Abstract
Proteolysis targeting chimeras (PROTACs) have gained tremendous interest in both the academic and pharmaceutical communities. This opens a new way to regulate the cellular protein homeostasis, especially for disease-related proteins. In this work, we designed and synthesized a series of MDM2 degraders based on ligands that were readily prepared by a four-component Ugi reaction. After extensive optimization based on anti-proliferation and MDM2 degradation, WB214 was identified as the most potent anti-proliferative agent in various leukemia cell lines. Surprisingly, our mechanistic investigations indicated that WB214 not only effectively induced the degradation of MDM2, but also led to the degradation of p53. Further studies revealed that WB214 degraded MDM2 as a molecular glue. WB214 and its related analogues did not bind to MDM2 in the p53 binding region and MDM2 was discovered as a novel neo-substrate of the E3 ligase cereblon. Finally, we found that WB214 could potently degrade GSPT1, which could rationalize the inhibition of cell growth. A selective degrader for GSPT1 over MDM2 was then developed through systematically varying different motifs.
Keywords: MDM2 degraders, molecular glue, PROTAC, GSPT1
Graphical Abstract
1. Introduction
Proteolytic targeting chimeras (PROTACs) have received significant attention as a new modality for the regulation of protein homeostasis. The initial proof-of-concept study of PROTAC was realized in 2001 [1]. PROTACs are heterobifunctional molecules with a linker between two ligands. Upon binding to its protein partners, a PROTAC can recruit the corresponding E3 ligase to the proximal position of the target protein, which then facilitates the ubiquitination and subsequent proteasomal degradation. PROTAC technology has achieved great progresses with the expanding of E3 ligase repertoire [2–12]. Among them, Von Hippel Lindau (VHL)[2] and cereblon (CRBN)[3] are the most commonly used E3 ligases.
CRBN is part of the CUL4–RBX1–DDB1 (known as CRL4) E3 ubiquitin ligase complex, which acts as a substrate receptor (also known as DCAF) to recognize the substrate protein to be degraded. A family of small molecules called immunomodulators (IMiDs) were identified as the ligands of CRBN, including thalidomide, lenalidomide and pomalidomide. Upon binding, CRBN can recruit a set of neo-substrates, leading to their proteolysis[13–20]. This novel mechanism-of-action (MOA) of IMiDs was termed as molecular glue, which induces a neo-interaction between CRBN E3 ligase and a target protein. In addition, indisulam and other aryl-sulfonamides, were also validated as molecular glues to induce the interaction between DCAF15 and RBM39, promoting the proteolytic degradation of RBM39[21,22]. Recently, two research groups independently discovered that small molecular inhibitors of CDK12 induced the degradation of its partner protein, cyclin K[23,24]. Mechanistically, cyclin K degradation induced by CDK12 inhibitors follows the MOA of molecular glue. Notably, CDK12-cyclin K complex binds directly to DDB1 in the presence of CDK12 inhibitors, rather than DCAFs. Overall, the development of molecular glues[25,26], along with PROTAC technology, provide new strategies to target disease-causing proteins, many of which are difficult to drug using traditional methods.
Recently, we developed a highly potent MDM2 PROTAC WB156 by linking a nutlin derivative with CRBN ligand lenalidomide (Figure 1A)[27]. Degrader WB156 depletes MDM2 efficiently and activates wild type p53 in leukemia cells, which further induces the cellular apoptosis. Despite of the high potency of WB156 for the degradation of MDM2, induction of p53, and anti-proliferation, it only works in a limited number of leukemia cell lines. We envisaged that the incorporation of a different MDM2 ligand may lead to the development of MDM2 degraders that would work for a broader scope of cancers. MDM2 inhibitors 1 and 2 (Figure 1A) can be easily prepared by a four-component Ugi reaction[28], which has the potential to generate a diverse range of structures by varying each of the four components. We then used these ligands as the binder for MDM2 to construct active MDM2 degraders. After extensive optimization based on anti-proliferation and MDM2 degradation, we developed WB214 as the most potent MDM2 degrader and anti-proliferative agent in various leukemia cell lines. Surprisingly, this new class of MDM2 degraders did not activate p53. Rather, WB214 and its related compounds induced the degradation of p53, which is completely opposite to our previously reported MDM2 degrader WB156. Follow-up studies revealed that this new class of compounds worked as a molecular glue to degrade both MDM2 and p53. In addition, WB214 also degrades GSPT1, which can then rationalize the cell growth inhibition despite the decrease of p53. Further investigations suggested that WB214 and its related analogues did not bind to MDM2 in the p53 binding region and that MDM2 appeared to be a novel neo-substrate of CRBN E3 ligase. The p53 protein is degraded as a bystander due to the direct interaction with MDM2.
Figure 1.
Design of MDM2 degraders. (A) Structure of MDM2 degrader WB156, Ugi ligands 1 and 2; (B) Co-crystal structure of Ugi ligand bound to the MDM2 binding site (PDB code: 3TJ2); (C) The strategy to construct MDM2 degraders from ligands derived from Ugi reaction and lenalidomide derivative.
2. Design of MDM2 degraders
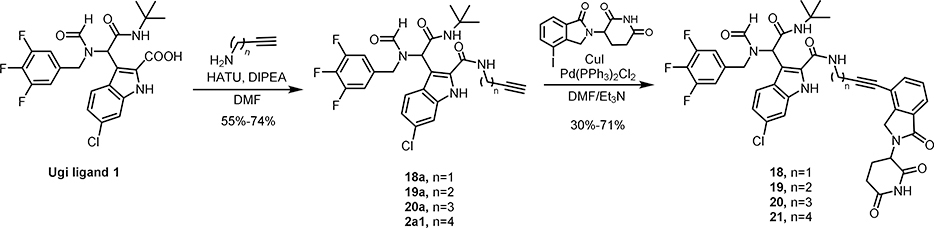
PROTAC molecules are made by linking the ligands of an E3 ligase and a target protein at the solvent exposing positions. The co-crystal structure of Ugi ligand and MDM2 (Figure 1B), shows that 4-chlorophenyl, tert-butyl amide and 6-chloroindole are all deeply buried in the binding pocket[28]. Both the formamide and ethyl ester of indole are pointing towards solvent. Accordingly, these two positions can be potential hubs to link with CRBN ligand (Figure 1C). We then prepared a library of MDM2 degraders by linking the MDM2 ligand derived from a four-component Ugi reaction and the CRBN ligand. The detailed synthesis can be found in Scheme 1–6.
Scheme 1.
The Synthesis of MDM2 degraders 3–15 with various length of flexible linkers linking out from formamide position.
Scheme 6.
The structural modification of WB214.
3. Chemical synthesis
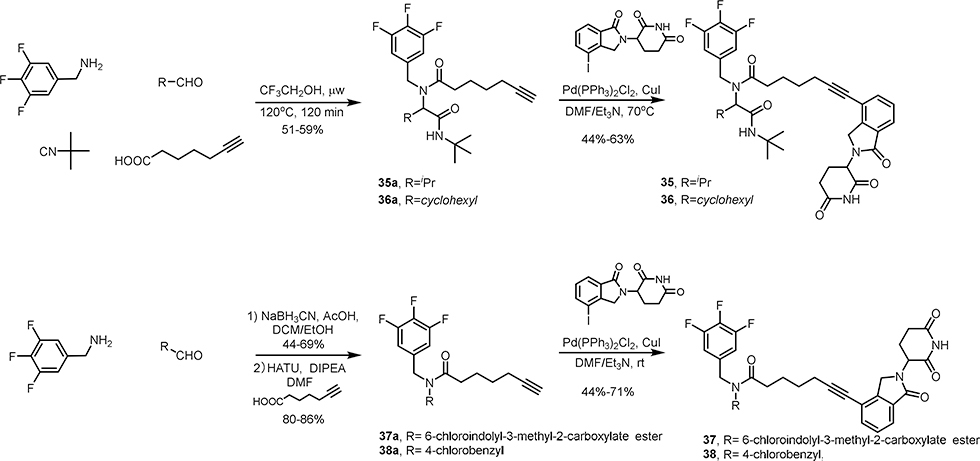
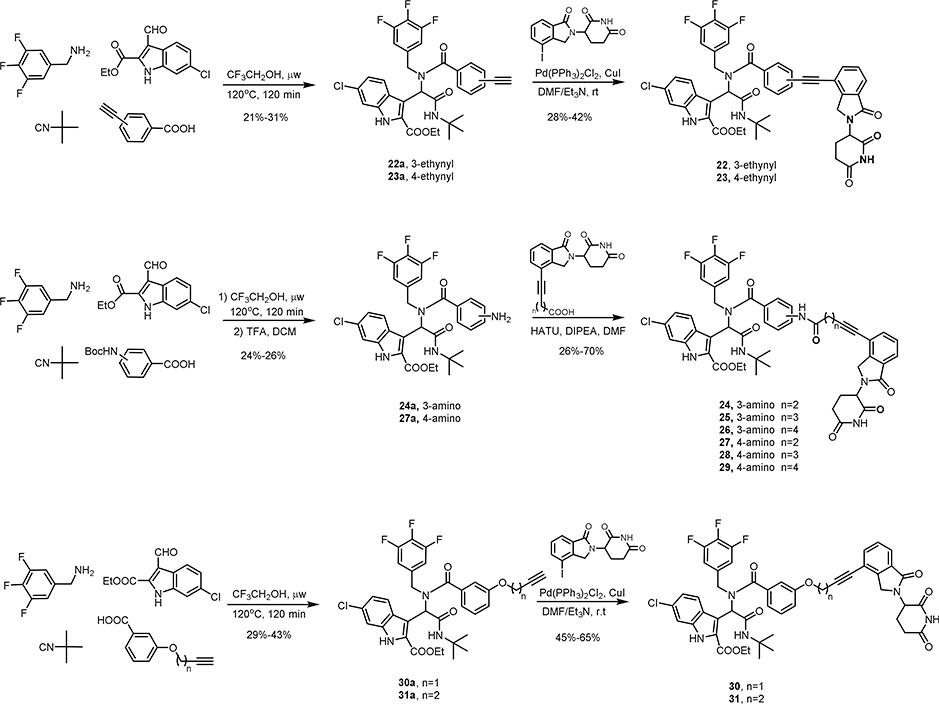
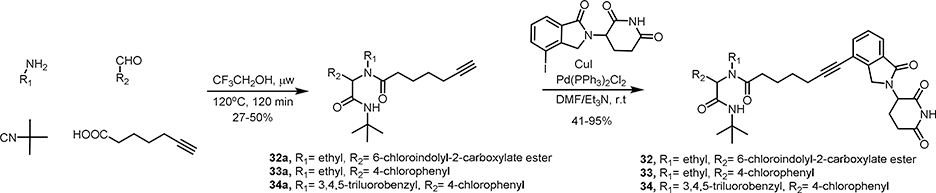
The synthesis of MDM2 degraders is relatively straightforward. Compounds 3–15 (Scheme 1), 22-23 (Scheme 4) and 30–36 (Scheme 4, 5 and 6) were prepared by a four-component Ugi reaction followed by a Sonogashira coupling. Compound 16 was synthesized by a Sonogashira coupling reaction from 11b, which was obtained by the hydrolysis of 11a. Compound 17 was synthesized by a Sonogashira coupling reaction from 11c, which was derived from 11b (Scheme 2). Compounds 18-21 were prepared by the amidation of Ugi ligand 1 and Sonogashira coupling reactions (Scheme 3). Compounds 24-29 were obtained by Ugi reaction, TFA-meditated Boc deprotection and amidation (Scheme 4). Compounds 37 and 38 were synthesized by a sequence of reductive amination, amidation and Sonogashira coupling reaction (Scheme 6).
Scheme 4.
The Synthesis of MDM2 degraders 22–31 with rigid linkers linking out from the formamide position.
Scheme 5.
The structural modification of WB214.
Scheme 2.
The Synthesis of acid and amide version of MDM2 degrader WB214
Scheme 3.
The Synthesis of MDM2 degraders 18–21 with various length of flexible linkers linking out from the acid position.
4. Biological result and discussion
4.1. MDM2 degraders display highly potent cell growth inhibition in RS4;11 leukemia cells
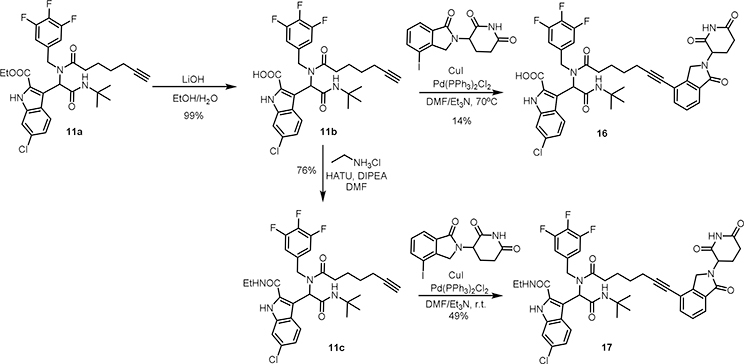
A set of bifunctional molecules 3–11 were prepared by linking a CRBN ligand to MDM2 ligand 1 at the formamide position, as shown in Scheme 1. The potency of cell growth inhibition of these compounds was then evaluated by a cell viability assay (Table 1). Compared to MDM2 ligand 1, most of the degraders exhibit inhibitory activity in RS4;11 leukemia cells with lower IC50s. When group R1 is switched from H to 4-chloro, and then to 3,4,5-trifluoro, the anti-proliferation effect is significantly increased, which can likely be attributed to the increasing hydrophobic interactions with MDM2 protein. Notably, compound 11 (WB214) with the longest linker, exhibits the most potent growth inhibition of RS4;11 cells with an IC50 of 1.2 nM. The length of linker plays an important role in the potency of PROTAC molecules[29,30]. Therefore, we prolonged the linker of WB214 to generate compounds 12–15. Compound 12 has slightly better potency than WB214. The potency of compounds 13, 14, 15 decrease along with the increasing linker length. Because of the hydrolysis liability of ester in cells, we also prepared compounds 16 and 17 by replacing the ester with acid and amide, respectively (Scheme 2). Compound 17 has similar growth inhibition potency to WB214. On the contrary, compound 16, the acid version of WB214, lost its potency completely. This is likely due to the poor cellular permeability, which is also correlated to the cellular inactivity of its parent ligand 2 [31].
Table 1.
Evaluation of cell growth inhibition of the synthesized MDM2 degraders in RS4;11 leukemia cells
 | ||||
|---|---|---|---|---|
| Cmp. No | R1 | R2 | n | IC50 (nM)a |
| 1b | 3,4,5-trifluoro | COOEt | - | 2700 |
| 3 | H | COOEt | 2 | 56 |
| 4 | H | COOEt | 3 | 7.6 |
| 5 | H | COOEt | 4 | 4.8 |
| 6 | 4-Cl | COOEt | 2 | 1403 |
| 7 | 4-Cl | COOEt | 3 | 5.3 |
| 8 | 4-Cl | COOEt | 4 | 1.3 |
| 9 | 3,4,5-trifluoro | COOEt | 2 | 95.9 |
| 10 | 3,4,5-trifluoro | COOEt | 3 | 3.2 |
| 11 (WB214) | 3,4,5-trifluoro | COOEt | 4 | 1.2 |
| 12 | 3,4,5-trifluoro | COOEt | 5 | 0.76 |
| 13 | 3,4,5-trifluoro | COOEt | 6 | 2.6 |
| 14 | 3,4,5-trifluoro | COOEt | 7 | 3.0 |
| 15 | 3,4,5-trifluoro | COOEt | 8 | 250 |
| 16 | 3,4,5-trifluoro | COOH | 4 | >10 μM |
| 17 | 3,4,5-trifluoro | CONHEt | 4 | 2.4 |
The cell growth inhibition (IC50) of the compounds were measured in RS4;11 leukemia cells by Alarma Blue assay.
The reported data of the cell growth inhibition (IC50) of Ugi ligand 1 in SJSA-1 cells by MTT assay.
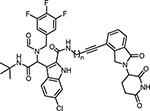
Besides linking out from the formamide position of ligand 1, another set of compounds (18–21) were prepared by tethering at the acid position of indole (Scheme 3). Similarly, cell viability assays were conducted in RS4;11 leukemia cells and the results were summarized in Table 2. For this series of compounds, compounds 18 and 19 with shorter linker lengths exhibit more potent inhibition of cell proliferation, though they are much weaker than WB214.
Table 2.
Evaluation of cell growth inhibition of the synthesized MDM2 degraders in RS4;11 leukemia cells
 | ||
|---|---|---|
| Cmp. No | n | IC50 (nM) a |
| 18 | 1 | 61 |
| 19 | 2 | 39 |
| 20 | 3 | 446 |
| 21 | 4 | 731 |
The cell growth inhibition (IC50) of the compounds were measured in RS4;11 leukemia cells by Alarma Blue assay.
4.2. Both MDM2 and p53 are degraded in a broad range of leukemia cell lines
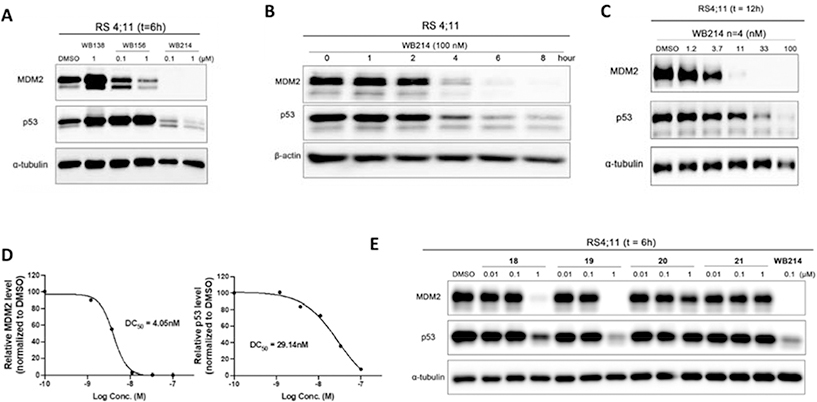
After evaluation of the cell growth inhibition of compounds 3–21, we then profiled the extent of MDM2 degradation induced by these compounds in RS4;11 cells by Western blot analysis (Figure S2). Among these, WB214 showed the most potent degradation efficacy.
As shown in Figure 2A, WB138 (MDM2 ligand of degrader WB156) and WB156 were used as controls. WB138 increases the levels of both p53 and MDM2, which is consistent with the activity of previous reports on MDM2 inhibitors[28,32–36]. WB156 showed effective depletion of MDM2 and activation of p53 (Figure 2A) as reported previously. We then evaluated MDM2 level after treating RS4;11 cells with WB214 for 6 h. Immunoblotting demonstrated that significant MDM2 degradation could be achieved, and it is correlated with its potency on cell growth inhibition. However, in contrast to WB156, WB214 also induces degradation of p53 (Figure 2A), which is completely unexpected. Other compounds (3–15) of this series were then screened by Western blot analysis and downregulation of p53 was observed in all cases, along with the degradation of MDM2 (Figure S2). The degradation induced by WB214 proceeded in a time-dependent manner (Figure 2B). Degradation of MDM2 and p53 was observed as early as 4 h post-treatment with 100 nM of WB214. At 8 h, almost all of the MDM2 was depleted. Furthermore, protein levels of both MDM2 and p53 were downregulated in the dose-dependent manner (Figure 2C), with a DC50 of 4.1 nM and 29 nM, respectively (Figure 2D).
Figure 2.
Western blot profiling of degradation in RS4;11 leukemia cells. (A) Immunoblot of MDM2 and p53 following treatment of cells with DMSO or indicated compounds for 6 h; (B) Immunoblot of MDM2, p53 following incubation with 100 nM WB214 for indicated times; (C) Immunoblot of MDM2 and p53 following 12 h incubation with DMSO or the indicated concentrations of WB214; (D) Band intensity quantification of blot C calculated by Image J, and bar graph plotted and fitted by GraphPad Prism; E) Immunoblot of MDM2 and p53 following treatment with DMSO or indicated compounds for 6 h.
Similarly, compounds 18–21, which have the linker between the CRBN ligand and the acid position of ligand 2, were incubated with RS4;11 cells. Western blotting analysis indicates that 19 exhibits the most degradation of MDM2 and p53 at 1 μM, although it is less effective than WB214 (Figure 2E).
As aforementioned, MDM2 degradation induced by WB156 only happened in a limited number of leukemia cell lines (RS4;11, MOLT-4 and NOMO-1). We then compared the MDM2 degradation profiles between WB156 and WB214 in a variety of cell lines (Figure S3 and Table S1). The results demonstrated that WB214 induced MDM2 degradation in a broad spectrum of cell lines, including TP53 wild type leukemia cells (RS4;11, MV-4–11, MOLM-13, MOLT-4), solid tumors cells (A549, HepG2 and WM115), and TP53 mutant cells (CCRF-CEM and NOMO-1).
4.3. WB214 works as a molecular glue for the degradation of MDM2
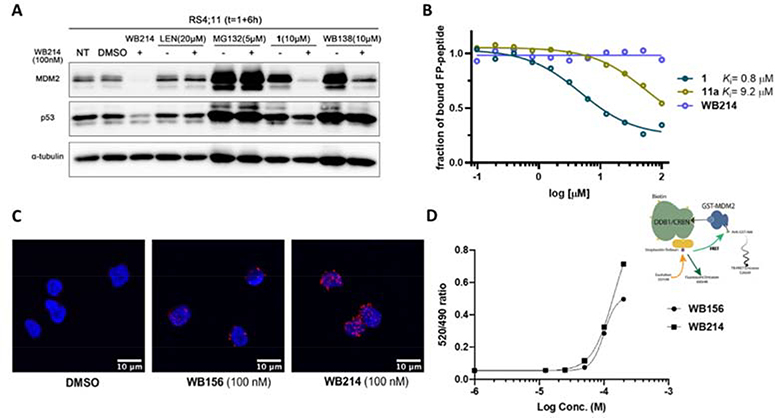
It is totally unexpected that the protein level of both MDM2 and p53 is downregulated by MDM2 degraders based on ligands derived from Ugi reactions. We then conducted a series of experiments to investigate the potential mechanism of action. To investigate the engagement of CRL4CRBN E3 ligase and the proteasome in the degradation of MDM2 and p53, RS4;11 cells were incubated with different inhibitors in the presence of WB214 (100 nM). As illustrated in Figure 3A, the degradation of MDM2 and p53 was abolished by the addition of 20 μM lenalidomide, which is a ligand of CRL4CRBN. This result validates the involvement of CRBN E3 ligase in the WB214 mediated degradation of MDM2 and p53. To confirm that the degradation was executed by proteasome, cells were co-treated with proteasome inhibitor MG132 (5 μM) and WB214. As demonstrated in Figure 3A, the degradation of MDM2 and p53 was blocked completely, indicating that the depletion of MDM2 and p53 induced by WB214 is dependent on the ubiquitin-proteasome system. Additionally, we incubated cells with MDM2 inhibitors in the presence of WB214 to study the engagement of MDM2. By co-treatment of cells with ligand 1 (10 μM) and WB214 (100 nM), p53 degradation was rescued. However, the degradation of MDM2 persisted. The addition of 10 μM WB138, the MDM2 ligand of WB156, yielded the same outcome. These co-treatment results revealed that p53 degradation is due to its direct association with MDM2 because compounds are known to block the interaction between MDM2 and p53 could abolish the degradation of p53 but not MDM2 by WB214[37]. Taken together, these data demonstrated that: 1) the MDM2-p53 complex degradation mediated by WB214 is through the ubiquitin-proteasome machinery; 2) p53 is a bystander during the degradation of MDM2 because of its direct association with MDM2; and 3) the WB214-CRBN complex does not bind to MDM2 in the p53 binding region, which is where MDM2 ligand 1 or WB138 binds to.
Figure 3.
Mechanistic study of MDM2-p53 degradation induced by WB214. (A) MDM2 and p53 degradation induced by WB214 is dependent on CRBN and the proteasome. Cells were pre-treated with CRBN ligand lenalidomide (20 μM), proteasome inhibitor MG132 (5 μM), MDM2 inhibitor Ugi ligand 1 (10 μM) or WB138 (10 μM) for 1 h, followed by treatment with DMSO or WB214 for 6 h; (B) Evaluation of the binding affinity of compounds 1,11a and WB214 to MDM2 by a competitive fluorescent polarization (FP) assay; (C) Study of the ternary complex formation induced by WB156 and WB214 at 100 nM using proximity ligation assay (PLA); (D) Study of the ternary complex formation by time-resolved fluorescence energy transfer (TR-FRET).
The co-treatment experiment (Figure 3A) shows that excessive amount of MDM2 inhibitors cannot abrogate WB214-mediated MDM2 degradation. We then further evaluated the binding affinity of WB214 to MDM2 by a competitive fluorescence polarization assay[33] (Figure 3B). Ligand 1 competes fluorescent peptide (PMDM6-F) off of MDM2 with a Ki value of 0.8 μM, which correlates with the reported literature value (Ki= 0.4 μM)[28]. However, compound 11a with the linker attached at the formamide position loses the binding affinity dramatically (Ki= 9.2 μM). For compound WB214, it does not compete off the fluorescent peptide at all, indicating that it does not bind to the p53 binding pocket of MDM2. In brief, chemical modification of ligand 1 at the formyl group appears to be detrimental to its binding to MDM2. Similar results were recently reported by others as well. The change of formyl group to methyl or acetyl, causes the loss of the binding affinity[38].
As shown above (Figure 3A), MDM2 degradation induced by WB214 occurs through the ubiquitin-proteosome pathway. Ternary complex formation is the prerequisite for the ubiquitin transfer and the subsequent proteasomal degradation. To investigate the formation of ternary complex, proximity ligation assay (PLA) was conducted to visualize in situ ternary complex formation in RS4;11 cells. In PLA, the proteins of interest are recognized by primary antibodies from two species, which are then recognized by detection antibodies (secondary antibodies against the two species) conjugated to specific oligonucleotides probe. If the two proteins of interest are at proximity (<40 nM), the two probes will be hybridized and amplified with fluorescently labeled oligonucleotides. PLA signals are typically a number of discrete fluorescent spots. Here we used primary antibodies against MDM2 and CRBN to determine their interaction (MDM2-compound-CRBN ternary complex) in the presence of candidate compounds. As shown in Figure 3C, the red dots indicate the formation of the ternary complex. Compared to WB156, ternary complex is formed more efficiently in WB214 treated cells, which is consistent with its high efficacy of MDM2 degradation. Orthogonally, time-resolved fluorescence energy transfer (TR-FRET) experiment was carried out for MDM2 protein and CRBN-DDB1 complex. Biotinylated DDB1/CRBN binds to streptavidin which is conjugated with donor fluorophore terbium. GST (Glutathione S-transferase) is fused to the N-terminal of MDM2 and GST antibody is conjugated with Alexa fluor 488, which is used as acceptor. The interaction between DDB1/CRBN and MDM2 in the presence of candidate compounds was indicated by the FRET ratio (520 nm/490 nm ratio). Both WB156 and WB214 could induce the formation of ternary complexes efficiently in a dose-dependent manner and WB214 induced CRBN-MDM2 interaction more potently (Figure 3D).
Collectively, these data indicate that, unlike WB156 (a bona fide MDM2 PROTAC), WB214 does not degrade MDM2 through the classical PROTAC mechanism. The MOA for WB214 is more consistent with molecular glue. In other words, WB214 only binds to MDM2 through its interaction with CRBN. We discovered here for the first time that MDM2 can be a neo-substrate of CRBN.
4.4. WB214 degrades GSPT1, which rationalizes the inhibition of cell growth
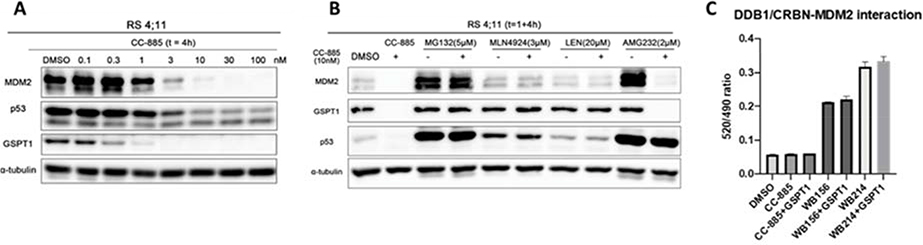
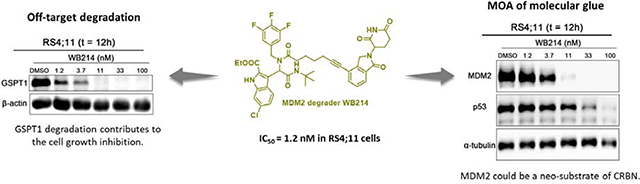
p53 is the guardian of cell defense and restoration of its activity can lead to the inhibition of cancer cell proliferation. However, with the degradation of p53, WB214 still exhibits highly potent cell growth inhibition. We hypothesize that an off-target effect may be responsible for the cell growth inhibition. To answer these questions, we evaluated the proteins with potential interactions with MDM2 or p53 and studied the effect of WB156 and WB214 on several known cell cycle effectors and apoptosis regulators (Figure S4). Our data clearly indicate that WB156 and WB214 have different mechanisms of action. However, this does not reveal the source of the anti-proliferation effect induced by WB214. As described before, in the presence of IMiDs, CRBN E3 ligase induces the proteasomal degradation of a set neo-substrates, such as IKZF1/3[13,39], CK1α[40] and GSPT1[15]. Among them, GSPT1 (G1 to S phase transition 1), also known as eRF3a, mediates stop codon recognition and nascent protein release from the ribosome through the interaction with a release factor, eRF1. GSPT1, as a neo-substrate of CRBN, can be efficiently degraded by CC-885[15] or ZXH1–161 (a lenalidomide derivative)[41], contributing significantly to the inhibition of leukemia cell proliferation. It has also been reported that GSPT1 is responsible for off-target effects for PROTAC molecules that recruit CRBN [42–44]. Crews and his co-workers demonstrated that the degradation of GSPT1 induced by thalidomide-based c-Met PROTAC molecule was observed by the proteomic analysis[42]. In addition, MI-389, initially designed as a receptor tyrosine kinases (RTK) degrader shows significant cell growth inhibition. Interestingly, it degrades GSPT1, rather than the intended target kinase[43]. Recently, the Wang’ group also serendipitously discovered a molecular glue (MG-227) of GSPT1 by simple structural modification of a bona fide MDM2 PROTAC molecule[39]. MG-227 displays only moderate MDM2 degradation effect but with highly potent cell growth inhibition in a p53-independent manner. By unbiased proteomic study, GSPT1 was identified as the target of MG-227. Collectively, GSPT1 is a common neo-substrate of CRBN and it contributes greatly to the inhibition of cell proliferation. Accordingly, we tested our compounds by Western blot to examine whether GSPT1 degradation happens in our case. As illustrated in Figure 4A, known GSPT1 degrader CC-885 was used as a positive control. All the tested compounds induce degradation of GSPT1 along with depletion of MDM2 and p53. WB214 exhibits the most potent GSPT1 degradation, in accordance with its most potent anti-proliferation activity. It depletes GSPT1 in a dose-dependent manner (DC50= 0.64 nM) as shown in Figure 4B and 4C.
Figure 4.
Western blot profiling of degradation in RS4;11 leukemia cells. (A) Immunoblot of MDM2, p53 and GSPT1 following treatment of cell with DMSO or indicated compounds for 6 h; (B) immunoblot of GSPT1 following 12 h incubation with DMSO or the indicated concentrations of WB214; (C) Band intensity of blot B calculated by Image J, and bar graph plotted and fitted by GraphPad Prism.
When cells were treated with CC-885, we observed the downregulation of MDM2 and p53. This has never been reported in the literature. It is unclear whether MDM2 and p53 are neo-substrates of CC-885 or indirect bystander of GSPT1. To clarify, we did further mechanistic studies as shown in Figure 5 and Figure S5. The degradation of MDM2 and GSPT1 by CC-885 is time- and dose-dependent (Figure 5A and Figure S5A). Moreover, co-treatment of CC-885 with MG132 (5μM), MLN4924 (3μM), or lenalidomide (20μM), abolished the effect of CC-885 for the degradation of GSPT1, MDM2, and p53, indicating that MDM2 degradation depends on the recruitment of CRBN E3 ligase and ubiquitin proteasome system (Figure 5B). Likewise, p53 degradation is due to its interaction with MDM2 as in the case of WB214, since the co-treatment of cells with AMG232 (a potent MDM2-p53 inhibitor) can completely block the degradation of p53. The mRNA levels of GSPT1, MDM2 and p53 were evaluated by qRT-PCR analysis and our results indicated that the degradation of these proteins induced by CC-885 is not regulated transcriptionally (Figure S5B).
Figure 5.
Mechanistic study of MDM2-p53 degradation induced by CC-885. (A) Immunoblot of MDM2, p53 and GSPT1 following incubation with CC-885 at indicated concentration for 4 h; (B) MDM2 and p53 degradation induced by CC-885 is dependent on CRBN and the proteasome. Cells were pre-treated with proteasome inhibitor MG132 (5 μM), MLN4924 (3 μM), CRBN ligand lenalidomide (20 μM) and MDM2 inhibitor AMG232 (2 μM) for 1 h, followed by treatment with DMSO or CC-885 for 4 h; (C) Study of the ternary complex formation between CRBN/DDB1-MDM2 by time-resolved fluorescence energy transfer (TR-FRET).
Ternary complex formation was examined by TR-FRET assay as illustrated in Figure 5C. Complex formation [MDM2-CC885-CRBN/DBB1] was not observed in the presence of CC-885. Moreover, in the presence of GSPT1, we still did not observe complex formation between MDM2 and CRBN. These data indicated that there was no direct interaction between MDM2 and CRBN/DDB1 in the presence of CC-885, and MDM2 degradation induced by CC-885 is likely an indirect effect. To further confirm the independence of degradation between GSPT1 and MDM2, we knocked down GSPT1 in A549 cells. MDM2 degradation was detected by treating cells with CC-885 or WB214, even after knocking down GSPT1 (Figure S5C). Our data suggest that the degradation of GSPT1 and MDM2 induced by CC-885 are independent of each other. The degradation of these two proteins by WB214 are also independent of each other.
4.5. Attempts for selective degradation of MDM2 or GSPT1
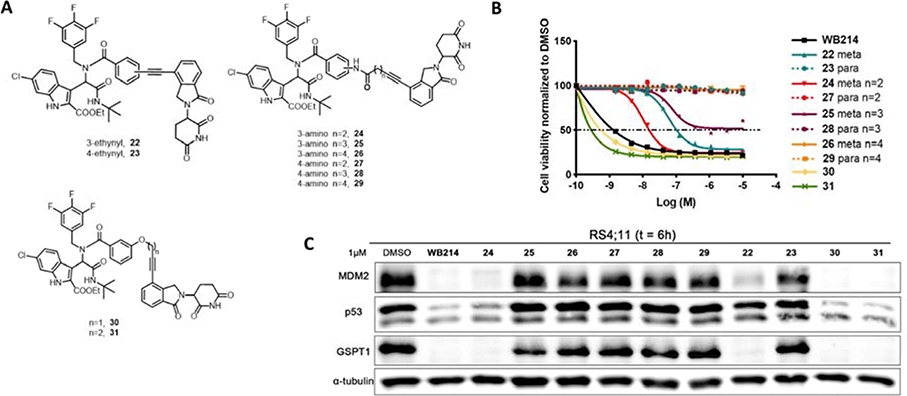
GSPT1 is not the intended target of our MDM2 degraders based on ligands derived from Ugi reaction and the degradation of GSPT1 is independent to MDM2 degradation. Since the linker plays a pivotal role in the formation of ternary complexes, we set out to perform the linker modification to MDM2 degraders to explore the possibility of selectively degrading one of them. Degrader WB214 has a relatively flexible alkyl linker. We first tried to increase the linker rigidity by introducing a phenyl ring as outlined in Figure 6A. Compound 22 and 23 were prepared by Ugi reaction and followed by Sonogashira coupling reactions. Both the 3-ethynyl (meta-) and 4-ethynyl (para-) were introduced to have different linker orientations. In cell viability assays and Western blots, we observed that meta-substituted compound 22 displayed cell growth inhibition and the degradation of MDM2 and p53 along with GSPT1 degradation. However, the para-substituted compound 23 lost its activity of cell growth inhibition and degradation. We then prolonged the linker length in compounds 24–29 (Figure 6A). Interestingly, none of the para-substituted compounds 27–29 showed any inhibition of cell growth (Figure 6B, dot line), nor did they induce degradation (Figure 6C). In contrast, compounds with 3-phenylamide linker showed potent anti-proliferation activity. Compound 24 with the shortest linker is the most potent one in this series and displays significant MDM2 degradation, as well as GSPT1 degradation. Since only the meta-substituted compounds were active, we then replaced the 3-phenylamide by 3-phenyl ether as shown in compounds 30 and 31. Although these two compounds exhibited the highest potency among the compounds with rigid linkers, even in comparison to WB214, there was no selective degradation between MDM2 and GSPT1.
Figure 6.
(A) Structures of compounds with rigid linkers; (B) Cell viability assay for the indicated compounds; (C) Immunoblot of MDM2, p53 and GSPT1 following treatment of cells with DMSO or indicated compounds for 6 h.
The introduction of rigid linkers fails to achieve selective degradation between MDM2 and GSPT1. We then started to modify the MDM2 ligand structure in WB214. As discussed previously, WB214 does not bind to the tripod binding pocket (Phe19, Leu26, Trp23) of MDM2, so we envisaged that the chemical structure modification of MDM2 ligand part of WB214 might discriminate the degradation of MDM2 and GSPT1.
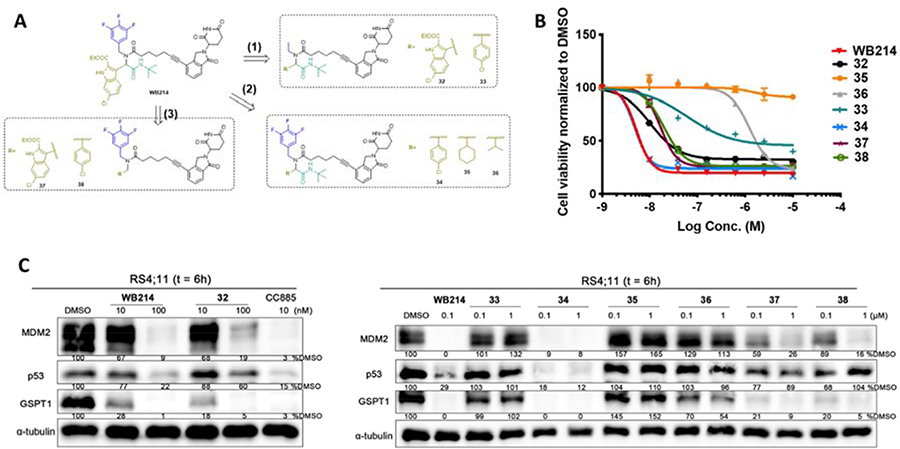
The structural modification of MDM2 ligand proceeded in three different ways (Figure 7A): (1) changing trifluorobenzyl to ethyl; (2) replacing indolyl by benzyl, cyclohexyl or isopropyl; and (3) trimming off the tert-butyl amide. By doing so, we discovered a compound that selectively degrades GSPT1 over MDM2. The detailed process and results are shown in Figure 7.
Figure 7.
(A) The strategy to structural modification of the MDM2 ligand; (B) Cell viability assay for indicated compounds; (C) Immunoblot of MDM2, p53, and GSPT1 following treatment of cells with DMSO or indicated compounds for 6 h.
Firstly, the aromatic trifluorobenzyl group of WB214 was changed into a simple ethyl group to give compound 32. Compound 32 showed weaker potency than WB214 in terms of inhibition of cell proliferation (Figure 7B). It degraded both MDM2 and GSPT1 (Figure 7C), though with slightly decreased potency. We then replaced the indolyl group by a 4-chloroplenyl group in both 32 and WB214 to create compounds 33 and 34, respectively. The potency of cell growth inhibition and degradation of 34 was similar to WB214. When treating cells with 33, the potency of cell inhibition and degradation was dramatically diminished. We then changed the aromatic 4-chlorophenyl of 34 to isopropyl or cyclohexyl, giving compounds 35 and 36, respectively. The cell viability assay showed that compound 35 with isopropyl group did not inhibit the proliferation of RS4;11 cells. Compound 36, however, could inhibit the cell proliferation with a IC50 value of 1.24 μM. Western blot analysis showed that neither MDM2 nor GSPT1 could be depleted by compound 35 in RS4;11 cells. Gratifyingly, compound 36 induced the selective degradation of GSPT1 over MDM2, though with the compromise of potency. We also made two compounds 37 and 38 based on WB214 and 34 by trimming off the tert-butyl amide group. Both compounds displayed comparable potency to WB214 and 34 with IC50 values of 16.9 nM and 19.7 nM, respectively. This indicates that tert-butyl amide is dispensable for the degradation and consequent inhibition of cell proliferation. Taken together, by varying different motifs in compound WB214, we discovered compound 36 that could selectively degrade GSPT1 over MDM2, though with weaker potency. Additionally, we investigated the required minimal structural requirement of MDM2 degraders. Further studies will be conducted to increase the potency and to discover compounds with preferential MDM2 degradation in the future.
5. Conclusion
In summary, we designed and prepared dozens of MDM2 degraders based on ligands derived from Ugi reactions. This work was exemplified by the discovery of WB214, the most potent MDM2 degrader which functions through a molecular glue type of mechanism of action to deplete both MDM2 and p53. Our mechanistic studies revealed that the degradation of neo-substrate GSPT1 could rationalize the inhibition of cell proliferation. Moreover, our study demonstrated that the degradation of MDM2 and GSPT1 induced by WB214 are not dependent upon each other. By modifying the structure of WB214, we also discovered a compound that can degrade GSPT1 over MDM2.
The discovery of new E3 ligases with small molecular ligands has fueled the promises of the therapeutic potential of PROTACs. However, possible off-target effects may arise from the E3 ligase ligand side, such as the degradation of GSPT1 in this work and others[42–44]. One lesson we learned from this work is that the incorporation of an E3 ligase ligand into heterobifunctional molecules can often still retain its degradation activity for neo-substrates and the anti-proliferation can be caused by off-target effect. The other lesson we learned is that the proteins (e.g. p53) that bind to the target protein (MDM2) may also be degraded as a bystander. Most neo-substrates reported so far are for degraders that recruit the CRBN E3 ligase. Nevertheless, with the expansion of E3 ligase repertoire, the off-target effect may occur more often.
6. Experimental section
6.1. Compounds synthesis
Unless otherwise noted, all reagents were purchased from commercial sources and used without further purification. Dry solvents were obtained from a solvent purification system. NMR spectra were recorded on Bruker AV-400 MHz in ppm (δ) downfield of TMS (δ = 0). Signal splitting patterns were described as singlet (s), doublet (d), triplet (t) or multiplet (m), with coupling constants (J) in hertz. Assignments were aided by COSY and HSQC experiments. High resolution mass spectra (HRMS) were performed by Analytical Instrument Center at the School of Pharmacy on a Bruker MaXis Ultra-High Resolution Quadrupole Time-of-Flight MS. The liquid chromatography–mass spectrometry (LC–MS) analysis of final products was processed on an Agilent 1290 Infinity II LC system using a Poroshell 120 EC-C18 column (5 cm × 2.1 mm, 1.9 μm) for chromatographic separation. Agilent 6120 Quadrupole LC/MS with multimode electrospray ionization plus atmospheric pressure chemical ionization was used for detection. The mobile phases were 5.0% methanol and 0.1% formic acid in purified water (A) and 0.1% formic acid in methanol (B). The gradient was held at 5% (0–0.2 min), increased to 100% at 2.5 min, then held at isocratic 100% B for 0.4 min, and then immediately stepped back down to 5% for 0.1 min re-equilibration. The flow rate was set at 0.8 mL/min. The column temperature was set at 40 °C. The LC-MS spectra can be found in the supporting information. The purities of all title compounds are ≥95%.
General Procedure for Preparing compounds 3–15
To a G10 microwave tube containing aldehyde (0.2 mmol, 1 equiv.) in trifluoroethanol (2 mL), amine (0.2 mmol, 1 equiv.) was added, followed by addition of acid (0.2 mmol, 1 equiv.) and isocyanide (0.2 mmol, 1 equiv.). The reaction was stirred at 120°C under the microwave irradiation for 90 min. TLC was used to monitor the reaction. When the reaction is done, the solvent was evaporated, and the residue was submitted to a silica gel flash column (Hexane/EtOAc: 3/1 – 2/1), giving the desired product 3a-15a with the yields of 26%−51%.
To a solution of 3a-15a (1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (2 equiv.) in dry DMF (3mL), copper iodide (0.2 equiv.) and Pd(PPh3)Cl2 (0.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (3 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compounds 3-15 as light-yellow foam with the yields of 15%−55%.
Ethyl 3-(1-(N-benzyl-5-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)pent-4-ynamido)-2-(tert-butylamino)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (3)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 3/1, here is the major rotamer): δ 9.02 (d, J = 14.5 Hz, 1H), 8.40 (d, J = 18.2 Hz, 1H), 7.92 – 7.88 (m, 1H), 7.78 (d, J = 7.4 Hz, 1H), 7.53 (d, J = 7.7 Hz, 1H), 7.42 (m, 1H), 7.27 (m, 1H), 7.13 (d, J = 8.8 Hz, 1H), 6.98 – 6.78 (m, 4H), 6.63 (d, J = 7.4 Hz, 2H), 5.47 (s, 1H), 5.25 – 5.17 (m, 1H), 4.80 (d, J = 18.0 Hz, 1H), 4.57 – 4.19 (m, 5H), 3.02 – 2.56 (m, 6H), 2.40 (m, 1H), 2.25 – 2.10 (m, 1H), 1.32 (t, J = 7.3 Hz, 3H), 1.29 (s, 9H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 3/1, here is the major rotamer): δ 171.6, 171.2, 169.5, 169.4, 169.1, 169.0, 160.6, 143.7, 137.7, 134.6, 131.7, 131.5, 128.3(2C), 127.8, 126.5, 125.7, 124.9(2C), 123.3, 122.8, 122.4, 119.4, 114.5, 114.4, 111.8, 95.2, 76.6, 61.6, 54.6, 51.8, 51.8, 49.5, 47.1, 32.9, 31.5, 28.7(3C), 23.3, 15.0, 14.3. HRMS (ESI): Calcd. For C42H42ClN5O7Na [M+Na]1+: 786.2665; Found: 786.2651. LC-MS purity: 96%.
Ethyl 3-(1-(N-benzyl-6-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hex-5-ynamido)-2-(tert-butylamino)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (4)
1H NMR (400 MHz, CDCl3, (400 MHz, CDCl3, a mixture of rotamers: 3/1, here is the major rotamer): δ 8.85 (d, J = 3.7 Hz, 1H), 7.92 – 7.87 (m, 1H), 7.77 (d, J = 6.7 Hz, 1H), 7.44 – 7.37 (m, 1H), 7.26 (s, 1H), 7.19 – 7.07 (m, 1H), 6.97 – 6.75 (m, 4H), 6.58 (t, J = 8.0 Hz, 2H), 5.43 (d, J = 17.6 Hz, 1H), 5.26 – 5.14 (m, 1H), 4.81 (dd, J = 17.9, 10.9 Hz, 1H), 4.56 – 4.14 (m, 5H), 2.94 – 2.35 (m, 7H), 2.26 – 1.99 (m, 3H), 1.41 (t, J = 7.1 Hz, 3H), 1.29 (s, 9H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 3/1, here is the major rotamer): δ 173.1, 171.4, 171.1, 169.5, 169.1, 169.0, 143.8, 135.7, 134.6, 134.3, 131.7, 131.5, 128.2, 127.8, 127.7, 127.1, 126.4, 124.8, 124.8, 123.2, 122.9, 122.5, 122.4, 119.4, 114.8, 111.7, 95.6, 61.6, 54.4, 51.9, 51.7, 49.7, 47.1, 34.5, 32.4, 31.6, 28.7(3C), 24.4, 23.3, 19.1, 14.4. HRMS (ESI): Calcd. For C43H44ClN5O7Na [M+Na]1+: 800.2821; Found: 800.2807. LC-MS purity: 96%.
Ethyl 3-(1-(N-benzyl-7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hept-6-ynamido)-2-(tert-butylamino)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (5)
1H NMR (400 MHz, CDCl3, (400 MHz, CDCl3, a mixture of rotamers: 3/1, here is the major rotamer): δ 9.03 – 8.77 (m, 2H), 7.93 (d, J = 8.9 Hz, 1H), 7.79 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 7.1 Hz, 1H), 7.42 (t, J = 7.6 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.17 – 7.08 (m, 1H), 6.97 – 6.91 (m, 4H), 6.65 – 6.54 (m, 2H), 5.70 (s, 1H), 5.30 – 5.22 (m, 1H), 4.78 (dd, J = 18.0, 11.2 Hz, 1H), 4.68 – 4.24 (m, 5H), 3.01 – 2.76 (m, 2H), 2.64 – 2.16 (m, 6H), 1.85 – 1.48 (m, 4H), 1.37 (t, J = 7.1 Hz, 3H), 1.31 (s, 9H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 3/1, here is the major rotamer)): δ 173.4, 172.8, 169.7, 169.6(2C), 169.1, 160.5, 144.7, 138.4, 135.7, 134.2, 133.6, 131.6, 128.2, 127.7, 126.9, 126.4, 125.7, 124.9, 123.1, 123.0, 122.3, 119.6, 111.7, 95.7, 61.5, 54.4, 51.8, 47.3, 34.5, 33.3, 31.9, 28.6(3C), 28.0, 27.6, 23.4, 19.3, 14.4. HRMS (ESI): Calcd. For C44H46ClN5O7Na [M+Na]1+: 814.2978; Found: 814.2961. LC-MS purity: 97%.
Ethyl 3-(2-(tert-butylamino)-1-(N-(4-chlorobenzyl)-5-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)pent-4-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (6)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 2/1, here is the major rotamer): δ 8.87 (d, J = 6.8 Hz, 1H), 8.16 (d, J = 15.8 Hz, 1H), 7.79 (d, J = 7.5 Hz, 2H), 7.54 (d, J = 7.5 Hz, 1H), 7.46 – 7.39 (m, 1H), 7.13 (t, J = 7.6 Hz, 2H), 6.95 – 6.85 (m, 3H), 6.57 (d, J = 8.2 Hz, 2H), 5.40 (s, 1H), 5.28 – 5.16 (m, 2H), 4.55 – 4.19 (m, 4H), 2.88 (m, 4H), 2.59 – 2.13 (m, 4H), 1.37 – 1.25 (m, 12H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 2/1, here is the major rotamer): δ 171.5, 171.0, 169.4, 169.3, 169.0, 168.8, 141.9, 136.4, 135.6, 135.0, 134.6, 132.2, 131.5, 130.2, 128.3, 127.9, 127.9, 127.8, 127.3, 126.3, 123.3, 123.1, 122.7, 117.8, 113.9, 111.8, 99.6, 95.1, 68.6, 61.6, 51.9, 49.0, 47.9, 47.1, 31.5, 29.7, 28.7(3C), 23.4, 15.6, 14.3. HRMS (ESI): Calcd. For C42H42ClN5O7 [M+H]1+: 798.2456; Found: 798.2455. LC-MS purity: >99%.
Ethyl 3-(2-(tert-butylamino)-1-(N-(4-chlorobenzyl)-6-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hex-5-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (7)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 2/1, here is the major rotamer): δ 9.03 (d, J = 7.1 Hz, 1H), 8.31 (d, J = 20.4 Hz, 1H), 7.79 (m, 2H), 7.44 – 7.35 (m, 1H), 7.21 – 7.07 (m, 3H), 6.94 – 6.75 (m, 3H), 6.54 (t, J = 8.4 Hz, 2H), 5.46 (d, J = 20.7 Hz, 1H), 5.23 (m, 2H), 4.55 – 4.20 (m, 4H), 3.01 – 2.76 (m, 2H), 2.70 – 2.31 (m, 6H), 2.27 – 1.97 (m, 2H), 1.40 (t, J = 7.1 Hz, 3H), 1.28 (s, 9H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 2/1, here is the major rotamer): δ 173.0, 171.6, 171.1, 169.6, 169.1, 169.0, 143.8, 135.7, 134.6, 134.2, 132.1, 131.5, 129.1, 128.8, 127.9, 127.3, 127.0, 126.2, 123.4, 123.2, 122.8, 122.5, 119.3, 114.8, 113.9, 111.9, 101.5, 94.9, 61.7, 54.4, 51.8, 47.0, 42.9, 35.2, 32.4, 31.5, 28.7(3C), 24.4, 23.4, 19.0, 14.4. HRMS (ESI): Calcd. For C43H44ClN5O7 [M+H]1+: 812.2612; Found: 812.2607. LC-MS purity: >99%.
Ethyl 3-(2-(tert-butylamino)-1-(N-(4-chlorobenzyl)-7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hept-6-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (8)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 2/1, here is the major rotamer): δ 8.87 (s, 1H), 8.49 (s, 1H), 7.93 (d, J = 8.9 Hz, 1H), 7.79 (d, J = 7.9, 2H), 7.53 (dd, J = 10.0, 5.1 Hz, 2H), 7.42 (t, J = 7.6 Hz, 1H), 7.18 – 7.07 (m, 1H), 6.91 (m, 3H), 6.52 (d, J = 8.6 Hz, 2H), 5.87 (s, 1H), 5.32 – 5.11 (m, 2H), 4.81 – 4.23 (m, 4H), 3.05 – 2.79 (m, 4H), 2.62 – 2.11 (m, 4H), 2.08 – 1.44 (m, 4H), 1.34 (m, 12H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 2/1, here is the major rotamer): δ 173.2, 173.0, 169.7, 169.6, 169.2, 169.1, 144.1, 137.0, 135.6, 134.2, 133.5, 131.9, 131.6, 129.0, 128.8, 128.2, 127.8, 126.7, 126.3, 125.6, 123.1, 122.9, 122.5, 119.6, 113.9, 111.7, 100.0, 95.6, 61.7, 54.0, 51.8, 51.6, 49.2, 47.3, 33.3, 28.7, 28.6(3C), 27.9, 27.5, 23.2, 19.3, 14.4. HRMS (ESI): Calcd. For C44H46ClN5O7 [M+H]1+: 826.2769; Found: 826.2749. LC-MS purity: 95%.
Ethyl 3-(2-(tert-butylamino)-1-(5-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)pent-4-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (9)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 1/1): δ 9.12 – 8.96 (m, 2H), 8.28 – 8.08 (m, 2H), 7.87 (d, J = 8.9 Hz, 1H), 7.79 (d, J = 7.7 Hz, 2H), 7.70 (d, J = 8.7Hz, 1H), 7.62 – 7.51 (m, 2H), 7.48 – 7.28 (m, 4H), 7.26 (d, 1H), 7.18 – 7.10 (m, 1H), 6.92 (d, J = 5.5 Hz, 1H), 6.44 (s, 1H), 6.33 (t, J = 7.3 Hz, 1H), 6.00 (q, J = 6.6 Hz, 1H), 5.50 (d, J = 18.4 Hz, 1H), 5.42 (d, J = 9.5 Hz, 1H), 5.34 – 5.14 (m, 3H), 4.72 (d, J = 18.3 Hz, 1H), 4.62 – 4.19 (m, 9H), 3.71 (dd, J = 15.9, 6.0 Hz, 1H), 2.96 – 2.63 (m, 10H), 2.59 – 2.37 (m, 3H), 2.22 (m, 2H), 1.44 – 1.23 (m, 24H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 1/1): δ 171.4, 171.4, 171.1, 171.1, 169.5, 169.4, 169.1, 169.00, 168.8, 168.7, 168.4, 168.3, 156.7, 143.9, 143.8, 135.7, 135.6, 135.6, 135.6, 135.5, 134.7, 134.5, 132.5, 132.2, 131.5, 128.3(2C), 127.8, 127.1, 127.0, 126.3, 125.4, 124.7, 123.3, 123.3, 123.3, 123.2, 122.9, 122.9, 122.4, 121.7, 121.7, 119.5, 119.2, 114.3, 113.9, 112.3, 112.0, 109.8, 109.6, 109.1, 109.0, 108.9, 108.8, 100.0, 99.8, 95.3, 94.7, 61.8, 61.8, 56.0, 54.4, 51.9, 51.8, 48.5, 47.1, 47.1, 47.0, 34.5, 32.9, 32.6, 31.5, 31.5, 29.7, 28.7(3C), 28.7(3C), 23.4, 23.3, 15.5, 14.8, 14.3, 14.2. HRMS (ESI): Calcd. For C42H40ClF3N5O7 [M+H]1+: 818.2563; Found: 818.2564. LC-MS purity: 96%.
Ethyl 3-(2-(tert-butylamino)-1-(6-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hex-5-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (10)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 1/1): δ 9.09 (m, 2H), 8.50 – 7.98 (m, 2H), 7.91 – 7.52 (m, 5H), 7.40 (m, 4H), 7.24 – 7.07 (m, 2H), 6.89 (d, J = 21.7 Hz, 1H), 6.81 (d, J = 8.3 Hz, 1H), 6.47 (s, 1H), 6.29 (br, 2H), 6.10 – 5.91 (m, 2H), 5.52 – 5.08 (m, 5H), 4.80 – 4.64 (m, 1H), 4.57 – 4.15 (m, 10H), 3.70 (dd, J = 16.0, 7.4 Hz, 1H), 3.01 – 2.75 (m, 4H), 2.53 (m, 10H), 2.27 – 1.98 (m, 5H), 1.42 (t, J = 7.1 Hz, 3H), 1.36 – 1.21 (m, 21H).13C NMR (101 MHz, CDCl3, a mixture of rotamers: 1/1): δ 170.0, 170.0, 169.5, 169.4, 169.1, 169.0, 169.0, 168.8, 168.4, 160.5, 156.2, 148.9, 148.8, 143.9, 143.7, 135.7, 135.6, 134.5, 134.4, 132.4, 132.2, 131.6, 131.4, 128.4, 128.3, 128.3, 127.8, 127.0, 126.7, 125.4, 124.8, 123.3, 123.2, 123.1, 122.9, 122.8, 122.5, 121.9, 119.6, 119.2, 113.9(2C), 112.3, 112.0, 109.7, 109.0, 108.8, 100.5, 100.0, 95.9, 95.1, 68.6, 62.0, 55.9, 54.3, 53.4, 52.0, 51.8, 48.7, 46.9, 34.5, 32.4, 31.6, 31.0, 29.7, 28.7, 28.6(3C), 28.5(3C), 28.4, 23.3, 19.2, 19.1, 14.8, 14.3, 14.2. HRMS (ESI): Calcd. For C43H42ClF3N5O7 [M+H]1+: 832.2719; Found: 832.2722. LC-MS purity: 95%.
Ethyl 3-(2-(tert-butylamino)-1-(7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (11, WB214)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 1/1): δ 8.91 (s, 1H), 8.79 (s, 1H), 8.44 (d, J = 16.0 Hz, 1H ), 7.89 (d, J = 8.7 Hz, 1H), 7.78 (d, J = 7.5 Hz, 2H), 7.73 (d, J = 8.8 Hz, 1H), 7.61 – 7.31 (m, 6H), 7.17 – 7.15 (d, J = 8.5 Hz, 1H), 6.95 (s, 1H), 6.80 (s, 1H), 6.47 (s, 1H), 6.29 (t, J = 7.4 Hz, 2H), 5.99 – 5.94 (m, 2H), 5.36–5.25 (m, 3H), 4.76 – 4.25 (m, 10H), 3.70 (m, 1H), 2.90 – 2.78 (m, 6H), 2.55 – 2.48 (m, 6H), 2.23–2.19 (m, 4H), 2.01 – 1.55 (m, 8H), 1.44 – 1.19 (m, 24H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 1/1): δ 173.1, 173.1, 171.4, 171.3, 170.0, 169.7, 169.4, 169.2, 169.1, 168.7, 160.6, 152.3, 150.0, 149.4, 148.9, 144.7, 135.7, 135.6, 135.2, 135.2, 134.2, 133.5, 132.1, 131.6, 131.5, 128.4, 128.3, 128.3, 128.2, 127.8, 126.9, 126.8, 126.8, 126.6, 125.4, 124.8, 123.1(2C), 123.0, 122.7, 122.5, 121.9, 119.5, 119.5, 115.6, 115.0, 114.7, 113.9, 112.2, 111.9, 109.7, 109.0, 108.8, 100.7, 100.0, 96.0, 95.5, 61.9, 61.9, 56.0, 53.9, 53.9, 52.3, 51.8, 51.7, 48.9, 48.7, 47.3, 34.5, 33.2, 32.2, 31.9, 31.5, 29.7, 28.7 (3C), 28.6(3C), 28.0, 27.5, 24.2, 23.3, 23.2, 19.2, 14.8, 14.3. HRMS (ESI): Calcd. For C44H44ClF3N5O7 [M+H]1+: 846.2876; Found: 846.2885. LC-MS purity: 95%.
Ethyl 3-(2-(tert-butylamino)-1-(8-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)oct-7-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (12)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 2/1): δ 7.81 (d, J = 8.7 Hz, 3H), 7.72 (d, J = 7.6 Hz, 3H), 7.63 – 7.54 (m, 3H), 7.52 – 7.44 (m, 3H), 7.43 – 7.41 (s, 2H), 7.36 (s, 1H), 7.20 – 7.17 (m, 2H), 7.11 (d, J = 8.8 Hz, 1H), 6.89 (d, J = 8.6 Hz, 1H), 6.55 (d, J = 2.3 Hz, 2H), 6.33 (m, 2H), 6.00 (m, 4H), 5.24 (d, J = 16.2 Hz, 2H), 5.20 – 5.10 (m, 3H), 4.81 (d, 1H), 4.64 – 4.44 (m, 6H), 4.43 – 4.25 (m, 7H), 3.81 (d, J = 16.1 Hz, 2H), 3.00 – 2.69 (m, 9H), 2.67 – 2.44 (m, 13H), 2.40 – 2.09 (m, 5H), 1.90 – 1.52 (m, 15H), 1.44 – 1.33 (m, 9H), 1.32 – 1.23 (m, 27H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 2/1): δ 175.1(2C), 173.2, 173.2, 170.7, 170.4, 170.1(2C), 169.7, 160.8, 151.1, 151.0, 148.7, 148.6, 144.0, 144.0, 136.3, 136.3, 134.3, 134.2, 131.5(2C), 130.9(2C), 128.2, 127.4(2C), 124.6(2C), 122.2, 122.2, 121.8(2C), 121.6(2C), 121.5(2C), 121.3(2C), 119.7, 119.6, 114.1, 114.1, 112.0, 111.8, 109.5, 109.3, 109.0, 108.8, 95.8, 95.8, 76.0, 76.0, 60.9(2C), 56.1(2C), 52.4, 52.3, 51.2, 51.2, 51.0, 51.0, 47.9, 47.7, 32.7, 32.6, 31.0(2C), 28.4, 28.3, 28.0(2C), 27.5(3C), 27.5(3C), 24.1(2C), 22.7, 22.7, 18.6, 18.6, 13.3(2C). HRMS (ESI): Calcd. For C45H46ClF3N5O7 [M+H]1+: 860.3038; Found: 860.3065. LC-MS purity: 95%.
Ethyl 3-(2-(tert-butylamino)-1-(9-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)non-8-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (13)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 2/1): δ 7.81 (m, 3H), 7.72 (m, 3H), 7.63 – 7.55 (m, 3H), 7.49 (t, J = 7.8 Hz, 3H), 7.42 (s, 2H), 7.38 – 7.34 (s, 1H), 7.18 (d, J = 8.8 Hz, 2H), 7.11 (d, J = 8.9 Hz, 1H), 6.87 (s, 1H), 6.54 (s, 2H), 6.33 (m, 2H), 6.06 – 5.90 (m, 4H), 5.25 (d, J = 16.1 Hz, 2H), 5.17 (dd, J = 13.3, 5.1 Hz, 3H), 4.78 (d, 1H), 4.57 – 4.44 (m, 6H), 4.36 (m,, 7H), 3.81 (d, J = 16.1 Hz, 2H), 3.03 – 2.72 (m, 9H), 2.63 – 2.42 (m, 13H), 2.28 (t, J = 7.4 Hz, 6H), 2.23 – 2.07 (m, 9H), 1.66 – 1.47 (m, 11H), 1.46 – 1.25 (m, 36H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 2/1): δ 176.3, 175.2, 174.6, 173.3, 173.2, 170.8, 170.7, 170.2, 170.1, 169.6, 160.8, 160.5, 151.5, 151.0, 148.7, 148.5, 144.0, 143.9, 136.4, 136.3, 136.3, 136.2, 134.3(2C), 134.3, 134.3, 131.5, 131.5, 130.9(2C), 130.7, 128.2, 128.2, 127.4, 125.2, 124.6, 122.2, 122.2, 121.8, 121.6, 121.5, 121.3, 119.7, 119.6, 114.2, 113.7, 112.0, 111.8, 109.5, 109.3, 109.0, 108.9, 95.9, 83.6, 76.0, 75.9, 68.0, 61.0, 60.8, 56.1, 56.1, 52.4, 52.3, 51.2, 51.0, 47.7, 47.1, 33.6, 32.8, 31.0, 28.7, 28.4, 28.3, 28.2, 28.2, 28.1, 28.0, 27.5(3C), 27.5(3C), 24.6, 22.7, 22.7, 18.7, 18.6, 13.3, 13.3. HRMS (ESI): Calcd. For C46H47ClF3N5O7 [M+H]1+: 874.3194; Found: 874.3221. LC-MS purity: 95%.
Ethyl 3-(2-(tert-butylamino)-1-(10-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)dec-9-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (14)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 2/1): δ 7.82 (d, J = 8.8 Hz, 2H), 7.73 (d, J = 7.6 Hz, 3H), 7.61 (t, J = 6.8 Hz, 3H), 7.49 (t, J = 7.7 Hz, 3H), 7.42 (s, 2H), 7.37 (s, 1H), 7.18 (d, J = 8.9, 1H), 7.11 (d, J = 8.9, 1H), 6.86 (s, 1H), 6.55 (s, 2H), 6.41 – 6.29 (m, 2H), 6.04 – 5.94 (m, 4H), 5.25 (d, J = 16.1 Hz, 2H), 5.17 (m, 3H), 4.80 (d, J = 18.8 Hz, 2H), 4.50 (m, 6H), 4.42 – 4.30 (m, 7H), 3.81 (d, J = 16.1 Hz, 2H), 3.00 – 2.73 (m, 10H), 2.62 – 2.42 (m, 15H), 2.37 – 2.13 (m, 5H), 1.86 – 1.42 (m, 24H), 1.43 – 1.21 (m, 36H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 2/1): δ 175.3, 175.2, 174.7, 173.2, 173.2, 170.8, 170.7, 170.2, 169.6(2C), 160.8, 160.5, 153.5, 153.0, 151.1, 150.9, 143.9, 143.9, 136.4, 136.3, 134.4, 134.3, 131.5(2C), 130.9, 130.7, 128.2(2C), 127.4(2C), 125.2(2C), 124.6(2C), 122.2(2C), 121.8(2C), 121.5(2C), 121.3(2C), 120.0, 119.7, 114.1, 113.6, 112.0, 111.8, 109.5, 109.2, 109.0, 108.8, 96.0, 96.0, 75.9, 75.8, 60.9, 60.8, 56.1(2C), 54.5, 54.5, 52.4(2C), 51.2, 51.2, 51.0, 51.0, 32.8, 32.7, 31.0(2C), 29.1, 28.5(2C), 28.5, 28.4, 28.3, 28.2, 27.5(3C), 27.5(3C), 24.5, 24.5, 22.7(2C), 18.7(2C), 13.3, 13.3. HRMS (ESI): Calcd. For C47H49ClF3N5O7 [M+H]1+: 888.3351; Found: 888.3370. LC-MS purity: 95%.
Ethyl 3-(2-(tert-butylamino)-1-(11-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)undec-10-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (15)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 2/1): δ 7.90 – 7.81 (m, 6H), 7.80 – 7.74 (m, 3H), 7.66 – 7.59 (m, 3H), 7.52 (t, J = 7.7 Hz, 3H), 7.45 (d, J = 1.9 Hz, 2H), 7.40 (d, J = 1.9 Hz, 1H), 7.21 (dd, J = 8.8, 1.9 Hz, 2H), 7.15 (dd, J = 8.8, 1.9 Hz, 1H), 6.90 (s, 1H), 6.57 (s, 2H), 6.43 – 6.36 (m, 2H), 6.03 (dd, J = 9.0, 6.6 Hz, 4H), 5.28 (d, J = 16.1 Hz, 2H), 5.25 – 5.16 (m, 3H), 4.83 (d, J = 18.4 Hz, 1H), 4.62 – 4.31 (m, 13H), 3.84 (d, J = 16.1 Hz, 2H), 3.04 – 2.76 (m, 8H), 2.63 – 2.46 (m, 12H), 2.33 (m, 1H), 2.22 (m, 3H), 1.86 – 1.26 (m, 72H).
13C NMR (101 MHz, CD3OD, a mixture of rotamers: 2/1): δ 175.3(2C), 174.7, 173.2, 170.7, 170.4, 170.2(2C), 169.6(2C), 160.8, 160.5, 151.2(2C), 151.0(2C), 148.7, 148.6, 146.7, 143.9(2C), 141.0, 136.4, 136.3, 136.3, 136.2, 134.4(2C), 131.5, 130.9, 130.7, 129.8, 128.2(2C), 127.4, 125.2, 124.6, 122.9, 122.2(2C), 121.8, 121.5(2C), 121.3, 119.7, 114.1, 113.6, 113.5, 112.0, 111.8, 109.6, 109.3, 109.1, 108.8, 96.0, 89.7, 75.8, 75.8(2C), 61.0, 60.8, 56.1, 54.5, 52.4, 52.3, 51.7, 51.2, 51.0, 47.5, 47.1, 33.0, 32.7, 31.0, 30.9, 29.1, 29.0, 29.0, 29.0, 28.8, 28.8, 28.6, 28.5, 28.3, 28.3, 27.5(3C), 27.5(3C), 24.9, 24.5, 22.7, 22.6, 18.7, 18.6, 13.4, 13.3. HRMS (ESI): Calcd. For C48H51ClF3N5O7 [M+H]1+: 902.3502; Found: 902.3537. LC-MS purity: 95%.
3-(2-(tert-butylamino)-1-(7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylic acid (16)
To a solution of 11a (177 mg, 0.294 mmol, 1 equiv.) in a mixture of EtOH (3 mL) and water (3 mL), LiOH (61 mg, 1.47 mmol, 5 equiv.) was added. The reaction mixture was stirred at room temperature for 12 h. The reaction was then acidified with 1 M HCl, and extracted with EtOAc. The collected organic phase was then dried over Na2SO4, and the solvent was evaporated to give compound 11b (169 mg, 99%), which was used for the next step without further purification.
To a solution of 11b (60 mg, 0.104 mmol, 1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (77 mg, 0.208 mmol, 2 equiv.) in dry DMF (5 mL), copper iodide (4 mg, 0.0208 mmol, 0.2 equiv.) and Pd(PPh3)Cl2 (7 mg, 0.0104 mmol.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (5 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. The reaction mixture was acidified by 1M HCl. The reaction was extracted with EtOAc. The collected organic phase was dried over Na2SO4. The solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compound 16 as the light-yellow foam with the yield of 14%. 1H NMR (400 MHz, CD3OD, a mixture of rotamers: 3/1): δ 7.80 (d, J = 8.8 Hz, 4H), 7.74 (d, J = 7.5 Hz, 4H), 7.62 (d, J = 7.7 Hz, 3H), 7.57 (dd, J = 7.3, 4.1 Hz, 1H), 7.51 (d, J = 7.6 Hz, 4H), 7.41 (t, J = 1.6 Hz, 3H), 7.36 (t, J = 1.9 Hz, 1H), 7.18 (td, J = 8.8, 1.9 Hz, 3H), 7.10 (dd, J = 8.8, 1.8 Hz, 1H), 6.91 (s, 1H), 6.60 (s, 1H), 6.43 – 6.31 (m, 2H), 6.10 – 5.98 (m, 6H), 5.31 – 5.12 (m, 7H), 4.80 (d, J = 18.1 Hz, 1H), 4.65 – 4.46 (m, 8H), 4.38 (d, J = 18.1 Hz, 1H), 3.84 (d, J = 16.1 Hz, 1H), 2.97 – 2.44 (m, 27H), 2.43 – 2.29 (m, 1H), 2.18 (m, 4H), 2.04 – 1.86 (m, 8H), 1.73 (m, 8H), 1.31 (s, 27H), 1.27 (s, 9H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 3/1): δ 174.9, 174.4, 173.5, 173.3, 170.7, 170.5, 170.2, 170.2, 169.7, 169.7, 161.9, 161.9, 151.2, 151.1, 148.8, 148.6, 144.2, 144.1, 144.1, 144.0, 136.2, 136.2, 134.3, 134.2, 131.6, 131.5, 130.6, 130.4, 128.4, 128.2, 125.4, 124.8, 122.3, 122.2, 121.7, 121.5, 121.5, 121.5, 121.4, 121.2, 119.6, 119.5, 113.5, 113.4, 111.9, 111.8, 109.5, 109.3, 109.0, 108.8, 95.4, 95.2, 76.5, 76.4, 56.1, 54.6, 52.3, 52.3, 51.2, 51.1, 48.3, 47.9(2C), 47.1, 32.7, 32.0, 31.1, 27.7, 27.6(3C), 27.5(3C), 24.2, 23.6, 23.5, 22.5, 18.5, 18.4, 18.3. HRMS (ESI): Calcd. For C42H40ClF3N5O7 [M+H]1+: 818.2563; Found: 818.2592. LC-MS purity: >99%.
3-(2-(tert-butylamino)-1-(7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamido)-2-oxoethyl)-6-chloro-N-ethyl-1H-indole-2-carboxamide (17)
To a solution of 11b (60 mg, 0.104 mmol, 1 equiv.) in DMF (5 mL), DIPEA (0.05 mL, 0.287 mmol, 3 equiv.) was added at 0ºC, followed by addition of HATU (73 mg, 0.191 mmol, 2 equiv.) After being stirred at room temp. for 30 min, ethylamine hydrochloride (12 mg, 0.143 mmol, 1.5 equiv.) was added. The reaction mixture was stirred overnight at room temp. Then the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water. The collected organic phase was dried over MgSO4, after the filtration and concentration, the residue was submitted to a silica gel chromatograph (DCM/MeOH: 1% to 5% MeOH in DCM), giving the desired compound 11c (44 mg, 76%).
To a solution of 11c (39 mg, 0.065 mmol, 1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (48 mg, 0.129 mmol, 2 equiv.) in dry DMF (3 mL), copper iodide (2.5 mg, 0.013 mmol, 0.2 equiv.) and Pd(PPh3)Cl2 (4.5 mg, 0.006 mmol.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (3 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. The solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compound 17 (27 mg, 49%) as light-yellow foam. 1H NMR (400 MHz, CD3OD, a mixture of rotamers: 2/1): δ 7.78 (d, J = 8.7 Hz, 3H), 7.71 (m, 3H), 7.64 – 7.54 (m, 3H), 7.53 – 7.43 (m, 3H), 7.36 (s, 2H), 7.33 (s, 1H), 7.19 (t, J = 8.9 Hz, 2H), 7.10 (d, J = 8.8 Hz, 1H), 6.91 (d, J = 5.2 Hz, 1H), 6.73 (d, J = 3.4 Hz, 2H), 6.41 (m, 2H), 6.13 (m, 4H), 5.20 (m, 6H), 4.78 (d, J = 18.5 Hz, 1H), 4.66 – 4.39 (m, 6H), 3.90 (d, J = 16.3 Hz, 2H), 3.45 – 3.24 (m, 6H), 3.04 – 2.42 (m, 24H), 2.26 – 2.14 (m, 3H), 2.06 – 1.84 (m, 6H), 1.78 (m, 4H), 1.65 (m, 2H), 1.36 – 1.23 (m, 27H), 1.20 (m, 9H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 2/1): δ 174.9, 174.5, 173.3, 173.3, 170.7, 170.7, 170.4, 170.3, 169.7, 169.7, 161.6, 161.4, 151.2, 151.2, 148.7, 148.6, 144.2, 144.1, 136.2, 135.9(2C), 134.3, 134.2, 131.6, 131.5, 131.3, 130.5, 130.4, 130.2, 129.9, 128.2(2C), 125.5, 124.9, 122.3(2C), 121.6, 121.5, 121.4, 121.3, 121.2, 119.6, 119.6, 112.3(2C), 111.6, 111.5, 109.5, 109.3, 109.1, 108.9, 95.5, 95.3, 76.5, 76.3, 56.1, 54.3, 52.3, 52.3, 51.2, 51.0, 48.3(2C), 47.6, 47.4, 47.0, 46.6, 34.3, 34.2, 32.1(2C), 31.1, 31.1, 27.7, 27.6(3C), 27.5(3C), 23.7, 23.5, 22.6, 22.5, 18.4, 18.3, 13.3(2C). HRMS (ESI): Calcd. For C44H45ClF3N6O6 [M+H]1+: 845.3036; Found: 845.3037. LC-MS purity: >99%.
General Procedure for preparing compounds 18–21
To a solution of Ugi ligand 1 (1.2 equiv.) in DMF (2mL), DIPEA (3 equiv.) was added at 0ºC, followed by addition of HATU (2 equiv.) After being stirred at room temp. for 30 min, a solution of amine (1 equiv.) in DMF was added. The reaction mixture was stirred for another 3 hr at room temp. Then the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water. The collected organic phase was dried over MgSO4, after the filtration and concentration, the residue was submitted to a silica gel chromatograph (Hexane/EtOAc: 1/1 to 1/2), giving the desired compound 18a-21a with the yields of 55%−74%.
To a solution of 18a-21a (1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (2 equiv.) in dry DMF (3mL), copper iodide (0.2 equiv.) and Pd(PPh3)Cl2 (0.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (3 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. After cooling down, the solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compounds 18-21 as light-yellow foam with the yields of 30%−71%.
3-(2-(tert-butylamino)-2-oxo-1-(N-(3,4,5-trifluorobenzyl)formamido)ethyl)-6-chloro-N-(3-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)prop-2-yn-1-yl)-1H-indole-2-carboxamide (18)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 3/1, here is the major isomer) δ 8.31 (s, 1H), 7.67 (dd, J = 10.7, 8.3 Hz, 2H), 7.58 (d, J = 7.7 Hz, 1H), 7.42 (t, J = 7.5 Hz, 1H), 7.27 (d, J = 10.0 Hz, 1H), 7.03 (d, J = 8.8 Hz, 1H), 6.42 (m, 2H), 6.19 – 6.08 (m, 2H), 5.14 – 4.99 (m, 1H), 4.91 (m, 1H), 4.60 – 4.02 (m, 7H), 2.78 (m, 1H), 2.66 (m, 1H), 2.40 (m, 1H), 2.07 (m, 1H), 1.16 (s, 9H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 3/1, here is the major isomer): δ 173.2, 170.7, 169.4, 169.4, 165.3, 161.7, 151.3, 149.0, 144.4, 136.1, 134.7, 131.7, 130.4, 129.8, 128.3, 125.6, 124.7, 123.2, 121.5, 121.4, 121.3, 118.3, 113.0, 111.7, 111.00, 109.7, 90.3, 77.9, 56.8, 52.3, 51.2, 48.9, 47.6, 45.6, 30.9, 29.2, 27.4(6C), 22.7. HRMS (ESI): Calcd. For C39H35ClF3N6O7 [M+H]1+: 775.2259; Found: 775.2321. LC-MS purity: 98%.
3-(2-(tert-butylamino)-2-oxo-1-(N-(3,4,5-trifluorobenzyl)formamido)ethyl)-6-chloro-N-(4-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)but-3-yn-1-yl)-1H-indole-2-carboxamide (19)
1H NMR (400 MHz, CD3OD, a mixture of rotamers :2/1): δ 8.45–8.39 (m, 3H), 7.81 – 7.68 (m, 6H), 7.58 (m, 3H), 7.47 (m, 3H), 7.39 (dd, J = 8.5, 1.8 Hz, 3H), 7.12 (m, 3H), 6.70 (d, J = 7.8 Hz, 1H), 6.50 (t, J = 7.6 Hz, 4H), 6.31 – 6.20 (m, 4H), 5.16 – 4.93 (m, 5H), 4.64 (d, J = 16.7 Hz, 1H), 4.47 (m, 6H), 4.35 (dd, J = 16.7, 4.3 Hz, 1H), 4.26 (dd, J = 15.8, 5.1 Hz, 2H), 3.60 – 3.36 (m, 6H), 2.92 – 2.63 (m, 12H), 2.54 – 2.35 (m, 3H), 2.19 – 2.04 (m, 3H), 1.35 – 1.23 (m, 27H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 2/1): δ 173.2, 173.1, 170.7, 170.7, 169.5, 169.4, 165.3, 165.3, 164.7, 164.6, 161.9, 161.4, 151.4, 151.3, 148.9, 148.8, 144.1, 144.0, 136.0, 134.5, 131.6, 131.5, 130.4, 130.0, 129.9, 128.2, 125.6, 124.8, 122.6, 121.5, 121.4, 119.0, 112.9, 112.9, 111.7, 111.5, 111.0, 110.9, 109.8, 109.6, 92.6, 92.5, 77.0, 76.9, 56.9, 56.9, 52.4, 52.3, 49.0, 49.0, 46.6, 45.7, 38.4, 38.4, 31.0, 27.5(3C), 27.5(3C), 22.7, 22.6, 19.6. HRMS (ESI): Calcd. For C40H37ClF3N6O6 [M+H]1+: 789.2415; Found: 789.2441. LC-MS purity: 95%.
3-(2-(tert-butylamino)-2-oxo-1-(N-(3,4,5-trifluorobenzyl)formamido)ethyl)-6-chloro-N-(5-(2-(2,6dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)pent-4-yn-1-yl)-1H-indole-2-carboxamide (20)
1H NMR (400 MHz, CD3OD, a mixture of rotamers :2/1): δ 8.42 (s, 1H), 8.39 (s, 2H), 7.79 – 7.68 (m, 6H), 7.61 – 7.53 (m, 3H), 7.49 – 7.40 (m, 3H), 7.40 – 7.31 (m, 3H), 7.13 (d, J = 8.8 Hz, 3H), 6.66 (d, J = 3.0 Hz, 1H), 6.57 – 6.46 (m, 4H), 6.30 – 6.11 (m, 4H), 5.16 (m, 3H), 4.97 (d, J = 15.7 Hz, 2H), 4.69 – 4.45 (m, 7H), 4.36 (d, J = 16.6 Hz, 1H), 4.29 (dd, J = 15.8, 3.5 Hz, 2H), 3.66 – 3.43 (m, 6H), 3.00 – 2.71 (m, 6H), 2.65 – 2.46 (m, 8H), 2.36 – 2.11 (m, 4H), 1.93 (m, 6H), 1.29 (s, 9H), 1.26 (s, 18H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 2/1): δ 173.3, 173.2, 170.8, 170.8, 169.6, 169.5, 169.5, 169.4, 165.3, 164.8, 162.1, 161.7, 151.4, 151.3, 148.9, 148.8, 144.1, 144.0, 135.9, 134.4, 134.3, 131.5, 130.3, 130.2, 130.1, 128.2, 125.6, 124.8, 122.4, 121.4, 121.3, 119.3, 112.4, 112.4, 111.6, 111.4, 111.0, 110.8, 109.8, 109.5, 94.8, 94.8, 76.4, 76.4, 56.8, 56.8, 52.4, 52.3, 52.2, 51.2, 48.9, 46.5, 45.7, 45.6, 38.4, 38.4, 31.0, 31.0, 28.0, 28.0, 28.0, 27.5(3C), 27.4(3C), 22.7, 16.4, 16.4. HRMS (ESI): Calcd. For C41H39ClF3N6O6 [M+H]1+: 803.2572; Found: 803.2594. LC-MS purity: 97%.
3-(2-(tert-butylamino)-2-oxo-1-(N-(3,4,5-trifluorobenzyl)formamido)ethyl)-6-chloro-N-(6-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hex-5-yn-1-yl)-1H-indole-2-carboxamide (21)
1H NMR (400 MHz, CD3OD, a mixture of rotamers :2/1): δ 8.37 (m, 3H), 7.87 – 7.65 (m, 6H), 7.60 (m, 3H), 7.53 – 7.41 (m, 3H), 7.37 (m, 3H), 7.13 (m, 3H), 6.64 (d, J = 4.8 Hz, 1H), 6.58 – 6.42 (m, 4H), 6.28 – 6.05 (m, 4H), 5.18 – 5.06 (m, 3H), 4.97 (d, J = 15.8 Hz, 2H), 4.64 (d, J = 16.6 Hz, 1H), 4.50 (m, 6H), 4.34 (d, J = 16.6 Hz, 1H), 4.25 (dd, J = 15.8, 7.0 Hz, 2H), 3.54 – 3.32 (m, 6H), 2.98 – 2.66 (m, 6H), 2.53 (m, 8H), 2.31 (m, 1H), 2.17 (m, 3H), 1.76 (m, 12H), 1.34 – 1.18 (m, 27H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers :2/1): δ 173.2, 173.1, 170.8, 170.7, 169.6, 169.6, 169.5, 169.3, 165.2, 164.7, 162.0, 161.5, 151.4, 151.3, 149.0, 148.8, 143.9, 143.9, 135.9, 134.4, 131.5, 130.3, 130.2, 130.0, 128.2, 125.6, 124.8, 122.3, 121.4, 121.3, 121.2, 119.5, 112.3, 111.6, 111.4, 111.1, 111.0, 110.9, 109.8, 109.5, 95.4, 95.4, 76.2, 76.1, 56.8, 56.7, 52.3, 52.2, 51.2, 51.2, 48.9, 48.9, 45.7, 45.6, 38.9, 38.8, 30.9, 28.4, 28.4, 28.2, 27.5(3C), 27.4(3C), 25.8, 25.7, 25.4, 22.7, 18.4, 18.0. HRMS (ESI): Calcd. For C42H41ClF3N6O6 [M+H]1+: 817.2728; Found: 817.2740. LC-MS purity: 96%.
General Procedure for Preparing compounds 22 and 23
To a G10 microwave tube containing aldehyde (0.2 mmol, 1 equiv.) in trifluoroethanol (2 mL), amine (0.2 mmol, 1 equiv.) was added, followed by addition of acid (0.2 mmol, 1 equiv.) and isocyanide (0.2 mmol, 1 equiv.). The reaction was stirred at 120°C under the microwave irradiation for 90 min. TLC was used to monitor the reaction. When the reaction is done, the solvent was evaporated, and the residue was submitted to a silica gel flash column (Hex/EA: 3/1 – 2/1), giving compounds 22a (21%) and 23a (31%).
To a solution of 22a and 23a (1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (2 equiv.) in dry DMF (3mL), copper iodide (0.2 equiv.) and Pd(PPh3)Cl2 (0.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (3 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. After cooling down, the solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compounds 22 (28%) and 23 (42%) as light-yellow foam.
Ethyl 3-(2-(tert-butylamino)-1-(3-((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)ethynyl)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (22)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 2/1): δ 9.24 (s, 1H), 9.07 (s, 2H), 8.37 (m, 3H), 7.92 – 7.42 (m, 30H), 7.37 (s, 3H), 7.22 (d, J = 9.1 Hz, 3H), 6.27 – 6.16 (m, 8H), 6.09 (s, 1H), 5.50 – 5.20 (m, 3H), 5.09 (t, J = 9.3 Hz, 2H), 4.59 (m, 2H), 4.52 – 4.22 (m, 6H), 3.97 (d, J = 16.2 Hz, 2H), 3.11 – 2.80 (m, 6H), 2.48 (m, 3H), 2.33 – 2.16 (m, 3H), 1.28 (m, 36H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 2/1): δ 171.2, 171.1, 170.5, 170.0, 170.0, 169.8, 169.5, 169.5, 168.9, 168.9, 159.8, 159.5, 148.9, 148.8, 143.6, 143.6, 136.9, 134.6, 134.5, 132.8, 132.7, 132.3, 131.8, 131.8, 130.2, 130.1, 129.6, 128.6(2C), 127.8, 127.7, 124.1, 123.1, 123.0, 122.5, 121.8, 118.6, 117.8, 114.4, 113.9, 112.1, 112.0, 110.0, 109.9, 85.6, 85.5, 81.6, 80.6, 61.8, 61.7, 60.4, 57.9, 57.8, 52.1, 52.0, 51.9, 51.8, 47.6, 47.0, 46.9, 34.5, 31.5, 28.7(3C), 28.6(3C), 23.4, 23.3, 14.9, 14.3. HRMS (ESI): Calcd. For C46H40ClF3N5O7 [M+H]1+: 866.2569, Found: 866.2582. LC-MS purity: 95%.
Ethyl 3-(2-(tert-butylamino)-1-(4-((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)ethynyl)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (23)
1H NMR (400 MHz, CDCl3, a mixture of rotamers: 2/1): δ 9.34 (s, 1H), 9.15 (s, 2H), 8.53 (s, 1H), 8.37 (s, 2H), 7.96 – 7.53 (m, 30H), 7.33 (s, 3H), 7.19 (d, J = 8.0 Hz, 3H), 6.30 – 6.00 (m, 9H), 5.66 – 5.08 (m, 5H), 4.73 – 4.20 (m, 8H), 3.98 (d, J = 15.9 Hz, 2H), 3.00 – 2.68 (m, 6H), 2.41 (m, 3H), 2.31 – 2.20 (m, 3H), 1.45 – 0.94 (m, 36H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers: 2/1): δ 172.4, 172.3, 171.1, 171.1, 170.0, 169.7, 169.4, 169.3, 168.8, 168.6, 160.2, 159.9, 151.3, 151.2, 148.9, 148.8, 143.5, 143.3, 135.6, 134.9, 134.8, 132.4, 132.3, 131.8, 131.5, 131.5, 128.8, 128.7, 127.8, 127.6, 127.5, 126.8, 124.9, 124.3, 124.2, 123.0, 121.9, 121.8, 118.4, 118.4, 113.9, 112.1, 112.0, 110.1, 109.9, 109.7, 86.4, 86.2, 81.9, 81.5, 61.9, 61.7, 60.4, 57.8, 57.7, 52.0, 51.9, 51.8, 51.6, 47.7, 46.9, 46.7, 34.5, 31.5, 28.5(3C), 28.3(3C), 23.4, 23.3, 14.8, 14.3. HRMS (ESI): Calcd. For C46H40ClF3N5O7 [M+H]1+: 866.2569, Found: 866.2589. LC-MS purity: 96%.
General Procedure for Preparing compounds 24a and 27a
To a G10 microwave tube containing aldehyde (0.2 mmol, 1 equiv.) in trifluoroethanol (2 mL), amine (0.2 mmol, 1 equiv.) was added, followed by addition of acid (0.2 mmol, 1 equiv.) and isocyanide (0.2 mmol, 1 equiv.). The reaction was stirred at 120°C under the microwave irradiation for 90 min. TLC was used to monitor the reaction. When the reaction is done, the solvent was evaporated, and the residue was submitted to a silica gel flash column (Hexane/EtOAc: 3/1–2/1). The obtained product was then redissolved in DCM, followed by addition of TFA. The reaction mixture was stirred at room temperature for 2 h. Saturated sodium bicarbonate was added to adjust pH to 12. The organic phase was then extracted with DCM. The combine organic phase was collected and dried over Na2SO4. After removal of solvent, compounds 24a (26%) and 27a (24%) were obtained.
To a solution of acid (1.2 equiv.) in DMF (2mL), DIPEA (3 equiv.) was added at 0ºC, followed by addition of HATU (2 equiv.) After being stirred at room temp. for 30 min, a solution of 24a or 27a (1 equiv.) in DMF was added. The reaction mixture was stirred for another 3 hr at room temp. Then the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water. The collected organic phase was dried over MgSO4, after the filtration and concentration, the residue was submitted to a silica gel chromatograph (Hex/EA: 1/1 to 1/2), giving the desired compound 24–29 with the yields of 26%−70%.
Ethyl 3-(2-(tert-butylamino)-1-(3-(5-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)pent-4-ynamido)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (24)
1H NMR (400 MHz, CD3OD, a mixture of rotamers, here is the major isomer): δ 7.89 – 7.78 (m, 2H), 7.74 – 7.67 (m, 2H), 7.59 (ddd, J = 7.7, 3.6, 1.1 Hz, 1H), 7.51 – 7.37 (m, 1H), 7.16 (dt, J = 8.9, 1.7 Hz, 1H), 6.23 – 6.13 (m, 3H), 5.37 (d, J = 15.9 Hz, 1H), 5.00 (m, 1H), 4.49 – 4.25 (m, 4H), 3.99 (d, J = 16.0 Hz, 1H), 2.95 – 2.65 (m, 6H), 2.28 (m, 1H), 2.16 – 1.96 (m, 1H), 1.39 – 1.29 (m, 3H), 1.13 (s, 9H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers, here is the major isomer): δ 173.7, 173.2, 170.9, 170.6, 170.4, 169.5, 160.2, 151.2, 148.6, 144.1, 138.7, 137.1, 136.2, 134.2, 131.6, 130.8, 128.7, 128.2, 127.3, 124.8, 122.5, 122.2, 122.0, 121.5, 120.9, 120.6, 118.6, 118.0, 114.1, 111.9, 109.9, 109.7, 94.3, 76.5, 61.0, 57.9, 52.1, 50.9, 47.9, 47.3, 35.4, 31.0, 27.4(3C), 22.6, 15.1, 13.3. HRMS (ESI): ): Calcd. For C49H44ClF3N6O8 [M+H]1+: 937.2939, Found: 937.2957. LC-MS purity: 96%.
Ethyl 3-(2-(tert-butylamino)-1-(3-(6-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hex-5-ynamido)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (25)
1H NMR (400 MHz, DMSO-d6, a mixture of rotamers: 3/1): δ 11.99 (s, 1H), 11.84 (s, 3H), 11.05 (s, 4H), 10.14 (m, 4H), 7.87 – 7.67 (m, 22H), 7.64 – 7.52 (m, 6H), 7.47 – 7.37 (m, 6H), 7.26 (dd, J = 16.0, 8.2 Hz, 4H), 7.16 (d, J = 9.1 Hz, 1H), 7.02 (d, J = 7.6 Hz, 1H), 6.82 (s, 1H), 6.50 (t, J = 8.0 Hz, 2H), 6.43 – 6.35 (m, 6H), 5.99 (s, 3H), 5.18 (m, 8H), 4.54 (m, 8H), 4.47 – 4.27 (m, 14H), 4.05 (d, J = 16.1 Hz, 3H), 3.05 – 2.89 (m, 8H), 2.87 (s, 1H), 2.79 (s, 3H), 2.64 (m, 9H), 2.15 – 1.81 (m, 14H), 1.48 (t, J = 7.1 Hz, 3H), 1.35 (t, J = 7.0 Hz, 6H), 1.28 (s, 9H), 1.23 (s, 27H). 13C NMR (101 MHz, DMSO-d6, a mixture of rotamers: 3/1): δ 173.3, 173.3, 172.3, 171.6, 171.4, 171.4, 171.1, 170.2, 168.1(2C), 162.8(2C), 160.5(2C), 150.9, 150.7, 148.4, 148.3, 144.3(2C), 139.8, 139.6, 139.6, 138.2, 137.6, 137.6, 137.4, 137.1, 136.6, 136.4, 134.6, 132.5, 132.4, 129.9, 129.8, 129.6, 129.1, 129.1, 128.7, 128.5, 127.6, 126.5, 125.2, 123.2, 123.2, 122.4, 122.4, 121.7, 121.7, 121.1, 121.1, 120.8, 119.2, 119.2, 118.7, 114.8, 112.4, 110.5, 110.3, 110.3, 110.2, 108.7, 102.5, 101.9, 96.1, 96.1, 77.3, 76.9, 61.4, 61.0, 57.9, 57.9, 52.1, 52.1, 51.0(2C), 47.9, 47.4, 31.7, 31.2, 29.0(2C), 28.7(6C), 24.5, 24.4, 22.8, 22.8, 18.9, 18.8, 14.8, 14.7. HRMS (ESI): Calcd. For C50H46ClF3N6O8 [M+H]1+: 951.3091; Found: 951.3122. LC-MS purity: 96%.
Ethyl 3-(2-(tert-butylamino)-1-(3-(7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hept-6-ynamido)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (26)
1H NMR (400 MHz, DMSO-d6, a mixture of rotamers: 4/1, here is the major isomers): δ 11.78 (s, 1H), 11.00 (s, 1H), 10.06 (s, 1H), 7.85 – 7.59 (m, 5H), 7.53 (m, 3H), 7.36 (d, J = 2.1 Hz, 1H), 7.18 (dd, J = 8.9, 2.0 Hz, 1H), 6.33 (dd, J = 9.2, 6.7 Hz, 2H), 5.94 (s, 1H), 5.20 – 5.08 (m, 2H), 4.54 – 4.42 (m, 1H), 4.40 – 4.21 (m, 3H), 3.99 (d, J = 16.1 Hz, 1H), 3.02 – 2.84 (m, 1H), 2.67 – 2.27 (m, 6H), 2.11 – 1.97 (m, 1H), 1.87 – 1.73 (m, 2H), 1.64 (m, 2H), 1.29 (t, J = 7.1 Hz, 3H), 1.17 (s, 9H). 13C NMR (101 MHz, DMSO-d6, a mixture of rotamers: 4/1, here is the major isomers): δ 173.3, 172.3, 171.6, 171.4, 170.2, 168.1, 160.5, 150.8, 148.3, 144.2, 137.5, 136.4, 134.5, 132.5, 129.8, 129.1, 127.6, 125.2, 123.1, 122.4, 121.7, 120.7, 119.3, 114.7, 112.4, 110.5, 110.3, 96.5, 77.1, 61.0, 57.9, 52.1, 51.0, 47.9, 47.4, 36.2, 31.7, 28.7(3C), 28.0, 24.8, 22.8, 19.0, 14.8. HRMS (ESI): Calcd. For C51H49ClF3N6O8 [M+H]1+: 965.3252; Found: 965.3284. LC-MS purity: >99%.
Ethyl 3-(2-(tert-butylamino)-1-(4-(5-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)pent-4-ynamido)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (27)
1H NMR (400 MHz, DMSO-d6, a mixture of rotamers: 6/1, here is the major isomers): δ 11.76 (s, 1H), 11.01 (s, 1H), 10.27 (s, 1H), 7.82 – 7.76 (m, 1H), 7.72 (d, J = 7.9 Hz, 2H), 7.65 – 7.58 (m, 2H), 7.55 – 7.48 (m, 3H), 7.35 (s, 1H), 7.19 (d, J = 8.4 Hz, 1H), 6.33 (t, J = 8.1 Hz, 2H), 5.97 (s, 1H), 5.11 (m, 2H), 4.48 – 4.20 (m, 4H), 4.02 (d, J = 16.1 Hz, 1H), 2.91 (d, J = 14.3 Hz, 1H), 2.86 – 2.74 (m, 2H), 2.73 – 2.56 (m, 3H), 2.35 (d, J = 13.6 Hz, 1H), 1.97 (m, 1H), 1.30 (t, J = 7.0 Hz, 3H), 1.22 (s, 9H). 13C NMR (101 MHz, CDCl3, DMSO-d6, a mixture of rotamers: 6/1, here is the major isomer): 173.3, 172.2, 171.4, 170.5, 170.1, 168.1, 160.4, 150.7, 148.2, 144.3, 140.7, 137.2, 136.3, 135.7, 134.4, 132.4, 131.5, 129.8, 129.1, 128.2, 127.6, 127.6, 125.2, 123.2, 122.3, 121.7, 119.0, 118.7, 115.0, 114.9, 112.4, 110.5, 110.3, 95.8, 77.0, 61.0, 58.0, 52.0, 51.1, 51.0, 47.3, 35.9, 31.6, 29.0, 28.8(3C), 22.8, 15.8, 14.8. HRMS (ESI): Calcd. For C49H44ClF3N6O8 [M+H]1+: 937.2934; Found: 937.2949. LC-MS purity: 96%.
Ethyl 3-(2-(tert-butylamino)-1-(4-(6-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hex-5-ynamido)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (28)
1H NMR (400 MHz, DMSO-d6, a mixture of rotamers: 5/1, here is the major isomers): δ 11.77 (s, 1H), 11.00 (s, 1H), 10.17 (s, 1H), 7.79 (d, J = 8.8 Hz, 1H), 7.69 (m, 4H), 7.52 (m, 3H), 7.36 (s, 1H), 7.19 (dd, J = 8.8, 2.0 Hz, 1H), 6.34 (dd, J = 9.3, 6.7 Hz, 2H), 5.98 (s, 1H), 5.22 – 5.03 (m, 2H), 4.57 – 4.18 (m, 4H), 4.03 (d, J = 16.1 Hz, 5H), 3.00 – 2.85 (m, 1H), 2.65 – 2.38 (m, 6H), 2.04 – 1.98 (m, 1H), 1.93 (t, J = 7.2 Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H), 1.25 (s, 9H). 13C NMR (101 MHz, DMSO-d6, a mixture of rotamers: 5/1, here is the major isomers): δ 173.3, 172.2, 171.4, 171.4, 170.6, 168.1, 160.5, 150.7, 148.3, 144.3, 141.0, 136.4, 135.9, 134.9, 134.6, 132.5, 131.3, 129.7, 129.0, 128.2, 127.6, 125.2, 123.2, 122.3, 121.7, 120.1, 119.2, 118.6, 115.0, 114.5, 112.4, 110.6, 110.4, 96.1, 77.3, 61.1, 58.0, 52.1, 51.1, 48.2, 47.5, 35.7, 31.7, 28.7(3C), 24.4, 22.8, 18.9, 14.8. HRMS (ESI): Calcd. For C50H46ClF3N6O8 [M+H]1+: 951.3091; Found: 951.3105. LC-MS purity: >99%.
Ethyl 3-(2-(tert-butylamino)-1-(4-(7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)hept-6-ynamido)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (29)
1H NMR (400 MHz, DMSO-d6, a mixture of rotamers: 5/1, here is the major isomers): δ 11.84 (s, 1H), 11.07 (s, 1H), 10.19 (s, 1H), 7.86 (d, J = 8.8 Hz, 1H), 7.80 – 7.65 (m, 4H), 7.64 – 7.53 (m, 3H), 7.42 (d, J = 2.0 Hz, 1H), 7.26 (dd, J = 8.9, 2.0 Hz, 1H), 6.40 (dd, J = 9.3, 6.7 Hz, 2H), 6.05 (s, 1H), 5.29 – 5.12 (m, 2H), 4.59 – 4.49 (m, 1H), 4.44 – 4.24 (m, 3H), 4.09 (d, J = 16.1 Hz, 1H), 3.06 – 2.90 (m, 1H), 2.71 – 2.41 (m, 6H), 2.09 (m, 1H), 1.86 (m, 2H), 1.71 (m, 2H), 1.37 (t, J = 7.0 Hz, 3H), 1.31 (s, 9H). 13C NMR (101 MHz, DMSO-d6, a mixture of rotamers: 5/1, here is the major isomers): δ 173.3, 172.2, 171.8, 171.4, 170.6, 168.1, 160.5, 150.6, 148.4, 144.3, 141.0, 137.3, 136.4, 134.5, 132.4, 131.3, 130.1, 129.7, 129.1, 128.2, 127.6, 126.3, 125.2, 123.1, 122.3, 121.7, 119.3, 118.9, 118.6, 115.0, 112.4, 110.6, 110.4, 96.5, 77.1, 61.1, 58.0, 52.1, 51.1, 48.2, 47.4, 36.3, 31.7, 28.7(3C), 28.1, 24.7, 22.8, 19.0, 14.8. HRMS (ESI): Calcd. For C51H49ClF3N6O8 [M+H]1+: 965.3252; Found: 965.3278. LC-MS purity: >99%.
General Procedure for Preparing compounds 30 and 31
To a G10 microwave tube containing aldehyde (0.2 mmol, 1 equiv.) in trifluoroethanol (2 mL), amine (0.2 mmol, 1 equiv.) was added, followed by addition of acid (0.2 mmol, 1 equiv.) and isocyanide (0.2 mmol, 1 equiv.). The reaction was stirred at 120°C under the microwave irradiation for 90 min. TLC was used to monitor the reaction. When the reaction is done, the solvent was evaporated, and the residue was submitted to a silica gel flash column (Hexane/EtOAc: 3/1 – 2/1), giving compounds 30a (43%) and 31a (29%).
To a solution of 30a and 31a (1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (2 equiv.) in dry DMF (3mL), copper iodide (0.2 equiv.) and Pd(PPh3)Cl2 (0.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (3 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. After cooling down, the solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compounds 30 (65%) and 31 (45%) as light-yellow foam.
Ethyl 3-(2-(tert-butylamino)-1-(3-((3-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)prop-2-yn-1-yl)oxy)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (30)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 4/1, here is the major isomer): δ 7.84 (dd, J = 8.8, 2.9 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.66 (d, J = 7.6 Hz, 1H), 7.58 – 7.27 (m, 7H), 7.18 (m, 2H), 6.20 (m, 3H), 5.37 (dd, J = 16.1, 8.4 Hz, 1H), 5.20 – 5.05 (m, 3H), 4.41 – 4.26 (m, 3H), 3.99 (t, J = 15.3 Hz, 1H), 2.92 – 2.49 (m, 3H), 2.16 – 1.96 (m, 1H), 1.36 (t, J = 7.1, 3H), 1.23 (s, 9H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 4/1): δ 173.6, 173.5, 173.2, 173.1, 170.6, 170.5, 169.2, 169.0, 160.3, 160.2, 157.3, 157.3, 151.2, 151.1, 148.7, 148.6, 144.7, 144.6, 137.6, 137.5, 136.2, 136.2, 136.0, 135.9, 134.3(2C), 131.7, 131.7, 130.9, 130.8, 129.9, 129.8, 129.3, 129.2, 128.3, 128.3, 127.4, 127.4, 125.2, 124.8, 123.5, 123.2, 121.8, 121.5(2C), 121.4, 119.6, 119.5, 117.8, 117.7, 116.7, 116.3, 114.0, 114.0, 112.4, 112.3, 111.9, 111.9, 111.8, 111.7, 109.9, 109.8, 109.6, 109.6, 89.5, 89.5, 82.4, 82.4, 61.0, 61.0, 60.8, 60.7, 58.0, 57.9, 55.9, 55.8, 52.3, 52.2, 51.2, 51.1, 51.0, 51.0, 30.9, 30.9, 27.6(3C), 27.4(3C), 22.6, 22.5, 13.3. HRMS (ESI): Calcd. For C47H42ClF3N5O8 [M+H]1+: 896.2674; Found: 896.2692. LC-MS purity: 97%.
Ethyl 3-(2-(tert-butylamino)-1-(3-((4-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)but-3-yn-1-yl)oxy)-N-(3,4,5-trifluorobenzyl)benzamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (31)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 6/1, here is the major isomer): δ 7.83 (dd, J = 8.9, 3.6 Hz, 1H), 7.77 – 7.71 (m, 1H), 7.62 (m, 1H), 7.54 – 7.46 (m, 1H), 7.45 – 7.23 (m, 5H), 7.14 (m, 3H), 6.25 – 6.11 (m, 3H), 5.37 (d, J = 15.9 Hz, 1H), 5.15 (dd, J = 13.4, 5.1 Hz, 1H), 4.46 (m, 2H), 4.40 – 4.23 (m, 2H), 4.01 (dd, J = 16.0, 4.8 Hz, 1H), 3.01 (m, 2H), 2.96 – 2.72 (m, 2H), 2.54 – 2.36 (m, 1H), 2.15 (m, 1H), 1.31 (t, J = 7.6 Hz, 3H), 1.21 (s, 9H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 6/1): δ 173.9, 173.8, 173.3, 173.1, 170.7, 170.5, 169.6, 169.5, 160.3, 160.2, 158.4, 158.4, 151.2, 151.1, 148.7, 148.6, 144.3, 144.3, 137.5, 137.5, 136.4, 136.2, 136.2, 135.9, 135.9, 134.2, 134.1, 131.6, 131.6, 130.9, 130.8, 129.8, 129.4, 129.4, 128.5, 128.3(2C), 128.2, 127.4, 127.4, 124.8, 124.7, 122.6, 122.5, 121.5, 121.5(2C), 119.3, 119.2, 119.2(2C), 118.5, 115.8, 115.7, 114.1, 114.0, 113.3, 113.2, 113.1, 111.9, 111.8, 109.9, 109.8, 109.7, 109.7, 100.0, 99.8, 92.8, 92.6, 76.9, 76.9, 66.1, 66.0, 60.8, 60.7, 58.0, 58.0, 52.3, 52.3, 51.0, 51.0, 31.0, 31.0, 27.5(3C), 27.4(3C), 22.7, 22.6, 19.9(2C), 13.3(2C). HRMS (ESI): Calcd. For C48H44ClF3N5O8 [M+H]1+: 910.2831; Found: 910.2897. LC-MS purity: 95%.
General Procedure for Preparing compounds 32–36
To a G10 microwave tube containing aldehyde (0.2 mmol, 1 equiv.) in trifluoroethanol (2 mL), amine (0.2 mmol, 1 equiv.) was added, followed by addition of acid (0.2 mmol, 1 equiv.) and isocyanide (0.2 mmol, 1 equiv.). The reaction was stirred at 120°C under the microwave irradiation for 120 min. TLC was used to monitor the reaction. When the reaction is done, the solvent was evaporated, and the residue was submitted to a silica gel flash column (Hexane/EtOAc: 3/1 – 2/1), giving compounds 32a-36a with the yields of 27%−59%.
To a solution of 32a-36a (1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (2 equiv.) in dry DMF (3mL), copper iodide (0.2 equiv.) and Pd(PPh3)Cl2 (0.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (3 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. After cooling down, the solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compounds 32–36 as light-yellow foam with the yields of 41%−95%.
Ethyl 3-(2-(tert-butylamino)-1-(7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-ethylhept-6-ynamido)-2-oxoethyl)-6-chloro-1H-indole-2-carboxylate (32)
1H NMR (400 MHz, CD3OD, a mixture of rotamers: 3/2): δ 7.79 (dd, J = 8.8, 2.2 Hz, 3H), 7.74 (m, 5H), 7.64 – 7.59 (m, 5H), 7.53 – 7.46 (m, 10H), 7.38 (d, J = 6.0 Hz, 2H), 7.16 – 7.05 (m, 5H), 6.72 (s, 3H), 6.47 (s, 2H), 5.16 (m, 5H), 4.64 – 4.20 (m, 20H), 3.73 (m, 2H), 3.51 – 3.40 (m, 3H), 3.26 (m, 3H), 3.02 (m, 2H), 2.96 – 2.36 (m, 35H), 2.18 (m, 5H), 1.92 (m, 10H), 1.73 (m, 10H), 1.45 – 1.20 (m, 60H), 0.62 (t, J = 7.0 Hz, 9H), 0.48 (t, J = 6.7 Hz, 6H). 13C NMR (101 MHz, CD3OD, a mixture of rotamers: 3/2): δ 173.8, 173.8, 173.7, 173.7, 173.4, 173.2, 170.7, 170.7, 169.7, 169.6, 161.0, 160.7, 144.2, 144.0, 136.6, 136.5, 134.3, 134.3, 131.5(2C), 130.9, 130.7, 128.2(2C), 125.3, 124.8, 122.3(2C), 122.0, 121.9, 121.4, 121.3, 121.1(2C), 119.6(2C), 114.9, 114.5, 112.0, 111.9, 95.6, 95.4, 76.1, 76.1, 60.9, 60.9, 52.3, 52.3, 51.0, 50.9, 40.6, 40.2, 32.3, 32.3, 31.1, 31.0, 27.9, 27.8, 27.5(3C), 27.5(3C), 24.7, 24.6, 23.8, 23.6, 22.6, 22.5, 18.6(2C), 18.4, 18.3, 14.6(2C), 13.4, 13.3, 13.0. HRMS (ESI): Calcd. For C39H45ClN5O7 [M+H]1+: 730.3008; Found: 730.3031. LC-MS purity: 97%.
N-(2-(tert-butylamino)-1-(4-chlorophenyl)-2-oxoethyl)-7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-ethylhept-6-ynamide (33)
1H NMR (400 MHz, CDCl3): δ 8.61 (s, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.35 (d, J = 7.6 Hz, 1H), 7.25 (m, 4H), 6.02 (s, 1H), 5.80 (d, J = 3.8 Hz, 1H), 5.19 (dd, J = 13.3, 5.0 Hz, 1H), 4.44 (dd, J = 17.0, 10.0 Hz, 1H), 4.30 (d, J = 4.0 Hz, 1H), 3.45 – 3.21 (m, 2H), 2.86 – 2.68 (m, 2H), 2.41 (m, 5H), 2.17 – 2.05 (m, 1H), 1.94 – 1.73 (m, 2H), 1.68 – 1.52 (m, 2H), 1.24 (d, J = 11.4 Hz, 9H), 0.75 (q, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 173.8, 172.0, 169.8, 169.2, 168.9, 158.9, 158.5, 143.9, 134.5, 134.4, 134.3, 131.5, 130.5, 129.0, 128.3, 123.2, 119.5, 116.3, 95.6, 61.1, 53.5, 51.8, 51.7, 47.2, 41.1, 32.9, 31.6, 28.6, 28.1, 24.8, 23.3, 19.3, 15.4. HRMS (ESI): Calcd. For C34H40ClN4O5 [M+H]1+: 619.2687; Found: 619.2698. LC-MS purity: 99%.
N-(2-(tert-butylamino)-1-(4-chlorophenyl)-2-oxoethyl)-7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamide (34)
1H NMR (400 MHz, CDCl3): δ 8.71 (s, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.65 – 7.58 (m, 1H), 7.57 – 7.49 (m, 1H), 7.49 – 7.37 (m, 2H), 7.24 (m, 2H), 6.56 (t, J = 7.2 Hz, 2H), 6.12 (s, 1H), 5.28 (dd, J = 13.1, 4.0 Hz, 1H), 4.71 (dd, J = 18.3, 7.1 Hz, 1H), 4.58 – 4.27 (m, 3H), 2.93 – 2.74 (m, 2H), 2.64 – 2.29 (m, 4H), 2.22 – 2.11 (m, 2H), 1.84 (m, 2H), 1.56 (m, 2H), 1.31 (d, J = 12.8 Hz, 9H). 13C NMR (101 MHz, CDCl3): δ 174.5, 172.2, 169.8, 169.1, 168.5, 152.2, 149.9, 144.0, 134.8, 134.2, 133.8, 132.8, 132.1, 132.0, 131.5, 130.7, 129.1, 128.6, 128.4, 128.3, 123.2, 119.4, 109.9, 109.7, 95.4, 60.3, 51.9, 48.2, 47.1, 33.4, 31.6, 28.5(3C), 27.8, 24.3, 23.2, 19.3. HRMS (ESI): Calcd. For C39H39ClF3N4O5 [M+H]1+: 735.2561; Found: 735.2574. LC-MS purity: 99%.
N-(1-(tert-butylamino)-3-methyl-1-oxobutan-2-yl)-7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamide (35)
1H NMR (400 MHz, CDCl3): δ 8.59 (s, 1H), 7.79 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 7.5 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 6.76 (t, J = 7.1 Hz, 2H), 6.41 (d, J = 37.1 Hz, 1H), 5.35 – 5.19 (m, 1H), 4.91 (m, 1H), 4.59 – 4.22 (m, 3H), 2.88 (m, 2H), 2.64 – 2.46 (m, 1H), 2.42 (t, J = 6.8 Hz, 2H), 2.34 – 2.15 (m, 3H), 2.06 (m, 1H), 1.87 – 1.68 (m, 2H), 1.60 – 1.50 (m, 2H), 1.28 (s, 9H), 0.96 (d, J = 6.4 HzZ, 3H), 0.82 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 174.6, 171.7, 169.7, 169.1, 169.0, 152.6, 150.0, 143.9, 135.0, 134.2, 132.1, 131.5, 128.6, 128.4, 123.3, 119.4, 110.0, 109.8, 95.3, 51.8, 51.6, 47.0, 46.2, 34.5, 33.5, 31.7, 28.4(3C), 28.1, 27.9, 24.5, 23.2, 19.3, 19.2, 18.7. HRMS (ESI): Calcd. For C36H42F3N4O5 [M+H]1+: 667.3107; Found: 667.3119. LC-MS purity: 98%.
N-(2-(tert-butylamino)-1-cyclohexyl-2-oxoethyl)-7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamide (36)
1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 10.0 Hz, 1H), 7.80 (d, J = 7.2 Hz, 2H), 7.53 (d, J = 7.1 Hz, 2H), 7.43 (t, J = 7.6 Hz, 2H), 6.80 – 6.69 (m, 2H), 6.31 (d, J = 42.1 Hz, 1H), 5.28 (m, 1H), 4.92 (dd, J = 17.6, 6.5 Hz, 1H), 4.61 (d, J = 9.4 Hz, 1H), 4.55 – 4.28 (m, 3H), 2.99 – 2.74 (m, 2H), 2.64 – 2.46 (m, 1H), 2.42 (t, J = 6.8 Hz, 2H), 2.31 – 2.14 (m, 2H), 2.09 – 1.85 (m, 2H), 1.83 – 1.59 (m, 8H), 1.51 (m, 3H), 1.27 (s, 9H), 1.20 – 1.05 (m, 1H), 1.07 – 0.63 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.5, 171.7, 169.6, 169.1, 168.8, 152.5, 150.0, 144.0, 135.1, 134.1, 132.1, 131.5, 128.6, 128.4, 123.3, 119.4, 110.0, 109.7, 95.3, 51.8, 51.7, 47.0, 37.0, 33.4, 31.7, 29.8, 29.1, 28.4(3C), 28.1, 27.9, 26.3, 25.7, 25.6, 24.6, 24.4, 23.2, 19.2. HRMS (ESI): Calcd. For C39H46F3N4O5 [M+H]1+: 707.3420; Found: 707.3431. LC-MS purity: 96%.
General Procedure for Preparing compounds 37 and 38
To a solution of trifluorobenzylamine (0.5 mmol, 1 equiv.) and 4-chlorobenzaldehye or ethyl 6-chloro-3-formyl-1H-indole-2-carboxylate (0.5 mmol, 1 equiv.) in DCM/EtOH (3 mL, 2/1), AcOH (0.5 mmol, 1 equiv.) was added. After being stirred at room temperature for 1 h, NaBH3CN (0.75 mmol, 1.5 equiv) was added. The reaction was stirred at room temperature for 2 h. The reaction was washed with Sat. NaHCO3 and brine. The collected organic phase was dried over MgSO4, and the solvent was evaporated. The residue was submitted to a silica gel chromatograph (DCM/MeOH: 5% to 40% EtOAc in Hexane), giving the desired compound with the yields of 44% and 68%.
To a solution of 4-pentynoic acid (1.2 equiv.) in DMF (2 mL), DIPEA (2 equiv.) was added at 0ºC, followed by addition of HATU (1.5 equiv.) After being stirred at room temp. for 30 min, the above synthesized compounds (1 equiv.) were added. The reaction mixture was stirred overnight at room temp. Then the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water. The collected organic phase was dried over MgSO4, after the filtration and concentration, the residue was submitted to a silica gel chromatograph (Hexane/EtOAc: 4/1), giving the desired compounds 37a (80%) and 38a (86%)
To a solution of 37a or 38a (1 equiv.) and 3-(4-iodo-1-oxoisoindolin-2-yl)piperidine-2,6-dione (2 equiv.) in dry DMF (3mL), copper iodide (0.2 equiv.) and Pd(PPh3)Cl2 (0.1 equiv.) were added. The solution was purged and refilled with argon for 3 times. Then triethylamine (3 mL) was added, and the solution was purged again with Argon. Then reaction mixture was stirred at room temperature overnight. After cooling down, the solvent was removed under reduced pressure and the black tar residue was submitted to a silica gel chromatograph (DCM/MeOH: 99/1–95/5, gradually), giving compounds 37 (44%) and 38 (71%) as light-yellow foam.
Ethyl 6-chloro-3-((7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamido)methyl)-1H-indole-2-carboxylate (37)
1H NMR (400 MHz, CDCl3, a mixture of rotamers with a ratio of 2/1): δ 9.57 (s, 1H), 9.45 (s, 2H), 8.73 (s, 2H), 8.64 (s, 1H), 7.77 (dd, J = 14.5, 8.0 Hz, 6H), 7.51 (d, J = 7.6 Hz, 3H), 7.46 – 7.31 (m, 6H), 7.07 (m, 3H), 6.74 – 6.47 (m, 6H), 5.34 – 5.13 (m, 9H), 5.03 (s, 2H), 4.57 – 4.25 (m, 12H), 4.20 (q, J = 7.1 Hz, 4H), 2.94 – 2.67 (m, 8H), 2.47 (m, 10H), 2.31 (t, J = 7.4 Hz, 4H), 2.25 – 2.13 (m, 2H), 1.99 – 1.80 (m, 6H), 1.71 (t, J = 7.5 Hz, 2H), 1.59 (t, J = 7.7 Hz, 4H), 1.31 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 7.1 Hz, 6H). 13C NMR (101 MHz, CDCl3, a mixture of rotamers with a ratio of 2/1): δ 173.5, 173.2, 171.8, 171.6, 169.8(2C), 169.4, 169.4, 161.6, 161.0, 152.7, 152.6, 150.2, 150.1, 143.8, 143.7, 139.9, 137.4, 136.2, 136.1, 134.6, 134.5, 134,2, 133.9, 132.1, 132.0, 131.4, 131.4, 128.4(2C), 125.8, 125.8, 125.3, 125.2, 123.3, 123.2, 122.9, 122.3, 122.0, 121.1, 119.4(2C), 118.2(2C), 116.8, 112.3, 111.7, 111.5, 109.9, 109.8, 109.7, 109.6, 95.5, 95.4, 61.6, 61.5, 51.9(2C), 50.7, 48.5, 47.2, 47.1, 42.7, 38.3, 33.2, 32.7, 31.5, 31.5, 28.1, 28.0, 24.5, 24.4, 23.2, 19.3(3C), 14.3, 14.0. HRMS (ESI): Calcd. For C39H35ClF3N4O6 [M+H]1+: 747.2197; Found: 747.2207. LC-MS purity: 99%.
N-(4-chlorobenzyl)-7-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-4-yl)-N-(3,4,5-trifluorobenzyl)hept-6-ynamide (38)
1H NMR (400 MHz, CDCl3): δ 8.37 (d, J = 15.0 Hz, 1H), 7.71 (d, J = 7.2 Hz, 1H), 7.45 (d, J = 7.5 Hz, 1H), 7.34 (t, J = 7.6 Hz, 1H), 7.23 (m, 2H), 7.04 (d, J = 8.2 Hz, 1H), 6.98 (d, J = 8.3 Hz, 1H), 6.77 – 6.71 (m, 1H), 6.70 – 6.63 (m, 1H), 5.16 (dd, J = 13.3, 5.0 Hz, 1H), 4.50 – 4.24 (m, 6H), 2.74 (dt, J = 11.8, 5.1 Hz, 2H), 2.37 (m, 5H), 2.11 (dt, J = 8.7, 3.4 Hz, 1H), 1.83 (m, 2H), 1.59 (m, 2H). 13C NMR (101 MHz, CDCl3): δ 173.3, 171.4, 169.7, 169.0, 143.8, 135.3, 134.5, 134.4, 134.3, 133.8, 133.6, 131.6, 129.5, 129.3, 129.0, 128.3, 127.6, 123.3, 119.4, 112.02, 110.3, 95.3, 51.8, 49.9, 47.9, 47.6, 47.1, 32.6, 31.5, 28.1, 24.4, 23.3, 19.3. HRMS (ESI): Calcd. For C34H30ClF3N3O4 [M+H]1+: 636.1877; Found: 636.1882. LC-MS purity: 97%.
6.2. Cell culture
The human acute leukemia RS4;11 cell line was purchased from American Type Culture Collection (ATCC, CRL-1873), and cultured in RPMI-1640 media (Corning) supplemented with 10% fetal bovine serum (FBS), 1% Sodium Pyruvate, 1% Penicillin/Streptomycin, and 10 mM HEPES at 37°C under a humidified 5% CO2 atmosphere. The experiments were performed by using cells within 20 passages after purchasing.
6.3. Cell viability assay
Cells were seeded in a 96-well cell culture plate at a density of 2×104 cells with 100 μL cell culture media overnight. The cells were then treated with the indicated dose of compounds, and incubated at 37°C under a humidified 5% CO2 atmosphere for 72 h. After the incubation, 10 μL Alamar Blue (0.5mg/mL) was added into each well and incubated overnight. The plate was scanned at 570 nm and 600 nm. The absorbance was normalized to the DMSO-treated cells, and the IC50 was calculated by non-linear regression analysis using GraphPad Prism 7 software.
6.4. Immunoblot assay
Cells were treated with the indicated concentrations of compounds under the indicated time. Cells were collected and lysed with RIPA buffer after being washed with cold PBS twice. The supernatant was collected after centrifugation at 16,000 × g at 4 °C for 15 min. The protein concentration was determined using BCA (Bicinchoninic Acid) assay. 20 μg total protein was loaded and then separated by SDS-PAGE. Protein was transferred to polyvinylidene fluoride (PVDF) membranes, then blocked with 5% non-fat milk, and probed by following primary antibodies: MDM2 (D1V2Z) (1:1000, CST), p53 (DO-7) (1:1000, CST), PARP (46D11) (1:1000, CST), Caspase-3 (1:1000, CST), p21 Waf1/Cip 1(12D1) (1:1000, CST), α-Tubulin(1:10000, R&D), GSPT1 (14980, 1:1000, CST), Rb (9309, 1:1000, CST), phospho-Rb (8180, 9301, CST), β-Actin (K1418, 1:1000, Santa Cruz). The membrane was washed with 1X TBS-T 3 times and incubated with HRP linked secondary antibodies for 1 h at room temperature. The membrane was incubated with ECL (Bio-rad) for 5 min. The membrane was visualized by using a Bio-Rad Chemi-Doc MP imaging system. The intensity of the band was measured by Image J software.
6.5. Fluorescent polarization binding assay
The FP assay was performed in black 384-well microtiter plates at a final volume of 20 μL per well. Assay plates were prepared by addition of 10 μL of assay buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 0.01% TX-100) containing 100 nM GST-MDM2 (1–118) (Signal Chem) and 5 μL of 5-FAM labeled PMDM6-F (20 nM in assay buffer) (AnaSpec) to each well. For testing, a dilution series of small molecules (spanning 40 μM to 0.39 μM in assay buffer with 10% DMSO, in 1:2-dilution) was added (5 μL) by direct addition to the assay plate giving a test dilution series spanning 10 μM to 97 nM with a final concentration of 2.5 % DMSO. The assay mixture was incubated for 0.5 h at r.t. and the fluorescence polarization signal was measured on a Pherastar FS plate reader fitted with a 493-nm excitation filter, 517-nm static and polarized filters. Technical triplicate data was normalized to the positive (100% inhibition) and negative (0% inhibition) controls on the corresponding row of the 384-well plate (the percentage inhibition = 100 × (sample result – negative control)/(positive control mean – negative control)). Two to seven independent experiments of normalized data were combined into a data set, and then fit using a non-linear regression in GraphPad Prism with the formula log(inhibitor) vs. response – Variable slope (four parameters). Additionally, the residuals of the curve fit were plotted to determine the fit of the theoretical curve using GraphPad Software.
6.6. Proximity ligation assay (PLA)
Cells were seeded in the chamber slide and treated with MG132 (5μM) and indicated compounds. Cells were fixed with 4% formaldehyde for 20 min and permeabilized with 0.1% Triton X-100 in PBS for 10 min, washed, and then blocked using 2% BSA in PBS for 1 h. To detect the interaction between MDM2 and CRBN, MDM2 (SC-965, Santa Cruz) and CRBN (71810, Cell Signaling Technology) antibody from two different species were used for the primary antibody incubation. Chamber slide was incubated with primary antibodies overnight at 4 °C. The two corresponding PLA probes were mixed and diluted (1:5) in the antibody diluent. The mixture was equilibrated at room temperature for 20 min. The samples were washed with PBS+0.05% Tween twice, and then incubated with PLA probes MINUS and PLUS for 1 h at 37 °C. Then the probes were ligated with two other circle-forming DNA oligonucleotides by ligase for 30 min at 37 °C (two PLA probes were hybridized and joined to a closed circle if they are in close proximity). The DNA circle was amplified using fluorescently labeled oligonucleotides and polymerase for 90 min at 37 °C. The slides were washed and mounted using ProLong Gold antifade reagent (Invitrogen) with DAPI. Fluorescent signal amplification was detected using a confocal microscope.
6.7. Time-resolved fluorescent energy transfer (TR-FRET) assay
Titrations of compounds to induce DDB1/CRBN-CC885-MDM2 complex were carried out by mixing 200 nM biotinylated DDB1/CRBN (R&D), 200 nM GST-tagged MDM2 (R&D), 2 nM terbium-coupled streptavidin (Cisbio) and 100nM anti-GST-488 antibody (Cell signaling technology) in an assay buffer containing 50 mM HEPES, 100 mM Sodium Chloride (NaCl), 0.1 % Triton X-100 and 0.1% Bovine Serum Albumin (BSA). Full-length human DDB1/CRBN and MDM2 were used in all TR-FRET assays. After dispensing the assay mixture, increasing concentrations of compounds were dispensed in a 384-well microplate normalized to 2% DMSO. Before TR-FRET measurements were conducted, the reactions were incubated for 15 min at room temperature. After excitation of terbium fluorescence at 337 nm, emission at 490 nm (terbium) and 520 nm (Alexa Fluor 488) were recorded with a 70-μs delay over 600 μs to reduce background fluorescence, and the reaction was followed over 60 cycles of each data point using a PHERAstar FS microplate reader (BMG Labtech). The TR-FRET signal of each data point was extracted by calculating the 520/490 nm ratio.
Supplementary Material
Highlights.
A series of MDM2 degraders were designed, synthesized and evaluated in cell-based assays.
Degrader WB214 inhibits the proliferation of RS4;11 leukemia cells with an IC50 of 1.2 nM.
Degrader WB214 degrades MDM2 as a molecular glue, which also leads to the degradation of p53.
Degradation of GSPT1 induced by WB214 contributes to the inhibition of cell proliferation.
A selective degrader for GSPT1 over MDM2 was discovered.
Acknowledgements
We thank University of Wisconsin Carbone Cancer Center’s (UWCCC) Consultation Panel for support of this project. This work is also supported in part by NIH P30 CA014520-UW Comprehensive Cancer Center Support (CCSG) and NIH training grant to I.T. (T32 GM008688). This study made use of the Medicinal Chemistry Center at UW-Madison instrumentation, funded by the UW School of Pharmacy and the Wisconsin Alumni Research Foundation (WARF).
Footnotes
Conflicts of interest
A provisional patent application on some of the compounds was filed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ, Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation, Proc. Natl. Acad. Sci 98 (2001) 8554–8559. 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buckley DL, Van Molle I, Gareiss PC, Tae HS, Michel J, Noblin DJ, Jorgensen WL, Ciulli A, Crews CM, Targeting the von Hippel–Lindau E3 Ubiquitin Ligase Using Small Molecules To Disrupt the VHL/HIF-1α Interaction, J. Am. Chem. Soc 134 (2012) 4465–4468. 10.1021/ja209924v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, Bradner JE, Phthalimide conjugation as a strategy for in vivo target protein degradation, Science. 348 (2015) 1376–1381. 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Itoh Y, Ishikawa M, Naito M, Hashimoto Y, Protein Knockdown Using Methyl Bestatin−Ligand Hybrid Molecules: Design and Synthesis of Inducers of Ubiquitination-Mediated Degradation of Cellular Retinoic Acid-Binding Proteins, J. Am. Chem. Soc 132 (2010) 5820–5826. 10.1021/ja100691p. [DOI] [PubMed] [Google Scholar]
- [5].Hines J, Lartigue S, Dong H, Qian Y, Crews CM, MDM2-Recruiting PROTAC Offers Superior, Synergistic Antiproliferative Activity via Simultaneous Degradation of BRD4 and Stabilization of p53, Cancer Res. 79 (2019) 251–262. 10.1158/0008-5472.CAN-18-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ward CC, Kleinman JI, Brittain SM, Lee PS, Chung CYS, Kim K, Petri Y, Thomas JR, Tallarico JA, McKenna JM, Schirle M, Nomura DK, Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications, ACS Chem. Biol 14 (2019) 2430–2440. 10.1021/acschembio.8b01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spradlin JN, Hu X, Ward CC, Brittain SM, Jones MD, Ou L, To M, Proudfoot A, Ornelas E, Woldegiorgis M, Olzmann JA, Bussiere DE, Thomas JR, Tallarico JA, McKenna JM, Schirle M, Maimone TJ, Nomura DK, Harnessing the anti-cancer natural product nimbolide for targeted protein degradation, Nat. Chem. Biol 15 (2019) 747–755. 10.1038/s41589-019-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li L, Mi D, Pei H, Duan Q, Wang X, Zhou W, Jin J, Li D, Liu M, Chen Y, In vivo target protein degradation induced by PROTACs based on E3 ligase DCAF15, Signal Transduct. Target. Ther 5 (2020) 1–3. 10.1038/s41392-020-00245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang X, Crowley VM, Wucherpfennig TG, Dix MM, Cravatt BF, Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16, Nat. Chem. Biol 15 (2019) 737–746. 10.1038/s41589-019-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tong B, Luo M, Xie Y, Spradlin JN, Tallarico JA, McKenna JM, Schirle M, Maimone TJ, Nomura DK, Bardoxolone conjugation enables targeted protein degradation of BRD4, Sci. Rep 10 (2020) 15543. 10.1038/s41598-020-72491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu M, Liu T, Jiao Q, Ji J, Tao M, Liu Y, You Q, Jiang Z, Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway, Eur. J. Med. Chem 146 (2018) 251–259. 10.1016/j.ejmech.2018.01.063. [DOI] [PubMed] [Google Scholar]
- [12].Ohoka N, Tsuji G, Shoda T, Fujisato T, Kurihara M, Demizu Y, Naito M, Development of Small Molecule Chimeras That Recruit AhR E3 Ligase to Target Proteins, ACS Chem. Biol 14 (2019) 2822–2832. 10.1021/acschembio.9b00704. [DOI] [PubMed] [Google Scholar]
- [13].Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA, Ebert BL, Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells, Science. 343 (2014) 301–305. 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krönke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, Chamberlain PP, Mani DR, Man HW, Gandhi AK, Svinkina T, Schneider RK, McConkey M, Järås M, Griffiths E, Wetzler M, Bullinger L, Cathers BE, Carr SA, Chopra R, Ebert BL, Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS, Nature. 523 (2015) 183–188. 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matyskiela ME, Lu G, Ito T, Pagarigan B, Lu C-C, Miller K, Fang W, Wang N-Y, Nguyen D, Houston J, Carmel G, Tran T, Riley M, Nosaka L, Lander GC, Gaidarova S, Xu S, Ruchelman AL, Handa H, Carmichael J, Daniel TO, Cathers BE, Lopez-Girona A, Chamberlain PP, A novel cereblon modulator recruits GSPT1 to the CRL4 CRBN ubiquitin ligase, Nature. 535 (2016) 252–257. 10.1038/nature18611. [DOI] [PubMed] [Google Scholar]
- [16].An J, Ponthier CM, Sack R, Seebacher J, Stadler MB, Donovan KA, Fischer ES, pSILAC mass spectrometry reveals ZFP91 as IMiD-dependent substrate of the CRL4 CRBN ubiquitin ligase, Nat. Commun 8 (2017) 1–11. 10.1038/ncomms15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Donovan KA, An J, Nowak RP, Yuan JC, Fink EC, Berry BC, Ebert BL, Fischer ES, Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome, ELife. 7 (2018) e38430. 10.7554/eLife.38430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Matyskiela ME, Zhu J, Baughman JM, Clayton T, Slade M, Wong HK, Danga K, Zheng X, Labow M, LeBrun L, Lu G, Chamberlain PP, Thompson JW, Cereblon Modulators Target ZBTB16 and Its Oncogenic Fusion Partners for Degradation via Distinct Structural Degrons, ACS Chem. Biol (2020). 10.1021/acschembio.0c00674. [DOI] [PubMed] [Google Scholar]
- [19].Yamamoto J, Suwa T, Murase Y, Tateno S, Mizutome H, Asatsuma-Okumura T, Shimizu N, Kishi T, Momose S, Kizaki M, Ito T, Yamaguchi Y, Handa H, ARID2 is a pomalidomide-dependent CRL4 CRBN substrate in multiple myeloma cells, Nat. Chem. Biol (2020) 1–10. 10.1038/s41589-020-0645-3. [DOI] [PubMed] [Google Scholar]
- [20].Yamanaka S, Murai H, Saito D, Abe G, Tokunaga E, Iwasaki T, Takahashi H, Takeda H, Suzuki T, Shibata N, Tamura K, Sawasaki T, PLZF is a new substrate of CRBN with thalidomide and 5-hydroxythalidomide, BioRxiv. (2020) 2020.02.28.969071. 10.1101/2020.02.28.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bussiere DE, Xie L, Srinivas H, Shu W, Burke A, Be C, Zhao J, Godbole A, King D, Karki RG, Hornak V, Xu F, Cobb J, Carte N, Frank AO, Frommlet A, Graff P, Knapp M, Fazal A, Okram B, Jiang S, Michellys P-Y, Beckwith R, Voshol H, Wiesmann C, Solomon JM, Paulk J, Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex, Nat. Chem. Biol 16 (2020) 15–23. 10.1038/s41589-019-0411-6. [DOI] [PubMed] [Google Scholar]
- [22].Faust TB, Yoon H, Nowak RP, Donovan KA, Li Z, Cai Q, Eleuteri NA, Zhang T, Gray NS, Fischer ES, Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15, Nat. Chem. Biol 16 (2020) 7–14. 10.1038/s41589-019-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Słabicki M, Kozicka Z, Petzold G, Li Y-D, Manojkumar M, Bunker RD, Donovan KA, Sievers QL, Koeppel J, Suchyta D, Sperling AS, Fink EC, Gasser JA, Wang LR, Corsello SM, Sellar RS, Jan M, Gillingham D, Scholl C, Fröhling S, Golub TR, Fischer ES, Thomä NH, Ebert BL, The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K, Nature. (2020) 1–5. 10.1038/s41586-020-2374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mayor-Ruiz C, Bauer S, Brand M, Kozicka Z, Siklos M, Imrichova H, Kaltheuner IH, Hahn E, Seiler K, Koren A, Petzold G, Fellner M, Bock C, Müller AC, Zuber J, Geyer M, Thomä NH, Kubicek S, Winter GE, Rational discovery of molecular glue degraders via scalable chemical profiling, Nat. Chem. Biol (2020) 1–9. 10.1038/s41589-020-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].den Besten W, Lipford JR, Prospecting for molecular glues, Nat. Chem. Biol (2020) 1–2. 10.1038/s41589-020-0620-z. [DOI] [PubMed] [Google Scholar]
- [26].Schreiber SL, The Rise of Molecular Glues, Cell. 184 (2021) 3–9. 10.1016/j.cell.2020.12.020. [DOI] [PubMed] [Google Scholar]
- [27].Wang B, Wu S, Liu J, Yang K, Xie H, Tang W, Development of selective small molecule MDM2 degraders based on nutlin, Eur. J. Med. Chem 176 (2019) 476–491. [DOI] [PubMed] [Google Scholar]
- [28].Huang Y, Wolf S, Koes D, Popowicz GM, Camacho CJ, Holak TA, Dömling A, Exhaustive Fluorine Scanning towards Potent p53-Mdm2 Antagonists, ChemMedChem. 7 (2012) 49–52. 10.1002/cmdc.201100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nowak RP, DeAngelo SL, Buckley D, He Z, Donovan KA, An J, Safaee N, Jedrychowski MP, Ponthier CM, Ishoey M, Zhang T, Mancias JD, Gray NS, Bradner JE, Fischer ES, Plasticity in binding confers selectivity in ligand-induced protein degradation, Nat. Chem. Biol 14 (2018) 706–714. 10.1038/s41589-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zorba A, Nguyen C, Xu Y, Starr J, Borzilleri K, Smith J, Zhu H, Farley KA, Ding W, Schiemer J, Feng X, Chang JS, Uccello DP, Young JA, Garcia-Irrizary CN, Czabaniuk L, Schuff B, Oliver R, Montgomery J, Hayward MM, Coe J, Chen J, Niosi M, Luthra S, Shah JC, El-Kattan A, Qiu X, West GM, Noe MC, Shanmugasundaram V, Gilbert AM, Brown MF, Calabrese MF, Delineating the role of cooperativity in the design of potent PROTACs for BTK, Proc. Natl. Acad. Sci 115 (2018) E7285–E7292. 10.1073/pnas.1803662115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Skalniak L, Twarda-Clapa A, Neochoritis CG, Surmiak E, Machula M, Wisniewska A, Labuzek B, Ali AM, Krzanik S, Dubin G, Groves M, Dömling A, Holak TA, A fluorinated indole-based MDM2 antagonist selectively inhibits the growth of p53wt osteosarcoma cells, FEBS J. 286 (2019) 1360–1374. 10.1111/febs.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA, In Vivo Activation of the p53 Pathway by Small-Molecule Antagonists of MDM2, Science. 303 (2004) 844–848. 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- [33].Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S, Structure-Based Design of Spiro-oxindoles as Potent, Specific Small-Molecule Inhibitors of the MDM2−p53 Interacdon, J. Med. Chem 49 (2006) 3432–3435. 10.1021/jm051122a. [DOI] [PubMed] [Google Scholar]
- [34].[DUMMY_SPACE]Ding Q, Zhang Z, Liu J-J, Jiang N, Zhang J, Ross TM, Chu X-J, Bartkovitz D, Podlaski F, Janson C, Tovar C, Filipovic ZM, Higgins B, Glenn K, Packman K, Vassilev LT, Graves B, Discovery of RG7388, a Potent and Selective p53–MDM2 Inhibitor in Clinical Development, J. Med. Chem 56 (2013) 5979–5983. 10.1021/jm400487c. [DOI] [PubMed] [Google Scholar]
- [35].Vu B, Wovkulich P, Pizzolato G, Lovey A, Ding Q, Jiang N, Liu J-J, Zhao C, Glenn K, Wen Y, Tovar C, Packman K, Vassilev L, Graves B, Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development, ACS Med. Chem. Lett 4 (2013) 466–469. 10.1021/ml4000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun D, Li Z, Rew Y, Gribble M, Bartberger MD, Beck HP, Canon J, Chen A, Chen X, Chow D, Deignan J, Duquette J, Eksterowicz J, Fisher B, Fox BM, Fu J, Gonzalez AZ, Gonzalez-Lopez De Turiso F, Houze JB, Huang X, Jiang M, Jin L, Kayser F, (Jim) Liu J, Lo M-C, Long AM, Lucas B, McGee LR, McIntosh J, Mihalic J, Oliner JD, Osgood T, Peterson ML, Roveto P, Saiki AY, Shaffer P, Toteva M, Wang Y, Wang YC, Wortman S, Yakowec P, Yan X, Ye Q, Yu D, Yu M, Zhao X, Zhou J, Zhu J, Olson SH, Medina JC, Discovery of AMG 232, a Potent, Selective, and Orally Bioavailable MDM2–p53 Inhibitor in Clinical Development, J. Med. Chem 57 (2014) 1454–1472. 10.1021/jm401753e. [DOI] [PubMed] [Google Scholar]
- [37].Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP, Structure of the MDM2 Oncoprotein Bound to the p53 Tumor Suppressor Transactivation Domain, Science. 274 (1996) 948–953. 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- [38].Neochoritis CG, Atmaj J, Twarda-Clapa A, Surmiak E, Skalniak L, Köhler L-M, Muszak D, Kurpiewska K, Kalinowska-Tłuścik J, Beck B, Holak TA, Dömling A, Hitting on the move: Targeting intrinsically disordered protein states of the MDM2-p53 interaction, Eur. J. Med. Chem 182 (2019) 111588. 10.1016/j.ejmech.2019.111588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Matyskiela ME, Zhang W, Man H-W, Muller G, Khambatta G, Baculi F, Hickman M, LeBrun L, Pagarigan B, Carmel G, Lu C-C, Lu G, Riley M, Satoh Y, Schafer P, Daniel TO, Carmichael J, Cathers BE, Chamberlain PP, A Cereblon Modulator (CC-220) with Improved Degradation of Ikaros and Aiolos, J. Med. Chem 61 (2018) 535–542. 10.1021/acs.jmedchem.6b01921. [DOI] [PubMed] [Google Scholar]
- [40].Petzold G, Fischer ES, Thomä NH, Structural basis of lenalidomide-induced CK1α degradation by the CRL4 CRBN ubiquitin ligase, Nature. 532 (2016) 127–130. 10.1038/nature16979. [DOI] [PubMed] [Google Scholar]
- [41].Powell CE, Du G, Che J, He Z, Donovan KA, Yue H, Wang ES, Nowak RP, Zhang T, Fischer ES, Gray NS, Selective Degradation of GSPT1 by Cereblon Modulators Identified via a Focused Combinatorial Library, ACS Chem. Biol 15 (2020) 2722–2730. 10.1021/acschembio.0c00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bondeson DP, Smith BE, Burslem GM, Buhimschi AD, Hines J, Jaime-Figueroa S, Wang J, Hamman BD, Ishchenko A, Crews CM, Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead, Cell Chem. Biol 25 (2018) 78–87.e5. 10.1016/j.chembiol.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ishoey M, Chorn S, Singh N, Jaeger MG, Brand M, Paulk J, Bauer S, Erb MA, Parapatics K, Müller AC, Bennett KL, Ecker GF, Bradner JE, Winter GE, Translation Termination Factor GSPT1 Is a Phenotypically Relevant Off-Target of Heterobifunctional Phthalimide Degraders, ACS Chem. Biol 13 (2018) 553–560. 10.1021/acschembio.7b00969. [DOI] [PubMed] [Google Scholar]
- [44].Yang J, Li Y, Aguilar A, Liu Z, Yang C-Y, Wang S, Simple Structural Modifications Converting a Bona fide MDM2 PROTAC Degrader into a Molecular Glue Molecule: A Cautionary Tale in the Design of PROTAC Degraders, J. Med. Chem 62 (2019) 9471–9487. 10.1021/acs.jmedchem.9b00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.