Abstract
Introduction and importance
Coronavirus Disease 2019 (COVID-19) has become a pandemic since the beginning of 2020. COVID-19 is also spreading very rapidly in Indonesia and so far, no definitive therapy has been found.
Case presentation
We report two cases of confirmed COVID-19 with moderate pneumonia, who received 400 ml of convalescent plasma and showed improvements in clinical, laboratory and radiological examinations.
Clinical discussion
Passive immunotherapy is generally more effective when given early. Plasma transfusion is more beneficial when given before clinical conditions become severe. Some studies have shown that therapy with convalescent plasma can contribute to a longer survival and a lower length of stay.
Conclusion
Convalescent plasma can be used as an adjunctive therapy option for patients with moderate COVID-19.
Keywords: COVID-19, Convalescent plasma, Adjunctive therapy treatment, Case report
Highlights
-
•
COVID-19.
-
•
Convalescent Plasma.
-
•
Alternative treatment.
1. Introduction
Coronavirus Disease 2019 (COVID-19) is an infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 or SARS-CoV-2. Since its identification, this virus has quickly spread throughout China and other countries in the world. As a result, COVID-19 has become a global pandemic since the beginning of 2020 and has infected more than 161 million people and caused almost 3,4 millions death in the world as of May 18, 2021. Indonesia alone has contributed more than 1.64 million people infected and over 44,500 deaths [[1], [2], [3]].
People who are infected with this virus will usually experience respiratory problems which can present as mild, moderate, or severe infection and sometimes require special care. Although there are currently proven vaccines or therapies for COVID-19, the clinical management protocol of the disease recommended by the World Health Organization (WHO) focuses on infection prevention, monitoring, and detection [1,4].
Convalescent plasma or immunoglobulin therapy has been previously used to increase the survival rate in patients with SARS. In 2014, the WHO recommended the use of convalescent plasma as an empirical treatment in several outbreaks including the SARS virus and Middle East Respiratory Syndrome (MERS) pandemic in the Middle East in 2015, hemorrhagic fever such as the West African Ebola outbreak in 2014, Human influenza-A (H1N1) in 2009, and avian influenza-A (H5N1) in 2019 [1].
Many studies have shown a significant improvement in patients’ condition after convalescent plasma administration. A study in South Korea showed two cases of patients with COVID-19 treated with convalescent plasma who recovered and showed improvement in oxygen level and chest X-ray findings with decreased viral load and inflammatory markers [5]. Another study in China on patients with severe COVID-19 also showed improvement in clinical outcomes after convalescent plasma administration and no adverse effects reported [6]. The results of a study by Shen et al. on COVID-19 patients with critical condition also showed an improvement in clinical status [7]. Despite the promising results of these studies, several limitations should be considered such as the absence of large-scale randomized clinical trials with control groups and the fact that in some of these studies plasma transfusions were given in conjunction with the administration of antiviral drugs, which can be considered as a confounding factor [8].
In addition, the safety of using convalescent plasma to treat COVID-19 patients has not been confirmed. The results of a study on 5000 patients with COVID-19 who received convalescence plasma showed that less than 1% experienced serious side effects [9].
In this study, we report the administration of convalescent plasma to patients with moderate positive COVID-19 condition. This study has been reported in line with the SCARE 2020 criteria [10].
2. Methods
2.1. Donor eligibility
2.1.1. Inclusion criteria for convalescent plasma donor
-
1)
Candidate donors are aged 17–60 years
-
2)
Prioritization of men or women who have never been pregnant.
-
3)
Donor has been declared as a discarded COVID-19 patient which has been confirmed from two consecutive negative results in naso-oropharyngeal swab PCR examination.
-
4)
Donor is declared free from transfusion transmitted infection (TTI), including hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), and Syphilis.
-
5)
Donor has ABO and rhesus blood group compatible with plasma recipient candidates: The SARS-CoV-2 specific serum antibody titer is greater than 1: 320.
2.1.2. Exclusion criteria for convalescent plasma donor
-
1)
The donor candidate does not agree to participate in the study.
-
2)
Donor with incomplete clinical information during COVID-19 treatment
-
3)
Incompatible result from cross-match test.
-
4)
Donor has Hb < 12.5, hypotension, or positive pregnancy test result.
2.2. Criteria for recipients
Inclusion criteria for recipients:
-
1)
≥ 18 years old
-
2)
Tested positive for COVID-19 through polymerase chain reaction (PCR) examination from naso-oropharyngeal smear.
-
3)
Experiencing moderate degrees of pneumonia
Criteria for moderate degrees in COVID-19 [4,11,12]:
-
1)
Signs and symptoms associated with pneumonia.
-
2)
Does not require oxygen supplementation therapy.
-
3)
Does not meet any of the criteria for severe pneumonia.
Criteria for severe degrees in COVID-19 [4,11,12]:
-
1)
Shortness of breath with a respiration rate of ≥30 times per minute.
-
2)
Oxygen saturation (SpO 2) ≤ 93% in room air.
-
3)
PaO2/FiO2 ratio ≤300 mmHg.
-
4)
Worsening chest X-ray lesions >50% within 24–48 hours.
2.3. Specifications of plasma product
2.3.1. Neutralizing antibody titer in plasma donor
In this protocol, we recommend that the titer of SARS-CoV-2 neutralizing antibody should be more than 1:320. According to the American Food and Drug Administration (FDA), a convalescent plasma titer level >1:320 can significantly reduce mortality by 37% [13].
2.3.2. Convalescent plasma withdrawal
Competent personnel must take and handle the convalescent plasma from donors. Plasma donors must have a matched ABO blood group, matched rhesus group, and compatible cross match with the recipients. Donors must also be free from infectious disease and must have feasible antibody titer. The plasma must be labeled for convalescent plasma transfusion purposes and must also be specially labeled for COVID-19 patients. Collection time must also be written in the label [14].
2.3.3. Plasmapheresis
Plasmapheresis is a procedure in which donor blood is inserted in a device that separates plasma from blood components [15]. In this protocol, 400 ml convalescent plasma will be processed by plasmapheresis and leukoreduction followed by pathogen inactivation procedures.
2.4. Dosage
Convalescent plasma should be transfused into patients with COVID-19 using standard transfusion equipment. One or two units of convalescent plasma (approximately 400 ml total) can be given in 1 or 2 days to an adult patient according to their condition at a slow rate and the patient should be monitored during plasma administration for early detection of transfusion reactions or other adverse effects, especially within the first 15–20 minutes. The transfusion procedure is completed within 1–4 hours. Post-transfusion, clinical and laboratory conditions must be monitored to evaluate the progression of the disease [1].
2.5. Clinical and laboratory parameters before and after convalescent plasma transfusions
Patient monitoring is very important to evaluate before and after convalescent plasma transfusion. It is aimed to determine its safety and efficacy, to monitor clinical outcomes and to improve knowledge and understanding of convalescent plasma effects.
2.5.1. Clinical parameters
Clinical parameters such as vital signs including body temperature, The Sequential Organ Failure Assessment Score (SOFA score), and PaO2/FiO2 parameters are needed to assess clinical outcome as well as help to evaluate if adverse reactions such as hypersensitivity type I occur. High SOFA scores are associated with worsening disease [4].
2.5.2. Laboratory parameters
Laboratory parameters include complete blood count (CBC), evaluation of lung and kidney function, and inflammatory factors: D-Dimer, C-reactive protein (CRP) and procalcitonin. Several other additional parameters involve imaging such as Acute Respiratory Distress Syndrome (ARDS) findings in chest x-ray.
3. Research registration
This study has been registered in the Research Registry with unique identifying number (UIN): researchregistry6830.
4. Case presentation
This serial study reports two cases of patients with COVID-19 who received convalescent plasma. All patient were hospitalized in a quarantined COVID 19 Intensive Care Unit (ICU).
5. First case
A 66-year-old woman came with complaints of right limb weakness, shortness of breath, and cough. The patient denied any complaints of fever. On physical examination of the lungs, it was found that there were decreases in right pulmonary vesicular sounds and paralysis of the right upper and lower extremities. In the chest x-ray, there was a picture of bilateral pneumonia, especially on the right. The RT-PCR examinations of nasopharyngeal and/or oropharyngeal swab specimens for COVID-19 were positive. Based on this examination, the patient was diagnosed with confirmed COVID-19.
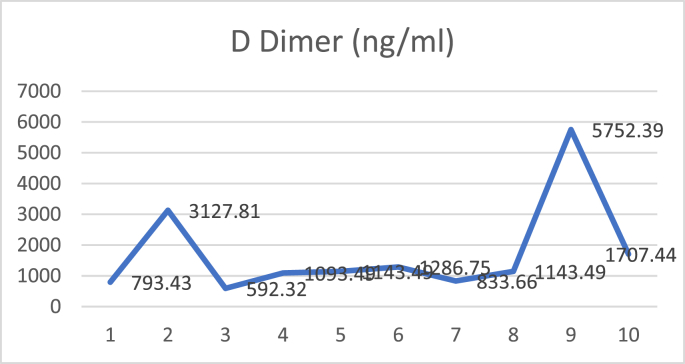
Patients received hydroxychloroquine therapy 400 mg every 24 hours, oseltamivir 75 mg per every 12 hours, N-acetylcysteine 5 g every 24 hours, azithromycin 500 mg every 24 hours, and vitamin C 2 g every 24 hours. Arixtra 2.5 mg every 24 hours was given because D-dimer was 3127 ng/ml. On the 7th day of hospitalization, chest X-ray showed there was an increase of right lung infiltrates, right upper lobe pulmonary atelectasis, and right pleural effusion. The PaO2/FiO2 ratio decreased from 265 to 116 without oxygen supplementation.
The patient then received 200 ml of convalescent plasma for 2 consecutive days on day 7 of treatment. The patient showed a marked improvement in respiratory distress after plasma transfusion. On the third day after plasma transfusion, complaints of shortness of breath decreased, the PaO2/FiO2 ratio increased to 200.9, and D-dimer decreased to 1286 ng/ml while chest X-ray showed persistent results. No side effects occurred with convalescent plasma therapy.
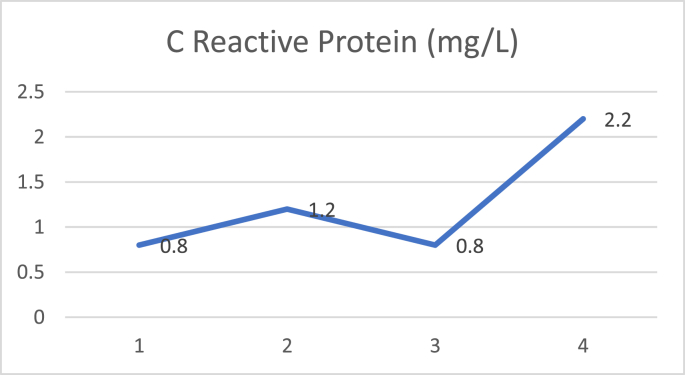
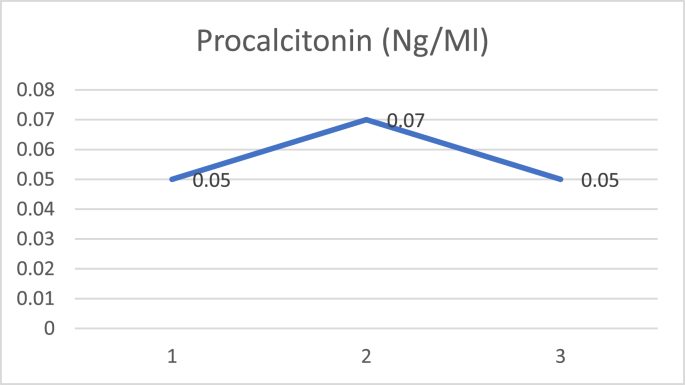
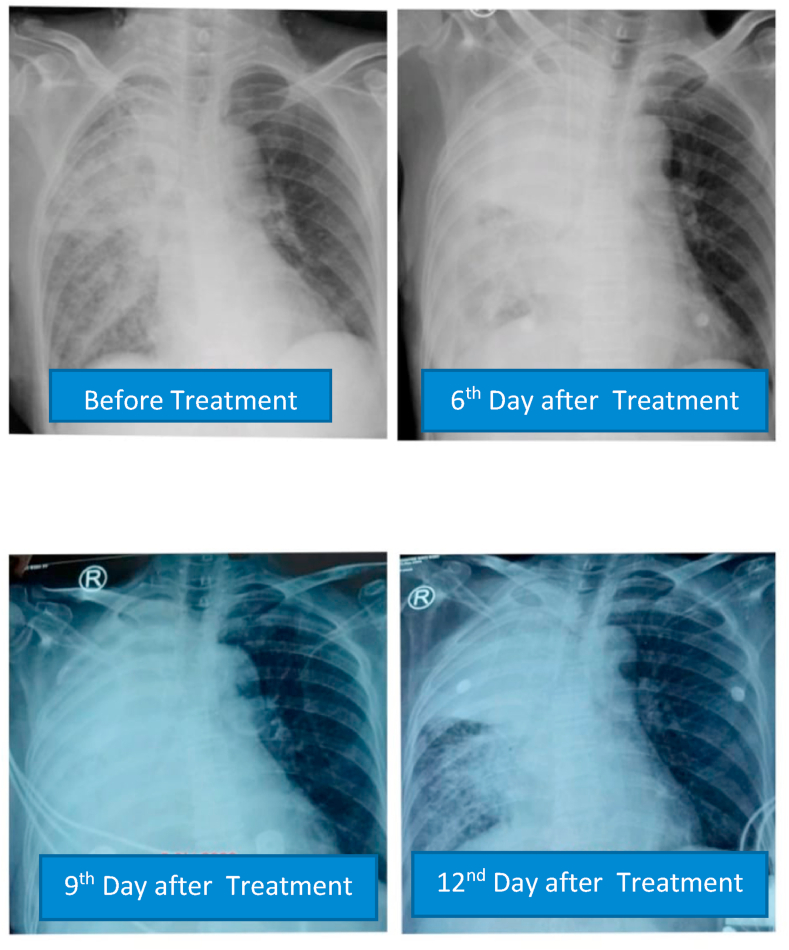
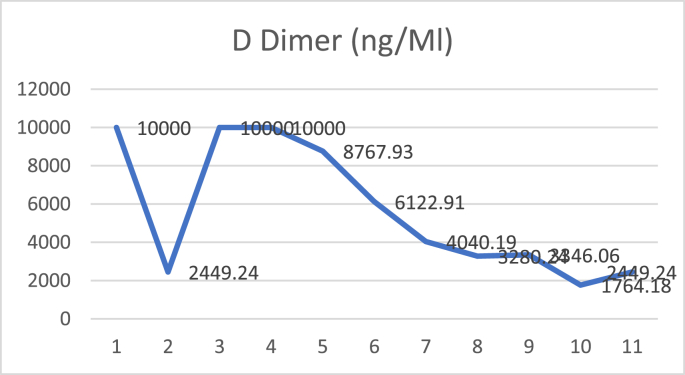
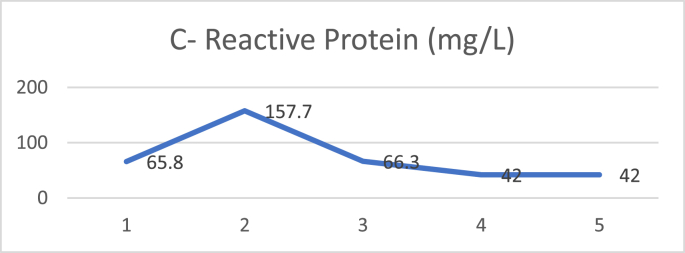
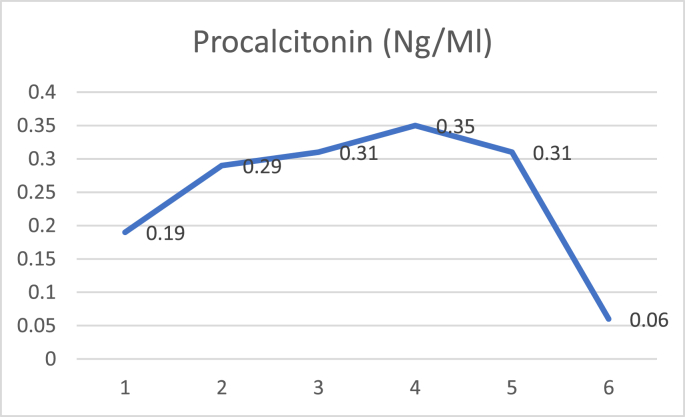
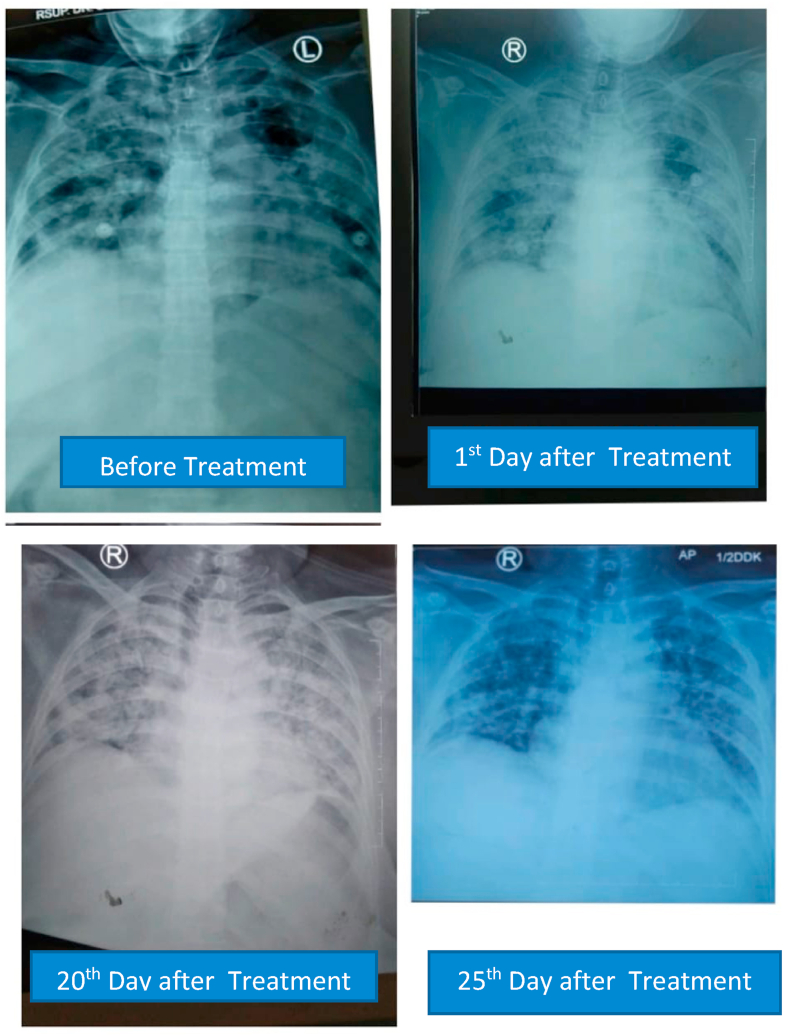
D dimer examination showed a value that fluctuated in levels that were more than normal during treatment (Fig. 1), and CRP examination during treatment was within normal limits (Fig. 2), while Procalcitonin examination during treatment was also within normal limits (Fig. 3). The chest X-ray showed improvement during treatment (Fig. 4).
Fig. 1.
D dimer.
Fig. 2.
C reactive protein.
Fig. 3.
Procalcitonin.
Fig. 4.
Serial postero-anterior chest X-ray.
The patient was discharged from the hospital after 22 days of treatment without any other complications. When the patient was discharged, all N, RdRp, and E genes were undetectable in upper respiratory tract secretions (CT cut-off value of 40).
6. Second case
A 56-year-old woman came to our hospital with a fever that had lasted for 3 days. In addition, there were also complaints of nausea and vomiting. SARS CoV-2 antibody IgM examination showed reactive results while IgG was non-reactive. On the chest X-ray, a typical COVID-19 pneumonia could be seen. The RT-PCR examinations of nasopharyngeal and/or oropharyngeal swab specimens were positive. Based on this examination, the patient was diagnosed with confirmed COVID-19.
Patients received hydroxychloroquine therapy 400 mg every 24 hours, oseltamivir 75 mg every 12 hours, azithromycin 500 mg every 24 hours, cefoperazone sulbactam 1 g every 8 hours, N-acetylcysteine 5 g every 24 hours, and vitamin C 2 g every 24 hours. After five days of treatment, complaints of shortness of breath increased with PaO2/FiO2 58.9 and D-Dimer level of >10,000 ng/ml. The patient received 200 ml of convalescent plasma for 2 consecutive days and 10,000 IU of heparin per 8 hours subcutaneously. The patient showed marked improvement in respiratory distress and febrile symptoms after plasma transfusion.
On the second day after plasma transfusion, there was improvement in the patient's fever condition, and an increase in the PaO2/FiO2 ratio while the chest X-ray showed no improvement nor deterioration. There were no significant acute side effects observed during convalescent plasma administration. D-dimer examination showed a decreasing trend after convalescent plasma administration (Fig. 5), and CRP examination during treatment showed a decreasing trend (Fig. 6), while procalcitonin assay during treatment was also within normal limits (Fig. 7). The chest X-ray showed improvement during treatment (Fig. 8).
Fig. 5.
D dimer.
Fig. 6.
C reactive protein.
Fig. 7.
Procalcitonin.
Fig. 8.
Serial postero-anterior chest X-rays.
7. Discussion
Passive immunotherapy is generally more effective when given early and can be effective even at low doses. Plasma transfusion is more beneficial when given before clinical conditions deteriorate [14]. Other literature also stated that earlier convalescent plasma transfusions provide good outcomes [8]. Additional studies have shown that therapy with convalescent plasma can contribute to a longer survival and a lower length of stay in the ICU [16].
In our study, there was a difference in time of administration of the convalescence plasma, the first patient on day 7 and the second patient on day 5, which was due to the availability that was not always ready every time it was needed. Also the first case did not received cefoperazone sulbactam, but the 2nd did because the results of the sputum culture of the 2nd case showed indications of Klebsiella pneumonia infection, Meanwhile, in the first patient, the bacterial culture did not grow positive results in the first patient.
Neutralizing antibodies produced by humoral immunity have an important role in reducing disease severity and viral replication, and can help in treating and eliminating the viral infections. If a specific neutralizing antibody of a virus is given to a person who is infected by the virus, this may decrease viral replication and the severity of disease [17]. Normally a peak in viral load occurs in the first week of infection and seroconversion in SARS-Cov 2 occurs 10–14 days after onset [18]. Many studies have shown that SARS-CoV-2 seroreactivity is present in the majority of patients who have recovered regardless of clinical severity and onset during infection. A study in China showed that seropositivity in 70 patients with confirmed COVID-19 reached 100% within 20 days of onset, 52% had antibody titers between 1:64–1:512 and 47% had titers 1:4000–1:40,000, in which the antibody titer peaks at 31–40 days and lasts 41–53 days from onset, then decreases slowly [19]. Another study stated that 39 from 40 patients who recovered from COVID-19 had received convalescent plasma and showed a SARS-Cov-2 antibody titer of more than 1:160, and only one had an antibody titer of 1:326. The results of another study found that only one out of 64 patients who donated convalescent plasma had a SARS-CovV-2 antibody titer of 1:160, while the others had a titer >1:320[20].
The assessment of viral neutralizing antibodies is an important parameter because it indicates the number of antibodies which can neutralize the virus. Titrations for neutralizing antibodies in plasma donor recipients should be able to identify the donor who has efficient neutralizing antibody titer which can be used to evaluate the efficacy of convalescent plasma transfusions.
C-Reactive Protein (CRP) values have been reported to be an important marker that changes significantly in patients with COVID-19. This increase may be related to the severity and progression of the disease. Results from studies in China show that the CRP value increases during the first 7 days while in the hospital, and this can be used as a parameter to predict the progression of the disease. In addition, another study reported that patients who died from COVID-19 had a CRP level of 10x higher than those who had recovered [21]. In the cases reported in this series, both patients also had high CRP levels. For the first case, there was a decrease in the CRP level after convalescent plasma transfusion. Whereas in the second case, the CRP level was still fluctuating even though convalescent plasma had been given. This might be influenced by the severity of the disease.
Recent reports have shown an increase in D-dimer in patients with COVID-19 (36–43%) with higher levels found in severe disease thus correlating with worse disease progression [22]. In this case, the D-dimers in both patients also increased significantly. Several studies in China also showed an increase in D-dimer 2.5–5x higher in severe disease cases [21,23,24]. The results of a study conducted by Zhou et al. revealed that deceased COVID-19 patients had a D-Dimer level 9x higher than those who recovered [25].
A study conducted in China on 10 COVID-19 patients with severe conditions showed clinical improvement, and decreased viral load until it turned negative on the 2nd and 3rd day after transfusion. Additionally, other good outcomes indicated that 3 patients were discharged from the hospital and 7 patients experienced improvement [6]. Another study in a patient with critical condition also showed a clinical improvement, and a decrease in viral load which turned negative within 12 days after transfusion and an increase in antibody titers to COVID-19 with 40–60 before transfusion and 80–320 on day 7 after transfusion [7]. Meanwhile, a randomized control trial showed that there was no significant difference in mortality rates for patients with severe and life-threatening levels, between patients who were given conventional plasma therapy and controls [26].
8. Conclusions
There are limitations to the current therapy for COVID-19, so convalescent plasma is being used as an adjunctive therapy option for COVID-19. However, more parameter data and long-term follow up are needed to assess the safety and effectiveness of convalescent plasma treatment.
Ethical approval
The study is exempt from ethical approval in our institution.
Funding
No source of funding.
Author contributions
AT,WSH, JAT, ZN, K, IJ, AS, AND, IS and SR concepted and designed this study, AT, ZN, AND, IS and SR analysized and interpretated of the data; AT, ZN, AND, IS and SR the drafted of the paper, AT, ZN, AND, IS and SR revised it critically for intellectual content; AT,WSH, JAT, ZN, K, IJ, AS, AND, IS and SR finalized approval of the version to be published; and that all authors agree to be accountable for all aspects of the work.
Declaration of competing interest
The authors declare no conflict of interest.
Research registration
This study has been registered in Research Registry with unique identifying number (UIN): researchregistry6830.
Guarantor
Achmad Thabrani is the guarantor and accepts full responsibility.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Consent
Written informed consent was obtained from the patient for publication of this study and accompanying images. A copy of written consent is available for review by Editor-in-Chief of this journal on request.
Acknowledgement
1. We thank the staff of Klinik Bahasa for their help during manuscript preparation.
2. We thank to dr Budi Santoso Sp. An(K) and Mr. Muhammad Yasir Sudarno, S. Kep.Ners for their help during study and manuscript preparation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102444.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chen L., Xiong J., Bao L.S.Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization W.H. Epidemiological update - 22 December. World Heal Organ. 2020;(December):1–3. [Google Scholar]
- 3.Dwi Putera D., Suci Hardianti M. Efficacy and safety of convalescent plasma therapy in patients with COVID-19: a rapid review of case series. J thee Med Sci (Berkala Ilmu Kedokteran) 2020;52:134–147. 03. [Google Scholar]
- 4.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance 28 January 2020. Who [Internet] 2020. WHO/2019-nCoV/clinical/2020.5%0ACC BY-NC-SA 3.0 IGO%0AWHO/2019-nCoV/clinical/2020.5%0ACC BY-NC-SA 3.0 IGO%0Ahttps://apps.who.int/iris/handle/10665/330893e January):10. Available from:
- 5.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J. Use of convalescent plasma therapy in two covid-19 patients with acute respiratory distress syndrome in Korea. J. Kor. Med. Sci. 2020;35(14) doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA, J. Am. Med. Assoc. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan M.O., Osman A.A., Elbasit H.E.A., Hassan H.E., Rufai H., Satti M.M.M. Convalescent plasma as a treatment modality for coronavirus disease 2019 in Sudan. Transfus. Apher. Sci. 2020;59(6) doi: 10.1016/j.transci.2020.102918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84(Cdc):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Burhan E., Susanto A.D., Nasution S.A. third ed. 2020. Pedoman Tatalaksana COVID-19. [Google Scholar]
- 12.Bodini G., Demarzo M.G., Casagrande E., De Maria C., Kayali S., Ziola S. Concerns related to COVID-19 pandemic among patients with inflammatory bowel disease and its influence on patient management. Eur. J. Clin. Invest. 2020;50(5):2020–2022. doi: 10.1111/eci.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA Fda Issues emergency use authorization for convalescent plasma as potential promising COVID–19 treatment, another achievement in administration's fight against pandemic. Fda [internet] 2020. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment 1–4. Available from:
- 14.Lanza F., Seghatchian J. Reflection on passive immunotherapy in those who need most: some novel strategic arguments for obtaining safer therapeutic plasma or autologous antibodies from recovered COVID-19 infected patients. Br. J. Haematol. 2020;190(1):e27–e29. doi: 10.1111/bjh.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz J., Padmanabhan A., Aqui N., Balogun R.A., Connelly-Smith L., Delaney M. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the writing committee of the American society for apheresis: the seventh special issue. J. Clin. Apher. 2016;31(3):149–162. doi: 10.1002/jca.21470. [DOI] [PubMed] [Google Scholar]
- 16.Erkurt M.A., Sarici A., Berber İ., Kuku İ., Kaya E., Özgül M. Life-saving effect of convalescent plasma treatment in covid-19 disease: clinical trial from eastern Anatolia. Transfus. Apher. Sci. 2020;59(5):1–6. doi: 10.1016/j.transci.2020.102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawar A.Y., Hiray A.P., Sonawane D.D., Bhambar R.S., Derle D.V., Ahire Y.S. Convalescent plasma: a possible treatment protocol for COVID- 19 patients suffering from diabetes or underlying liver diseases. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):665–669. doi: 10.1016/j.dsx.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Q.L., Yu Z.J., Gou J.J., Li G.M., Ma S.H., Zhang G.F. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J. Infect. Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Guo X., Xin Q., Pan Y., Hu Y., Li J. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin. Infect. Dis. 2020;71(10):2688–2694. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L., Yang R., Wang J., Lv Q., Ren M., Zhao L. Feasibility of a pilot program for COVID-19 convalescent plasma collection in Wuhan, China. Transfusion. 2020;60(8):1773–1777. doi: 10.1111/trf.15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA, J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 23.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.