Abstract
In this study, we have successfully synthesized magnetic nanoparticles (MNPs), functionalised them by silanization and used them for the covalent immobilization of a recombinant small laccase (rSLAC) from Streptomyces coelicolor. The immobilized recombinant laccase (MNP-rSLAC) was subsequently used for the treatment of phenol, 4-chlorophenol (4-CP) and 4-fluorophenol (4-FP). The enzyme completely degraded 80 µg/mL of the selected phenolic compounds within 2 h in the presence of a natural mediator, acetosyringone. The MNP-rSLAC retained > 73% of initial activity (2,6-dimethoxyphenol as substrate) after 10 catalytic cycles and could be easily recovered from the reaction mixture by the application of magnetic field. Furthermore, immobilised rSLAC exhibited better storage stability than its free counterpart. The Michaelis constant (Km) value for the immobilised rSLAC was higher than free rSLAC, however the maximum velocity (Vmax) of the immobilised SLAC was similar to that of the free rSLAC. Growth inhibition studies using Escherichia coli showed that rSLAC-mediated treatment of phenolic compounds reduced the toxicity of phenol, 4-CP and 4-FP by 90, 60 and 55%, respectively. Interestingly, the presence of selected metal ions (Co2+, Cu2+, Mn2+) greatly enhanced the catalytic activity of rSLAC and MNP-rSLAC. This study indicates that immobilized small laccase (MNP-rSLAC) has potential for treating wastewater contaminated with phenolic compounds.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02854-0.
Keyword: Bioremediation, Immobilization, Magnetic nanoparticles, Phenolic pollutants, Small laccase
Introduction
The extensive use of phenolic compounds mainly as herbicides, flame retardants, pesticides, biocides and wood preservatives (Dai et al. 2015) has led to their detection in surface water. Removal of these compounds from the contaminated water is necessary due to their low biodegradability (Pera-Titus et al. 2004) and toxicity (Liu et al. 2012) as well as the pressure to comply with stringent standards against their disposal (Karam and Nicell 1997). Laccases have been extensively investigated as potential candidates for the remediation of several pollutants (Srinivasan et al. 2019, 2020). Laccases are multi-copper oxidases which have been gaining a lot of attention mainly due to their ability to catalyse the oxidation of a broad range of substrates without the aid of any cofactors (Upadhyay et al. 2016). They use molecular oxygen as an electron acceptor during substrate oxidation, leading to the formation of reactive radicals. The radicals may polymerise and precipitate out of the solution which facilitates the removal of the pollutant. Alternatively, they can cleave covalent bonds such as C–C, C–O, sometimes assisted by oxidised mediators, leading to the breakdown of pollutants (Huber et al. 2018; Kudanga et al. 2011). In this sense, laccases, due to their relative promiscuity, can be applied in the bioremediation of several pollutants (Zdarta et al. 2018), including phenolic pollutants. However, there are a number of limitations associated with their use as free enzymes which include large consumption, difficulty in separation from the reaction mixture and low recycling capability (Fernandes et al. 2017; Ranjan et al. 2018). Furthermore, low stability and potential contamination of the free enzyme in wastewater (Rotková et al. 2009) restricts the industrial use of free laccase in wastewater treatment plants (WWTPs).
Alternatively, enzyme immobilisation can solve the challenges associated with free enzymes and enables enzyme recovery, reusability and tolerance towards extreme conditions (Gianolini et al. 2020). Enzyme immobilization can be achieved by physical adsorption or covalent bonding (Mohamad et al. 2015). Adsorption is a relatively simple method, however, leaching of the enzyme from the immobilized matrix after a few cycles limits its application (Sheldon and Woodley 2018). In contrast, covalent binding provides an advantage over the adsorption method by stabilising strong bonding between the enzyme and the matrix via a chemical reaction, thereby minimising enzyme leaching (Faridi et al. 2017). Among the several immobilization matrices that have been studied, magnetic nanoparticles (MNPs) have proven to be a promising candidate for the immobilization of enzymes (Ranjan et al. 2018). They possess unique properties such as superparamagnetism, low toxicity, large surface area-to-volume ratio, biocompatibility, and ease of separation by the application of an external magnetic field (Fortes et al. 2017; Mohamed et al. 2017; Pereira et al. 2017; Selvam et al. 2016; Srinivasan et al. 2020). However, formation of agglomerates due to magnetic dipole interactions (Villa et al. 2016) and the unstable nature (in acidic medium) of naked iron magnetic particles, could greatly affect their longevity and reusability.
Functionalisation via chemicals and biological materials could enhance and optimise the surface properties of MNPs such as dispersibility, biocompatibility and biodegradability (Xu et al. 2014). In addition, surface functionalisation provides the anchoring surface which mediates the attachment of functional groups, leading to an engineered surface with a selective affinity towards diverse bio-compounds (Seyed et al. 2015). In one recent study, polydopamine (PDA) coating was used to functionalize Fe3O4 nanoparticles for immobilization of a fungal laccase (Zhang et al. 2017). However, the high cost of dopamine could potentially impact its usage in industrial catalysis (Wang et al. 2016). Silanization is a viable alternative owing to its responsivity, high stability, low cytotoxicity as well as the ease of surface chemical transformation. In addition, no specialized equipment is required, making silanization an ideal method for functionalization (Faridi et al. 2017). Despite these favourable properties, silanization has not been used in the immobilization of laccase.
Therefore, the aim of this study was to investigate silanization for surface functionalization of MNPs followed by immobilization of rSLAC onto the functionalized MNPs. The immobilized rSLAC was then applied in the treatment of selected phenolic pollutants.
Materials and methods
Materials
Phenol, 4-chlorophenol (4-CP), 4-fluorophenol (4-FP), 4-aminoantipyrine (4-AAP), potassium ferricyanide, 2,6-dimethoxyphenol (2,6-DMP) and acetosyringone (AS) were purchased from Sigma-Aldrich (South Africa). Orthophosphoric acid and acetonitrile were purchased from Merck (South Africa). All chemicals were of analytical grade and were used without any further purification. The rSLAC was produced and purified as previously described (Yadav et al. 2018).
Determination of laccase activity and protein concentration
Laccase activity was determined using 2,6-DMP as a substrate, as previously described (Yadav et al. 2018). The oxidation of 2,6-DMP (2 mM) was analysed by measuring the increase in absorbance at 477 nm (ε = 14,800 M−1 cm−1) in Tris–HCl buffer (20 mM, pH 8.0). One unit of laccase activity was defined as the amount of enzyme required for the oxidation of 1 µmol of substrate per minute. Protein concentration was determined by the Lowry method (Lowry et al. 1951) using bovine serum albumin as a standard.
Synthesis and silanization of MNPs
MNPs were synthesized by a chemical co-precipitation method as previously described (Ranjan et al. 2017). Synthesized MNPs were collected by the application of a magnetic field and washed with ethanol followed by Milli-Q water. Finally, MNPs were dried under vacuum overnight at 60 °C. Surface modification of the dried MNPs was carried out using (3-aminopropyl)-triethoxysilane (APTES) as described by Faridi et al. (2017). One gram of MNPs were dispersed in a mixture of 20% (w/v) APTES and 4 mL glycerol, and the resulting mixture was heated at 90 °C for 2 h under nitrogen sparging and stirring. The mixture was then cooled, sonicated for 10 min and the modified MNPs collected using a magnet followed by washing with ethanol and Milli-Q water and dried overnight under vacuum at 60 °C.
Immobilization of rSLAC on modified MNPs
The carboxyl groups of rSLAC were treated with 3-(3 dimethylaminopropyl) N′-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS) as described by Faridi et al. (2017). Briefly, 3 mg of EDC was added to a 5 mL solution of rSLAC and the mixture was incubated at room temperature (22 ± 3 °C) for 1 h with gentle shaking followed by the addition of 3 mg of NHS. Thereafter, 10 mg of MNPs were added to the mixture and the incubation was continued for a further 3 h. The binding efficiency of rSLAC onto silanized MNPs was determined by the difference in concentration of the free enzyme after immobilization against the initial enzyme concentration (Faridi et al. 2017).
Characterization of MNPs
The presence of functional groups on the surface of MNPs was assessed by FT-IR spectroscopy (Perkin Elmer, USA). The morphology of MNPs was studied by field emission scanning electron microscopy (FE-SEM) using a Zeiss Gemini instrument (Germany).
Biochemical and kinetic characterization of free and immobilized rSLAC
The optimum pH for the activity of free and immobilized rSLAC was determined by conducting enzyme assays in the pH range 5.0–9.0, in 1.0 pH value increments [20 mM sodium acetate buffer (pH 5.0), 20 mM Tris–HCl buffer (pH 6.0–8.0) and 20 mM glycine–NaOH buffer (pH 9.0)] at 80 °C. Optimum temperature for free and immobilized rSLAC was determined by performing enzyme assays at various temperatures (40 to 100 °C) at pH 8.0. The enzyme activity was presented in terms of relative activity, with the highest catalytic activity set at 100%.
Kinetic parameters (Km and kcat) were determined using 2,6-DMP (50–2000 µM) as a substrate for the immobilized rSLAC and compared with the free rSLAC (Yadav et al. 2018). The reactions were carried out in Tris–HCl buffer (20 mM, pH 8.0) and oxidation of 2,6-DMP was determined at 477 nm (ε = 14,800 M−1 cm−1). The experiments were performed in triplicate.
Effect of metal ions and organic solvents on the catalytic activity of free and immobilized rSLAC
The effect of different metal ions (Li+, Co2+, Cu2+, Mn2+, Ca2+, Mo6+, Al3+, Mg2+) on the catalytic activity of free and immobilized rSLAC was evaluated. Initially, enzymes were pre-incubated in presence of 10 and 30 mM of individual metal ions for 30 min (Sondhi et al. 2014) and thereafter enzyme activity was measured using 2,6-DMP as substrate as previously described. The enzyme activities of free and immobilized rSLAC without metal ions were considered as controls (100% activity). Similarly, the enzyme (free or immobilized) was pre-incubated for 30 min in organic solvents (20 and 50% of methanol, ethanol, acetone, dioxane and DMSO) and the residual activity determined using 2,6 DMP as substrate. All experiments were performed in triplicate.
Storage stability and reusability of the immobilized rSLAC
The storage stability of the free and immobilized rSLAC was evaluated by storing the enzyme in Tris–HCl buffer (20 mM, pH 8) at 4 °C for 30 days. Sampling was done after every 6 d and the enzyme activity was determined using 2,6-DMP as a substrate. Enzyme activity at time zero was taken as 100% relative activity.
The reusability of the immobilized rSLAC was evaluated by recovering the MNP-rSLAC with a magnet after each cycle and washing three times with Tris–HCl buffer (20 mM, pH 8.0) to prepare for the subsequent cycle. Enzyme activity was determined using 2,6-DMP as the substrate. The enzyme activity for the first cycle was considered as 100% and relative activities of the subsequent cycles were calculated with respect to the activity of the first cycle.
Treatment of phenol, 4-CP and 4-FP by free and immobilized rSLAC
The rSLAC (2 U) was added to a reaction mixture (1 mL total volume) consisting of Tris–HCl buffer (50 mM, pH 8.0), phenols or halogenated phenols (80 µg/mL each), and AS (8 µg/mL) as a mediator, and the mixture was incubated for 4 h at 37 °C in the dark with gentle shaking (50 rpm). A negative control was also set in parallel where a denatured enzyme (in case of free rSLAC) and bare MNPs (in case of immobilized rSLAC), were used and incubated under identical conditions. Samples were collected every 10 min to analyse the residual concentrations of phenolic compounds by colorimetric and HPLC analysis. Furthermore, the effects of pH (4.0–8.0), mediator concentrations (2–10 µg/mL) and reaction time, on the degradation of phenolic compounds (80 µg/mL each), were studied.
Analysis of the residual phenolic compounds by a colorimetric method
Residual phenolic compound concentrations were measured using a simple colorimetric assay as described by Modaressi et al. (2005). The assay is based on the electrophilic attack of the phenolic compound by a primary amine (4-AAP) under alkaline conditions, and its subsequent oxidation by potassium ferricyanide to a red quinone-type dye (Abdollahi et al. 2018). The reaction mixture consisted of 700 µL of Tris–HCl buffer (100 mM, pH 8), 300 µL laccase treated phenolic compounds, 10 µL 4-AAP (0.1 M) and 10 µL potassium ferricyanide solution (0.2 M) and was incubated at 25 °C for 15 min, with gentle shaking (50 rpm). The extent of degradation of the compounds was determined spectrophotometrically as the relative decrease of the maximum absorbance at 510 nm. Percentage degradation of the phenolic compounds was calculated according to Singh et al. (2017) as follows:
where Ai is the initial absorbance of the phenolic compounds and Af is the final absorbance of the phenolic compounds (after rSLAC treatment).
Analysis of the residual phenolic compounds by HPLC
HPLC analysis was performed using a Shimadzu HPLC system from Dionex (Massachusetts, USA) equipped with a P580 pump, an ASI-100 autosampler and a PDA-100 photodiode array detector. Chromatographic separation was achieved on C18 column (Sunfire, Waters, Ireland) using 0.1% orthophosphoric acid (A) and acetonitrile (B) as mobile phase, flow rate 1.0 mL/min, injection volume of 10 µL and an oven temperature of 40 °C. The gradient was set up as follows: 2% B to 100% B (0–20 min), 100% B to 2% B (20–22 min), 2% B (22–30 min). The peaks for phenolic compounds as well as the transformation products were observed at 254 nm.
Toxicity analysis
Reduction in toxicity of phenolic compounds after rSLAC treatment was monitored by bacterial growth inhibition studies as described by Murugesan et al. (2010) using Escherichia coli ATCC 25922 as a test organism. Individual phenolic compounds (80 µg, total volume of 900 µL) and their degradation products (total volume of 900 µL) were incubated separately in sterile nutrient broth (9 mL) with 100 µL of 0.1 OD600 overnight culture of E. coli ATCC 25922. The test tubes were incubated with shaking (200 rpm) in the dark at 37 °C and bacterial growth was monitored spectrophotometrically at 600 nm. Results were presented as percentage growth inhibition relative to the control sample (containing 9 mL nutrient broth 100 µL of 0.1 OD600 overnight culture of E. coli ATCC 25922 and 900 µL saline).
Results and discussion
Characterization of MNPs and immobilized MNPs
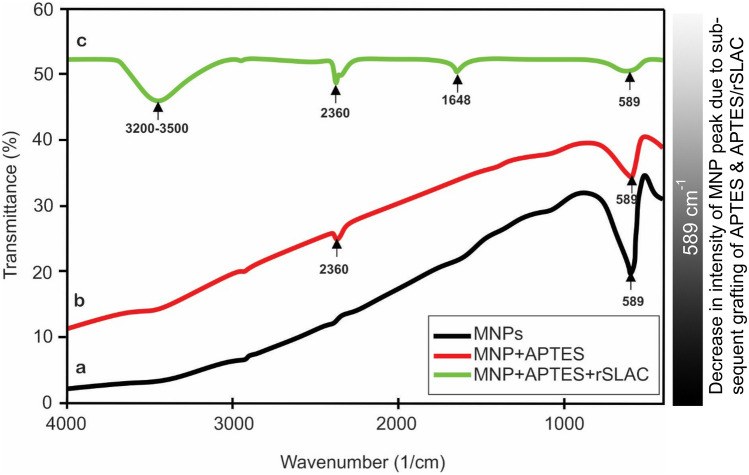
FE-SEM images of bare and silanized MNPs showed no evidence of morphological changes after silanization. Further, FE-SEM images of MNPs also revealed that their diameter was in the range of 50–200 nm (Fig. 1). Fig. S4 shows the typical High-Resolution Transmission Electron Microscopy (HR-TEM) image of MNPs, whereby MNPs appear to be nearly spherical in shape and exhibits a homogenous distribution, along with a noticeable agglomeration owing to the attractive forces between them. FT-IR spectra confirmed the presence of characteristic peaks for MNP, MNP/APTES and MNP/APTES/rSLAC (Fig. 2). A high-intensity peak at 589 cm−1 was observed in uncoated nanoparticles, which is a characteristic peak for MNPs (Zhang et al. 2007). Interestingly, we also observed that the intensity of MNP peaks (589 cm−1) decreased after subsequent grafting of APTES and APTES/rSLAC, confirming the successful grafting of APTES and rSLAC onto the MNPs (Fig. 2). The appearance of a peak in the region of 2360 cm−1 is due to Si–H silane (Fig. 2b, c), which further confirmed the grafting of APTES onto MNPs. The appearance of a peak at 1648 cm−1 in MNP/APTES/rSLAC (Fig. 2c) could be due to the CO-NH2 group, which confirmed the formation of bond between enzyme and Si-MNP (Faridi et al. 2017). The appearance of a broad peak obtained at 3200–3500 cm−1 in all three spectra depicts the presence of hydroxyl groups on the surface of nanoparticles (Ma et al. 2003). Furthermore, we also noticed that the peak intensity at 3200–3500 cm−1 has subsequently increased after grafting of APTES and APTES/rSLAC onto MNPs, which could be due to the physical and chemical adsorption of water molecules after each coating. Thus, peaks shown by FT-IR confirmed the successful synthesis of MNPs and grafting of APTES and rSLAC onto the surface of nanoparticles.
Fig. 1.

FE-SEM images showing surface morphology of a bare and b functionalized MNPs
Fig. 2.
FTIR spectra of MNPs (black), MNP + APTES (red) and MNP + APTES + rSLAC (green)
Effect of pH and temperature on the catalytic activity of free and immobilized rSLAC
The catalytic activity of free and immobilized rSLAC was evaluated in the pH range 5.0—9.0 and maximum activity was observed at pH 8.0 for both free and immobilized enzyme (Fig. 3a). However, the immobilized enzyme showed higher catalytic activity at pH 5.0 and 6.0 compared to the free enzyme. The enhanced catalytic activity over a broad pH range could be attributed to the MNP support. Similar results have been observed by Zhang et al. (2017), who also reported that the immobilized enzyme showed a broader pH-activity profile. The optimum temperature for both enzymes was 80 °C (Fig. 3b). It was also observed that the immobilized rSLAC retained higher catalytic activity (~ 20%) at 100 °C compared to the free enzyme, which suggests enhanced thermostability after immobilization. Elevated temperature could change the conformation and enzyme–substrate interaction of free enzymes, causing a negative effect on catalytic performance. On the other hand, immobilization can provide a protective effect against increase in temperature by reducing the conformational flexibility of enzymes. Therefore, enhanced thermostability of rSLAC immobilized on modified MNP could be attributed to the enzyme rigidity owing to the anchorage between the support and protein, promoted by covalent bond formation (Costa et al. 2019).
Fig. 3.
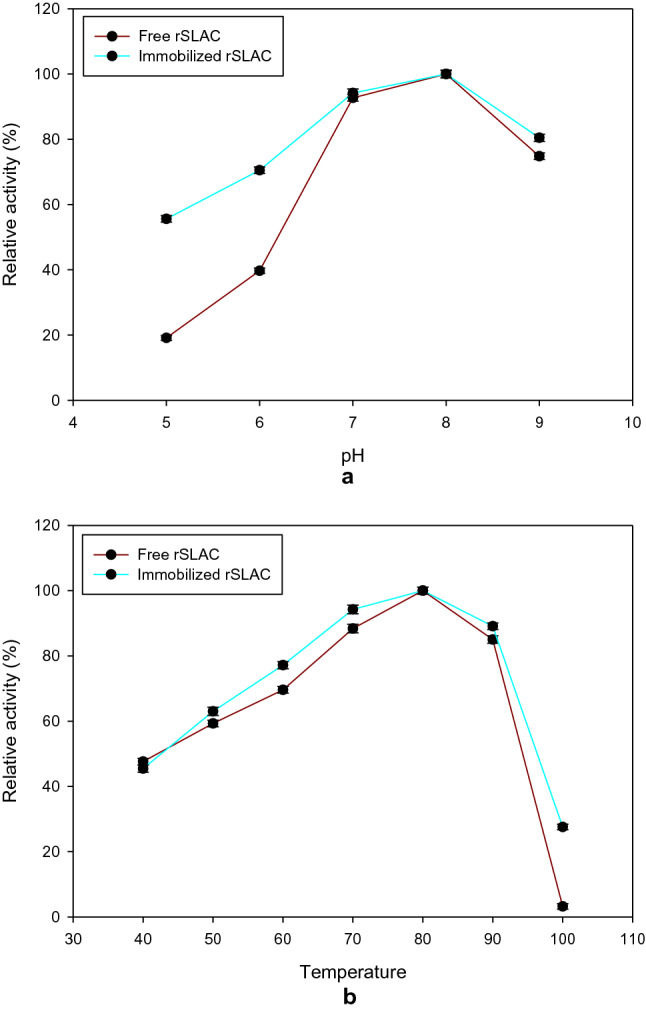
Effect of (a) pH and (b) temperature on the catalytic activity of free  and immobilized
and immobilized  rSLAC. The effect of pH was assessed using different buffers [20 mM sodium acetate buffer (pH 5.0), 20 mM Tris–HCl buffer (pH 6.0–8.0) and 20 mM glycine–NaOH buffer (pH 9.0)] at 80 °C. Temperature optimisation was carried out by performing laccase assays at different temperatures (40 to 100 °C) at pH 8.0 using 2,6 DMP as a substrate. Values are means ± standard deviation (n = 3)
rSLAC. The effect of pH was assessed using different buffers [20 mM sodium acetate buffer (pH 5.0), 20 mM Tris–HCl buffer (pH 6.0–8.0) and 20 mM glycine–NaOH buffer (pH 9.0)] at 80 °C. Temperature optimisation was carried out by performing laccase assays at different temperatures (40 to 100 °C) at pH 8.0 using 2,6 DMP as a substrate. Values are means ± standard deviation (n = 3)
Determination of kinetic parameters for free and immobilized rSLAC
The Michaelis constant (Km) for the free and immobilized enzymes were 54.05 and 54.17 µM, respectively while the maximum velocities (Vmax) were 25.88 and 26.26 µM mg−1 min−1, respectively. The slight increase in Km value for the immobilized rSLAC could be due to the diffusional limitation, which resulted in a lower enzyme–substrate affinity. An increase in Km values after immobilization of laccase has also been reported in several studies (Wang et al. 2010, 2013). Comparable Vmax values of free and immobilized rSLAC suggest that the immobilization process did not compromise catalytic activity.
Effect of metal ions and organic solvents on free and immobilized rSLAC
Heavy metal ions are common environmental pollutants and are often found in industrial wastewater (Zeng et al. 2017). These metal ions could activate or inhibit the catalytic activity of enzymes (Couto et al. 2005), which can affect the enzymatic remediation process. Therefore, the effects of different metal ions on the catalytic activity of free and immobilized rSLAC were evaluated. Interestingly, it was observed that Co2+, Cu2+ and Mn2+ ions greatly stimulated the catalytic activity of free and immobilized rSLAC (Table 1), where the order of enhancement in rSLAC activity was Co2+ > Mn2+ > Cu2+. Activation of rSLAC by Cu2+ is not surprising as copper is an essential component of the active site (Chakroun et al. 2010; Lu et al. 2012a, b). The results are consistent with those obtained using a recombinant polyphenol oxidase, where Co2+ stimulated a higher catalytic efficiency compared to Cu2+ (Moon et al. 2018). In addition, apart from copper, manganese also has a known role in inducing the transcription and activity of laccases (Baldrian et al. 2000). However, the other metal ions only slightly improved the activity of the enzymes, with Al3+ reducing activity at 30 mM (Table 1). Enhanced catalytic activity of laccase by several metal ions viz., Mn2+, Mg2+, Mo6+, Ca2+ has also been reported in a previous study (Zhao et al. 2012). The negative effect of Al3+ observed here is consistent with a previous report where the suppressive effect of this trivalent metal ion towards laccase-Cu2+ inhibited the degradation of 4-nitrophenol (Lu et al. 2012a, b). Overall, our results suggest that the presence of some metal ions in wastewater could facilitate the remediation of phenolic compounds.
Table 1.
Effect of different metal ions on catalytic activity of free and immobilized rSLAC
| Metal ions | Concentrations (mM) | Relative activity (%) | |
|---|---|---|---|
| Free rSLAC | MNP-rSLAC | ||
| Control | – | 100 ± 0.16 | 100 ± 1.62 |
| Li+ |
10 30 |
104 ± 0.58 114 ± 2.08 |
103 ± 1.53 112 ± 1.17 |
| Co2+ |
10 30 |
563 ± 2.00 604 ± 2.08 |
556 ± 2.52 598 ± 1.54 |
| Cu2+ |
10 30 |
179 ± 2.07 253 ± 2.59 |
175 ± 2.06 250 ± 3.04 |
| Mn2+ |
10 30 |
315 ± 2.04 333 ± 2.53 |
311 ± 3.52 329 ± 1.50 |
| Ca2+ |
10 30 |
120 ± 3.08 141 ± 2.02 |
116 ± 2.40 138 ± 2.50 |
| Mo6+ |
10 30 |
126 ± 2.48 137 ± 2.10 |
128 ± 1.24 136 ± 1.56 |
| Al3+ |
10 30 |
117 ± 0.82 94 ± 1.57 |
119 ± 2.08 96 ± 1.58 |
| Mg2+ |
10 30 |
114 ± 2.59 119 ± 1.55 |
112 ± 1.54 121 ± 1.18 |
All values are means ± SD (n = 3)
Laccase activity in the absence of metal ions was considered as 100%
Organic solvents have been known to inhibit enzyme activity via protein unfolding (Afreen et al. 2017). Results on the effect of different organic solvents showed that catalytic activities of free and immobilized rSLAC were not affected by the solvents tested (at 20 and 50%) except for DMSO (Table 2). The activities of both the free and immobilized enzymes were significantly increased in the presence of dioxane (Table 2). To the best of our knowledge, the stimulatory effect of dioxane has not been reported, which could be a novel feature of rSLAC. The rSLAC retained full activity at 20 and 50% methanol, ethanol and acetone whilst a minor effect was observed with DMSO (2% inhibition at 20% DMSO and 8% inhibition at 50% DMSO). In a previous study with rCotA laccase, however, DMSO significantly inhibited the enzyme (62% inhibition with 10% DMSO) (Wang et al. 2015) which indicates that the rSLAC has a much better tolerance to DMSO. As some of the pollutants, such as the phenolics studied here, polycyclic aromatic hydrocarbons (PAHs), organophosphorus pesticides and azo dyes, are only soluble in organic solvents, the use of immobilized rSLAC could be useful for their detoxification.
Table 2.
Effect of different organic solvents on catalytic activity of free and immobilized rSLAC
| Organic solvent | Concentration (%, v/v) | Relative activity | |
|---|---|---|---|
| Free rSLAC | MNP-rSLAC | ||
| Control | – | 100 ± 1.16 | 100 ± 1.62 |
| Methanol |
20 50 |
110 ± 3.08 104 ± 1.58 |
109 ± 1.56 106 ± 1.54 |
| Ethanol |
20 50 |
115 ± 2.06 109 ± 2.56 |
113 ± 1.57 110 ± 3.62 |
| Acetone |
20 50 |
109 ± 2.54 101 ± 2.06 |
107 ± 2.07 103 ± 2.53 |
| Dioxane |
20 50 |
123 ± 1.74 198 ± 3.05 |
122 ± 2.48 194 ± 2.58 |
| DMSO |
20 50 |
98 ± 2.00 92 ± 1.18 |
99 ± 3.06 98 ± 1.16 |
All values are means ± SD (n = 3)
Laccase activity in the absence of organic solvents was considered as 100%
Storage stability and recycling efficiency of immobilized rSLAC
The use of enzymes in an industrial application is greatly affected by the cost of their production, thus, enzymes with long-term storage stability and high recycling efficiency are preferred (Alex et al. 2014). To this end, storage stability and reusability of immobilized rSLAC were assessed. The free and immobilized enzymes were stored in Tris–HCl buffer (20 mM, pH 8) at 4 °C and their activities monitored every 6 days over a period of 30 days. Both enzymes retained ≥ 95% of initial activity (Fig. 4a).
Fig. 4.
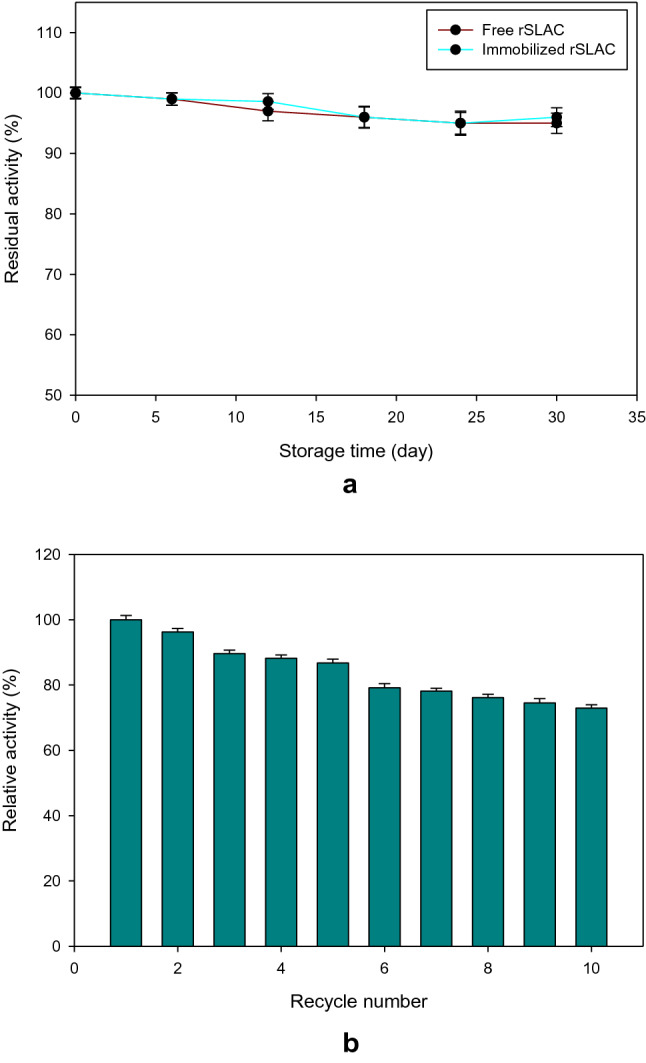
a Storage stability of free  and immobilized
and immobilized  rSLAC. This was evaluated by keeping both free and MNP-rSLAC in 20 mM Tris–HCl buffer (pH 8.0) at 4 °C for a period of 30 days. b Reusability of immobilized rSLAC (MNP-rSLAC) was monitored over 10 reaction cycles. Values are means ± standard deviation (n = 3)
rSLAC. This was evaluated by keeping both free and MNP-rSLAC in 20 mM Tris–HCl buffer (pH 8.0) at 4 °C for a period of 30 days. b Reusability of immobilized rSLAC (MNP-rSLAC) was monitored over 10 reaction cycles. Values are means ± standard deviation (n = 3)
The recycling efficiency of immobilized rSLAC was evaluated in a batch operation mode for 10 cycles, using 2,6-DMP as a substrate. The MNP-rSLAC retained > 73% of its initial activity after 10 consecutive cycles (Fig. 4b). The decrease in laccase activity after repeated cycles could be due to the leaching of the enzyme from the immobilized support or accumulation of reaction products that may reduce mass transfer efficiency and make the enzyme less accessible by its products. A fungal laccase immobilized on MNPs functionalised by PDA retained 70% of its initial activity after 10 catalytic cycles (Zhang et al. 2017). This implies that silanization, with favourable properties outlined earlier, could be a viable alternative functionalisation approach. The remarkable storage stability along with the reusability of the MNP-rSLAC during 10 successive cycles, emphasises its potential for large-scale application.
Transformation of phenol, 4-CP and 4-FP by rSLAC
To enhance transformation, the effects of various parameters, such as pH, mediator concentrations and time, on the degradation of the phenolic compounds, were investigated.
Effect of pH on degradation of phenolic compounds
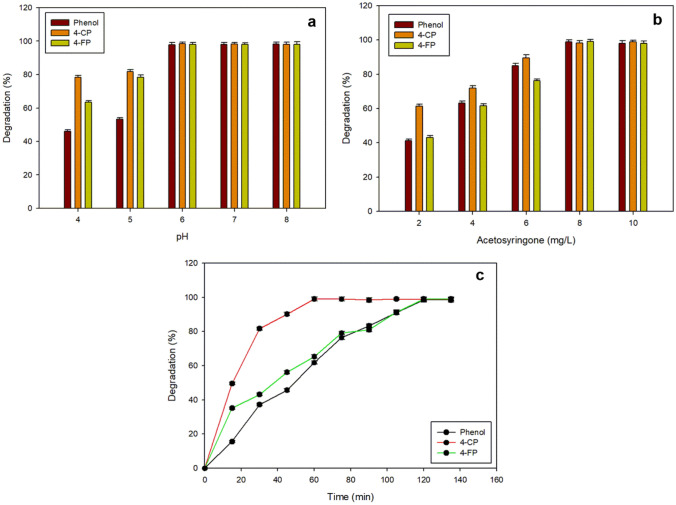
pH is one of the critical factors for any enzyme-catalysed reaction. Therefore, the effect of pH on rSLAC-mediated degradation of phenol, 4-CP and 4-FP was evaluated in the pH range of 4.0–8.0. Interestingly, it was observed that ≥ 98% degradation was achieved over a wide pH range of pH 6.0–8.0 (Fig. 5a). However, the degradation of phenolic compounds was relatively low at acidic pH; it increased substantially in the alkaline range (Fig. 5a). Since the pH of wastewater effluents are in the range of 5.5–9.8 (Gaitan et al. 2011; Nair et al. 2013), the application of rSLAC in real wastewater conditions could be feasible.
Fig. 5.
Effect of pH (a), acetosyringone concentration (b) and time (c) on the transformation of phenol, 4-CP and 4-FP. a Effect of pH on the transformation of phenol, 4-CP and 4-FP. The reaction mixture contained 80 mg/L of each phenolic compound in different pH buffers and laccase (2 U/mL), and was incubated at 37 °C for 4 h. b Effect of mediator (acetosyringone) concentration (2–10 mg/L) on the transformation of phenol, 4-CP and 4-FP using rSLAC. Reaction was carried out in Tris–HCl buffer (50 mM, pH 8.0) with 80 mg/L of phenol, 4-CP, 4-FP and laccase 2 U/mL. c-Time dependent degradation profile of phenol, 4-CP and 4-FP using rSLAC in the presence of a redox mediator, acetosyringone. Reaction conditions: 80 mg/L phenol, 4-CP or 4-FP, 2U rSLAC, 8 µg/mL acetosyringone, 37 °C, pH 8. The residual concentrations of phenol, 4-CP and 4-FP were determined colorimetrically (510 nm). Data is presented as the mean ± SD (n = 3)
Effect of mediator concentration on degradation of phenolic compounds
The inclusion of mediators has been the general approach in enhancing laccase-mediated transformation of recalcitrant compounds (Murugesan et al. 2010). Therefore, the effect of different mediators such as acetosyringone (natural) and 1-hydroxybenzotrialzole (synthetic), on the degradation of phenolic compounds was evaluated. Both mediators showed similar effects (data not shown), therefore further experiments were carried out with the natural mediator due to its eco-friendly properties (Kunamneni et al. 2008). It was observed that complete degradation was achieved at 8 mg/L concentration of acetosyringone (Fig. 5b). A previous study used a much higher concentration of artificial mediator (ABTS; 642.5 mg/L) to achieve the complete degradation of 4-CP (64.28 mg/L) (Chakroun et al. 2010).
Effect of time on degradation of phenolic compounds
The transformation of phenol, 4-CP and 4-FP were performed using the purified rSLAC (2 U or 40 µg/mL) at optimal pH and mediator concentration. Remarkably, ≥ 98% degradation of all the phenolic compounds was achieved within 2 h (Fig. 5c). Moreover, degradation of 61, 98 and 65% was observed for phenol, 4-CP and 4-FP, respectively, in only 1 h of reaction time. However, in a previous study, 100% degradation of 2-chlorophenol was achieved in 4 h using 10 U of laccase (Gaitan et al. 2011). Similarly, Zhang et al. (2017) achieved 86% degradation of 4-CP in 2 h using 250 mg/L of immobilized laccase. The requirement of the low amount of laccase for the complete degradation of phenolic compounds, in this study, could be attributed to the high catalytic efficiency of the rSLAC, as previously reported (Yadav et al. 2018). In addition, we also noticed that the degradation of halogenated phenols was attained in less time compared to the non-halogenated phenol (Fig. 5c). This is consistent with previous reports where 4-chlorophenol was degraded much faster than phenol (Arica et al. 2009; Liu et al. 2012).
HPLC analysis of transformation products
The transformation of phenol, 4-CP and 4-FP by rSLAC was further analysed by HPLC. The retention time for phenol was tR = 8.7 min; the peak completely disappeared after laccase-catalysed transformation and several transformation products were formed which eluted at longer retention times (Fig. S1c). The retention times for the elution of 4-CP and 4-FP were observed at tR = 10.6 and tR = 9.6 min, respectively, in control samples (Fig. S2a and S3a). Similarly, the peaks for 4-CP and 4-FP disappeared in test samples and multiple peaks appeared after delayed retention times (Fig. S2c and S3c). The delayed retention times observed in all test samples could be due to the formation of oligomeric products (Cabana et al. 2007). Similar findings were observed in laccase-mediated transformation of triclosan (Murugesan et al. 2010).
Toxicity analysis
Toxicity analysis of phenolic compounds and their products were assessed by performing the bacterial growth inhibition studies using E. coli ATCC 25922 as a test organism. It is well known that phenolic compounds increase bacterial cell membrane permeability which leads to the release of their cell wall components and ultimately cell death (Denyer 1995; Mcdonnell and Russell 1999). In the present study, 80 µg/mL of the phenolic compounds used (phenol, 4-CP and 4-FP) completely inhibited the growth of E. coli ATCC 25922 (Fig. 6). However, the transformation products inhibited the bacterial growth by 10, 40 and 45% for phenol, 4-CP and 4-FP, respectively. This suggests that the toxicity of phenol, 4-CP and 4-FP transformation products were reduced by 90, 60 and 55%, respectively. The halogenated compounds viz., 4-CP and 4-FP showed higher toxicity compared to the phenol. Higher toxicity of halogenated phenolic compounds was also observed in a previous study, where it was reported that an increase in the number of chlorine atoms was associated with an increase in bacterial growth inhibition (Wang et al. 2008). On the other hand, it was observed in a previous study that degradation products were more toxic than the parental compounds (Lu et al. 2015). In this study, we observed that after the rSLAC treatment of phenolic compounds the products were precipitated out of the reaction mixture, which could be due to the polymerisation of the products. This could provide an additional advantage for the physical separation of the polymerized products from a wastewater sample using filtration methods. Separation of precipitates by filtration methods has also been previously reported (Abdollahi et al. 2018).
Fig. 6.
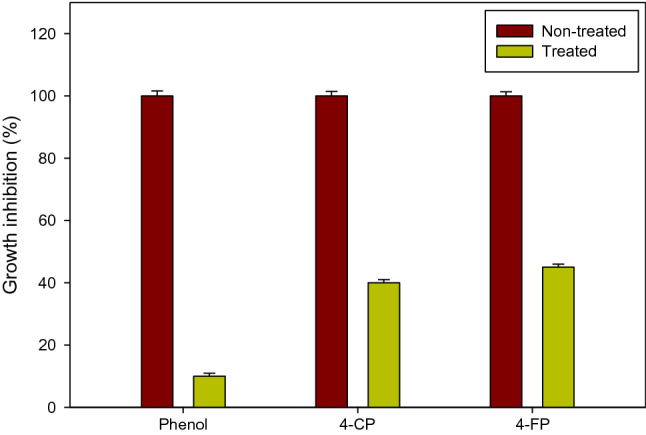
Effect of rSLAC treatment of phenolic compounds on bacterial growth inhibition. Toxicity analyses of the degradation products of phenol, 4-chlorophenol (4-CP) and 4-fluorophenol (4-FP) were tested using E. coli ATCC 25922 as reference organism  denotes untreated samples (no enzyme) and
denotes untreated samples (no enzyme) and  shows rSLAC-treated samples. Data is presented as the mean ± SD (n = 3)
shows rSLAC-treated samples. Data is presented as the mean ± SD (n = 3)
Conclusion
In this study, rSLAC was for the first time successfully immobilized onto MNPs functionalised by silanization. Enzyme immobilisation was confirmed by SEM and FTIR. The immobilised enzyme retained 73% of initial activity after 10 cycles, exhibited improved storage stability, and showed enhanced activity when exposed to selected metal ions. The enzyme showed great potential for the removal of phenolic compounds within a wide pH range, significantly reducing the toxicity of the compounds. In the presence of a natural mediator, acetosyringone, ≥ 98% transformation of phenol and halogenated phenols was achieved within 2 h. These findings suggest that immobilized rSLAC could be a candidate for the sustainable bioremediation of phenolic compounds in wastewater.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the National Research Foundation (South Africa) for financial support (Grant 105889 and 112099). Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto. Financial support from the Council of Scientific and Industrial Research (CSIR) for student (Deepti Yadav) scholarship is gratefully acknowledged.
Author contribution statement
DY—Investigation, Methodology, Writing—original draft; BR—Investigation, Methodology; NM—Conceptualisation, Supervision; MLR-H—Conceptualisation, Supervision, Writing—review and editing; TK—Conceptualisation, Supervision, Writing-review and editing, Funding acquisition.
Funding
The authors acknowledge the National Research Foundation (South Africa) for financial support (Grant 105889 and 112099). Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto. Financial support from the Council of Scientific and Industrial Research (CSIR) for student (Deepti Yadav) scholarship, is gratefully acknowledged.
Availability of data and material
Data will be made available on request.
Code availability
N/A
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethics approval:
This article does not contain any studies with human participants or animals.
Consent to participate
N/A
Consent for publication
All the authors have seen and approved to the publication of the manuscript in 3 Biotech.
References
- Afreen S, Shamsi TN, Baig MA, Ahmad N, Fatima S, Qureshi MI, Hassan MI, Fatma T. A novel multicopper oxidase (laccase) from cyanobacteria: purification, characterization with potential in the decolorization of anthraquinonic dye. PLoS ONE. 2017;12(4):e0175144. doi: 10.1371/journal.pone.0175144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex D, Mathew A, Sukumaran RK. Esterases immobilized on aminosilane modified magnetic nanoparticles as a catalyst for biotransformation reactions. Bioresour Technol. 2014;167:547–550. doi: 10.1016/j.biortech.2014.05.110. [DOI] [PubMed] [Google Scholar]
- Arıca MY, Altıntas B, Bayramoğlu G. Immobilization of laccase onto spacer-arm attached non-porous poly (GMA/EGDMA) beads: application for textile dye degradation. Bioresour Technol. 2009;100(2):665–669. doi: 10.1016/j.biortech.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Abdollahi K, Yazdani F, Panahi R, Mokhtarani B. Biotransformation of phenol in synthetic wastewater using the functionalized magnetic nano-biocatalyst particles carrying tyrosinase. 3 Biotech. 2018;8(10):419. doi: 10.1007/s13205-018-1445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrian P, Gabriel J, Nerud F, Zadražil F. Influence of cadmium and mercury on activities of ligninolytic enzymes and degradation of polycyclic aromatic hydrocarbons by Pleurotus ostreatus in soil. Appl Environ Microbiol. 2000;66(6):2471–2478. doi: 10.1128/aem.66.6.2471-2478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabana H, Jiwan JL, Rozenberg R, Elisashvili V, Penninckx M, Agathos SN, Jones JP. Elimination of endocrine disrupting chemicals nonylphenol and bisphenol A and personal care product ingredient triclosan using enzyme preparation from the white rot fungus Coriolopsis polyzona. Chemosphere. 2007;67(4):770–778. doi: 10.1016/j.chemosphere.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Chakroun H, Mechichi T, Martinez MJ, Dhouib A, Sayadi S. Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: application on bioremediation of phenolic compounds. Process Biochem. 2010;45(4):507–513. doi: 10.1016/j.procbio.2009.11.009. [DOI] [Google Scholar]
- Costa JB, Lima MJ, Sampaio MJ, Neves MC, Faria JL, Morales-Torres S, Tavares AP, Silva CG. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem Eng J. 2019;355:974–985. doi: 10.1016/j.cej.2018.08.178. [DOI] [Google Scholar]
- Couto SR, Sanromán MA, Guebitz GM. Influence of redox mediators and metal ions on synthetic acid dye decolourization by crude laccase from Trametes hirsuta. Chemosphere. 2005;58(4):417–422. doi: 10.1016/j.chemosphere.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Dai Y, Song Y, Wang S, Yuan Y. Treatment of halogenated phenolic compounds by sequential tri-metal reduction and laccase-catalytic oxidation. Water Res. 2015;71:64–73. doi: 10.1016/j.watres.2014.12.047. [DOI] [PubMed] [Google Scholar]
- Dehnavi SM, Pazuki G, Vossoughi M. PEGylated silica-enzyme nanoconjugates: a new frontier in large scale separation of α-amylase. Sci Rep. 2015;5(1):1–9. doi: 10.1038/srep18221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer SP. Mechanisms of action of antibacterial biocides. Int Biodeter Biodegr. 1995;36(3–4):227–245. doi: 10.1016/0964-8305(96)00015-7. [DOI] [Google Scholar]
- Faridi S, Bose H, Satyanarayana T. Utility of immobilized recombinant carbonic anhydrase of Bacillus halodurans TSLV1 on the surface of modified iron magnetic nanoparticles in carbon sequestration. Energy Fuels. 2017;31(3):3002–3009. doi: 10.1021/acs.energyfuels.6b02777. [DOI] [Google Scholar]
- Fernandes RA, Daniel-da-Silva AL, Tavares AP, Xavier AM. EDTA-Cu (II) chelating magnetic nanoparticles as a support for laccase immobilization. Chem Eng Sci. 2017;158:599–605. doi: 10.1016/j.ces.2016.11.011. [DOI] [Google Scholar]
- Fortes CC, Daniel-da-Silva AL, Xavier AM, Tavares AP. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem Eng Proc. 2017;117:1–8. doi: 10.1016/j.cep.2017.03.009. [DOI] [Google Scholar]
- Gaitan IJ, Medina SC, González JC, Rodríguez A, Espejo ÁJ, Osma JF, Sarria V, Alméciga-Díaz CJ, Sánchez OF. Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens. Bioresour Technol. 2011;102(3):3632–3635. doi: 10.1016/j.biortech.2010.11.040. [DOI] [PubMed] [Google Scholar]
- Gianolini JE, Britos CN, Mulreedy CB, Trelles JA. Hyperstabilization of a thermophile bacterial laccase and its application for industrial dyes degradation. Biotech. 2020;10:288. doi: 10.1007/s13205-020-02277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Bleymaier K, Pellis A, Vielnascher R, Daxbacher A, Greimel KJ, Guebitz GM. Laccase catalyzed elimination of morphine from aqueous systems. N Biotechnol. 2018;42:19–25. doi: 10.1016/j.nbt.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Karam J, Nicell JA. Potential applications of enzymes in waste treatment. J Chem Technol Biotechnol. 1997;69(2):141–153. doi: 10.1002/(SICI)1097-4660(199706)69:2<141::AID-JCTB694>3.0.CO;2-U. [DOI] [Google Scholar]
- Kudanga T, Nyanhongo GS, Guebitz GM, Burton S. Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzyme Microb Technol. 2011;48(3):195–208. doi: 10.1016/j.enzmictec.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Kunamneni A, Camarero S, García-Burgos C, Plou FJ, Ballesteros A, Alcalde M. Engineering and applications of fungal laccases for organic synthesis. Microb Cell Fact. 2008;7(1):32. doi: 10.1186/1475-2859-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zeng Z, Zeng G, Tang L, Pang Y, Li Z, Liu C, Lei X, Wu M, Ren P, Liu Z. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour Technol. 2012;115:21–26. doi: 10.1016/j.biortech.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/0304-3894(92)87011-4. [DOI] [PubMed] [Google Scholar]
- Lu C, Cao L, Liu R, Lei Y, Ding G. Effect of common metal ions on the rate of degradation of 4-nitrophenol by a laccase-Cu2+ synergistic system. J Environ Manage. 2012;113:1–6. doi: 10.1016/j.jenvman.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Lu J, Shao J, Liu H, Wang Z, Huang Q. Formation of halogenated polyaromatic compounds by laccase catalyzed transformation of halophenols. Environ Sci Technol. 2015;49(14):8550–8557. doi: 10.1021/acs.est.5b02399. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhao M, Wang TN, Zhao LY, Du MH, Li TL, Li DB. Characterization and dye decolorization ability of an alkaline resistant and organic solvents tolerant laccase from Bacillus licheniformis LS04. Bioresour Technol. 2012;115:35–40. doi: 10.1016/j.biortech.2011.07.111. [DOI] [PubMed] [Google Scholar]
- Ma M, Zhang Y, Yu W, Shen HY, Zhang HQ, Gu N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf A Physicochem Eng. 2003;212(2–3):219–226. doi: 10.1016/S0927-7757(02)00305-9. [DOI] [Google Scholar]
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–179. doi: 10.1007/s13398-014-0173-7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modaressi K, Taylor KE, Bewtra JK, Biswas N. Laccase-catalyzed removal of bisphenol-A from water: protective effect of PEG on enzyme activity. Water Res. 2005;39(18):4309–4316. doi: 10.1016/j.watres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mohamad NR, Marzuki NH, Buang NA, Huyop F, Wahab RA. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip. 2015;29(2):205–220. doi: 10.1080/13102818.2015.1008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Najavand S, Pazhang M. Immobilization of endoglucanase Cel9A on chitosan nanoparticles leads to its stabilization against organic solvents: the use of polyols to improve the stability. 3 Biotech. 2019;9(7):269. doi: 10.1007/s13205-019-1794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed SA, Al-Harbi MH, Almulaiky YQ, Ibrahim IH, El-Shishtawy RM. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron J Biotechnol. 2017;27:84–90. doi: 10.1016/j.ejbt.2017.03.010. [DOI] [Google Scholar]
- Moon SJ, Kim HW, Jeon SJ. Biochemical characterization of a thermostable cobalt-or copper-dependent polyphenol oxidase with dye decolorizing ability from Geobacillus sp. JS12. Enzyme Microb Technol. 2018;118:30–36. doi: 10.1016/j.enzmictec.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Murugesan K, Chang YY, Kim YM, Jeon JR, Kim EJ, Chang YS. Enhanced transformation of triclosan by laccase in the presence of redox mediators. Water Res. 2010;44(1):298–308. doi: 10.1016/j.watres.2009.09.058. [DOI] [PubMed] [Google Scholar]
- Nair RR, Demarche P, Agathos SN. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. N Biotechnol. 2013;30(6):814–823. doi: 10.1016/j.nbt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Pera-Titus M, García-Molina V, Baños MA, Giménez J, Esplugas S, Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl Catal B-Environ. 2004;47(4):219–256. doi: 10.1016/j.apcatb.2003.09.010. [DOI] [Google Scholar]
- Pereira L, Dias P, Soares OS, Ramalho PS, Pereira MF, Alves MM. Synthesis, characterization and application of magnetic carbon materials as electron shuttles for the biological and chemical reduction of the azo dye Acid Orange 10. Appl Catal B-Environ. 2017;212:175–184. doi: 10.1016/j.apcatb.2017.04.060. [DOI] [Google Scholar]
- Ranjan B, Pillai S, Permaul K, Singh S. A novel strategy for the efficient removal of toxic cyanate by the combinatorial use of recombinant enzymes immobilized on aminosilane modified magnetic nanoparticles. Bioresour Technol. 2018;253:105–111. doi: 10.1016/j.biortech.2017.12.087. [DOI] [PubMed] [Google Scholar]
- Rotková J, Šuláková R, Korecká L, Zdražilová P, Jandová M, Lenfeld J, Horák D, Bílková Z. Laccase immobilized on magnetic carriers for biotechnology applications. J Magn Magn Mater. 2009;321(10):1335–1340. doi: 10.1016/j.jmmm.2009.02.034. [DOI] [Google Scholar]
- Selvam K, Govarthanan M, Senbagam D, Kamala-Kannan S, Senthilkumar B, Selvankumar T. Activity and stability of bacterial cellulase immobilized on magnetic nanoparticles. Chinese J Catal. 2016;37(11):1891–1898. doi: 10.1016/S1872-2067(16)62487-7. [DOI] [Google Scholar]
- Sheldon RA, Woodley JM. Role of biocatalysis in sustainable chemistry. Chem Rev. 2018;118(2):801–838. doi: 10.1021/acs.chemrev.7b00203. [DOI] [PubMed] [Google Scholar]
- Singh S, Mishra R, Sharma RS, Mishra V. Phenol remediation by peroxidase from an invasive mesquite: turning an environmental wound into wisdom. J Hazard Mater. 2017;334:201–211. doi: 10.1016/j.jhazmat.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Sondhi S, Sharma P, Saini S, Puri N, Gupta N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS ONE. 2014;9(5):e96951. doi: 10.1371/journal.pone.0096951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P, Selvankumar T, Kamala-Kannan S, Mythili R, Sengottaiyan A, Govarthanan M, Senthilkumar B, Selvam K. Production and purification of laccase by Bacillus sp. using millet husks and its pesticide degradation application. 3 Biotech. 2019;9(11):396. doi: 10.1007/s13205-019-1900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P, Selvankumar T, Paray BA, Rehman MU, Kamala-Kannan S, Govarthanan M, Kim W, Selvam K. Chlorpyrifos degradation efficiency of Bacillus sp. laccase immobilized on iron magnetic nanoparticles. 3 Biotech. 2020;10(8):1–11. doi: 10.1007/s13205-020-02363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay P, Shrivastava R, Agrawal PK. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech. 2016;6(1):15. doi: 10.1007/s13205-015-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa S, Riani P, Locardi F, Canepa F. Functionalization of Fe3O4 NPs by silanization: use of amine (APTES) and thiol (MPTMS) silanes and their physical characterization. Materials. 2016;9(10):826. doi: 10.3390/ma9100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Guo C, Yang LR, Liu CZ. Magnetic mesoporous silica nanoparticles: fabrication and their laccase immobilization performance. Bioresour Technol. 2010;101(23):8931–8935. doi: 10.1016/j.biortech.2010.06.115. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang XJ, Zhao JF, Chen L. Toxicity assessment of heavy metals and organic compounds using Cell Sense biosensor with E. coli. Chin Chem Lett. 2008;19(2):211–214. doi: 10.1016/j.cclet.2007.10.053. [DOI] [Google Scholar]
- Wang L, Shi Y, Sa R, Ning N, Wang W, Tian M, Zhang L. Surface modification of aramid fibers by catechol/polyamine codeposition followed by silane grafting for enhanced interfacial adhesion to rubber matrix. Ind Eng Chem Res. 2016;55(49):12547–12556. doi: 10.1021/acs.iecr.6b03177. [DOI] [Google Scholar]
- Wang TN, Lu L, Wang JY, Xu TF, Li J, Zhao M. Enhanced expression of an industry applicable CotA laccase from Bacillus subtilis in Pichia pastoris by non-repressing carbon sources together with pH adjustment: recombinant enzyme characterization and dye decolorization. Process Biochem. 2015;50(1):97–103. doi: 10.1016/j.procbio.2014.10.009. [DOI] [Google Scholar]
- Wang Y, Chen X, Liu J, He F, Wang R. Immobilization of laccase by Cu2+ chelate affinity interaction on surface-modified magnetic silica particles and its use for the removal of 2, 4-dichlorophenol. Environ Sci Pollut Res. 2013;20(9):6222–62231. doi: 10.1007/s11356-013-1661-6. [DOI] [PubMed] [Google Scholar]
- Xu J, Sun J, Wang Y, Sheng J, Wang F, Sun M. Application of iron magnetic nanoparticles in protein immobilization. Molecules. 2014;19(8):11465–11486. doi: 10.3390/molecules190811465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D, Ranjan B, Mchunu N, Le Roes-Hill M, Kudanga T. Secretory expression of recombinant small laccase from Streptomyces coelicolor A3 (2) in Pichia pastoris. Int J Biol Macromol. 2018;108:642–649. doi: 10.1016/j.ijbiomac.2017.11.169. [DOI] [PubMed] [Google Scholar]
- Zdarta J, Antecka K, Frankowski R, Zgoła-Grześkowiak A, Ehrlich H, Jesionowski T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci Total Environ. 2018;615:784–795. doi: 10.1016/j.scitotenv.2017.09.213. [DOI] [PubMed] [Google Scholar]
- Zeng S, Qin X, Xia L. Degradation of the herbicide isoproturon by laccase-mediator systems. Biochem Eng J. 2017;119:92–100. doi: 10.1016/j.bej.2016.12.016. [DOI] [Google Scholar]
- Zhang D, Deng M, Cao H, Zhang S, Zhao H. Laccase immobilized on magnetic nanoparticles by dopamine polymerization for 4-chlorophenol removal. Green Energy Environ. 2017;2(4):393–400. doi: 10.1016/j.gee.2017.04.001. [DOI] [Google Scholar]
- Zhang JL, Srivastava RS, Misra RD. Core− shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir. 2007;23(11):6342–6351. doi: 10.1021/la0636199. [DOI] [PubMed] [Google Scholar]
- Zhao D, Zhang X, Cui D, Zhao M. Characterisation of a novel white laccase from the deuteromycete fungus Myrothecium verrucaria NF-05 and its decolourisation of dyes. PLoS ONE. 2012;7(6):e38817. doi: 10.1371/journal.pone.0038817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.
N/A