Abstract
One major goal in tissue engineering is to create functional materials, mimicking scaffolds in native tissues, to modulate cell function for tissue repair. Collagen is the most abundant structural protein in human body. Though collagen I (COLI) and collagen III (COLIII) are the predominant collagen types in connective tissues and they form stable hybrid fibrils at varied ratios, cell responses to the hybrid matrices are underinvestigated. In this work, we aim to explicate the distinctive roles of COLI and COLIII in fibroblast activation. Unidirectionally aligned COLI, COLIII and COLI-COLIII hybrid nanofibrils were generated via epitaxial growth of collagen on mica. AFM analyses revealed that, with the increase of COLI/COLIII ratio, the fibril width and stiffness increased and the binding affinity of cells to the matrix decreased. A hybrid matrix was found to activate fibroblasts the most effectively, characterized by extensive cell polarization with rigid stress fiber bundles and high α-SMA expression, and by the highest-level of collagen synthesis. It is ascribed to the fine balance between biochemical and biophysical cues achieved on the hybrid matrix. Thus, matrices of aligned COLI-COLIII hybrid fibrils and their derived multifunctional composites can be good candidates of implantation scaffolds for tissue regeneration.
Keywords: Collagen I, Collagen III, Nanofibrils, Fibroblast Activation, Cell-Matrix Interaction, Collagen Synthesis
1. Introduction
Collagen is the main component of extracellular matrix (ECM) in native tissues. It provides mechanical stability, elasticity and strength to the organs [1]. The fibrillar collagen I (COLI) and collagen III (COLIII) are the predominant types of collagen in connective tissues. COLI is a heterotrimer that consists of two α1 chains and one α2 chain. It offers high tensile strength and rigidity of the fibril matrix. COLIII is a homotrimer containing three α1 chains, and is often found in close association with COLI to form COLI-rich hybrid fibrils to increase the tissues’ flexibility and distension [1,2]. The hybrid fibrils were reported to have a COLI:COLIII ratio of 2:1 to 3:1 in skin, cardiac and vascular tissues [1,3]. In scar tissues, the ratio was found much higher (5:1) [4]. While the fibril bundles form meshed network in large scale, the fibrils are locally aligned on the nanoscopic to microscopic scale to support cell activities.
Cells interact with ECM through integrin, which is composed of two non-covalently bound α and β subunits [5]. Among the various heterodimers, α1β1-integrin was identified a collagen receptor that regulates collagen synthesis and cell growth [6,7]. Since COLI and COLIII differ in molecular structure, their binding affinity to α1β1-integrin may differ. Fibroblasts synthesize the ECM proteins, including COLI and COLIII. They are spindle-shaped, locally align with collagen fibrils in connective tissues to maintain and remodel the structural framework, and play critical roles in wound healing and tissue regeneration [8]. It was reported that the fibroblasts’ collagen productivity is affected by external mechanical stress through mechanotransduction pathway [8]; an aligned collagen matrix provides structural and signaling cues to regulate cell functions [9–12]. Thus, both the biochemical composition and biophysical properties of a matrix can impact fibroblasts’ collagen productivity. Though COLI and COLIII co-exist in connective tissues and the ratio differs in different tissue types, studies of fibroblast responses to aligned COLI-COLIII hybrid scaffolds were rarely reported.
In this study, we generated aligned COLI and COLIII fibril matrices using a simple epitaxial growth method, in which collagen molecules were guided by the crystalline orientation of mica and self-assemble into unidirectionally aligned nanofibrils [13]. Primary fibroblasts extracted from soft connective tissues were used in the study to delve into the cell-matrix interaction on the scale that the interaction takes place and to offer new insights into the matrix mediated fibroblast activation. The COLI, COLIII and COLI-COLIII hybrid fibrils, their affinity to α1β1-integrin and their ability to activate fibroblasts for collagen synthesis have been examined nanoscopically by AFM in combination with protein and gene level analyses. The results revealed that a COLI-COLIII hybrid matrix is favorable due to the balanced biochemical and biophysical cues, which play critical roles in regulating fibroblasts’ collagen productivity. Such prospects inspire the exploration of COLI-COLIII hybrid fibril based implantable materials for connective tissue repair and regeneration.
2. Materials and methods
2.1. Preparation of collagen matrices
COLI and COLIII proteins derived from bovine tendon were purchased from EMD Millipore (Billerica, MA, USA). A stock solution of 1.5 mg/mL was prepared by dissolving the protein powder in 0.1% acetic acid, then diluted to 35 μg/ml in 10[ISP]× PBS buffer containing 1N NaOH to adjust the pH to 9 [8,13]. Following the previously established protocol [10,11,13], unidirectionally aligned collagen fibrils were achieved on a freshly cleaved surface of a Muscovite mica disk (Ted Pella, Inc., Redding CA). In addition to pure COLI and pure COLIII, matrices were also prepared from COLI and COLIII mixture at ratios of 1:1 (COLI-COLIII (1:1)) and 2:1 (COLI-COLIII (2:1)) for comparison.
2.2. AFM imaging and fibril/cell stiffness measurements
Both AFM imaging and stiffness measurements were carried out in 1× PBS buffer using Si3N4 tips with a spring constant of 0.030 ± 0.002 N/m, calibrated by reference cantilevers with known spring constants [12,13]. A multimode Nanoscope IIIa AFM (Veeco Metrology, Santa Barbara, CA), equipped with a J-scanner, was utilized in this study. Images of various matrices were collected in fluid tapping mode, with topographic and amplitude images captured simultaneously. In collecting images and elasticity maps of cells on the matrices, the cells were gently fixed with 4% paraformaldehyde (PFA) for three minutes.
The stiffness of various matrices and cells, characterized by the Young’s Modulus (E-value), was derived from the force maps (16 pixels per line) collected in fluid contact mode in 1× PBS buffer. From each force-distance curve, the E value was derived using the Hertzian model with the AFM tip modeled as a nano-indenter to probe a one-dimensional material for fibrous matrices or a flat, infinite surface for a gelatin surface and the cells [14,15].
2.3. Fibroblast cell culture and characterization
Vaginal fibroblasts, extracted from the biopsy harvested at the posterior vaginal fornix of an individual who had total hysterectomy following the approved IRB protocol (#EH15-340) [2,11], was used as a cell model in this study. The cells were cultured following an established protocol [11,12]. At confluence, the cells were trypsinized and plated on target matrices at a seeding density of 5000 cells/cm2 for various studies.
Cell polarity was characterized by the cell length-to-width ratio (Table S1), which was evaluated based on optical images [10–12]. To examine fibroblast activation, the cells were stained against α-smooth muscle actin (α-SMA). The immunofluorescent images were analyzed quantitatively by ImageJ (See Supplementary Materials).
2.4. AFM probe functionalization and binding strength measurement
α1β1-integrin (B&D Systems, MN) was functionalized on gold-coated AFM probes (Nanoworld, Switzerland) via a crosslinker, N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP, Soltec Ventures, MA), as routinely performed in our lab [12,13]. Force-distance curves were then recorded at a z-scan rate of 3 μm/s and a trigger force of 4.6 nN. The binding strength was quantitatively determined from the adhesion force measured during probe retraction from the surface. In control, BSA modified AFM probe was employed in parallel experiments to evaluate the level of nonspecific interaction.
Cell adhesion was similarly measured between a cell-modified AFM probe and various matrices (Fig. S1). The adhesion forces were derived from the retraction curves.
2.5. RT-qPCR analysis
Total RNA was extracted from fibroblasts after cultured on various matrices for two days. Reverse-transcription was carried out using a SuperScript® III kit (Invitrogen, Carlsbad, CA). RT-qPCR was performed using an ABI Prism 700 (Applied Biosystem, Foster City, CA) with TaqMan® Gene Expression Assays (Life Technology, Madison, WI). Primers against the following genes were used in this study: COLI (Hs00164404_m1), COLIII (Hs00164103_m1), and ACTA2 (Hs00426835_g1). GAPDH (Hs99999905_m1) was used as an endogenous reference. Data analysis was performed using the 2−ΔΔCT method for relative quantification based on three replicates. Student’s t-test was performed for statistical analysis.
3. Results
3.1. Morphology and stiffness of collagen fibrils
Figure 1a–e shows the AFM images of collagen fibrils at various COLI/COLIII ratios formed on mica, with the gelatin coated substrate as a control. All the fibrils exhibit unidirectional alignment and unform surface coverage. The characteristic collagen D-period (inset of Fig. 1b), which signifies the proper assembly of collagen molecules into fibrils, was measured to be 68 ± 1 nm on all the collagen matrices (p > 0.05, no significant difference). With the increase of COLI/COLIII ratio, the fibril diameter increased from 86 ± 14 nm for pure COLIII to 126 ± 11 nm for pure COLI (Table S1). This is consistent with the notion that COLIII has the function of regulating the lateral assembly of collagen fibrils, hence, limiting fibril diameter due to the slower process of COLIII procollagen than COLI procollagen [2,16].
Figure 1.
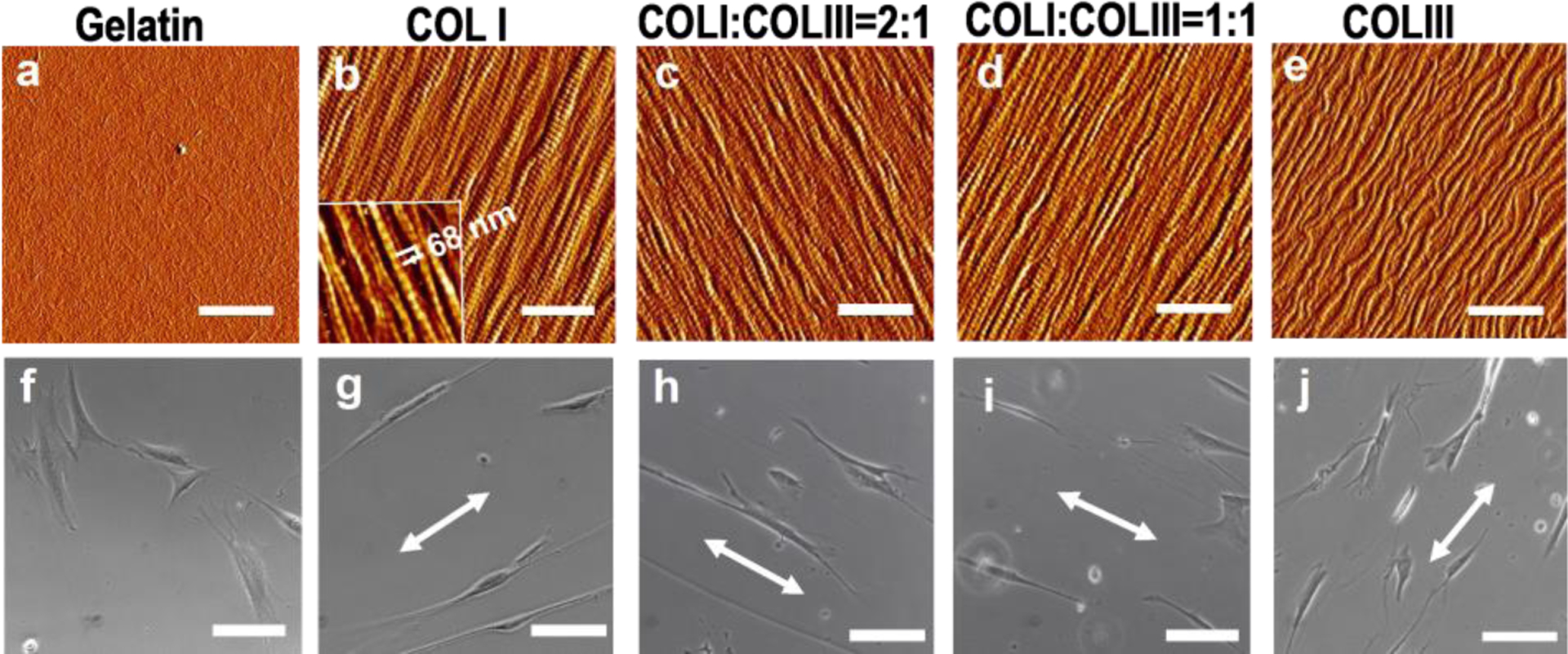
Unidirectionally aligned collagen fibrils and the induced cell polarization. (a–e) AFM images of gelatin and collagen fibrils on mica at various COLI/COLIII ratios (bar size: 1 μm): (a) Gelatin; (b) Pure COLI; (c) COLI: COLIII=2:1; (d) COLI: COLIII=1:1; (e) Pure COLIII. The inset of (b) is the high-resolution image illustrating the characteristic collagen D-period. (f–j) Optical images of fibroblasts 24 h post-plating on the respective matrices (bar size: 100 μm). The arrows indicate the direction of fibril alignment.
Young’s modulus (E-value) of the prepared fibrils was evaluated by the nano-indentation method. For fibrils at COLI/COLIII ratio of 1:0, 2:1, 1:1 and 0:1, the E-value is 0.26 ± 0.06 MPa, 0.21 ± 0.05 MPa, 0.19 ± 0.06MPa and 0.06 ± 0.03 MPa, respectively (Table S1). The E-value of a homogenous gelatin surface was measured to be 0.08 ± 0.04 MPa, as soft as the pure COLIII fibrils. Noticeably, pure COLI fibrils and COLI-COLIII hybrid fibrils are much stiffer than pure COLIII fibrils.
3.2. Fibroblast polarization on fibril matrices
The morphology of fibroblasts was examined 24 h post-plating on various matrices. As shown Figure. 1g–j, the cells elongated in the direction of fibril alignment. The COLI-COLIII (2:1) hybrid fibrils induced the highest-level cell polarization, giving rise to the cell length-to-width ratio of 35.6±7.9. The ratio is 1.4, 2.5 and 4.1 times higher than that of cells on pure COLI, COLI: COLIII (1:1) and pure COLIII fibrils (see data in Table S1). On the featureless gelatin surface, cells spread in random directions and didn’t develop the bipolar shape (Fig. 1f). The result suggests that the aligned fibrils played an essential role in cell polarization; though pure COLI fibrils are the stiffest, COLI-COLIII (2:1) fibrils outperform other fibril types in mediating fibroblast polarization. We surmise that factors other than matrix stiffness play roles in determining the level of cell polarization.
3.3. Binding strength of α1β1-integrin to COLI and COLIII
We applied the α1β1-integrin functionalized AFM probes to evaluate the strength of specific interaction between α1β1-integrin and COLI or COLIII fibrils (Fig. 2a). The level of non-specific interaction was evaluated using a BSA modified AFM probe (Fig. 2d). As shown in the histogram, the most probable force appears at 65 pN, and 80% of the non-specific interaction forces fall below 95 pN. The distribution of the measured binding forces between α1β1-integrin and COLI or COLIII fibrils were summarized in Figure 2b and 2c. By Gaussian fitting, we identified four peaks in each histogram, and the forces at the 2nd, 3rd and 4th peaks are multiples of that at the 1st peak. Additionally, all these peak values are above the non-specific interaction level. We infer 0.126 nN or 0.165 nN is the binding force between a single-pair of α1β1-integrin and COLI or α1β1-integrin and COLIII [17–19]. It implies that COLIII binds to α1β1-integrin stronger than COLI does. It is consistent with the report by P. Nykvist et al., who found a higher percentage of α1β1-integrin transfected cells attached to COLIII than to COLI matrices [20]. To our knowledge, this is the first study to compare the strength of α1β1-integrin binding to COLI and to COLIII fibrils quantitatively at the molecular level.
Figure 2.
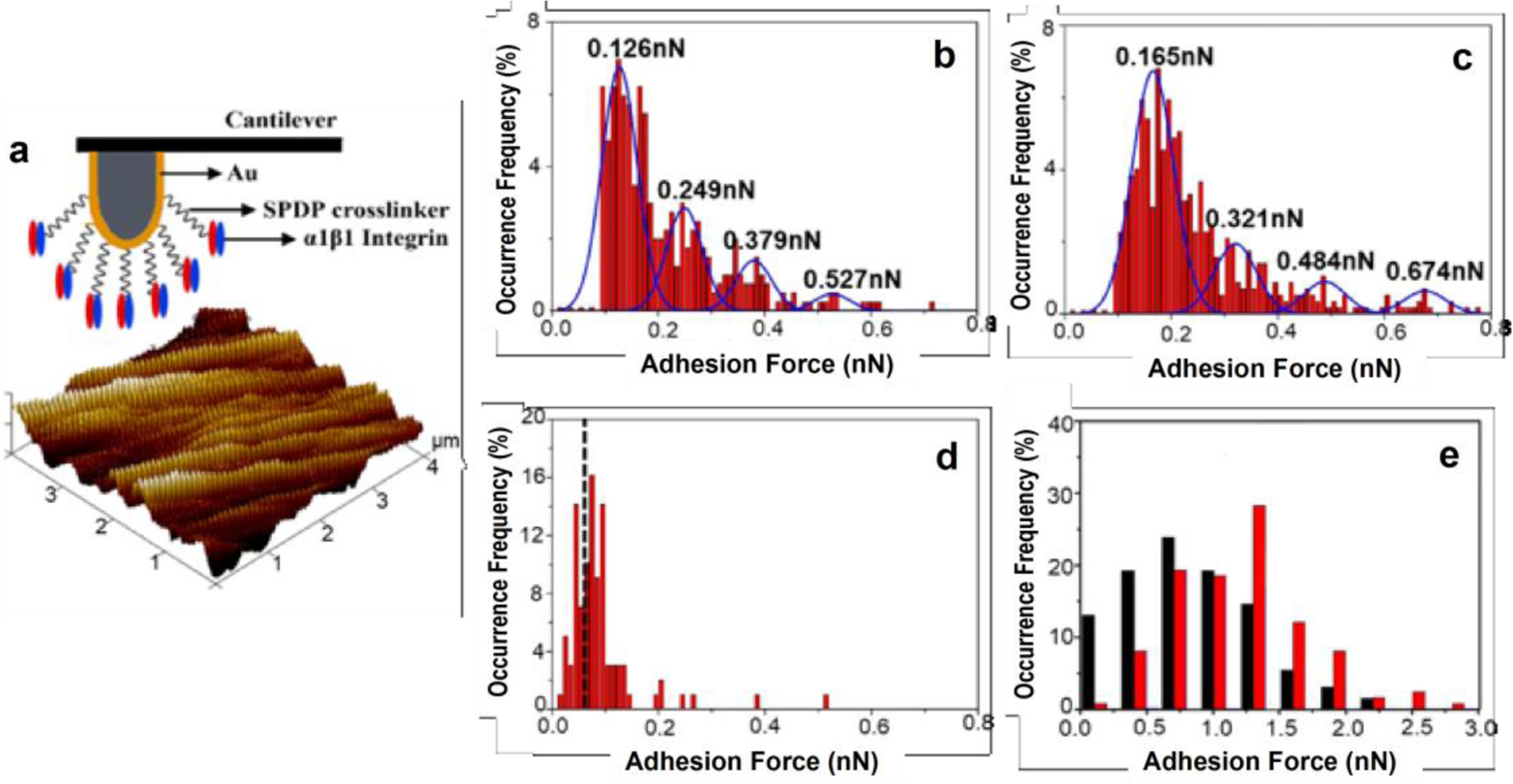
Binding force measurements on various matrices. (a) Functionalization of α1β1-integrin on an AFM probe to measure its binding force to a collagen matrix. (b–d) Histograms showing the distribution of binding forces measured between α1β1-integrin and COLI (b), COLIII (c) or a control BSA coated surface (d). Gaussian fitting peaks are shown in (b,c). (e) Histograms showing the distribution of adhesion forces measured between a whole cell and a COLI (black) or COLIII (red) matrix.
We also attached a single fibroblast on an AFM probe to evaluate the cell adhesion to the collagen matrices (Fig. S1). As shown in Figure 2e, cells showed marked preference to adhere to COLIII fibrils (1.23 ± 0.04 nN) than to COLI fibrils (0.86 ± 0.04 nN), and the difference is statistically significant (p < 0.001). It supports that α1β1-integrin is the essential cell surface receptor that mediates fibroblast adhesion to collagen matrices. The fibroblasts demonstrated a medium level adhesion to COLI-COLIII (2:1) fibrils (1.05 ± 0.05 nN).
3.4. Cytoskeletal structure and local stiffness of fibroblasts on various matrices
Activation of fibroblast is accompanied by the cell’s increased contractility, characterized by high level α-SMA expression and relevant to the structure and stiffness of actin filaments in the cells [21]. Fibroblasts grown on gelatin, COLI, COLIII and COLI-COLIII (2:1) matrices were stained against DAPI (blue) and α-SMA (green) (Fig. 3a–d). Actin filaments of cells on the aligned collagen fibrils are parallel to each other and well oriented, in contrast to the filaments stretched in various directions in cells on gelatin. Quantitative analysis by ImageJ suggests that cells on the COLI-COLIII hybrid matrix expressed α-SMA at the highest-level and cells on the pure COLI fibrils expressed α-SMA at a slightly lower level. However, cells on pure COLIII and featureless gelatin expressed α-SMA 34% and 60% lower (Table S1). This is consistent with that COLI-COLIII hybrid fibrils induced the highest-level of cell polarization.
Figure 3.
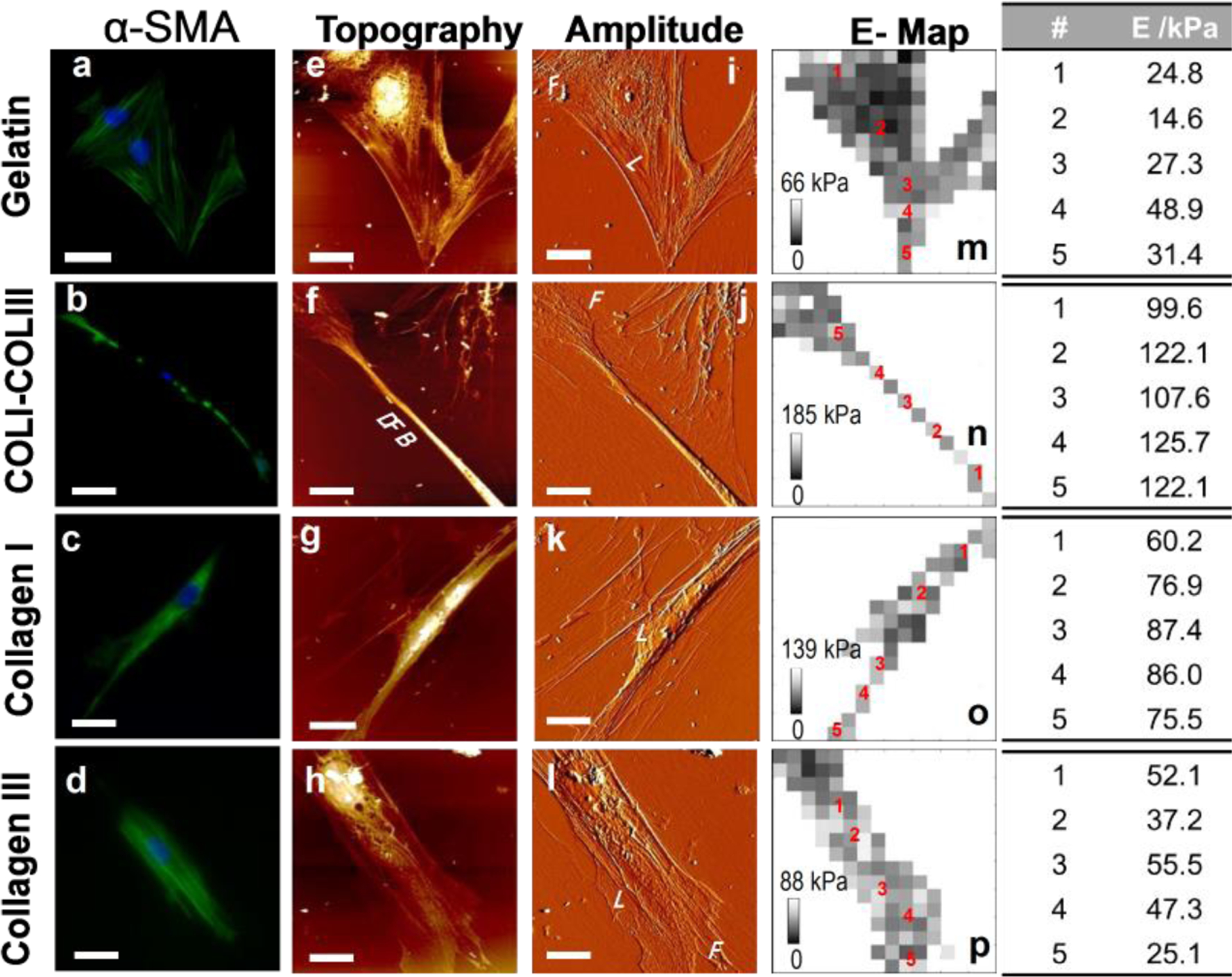
Cytoskeletal structure and local elasticity of fibroblasts on various matrices. (a–d) Immunostaining of α-SMA (green) and nuclei (blue) of fibroblasts on various matrices. Bar size: 56 μm on (b); 25 μm on the rest. (e–l) Corresponding AFM topographic (e–h) and amplitude (i–l) images of fibroblasts on various matrices. (m–p) E-maps derived from the force maps collected at the same regions of the AFM images in (e–l). Bar size: (e, i, m) 26 μm; (f, j, n) 20 μm; (g, k, o) 25 μm; (h, i, p) 20 μm.
AFM topographic and amplitude images (Fig. 3e–l) were collected in accordance to the immunofluorescent images (Fig. 3a–d). Aligned collagen fibrils are visible in the amplitude images (Fig. 3i–l). Similar to our previous observations [13], cells demonstrate sheet-like lamellipodia (L) and finger-like filopodia (F), which function as pathfinders to probe the environment for cues. On gelatin, a cell tends to develop lamellipodia around the cell body and develop a number of filopodia at the periphery leading to actin filaments stretching in random directions. The aligned collagen fibrils appear to coax the actin filaments stretching in the direction of the fibril alignment. On the soft COLIII fibrils, the presence of lamellipodia along the sides of a cell causes the cell to laterally spread and deform the soft fibrils. On both COLI and COLI-COLIII hybrid fibrils, the presence of dense actin filaments is evident (Fig. 3b,c). Noticeably, cells on COLI-COLIII hybrid fibrils develop long and dense filament bundles (DFB) with a narrow cell body.
To evaluate the stiffness of actin filaments in cells on various matrices, we collected the cell elasticity maps (Fig. 3m–p) obtained at the same regions as the high-resolution images were collected. The bright (dark) contrast on the E map indicates the high (low) E values of the local regions. To compare the local cell elasticity, we chose 5 spots for each cell, spanning from the nucleus to the filament (Fig. 3 right panel). The cell body near the nucleus (Point 1) is typically softer due to the lack of stress fibers. At the center of the filaments (Point 2), the fibroblast cytoskeleton is 2.5 times stiffer on COLIII than on gelatin, but is 5.3 times and 8.4 times stiffer on COLI and COLI-COLIII hybrid fibrils than on gelatin. Along the thin and dense filaments (Points 3–5), the variation of E-value is relatively small. However, the dense filaments of the cell on COLI-COLIII hybrid fibrils are markedly stiffer than those on other substrates. Taken together, COLI-COLIII (2:1) fibrils most effectively mediated the transition of fibroblast to contractile myofibroblast.
3.5. Gene expression analysis of fibroblasts grown on various matrices
RT-qPCR analysis was carried out to examine collagen synthesis by fibroblasts cultured on various matrices. As shown in Figure 4a, cells cultured on pure COLI, pure COLIII and gelatin substrates demonstrated similar level of collagen productivity (p>0.05). Cells on COLI-COLIII fibril matrix synthesized COLI and COLIII at a much higher level. The COLI/COLIII ratio was derived from COLI and COLIII expressions in cells on each matrix (Fig. 4b). While cells on pure COLI and pure COLIII matrices synthesized COLI and COLIII at a similar ratio (1.73 for COLI matrix and 1.74 for COLIII matrix), interestingly, the cells on COLI-COLIII (2:1) fibrils synthesized collagen at a COLI/COLIII ratio of 2.8. On the other hand, cells on COLI and COLI-COLIII hybrid fibrils expressed ACTA2 at a higher level than cells on gelatin or COLIII fibrils, consistent with the higher α-SMA expression at the protein level. The results suggest that the fibroblasts activated on the COLI-COLIII hybrid fibrils are the most productive in collagen.
Figure 4.
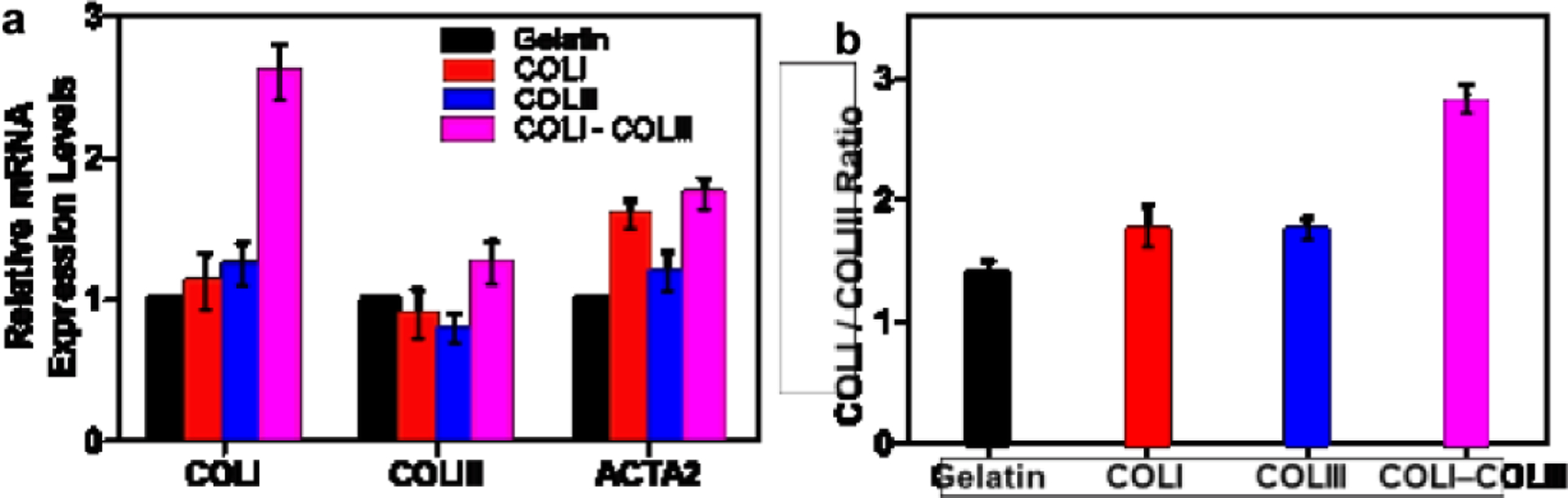
Gene expression profiles of fibroblasts grown on various matrices. (a) COLI, COLIII and ACTA2 expressions in cells on gelatin, COLI, COLIII and COLI-COLIII (2:1) matrices; (b) COLI/COLIII ratio for cells on each matrix type.
4. Discussion
One strategy of tissue engineering is the transplantation of in vitro grown cells on an implantable matrix for tissue reconstruction and regeneration. For connective tissue repair, fibroblast is frequently chosen as the cell source due to its abundance, easy collection and culture. A potential implant construct is expected to effectively activate fibroblasts grown on it to enhance collagen production for tissue regenerate regeneration [4,22]. Other than synthetic polymers, COLI matrix has been frequently used in such efforts. In this study for the first time, we have shown that aligned COLI-COLIII hybrid fibril matrices are more effective on boosting the fibroblasts’ collagen productivity.
Fibroblast activation requires both a stressed, mechanoresistant matrix and active TGFβ1 signaling. Collagen – α1β1-integrin specific interaction induces the formation of focal adhesion complexes, which assist in targeting the location of actin filaments and signaling components [23]. With the aligned collagen fibrils, the cells deposit the focal adhesions anisotropically, causing the cytoskeleton structure to stretch in the direction of fibril alignment. A stiffer matrix exerts a higher force to the cells through the focal adhesions, inducing a greater level of cytoskeletal tension, hence, a greater level of cell polarization and contractility to promote the conversion of fibroblast to myofibroblast. On the other hand, mechanically strong fibrils are hard to deform or break when the cells migrate and contract on them, therefore, can mediate the mechanotransductive signals more efficiently [9–13]. Evidently, cells on pure COLI and COLI-COLIII hybrid fibrils demonstrated much better polarization than cells on pure COLIII (Fig. 1). The cells were more adhesive to the COLIII fibrils than to the stiffer COLI and COLI-COLIII fibril matrices, evidenced by the measured adhesion forces (Fig. S1) and the wider extension of lamellipodia in cells on the COLIII substrates (Fig. 3). Excessive cell adhesion can impede cell contraction, which is essential for fibroblast activation. Concerning the contribution of physical cues derived from fibril alignment, matrix stiffness and cell adhesion, COLI and COLI-COLIII hybrid fibrils are favorable over COLIII fibrils for fibroblast activation.
Fibroblast activation is mediated by active TGFβ1 signaling which requires β1 integrin binding to collagen specifically [24,25]. Different from COLIII which is a homotrimer of three α1 chains, COLI is a heterotrimer that consists of two α1 chains and one α2 chain. We found the binding strength of α1β1-integrin to COLIII is stronger than to COLI by 30% (Fig. 2). This is likely due to the presence of the GROGER sequence in α2 chain, making α2 chain’s binding to β1 integrin weaker [24]. The strong affinity between COLIII-α1β1-integrin suggests that COLIII is favored over COLI in transmitting intracellular signals. It is consistent with the report by Volk et al [26] that COLIII modulates TGFβ signaling to boost fibroblasts’ collagen synthesis.
Collectively, a COLI-COLIII hybrid matrix outperforms a pure COLIII matrix in supporting cell polarization and contraction; it outperforms a pure COLI matrix in offering a stronger collagen-α1β1-integrin binding to mediate the intracellular signal transmission. Consequently, the COLI-COLIII fibrils induced the fibroblast activation the most effectively, characterized by the presence of rigid, dense stress fibers expressing α-SMA at a high level. These stress fiber bundles can enhance the cells’ contractility to induce β1 integrin-mediated activation of latent TGFβ1 [6] which signals for the improved collagen production as observed (Fig. 4). Not only the activated fibroblasts showed increased collagen synthesis on the COLI-COLIII fibril matrix, they synthesized collagen at an increased COLI/COLIII ratio, implying the cells’ tendency to remodel the matrix that they reside to achieve the inner- and extra-cellular balance of ECM composition and biomechanics [27].
A potential implant construct for tissue regeneration is expected to mimic both the biochemical composition and the biophysical features of collagen in native tissues to activate fibroblast for improved collagen synthesis. Instead of pure COLI, as used in many researches, our study shows that COLI-COLIII hybrid fibrils can be a better base-material to develop biocompatible, multifunctional, composite materials for tissue engineering. The fibrils generated on mica are well aligned, and the fibril dimension and composition can be tuned by varying the protein concentration, ratio and reaction conditions. While they serve as great models in in vitro studies, they are not implantable due to the attachment to mica. The results from this research provide the basis of generating implantable materials with alternative techniques, such as electrospinning [10–12], which prepares free-standing protein fibers with good alignment.
Supplementary Material
Highlights.
Collagen I and collagen III have distinctive roles in fibroblast activation.
Unidirectionally aligned collagen nano-fibrils prompted cell polarization.
The stiffer COLI fibrils exert a higher force to cells.
α1β1-integrin binds stronger to COLIII than to COLI.
COLI-COLIII hybrid fibrils promoted collagen synthesis the most effectively.
Acknowledgement
We thank Dr. Svjetlana Lozo of NorthShore Hospital for providing the tissue specimen. This research was partially supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R15HD096410 and the pilot grant of Pritzker Institute of Biomedical Science and Engineering at IIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Gelse K, Pöschl E, Aigner T, Collagens--structure, function, and biosynthesis, Advanced drug delivery reviews, 55 (2003) 1531–1546. [DOI] [PubMed] [Google Scholar]
- [2].Kim T, Sridharan I, Ma Y, Zhu B, Chi N, Kobak W, Rotmensch J, Schieber JD, Wang R, Identifying distinct nanoscopic features of native collagen fibrils towards early diagnosis of pelvic organ prolapse, Nanomedicine : nanotechnology, biology, and medicine, 12 (2016) 667–675. [DOI] [PubMed] [Google Scholar]
- [3].Hance AJ, Crystal RG, Rigid control of synthesis of collagen types I and III by cells in culture, Nature, 268 (1977) 152–154. [DOI] [PubMed] [Google Scholar]
- [4].Cheng W, Yan-hua R, Fang-gang N, Guo-an Z, The content and ratio of type I and III collagen in skin differ with age and injury, African Journal of Biotechnology, 10 (2013) 2524–2529. [Google Scholar]
- [5].Widgerow AD, Bioengineered matrices—part 2: focal adhesion, integrins, and the fibroblast effect, Annals of plastic surgery, 68 (2012) 574–578. [DOI] [PubMed] [Google Scholar]
- [6].Pozzi A, Wary KK, Giancotti FG, Gardner HA, Integrin α1β1 Mediates a Unique Collagen-dependent Proliferation Pathway In Vivo, Journal of Cell Biology, 142 (1998) 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gardner H, Integrin α1β1, Adv Exp Med Biol, 819 (2014) 21–39. [DOI] [PubMed] [Google Scholar]
- [8].Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW, Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast, Biomaterials, 26 (2005) 1261–1270. [DOI] [PubMed] [Google Scholar]
- [9].Sridharan I, Kim T, Strakova Z, Wang R, Matrix-specified differentiation of human decidua parietalis placental stem cells, Biochemical and biophysical research communications, 437 (2013) 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhu B, Li W, Lewis RV, Segre CU, Wang R, E-spun composite fibers of collagen and dragline silk protein: fiber mechanics, biocompatibility, and application in stem cell differentiation, Biomacromolecules, 16 (2015) 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chi N, Wang R, Electrospun protein-CNT composite fibers and the application in fibroblast stimulation, Biochemical and biophysical research communications, 504 (2018) 211–217. [DOI] [PubMed] [Google Scholar]
- [12].Chi N, Zheng S, Clutter E, Wang R, Silk-CNT Mediated Fibroblast Stimulation toward Chronic Wound Repair, Recent Prog Mater, 1 (2019) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li W, Zhu B, Strakova Z, Wang R, Two-way regulation between cells and aligned collagen fibrils: local 3D matrix formation and accelerated neural differentiation of human decidua parietalis placental stem cells, Biochemical and biophysical research communications, 450 (2014) 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Touhami A, Nysten B, Dufrêne YF, Nanoscale Mapping of the Elasticity of Microbial Cells by Atomic Force Microscopy, Langmuir : the ACS journal of surfaces and colloids, 19 (2003) 4539–4543. [Google Scholar]
- [15].Heim AJ, Matthews WG, Koob TJ, Determination of the elastic modulus of native collagen fibrils via radial indentation, Applied Physics Letters, 89 (2006) 181902. [Google Scholar]
- [16].Lapiere CM, Nusgens B, Pierard GE, Interaction between collagen type I and type III in conditioning bundles organization, Connective tissue research, 5 (1977) 21–29. [DOI] [PubMed] [Google Scholar]
- [17].Li Z, Qiu D, Sridharan I, Qian X, Zhang H, Zhang C, Wang R, Spatially Resolved Quantification of E-Cadherin on Target hES Cells, The Journal of Physical Chemistry B, 114 (2010) 2894–2900. [DOI] [PubMed] [Google Scholar]
- [18].Kim T, Sridharan I, Zhu B, Orgel J, Wang R, Effect of CNT on collagen fiber structure, stiffness assembly kinetics and stem cell differentiation, Materials Science and Engineering: C, 49 (2015) 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reddy CV, Malinowska K, Menhart N, Wang R, Identification of TrkA on living PC12 cells by atomic force microscopy, Biochimica et biophysica acta, 1667 (2004) 15–25. [DOI] [PubMed] [Google Scholar]
- [20].Nykvist P, Tu H, Ivaska J, Käpylä J, Pihlajaniemi T, Heino J, Distinct recognition of collagen subtypes by a (1) b (1) and a (2) b (1) integrins. a (1) b (1) mediates cell adhesion to type XIII collagen, J. Biol. Chem, 275 (2000) 8255–8261. [DOI] [PubMed] [Google Scholar]
- [21].Carlson MA, Longaker MT, The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence, Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society, 12 (2004) 134–147. [DOI] [PubMed] [Google Scholar]
- [22].Klinge U, Si Z, Zheng H, Schumpelick V, Bhardwaj R, Klosterhalfen B, Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias, Journal of Investigative Surgery, 14 (2001) 47–54. [DOI] [PubMed] [Google Scholar]
- [23].Arjonen A, Kaukonen R, Ivaska J, Filopodia and adhesion in cancer cell motility, Cell Adh Migr, 5 (2011) 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim JK, Xu Y, Xu X, Keene DR, Gurusiddappa S, Liang X, Wary KK, Höök M, A novel binding site in collagen type III for integrins α1β1 and α2β1, J. Biol. Chem, 280 (2005) 32512–32520. [DOI] [PubMed] [Google Scholar]
- [25].Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A, Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model, Arthritis and rheumatism, 60 (2009) 2817–2821. [DOI] [PubMed] [Google Scholar]
- [26].Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL, Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing, Cells Tissues Organs, 194 (2011) 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stallmach A, Schuppan D, Riese HH, Matthes H, Riecken EO, Increased collagen type III synthesis by fibroblasts isolated from strictures of patients with Crohn’s disease, Gastroenterology, 102 (1992) 1920–1929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


