Abstract
Objective:
Prospective data have demonstrated the efficacy of bevacizumab monotherapy in the treatment of advanced endometrial cancer. Bevacizumab is used off-label, and real-world data regarding the role of bevacizumab in endometrial cancer treatment are scant. In this largest single-institution retrospective study of its kind, we report our experience with bevacizumab monotherapy in the treatment of advanced/recurrent endometrial cancer.
Methods:
All eligible patients (n=101) had histologically confirmed endometrial cancer and were treated with bevacizumab at our institution from 2004–2017. Demographic data and tumor characteristics were obtained through chart review. Primary objective was response to therapy determined by Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
Results:
Analysis included 13 grade 1/2 endometrioid, 15 grade 3 endometrioid, 44 serous, 8 carcinosarcoma, and 21 other/mixed histologies. No patients achieved complete (CR) or partial (PR) responses; 19 achieved stable disease (SD). The clinical benefit rate (CBR; CR+PR+SD) was 19% (95% CI: 12–28%). The CBRs were 7%, 17%, 21%, and 23% for patients with 1, 2, 3, and ≥4 prior treatment lines. Median PFS ranged from 2.6 months (2 lines) to 4.9 months (≥4 lines). The 3-year OS rate was 58% (95% CI: 47–67%). The median OS was 3.4 years (95% CI: 2.9–4.2), ranging from 2.5 years (2 lines) to 4.5 years (≥4 lines). The most common treatment-related adverse event was hypertension; 35 (78%) of 45 were grade 1 or 2.
Conclusions:
In heavily pretreated advanced endometrial cancer, bevacizumab was associated with modest clinical efficacy and remains a viable palliative option in this setting.
Keywords: bevacizumab, endometrial cancer, survival analysis, vascular endothelial growth factor, VEGF, cancer therapy
Introduction
Endometrial cancer is the most common gynecologic malignancy in the United States, with more than 65,000 newly diagnosed cases and approximately 13,000 deaths per year [1]. Relative to other gynecological malignancies, the incidence and mortality rates of endometrial cancer have been rising alarmingly. The death rate of endometrial cancer, which had previously increased at 0.3% a year from 1997–2008, has now accelerated to a 1.9% increase per year since 2008. For patients with advanced disease, treatment options are generally limited, and the 5-year surivival rate is approximately 17% [1]. Further consideration and exploitation of the molecular underpinnings and tumor microenvironment is necessary to determine the optimal treatments for the various molecular phenotypes of endometrial cancer.
The Cancer Genome Atlas (TCGA) has improved our molecular understanding of endometrial cancer and has galvanized efforts to rationally target the driver oncogenic processes across various subtypes [2]. Numerous practice-changing advancements have recently occurred, including the approval of pembrolizumab in MSI-H or mismatch-repair deficient (dMMR) endometrial cancer, and the accelerated approval of lenvatinib and pembrolizumab combination therapy for microsatellite stable (MSS) endometrial cancer [3–5]. As the majority of recurrent endometrial cancers are MSS, the combination of lenvatinib and pembrolizumab has been an important step forward in the management of these patients. However, this combination is associated with a high incidence of adverse events. Data from a phase 2 study showed that 67% of patients on this combination developed grade 3 or 4 treatment-related adverse events; furthermore, 70% and 63% required dose interruptions or dose reductions, respectively [4, 6]. These therapeutic limitations highlight the need for the further investigation and discovery of well-tolerated therapies for the management of advanced endometrial cancer.
Bevacizumab is a humanized monoclonal antibody targeting vascular endothelial growth factor A (VEGF-A). In combination with chemotherapy, bevacizumab is FDA approved for the treatment of ovarian cancer after initial surgical resection, as well as for platinum-sensitive and platinum-resistant, recurrent ovarian cancer [7–11]. Combined with cisplatin and paclitaxel, bevacizumab is also FDA approved for the treatment of persistent or metastatic cervical cancer [12]. As monotherapy, bevacizumab has demonstrated activity on par with palliative chemotherapy in advanced ovarian, cervical, and endometrial cancers [13–16]. Findings from the phase 2 MITO END-2 study showed that bevacizumab in combination with carboplatin and paclitaxel was associated with an increased response rate (74% versus 53% with carboplatin and paclitaxel alone), and although progression-free survival (PFS) with bevacizumab was 13.7 months (versus 10.5 months), the difference was not statistically significant (HR: 0.85; CI: 0.5–1.3) [17]. A similar response rate of 73% was seen in a single-arm phase 2 trial of the carboplatin, paclitaxel and bevacizumab combination, with a median PFS of 18 months [18]. Results from the phase 2 GOG-86P trial showed no difference in PFS between the bevacizumab combination and historical controls. While there was a significant improvement in overall survival (OS), this result should be interpreted with caution given the lack of improvement in response rate and PFS [19]. In practice, bevacizumab monotherapy is used off-label for the treatment of advanced or recurrent endometrial cancer based on the findings of a phase 2 study that demonstrated a response rate of 13.5% and 6-month PFS rate of 40% [16].
To date, there have not been any prospective confirmatory phase 3 studies to demonstrate efficacy of bevacizumab monotherapy in advanced endometrial cancer. In this retrospective study, we sought to further characterize a single academic instutition’s clinical experience with bevacizumab monotherapy in the treatment of advanced or recurrent endometrial cancer.
Methods
Upon Institutional Review Board approval, we searched our institutional database at Memorial Sloan Kettering Cancer Center (MSK) to identify all patients who were diagnosed with endometrial cancer and received their first dose of bevacizumab treatment between January 2004 and December 2017. We retrospectively analyzed MSK’s electronic medical records to collect data on patient, tumor, and treatment characteristics. Prior chemotherapy regimens and radiation therapy treatments were also noted. Adverse events related to bevacizumab were identified by review of clinical notes and graded based on the Common Terminology Criteria for Adverse Events 5.0.
The data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at MSK [20, 21]. The following histologic subtypes were included: endometrioid, serous, clear cell, carcinosarcoma, and other/mixed histologies. All included patients had at least one “target lesion” per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. All radiologic assessments were completed by blinded radiologist review per RECIST 1.1 [22]. Response was evaluated by retrospectively re-assessing each patient’s scans using RECIST 1.1 criteria. Due to the retrospective nature of the study, patients did not have uniform follow-up time points; however, the follow-up time points were generally similar given they were conducted either routinely or for clinical need at the time. All patients had baseline and follow-up computed tomography (CT) or magnetic resonance imaging (MRI) available for review.
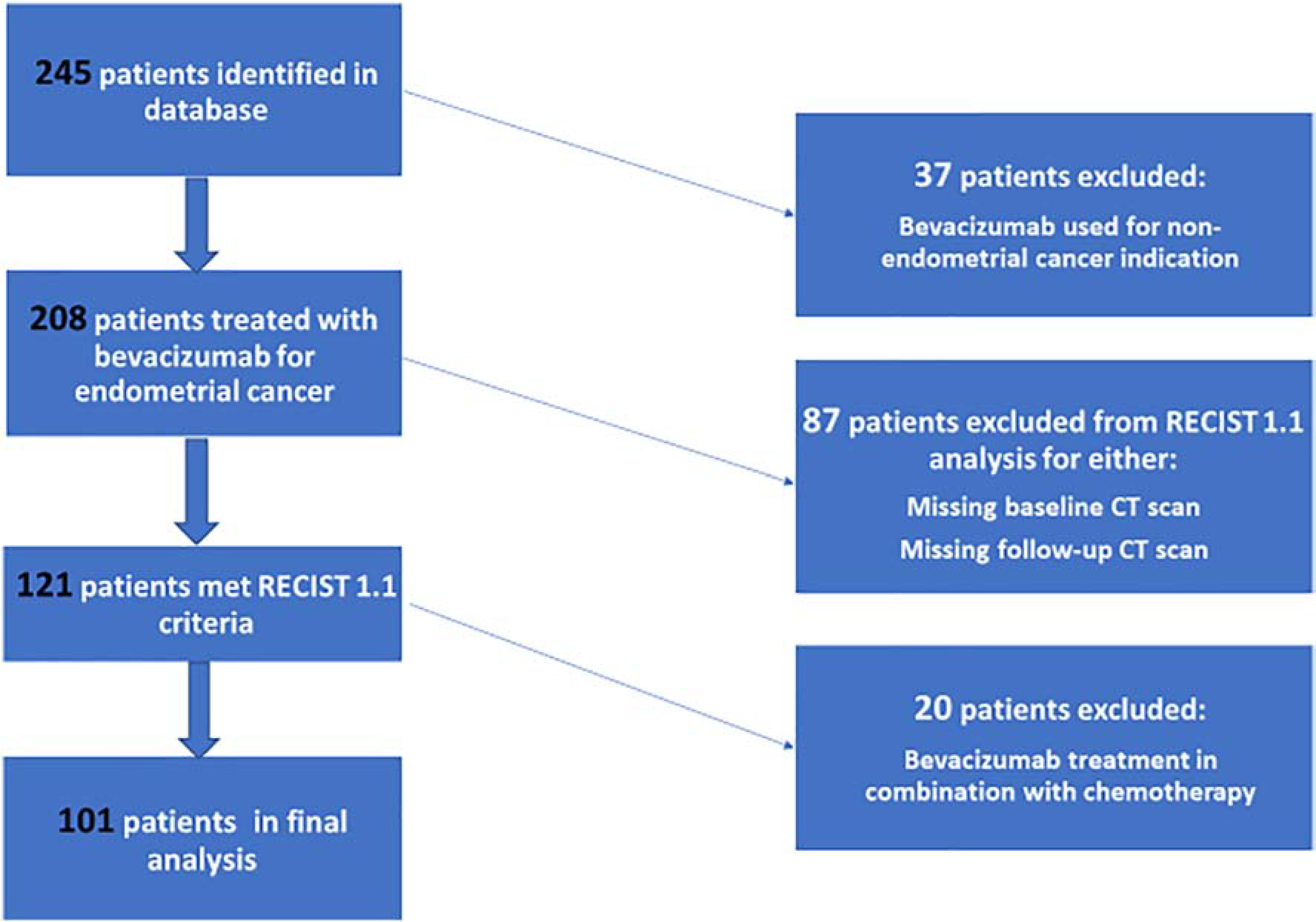
This single-institution, retrospective analysis had the following efficacy endpoints: overall response rate (ORR), clinical benefit rate (CBR), duration of response (DOR), PFS, and OS. Response was defined as a complete response (CR) or partial response (PR). Reasons for exclusion from the final efficacy analysis are shown in Figure 1. CBR was defined as CR, PR or stable disease (SD) at any time point, without a prespecified duration. Two-sided 95% confidence intervals (CIs) were calculated for CBR and PFS. The 95% CI for PFS was constructed using Kaplan-Meier methodology, the Greenwood formula was used to calculate the variance of Kaplan-Meier point estimates, and the 95% CI for CBR was estimated using exact binomial proportion [23, 24]. PFS was defined as the time from the patient’s first bevacizumab treatment to the date of progression, death, or last follow-up. OS was defined as the time from the patient’s date of diagnosis to death or last follow-up. The PFS, median PFS, OS, and median OS rates were estimated by the Kaplan–Meier method. Due to the vastly heterogeneous timing of when bevacizumab was used as a line of therapy, the main analysis was also performed separately by groups: 1 prior line of therapy (Group 1), 2 prior lines (Group 2), 3 prior lines (Group 3), and ≥4 prior lines (Group 4).
Figure 1: Patient Inclusion Flow Chart.
Results
Patient characteristics
A total of 245 patients with endometrial cancer who had received bevacizumab at MSK were identified. Patients with concomitant malignancy (other than non-melanoma skin cancer) within 3 years of their endometrial cancer diagnosis and those who received bevacizumab for another malignancy (n=37) were excluded from analysis. Patients who had incomplete baseline or follow-up imaging while on bevacizumab treatment (n=87) and those who underwent bevacizumab treatment in conjunction with chemotherapy (n=20) were excluded from analysis. A total of 101 patients were evaluated (Figure 1). Eighty-five patients (84%) started bevacizumab at a dose of 15 mg/kg, 9 (9%) started at 10 mg/kg, and 7 (7%) started at 7.5 mg/kg, with dosing every 3 weeks.
Patient characteristics are outlined in Table 1. The median age of diagnosis was 65 years. Sixty-three patients were White, 20 were Black, 9 were Asian, and 4 were Hispanic. Serous histology was observed in 44 patients (44%), followed by other/mixed histologies (21%), grade 3 endometrioid (15%), grade 1–2 endometrioid (13%), and uterine carcinosarcoma (8%). Forty-six patients (46%) had stage IV disease at the time of initial diagnosis. Most patients had an Eastern Cooperative Oncology Group (ECOG) score of 0 (32%) or 1 (60%). Patients had received a median of 3 lines of prior therapy before initiating bevacizumab treatment (range, 1–13), and approximately half (53%) had received prior radiation therapy.
Table 1:
Patient Characteristics and Treatment History (N=101)
| Characteristic | No. of Patients |
|---|---|
| Median Age at Diagnosis, years (range) | 65 (41–77) |
| Performance Status | |
| ECOG 0 | 32 (32%) |
| ECOG 1 | 60 (60%) |
| ECOG 2 | 8 (8%) |
| Unknown | 1 |
| Race | |
| White | 63 (62%) |
| Black | 20 (20%) |
| Asian | 9 (9%) |
| Hispanic | 4 (4%) |
| Other | 2 (2%) |
| Unknown | 3 (3%) |
| Histology | |
| Serous | 44 (44%) |
| Carcinosarcoma | 8 (8%) |
| Grade 3 Endometrioid | 15 (15%) |
| Grade 1–2 Endometrioid | 13 (13%) |
| Other/mixed | 21 (21%) |
| Median Age at 1st Bevacizumab Treatment, years (range) | 67 (43–79) |
| Stage | |
| I | 25 (25%) |
| II | 5 (5%) |
| III | 24 (24%) |
| IV | 46 (46%) |
| Unknown | 1 |
| Median Lines of Treatment (range) | 3 (1–13) |
| Prior Lines of Treatment | |
| Group 1 (1) | 14 (14%) |
| Group 2 (2) | 29 (29%) |
| Group 3 (3) | 28 (28%) |
| Group 4 (≥4) | 30 (30%) |
| Prior Radiation Therapy | |
| No | 47 (47%) |
| Yes | 54 (53%) |
ECOG, Eastern Cooperative Oncology Group
Of the entire 101-patient group, 63 patients had MSS disease, including 1 patient with dMMR grade 1 endometroid adenocarcinoma (low purity sample). Microsatellite status was unknown in 36 patients, and 1 patient with serous endometrial cancer had MSI-H disease (MMR proficient on immunohistochemistry [IHC]). MMR proficiency was identified in 46 patients. MMR deficiency was identified in 1 patient (low tumor purity), and MMR IHC status was unknown in 54 patients.
Patients were included regardless of prior lines of therapy and were separated into cohorts based on prior treatment history. Fourteen patients (14%) had received 1 prior line of therapy, 29 (29%) had received 2 prior lines, 28 (28%) had received 3 prior lines, and 30 (30%) had received ≥4 prior lines of therapy. Patient characteristics were similar for all four treatment groups (Table 1).
Efficacy
There were no CRs or PRs; 19 patients achieved SD as best overall response. The CBR for the entire cohort (n=101) was 19% (95% CI: 12–28%). Given the large variation in the number of prior treatments for this cohort (range, 1–13), we evaluated efficacy outcomes based on the number of prior treatment regimens (Groups 1–4). The CBR increased with each additional line of therapy—7%, 17%, 21%, and 23%, respectively. Clinical benefit was observed in patients with serous (n=9, 47%), other/mixed histologies (n=4, 21%), grade 1/2 endometrioid (n=3, 16%), grade 3 endometrioid (n=2, 11%), and carcinosarcoma (n=1, 5%). The DOR was longest in Group 1 (6.5 months) and shortest in Group 4 (3.2 months).
Median PFS ranged from 2.6 months (Group 2) to 4.9 months (Group 4) (Figure 2). For the entire cohort, the 3-year OS rate was 58% (95% CI: 47–67%) and the median OS was 3.4 years (95% CI: 2.9–4.2) (Figure 3). The median OS for Groups 1–4 were 2.9, 2.5, 4.1, and 4.5 years, respectively (Figure 4).
Figure 2: Median Progression-Free Survival by Treatment Groups*.
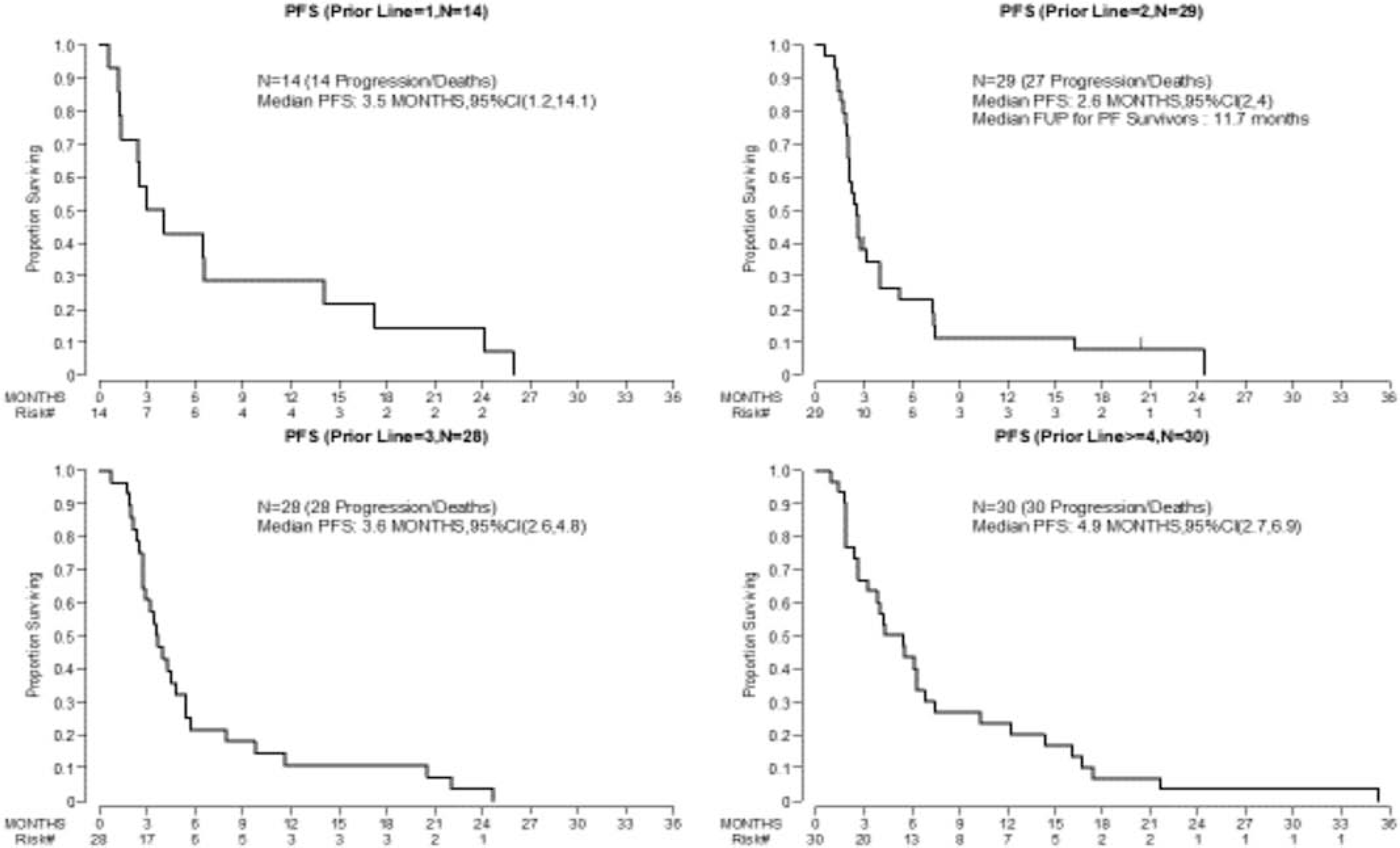
*group is defined as the number of prior lines of treatment: (1) one prior line of therapy, (2) two prior lines of therapy, (3) three prior lines of therapy, and (4) ≥ four lines of therapy. PFS, progression free survival; CI, confidence interval
Figure 3: Overall Survival Curve for the Entire Cohort (N=101).
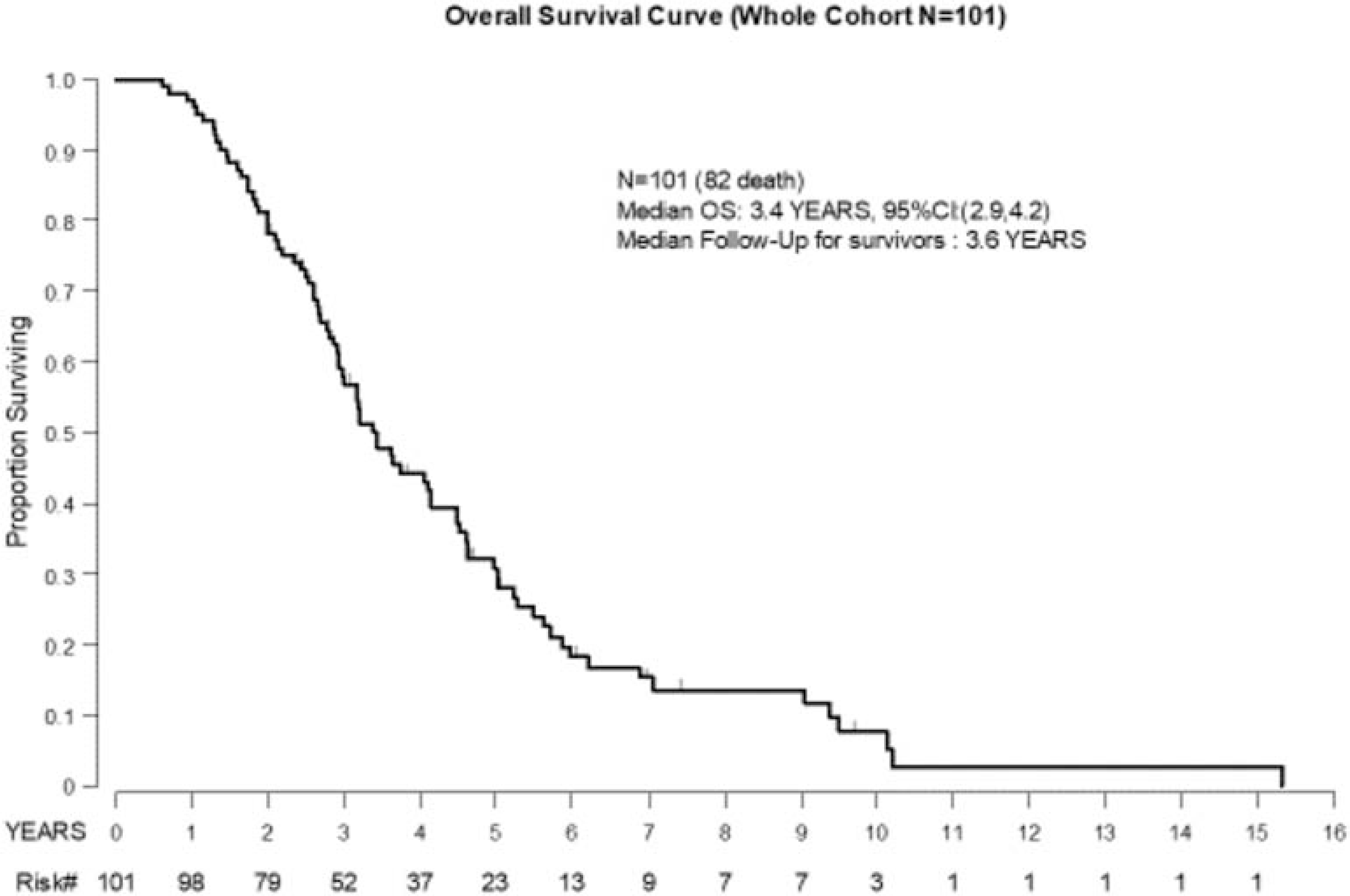
Figure 4: Median Overall Survival by Treatment Groups*.
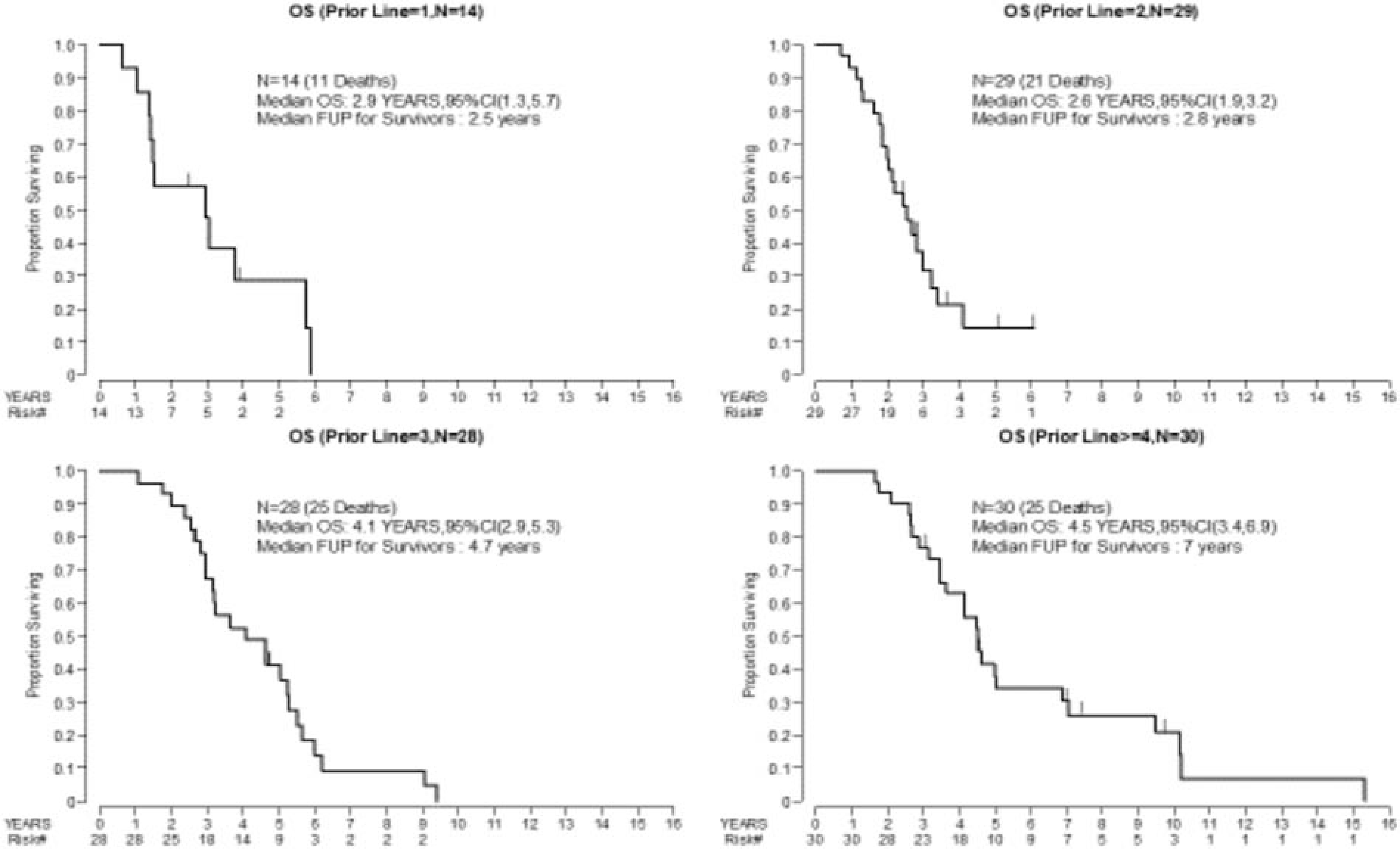
*group is defined as the number of prior lines of treatment: (1) one prior line of therapy, (2) two prior lines of therapy, (3) three prior lines of therapy, and (4) ≥ four lines of therapy. OS, overall survival; CI, confidence interval
Further analyses of stage at diagnosis (stage I/II versus stage III/IV) and history of prior radiation therapy did not reveal any differences in PFS or OS. Across all treatment groups, there were no differences in PFS or OS in patients with disease of serous histology versus other histologies.
Toxicity
Treatment-related adverse events are listed in Table 3. The most common treatment-related adverse event was hypertension in 45 patients (35 with grade 1–2 and 10 with grade 3 toxicity). The most common grade 3 toxicity was hypertension, which was managed with antihypertensive dose adjustment or with additional antihypertensives. Two patients with grade 2 hypertension required a bevacizumab dose to be witheld, and 1 patient with grade 2 hypertension required a bevacizumab dose reduction. Patients with hypertension were largely asymptomatic. Three patients had bowel obstruction, all of which could be attributed to disease progression. Two patients were managed conservativly, with improvement in symptoms, and 1 patient had a duodenal stent placed. Grade 1 proteinuria was identified on urinanalysis in 1 patient who also had hypertension while on bevacizumab treatment. One patient had a stroke while being treated for hypertension. Two patients had lower gastrointestinal bleeding, one of whom had a history of hemorrhoids and was taking oral anticoagulant medication. The other patient discontinued treatment after developing rectal bleeding; this patient was found to have an ulcerative mass involving the sigmoid colon. In total, grade 3 toxicities were observed in 13 patients (13%), which included 10 patients with grade 3 hypertension and 3 patients with bowel obstruction.
Table 3:
Treatment-Related Significant Adverse Events
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Total Number of Patients (N=101) |
|---|---|---|---|---|
| Proteinuria | 1 (1%) | 0 | 0 | 1 (1%) |
| Bowel obstruction | 0 | 0 | 3 (3%) | 3 (3%) |
| Hypertension | 14 (14%) | 21 (21%) | 10 (10%) | 45 (45%) |
| Stroke | 0 | 1 (1%) | 0 | 1 (1%) |
| Thrombosis | 0 | 2 (2%) | 0 | 2 (2%) |
| Gastrointestinal bleeding | 1 (1%) | 1 (1%) | 0 | 2 (2%) |
| Edema | 0 | 1 (1%) | 0 | 1 (1%) |
Discussion
Here we report on the largest retrospective cohort of patients with advanced endometrial cancer treated with bevacizumab monotherapy. We found that in this heavily pretreated cohort, bevacizumab monotherapy resulted in a CBR of 19% and was overall well tolerated.
To date, there has been only one prospective phase 2 trial of bevacizumab monotherapy (15 mg/m2 every 3 weeks) for endometrial cancer treatment, and the response rate for the study was 13.5% (including 1 CR and 6 PRs) [16]. Median PFS was 4.2 months, OS was 10.5 months, and the 6-month PFS rate was 40.4% [16]. In comparison, our cohort included patients who had received more extensive prior treatment; patients were previously treated with a median of 3 lines of therapy, and 30 patients had received ≥4 lines. The group of patients who had received ≥4 lines of prior treatment had the longest PFS at 4.9 months (95% CI: 2.7–6.9), which was comparable to that of the phase 2 trial, and the 6-month PFS rate was 43%. Interestingly, the CBR in our study was higher in the more heavily pretreated groups—23% (95% CI: 9.9–42%) for Group 4 and 7% (95% CI: 0.2–34%) for Group 1. The higher CBR in patients with extensive prior treatment histories was unexpected and difficult to attribute to bevacizumab treatment. Given what we know regarding the molecular classification of endometrial cancer and its responses to treatment, it would be important to also better understand the biological milieu of these groups of patients.
The retrospective design of this study poses some limitations and challenges. Although we systemically applied RECIST criteria to previously completed imaging studies, the time points of imaging follow-up were not completely uniform. Because of this, there was no consistent duration of disease stability defined. As with all retrospective work, conclusions regarding efficacy of treatment are not without limitations; however, we applied prospective trial design principles to our retrospective work. We evaluated patients who received bevacizumab monotherapy, ensured appropriate and consistent imaging, applied standardized RECIST criteria, and classified treatment-related events using CTCAE version 5.0. These parameters have the potential to exclude many other patients in our institution who had received bevacizumab for the treatment of their endometrial cancer. Patients had received bevacizumab at various time points in their disease course. The timing of the imaging was not standardized, and many had imaging >6 months from the start of their bevacizumab treatment, which can contribute to a higher PFS at 6 months compared to CBR. This heterogeneity, which reflects clinical practice, makes it challenging to extrapolate the exact timing when clinicians should consider bevacizumab treatment for endometrial cancer and the optimal timing to evaluate radiographic response. Furthermore, due to the retrospective nature of our study, it is difficult to ascertain whether the improved CBR is related to the underlying natural progression of extensively pretreated endometrial cancer or to the biological activity of bevacizumab. In our retrospective study, of the 19 patients who achieved a clinical benefit, 9 (47%) had disease of serous histology; in the prospective study, 4 of the 7 treatment responses were achieved in patients with serous histology (1 CR and 3 PRs) [16]. There remains a suggestion that serous disease may derive clinical benefit from bevacizumab treatment; however, this has not been studied or confirmed.
At this time, there are no predictive biomarkers to delineate which patients will derive benefit from bevacizumab treatment. Increased VEGF expression is associated with higher-stage disease and a 19-fold higher risk of death compared to low VEGF expression [25]. Associations have also been observed between bevacizumab treatment of high VEGF-A staining samples and reduced risk of death (HR: 0.35; 95% CI: 0.153–0.797) [16]. Results from GOG-86P suggested an improved PFS for patients with CTNNB1 mutations treated with the addition of bevacizumab (HR: 0.73; 95% CI: 0.60–0.91) [19]. Other studies of VEGF inhibitors such as cabozantinib have also suggested that tumors with CTNNB1 may have increased responses [26]. CTNNB1-mutated cell lines are associated with higher VEGF-A expression compared to CTNNB1 wildtype cell lines [27]. In our patient cohort, of the 19 patients who achieved SD as best response, 8 had undergone next-generation sequencing with MSK-IMPACT (MSK-Integrated Mutation Profiling of Actionable Cancer Targets), and none had a CTNNB1 mutation [28].
Various VEGF targeting therapies, such as lenvatinib, cabozantinib, cediranib and aflibercept, have also been prospectively studied as monotherapies for endometrial cancer [26, 29–31]. Lenvatinib (multikinase inhibitor of VEGFR1, VEGFR2, and VEGFR3) demonstrated an ORR of 14.3%, although 59% of patients experienced a grade ≥3 treatment-related adverse event; 53% of patients required a dose interruption and 18% discontinued treatment [29]. Cabozantinib (multikinase inhibitor of MET, VEGFR2, RET, and AXL) demonstrated an ORR of 14%; however, 21% of patients discontinued treatment due to an adverse event [26]. Cediranib (multikinase inhibitor of all VEGFRs, platelet-derived growth factor [PDGF], and fibroblast growth factor [FGF]) and aflibercept (VEGF ligand binding decoy receptor) demonstrated modest response rates of 12.5% and 7%, respectively; however, patients experienced a wide range of associated toxicities, from colonic perforation to posterior leukoencephalopathy [30, 31].
Bevacizumab has shown comparable activity as these VEGF agents but with a more acceptable toxicity profile, as seen in the prospective study and our retrospective work. In our retrospective trial, the rate of reported proteinuria was low at 1%, which may have been due to underreporting; however, in a prospective phase 2 trial with bevacizumab in endometrial cancer 2 (5%) of 52 patients developed grade 2–4 proteinuria [16]. Hypertension of all grades was seen in 45% of patients, but the majority were grade 1–2, and only 10 (10%) of 101 patients experienced grade 3 hypertension. Comparatively, in the prospective phase 2 trial, 17% of patients developed hypertension and 8% (4/52) developed grade 2 hypertension [16]. In this heavily pretreated population, bevacizumab shows favorable tolerability.
Tumor angiogenesis in endometrial cancer involves complex interactions between the immune response and tumor microenvironment, and although these mechanisms are not entirely clear, this has prompted further investigation into combination therapies. Recently, the combination of lenvatinib plus pembrolizumab (an anti–programmed cell death protein 1 [PD-1] antibody) has dramatically changed the treatment landscape for endometrial cancer, with an ORR (CR+PR) of 38%, and is currently the only FDA approved second-line therapy for recurrent MSS endometrial cancer [6]. The clinical efficacy of this combination highlights the additive relationship between VEGF and PD-1/programmed death-ligand 1 (PD-L1) inhibition and has prompted future studies to investigate the combination of atezolizumab (PD-L1) and bevacizumab (NCT03526432). Additionally, it has been postulated that bevacizumab leads to homologous repair defects and therefore enhances poly (ADP-ribose) polymerase (PARP) sensitivity [32]. Combinations of bevacizumab with PARP inhibition may be a promising avenue of research. Similarly, studies are investigating bevacizumab with rucaparib (PARP inhibitor) (NCT03476798), as well as the combination of atezolizumab, bevacizumab, and rucaparib (NCT03694262).
In conclusion, our retrospective study of bevacizumab monotherapy in the treatment of advanced or recurrent endometrial cancer supports the prospective data. Our findings demonstrate that in heavily pretreated endometrial cancer, bevacizumab displays modest activity and is well tolerated. Patients with recurrent endometrial cancer have few therapeutic options, and future prospective studies with bevacizumab should carefully incorporate the molecular classification of endometrial cancer and consider novel combination strategies with PD-1 inhibition and PARP inhibitors to improve outcomes.
Table 2:
Efficacy Analysis by Treatment Groups*
| Group 1 n=14 | Group 2 n=29 | Group 3 n=28 | Group 4 n=30 | |
|---|---|---|---|---|
|
CBR, % 95% CI |
7% (0.2–34%) | 17 % (5.8–36%) | 21% (8.3–41%) | 23% (9.9–42%) |
|
Median PFS, months 95% CI |
3.5 (1.2–14) | 2.6 (2–4) | 3.5 (2.6–4.8) | 4.9 (2.7–6.9) |
|
PFS rate at 6 months 95% CI |
40% (19–60%) | 23% (9.5–39%) | 21% (8.7–38%) | 43% (26–60%) |
|
DOR, months 95% CI |
6.5 (2.2–NE) | 5.6 (2.1–15) | 3.4 (2.4–8.3) | 3.2 (2.3–6.4) |
|
Median OS, years 95% CI |
2.9 (1.3–5.7) | 2.5 (2–3) | 4.1 (2.9–5.3) | 4.5 (3.4–6.9) |
|
3-year OS rate, % 95% CI |
48% (20–71%) | 32% (15–51%) | 67% (47–82%) | 77% (57–88%) |
group is defined as the number of prior lines of treatment: (1) one prior line of therapy, (2) two prior lines of therapy, (3) three prior lines of therapy, and (4) ≥ four lines of therapy.
CBR, clinical benefit rate; PFS, progression-free survival; DOR, duration of response; OS, overall survival; CI, confidence interval; NE, not estimable
Highlights.
Bevacizumab had a clinical benefit rate of 19% in pretreated advanced endometrial cancer patients
Bevacizumab is well tolerated in heavily treated endometrial cancer and should be considered as a palliative therapy
Future trials with bevacizumab should incorporate the molecular classification of endometrial cancer
Bevacizumab should be considered in novel combinations with immunotherapy/PARP inhibitors to potentially improve outcomes
Acknowledgments
Funding:
Funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflict of Interest Statement
Outside the submitted work, Dr. Rubinstein reports a 2019 ASCO Young Investigator Award; Dr. Lakhman is a shareholder of Y-mAbs Therapeutics, Inc.; Dr. Iasonos reports consulting fees from Mylan; and Dr. Makker reports personal fees from ArQule, Eisai, Karyopharm, Merck, Clovis, and IBM Watson, as well as grants (institutional funding support) from Merck, Eisai, Karyopharm, AstraZeneca, Clovis, Moreo, Takeda, Genentech, and Zymeworks. The other authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, et al. , Cancer Statistics, 2021. CA Cancer J Clin, 2021. 71(1): p. 7–33. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research, N., et al. , Integrated genomic characterization of endometrial carcinoma. Nature, 2013. 497(7447): p. 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz LA, et al. , Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. 2017. 35(15_suppl): p. 3071–3071. [Google Scholar]
- 4.Makker V, et al. , Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol, 2019. 20(5): p. 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marabelle A, et al. , Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol, 2020. 21(10): p. 1353–1365. [DOI] [PubMed] [Google Scholar]
- 6.Makker V, et al. , Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. Journal of Clinical Oncology, 2020. 38(26): p. 2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tewari KS, et al. , Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. Journal of Clinical Oncology, 2019. 37(26): p. 2317–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghajanian C, et al. , OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol, 2012. 30(17): p. 2039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman RL, et al. , A phase III randomized controlled trial of secondary surgical cytoreduction (SSC) followed by platinum-based combination chemotherapy (PBC), with or without bevacizumab (B) in platinum-sensitive, recurrent ovarian cancer (PSOC): A NRG Oncology/Gynecologic Oncology Group (GOG) study. Journal of Clinical Oncology, 2018. 36(15_suppl): p. 5501–5501. [Google Scholar]
- 10.Pujade-Lauraine E, et al. , Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol, 2014. 32(13): p. 1302–8. [DOI] [PubMed] [Google Scholar]
- 11.Perren TJ, et al. , A Phase 3 Trial of Bevacizumab in Ovarian Cancer. New England Journal of Medicine, 2011. 365(26): p. 2484–2496. [DOI] [PubMed] [Google Scholar]
- 12.Tewari KS, et al. , Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). The Lancet, 2017. 390(10103): p. 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger RA, et al. , Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. New England Journal of Medicine, 2011. 365(26): p. 2473–2483. [DOI] [PubMed] [Google Scholar]
- 14.Emile G, et al. , A clinical experience of single agent bevacizumab in relapsing ovarian cancer. Gynecol Oncol, 2013. 129(3): p. 459–62. [DOI] [PubMed] [Google Scholar]
- 15.Monk BJ, et al. , Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol, 2009. 27(7): p. 1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghajanian C, et al. , Phase II Trial of Bevacizumab in Recurrent or Persistent Endometrial Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology, 2011. 29(16): p. 2259–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorusso D, et al. , Carboplatin-paclitaxel compared to Carboplatin-Paclitaxel-Bevacizumab in advanced or recurrent endometrial cancer: MITO END-2 - A randomized phase II trial. Gynecol Oncol, 2019. 155(3): p. 406–412. [DOI] [PubMed] [Google Scholar]
- 18.Simpkins F, et al. , A phase II trial of paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma (EMCA). Gynecol Oncol, 2015. 136(2): p. 240–5. [DOI] [PubMed] [Google Scholar]
- 19.Aghajanian C, et al. , A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecologic Oncology, 2018. 150(2): p. 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, et al. , Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009. 42(2): p. 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, et al. , The REDCap consortium: Building an international community of software platform partners. J Biomed Inform, 2019. 95: p. 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 23.Kalbfleisch JD, & Prentice RL, The statistical analysis of failure time data. 1980. [Google Scholar]
- 24.CLOPPER CJ and PEARSON ES, THE USE OF CONFIDENCE OR FIDUCIAL LIMITS ILLUSTRATED IN THE CASE OF THE BINOMIAL. Biometrika, 1934. 26(4): p. 404–413. [Google Scholar]
- 25.Kamat AA, et al. , Clinical and Biological Significance of Vascular Endothelial Growth Factor in Endometrial Cancer. Clinical Cancer Research, 2007. 13(24): p. 7487–7495. [DOI] [PubMed] [Google Scholar]
- 26.Dhani NC, et al. , Phase II Trial of Cabozantinib in Recurrent/Metastatic Endometrial Cancer: A Study of the Princess Margaret, Chicago, and California Consortia (NCI9322/PHL86). Clinical Cancer Research, 2020. 26(11): p. 2477–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger AA, Jelinic P, and Levine DA, Mechanisms of response to anti-angiogenesis therapy in CTNNB1-mutated endometrial cancers. Gynecologic Oncology, 2019. 154: p. 50. [Google Scholar]
- 28.Cheng DT, et al. , Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn, 2015. 17(3): p. 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vergote I, et al. , Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol Oncol, 2020. 156(3): p. 575–582. [DOI] [PubMed] [Google Scholar]
- 30.Bender D, et al. , A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol, 2015. 138(3): p. 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman RL, et al. , A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecologic oncology, 2012. 127(3): p. 538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan N and Bristow RG, “Contextual” Synthetic Lethality and/or Loss of Heterozygosity: Tumor Hypoxia and Modification of DNA Repair. Clinical Cancer Research, 2010. 16(18): p. 4553–4560. [DOI] [PubMed] [Google Scholar]


