Abstract
The Research Domain Criteria (RDoC) and the Hierarchical Taxonomy of Psychopathology (HiTOP) represent major dimensional frameworks proposing two alternative approaches to accelerate progress in the way psychopathology is studied, classified, and treated. RDoC is a research framework rooted in neuroscience aiming to further the understanding of transdiagnostic biobehavioral systems underlying psychopathology and ultimately inform future classifications. HiTOP is a dimensional classification system, derived from the observed covariation among symptoms of psychopathology and maladaptive traits, which seeks to provide more informative research and treatment targets (i.e., dimensional constructs and clinical assessments) than traditional diagnostic categories. This article argues that the complementary strengths of RDoC and HiTOP can be leveraged in order to achieve their respective goals. RDoC’s biobehavioral framework may help elucidate the underpinnings of the clinical dimensions included in HiTOP, whereas HiTOP may provide psychometrically robust clinical targets for RDoC-informed research. We present a comprehensive mapping between dimensions included in RDoC (constructs and subconstructs) and HiTOP (spectra and subfactors) based on narrative review of the empirical literature. The resulting RDoC-HiTOP interface sheds light on the biobehavioral correlates of clinical dimensions and provides a broad set of dimensional clinical targets for etiological and neuroscientific research. We conclude with future directions and practical recommendations for using this interface to advance clinical neuroscience and psychiatric nosology. Ultimately, we envision that this RDoC-HiTOP interface has the potential to inform the development of a unified, dimensional, and biobehaviorally-grounded psychiatric nosology.
1. Introduction
Accurately classifying mental disorders and elucidating their etiologies remain among the greatest challenges of modern clinical psychology and psychiatry. Ongoing efforts seek to refine psychiatric nosology, but rates of misdiagnosis remain high (Regier et al., 2013) and available categorical classification systems, including the Diagnostic and Statistical Manual of Mental Disorders (DSM) (APA, 2013) and the International Classification of Diseases (ICD) (WHO, 1992), carry limited clinical utility for prognosis and treatment (Conway et al., 2019). With regard to etiology, we lack a complete understanding of the causes of any mental disorder, despite considerable efforts in elucidating their genetic (Waszczuk et al., 2020) and neural (Thompson et al., 2020) bases.
These two issues (i.e., diagnostic classification and etiology) are strongly interdependent. On the one hand, developing a refined taxonomy of clinical problems requires a better understanding of the antecedents and processes that lead to different forms psychopathology in order to develop better treatments. On the other hand, progress in understanding the etiology of mental illnesses is hindered by common misdiagnosis and limited validity of diagnoses based on traditional nosologies. Integrating advances in empirical research on diagnostic classification and etiology has the potential to accelerate progress in these fields (Latzman et al., 2020; Waszczuk et al., 2020).
This article proposes an interface to advance these two fronts by leveraging two major initiatives that are using dimensional approaches to promote a radical change in the way mental illness is studied and classified: the Research Domain Criteria (RDoC) framework by the National Institute of Mental Health (NIMH) (Cuthbert & Insel, 2013; Kozak & Cuthbert, 2016) and the Hierarchical Taxonomy of Psychopathology (HiTOP) (Kotov et al., 2017; Kotov et al., 2021; Krueger et al., 2018). We start with an overview of the challenges of psychiatric nosologies and the solutions offered by RDoC and HiTOP, highlighting the complementary nature of these frameworks and providing rationale for an interface that bridges between them. We then present a comprehensive map of the interface linking between RDoC constructs and HiTOP spectra based on narrative review of the empirical literature. We conclude with examples of integrative research linking biobehavioral systems with dimensions of psychopathology, and with recommendations for implementing this approach in future studies with the goal to promote progress in elucidating the causes of psychopathology and improving future classifications.
2. Dimensional classifications of psychopathology
Extensive evidence points to major limitation of categorical classifications that make them a poor guide for research and clinical practice, as explained in detail elsewhere (Cuthbert & Insel, 2013; Kotov et al., 2017). First, categorical diagnoses do not adequately reflect the extensive evidence that forms of psychopathology and their underlying processes are continuous in nature (Cuthbert & Insel, 2013; Markon & Krueger, 2005). The imposition of artificial categories can lead to low diagnostic reliability (Markon et al., 2011), diagnostic instability (due to symptoms presenting just above or below the clinical cut-off), and failure to recognize subthreshold presentations that can be associated with poor functional outcomes and increased risk for more severe psychopathology (Shankman et al., 2009). Second, traditional diagnoses are based on subjective reports or observations of symptoms and do not consider underlying etiological and pathophysiological mechanisms that largely cut across diagnostic boundaries, although this information may be important for selecting effective treatments (Bzdok & Meyer-Lindenberg, 2018). Third, traditional classifications focus on individual diagnoses and ignore widespread comorbidity and developmental continuity between disorders (Caspi et al., 2020), which can substantially undermine prediction of illness course and correct treatment decisions (McIntyre et al., 2012). Fourth, DSM and ICD do not provide tools to consider the extensive heterogeneity within each diagnosis, which makes individuals with the same diagnosis greatly differ from one another and can produce variable treatment response (Fried, 2017). Fifth, overlap in symptoms across different diagnostic categories (e.g., distractibility, anhedonia) can make differential diagnosis especially difficult and contributes to misdiagnosis (Asherson et al., 2014).
Although DSM-5 and ICD-11 are making progress in addressing some of these limitations (Clark et al., 2017), they remain largely categorical. Two major dimensional frameworks, RDoC and HiTOP, have emerged in the last decade to address the limitations of categorical classifications with two different strategies.
2.1. What is RDoC?
The RDoC framework was developed by the National Institute of Mental Health in 2009 to guide research on the neurobiological bases of psychopathology organized around biobehavioral dimensions, and ultimately inform efforts to refine psychiatric classifications (Cuthbert & Insel, 2013; Insel et al., 2010). The approach proposed by RDoC mainly addresses the first (dimensional nature of psychopathology), second (lack of consideration of underlying underpinnings), and fourth (within-disorder heterogeneity) aforementioned limitations of categorical systems. RDoC tackles these limitations by encouraging research on fundamental dimensional biobehavioral systems (e.g., cognition, social processes) cutting across traditional boundaries, as well as on their associations with behavioral manifestations, rather than diagnostic categories, in heterogeneous clinical populations (Cuthbert & Insel, 2013; Kozak & Cuthbert, 2016). A more explicit consideration of the dimensional and heterogeneous nature of psychopathology and its underpinnings is expected to elucidate the processes underlying mental health problems and, in turn, inform their future classification (Cuthbert & Insel, 2013).
The RDoC framework is operationalized in the RDoC matrix (https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/constructs/rdoc-matrix.shtml) (Figure 1A). The matrix columns represent eight units of analysis (genes, molecules, cells, circuits, physiology, behavior, paradigms, and self-reports), and the rows represent constructs and subconstructs, representing functional dimensions characterized by specific elements within each units of analysis. Constructs are grouped into six higher-level domains: Negative Valence Systems, Positive Valence Systems, Cognitive Systems, Systems for Social Processes, Arousal/Regulatory Systems, and Sensorimotor Systems. Domains, constructs/subconstructs, and units of analysis were defined based on consensus of experts from relevant fields who reviewed the scientific knowledge about major systems relevant to typical and atypical human behavior (Clark et al., 2017; Kozak & Cuthbert, 2016).
Figure 1.
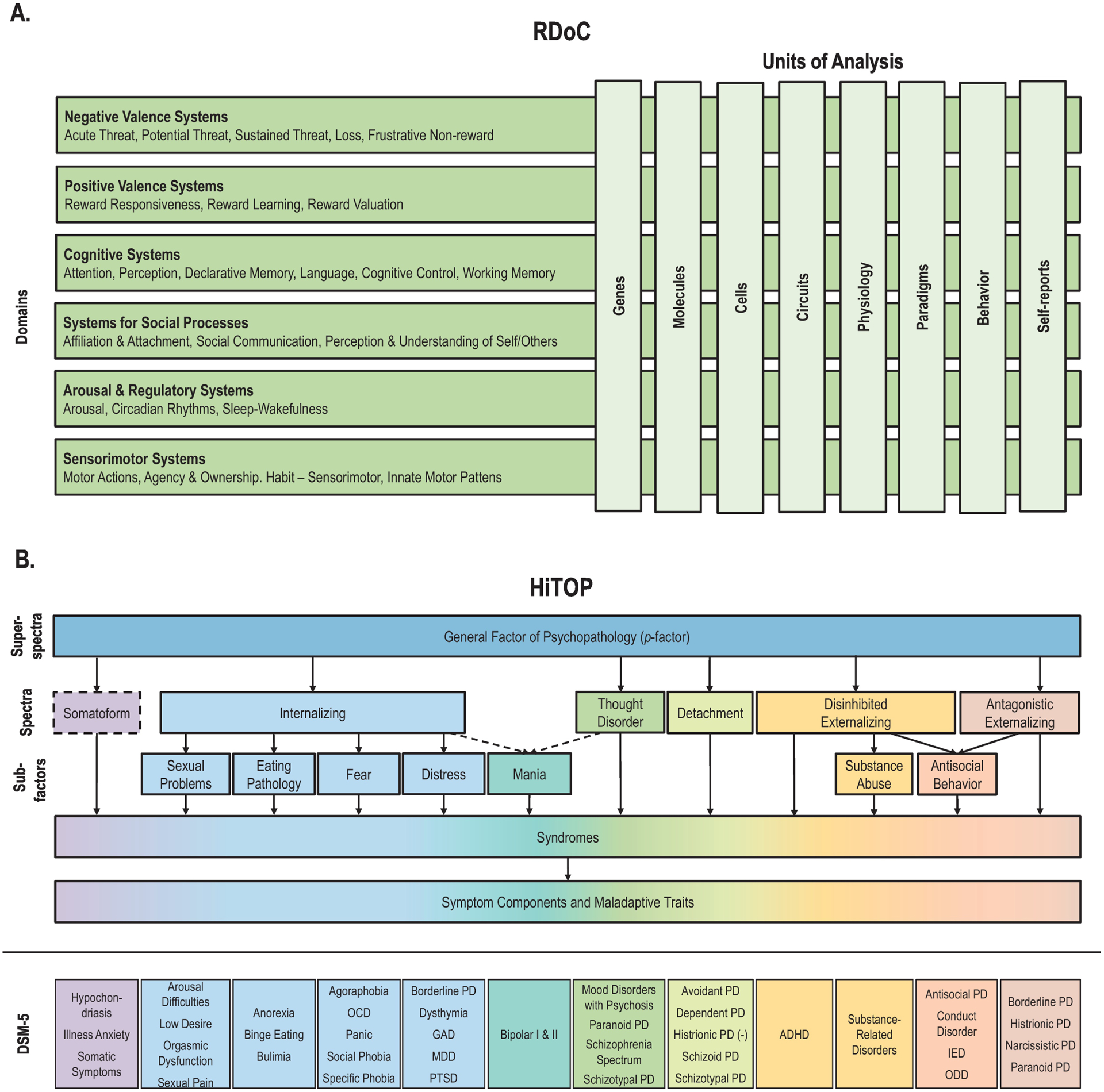
Research Domain Criteria (RDoC) and Hierarchical Taxonomy of Psychopathology (HiTOP).
Notes: A. The RDoC matrix includes six domains (rows) and their respective constructs (some of which also include subconstructs, not shown here), which can be characterized on the basis of eight units of analysis (columns). B. HiTOP dimensions span from super-spectra (broader and more general) to symptoms components and maladaptive traits (narrower and more specific). Dashed lines indicate provisional elements requiring more study. DSM diagnoses are not part of the HiTOP model and are only included here to allow mapping of their symptoms and signs onto HiTOP. DSM diagnoses mapping onto more than one HiTOP spectrum or subfactor are listed in multiple places. (−) indicates negative association between histrionic personality and the Detachment spectrum.
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; GAD, generalized anxiety disorder; IED, intermittent explosive disorder; MDD, major depressive disorder; OCD, obsessive–compulsive disorder; ODD, oppositional defiant disorder; PD, personality disorder; PTSD, posttraumatic stress disorder.
2.2. What is HiTOP?
HiTOP is a system developed by a consortium of scientists studying psychiatric nosology (Kotov et al., 2018; Kotov et al., 2017) that proposes a hierarchical dimensional classification of mental health problems. The HiTOP model was constructed based on extensive research using factor analysis and latent class analysis with the goal of organizing psychopathology according to the natural covariance structure of signs, symptoms, maladaptive behaviors and traits, following a long-established quantitative approach to nosology (Achenbach, 1966; Kotov et al., 2017). A major motivation of HiTOP is to reorganize and reconceptualize clinical classification in order to improve clinical practice and inform treatment and prevention, as discussed in detail elsewhere (Mullins-Sweatt et al., 2020; Ruggero et al., 2019; https://hitop.unt.edu/clinical-tools).
HiTOP’s approach primarily aims to address the first (dimensional nature of psychopathology), third (widespread comorbidity), fourth (within-disorder heterogeneity) and fifth (symptom overlap) aforementioned limitations of current categorical systems. The model seeks to resolve these issues by delineating broader dimensions that explain psychiatric comorbidity as well as specific dimensions that accommodate within-disorder heterogeneity and symptom overlap between different conditions (Kotov et al., 2020; Kotov et al., 2017; Krueger et al., 2018). The hierarchical organization of HiTOP describes psychopathology at different levels of breadth and specificity (Figure 1B): components are specific dimensions of symptoms and maladaptive behavior (e.g., dysphoria, suicidal ideation); highly correlated components form broader dimensional syndromes (e.g., depression); closely related syndromes define subfactors (e.g., distress); and related subfactors form spectra (e.g., internalizing). More general dimensions also have been described, such as the general factor of psychopathology or p-factor (Caspi & Moffitt, 2018; Lahey et al., 2012). Six spectra have been included in HiTOP so far: Internalizing, Disinhibited Externalizing, Antagonistic Externalizing, Thought Disorder, Detachment, and Somatoform. Seven subfactors have been identified within these spectra (Kotov et al., 2017). Of note, traditional diagnostic categories are not part of HiTOP, as the model seeks to include and reorganize symptoms that constitute these diagnoses.
The model currently incorporates the most common forms of psychopathology, as well as a number of rare conditions (Figure 1B). Conditions that have not been examined in rigorous quantitative research of this type (e.g., most neurodevelopmental problems) are not yet in the system, but are the focus of ongoing efforts (Michelini et al., 2019). The dimensions in the models have been validated with regard to etiology (e.g., genetic and neural underpinnings), predictive validity, and utility for clinical practice (Conway et al., 2019; Kotov et al., 2017; Ruggero et al., 2019; Waszczuk et al., 2020). Although etiological or biological parameters are currently not included, it is expected that empirically-derived and reliable dimensions will have more coherent etiology than traditional categories, thereby producing stronger and more specific profiles of risk factors, biomarkers, and outcomes, as described in detail elsewhere (Kotov et al., 2020; Krueger et al., in press; Watson et al., in press).
2.3. Similarities and differences between RDoC and HiTOP?
Before describing how RDoC and HiTOP are interconnected, we note key similarities and differences between the two approaches. RDoC and HiTOP share core features:
Intent to move away from diagnostic categories: Both frameworks recognize the aforementioned limitations of diagnostic categories and emphasize the need for a dimensional approach to psychiatry.
Work-in-progress approach: Both frameworks are designed to evolve with new evidence, as overseen respectively by the National Advisory Mental Health Council Workgroup on Changes to the RDoC Matrix and the HiTOP Revisions Workgroup. For example, the RDoC Sensorimotor Systems domain involved in control and execution of motor behaviors was added in January 2019 (NIMH, 2019).
Notable differences, however, also exist:
Delineation of dimensions: RDoC domains and constructs were rationally defined based on consensus of experts regarding biobehavioral systems relevant to mental health. HiTOP dimensions reflect a replicated empirical structure of psychopathology based on covariation among signs, symptoms, diagnoses, and maladaptive behaviors.
Content and units of analysis: RDoC spans from genes to brain to behavior, placing particular emphasis on neurobiology (e.g., circuits), whereas HiTOP focuses on signs, symptoms, diagnoses, and maladaptive behaviors.
Current gaps and limitations: The RDoC matrix includes, alongside units of analyses relevant to studying to neural and physiological correlates, units called behavior and self-report, but at present these units do not intend to include the majority of signs, symptoms, and behaviors requiring clinical attention (e.g., suicidality, hazardous alcohol use) (Kozak & Cuthbert, 2016; Patrick & Hajcak, 2016). Furthermore, many self-report, behavioral, and task exemplars included in RDoC have inadequate or unclear psychometric properties and were not developed to operationalize RDoC constructs as RDoC defines them (Patrick & Hajcak, 2016; Watson et al., 2017). Consequently, RDoC is currently designed as a research framework for translational and clinically-relevant research, but has limited application in clinical practice (Clark et al., 2017; Kozak & Cuthbert, 2016). In contrast, HiTOP dimensions are based on descriptions of clinical phenomena, assessed with clinical interviews, observer-reports, and self-reports (Kotov et al., 2017), but do not take into account their underlying underpinnings. As such, the system remains agnostic with regard to the etiology of psychopathology, even though clarifying the underpinnings of clinical problems included in HiTOP is important for aiding diagnostic and treatment decisions.
3. A new interface linking RDoC and HiTOP
The complementary nature and strengths of RDoC and HiTOP can be leveraged to advance understanding of psychopathology and inform clinical practice (Latzman et al., 2020). Since RDoC currently lacks direct clinical applicability, HiTOP may aid RDoC-informed research by providing psychometrically robust clinical targets. Additionally, since HiTOP does not consider the etiology of psychopathology, RDoC provides a solid transdiagnostic framework for elucidating the underpinnings of the clinical problems in HiTOP beyond what is possible using current diagnoses. The specific aim of the present narrative literature review is to delineate an interface between the two systems. The interface supported by our narrative review can help build a “common language” between RDOC and HiTOP, provoke novel hypotheses for future studies, and encourage future systematic reviews or meta-analyses focused on specific connections. Ultimately, bridging between RDoC and HiTOP may accelerate etiological and translational neuroscience research and inform future clinically-useful classifications that explicitly include psychopathological underpinnings. This will facilitate development of novel treatments targeting mechanisms and with potential effects on a broader range of conditions subsumed by more general dimensions, which are expected to be more efficient for treating commonly co-occurring problems (Barlow et al., 2017; Bzdok & Meyer-Lindenberg, 2018).
Given the broad literature covered by our review, a systematic review of the myriad possible connections between RDoC and HiTOP dimensions was unfeasible and beyond the scope of our article, in line with previous narrative reviews on RDoC and DSM diagnoses (Koudys et al., 2019; Lebowitz et al., 2018). Rather, we present connections between RDoC and HiTOP by reviewing available empirical articles or reviews that focused on markers of these two frameworks, helping us outline an interface between them. We delineated links between RDoC and HiTOP at the most specific level possible: subconstructs or constructs for RDoC and spectra or subfactors for HiTOP (as research on associations of more specific HiTOP elements with RDoC is very limited). With regard to RDoC, we focused on studies investigating elements from the self-reports, paradigms, behaviors, physiology, or circuits RDoC units of analysis, included in the matrix as of April 2020 (other units have not been sufficiently studied together with HiTOP dimensions). These elements of the RDoC matrix were used as exemplars of markers of specific constructs/subconstructs and to distinguish between different constructs/subconstructs within the same domain (e.g., Acute Threat vs. Potential Threat). In the instance of RDoC constructs/subconstructs including elements that are common across several RDoC constructs and domains (mostly from circuits and physiology units of analysis, e.g., amygdala, hypothalamic–pituitary–adrenal axis), we focused on other units of analyses (e.g., behavior and self-report) that could allow us to draw more specific associations. To operationalize HiTOP dimensions, we focused on psychopathology constructs conformant with latent dimensions included in the HiTOP system (i.e., derived through latent variable modeling) or measured by scales capturing symptom or trait dimensions included in HiTOP (Kotov et al., 2017). We further considered empirical studies or reviews focused on multiple categorical disorders that fall under one or more spectra (e.g., studies of various internalizing and/or psychotic disorders). Studies including cases of one disorder and controls were considered if they examined continuous associations between RDoC and HiTOP markers, but not if they only reported case-control comparisons, as the latter provide limited information about RDoC and HiTOP dimensions.
Some caveats to the literature review should be considered. First, although HiTOP includes most forms of psychopathology, it does not yet cover some conditions, such as autism and learning, motor, or intellectual difficulties, which are therefore not reviewed here. Second, HiTOP provisionally includes a Somatoform spectrum, but this dimension is relatively understudied, especially with regard to its behavioral-neural correlates, and thus not included in our review. Third, since the psychopathological correlates of the RDoC Sensorimotor domain have been rarely investigated to date, it was not possible to identify associations with HiTOP dimensions.
4. Literature review
Our review identified numerous HiTOP correlates of each RDoC construct/subconstruct.
4.1. Negative Valence Systems (Supplementary Figure 1)
Negative Valence Systems include five constructs involved in response to aversive situations.
4.1.1. Acute Threat (Fear)
Markers of RDoC Acute Threat show robust associations with several forms of psychopathology (i.e., panic disorder, agoraphobia, social phobia, specific phobias, obsessive-compulsive disorder) included in HiTOP Fear, a subfactor of the Internalizing spectrum. Several psychophysiological markers, including increased skin conductance and heart rate in the context of defensive reactivity (e.g., exposure to an acute threat), have been related to panic attacks and agoraphobia (Hamm et al., 2016). One study further found that a latent acute-threat factor—defined by self-rated trait fearfulness and physiological reactions potentiated by aversive pictures—was strongly associated with fear disorders, especially phobias (Yancey et al., 2016). At the neural level, some studies have found stronger associations of hyperactivation of the fear circuit (amygdala, insula, anterior cingulate and prefrontal cortex) with fear disorders (e.g., panic disorder and phobias) than with distress disorders (e.g., generalized anxiety disorder and posttraumatic stress disorder) (Brühl et al., 2014; Duval et al., 2015). Alterations in behavioral markers of RDoC Acute Threat, such as increased avoidance of an acute threat (‘flight’ response) and freezing, are also common markers of fear disorders (Blanchard et al., 2011; Corr & Cooper, 2016). Among paradigms, the CO2 challenge has been shown to capture individual differences in panic symptoms (Battaglia et al., 2014; Rapee et al., 1992). Finally, the association between RDoC Acute Threat and HiTOP Fear is supported by evidence showing that the four dimensions of feared stimuli included in the Fear Survey Schedule (a self-report marker of RDoC Acute Threat) are consistent with the forms of psychopathology included in the Fear subfactor (Beck et al., 1998).
Of note, the association between RDoC Acute Threat and HiTOP Fear appears less supported by studies using fear conditioning paradigms. Multiple anxiety disorders (falling under both Fear and Distress within HiTOP) are associated with increased fear responses only to stimuli associated with safety (i.e., CS−), as opposed to actual danger (i.e., CS+), suggesting that these conditions may not be associated with responses to immediate threat (Duits et al., 2015). It may be argued, however, that fear conditioning paradigms measure processes related to fear acquisition and learning, rather than acute response to threat. Research examining multiple markers of RDoC Acute Threat is needed to clarify the potential sources of these inconsistencies.
4.1.2. Potential Threat (Anxiety)
Substantial evidence indicates an association between RDoC Potential Threat and HiTOP Fear. Enhanced startle potentiation to uncertain threat has been consistently associated with fear disorders (e.g., social anxiety disorder, specific phobia) (Gorka et al., 2017; Yancey et al., 2015). In a study of adults with various internalizing conditions, startle potentiation to uncertain threat was more strongly associated with fear disorders than distress disorders (e.g., generalized anxiety disorder, major depression) (Gorka, Lieberman, Shankman, & Phan, 2017).). Neuroimaging evidence further suggests common neural bases during processing of threatening stimuli (exaggerated anterior insula reactivity during unpredictable threat and brainstem reactivity during predictable threat) across individuals with internalizing conditions, although brainstem activity during unpredictable threat correlated significantly with a fear dimension, but not with a distress/misery dimension (Radoman et al., 2019). Further, self-report markers of RDoC Potential Threat, such as the Fear of Negative Evaluation scale and the Behavioral Inhibition System scale, show associations with conditions included in HiTOP Fear (Carleton et al., 2011; Corr & Cooper, 2016).
Other studies suggest a differential association of RDoC Potential Threat with the HiTOP Fear and Distress subfactors. Potentiated startle responses may be blunted at high levels of the HiTOP Distress subfactor (McTeague & Lang, 2012; Vaidyanathan et al., 2012; Yancey et al., 2015). An RDoC-informed study showed that a distress/negative affectivity factor was negatively associated with defensive physiological reactivity (startle and heart rate reactivity during fear imagery) (Lang et al., 2016). Similarly, a recent meta-analysis found induced anxiety (e.g., adaptive response to unpredictable threats) and specific phobia, social phobia, and panic disorder (i.e., fear disorders) share similar patterns of hyperactivation in insula, cingulate and prefrontal regions (Chavanne & Robinson, 2021). Conversely, little convergence was found between the underpinnings of induced anxiety and those of PTSD and GAD (i.e., distress disorders), with the latter disorder showing deactivation in insula and cingulate cortex. These findings using physiological and neural markers appear in contrast with evidence on self-report markers of RDoC Potential Threat (anxiety sensitivity and intolerance to uncertainty) suggesting a positive relationship with HiTOP Distress. A meta-analysis on anxiety sensitivity found positively associations with both distress and fear factors derived from associations among internalizing disorders (Naragon-Gainey, 2010). Increased intolerance to uncertainly has also been related with both distress and fear psychopathology (Carleton et al., 2012; Correa et al., 2019), although some evidence suggests a stronger positive relationship with depressive symptoms (Sexton et al., 2003), which is subsumed under HiTOP Distress. Further research is needed to clarify whether different markers of RDoC Potential Threat show differential associations with HiTOP Distress and show more specific associations with certain aspects of HiTOP Internalizing.
4.1.3. Sustained Threat
RDoC Sustained Threat appears robustly linked with HiTOP Distress, as individuals with high sustained threat show high levels of distress symptoms, especially depression and posttraumatic stress (Pryce et al., 2011; Spinhoven et al., 2016). At the neural level, altered neurocircuitry of sustained threat (e.g., amygdala reactivity) has been associates with depression (Henje Blom et al., 2016; Ross et al., 2017). Further, distress psychopathology has been linked with RDoC behavioral markers (e.g., punishment sensitivity, helpless behavior, anxious arousal, and attention bias) (Gentes et al., 2015; Gibb et al., 2016; Harnett, Loxton, & Jackson, 2013; Pryce et al., 2011; Torrubia, Ávilab, Moltób, & Caseras, 2001) and self-report markers (Childhood Trauma Questionnaire) (Spinhoven et al., 2016) of Sustained Threat.
4.1.4. Loss
RDoC Loss also appears robustly linked with HiTOP Distress. This association is supported by studies of the behavioral manifestations of Loss, such as shame, guilt, sadness, worry, hopelessness, and rumination, with show clear links with distress symptoms, especially depression (Grierson et al., 2016; Mazzer et al., 2019; Robinaugh & McNally, 2010; Watson et al., 2012; Watson et al., 2017). This link to HiTOP Distress is also supported by markers in the circuits unit of analysis, such as habit system and reward circuit, which are key to Positive Valence Systems. This evidence is thus discussed more in detailed in section 4.2.
4.1.5. Frustrative Non-Reward
RDoC Frustrative Non-Reward has few markers that apply to humans. Research on behavioral and self-report markers (e.g., Questionnaire of Daily Frustrations) indicate positive associations with depression (Baars et al., 2011) and aggression (Baars et al., 2011; Geniole et al., 2017; Smeets et al., 2017). These studies suggest emerging links of RDoC Frustrative Non-Reward, respectively, with the HiTOP Distress subfactor of the Internalizing spectrum and the HiTOP Antisocial Behavior subfactor of the Disinhibited and Antagonistic Externalizing spectra.
4.2. Positive Valence Systems (Supplementary Figure 2)
Positive Valence Systems are primarily involved in positive or appetitive motivational contexts and comprises three broad constructs, each with three subconstructs.
4.2.1. Reward Responsiveness
The RDoC Reward Anticipation subconstruct is associated with the HiTOP Disinhibited Externalizing spectrum and its Substance Abuse subfactor. Neuroimaging studies using monetary incentive delay (MID) tasks have revealed atypical functioning and/or functional connectivity between the ventral striatum and prefrontal structures (i.e., anterior and ventromedial prefrontal cortex) in relation to symptoms of impulsivity, inattention, and hyperactivity (Hahn et al., 2009; Plichta & Scheres, 2014; Stark et al., 2011). Further, reduced anticipatory signaling has been observed alongside disruption in mesolimbic-dopaminergic systems in addiction-based psychopathology (Balodis & Potenza, 2015; Luijten et al., 2017; Robinson & Berridge, 2001). This RDoC subconstruct also appears linked to the HiTOP Thought Disorder spectrum, as reduced anticipatory signaling in the ventral striatum is associated with negative symptoms of schizophrenia (Radua et al., 2015) Associations of MID-related dysfunctional anticipatory reward processing (decreased caudate activation, increased activation in the middle frontal gyrus, anterior cingulate cortex [ACC] and frontal lobe) with depressed mood further suggests an emerging link to the HiTOP Distress subfactor (Dillon et al., 2014; Zhang et al., 2013).
Markers of RDoC Initial Response to Reward demonstrate a robust link to the HiTOP Distress subfactor, based on associations of depressed mood with blunted event-rated potentials (ERPs) and behavior during simple guessing tasks (Baskin-Sommers & Foti, 2015; Foti & Hajcak, 2009; Nelson et al., 2016; Nielson et al., 2021; Weinberg et al., 2015). For example, blunted reward positivity prospectively predicts increases in depressive symptoms (Nelson et al., 2016). Further, depressive symptoms are associated with a decreased responsiveness and failure to alter behavioral responses following reward attainment (Foti & Hajcak, 2009). An additional association between RDoC Initial Response to Reward and the HiTOP Substance Abuse subfactor is supported by evidence that reduced or absent activations in the orbitofrontal cortex (OFC), amygdala, and striatum, as well as sensory processing regions, in response to monetary reward or verbal/visual feedback is related with addiction (Diekhof et al., 2008; Koob & Volkow, 2016; Luijten et al., 2017; Redish et al., 2008; Volkow et al., 2010). Further links can be delineated with the HiTOP Disinhibited Externalizing spectrum, via positive associations between psychophysiological markers of reward sensitivity and disinhibitory traits, inattention and hyperactivity-impulsivity (Carlson et al., 2013; Luman et al., 2005) (although negative associations have also been reported (Babinski et al., 2019; Plichta & Scheres, 2014)), and to the HiTOP Antisocial Behavior subfactor, based on reward-driven antisocial and callous-aggressive tendencies (Byrd et al., 2014; Carlson et al., 2013; Frick & White, 2008).
Finally, since markers of RDoC Reward Satiation have not yet been reliably linked to psychopathology, it was not possible to identify links to HiTOP.
4.2.2. Reward Learning
The RDoC Probabilistic and Reinforcement Learning subconstruct shows an association with the HiTOP Distress subfactor. Using probabilistic reward tasks (e.g., Pizzagalli et al., 2009), blunted reward learning has been observed in distress disorders (major depressive disorder, post-traumatic stress disorder, generalized anxiety disorder), as indicated by poor or inflexible modulation of behavior (Dillon et al., 2014; Halahakoon et al., 2020; Morris et al., 2015) and reduced nucleus accumbens and ACC activity (Baskin-Sommers & Foti, 2015). An additional link to the HiTOP Substance Abuse subfactor is supported by robust associations of neural mechanisms of disrupted reward learning (activity within the prefrontal cortex, dorsal striatum, and basolateral amygdala) (Hyman et al., 2006; Redish et al., 2008; Wassum & Izquierdo, 2015) and distorted Pavlovian conditioning (Baskin-Sommers & Foti, 2015; Bevins & Murray, 2011; MacRae et al., 1987) with addiction. This subconstruct also shows an association, although potentially weaker, with the HiTOP Thought Disorder spectrum, via research demonstrating that abnormal neural responses in reward learning via meso-corticolimbic and meso-striatal function (Radua et al., 2015; Ziauddeen & Murray, 2010), rigid reward learning (Deserno et al., 2013), and impaired reversal learning (Ziauddeen & Murray, 2010) are associated with psychotic symptoms.
RDoC Reward Prediction Error is robustly linked to the HiTOP Substance Abuse subfactor. At the neural level, impaired fronto-striatal connectivity (Park et al., 2010) and increased activity of dopaminergic neurons and projections within the striatum, amygdala, and orbitofrontal cortex (OFC; Keiflin & Janak, 2015; Schultz, 2011; Volkow et al., 2010) have been observed in substance use populations in the context of reward prediction. This association is also supported by increases in sign-tracking behavior (Redish et al., 2008), and self-reported craving (Chase et al., 2011; Serre et al., 2015). An additional link may be delineated with the HiTOP Antisocial Behavior subfactor, as indicated by associations of aberrant dopaminergic functioning and neural circuitry to expectancy violations (e.g., OFC, ACC, amygdala) with disruptive behaviors (Blair et al., 2013; Estrada et al., 2019; Sonuga-Barke et al., 2016). Further, some evidence suggests that reduced activity in the dopaminergic frontal, striatal, and limbic system underlying prediction error are associated with psychotic symptoms, suggesting an emerging link to HiTOP Thought Disorder (Corlett & Fletcher, 2012; Radua et al., 2015).
Finally, the RDoC Habit subconstruct also appears linked to the HiTOP Substance Abuse subfactor, as the development of substance-related habits and compulsive behaviors are robustly associated with increased activity in the dorsal striatum and its dopaminergic projections (Everitt & Robbins, 2005; Newlin & Strubler, 2007; Ostlund & Balleine, 2008; Sjoerds et al., 2013; Yin et al., 2008).
4.2.3. Reward Valuation
The RDoC Reward (probability) subconstruct shows a robust link with the HiTOP Antisocial Behavior subfactor, as externalizing behavior is associated with impaired probability discounting, thereby increasing the likelihood of riskier behavioral choices (Olson et al., 2007; Sparks et al., 2014). An additional link can be drawn to the HiTOP Substance Abuse subfactor via a short-term probability discounting process, though findings are mixed and careful consideration should be given to the distinction between probabilistic discounting versus delay discounting (discussed below) in addiction (Redish et al., 2008; Yi et al., 2010).
The RDoC Delay subconstruct demonstrates robust links to the HiTOP Disinhibited Externalizing spectrum and its Substance Abuse and Antisocial Behavior subfactors, respectively supported by associations of greater delay discounting with impulsivity and inattention (Jackson & MacKillop, 2016; Patros et al., 2016; Pauli-Pott & Becker, 2011), substance use (Bickel & Marsch, 2001; Redish et al., 2008; Reynolds, 2006; Yi et al., 2010), and antisocial behavior (Bobova et al., 2009; Dai et al., 2016; Sparks et al., 2014; Woltering et al., 2016).
Research on markers of the RDoC Effort subconstruct reveals an association with HiTOP Substance Abuse, as indicated by associations of addiction with heightened self-reported drive and rash effort-based decision making (Dawe et al., 2004; Franken, 2002), as well as increased fronto-striatal activity associated with effort calculation and expenditure (Redish et al., 2008; Volkow et al., 2010). Further, self-report and task-based behavioral markers link this RDoC subconstruct to disorders and symptoms pertaining to HiTOP Distress via deficient willingness to exert effort and decreased drive (Culbreth, Moran, & Barch, 2018). Indeed, the Effort-Expenditure for Rewards Task (EEfRT) probes effort-based decision making which show robust associations to depressive symptoms, particularly anhedonia (Dillon et al., 2014; Treadway et al., 2009). Finally, an additional link can be delineated with HiTOP Thought Disorder via associations of negative symptoms of schizophrenia with impaired effort-based decision making (Green et al., 2015; Reddy et al., 2016) and effort-cost computations (Fervaha et al., 2013).
4.3. Cognitive Systems (Supplementary Figure 3)
Cognitive Systems cover six cognitive constructs and their subconstructs.
4.3.1. Attention
Existing research indicates a robust association between markers of RDoC Attention and the HiTOP Disinhibited Externalizing spectrum. With regard to neural circuitry, an imbalance (i.e., reduced anti-correlation) between task positive network (TPN) and default mode network (DMN) is considered a key correlate of inattentive and hyperactive-impulsive symptoms (Gerrits et al., 2019; Sonuga-Barke & Castellanos, 2007). Further, increased intra-individual variability in response times, thought to reflect attentional lapses and to also arise from TPN-DMN imbalance (Sonuga-Barke & Castellanos, 2007), is robustly associated with inattentive, hyperactive-impulsive, and externalizing symptoms (Agnes Brunnekreef et al., 2007; Bastiaansen et al., 2015; Kuntsi et al., 2014; Michelini et al., 2018). The link between RDoC Attention and the Disinhibited Externalizing spectrum is also support by studies indicating a link to the Substance Abuse subfactor of this spectrum, based on associations between attentional impairments and substance use in cross-sectional (Indlekofer et al., 2009; Thoma et al., 2011) and prospective longitudinal studies (Tapert et al., 2002). Another robust association can be delineated between RDoC Attention and HiTOP Thought Disorder. Neural markers of attentional dysfunction (e.g., ERPs reflecting atypical gating, ventral attention network) have been associated with delusional ideation (Galderisi et al., 2014; Lavigne et al., 2020). Increased attentional lapses, distractibility, and impairments in attentional task performance are also robustly associated with symptoms of schizophrenia (Guastella et al., 2013) and positive schizotypy (Marsh et al., 2017; Mohanty et al., 2008; Siddi et al., 2017). The association of attentional lapses, distractibility with manic symptoms further suggests an emerging association between RDoC Attention and the HiTOP Mania subfactor (Glaus et al., 2018; Pagliaccio et al., 2017), which in HiTOP is provisionally connected to both the Thought Disorder and Internalizing spectra.
Additional evidence indicates that RDoC Attention may be linked with HiTOP Detachment, based on associations of poor performance in sustained attention tasks with negative schizotypy (Moreno-Samaniego et al., 2017; Siddi et al., 2017). Another emerging association, although potentially weaker, is with the HiTOP Distress subfactor, supported by associations of attentional markers (e.g., increased reaction time variability, distractibility) with depression and suicidality (Hsu et al., 2019; Keller et al., 2019; van Deurzen et al., 2012).
4.3.2. Perception
RDoC Perception shows a clear association with HiTOP Thought Disorder. This link is supported, at the neural level, by associations of attenuated auditory and visual ERPs (e.g., reduced mismatch negativity, P50, N1, P2, P3) with symptoms of schizophrenia, positive schizotypy, and high psychosis risk (Earls et al., 2016; Pokorny et al., 2019; Premkuma et al., 2014). Low performance on a visual integration task (Jittered Orientation Visual Integration task) has also been associated with positive and disorganized schizotypy (Panton et al., 2018). Further, this link is supported by evidence of visual and/or auditory perceptual abnormalities (e.g., hallucinations) in individuals with psychotic disorders and at high psychosis risk (Baumeister et al., 2017; Keane et al., 2018; Lehembre-Shiah et al., 2017; Panton et al., 2016).
Although limited evidence exists to link RDoC Olfactory/Somatosensory/Multimodal Perception with HiTOP, associations between olfactory perceptual deficits and psychotic symptoms suggest an emerging link with HiTOP Thought Disorder (Kamath et al., 2018; Kastner et al., 2013).
4.3.3. Declarative Memory
RDoC Declarative Memory shows robust associations with HiTOP Thought Disorder, based on studies linking behavioral deficits and atypical hippocampal function and circuitry with psychotic disorders and psychosis risk (Cirillo & Seidman, 2003; Ivleva et al., 2012; Seabury & Cannon, 2020; Stone & Hsi, 2011). Negative associations between behavioral markers of episodic memory and depressive symptoms further indicate a potential link with the HiTOP Distress subfactor (Huber et al., 2019; Talarowska et al., 2016).
4.3.4. Language
RDoC Language shows a robust link with HiTOP Thought Disorder, as indicated by associations of alterations in brain circuits underlying verbal fluency, language, and sematic systems (Costafreda et al., 2011; Sumner et al., 2018) and behavioral markers of reduced verbal fluency (Docherty et al., 2011; Krabbendam et al., 2005; Siddi et al., 2017) with psychotic symptoms. Further, associations between low verbal task performance and negative schizotypy (Siddi et al., 2017) suggest an additional tentative link with HiTOP Detachment.
4.3.5. Cognitive Control
The RDoC Goal Selection, Updating, Representation, and Maintenance subconstruct, which shares several elements with the RDoC Attention construct, shows robust associations with the HiTOP Disinhibited Externalizing spectrum. This link is based on associations of self-reported executive functions (e.g., Behavior Rating Inventory of Executive Function) with greater disinhibitory traits (Long et al., 2015; Teivaanmaki et al., 2019) and inattentive and hyperactive-impulsive symptoms (Mahone et al., 2002; Mahone & Hoffman, 2007). This link is also supported for the Substance Abuse subfactor of this HiTOP spectrum, based on associations between difficulties maintaining goal-directed behavior (e.g., indexed by executive control over incentive salience and PFC activity) and addiction (Koob & Volkow, 2016). This RDoC subconstruct further shows robust associations with HiTOP Thought Disorder, based on by studies indicating atypical fronto-thalamo-parietal circuitry across psychotic disorders (Lencer et al., 2019) and reporting associations of impaired goal selection and updating with psychotic disorders (Barch & Sheffield, 2014) and positive schizotypy (Steffens et al., 2018). Additionally, a meta-analysis showing an association, albeit of small size, between updating and negative schizotypy supports a further link with HiTOP Detachment (Steffens et al., 2018).
The RDoC Response Selection and Inhibition/Suppression subconstruct shows a robust link with HiTOP Thought Disorder, based on studies reporting associations of altered neural circuitry (e.g., reduced dorsolateral prefrontal activity) (Bourque et al., 2017; Van Voorhis et al., 2019) and behavioral performance impairments (Ethridge et al., 2014; Huddy et al., 2013; Wright et al., 2014) during response inhibition tasks (e.g., in Go/No-Go and Stroop tasks) with psychotic disorders and psychotic symptoms. Another robust link can be drawn to the HiTOP Disinhibited Externalizing spectrum, as atypical brain activity (e.g., reduced event-related theta power) and behavioral markers (e.g., prepotent inhibition, impulsive errors) of response inhibition have been related to disinhibitory traits, inattention, and hyperactivity-impulsivity (Crosbie et al., 2013; Leshem, 2016; Venables et al., 2018; Weafer et al., 2015). Studies on behavioral and neural markers of response inhibition also indicate an association between RDoC Response Selection and Inhibition/Suppression and the Substance Abuse (Smith et al., 2014; Squeglia et al., 2014; Wong et al., 2006) and the Antisocial Behavior (Agnes Brunnekreef et al., 2007; Castellanos-Ryan et al., 2014; Heritage & Benning, 2013) subfactors of the HiTOP Disinhibited Externalizing spectrum. Additional links, potentially of smaller magnitude, may be delineated with the HiTOP Distress subfactor (Macatee et al., 2018; van Deurzen et al., 2012; Venables et al., 2017) and HiTOP Detachment (Steffens et al., 2018) based, respectively, on associations between impaired behavioral markers of response inhibition with distress symptoms (e.g., distress intolerance, affective problems) and negative schizotypy.
The RDoC Performance Monitoring subconstruct shows a robust link to HiTOP Fear, based on the self-reported Yale–Brown Obsessive Compulsive Scale (Y-BOCS) and associations of higher error-related negativity (ERN) amplitude during performance monitoring tasks (e.g., Flankers) with OCD traits (Meyer & Hajcak, 2019; Riesel et al., 2017; Storch et al., 2017; Weinberg et al., 2016) and social anxiety/shyness (Meyer & Hajcak, 2019; Meyer & Klein, 2018). Another well-supported link is with the HiTOP Disinhibited Externalizing spectrum, via associations of reduced post-error slowing, ERN amplitude, and lower insula and anterior cingulate activity during error processing with measures of disinhibition, impulsivity, and inattention and hyperactivity (Carroll et al., 2015; Hill et al., 2016; Kessel et al., 2016; Meyer & Hajcak, 2019; Michelini et al., 2018; Pasion & Barbosa, 2019; Taylor et al., 2018). A blunted ERN has also been linked to positive symptoms (but not negative symptoms) (Morris et al., 2008), and negative symptoms (but not psychotic or disorganized symptoms) in individuals with psychotic disorders (Foti et al., 2012; Foti et al., 2016). These mixed findings warrant further research and support a potential link with HiTOP Thought Disorder.
4.3.6. Working Memory
We examined associations with the broader RDoC Working Memory construct, rather than its subconstructs, since the elements contained within each subconstruct overlap with one another and it is difficult to distinguish them. Available evidence supports a robust association with the HiTOP Disinhibited Externalizing spectrum, based on associations of neural (e.g., reduced theta power) and behavioral performance markers during working memory and interference control tasks with trait impulsivity and inattentive and hyperactive-impulsive symptoms (Jang et al., 2020; Lenartowicz et al., 2019; Michelini et al., 2018; Polner et al., 2015). Similar associations of behavioral markers with substance use escalation and externalizing problems further suggest links, respectively, with the Substance Abuse (Khurana et al., 2013; Peeters et al., 2014; Squeglia et al., 2014) and Antisocial Behavior (Agnes Brunnekreef et al., 2007; Huang-Pollock et al., 2017) subfactors of this HiTOP spectrum.
Another robust association can be delineated with HiTOP Thought Disorder. This link is supported by associations between atypical neural activity (e.g., ventrolateral PFC) and psychotic symptoms (Pu et al., 2016; Sabharwal et al., 2016) during working memory tasks. Impaired behavioral performance markers during working memory and interference control tasks (e.g., n-back and Stroop) are also associated with psychotic disorders and positive schizotypy (Ettinger et al., 2018; Gold et al., 2019; Lencz et al., 2003; Sabharwal et al., 2016; Schmidt-Hansen & Honey, 2009; Seidman et al., 2016; Wolf et al., 2015; Xie et al., 2018). Initial evidence that working memory impairments are associated with high negative schizotypy also supports a possible link to the HiTOP Detachment spectrum (Xie et al., 2018).
4.4. Social Processes (Supplementary Figure 4)
Social Processes systems mediate responses to interpersonal settings and includes four constructs.
4.4.1. Affiliation and Attachment
Markers of RDoC Affiliation and Attachment demonstrate associations with several HiTOP spectra and subfactors. A robust link can to drawn to HiTOP Distress, as greater insecurity and impairment measured via behavioral paradigms (e.g., Strange Situation) and self-report is related to greater distress-based symptom severity (Colonnesi et al., 2011; Davila et al., 2005; Lee & Hankin, 2009; Madigan et al., 2013). Stronger parental attachment is associated with reduced amygdala reactivity and fronto-amygdala connectivity patterns associated with lower anxiety (Lebowitz et al., 2018). Greater self-reported attachment insecurity indicates a link to HiTOP Thought Disorder, via greater positive, negative, and affective symptoms (Berry et al., 2008; Gumley et al., 2014; Korver-Nieberg et al., 2014), and to HiTOP Detachment, as evidenced by insecure attachment associated with personality pathology and social impairment (Davila et al., 2005; Fossati et al., 2017; Levy et al., 2015; Lorenzini & Fonagy, 2013; Sinha & Sharan, 2007; West et al., 1994). Further, a robust links to the HiTOP Antagonistic Externalizing spectrum and the Antisocial Behavior subfactor are supported by associations of greater attachment insecurity and poor peer affiliation with antagonistic dispositional traits (Davila et al., 2005; Levy et al., 2015; Lorenzini & Fonagy, 2013; Nakash-Eisikovits et al., 2002; Sinha & Sharan, 2007) and disinhibitory and callous/aggressive psychopathology (Davila et al., 2005; Fearon et al., 2010; Hoeve et al., 2012; Laird et al., 2001; Van Ijzendoorn, 1997).
4.4.2. Social Communication
The RDoC Reception of Facial Communication subconstruct is associated with HiTOP Thought Disorder via significant neural disruptions, particularly under-recruitment of the amygdala, fusiform gyrus, and the ventral temporal–basal ganglia–prefrontal cortex system (Chan et al., 2010; Collin et al., 2013; Delvecchio et al., 2013; Habel et al., 2010), and impaired identification (Barkl et al., 2014; Chan et al., 2010; Collin et al., 2013; Kohler et al., 2010) of facial emotional states. Another robust link to HiTOP Detachment is supported by impaired facial emotion recognition associated with interpersonal detachment (Abbott & Green, 2013; Germine & Hooker, 2011) and negative schizotypy (Morrison et al., 2013; Rosell et al., 2014; Statucka & Walder, 2017; Williams et al., 2007). The HiTOP Antisocial Behavior subfactor also shows a well-supported link, as antisocial, disruptive, and callous-unemotional traits are associated with reduced ability to recognize facial emotions and amygdala dysfunction (Collin et al., 2013; da Costa et al., 2018; Marsh & Blair, 2008; Marsh et al., 2008). A further link can be delineated with HiTOP Distress via dysfunctional neural activation, impaired facial recognition, and attentional biases in individuals with depressive and anxious psychopathology (Bistricky et al., 2011; Bourke et al., 2010; Demenescu et al., 2010; Kohler et al., 2011). Finally, studies on markers of behavioral recognition paradigms and aberrant neural activation suggest an emerging link to HiTOP Mania (Chen et al., 2011; Delvecchio et al., 2013).
RDoC Production of Facial Communication shows a potential link to HiTOP Thought Disorder, as blunted spontaneous and imitative facial emotional expressiveness are associated with negative psychotic symptoms (Kring, 1999; Mandal et al., 1998). Research also suggests an association with the HiTOP Antisocial Behavior subfactor, as reduced reciprocal facial expressivity and mimicry in response to angry stimuli, assessed via observation (Cole et al., 1994; Eisenberg et al., 2001) and facial EMG (de Wied et al., 2010), have been associated with disruptive behaviors and externalizing tendencies.
With regard to the RDoC Reception of Non-Facial Communication subconstruct, a robust association with the HiTOP Thought Disorder is supported via studies showing that psychotic disorders are associated with impaired comprehension of emotional prosody (Hoekert et al., 2007; Lin et al., 2018), humor, sarcasm, and irony (Edwards et al., 2002; Mitchell & Crow, 2005).
Finally, at the behavioral level, RDoC Production of Non-Facial Communication is linked to HiTOP Thought Disorder as negative symptoms of psychosis are associated with impaired production of affective speech prosody (Hoekert et al., 2007; Mitchell & Crow, 2005).
4.4.3. Perception and Understanding of Self
The RDoC Agency subconstruct shows a primarily link to HiTOP Thought Disorder, based on studies of self-recognition (i.e., hallucinations., source-monitoring; Brookwell et al., 2013; Waters et al., 2012) and self-awareness (Hur et al., 2014).
The RDoC Self-Knowledge subconstruct is reliably linked to the Distress subfactor of the HiTOP Internalizing spectrum. Longitudinal and meta-analytic studies demonstrate associations of distress disorders, traits or symptoms with self-reported emotional awareness (Rieffe & De Rooij, 2012; Sendzik, Ö Schäfer, et al., 2017) and alexithymia (difficulty in naming and describing one’s emotions) (Leweke et al., 2012; Li et al., 2015). Further, the well-supported relationship of alexithymia (O’Driscoll et al., 2014; van ‘t Wout et al., 2004) and impaired self-monitoring (Farrer & Franck, 2007; Stephan et al., 2009) with psychotic symptoms indicate an additional link to HiTOP Thought Disorder. A further robust link is supported for HiTOP Detachment via self-reported alexithymia (Bach et al., 1994; Vanheule et al., 2007) and poor self-insight (Bach et al., 1994; Lim et al., 2019). Finally, emerging evidence supports a link between RDoC Self-Knowledge and HiTOP Fear, as individuals with fear psychopathology demonstrate poor emotion knowledge (O’Toole et al., 2013; Sendzik, J, et al., 2017; Summerfeldt et al., 2011).
4.4.4. Perception and Understanding of Others
The few markers of RDoC Animacy Perception suggest a link to Thought Disorder, based on evidence suggesting impaired processing of biological motion (Okruszek & Pilecka, 2017).
A link between RDoC Action Perception and HiTOP Antisocial Behavior is supported by impaired facial mimicry and preference within empathic paradigms, as well as poor directed gaze following (Bedford et al., 2017; de Wied et al., 2010; Wagner et al., 2016). HiTOP Thought Disorder also shows an association with this subconstruct, as evidenced by dysfunctional mirror neuron activity (Mehta et al., 2014; Minichino & Cadenhead, 2016) and action blindness (i.e., inability to identify what actions an agent is executing) (Liepelt et al., 2012).
RDoC Understanding Mental States shows a reliable link to Thought Disorder, as psychotic symptom severity is associated with impaired self-report affective empathy (Bonfils et al., 2016, 2017) and cognitive empathy during theory of mind tasks (Bora et al., 2009; Harrington et al., 2005). An additional robust link to HiTOP Antisocial Behavior is supported by associations of impaired empathic functioning with externalizing, antisocial, and callous-unemotional traits (de Wied et al., 2010; Hawes & Dadds, 2012; Miller & Eisenberg, 1988; Moul et al., 2018). Indeed, such empathic deficits are thought to be at the core of traits or symptoms pertaining to antisocial tendencies (Frick & White, 2008). This construct further shows a well-supported link with HiTOP Detachment, as affective and cognitive empathy partially mediates between negative schizotypy and social functioning (Henry et al., 2008; Wang et al., 2013) and self-reported detachment has been related to impaired social cognition (Fossati et al., 2017). Finally, this subconstruct may be linked to HiTOP Distress via impaired affective and cognitive empathy associated with depressive symptoms (Schreiter et al., 2013; Tone & Tully, 2014; Zahn-Waxler & Van Hulle, 2011), though associations with other distress-related symptoms remain unclear.
4.5. Arousal and Regulatory Systems (Supplementary Figure 5)
Arousal and Regulatory Systems are primarily involved in activating neural systems in response to various contexts and providing homeostatic regulation of energy balance and sleep.
4.5.1. Arousal
The RDoC Arousal construct shows a robust positive association with the HiTOP Distress subfactor, based on associations of measures of increased pupil dilatation during video-clips eliciting internalizing reactions (McCord et al., 2017), psychomotor agitation (Leventhal et al., 2008; Parker et al., 1993), and high cortisol productivity (Kircanski et al., 2017) with distress symptomatology (e.g., hopelessness, demoralization, stress/worry, generalized anxiety). High RDoC Arousal is also linked to HiTOP Fear via studies showing associations between skin conductance and social anxiety (Dieleman et al., 2015; Panayiotou et al., 2017). Another robust link to the HiTOP Disinhibited Externalizing spectrum is supported by evidence that skin conductance during a low-demand attentional task are related with inattention and hyperactivity/impulsivity (independently from antisocial behaviors) (Du Rietz et al., 2019), consistent with theoretical accounts proposing hypo-arousal as a core underpinning of these symptoms (Hegerl & Hensch, 2014; Sergeant, 2005). Reduced arousal in individuals with psychopathic traits, indexed by low cortisol productivity, stress reactivity, and skin conductance levels (Lorber, 2004; Shirtcliff et al., 2009), further indicate an association with the HiTOP Antagonistic Externalizing spectrum. The Antisocial Behavior subfactor at the intersection of the HiTOP Disinhibited and Antagonistic spectra also shows robust associations, as indicated by links of low skin conductance with conduct problems (Lorber, 2004), and of low pupil dilation during video-clips eliciting externalizing reactions with antisocial behavior and aggression (McCord et al., 2017). Finally, evidence of enhanced behavioral activation and hypo-arousal during mania suggests a potential link of RDoC Arousal with HiTOP Mania (Geissler et al., 2014; Hegerl & Hensch, 2014).
4.5.2. Circadian Rhythms
The RDoC Circadian Rhythms construct comprises elements such as morningness/eveningness, which refers to when people have their peak arousal levels. Since eveningness is linked to most forms of psychopathology (Fabbian et al., 2016; Gau et al., 2007; Hsu et al., 2012; Lemoine et al., 2013; Li et al., 2018; Schlarb et al., 2014), we suggest a tentative link to the general factor of psychopathology, or p-factor, which warrants further research.
4.5.3. Sleep-Wakefulness
The RDoC Sleep-Wakefulness construct shows a robust link with the HiTOP Distress subfactor, based on associations of insomnia and low sleep quality with depression and anxiety (Baglioni et al., 2011; Goldman-Mellor et al., 2014; Gregory et al., 2011; Madrid-Valero et al., 2019; Sawyer et al., 2015). A more tentative link can be identified with the HiTOP Disinhibited Externalizing spectrum, its Substance Abuse subfactor, and the Antisocial Behavior Subfactor, as lower sleep quality is associated with inattentive and hyperactive-impulsive symptoms (Gregory et al., 2017), substance abuse (Pieters et al., 2015), and externalizing problems (Denis et al., 2017). Notably, the latter study did not find any association with callous-unemotional traits, suggesting the association is specific to the HiTOP Disinhibited Externalizing spectrum, rather than shared across HiTOP Externalizing spectra (Denis et al., 2017). Finally, dysregulated wakefulness during manic episodes suggest an additional, tentative link to the HiTOP Mania subfactor (Hegerl et al., 2010; Robillard et al., 2016).
5. RDoC-HiTOP interface summary and general discussion
In the previous section, we have delineated an interface between RDoC and HiTOP frameworks by identifying connections between their dimensions based on available literature. Our review indicates a large number of associations of between RDoC constructs/subconstructs and HiTOP spectra/subfactors, which are of varying strength and documented by a varying number of studies. More associations are expected to emerge in future research informed by this interface. Figure 2 illustrates the most robust and consistent connections on the level of RDoC domains and HiTOP spectra. Multiple explanations exist for this large number of associations. First, the domains included in RDoC were designed to be transdiagnostic and capture major biobehavioral systems relevant to multiple forms of psychopathology (Kozak & Cuthbert, 2016), thus associations of varying strength and direction are expected among most RDoC and HiTOP dimensions. Second, the substantial correlations among psychopathology dimensions likely contributes to widespread associations with RDoC, consistent with high psychiatric comorbidity and limited specificity of biomarkers (Caspi et al., 2020). Third, there is evidence of etiological heterogeneity underlying psychopathology, with multiple etiological pathways contributing to similar clinical manifestations in different individuals (Kendler, 2019). Fourth, the biobehavioral correlates of a given HiTOP dimension may change across development (e.g., some associations may be more prominent in children than adults) (Tseng et al., 2019), thus studies of different developmental periods may suggest different links.
Figure 2.
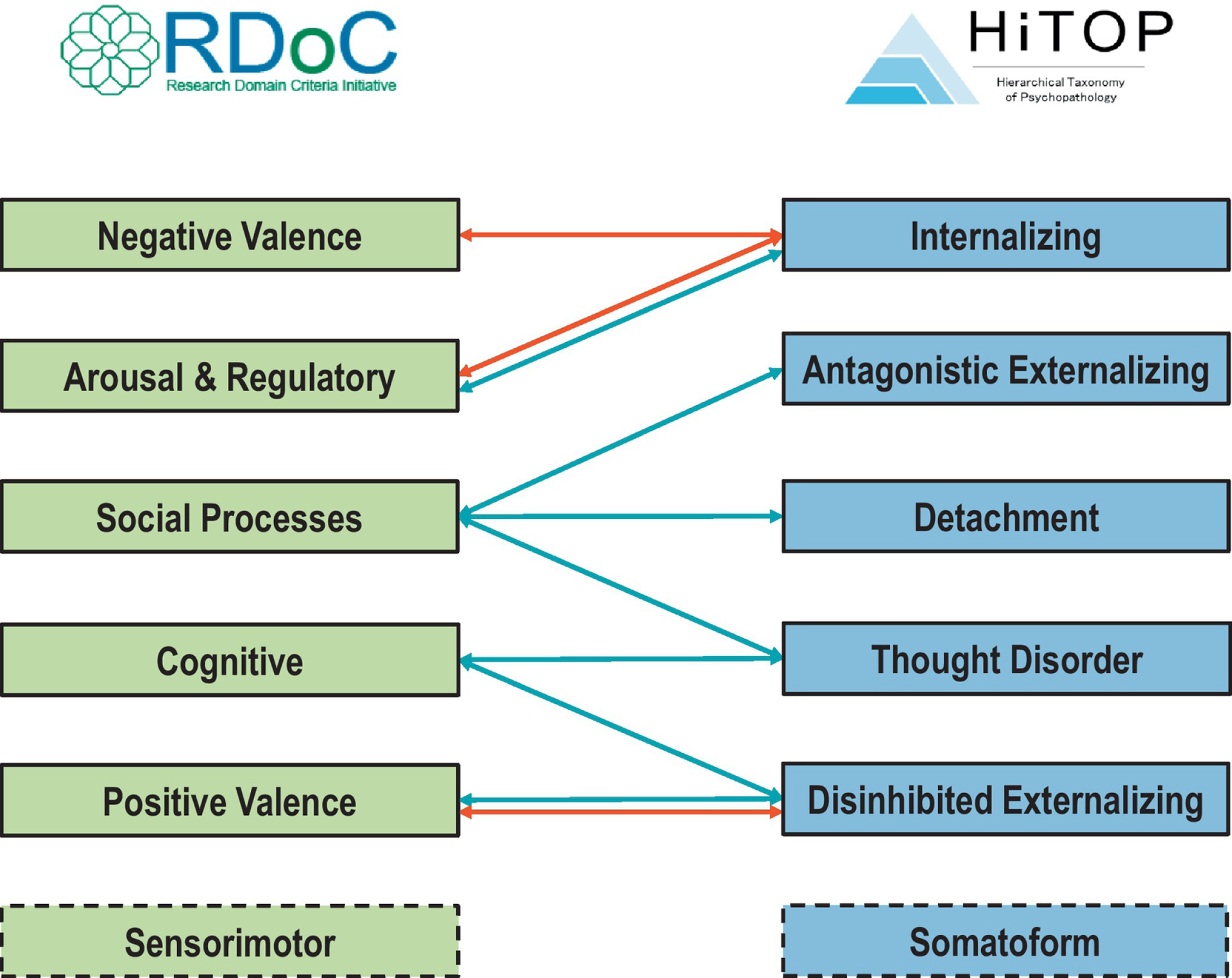
Overview of top associations between the Research Domain Criteria (RDoC) and the Hierarchical Taxonomy of Psychopathology (HiTOP).
Notes: Arrows show the most consistent associations between RDoC domains and HiTOP spectra (i.e., those depicting robust associations between more than one third of RDoC constructs within a domain with a HiTOP spectrum or its subfactors). Full details of associations between RDoC constructs/subconstructs and HiTOP spectra/subfactors are given in text and Supplementary Figures. Red = positive association; Blue = negative association. Associations that differed in sign across RDoC constructs within the domain are depicted with double arrows.
Nevertheless, some connections stood out because of their consistent effects across multiple constructs within each RDoC domain (Figure 2). RDoC Negative Valence Systems were robustly and positively linked to the HiTOP Internalizing spectrum, in line with a vast literature linking negative affectivity with internalizing psychopathology (Chorpita & Barlow, 1998; Clark & Watson, 1991; Hur et al., 2019). RDoC Arousal and Regulatory Systems was also linked to HiTOP Internalizing, but with positive associations with Arousal and negative with Sleep/Wakefulness, consistent with the association between poor sleep and hyperarousal (Kalmbach et al., 2018). RDoC Social Processes were negatively associated with HiTOP Thought Disorder, Detachment, and Antagonistic Externalizing spectra, in line with the prominent role of social functioning in the forms of psychopathology that they subsume (Green et al., 2019; Widiger et al., 2019). RDoC Cognitive Systems dysfunction was robustly linked to HiTOP Thought Disorder and Disinhibited Externalizing spectra, consistent with multiple theoretical models (Baskin-Sommers & Newman, 2013; Green et al., 2019). RDoC Positive Valence Systems showed strong negative links with HiTOP Disinhibited Externalizing, except for Substance Abuse, which showed some positive associations. These opposite links may suggest that substance use show specific correlates (e.g., enhanced Effort and Habit) that distinguish them from other dimensions of the HiTOP Disinhibited Externalizing spectrum.
Overall, our findings highlight that, although the interplay between biobehavioral and clinical features is complex, a number of core connections can be identified, which may serve as the foundation of a unified nosology that systematically describes both clinical presentation and its etiological underpinnings.
6. Practical recommendations and future directions
In this article we have provided a detailed map (section 4, Supplementary Figures 1–5) as well as an overarching summary (section 5, Figure 2) of an interface between RDoC and HiTOP. We have delineated associations between transdiagnostic biobehavioral systems included in RDoC and various clinical phenomena organized in the HiTOP model, but also highlighted more tentative associations where additional research is needed. The identified connections between RDoC and HiTOP dimensions can guide design of applied studies seeking to elucidate the underpinnings of clinical problems using RDoC domains or to validate HiTOP dimensions using etiological and neuroscientific approaches. On the one hand, latent HiTOP dimensions (i.e., obtained through factor analytic approaches) and assessments recommended by HiTOP (lists for research and clinical applications, respectively, are available in Kotov et al., 2017 and at https://hitop.unt.edu/clinical-tools/hitop-friendly-measures) provide more psychometrically-robust clinical targets than diagnostic categories for RDoC-informed studies. HiTOP-conformant measures may also supplement RDoC’s self-report and behavior markers, since the former are more clearly tied to clinical phenomena. On the other hand, RDoC provides a detailed framework to establish the validity of HiTOP dimensions with respect to coherent associations with core biobehavioral and neural dimensions across multiple units of analysis. This will promote a better understanding of the underpinnings of HiTOP dimensions and inform modifications of the model. Together, future efforts using this interface hold promise for promoting future dimensional classifications informed by the taxonomy of clinical problems and by their etiological underpinnings.
A number of links that seem likely for theoretical and empirical reasons were indicated as emerging or tentative to provoke further research, or not included because of a lack of strong studies investigating RDoC and HiTOP markers. For example, we expect HiTOP Detachment to show a negative association with RDoC Positive Valence Systems. Considerable research indicates that Detachment is equivalent to the low pole of the extraversion dimension in normal personality (Suzuki et al., 2015), which is associated with variation in the dopaminergic reward system that is central to Positive Valence Systems (Smillie et al., 2019). Further, emerging evidence suggests that negative symptoms of schizophrenia (currently under Thought Disorder), which have also been linked to deficits in reward processing (Reddy et al., 2016), should instead be located under Detachment (Cicero et al., 2019). Detachment thus likely reflects low sensitivity to reward, suggesting a link to Positive Valence Systems.
An important future direction will be to establish the specificity of the associations delineated in this interface, since most of the studies reviewed examined only a limited set of measures consistent with RDoC and HiTOP dimensions. Assessing multiple HiTOP spectra in any given study would allow researchers to test the specificity of the hypothesized associations with clinical features (i.e., discriminant validity) by showing that an RDoC construct is more strongly associated with one HiTOP spectrum than another. Even within a given spectrum, there may be differential associations of RDoC constructs with different subfactors and specific symptom dimensions. For example, the associations identified between RDoC Positive Valence Systems constructs and HiTOP Distress are based on studies on depressive symptoms, but links to other forms of Distress (e.g., generalized anxiety) are currently less researched. Future research is needed to clarify whether the link of RDoC Positive Valence Systems with HiTOP Distress extends also to other forms of internalizing psychopathology or is specific to depressive symptoms. Specific associations may be identified with assessments that capture several levels of the HiTOP hierarchy, for example through total scale and subscale scores of broad assessments. Similarly, assessing multiple RDoC domains will be essential to identify the relative contribution of core biobehavioral systems to each HiTOP dimension and elucidate their underpinnings.
The evidence supporting this interface comes from studies on community samples, heterogeneous clinical samples, or clinical samples recruited based on a clinical diagnosis but providing dimensional ratings. Stronger associations between RDoC and HiTOP dimensions may be expected from clinical samples due to greater variability in the clinical range (Sackett & Yang, 2000). Moving forward, as recommended by both RDoC and HiTOP, studies leveraging this interface should focus on demographically diverse population-based samples or samples from mixed patient populations. Researchers may wish to take steps to oversample participants in the range of high risk on the dimensions of interest. These designs are preferable to case-control design, in which the differences between cases and controls on the dimensions of interest are confounded by many extraneous factors (Preacher et al., 2005). Researchers working with existing data can operationalize HiTOP dimensions through latent variable modeling, which is optimal for studying dimensional constructs, but requires large samples. For most applications, samples of at least 300 participants are needed. In smaller samples, dimensional measures based on self- or other-reports consistent with HiTOP, or dimensional scores of symptom severity from clinical interviews, can be used as observed variables, but binary diagnoses should be avoided.
One good example of research that can be done at the interface of HiTOP and RDoC is a study of the IMAGEN sample (N=1700) that investigated neural and cognitive correlates of externalizing problems (Castellanos-Ryan et al., 2014). Using two diagnostic variables (ADHD and conduct disorder) and various self- and parent-reported symptoms and behaviors, such as age at drinking onset, this study reports a latent variable model incorporating three factors resampling the HiTOP Disinhibited Externalizing spectrum and its Substance Abuse and Antisocial Behavior subfactors. Two paradigms listed in the RDoC matrix (under Cognitive and Positive Valence Systems) were administered during fMRI, enabling analysis of associations of the externalizing factors with behavioral and neural variation in response inhibition and reward processing. Results showed similar effects for some biobehavioral variables across the three externalizing factors, but differential effects for others. For example, increased delay discounting was associated with all three factors, but increased OFC activation during reward anticipation was associated only with the substance abuse factor. This study highlights that diagnostic categories can potentially be incorporated into a HiTOP-conformant analysis if there are enough variables to allow diagnoses to be used as indicators of latent variables. Other notable examples are studies using the Philadelphia Neurodevelopmental Cohort (Kaczkurkin et al., 2019) and the Adolescent Brain Cognitive Development study (Karcher et al., 2020).
7. Conclusions
The dimensional approach to clinical research endorsed by both RDoC and HiTOP is the most promising way forward. It requires larger samples and different sampling designs than those with which many clinical researchers are familiar, but the payoff will be more effective scientific research, illuminating the etiology and clinical manifestations of psychopathology. The RDoC-HiTOP interface delineated in this article provides a synergistic approach that brings these dimensional frameworks together, integrates efforts made on both etiological and nosological fronts, and offers a guide for future research. Ultimately, we envision that this interface will contribute to the development of a unified, dimensional, and biobehaviorally-grounded psychiatric nosology.
Supplementary Material
Highlights.
This article provides a narrative review outlining an interface connecting RDoC and HiTOP dimensions
RDoC provides a solid transdiagnostic framework for elucidating the underpinnings of HiTOP dimensions
HiTOP may aid RDoC-informed research by providing psychometrically robust clinical targets
Leveraging the complementary strengths of RDoC and HiTOP may help advance clinical neuroscience and psychopathology research
This interface may facilitate the development of future biobehaviorally-grounded classifications of psychopathology
Role of Funding Sources
Dr. Michelini was funded by a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation (grant number 28566). Dr. Kotov was funded by the National Institute of Mental Health (grant number R01MH122537).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors report no conflict of interest.
References
- Abbott GR, & Green MJ (2013). Facial affect recognition and schizotypal personality characteristics. Early Interv Psychiatry, 7(1), 58–63. 10.1111/j.1751-7893.2012.00346.x [DOI] [PubMed] [Google Scholar]
- Achenbach TM (1966). The classification of children’s psychiatric symptoms: a factor-analytic study. Psychol Monogr, 80(7), 1–37. [DOI] [PubMed] [Google Scholar]
- Agnes Brunnekreef J, De Sonneville LM, Althaus M, Minderaa RB, Oldehinkel AJ, Verhulst FC, & Ormel J (2007). Information processing profiles of internalizing and externalizing behavior problems: evidence from a population-based sample of preadolescents. J Child Psychol Psychiatry, 48(2), 185–193. 10.1111/j.1469-7610.2006.01695.x [DOI] [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders (5th Edition ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Asherson P, Bushe C, Saylor K, Tanaka Y, Deberdt W, & Upadhyaya H (2014). Efficacy of atomoxetine in adults with attention deficit hyperactivity disorder: an integrated analysis of the complete database of multicenter placebo-controlled trials. J Psychopharmacol, 28(9), 837–846. 10.1177/0269881114542453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars MY, Muller MJ, Gallhofer B, & Netter P (2011). Depressive and aggressive responses to frustration: development of a questionnaire and its validation in a sample of male alcoholics. Depress Res Treat, 2011, 352048. 10.1155/2011/352048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski DE, Kujawa A, Kessel EM, Arfer KB, & Klein DN (2019). Sensitivity to Peer Feedback in Young Adolescents with Symptoms of ADHD: Examination of Neurophysiological and Self-Report Measures. J Abnorm Child Psychol, 47(4), 605–617. 10.1007/s10802-018-0470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, de Zwaan M, Ackard D, Nutzinger DO, & Mitchell JE (1994). Alexithymia: relationship to personality disorders. Compr Psychiatry, 35(3), 239–243. 10.1016/0010-440x(94)90197-x [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, & Riemann D (2011). Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord, 135(1–3), 10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Balodis IM, & Potenza MN (2015). Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biol Psychiatry, 77(5), 434–444. 10.1016/j.biopsych.2014.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, & Sheffield JM (2014). Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry, 13(3), 224–232. 10.1002/wps.20145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Farchione TJ, Bullis JR, Gallagher MW, Murray-Latin H, Sauer-Zavala S, Bentley KH, Thompson-Hollands J, Conklin LR, Boswell JF, Ametaj A, Carl JR, Boettcher HT, & Cassiello-Robbins C (2017). The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders Compared With Diagnosis-Specific Protocols for Anxiety Disorders: A Randomized Clinical Trial. JAMA Psychiatry, 74(9), 875–884. 10.1001/jamapsychiatry.2017.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. Int J Psychophysiol, 98(2 Pt 2), 227–239. 10.1016/j.ijpsycho.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Newman JP (2013). Differentiating the cognition-emotion interactions that characterize psychopathy versus externalizing. Handbook of cognition and emotion, 501–520. [Google Scholar]
- Bastiaansen JA, van Roon AM, Buitelaar JK, & Oldehinkel AJ (2015). Mental health problems are associated with low-frequency fluctuations in reaction time in a large general population sample. The TRAILS study. Eur Psychiatry, 30(2), 347–353. 10.1016/j.eurpsy.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, D’Amato F, & Kinkead R (2014). Early-life risk factors for panic and separation anxiety disorder: insights and outstanding questions arising from human and animal studies of CO2 sensitivity. Neurosci Biobehav Rev, 46 Pt 3, 455–464. 10.1016/j.neubiorev.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Baumeister D, Sedgwick O, Howes O, & Peters E (2017). Auditory verbal hallucinations and continuum models of psychosis: A systematic review of the healthy voice-hearer literature. Clin Psychol Rev, 51, 125–141. 10.1016/j.cpr.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JG, Carmin C& Henninger N.(1998). The utility of the Fear Survey Schedule-III: an extended replication. J Anxiety Disord, 12(3), 177–182. doi: 10.1016/s0887-6185(98)00007-3 [DOI] [PubMed] [Google Scholar]
- Bedford R, Wagner NJ, Rehder PD, Propper C, Willoughby MT, & Mills-Koonce RW (2017). The role of infants’ mother-directed gaze, maternal sensitivity, and emotion recognition in childhood callous unemotional behaviours. Eur Child Adolesc Psychiatry, 26(8), 947–956. 10.1007/s00787-017-0967-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry K, Barrowclough C, & Wearden A (2008). Attachment theory: a framework for understanding symptoms and interpersonal relationships in psychosis. Behav Res Ther, 46(12), 1275–1282. 10.1016/j.brat.2008.08.009 [DOI] [PubMed] [Google Scholar]
- Bevins RA, & Murray JE (2011). Internal stimuli generated by abused substances: Role of Pavlovian conditioning and its implications for drug addiction. In Associative learning and conditioning theory: Human and non-human applications. Oxford University Press. [Google Scholar]
- Bickel WK, & Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction, 96(1), 73–86. 10.1046/j.1360-0443.2001.961736.x [DOI] [PubMed] [Google Scholar]
- Bistricky SL, Ingram RE, & Atchley RA (2011). Facial affect processing and depression susceptibility: cognitive biases and cognitive neuroscience. Psychol Bull, 137(6), 998–1028. 10.1037/a0025348 [DOI] [PubMed] [Google Scholar]
- Blair RJR, White SF, Meffert H, & Hwang S (2013). Disruptive behavior disorders: taking an RDoC (ish) approach. In The Neurobiology of Childhood (pp. 319–336). Springer. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, & Blanchard RJ (2011). Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev, 35(4), 991–998. 10.1016/j.neubiorev.2010.10.016 [DOI] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, & Lucas J (2009). Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol, 17(1), 51–61. 10.1037/a0014503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfils KA, Lysaker PH, Minor KS, & Salyers MP (2016). Affective empathy in schizophrenia: a meta-analysis. Schizophr Res, 175(1–3), 109–117. 10.1016/j.schres.2016.03.037 [DOI] [PubMed] [Google Scholar]
- Bonfils KA, Lysaker PH, Minor KS, & Salyers MP (2017). Empathy in schizophrenia: A meta-analysis of the Interpersonal Reactivity Index. Psychiatry Res, 249, 293–303. 10.1016/j.psychres.2016.12.033 [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, & Pantelis C (2009). Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res, 109(1–3), 1–9. 10.1016/j.schres.2008.12.020 [DOI] [PubMed] [Google Scholar]
- Bourke C, Douglas K, & Porter R (2010). Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry, 44(8), 681–696. 10.3109/00048674.2010.496359 [DOI] [PubMed] [Google Scholar]
- Bourque J, Spechler PA, Potvin S, Whelan R, Banaschewski T, Bokde A, Bromberg U, Büchel C, Quinlan EB, Desrivières S, Flor H, Frouin V, Gowland P, Heinz A, Ittermann B, Martinot JL, Paillère-Martinot ML, McEwen SC, Nees F, Orfanos DP, … IMAGEN Consortium (2017). Functional Neuroimaging Predictors of Self-Reported Psychotic Symptoms in Adolescents. The American journal of psychiatry, 174(6), 566–575. 10.1176/appi.ajp.2017.16080897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookwell ML, Bentall RP, & Varese F (2013). Externalizing biases and hallucinations in source-monitoring, self-monitoring and signal detection studies: a meta-analytic review. Psychol Med, 43(12), 2465–2475. 10.1017/s0033291712002760 [DOI] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, & Weidt S (2014). Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neuroscience and biobehavioral reviews, 47. 10.1016/j.neubiorev.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Byrd AL, Loeber R, & Pardini DA (2014). Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clin Child Fam Psychol Rev, 17(2), 125–156. doi: 10.1007/s10567-013-0159-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, & Meyer-Lindenberg A (2018). Machine Learning for Precision Psychiatry: Opportunities and Challenges. Biol Psychiatry Cogn Neurosci Neuroimaging, 3(3), 223–230. 10.1016/j.bpsc.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Carleton RN, Collimore KC, McCabe RE, & Antony MM (2011). Addressing revisions to the Brief Fear of Negative Evaluation scale: measuring fear of negative evaluation across anxiety and mood disorders. J Anxiety Disord, 25(6), 822–828. 10.1016/j.janxdis.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, & Asmundson GJ (2012). Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. J Anxiety Disord, 26(3), 468–479. 10.1016/j.janxdis.2012.01.011 [DOI] [PubMed] [Google Scholar]
- Carlson SR, Pritchard AA, & Dominelli RM (2013). Externalizing behavior, the UPPS-P impulsive behavior scale and reward and punishment sensitivity. Personality and individual differences, 54(2), 202–207. [Google Scholar]
- Carroll A, Sutherland M, Salmeron B, Ross T, & Stein E (2015). Greater externalizing personality traits predict less error-related insula and anterior cingulate cortex activity in acutely abstinent cigarette smokers. Addiction biology, 20(2). 10.1111/adb.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, Harrington H, Hogan S, Poulton R, Ramrakha S, Rasmussen LJH, Reuben A, Richmond-Rakerd L, Sugden K, Wertz J, Williams BS, & Moffitt TE (2020). Longitudinal Assessment of Mental Health Disorders and Comorbidities Across 4 Decades Among Participants in the Dunedin Birth Cohort Study. JAMA Netw Open, 3(4), e203221. 10.1001/jamanetworkopen.2020.3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, & Moffitt TE (2018). All for One and One for All: Mental Disorders in One Dimension. Am J Psychiatry, 175(9), 831–844. 10.1176/appi.ajp.2018.17121383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Buchel C, Flor H, Fauth-Buhler M, Frouin V, Gallinat J, Gowland P, Heinz A, Lawrence C, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Schumann G, Garavan H, & Conrod PJ (2014). Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry, 171(12), 1310–1319. 10.1176/appi.ajp.2014.13111499 [DOI] [PubMed] [Google Scholar]
- Chan RC, Li H, Cheung EF, & Gong QY (2010). Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res, 178(2), 381–390. 10.1016/j.psychres.2009.03.035 [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, & Hogarth L (2011). The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry, 70(8), 785–793. 10.1016/j.biopsych.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavanne AV, & Robinson OJ (2021). The Overlapping Neurobiology of Induced and Pathological Anxiety: A Meta-Analysis of Functional Neural Activation. The American journal of psychiatry, 178(2), 156–164. 10.1176/appi.ajp.2020.19111153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, & Bullmore ET (2011). A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord, 13(1), 1–15. 10.1111/j.1399-5618.2011.00893.x [DOI] [PubMed] [Google Scholar]
- Chorpita BF, & Barlow DH (1998). The development of anxiety: the role of control in the early environment. Psychol Bull, 124(1), 3–21. 10.1037/0033-2909.124.1.3 [DOI] [PubMed] [Google Scholar]
- Cicero DC, Jonas KG, Li K, Perlman G, & Kotov R (2019). Common Taxonomy of Traits and Symptoms: Linking Schizophrenia Symptoms, Schizotypy, and Normal Personality. Schizophr Bull, 45(6), 1336–1348. 10.1093/schbul/sbz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo MA, & Seidman LJ (2003). Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev, 13(2), 43–77. 10.1023/a:1023870821631 [DOI] [PubMed] [Google Scholar]
- Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, & Reed GM (2017). Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol Sci Public Interest, 18(2), 72–145. 10.1177/1529100617727266 [DOI] [PubMed] [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol, 100(3), 316–336. 10.1037//0021-843x.100.3.316 [DOI] [PubMed] [Google Scholar]
- Cole PM, Zahn-Waxler C, & Smith KD (1994). Expressive control during a disappointment: Variations related to preschoolers’ behavior problems. Developmental psychology, 30(6), 835. [Google Scholar]
- Collin L, Bindra J, Raju M, Gillberg C, & Minnis H (2013). Facial emotion recognition in child psychiatry: a systematic review. Res Dev Disabil, 34(5), 1505–1520. 10.1016/j.ridd.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Colonnesi C, Draijer EM, Jan JMSG, Van der Bruggen CO, Bogels SM, & Noom MJ (2011). The relation between insecure attachment and child anxiety: a meta-analytic review. J Clin Child Adolesc Psychol, 40(4), 630–645. 10.1080/15374416.2011.581623 [DOI] [PubMed] [Google Scholar]
- Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, Mullins-Sweatt SN, Shackman AJ, Skodol AE, South SC, Sunderland M, Waszczuk MA, Zald DH, Afzali MH, Bornovalova MA, Carragher N, Docherty AR, Jonas KG, Krueger RF, Patalay P, … Eaton NR (2019). A Hierarchical Taxonomy of Psychopathology Can Transform Mental Health Research. Perspectives on psychological science : a journal of the Association for Psychological Science, 14(3), 419–436. 10.1177/1745691618810696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, & Fletcher PC (2012). The neurobiology of schizotypy: fronto-striatal prediction error signal correlates with delusion-like beliefs in healthy people. Neuropsychologia, 50(14), 3612–3620. 10.1016/j.neuropsychologia.2012.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr PJ, & Cooper AJ (2016). The Reinforcement Sensitivity Theory of Personality Questionnaire (RST-PQ): Development and validation. Psychol Assess, 28(11), 1427–1440. 10.1037/pas0000273 [DOI] [PubMed] [Google Scholar]
- Correa KA, Liu H, & Shankman SA (2019). The role of intolerance of uncertainty in current and remitted internalizing and externalizing psychopathology. J Anxiety Disord, 62, 68–76. 10.1016/j.janxdis.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Picchioni M, Toulopoulou T, McDonald C, Kravariti E, Walshe M, Prata D, Murray RM, & McGuire PK (2011). Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry, 11, 18. 10.1186/1471-244x-11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie J, Arnold P, Paterson A, Swanson J, Dupuis A, Li X, Shan J, Goodale T, Tam C, Strug LJ, & Schachar RJ (2013). Response inhibition and ADHD traits: correlates and heritability in a community sample. J Abnorm Child Psychol, 41(3), 497–507. 10.1007/s10802-012-9693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med, 11, 126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa HP, Vrabel JK, Zeigler-Hill V, & Vonk J (2018). DSM-5 pathological personality traits are associated with the ability to understand the emotional states of others. Journal of Research in Personality, 75, 1–11. 10.1016/j.jrp.2018.05.001 [DOI] [Google Scholar]
- Dai J, Gunn RL, Gerst KR, Busemeyer JR, & Finn PR (2016). A random utility model of delay discounting and its application to people with externalizing psychopathology. Psychol Assess, 28(10), 1198–1206. 10.1037/pas0000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila J, Ramsay M, Stroud CB, & Steinberg SJ (2005). Attachment as vulnerability to the development of psychopathology. Development of psychopathology: A vulnerability-stress perspective, 215–242. [Google Scholar]
- Dawe S, Gullo MJ, & Loxton NJ (2004). Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addict Behav, 29(7), 1389–1405. 10.1016/j.addbeh.2004.06.004 [DOI] [PubMed] [Google Scholar]
- de Wied M, Gispen-de Wied C, & van Boxtel A (2010). Empathy dysfunction in children and adolescents with disruptive behavior disorders. Eur J Pharmacol, 626(1), 97–103. 10.1016/j.ejphar.2009.10.016 [DOI] [PubMed] [Google Scholar]
- Delvecchio G, Sugranyes G, & Frangou S (2013). Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med, 43(3), 553–569. 10.1017/s0033291712001432 [DOI] [PubMed] [Google Scholar]
- Demenescu LR, Kortekaas R, den Boer JA, & Aleman A (2010). Impaired attribution of emotion to facial expressions in anxiety and major depression. PLoS One, 5(12), e15058. 10.1371/journal.pone.0015058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis D, Akhtar R, Holding BC, Murray C, Panatti J, Claridge G, Sadeh A, Barclay NL, O’Leary R, Maughan B, McAdams TA, Rowe R, Eley TC, Viding E, & Gregory AM (2017). Externalizing Behaviors and Callous-Unemotional Traits: Different Associations With Sleep Quality. Sleep, 40(8). 10.1093/sleep/zsx070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deserno L, Boehme R, Heinz A, & Schlagenhauf F (2013). Reinforcement learning and dopamine in schizophrenia: dimensions of symptoms or specific features of a disease group? Front Psychiatry, 4, 172. 10.3389/fpsyt.2013.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, & Gruber O (2008). Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev, 59(1), 164–184. 10.1016/j.brainresrev.2008.07.004 [DOI] [PubMed] [Google Scholar]
- Dieleman GC, Huizink AC, Tulen JH, Utens EM, Creemers HE, van der Ende J, & Verhulst FC (2015). Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology, 51, 135–150. 10.1016/j.psyneuen.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, & Pizzagalli DA (2014). Peril and pleasure: an RDoC-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety, 31(3), 233–249. 10.1002/da.22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty AR, Berenbaum H, & Kerns JG (2011). Alogia and formal thought disorder: differential patterns of verbal fluency task performance. J Psychiatr Res, 45(10), 1352–1357. 10.1016/j.jpsychires.2011.04.004 [DOI] [PubMed] [Google Scholar]
- Du Rietz E, James SN, Banaschewski T, Brandeis D, Asherson P, & Kuntsi J (2019). Autonomic arousal profiles in adolescents and young adults with ADHD as a function of recording context. Psychiatry Res, 275, 212–220. 10.1016/j.psychres.2019.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, van den Hout MA, & Baas JM (2015). Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression and anxiety, 32(4). 10.1002/da.22353 [DOI] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, & Liberzon I (2015). Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and clinical risk management, 11. 10.2147/TCRM.S48528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls HA, Curran T, & Mittal V (2016). A Meta-analytic Review of Auditory Event-Related Potential Components as Endophenotypes for Schizophrenia: Perspectives From First-Degree Relatives. Schizophr Bull, 42(6), 1504–1516. 10.1093/schbul/sbw047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, & Pattison PE (2002). Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev, 22(6), 789–832. 10.1016/s0272-7358(02)00130-7 [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Losoya S, Fabes RA, Guthrie IK, Reiser M, Murphy B, Shepard SA, Poulin R, & Padgett SJ (2001). Parental socialization of children’s dysregulated expression of emotion and externalizing problems. J Fam Psychol, 15(2), 183–205. 10.1037//0893-3200.15.2.183 [DOI] [PubMed] [Google Scholar]
- Estrada S, Tillem S, Stuppy-Sullivan A, & Baskin-Sommers A (2019). Specifying the Connection Between Reward Processing and Antisocial Psychopathology Across Development. The Oxford Handbook of Positive Emotion and Psychopathology, 312. [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Hill SK, Keefe RS, Gershon ES, Pearlson GD, Tamminga CA, Keshavan MS, & Sweeney JA (2014). Behavioral response inhibition in psychotic disorders: diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr Res, 159(2–3), 491–498. 10.1016/j.schres.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Aichert DS, Wostmann N, Dehning S, Riedel M, & Kumari V (2018). Response inhibition and interference control: Effects of schizophrenia, genetic risk, and schizotypy. J Neuropsychol, 12(3), 484–510. 10.1111/jnp.12126 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci, 8(11), 1481–1489. 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Fabbian F, Zucchi B, De Giorgi A, Tiseo R, Boari B, Salmi R, Cappadona R, Gianesini G, Bassi E, Signani F, Raparelli V, Basili S, & Manfredini R (2016). Chronotype, gender and general health. Chronobiol Int, 33(7), 863–882. 10.1080/07420528.2016.1176927 [DOI] [PubMed] [Google Scholar]
- Farrer C, & Franck N (2007). Self-Monitoring in Schizophrenia [Text]. Current Psychiatry Reviews, 3(4), 243–251. [Google Scholar]
- Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley AM, & Roisman GI (2010). The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: a meta-analytic study. Child Dev, 81(2), 435–456. 10.1111/j.1467-8624.2009.01405.x [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, & Remington G (2013). Neural substrates underlying effort computation in schizophrenia. Neurosci Biobehav Rev, 37(10 Pt 2), 2649–2665. 10.1016/j.neubiorev.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Fossati A, Somma A, Krueger RF, Markon KE, & Borroni S (2017). On the relationships between DSM-5 dysfunctional personality traits and social cognition deficits: A study in a sample of consecutively admitted Italian psychotherapy patients. Clin Psychol Psychother, 24(6), 1421–1434. 10.1002/cpp.2091 [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biol Psychol, 81(1), 1–8. 10.1016/j.biopsycho.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Bromet E, & Hajcak G (2012). Beyond the broken error-related negativity: functional and diagnostic correlates of error processing in psychosis. Biol Psychiatry, 71(10), 864–872. 10.1016/j.biopsych.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Perlman G, Hajcak G, Mohanty A, Jackson F, & Kotov R (2016). Impaired error processing in late-phase psychosis: Four-year stability and relationships with negative symptoms. Schizophr Res, 176(2–3), 520–526. 10.1016/j.schres.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IHA (2002). Behavioral approach system (BAS) sensitivity predicts alcohol craving. Personality and Individual Differences, 32(2), 349–355. 10.1016/S0191-8869(01)00030-7 [DOI] [Google Scholar]
- Frick PJ, & White SF (2008). Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry, 49(4), 359–375. 10.1111/j.1469-7610.2007.01862.x [DOI] [PubMed] [Google Scholar]
- Fried EI (2017). Moving forward: how depression heterogeneity hinders progress in treatment and research. Expert Rev Neurother, 17(5), 423–425. 10.1080/14737175.2017.1307737 [DOI] [PubMed] [Google Scholar]
- Galderisi S, Vignapiano A, Mucci A, & Boutros N (2014). Physiological correlates of positive symptoms in schizophrenia. Current topics in behavioral neurosciences, 21. 10.1007/7854_2014_322 [DOI] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, & Cheng AT (2007). Association between morningness-eveningness and behavioral/emotional problems among adolescents. J Biol Rhythms, 22(3), 268–274. 10.1177/0748730406298447 [DOI] [PubMed] [Google Scholar]
- Geissler J, Romanos M, Hegerl U, & Hensch T (2014). Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten Defic Hyperact Disord, 6(3), 159–173. 10.1007/s12402-014-0144-z [DOI] [PubMed] [Google Scholar]
- Geniole SN, MacDonell ET, & McCormick CM (2017). The Point Subtraction Aggression Paradigm as a laboratory tool for investigating the neuroendocrinology of aggression and competition. Horm Behav, 92, 103–116. 10.1016/j.yhbeh.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Gentes E, Dennis PA, Kimbrel NA, Kirby AC, Hair LP, Beckham JC, & Calhoun PS (2015). Latent Factor Structure of DSM-5 Posttraumatic Stress Disorder. Psychopathology review, 2(1). 10.5127/pr.035914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germine LT, & Hooker CI (2011). Face emotion recognition is related to individual differences in psychosis-proneness. Psychol Med, 41(5), 937–947. 10.1017/s0033291710001571 [DOI] [PubMed] [Google Scholar]
- Gerrits B, Vollebregt M, Olbrich S, van Dijk H, Palmer D, Gordon E, Pascual-Marqui R, Kessels R, & Arns M (2019). Probing the “Default Network Interference Hypothesis” With EEG: An RDoC Approach Focused on Attention. Clinical EEG and neuroscience, 50(6). 10.1177/1550059419864461 [DOI] [PubMed] [Google Scholar]
- Gibb BE, McGeary JE, & Beevers CG (2016). Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. Am J Med Genet B Neuropsychiatr Genet, 171b(1), 65–80. 10.1002/ajmg.b.32383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaus J, Van Meter A, Cui L, Marangoni C, & Merikangas KR (2018). Factorial structure and familial aggregation of the Hypomania Checklist-32 (HCL-32): Results of the NIMH Family Study of Affective Spectrum Disorders. Compr Psychiatry, 84, 7–14. 10.1016/j.comppsych.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Barch DM, Feuerstahler LM, Carter CS, MacDonald AW, Ragland JD, Silverstein SM, Strauss ME, & Luck SJ (2019). Working Memory Impairment Across Psychotic disorders. Schizophr Bull, 45(4), 804–812. 10.1093/schbul/sby134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Mellor S, Gregory AM, Caspi A, Harrington H, Parsons M, Poulton R, & Moffitt TE (2014). Mental health antecedents of early midlife insomnia: evidence from a four-decade longitudinal study. Sleep, 37(11), 1767–1775. 10.5665/sleep.4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, & Phan KL (2017). Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders. J Abnorm Psychol, 126(1), 8–18. 10.1037/abn0000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Barch DM, & Gold JM (2015). Effort-Based Decision Making: A Novel Approach for Assessing Motivation in Schizophrenia. Schizophr Bull, 41(5), 1035–1044. 10.1093/schbul/sbv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, & Lee J (2019). Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry, 18(2), 146–161. 10.1002/wps.20624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Agnew-Blais JC, Matthews T, Moffitt TE, & Arseneault L (2017). Associations between ADHD and sleep quality: Longitudinal analyses from a nationally-representative cohort of twins. J Clin Child Adolesc Psychol, 46(2), 284–294. 10.1080/15374416.2016.1183499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Buysse DJ, Willis TA, Rijsdijk FV, Maughan B, Rowe R, Cartwright S, Barclay NL, & Eley TC (2011). Associations between sleep quality and anxiety and depression symptoms in a sample of young adult twins and siblings. J Psychosom Res, 71(4), 250–255. 10.1016/j.jpsychores.2011.03.011 [DOI] [PubMed] [Google Scholar]
- Grierson AB, Hickie IB, Naismith SL, & Scott J (2016). The role of rumination in illness trajectories in youth: linking trans-diagnostic processes with clinical staging models. Psychol Med, 46(12), 2467–2484. 10.1017/s0033291716001392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Hermens DF, Van Zwieten A, Naismith SL, Lee RS, Cacciotti-Saija C, Scott EM, & Hickie IB (2013). Social cognitive performance as a marker of positive psychotic symptoms in young people seeking help for mental health problems. Schizophr Res, 149(1–3), 77–82. 10.1016/j.schres.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Gumley AI, Taylor HE, Schwannauer M, & MacBeth A (2014). A systematic review of attachment and psychosis: measurement, construct validity and outcomes. Acta Psychiatr Scand, 129(4), 257–274. 10.1111/acps.12172 [DOI] [PubMed] [Google Scholar]
- Habel U, Chechko N, Pauly K, Koch K, Backes V, Seiferth N, Shah NJ, Stocker T, Schneider F, & Kellermann T (2010). Neural correlates of emotion recognition in schizophrenia. Schizophr Res, 122(1–3), 113–123. 10.1016/j.schres.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis A, Plichta M, Heinzel S, Polak T, Lesch K, Breuer F, Jakob P, & Fallgatter A (2009). Neural response to reward anticipation is modulated by Gray’s impulsivity. NeuroImage, 46(4). 10.1016/j.neuroimage.2009.03.038 [DOI] [PubMed] [Google Scholar]
- Hamm AO, Richter J, Pane-Farre C, Westphal D, Wittchen HU, Vossbeck-Elsebusch A… Deckert J. (2016). Panic disorder with agoraphobia from a behavioral neuroscience perspective: Applying the research principles formulated by the Research Domain Criteria (RDoC) initiative. Psychophysiology, 53(3), 312–322. doi: 10.1111/psyp.12553 [DOI] [PubMed] [Google Scholar]
- Halahakoon D, Kieslich K, O’Driscoll C, Nair A, Lewis G, & Roiser J (2020). Reward-Processing Behavior in Depressed Participants Relative to Healthy Volunteers: A Systematic Review and Meta-analysis. JAMA psychiatry, 77(12). 10.1001/jamapsychiatry.2020.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett PH, Loxton NJ, & Jackson CJ (2013). Revised reinforcement sensitivity theory: Implications for psychopathology and psychological health. Personality and Individual Differences, 54(3), 432–437. [Google Scholar]
- Harrington L, Siegert RJ, & McClure J (2005). Theory of mind in schizophrenia: a critical review. Cogn Neuropsychiatry, 10(4), 249–286. 10.1080/13546800444000056 [DOI] [PubMed] [Google Scholar]
- Hawes DJ, & Dadds MR (2012). Revisiting the role of empathy in childhood pathways to antisocial behavior. Emotions, imagination, and moral reasoning, 45–70. [Google Scholar]
- Hegerl U, & Hensch T (2014). The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev, 44, 45–57. 10.1016/j.neubiorev.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Hegerl U, Himmerich H, Engmann B, & Hensch T (2010). Mania and attention-deficit/hyperactivity disorder: common symptomatology, common pathophysiology and common treatment? Curr Opin Psychiatry, 23(1), 1–7. 10.1097/YCO.0b013e328331f694 [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Ho TC, Connolly CG, LeWinn KZ, Sacchet MD, Tymofiyeva O, Weng HY, & Yang TT (2016). The neuroscience and context of adolescent depression. Acta Paediatr, 105(4), 358–365. 10.1111/apa.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Bailey PE, & Rendell PG (2008). Empathy, social functioning and schizotypy. Psychiatry Res, 160(1), 15–22. 10.1016/j.psychres.2007.04.014 [DOI] [PubMed] [Google Scholar]
- Heritage AJ, & Benning SD (2013). Impulsivity and response modulation deficits in psychopathy: evidence from the ERN and N1. J Abnorm Psychol, 122(1), 215–222. 10.1037/a0030039 [DOI] [PubMed] [Google Scholar]
- Hill KE, Samuel DB, & Foti D (2016). Contextualizing individual differences in error monitoring: Links with impulsivity, negative affect, and conscientiousness. Psychophysiology, 53(8), 1143–1153. 10.1111/psyp.12671 [DOI] [PubMed] [Google Scholar]
- Hoekert M, Kahn RS, Pijnenborg M, & Aleman A (2007). Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res, 96(1–3), 135–145. 10.1016/j.schres.2007.07.023 [DOI] [PubMed] [Google Scholar]
- Hoeve M, Stams GJ, van der Put CE, Dubas JS, van der Laan PH, & Gerris JR (2012). A meta-analysis of attachment to parents and delinquency. J Abnorm Child Psychol, 40(5), 771–785. 10.1007/s10802-011-9608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Gau SS, Shang CY, Chiu YN, & Lee MB (2012). Associations between chronotypes, psychopathology, and personality among incoming college students. Chronobiol Int, 29(4), 491–501. 10.3109/07420528.2012.668995 [DOI] [PubMed] [Google Scholar]
- Hsu KJ, Forgeard M, Stein AT, Beard C, & Bjorgvinsson T (2019). Examining differential relationships among self-reported attentional control, depression, and anxiety in a transdiagnostic clinical sample. J Affect Disord, 248, 29–33. 10.1016/j.jad.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C, Shapiro Z, Galloway-Long H, & Weigard A (2017). Is poor working memory a transdiagnostic risk factor for psychopathology? J Abnorm Child Psychol, 45(8), 1477–1490. 10.1007/s10802-016-0219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber RS, Hodgson R, & Yurgelun-Todd DA (2019). A qualitative systematic review of suicide behavior using the cognitive systems domain of the research domain criteria (RDoC) framework. Psychiatry Res, 282, 112589. 10.1016/j.psychres.2019.112589 [DOI] [PubMed] [Google Scholar]
- Huddy VC, Clark L, Harrison I, Ron MA, Moutoussis M, Barnes TR, & Joyce EM (2013). Reflection impulsivity and response inhibition in first-episode psychosis: relationship to cannabis use. Psychol Med, 43(10), 2097–2107. 10.1017/s0033291712003054 [DOI] [PubMed] [Google Scholar]
- Hur J, Stockbridge M, Fox A, & Shackman A (2019). Dispositional negativity, cognition, and anxiety disorders: An integrative translational neuroscience framework. Progress in brain research, 247. 10.1016/bs.pbr.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur JW, Kwon JS, Lee TY, & Park S (2014). The crisis of minimal self-awareness in schizophrenia: a meta-analytic review. Schizophr Res, 152(1), 58–64. 10.1016/j.schres.2013.08.042 [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, & Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci, 29, 565–598. 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Indlekofer F, Piechatzek M, Daamen M, Glasmacher C, Lieb R, Pfister H, Tucha O, Lange KWU, & Schütz CG (2009). Reduced memory and attention performance in a population-based sample of young adults with a moderate lifetime use of cannabis, ecstasy and alcohol. J Psychopharmacol, 23(5), 495–509. 10.1177/0269881108091076 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, & Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Shohamy D, Mihalakos P, Morris DW, Carmody T, & Tamminga CA (2012). Memory generalization is selectively altered in the psychosis dimension. Schizophr Res, 138(1), 74–80. 10.1016/j.schres.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JN, & MacKillop J (2016). Attention-Deficit/Hyperactivity Disorder and Monetary Delay Discounting: A Meta-Analysis of Case-Control Studies. Biol Psychiatry Cogn Neurosci Neuroimaging, 1(4), 316–325. 10.1016/j.bpsc.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K, Kim M, & Kim D (2020). The Dynamic Properties of a Brain Network During Spatial Working Memory Tasks in College Students With ADHD Traits. Frontiers in human neuroscience, 14. 10.3389/fnhum.2020.580813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, Rosen AFG, Ciric R, Xia CH, Cui Z, Sharma A, Wolf DH, Ruparel K, Pine DS, Shinohara RT, Roalf DR, Gur RC, Davatzikos C, Gur RE, & Satterthwaite TD (2019). Evidence for Dissociable Linkage of Dimensions of Psychopathology to Brain Structure in Youths. Am J Psychiatry, 176(12), 1000–1009. 10.1176/appi.ajp.2019.18070835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach DA, Cuamatzi-Castelan AS, Tonnu CV, Tran KM, Anderson JR, Roth T, & Drake CL (2018). Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep, 10, 193–201. 10.2147/NSS.S138823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Lasutschinkow P, Ishizuka K, & Sawa A (2018). Olfactory Functioning in First-Episode Psychosis. Schizophr Bull, 44(3), 672–680. 10.1093/schbul/sbx107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Michelini G, Kotov R, & Barch DM (2020). Associations Between Resting-State Functional Connectivity and a Hierarchical Dimensional Structure of Psychopathology in Middle Childhood. Biol Psychiatry Cogn Neurosci Neuroimaging. 10.1016/j.bpsc.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner A, Malzahn D, Begemann M, Hilmes C, Bickeboller H, & Ehrenreich H (2013). Odor naming and interpretation performance in 881 schizophrenia subjects: association with clinical parameters. BMC Psychiatry, 13, 218. 10.1186/1471-244x-13-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane BP, Cruz LN, Paterno D, & Silverstein SM (2018). Self-Reported Visual Perceptual Abnormalities Are Strongly Associated with Core Clinical Features in Psychotic Disorders. Front Psychiatry, 9, 69. 10.3389/fpsyt.2018.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiflin R, & Janak PH (2015). Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron, 88(2), 247–263. 10.1016/j.neuron.2015.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AS, Leikauf JE, Holt-Gosselin B, Staveland BR, & Williams LM (2019). Paying attention to attention in depression. Transl Psychiatry, 9(1), 279. 10.1038/s41398-019-0616-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS (2019). From Many to One to Many-the Search for Causes of Psychiatric Illness. JAMA Psychiatry. 10.1001/jamapsychiatry.2019.1200 [DOI] [PubMed] [Google Scholar]
- Kessel EM, Meyer A, Hajcak G, Dougherty LR, Torpey-Newman DC, Carlson GA, & Klein DN (2016). Transdiagnostic factors and pathways to multifinality: The error-related negativity predicts whether preschool irritability is associated with internalizing versus externalizing symptoms at age 9. Dev Psychopathol, 28(4pt1), 913–926. 10.1017/s0954579416000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, & Hurt H (2013). Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction, 108(3), 506–515. 10.1111/add.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, LeMoult J, Ordaz S, & Gotlib IH (2017). Investigating the nature of co-occurring depression and anxiety: Comparing diagnostic and dimensional research approaches. J Affect Disord, 216, 123–135. 10.1016/j.jad.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, & Moberg PJ (2011). Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res, 188(3), 303–309. 10.1016/j.psychres.2011.04.019 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 3(8), 760–773. 10.1016/s2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver-Nieberg N, Berry K, Meijer CJ, & de Haan L (2014). Adult attachment and psychotic phenomenology in clinical and non-clinical samples: a systematic review. Psychol Psychother, 87(2), 127–154. 10.1111/papt.12010 [DOI] [PubMed] [Google Scholar]
- Kotov R, Jonas KG, Carpenter WT, Dretsch MN, Eaton NR, Forbes MK, Forbush KT, Hobbs K, Reininghaus U, Slade T, South SC, Sunderland M, Waszczuk MA, Widiger TA, Wright AGC, Zald DH, Krueger RF, Watson D, & HiTOP Utility Workgroup (2020). Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry, 19(2), 151–172. 10.1002/wps.20730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, & Watson D (2018). A paradigm shift in psychiatric classification: the Hierarchical Taxonomy Of Psychopathology (HiTOP). World Psychiatry, 17(1), 24–25. 10.1002/wps.20478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, Brown TA, Carpenter WT, Caspi A, Clark LA, Eaton NR, Forbes MK, Forbush KT, Goldberg D, Hasin D, Hyman SE, Ivanova MY, Lynam DR, Markon K, Miller JD, … Zimmerman M (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of abnormal psychology, 126(4), 454–477. 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Cicero DC, Conway CC, DeYoung CG, Eaton NR, Forbes MK, Hallquist MN, Latzman RD, Mullins-Sweatt SN, Ruggero CJ, Simms LJ, Waldman ID, Waszczuk MA, & Wright A (2021). The Hierarchical Taxonomy of Psychopathology (HiTOP): A Quantitative Nosology Based on Consensus of Evidence. Annual review of clinical psychology. Advance online publication. 10.1146/annurev-clinpsy-081219-093304 [DOI] [PubMed] [Google Scholar]
- Koudys J, Traynor J, Rodrigo A, Carcone D, & Ruocco A (2019). The NIMH Research Domain Criteria (RDoC) Initiative and Its Implications for Research on Personality Disorder. Current psychiatry reports, 21(6). 10.1007/s11920-019-1023-2 [DOI] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology, 53(3), 286–297. 10.1111/psyp.12518 [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Myin-Germeys I, Hanssen M, & van Os J (2005). Familial covariation of the subclinical psychosis phenotype and verbal fluency in the general population. Schizophr Res, 74(1), 37–41. 10.1016/j.schres.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Kring AM (1999). Emotion in schizophrenia: Old mystery, new understanding. Current Directions in Psychological Science, 8(5), 160–163. [Google Scholar]
- Krueger RF, Hobbs KA, Conway CC, Dick DM, Dretsch MN, Eaton ER, Forbes MK, Forbush KT, Keyes KM, Latzman RD, Michelini G, Patrick CJ, Sellbom M, Slade T, South SC, Sunderland M, Tackett J, Waldman I, Waszczuk MA, Wright AGC, Zald DH, Watson D, Kotov R, & HiTOP Utility Workgroup (in press). Validity and utility of the Hierarchical Taxonomy of psychopathology (HiTOP): II. Externalizing superspectrum. World Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Kotov R, Watson D, Forbes MK, Eaton NR, Ruggero CJ, Simms LJ, Widiger TA, Achenbach TM, Bach B, Bagby RM, Bornovalova MA, Carpenter WT, Chmielewski M, Cicero DC, Clark LA, Conway C, DeClercq B, DeYoung CG, Docherty AR, … Zimmermann J (2018). Progress in achieving quantitative classification of psychopathology. World Psychiatry, 17(3), 282–293. 10.1002/wps.20566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Pinto R, Price TS, van der Meere JJ, Frazier-Wood AC, & Asherson P (2014). The separation of ADHD inattention and hyperactivity-impulsivity symptoms: pathways from genetic effects to cognitive impairments and symptoms. J Abnorm Child Psychol, 42(1), 127–136. 10.1007/s10802-013-9771-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, & Rathouz PJ (2012). Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol, 121(4), 971–977. 10.1037/a0028355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird RD, Jordan KY, Dodge KA, Pettit GS, & Bates JE (2001). Peer rejection in childhood, involvement with antisocial peers in early adolescence, and the development of externalizing behavior problems. Dev Psychopathol, 13(2), 337–354. 10.1017/s0954579401002085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, & Bradley MM (2016). RDoC, DSM, and the reflex physiology of fear: A biodimensional analysis of the anxiety disorders spectrum. Psychophysiology, 53(3), 336–347. 10.1111/psyp.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzman RD, DeYoung CG, & HiTOP Neurobiological Foundations Workgroup (2020). Using empirically-derived dimensional phenotypes to accelerate clinical neuroscience: the Hierarchical Taxonomy of Psychopathology (HiTOP) framework. Neuropsychopharmacology. 10.1038/s41386-020-0639-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne K, Menon M, & Woodward T (2020). Functional Brain Networks Underlying Evidence Integration and Delusions in Schizophrenia. Schizophrenia bulletin, 46(1). 10.1093/schbul/sbz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz ER, Gee DG, Pine DS, & Silverman WK (2018). Implications of the Research Domain Criteria project for childhood anxiety and its disorders. Clinical psychology review, 64. 10.1016/j.cpr.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, & Hankin BL (2009). Insecure attachment, dysfunctional attitudes, and low self-esteem predicting prospective symptoms of depression and anxiety during adolescence. J Clin Child Adolesc Psychol, 38(2), 219–231. 10.1080/15374410802698396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehembre-Shiah E, Leong W, Brucato G, Abi-Dargham A, Lieberman JA, Horga G, & Girgis RR (2017). Distinct Relationships Between Visual and Auditory Perceptual Abnormalities and Conversion to Psychosis in a Clinical High-Risk Population. JAMA Psychiatry, 74(1), 104–106. 10.1001/jamapsychiatry.2016.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine P, Zawieja P, & Ohayon MM (2013). Associations between morningness/eveningness and psychopathology: an epidemiological survey in three in-patient psychiatric clinics. J Psychiatr Res, 47(8), 1095–1098. 10.1016/j.jpsychires.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Truong H, Salgari GC, Bilder RM, McGough J, McCracken JT, & Loo SK (2019). Alpha modulation during working memory encoding predicts neurocognitive impairment in ADHD. J Child Psychol Psychiatry, 60(8), 917–926. 10.1111/jcpp.13042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer R, Yao L, Reilly J, Keedy S, McDowell J, Keshavan M, Pearlson G, Tamminga C, Gershon E, Clementz B, Lui S, & Sweeney J (2019). Alterations in intrinsic fronto-thalamo-parietal connectivity are associated with cognitive control deficits in psychotic disorders. Human brain mapping, 40(1). 10.1002/hbm.24362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, & Lieberman JA (2003). Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch Gen Psychiatry, 60(3), 238–243. 10.1001/archpsyc.60.3.238 [DOI] [PubMed] [Google Scholar]
- Leshem R (2016). Relationships between trait impulsivity and cognitive control: the effect of attention switching on response inhibition and conflict resolution. Cogn Process, 17(1), 89–103. 10.1007/s10339-015-0733-6 [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Pettit JW, & Lewinsohn PM (2008). Characterizing major depression phenotypes by presence and type of psychomotor disturbance in adolescents and young adults. Depress Anxiety, 25(7), 575–592. 10.1002/da.20328 [DOI] [PubMed] [Google Scholar]
- Levy KN, Johnson BN, Clouthier TL, Scala J, & Temes CM (2015). An attachment theoretical framework for personality disorders. Canadian Psychology/Psychologie Canadienne, 56(2), 197. [Google Scholar]
- Leweke F, Leichsenring F, Kruse J, & Hermes S (2012). Is alexithymia associated with specific mental disorders? Psychopathology, 45(1), 22–28. 10.1159/000325170 [DOI] [PubMed] [Google Scholar]
- Li S, Zhang B, Guo Y, & Zhang J (2015). The association between alexithymia as assessed by the 20-item Toronto Alexithymia Scale and depression: A meta-analysis. Psychiatry Res, 227(1), 1–9. 10.1016/j.psychres.2015.02.006 [DOI] [PubMed] [Google Scholar]
- Li SX, Chan NY, Man Yu MW, Lam SP, Zhang J, Yan Chan JW, Li AM, & Wing YK (2018). Eveningness chronotype, insomnia symptoms, and emotional and behavioural problems in adolescents. Sleep Med, 47, 93–99. 10.1016/j.sleep.2018.03.025 [DOI] [PubMed] [Google Scholar]
- Liepelt R, Schneider JC, Aichert DS, Wostmann N, Dehning S, Moller HJ, Riedel M, Dolk T, & Ettinger U (2012). Action blind: disturbed self-other integration in schizophrenia. Neuropsychologia, 50(14), 3775–3780. 10.1016/j.neuropsychologia.2012.10.027 [DOI] [PubMed] [Google Scholar]
- Lim DSH, Gwee AJ, & Hong RY (2019). Associations between the DSM-5 section III trait model and impairments in functioning in Singaporean college students. Journal of personality disorders, 33(3), 413–431. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ding H, & Zhang Y (2018). Emotional Prosody Processing in Schizophrenic Patients: A Selective Review and Meta-Analysis. J Clin Med, 7(10). 10.3390/jcm7100363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EC, Hill J, Luna B, Verhulst B, & Clark DB (2015). Disruptive behavior disorders and indicators of disinhibition in adolescents: The BRIEF-SR, anti-saccade task, and D-KEFS color-word interference test. J Adolesc, 44, 182–190. 10.1016/j.adolescence.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber MF (2004). Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychol Bull, 130(4), 531–552. 10.1037/0033-2909.130.4.531 [DOI] [PubMed] [Google Scholar]
- Lorenzini N, & Fonagy P (2013). Attachment and Personality Disorders: A Short Review. FOCUS, 11(2), 155–166. 10.1176/appi.focus.11.2.155 [DOI] [Google Scholar]
- Luijten M, Schellekens AF, Kuhn S, Machielse MW, & Sescousse G (2017). Disruption of Reward Processing in Addiction : An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry, 74(4), 387–398. 10.1001/jamapsychiatry.2016.3084 [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, & Sergeant JA (2005). The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev, 25(2), 183–213. 10.1016/j.cpr.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Macatee RJ, Albanese BJ, Clancy K, Allan NP, Bernat EM, Cougle JR, & Schmidt NB (2018). Distress intolerance modulation of neurophysiological markers of cognitive control during a complex go/no-go task. J Abnorm Psychol, 127(1), 12–29. 10.1037/abn0000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae JR, Scoles MT, & Siegel S (1987). The contribution of Pavlovian conditioning to drug tolerance and dependence. Br J Addict, 82(4), 371–380. 10.1111/j.1360-0443.1987.tb01493.x [DOI] [PubMed] [Google Scholar]
- Madigan S, Atkinson L, Laurin K, & Benoit D (2013). Attachment and internalizing behavior in early childhood: a meta-analysis. Dev Psychol, 49(4), 672–689. 10.1037/a0028793 [DOI] [PubMed] [Google Scholar]
- Madrid-Valero JJ, Ronald A, Shakeshaft N, Schofield K, Malanchini M, & Gregory AM (2019). Sleep quality, insomnia and internalising difficulties in adolescents: insights from a twin study. Sleep. 10.1093/sleep/zsz229 [DOI] [PubMed] [Google Scholar]
- Mahone EM, Cirino PT, Cutting LE, Cerrone PM, Hagelthorn KM, Hiemenz JR, Singer HS, & Denckla MB (2002). Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Archives of Clinical Neuropsychology, 17(7), 643–662. 10.1016/S0887-6177(01)00168-8 [DOI] [PubMed] [Google Scholar]
- Mahone EM, & Hoffman J (2007). Behavior ratings of executive function among preschoolers with ADHD. Clin Neuropsychol, 21(4), 569–586. 10.1080/13854040600762724 [DOI] [PubMed] [Google Scholar]
- Mandal MK, Pandey R, & Prasad AB (1998). Facial expressions of emotions and schizophrenia: a review. Schizophr Bull, 24(3), 399–412. 10.1093/oxfordjournals.schbul.a033335 [DOI] [PubMed] [Google Scholar]
- Markon KE, Chmielewski M, & Miller CJ (2011). The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychol Bull, 137(5), 856–879. 10.1037/a0023678 [DOI] [PubMed] [Google Scholar]
- Markon KE, & Krueger RF (2005). Categorical and continuous models of liability to externalizing disorders: a direct comparison in NESARC. Arch Gen Psychiatry, 62(12), 1352–1359. 10.1001/archpsyc.62.12.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, & Blair RJ (2008). Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci Biobehav Rev, 32(3), 454–465. 10.1016/j.neubiorev.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, & Blair RJ (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry, 165(6), 712–720. 10.1176/appi.ajp.2007.07071145 [DOI] [PubMed] [Google Scholar]
- Marsh JE, Vachon F, & Sorqvist P (2017). Increased distractibility in schizotypy: Independent of individual differences in working memory capacity? Q J Exp Psychol (Hove), 70(3), 565–578. 10.1080/17470218.2016.1172094 [DOI] [PubMed] [Google Scholar]
- Mazzer K, Boersma K, & Linton SJ (2019). A longitudinal view of rumination, poor sleep and psychological distress in adolescents. J Affect Disord, 245, 686–696. 10.1016/j.jad.2018.11.053 [DOI] [PubMed] [Google Scholar]
- McCord DM, Achee MC, Cannon EM, Harrop TM, & Poynter WD (2017). Using the Research Domain Criteria Framework to Explore Associations Between MMPI-2-RF Constructs and Physiological Variables Assessed by Eye-Tracker Technology. J Pers Assess, 99(4), 363–374. 10.1080/00223891.2016.1228067 [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Rosenbluth M, Ramasubbu R, Bond DJ, Taylor VH, Beaulieu S, & Schaffer A (2012). Managing medical and psychiatric comorbidity in individuals with major depressive disorder and bipolar disorder. Ann Clin Psychiatry, 24(2), 163–169. [PubMed] [Google Scholar]
- McTeague LM, & Lang PJ (2012). The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety, 29(4), 264–281. 10.1002/da.21891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta UM, Thirthalli J, Aneelraj D, Jadhav P, Gangadhar BN, & Keshavan MS (2014). Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophr Res, 160(1–3), 9–19. 10.1016/j.schres.2014.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, & Hajcak G (2019). A review examining the relationship between individual differences in the error-related negativity and cognitive control. Int J Psychophysiol, 144, 7–13. 10.1016/j.ijpsycho.2019.07.005 [DOI] [PubMed] [Google Scholar]
- Meyer A, & Klein DN (2018). Examining the relationships between error-related brain activity (the ERN) and anxiety disorders versus externalizing disorders in young children: Focusing on cognitive control, fear, and shyness. Compr Psychiatry, 87, 112–119. 10.1016/j.comppsych.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Michelini G, Barch DM, Tian Y, Watson D, Klein DN, & Kotov R (2019). Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Transl Psychiatry, 9(1), 261. 10.1038/s41398-019-0593-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini G, Cheung CHM, Kitsune V, Brandeis D, Banaschewski T, McLoughlin G, Asherson P, Rijsdijk F, & Kuntsi J (2018). The Etiological Structure of Cognitive-Neurophysiological Impairments in ADHD in Adolescence and Young Adulthood. J Atten Disord, 1087054718771191. 10.1177/1087054718771191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PA, & Eisenberg N (1988). The relation of empathy to aggressive and externalizing/antisocial behavior. Psychol Bull, 103(3), 324–344. 10.1037/0033-2909.103.3.324 [DOI] [PubMed] [Google Scholar]
- Minichino A, & Cadenhead K (2016). Mirror Neurons in Psychiatric Disorders: from Neuroception to Biobehavioral System Dysregulation [News]. Neuropsychopharmacology, 42(1), 366–366. 10.1038/npp.2016.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RL, & Crow TJ (2005). Right hemisphere language functions and schizophrenia: the forgotten hemisphere? Brain, 128(Pt 5), 963–978. 10.1093/brain/awh466 [DOI] [PubMed] [Google Scholar]
- Mohanty A, Heller W, Koven NS, Fisher JE, Herrington JD, & Miller GA (2008). Specificity of emotion-related effects on attentional processing in schizotypy. Schizophr Res, 103(1–3), 129–137. 10.1016/j.schres.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Samaniego L, Gaviria AM, Vilella E, Valero J, & Labad A (2017). Schizotypal traits and cognitive performance in siblings of patients with psychosis. Psychiatry Res, 258, 551–556. 10.1016/j.psychres.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, & Holroyd CB (2008). Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res, 99(1–3), 274–285. 10.1016/j.schres.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Vaidyanathan U, & Cuthbert BN (2015). Psychophysiological science and the research domain criteria: A commentary. Int J Psychophysiol, 98(2 Pt 2), 378–380. 10.1016/j.ijpsycho.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SC, Brown LA, & Cohen AS (2013). A multidimensional assessment of social cognition in psychometrically defined schizotypy. Psychiatry Res, 210(3), 1014–1019. 10.1016/j.psychres.2013.08.020 [DOI] [PubMed] [Google Scholar]
- Moul C, Hawes DJ, & Dadds MR (2018). Mapping the developmental pathways of child conduct problems through the neurobiology of empathy. Neurosci Biobehav Rev, 91, 34–50. 10.1016/j.neubiorev.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Mullins-Sweatt SN, Hopwood CJ, Chmielewski M, Meyer NA, Min J, Helle AC, & Walgren MD (2020). Treatment of personality pathology through the lens of the hierarchical taxonomy of psychopathology: Developing a research agenda. Personal Ment Health, 14(1), 123–141. 10.1002/pmh.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakash-Eisikovits O, Dutra L, & Westen D (2002). Relationship between attachment patterns and personality pathology in adolescents. J Am Acad Child Adolesc Psychiatry, 41(9), 1111–1123. 10.1097/00004583-200209000-00012 [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K (2010). Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychol Bull, 136(1), 128–150. 10.1037/a0018055 [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2016). Blunted Neural Response to Rewards as a Prospective Predictor of the Development of Depression in Adolescent Girls. Am J Psychiatry, 173(12), 1223–1230. 10.1176/appi.ajp.2016.15121524 [DOI] [PubMed] [Google Scholar]
- Newlin DB, & Strubler KA (2007). The habitual brain: an “adapted habit” theory of substance use disorders. Subst Use Misuse, 42(2–3), 503–526. 10.1080/10826080601144606 [DOI] [PubMed] [Google Scholar]
- Nielson DM, Keren H, O’Callaghan G, Jackson SM, Douka I, Vidal-Ribas P, Pornpattananangkul N, Camp CC, Gorham LS, Wei C, Kirwan S, Zheng CY, & Stringaris A (2021). Great Expectations: A Critical Review of and Suggestions for the Study of Reward Processing as a Cause and Predictor of Depression. Biol Psychiatry, 89(2), 134–143. 10.1016/j.biopsych.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH (2019). Sensorimotor Domain Added to the RDoC Framework. https://www.nimh.nih.gov/news/science-news/2019/sensorimotor-domain-added-to-the-rdoc-framework.shtml
- O’Driscoll C, Laing J, & Mason O (2014). Cognitive emotion regulation strategies, alexithymia and dissociation in schizophrenia, a review and meta-analysis. Clin Psychol Rev, 34(6), 482–495. 10.1016/j.cpr.2014.07.002 [DOI] [PubMed] [Google Scholar]
- O’Toole MS, Hougaard E, & Mennin DS (2013). Social anxiety and emotion knowledge: a meta-analysis. J Anxiety Disord, 27(1), 98–108. 10.1016/j.janxdis.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Okruszek L, & Pilecka I (2017). Biological motion processing in schizophrenia - Systematic review and meta-analysis. Schizophr Res, 190, 3–10. 10.1016/j.schres.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, & Luciana M (2007). Adolescents’ performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Pers Individ Dif, 43(7), 1886–1897. 10.1016/j.paid.2007.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, & Balleine BW (2008). On habits and addiction: An associative analysis of compulsive drug seeking. Drug Discov Today Dis Models, 5(4), 235–245. 10.1016/j.ddmod.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Wiggins JL, Adleman NE, Harkins E, Curhan A, Towbin KE, Brotman MA, Pine DS, & Leibenluft E (2017). Behavioral and Neural Sustained Attention Deficits in Bipolar Disorder and Familial Risk of Bipolar Disorder. Biol Psychiatry, 82(9), 669–678. 10.1016/j.biopsych.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotou G, Karekla M, Georgiou D, Constantinou E, & Paraskeva-Siamata M (2017). Psychophysiological and self-reported reactivity associated with social anxiety and public speaking fear symptoms: Effects of fear versus distress. Psychiatry Res, 255, 278–286. 10.1016/j.psychres.2017.05.044 [DOI] [PubMed] [Google Scholar]
- Panton KR, Badcock DR, & Badcock JC (2016). A Metaanalysis of Perceptual Organization in Schizophrenia, Schizotypy, and Other High-Risk Groups Based on Variants of the Embedded Figures Task. Front Psychol, 7, 237. 10.3389/fpsyg.2016.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panton KR, Badcock JC, Dickinson JE, & Badcock DR (2018). Poorer Integration of Local Orientation Information Occurs in Students With High Schizotypal Personality Traits. Front Psychiatry, 9, 518. 10.3389/fpsyt.2018.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, & Heinz A (2010). Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci, 30(22), 7749–7753. 10.1523/jneurosci.5587-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Hadzi-Pavlovic D, Brodaty H, Boyce P, Mitchell P, Wilhelm K, Hickie I, & Eyers K (1993). Psychomotor disturbance in depression: defining the constructs. J Affect Disord, 27(4), 255–265. 10.1016/0165-0327(93)90049-p [DOI] [PubMed] [Google Scholar]
- Pasion R, & Barbosa F (2019). ERN as a transdiagnostic marker of the internalizing-externalizing spectrum: A dissociable meta-analytic effect. Neurosci Biobehav Rev, 103, 133–149. 10.1016/j.neubiorev.2019.06.013 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, & Hajcak G (2016). Reshaping clinical science: Introduction to the Special Issue on Psychophysiology and the NIMH Research Domain Criteria (RDoC) initiative. Psychophysiology, 53(3), 281–285. 10.1111/psyp.12613 [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, & Hudec KL (2016). Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin Psychol Rev, 43, 162–174. 10.1016/j.cpr.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U, & Becker K (2011). Neuropsychological basic deficits in preschoolers at risk for ADHD: a meta-analysis. Clin Psychol Rev, 31(4), 626–637. 10.1016/j.cpr.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Peeters M, Monshouwer K, Janssen T, Wiers RW, & Vollebergh WA (2014). Working memory and alcohol use in at-risk adolescents: a 2-year follow-up. Alcohol Clin Exp Res, 38(4), 1176–1183. 10.1111/acer.12339 [DOI] [PubMed] [Google Scholar]
- Pieters S, Burk WJ, Van der Vorst H, Dahl RE, Wiers RW, & Engels RC (2015). Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J Youth Adolesc, 44(2), 379–388. 10.1007/s10964-014-0213-9 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, & Fava M (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry, 166(6), 702–710. 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, & Scheres A (2014). Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev, 38, 125–134. 10.1016/j.neubiorev.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny V, Lano T, Schallmo M, Olman C, & Sponheim S (2019). Reduced influence of perceptual context in schizophrenia: behavioral and neurophysiological evidence. Psychological medicine. 10.1017/S0033291719003751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polner B, Aichert D, Macare C, Costa A, & Ettinger U (2015). Gently restless: association of ADHD-like traits with response inhibition and interference control. Eur Arch Psychiatry Clin Neurosci, 265(8), 689–699. 10.1007/s00406-014-0531-7 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, MacCallum RC, & Nicewander WA (2005). Use of the extreme groups approach: a critical reexamination and new recommendations. Psychol Methods, 10(2), 178–192. 10.1037/1082-989X.10.2.178 [DOI] [PubMed] [Google Scholar]
- Premkuma r. P., Onwumere J, Wilson D, Sumich A, Castro A, Kumari V, & Kuipers E (2014). Greater positive schizotypy relates to reduced N100 activity during rejection scenes. Neuropsychologia, 61. 10.1016/j.neuropsychologia.2014.06.031 [DOI] [PubMed] [Google Scholar]
- Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, & Meinlschmidt G (2011). Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther, 132(3), 242–267. 10.1016/j.pharmthera.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Pu S, Nakagome K, Itakura M, Iwata M, Nagata I, & Kaneko K (2016). The association between cognitive deficits and prefrontal hemodynamic responses during performance of working memory task in patients with schizophrenia. Schizophrenia research, 172(1–3). 10.1016/j.schres.2016.01.045 [DOI] [PubMed] [Google Scholar]
- Radoman M, Phan KL, & Gorka SM (2019). Neural correlates of predictable and unpredictable threat in internalizing psychopathology. Neurosci Lett, 701, 193–201. 10.1016/j.neulet.2019.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, & Fusar-Poli P (2015). Ventral Striatal Activation During Reward Processing in Psychosis: A Neurofunctional Meta-Analysis. JAMA Psychiatry, 72(12), 1243–1251. 10.1001/jamapsychiatry.2015.2196 [DOI] [PubMed] [Google Scholar]
- Rapee RM, Brown TA, Antony MM, & Barlow DH (1992). Response to hyperventilation and inhalation of 5.5% carbon dioxide-enriched air across the DSM-III-R anxiety disorders. J Abnorm Psychol, 101(3), 538–552. 10.1037//0021-843x.101.3.538 [DOI] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, & Green MF (2016). Motivational Deficits and Negative Symptoms in Schizophrenia: Concepts and Assessments. In Simpson EH & Balsam PD (Eds.), Behavioral Neuroscience of Motivation (pp. 357–373). Springer International Publishing. 10.1007/7854_2015_379 [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, & Johnson A (2008). A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci, 31(4), 415–437; discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, & Kupfer DJ (2013). DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry, 170(1), 59–70. 10.1176/appi.ajp.2012.12070999 [DOI] [PubMed] [Google Scholar]
- Reynolds B (2006). A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol, 17(8), 651–667. 10.1097/FBP.0b013e3280115f99 [DOI] [PubMed] [Google Scholar]
- Rieffe C, & De Rooij M (2012). The longitudinal relationship between emotion awareness and internalising symptoms during late childhood. Eur Child Adolesc Psychiatry, 21(6), 349–356. 10.1007/s00787-012-0267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesel A, Goldhahn S, & Kathmann N (2017). Hyperactive performance monitoring as a transdiagnostic marker: Results from health anxiety in comparison to obsessive-compulsive disorder. Neuropsychologia, 96, 1–8. 10.1016/j.neuropsychologia.2016.12.029 [DOI] [PubMed] [Google Scholar]
- Robillard R, Hermens DF, Lee RS, Jones A, Carpenter JS, White D, Naismith SL, Southan J, Whitwell B, Scott EM, & Hickie IB (2016). Sleep-wake profiles predict longitudinal changes in manic symptoms and memory in young people with mood disorders. J Sleep Res, 25(5), 549–555. 10.1111/jsr.12413 [DOI] [PubMed] [Google Scholar]
- Robinaugh DJ, & McNally RJ (2010). Autobiographical memory for shame or guilt provoking events: association with psychological symptoms. Behav Res Ther, 48(7), 646–652. 10.1016/j.brat.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2001). Incentive-sensitization and addiction. Addiction, 96(1), 103–114. 10.1046/j.1360-0443.2001.9611038.x [DOI] [PubMed] [Google Scholar]
- Rosell DR, Futterman SE, McMaster A, & Siever LJ (2014). Schizotypal personality disorder: a current review. Curr Psychiatry Rep, 16(7), 452. 10.1007/s11920-014-0452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Foster SL, & Ionescu DF (2017). The Role of Chronic Stress in Anxious Depression. Chronic Stress (Thousand Oaks), 1, 2470547016689472. 10.1177/2470547016689472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero CJ, Kotov R, Hopwood CJ, First M, Clark LA, Skodol AE, Mullins-Sweatt SN, Patrick CJ, Bach B, Cicero DC, Docherty A, Simms LJ, Bagby RM, Krueger RF, Callahan JL, Chmielewski M, Conway CC, De Clercq B, Dornbach-Bender A, Eaton NR, … Zimmermann J (2019). Integrating the Hierarchical Taxonomy of Psychopathology (HiTOP) into clinical practice. Journal of consulting and clinical psychology, 87(12), 1069–1084. 10.1037/ccp0000452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharwal A, Szekely A, Kotov R, Mukherjee P, Leung HC, Barch DM, & Mohanty A (2016). Transdiagnostic neural markers of emotion-cognition interaction in psychotic disorders. J Abnorm Psychol, 125(7), 907–922. 10.1037/abn0000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett PR, & Yang H (2000). Correction for range restriction: an expanded typology. J Appl Psychol, 85(1), 112–118. 10.1037/0021-9010.85.1.112 [DOI] [PubMed] [Google Scholar]
- Sawyer A, Fisher A, Llewellyn C, & Gregory AM (2015). Self-reported sleep quality, weight status and depression in young adult twins and siblings. BMC Obes, 2, 50. 10.1186/s40608-015-0079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlarb AA, Sopp R, Ambiel D, & Grunwald J (2014). Chronotype-related differences in childhood and adolescent aggression and antisocial behavior--a review of the literature. Chronobiol Int, 31(1), 1–16. 10.3109/07420528.2013.829846 [DOI] [PubMed] [Google Scholar]
- Schmidt-Hansen M, & Honey RC (2009). Working memory and multidimensional schizotypy: dissociable influences of the different dimensions. Cogn Neuropsychol, 26(7), 655–670. 10.1080/02643291003644501 [DOI] [PubMed] [Google Scholar]
- Schreiter S, Pijnenborg GH, & Aan Het Rot M (2013). Empathy in adults with clinical or subclinical depressive symptoms. J Affect Disord, 150(1), 1–16. 10.1016/j.jad.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Schultz W (2011). Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron, 69(4), 603–617. 10.1016/j.neuron.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Seabury R, & Cannon T (2020). Memory Impairments and Psychosis Prediction: A Scoping Review and Theoretical Overview. Neuropsychology review, 30(4). 10.1007/s11065-020-09464-2 [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, & Woods SW (2016). Association of Neurocognition With Transition to Psychosis: Baseline Functioning in the Second Phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry, 73(12), 1239–1248. 10.1001/jamapsychiatry.2016.2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendzik L, J OS, A CS, Naumann E, & Tuschen-Caffier (2017). Emotional Awareness in Depressive and Anxiety Symptoms in Youth: A Meta-Analytic Review. J Youth Adolesc, 46(4), 687–700. 10.1007/s10964-017-0629-0 [DOI] [PubMed] [Google Scholar]
- Sendzik L, Ö Schäfer J, C Samson A, Naumann E, & Tuschen-Caffier B (2017). Emotional Awareness in Depressive and Anxiety Symptoms in Youth: A Meta-Analytic Review. J Youth Adolesc, 46(4), 687–700. 10.1007/s10964-017-0629-0 [DOI] [PubMed] [Google Scholar]
- Sergeant JA (2005). Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry, 57(11), 1248–1255. 10.1016/j.biopsych.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Swendsen J, & Auriacombe M (2015). Ecological momentary assessment in the investigation of craving and substance use in daily life: a systematic review. Drug Alcohol Depend, 148, 1–20. 10.1016/j.drugalcdep.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Sexton KA, Norton PJ, Walker JR, & Norton GR (2003). Hierarchical model of generalized and specific vulnerabilities in anxiety. Cogn Behav Ther, 32(2), 82–94. 10.1080/16506070302321 [DOI] [PubMed] [Google Scholar]
- Shankman SA, Lewinsohn PM, Klein DN, Small JW, Seeley JR, & Altman SE (2009). Subthreshold conditions as precursors for full syndrome disorders: a 15-year longitudinal study of multiple diagnostic classes. J Child Psychol Psychiatry, 50(12), 1485–1494. 10.1111/j.1469-7610.2009.02117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, & Zahn-Waxler C (2009). Neurobiology of Empathy and Callousness: Implications for the Development of Antisocial Behavior. Behav Sci Law, 27(2), 137–171. 10.1002/bsl.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddi S, Petretto DR, & Preti A (2017). Neuropsychological correlates of schizotypy: a systematic review and meta-analysis of cross-sectional studies. Cogn Neuropsychiatry, 22(3), 186–212. 10.1080/13546805.2017.1299702 [DOI] [PubMed] [Google Scholar]
- Sinha P, & Sharan P (2007). Attachment and Personality Disorders. Journal of Indian Association for Child and Adolescent Mental Health, 3(4), 105–112. [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman AT, Penninx BW, & Veltman DJ (2013). Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry, 3, e337. 10.1038/tp.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets KC, Oostermeijer S, Lappenschaar M, Cohn M, van der Meer JM, Popma A, Jansen LM, Rommelse NN, Scheepers FE, & Buitelaar JK (2017). Are Proactive and Reactive Aggression Meaningful Distinctions in Adolescents? A Variable- and Person-Based Approach. J Abnorm Child Psychol, 45(1), 1–14. 10.1007/s10802-016-0149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie LD, Jach HK, Hughes DM, Wacker J, Cooper AJ, & Pickering AD (2019). Extraversion and reward-processing: Consolidating evidence from an electroencephalographic index of reward-prediction-error. Biol Psychol, 146, 107735. 10.1016/j.biopsycho.2019.107735 [DOI] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, & Iredale JM (2014). Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend, 145, 1–33. 10.1016/j.drugalcdep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, & Castellanos FX (2007). Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev, 31(7), 977–986. 10.1016/j.neubiorev.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Cortese S, Fairchild G, & Stringaris A (2016). Annual Research Review: Transdiagnostic neuroscience of child and adolescent mental disorders--differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. J Child Psychol Psychiatry, 57(3), 321–349. 10.1111/jcpp.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JC, Isen JD, & Iacono WG (2014). Preference on cash-choice task predicts externalizing outcomes in 17-year-olds. Behav Genet, 44(2), 102–112. 10.1007/s10519-013-9638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P, Elzinga BM, Van Hemert AM, de Rooij M, & Penninx BW (2016). Childhood maltreatment, maladaptive personality types and level and course of psychological distress: A six-year longitudinal study. J Affect Disord, 191, 100–108. 10.1016/j.jad.2015.11.036 [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Nguyen-Louie TT, & Tapert SF (2014). Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology, 28(5), 782–790. 10.1037/neu0000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Bauer E, Merz CJ, Zimmermann M, Reuter M, Plichta MM, Kirsch P, Lesch KP, Fallgatter AJ, Vaitl D, & Herrmann MJ (2011). ADHD related behaviors are associated with brain activation in the reward system. Neuropsychologia, 49(3), 426–434. 10.1016/j.neuropsychologia.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Statucka M, & Walder D (2017). Facial Affect Recognition and Social Functioning Among Individuals With Varying Degrees of Schizotypy. Psychiatry research, 256. 10.1016/j.psychres.2017.06.040 [DOI] [PubMed] [Google Scholar]
- Steffens M, Meyhofer I, Fassbender K, Ettinger U, & Kambeitz J (2018). Association of Schizotypy With Dimensions of Cognitive Control: A Meta-Analysis. Schizophr Bull, 44(suppl_2), S512–s524. 10.1093/schbul/sby030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, & Frith CD (2009). Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull, 35(3), 509–527. 10.1093/schbul/sbn176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, & Hsi X (2011). Declarative memory deficits and schizophrenia: problems and prospects. Neurobiol Learn Mem, 96(4), 544–552. 10.1016/j.nlm.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Storch EA, Nadeau JM, De Nadai AS, Cepeda SL, Riemann BC, Seibell P, & Kay B (2017). Symptom correspondence between clinicians and patients on the Yale-Brown Obsessive Compulsive Scale. Compr Psychiatry, 73, 105–110. 10.1016/j.comppsych.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Summerfeldt LJ, Kloosterman PH, Antony MM, McCabe RE, & Parker JDA (2011). Emotional intelligence in social phobia and other anxiety disorders. Journal of Psychopathology and Behavioral Assessment, 33(1), 69–78. [Google Scholar]
- Sumner PJ, Bell IH, & Rossell SL (2018). A systematic review of task-based functional neuroimaging studies investigating language, semantic and executive processes in thought disorder. Neurosci Biobehav Rev, 94, 59–75. 10.1016/j.neubiorev.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Samuel DB, Pahlen S, & Krueger RF (2015). DSM-5 alternative personality disorder model traits as maladaptive extreme variants of the five-factor model: An item-response theory analysis. J Abnorm Psychol, 124(2), 343–354. 10.1037/abn0000035 [DOI] [PubMed] [Google Scholar]
- Talarowska M, Berk M, Maes M, & Gałecki P (2016). Autobiographical Memory Dysfunctions in Depressive Disorders. Psychiatry and clinical neurosciences, 70(2). 10.1111/pcn.12370 [DOI] [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, & Brown SA (2002). Attention dysfunction predicts substance involvement in community youths. J Am Acad Child Adolesc Psychiatry, 41(6), 680–686. 10.1097/00004583-200206000-00007 [DOI] [PubMed] [Google Scholar]
- Taylor JB, Visser TAW, Fueggle SN, Bellgrove MA, & Fox AM (2018). The error-related negativity (ERN) is an electrophysiological marker of motor impulsiveness on the Barratt Impulsiveness Scale (BIS-11) during adolescence. Dev Cogn Neurosci, 30, 77–86. 10.1016/j.dcn.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teivaanmaki S, Huhdanpaa H, Kiuru N, Aronen ET, Narhi V, & Klenberg L (2019). Heterogeneity of executive functions among preschool children with psychiatric symptoms. Eur Child Adolesc Psychiatry. 10.1007/s00787-019-01437-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, Tonigan JS, & Yeo RA (2011). Adolescent substance abuse: the effects of alcohol and marijuana on neuropsychological performance. Alcohol Clin Exp Res, 35(1), 39–46. 10.1111/j.1530-0277.2010.01320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Jahanshad N, Ching C, Salminen LE, Thomopoulos SI, Bright J, Baune BT, Bertolín S, Bralten J, Bruin WB, Bülow R, Chen J, Chye Y, Dannlowski U, de Kovel C, Donohoe G, Eyler LT, Faraone SV, Favre P, Filippi CA, … ENIGMA Consortium (2020). ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Translational psychiatry, 10(1), 100. 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone EB, & Tully EC (2014). Empathy as a “risky strength”: a multilevel examination of empathy and risk for internalizing disorders. Dev Psychopathol, 26(4 Pt 2), 1547–1565. 10.1017/s0954579414001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Ávilab C, Moltób J, & Caseras X (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, & Zald DH (2009). Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One, 4(8), e6598. 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WL, Deveney CM, Stoddard J, Kircanski K, Frackman AE, Yi JY, Hsu D, Moroney E, Machlin L, Donahue L, Roule A, Perhamus G, Reynolds RC, Roberson-Nay R, Hettema JM, Towbin KE, Stringaris A, Pine DS, Brotman MA, & Leibenluft E (2019). Brain Mechanisms of Attention Orienting Following Frustration: Associations With Irritability and Age in Youths. Am J Psychiatry, 176(1), 67–76. 10.1176/appi.ajp.2018.18040491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Nelson LD, & Patrick CJ (2012). Clarifying domains of internalizing psychopathology using neurophysiology. Psychol Med, 42(3), 447–459. 10.1017/s0033291711001528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ‘t Wout M, Aleman A, Kessels RP, Laroi F, & Kahn RS (2004). Emotional processing in a non-clinical psychosis-prone sample. Schizophr Res, 68(2–3), 271–281. 10.1016/j.schres.2003.09.006 [DOI] [PubMed] [Google Scholar]
- van Deurzen PA, Buitelaar JK, Agnes Brunnekreef J, Ormel J, Minderaa RB, Hartman CA, Huizink AC, Speckens AE, Oldehinkel AJ, & Slaats-Willemse DI (2012). Response time variability and response inhibition predict affective problems in adolescent girls, not in boys: the TRAILS study. Eur Child Adolesc Psychiatry, 21(5), 277–287. 10.1007/s00787-012-0260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ijzendoorn MH (1997). Attachment, emergent morality, and aggression: Toward a developmental socioemotional model of antisocial behaviour. Int J Behav Dev, 21(4), 703–728. [Google Scholar]
- Van Voorhis A, Kent J, Kang S, Goghari V, MacDonald A, & Sponheim S (2019). Abnormal neural functions associated with motor inhibition deficits in schizophrenia and bipolar disorder. Human brain mapping, 40(18). 10.1002/hbm.24780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheule S, Vandenbergen J, Desmet M, Rosseel Y, & Insleghers R (2007). Alexithymia and core conflictual relationship themes: a study in a chronically fatigued primary care population. Int J Psychiatry Med, 37(1), 87–98. 10.2190/P474-62H5-2250-W343 [DOI] [PubMed] [Google Scholar]
- Venables NC, Foell J, Yancey JR, Kane MJ, Engle RW, & Patrick CJ (2018). Quantifying Inhibitory Control as Externalizing Proneness: A Cross-Domain Model. Clinical Psychological Science, 6(4), 561–580. [Google Scholar]
- Venables NC, Hicks BM, Yancey JR, Kramer MD, Nelson LD, Strickland CM, Krueger RF, Iacono WG, & Patrick CJ (2017). Evidence of a prominent genetic basis for associations between psychoneurometric traits and common mental disorders. Int J Psychophysiol, 115, 4–12. 10.1016/j.ijpsycho.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, & Baler R (2010). Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays, 32(9), 748–755. 10.1002/bies.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, Mills-Koonce WR, Propper CB, Willoughby MT, Rehder PD, Moore GA, & Cox MJ (2016). Associations between Infant Behaviors during the Face-To-Face Still-Face Paradigm and Oppositional Defiant and Callous-Unemotional Behaviors in Early Childhood. J Abnorm Child Psychol, 44(8), 1439–1453. 10.1007/s10802-016-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neumann DL, Shum DH, Liu WH, Shi HS, Yan C, Lui SS, Zhang Q, Li Z, Cheung EF, & Chan RC (2013). Cognitive empathy partially mediates the association between negative schizotypy traits and social functioning. Psychiatry Res, 210(1), 62–68. 10.1016/j.psychres.2013.03.015 [DOI] [PubMed] [Google Scholar]
- Wassum KM, & Izquierdo A (2015). The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev, 57, 271–283. 10.1016/j.neubiorev.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Eaton NR, Krueger RF, Shackman AJ, Waldman ID, Zald DH, Lahey BB, Patrick CJ, Conway CC, Ormel J, Hyman SE, Fried EI, Forbes MK, Docherty AR, Althoff RR, Bach B, Chmielewski M, DeYoung CG, Forbush KT, Hallquist M, … Kotov R (2020). Redefining phenotypes to advance psychiatric genetics: Implications from hierarchical taxonomy of psychopathology. Journal of abnormal psychology, 129(2), 143–161. 10.1037/abn0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F, Allen P, Aleman A, Fernyhough C, Woodward TS, Badcock JC, Barkus E, Johns L, Varese F, Menon M, Vercammen A, & Laroi F (2012). Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull, 38(4), 683–693. 10.1093/schbul/sbs045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Levin-Aspenson HF, Waszczuk MA, Conway CC, Dalgleish T, Dretsch MN, Eaton NR, Forbes MK, Forbush KT, Hobbs KA, Michelini G, Nelson BD, Sellbom M, Slade T, South SC, Sunderland M, Waldman I, Witthöft M, Wright AGC, Kotov R, Krueger RF, & HiTOP Utility Workgroup (in press). Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, Stasik SM, & Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
- Watson D, Stanton K, & Clark LA (2017). Self-report indicators of negative valence constructs within the research domain criteria (RDoC): A critical review. J Affect Disord, 216, 58–69. 10.1016/j.jad.2016.09.065 [DOI] [PubMed] [Google Scholar]
- Weafer J, Dzemidzic M, Eiler W, Oberlin B, Wang Y, & Kareken D (2015). Associations between regional brain physiology and trait impulsivity, motor inhibition, and impaired control over drinking. Psychiatry research, 233(2). 10.1016/j.pscychresns.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Hajcak G, & Shankman SA (2015). Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. J Abnorm Psychol, 124(4), 878–889. 10.1037/abn0000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, & Hajcak G (2016). Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372–385. 10.1111/psyp.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M, Rose MS, & Sheldon-Keller A (1994). Assessment of patterns of insecure attachment in adults and application to dependent and schizoid personality disorders. Journal of Personality Disorders, 8(3), 249–256. [Google Scholar]
- WHO. (1992). The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. (10th Edition ed.). World Health Organization. [Google Scholar]
- Widiger TA, Sellbom M, Chmielewski M, Clark LA, DeYoung CG, Kotov R, Krueger RF, Lynam DR, Miller JD, & Mullins-Sweatt S (2019). Personality in a hierarchical model of psychopathology. Clinical Psychological Science, 7(1), 77–92. [Google Scholar]
- Williams BT, Henry JD, & Green MJ (2007). Facial affect recognition and schizotypy. Early Intervention in Psychiatry, 1(2), 177–182. [Google Scholar]
- Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, Jackson CT, Prabhakaran K, Bilker WB, Hakonarson H, Gur RC, & Gur RE (2015). Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry, 72(5), 456–465. 10.1001/jamapsychiatry.2014.3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering S, Lishak V, Hodgson N, Granic I, & Zelazo PD (2016). Executive function in children with externalizing and comorbid internalizing behavior problems. J Child Psychol Psychiatry, 57(1), 30–38. 10.1111/jcpp.12428 [DOI] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, & Adams K (2006). Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev, 77(4), 1016–1033. 10.1111/j.1467-8624.2006.00916.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, & Schachar R (2014). Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. J Abnorm Psychol, 123(2), 429–439. 10.1037/a0036295 [DOI] [PubMed] [Google Scholar]
- Xie W, Cappiello M, Park HB, Deldin P, Chan RCK, & Zhang W (2018). Schizotypy is associated with reduced mnemonic precision in visual working memory. Schizophr Res, 193, 91–97. 10.1016/j.schres.2017.07.046 [DOI] [PubMed] [Google Scholar]
- Yancey J, Vaidyanathan U, & Patrick C (2015). Aversive startle potentiation and fear pathology: Mediating role of threat sensitivity and moderating impact of depression. International journal of psychophysiology, 98(2 Pt 2). 10.1016/j.ijpsycho.2014.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, & Patrick CJ (2016). Psychoneurometric operationalization of threat sensitivity: Relations with clinical symptom and physiological response criteria. Psychophysiology, 53(3), 393–405. 10.1111/psyp.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Mitchell SH, & Bickel WK (2010). Delay discounting and substance abuse-dependence.
- Yin HH, Ostlund SB, & Balleine BW (2008). Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci, 28(8), 1437–1448. 10.1111/j.1460-9568.2008.06422.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, & Van Hulle C (2011). Empathy, guilt, and depression. Pathological altruism, 321–344. [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, & Wang J (2013). The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord, 151(2), 531–539. 10.1016/j.jad.2013.06.039 [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, & Murray GK (2010). The relevance of reward pathways for schizophrenia. Curr Opin Psychiatry, 23(2), 91–96. 10.1097/YCO.0b013e328336661b [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


