2015). The density scan in Figure 2g, taken at the level indicated in Figure 2a, illustrates that the labeling density decreases laterally in V1, but unlike in normal young rats (Figure 1h), it reaches the lateral V1 border, or leaves at most a very narrow low density gap. Contralaterally, the labeling was homogeneously distributed throughout V1, including the LS (Figure 2c,f)). In all cases, restricted regions of reduced labeling corresponding to territory innervated by the missing eye were not observed.
Figure 2.
Retino-geniculo-cortical projections from the remaining eye in rats monocularly enucleated at P7 (MEP7) and perfused before the onset of the critical period. The age at perfusion is indicated by the second number above each case. (a),(d),(e) Ipsilateral projections in cases perfused at P18 (a) and P20 (d)(e). The thin line in (a) illustrates the region used for calculating the patch index and the %V1 occupied by ipsilateral eye projections (see Materials and Methods). (b) White dots indicate myelin pattern used to delineate the V1 border in (a). (c),(f) Distribution of WGA-HRP labeling in V1 contralateral to the injection for cases shown in (a) and (e), respectively. Note that areas of reduced labeling corresponding to ipsilateral territory serving the enucleated orbit are not observed. (g) Density scan at the level indicated in (a). (h) Ipsilateral (left) and contralateral (right) labeling in the LGN of a rat monocularly enucleated at P7 and perfused at P18. Arrow indicates dorsomedial region that is densely labeled after intraocular injections of WGA-HRP into the ipsilateral eye. In the contralateral LGN, note that an area of reduced labeling corresponding to ipsilateral territory serving the enucleated orbit is not observed. The asterisks in (d) and (e) indicate areas of artifactual labeling that were not included in the measurements. ME = monocular enucleation. Scale bars = 1.0 mm. Other conventions as in Figure 1.
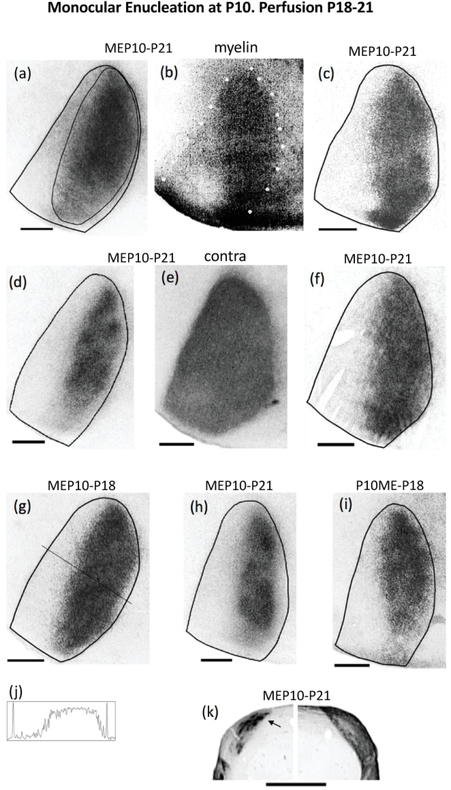
Because we did not observe a clear pattern of ODCs in normal pups at P7, it is possible that ODCs are still forming at this age, and that the abnormally broad ipsilateral labeling patterns we observed in rats monocularly enucleated at P7 reflect an interruption of normal ODC development. We therefore monocularly enucleated rats at P10, when ODCs are clearly visible (Figure 1e,f), and perfused them at P18-P21 (n = 7), at about the beginning of the critical period for ocular dominance plasticity (Fagiolini et al., 1994). Figure 3 shows the labeling patterns in all 7 rats analyzed. Figure 3b shows the myelin pattern used to delineate the V1 border in Figure 3a. In all these cases, the labeling ipsilateral to the remaining eye was distributed in a broad area that, on average, occupied 45.32 % of the area of V1 (SEM = 3.45, n = 7). The labeling extended laterally, in some cases markedly reducing the width of LS, or nearly obliterating it altogether (e.g., Figure 3a,f), whereas in some cases LS was clearly visible (e.g., Figure 3d,h). In some cases, the labeling was distributed uniformly (Figure 3a,f,g), whereas in other cases the distribution was less uniform, with areas of greater label density separated by areas less densely labeled (Figure 3c,d,h,i). The average patch index was 31.71 (SEM= 2.12, n = 7). The density scan in Figure 3j, taken at the level indicated in Figure 3g, illustrates that the labeling density decreases laterally in V1, leaving a narrow low density gap that in most cases is wider than that in rats monocularly enucleated at P7 (cf. Figure 2). Contralaterally, in all cases, the labeling was homogeneously distributed throughout V1, including the LS (e.g., Figure 3e). In all 7 cases, restricted regions of reduced labeling corresponding to territory innervated by the missing eye were not observed. We obtained similar results in rats monocularly enucleated at P12 and perfused before the peak of the critical period (n = 4, data not shown).
Figure 3.
Retino-geniculo-cortical projections from the remaining eye in rats monocularly enucleated at P10 and perfused before the onset of the critical period. Seven cases are shown: (a)(c)(d)(f)(g)(h)(i). (a),(c),(d),(f),(h) Cases perfused at P21. (g),(i) Cases perfused at P18. The thin line in (a) illustrates the region used for calculating the patch index and the %V1 occupied by ipsilateral eye projections. (b) White dots indicate myelin pattern used to delineate the V1 border in (a). (e) Distribution of WGA-HRP labeling in V1 contralateral to the injection for the case shown in (d). Note that areas of reduced labeling corresponding to ipsilateral projections from the enucleated orbit are not observed. (j) Density scan at the level indicated in (g). (k) Ipsilateral (left) and contralateral (right) labeling in the LGN of a rat monocularly enucleated at P10 and perfused at P21. Arrow indicates dorsomedial region that is densely labeled after intraocular injections of WGA-HRP into the ipsilateral eye. Note that an area of reduced labeling corresponding to territory innervated by ipsilateral projections from the enucleated orbit is observed in the LGN contralateral to the injection. Scale bars = 1.0 mm. Other conventions as in Figures 1, 2.
3.2.1. Retino-geniculate projections
In rats monocularly enucleated at P7 and P10 and perfused at P18–21, the ipsilateral retino-geniculate projection from the remaining eye expanded (Figure 2h, 3k, respectively) compared to the ipsilateral LGN labeling in normal young (cf. Figure 1j) and adult rats (cf. Figure 1i), consistent with a study of the retino-geniculate projections in mice enucleated at P10 (Hayakawa & Kawasaki, 2010). The labeled projections expanded ventrally in the LGN, into regions projecting medially in V1 (Lewis & Olavarria, 1995), as well as dorsomedially, into regions that project to the LS (Lewis & Olavarria, 1995; Olavarria & Hiroi, 2003) (arrows in Figures 2h, 3k). Thus, the topography of the V1 expansion in these rats seems to mirror the topography of the expansion in the LGN. In the contralateral LGN of rats enucleated at P7, the retinal projection is densely distributed throughout the LGN, and, as in contralateral V1 (Figure 2c,f), no area of reduced labeling corresponding to the ipsilateral projection from the missing eye is observed (Figure 2h). In contrast, an area of reduced labeling in the contralateral LGN was observed in all rats monocularly enucleated at P10 (Figure 3k), whereas no such areas were observed in contralateral V1 (Figure 3e), suggesting that the geniculo-cortical projection is more plastic then the retino-geniculate projection.
In summary, the pattern of ocular dominance columns is clearly visible at P10, but monocular enucleation at this age induces a virtually complete desegregation of ODCs ipsilateral to the remaining eye, and more than doubles the area of ipsilateral eye projections in V1. These results provide evidence that ODCs in rats are highly susceptible to monocular enucleation during the precritical period.
3.3. ODC plasticity during the critical period: monocular enucleation and monocular deprivation by eyelid suture
3.3.1. Effects of monocular enucleation during the critical period
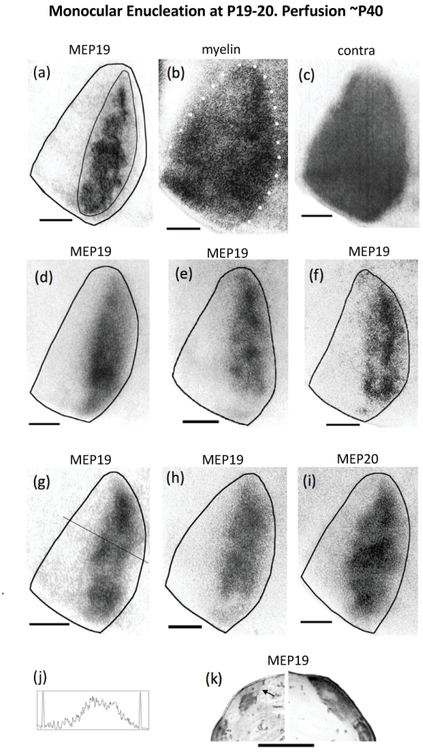
To investigate whether monocular enucleation near the beginning of the critical period has effects comparable to those we observed in rats monocularly enucleated around P10 and perfused before the onset of the critical period, we monocularly enucleated rats at P19-P20 (n = 8), and perfused them at adulthood (P >40). Figure 4 shows the V1 labeling patterns in 7 cases. The border of V1 was drawn based on the myelin pattern, as illustrated in Figure 4b. Comparisons of the WGA-HRP labeling patterns show common features across cases, as well as some variability. As illustrated in Figure 4 d,e,g,h,i, the labeling in V1 accumulates in regions of varying density, without forming separate, discrete ODCs as is the case in normal young rats (cf. Figure 1b,e,f) and in normal adult rats (cf. Figure 1a). In some cases (Figure 4a,f), the labeling coalesces into long strips that run almost the entire anteroposterior length of the CS. Reflecting these variations in labeling density, the average patch index increased to 42.14 (SEM = 2.52, n = 8), significantly higher (p < 0.05) than that for rats monocularly enucleated at P10 (31.71, SEM = 2.12, n = 7), but significantly smaller (p < 0.01) than that for normal adults. Compared to rats monocularly enucleated at P7 and P10 (cf. Figures 2,3), the labeling does not invade as far medial and as far lateral in V1 (Figure 4), which results in a smaller fraction of the area of V1 occupied by the labeling (33.42% SEM = 2.87, n = 7). This value is significantly smaller (p <0.05) than the area of V1 occupied in rats monocularly enucleated at P10, but significantly larger (p < 0.01) than the area of V1 occupied by ipsilateral projections in normal adult rats. In contrast to rats monocularly enucleated at P7 and P10, in which the labeled region extended laterally into the LS, leaving at most narrow islands of reduced labeling, a strip of cortex corresponding to LS was clearly observed in all rats monocularly enucleated at P19-P20, which was comparable in width to LS in normal young rats and normal adult rats (cf Figure 1). The density scan in Figure 4j, taken at the level indicated in Figure 4g, illustrates that the lateral decrease in labeling density is less abrupt than in rats monocularly enucleated at P10, thus increasing the width of the low-density gap (cf. Figure 3). Contralaterally, the labeling was homogeneously distributed throughout V1, including the CS and LS (illustrated in Figure 4c, same case as in Figure 4a). Restricted regions of reduced or absent labeling corresponding to territory innervated by the missing eye were not observed in any of the cases analyzed (n = 8).
Figure 4.
Retino-geniculo-cortical projections from the remaining eye in rats monocularly enucleated at the beginning of the critical period and perfused at adulthood. Seven cases shown: (a)(d)(e)(f)(g)(h)(i). The thin line in (a) illustrates the region used for calculating the patch index and the %V1 occupied by ipsilateral eye projections. (b) White dots indicate myelin pattern used to delineate the V1 border in (a). (c) Distribution of WGA-HRP labeling in V1 contralateral to the injection for the case shown in (a),(b). Note that areas of reduced labeling corresponding to ipsilateral projections from the enucleated orbit are not observed. (j) Density scan at the level indicated in (g). (k) Ipsilateral (left) and contralateral (right) labeling in the LGN of a rat monocularly enucleated at P19 and perfused at P46. Arrow indicates dorsomedial region that remains unlabeled after intraocular injections of WGA-HRP into the ipsilateral eye. Note that an area of reduced labeling that likely corresponds to territory innervated by ipsilateral projections from the enucleated orbit is observed in the LGN contralateral to the injection. Scale bars = 1.0 mm. Other conventions as in Figure 1.
Comparing these results with those we obtained from rats monocularly enucleated around P10 and perfused before the activation of the critical period indicates that the susceptibility of rat ODCs to monocular enucleation is higher during the precritical period than during the critical period.
3.3.2. Effects of monocular deprivation by eyelid suture during the critical period
3.3.2.1. Electrophysiological recordings
In a separate group of monocularly deprived rats, we recorded visually evoked responses in the hemisphere contralateral to the eye sutured shut to assess the physiological effects of monocular deprivation (see Materials and Methods). We compared these data to data from normal adult Long Evans rats obtained similarly (Andelin et al., 2020). To determine the ocular preference of evoked responses at each recording site, a contralateral bias index (CBI) was calculated from the counts of action potentials recorded for each trial. This index, defined as CBI = (Rcontra −Ripsi) / (Rcontra + Ripsi), ranges from −1 (purely ipsilateral response) to +1 (purely contralateral response). Values ~ 0 indicate balanced binocular responses (see Materials and Methods).
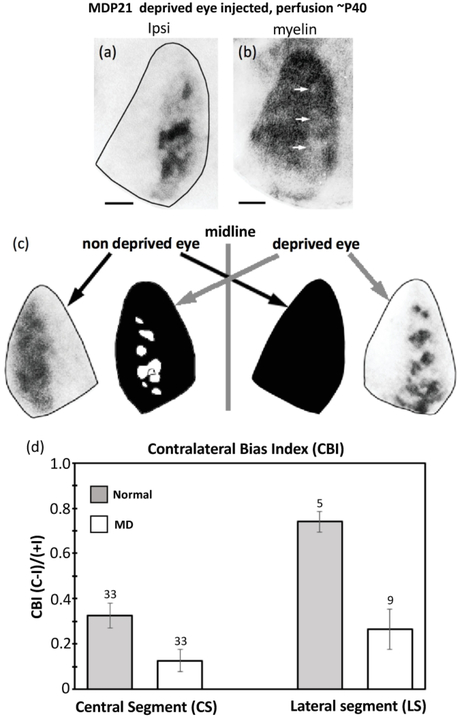
Consistent with previous studies (Fagiolini et al., 1994), the contralateral eye exerts some dominance in the CS of normal rats (CBI = 0.33, SEM = 0.053, n = 33), whereas in the CS of the hemisphere ipsilateral to the non-deprived eye, the responses became more binocular (CBI = 0.126, SEM = 0.049, n = 33) indicating a shift in ocular dominance preference towards ipsilateral eye responses (see histogram in figure below). This shift towards the ipsilateral eye is significant (p = 0.008), and in agreement with previous studies of the effect of monocular deprivation during the critical period in rats (Fagiolini et al., 1994), and mice (Gordon & Stryker, 1996). These results confirm that our procedures to induce monocular deprivation for an extend period of time were successful. In the LS of normal adult rats, responses were strongly dominated by the contralateral eye (CBI =0.74, SEM = 0.045, n = 5), in agreement with previous reports indicating that the LS in normal Long Evans rats is strongly dominated by the contralateral eye (Laing et al. 2015; Andelin et al., 2020). However, contrary to our expectations, in the LS ipsilateral to the non-deprived eye, we observed a significant (p = 0.0027) shift towards ipsilateral eye responses (CBI= 0.265, SEM = 0.089, n = 9) (see histogram in figure below). In the Discussion, we propose a possible mechanism involving callosal connections in this ipsilateral eye shift in the LS ipsilateral to the non-deprived eye, and analyze its potential implications.
3.3.2.2. Injection of WGA-HRP into the non-deprived eye
We next studied the effects of monocular deprivation by eyelid suture on the pattern of ODCs to determine whether ODCs in the rat are susceptible to imbalance of visual input between the eyes during the critical period, as in other species (Hubel et al., 1970; Shatz and Stryker, 1978; LeVay et al., 1980; Horton and Hocking, 1997a). The eyes to be deprived were sutured at P20-P22, and the rats were perfused at least 3 weeks later. The lids were inspected daily to ensure that windows were not present. In one group of rats we injected WGA-HRP into the non-deprived eye, while in another group we injected the deprived eye. Both hemispheres were analyzed in each group.
We first examined the labeling patterns in the hemisphere ipsilateral to the non-deprived eye (Figure 5) and compared them to the patterns we observed following monocular enucleation at the beginning of the critical period (Figure 4). We wanted to determine whether ODCs are less susceptible to monocular deprivation than to monocular enucleation during the critical period, as shown in monkeys (Horton & Hocking, 1998).
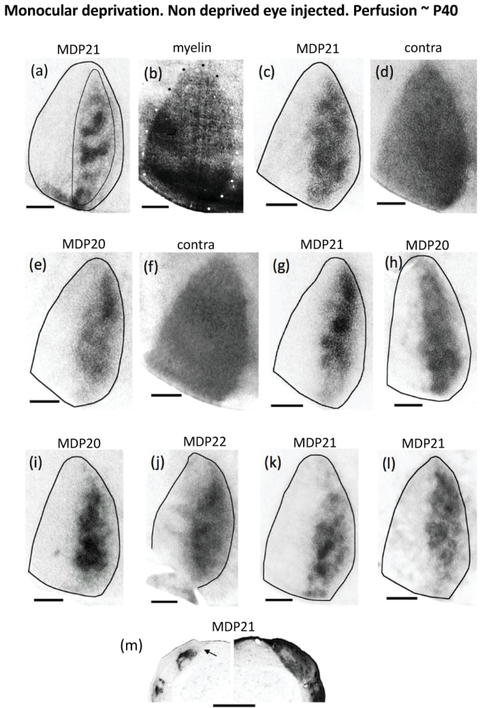
Figure 5.
Retino-geniculo-cortical projections from the non-deprived eye in rats monocularly deprived at the beginning of the critical period (P20-P22) and perfused at adulthood. Nine cases shown: (a)(c)(e)(g)(h)(i)(j)(k)(l). The thin line in (a) illustrates the region used for calculating the patch index and the %V1 occupied by ipsilateral eye projections. (b) White and black dots indicate myelin pattern used to delineate the V1 border in (a). (d),(f) Distribution of WGA-HRP labeling in V1 contralateral to the injection for the cases shown in (c) and (e), respectively. Note that areas of reduced labeling corresponding to ipsilateral projections from the deprived eye are not observed. (m) Ipsilateral (left) and contralateral (right) labeling in the LGN of a rat monocularly deprived at P21 and perfused at adulthood. Arrow indicates dorsomedial region that remains unlabeled after intraocular injections of WGA-HRP into the ipsilateral eye. Note that an area of reduced labeling that likely corresponds to territory innervated by ipsilateral projections from the deprived eye is observed in the LGN contralateral to the injection. Scale bars = 1.0 mm. Other conventions as in Figure 1.
Figure 5 shows the labeling pattern from 9 cases. The border of V1 was drawn based on the myelin pattern, as illustrated in Figure 5b. Except for the case in Figure 5a, which shows well defined ipsilateral eye bands, the labeling patterns in the other 8 cases resemble the pattern observed following monocular enucleation at the beginning of the critical period (cf. Figure 4). The labeling patterns appear as areas of dense labeling immersed in large areas (often encompassing the entire CS) of less dense labeling, where segregation into distinct, isolated ODCs is not observed. Consistent with this, the fraction of the area of V1occupied by the labeled area (28.51%, SEM = 1.82, n = 9) is significantly larger (p < 0.05) than the area of V1 occupied by ipsilateral projections in normal adult rats (18.96, SEM = 1.95, n = 4), whereas the patch index (45.56, SEM = 1.57, n = 9) is significantly smaller than that in normal adult rats (64.51, SEM = 0.97, n = 5). The labeled region ipsilateral to the non-deprived eye does not expand into the MS, and laterally, a strip of cortex with little or no labeling corresponding to LS can be observed in all cases. Comparison of the fraction of the area of V1 occupied by the labeled area (28.51% SEM = 1.82, n = 9), and the patch index (45.56, SEM = 1.57, n = 9) to corresponding values in rats monocularly enucleated at the beginning of the critical period (33.42 %, SEM = 2.87; 42.14, SEM = 2.52, respectively) suggests that the ipsilateral projection from the non-deprived eye to V1 is more segregated than the ipsilateral projection from the remaining eye in rats monocularly enucleated at the onset of the critical period. However, the differences in the corresponding values do not reach statistical significance (p < 0.05). Thus, our results suggest that ODCs in rats are similarly susceptible to both monocular enucleation and monocular deprivation during the critical period.
Contralaterally, the labeling was homogeneously distributed throughout V1, including the LS (illustrated in Figure 5d, same case as in Figure 5c, and Figure 5f, same case as in Figure 5e). The pattern of contralateral labeling was carefully examined in all cases (n = 9). Restricted regions of reduced or no labeling corresponding to territory innervated by the deprived eye were not observed in any of the cases analyzed.
3.3.2.3. Injection of WGA-HRP into the deprived eye
We analyzed the pattern in 10 rats that received an injection of WGA-HRP into the deprived eye (7 cases are shown in Figure 6, and an additional case is shown in Figure 7a). Comparison of these labeling patterns with those following injections in the non-deprived eye (Figure 5) reveals clear differences in the hemispheres ipsilateral to the injections. Unlike the relative widespread distribution of labeling in the CS in V1 ipsilateral to the non-deprived eye (Figure 5), in V1 ipsilateral to the injection into the deprived eye (Figure 6), the labeling accumulates in discrete patches separated by areas where the labeling is significantly reduced or at background levels (Figure 6a,e,h,k,i,m,n). As is clearly visible in all cases, the labeling does not extend into the MS and the LS. Both the fraction of V1 occupied by the labeling (20.96%, SEM = 2.01, n = 10) and the patch index (60.16, SEM = 1.46, n = 10) are significantly different (p < 0.05) from the corresponding values in the hemisphere ipsilateral to the non-deprived eye (28.51%, SEM = 1.82, n = 9; 45.56, SEM = 1.57, n = 9, respectively) (Figure 8a,b). Overall, the patterns in the hemisphere ipsilateral to the deprived eye (Figure 6) closely resemble those observed in normal adult rats and normal young rats (cf. Figure 1). This is also reflected in the fact that both the fraction of V1 occupied by the labeled area, and the patch index are not significantly different (p > 0.05) from the corresponding values calculated in normal adults (18.96 %, SEM = 1.95, n = 4; 64.51, SEM = 0.97, n = 5) (Figure 8). The density scan in Figure 6o (taken at the level indicated in Figure 6m) illustrates that the definition of the patches in the hemisphere ipsilateral resembles the definition of patches in normal young rats (cf. Figure 1g). Finally, examining sections through layer IV scanned to reveal the V1 myelin pattern in the hemisphere ipsilateral to the deprived eye, in one case we observed areas of reduced density (arrows in Figure 7b) that appear to correspond to the pattern of ipsilateral ODCs in the same case (Figure 7a). This observation compares to that by Horton and Hockey (1997b), who reported that patterns of light columns seen in unstained section through V1 in monocularly enucleated macaque monkeys matched columns serving the missing eye after cytochrome oxidase staining.
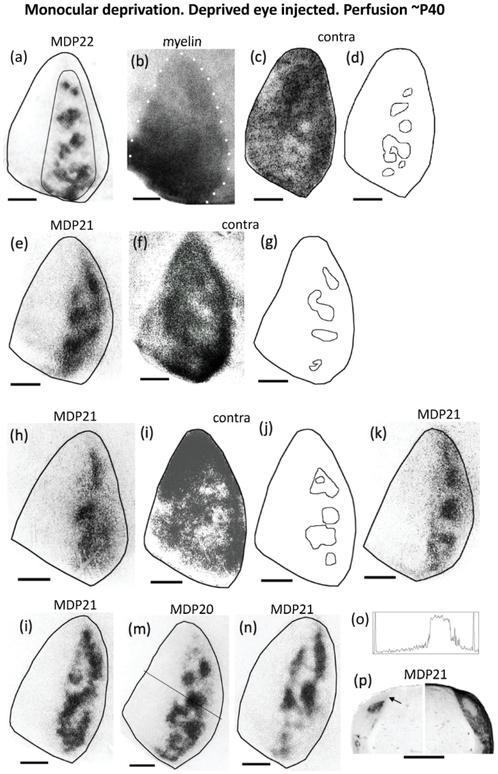
Figure 6.
Retino-geniculo-cortical projections from the deprived eye in rats monocularly deprived at the beginning of the critical period (P20-P22) and perfused at adulthood. Seven cases shown: (a)(e)(h)(k)(l)(m)(n). The thin line in (a) illustrates the region used for calculating the patch index and the %V1 occupied by ipsilateral eye projections. (b) White dots indicate myelin pattern used to delineate the V1 border in (a). (c),(f),(i) Distribution of WGA-HRP labeling in V1 contralateral to the injection for the cases shown in (a),(e),(h), respectively. Note the areas of reduced or absent labeling likely corresponding to territory serving ipsilateral projections from the non-deprived eye. Respective outlines of these areas are shown in (d),(g),(j). (o) Density scan at the level indicated in (m). The drop in labeling density is abrupt on both the medial and lateral sides, as it occurs in normal young rats (cf. Figure 1g). (p) Ipsilateral (left) and contralateral (right) labeling in the LGN of a rat monocularly deprived at P21 and perfused at adulthood. Arrow indicates dorsomedial region that remains unlabeled after tracer injections into the ipsilateral eye. In the LGN contralateral to the injection, note the area of reduced labeling corresponding to territory innervated by ipsilateral projections from the non-deprived eye. Scale bars = 1.0 mm. Other conventions as in Figure 1.
Figure 7.
(a) Ocular dominance columns in the hemisphere ipsilateral to the deprived eye appear to correlate with areas of reduced myelin density [arrows in (b)] in some monocularly deprived rats. Scale bars = 1.0 mm. (c) Schematic diagram of the relationship between projections from the deprived and non-deprived eye in V1 of rats monocularly deprived at the beginning of the critical period and perfused at adulthood. Contralateral projections are represented in black. Note that the contralateral projections from the non-deprived eye are distributed throughout V1, invading and intermixing with the ipsilateral ODCs serving the deprived eye. In contrast, little or no intermixing of contralateral and ipsilateral projections occurs in the hemisphere ipsilateral to the non-deprived eye. (d) Quantitative analysis of binocularity in central and lateral segments of V1 in Long Evans and in V1 ipsilateral to the non-deprived eye in monocularly deprived rats. In the central segment, the CBI shifts from moderate dominance by the contralateral eye in normal rats (CBI = 0.33, n = 33 recording sites) to more binocular responses in monocularly enucleated rats (CBI = 0.12, n = 33 recording sites). In the lateral segment, there is a sizable shift from strong dominance by the contralateral eye in normal rats (CBI = 0.74, n = 5 recording sites) to more binocular responses in monocularly enucleated rats (CBI = 0.26, n = 9 recording sites). Number over the bars indicate recording sites. Error bars indicate SEM.
Figure 8.
Patch index and %V1 occupied by ipsilateral projections from the injected eye. (a) Comparison of the patch indices for groups studied during the precritical and critical periods. (b) Comparison of the %V1 occupied by the ipsilateral projections from the injected eye for groups studied during the precritical and critical periods. ME: monocularly enucleated; MD: monocularly deprived by eyelid suture. Numbers above the bars indicate number of cases in each group of animals. Error bars indicate SEM.
In the hemisphere contralateral to the deprived eye, the labeling was reduced or absent in several patches located in a region corresponding to the CS. These regions were observed in 4 cases, three of which are illustrated in Figure 6c,f,i. Outlines of these regions are shown next to the patterns (Figure 6d,g,j, respectively). In normal adult rats, similar regions with little or no labeling in the hemisphere contralateral to the WGA-HRP injection correspond to territories innervated by the ipsilateral eye (Laing et al., 2015), so it is likely that the regions illustrated in Figure 6 correspond, at least partially, to territory innervated by ipsilateral projections from the non-deprived eye. After careful search for these areas in all our material, with the exception of normal animals, we only observed such regions in the hemisphere contralateral to the deprived eye.
In summary, ipsilateral projections from the deprived eye form ODCs that closely resemble those in normal rats, whereas ipsilateral projections from the non-deprived eye expand and accumulate into areas of dense labeling immersed in large areas of less dense labeling, so that segregation into distinct, isolated ODCs is not observed. Contralateral projections from the non-deprived eye expand and occupy the entire V1, invading and intermixing with the ODCs serving the deprived eye. In contrast, in many cases, contralateral projections from the deprived eye spare, at least partially, the territory innervated by ipsilateral projections from the non-deprived eye. These relationships between the bilateral projections from the deprived and non-deprived eye are represented schematically in Figure 7c. Compared to normal adult rats, our results indicate that both the ipsilateral and contralateral projections from the non-deprived eye expand. On the other hand, the ipsilateral projection from the deprived eye does not appear to shrink, whereas changes in the contralateral projection, if they exist, are probably small.
3.3.2.4. Retino-geniculate and retino-geniculo-cortical projections following monocular enucleation and monocular deprivation during the critical period
Following monocular enucleation or monocular deprivation at the beginning of the critical period, the size and location of the ipsilateral retino-geniculate projection from the remaining eye, or from either the non-deprived or deprived eye (Figures 4k, 5m, 6p, respectively) roughly resemble the size and location of the corresponding ipsilateral retino-geniculate projection in normal adult rats (cf. Figure 1i). Of note is that the dorsomedial LGN region remains unlabeled in these three groups (arrows in Figures 4k, 5m, 6p), consistent with the fact that a lateral cortical strip in V1 corresponding to the LS is largely devoid of ipsilateral eye labeling in all cases in these three groups (Figures 4,5,6). In these three experimental groups, the contralateral retino-geniculate projection does not invade ipsilateral eye territories (Figures 4k, 5m, 6p). Interestingly however, the contralateral retino-geniculo-cortical projections from either the remaining eye (Figure 4c) or from the non-deprived eye (Figure 5d,f) do invade ipsilateral eye territories in V1. In these cases, as pointed out above for rats monocularly enucleated at P10 (Figure 3), it appears that the retino-geniculate projections are less plastic than the geniculo-cortical projections.
3.4. 3D surface plots of labeling density distribution in V1
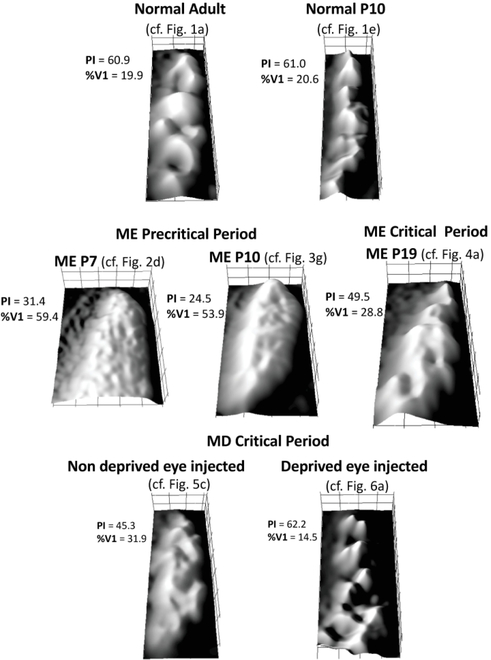
An overall view of the different patterns of WGA-HRP labeling in V1 in the various experimental groups in this study is presented in Figure 9. This Figure shows 3D surface plots of the labeling density distribution of ipsilateral projections from the injected eye in a representative case from each group. For each panel, the location of the original figure is indicated, as well as the values of patch index and %V1 occupied by the ipsilateral projections from the eye injected with WGA-HRP.
Figure 9.
3D surface plots of the labeling density distribution of ipsilateral projections from the injected eye to V1 in a representative case from each experimental group in this study. ME: monocularly enucleated; MD: monocularly deprived by eyelid suture. For each plot, the location of the original case is indicated, as well as the values for patch index and %V1 occupied by ipsilateral eye projections.
3.5. Development and plasticity of callosal patches in V1
3.5.1. Callosal patches are visible by postnatal day10 in the central segment of V1.
Callosal patterns in V1 of one hemisphere were revealed following multiple intracortical injections of HRP into occipital cortex of the opposite hemisphere. Previous studies at both light and electron microscopic levels have demonstrated that HRP is transported both anterogradely and retrogradely (Trojanowski, Gonatas, & Gonatas, 1981). Uptake and transport to the tracer led to extensive labeling of ipsilateral visual thalamic nuclei and superior colliculus (Olavarria and Van Sluyters, 1985). The tangential pattern of callosal connections in V1 in three normal adult rats is shown in Figure 10a,b,c. The pattern is characterized by a band of callosal connections, 0.2–0.3 mm wide, occupying the lateral segment (LS) of V1, and a group of densely labeled patches occupying the central segment (CS), outlined by a thin line in Figure 10a. These patches vary in size and number. In some cases, they are arranged in a single anteroposterior row (e.g., Figure 10b), or in parallel rows (e.g., Figure 10a). In both the LS and CS, callosal cells and terminations are densely aggregated in layers II-III, Va and Vc-VIa, and less densely in layer IV and the remaining portions of layers V and VI (Olavarria & Van Sluyters, 1985). Further medially, in the monocular segment (MS), no or few callosal connections are observed, except in layers Vc-VIa (Olavarria & Van Sluyters, 1983). This laminar distribution of callosal connections is also present in V1of adult rats enucleated early in life (Olavarria et al., 1987). In all cases in this study, we analyzed the patterns of callosal connections in tangential sections taken at levels above layers Vc-VIa, therefore avoiding the widespread distribution of callosal connections in these layers. The callosal patterns were similar in sections taken through layers II-III and Va.
Figure 10.
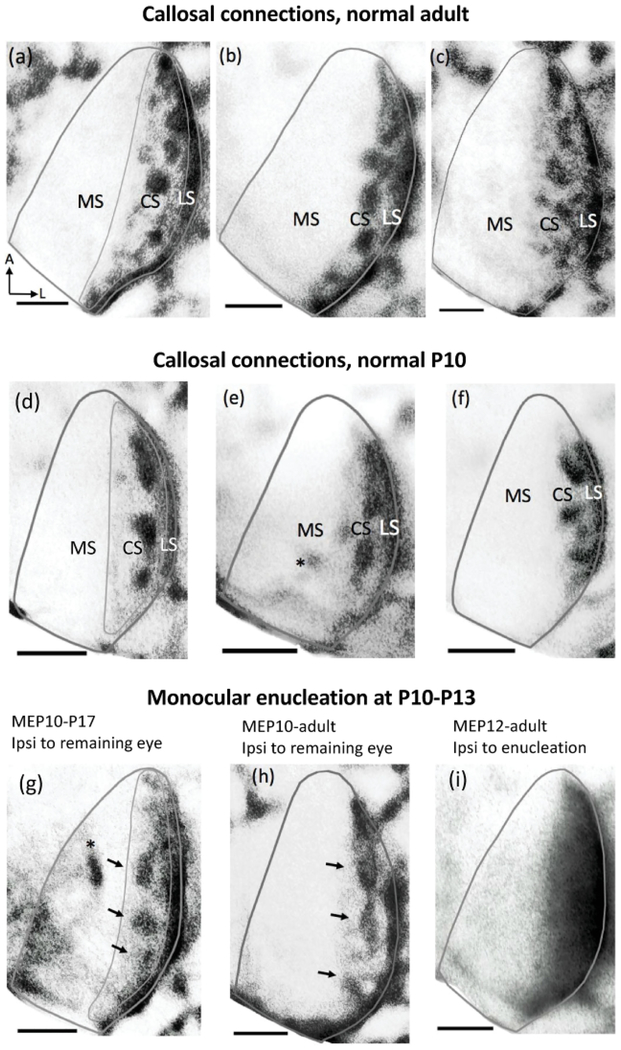
Pattern of callosal patches in V1 of normal adult, normal P10 and in rats monocularly enucleated at P10-P13. The callosal pattern was revealed following intracortical Injections of horseradish peroxidase (HRP) in the opposite hemisphere. The thin lines in (a),(d),(g) illustrate the region used for analysis. This region does not include the band of callosal labeling at the 17/18a border. (a),(b),(c) Callosal patterns in the right hemisphere of normal adult rats. Bottom, left arrows in (a) indicate anterior (A) and lateral (L). Note the distinctly labeled patches in the CS of V1 and the densely labeled band in the LS. (d),(e),(f) Callosal patterns in V1 of rats perfused at P10. Note that both the callosal patches in the CS and the band of callosal connections in the LS are visible at this age. Asterisk in (e) indicates artifact. (g),(h) Callosal patterns in the hemisphere ipsilateral to the remaining eye. Arrows indicate callosal patches. Asterisk in (g) indicates artifact. (i) Dense HRP-labeling is widely distributed over the lateral half of V1 in the hemisphere ipsilateral to the enucleation. Scale bars = 1.0 mm. Other conventions as in Figures 1.
Similar to normal adult rats (n = 5) (Figure 10a,b,c), in normal pups perfused at P10 (n = 7), callosal connections accumulate forming several patches arranged within a region corresponding to the CS (outlined in Figure 10d), and are readily differentiated from a dense band of callosal connections located more laterally in a region corresponding to the LS (Figure 10d,e,f). These observations are consistent with reports that the pattern of callosal connections revealed with HRP in rat V1 resembles the mature pattern by the middle of the second week of life (Olavarria and Van Sluyters, 1985).
3.5.2. Effects of monocular enucleation at P10 on callosal patches
In rats monocularly enucleated at P10-P13 (n = 4) and perfused either at P17 (Figure 10g), or as adult (Figure 10h), callosal patches were present in a region corresponding to the CS in the hemisphere ipsilateral to the remaining eye (arrows in Figure 10g,h). Further laterally, a densely labeled callosal band was observed in a region corresponding to the LS. However, callosal patches were not observed in the hemisphere ipsilateral to the enucleated orbit in rats monocularly enucleated at P10-P12 (Figure 10i, n = 3), indicating that following monocular enucleation after callosal patches have formed, these are maintained in the hemisphere ipsilateral to the remaining eye, but desegregate in the hemisphere ipsilateral to the enucleated orbit.
Thus, following monocular enucleation at around P10, ipsilateral ODCs desegregate in the hemisphere ipsilateral to the remaining eye (Figure 3), whereas callosal patches remain in this hemisphere (Figure 10g, h). These results indicate that the spatial correspondence between ipsilateral ODCs and callosal patches present in normal rats is severely altered in rats monocularly enucleated around P10.
3.5.3. Effect of monocular enucleation at the beginning of the critical period
We next examined the patterns of callosal connections in rats monocularly enucleated close to the onset of the critical period and perfused at adulthood. Figure 11a,b,c shows the patterns of callosal connections in the hemisphere ipsilateral to the remaining eye in three rats monocularly enucleated at P19-P21 and perfused at ~P40. Similar to our results from rats enucleated at P10-P13 (Figure 10g,h), callosal patches were present in a region corresponding to the CS (outlined in Figure 11a) in the hemisphere ipsilateral to the remaining eye, and a densely labeled callosal band was located in a region corresponding to the LS. Figure 11d illustrates the myelin pattern used to trace the V1 border for the case in Figure 11c. In contrast, in the hemisphere ipsilateral to the enucleated orbit, callosal patches were not observed. Instead, fine and coarse reaction product was distributed homogeneously over the lateral half of V1, giving it a granular appearance (Figure 11e,f,g). Observation at higher magnification reveals that the granules correspond to retrogradely labeled cells (Figure 11h, data from case 11f). It is possible that the granular appearance at low magnification reflects the decrease in the density of labeled cells, which, due to the desegregation of callosal patches, are now distributed over the entire CS.
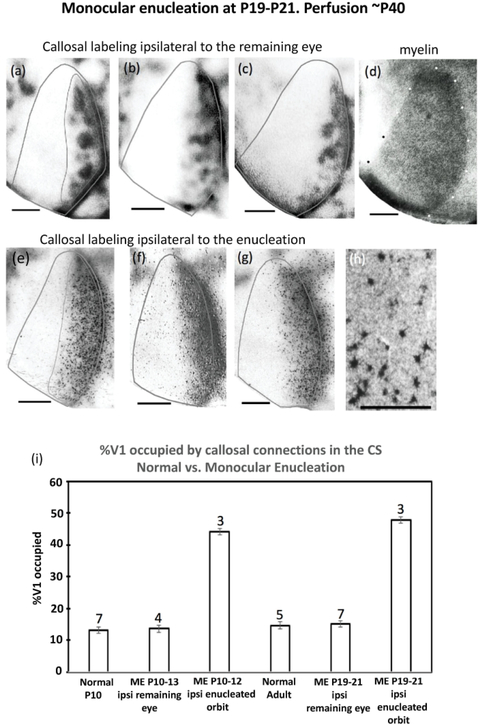
Figure 11.
Effect on the callosal pattern of monocular enucleation at P19-P21 and perfusion at adulthood. (a),(b),(c) Labeling ipsilateral to the remaining eye. Note the callosal patches in the region corresponding to the CS, and the densely labeled band in the region corresponding to the LS. (d) V1 myelin pattern (white dots) for case in (c). (e),(f),(g) HRP labeling ipsilateral to the enucleated orbit. Note that no callosal patches are observed. h) High magnification view of retrogradely labeled callosal cells from case in (f). The thin lines in (a),(e) illustrate the region used for analysis. This area does not include the band of callosal labeling at the 17/18a border. (i) Comparison of the %V1 occupied by callosal connections in the central segment of either the hemisphere ipsilateral to the remaining eye, or ipsilateral to the enucleated orbit following monocular enucleation at P10–13 or P19-P21. ME = monocular enucleation. Numbers above the bars indicate number of cases in each group of animals. Error bars indicate SEM. Scale bar in (h) = 100 um, other scale bars = 1.0 mm.
Figure 11i compares the % of V1 occupied by callosal connections in the CS in normal P10 (n = 7) and adult rats (n = 5) to that in rats monocularly enucleated either at P10-P13 or P19-P21. We found that the % of V1 occupied by callosal patches in the hemisphere ipsilateral to the remaining eye in rats monocularly enucleated at P10-P13 (13.54%, SEM = 0.57, n = 4), and in rats monocularly enucleated at P19–21 (15.02%, SEM= 0.91, n = 7) was not significantly different (p > 0.05) from the % of V1 occupied by callosal patches in normal P10 (13.18, SEM 0.57, n = 7) and normal adult (14.67, SEM = 1.15, n = 5) rats. However, because of the desegregation of callosal patches, the % of V1 occupied by callosal connections in the CS increased significantly compared to controls (p << 0.05) in the hemisphere ipsilateral to the enucleated orbit in rats enucleated at P10-P12 (44.14, SEM = 2.19, n = 3), and at P19-P21 (47.86, SEM = 1.89, n = 3).
3.5.4. Effect of monocular eyelid suture at the beginning of the critical period
Our analysis of ODCs showed that monocular enucleation and monocular deprivation during the critical period exerted comparable effects on the projections from the remaining eye and from the non-deprived eye, respectively. Indeed, compared to normal adult controls, both projections expanded in ipsilateral V1 (Figure 8, bottom panel), as well as in contralateral V1 (Figure 4c, and 5d,f, respectively). We therefore expected that the effects of monocular deprivation on callosal patches would be similar to those observed following monocular enucleation during the critical period (Figure 11), namely, we expected maintenance of callosal patches in the hemisphere ipsilateral to the non-deprived eye, and desegregation of callosal patches in the hemisphere ipsilateral to the deprived eye. This prediction was only partly correct: we observed callosal patches in both hemispheres. Figure 12a,b,c shows patterns of callosal connections in V1 in the hemisphere ipsilateral to the non-deprived eye, and Figure 12d,e,f shows patterns of callosal connections in V1 in the hemisphere ipsilateral to the deprived eye. On average, the patch indices for the hemisphere ipsilateral to the non-deprived eye (62.58, SEM = 2.42, n = 5), and ipsilateral to the deprived eye (60.55, SEM = 1.29, n = 7) were not significantly different from each other (p > 0.05), nor from the patch index for normal adult rats (61.77, SEM = 2.84, n = 5) (Figure 12g). Similarly, the % of V1 occupied by callosal patches in the CS was somewhat greater in the hemisphere ipsilateral to the non-deprived eye (17.25, SEM= 2.19, n = 5) than in the hemisphere ipsilateral to the deprived eye (14.41, SEM= 1.18, n = 7), but these values were not significantly different (p > 0.05) from each other, nor from that for normal adult rats (14.67, SEM = 1.15, n = 5) (Figure 12h).
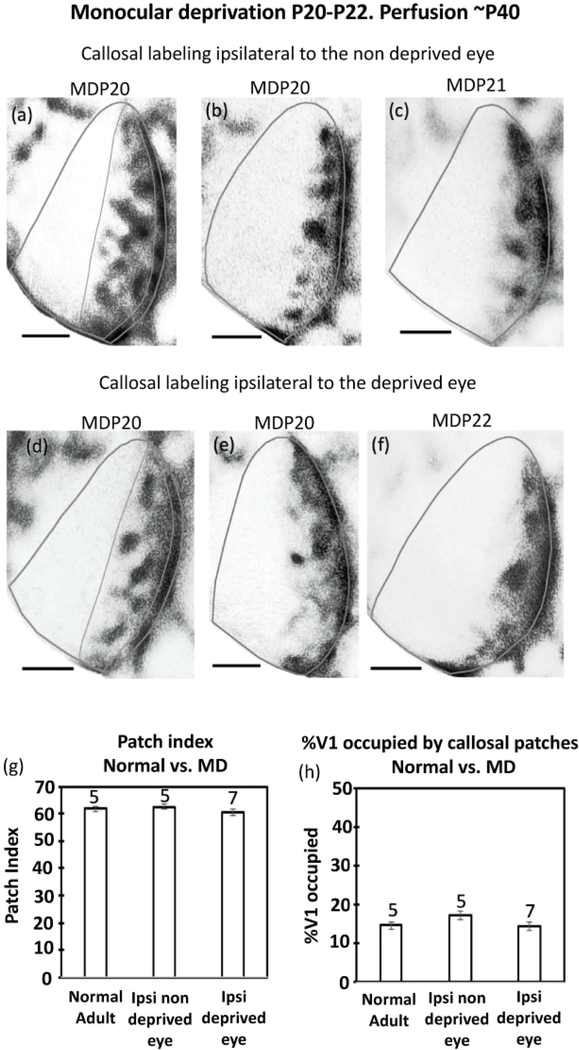
Figure 12.
Effect on the callosal pattern of monocular deprivation at P20-P22 and perfusion at adulthood. (a),(b),(c) Labeling ipsilateral to the non deprived eye. (d),(e),(f) Labeling ipsilateral to the deprived eye. The thin lines in (a),(d) illustrate the region used for analysis. This area does not include the band of callosal labeling at the 17/18a border. (g) Comparison of patch indices in the central segment in normal adult, in the hemisphere ipsilateral to the non deprived eye, and in the hemisphere ipsilateral to the deprived eye. (h) Comparison of the %V1 occupied by callosal patches in normal adult, in the hemisphere ipsilateral to the non deprived eye, and in the hemisphere ipsilateral to the deprived eye. Numbers above the bars indicate number of cases in each group of animals. Error bars indicate SEM. MD = monocular deprivation. Scale bars = 1.0 mm.
In summary, comparing our results from monocularly enucleated and monocularly deprived rats reveals similarities and sharp differences. Callosal patches are maintained in the hemisphere ipsilateral to the remaining aye, and also in the hemisphere ipsilateral to the non deprived eye. In contrast, callosal patches desegregate in the hemisphere contralateral to the remaining eye, but they are maintained in the hemisphere contralateral to the non deprived eye.
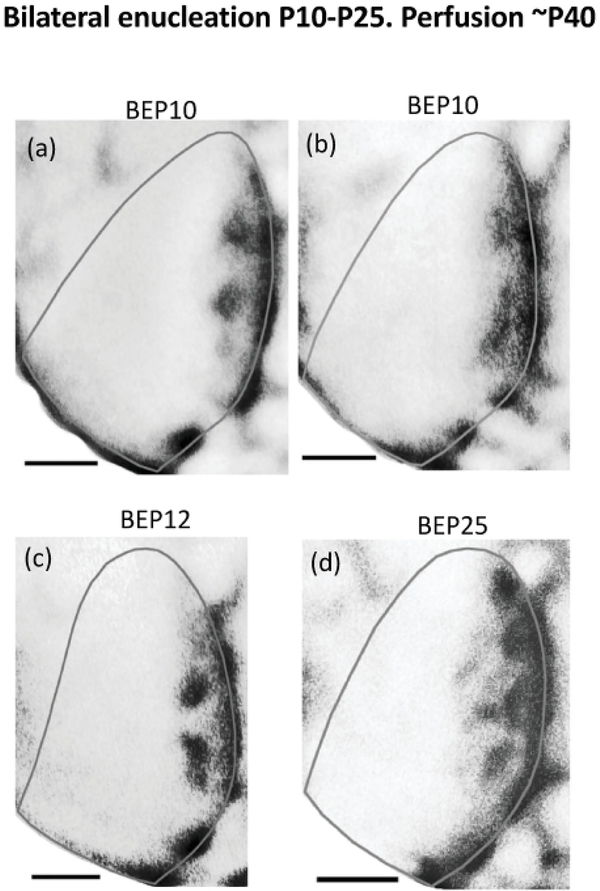
3.5.5. Callosal patches are resistant to binocular enucleation
Callosal patches are in close spatial correlation with ipsilateral ODCs in the CS of normal rats (Laing et al., 2015). We found that callosal patches desegregate in the hemisphere ipsilateral to the enucleated orbit in rats monocularly enucleated at 10-P13 (Figure 10i) or P19–21(Figure 11e,f,g), suggesting that desegregation of callosal patches may be triggered by the removal of ipsilateral eye input. Weakening of ipsilateral eye input by eyelid suture is not sufficient to trigger desegregation of callosal patches (Figure 12d,e,f). We tested the idea that maintenance of callosal patches may require the presence of projections from the ipsilateral eye by examining the effect of bilateral enucleation on the distribution of callosal connections in V1. We found that callosal patches are present following bilateral enucleation at P10 (Figure 13a,b, n = 2), at P12 (Figure 13c, n = 3), and at P25 (Figure 13d, n = 4), near the peak of the critical period (Fagiolini et al., 1994). These results indicate that removal of ipsilateral eye projections is not sufficient to trigger desegregation of callosal patches, and that other factors, discussed below, are probably involved.
Figure 13.
Callosal pattern in the right hemisphere in rats binocularly enucleated at, or after, P10 and perfused at adulthood. Note the distinctly labeled patches in a region corresponding to the central segment of V1, and the densely-labeled band in a region corresponding to the lateral segment. Scale bars = 1 mm.
4. DISCUSSION
4.1. Visual experience is not necessary for ODC development in the rat
We found that ODCs are visible as early as P10. At this age, retino-geniculate projections have largely segregated into ipsilateral and contralateral domains (Manford et al., 1984), and spindle bursts can be evoked in the visual cortex by light stimulation (Hanganu et al., 2006). Rat ODCs emerge a few days before eye opening, indicating that, as in ferrets (Crowley and Katz, 2000) and monkeys (Rakic, 1976; Horton and Hocking, 1996), visual experience is not necessary for ODC development. Based on the distribution of WGA-HRP labeling, we were able to identify in young rats the tripartite subdivision of V1 described in adult Long Evans rats (Laing et al., 2015).
4.2. ODCs in rats are highly plastic during the precritical period
In rats monocularly enucleated at P10 and perfused ~P20, we observed a nearly complete desegregation of ODCs ipsilateral to the remaining eye, and the fraction of V1 occupied by the labeled area increased to more than twice the fraction occupied by the labeled area in normal young and adult rats. These results show that rat ODCs are highly susceptible to imbalances of retinal activity induced by monocular enucleation during the precritical period.
Our results contrast with those of Crowley and Katz (2000), who reported that ODCs are unaffected by monocular enucleation in ferrets perfused before the onset of the critical period. In ferrets, ODCs are apparent by P17 (Crowley and Katz, 2000), and the physiologically defined critical period begins by P33 (Issa et al., 1999). Crowley and Katz (2000) monocularly enucleated ferrets between P7 and P14, at P14–18 they injected the tracer biotinylated dextran amine (BDA) into single geniculate layers, and perfused the animals at P7-P21, before the onset of the critical period. These animals sustained 4 to 14 days of unbalanced retinal activity during the initial establishment of ODCs. They report that the patterns of ODCs, reconstructed from series of coronal sections, were not significantly different from the patterns reconstructed in normal adult ferrets. They concluded that ODCs in the ferret are resistant to experience-dependent competitive interactions during the period preceding the critical period and proposed that the establishment and plasticity of ODCs are temporally and mechanistically distinct phases of visual development. It is unfortunate that they did not allow some enucleated ferrets to survive into the critical period to document the plastic effects of monocular enucleation on the pattern of ODCs during the period of ocular dominance plasticity. To our knowledge, information regarding whether ferret ODCs are susceptible or resistant to monocular enucleation during the critical period is not yet available.
The different outcome of our study may be due to species differences, or differences in the procedures used to reveal and analyze the pattern of ODCs: injections of BDA into geniculate layers and analysis in coronal sections vs. intraocular injections of WGA-HRP and analysis in tangential sections of the flattened cortex. Injections into the LGN layers are not commonly used to reveal ODCs (Shook and Chalupa, 1986), and have the drawback that they restrict the analysis of the ODC pattern to only a small region of V1. Crowley and Katz (2000) chose this approach to avoid the spillover problem presented by the transneuronal tracer tritiated proline, especially in young animals. WGA-HRP does not have a spillover problem and injecting it intraocularly can potentially label the entire geniculo-cortical projection. This method has been widely used in studies of the patterns of ODCs in both young and adult specimens of several species, including the rat (Laing et al., 2015), mouse (Antonini et al., 1999), cat (Anderson et al., 1988), ferret (Ruthazer et al., 1999), and monkey (Spatz 1989; Horton and Hocking, 1996).
4.3. The plastic effect of monocular enucleation on ODCs is smaller during the critical period than during the precritical period
Our observation that ODCs are susceptible to the effects of monocular enucleation during the critical period is consistent with previous studies (Horton and Hocking, 1998). However, we found that ODCs are also susceptible, and to a greater extent, to monocular enucleation during the precritical period. During the precritical period, we observed a nearly complete desegregation of ODCs, and a large expansion of the labeled area in V1, whereas during the critical period, the expansion was smaller and some structure was apparent in the distribution of the labeling, in the form of patches or stripes of higher density labeling (cf, Figures 2,3,4). These differences suggest that the effects of monocular enucleation during the critical period may reflect the waning of the process operating during the precritical period. In the contralateral projection of rats monocularly enucleated in either period, unlabeled regions corresponding to projections from the missing eye were not observed. In contrast, in monocularly enucleated monkeys, even in the cases with the greatest effect, narrow or fragmented areas corresponding to territories innervated by the missing eye remain (Horton and Hocking, 1998).
4.4. Monocular enucleation vs. monocular deprivation
Upon comparing the effects of monocular enucleation and monocular deprivation during the critical period, we found, rather surprisingly, that the patch index and fraction of V1 covered by the labeling were similar for both the ipsilateral projection from the remaining eye and the ipsilateral projection from the non-deprived eye. The contralateral projections were equally similar in that the labeling comprised the entire V1 without areas of reduced labeling corresponding to territories innervated by the missing or the deprived eye. These observations contrast with previous studies in monkey showing that ODCs are more severely affected by monocular enucleation than by eyelid suture (Horton and Hocking, 1998).
4.5. Potential contribution of callosal connections to the effects of monocular deprivation
As expected, in monocularly deprived rats we observed a shift in binocularity towards ipsilateral eye responses in the hemisphere ipsilateral to the non-deprived eye (Fagiolini et al., 1994; Gordon and Stryker, 1996). Surprisingly however, an increase in ipsilateral eye responses was also observed in the LS ipsilateral to the non-deprived eye. In normal rats, the LS is nearly exclusively dominated by the contralateral eye (Laing et al., 2015). Figure 14a illustrates that in normal rats the LS in the left hemisphere (blue strip) cannot receive transcallosal ipsilateral eye input from the retinotopically matched nasal retina (colored yellow) because the nasal retina projects to regions in the contralateral V1 (colored yellow) that are deprived of callosal connections. Indeed, callosal connections are restricted to patches that overlap with ipsilateral ODCs (colored blue) (Laing et al., 2015). How do ipsilateral eye inputs reach the LS ipsilateral to the non-deprived eye? One possibility is that LS receives direct ipsilateral retino-geniculo-cortical projections from the non-deprived eye, but this idea is not supported by our observation that a region corresponding to the LS remains unlabeled following injections of WGA-HRP into the non-deprived eye (Figure 5). Another possibility is suggested by our finding that contralateral retinal projection from the non-deprived eye (yellow in Figure 14b) invade into, and intermix with ipsilateral eye projections (blue) innervating deprived eye ODCs (green = yellow+blue). We propose that this intermixing in the CS enables contralateral projections from the non-deprived eye (yellow in Figure 14b) to return via the callosum as ipsilateral eye input to the LS ipsilateral to the non-deprived eye (green), thereby increasing the binocularity of LS in this hemisphere. As in normal rats, ODCs ipsilateral to the deprived eye (Figure 6) likely overlap with discrete callosal patches (Figure 12d,e,f), and the CS in one hemisphere connects reciprocally with the contralateral LS by means of asymmetric, but topographically corresponding, callosal projections (Lewis and Olavarria, 1995). Although the transcallosal input is depicted as binocular (green) in Figure 14b, it is likely that it is mostly ipsilateral because the input from the deprived eye (blue) is weak due to the deprivation. A similar situation occurs in albino rats (Andelin et al., 2020). Instead of segregating into ODCs, ipsilateral eye projections intermix with contralateral eye projections throughout the CS, which enables callosal projections to relay indirect ipsilateral eye input to the LS (see Figure 5 in Andelin et al., 2020).
Figure 14.
Potential contribution of callosal connections to the effect of monocular deprivation assessed in the hemisphere ipsilateral to the non-deprived eye. In Long Evans rats, callosal connections connect asymmetric, but topographically corresponding, loci in V1, such that opposite central and lateral segments connect reciprocally with each other (Lewis & Olavarria, 1995). (a) In normal Long Evans rats, ODCs and patchy callosal connections prevent the passage of ipsilateral eye input to the lateral segment. The left lateral segment (blue) cannot receive transcallosal input from the left, ipsilateral nasal retina (yellow) because the contralaterally dominated territory in the right central segment (yellow) is deprived of callosal connections (callosal patches overlap with ipsilateral ODCs, colored blue, Laing et al., 2015). (b) In contrast, in the hemisphere ipsilateral to the deprived eye, contralateral projections from the non-deprived eye (yellow) intermix with ODCs (circles) innervated by projections (blue) from the deprived eye (yellow+blue = green). Due to the intermixing, and because ODCs correlate with patches of callosal connections (Laing et al. 2015), the LS in the hemisphere ipsilateral to the non-deprived eye can now receive ipsilateral input from the non-deprived eye (yellow) via the callosum. Although the transcallosal input is depicted as binocular (green), it is likely that it is mostly ipsilateral because the input from the deprived eye (blue) is weak due to the deprivation. Inhibiting callosal communication would reduce ipsilateral eye responses in the hemisphere ipsilateral to the non-deprived eye, thereby reducing the effect of monocular deprivation in that hemisphere (Restani et al., 2009). CS, central segment; LS, lateral segment; MS, medial segment.
Direct evidence that the callosal input contributes to the shift towards the ipsilateral eye in the hemisphere ipsilateral to the non-deprived eye was presented by Restani et al. (2009), who found that inhibiting callosal communication with muscimol reduces ipsilateral eye responses in the hemisphere ipsilateral to the non-deprived eye, thereby reducing the effect of monocular deprivation. Our hypothesis raises the possibility that at least some of the shift towards the ipsilateral eye observed in monocularly deprived rats is due to transcallosal transfer of ipsilateral eye input to the hemisphere ipsilateral to the non-deprived eye. Indeed, because the area size of LS in the hemisphere ipsilateral to the non-deprived eye is at least 25–30% of the area of CS (Figure 5), pooling responses from CS and LS would result in an overall increase of ipsilateral eye responses in V1 of this hemisphere.
Restani et al. (2009) also reported that visual responses in the hemisphere ipsilateral to the deprived eye were strongly dominated by the contralateral, non-deprived eye, and that inhibition of callosal projections with muscimol reduced contralateral eye responses and increased binocularity. It is possible that at least some of this enhanced contralateral eye input is relayed via the callosum from the CS in the hemisphere ipsilateral to the non-deprived eye to the LS in the hemisphere ipsilateral to the deprived eye (this callosal connection is not represented in Figure 14b to keep the figure simple). It is interesting to note that while our results and those of Restani et al. (2009) support the idea that callosal connections contribute to the changes in binocularity observed in MD rats, it is still debated whether the callosum plays an important role in the binocularity of V1 in normal animals. Some studies report that binocularity in V1 does not depend on callosal connections (cat: Minciacchi and Antonini, 1984; Conde-Ocazionez et al., 2018; rat: Laing et al., 2015), whereas other studies conclude the opposite (Restani et al., 2009, reviewed in Olavarria, 2002).
4.6. Comparison with cats
In cats, patchy callosal connections in V1 have been described in studies of the distribution of retrogradely labeled callosal neurons and anterogradely labeled callosal axons (Boyd and Matsubara, 1994; Olavarria, 2001; Houzel et al., 1994; Aggoun-Zouaoui et al., 1996, Zufferey et al., 1999). As in rats, callosal patches in cats overlap with contralateral eye domains in the transition zone (homologous to the LS), and with ipsilateral ODCs in more medial regions of V1 (Olavarria, 2001). Consistent with our finding in rats, the overlap between ipsilateral and contralateral ODCs is greater in monocularly deprived cats than in normal cats (Shatz and Stryker, 1978; Schmidt et al., 2002), suggesting that callosal connections in cats may also contribute to some extent to the effects of monocular deprivation. Moreover, Aggoun-Zouaoui et al. (1996) reported that callosal axon “columns” appear by P14, about the time ODCs are visible in the cat (Crair et al., 2001), at least a week before the onset of the critical period. This provides an opportunity for studying whether the precritical and critical period plasticity of ODCs and associated callosal patches in cats resemble our findings in rats.
4.7. Comparison with mice
In mice, projections from both eyes intermix in the binocular region of V1 without segregating into ODCs (Antonini et al., 1999). Because this intermixing would allow the transcallosal passage of ipsilateral eye input to the LS (Andelin et al., 2020), it is likely that the LS in mice is binocular, whereas the LS in rats is strongly dominated by input from the contralateral eye, (Laing et al, 2015, Andelin et al., 2020). The effects of monocular deprivation in mice and rats seem to differ in some respects. We found that ipsilateral projections from the non-deprived eye expand in V1, whereas ipsilateral projections from the deprived eye segregate into ODCs similar to those in normal rats. In mice, Antonini et al. (1999) reported that after injections of WGA-HRP into the deprived eye, the labeling in the ipsilateral hemisphere was pale and spatially restricted compared with normal animals, whereas after injections into the non-deprived eye, the labeling in the ipsilateral hemisphere was as strong as in normal mice. Drager (1978), using tritiated proline, found that the ipsilateral projections from either the deprived or non-deprived eye showed no obvious abnormality. Additional physiological experiments in mice showed that, although present, ipsilateral projections from the deprived eye become largely nonfunctional (Drager, 1978; Gordon and Stryker, 1996; Antonini et al., 1999). Similarly, in rats, visual responses in V1 ipsilateral to the deprived eye are strongly dominated by the contralateral, non-deprived eye (Restani et al., 2009), suggesting that ipsilateral projections from the deprived eye are severely weakened, even though they segregate into patches that resemble normal ODCs (Figure 6). Finally, it would be of interest to study the effect of visual deprivation on the size and organization of geniculo-cortical axon arbors in rats and compare them with the results of previous studies in mice (Antonini et al., 1999).
4.8. Callosal patches in V1 are less plastic than ODCs
Previous studies have proposed that bilateral projections from the temporal retina specify the close spatial and functional association that exists between ipsilateral eye domains and callosal patches in the CS, and between contralateral eye domains and the densely labeled callosal band in the LS (Olavarria, 1996, 2001, 2002; Laing et al., 2015). Our finding that both ODCs and callosal patches are visible as early as P10 is consistent with this idea. However, in spite of the close correlation between ODCs and callosal patches, we observed that the plasticity of callosal patches follows a trajectory that is strikingly different from that of ODCs. Whereas ODCs ipsilateral to the remaining eye undergo desegregation and expansion in rats monocularly enucleated either at P10 or ~P20, callosal patches persist in the hemisphere ipsilateral to the remaining eye in similarly treated rats. Moreover, monocular eye lid suture during the critical period led to a significant expansion of the ipsilateral projection from the non-deprived eye, but callosal patches ipsilateral to the non-deprived eye were no different than patches in normal adult rats. These results imply that both monocular enucleation and monocular deprivation after ODCs and callosal patches form disrupt the close spatial coupling between ipsilateral ODCs and callosal patches observed in normal rats (Laing et al., 2015). However, callosal patches desegregated in the hemisphere ipsilateral to the enucleated orbit in rats monocularly enucleated either at ~P10 or ~P20, indicating that callosal patches ipsilateral to the enucleated orbit are highly susceptible to the imbalance of retinal input produced by monocular enucleation.
4.9. Callosal patches desegregate in the hemisphere ipsilateral to the enucleated orbit, but not in bilateral enucleated rats
Our finding that callosal patches desegregate in the hemisphere ipsilateral to the enucleated orbit in rats monocularly enucleated either at ~P10 or ~P20 suggested that maintenance of callosal patches requires the presence of ipsilateral eye projections. However, callosal patches were present in rats binocularly enucleated at P10 or later, indicating that maintenance of callosal patches after they form does not require retinal input in these rats. Searching for factors potentially involved in the desegregation of callosal patches ipsilateral to the enucleated orbit, we noted that retinofugal projections from the remaining eye were homogeneously distributed throughout contralateral V1, invading the territories serving the missing ipsilateral eye projections (Figure 3e, 4c), which, at the time of the enucleation correlate with callosal patches. We propose that this contralateral eye invasion of territory occupied by callosal patches destabilizes the callosal patches, leading to their desegregation. The absence of contralateral eye projections in binocularly enucleated rats would explain why callosal patches do not desegregate in these rats. However, in rats monocularly deprived by eyelid suture, the contralateral projection from the non-deprived eye also invades the territories serving the projections from the ipsilateral, deprived, eye (Figure 5d,f), but the callosal patches in these territories do not desegregate (Figure 12,d,e,f). To explain this observation, we further propose that ipsilateral eye projections, even those from a deprived eye, can protect the ipsilateral callosal patches from the destabilizing effect of invasive contralateral eye projections, thus preventing their desegregation. Based on these observations, summarized in Table 1, we propose the following rule: Ipsilateral eye projections are not required for the maintenance of callosal patches, except in the presence of invasive projections from the contralateral eye. This hypothesis predicts that desegregation of callosal patches in rats monocularly enucleated at P10–20 would be prevented by transecting the optic radiations contralateral to the remaining eye. It will be interesting to study the mechanisms by which contralateral eye projections destabilize and desegregate callosal patches, and how ipsilateral eye projections prevent this desegregation.
Table 1.
Maintenance or desegregation of V1 callosal patches
| Invasive projection from contra eye? | Ipsilateral eye projection present? | Maintenance of callosal patches after P10? | |
|---|---|---|---|
| HEMISPHERE | |||
| Normal | NO | YES | YES |
| ME: ipsi to remaining eye | NO | YES | YES |
| ME: ipsi to enucleated orbit | YES | NO | NO: desegregation |
| MD: ipsi to non-deprived eye | NO | YES | YES |
| MD: ipsi to deprived eye | YES | YES | YES |
| Binocular enucleation | NO | NO | YES |
Table 1 Ipsilateral eye projections are not required for the maintenance of callosal patches, except in the presence of invasive projections from the contralateral eye (as those present in the hemisphere ipsilateral to the enucleated orbit in ME rats, and in the hemisphere ipsilateral to the deprived eye in MD rats). ME = monocular enucleation; MD = monocular deprivation.
4.10. Same insults at different developmental periods elicit different plastic responses from visual callosal connections.
Previous studies in rodents have shown that, when performed at birth, enucleation of one or both eyes induce marked anomalies in the tangential distribution of callosal connections within V1 (Cusick & Lund, 1982; Olavarria et al., 1987; see review by Restani & Caleo, 2016). These anomalies are no longer observed in rats enucleated at P6. Instead, callosal connections are distributed homogeneously in the lateral half of V1, without segregating into patches (Olavarria et al., 1987). Adding to these periods of differential plastic expression, we now show that, once formed, callosal patches become resistant to enucleation of one or both eyes, except in the hemisphere ipsilateral to the enucleated orbit in monocularly enucleated rats.
5. CONCLUSION
Our results show that ODCs in Long Evans rats respond to monocular deprivation during the critical period in a manner consistent with previous studies in other species. A novel finding is that ODCs are highly susceptible to monocular enucleation during the precritical period for ocular dominance plasticity, even more so than during the critical period. In sharp contrast, although closely associated with ODCs, callosal patches in V1 are resistant to monocular deprivation and to enucleation of one or both eyes, with the exception that they desegregate in the hemisphere ipsilateral to the enucleated orbit in monocularly enucleated rats. Our findings reveal intriguing plastic interactions between eye-specific cortical projections and callosal connections, some of which raise the possibility that callosal connections contribute to the effect of monocular deprivation assessed in the hemisphere ipsilateral to the non-deprived eye, and that ipsilateral projections from the deprived eye are capable of preventing the desegregation of callosal patches. It will be of interest to study ODCs and callosal patch interactions in other species and explore their potential clinical implications. As knowledge of the rat genome progresses and the arsenal of genetic tools increases, rat ocular dominance columns and callosal patches will offer valuable models for studies of the anatomical, physiological and molecular bases of plasticity in the mammalian visual system.
ACKNOWLEDGMENTS
This work was supported in part by a Royalty Research Fund award, University of Washington to JFO, and by National Institutes of Health grant R01NSO70022. AKA & RJL were supported by National Institute of Health Vision Training Grant T32 EY7031. We thank Misha Hashemi, Baihan Lin, Zane Doyle, CL Moss and HC Lu for participating in some experiments.
Footnotes
CONFLICT OF INTEREST
Authors declare no conflict of interest
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aggoun-Zouaoui D, Kiper DC, & Innocenti GM (1996). Growth of callosal terminal arbors in primary visual areas of the cat. European Journal of Neuroscience, 8(6), 1132–1148. [DOI] [PubMed] [Google Scholar]
- Andelin AK, Doyle Z, Laing RJ, Turecek J, Lin B, & Olavarria JF (2020). Influence of ocular dominance columns and patchy callosal connections on binocularity in lateral striate cortex: Long Evans vs. Albino Rats. The Journal of Comparative Neurology, 528(4), 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PA, Olavarria J, & Van Sluyters RC (1988). The overall pattern of ocular dominance bands in cat visual cortex. The Journal of Neuroscience, 8(6), 2183–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, & Stryker MP (1999). Anatomical correlates of functional plasticity in mouse visual cortex. The Journal of Neuroscience, 19(11), 4388–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J, & Matsubara J (1994). Tangential organization of callosal connectivity in the cat’s visual cortex. The Journal of Comparative Neurology, 347(2), 197–210. [DOI] [PubMed] [Google Scholar]
- Conde-Ocazionez SA, Jungen C, Wunderle T, Eriksson D, Neuenschwander S, & Schmidt KE (2018). Callosal influence on visual receptive fields has an ocular, an orientation-and direction bias. Frontiers in System Neuroscience, 12(11), 1–13. 10.3389/fnsys.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair CM, Horton JC, Antonini A, & Stryker MP (2001). Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age. The Journal of Comparative Neurology, 430 (2), 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JC, & Katz LC (2000). Early development of ocular dominance columns. Science, 290 (5495), 1321–1324. [DOI] [PubMed] [Google Scholar]
- Cusick CG, & Lund RD, (1982) Modification of visual callosal projections in rats. The Journal of Comparative Neurology, 212(4), 385–398. [DOI] [PubMed] [Google Scholar]
- Drager UC (1978). Observations on monocular deprivation in mice. Journal of Neurophysiology, 41(1), 28–42. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domeneci L, & Maffei L (1994). Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Visual Research 34(6), 709–720. [DOI] [PubMed] [Google Scholar]
- Finney EM, & Shatz CJ (1998). Establishment of patterned thalamocortical connections does not require nitric oxide synthase. The journals of Neuroscience, 18(21), 8826–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, & Stryker MP (1996). Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. The Journal of Neuroscience, 16(10), 3274–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, & Khazipov R (2006). Retinal waves trigger spindle bursts in the neonatal rat visual cortex. The journals of Neuroscience, 26(25), 6728–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa I, & Kawasaki H (2010). Rearrangement of retinogeniculate projection patterns after eye-specific segregation in mice. PLoS One 5(6):e11001.doi: 10.1371/journal.pone.0011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, & Hocking DR (1996). An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. The Journal of Neuroscience, 16(5), 1791–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, & Hocking DR (1997a). Timing of the critical period for plasticity of ocular dominance columns in macaque striate cortex. The Journal of Neuroscience, 17(10), 3684–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, & Hocking DR (1997b). Myelin patterns in V1 and V2 of normal and monocularly enucleated monkeys. Cerebral Cortex, 7(2)166–177. [DOI] [PubMed] [Google Scholar]
- Horton JC, & Hocking DR (1998). Effect of early monocular enucleation upon ocular dominance columns and cytochrome oxidase activity in monkey and human visual cortex. Visual Neuroscience, 15(2), 289–303. [DOI] [PubMed] [Google Scholar]
- Houzel J-C, Milleret C, & Innocenti G (1994) Morphology of Callosal Axons Interconnecting Areas 17 and 18 of the Cat. European Journal of Neuroscience, 6(6), 898–917. [DOI] [PubMed] [Google Scholar]
- Hubel DH, & Wiesel TN, (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology, 206(2), 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, & Stryker MP (1999). The critical period for ocular dominance plasticity in the ferret’s visual cortex. The Journal of Neuroscience, 19(16), 6965–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya SK, & van Hoesen GW (1982). WGA-HRP as a transneuronal marker in the visual pathways of monkey and rat. Brain Research, 236(1), 199–204. [DOI] [PubMed] [Google Scholar]
- Kageyama GH, Gallivan ME, Gallardo KA, & Robertson RT (1990). Relationships between patterns of acetylcholinesterase activity and geniculocortical terminal fields in developing and mature rat visual cortex. Developmental Brain Research, 53(1), 139–144. [DOI] [PubMed] [Google Scholar]
- Kageyama HT, & Robertson RT (1993). Development of geniculocortical projections to visual cortex in rat: evidence early ingrowth and synaptogenesis. The The Journal of Comparative Neurology, 335(1), 123–148. [DOI] [PubMed] [Google Scholar]
- Laing RJ, Bock AS, Lasiene J, & Olavarria JF (2012). Role of retinal input on the development of striate–extrastriate patterns of connections in the rat. The Journal of Comparative Neurology, 520(1), 3256–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing RJ, Turecek J, Takahata T, & Olavarria JF (2015). Identification of eye-specific domains and their relation to callosal connections in primary visual cortex of Long Evans rats. Cerebral Cortex, 25(10), 3314–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, & Shatz CJ (1978) Ocular dominance columns and their development in layer IV of the cat’s visual cortex: a quantitative study. The Journal of Comparative Neurology, 179(1), 223–244. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, & Hubel DH (1980). The development of ocular dominance columns in normal and visually deprived monkeys. The Journal of Comparative Neurology, 191(1), 1–51. [DOI] [PubMed] [Google Scholar]
- Lewis JW, & Olavarria JF (1995). Two rules for callosal connectivity in striate cortex of the rat. The Journal of Comparative Neurology, 361(1), 119–137. [DOI] [PubMed] [Google Scholar]
- Manford M, Campbell G, & Lieberman AR (1984). Postnatal development of ipsilateral retino-geniculate projections in normal albino rats and the effects of removal of one eye 1087 at birth. Anatomy and Embriology, 170(1), 71–78. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1978). Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: A non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. Journal of Histochemistry and Cytochemistry, 26(2), 106–117. [DOI] [PubMed] [Google Scholar]
- Minciacchi D, & Antonini A (1984). Binocularity in the visual cortex of the adult cat does not depend on the integrity of the corpus callosum. Behavioral Brain Research, 13(2), 183–192. [DOI] [PubMed] [Google Scholar]
- Olavarria JF (1996). Non-mirror symmetric patterns of callosal linkages in areas 17 and 18 in cat visual cortex. The Journal of Comparative Neurology, 366(4), 643–655. [DOI] [PubMed] [Google Scholar]
- Olavarria JF (2001). Callosal connections correlate preferentially with ipsilateral cortical domains in cat areas 17 and 18, and with contralateral domains in the 17/18 transition zone. The Journal of Comparative Neurology, 433(4), 441–457. [DOI] [PubMed] [Google Scholar]
- Olavarria JF (2002). Influence of topography and ocular dominance on the functional organization of callosal connections in cat striate cortex. In Payne B & Peters A (Eds.), The cat primary visual cortex (pp. 259–294). New York, NY: Academic Press. [Google Scholar]
- Olavarria JF, & Hiroi R (2003). Retinal influences specify cortico-cortical maps by postnatal day six in rats and mice. The Journal of Comparative Neurology, 459(2), 156–172. [DOI] [PubMed] [Google Scholar]
- Olavarria J, & Van Sluyters RC (1983). Widespread callosal connections in infragranular visual cortex of the rat. Brain Research, 279(1–2), 233–237. [DOI] [PubMed] [Google Scholar]
- Olavarria JF, & Van Sluyters RC (1985). Organization and postnatal development of callosal connections in the visual cortex of the rat. The Journal of Comparative Neurology, 239(1), 1–26. [DOI] [PubMed] [Google Scholar]
- Olavarria J, Malach R, & Van Sluyters RC (1987). Development of visual callosal connections in neonatally enucleated rats. The Journal of Comparative Neurology, 260(3), 321–348. [DOI] [PubMed] [Google Scholar]
- Pinazo-Durán MD, Pons-Vázquez S, Gallego-Pinazo R, Galbis Estrada C, Zanón-Moreno V, Vila Bou V, & Sanz Solana P (2011). Thyroid hormone deficiency disrupts rat eye neurodevelopment. Brain Research, 1392, 16–26. [DOI] [PubMed] [Google Scholar]
- Rakic P (1976). Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature, 26(5560), 467–471. [DOI] [PubMed] [Google Scholar]
- Restani L, Cerri C, Pietrasanta M, Gianfranceschi L, Maffei L, & Caleo M (2009). Functional masking of deprived eye responses by callosal input during ocular dominance plasticity. Neuron, 64(5)707–718. [DOI] [PubMed] [Google Scholar]
- Restani L, & Caleo M (2016). Reorganization of visual callosal connections following alterations of retinal input and brain damage. Frontiers in Systems Neuroscience, doi: 10.3389/fnsys.2016.00086. eCollection 2016., 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, & Warner CL (1974). Comparison of Weigert stained sections with unfixed, unstained sections for study of myelin sheaths. Proceedings of the National Academy of Sciences of the United States of America, 71(3), 598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Basker GE, & Stryker MP (1999). Development and organization of ocular dominance bands in primary visual cortex of the sable ferret. The Journal of Comparative Neurology, 407(2), 151–165. [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, & Stryker MP (1978) Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. Journal of Physiology (Lond), 281:267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KE, Stephan M, Singer W, & Löwel S (2002). Spatial analysis of ocular dominance patterns in monocularly deprived cats. Cerebral Cortex, 12(8), 783–96. [DOI] [PubMed] [Google Scholar]
- Shook BL, & Chalupa LM (1986) Organization of geniculocortical connections following prenatal interruption of binocular interactions. Brain Research, 393(1), 47–62. [DOI] [PubMed] [Google Scholar]
- Spatz WB (1989). Loss of ocular dominance columns with maturity in the monkey, Callithrix jacchus. Brain Research, 488(1–2), 376–380. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Gonatas JO, & Gonatas NK (1981). A light and electron microscopic study of the intraneuronal transport of horseradish peroxidase and wheat germ agglutinin-peroxidase conjugates in the rat visual system. Journal of Neurocytology, 10(3), 441–456. [DOI] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, & Finlay BL (2013). Modeling transformations of neurodevelopmental sequences across mammalian species. The Journal of Neuroscience, 33(17), 7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey PD, Jin F, Nakamura H, Tettoni L, & Innocenti GM (1999), The role of pattern vision in the development of cortico-cortical connections. European Journal of Neuroscience, 11(8), 2669–2688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.