Abstract
Objective :
This article will familiarize the reader with the most common cutaneous adverse events with immune checkpoint inhibitors, as well as their grading and treatment.
Data Sources :
Recent research articles, relevant review articles, and case series/reports in English from PubMed database mostly from 2010 onwards.
Study Selections :
Most data are from retrospective studies and case series. Older studies regarding mechanism were included if they were of particular importance. Results : An understanding of this review should enable the reader to identify specific skin disorders in patients receiving immune checkpoint inhibitors (CPI), grade the adverse event, and be able to treat or refer the patient as needed.
Conclusion :
Allergists/immunologists need to be familiar with these immune-related adverse cutaneous adverse events as their incidence will increase with the ever-expanding use of CPI and in particular as patients will certainly continue to be referred suspecting drug allergies.
Keywords: immune checkpoint inhibitors, immune-related cutaneous adverse events, pruritus, dermatitis, depigmentation, drug reactions
Introduction
Immune checkpoint inhibition (CPI) with monoclonal antibodies targeting cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed death ligand (PD-L1) has shown promise as anticancer strategy.1 The CTLA-4 and PD-1/PDL-1 pathways are important at different points in the activation of an immune response. CTLA-4 downregulates T cell activation in lymphoid tissue, stopping autoreactive T cells at early stages of naïve T cell activation in lymph nodes, while the PD-1/PD-L1 inhibitors act later in the immune response pathway regulating activated T-cells peripherally, primarily in the tumor microenvironment. Thus, by downregulating T cell activation, immune checkpoints are important in self-antigen tolerance of cancer cells.2 By activating cytotoxic T cells, this therapy shifts the immune system toward anti-tumor activity. However, this therapy is associated with diverse autoimmune and auto-inflammatory phenomena termed immune-related adverse events (irAEs).1
Immune-related adverse events are very common and have the potential to impact treatment efficacy through dose-limiting effects.1 Adverse events affect multiple organs including the thyroid, lungs, GI, liver, eyes, and pancreas.2 Most irAEs are mild and can be reversed within two weeks if adequately treated.3 However, if severe AEs do occur, they may require permanent discontinuation of an otherwise effective therapy.
Cutaneous adverse events are the earliest and most common adverse events in these patients (Figure 1).1 Immune-related cutaneous adverse events (ircAEs) are reported in 90% of patients treated with CTLA-4 inhibitors, 70% with PD-1/PD-L1 inhibitors, and in almost all patients receiving combined therapies.4 While the most common ircAEs include nonspecific maculopapular rash, vitiligo, and lichenoid dermatitis, other rarer ircAEs include bullous dermatosis and psoriasiform dermatitis. Severe cutaneous complications include drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN). Most cutaneous adverse events resolve with mild to moderate potency topical corticosteroids but occasionally require systemic corticosteroids or biologic drugs including anti-tumor necrosis factor (TNF)-alpha agents.5 Because CTLA-4 and PD-1/L1 inhibitors act differently, if there is intolerance to one, the patient may be able to be challenged with the other.1 Due to the potential impact on therapy, other etiologies for the cutaneous adverse events should be excluded, such as infection, systemic disease, and adverse effects of other drugs, before concluding the reaction is due to the checkpoint inhibitor.
Figure 1:
Checkpoint proteins such as PD-1 and CTLA-4 on T cells and PD-L1 on tumor cells keep immune responses at bay. Immune checkpoint inhibitors block the binding of PD-L1 to PD1, which allows the T cells to kill tumor cells. It also causes side effects in multiple organs including the skin.
Given the increasing use of CPI for the treatment of various cancers, the practicing allergist can expect consults for ircAEs. Clinical severity of adverse events in oncology, including skin events, is assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) criteria, which grades adverse event severity on a scale of 1–5 (Table 1, Figure 2).6 Of note, not all cutaneous adverse events reach a high-grade AE status. Whereas alopecia will be graded either as grade 1 in case of less than 50% hair loss, 50–100% hair loss would be grade 2 and does not reach a high grade. Bullous ircAE can reach grades 4 and 5, notably in cases of TEN (Table 1). However, for most ircAE, involvement of less than 10% body surface area (BSA) will be grade 1, up to 30% BSA will be grade 2, and above that will be grade 3. For detailed guidance on grading by type of cutaneous adverse event, the CTCAE by Chen and colleagues in 2012 should be consulted.7
Table 1:
Grading of cutaneous adverse events according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE)6
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|
| Overall | Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated | Moderate; minimal, local or noninvasive intervention indicated; limiting age- appropriate instrumental activities of daily living (ADL) | Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death related to adverse event |
| Pruritus | Mild or localized; topical intervention indicated | Widespread and intermittent; skin changes from scratching; oral intervention indicated; limiting instrumental ADL | Widespread and constant; limiting self-care ADL or sleep; systemic corticosteroid or immunosuppressive therapy indicated | X | X |
| Maculopapular Rash | Macules/papules covering <10% BSA with or without symptoms | Macules/papules covering 10–30% BSA with or without symptoms; limiting instrumental ADL; rash covering >30% BSA with or without mild symptoms | Macules/papules covering >30% BSA with moderate or severe symptoms; limiting self-care ADL | X | X |
| Lichenoid reactions | Covering < 10% BSA and no associated symptom | Covering 10–30% BSA and associated with erythema or pruritus; limiting instrumental ADL | Covering >30% BSA and associated with pruritus; limiting self-care ADL | X | X |
| Vitiligo-like depigmentation | Hypopigmentation or depigmentation covering <10% BSA; no psychosocial impact | Hypopigmentation or depigmentation covering >10% BSA; associated with psychosocial impact | x | X | X |
| Blistering adverse events | Asymptomatic; blisters covering < 10% BSA | Blisters covering 10–30% BSA;painful blisters; limiting instrumental ADL | Blisters covering >30% BSA; limiting self-care ADL | Blisters covering >30% BSA; associated with fluid or electrolyte abnormalities; ICU care or burn unit indicated | Death |
| Stevens-Johnson syndrome | X | X | Skin sloughing covering <10% BSA with associated signs (erythema, purpura, epidermal detachment, and mucous membrane detachment) | Skin sloughing covering 10-30% BSA with associated signs (like in grade 3) | Death |
| Toxic epidermal necrolysis | X | X | X | Skin sloughing covering >=30% BSA with associated symptoms (erythema, purpura or epidermal detachment) | Death |
| Alopecia | Hair loss less than 50% normal for that individual, not obvious from a distance | Hair loss >= 50% of normal, readily apparent to others and requiring a wig | X | X | X |
| Nail changes | Clinical or diagnostic observation of discoloration; asymptomatic separation of the nail bed from the nail plate or nail loss | Symptomatic separation of the nail bed; limiting instrumental ADL | X | X | X |
Figure 2:
Calculation of body surface area by age (rule of nines).65
Cutaneous Eruptions
Maculopapular Rash
Maculopapular rashes are the most frequent ircAE observed, present in up to 25% of patients receiving treatment with anti-CTLA-4 or combination therapy of anti-CTLA-4 and antiPD-1/PD-L1 and 15% of patients treated with anti-PD-1 monotherapy.8 Lesions typically appear after 3–6 weeks of treatment, seem to be dose-dependent, and can worsen after each cycle of treatment.9 Patients can have nonspecific, morbilliform, blanchable pink coalescent macules and papules that occur mainly on the trunk and extremities, sparing the face and palmoplantar surfaces (Figure 3).10 The rash is generally pruritic and similar to the morbilliform drug eruption seen with antibiotics.8 An affiliated increase in peripheral blood eosinophil count has been reported.2 This rash is mostly self-limited but can also be the first signs of a higher grade reaction, such as a lichenoid reaction, psoriasis, bullous pemphigoid, or drug reaction with eosinophilia (DRESS).10
Figure 3:
Maculopapular rash in a patient on anti-PD-1 therapy.
Early recognition and treatment are necessary to continue oncologic therapy at an effective dose.2 These rashes can be long lasting and regress slowly, with resolution in most patients within 3 to 10 weeks.12
Lichenoid Reactions
Lichenoid reactions are the most well-characterized ircAE in patients receiving anti-PD-1 therapy, affecting 20% of patients.13 This rash tends to occur later than maculopapular rashes, with an average onset at 6–12 weeks after initiation of CPI.14 Lichenoid reactions are pruritic and vary dramatically in presentation and clinical manifestations. Some are similar to lichen planus with flat-topped, polygonal, pink-violaceous papules and visible, reticulated white streaks (known as Wickham’s striae). Others are hypertrophic or papulosquamous with a network of white scales (Figure 4).2,8 Lesions are usually on the chest and back but can involve limbs, palmoplantar, and mucosal surfaces.15 Lichen planus pemphigoides or lichen sclerosus et atrophicus have been described, as well as muscosal (genital and oral) and nail involvement.2,16 Histology shows a band-like lymphocytic infiltrate that is either mixed CD4+/CD8+ or predominant CD4+ T cell.2 This histology can also be associated with spongiotic changes together with an epidermal eosinophil infiltrate.15 In a case series of five patients, CD163-positive cells were seen in four patients compared to three patients with idiopathic lichen planus, thus suggesting a macrophage-monocyte lineage.17 Eruptions are usually mild and can be managed with topical steroids. Rarely systemic steroids, phototherapy, or acitretin are needed. Again, most cases of these AEs are of low grade and the immune checkpoint blockade can be maintained.15
Figure 4:
Lichenoid reaction in a patient on anti-PD-1 therapy.
Blistering Adverse Events
Bullous pemphigoid (BP) is an uncommon autoimmune disorder with an approximate incidence of 12.1 per million per year in a general European population, rising to 163 per million per year in Swiss patients aged 90 or above.18 While CPI therapy with underlying malignancies was found to be associated with mucous membrane pemphigoid, such a correlation has not been established with bullous pemphigoid, despite occasional reports.19 In a retrospective analysis, 1% of patients receiving PD-1 or PD-L1 inhibitors developed bullous pemphigoid.20 Furthermore, a disproportionality analysis found elevated proportional reporting ratios for patients treated with the PD-1 inhibitors pembrolizumab and nivolumab.21 Lesions may appear quickly or after several months of treatment with a mean onset of 14 weeks after treatment initiation.11 Initially, pruritus and nonspecific maculopapular eruptions are often seen, followed by tense bullae (blisters filled with serous or hemorrhagic fluid) overlying edematous pink urticarial plaques (Figure 5).22 The trunk and extremities are usually involved; mucosal involvement is unusual.23 Early-stage bullous disease, also called prebullous disease, should be suspected in any type of pruritic skin lesion appearing during CPI that is refractory to topical corticosteroids.24 The diagnosis is confirmed by skin biopsy for both dermatopathologic histology and direct immunofluorescence (DIF). While subepidermal cleavage can be seen histologically, DIF identifies characteristic IgG and C3 deposits along the dermo-epidermal junction. Whereas IgG autoantibodies against bullous pemphigoid antigen 180 (BP180) and BP230 are routinely identified in serum enzyme-linked immunosorbent assay (ELISA) in both idiopathic and CPI associated BP, BP180 has interestingly also been identified as ircAE predictive factor in patients with non-small-cell lung carcinoma (NSCLC) undergoing PD-1 based CPI.20,23,25 Monitoring for these circulating antibodies is helpful to understand disease severity and monitor treatment response. Since linear deposits of IgG and C3 at the dermo-epidermal junction are not specific for BP (it can be seen in cicatricial pemphigoid and epidermolysis bullosa acquisita), it is important to use direct immunofluorescence on salt split skin as there will be IgG deposition on the blister roof in bullous pemphigoid.1 The mechanism underlying bullous pemphigoid in CPI has not been characterized yet but is hypothesized to be secondary to B-cell activation, suggesting a humoral component to its development.23 The presence of lesions several months after the discontinuation of anti-PD-1 treatment has also been reported and suggested to be associated with a better clinical cancer outcome.23
Figure 5:
Bullous pemphigoid in a patient on anti-PD-1 therapy.
Psoriasiform Dermatitis
Psoriasiform dermatitis has also been reported in melanoma patients treated with PD-1/PD-L1 inhibitors. Similar to vitiligo, the eruption is related to tumor response. It has a mean onset of three weeks with a range of days to months.26 Lesions present indistinguishably from psoriasis vulgaris as sharply bordered, scaly, erythematous plaques, and are mainly located on the trunk and limbs (Figure 6). Similar to non-CPI-associated psoriasis, hypogranulosis, parakeratosis, acanthosis, dilated superficial dermal capillaries, perivascular lymphocytic infiltration, and neutrophils within or beneath the stratum corneum are seen on skin biopsy.26 Increases in IL-17, produced by Th17-delineated T cells, are key to the pathogenesis of psoriasis. Increased levels of interferon-gamma, tumor necrosis factor-alpha and interleukins 2, 6, and 17 are common.27
Figure 6:
Psoriasiform dermatitis in a patient on anti-PD-1 therapy.
Pruritus on Unchanged/Normal-Appearing Skin
Pruritus is one of the most common side effects of CPIs and can negatively impact the quality of life. It affects 14–47% of patients on CPI therapy and can be severe in 1–3% of patients.2 It can develop with or without rash. It most commonly involves the torso and extremities and usually spares the head and neck.28 The most effective prevention is through gentle skincare, including proper cleaning and moisturizer.29
Vitiligo-Like Depigmentation
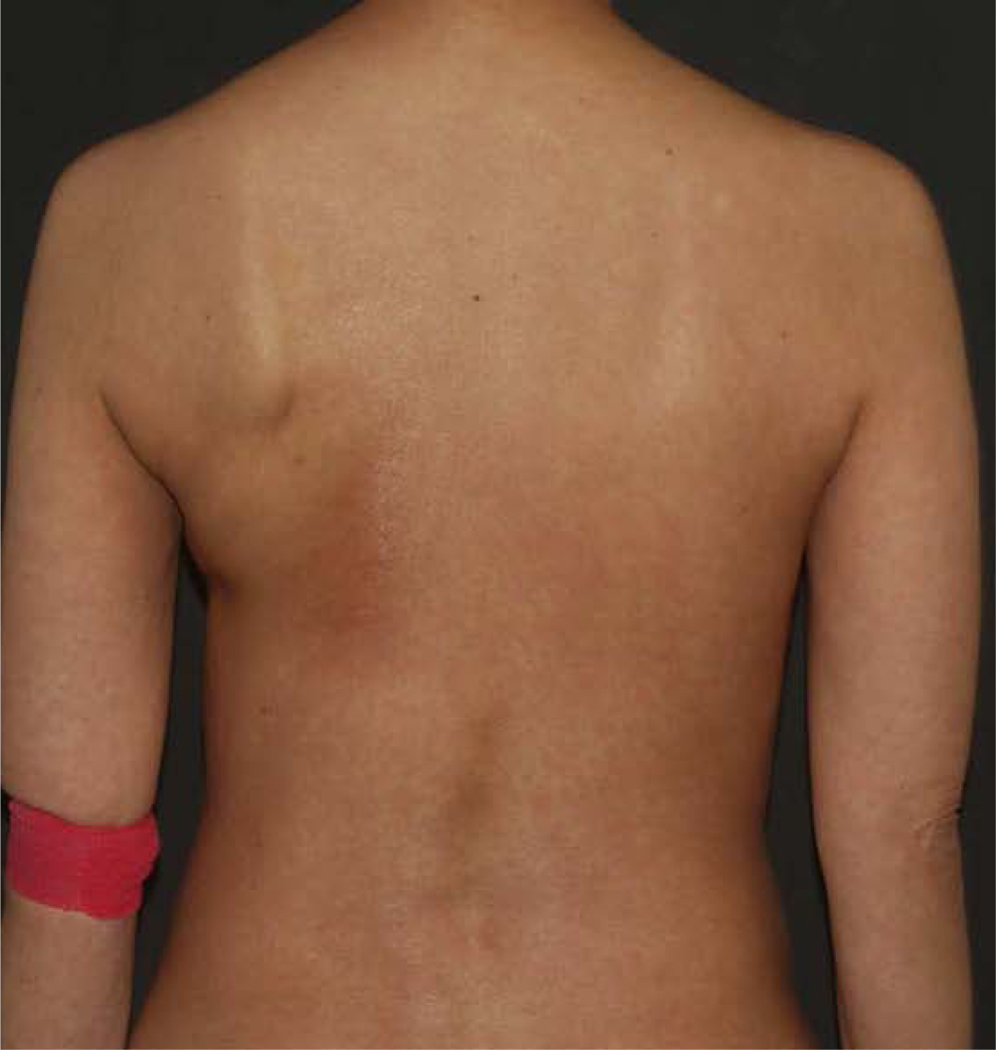
Vitiligo is a common cutaneous adverse event characterized by a loss of functional melanocytes in the epidermis. In the treatment of melanoma, it may be seen in up to 11% of patients treated with anti-CTLA-4 drugs, and in up to 25% of patients treated with anti-PD-1 agents.30 The event leading to vitiligo starts as an inflammatory phase, with a medium time to onset of 31 weeks and may progress while on therapy.30 It does not seem to be dose related.31 It commonly presents in a bilateral, symmetrical, and photo distribution affecting sun exposed areas such as the face, dorsum of the hands, forearms, neck, and may spread (Figure 7). However, focal or localized vitiligo has also been described.13 Lack of pigmentation in hair of the scalp, eyebrows, eyelash, and body hair may occur.30
Figure 7:
Vitiligo-like depigmentation in a patient on combination anti-CTLA-4 and anti-PD-1 therapy.
Interestingly, CPI induced vitiligo as an ircAE may present as a distinct pathophysiologic mechanism since the lesions differ from vitiligo clinically, as they present in more ultraviolet exposed areas and the lesions are not symmetrical.2,16 Histologically, it also differs from vitiligo as there is an overexpression of C-X-C motif chemokine receptor 3 (CXCR3) by CD8+ T lymphocytes.30 After treatment with anti-CTLA-4, immunohistochemical staining shows CD4 and melanin-specific CD8+ T cells near apoptotic melanocytes. Thus, it is believed that CTLA-4 antibodies stimulate an immune response against melanocytes.1
Vitiligo most often persists after CPI treatment discontinuation.32 This adverse event is more common in patients with melanoma than with other patients, supporting a shared antigen thesis. The development of vitiligo in patients treated with CPIs for advanced melanoma has been associated with both an objective response to therapy and an improved overall survival rate.33 In fact, doubling of progression-free survival, and four-fold increases in overall survival are associated with this ircAE, demonstrating that ircAEs may correlate with an enhanced antitumor response.34
Life-Threatening Cutaneous Drug Reactions
Life-threatening cutaneous drug reactions can occur with immune CPI. They are usually reversible with the discontinuation of immunotherapy and systemic treatment. DRESS presents initially with an extensive morbilliform rash associated with peripheral eosinophilia, fever, and involvement of organs other than skin and blood including liver, kidneys or lung, facial edema, and lymphadenopathy.35
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) cover a spectrum of acutely life-threatening dermatologic diseases. The mortality rate for SJS is 10% and 50% for TEN.36 Of note, TEN is a key predictive prognostic factor for lethal outcome.37 Clinically, along with mucous membrane involvement, skin lesions are painful and evolve from dusky erythematous maculae and flask blistering due to epidermal detachment. Tangential pressure leads to detachment (Nikolsky phenomenon).38 Full-thickness epidermal necrosis is seen on biopsy.1 In one case report, a dense CD8+ T cell infiltrate was seen within the dermo-epidermal junction.39 This case report also noted lymphocytes and keratinocytes with higher PD-L1 expression.2 Classically, extremely scarce immune infiltrates are expected in TEN as the keratinocyte apoptosis is an extremely rapid process. Interestingly, upregulation of inflammatory chemokines like chemokine ligand 9 (CXCL9), CXCL10, CXCL11, cytotoxic mediators like perforin 1 (PRF1) and granzyme B (GZMB), as well as proapoptotic molecules like fas ligand (FASLG) have been seen in both CPI-induced skin eruptions as well as life-threatening cutaneous reactions to the drug.40
Hair and Nail Abnormalities
Alopecia has been noted with CLTA-4 and PD-1 inhibitor treatment. At 1–2%, the incidence is low.41 It develops 3–6 months after treatment initiation and has been noted as focal (alopecia areata involving scalp hair, eyebrows, or beard) or a more generalized universalis type.2 On biopsy, peritubular swarm-like CD4+ lymphocytic infiltrates are seen (with scant CD8+ cells), which is consistent with idiopathic alopecia areata.27 Alopecia is graded by the NCI CTCAE as grade 1 with hair loss less than 50% of normal for that individual, not obvious from a distance, and grade 2 with hair loss ≥ 50% of normal, is readily apparent to others and requires a wig. Patches of gray regrown hair, as well as changes in the texture of the hair, have been observed.41 Furthermore, Rivera et al noted 14 patients treated with anti-PD-1/PD-L1 therapies for lung cancer who had diffuse hair repigmentation.42
Nail changes have been rarely seen, however, there have been a few cases of nail dystrophy associated with onychomadesis or proximal onychoschizia. The nail changes are most likely psoriatic or lichenoid.2
Oral Lesions
Oral lichenoid reactions have been seen in an isolated manner, but also with skin, nail, or genital lichenoid lesions. On physical exam, the most common findings are Wickham’s striae. Plaque-like, ulcerative, atrophic and erythematous lesions have also been described.2 Similar to xerostomia, these lesions are mostly low-grade and self-limited.2 Histologically, a T cell infiltrate is seen with a CD4/CD8 ratio of 1.43
Mechanistic Understanding of IRCAE
With an increasing incidence of ircAE due to the increasing numbers of patients treated with CPI, the body of knowledge about underlying mechanisms is growing. Pathophysiological understanding of certain toxicities is relatively advanced, especially gastrointestinal and cardiac toxicities.44 However, the mechanistic understanding of ircAEs is incomplete. While certain mechanisms have been identified as specifically involved in certain reaction patterns, the most important question remains how tightly and how exactly ircAEs are linked to on-target effects of CPI with the goal being to uncouple these effects. The focus of attention so far has been on approaches assuming shared epitopes between the underlying tumor and the skin as the cause of the ircAE. As an example, a high frequency of shared epitopes between NSCLC and skin have been identified. Patients treated with anti-PD-1 or anti-PD-L1 agents developed ircAE more frequently when their NSCLC response was also favorable.45 Fairfax and colleagues demonstrated a predictive value of certain T-cell receptor (TCR) repertoires in patients undergoing CPI therapy.46 Furthermore, higher incidences of ircAE have been reported with certain richer TCRVbeta sequences.47 A study in patients with castration-resistant prostate cancer provided similar results, finding an association of increased CD4 and CD8 diversity with ircAE.48
Another interesting find is based on the comparison found in various types of ircAE in melanoma patients treated with PD-1 inhibitors to non-CPI-related maculopapular rashes, SJS/TEN, and cutaneous graft versus host disease. The overlap was most important when compared with SJS/TEN, as apoptotic keratinocytes and a case of epidermal full-thickness necrosis (both histopathological features of SJS/TEN) were identified.49 Additionally, a prospective study identified elevated baseline BP180 to be associated with increased frequency of ircAE with CPI. Interestingly, this was not restricted to blistering ircAE.50
Psoriasiform ircAE develop most often as a flareup in patients with preexisting psoriasis, or a personal or family history of psoriasis. A detailed understanding of the respective triggers and mechanisms remain to be elucidated. Overall the pathophysiology seems similar or identical to sporadic psoriasis.51 This notion is supported by case reports reporting successful treatment with drugs targeted to established drivers of psoriasis, such as the IL-17 inhibitor secukinumab.52
Multiple investigations have been undertaken as vitiligo is one of the more frequent ircAE, a fact known since the first clinical trials of ipilimumab in metastatic melanoma. Of note, vitiligo is known to spontaneously appear in patients with melanoma and has also occurred more frequently in experimental treatments with melanoma vaccines.53 Although most frequent in patients with underlying melanoma, vitiligo is rarely seen in other patients treated with CPI.54,55 The current understanding of the underlying mechanism is around T-cell-mediated toxicity, notably infiltration of CD8+ skin-homing T-lymphocytes.56 Additional findings include elevated serum CXCL10 in melanoma patients being associated with an increased incidence of CPI-related depigmentation. The measured levels were also higher than those found in patients with idiopathic vitiligo or in healthy controls.30
IrcAE seems to share key mechanisms with non-CPI-related dermatological entities. However, further investigation into the respective differences and possible shared characteristics of ircAEs will allow for not only improved understanding but, more importantly, targeted treatment and possibly development of less toxic CPIs.
Approach to Treatment
Every patient presenting with a skin condition during CPI should receive a thorough examination of the entire skin, including accessible mucosa. Furthermore, the patient needs to be assessed for associated findings such as fever or lymphadenopathy. To determine a relationship to CPI, other reasonable etiologies (including independent dermatoses, notably infections, other systemic diseases, or paraneoplastic dermatoses) need to be ruled out. Whenever possible, the type of ircAE should be determined since treatment recommendations are generally aligned with the corresponding non-CPI-associated dermatoses.
Mild (grade 1) events can be managed by the treating oncologist or general practitioner.4 Special precautions are warranted in bullous ircAE and with any suspicion of SJS/TEN; specifically, an urgent dermatology referral is required. Photo documentation is recommended before initiating a treatment with emollients, mild (desonide 0.05% ointment, hydrocortisone 2.5% ointment) to medium strength (triamcinolone 0.1% ointment) topical corticosteroids, and/or oral antihistamines if the lesions are pruritic. Medium to high potency (mometasone furoate 0.1% ointment, betamethasone diproprionate 0.05% ointment) topical steroids can be used on the body and lower potency topical steroids on the face. CPI should not be interrupted.
For grade 2 ircAEs, the same treatment as for grade 1 ircAE is indicated, with a preference for higher potency topical steroids. In general, a dermatology referral is recommended and a skin biopsy should be considered. With appropriate treatment and regular assessment, CPI therapy can be continued. In case of persisting or worsening ircAE, CPI should be held until the ircAE improves to grade 1.
Any grade 3 ircAE should warrant a same-day dermatology referral and CPI therapy should be held and resumed no earlier than upon improvement to grade 1 or possibly grade 2. Treatment should include systemic corticosteroids, such as 0.5–1mg/kg/day prednisone daily for 3 days with a taper over 10–14 days, or if more severe, 0.5–1 mg/kg/day IV methylprednisolone followed by the oral equivalent tapered over 2–4 weeks upon response.
The occurrence of a grade 4 ircAE will require dermatological hospitalization, possible intensive care, and permanent discontinuation of the CPI. Other types of CPI should only be introduced after a very careful weighing of risks and benefits and no earlier than upon improvement of the AE to grade 1 (Table 1).4
For pruritus, gamma-aminobutyric acid (GABA) analogs (gabapentin, pregabalin) have been used as treatment with moderate success.1 One study showed GABA analogs and neurokinin-1 receptor antagonist to be superior to topical corticosteroids and oral antihistamines.12 Other treatments include antidepressants and systemic corticosteroids.29
Mild bullous pemphigoid may respond to potent topical corticosteroids, however, most bullous pemphigoid treatment involves interruption of CPI therapy and initiation of systemic steroids.57 In idiopathic bullous pemphigoid, oral doxycycline has been shown to be equivalent to systemic steroids and is preferred due to its superior safety profile. No data using doxycycline is available for CPI-associated BP.58 In some refractory cases, rituximab has been used successfully.59 One review notes omalizumab for bullous eruptions with high IgE levels.60
For psoriasiform dermatitis, treatment includes high potency topical corticosteroids, vitamin D3 analogues, narrow-band ultraviolet B phototherapy, methotrexate, and either retinoids or biologics.12
With SJS and TEN being potentially fatal, CPI therapy must be stopped immediately and intensive care (burn unit) is indicated. Systemic corticosteroids or intravenous immunoglobulins are commonly used based on the assumption that the FASLG is the key mediator of keratinocyte apoptosis.61
For alopecia, treatment options include intralesional topical steroids, systemic steroids, and topical immunotherapy with diphenylcyclopropenone (DPCP), an obligatory contact sensitizer, amongst others.62
For oral mucosa involvement, high potency topical corticosteroids are the mainstay of treatment.
Overall it is essential to pay close attention to the skin in regular intervals in patients treated with CPI. Early and appropriate management can avoid treatment delays and interruptions. Furthermore, CPI treatment should not be interrupted without a recommendation from an experienced dermatologist.
Conclusion
Checkpoint inhibitors are a breakthrough in cancer therapy and have shown promise in treating melanoma and other solid and hematologic malignancies. Immune-related adverse events are very common. They impact the quality of life in these patients and may affect treatment efficacy through dose limitations or discontinuation. Cutaneous toxicities are some of the most frequent adverse events. Dermatologic safety profiles for CTLA- 4 and PD-1/PD-L1 inhibitors are relatively similar, with increased toxicity with combined treatment.2 While the most common ircAEs include nonspecific maculopapular rashes, vitiligo, and lichenoid dermatitis, other ircAEs include bullous dermatosis and psoriasiform dermatitis. Pruritus is common and has a significant impact on the quality of life. Severe cutaneous complications like DRESS, SJS, and TEN are rare but can limit the use of immune CPI.1 While ircAEs are thought to be due to over activation of the immune system secondary to the same mechanism by which immune checkpoint inhibitors create their anti-tumor effect, the mechanistic understanding is limited. This correlation is most established in vitiligo during the treatment of melanoma. It is likely that additional ircAEs will be identified as positive prognostic markers. However, further studies are needed to establish the prognostic value of other cutaneous reactions such as morbilliform exanthems, lichenoid, and bullous pemphigoid, as well as to stratify which patients are at greatest risk for the development of these ircAEs. Both CTLA-4 and PD-1/L1 inhibitors acting at different stages of T cell activation can result in a multitude of ircAEs, pointing to T lymphocytes playing a key role.8
While high dose systemic corticosteroids are mostly effective in alleviating and eliminating symptoms, there have been a few reports of events continuing even after administration of steroids.64,65 There are limited reports on the use of other therapies, like biologics to treat these steroid refractory adverse events. Further studies are needed to understand the underlying mechanism of these ircAEs to recommend targeted therapies in the future.
Acknowledgments
Funding Source: This research was funded in part through NIAMS/NIH 1U01AR077511 and NIH/NCI Cancer Center Support Grant P30 CA008748. The authors acknowledge Nicole Meiklejohn for her outstanding assistance with preparing this manuscript and for creating Figure 1.
Abbreviations:
- BSA
body surface area
- BP
bullous pemphigoid
- CXCL
chemokine ligand
- CXCR
CXC motif chemokine receptor
- CTLA4
cytotoxic T lymphocyte-associated antigen-4
- DPCP
diphenylcyclopropenone
- DIF
direct immunofluorescence
- DRESS
drug reactions with eosinophilia and systemic symptoms
- ELISA
enzyme-linked immunosorbent assay
- FASLG
fas ligand
- GABA
gamma-aminobutyric acid
- GZMB
granzyme B
- CPI
immune checkpoint inhibition/inhibitor
- irAEs
immune-related adverse events
- ircAEs
immune-related adverse cutaneous adverse events
- NCI-CTCAE
National Cancer Institute’s Common Terminology Criteria for Adverse Events
- NSCLC
non-small-cell lung cancer
- PRF1
perforin 1
- PD-1
programmed cell death protein 1
- PD-L1
programmed death ligand
- SJS
Stevens-Johnson syndrome
- TCR
T-cell receptor
- TEN
toxic epidermal necrolysis
- TNF
tumor necrosis factor
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Geisler AN, Phillips GS, Barrios DM, et al. CME Part II: Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. [DOI] [PubMed] [Google Scholar]
- 3.Inno A, Metro G, Bironzo P, et al. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori. 2017;103(5):405–421. [DOI] [PubMed] [Google Scholar]
- 4.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer.2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Common terminology criteria for adverse events (CTCAE). Version 5.0. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Accessed November 11, 2020.
- 7.Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. 2012;67(5):1025–1039. [DOI] [PubMed] [Google Scholar]
- 8.Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93(1):123–132. [PMC free article] [PubMed] [Google Scholar]
- 9.Minkis K, Garden BC, Wu S, Pulitzer MP, Lacouture ME. The risk of rash associated with ipilimumab in patients with cancer: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2013;69(3):e121–128. [DOI] [PubMed] [Google Scholar]
- 10.Gravalos C, Sanmartin O, Gurpide A, et al. Clinical management of cutaneous adverse events in patients on targeted anticancer therapies and immunotherapies: a national consensus statement by the Spanish Academy of Dermatology and Venereology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2019;21(5):556–571. [DOI] [PubMed] [Google Scholar]
- 11.Phillips GS, Wu J, Hellmann MD, et al. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol. 2019;37(30):2746–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(3):455–461 e451. [DOI] [PubMed] [Google Scholar]
- 13.Tetzlaff MT, Nagarajan P, Chon S, et al. Lichenoid dermatologic toxicity from immune checkpoint blockade therapy: a detailed examination of the clinicopathologic features. Am J Dermatopathol. 2017;39(2):121–129. [DOI] [PubMed] [Google Scholar]
- 14.Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152(10):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 16.Schaberg KB, Novoa RA, Wakelee HA, et al. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J Cutan Pathol. 2016;43(4):339–346. [DOI] [PubMed] [Google Scholar]
- 17.Marazza G, Pham HC, Scharer L, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol. 2009;161(4):861–868. [DOI] [PubMed] [Google Scholar]
- 18.Chen CT, Hu HY, Chang YT, Li CP, Wu CY. Cancer is not a risk factor for bullous pemphigoid: 10-year population-based cohort study. Br J Dermatol. 2019;180(3):553–558. [DOI] [PubMed] [Google Scholar]
- 19.Siegel J, Totonchy M, Damsky W, et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: a retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. 2018;79(6):1081–1088. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal P. Disproportionality analysis of bullous pemphigoid adverse events with PD-1 inhibitors in the FDA adverse event reporting system. Expert Opin Drug Saf. 2019;18(7):623–633. [DOI] [PubMed] [Google Scholar]
- 21.Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44(2):158–176. [DOI] [PubMed] [Google Scholar]
- 22.Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57(6):664–669. [DOI] [PubMed] [Google Scholar]
- 23.Naidoo J, Schindler K, Querfeld C, et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez AT, Geskin L. A case of nivolumab-induced bullous pemphigoid: review of dermatologic toxicity associated with programmed cell death protein-1/programmed death ligand-1 inhibitors and recommendations for diagnosis and management. Oncologist. 2018;23(10):1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasan Ali O, Bomze D, Ring SS, et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J Am Acad Dermatol. 2020;82(4):854–861. [DOI] [PubMed] [Google Scholar]
- 26.Kaunitz GJ, Loss M, Rizvi H, et al. Cutaneous eruptions in patients receiving immune checkpoint blockade: clinicopathologic analysis of the nonlichenoid histologic pattern. Am J Surg Pathol. 2017;41(10):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol. 2020;83(4):1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips GS, Freites-Martinez A, Wu J, et al. Clinical characterization of immunotherapy-related pruritus among patients seen in 2 oncodermatology clinics. JAMA Dermatol. 2019;155(2):249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Lacouture ME. Pruritus associated with targeted anticancer therapies and their management. Dermatol Clin. 2018;36(3):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsabal M, Marti A, Jacquemin C, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. 2017;76(5):863–870. [DOI] [PubMed] [Google Scholar]
- 31.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. [DOI] [PubMed] [Google Scholar]
- 34.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. [DOI] [PubMed] [Google Scholar]
- 35.Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588–597. [DOI] [PubMed] [Google Scholar]
- 36.Chen CB, Wu MY, Ng CY, et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag Res. 2018;10:1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. [DOI] [PubMed] [Google Scholar]
- 38.Bolognia JSJ, Cerroni L. Dermatology: 2- Volume Set 4th Edition. Elsevier; 2017. [Google Scholar]
- 39.Vivar KL, Deschaine M, Messina J, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44(4):381–384. [DOI] [PubMed] [Google Scholar]
- 40.Goldinger SM, Stieger P, Meier B, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016;22(16):4023–4029. [DOI] [PubMed] [Google Scholar]
- 41.Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176(6):1649–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti-programmed cell death 1 and anti-programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153(11):1162–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sibaud V, Eid C, Belum VR, et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: clinicopathological findings. J Eur Acad Dermatol Venereol. 2017;31(10):e464–e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fessas P, Possamai LA, Clark J, et al. Immunotoxicity from checkpoint inhibitor therapy: clinical features and underlying mechanisms. Immunology. 2020;159(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berner F, Bomze D, Diem S, et al. Association of checkpoint inhibitor–induced toxic effects with shared cancer and tissue antigens in non–small cell lung cancer. JAMA Oncology. 2019;5(7):1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairfax BP, Taylor CA, Watson RA, et al. Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nature Medicine. 2020;26(2):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clinical Cancer Research. 2014;20(9):2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh DY, Cham J, Zhang L, et al. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Research. 2017;77(6):1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldinger SM, Stieger P, Meier B, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clinical Cancer Research. 2016;22(16):4023–4029. [DOI] [PubMed] [Google Scholar]
- 50.Hasan Ali O, Bomze D, Ring SS, et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. Journal of the American Academy of Dermatology. 2020;82(4):854–861. [DOI] [PubMed] [Google Scholar]
- 51.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. European Journal of Cancer. 2016;60:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monsour EP, Pothen J, Balaraman R. A novel approach to the treatment of pembrolizumab-induced psoriasis exacerbation: a case report. Cureus. 2019;11(10):e5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsao H, Millman P, Linette GP, et al. Hypopigmentation associated with an adenovirus-mediated gp100/MART-1-transduced dendritic cell vaccine for metastatic melanoma. Arch Dermatol. 2002;138(6):799–802. [DOI] [PubMed] [Google Scholar]
- 54.Kosche C, Mohindra N, Choi JN. Vitiligo in a patient undergoing nivolumab treatment for non-small cell lung cancer. JAAD Case Rep. 2018;4(10):1042–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lolli C, Medri M, Ricci M, et al. Vitiligo-like lesions in a patient treated with nivolumab for renal cell carcinoma. Medicine (Baltimore). 2018;97(52):e13810-e13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Gal FA, Avril MF, Bosq J, et al. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117(6):1464–1470. [DOI] [PubMed] [Google Scholar]
- 57.Mochel MC, Ming ME, Imadojemu S, et al. Cutaneous autoimmune effects in the setting of therapeutic immune checkpoint inhibition for metastatic melanoma. J Cutan Pathol. 2016;43(9):787–791. [DOI] [PubMed] [Google Scholar]
- 58.Williams HC, Wojnarowska F, Kirtschig G, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet. 2017;389(10079):1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sowerby L, Dewan AK, Granter S, Gandhi L, LeBoeuf NR. Rituximab treatment of nivolumab-induced bullous pemphigoid. JAMA Dermatol. 2017;153(6):603–605. [DOI] [PubMed] [Google Scholar]
- 60.Kremer N, Snast I, Cohen ES, et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol. 2019;20(2):209–216. [DOI] [PubMed] [Google Scholar]
- 61.Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63(2):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42(4):549–566; quiz 567–570. [PubMed] [Google Scholar]
- 63.Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569(7756):428–432. [DOI] [PubMed] [Google Scholar]
- 64.Gao L, Yang X, Yi C, Zhu H. Adverse Events of Concurrent Immune Checkpoint Inhibitors and Antiangiogenic Agents: A Systematic Review. Front Pharmacol. 2019;10:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Assessing Burns and Planning Resuscitation: The Rule of Nines. https://www.uwhealth.org/emergency-room/assessing-burns-and-planning-resuscitationthe-rule-of-nines/12698. Accessed November 11, 2020.