Abstract
Study objective
Emergency departments (EDs) often serve vulnerable populations who may lack primary care and have suffered disproportionate COVID-19 pandemic effects. Comparing patients having and lacking a regular source of medical care and other ED patient characteristics, we assessed COVID-19 vaccine hesitancy, reasons for not wanting the vaccine, perceived access to vaccine sites, and willingness to get the vaccine as part of ED care.
Methods
This was a cross-sectional survey conducted from December 10, 2020, to March 7, 2021, at 15 safety net US EDs. Primary outcomes were COVID-19 vaccine hesitancy, reasons for vaccine hesitancy, and sites (including EDs) for potential COVID-19 vaccine receipt.
Results
Of 2,575 patients approached, 2,301 (89.4%) participated. Of the 18.4% of respondents who lacked a regular source of medical care, 65% used the ED as their usual source of health care. The overall rate of vaccine hesitancy was 39%; the range among the 15 sites was 28% to 58%. Respondents who lacked a regular source of medical care were more commonly vaccine hesitant than those who had a regular source of medical care (47% versus 38%, 9% difference, 95% confidence interval 4% to 14%). Other characteristics associated with greater vaccine hesitancy were younger age, female sex, Black race, Latinx ethnicity, and not having received an influenza vaccine in the past 5 years. Of the 61% who would accept a COVID-19 vaccine, 21% stated that they lacked a primary physician or clinic at which to receive it; the vast majority (95%) of these respondents would accept the COVID-19 vaccine as part of their care in the ED.
Conclusion
ED patients who lack a regular source of medical care are particularly hesitant regarding COVID-19 vaccination. Most COVID-19 vaccine acceptors would accept it as part of their care in the ED. EDs may play pivotal roles in COVID-19 vaccine messaging and delivery to highly vulnerable populations.
Editor’s Capsule Summary.
What is already known on this topic
Improving COVID-19 vaccination access during care may enhance personal and collective immunity.
What question this study addressed
What could be acceptance and hesitancy concerns for COVID-19 vaccinations if offered to emergency department (ED) patients already seeking care?
What this study adds to our knowledge
After receiving 2,301 survey responses (89.4% of all approached) at 15 safety net hospitals over 3 months starting December 2020, 65% stated they used the ED as a regular care source and 39% reported hesitancy concerns, with varying factors altering that observation in subgroups.
How this is relevant to clinical practice
While EDs are a potential vaccination resource site, many patient factors will likely dampen the impact of ED-based vaccination programs.
Introduction
Background
The greatest public health crisis of the past century, the spread of SARS-CoV-2 and the resulting COVID-19 pandemic has led to more than 500,000 deaths in the United States as of February 23, 2021.1 While community lockdowns, social distancing, contact tracing, and mask wearing have had varied success in stemming the spread of COVID-19, adherence to these interventions has waned over time, and these measures are essentially bridges to the ultimate mitigation measure—broad population COVID-19 immunization.2, 3, 4, 5, 6
The strength of immunization for pandemic mitigation is predicated on broad acceptance and administration of COVID-19 vaccines to a majority of the population. To achieve herd immunity from COVID-19 infection, experts have estimated that approximately 67% to 90% of the population must be immune (by either vaccination or natural infection).7 , 8 With approximately one third of the population saying that they will not accept a COVID-19 vaccine, hesitancy is a major barrier to reaching this target in the United States.9, 10, 11, 12, 13, 14, 15, 16 The major limitation of prior investigations of COVID-19 vaccine hesitancy is that they have been primarily conducted online or by telephone, sampling methods that often miss medically underserved or disadvantaged populations who may be at the greatest risk from COVID-19 infection.17, 18, 19 They also may not reflect the attitudes of patients during true, in-person health care encounters, when they might actually receive a vaccine.
The emergency department (ED) has been commonly described by policymakers as “the safety net of the safety net.”20 With approximately 140 million visits in the United States annually, EDs serve as the primary (and often only) health care access point for up to one fifth of the population, including a number of vulnerable groups—immigrants, persons experiencing homelessness, the impoverished, and the uninsured, many of whom fall into high-risk categories for poor outcomes from COVID-19 infection.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Minorities, especially Blacks and Latinos who have suffered disproportionate morbidity and mortality during the pandemic, also receive high amounts of primary health care through EDs.21, 22, 23, 24, 25, 26, 27, 28
Importance and Goals of This Investigation
The overall premise underlying this research is that efforts toward vaccination-based herd immunity, prevention of disease in high-risk, vulnerable groups, and equitable distribution of the COVID-19 vaccine must go where vulnerable individuals go for care and consider ED-based vaccine messaging and administration programs—analogous to other programs pioneered in the early 1990s in which EDs provide influenza and pneumococcal vaccines.32, 33, 34, 35, 36, 37 In this in-person survey study of patients conducted in a real-world, health care safety net setting (patient visits to 15 EDs across the United States), we assessed the need for such ED-based programs. Comparing patients who have and lack a regular source of medical care and delineating a group that uses the ED as their usual source of care, we assessed COVID-19 vaccine hesitancy rates, reasons for vaccine hesitancy, and willingness to get the COVID-19 vaccine as part of ED care.
Materials and Methods
Design and Setting
From December 20, 2020, to March 7, 2021, we conducted this cross-sectional survey study of ED patients during their visits to 15 safety net EDs in 14 US cities (San Francisco, CA; Oakland, CA; Fresno, CA; Sylmar, CA; Seattle, WA; Iowa City, IA; Detroit, MI; Ann Arbor, MI; New Orleans, LA; Philadelphia, PA; Durham, NC; Baltimore, MD; Camden, NJ; and Boston, MA). The median annual number of visits to these EDs was 77,000 (range 45,000 to 120,000). We obtained institutional review board approval to conduct this survey study by scripted verbal consent at all study sites. We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Survey Instrument Development
The lead investigator reviewed existing literature on vaccine hesitancy and consulted with experts in survey development at the University of California San Francisco (UCSF) to generate an initial survey template. Questions about vaccine hesitancy were adapted from previously published instruments.9, 10, 11, 12, 13, 14, 15, 16 We reviewed this initial template with a focus group of 8 community participants from the UCSF COVID-19 Patient Community Advisory Board to gain recommendations about survey questions’ relevance, wording, cultural sensitivity, comprehension, and length; we also sought input on overall study procedures in terms of survey location, timing, languages needed, and delivery. Investigators on the research team reviewed and edited the template according to focus group recommendations, and we again presented this second iteration to the Patient Community Advisory Board for final review and editing. We pilot tested the final instrument on 6 ED patients at the core site and found excellent comprehension and response consistency (Appendix E1, available at http://www.annemergmed.com).
Selection of Participants: Inclusions, Exclusions, and Survey Administration
Because of differing times of institutional review board approval receipt, the sites began enrollment at different times from December 10, 2020, to January 21, 2021; each site targeted enrollment of 150 patients over a 5-week period. We enrolled adult patients (>18 years of age) using convenience sampling according to the availability of study personnel (typically 4- to 6-hour time blocks), excluding patients with any of the following characteristics: major trauma, transfer from another facility, incarceration, psychiatric hold, intoxication, altered mental status, critical illness, or temporary visit from another country. Research personnel reviewed triage logs and ED electronic health record census boards to identify potentially eligible participants. At 11 sites, all surveys were conducted in person; given constraints for in-person surveys during the pandemic, 3 sites used a second mechanism of calling into telephones in ED patient rooms to conduct the survey, and 1 site called patients immediately after ED discharge. After scripted verbal consent, research personnel read survey questions to participants directly from data collection forms and tablets in their preferred language. Before questions about COVID-19 vaccines, we presented this statement: “It is likely that one or more vaccines for COVID-19 will be available in the spring or summer of next year. While these vaccines cannot assure complete protection, they will decrease your and your family members’ likelihood of becoming infected with COVID-19. These vaccines will likely be provided free of charge.”
Definitions and Primary Outcomes
Vaccine hesitancy was defined as a “no” or “unsure” response to the question, “Would you accept the COVID-19 vaccine when it becomes available?” After survey completion, we categorized participants into either the “have regular source of medical care” or the “lack regular source of medical care” group by their response to this yes/no question: “Do you have a regular clinic or doctor for medical care?” We further delineated patients who used the ED as their usual source of care by their answers to this multiple-choice question: “If NO to regular doctor, where do you usually go when you are sick or need medical advice?”
Our primary outcomes were responses to key survey questions regarding acceptance versus nonacceptance (hesitancy) of the COVID-19 vaccine, reasons for vaccine hesitancy, sites for potential receipt of the COVID-19 vaccine, and acceptability of the COVID-19 vaccine as part of care in the ED.
Data Analysis
We summarized patient characteristics as raw counts and frequency percentages and aggregate key survey question responses as percentages with 95% confidence intervals (CIs), excluding nonresponses to individual questions in proportion denominators. To assess differences in vaccine hesitancy between groups (have versus lack regular source of medical care, male verses female sex, Black versus White race, Latinx versus White/nonLatinx ethnicity, homeless versus housed, and having received an influenza vaccine in the past 5 years versus not having received an influenza vaccine), we compared 95% CIs around differences in proportions. We used a 2-sample Wilcoxon rank sum test to test for differences in vaccine hesitancy for the nonnormally distributed age characteristic. We further stratified vaccine hesitancy by the 15 sites, along with data regarding having a regular source of medical care and ED usual source of care for context. We stratified reasons for vaccine hesitancy according to whether respondents had a regular source of medical care.
In our a priori sample size calculation, we determined that we would need to enroll 2,144 patients to attain a 2% margin of error around point estimates of primary outcome questions; therefore, we sought 150 respondents at each of the 15 sites. We managed data using REDCap38 and conducted analyses using Stata v16.1 (StataCorp LLC, College Station, TX).
Results
Of 2,575 patients approached, 2,301 (89.4%) participated; 339 (14.7%) had been previously diagnosed with COVID-19. Most respondents (81%) had primary care doctors or clinics. Of the 19% who lacked primary care, 65% used the ED as their usual source of health care and 26% went to an urgent care center or clinic. Compared to patients who had primary care, patients who did not have primary care were younger (median age 36 versus 52 years, P<.001), were more often men (67% versus 47%, P<.001), were more commonly Latinx (37% versus 21%, P<.001), were more commonly homeless (10% versus 2%, P<.001), were more commonly uninsured (38% versus 7%, P<.001), less commonly spoke English as their primary language (71% versus 83%, P<.001), and had less commonly received an influenza vaccine in the past 5 years (46% versus 74%, P<.001).
COVID-19 Vaccine Hesitancy
The overall rate of vaccine hesitancy was 39% (95% CI 37% to 41%). Respondents who lacked a regular source of medical care were more commonly vaccine hesitant than those who had a regular source of medical care (47% versus 38%, 9% difference, 95% CI 4% to 14%); likewise, the subgroup of respondents who reported the ED as their usual source of care was more vaccine hesitant than those who had a regular source of medical care (46% versus 38%, difference 8%, 95% CI 2% to 14%). Other characteristics associated with greater vaccine hesitancy were younger age (median 40 years versus 52 years, P<.0001), female sex (45% versus 33%, difference 12%, 95% CI 8% to 16%), Black race (54% versus 30%, difference 24%, 95% CI 19% to 29%), Latinx ethnicity (39% versus 30%, difference 9%, 95% CI 4% to 14%), and not having received an influenza vaccine in the past 5 years (58% versus 31%, difference 27%, 95% CI 23% to 32%). Homelessness and uninsured status were not associated with greater vaccine hesitancy. Fewer vaccine-hesitant respondents reported that some or all of their family members would accept the COVID-19 vaccine if it was offered to them (29% versus 75%, 46% difference, 95% CI 42% to 50%) (Table 1 ).
Table 1.
Vaccine hesitancy stratified by participant characteristics.
| Characteristic | All (2,301) | Will Accept Vaccine (1,381, 60%) | Vaccine Hesitant (900, 39%) |
|---|---|---|---|
| Age in years, median (IQR) | 48 (34–61) | 52 (38–64) | 40 (29–55) |
| Sex | N (%) | N [%, 95% CI] or N (%) | N [%, 95% CI] or N (%) |
| Male | 1,147 (50) | 761 [66%, 95% CI 64–69] | 384 [33%, 95% CI 31–36] |
| Female | 1,131 (49) | 618 [55%, 95% CI 52–58] | 512 [45%, 95% CI 42–48] |
| Have a regular source of medical care | 1,859 (81) | 1,159 [62%, 95% CI 60–65] | 698 [38%, 95% CI 35–40] |
| Lack regular source of medical care | 424 (18) | 227 [54%, 95% CI 49–58] | 197 [46%; 95% CI 42–51] |
| ED usual source of care | 275 (12) | 150 [55%, 95% CI 49–61] | 125 [45%, 95% CI 40–52] |
| Race/Ethnicity | |||
| Black | 700 (30) | 324 [46%, 95% CI 43–50] | 376 [54%, 95% CI 50–57] |
| Asian | 106 (5) | 74 (70) | 32 (30) |
| Latinx | 538 (23) | 326 [61%, 95% CI 56–65] | 209 [39%, 95% CI 35–43] |
| Middle Eastern | 28 (1) | 14 (50) | 14 (50) |
| Native American | 27 (1) | 13 (48) | 14 (52) |
| Native Hawaiian/Pacific Islander | 8 (0.3) | 6 (75) | 2 (25) |
| White (nonLatinx) | 900 (39) | 631 [70%, 95% CI 67–73] | 268 [30%, 95% CI 27–33] |
| Other | 70 (3) | 42 (60) | 28 (40) |
| Homeless | 84 (4) | 53 [63%, 95% CI 52–73] | 31 [37%, 95% CI 27–48] |
| Housed | 2,202 (96) | 1,332 [60%, 95% CI 58–63] | 867 [39%, 95% CI 37–41] |
| Health insurance types | |||
| Private | 871 (38) | 578 (66) | 293 (34) |
| Medicaid | 653 (28) | 350 (54) | 302 (46) |
| Medicare | 539 (24) | 372 (69) | 167 (31) |
| ACA/ObamaCare | 108 (5) | 65 (60) | 43 (40) |
| Kaiser Permanente | 22 (1) | 12 (55) | 10 (45) |
| Veterans administration | 17 (0.7) | 8 (47) | 9 (53) |
| Other | 44 (2) | 23 (52) | 21 (48) |
| Uninsured | 286 (12) | 164 [57%, 95% CI 51–63] | 121 [42%, 95% CI 37–48] |
| Primary language | |||
| English | 1,851 (80) | 1,095 (59) | 754 (41) |
| Spanish | 344 (15) | 235 (68) | 108 (31) |
| Cantonese/Mandarin | 16 (0.7) | 12 (75) | 4 (25) |
| Other | 79 (3) | 47 (59) | 32 (41) |
| Have you had an influenza vaccine in the last 5 years? | |||
| Yes | 1,561 (68) | 1,076 [69%, 95% CI 67–71] | 483 [31%, 95% CI 29–33] |
| No | 645 (28) | 268 [42%, 95% CI 38–46] | 376 [58%, 95% CI 54–62] |
| Would your family accept a COVID-19 vaccine? | |||
| Yes/some | 1,529 (66) | 1,143 (75) | 385 (25) |
| No/unsure | 693 (30) | 201 (29) | 492 (71) |
IQR, interquartile range; ACA, Affordable Care Act.
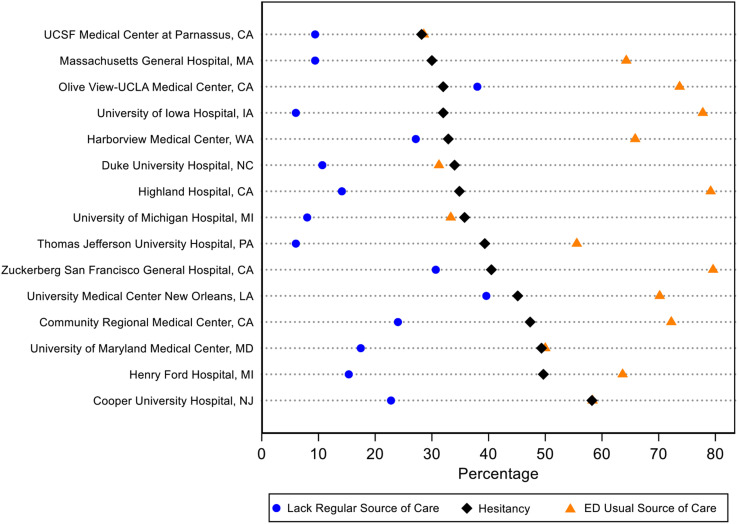
The lowest rate of vaccine hesitancy (28%) was at the UCSF Parnassus ED (San Francisco, CA), which also had the lowest rate of patients who reported the ED as their usual source of care. At the county hospital ED in the same city, Zuckerberg San Francisco General, the hesitancy rate was 40%. The highest vaccine hesitancy rate (58%) was at the Cooper University Hospital site (Camden, NJ) (Figure ).
Figure.
Vaccine hesitancy, lack of regular source of medical care, and ED as usual source of medical care, stratified by 15 study sites.
Reasons for Vaccine Hesitancy
The 3 primary reasons for vaccine hesitancy were similar for those with and without a source of regular medical care: concerns about side effects and safety (65%), need for more information (47%), and having heard stories in the media or online (24%). The fourth most common reason for respondents who had primary care was “don’t believe the vaccine will work”; in respondents who lacked primary care, the fourth most common reason was “not worried about getting COVID-19 infection” (Table 2 ).
Table 2.
Declared reasons for vaccine hesitancy.
| What are Reasons for not Accepting a COVID-19 Vaccine? n (%) |
All N=900 | Have Primary Care/Clinic N=698 | Lack Primary Care/Clinic N=197 |
|---|---|---|---|
| Have concerns about side effects and safety | 582 (65%) | 470 (67%) | 110 (56%) |
| Need more information about the vaccine | 421 (47%) | 324 (46%) | 94 (48%) |
| Heard media stories that gave me doubt about vaccines | 218 (24%) | 167 (24%) | 50 (25%) |
| Don't believe the vaccine will work | 111 (12%) | 81 (12%) | 29 (15%) |
| Not worried about getting COVID-19 infection | 86 (10%) | 55 (8%) | 31 (16%) |
| Already had COVID-19 infection | 34 (4%) | 25 (4%) | 9 (5%) |
| Other | 180 (20%) | 136 (20%) | 44 (22%) |
Sites for Receipt of COVID-19 Vaccine
Of the 1,392 (61%) respondents who stated they would accept the COVID-19 vaccine, 1,100 (79%) had a primary clinic at which to get it. The vast majority of all vaccine acceptors (93%) and patients who reported the ED as their usual source of care (95%) reported that they would accept the COVID-19 vaccine as part of their care in the ED.
Limitations
Our study is subject to the limitations inherent to survey-type research—most notably, various elements of spectrum bias. All of the sites in this study were urban EDs affiliated with academic medical institutions; our findings may not apply to rural, nonacademic ED populations. We only surveyed people who actually came to the EDs; people who have the greatest distrust of health care and highest vaccine hesitancy may be less likely to come to an ED (or any health care facility) for care. We had numerous exclusion criteria for this study, including critical illness, major trauma, and altered mental status, which limit the applicability of our findings to the fully alert and less-ill segment of the ED population. Nevertheless, most of these excluded patients would be unable to meaningfully participate in COVID-19 vaccine messaging programs; they would likewise be ineligible to receive COVID-19 vaccines in the ED. In other words, our sampling precisely reflects the population that would practically benefit from ED-based COVID-19 interventions.
Although we employed best practice methods for survey development and standard questions to assess vaccine hesitancy, our instrument was not independently validated. Despite using neutral tones and reading questions directly from survey instruments, we may have induced a social desirability bias in participant responses, which would likely inflate rates of vaccine acceptance.
Discussion
Conducted in a real-world, health care safety net setting, the Rapid Evaluation of COVID-19 Vaccination in EDs for Underserved Patients study defines populations who are distinctly vulnerable to vaccine hesitancy and poor health care access for receipt of vaccines—those who lack a regular source of medical care and whose primary health care access is through EDs. Nearly half of these groups were COVID-19-vaccine hesitant, and over one fifth of those who would accept a COVID-19 vaccine reported that they did not have a clinic or physician they could visit to readily get it. Notably, two thirds of patients who lacked a regular source of medical care and used the ED as a usual source of care in our EDs were Black or Latinx, racial and ethnic groups that have experienced disproportionately high morbidity and mortality during the COVID-19 pandemic. Supporting the notion that these populations have traditionally suffered other vaccine-related health care disparities is our finding that less than half had received an influenza vaccine in the past 5 years.
Toward herd immunity and greater acceptance and delivery of the COVID-19 vaccine in vulnerable populations, our research highlights an ideal site for interventions—the ED. Homelessness, poverty, language difficulties, and other factors may render traditional internet-, television-, radio-, and social media-based vaccine hesitancy messaging platforms ineffective for these groups. Even in those who do have access to media, messages that are directed at socioeconomically dissimilar populations may not resonate with them or convince them to accept the COVID-19 vaccine. Side effects and misinformation were commonly reported concerns among vaccine-hesitant responders in our study, and approximately half stated that they wanted more information about the vaccines. Considering that nearly two thirds of respondents who lacked a regular source of medical care stated that their usual care occurred in EDs, ED health care personnel may become their de facto primary care providers and consequently serve as their best trusted messengers to promote COVID-19 vaccine acceptance.
Similarly, the ED has great potential to overcome the other barrier to COVID-19 vaccination for these groups—perceived lack of a health care site for receipt of the vaccine. Because most sites in this study conducted their surveys when vaccines were available only to health care workers and nursing home residents, we did not ask questions about attempts to obtain the COVID-19 vaccine. Nevertheless, the vast majority of respondents who had received an influenza vaccine had received it at their primary care clinic, and many vaccine acceptors reported not having a clinic at which to get the COVID-19 vaccine. Furthermore, current internet-based signups and drive-through mass vaccinations may not be feasible mechanisms for COVID-19 vaccination of many vulnerable populations who lack internet access and cars.39, 40, 41 In addition to messaging about safety and efficacy of the vaccine, ED providers can inform patients where, when, and how they can get the COVID-19 vaccine, perhaps assisting them in scheduling appointments prior to discharge from the ED.
An even more ambitious role for the ED is as a site for actual COVID-19 vaccine administration, analogous to the current practice in many EDs of providing influenza vaccines to their patients as part of their ED care for other problems. Almost all vaccine acceptors, including 95% of patients who reported the ED as their usual source of care, stated that they would accept a COVID-19 vaccine as part of their ED care. Some EDs have already adopted the practice of using “end-of-the-day” leftover supplies of vaccines from their affiliated hospital vaccine sites.42 In terms of practicality of broader ED-based immunization programs, single-dose (Johnson and Johnson) vaccines would alleviate the problems of scheduling return visits for a second COVID-19 injection.43 One-shot, ED-based COVID-19 vaccine delivery programs may be particularly useful for homeless persons, many of whom derive most of their health care and, at times, subsistence needs in EDs, as well as for others who do not have pre-established hospital connections.31 , 44 , 45 Undocumented immigrants, many of whom fear discovery and deportation when providing personal information,46 would also benefit from a limited, one-time interaction for COVID-19 vaccination in the ED. Although feasibility constraints and interference with critical ED workflow preclude converting EDs into mass vaccination sites for the general population, opportunistically vaccinating patients while they are already in EDs for other reasons could leverage EDs’ great visit volumes (139 million visits in 201721) and lead to substantially greater COVID-19 vaccine delivery to vulnerable populations whose only health care occurs there. In a survey that our team conducted with the American College of Emergency Physicians, a majority of ED medical directors indicated support for such adjunctive ED-based COVID-19 immunization programs.47
As compared to other online- and telephone-based investigations of the national landscape regarding vaccine hesitancy, our high-response-rate, in-person survey of all eligible ED patients has several notable advantages. First, it reduces the sampling bias inherent in other methods, allowing for the potential inclusion of those who do not have internet access and those who do not respond to telephone surveys. Second, views expressed in true health care environments are likely to represent true health care decisions more accurately than those expressed over the phone or through anonymous internet surveys. Finally, our site stratification provides granular information that may inform local efforts to address vaccine hesitancy at specific institutions.
Our findings are similar to those of other surveys with regard to the higher rates of vaccine hesitancy in Blacks and Latinos.11, 12, 13, 14 This vaccine hesitancy gap threatens to further exacerbate the existing disproportionate effects of the COVID-19 pandemic on Blacks, Latinos, and other vulnerable communities.48, 49, 50, 51, 52 Despite having over twice the age-adjusted death rates of Whites, Blacks and Latinos have had approximately half the vaccination rates.53 , 54 Of the 57 million people in the United States who had received a COVID-19 vaccine as of March 6, 2021, only 7% were Black and 8.5% were Latinx, compared to 65.3% non-Hispanic Whites.54 Given that they serve high proportions of Blacks and Latinos, EDs are uniquely positioned to address COVID-19 and other health care disparities. In terms of other characteristics, younger age, female sex, and not having previously received influenza vaccines were powerful predictors of vaccine hesitancy, but homelessness and uninsured status were not.
The reasons for COVID-19 vaccine hesitancy found in our study mirror those reported in research regarding vaccines in the setting of other infectious disease outbreaks.55 , 56 In a survey conducted in Detroit during a hepatitis A outbreak, 23% of homeless individuals reported hesitation in receiving hepatitis A immunization, citing safety and efficacy concerns as well as mistrust of the intentions of health care providers and vaccine manufacturers.55 Among a predominantly Latinx population of homeless individuals in New York City, concerns regarding becoming ill as a result of the vaccine was the most common reason cited for not receiving the influenza vaccination during the 2018 to 2019 influenza season.56
In conclusion, we have identified populations—those who lack a regular source of medical care and whose principal health care access occurs in EDs—that are particularly vulnerable to vaccine hesitancy and perceive limited access to sites for receipt of the COVID-19 vaccine. National programs for ED-based COVID-19 vaccine messaging and vaccine delivery should be considered for these highly vulnerable populations.
Acknowledgment
The REVVED UP Investigators: Graham Nichol, MD, MPH, Blair A. Parry, Alaina Hunt, BA, Morgan Kelly, BS, Breena R. Taira, MD, MPH, Michael Pham, Joshua Tiao, MD, Kyra Lasko, Mayuri Aivale, MPH, Alex Farthing, BA, Nicole Byl, BA, Virginia Chan, BS, Nancy Anaya, MD, Angela H. Wong, BA, Bhanu Chadalawada, MS, Anna Tupetz, PT, DPT.
Footnotes
Please see page 503 for the Editor’s Capsule Summary of this article.
Supervising editor: Donald M. Yealy, MD. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
Author contributions: RMR and JT designed the study and performed data analysis. All authors were responsible for study implementation, data acquisition, and manuscript preparation. RMR takes responsibility for the paper as a whole.
All authors attest to meeting the four ICMJE.org authorship criteria:(1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fundingandsupport: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (seewww.icmje.org). The authors have stated that no such relationships exist.
Readers: click on the link to go directly to a survey in which you can provide feedback to Annals on this particular article.
A podcast for this article is available at www.annemergmed.com.
Contributor Information
The REVVED UP Investigators:
Graham Nichol, Blair A. Parry, Alaina Hunt, Morgan Kelly, Breena R. Taira, Michael Pham, Joshua Tiao, Kyra Lasko, Mayuri Aivale, Alex Farthing, Nicole Byl, Virginia Chan, Nancy Anaya, Angela H. Wong, Bhanu Chadalawada, and Anna Tupetz
Supplementary Data
References
- 1.United States COVID-19 Cases, Deaths, and Laboratory Testing (NAATs) by State, Territory, and Jurisdiction Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
- 2.Ngonghala C.N., Iboi E., Eikenberry S., et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel Coronavirus. Math Biosci. 2020;325:108364. doi: 10.1016/j.mbs.2020.108364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyu W., Wehby G.L. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US. Health Aff (Millwood) 2020;39:1419–1425. doi: 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- 4.Crane MA, Shermock KM, Omer SB, et al. Change in reported adherence to nonpharmaceutical interventions during the COVID-19 pandemic, April-November 2020. JAMA. Published online January 22, 2021. https://doi.org/10.1001/jama.2021.0286 [DOI] [PMC free article] [PubMed]
- 5.Pandemic fatigue: reinvigorating the public to prevent COVID-19 World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/335820/WHO-EURO-2020-1160-40906-55390-eng.pdf Accessed February 5, 2021.
- 6.Paltiel A.D., Schwartz J.L., Zheng A., et al. Clinical outcomes of a COVID-19 vaccine: implementation over efficacy. Health Aff (Millwood) 2021;40:42–52. doi: 10.1377/hlthaff.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frederiksen L.S.F., Zhang Y., Foged C., et al. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front Immunol. 2020;11:1817. doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontanet A., Cauchemez S. COVID-19 herd immunity: where are we? Nat Rev Immunol. 2020;20:583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KFF COVID-19 vaccine monitor: December 2020. Hamel L., Kirzinger A., Munana C., et al. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
- 10.McAteer J., Yildirim I., Chahroudi A. The vaccines Act: deciphering vaccine hesitancy in the time of COVID-19. Clin Infect Dis. 2020;71:703–705. doi: 10.1093/cid/ciaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szilagyi P.G., Thomas K., Shah M.D., et al. National trends in the US public’s likelihood of getting a COVID-19 vaccine–April 1 to December 8, 2020. JAMA. 2020;325:396–398. doi: 10.1001/jama.2020.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38:6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black Americans face higher COVID-19 risks, are more hesitant to trust medical scientists, get vaccinated. Gramlich J., Funk C. https://www.pewresearch.org/fact-tank/2020/06/04/black-americans-face-higher-covid-19-risks-are-more-hesitant-to-trust-medical-scientists-get-vaccinated/ Accessed February 5, 2021.
- 14.Fisher K.A., Bloomstone S.J., Walder J., et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann Intern Med. 2020;173:964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Largent E.A., Persad G., Sangenito S., et al. US public attitudes toward COVID-19 vaccine mandates. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.33324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gostin L.O., Salmon D.A., Larson H.J. Mandating COVID-19 vaccines. JAMA. 2021;325:532–533. doi: 10.1001/jama.2020.26553. [DOI] [PubMed] [Google Scholar]
- 17.Safi A.G., Reyes C., Jesch E., et al. Comparing in person and internet methods to recruit low-SES populations for tobacco control policy research. Soc Sci Med. 2019;242:112597. doi: 10.1016/j.socscimed.2019.112597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMorris B.J., Petrie R.S., Catalano R.F., et al. Use of web and in-person survey modes to gather data from young adults on sex and drug use: an evaluation of cost, time, and survey error based on a randomized mixed-mode design. Eval Rev. 2009;33:138–158. doi: 10.1177/0193841X08326463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonevski B., Randell M., Paul C., et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42. doi: 10.1186/1471-2288-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellermann A.L., Hsia R.Y., Yeh C., et al. Emergency care: then, now, and next. Health Aff (Millwood) 2013;32:2069–2074. doi: 10.1377/hlthaff.2013.0683. [DOI] [PubMed] [Google Scholar]
- 21.National Hospital Ambulatory Medical Care Survey: 2017 Emergency Department Summary Tables Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf Accessed January 23, 2021.
- 22.Lane B.H., Mallow P.J., Hooker M.B., et al. Trends in United States emergency department visits and associated charges from 2010 to 2016. Am J Emerg Med. 2020;38:1576–1581. doi: 10.1016/j.ajem.2019.158423. [DOI] [PubMed] [Google Scholar]
- 23.HCUP Fast Stats - Trends in emergency department visits Agency for Healthcare Research and Quality. https://www.hcup-us.ahrq.gov/faststats/NationalTrendsEDServlet
- 24.Weber E.J., Showstack J.A., Hunt K.A., et al. Does lack of a usual source of care or health insurance increase the likelihood of an emergency department visit? Results of a national population-based study. Ann Emerg Med. 2005;45:4–12. doi: 10.1016/j.annemergmed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Health, United States 2017 – Data Finder. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/hus/contents2017.htm
- 26.Walls C.A., Rhodes K.V., Kennedy J.J. The emergency department as usual source of medical care: estimates from the 1998 National Health Interview Survey. Acad Emerg Med. 2002;9:1140–1145. doi: 10.1111/j.1553-2712.2002.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 27.Arnett M.J., Thorpe R.J., Jr., Gaskin D.J., et al. Race, medical mistrust, and segregation in primary care as usual source of care: findings from the Exploring Health Disparities in Integrated Communities study. J Urban Health. 2016;93:456–467. doi: 10.1007/s11524-016-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang N., Stein J., Hsia R.Y., et al. Trends and characteristics of US emergency department visits, 1997-2007. JAMA. 2010;304:664–670. doi: 10.1001/jama.2010.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez R.M. Commentary: embracing the problem of subsistence needs in the emergency department. Ann Emerg Med. 2019;74:S28–S30. doi: 10.1016/j.annemergmed.2019.08.438. [DOI] [PubMed] [Google Scholar]
- 30.Wilson K.M., Klein J.D. Adolescents who use the emergency department as their usual source of care. Arch Pediatr Adolesc Med. 2000;154:361–365. doi: 10.1001/archpedi.154.4.361. [DOI] [PubMed] [Google Scholar]
- 31.Raven M.C., Tieu L., Lee C.T., et al. Emergency department use in a cohort of older homeless adults: results from the HOPE HOME study. Acad Emerg Med. 2017;24:63–74. doi: 10.1111/acem.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez R.M., Baraff L.J. Emergency department immunization of the elderly with pneumococcal and influenza vaccines. Ann Emerg Med. 1993;22:1729–1732. doi: 10.1016/s0196-0644(05)81313-7. [DOI] [PubMed] [Google Scholar]
- 33.Wrenn K., Zeldin M., Miller O. Influenza and pneumococcal vaccination in the emergency department: is it feasible? J Gen Intern Med. 1994;9:425–429. doi: 10.1007/BF02599056. [DOI] [PubMed] [Google Scholar]
- 34.Kapur A.K., Tenenbein M. Vaccination of emergency department patients at high risk for influenza. Acad Emerg Med. 2000;7:354–358. doi: 10.1111/j.1553-2712.2000.tb02237.x. [DOI] [PubMed] [Google Scholar]
- 35.Slobodkin D., Zielske P.G., Kitlas J.L., et al. Demonstration of the feasibility of emergency department immunization against influenza and pneumococcus. Ann Emerg Med. 1998;32:537–543. [PubMed] [Google Scholar]
- 36.Stack S.J., Martin D.R., Plouffe J.F. An emergency department-based pneumococcal vaccination program could save money and lives. Ann Emerg Med. 1999;33:299–303. doi: 10.1016/s0196-0644(99)70366-5. [DOI] [PubMed] [Google Scholar]
- 37.Martin D.R., Brauner M.E., Plouffe J.F. Influenza and pneumococcal vaccinations in the emergency department. Emerg Med Clin North Am. 2008;26:549–570. doi: 10.1016/j.emc.2008.02.004. xi. [DOI] [PubMed] [Google Scholar]
- 38.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.How to Get a COVID-19 Vaccine in Los Angeles without a car. Rylah J.B. https://www.welikela.com/how-to-get-a-covid-19-vaccine-in-los-angeles-without-a-car/ Accessed January 27, 2021.
- 40.How inequity gets built into America's vaccination system. Jameel M., Chen C. https://www.propublica.org/article/how-inequity-gets-built-into-americas-vaccination-system Accessed March 4, 2021.
- 41.In Mississippi, Black residents are desperate to get vaccinated. But they face access barriers. Harris B. https://www.nbcnews.com/news/us-news/mississippi-black-residents-are-desperate-get-vaccinated-they-face-access-n1256652 Accessed February 11, 2021.
- 42.UNC Health turns to Twitter to make sure leftover COVID vaccine doses are used. Wagner A. https://www.newsobserver.com/news/coronavirus/article250183070.html
- 43.Oliver S.E., Gargano J.W., Scobie H., et al. The Advisory Committee on immunization practices’ interim recommendation for use of Janssen COVID-19 vaccine–United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:329–332. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez R.M., Fortman J., Chee C., et al. Food, shelter and safety needs motivating homeless persons' visits to an urban emergency department. Ann Emerg Med. 2009;53:598–602. doi: 10.1016/j.annemergmed.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 45.Covid-19 vaccine is a struggle for those with no hospital connection. Findell E. https://www.wsj.com/articles/covid-19-vaccine-is-a-struggle-for-those-with-no-hospital-connection-11614508201
- 46.Maldonado C.Z., Rodriguez R.M., Torres J.R., et al. Fear of discovery among Latino immigrants presenting to the emergency department. Acad Emerg Med. 2013;20:155–161. doi: 10.1111/acem.12079. [DOI] [PubMed] [Google Scholar]
- 47.ED medical directors share COVID-19 needs in survey American College of Emergency Physicians. https://www.acep.org/corona/COVID-19-alert/covid-19-articles/ed-medical-directors-share-covid-19-needs-in-survey/
- 48.Karmakar M., Lantz P.M., Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bibbins-Domingo K. This time must be different: disparities during the COVID-19 pandemic. Ann Intern Med. 2020;173:233–234. doi: 10.7326/M20-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore J.T., Ricaldi J.N., Rose C.E., et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5-18, 2020-22 states, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooper M.W., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The color of coronavirus: COVID-19 deaths analyzed by race and ethnicity APM Research Lab. https://www.apmresearchlab.org/covid/deaths-by-race
- 53.Latest data on COVID-19 vaccinations Race/Ethnicity. Ndugga N., Pham O., Hill L., et al. https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/
- 54.Demographic characteristics of people receiving COVID-19 vaccinations in the United States Centers of Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic Accessed March 10, 2021.
- 55.Buechler C.R., Ukani A., Elsharawi R., et al. Barriers, beliefs, and practices regarding hygiene and vaccination among the homeless during a hepatitis A outbreak in Detroit, MI. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G., Kazmi M., Chen D., et al. Identifying associations between influenza vaccination status and access, beliefs, and sociodemographic factors among the uninsured population in Suffolk County, NY. J Community Health. 2020;45:1236–1241. doi: 10.1007/s10900-020-00873-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.