Abstract
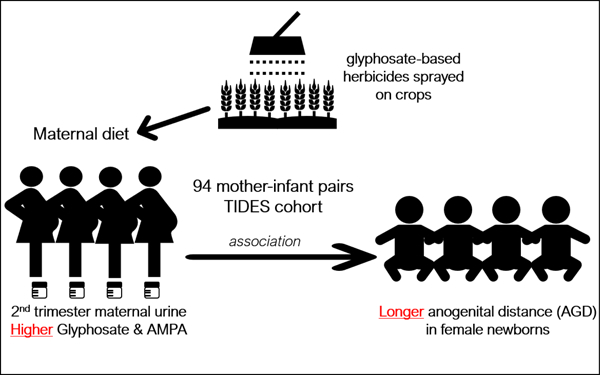
Human exposure to glyphosate has become ubiquitous because of its increasing agricultural use. Recent studies suggest endocrine disrupting effects of glyphosate. Specifically, in our work in rodents, low-dose early-life exposure to Roundup® (glyphosate-based herbicide) lengthened anogenital distance (AGD) in male and female offspring. AGD is a marker of the prenatal hormone milieu in rodents and humans. The relationship between glyphosate exposure and AGD has not been studied in humans. We conducted a pilot study in 94 mother-infant pairs (45 female and 49 male) from The Infant Development and the Environment Study (TIDES). For each infant, two AGD measurements were collected after birth; the anopenile (AGD-AP) and anoscrotal (AGD-AS) distances for males, and anoclitoral (AGD-AC) and anofourchette distances (AGD-AF) for females. We measured levels of glyphosate and its degradation product aminomethylphosphonic acid (AMPA) in 2nd trimester maternal urine samples using ultra-high-performance liquid chromatography-tandem mass spectrometry. We assessed the relationship between exposure and AGD using sex-stratified multivariable linear regression models. Glyphosate and AMPA were detected in 95% and 93% of the samples (median 0.22 ng/mL and 0.14 ng/mL, respectively). Their concentrations were moderately correlated (r=0.55, p=5.7x10−9). In female infants, high maternal urinary glyphosate (above the median) was associated with longer AGD-AC (β=1.48, 95%CI (−0.01, 3.0), p=0.05), but this was not significant after covariate adjustment. Increased AMPA was associated with longer AGD-AF (β=1.96, 95%CI (0.44, 3.5), p=0.01) after adjusting for infant size and age at AGD exam. No associations were detected in male offspring. These preliminary findings partially reproduce our previous results in rodents and suggest that glyphosate is a sex-specific endocrine disruptor with androgenic effects in humans. Given the increasing glyphosate exposures in the US population, larger studies should evaluate potential developmental effects on endocrine and reproductive systems.
Keywords: glyphosate, AMPA, herbicides, anogenital distance, endocrine disrupting chemicals
Graphical Abstract
1. Introduction
Glyphosate is the active ingredient of broad-spectrum glyphosate-based herbicides (GBHs) which are the most frequently used herbicides worldwide (Benbrook, 2016). Glyphosate was first commercialized as Roundup® in 1974, and its initial use in agriculture was low and limited to pre-harvest spray to kill weeds. However, since the introduction of genetically engineered glyphosate-tolerant crops to the U.S. market in 1996, the agricultural use of GBHs has increased 300-fold (from 0.36 million kilograms (kg) in 1974 to 113.4 million in 2014) (Benbrook, 2016). Residues of glyphosate and its primary metabolite aminomethylphosphonic acid (AMPA) are commonly detected in air (Chang et al., 2011), soil (Battaglin et al., 2014), water (Medalie et al., 2020) and food (FDA, 2019; Kolakowski et al., 2020; Ledoux et al., 2020; Zoller et al., 2018). Human glyphosate exposure in the general population is widespread, usually through diet (Fagan et al., 2020); with recent studies reporting increasing glyphosate and AMPA levels in urine samples from adults in the general population (Conrad et al., 2017; Mills et al., 2017). Urinary glyphosate levels are considered good exposure markers because glyphosate is highly hydrophilic, does not bio-accumulate and is poorly metabolized (mainly to AMPA)(EFSA, 2015).
Glyphosate is an organophosphorus compound that inhibits the 5-enolpyruvylshikimate-3-phosphate synthase (EPSP), an enzyme involved in the shikimate pathway of aromatic amino acids biosynthesis present in plants, bacteria and fungi, but absent in mammals that obtain these amino acids from diet (Mir et al., 2015). Thus, glyphosate was initially considered “safe” to humans. However, in recent years multiple studies have challenged this assumption and described adverse effects of either glyphosate or GBHs (Myers et al., 2016). Possible carcinogenic effects have been highly debated after the International Agency for Research on Cancer (IARC) classified glyphosate as a “probable human carcinogen” (EFSA, 2015; EPA, 2016; IARC, 2017; Portier et al., 2016). Other studies using model systems suggest that glyphosate and GBHs can have endocrine and reproductive activity (reviewed in (Ingaramo et al., 2020; Muñoz et al., 2020)). In our own study of Sprague-Dawley rats, gestational and early-life low-dose (1.75 mg/kg/day) exposure to glyphosate and Roundup® suggested androgenic-like effects in offspring. In Roundup® exposed female pups, we observed delayed age at first estrous, increased testosterone and anogenital distance (AGD). In males, our findings showed increased AGD in both glyphosate and Roundup® exposed pups (Manservisi et al., 2019). The glyphosate dose used in these experiments is considered to be “safe” in humans, i.e. the US Acceptable Daily Intake (ADI) of 1.75 mg/kg bw/day, defined as the chronic Reference Dose determined by the United States Environmental Protection Agency (US EPA) (EPA, 1993).
AGD, an early-life biomarker of fetal androgen exposure in multiple species has been used as a reproductive toxicity endpoint to evaluate chemicals in animal studies by the US EPA(EPA, 1996). AGD length is influenced by body size and is longer in males than females in most mammalian species (Schwartz et al., 2019; Swan and Kristensen, 2018). Early in development androgens regulate masculinization of the genital tract; disruptions during this critical window can lead to shorter AGD (feminized) and reproductive tract abnormalities in males (Macleod et al., 2010; van den Driesche et al., 2011). In females, in utero androgen exposure masculinizes (lengthens) AGD and other reproductive organs (Abbott et al., 2017; Dean et al., 2012; Hotchkiss et al., 2007; Ramezani Tehrani et al., 2014; Wolf et al., 2002). The most frequently used AGD measures in human males are the shorter anoscrotal distance (AGD-AS) and the longer anopenile distance (AGD-AP). In females, the shorter measure is the anofourchette distance (AGD-AF) and the longer is the anoclitoral distance (AGD-AC) (Sathyanarayana et al., 2015). Multiple epidemiological studies have shown that AGD measurements in infants are sensitive to in utero exposures to endocrine disrupting chemicals (EDCs) (Swan and Kristensen, 2018). Given our previous study on the effects of Roundup® on AGD in rats, we sought to investigate the relationship between infant AGD and glyphosate and AMPA concentrations in 2nd trimester maternal urine in a human population.
2. Methods
2.1. Study population
This study is nested within The Infant Development and the Environment Study (TIDES), a multicenter pregnancy cohort designed to investigate prenatal exposure to endocrine disrupting chemicals (EDCs) in relation to reproductive development. Between August 2010 and August 2012, women were recruited in the 1st trimester of pregnancy at four university-based medical centers: University of California, San Francisco, (UCSF), University of Rochester Medical Center (URMC), University of Minnesota (UMN) and the University of Washington (UW). Eligible participants included any pregnant woman (<13 weeks of gestation) over 18 years old, without major medical complications, able to read and write English (or Spanish at the UCSF center) and planning to deliver at a study center hospital. In each trimester, study participants provided urine samples and completed questionnaires that collected demographic, health, lifestyle and reproductive history information. Urine samples were collected in polypropylene cups, specific gravity (SpG) was measured within 30 minutes of collection using a hand-held refractometer (National Instrument Company, Inc., USA). Samples were stored at −80°C. The Institutional Review Boards (IRB) of each participating center approved all the study protocols and all participants signed an informed consent prior to the start of the study. For this pilot, we randomly selected 100 participants with a second trimester urine samples and birth exam. After removing premature infants, the analytic sample consisted of 94 term births (49 males and 45 females).
2.2. Infant anogenital distance (AGD)
Before hospital discharge, the TIDES team performed a newborn physical examination usually on the 1st or 2nd day of life. For infants born preterm or with medical conditions, examinations were delayed until clinically indicated. During physical examination, experienced examiners measured weight and length and conducted a comprehensive genital exam following methods described previously (Sathyanarayana et al., 2015; Swan et al., 2005). Two measures of AGD (AGD-AP and AGD-AS) and one measure of penile width (PW) were obtained on all male infants and two measures of AGD (AGD-AF and AGD-AC) were obtained on all females following a standard protocol (Sathyanarayana et al., 2015; Swan et al., 2015). AGD was measured from the center of the anus to a genital landmark. In males the genital landmarks are (i) the anterior base of the penis where the penile tissue meets the pubic bone (AGD-AP) and (ii) the base of the scrotum where the skin changes from rugated to smooth (AGD-AS). In females, the genital landmarks are (i) the anterior tip of the clitoral hood (AGD-AC) and (ii) the base of the posterior fourchette where skin folds fuse (AGD-AF). In previous TIDES analyses, we identified a number of covariates potentially predictive of AGD and PW including: infant’s age at exam, gestational age at birth, maternal age and clinical center (Sathyanarayana et al., 2015). We used weight-for-age percentiles for birth exam (WTPCT) calculated from World Health Organization (WHO) standard curves (WHO, 2009) to adjust for infant body size. These Z-scores provide the best predictor of genital measurements among the estimates of body size that we considered (including weight, weight-for-age and length-for-age Z-scores) and are independent of age. Maternal ethnicity, education and smoking were also considered, but did not alter effect estimates by >10% and were not included in final models (Sathyanarayana et al., 2015; Swan et al., 2015).
2.3. Urinary glyphosate and AMPA analyses
The urinary concentrations (ng/mL) of glyphosate and its metabolite AMPA were measured using ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) on the Vanquish UHPLC coupled to a TSQ Altis triple quadrupole mass spectrometer (ThermoFisher, San Jose, CA). Measurements were performed at the Collaborative Center for Translational Mass Spectrometry (CCTMS) (TGen, Phoenix, AZ). Samples were prepared following protocols previously described (Jensen et al., 2016). The assays were validated over a linear range of 0 to 5 ng/mL of glyphosate and AMPA concentrations (coefficient of determination R2 > 0.99) (Figure S1). Urinary creatinine levels (mg/dL) were also measured as part of the analytical methods to determine sample dilutions and align injection volume, high creatinine samples (>160 mg/dL) were diluted 2-fold with water. The injection volume for these samples were kept the same as for those with medium creatinine concentrations. The samples with low creatinine (0 to 40 mg/dL) and medium creatinine (40– 160 mg/dL) were injected at 100 µL and 50 µL, respectively. For glyphosate, the limit of detection (LOD) and limit of quantitation (LOQ) were 0.014 and 0.041 ng/mL respectively. The AMPA assay limits were: LOD 0.013 and LOQ: 0.04 ng/mL. Glyphosate and/or AMPA values below the LOD were replaced with LOD/√2, a common imputation method used for non-detectable measurements (Hornung and Reed, 1990). To account for urinary dilution, we measured specific gravity (SpG) and adjusted glyphosate measurements using the following formula: SpG-adj-Gly = Gly[1.014–1/(SpG-1)], where SpG-adj-Gly is the SpG adjusted glyphosate concentration (ng/mL), Gly is the observed glyphosate concentration and 1.014 is the mean SpG for all the TIDES samples. AMPA measurements were also adjusted using this method (Boeniger et al., 1993).
2.4. Statistical methods
We first calculated univariate summary statistics and examined the distributions of specific gravity adjusted glyphosate and AMPA concentrations (ng/mL). Since these exhibited right skewed distributions, measurements were log-transformed for use in parametric analyses. Next, we examined relationships between exposure variables and TIDES covariates of interest using Pearson correlations for continuous variables and Student’s t-test or ANOVA for categorical variables. Scatterplots and boxplots were used to display results of bivariate analyses. Because AGD measurements are sex-specific we modelled exposure-outcome relationships in sex-stratified analyses. Maternal urinary exposure concentrations were examined as continuous as well as dichotomous (above and below the median) variables. We examined relationships between infant AGD and maternal glyphosate and AMPA urinary concentrations using sex-stratified linear regression models. We fitted unadjusted models and models adjusted for infant age and weight-for-length Z-score (a measure of body size for age) at AGD exam; two covariates shown to influence AGD in previous TIDES analyses (Swan et al., 2015). We also constructed fully adjusted models including gestational age at birth, age at exam, weight-for-length Z-score, maternal age, time of day of urine collection, study center, maternal smoking, alcohol use and education level; shown in the supplementary material. Statistical analyses were conducted with R (version 4.0.0) (Team, 2019b) and RStudio (version 1.2.5033) (Team, 2019a). All tests were two-sided and p-value <0.05 were considered statistically significant.
3. Results
This analysis included 94 term infants among 100 randomly sampled TIDES participants with available 2nd trimester maternal urine samples and birth exam (Table 1). The clinical and demographic characteristics of the entire TIDES cohort have been described previously (Swan et al., 2015) and are similar to the subset in this analysis (Table S1). Participants were predominantly white (67%) with an average maternal age of 31 years (standard deviation (SD) 6 years). Maternal urine samples used in these analyses were collected on average at 21 weeks of gestational age (range: 14.7 – 28.9). All the infants included in this analysis were born at term (≥ 37 weeks); the median gestational age at birth was 39 weeks (range: 37 – 42). AGD was measured within the first week of life for the majority (89%) of the newborns (range: 0 – 9.3) and at this time the median weight for length z-score was −0.1 (range: −3.4 – 2.1) likely due to standard early postnatal water weight loss (Bertini et al., 2015). Among the 45 female infants, the mean AGD-AF was 16.3 mm (SD: 2.8) and the mean AGD-AC was 37.3 mm (SD: 2.5). In males (n = 49), the means for AGD-AS and AGD-AP were 25.2 mm (SD: 4.3) and 49.7 mm (SD: 5), respectively (Table 1). All AGD measurements were normally distributed (all p-values > 0.05, Shapiro–Wilk test). Similar to the results of the entire TIDES cohort (Swan et al., 2015), the subset in this study showed a moderate correlation between male AGD measurements (r=0.63, p=9×10−7), but in females AGD-AF and AGD-AC (r=0.24, p=0.12) were not correlated. The distributions of maternal urinary glyphosate and AMPA concentrations are shown in Table 2. Glyphosate concentrations ranged from 0.01 to 1.9 ng/mL with a median of 0.22 ng/mL; 95% of samples had detected levels of glyphosate (LOD 0.014 ng/mL) and 93% for AMPA (LOD 0.013 ng/mL) which had a lower concentration compared to glyphosate with a median of 0.14 ng/mL (range: 0.01 – 6). Maternal urinary glyphosate and AMPA concentrations showed a moderate positive correlation (Pearson’s r =0.55, p=5.7×10−9, Figure 1). The results of the bivariate analyses between continuous exposure measures (glyphosate or AMPA) and relevant maternal and infant characteristics (other than AGD) are shown in Table S2 and S3. Urinary AMPA concentration was significantly correlated with maternal age and time of day of urine sample collection p=0.04 and p=0.01, respectively (Table S2). Urinary glyphosate differed by season of urine collection (p=0.02). The levels of glyphosate or AMPA did not differ between study centers (Figure S2), maternal smoking status, alcohol use, ethnicity, or maternal education (Table S3).
Table 1.
Characteristics of the study population (N=94)
| N (%) | Mean | SD | Min. | P5 | P25 | P50 | P75 | P95 | Max. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal age (yrs) | 94 | 31.2 | 5.8 | 18.5 | 20.7 | 27.3 | 31.6 | 35.3 | 39.9 | 43.8 |
| Birthweight (kg) | 93 | 3.5 | 0.5 | 2.0 | 2.6 | 3.2 | 3.5 | 3.7 | 4.3 | 5.4 |
| Gestational age at birth (weeks) | 94 | 39.6 | 1.2 | 37.0 | 37.4 | 39.0 | 39.4 | 40.6 | 41.3 | 42.3 |
| Weight for length z-score | 94 | −0.3 | 1.2 | −3.4 | −2.4 | −1.0 | −0.1 | 0.6 | 1.5 | 2.1 |
| Age at exam (weeks) | 94 | 0.7 | 1.8 | 0.0 | 0.0 | 0.1 | 0.1 | 0.3 | 5.7 | 9.3 |
| Gestational age at urine | ||||||||||
| collection (weeks) | 94 | 20.8 | 3.3 | 14.7 | 16.2 | 18.9 | 20.0 | 23.1 | 27.1 | 28.9 |
| Time of urine collection (hours) | 93 | 11.9 | 2.8 | 6.0 | 8.6 | 9.5 | 11.2 | 14.9 | 16.2 | 17.0 |
| AGD-AF | 45 | 16.3 | 2.8 | 10.8 | 12.5 | 14.6 | 15.8 | 18.2 | 21.2 | 22.9 |
| AGD-AC | 45 | 37.3 | 2.5 | 31.6 | 33.2 | 35.4 | 37.8 | 39.2 | 41.4 | 41.8 |
| AGD-AS | 49 | 25.2 | 4.3 | 16.1 | 18.7 | 22.3 | 24.8 | 27.2 | 32.9 | 38.4 |
| AGD-AP | 49 | 49.7 | 5.0 | 41.9 | 42.6 | 46.1 | 49.3 | 51.9 | 59.0 | 65.4 |
| Infant sex | ||||||||||
| Female | 45 (47.9) | |||||||||
| Male | 49 (52.1) | |||||||||
| Infant race | ||||||||||
| Black or African-American | 18 (19.1) | |||||||||
| Other | 13 (13.8) | |||||||||
| White | 63 (67.0) | |||||||||
| Study center | ||||||||||
| UCSF | 20 (21.3) | |||||||||
| UMN | 25 (26.6) | |||||||||
| UR | 32 (34.0) | |||||||||
| UW | 17 (18.1) | |||||||||
| Urine collection season | ||||||||||
| Winter | 21 (22.3) | |||||||||
| Spring | 30 (31.9) | |||||||||
| Summer | 22 (23.4) | |||||||||
| Autumn | 21 (22.3) | |||||||||
| Maternal smoking | ||||||||||
| No | 80 (85.1) | |||||||||
| Yes | 10 (10.6) | |||||||||
| Maternal alcohol use | ||||||||||
| No | 88 (93.6) | |||||||||
| Yes | 2 (2.1) | |||||||||
| Maternal Education | ||||||||||
| Less than college degree | 27 (28.7) | |||||||||
| College degree or more | 67 (71.3) |
P, percentile; Min, minimum; Max., maximum; UCSF, University of California San Francisco; URMC, University of Rochester Medical Center; UW, University of Washington. Missing data: one observation (time of urine collection); four observations (maternal smoking) and four observations (alcohol use).
Table 2.
Summary statistics for glyphosate and AMPA measurements (ng/mL) in maternal urine
| N | % below* | Mean | SD | Min. | P5 | P25 | P50 | P75 | P95 | Max. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SpG-adj glyphosate | 94 | 0 | 0.33 | 0.30 | 0.01 | 0.03 | 0.12 | 0.22 | 0.52 | 0.86 | 1.90 |
| SpG-adj glyphosate >LOD | 89 | 5.3 | 0.34 | 0.31 | 0.02 | 0.04 | 0.13 | 0.23 | 0.54 | 0.87 | 1.90 |
| SpG-adj glyphosate >LOQ | 80 | 14.8 | 0.38 | 0.31 | 0.05 | 0.08 | 0.15 | 0.27 | 0.55 | 0.88 | 1.90 |
| SpG-adj AMPA | 94 | 0 | 0.31 | 0.66 | 0.01 | 0.02 | 0.08 | 0.14 | 0.28 | 1.06 | 6.01 |
| SpG-adj AMPA >LOD | 87 | 7.4 | 0.33 | 0.69 | 0.02 | 0.03 | 0.09 | 0.16 | 0.29 | 1.07 | 6.01 |
| SpG-adj AMPA >LOQ | 71 | 24.4 | 0.40 | 0.75 | 0.04 | 0.07 | 0.11 | 0.22 | 0.39 | 1.10 | 6.01 |
Percent of samples below the limit of detection (LOD); glyphosate 0.014 ng/mL; AMPA 0.013 ng/mL); or the limit of quantification (LOQ); glyphosate 0.041 ng/mL; AMPA 0.04 ng/mL.
P, percentile; Min, minimum; Max., maximum; SpG-adj, specific gravity-adjusted; SD, standard deviation.
Figure 1.
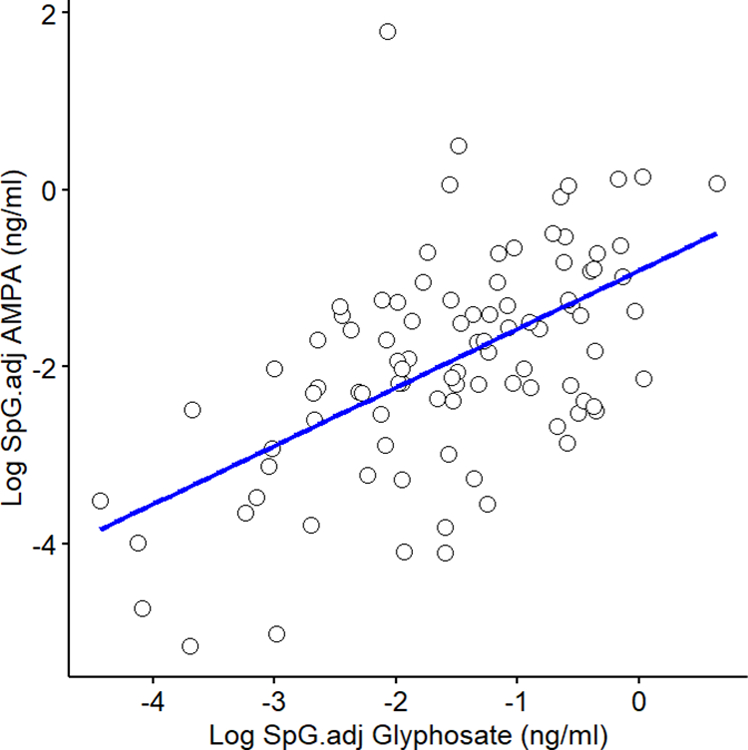
Scatterplot of maternal urinary glyphosate and AMPA concentrations (natural log transformed and SpG adjusted). Pearson’s r =0.55, p=5.7×10−9.
Scatterplots between glyphosate and AMPA in relation to AGD measurements are shown in Figure S3 (females) and Figure S4 (males). In females, the correlations between maternal glyphosate and AMPA and AGD-AF showed positive coefficients, yet not statistically significant (glyphosate: r =0.26, p=0.08; AMPA r = 0.19, p=0.2). Male AGD measurements were not correlated with exposure. After dichotomizing maternal glyphosate and AMPA exposure at their median values (for all infants); female AGD-AF was longer in the high AMPA group (p=0.01) and AGD-AC was longer in the high glyphosate group (p=0.05) (Figure S5). By contrast, male AGD measures were similar in the dichotomized glyphosate and AMPA exposure groups (Figure S6).
Next, we performed sex-stratified crude and adjusted linear regression models to examine glyphosate and AMPA concentration in relation to AGD. Multivariable models included adjustment for infant age and weight-for-length z-score age at AGD exam. Table 3 and 4 display crude and adjusted linear models in female and male infants, respectively. Fully adjusted models including adjustment for gestational age at birth, age at exam, weight-for-length Z-score, maternal age, time of day of urine collection, study center, maternal smoking, alcohol use and education level) are shown in Table S3 (females) and Table S4 (males).
Table 3.
Multivariable linear regression models between maternal urinary glyphosate or AMPA and female AGD measures (N=45)
| AGD-AF | AGD-AC | |||||||
|---|---|---|---|---|---|---|---|---|
| continuous exposure | Beta | SE | p | 95%CI | Beta | SE | p | 95%CI |
| Log SpG-adj glyphosatea | 0.62 | 0.35 | 0.09 | (−0.09, 1.33) | 0.11 | 0.33 | 0.73 | (−0.55, 0.77) |
| Log SpG-adj glyphosateb | 0.47 | 0.35 | 0.18 | (−0.23, 1.17) | 0.10 | 0.33 | 0.76 | (−0.56, 0.76) |
| Log SpG-adj AMPAa | 0.39 | 0.30 | 0.20 | (−0.22, 1.00) | −0.18 | 0.27 | 0.52 | (−0.73, 0.37) |
| Log SpG-adj AMPAb | 0.40 | 0.29 | 0.17 | (−0.18, 0.98) | −0.11 | 0.27 | 0.70 | (−0.65, 0.44) |
| categorical exposure | Beta | SE | p | 95%CI | Beta | SE | p | 95%CI |
| Glyphosate highc | 1.16 | 0.85 | 0.18 | (−0.55, 2.9) | 1.48 | 0.74 | 0.05 | (−0.01, 3.0) |
| Glyphosate highd | 0.75 | 0.82 | 0.37 | (−0.91, 2.4) | 1.35 | 0.74 | 0.07 | (−0.13, 2.8) |
| AMPA highc | 2.06 | 0.80 | 0.01 | (0.46, 3.7) | 0.44 | 0.76 | 0.56 | (−1.09, 2.0) |
| AMPA highd | 1.96 | 0.75 | 0.01 | (0.44, 3.5) | 0.27 | 0.75 | 0.72 | (−1.23, 1.8) |
SE, standard error; Log, natural log; SpG-adj, specific gravity-adjusted
unadjusted continuous exposure models
continuous exposure models adjusted for infant age (weeks) and weight-for-length Z-score at AGD exam
unadjusted models (exposure dichotomized at the median)
models (exposure dichotomized at the median) adjusted for infant age (weeks) and weight-for-length Z-score at AGD exam
Table 4.
Multivariable linear regression models between maternal urinary glyphosate or AMPA and male AGD measures (N=49)
| AGD-AS | AGD-AP | |||||||
|---|---|---|---|---|---|---|---|---|
| continuous exposure | Beta | SE | p | 95%CI | Beta | SE | p | 95%CI |
| Log SpG-adj glyphosatea | 0.26 | 0.73 | 0.72 | (−1.20, 1.72) | 0.57 | 0.83 | 0.50 | (−1.10, 2.24) |
| Log SpG-adj glyphosateb | 0.37 | 0.68 | 0.59 | (−0.99, 1.73) | 0.78 | 0.69 | 0.26 | (−0.61, 2.16) |
| Log SpG-adj AMPAa | −0.11 | 0.60 | 0.86 | (−1.31, 1.09) | −0.26 | 0.69 | 0.71 | (−1.64, 1.12) |
| Log SpG-adj AMPAb | −0.31 | 0.56 | 0.58 | (−1.43, 0.82) | −0.52 | 0.57 | 0.36 | (−1.68, 0.63) |
| categorical exposure | Beta | SE | p | 95%CI | Beta | SE | p | 95%CI |
| Glyphosate highc | 0.91 | 1.26 | 0.48 | (−1.6, 3.4) | 0.84 | 1.45 | 0.57 | (−2.1, 3.8) |
| Glyphosate highd | 1.06 | 1.18 | 0.37 | (−1.3, 3.4) | 1.19 | 1.21 | 0.33 | (−1.2, 3.6) |
| AMPA highc | 1.09 | 1.25 | 0.38 | (−1.4, 3.6) | 0.86 | 1.44 | 0.55 | (−2.0, 3.8) |
| AMPA highd | 0.21 | 1.21 | 0.86 | (−2.2, 2.6) | −0.60 | 1.24 | 0.63 | (−3.1, 1.9) |
SE, standard error; Log, natural log; SpG-adj, specific gravity-adjusted
unadjusted continuous exposure models
continuous exposure models adjusted for infant age (weeks) and weight-for-length Z-score at AGD exam
unadjusted models (exposure dichotomized at the median)
models (exposure dichotomized at the median) adjusted for infant age (weeks) and weight-for-length Z-score at AGD exam
In females, most of the models (crude or adjusted) with maternal glyphosate or AMPA as continuous exposures displayed positive beta coefficients with AGD measurements, but these were not statistically significant (Table 3, top panel). Linear models using dichotomous (below and above the median) exposure variables in female infants are shown in the bottom panel of Table 3. Female AGD-AC was longer in non-adjusted models (β=1.48, 95%CI (−0.01, 3), p=0.05), but this was not the case after adjustment for infant age and weight-for-length Z-score at AGD exam (β=1.35, 95%CI (−0.13, 2.8), p=0.07). High AMPA exposure was significantly associated with increasing AGD-AF in crude (β=2.06, 95%CI (0.46, 3.7), p=0.01) and adjusted (β=1.96, 95%CI (0.44, 3.5), p=0.01) models, but not with longer measurement of AGD (AGD-AC). In male infants, we did not detect any significant associations with glyphosate or AMPA in continuous (Table 4, top panel) or dichotomized (Table 4, bottom panel) exposure models.
4. Discussion
This work is the first human study to investigate prenatal urinary levels of glyphosate in relation to newborn AGD. Our results are partially consistent with previous findings in rodents (Manservisi et al., 2019), and suggest that glyphosate may function as an endocrine disruptor with possible androgen-like effects on humans in a sex-specific manner. Importantly, these data provide further evidence that glyphosate exposure is ubiquitous in the general US population given that almost all maternal urine samples had detectable levels of glyphosate and AMPA. Few epidemiological studies have assessed individual levels of glyphosate exposure in the general population (Gillezeau et al., 2019). Two recent studies reported increases in detection and urinary glyphosate levels over the last two decades in the U.S.(Mills et al., 2017) and Germany (Conrad et al., 2017). We detected glyphosate and AMPA in the great majority of the TIDES participants (>90%), with mean levels of 0.33 ng/mL and 0.31 ng/mL, respectively. Even though the assays LODs in our study are lower (0.014 and 0.013 ng/mL) than those of previous studies (range 0.02 to 0.5 ng/mL) (Gillezeau et al., 2019), our findings are similar to recent studies in the U.S. population. A study in 40 lactating women from the Pacific Northwest reported a 93% glyphosate detection rate (LOD 0.02 ng/mL) and average glyphosate levels of 0.28 ng/mL (McGuire et al., 2016). Similarly, a study in pregnant women in Indiana reported detectable glyphosate in 93% of the samples, albeit with higher mean levels (3.4 ng/mL) and LOD (0.1 ng/mL). Another study in California reported a detection rate of 70% (LOD 0.03 ng/mL) and similar average glyphosate urinary levels (0.31 ng/mL). In contrast, a recent study in children (newborns to 8 years) from Seattle and New York, reported a low detection rate of 11.1% (12/108, mean 0.278 ng/mL) in urine. This could be related to sampling year, or to lower sensitivity of the analytical assay used in that study (LOD = 0.1 ng/mL) (Trasande et al., 2020). Similar to the U.S., few studies have evaluated urinary levels of glyphosate in non-occupational settings in Europe (Gillezeau et al., 2019), though data from the two largest published studies from Germany (Conrad et al., 2017; Soukup et al., 2020) suggest that the detection rates (30–40%) and average glyphosate levels (≈0.16 ng/mL) are lower compared to the U.S. Outside North America and Europe, there are even fewer glyphosate studies (Gillezeau et al., 2019). Of note is one investigation in 82 pregnant women living in agricultural regions in Thailand, that detected glyphosate in 53% of the maternal serum (median: 17.5 ng/mL) and importantly in 49% (37/75) of umbilical cord serum samples (median: 0.2 ng/mL), suggesting that glyphosate is crossing the placenta (Kongtip et al., 2017).
Our results also show a moderate correlation (r = 0.5) between urinary glyphosate and AMPA measurements, similar to results described in two studies in German adults in 2017 (rho = 0.50, P ≤ 0.001, n = 399) (Conrad et al., 2017) and in 2020 (rho = 0.26, P ≤ 0.0001, n = 76) (Soukup et al., 2020). However, a 2013 unpublished report (Hoppe, 2013) described that glyphosate and AMPA urinary levels did not correlate well in 182 adults from 18 European countries (coefficient not reported). Evidence from humans suggests that the rate of conversion of glyphosate to AMPA in humans is low (Zoller et al., 2020). Thus, the observed correlation could be due to similar levels of these exposures in food (Connolly et al., 2020). However, since we did not directly measure their levels in diet, we cannot assess the level of conversion from glyphosate to AMPA. Such knowledge is scarce in humans and requires further investigation.
Some reports of model systems have described endocrine disrupting effects of glyphosate or GBH exposure (Ingaramo et al., 2020; Muñoz et al., 2020). In vitro, GBH exposure decreases or inhibits the activity of the placental aromatase, an important enzyme that converts testosterone into estradiol to protect the fetus from excess androgens (Benachour et al., 2007; Gasnier et al., 2009; Richard et al., 2005). In rats, perinatal (first week) GBH exposure (2 mg/kg/BW) disrupts uterine development (Guerrero Schimpf et al., 2017) and dysregulates estrogen and progesterone receptor signaling (Guerrero Schimpf et al., 2018; Ingaramo et al., 2017). Moreover, gestational exposure to GBHs induces reproductive abnormalities in female rat offspring (reduced number of implantation sites and increased rates of preimplantation embryo loss)(Milesi et al., 2018). More recently, the same group reported that GBH exposure also alters uterine development in lambs (Alarcón et al., 2019). However, the glyphosate and GBHs evaluations by regulatory agencies in the US and Europe concluded that the evidence did not support endocrine disruption or effects on AGD (EFSA, 2015; EPA, 2015). AMPA is even less studied than glyphosate. To date, there is no direct evidence in animals or in humans that AMPA itself has endocrine or other health effects. Further studies are needed to conclusively assess the potential endocrine disrupting effects of glyphosate, AMPA and GBHs.
In this study, we detected associations between 2nd trimester maternal urine high glyphosate exposure and longer AGD-AC and high AMPA and longer AGD-AF length in female infants. These results are in part consistent with our previous observations in female Sprague-Dawley rats, in which gestational and early-life exposure to Roundup® resulted in longer AGD at postnatal day 4. However, female rat pups exposed to glyphosate had longer mean AGD than controls, but this result was not statistically significant. In this study, we did not detect associations between glyphosate or AMPA and AGD in male infants. This contrasts with our previous results in which exposure (gestation and early-life) to glyphosate and Roundup® resulted in longer AGD in male rat pups (Manservisi et al., 2019). In female mice pups, maternal androgen exposure (dihydrotestosterone) has masculinizing effects even if the exposure occurs before or after the masculinization programming window (MPW), whereas male pups are unaffected suggesting that females are more sensitive to exogenous androgens (Dean et al., 2012; Holland et al., 2019). To the best of our knowledge, the relation between gestational GBH exposure and AGD has not been investigated in other animal studies, outside of our previous work (Manservisi et al., 2019), nor in human population studies. More research is needed to comprehensively characterize possible androgenic effects of glyphosate and/or GBHs on AGD and other reproductive traits.
Our study has multiple strengths. It is the first human study to investigate maternal urinary level of glyphosate in relation to newborn AGD. We leveraged a unique multicenter study representing diverse geographic regions across the U.S. TIDES was specifically designed to examine the role of in utero environmental exposures on reproductive outcomes, study protocols were standardized to collect reliable AGD measurements shortly after birth. Our analytical methods of glyphosate and AMPA are robust and sensitive.
We acknowledge that this study has several limitations. The size of our sample is small which limited our statistical power. The study is observational; therefore, causality cannot be determined. Maternal glyphosate and AMPA were measured in a single second trimester urine sample which does not coincide with the MPW. In this non-agriculturally exposed population glyphosate and/or GBH exposures are likely to have been through diet, but we do not have data on exposure sources or levels throughout pregnancy. Nevertheless, exposure to pure glyphosate is unlikely, since this is applied to crops in GBH formulations that contain other “inert” ingredients not listed on the commercial product. Thus, comprehensive quantification of GBH additives is not possible and we used urinary glyphosate and AMPA as biomarkers of exposure. The effects of formulation of GBHs should also be addressed in future studies. Whilst the current study is the first to explore glyphosate and AMPA in relation to a marker sensitive to steroid hormone disruption, future studies should examine possible combined effects with other endocrine disrupting chemical exposures.
In conclusion, current findings, together with our prior animal study (Manservisi et al., 2019), suggest a link between GBH exposure in early-life and longer AGD. This is the first human study reporting associations between maternal glyphosate and AMPA exposure and female AGD measures. Given that human exposures to glyphosate have become ubiquitous, larger studies are warranted to investigate the relation between GBHs and other endocrine/reproductive outcomes and evaluate possible programming consequences to long-term health.
Supplementary Material
Table S1. Population characteristics of the overall TIDES study (N=758) and this study (n=94)
Table S2. Maternal urinary glyphosate and AMPA and continuous covariates.
Table S3. Maternal urinary glyphosate and AMPA and categorical covariates.
Table S4. Fully adjusted multivariable linear regression models between maternal urinary glyphosate or AMPA and female AGD measures (N=43)
Table S5. Fully adjusted multivariable linear regression models between maternal urinary glyphosate or AMPA and male AGD measures (N=46)
Figure S1. Linearity of calibration curve, a) glyphosate and b) AMPA ranging from 0 to 5 ng/mL in urine. The area ratio represents ratio of variable concentration of glyphosate and AMPA to their respective internal standards (13C215N-Glyphosate and D213C15N-AMPA)
Figure S2. Boxplots examining the relation between urinary natural log SpG-adjusted concentration of glyphosate (A) or AMPA (B) between TIDES study centers. UCSF, University of California San Francisco; URMC, University of Rochester Medical Center; UW, University of Washington.
Figure S3. Scatterplots examining the relation between urinary natural log SpG-adjusted concentration of glyphosate or AMPA (c-d) and AGD measurements (AGD-AF and AGD-AC) in female infants (n = 45).
Figure S4. Scatterplots examining the relation between urinary natural log SpG-adjusted concentration of glyphosate or AMPA (c-d) and AGD measurements (AGD-As and AGD-AP) in male infants (n = 49).
Figure S5. Boxplots examining the relation between dichotomized (on the median) maternal urinary glyphosate or AMPA (c-d) and AGD measurements (AGD-AF and AGD-AC) in female infants (n = 45).
Figure S6. Boxplots examining the relation between dichotomized (on the median) maternal urinary glyphosate or AMPA (c-d) and AGD measurements (AGD-AS and AGD-AP) in male infants (n = 49).
Highlights.
Glyphosate-based herbicides are widely used worldwide.
Glyphosate and AMPA detected in >90% of 2nd trimester maternal urine samples.
High maternal glyphosate/AMPA associated with longer female anogenital distance.
Glyphosate could act as a sex-specific endocrine disruptor.
Acknowledgements
The authors thank the TIDES Study Team: Coordinating Center (Fan Liu, Erica Scher), UCSF (Marina Stasenko, Erin Ayash, Melissa Schirmer, Jason Farrell, Mari-Paule Thiet, Laurence Baskin); UMN (Heather L. Gray Chelsea Georgesen, Brooke J. Rody, Carrie A. Terrell, Kapilmeet Kaur); URMC (Erin Brantley, Heather Fiore, Lynda Kochman, Lauren Parlett, Jessica Marino, William Hulbert, Robert Mevorach, Eva Pressman); UW/SCH (Kristy Ivicek, Bobbie Salveson, Garry Alcedo) and the families who participated in the study. The Ramazzini institute authors thank the “Coopfond Fondo Mutualistico Legacoop” for their support.
Funding
This study was supported by NIEHS R01ES016863-04 and R01ES016863-02S4 fund the TIDES study. The Mount Sinai Transdisciplinary Center on Early Environmental Exposures NIEHS P30ES023515. The NIEHS Center for Environmental Exposures and Disease (CEED) P30 ES005022; NICHD K99HD097286 funds C. Lesseur.
Abbreviations:
- AGD
anogenital distance
- AGD-AF
anogenital distance from the anus to the fourchette
- AGD-AC
anogenital distance from the anus to the clitoris
- AGD-AS
anogenital distance from the anus to the scrotum
- AGD-AP
anogenital distance from the anus to the penis
- AMPA
aminomethylphosphonic acid
- GBHs
glyphosate-based herbicides
- LOD
Limit of detection
- LOQ
Limit of quantification
- MPW
male programming window
- SD
standard deviation
- SE
standard error
- SpG
specific gravity
- TIDES
The Infant Development and the Environment Study
- UPLC-MS/MS
ultra-high-performance liquid chromatography-tandem mass spectrometry
- UCSF
University of California, San Francisco
- URMC
University of Rochester Medical Center
- UMN
University of Minnesota
- UW
University of Washington
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
The TIDES study protocols were approved by the institutional review boards (IRB) of each study center (University of California, San Francisco, University of Rochester Medical Center, University of Minnesota, and University of Washington/Seattle Children’s Hospital). Prior to study implementation and all subjects provided signed informed consent before starting any study activities. IRB approval was also obtained at the Icahn School of Medicine at Mount Sinai, which serves as the TIDES Coordinating Center since 2011.
Declaration of competing interest
The authors declare that they have no competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbott DH, et al. , 2017. Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Hum Reprod. 32, 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón R, et al. , 2019. Neonatal exposure to a glyphosate-based herbicide alters the histofunctional differentiation of the ovaries and uterus in lambs. Mol Cell Endocrinol. 482, 45–56. [DOI] [PubMed] [Google Scholar]
- Battaglin WA, et al. , 2014. Glyphosate and Its Degradation Product AMPA Occur Frequently and Widely in U.S. Soils, Surface Water, Groundwater, and Precipitation. JAWRA Journal of the American Water Resources Association. 50, 275–290. [Google Scholar]
- Benachour N, et al. , 2007. Time- and dose-dependent effects of roundup on human embryonic and placental cells. Arch Environ Contam Toxicol. 53, 126–33. [DOI] [PubMed] [Google Scholar]
- Benbrook CM, 2016. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur. 28, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini G, et al. , 2015. Physiological weight loss chart helps to identify high-risk infants who need breastfeeding support. Acta Paediatr. 104, 1024–7. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, et al. , 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 54, 615–27. [DOI] [PubMed] [Google Scholar]
- Chang FC, et al. , 2011. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ Toxicol Chem. 30, 548–55. [DOI] [PubMed] [Google Scholar]
- Connolly A, et al. , 2020. Human Biomonitoring of Glyphosate Exposures: State-of-the-Art and Future Research Challenges. Toxics. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad A, et al. , 2017. Glyphosate in German adults - Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int J Hyg Environ Health. 220, 8–16. [DOI] [PubMed] [Google Scholar]
- Dean A, et al. , 2012. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int J Androl. 35, 330–9. [DOI] [PubMed] [Google Scholar]
- EFSA, 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. The European Food Safety Authority (EFSA) Journal. 13, 4302-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, 1993. Re-registration eligibility decision (red) glyphosate:Epa-738-r-93-014.
- EPA, 1996. Guidelines for Reproductive Toxicity Risk Assessment. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, 630/R-96/009. 56274–56322. [Google Scholar]
- EPA, 2016. Glyphosate issue paper: evaluation of carcinogenic potential. United States Environmental Protection Agency. [Google Scholar]
- EPA, U. S., Weight of evidence analysis of potential interaction with the estrogen, androgen or thyroid pathways. In: O. o. P. Program, (Ed.). U.S. Enviromental Protection Agency, 2015. [Google Scholar]
- Fagan J, et al. , 2020. Organic diet intervention significantly reduces urinary glyphosate levels in U.S. children and adults. Environ Res. 109898. [DOI] [PubMed]
- FDA, U. S., Pesticide Residue Monitoring Program Fiscal Year 2017 Pesticide Report. United States Food and Drug Administration, 2019. [Google Scholar]
- Gasnier C, et al. , 2009. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology. 262, 184–91. [DOI] [PubMed] [Google Scholar]
- Gillezeau C, et al. , 2019. The evidence of human exposure to glyphosate: a review. Environ Health. 18, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero Schimpf M, et al. , 2017. Neonatal exposure to a glyphosate based herbicide alters the development of the rat uterus. Toxicology. 376, 2–14. [DOI] [PubMed] [Google Scholar]
- Guerrero Schimpf M, et al. , 2018. Glyphosate-based herbicide enhances the uterine sensitivity to estradiol in rats. J Endocrinol. [DOI] [PubMed]
- Holland S, et al. , 2019. The influence of maternal androgen excess on the male reproductive axis. Sci Rep. 9, 18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe HW, 2013. Determination of glyphosate residues in human urine samples from 18 European countries. Medical Laboratory Bremen. [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 5, 46–51. [Google Scholar]
- Hotchkiss AK, et al. , 2007. Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol Sci. 96, 335–45. [DOI] [PubMed] [Google Scholar]
- IARC, 2017. IARC Monographs on the evaluation of carcinogenic risks to humans—volume 112: some organophosphate insecticides and herbicides. International Agency for Research on Cancer (IARC) [PubMed] [Google Scholar]
- Ingaramo P, et al. , 2020. Are glyphosate and glyphosate-based herbicides endocrine disruptors that alter female fertility? Molecular and Cellular Endocrinology. 110934. [DOI] [PubMed]
- Ingaramo PI, et al. , 2017. Neonatal exposure to a glyphosate-based herbicide alters uterine decidualization in rats. Reprod Toxicol. 73, 87–95. [DOI] [PubMed] [Google Scholar]
- Jensen PK, et al. , 2016. Validation of reliable and selective methods for direct determination of glyphosate and aminomethylphosphonic acid in milk and urine using LC-MS/MS. J Environ Sci Health B. 51, 254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakowski BM, et al. , 2020. Analysis of Glyphosate Residues in Foods from the Canadian Retail Markets between 2015 and 2017. Journal of Agricultural and Food Chemistry. 68, 5201–5211. [DOI] [PubMed] [Google Scholar]
- Kongtip P, et al. , 2017. Glyphosate and Paraquat in Maternal and Fetal Serums in Thai Women. J Agromedicine. 22, 282–289. [DOI] [PubMed] [Google Scholar]
- Ledoux ML, et al. , 2020. Penetration of glyphosate into the food supply and the incidental impact on the honey supply and bees. Food Control. 109, 106859. [Google Scholar]
- Macleod DJ, et al. , 2010. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 33, 279–87. [DOI] [PubMed] [Google Scholar]
- Manservisi F, et al. , 2019. The Ramazzini Institute 13-week pilot study glyphosate-based herbicides administered at human-equivalent dose to Sprague Dawley rats: effects on development and endocrine system. Environ Health. 18, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire MK, et al. , 2016. Glyphosate and aminomethylphosphonic acid are not detectable in human milk. Am J Clin Nutr. 103, 1285–90. [DOI] [PubMed] [Google Scholar]
- Medalie L, et al. , 2020. Influence of land use and region on glyphosate and aminomethylphosphonic acid in streams in the USA. Sci Total Environ. 707, 136008. [DOI] [PubMed] [Google Scholar]
- Milesi MM, et al. , 2018. Perinatal exposure to a glyphosate-based herbicide impairs female reproductive outcomes and induces second-generation adverse effects in Wistar rats. Arch Toxicol. 92, 2629–2643. [DOI] [PubMed] [Google Scholar]
- Mills PJ, et al. , 2017. Excretion of the Herbicide Glyphosate in Older Adults Between 1993 and 2016. Jama. 318, 1610–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir R, et al. , 2015. The shikimate pathway: review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit Rev Microbiol. 41, 172–89. [DOI] [PubMed] [Google Scholar]
- Muñoz JP, et al. , 2020. Glyphosate and the key characteristics of an endocrine disruptor: A review. Chemosphere. 128619. [DOI] [PubMed]
- Myers JP, et al. , 2016. Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health. 15, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier CJ, et al. , 2016. Differences in the carcinogenic evaluation of glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA). J Epidemiol Community Health. 70, 741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani Tehrani F, et al. , 2014. The time of prenatal androgen exposure affects development of polycystic ovary syndrome-like phenotype in adulthood in female rats. Int J Endocrinol Metab. 12, e16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, et al. , 2005. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect. 113, 716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, et al. , 2015. Anogenital distance and penile width measurements in The Infant Development and the Environment Study (TIDES): methods and predictors. J Pediatr Urol. 11, 76.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CL, et al. , 2019. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch Toxicol. 93, 253–272. [DOI] [PubMed] [Google Scholar]
- Soukup ST, et al. , 2020. Glyphosate and AMPA levels in human urine samples and their correlation with food consumption: results of the cross-sectional KarMeN study in Germany. Arch Toxicol. [DOI] [PMC free article] [PubMed]
- Swan SH, Kristensen DM, 2018. Anogenital Distance: A Marker of Steroidal Endocrine Disruption. Human Reproduction. 30, 963b972. [Google Scholar]
- Swan SH, et al. , 2005. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 113, 1056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, et al. , 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 30, 963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R., RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, 2019a. [Google Scholar]
- Team, R. D. C., R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2019b. [Google Scholar]
- Trasande L, et al. , 2020. Glyphosate exposures and kidney injury biomarkers in infants and young children. Environ Pollut. 256, 113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S, et al. , 2011. Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. Int J Androl. 34, e578–86. [DOI] [PubMed] [Google Scholar]
- WHO, WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference. WHO Press, 2009. [Google Scholar]
- Wolf CJ, et al. , 2002. Effects of prenatal testosterone propionate on the sexual development of male and female rats: a dose-response study. Toxicol Sci. 65, 71–86. [DOI] [PubMed] [Google Scholar]
- Zoller O, et al. , 2018. Glyphosate residues in Swiss market foods: monitoring and risk evaluation. Food Addit Contam Part B Surveill. 1–9. [DOI] [PubMed] [Google Scholar]
- Zoller O, et al. , 2020. Urine glyphosate level as a quantitative biomarker of oral exposure. Int J Hyg Environ Health. 228, 113526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Population characteristics of the overall TIDES study (N=758) and this study (n=94)
Table S2. Maternal urinary glyphosate and AMPA and continuous covariates.
Table S3. Maternal urinary glyphosate and AMPA and categorical covariates.
Table S4. Fully adjusted multivariable linear regression models between maternal urinary glyphosate or AMPA and female AGD measures (N=43)
Table S5. Fully adjusted multivariable linear regression models between maternal urinary glyphosate or AMPA and male AGD measures (N=46)
Figure S1. Linearity of calibration curve, a) glyphosate and b) AMPA ranging from 0 to 5 ng/mL in urine. The area ratio represents ratio of variable concentration of glyphosate and AMPA to their respective internal standards (13C215N-Glyphosate and D213C15N-AMPA)
Figure S2. Boxplots examining the relation between urinary natural log SpG-adjusted concentration of glyphosate (A) or AMPA (B) between TIDES study centers. UCSF, University of California San Francisco; URMC, University of Rochester Medical Center; UW, University of Washington.
Figure S3. Scatterplots examining the relation between urinary natural log SpG-adjusted concentration of glyphosate or AMPA (c-d) and AGD measurements (AGD-AF and AGD-AC) in female infants (n = 45).
Figure S4. Scatterplots examining the relation between urinary natural log SpG-adjusted concentration of glyphosate or AMPA (c-d) and AGD measurements (AGD-As and AGD-AP) in male infants (n = 49).
Figure S5. Boxplots examining the relation between dichotomized (on the median) maternal urinary glyphosate or AMPA (c-d) and AGD measurements (AGD-AF and AGD-AC) in female infants (n = 45).
Figure S6. Boxplots examining the relation between dichotomized (on the median) maternal urinary glyphosate or AMPA (c-d) and AGD measurements (AGD-AS and AGD-AP) in male infants (n = 49).


