Abstract
Background
Heart failure (HF) patients with CRT devices are a vulnerable patient population during the Coronavirus Disease 2019 (COVID-19) Pandemic. It is important to develop innovative virtual care models to deliver multidisciplinary care while minimizing the risk of SARS-CoV2 exposure.
Objective
We aim to provide a description of how HF patients with CRT devices were assessed and managed in our virtual multidisciplinary clinic during the COVID-19 Pandemic. Clinical outcomes between this group of patients seen in virtual clinic and a historical cohort followed by in-person multi-disciplinary clinic prior to the pandemic were compared.
Method
This is a retrospective cohort study of HF patients with CRT implants who were seen in the virtual multidisciplinary clinic from March 18th, 2020 to May 27th, 2020 (Virtual Visit Group, N = 43). A historical cohort of HF patients with CRT devices seen in the ReACT clinic in person during the same calendar time period in 2019 was used as a control group (In-Person Visit Group, N = 39). Both groups were followed until July 1st of the same calendar year (2020 or 2019) for clinical events. The primary outcome measure was a combined outcome of all-cause mortality and HF- or device-related hospitalizations during follow-up. The secondary outcome measures included patient satisfaction, COVID-19 infection, and other cardiovascular events.
Results
In the Virtual-Visit Group, 21 patients (48.8%) had their initial ReACT clinic visit (first visit after CRT implant) as a virtual visit; 22 patients (51.2%) had prior in-person ReACT clinic visits before the first virtual visit. During the virtual visits, 12 patients had either potential cardiac symptoms or significant device interrogation findings that required clinical intervention. In post-virtual clinic patient satisfaction survey, all 22 patients surveyed (100%) reported being very satisfied or satisfied with the overall experience of the virtual clinic, and every patient (100%) said they would like to use telemedicine again. During a median follow-up period of 82 days (interquartile range [IQR] 61–96 days), one patient died from pneumonia of unclear etiology at an outside hospital, without documentation of COVID-19 positivity. No patient was hospitalized for HF- or arrhythmia-related complications. No patient was diagnosed with COVID-19. Compared with the In-Person Visit Group, there was no significant increase in mortality or major cardiovascular events in the Virtual-Visit Group (2.3% versus 5.1%, P = 0.60).
Conclusions and Relevance
Virtual multidisciplinary care was feasible for HF patients with cardiac resynchronization therapy devices and achieved good patient satisfaction. Virtual care was not associated with short-term increase in adverse events for HF patients with CRT device during the COVID-19 Pandemic. This virtual care model could help promote the adoption of digital health methodology for high-risk patients with multiple cardiac comorbidities.
Keywords: CRT, Telemedicine, Multidisciplinary care, Patient outcomes
1. Introduction
The COVID-19 pandemic has prompted the widespread adoption and rapid implementation of telemedicine. The imperative of social distancing in the face of a highly infectious virus has transformed ambulatory care and necessitated the use of virtual communication platforms between providers and patients [1]. Heart failure (HF) patients with implanted cardiac resynchronization therapy (CRT) devices constitute a unique patient population that are both particularly vulnerable, due to their underlying cardiovascular disease, and require care by multiple subspecialists, including HF and cardiac electrophysiology providers. They are therefore potentially well-positioned for transitioning into the virtual care model with existing implantable devices and remote monitoring infrastructure.
The Resynchronization and Advanced Cardiac Therapeutics (ReACT) Program at Massachusetts General Hospital (MGH) is an established multidisciplinary program including subspecialists from the HF, electrophysiology, and echocardiography service aiming to optimize care for HF patients with CRT devices [2]. Through this program, integrated care is delivered to CRT patients at 1 month, 3 months, and 6 months post-CRT implant with occasional follow up at later time points if needed. Patients graduate after these 3 visits and follow up with their HF cardiologist and/or electrophysiologist. A prior study has shown that this coordinated, multi-disciplinary approach, that included optimization of device programming with electrocardiographic or echocardiographic guidance, guideline-directed medication titration and close coordination among the HF and electrophysiology specialists for outpatient diuretic management has led to 38% reduction in the risk for HF hospitalization, cardiac transplantation and all-cause mortality over a two-year period [2]. During the COVID-19 pandemic, we have taken this established multidisciplinary care approach on a virtual platform to deliver high quality patient care in a safe and effective manner. In this study, we provide a detailed overview of this transition and evaluate the impact of implementing the virtual multidisciplinary care model for HF patients with CRT devices.
2. Methods
2.1. Study population
We retrospectively constructed a cohort of all consecutive HF patients with CRT devices seen at the virtual multidisciplinary clinic in our hospital between March 18th, 2020 and May 27th, 2020. The dates were contemporaneous with the ‘shutdown’ of the hospital to outpatient clinic visits and the subsequent allowance of outpatient clinic visits following decline in COVID-19 cases. The virtual clinic is part of the existing ReACT program, an integrated multidisciplinary clinic in our hospital for HF patients with CRT devices. A total of 45 patients were seen at the virtual ReACT clinic during this period. Forty-three patients were eligible for this study, while 2 patients were excluded for not having CRT devices. These patients constituted the Virtual Visit Group (N = 43) and were compared with patients seen at the ReACT clinic during the same calendar time period in 2019 (In-Person Visit Group, N = 39), prior to the COVID-19 Pandemic.
2.2. The virtual multidisciplinary clinic
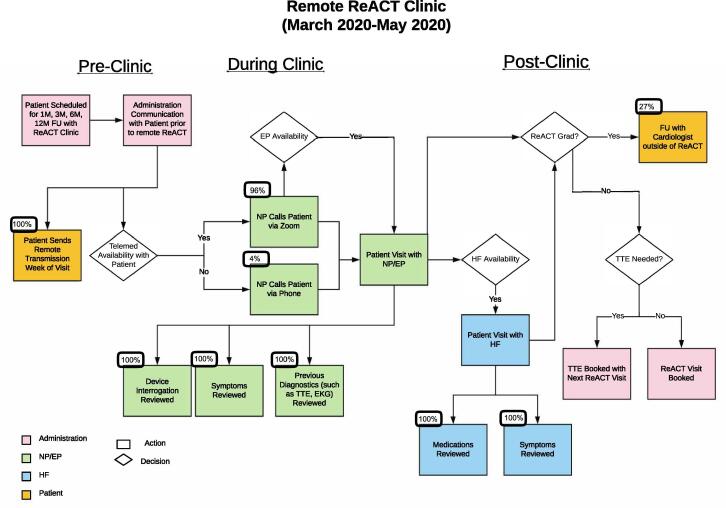
As described in previous reports [2], the ReACT clinic in our hospital was first established in November 2005 to provide multidisciplinary care to HF patients with CRT devices. The ReACT program, designed to integrate all the available electrical therapies for HF patients in one setting, was developed in 2015 and built upon the existing CRT clinic. To establish a new virtual model for patient care during the COVID-19 pandemic, the ReACT team carefully assessed the existing program and established the following workflow for virtual ReACT clinic (Fig. 1):
-
1.
Before each visit, the patient was contacted to confirm the virtual visit’s time and modality (by a Zoom interface integrated with our Epic electronic medical record or by telephone) and was instructed to send a remote interrogation the week of their visit. The remote interrogation was made available to the ReACT clinic practitioner to discuss with the patient.
-
2.
During the visit, the nurse practitioner and electrophysiologist would review patients’ self-measured vital signs, symptoms, clinical events, medications, device interrogation data, along with the other diagnostic information including any prior transthoracic echocardiogram (TTE), cardiac MRI, or Holter monitoring, etc. The nurse practitioner and electrophysiologist would assess whether there was any need for further diagnostic evaluation, adjustment for the CRT device or medication changes.
-
3.
Depending on availability, the HF specialist would either join during the same call or set up a separate virtual visit for HF management on the same day.
-
4.
All clinical providers would discuss the patients seen on a teleconference at the end of the clinic visit.
-
5.
In comparison to the regular in-person visits to the ReACT clinic, there was no physical exam, 12-lead electrocardiogram (EKG), in-person device interrogation, 6-minute-walk test or in-clinic TTE, which in the past had been used to adjust CRT device settings if deemed necessary by the supervising electrophysiologist.
Fig. 2.
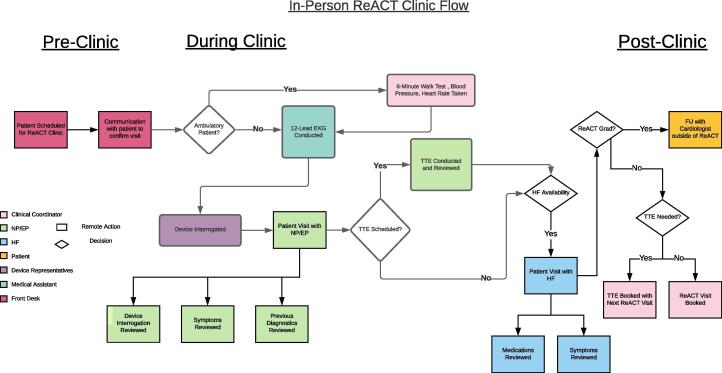
In-Person ReACT Clinic Workflow This figure demonstrates the flow of the in-person remote ReACT clinic. The color legend shows all members involved: the clinical coordinator, NP/EP, HF, patient, device representative, medical assistant, and front desk.
Fig. 1.
Remote ReACT Clinic Workflow This figure demonstrates the flow of the remote ReACT clinic, divided in the pre, during, and post clinic phases. See legend for color correspondence with role as well as shape correspondence with decision or action. This clinic flow was rapidly adapted from the in-person flow shown in Fig. 2. Roles in administration (front desk), NP/EP, HF, and patient.
2.3. Data collection procedures
The collection of data for all patients seen in the ReACT clinic had been approved previously by our institutional IRB committee. A patient identification log was generated that included all consecutive HF patients with CRT devices seen at the ReACT program between March 18th, 2020 and May 27th, 2020 (Virtual Visit Group), as well as between March 18th, 2019 and May 27th, 2019 (In-Person Visit Group). Clinical events between March 18th, 2020 and July 1st, 2020 (Virtual Visit Group) and March 18th, 2019 and July 1st, 2019 (In-Person Visit Group) were included in the analysis. Each patient was given a unique identifier. Each medical record was reviewed and an initial data collection form was completed by a trained data collector. Patient demographics, self-reported vitals and symptoms, comorbidities, device interrogations, medications, hospitalizations and mortality data were collected from electronic medical records. The hospitalizations and mortality data were adjudicated by a second data collector blinded to the patient’s group, and it was noted that every patient had seen or spoken to a provider at MGH at or after July 1st, 2020 to confirm any clinical events in the study period. A patient satisfaction survery of the Virtual Visit Group was conducted over phone call by ReACT clinic staff after the virtual visit as part of a quality improvement effort to be presented at monthly Quality Improvement and Safety meetings of the Cardiac Arrhythmia Service. The survey questions were modified from the Telehealth Satisfaction Scale (TeSS) [3].
2.4. Statistical analysis
Categorical data were expressed as number and percentage, and continuous data as mean ± standard deviation or median (interquartile range). Chi-square test or Fisher’s exact test (if the expected count in any cell was < 5) was used for categorical data; student’s t-test was used for continuous data. All tests were two sided and p-value of < 0.05 was considered statistically significant. Statistical analyses were conducted with Microsoft Excel 2011 software version 14.2.0 (Microsoft Corp, Redmond, WA).
3. Results
3.1. Patient demographics
During the period of March 18th, 2020 to May 27th, 2020, a total of 43 HF patients with CRT devices were seen at the virtual multidisciplinary clinic of the ReACT program (Virtual Visit Group). In comparison, between March 18th, 2019 to May 27th, 2019, 39 HF patients with CRT devices were seen at the in-person clinic of the ReACT program (In-Person Group). In the Virtual Visit Group, 21 patients (48.8%) had their first-ever ReACT clinic visit as a virtual visit; 22 patients (51.2%) had prior in-person ReACT clinic visits before the first virtual visit. Patient characteristics can be seen in Table 1. The mean age was 70.4 ± 13.9 years; 13 patients (27.9%) were women; 17 patients (39.5%) had ischemic cardiomyopathy and 27 patients (62.8%) had left bundle branch block pattern on the baseline EKG. The average left ventricular ejection fraction was 28.2 ± 10.4% and the mean NYHA functional class was 2.1 ± 0.7. These measures were not significantly different from the In-Person Visit Group (P > 0.05 for all). The prevalence of atrial fibrillation (55.8%), hypertension (83.7%), diabetes (23.3%) and chronic kidney disease (32.6%) in the Virtual Visit Group was also similar to the In-Person Visit Group (P > 0.05 for all). The use of loop diuretics (69.8% vs. 41.0%, P = 0.01) and spironolactone (34.9% vs. 15.4%, P = 0.05) were more common in the Virtual Visit Group. The use of ACE inhibitors/angiotensin receptor blocker or sacubitril/valsartan was not significantly different between the groups; it should be noted that more patients were on sacubitril/valsartan in the Virtual group, reflecting wider usage and market adoption over time. Overall, patient demographics of the Virtual Visit Group were comparable to the historic In-Person Visit Group.
Table 1.
Patient Demographics.
| Variable | Virtual Visit Group (n = 43) | In-Person Visit Group (n = 39) | P-Value |
|---|---|---|---|
| Baseline Characteristics | |||
| Age (SD) | 70.4 (13.9) | 71.0 (13.9) | 0.85 |
| Female (%) | 13 (27.9) | 10 (25.6) | 0.82 |
| NYHA (SD) | 2.1 (0.7) | 2.1 (0.6) | 1.00 |
| Ischemic cardiomyopathy (%) | 17 (39.5) | 19 (48.7) | 0.40 |
| Baseline LVEF% (SD) | 28.2 (10.4) | 31.2 (11.5) | 0.22 |
| Left bundle branch block (%) | 27 (62.8) | 17 (43.6) | 0.08 |
| Comorbidities | |||
| Atrial Fibrillation (%) | 24 (55.8) | 16 (41.0) | 0.18 |
| Hypertension (%) | 36 (83.7) | 30 (76.9) | 0.44 |
| Diabetes Mellitus (%) | 10 (23.3) | 8 (20.5) | 0.76 |
| Chronic Kidney Disease (%) | 14 (32.6) | 12 (30.8) | 0.86 |
| Medications | |||
| ACE Inhibitor or ARB (%) | 18 (41.9) | 20 (51.3) | 0.51 |
| Sacubitril / Valsartan (%) | 16 (37.2) | 10 (25.6) | 0.34 |
| Beta-Blocker (%) | 38 (88.4) | 31 (79.5) | 0.27 |
| Loop Diuretics (%) | 32 (69.8) | 16 (41.0) | 0.01 |
| Spironolactone (%) | 15 (34.9) | 6 (15.4) | 0.05 |
3.2. Clinical findings and interventions during virtual multidisciplinary Visit
Of the 43 patients who presented for virtual multidisciplinary visits, 20 patients (46.5%) reported their self-recorded weight and 11 patients (25.6%) reported self-recorded blood pressure (Table 2). During the virtual visits, 12 patients had either potential cardiac symptoms or significant device interrogation findings that required clinical intervention, including medication changes and/or in-person device adjustments. Seven patients reported potential cardiac symptoms, including 3 patients (7.0%) with dyspnea on exertion, 2 (4.7%) with palpitations, 2 (4.7%) with fatigue, 1 (2.3%) with dizziness, 1 (2.3%) with recurrent belching and nausea (Table 2). Device interrogation showed the mean percentage of biventricular pacing was 95.2%, with 33 patients (76.7%) having at least 95% biventricular pacing (Table 2). One patient who developed recurrent belching and nausea was suspected to have phrenic nerve stimulation on device interrogation. In addition, 1 patient developed new onset of atrial fibrillation, and 3 patients had frequent premature ventricular contractions. HeartLogic score was recorded for 19 patients with Boston Scientific devices, while OptiVol fluid index was collected for 20 patients with Medtronic devices. Two patients had evidence of fluid overload on device diagnostics (one with HeartLogic score ≥ 16 and the other with rising OptiVol fluid index) (Table 2, Supplemental Table 1).
Table 2.
Clinical Findings during Virtual Multidisciplinary Visit.
| Patient Reported Vitals and Symptoms (N = 43, %) | |
|---|---|
| Patients who recorded weight | 20 (46.5) |
| Patients who recorded blood pressure | 11 (25.6) |
| Chest pain | 0 (0.0) |
| Shortness of breath | 3 (7.0) |
| Fatigue | 4 (9.3) |
| Palpitations | 2 (4.7) |
| Syncope or Pre-syncope | 1 (2.3) |
| Lower extremity edema | 0 (0.0) |
| Phrenic nerve stimulation | 1 (2.3) |
| Remote Device Interrogations (N = 43, %) | |
| DDD or DDDR mode | 33 (76.7) |
| VVI or VVIR mode | 10 (23.3) |
| Percentage of BiV pacing (SD) | 95.17 (10.0) |
| Presence of AF | 10 (23.7) |
| Thoracic impedance (N = 35, %) | |
| Stable | 23 (65.7) |
| Increasing | 10 (28.6) |
| Decreasing | 2 (5.7) |
| Activity level (N = 39, %) | |
| <1 h daily | 13 (33.3) |
| 1–2 h daily | 17 (43.6) |
| 2–4 h daily | 4 (10.3) |
| >4 h daily | 5 (12.8) |
| Device fluid diagnostics (N = 32, %) | |
| HeartLogic score ≥ 16 | 1 (3.1) |
| Rising Optivol fluid index | 1 (3.1) |
Clinical findings during the virtual visits led to further evaluations and interventions. For example, the patient with suspected phrenic nerve stimulation underwent reprogramming of LV pacing polarity after they were brought in for an in-person visit, while a second patient noted to have LV non-capture (Table 3) was scheduled for an elective admission for lead extraction and placement of a new coronary sinus lead. One patient with clinical signs of volume overload and rising OptiVol fluid index had outpatient adjustment of diuretic doses and was started on sacubitril-valsartan. At the other extreme, several patients with a high level of physical activity noted on device interrogation, along with a history of clinical improvement were considered to be ‘responders’ to CRT and no adjustments were made to their medications or device settings. Details of the clinical findings and corresponding interventions for each patient are shown in Table 3. During the study period, 12 patients (28%) graduated from virtual multidisciplinary clinic and started regular cardiology follow-up.
Table 3.
Clinical Interventions Based on Findings During Virtual or In-Person Visit.
| Patient ID | Clinical Findings | Interventions |
|---|---|---|
| 3 | Worsened dyspnea and weight gain, with rising OptiVol fluid index to 80, frequent PVCs | Adjusted diuretic dose, added sacubitril-valsartan |
| 6 | Dizziness | Discontinued spironolactone |
| 8 | Repeated belching and nausea | Diagnosed phrenic nerve stimulation; reprogrammed LV pacing polarity during subsequent in-person visit |
| 9 | New AF | Started apixaban |
| 16* | Frequent PVCs | Continued to monitor |
| 19 | Frequent PVCs | Increased metoprolol dose |
| 23 | Fatigue and decline in stamina | Lead extraction was performed for LV lead noncapture and a new LV lead was implanted. |
| 38 | Frequent PVCs | Ordered Holter monitor which showed PVC burden of only 1.6% |
| 39 | HeartLogic score ≥ 16 | Patient was asymptomatic with stable weight, so no further intervention was taken, but was flagged for close follow-up |
| 40 | Decreased activity tolerance, AF with rapid ventricular rate | Increased metoprolol dose for rate control |
| 41 | Palpitations | Started mexiletine for suppression of PVCs |
| 42 | Dyspnea | Continued to follow as it was chronic and likely related to severe mitral regurgitation |
Patient No.16 passed away during the follow-up period after being admitted to OSH for “suspected pneumonia”, without mentioning the result of COVID-19 test.
3.3. Patient satisfaction survey after virtual multidisciplinary Visit
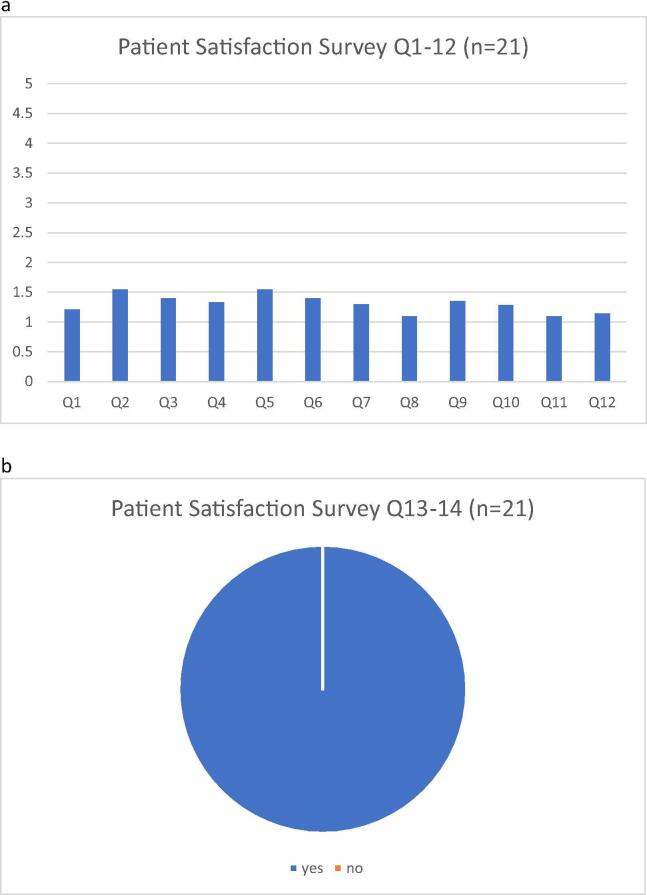
In the patient satisfaction survey conducted for the Virtual-Visit Group, 21 patients (48.8%) were reached by the clinic staff over phone call to answer all or some of the questions on the questionnaire (Table 4). Twenty-two patients (51.2%) either could not be reached over the phone or declined to answer the survey questions. Among patients who answered survey questions, majority of them (>90%) reported either very satisfied or satisfied with the ease of using telehealth and the clinical care they received during the virtual clinic (Table 4, and Fig. 3); every patient (100%) said they would like to use telemedicine again.
Table 4.
Patient Satisfaction Survey For Virtual Clinic Patients.
| Very Satisfied | Satisfied | Fair | Not Satisfied | Poor | NA | |
|---|---|---|---|---|---|---|
| 1. The length of time to get an appointment with telehealth? | 16 | 2 | 1 | 0 | 0 | 22 |
| 2. The ease of getting to the telehealth site? | 13 | 4 | 2 | 0 | 0 | 24 |
| 3. The length of time waiting in the office at telehealth? | 14 | 4 | 2 | 0 | 0 | 23 |
| 4. The length of time with the specialists you saw? | 16 | 3 | 2 | 0 | 0 | 22 |
| 5. The explanation of your condition by the specialist? | 12 | 6 | 1 | 0 | 0 | 24 |
| 6. The explanation of your treatment by the specialist including medications added or changed? | 14 | 4 | 2 | 0 | 0 | 23 |
| 7. The thoroughness, carefulness, and skillfulness of the specialists you saw? | 16 | 2 | 2 | 0 | 0 | 23 |
| 8. The courtesy, respect, sensitivity, and friendliness of the specialists you saw? | 19 | 2 | 0 | 0 | 0 | 22 |
| 9. The ease of connection with the device clinic for monitoring your device? | 12 | 4 | 1 | 0 | 0 | 26 |
| 10. How well the staff here answered your questions about your pacemaker or ICD? | 16 | 4 | 1 | 0 | 0 | 22 |
| 11. How well the staff treated you with respect? | 19 | 2 | 0 | 0 | 0 | 22 |
| 12. Your overall treatment experience at ReACT telehealth with seeing multiple providers in one telehealth visit? | 18 | 3 | 0 | 0 | 0 | 22 |
| Yes | No | |||||
| 13. Would you use Telehealth again? (yes or no) | 21 | 0 | 22 | |||
| 14. Would you recommend telehealth to another person (yes or no) | 21 | 0 | 22 |
(NA = Not able to reach patient or patient did not answer the questions)
Fig. 3.
a/b: Patient Satisfaction Survey Results These figures demonstrate the results of the patient satisfaction survey, adapted from the HTF 402 National First Nations Telehealth Research Project. For Q1-12, a “1″ rating indicated excellent and a “5” rating indicated poor. For Q13-14, every patient responded “yes” to would use telehealth again and to recommend it to someone else.
3.4. Clinical events during Follow-up
For the Virtual-Visit Group, during a median follow-up period of 82 days (IQR 61–96 days), no patients had hospitalization for HF- or arrhythmia-related issues, although as noted above, one patient was found to have LV non-capture leading to an elective procedure for lead extraction and replacement of the coronary sinus lead. While no patients had a confirmed diagnosis of COVID-19, one patient passed away after being diagnosed with pneumonia at an outside hospital without COVID testing being noted. One patient had upper respiratory infection symptoms and was instructed to self-quarantine without being tested for COVID-19, and one patient had a hospital admission for a leg ulcer needing vascular surgery intervention. No patients had other major cardiac events. In comparison, the In-Person Visit Group had two patients admitted for HF during a median follow-up period of 66 days (IQR 45–80 days), otherwise no major cardiac events. Compared with the In-Person Visit Group, there was no significant increase in mortality or major cardiovascular events in the Virtual-Visit Group (2.3% versus 5.1%, P = 0.60).
4. Discussion
While other studies have described the use of telemedicine for patient care during the COVID-19 pandemic [4], [5], to our knowledge, this is the first study to evaluate the adoption of virtual multidisciplinary clinic for HF patients with CRT devices during the COVID-19 Pandemic [6]. The study demonstrated that the virtual clinic achieved good patient satisfaction and did not lead to increased cardiac events or mortality for HF patients with CRT devices during a short follow-up period. Communication via the virtual platform and remote device interrogation promptly triggered appropriate clinical intervention, as detailed in Table 3. No major adverse event including cardiovascular death, cardiac hospitalization or confirmed COVID-19 infection occurred during the follow-up period.
The mandate of social distancing to curb virus transmission in the healthcare setting during the COVID-19 pandemic has accelerated the transition of routine clinics into virtual visits [1]. Recent studies have reported 41–62% of reduction in acute HF hospitalizations during the COVID-19 pandemic [7], [8], possibly due to HF patients avoiding or delaying care due to fear of COVID-19. The short-term results of our study would therefore need to be interpreted with caution. Nevertheless, our study showed that at least for a short period, this transition could be carried out with existing clinical staffs and infrastructure without compromising clinical outcomes.
HF patients with recently implanted CRT devices are often challenging to manage due to their cardiac and non-cardiac comorbidities. The virtual multidisciplinary clinic is an extension of the well-established ReACT program specifically designed for these high-risk patients. Our experience has shown that this multidisciplinary approach could significantly improve the post-device implant care through optimizing device programming and pre-emptively intervening upon potential non-responders of CRT [2]. Studies have also shown that remote monitoring of implantable defibrillators could achieve similar clinical outcomes compared with in-person device follow-ups [9], [10]. It is encouraging to see that via virtual visits and remote monitoring, the majority of the functions of the ReACT clinic could be achieved on a virtual platform. Such experiences could be valuable for the future design and implementation of a virtual care model for high-risk patients with multiple cardiac comorbidities.
In the meantime, we should also recognize that the current virtual platform has not fully satisfied the clinical needs of our patient population. For example, only 46.5% of patients reported weight and merely 25.6% of patients reported blood pressure during the virtual visit. Weight and blood pressure management are fundamental for the care of HF patients. EKG and echocardiographic testing have been critical for evaluation of CRT performance in this clinic, and whether the lack of these modalities translate to worse longer-term outcomes is not clear. Although there are many platforms for patients to monitor these parameters at home, an apparent gap exists in how to integrate these platforms into our virtual care flow [11]. For physical exam, the device pocket, lower extremity edema and jugular venous distention, etc. could all be visualized through live video streaming; while a digital stethoscope has been developed for the auscultation of the heart and lungs during virtual visit but was not performed here [11].
It is important to note that the utilization rate of guideline-directed medical therapy (GDMT) particularly the adoption of Angiotensin Receptor Neprilysin Inhibitors (ARNI) for HF was not ideal in our cohort, partly because many patients in our cohort were followed by local cardiologists and the penetrance of guideline-directed medical therapy in the community would take time to improve. Also, the rate of baseline HF medication use in our paper only reflected one time point prior to the procedure, while the longitudinal trend had directed towards improved utilization rate of GDMT. For example, the rate of vasodilator use (including ACEI/ARB, ARNI) in our cohort was 79.1% in the Virtual Visit group in 2020, which was improved from 76.9% the In-Person Visit group in 2019, primarily driven by the increased adoption of ARNI to 37.2% in 2020 from 25.6% in 2019. Still, some patients may not be able to tolerate sacubitril-valsartan due to hypotension or kidney disease. In addition, some of the HF medications, including sacubitril-valsartan, had expensive copays that patient might have difficulty paying for the drugs, but which have become more accessible over the past year.
Some device algorithms such as the Medtronic OptiVol or the Boston Scientific HeartLogic algorithm integrates data from a diverse set of implanted sensors in the CRT device to give the clinician a metric to follow in terms of heart congestion [12], [13], [14]. In certain cases, these device diagnostic data could provide objective evidence of clinical changes especially when in-person physical exam is not available [12], [13], [14]. At the present time, no prospective studies have shown that the use of OptiVol or HeartLogic could improve HF outcomes. How to integrate these technological solutions into an effective virtual platform requires further study, but we provide examples where device diagnostics led to further interventions, alteration in the type of follow-up, or were helpful in confirming a favorable response to CRT. Twelve-lead EKG, one of the most important diagnostic modalities for the CRT clinic, is another missing part in the virtual clinic. Currently, most application-based EKG monitoring or wearable devices can only take single-lead EKG [11]. Whether these single-lead EKG data could help with remote CRT evaluation is unknown. Interestingly, a recent study reported using Apple Watch to record single-lead EKGs at 3 different positions to achieve more accurate assessment of QT interval [15]. Similar techniques may be applied to remote CRT evaluation in the future. In-person visits are also necessary to obtain TTEs to evaluate ventricular function and identify dyssynchrony. Therefore, in the foreseeable future, the long-term management of HF patients with CRT devices will likely require a combination of virtual and in-person visits.
The COVID-19 pandemic has accelerated the transition from the traditional model to a virtual care model that leverages the advances in remote monitoring, wearable devices, smartphone and video streaming platforms in a favorable policy environment [1], [16]. At the present time, this transition is still in an early phase and much work is needed to effectively integrate the technological solutions into a virtual care platform. Data is also lacking on the impact of such a major transition and whether the new model could achieve equivalent or even superior clinical outcomes. It is foreseeable that some of the shifts during the COVID-19 pandemic will stay for the long-term, and a hybrid model involving both virtual and in-person care will likely become a new norm.
4.1. Limitations
Our study is a single-center, retrospective cohort study with a relatively small sample size and short follow-up period. Additionally, we were only able to reach half the patients for a follow-up patient survey. Although a historic cohort was used for comparison, no conclusion can be drawn on whether the virtual multi-disciplinary clinic is equivalent, superior or inferior to the in-person ReACT clinics. Prospective randomized studies to directly compare virtual or hybrid care model versus in-person only model are needed, but beyond the scope of this initial feasibility study. Further, the patient population of our hospital may not be representative of the general patient population, so the safety and efficacy outcome of the virtual visits may not apply to other HF patients with CRT devices. In addition, passive endpoint assessment from one hospital system’s electronic health record may be insufficient to identify hospitalizations and mortality, although this was somewhat mitigated by the fact that all of patients had subsequent clinical visits or telephone contact with providers documented in the EMR to corroborate the lack of clinical endpoints. As mentioned above, the current virtual multidisciplinary clinic is a developing and incomplete model, for which further improvement is needed.
5. Conclusions
The study demonstrated that virtual multidisciplinary clinic was not associated with short-term increase in adverse events for HF patients with CRT devices during the COVID-19 pandemic. Communication via the virtual platform and remote device interrogation promptly triggered appropriate clinical intervention. While this pilot venture was promising, at this point, no definitive conclusions can be drawn about the long-term sustainability of the virtual clinic model or whether virtual clinic is equivalent, superior or inferior to the in-person clinic. In the foreseeable future, a combination of virtual and in-person clinic is likely required to meet the clinical needs of HF patients with CRT devices and other patients with complex cardiac comorbidities. Further studies are required on how to integrate the various technological advances into a unified virtual platform to achieve optimal patient outcome.
Disclosures/Funding
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100811.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for Cardiac Electrophysiology During the COVID-19 Pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. Published online April 1, 2020. doi:10.1016/j.hrthm.2020.03.028 [DOI] [PMC free article] [PubMed]
- 2.Altman R.K., Parks K.A., Schlett C.L. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J. 2012;33(17):2181–2188. doi: 10.1093/eurheartj/ehs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan D.G., Kosteniuk J., Stewart N., O’Connell M.E., Karunanyake C., Beever R. The Telehealth Satisfaction Scale (TeSS): Reliability, validity, and satisfaction with telehealth in a rural memory clinic population. Telemed J E Health. 2014;20(11):997–1003. doi: 10.1089/tmj.2014.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberly Lauren A., Khatana Sameed Ahmed M., Nathan Ashwin S. Telemedicine Outpatient Cardiovascular Care During the COVID-19 Pandemic. Circulation. 2020;142(5):510–512. doi: 10.1161/CIRCULATIONAHA.120.048185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aws Almufleh, Givertz Michael M. Virtual Health During a Pandemic. Circulation. Heart Failure. 2020;13(8):e007317. doi: 10.1161/CIRCHEARTFAILURE.120.007317. [DOI] [PubMed] [Google Scholar]
- 6.DeFilippis E.M., Reza N., Donald E., Givertz M.M., Lindenfeld J., Jessup M. Considerations for Heart Failure Care During the COVID-19 Pandemic. JACC Heart Fail. 2020;8(8):681–691. doi: 10.1016/j.jchf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toner L., Koshy A.N., Ko J., Driscoll A., Farouque O. Clinical Characteristics and Trends in Heart Failure Hospitalizations. JACC Heart Fail. 2020;8(10):872–875. doi: 10.1016/j.jchf.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox Z.L., Lai P., Lindenfeld J. Decreases in acute heart failure hospitalizations during COVID-19. Eur J Heart Fail. 2020;22(6):1045–1046. doi: 10.1002/ejhf.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parthiban N., Esterman A., Mahajan R. Remote Monitoring of Implantable Cardioverter-Defibrillators: A Systematic Review and Meta-Analysis of Clinical Outcomes. J Am Coll Cardiol. 2015;65(24):2591–2600. doi: 10.1016/j.jacc.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Hindricks G., Taborsky M., Glikson M. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384(9943):583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 11.Sana F., Isselbacher E.M., Singh J.P., Heist E.K., Pathik B., Armoundas A.A. Wearable Devices for Ambulatory Cardiac Monitoring: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(13):1582–1592. doi: 10.1016/j.jacc.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C.-M., Wang L., Chau E. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841–848. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 13.Vollmann D., Nägele H., Schauerte P. Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J. 2007;28(15):1835–1840. doi: 10.1093/eurheartj/ehl506. [DOI] [PubMed] [Google Scholar]
- 14.Boehmer J.P., Hariharan R., Devecchi F.G. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results From the MultiSENSE Study. JACC Heart Fail. 2017;5(3):216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Marc Strik, Théo Caillol, Daniel Ramirez F. Validating QT-Interval Measurement Using the Apple Watch ECG to Enable Remote Monitoring During the COVID-19 Pandemic. Circulation. 2020;142(4):416–418. doi: 10.1161/CIRCULATIONAHA.120.048253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorodeski E.Z., Goyal P., Cox Z.L. Virtual Visits for Care of Patients with Heart Failure in the Era of COVID-19: A Statement from the Heart Failure Society of America. Journal of Cardiac Failure. 2020;26(6):448–456. doi: 10.1016/j.cardfail.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.