Abstract
Due to the advantages in efficacy and safety compared with traditional chemotherapy drugs, targeted therapeutic drugs have become mainstream cancer treatments. Since the first tyrosine kinase inhibitor imatinib was approved to enter the market by the US Food and Drug Administration (FDA) in 2001, an increasing number of small-molecule targeted drugs have been developed for the treatment of malignancies. By December 2020, 89 small-molecule targeted antitumor drugs have been approved by the US FDA and the National Medical Products Administration (NMPA) of China. Despite great progress, small-molecule targeted anti-cancer drugs still face many challenges, such as a low response rate and drug resistance. To better promote the development of targeted anti-cancer drugs, we conducted a comprehensive review of small-molecule targeted anti-cancer drugs according to the target classification. We present all the approved drugs as well as important drug candidates in clinical trials for each target, discuss the current challenges, and provide insights and perspectives for the research and development of anti-cancer drugs.
Subject terms: Drug development, Drug discovery
Introduction
Drug treatment together with surgical operation, radiotherapy and biotherapy constitute the main approaches to cancer treatment. For a long time, chemotherapy, which is a method of killing tumor cells and/or inhibiting the growth and proliferation of tumor cells by chemical drugs, was the only approach to cancer drug therapy. The biggest characteristic of chemotherapy is the inability to distinguish between cancer cells and normal cells, resulting in significant toxicity and side effects. Over the past two decades, there has been a tremendous shift in cancer treatment, from broad-spectrum cytotoxic drugs to targeted drugs.1 Compared with traditional chemotherapy drugs, targeted drugs can specifically target cancer cells but spare normal cells, hence having high potency and low toxicity. Encouraged by the approval of the first small-molecule tyrosine kinase inhibitor (TKI) imatinib for clinical use by the US Food and Drug Administration (FDA) in 2001,2 targeted drugs have rapidly developed and entered a golden period of development. In the past 20 years, there has been a significant increase in FDA-approved targeted drugs for cancer treatment.
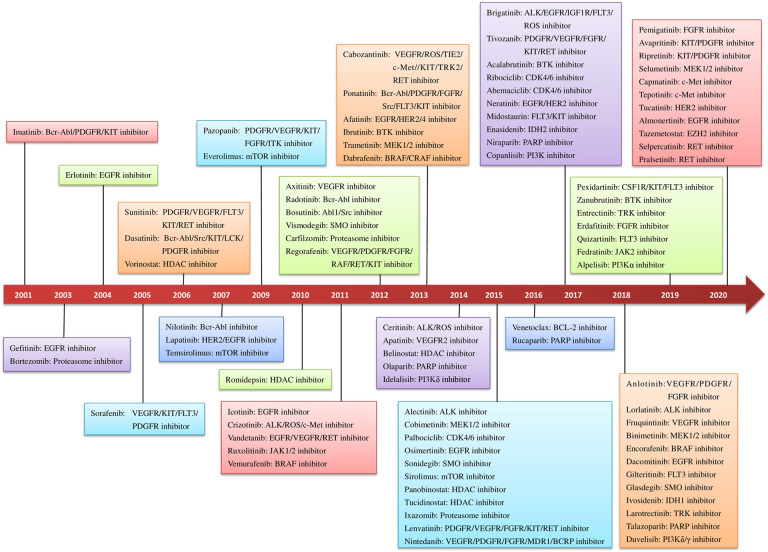
Targeted drugs can be roughly classified into two categories: small molecules and macromolecules (e.g., monoclonal antibodies, polypeptides, antibody–drug conjugates, and nucleic acids).3,4 Compared with macromolecule drugs, small-molecule targeted drugs have advantages in some aspects such as the pharmacokinetic (PK) properties, costs, patient compliance, and drug storage and transportation (Supplementary Table S1). Despite challenged by macromolecule drugs represented by monoclonal antibodies in recent years, small-molecule targeted drugs still gain great development. To date, there are a total of 89 anti-cancer small molecules approved in the United States and China. Figure 1 summarizes the small-molecule anti-cancer drugs approved by the US FDA and National Medical Products Administration (NMPA) of China since 2001. The targets of these drugs cover a large scope including kinases, epigenetic regulatory proteins, DNA damage repair enzymes, and proteasomes. It is undeniable that small-molecule targeted anti-cancer drugs still face many challenges such as low response rate and drug resistance.
Fig. 1.
Timeline for the approval of small-molecule targeted anti-cancer drugs
To better promote the development of small-molecule targeted anti-cancer drugs, we will conduct a comprehensive review for them. In order to facilitate the description, protein targets of the approved agents will be taken as a clue. For each target, marketed small-molecule drugs and important drug candidates in clinical trials will be presented. Finally, an analysis of the current challenges in the field and a future perspective will also be given.
Kinase inhibitors
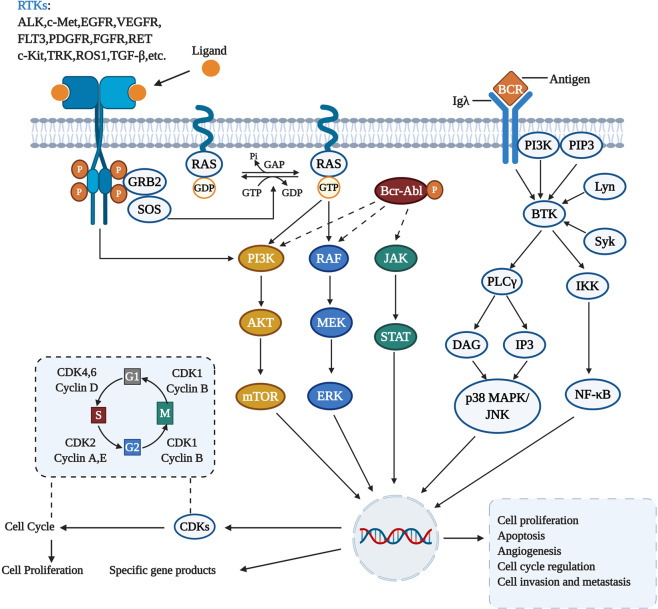
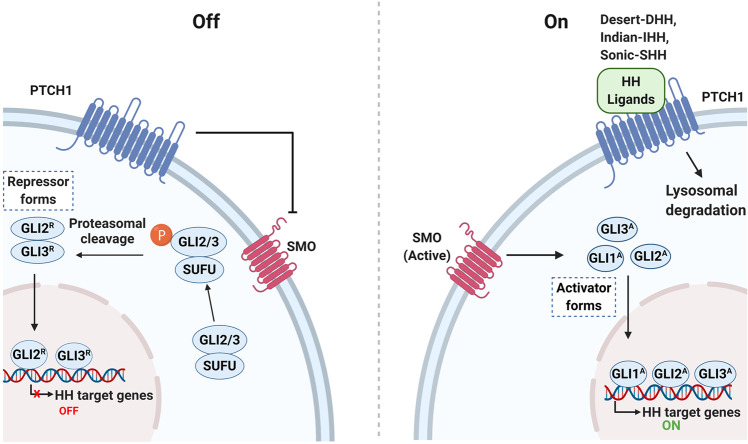
Protein kinase is a kind of enzyme that catalyzes the transfer of γ-phosphate group from ATP to protein residues containing hydroxyl groups. It has an important role in cell growth, proliferation, and differentiation (Fig. 2).5 The human kinome comprises ~535 protein kinases.6 According to the substrate residues, protein kinases can be classified as tyrosine kinases (including both receptor and non-receptor tyrosine kinases), serine/threonine kinases, and tyrosine kinase-like enzymes. Dysregulation of protein kinases is linked to various diseases, particularly cancer. Protein kinases are the most widely studied tumor therapeutic targets. Currently, a large number of protein kinase inhibitors have been reported. These kinase inhibitors can be classified into different categories by using many ways. Here we adopted an integrated classification system proposed by Roskoski, which is one of the most widely used methods.7 According to this classification system, protein kinase inhibitors are classified into six types (Type-I–VI). Type-I inhibitors bind to the active conformation of the kinase (DFG-Asp in, αC-helix in). Type-I½ inhibitors bind to a DFG-Asp in inactive kinase conformation with αC-helix out, while type-II inhibitors bind to a DFG-Asp out inactive conformation. These types of inhibitors occupy part of the adenine binding pocket and form hydrogen bonds with the hinge region connecting the small and large lobes of the enzyme. Among them, type-I½ and type-II antagonists can be further divided into A and B subtypes. Type A inhibitors extend past the Sh2 gatekeeper residue into the back cleft, while type B inhibitors fail to extend into the back cleft. The possible importance of this difference is that type A inhibitors have longer residence times compared with type B inhibitors when binding to their targets. Type III and type IV kinase inhibitors are allosteric in nature. Type III inhibitors restrain kinase activity by binding to an allosteric site, which is in the cleft between the small and large kinase lobes adjacent to the ATP-binding pocket. Contrariwise, type IV inhibitors bind outside of the cleft. Moreover, the bivalent molecules that span two distinct regions of the kinase domain are type V inhibitors. Type-I–V inhibitors are all reversible. In contrast, compounds that bind covalently with the kinase active site are called type VI inhibitors (irreversible kinase inhibitors).
Fig. 2.
Activation of different protein kinase-dependent pathways. The set of RTKs influences a small number of intermediaries, such as phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinases (MAPK), thereby activating the complex signaling networks that are related to cell proliferation, differentiation, adhesion, apoptosis, and migration. The aggregation, activation, and depolymerization of the periodic CDK-cyclin complex are critical events driving cell cycle turnover. Figure created with BioRender.com
Receptor tyrosine kinase inhibitors
ALK inhibitors
Anaplastic lymphoma kinase (ALK) encoded by the ALK gene is a single transmembrane tyrosine kinase of the insulin receptor family.8 ALK can activate multiple downstream signaling pathways and has an important role in the development of the nervous system.9 Constitutive activation of ALK through point mutations or chromosomal rearrangements has been identified in multiple human cancers such as anaplastic large cell lymphoma, diffuse large B-cell lymphoma (DLBCL),10 inflammatory myofibroblastic tumor,11 and non-small cell lung cancer (NSCLC).12 Fusion of echinoderm microtubule-associated protein-like 4 with ALK (EML4-ALK) in NSCLC was identified in 2007 by Soda et al.12 this rearrangement of the ALK gene has been detected in ~3–7% of patients with NSCLC. EML4-ALK gene fusion is initiated by inversion in the short arm of chromosome 2, which juxtaposes the N-terminal of the EML4 promoter and the kinase domain of the ALK gene, ultimately leading to ligand-independent constitutive activation of ALK and promoting cancer cell proliferation and survival. Several other ALK gene fusions, such as NPM-ALK, ATIC-ALK, and RANBP2-ALK, have also been discovered;13–15 these rearrangements define a specific subgroup of cancerous patients that can be treated with selective ALK inhibitors.16
Crizotinib approved in 2011 is a first-generation ALK inhibitor targeting multiple tyrosine kinases including ALK, cellular-mesenchymal-epithelial transition factor (c-Met), and proto-oncogene tyrosine-protein kinase reactive oxygen species (ROS) (Table 1).17 Two randomized phase III trials (NCT00932893, NCT01154140) established the superiority of crizotinib over chemotherapy in patients with advanced ALK-rearranged NSCLC, and it is now the standard drug therapy for metastatic ALK-positive NSCLC.18,19 Unfortunately, most patients develop resistant mutations to crizotinib within 12 months, especially L1196M and G1269A mutations, which can lead to relapse.20 The central nervous system (CNS) is the most common relapse site in patients with NSCLC treated with crizotinib, probably because of its poor blood–brain barrier (BBB) permeability.21 The second-generation ALK inhibitors ceritinib,22 alectinib,23 and brigatinib24 were subsequently developed for the treatment of crizotinib-resistant ALK-positive NSCLC, all of which are multikinase inhibitors (Table 1). Ceritinib is more potent than crizotinib and has doubled progression-free survival (PFS) compared with chemotherapy in clinical studies.22 Alectinib has advantages over both crizotinib and ceritinib, and has shown inhibitory activity against several crizotinib or ceritinib-resistant ALK mutations such as L1196M, G1269A, C1156Y, and F1174L.25 This agent is not a substrate of P-glycoprotein and can cross the BBB and effectively prevent the progression of CNS metastases. It was approved for NSCLC treatment in 2015 and recommended as first-line therapy for patients with ALK fusion-positive NSCLC in 2017. Moreover, brigatinib was granted accelerated approval by the FDA in 2017 as second-line therapy for patients with ALK-positive metastatic NSCLC, based on the considerable systemic and intracranial responses in clinical trials.26 Similar to the experience with crizotinib, novel resistance mechanisms were observed in patients who relapsed after treatment with second-generation ALK inhibitors. Secondary ALK kinase domain mutations, such as the G1202R, V1180L, and I1171T mutants, are the most common resistance mechanisms.27 Lorlatinib is an oral ATP-competitive brain penetrant inhibitor of ALK/ROS1 approved in 2018.28,29 As a third-generation ALK inhibitor, all recognized ALK mutations (except L1198F mutation) can be targeted by lorlatinib.29 Interestingly, lorlatinib is structurally distinct from most second-generation ALK inhibitors but has the same structural basis as crizotinib. However, patients harboring the L1198F mutation, which confers resistance to lorlatinib, have reported re-sensitivity to crizotinib.30 This result indicates that retreatment under molecular guidance should be considered as a clinically meaningful approach for ALK-positive NSCLC.
Table 1.
Properties of approved small-molecule inhibitors of receptor tyrosine kinases
| Chemical structure | Name | Targets | Approved indications (year) | Corporation |
|---|---|---|---|---|
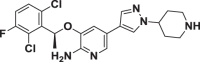 |
Crizotinib (Xalkori) | ALK/ROS/c-Met | NSCLC (2011) | Pfizer |
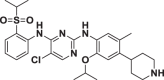 |
Ceritinib (Zykadia) | ALK/ROS | NSCLC (2014) | Novartis |
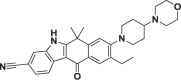 |
Alectinib (Alecensa) | ALK | NSCLC (2015) | Roche/Chugai |
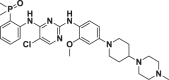 |
Brigatinib (Alunbrig) | ALK/ROS/ IGF1R/EGFR/FLT3 | NSCLC (2017) | Ariad |
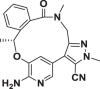 |
Lorlatinib (Lorbrena) | ALK | NSCLC (2018) | Pfizer |
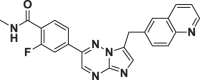 |
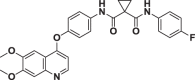
Capmatinib (Tabrecta) | c-Met | NSCLC (2020) | Novartis |
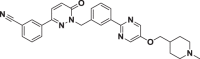 |
Tepotinib (Tepmetko) | c-Met | NSCLC (2020) | Merck |
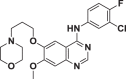 |
Gefitinib (Iressa) | EGFR | NSCLC (2003) | AstraZeneca |
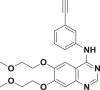 |
Erlotinib (Tarceva) | EGFR | NSCLC (2004) Pancreatic cancer (2005) | Roche/Astellas |
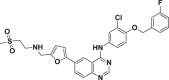 |
Lapatinib (Tykerb) | EGFR/HER2 | Breast cancer (2007) | Novartis |
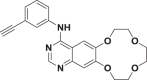 |
Icotinib (Conmana) | EGFR | NSCLC (2011) | Betta |
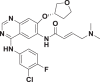 |
Afatinib (Gilotrif) | EGFR/HER2/HER4 | NSCLC (2013) | Boehringer Ingelheim |
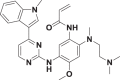 |
Osimertinib (Tagrisso) | EGFR | NSCLC (2015) | AstraZeneca |
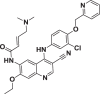 |
Neratinib (Nerlynx) | HER2/HER4/EGFR | Breast cancer (2017) | Puma Biotech |
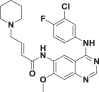 |
Dacomitinib (Vizimpro) | EGFR | NSCLC (2018) | Pfizer |
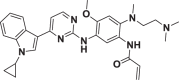 |
Almonertinib (Ameile) | EGFR | NSCLC (2020) | Hansoh |
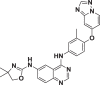 |
Tucatinib (Tukysa) | HER2 | Breast cancer (2020) | Seattle Genetics |
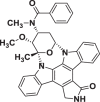 |
Midostaurin (Rydapt) | FLT3/c-Kit | AML (2017) | Novartis |
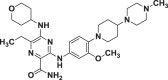 |
Gilteritinib (Xospata) | FLT3/AXL | AML (2018) | Kotobuki/Astellas |
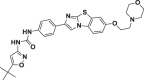 |
Quizartinib (Vanflyta) | FLT3 | AML (2019) | Daiichi Sankyo |
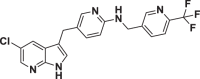 |
Pexidartinib (Turalio) | CSF1R/c-Kit/FLT3 | TGCT (2019) | Daiichi Sankyo |
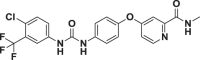 |
Sorafenib (Nexavar) | c-Kit/FLT3/RET/PTC/ VEGFR-1/2/3/PDGFR-β |
RCC (2005) HCC (2007) DTC (2013) Thyroid cancer (2014) |
Bayer |
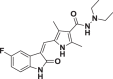 |
Sunitinib (Sutent) | PDGFR-α/β/VEGFR-1/2/3/CSF1R/c-Kit/RET/FLT3 |
RCC (2006) GIST (2006) Pancreas neuroendocrine tumor (2011) |
Pfizer |
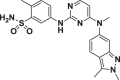 |
Pazopanib (Votrient) | PDGFR-β/VEGFR-1/2/3/ FGFR-1/3/c-Kit/Itk/Lck/c-GSK |
RCC (2009) STS (2012) |
Novartis |
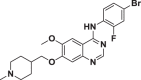 |
Vandetanib (Caprelsa) | EGFR/VEGFR/RET/BRK/TIE2/EPH | MTC (2011) | Genzyme |
 |
Axitinib (Inlyta) | VEGFR-1/2/3 | RCC (2012) | Pfizer |
 |
Cabozantinib (Cometriq) | VEGFR-1/2/3/TYRO3/ROS/TIE2/c-Met/HGFR/c-Kit/TRK2/RET |
MTC (2013) RCC (2016) HCC (2019) |
Exelixis |
 |
Regorafenib (Stivarga) | VEGFR-1/2/3/PDGFR-α/β/FGFR-1/2/RAF/RET/c-Kit |
CRC (2012) GIST (2013) HCC (2017) |
Bayer |
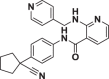 |
Apatinib (Aitan) | VEGFR-2/Src/c-Kit | Gastric cancer (2014) | Hengrui Medicine |
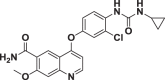 |
Lenvatinib (Lenvima) | PDGFR-α/VEGFR-1/2/3/FGFR-1/2/3/4/c-Kit/RET |
DTC (2015) Thyroid cancer (2015) RCC (2016) HCC (2018) Endometrial carcinoma (2019) |
Eisai |
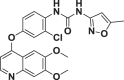 |
Tivozanib (Fotivda) | PDGFR-α/VEGFR-1/2/3/FGFR-1/2/3/4/c-Kit/RET | RCC (2017) | Eusa |
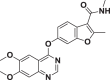 |
Fruquintinib (Elunate) | VEGFR-1/2/3 | CRC (2018) | Chi-Med/Lilly |
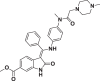 |
Nintedanib (Ofev) | VEGFR-1/2/3/PDGFR-α/β/ MDR1/BCRP/FGFR-1/3 | NSCLC (2015) | Boehringer Ingelheim |
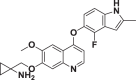 |
Anlotinib (Focus V) | VEGFR-2/3/PDGFR-β/FGFRs |
NSCLC (2018) STS (2019) SCLC (2020) |
Chia Tai Tianqing |
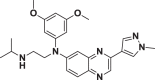 |
Erdafitinib (Balversa) | FGFR-1/2/3/4 | Urothelial carcinoma (2019) | Janssen |
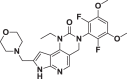 |
Pemigatinib (Pemazyre) | FGFR-1/2/3/4 | Cholangiocarcinoma (2020) | Incyte |
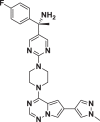 |
Avapritinib (Ayvakit) | c-Kit/PDGFR-α | GIST (2020) | Blueprint |
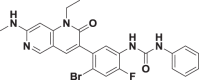 |
Ripretinib (Qinlock) | c-Kit/PDGFR-α | GIST (2020) | Deciphera |
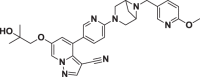 |
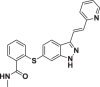
Selpercatinib (Retevmo) | RET |
NSCLC (2020) MTC (2020) Thyroid cancer (2020) |
Loxo |
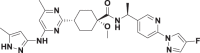 |
Pralsetinib (Gavreto) | RET |
NSCLC (2020) MTC (2020) Thyroid cancer (2020) |
Blueprint Medicines |
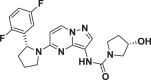 |
Larotrectinib (Vitrakvi) | TRKA/B/C | Solid tumors with NTRK fusion (2018) | Bayer |
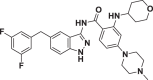 |
Entrectinib (Rozlytrek) | TRKA/B/C/ROS1/ALK | Solid tumors with NTRK fusion (2019) | Roche |
Currently, there are still some ALK inhibitors under clinical investigation, such as the pan-TKIs entrectinib,31 belizatinib,32 and repotrectinib,33 which target oncogenic rearrangements in ALK, ROS, and tropomyosin receptor kinase (TRK).34 Among them, entrectinib was approved for the treatment of TRK fusion solid tumors in 2019. The clinical use of these drugs as ALK inhibitors is still under evaluation.31 The aminopyrazine derivative ensartinib is a newly developed second-generation ALK inhibitor. It exhibits efficacy in crizotinib-naive and crizotinib-resistant patients with ALK-positive, locally advanced or metastatic NSCLC, as well as patients with brain metastases.34 A phase III study is ongoing to compare ensartinib with crizotinib for the first-line treatment of ALK-positive NSCLC (NCT02767804). Moreover, CEP-37440 is an orally administered inhibitor of ALK and focal adhesion kinase. A phase I trial (NCT01922752) of this agent has been performed in patients with solid tumors, but no preliminary data are available.35
Drug-resistant mutations are major obstacles that limit the clinical efficacy of ALK inhibitors. To date, ALK inhibitors have been developed to the third generation. Rational sequential therapy (using first-, second-, and third-generation ALK inhibitors for NSCLC therapy sequentially according to ALK gene mutations) can effectively overcome drug resistance and improve the survival of applicable patients. Of note is that retreatment with crizotinib can benefit patients harboring the ALK L1198F mutation, which is resistant to the latest generation of ALK inhibitors.36 For resistance arising from bypass activation, combining ALK inhibitors with other targeted therapies such as a mitogen-activated protein kinase (MAPK) inhibitor,37,38 cyclin-dependent kinase (CDK) inhibitor (ceritinib with ribociclib, NCT02292550), mammalian target of rapamycin (mTOR) inhibitor (ceritinib with everolimus, NCT02321501), and heat-shock protein 90 inhibitor has been assessed in a number of trials.39 Since the expression of programmed death-ligand 1 is reportedly associated with EML4-ALK, combined treatments of ALK and immune checkpoint inhibitors have also been evaluated in ALK-positive NSCLC.38 In addition to kinase inhibitors, degrading carcinogenic proteins using proteolysis targeting chimera (PROTAC) technology is an effective anti-cancer strategy. The PROTACs MS4077 and MS4078 designed by Zhang et al. have shown great potency in reducing ALK fusion protein in preclinical studies, suggesting a new approach to drug discovery targeting ALK.40
c-Met inhibitors
Cellular-mesenchymal-epithelial transition factor (c-Met), also known as hepatocyte growth factor receptor (HGFR), is encoded by the MET proto-oncogene located on chromosome 7q21-31.41,42 Under normal physiology, the binding of c-Met to its sole ligand HGF initiates the activation of the HGF/c-Met signaling pathway, which further activates several downstream signals including the PI3K/AKT, MAPK, STAT, and NF-κB pathways, and has a central role in a variety of cytoplasmic and nuclear processes, such as cell proliferation, survival, invasion, motility, scattering, angiogenesis, and epidermal–mesenchymal transition.42–44 These normal regulatory functions mainly occur during embryonic development, wound healing, and post-injury tissue regeneration. However, aberrant activation of c-Met signaling caused by MET amplification, mutation, inadequate degradation, transcriptional deregulation, or aberrant HGF autocrine or paracrine has been implicated in the development of various solid tumors.44,45 c-Met overexpression has also been reported to be related to poor prognosis and resistance to cytotoxic and molecular targeted therapy, especially for patients treated with EGFR inhibitors, in which MET amplification accounts for ~20% of resistant cases.46 Activating MET mutations usually occur in the semaphoring domain (e.g., E168D) and juxtamembrane domain (e.g., T1010I, P1009S, skipping mutation) of exons 14, 18, and 19. Of these, c-Met exon 14 skipping mutations promote its oncogenic activity by suppressing c-Met receptor degradation.43,45,47 These mutations are rare in patients with primary tumors but common in advanced cancers with metastases, especially in lung adenocarcinoma, brain gliomas, and renal cell carcinoma (RCC).47,48
During the last decade, great progress has been made in antitumor therapy targeting the HGF/c-Met signaling pathway. The early developed c-Met inhibitors were multikinase inhibitors. As early as 2011 and 2012, two multitarget c-Met inhibitors, crizotinib and cabozantinib, were approved for the treatment of NSCLC and medullary thyroid cancer (MTC) as well as RCC, respectively.49,50 However, the indications are not based on their ability to target c-Met but are due to the inhibitory effect of crizotinib on the ALK fusion protein and the multikinase inhibitory activity of cabozantinib. The development of selective c-Met inhibitors has progressed rapidly in recent years, and two highly selective c-Met inhibitors, capmatinib and tepotinib, were approved in the first half of 2020 (Table 1). Capmatinib (INCB28060) is an oral competitive c-Met inhibitor with ≥10,000-fold selectivity for c-Met compared with other kinases and potently inhibits c-Met activity at picomolar concentrations.51 In the GEOMETRY mono-1 trial (NCT02414139) conducted in patients with MET exon 14 skipping mutations, capmatinib exhibited a high objective response rate (ORR) and relatively durable responses in both previously treated and newly diagnosed patients, including those with brain metastases.52 Combination therapy of capmatinib and gefitinib was also evaluated in a phase II trial in NSCLC patients with disease progression after gefitinib treatment (NCT01610336). A disease control rate of 80% was achieved in 65 subjects, and more responses were observed in patients with MET amplification.53 Similar results were reported in the combination therapy of capmatinib with other EGFR inhibitors, such as erlotinib.54 Due to its significant efficacy compared to existing therapies, capmatinib granted a breakthrough therapy designation by the FDA for NSCLC patients harboring MET exon 14 skipping mutations in 2019 and was approved for this indication on May 6, 2020. Tepotinib (EMD1214063), developed by Merck, has more than 1000-fold selectivity for c-Met.55 Clinical trials of tepotinib (NCT04647838 and NCT03940703) also showed significant effectiveness in the treatment of cancer patients harboring MET mutations and in combination therapy with EGFR TKIs.56 It has been approved by the Ministry of Health, Labour and Welfare (MHLW) of Japan for the treatment of unresectable, advanced, or recurrent NSCLC in patients with skipping mutations in MET exon 14.57
There are also many small-molecule c-Met inhibitors at different stages of clinical trials. Representative multikinase c-Met inhibitors include foretinib (XL880/GSK1363089), glesatinib (MGCD265), BMS-777607, and S49076, which target c-Met/RON/VEGFR-2/KIT/TIE2/PDGFR, c-Met/TIE2/RON/VEGFR-1/2/3, c-Met/RON/AXL, and c-Met/AXL/MER/FGFR, respectively.58–61 Several clinical trials were carried out to test their efficacy for cancer therapy, but some of the results have not been disclosed. In a phase I trial for NSCLC patients who progressed after chemotherapy (NCT01068587), combined foretinib with erlotinib could achieve a response rate of 17.8% in the evaluated patients, and the clinical response was closely associated with baseline c-Met expression.58 In a phase I trial conducted in patients with advanced solid tumors (ISRCTN00759419), S49076 was administered orally once daily or twice daily in continuous 21-day cycles at escalating doses, followed by an expansion phase at the RP2D. The results showed that 83 patients (81.4%) had side effects, and 93% of them were grade 1–2. Nine patients had more than 6 months of stable disease, and the overall clinical benefit rate was 23%.61 In addition, several c-Met-specific inhibitors are also undergoing clinical research. Tivantinib is a non-ATP-competitive inhibitor of c-Met. In phase II randomized open-label study conducted in previously treated locally advanced or metastatic NSCLC patients (NCT00777309), the combination of tivantinib and erlotinib showed an ORR of 10% vs. 7% of the control arm and a median PFS of 3.8 vs. 2.3 months in the control arm.62 Volitinib (savolitinib) selectively inhibits c-Met activity in an ATP-dependent manner. The tolerability and safety of volitinib as monotherapy or in combination with gefitinib in NSCLC patients with mutant or wild-type EGFR have been studied in several phase I trials (NCT01773018, NCT02374645).63 It was also assessed in combination with osimertinib for patients with resistant NSCLC harboring the T790M mutation in a phase II clinical study (NCT02143466). The preliminary results showed that the combination of volitinib plus osimertinib had reliable safety and effectiveness. SAR125844 derived from triazolopyridazine is an effective specific c-Met inhibitor with an IC50 value of 4 nM.64 A phase I trial (NCT01657214) of SAR125844 displayed encouraging anti-NSCLC activity in patients with MET amplification, and the drug was well tolerated.65 More than a dozen c-Met inhibitors are currently under clinical assessment in China for the treatment of various malignancies either alone or in combination, such as bozitinib, ningetinib, glumetinib, and kanitinib. Some of them are undergoing phase II/III clinical trials and have great potential for approval in the near future.66
In addition to selective and non-selective small-molecule c-Met inhibitors, monoclonal antibodies against HGF ligand and c-Met are also effective strategies targeting the HGF/c-Met axis. One of the major challenges for the clinical use of these c-Met inhibitors is to distinguish the applicable population that is most likely to derive benefits from HGF/c-Met targeted therapy. It has been reported that c-Met expression detected by immunohistochemistry cannot accurately define the potential benefit patients.67 Biomarkers or biomarker combinations, such as MET mutations, MET gene amplification, and HGF expression, should be evaluated in cohorts receiving anti-c-Met targeted therapy to identify potential predictors of efficacy for specific c-Met inhibitors.67,68 For example, the MET exon 14 skipping mutation is a biomarker predicting the response to capmatinib and tepotinib. Drug resistance is another issue that needs to be considered in the development and clinical use of c-Met inhibitors. Multiple mechanisms have been identified to contribute to resistance to HGF/c-Met targeted therapy, including amplification of MET or KRAS, MET secondary mutations, induction of HGF secretion, and increased bypass activation.67,69,70 The clinical response to currently approved c-Met inhibitors is largely restricted to patients with MET amplification or exon 14 deletion. NSCLC patients who are resistant to EGFR inhibitors mediated by c-Met overexpression can also respond to c-Met inhibitors. However, inhibition of either c-Met or its ligand alone has not been proven to be potent in unselected cancer patients. Rationally designed combination strategies, such as combining c-Met inhibitors with HGF neutralizing antibodies, and cooperatively targeting upstream, downstream or parallel signaling, can not only improve the clinical benefits but also overcome drug resistance to c-Met inhibitors. Such strategies may also benefit a wide range of patients who lack MET gene abnormalities.
EGFR inhibitors
The epidermal growth factor receptor (EGFR) is a transmembrane protein implicated in a wide range of biological processes. Members of this family also include ERBB2/HER2, ERBB3/HER3, and ERBB4/HER4, which are structurally similar and consist of an extramembrane ligand-binding region, a single-stranded transmembrane region, and a highly conserved intra EGFR membrane tyrosine kinase region.71,72 When the EGFR extracellular domain binds to its ligand, such as EGF and TGF-α, EGFR dimerizes and autophosphorylates, thereby activating downstream intracellular signaling cascades, which are closely related to cell proliferation, survival, and apoptosis.72 Abnormal activation of EGFR mutations is an important contributor to the tumorigenesis of multiple cancer types, especially lung cancer, breast cancer, and pancreatic cancer.73–75
As shown in Table 1, several EGFR TKIs are clinically available. The first generation of EGFR TKIs, such as gefitinib, erlotinib, and icotinib, are reversible inhibitors with a quinazoline structure. These drugs are highly effective in NSCLC patients harboring EGFR-activating mutations (exon 19 deletion and exon 21 L858R).74,76,77 They demonstrated a significant PFS benefit over platinum doublet chemotherapy in the clinic. In addition, erlotinib has also been used in combination with gemcitabine for the clinical treatment of pancreatic cancer.78 The EGFR L858R/T790M dual mutation is the major cause of treatment failure (>50%) after taking the first generation of EGFR inhibitors.79 The second-generation irreversible EGFR-TKIs afatinib and dacomitinib are designed to conquer the T790M mutation.80,81 They can covalently bind to the ATP-binding pocket of EGFR and show stronger pharmacological activity than gefitinib. However, they also strongly inhibit wild-type EGFR and cause severe rash and diarrhea, thereby limiting their clinical doses. Therefore, these agents are only used for NSCLC patients harboring EGFR-sensitive mutations but could not benefit sufferers harboring the T790M mutant.79,80,82 Novel pyrimidine-based third-generation EGFR TKIs have inhibitory effects on EGFR-activating mutations and the T790M mutation specifically but show weak inhibitory activity on wild-type EGFR. Osimertinib is the first approved third-generation EGFR inhibitor and can achieve a PFS of over 10 months in patients harboring the EGFR T790M mutation.83 Almonertinib, developed by Hansoh Pharma, is an analog of osimertinib. This drug also showed significant anti-cancer effects in resistant patients with NSCLC in clinical trials, and has been approved for NSCLC therapy by the NMPA recently.84 The success of osimertinib and almonertinib in overcoming acquired resistance is mainly attributed to their high potency and selectivity against the EGFR T790M mutation.
Lapatinib and neratinib, which are clinically available for patients with breast cancer, are dual-target inhibitors that inhibit the activities of both EGFR and HER2 (Table 1). Among them, lapatinib is a reversible TKI and is mainly used in combination with capecitabine for the treatment of advanced or metastatic breast cancers that show HER2 overexpression and have previously received treatment by anthracycline, paclitaxel, or Herceptin.85 Neratinib is an irreversible inhibitor mainly used in breast cancer patients who have completed standard Herceptin-assisted treatment and are currently without but at high risk of progression.86 Besides, tucatinib (irbinitinib) is a potent and selective HER2 inhibitor with an IC50 of 8 nM. This is a newly approved HER2 inhibitor, and is also used for the treatment of patients with advanced unresectable or metastatic HER2-positive breast cancer.87
In addition, many other EGFR inhibitors are undergoing clinical trials. Typically, olmutinib is an irreversible anilino-thienopyrimidine inhibitor of EGFR that shows high inhibitory activity against the L858R/T790M dual mutation or exon 19 deletion.88 Phase I and phase II trials (NCT01588145, NCT02444819, and NCT02485652) have been conducted to evaluate the efficacy and safety of olmutinib alone or in combination with drugs such as afatinib, bevacizumab, or pembrolizumab on NSCLC patients.89 So far this drug is only clinically available in South Korea, and has not been approved in other countries due to the potential serious side effects, such as Stevens-Johnson syndrome.88,90 Avitinib is an irreversible pyrrolopyrimidine derivative that is evaluated clinically for the treatment of T790M mutant NSCLC (NCT03574402).91 Its inhibitory activity was 300 times higher on the T790M mutant than on wild-type EGFR. Pelitinib is an irreversible fluroanilino-quinoline EGFR inhibitor. This agent has been assessed in phase II clinical trials (NCT00072748, NCT00072748) for patients with NSCLC or colorectal carcinoma.92 Moreover, the third-generation EGFR inhibitor furmonertinib (alflutinib) developed by Allist Pharmaceuticals is being evaluated for the treatment of NSCLC in several clinical trials (NCT02973763, NCT03452592, and NCT03787992).
Acquired resistance to EGFR TKIs develops after 9–14 months of treatment. The main causes of drug resistance include EGFR secondary mutation, activation of alternative pathways, and phenotypic transformation, especially the former. The acquired EGFR C797S mutation mediates resistance to third-generation EGFR inhibitors in ∼40% of osimertinib-treated NSCLC patients.93 Both cis and trans mutations of C797S and T790M were observed in resistant cases. If EGFR C797S and T790M mutations occur in trans, the combination of first- and third-generation EGFR TKIs has been reported to be an effective treatment strategy.94 If they occur in cis, the patients are resistant to all approved EGFR TKIs; this is also a focus for research on fourth-generation EGFR TKIs.94 Jia et al. reported an allosteric inhibitor EAI045 that targets both C797 and T790M mutations but spares wild-type EGFR. A combination of EAI045 and cetuximab is effective in mouse models of lung cancer driven by EGFR (L858R/T790M) and by EGFR (L858R/T790M/C797S) mutants.95 Moreover, Shen et al. designed and synthesized a series of 5-methylpyrimidopyridone derivatives as EGFR (L858R/T790M/C797S) inhibitors.96,97 The representative compound 8r-B inhibited EGFR (L858R/T790M/C797S) mutant with an IC50 of 27.5 nM.97 Further PK-oriented optimization of 8r-B is ongoing.
FLT3 inhibitors
Fms-like tyrosine kinase 3 (FLT3), which is widely expressed in hematopoietic stem and progenitor cells, is a transmembrane protein encoded by the proto-oncogene FLT3. It belongs to the type III RTK family, which also includes PDGFR, FMS, and KIT. All of them consist of an extracellular ligand-binding domain, a single transmembrane hydrophobic alpha helix region, and an intracellular kinase domain. FLT3 is activated by binding to the ligands, which results in its dimerization and conformational changes. Subsequent autophosphorylation of FLT3 triggers signal transduction, activating intracellular signaling cascades such as PI3K/AKT/mTOR, RAS/RAF/MAPK, and JAK/STAT,98,99 which are closely related to cell proliferation, differentiation, survival, and apoptosis. FLT3 is widely overexpressed in patients with acute myeloid leukemia (AML), and its mutations lead to the constitutive activation of downstream signals.100,101 Internal tandem duplication (ITD) mutations in FLT3 (FLT3-ITD) are detected in ~25% of AML patients, and point mutations in the tyrosine kinase domain (TKD) are observed in 7–10% of patients.102 These mutations have been identified to be involved in the occurrence of leukemia. Due to the established pathogenetic and prognostic roles of FLT3-ITD and FLT3-TKD in AML, several FLT3 inhibitors have been developed for AML therapy.
The first-generation FLT3 inhibitors, including sorafenib, sunitinib, midostaurin, tandutinib, and lestaurtinib, are multikinase inhibitors.103–105 They are not specific for FLT3 and have inhibitory activity against various other RTKs, such as PDGFR, KIT, VEGFR, RAF, or JAK2. The clinical efficacy of most of these inhibitors as monotherapy for AML was unimpressive, and their off-target inhibition also increased adverse events.106 Therefore, clinical studies on first-generation FLT3 inhibitors for AML monotherapy were discontinued except for midostaurin. A randomized phase III trial (RATIFY study, NCT00651261) showed that the addition of midostaurin to cytarabine chemotherapy significantly improved overall survival (OS) for FLT3-mutated AML patients.104 Based on the beneficial results of the RATIFY study, midostaurin was approved by the US FDA for combination therapy with standard chemotherapy in 2017 (Table 1). Distinguishingly, pexidartinib (Turalio) is also an orally bioavailable multitarget inhibitor, with IC50 values of 9, 12, and 17 nM against FLT3-ITD, c-Kit, and colony-stimulating factor 1 receptor (CSF1R), respectively. However, it is not used clinically for AML therapy but approved for the treatment of adult patients with tenosynovial giant cell tumors (TGCTs). This indication is based on its inhibitory effect on CSF1R, which is frequently overexpressed in TGCTs.107 Second-generation FLT3 inhibitors developed by rational drug design are more potent and specific and have less toxicity related to off-target effects. Gilteritinib is the first approved second-generation FLT3 inhibitor and is also the first effective FLT3 inhibitor for AML monotherapy (Table 1).108 A randomized open-label phase III trial (ADMIRAL study, NCT02421939) showed that the median OS was significantly longer in the gilteritinib monotherapy group (9.3 months) than in conventional chemotherapy-treated patients (5.6 months) (p < 0.001). It was approved for the treatment of relapsed or refractory AML patients with FLT3 mutations in 2018. Quizartinib was screened by the KinomeScan technique to improve the affinity and specificity to FLT3 kinase, and it showed strong activity and selectivity against FLT3-ITD but not TKD.109 The clinical efficacy of quizartinib was also superior to conventional chemotherapy (NCT00989261); therefore, it was approved for relapsed or refractory AML patients with FLT3-ITD mutations in 2019.110
Currently, many promising FLT3 inhibitors are still under clinical evaluation. Crenolanib, originally developed as an inhibitor of PDGFR, is also a second-generation FLT3 inhibitor with inhibitory activity against both FLT3-ITD and FLT3 D835 mutations.111 Crenolanib development focused on assessing the combination effects of this drug with conventional chemotherapy in terms of first-line and relapse treatment. Several clinical trials are underway to evaluate the clinical efficacy of crenolanib, including a randomized phase III trial evaluating the potency of crenolanib in combination with induction chemotherapy for relapsed or refractory FLT3-mutated AML patients (NCT02298166) and a multicentre phase III trial comparing the effects of crenolanib with midostaurin during induction chemotherapy and consolidation therapy for newly diagnosed FLT3-mutated AML patients (NCT03258931). SKLB-1028 is a multitarget inhibitor with FLT3 inhibitory activity.112 A phase I trial (NCT02859948) was conducted to evaluate the safety, tolerability and pharmacokinetic characteristics of SKLB-1028 in FLT3 mutant AML subjects.113 Moreover, it has been reported that SKLB-1028 also has inhibitory activity on FLT3 secondary mutations such as FLT3-D835Y and FLT3-F691L; therefore, it is considered a potential therapeutic drug for resistant AML patients harboring the corresponding mutations.114 The Bcr-Abl1 inhibitor ponatinib is currently approved for chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) therapy, and it is also a FLT3 inhibitor with potent inhibition of FLT3-ITD.115 Phase I/II studies are ongoing to evaluate the efficacy and safety of ponatinib in combination with cytarabine for AML patients with the FLT3-ITD mutation (NCT02428543) and its monotherapy or combination with azacitidine for untreated AML patients who are unfit for conventional chemotherapy (NCT02829840). In addition, the anti-AML activity and safety of several other multikinase inhibitors with FLT3 inhibitory activity, such as AT-9283, ENMD-981693, 4SC-203, cabozantinib, and CR-4, are also being evaluated in the clinic (NCT01054937, NCT01961765).116
Primary and secondary resistance is a challenging issue for TKI treatment, including FLT3 inhibitors, which results in the limited and transient efficacy of FLT3 inhibitors. Primary resistance to FLT3 inhibitors involves insensitive FLT3 mutations, expression of FGF2 or CYP3A4 in the bone marrow microenvironment, upregulation of anti-apoptotic proteins MCL-1, BCL-XL, or BCL-2, and other activated signals. Acquired resistance includes TKD secondary mutations at the activating loop residues (e.g., D835, D839, I836, and Y842) or the gatekeeper site F691,117,118 autocrine FLT3 signaling, and activation of alternative pathways. The understanding of the molecular mechanisms associated with resistance to FLT3 inhibitors lays a foundation for establishing strategies to overcome and reduce resistance. Many combination strategies have been evaluated to improve the treatment outcome, such as the combination with epigenetic therapy (e.g., HDAC inhibitors), proteasome inhibitors, and inhibitors targeting independent signaling pathways or downstream pathways of FLT3-ITD (e.g., STAT5 inhibitor, CDK4/6 inhibitors, PI3K/mTOR inhibitors).119–123 Another strategy is to develop irreversible FLT3 inhibitors. FF-10101 is a covalent-binding FLT3 inhibitor and can maintain the ability to bind FLT3 in either an active or inactive conformation.124 The irreversible binding of FF-10101 provides potent inhibitory effects on multiple secondary mutations, such as F691L gatekeeper mutation. A phase I/IIa study (NCT03194685) was conducted to assess its safety, tolerability, pharmacokinetics, and efficacy in subjects with relapsed or refractory AML. Meanwhile, a variety of novel FLT3 inhibitors, including 7 h,125 PLX3379,126 and MZH29,127 are in the preclinical research and development stage. They are potential next-generation FLT3 inhibitors to conquer secondary mutation-mediated resistance.
VEGFR/FGFR/PDGFR inhibitors
Angiogenesis is a complex process through which new blood vessels form from pre-existing vessels.128 In physiological circumstances, angiogenesis is strictly regulated by various endogenous pro-angiogenic and anti-angiogenic factors.129 Aberrant angiogenesis exists in a wide range of diseases including arthritis, retinopathies, atherosclerosis, endometriosis, and cancer.130–132 In 1971, Judah Folkman raised the hypothesis that solid tumors cause new blood vessel growth (angiogenesis) in the tumor microenvironment by secreting pro-angiogenic factors, initiating the research between angiogenesis and cancer.133 Angiogenesis is critical for the development and subsequent growth of human solid tumors; otherwise, tumor size will not exceed 1–2 mm.134 Tumors require new blood capillaries to provide nutrient and oxygen, remove metabolic waste, and facilitate the formation of metastases.135,136 As an increasing number of tumor angiogenesis-related genes, transcription factors, signaling pathways, and their mechanisms of action have been revealed, anti-angiogenesis has become an attractive strategy for cancer therapy.137,138 Well-known pro-angiogenic factors mediating the angiogenic switch include vascular endothelial growth factor (VEGF),139 basic fibroblast growth factor (bFGF),140 platelet-derived growth factor (PDGF),141 transforming growth factor (TGF),142 insulin-like growth factor, epidermal growth factor (EGF),143 and angiopoietin.144 In the past few years, efforts to develop anti-angiogenic treatments have mainly focused on inhibiting the activities of their receptors such as VEGF receptors (VEGFR-1-3), FGF receptors (FGFR1–4), PDGF receptors (PDGFRα and PDGFRβ), and TGF-β receptors (TGF-βRI, TGF-βRII, and TGF-βRIII).131,145,146
Currently, more than 10 anti-angiogenic TKIs have been approved by the FDA and NMPA of China for the treatment of multiple solid malignancies, and most of them are multikinase inhibitors (Table 1). Sorafenib can inhibit a number of receptor tyrosine kinases (RTKs) including VEGFR-1/2/3, c-Kit, FLT3, RET, PDGFRβ, and RAF, and is the first approved anti-angiogenic inhibitor.147 It was initially approved for the treatment of advanced RCC in 2005. Subsequently, the FDA-approved sorafenib for the treatment of advanced hepatocellular carcinoma (HCC) in 2007 based on encouraging results from the SHARP trial, and for differentiated thyroid carcinoma (DTC) in 2013 based on beneficial results from the DECISION trial.148,149 Sorafenib is also the first small-molecule targeted drug to be approved for these three cancer indications. The multikinase inhibitor lenvatinib approved in 2015 has the same clinical indications as sorafenib, and they are currently the only two targeted agents used clinically for the first-line treatment of HCC.148,150–152 Other approved anti-angiogenic inhibitors for the first- or second-line treatment of RCC or DTC include sunitinib153 (2006), pazopanib154 (2009), axitinib155 (2012), cabozantinib156 (2016), and tivozanib157 (2017). Among them, sunitinib, an indol-2-one multikinase inhibitor targeting VEGFR-1/2/3, PDGFRα/β, c-Kit, CSF1R, RET, and FLT3, is the second approved anti-angiogenic TKI, and was simultaneously approved for two distinct indications including RCC and imatinib-resistant gastrointestinal stromal tumor (GIST).158,159 The anilinoquinazoline derivative vandetanib inhibits the activities of EGFR, VEGFR-2/3, RET, BRK, TIE2, and EPH. It is the first drug to be approved for the treatment of adult patients with metastatic MTC by the FDA.160 However, this indication is most likely attributed to its inhibitory effect on RET, a tyrosine kinase hyperactivated by mutations in MTC.161 Another anti-angiogenic inhibitor used for the clinical treatment of MTC is cabozantinib, which also has high RET inhibitory activity.162 Relatedly, the FDA-approved two highly specific RET inhibitors (selpercatinib and pralsetinib) in 2020. Both of them show a wide range of therapeutic effects on RET-driven (RET mutation or RET fusion-positive) malignancies in clinical trials and have been approved for the treatment of advanced or metastatic RET-mutant MTC, RET fusion-positive NSCLC, and radioactive iodine-refractory thyroid cancer (Table 1).163,164 Regorafenib developed by Bayer is a fluoro-derivative of sorafenib with activity against multiple kinases including VEGFR-1/2/3, PDGFRα/β, FGFR1/2, BRAF, c-Kit, and RET.165 It has shown clinical effectiveness for patients with metastatic colorectal cancer (mCRC), who progress after prior standard treatment (NCT01103323), and received FDA approval in 2012.166 Afterward, the FDA expanded its indication to advanced GIST in 2013 based on the results of GRID clinical trial (NCT01271712). In this phase III study, although no difference was observed in the OS between regorafenib and placebo groups (hazard ratio = 0.77, p = 0.199), the PFS was significantly improved to 4.8 months in the treatment group, and the placebo arm was just 0.9 months.167 The indolinone derivative nintedanib targets VEGFR-1/2/3, FGFR1/2, and PDGFRα/β.168 It was initially used clinically for the treatment of idiopathic pulmonary fibrosis169 and the combination therapy of nintedanib and docetaxel was approved as a second-line treatment for patients with NSCLC in the same year by the European Medicines Agency (EMA) but not the FDA.170 In the past decade, Chinese researchers have also made great progress in developing anti-angiogenic drugs, and several have been approved by the NMPA of China. Apatinib developed by Hengrui Medicine inhibits the activities of VEGFR-2, c-Src, and c-Kit simultaneously and was approved for the treatment of advanced gastric cancer in October 2014.171 The multitarget inhibitor anlotinib is developed by Chiatai Tianqing.172 This anti-angiogenic agent has been used for the treatment of several malignant tumors including NSCLC, soft tissue sarcoma (STS), and small cell lung cancer (SCLC).173 These two drugs have been identified as orphan drugs by the FDA, but have not been launched in the United States. Fruquintinib developed by Hutchison Whampoa Limited is a potent small-molecule inhibitor of VEGFR-1/2/3.174 The FRESCO trial, a randomized double-blind phase III study (NCT02314819), laid the foundation for the approval of fruquintinib in patients with mCRC in 2018.175 Its effectiveness and safety are being explored in a phase I trial conducted in a non-Chinese population in the United States.176
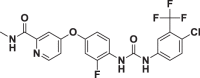
In addition to anti-angiogenic effects, several selective FGFR or PDGFR inhibitors have also been approved recently, mainly functioning as therapeutic agents for FGFR or PDGFR-driven malignancies (Table 1). Erdafitinib is an orally potent pan-FGFR inhibitor with IC50 values of 1.2, 2.5, 3.0, and 5.7 nM against FGFR1, 2, 3, and 4, respectively.177 As the first approved FGFR-selective inhibitor, it has been used for the second-line treatment of locally advanced or metastatic urothelial carcinoma. Meanwhile, it is undergoing clinical development as a treatment for other malignancies including NSCLC, gastric cancer, prostate cancer, cholangiocarcinoma, esophageal cancer, and lymphoma.178 Pemigatinib, also known as INCB054828, is an orally potent FGFR-selective inhibitor with IC50 values in the nanomolar range against FGFR1-3. Based on the results of FIGHT-202 (NCT02924376), a phase II, open-label, single-arm, multicenter study to evaluate the efficacy and safety of pemigatinib in cholangiocarcinoma subjects, pemigatinib received accelerated approval in April 2020 for the treatment of patients with previously treated, advanced/metastatic, or surgically unresectable cholangiocarcinoma harboring FGFR2 fusions or other rearrangements.179 This is also the first approved targeted treatment for cholangiocarcinoma.180 Pemigatinib is being evaluated for clinical use in several other FGFR-driven cancers and received orphan designation in August 2019 by the FDA for the treatment of myeloid/lymphoid neoplasms with eosinophilia and rearrangement of FGFR1 or PDGFRα/β.179 Avapritinib, developed by Blueprint Medicines, is an effective and selective inhibitor of PDGFRα and KIT activation loop mutants, which has recently been approved in the United States for adults with unresectable or metastatic GIST harboring PDGFRα exon 18 mutations.181 GIST can be classified according to different molecular subtypes, and KIT or PDGFRα mutated GISTs are important subgroups that commonly arise in the stomach. Five TKIs of PDGFRs or KIT (imatinib, sunitinib, regorafenib, avapritinib, and ripretinib) are currently used clinically for GIST therapy.182 As target-specific inhibitors, avapritinib and ripretinib have shown extensive inhibitory effects on KIT or PDGFRα mutant GIST, and are potent for patients harboring primary or secondary resistant mutations, including the PDGFRα D842V mutant.183–185
Efforts are being made to expand many of the above-mentioned approved VEGFR, FGFR, or PDGFR inhibitors to other cancer indications. Meanwhile, a large number of novel inhibitors are also being developed, many of which have entered clinical trials. Several representative drug candidates with the potential to receive approval in the near future are presented here. Cediranib developed by AstraZeneca is a potent multikinase inhibitor targeting VEGFR-1/2/3, PDGFRα/β, and c-Kit.186 However, AstraZeneca discontinued the development of cediranib for the treatment of mCRC, NSCLC, and recurrent glioblastoma due to the mediocre results from phase III clinical trials (NCT00399035, NCT00795340, NCT00777153) in these indications. At present, it shows new hope in the combination therapy of ovarian cancer, and two phase III trials (NCT03278717 and NCT02446600) are ongoing to compare the efficacy of cediranib plus olaparib with olaparib alone or in patients with ovarian cancer.187 Dovitinib, also named CHIR-258, is a multitargeted anti-angiogenic inhibitor with IC50s of 10/13/8, 2, 1, 8/9, and 27/210 nM for VEGFR-1/2/3, c-Kit, FLT3, FGFR1/3, and PDGFRα/β, respectively.188 In an open-label, randomized, phase III study to compare the safety and efficacy of dovitinib vs. sorafenib in patients with metastatic RCC (NCT01223027), there was no difference in PFS between these two drugs in third-line treatment.189 It received orphan drug designation for the treatment of adenoid cystic carcinoma in 2019. Motesanib is an orally administered multikinase inhibitor of VEGFR-1/2/3, PDGFRα/β, c-Kit, and RET, and was considered a potent anti-NSCLC drug in Asian patients based on the MONET1 trial.190 However, the results of a later phase III trial (NCT02629848) evaluating the efficacy of motesanib plus paclitaxel and carboplatin were disappointing. Motesanib plus paclitaxel/carboplatin did not significantly improve PFS vs. placebo plus paclitaxel/carboplatin (median PFS: 6.1 verse 5.6 months) in East Asian patients with stage IV/recurrent non-squamous NSCLC.191 Nevertheless, it showed marked anti-cancer effects in patients with advanced thyroid cancer in two phase II studies (NCT00121628, NCT02084732).192,193 Recently, a phase II study (NCT00121628) assessing the efficacy of motesanib in low-grade neuroendocrine tumors (NETs) also achieved satisfactory treatment results with a 4-month PFS of 78.5%, and the median PFS of all patients was 8.7 months.194 Sulfatinib is a potent inhibitor against VEGFR-1/2/3, FGFR1, and CSF1R with IC50 values in the range of 1–24 nM.195 An encouraging antitumor activity and acceptable safety profile were observed in a phase I trial (NCT02133157), particularly in NETs.196 It is currently being evaluated in advanced NETs in two phase III studies (NCT02589821 and NCT02588170).197 Crenolanib is an orally bioavailable TKI of PDGFRα/β and FLT3.198 A multicenter, randomized, double-blinded, phase III trial (NCT02847429) was conducted to assess the efficacy of oral crenolanib vs. oral placebo in combination with best supportive care in subjects with advanced or metastatic GIST with PDGFRα D842V mutation.199 In addition, due to its high inhibitory activity on both FLT3-ITD and FLT3-TKD mutant subtypes, which are important therapeutic targets for AML, crenolanib is also evaluated in a phase III randomized multicenter study conducted in AML subjects with FLT3 mutation (NCT03258931).200 Lucitanib, developed by Shanghai HaiHe Biopharma, potently and selectively inhibits VEGFR-1, VEGFR-2, VEGFR3, FGFR1, and FGFR2 with IC50 values of 7, 25, 10, 17.5, and 82.5 nM, respectively.201 A multicenter phase III study is being conducted to evaluate the efficacy of lucitanib in combination with carboplatin plus etoposide in untreated participants with extensive-stage SCLC (NCT04254471). Brivanib, in particular, is an orally active L-alanine ester prodrug that inhibits VEGFR-2 with an IC50 of 25 nM, and has moderate potency against VEGFR-1 and FGFR1.202 It is currently being evaluated in a phase III clinical trial on subjects with advanced HCC, who have failed or are intolerant to sorafenib (NCT00858871).203,204 Meanwhile, multiple noncovalent FGFR-selective inhibitors (pan-FGFR inhibitors) have also advanced to phase III trials, including the well-known AZD4547205 and infigratinib,206 both of which are typical type-I inhibitors. The former is being assessed for the treatment of patients with stage IV SCLC in phase II/III trial (NCT02965378), and the latter was granted orphan drug designation by the FDA for the treatment of cholangiocarcinoma in 2019.207 In addition, targeting TGF-β signaling is also a potential strategy for anti-angiogenic therapy.208 However, no TGF-βR inhibitor has been approved for clinical use, and several drug candidates are undergoing evaluation in clinical trials including galunisertib, vactosertib, LY-3200882, PF-06952229, YL-13027, and GFH018.209–211 Galunisertib, a selective TGF-βR type-I (TGF-βRI) kinase inhibitor with an IC50 of 56 nM, is the most advanced drug candidate among them.209,212 It has been evaluated clinically in several solid tumors including HCC, glioma, and glioblastoma multiforme, and achieved remarkable effect in the treatment of HCC. The median OS of galunisertib monotherapy for HCC can reach 16.8 months.213,214
The concept of “starving tumors to death” by inhibiting tumor angiogenesis has promoted the development of anti-angiogenic therapy.215 However, many anti-angiogenesis drugs produced only modest survival benefits for cancer patients in clinical trials.216,217 One of the reasons can be explained by vascular normalization; this theory emphasizes that anti-angiogenesis agents mainly selectively block the formation of immature blood vessels rather than the mature and functional vasculatures. Therefore, anti-angiogenesis treatment alone is generally ineffective unless combined with chemotherapy.218,219 On the other hand, tumor angiogenesis is regulated by multiple signaling pathways, and many interconnected pathways can compensate for the effect of single inhibition of one of these signals, such as the VEGFR pathway. This is why the approved small-molecule anti-angiogenesis TKIs are mostly multitarget inhibitors and only several selective inhibitors are used clinically for cancer patients with specific mutations. This also indicates the importance of combination therapy in the clinical use of anti-angiogenesis agents.220,221 Accumulating evidences have demonstrated that anti-angiogenic therapy can not only inhibit the formation of neo-vascular, but also regulate the immune microenvironment, which provides a theoretic basis for the combination of anti-angiogenesis agents with immunotherapy.221 Hundreds of clinical studies have been designed to evaluate such combination strategies. Encouragingly, the combination of axitinib with PD-1 antibody pembrolizumab has been approved for the treatment of patients with advanced RCC in 2019.222 Another challenge of anti-angiogenic therapy is the lack of more personalized use of existing anti-angiogenesis agents. This is also a significant feature that distinguishes most anti-angiogenesis TKIs from other molecular targeted therapies; the former is given to unselected patients within approved indications, while the latter are used in proper patients selected by robust biomarkers, which markedly improve their clinical benefits. Most efforts have been made to identify molecular biomarkers for anti-angiogenesis agents, such as expression of VEGF and FGF in blood and tumors,223 tumor perfusion status,224 and other angiogenic factors, but none of them have yet been validated for routine clinical use. Recently, several studies have suggested that anti-angiogenesis-related side effects, such as hypothyroidism, high blood pressure, or hand-foot syndrome, may be associated with the antitumor efficacy of angiogenesis inhibitors.223,224 As the lack of reliable predictive biomarkers in the clinic, these side effects may contribute to clinical decision, but further clinical verification is still needed.
TRK inhibitors
The tropomyosin receptor kinase (TRK) family is composed of three members, TRKA, TRKB, and TRKC, which are encoded by the neurotrophic tyrosine receptor kinase (NTRK) genes NTRK1, NTRK2, and NTRK3, respectively.225 To activate TRK receptors, neurotrophins (TRK ligands) bind to the extracellular domain of the receptors, stimulating homodimerization and autophosphorylation of TRK proteins, thereby activating downstream signaling pathways, such as RAS/MAPK/ERK, PI3K/AKT, and PLCγ. NTRK gene rearrangements containing a kinase domain of one of the three TRKs and a dimerization domain of another gene generate fusion proteins and result in aberrant activation of TRKs, which have been identified as oncogenic drivers of various cancers.226–228 Therefore, TRKs are emerging as important targets for cancer therapy. The rearrangements of NTRK genes occur in only 1% of all malignancies; they have been widely detected at low frequencies in some common cancers, such as lung cancer, thyroid carcinoma, glioblastoma, and colorectal cancer. However, in several rare pediatric and adult cancer types, including infantile fibrosarcoma, secretory breast carcinoma, and salivary gland secretory carcinoma, NTRK gene rearrangements are common.
The discovery of TRK inhibitors renewed interest in NTRK gene rearrangements as oncogenes. Currently, two first-generation TRK inhibitors are available for clinical cancer treatment (Table 1). Larotrectinib is the first approved selective oral pan-TRK inhibitor with high potency against TRKA, TRKB, and TRKC.229 Entrectinib (RXDX-101/NMS-E628) is a potent multikinase inhibitor targeting TRKA/B/C, ROS1, and ALK.230 Both agents received the FDA breakthrough therapy identification; this breakthrough designation highlights the efficacy of TRK inhibitors in various cancers that have the same mutation, regardless of cancer type and patient age. Based on the tumor-agnostic efficacy of the “basket trail” conducted in diverse NTRK fusion-positive cancers, larotrectinib and entrectinib granted FDA approval for the treatment of adult and pediatric patients with TRK fusion solid tumors. In clinical use, NTRK gene fusions should be diagnosed to select patients for targeted TRK therapy. Remarkably, both larotrectinib and entrectinib displayed activity against CNS tumors with NTRK fusions, indicating the ability for BBB penetration.231,232 When referring to the adverse events of first-generation TRK inhibitors, it should be noted that both agents have favorable overall safety profiles compared to other small-molecule TKIs. These drugs are generally well-tolerated in patients, with low incidences of dose reductions, discontinuations, and grade 3–4 adverse events.225
Recently, there have been several small-molecule TRK inhibitors in different stages of clinical research, some of which target multiple kinases, such as cabozantinib (targeting c-Met, RET, VEGFR-2, ROS1, ALK, and TRK),233 merestinib (targeting c-Met, TEK, ROS1, and TRK),234 belizatinib (targeting ALK and TRK),235 sitravatinib (targeting c-Met, RET, AXL, and TRK),236 altiratinib (targeting c-Met, TIE2, VEGFR-2, FLT3, and TRK),237 and DS-6051b (targeting ROS and TRK).238 These inhibitors displayed varying degrees of inhibitory activity against TRK. Some of them have been approved for indications other than TRK fusion tumors; for example, cabozantinib was approved as an anti-angiogenic inhibitor for the treatment of patients with advanced RCC in 2016, and data are limited on its efficacy against NTRK fusions. However, with the increasing interest in TRK as a cancer therapy target, an increasing number of clinical trials have been performed to evaluate the effects of these inhibitors in patients with TRK fusion-positive tumors. In addition, a phase I study was carried out to assess the safety, PKs, and PDs of the selective TRK inhibitor PLX7486 as a single agent in patients with any histological solid tumors with activating NTRK point or NTRK fusion mutations (NCT01804530).239 However, the results were not disclosed.
Acquired resistance to TKI treatment can be mediated by on-target mutations or off-target (bypass activation) mechanisms. Until now, on-target mutations in the kinase domain of NTRK fusion have been the only resistance mechanism of first-generation TRK inhibitors, which can result in amino acid substitutions of the solvent front, activation loop xDFG motif, and gatekeeper residues in the kinase domain of TRK fusion proteins, interfering with TRK inhibitor binding.225 The first resistance case to TRK inhibition was discovered in a colorectal cancer patient treated with entrectinib, and two acquired resistance mutations, TRKA G595R and TRKA G667C, were detected in the plasma cfDNA of this patient.240 Several other resistant mutations were subsequently identified in patients resistant to larotrectinib and entrectinib, including the acquired TRKC G623R substitution, A608D mutation and gatekeeper F589L substitution in TRKA, and the substitutions involving the xDFG site of TRKA (G667S) and TRKC (G696A).229,241 Fortunately, next-generation TRK inhibitors are currently in development to overcome acquired resistance to larotrectinib and entrectinib. In particular, selitrectinib (LOXO-195), repotrectinib (TPX-0005), and ONO-5390556 have demonstrated nanomolar inhibitory activity against the TRK mutants mentioned above.242 Among them, the safety and efficacy of selitrectinib and repotrectinib are currently under assessment in phase I/II trials (NCT04275960, NCT04094610).
Non-receptor tyrosine kinase inhibitors
Bcr-Abl1 inhibitors
c-Abl is encoded by the abelson murine leukemia 1 (ABL1) gene on chromosome 9 and belongs to the Abl family of non-receptor tyrosine kinase; it has been implicated in a range of cellular processes including the regulation of cell differentiation, cell cycle, and survival. Philadelphia (Ph) chromosome translocation results in the molecular juxtaposition of ABL1 and the breakpoint cluster region (BCR) of chromosome 22, forming an aberrant BCR-ABL fusion gene on chromosome 22.243 This gene encodes a 210 kDa oncoprotein (p210 Bcr-Abl1) that is capable of autophosphorylation and constitutively activates the downstream pathway, thereby driving the uncontrolled proliferation of leukemia cells in almost all cases of CML and ~20% of patients with ALL.244–246 The BCR-ABL fusion gene was identified as a specific biomarker for diagnosis and prediction of response to treatment, while Bcr-Abl1 fusion tyrosine kinase is considered to be a susceptible target for certain leukemias.
As indicated in Table 2, imatinib is the first approved Bcr-Abl1 inhibitor as well as the first approved small-molecule TKI, which launches a new era of tumor-targeted therapy. This agent has shown striking activity in patients with chronic phase CML (CML-CP) and Ph+ ALL.247 A 5-year follow-up study conducted in patients with CML-CP receiving interferon or imatinib treatment showed that the OS and PFS of patients taking imatinib could reach 89% and 93%, respectively.248 The introduction of imatinib for the treatment of CML patients with Ph chromosome translocation provides a proof-of-principle for using aberrant kinases as therapeutic targets. Currently, this drug represents the gold therapeutic standard in patients with CML in the clinical setting. Although treatment with imatinib has achieved exciting results, drug resistance caused by point mutations in the kinase domain of BCR-ABL has frequently emerged such as G250E, Q252H, Y253H/F, and E255K/V mutations located in the P loop region, T315I mutation in the ATP-binding region, and H395P/R mutation in the activation region.248–251 Point mutations decrease the affinity of imatinib to the Bcr-Abl1 kinase domain, resulting in reduced imatinib inhibitory activity.249,252 The increasing recognition of imatinib resistance stimulates the development of second-generation Bcr-Abl1 inhibitors including dasatinib, nilotinib, bosutinib, and radotinib, which were approved in 2006, 2007, 2012, and 2012, respectively.253–256 Both dasatinib and bosutinib are oral dual Src/Abl1 kinase inhibitors, and the former is ~300-fold more potent than imatinib. Nilotinib, an aniline pyrimidine derivative developed from imatinib by crystallographic analysis and structural modification, has better lipophilicity and solubility and ~30-fold higher potency. Radotinib is the structural analog of nilotinib and is used as a second-line treatment in the clinic. These inhibitors can suppress most clinically relevant BCR-ABL mutants, except T315I gatekeeper mutation, which occurs in up to 20% of patients with resistant CML.256,257 Ponatinib is a third-generation Bcr-Abl1 inhibitor with activity against T315I mutation.258 The binding pattern of ponatinib is similar to imatinib, except that the carbon-carbon triple bond extending from the purine of ponatinib enforces compatibility with T315I residue. It is currently approved for the treatment of patients with CML or ALL that are either resistant or unable to tolerate other Bcr-Abl1 inhibitors. In addition, due to the multitarget properties of imatinib, dasatinib, and nilotinib, they were also evaluated clinically for the treatment of some solid tumors. Among them, imatinib was approved for GIST therapy in 2003 (Table 2).
Table 2.
Properties of approved small-molecule inhibitors of non-receptor tyrosine kinases
| Chemical structure | Name | Targets | Approved indications (year) | Corporation |
|---|---|---|---|---|
| Imatinib (Gleevec) | Bcr-Abl/PDGFR-β/c-Kit |
CML (2001) GIST (2003) ALL (2006) |
Novartis | |
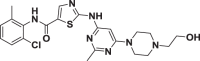 |
Dasatinib (Spraysel) | Bcr-Abl/Src/c-Kit/LCK/PDGFR-β |
CML (2006) ALL (2006) |
Bristol-Myers Squibb |
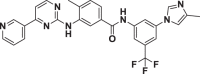 |
Nilotinib (Tasigna) | Bcr-Abl/DDR1/2 | CML (2007) | Novartis |
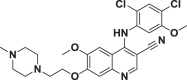 |
Bosutinib (Bosulif) | Abl1/Src | CML (2012) | Pfizer |
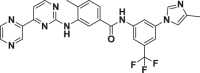 |
Radotinib (Supect) | Bcr-Abl | CML (2012) | IL-Yang |
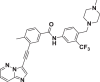 |
Ponatinib (Iclusig) | Bcr-Abl /PDGFR-α/VEGFR-2/FGFR-1/Src/FLT3/c-Kit |
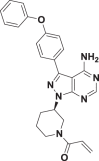
CML (2013) ALL (2013) |
Incyte/Takeda |
 |
Ibrutinib (Imbruvica) | BTK |
MCL (2013) CLL (2014) WM (2015) SLL (2016) MZL (2017) |
AbbVie/ Johnson & Johnson |
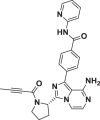 |
Acalabrutinib (Calquence) | BTK | MCL (2017) | AstraZeneca |
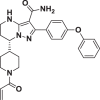 |
Zanubrutinib (Brukinsa) | BTK | MCL (2019) | BeiGene |
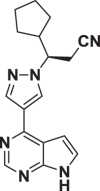 |
Ruxolitinib (Jakafi) | JAK1/2 | Myelofibrosis (2011) | Incyte/Novartis |
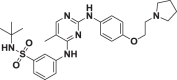 |
Fedratinib (Inrebic) | JAK2 | Myelofibrosis (2019) | Impact |
Research on the development of novel Bcr-Abl1 inhibitors against drug-resistant mutations is ongoing. To date, up to 13 inhibitors have entered clinical trials. Typically, asciminib (ABL001) is a potent and selective allosteric Abl1 inhibitor, which binds to the myristoyl pocket of Abl1 and induces the formation of an inactive kinase conformation.259 Phase I clinical trials are being conducted to evaluate the efficacy and safety of this drug alone or in combination with dasatinib and prednisone in patients with CML or BCR-ABL-positive B-cell ALL (NCT02081378, NCT03595917). Meanwhile, it is also evaluated in phase III clinical trial to compare its efficacy with bosutinib in patients with CML-CP (NCT03106779). Rebastinib (DCC-2036) is a potent conformational control inhibitor, designed to conquer Bcr-Abl1 resistant mutations, mainly T315I.260 It induces the kinase to a catalytically inactive state, regardless of gatekeeper mutations. Clinically, a phase I dose-finding study of rebastinib in patients with relapsed CML has been completed (NCT00827138); however, the clinical benefit was considered insufficient to support its continued use in leukemia treatment since the advent of ponatinib. Bafetinib (INNO-406) was developed to expand the susceptibility spectrum of mutations to TKIs and increase the selectivity to Bcr-Abl1 to reduce adverse reactions.261 In a phase I trial (NCT00352677), bafetinib as second-line treatment can achieve complete cytogenetic response in 19% of patients with CML and Ph+ ALL that is resistant or intolerant to imatinib, indicating its potential clinical efficacy.
Outcomes for patients with CML have been greatly improved since the clinical application of Bcr-Abl1 TKIs. However, despite the high initial response rate of these inhibitors, drug resistance and adverse events are two main problems influencing the achievement of the best response and quality of life for patients. Sequential therapy with Bcr-Abl1 inhibitors leads to the continuous acquisition of novel mutations, especially compound mutants, which refers to the accumulation of more than one mutation in the same allele. Ponatinib is the latest generation of Bcr-Abl1 inhibitor, and patients treated with this drug have developed new resistance mutations, such as T315M single-point mutation and complex mutations T315I/E255V and E255V/Y253H.262–264 Overcoming these resistance mutations requires the development of next-generation inhibitors and combination therapy strategies. Meanwhile, it has also been reported that some mutations are sensitive to the second-generation inhibitors, such as T315A to nilotinib, E255K/V and Y253H to dasatinib and bosutinib, and patients harboring such mutations can be retreated with these drugs. The clinical use of ponatinib is associated with cardiovascular events, and concerns about arterial thrombosis may limit its treatment in some patients with T315I mutations. For these patients, omacetaxine mepesuccinate approved by the FDA in 2012 is a proper treatment option.265,266 It has shown encouraging therapeutic results in patients harboring T315I mutation and is tolerable without cardiovascular toxicity. Moreover, some patients with GIST and systemic mastocytosis can benefit from the treatment of imatinib, dasatinib, or nilotinib due to the broad-spectrum selectivity of them against c-Kit, PDGFR, or Src.267,268 Mutations of these kinases can also drive the selection of appropriate inhibitors. An in vitro study showed that imatinib-resistant mutations PDGFRα D842V and c-Kit D816V that commonly occur in GISTs and mastocytosis, respectively, could be strongly inhibited by dasatinib.269
BTK inhibitors
The B-cell receptor (BCR) pathway has a key role in the progression of a variety of B-cell malignancies.270 Abnormal activation of BCR signaling has been identified in multiple heterogeneous hematologic malignancies, including B-cell non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), follicular lymphoma, Waldenstrom’s macroglobulinemia (WM), and DLBCL.271 Bruton’s agammaglobulinemia tyrosine kinase (BTK), a crucial component of the BCR pathway, belongs to the non-receptor tyrosine kinase of the TEC family, which contains four other members: tyrosine kinase expressed in hepatocellular carcinoma (TEC), interleukin-2-inducible T-cell kinase (ITK), resting lymphocyte kinase (RLK/TXK), and bone marrow expressed kinase (BMX).272 BTK is abundantly expressed in B-cell leukemias and lymphomas and functions as a vital regulator of cell proliferation and survival in various B-cell malignancies.273 Inhibiting BTK is considered an effective therapeutic strategy for some hematologic malignancies.274
Ibrutinib is the first-generation BTK inhibitor and has been proven to be superior to standard chemotherapy in multiple studies including older patients with significant comorbidity. It is an irreversible small-molecule inhibitor that covalently binds to Cys-481 within the ATP-binding pocket of BTK. Based on the high response rates and durable responses of its monotherapy275 or in combination with anti-CD20 antibody,276 ibrutinib has been approved by the FDA for the treatment of MCL, CLL, WM, SLL, and MZL between 2013 and 2017 (Table 2).277–279 The clinical efficacy of ibrutinib in the treatment of DLBCL, refractory/recurrent primary central nervous system lymphoma, and secondary central nervous system lymphoma is still undergoing evaluation to expand its indications.280 Despite the clinical achievement of ibrutinib, side effects including arthralgia, atrial fibrillation, pneumonitis and rash have also been reported and limit its clinical use. Most of the toxicity of ibrutinib is due to its off-target activities against four other TEC family kinases, EGFR, HER2, and Janus kinase 3 (JAK3).281 Particularly, in combination therapy with the CD20 antibody rituximab, off-target inhibition of ITK by ibrutinib led to an antagonistic effect on antibody-dependent cell-mediated cytotoxicity, influencing the combined effects.282 The off-target activity of ibrutinib triggered the development of more selective second-generation BTK inhibitors. Acalabrutinib283 and zanubrutinib284 are currently approved second-generation BTK inhibitors (Table 2). Similar to ibrutinib, they are irreversible inhibitors and form covalent bonds with the Cys-481 residue of the BTK active site.284 And their selectivity is significantly improved. Acalabrutinib inhibited BTK with an IC50 of 3 nM and had less off-target activity on EGFR, ITK, or TEC;285 zanubrutinib had similar inhibitory activity to ibrutinib against BTK, but its IC50s on TEC, ITK, EGFR, HER2, and JAK3 were 2–70 times higher than those of ibrutinib.286 Currently, the FDA has approved them for the treatment of adult MCL patients who have received at least one prior therapy.287 Both of them are still evaluated in the clinic for the treatment of some other malignancies, such as NHL,288 multiple myeloma (MM),289 and ovarian cancer.290
Several promising BTK irreversible inhibitors are under clinical evaluation.291 Tirabrutinib (ONO-4059) is a highly selective covalent inhibitor of BTK with an IC50 of 2.2 nM. In a phase I trial conducted in patients with B-cell malignancies (NCT02457559), tirabrutinib showed significant potency on patients in the CLL group. Ninety-six percent of CLL patients responded to tirabrutinib, and all of the evaluated CLL patients harboring del 17p or TP53 mutations without del 17p responded.292 Moreover, a phase II trial (NCT02968563) is underway to assess the efficacy and safety of tirabrutinib in combination with the PI3K inhibitor idelalisib and the anti-CD20 antibody obinutuzumab.293 Spebrutinib (CC-292/AVL-292) is also a second-generation BTK inhibitor and inhibits BTK activity with an IC50 of 0.5 nM.294 The results of phase I studies (NCT01692184, NCT01732861, and NCT01351935) showed that spebrutinib was safe and well-tolerated following once-daily administration in patients with relapsed or refractory CLL/SLL, WM, and NHL.295 Despite its high in vitro activity, spebrutinib exhibited inferior clinical efficacy compared with the approved BTK inhibitors. The reasons for the suboptimal effect are not fully understood, but the highly variable PK and pharmacodynamics (PD) seem to limit spebrutinib to continuously reach the in vivo targets.296 In addition, due to the critical role of BTK in the development and function of B cells, BTK has also been confirmed as a potential therapeutic target for autoimmune disorders. Several BTK inhibitors including evobrutinib,297 spebrutinib,294 branebrutinib, 298 and HM71224299 have been assessed in clinical trials for the treatment of autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythaematosus, and remitting multiple sclerosis.
Both the first and second generations of BTK inhibitors covalently bind to the sulfhydryl group of Cys-481 in the active site of BTK. Cys-481 is reported to be the frequently mutated residue of BTK in cases of resistance to irreversible BTK inhibitors. Several mutants, including C481S, C481R, C481F, C481Y, and C481T, have been identified in resistant patients, especially C481S, which results in the vast majority of drug resistance.300 Moreover, PLCγ2 mutations have also been implicated in BTK inhibitor resistance. As a downstream protein of BTK, PLCγ2 mutations drive continued signaling regardless of BTK activity.301 Developing noncovalent BTK inhibitors is one of the available strategies to overcome resistance caused by Cys-481 mutations. Vecabrutinib,302 LOXO-305,303 fenebrutinib304 and ARQ-531305 are all noncovalent reversible inhibitors of BTK undergoing clinical evaluation. These agents inhibit BTK activity without forming covalent bonds with the Cys-481 residue; therefore, they have the same inhibitory effects on either wild-type BTK or its mutants. Another strategy is to target other components of the BCR pathway, such as inhibition of the upstream signaling pathways LYN or SYK, which can restrain BTK phosphorylation and conquer resistance caused by PLCγ2 mutations. In addition, the combined treatment of BTK inhibitors and other targeted agents is also considered a potential strategy for patients resistant to BTK inhibitor monotherapy.306
JAK inhibitors
Janus kinases (JAKs) belong to the family of non-receptor tyrosine kinases and are composed of four isoforms, JAK1, JAK2, JAK3, and TYK2, with up to 70% homology.307,308 JAK1, JAK2, and TYK2 are widely distributed in various tissues and cells, while JAK3 is only expressed in the bone marrow and lymphatic-derived cells.309 JAKs are able to transfer extracellular signals to the nucleus and mediate DNA transcription and protein expression.310 Receptor-coupled JAKs can be activated when inflammatory cytokines such as interleukin and interferon bind to cytokine receptors.311 Then JAKs catalyze the phosphorylation of receptor tyrosine residues and recruit and phosphorylate downstream signal transducer and activator of transcription (STAT) proteins. Activated STAT proteins promote their translocation to the nucleus and regulation of target-gene transcription and expression. Distinct cytoplasmic domains of cytokine receptors activate different JAKs and STATs.312 The JAK/STAT pathway runs downstream of more than 50 cytokines and growth factors and is considered to be the central communication node for the immune system.JA-7 Given the important role of the JAK/STAT pathway in cytokine signal transduction, targeting JAK/STAT is considered a promising strategy for the treatment of multiple autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus. Additionally, STAT signals (e.g., STAT3, STAT5, or STAT6) have been found to be frequently activated in malignant tumors, especially hematopoietic cancers and are involved in cell proliferation, survival, invasion, or inflammation.313 As the critical upstream protein of STAT signals, JAKs are also potential targets for cancer treatment. The application of JAK inhibitors in cancer is mainly focused on hematologic malignancies.314
Thus far, four JAK inhibitors have been approved for clinical use. As shown in Table 2, ruxolitinib is the first launched JAK inhibitor and selectively targets JAK1 and JAK2 with moderate activity against TYK2. It was approved for the treatment of myelofibrosis (a myeloproliferative neoplasm), polycythemia vera, and bone marrow cancer in 2011 by the FDA.315 The recently approved fedratinib is a selective JAK2 inhibitor and is used for myelofibrosis treatment in the clinic.316 Meanwhile, it has been reported that fedratinib also showed efficacy in the treatment of NSCLC in preclinical studies. It can reverse the resistance of NSCLC cells to erlotinib and abrogate PD-L1 expression, which mediates immune checkpoint blockade therapy in NSCLC.317 Another two approved JAK inhibitors are tofacitinib and baricitinib.318,319 They are all used clinically for the treatment of autoimmune diseases.313
Recently, the role of several new JAK inhibitors in cancer treatment is undergoing clinical evaluation. WP1066 is a novel JAK2 and STAT3 inhibitor with little activity against JAK1 and JAK3. A phase I trial of WP1066 was performed to evaluate its effects in the treatment of melanoma and glioblastoma (NCT01904123).320 Gandotinib (LY2784544) is an orally potent inhibitor of JAK1 and JAK2.274 Clinical studies have demonstrated that gandotinib has adequate efficacy, safety, and tolerability profiles in patients with myeloproliferative neoplasms (NCT01594723). Lestaurtinib (CEP701) is a multikinase inhibitor targeting JAK2, FLT3, and neurotropin receptor TrkA. The use of lestaurtinib to treat multiple cancers, including AML, Hodgkin lymphoma, neuroblastoma, prostate cancer, and myeloproliferative disorders, has been reported in experimental or clinical studies.321 A phase II study (NCT00668421) exhibited moderate efficacy and moderate but frequent gastrointestinal toxicity of lestaurtinib in myelofibrosis patients.322 Further studies are still needed to assess its clinical benefits. INCB039110 is an effective JAK1 inhibitor with >20-fold selectivity over JAK2 and >100-fold over JAK3 and TYK2.323 In an open-label phase II trial (NCT01633372), myelofibrosis-related symptoms were obviously ameliorated after INCB039110 treatment. Moreover, pacritinib (SB1518) is a dual JAK2 and FLT3 inhibitor that can inhibit JAK2 and JAK2 V617F mutations as well as FLT3 and FLT3-D835Y mutations. It has been clinically tested in myelofibrosis and AML patients (NCT03645824, NCT02532010), and has shown efficacy for myelofibrosis therapy.324
JAK inhibitors are effective for the management of immune‐mediated diseases (rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis),313 myelofibrosis,325 and polycythaemia vera; these diseases generally respond well to JAK inhibitors. A large number of clinical trials related to JAK inhibitors are still in progress.326 With further understanding of the clinical potential of JAK inhibitors, their indications will also be expanded.327 For example, the combination of JAK inhibitors with PD-L1 monoclonal antibodies or inhibitors of relevant kinases such as STAT inhibitors in cancer treatment has a certain theoretical basis and data support.328 In addition, side effects should be considered in the clinical use of JAK inhibitors. The adverse reactions related to JAK inhibitors include infection, neutropenia, venous thromboembolism, anemia, hypercholesterolemia, and even malignancy. Susceptibility to various infections is the main side effect after treatment with these inhibitors. Therefore, reliable prevention and monitoring strategies are needed during the medication process.329,330
Serine/theonine kinase inhibitors
BRAF/MEK/ERK inhibitors
Rat sarcoma virus (RAS)-rapidly accelerated fibrosarcoma (RAF)-mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) signaling is a well-established pathway that controls cell growth, proliferation, and survival in normal cells and cancer cells.331,332 Core components of RAS-RAF-MAPK-ERK signaling include the small GTPase RAS, the serine/threonine kinase RAF, the protein kinases MEK1/2 and ERK1/2. RAF, including ARAF, BRAF, and CRAF, is the direct downstream of RAS, which serves as a transducer of extracellular stimuli.332 RAF activates the dual-specificity protein kinase MEK1/2, which subsequently phosphorylates ERK1/2. Activated ERK phosphorylates multiple substrates in the cytosol and nucleus, and then promote cell proliferation and survival.332 Dysregulation of RAS/RAF/MEK/ERK signaling can be observed in a large number of cancers, which is most commonly due to mutations in RAS or BRAF.332,333 BRAF mutations (mostly BRAF V600E) have been identified in ~40–50% of melanomas and 6% of other malignancies, which results in several-fold hyperactivation of BRAF and continuous activation of downstream MEK and ERK.333,334 Due to the picomolar affinities of RAS to GTP, RAS was taken as an undruggable target until the appearance of irreversible small-molecule inhibitors of KRAS G12C in recent years.335 Therefore, BRAF and the downstream kinases MEK1/2 and ERK are considered attractive therapeutic targets for malignant tumors, especially melanoma.
Small-molecule BRAF inhibitors can be classified into two types. Type-I inhibitors stabilize the kinase in its active (DFG-in) conformation by occupying the ATP-binding pocket.336 The FDA has approved three type-I competitive BRAF inhibitors for the treatment of non-resectable BRAF V600E/K/D mutant melanoma as single agents or combined with MEK inhibitors including vemurafenib in 2011,337 dabrafenib in 2013,338 and encorafenib in 2018 (Table 3).339 Type-II BRAF inhibitors bind to the adjacent hydrophobic sites of ATP-binding pocket and stabilize the kinase in its inactive (DFG-out) conformation.336 Sorafenib, a representative type-II pan-RAF inhibitor, is a multikinase inhibitor. This agent was initially developed as a RAF kinase inhibitor; however, it also showed inhibitory activity against VEGFR-1/2/3, PDGFR, FLT1, KIT, and RET. It is currently approved by FDA for the treatment of unresectable HCC, advanced RCC, and thyroid carcinoma refractory to radioactive iodine, but not for melanoma due to the unsatisfactory results of clinical trials.340
Table 3.
Properties of approved small-molecule inhibitors of serine/theonine kinases
| Chemical structure | Name | Targets | Approved indications (year) | Corporation |
|---|---|---|---|---|
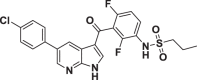 |
Vemurafenib (Zelboraf) | BRAF | Melanoma (2011) | Roche/Genentech |
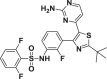 |
Dabrafenib (Tafinlar) | BRAF/CRAF |
Melanoma (2013) NSCLC (2017) ATC (2018) |
Novartis/GlaxoSmithkline |
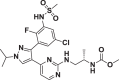 |
Encorafenib (Braftovi) | BRAF | Melanoma (2018) | Array |
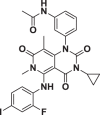 |
Trametinib (Mekinist) | MEK1/2 |
Melanoma (2013) NSCLC (2017) ATC (2018) |
Novartis/GlaxoSmithkline |
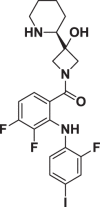 |
Cobimetinib (Cotellic) | MEK1/2 | Melanoma (2015) | Roche/Genentech/ Exelixis |
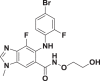 |
Binimetinib (Balimek) | MEK1/2 | Melanoma (2018) | Array |
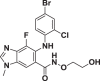 |
Selumetinib (Koselugo) | MEK1/2 |
Neurofibromatosis type 1 (2020) Plexiform neurofibromas (2020) |
AstraZeneca/Merck |
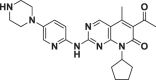 |
Palbociclib (Aiboxin) | CDK4/6 | Breast cancer (2015) | Pfizer |
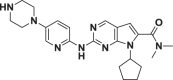 |
Ribociclib (Kisqali) | CDK4/6 | Breast cancer (2017) | Novartis |
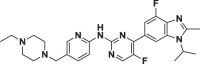 |
Abemaciclib (Verzenio) | CDK4/6 | Breast cancer (2017) | Lilly |
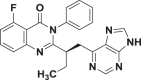 |
Idelalisib (Zydelig) | PI3Kδ |
CLL (2014) Follicular lymphoma (2014) |
Gilead |
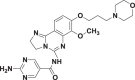 |
Copanlisib (Aliqopa) | Pan-PI3K | Follicular lymphoma (2017) | Bayer |
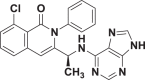 |
Duvelisib (Copiktra) | PI3Kγ/δ |
CLL (2018) SLL (2018) Follicular lymphoma (2018) |
Verastem |
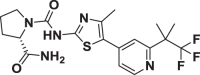 |
Alpelisib (Piqray) | PI3Kα | Breast cancer (2019) | Novartis |
 |
Temsirolimus (Torisel) | mTOR | RCC (2007) | Pfizer |
 |
Everolimus (Afinitor) | mTOR |
RCC (2009) Pancreatic cancer (2011) Breast cancer (2012) |
Novartis |
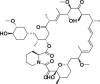 |
Sirolimus (Rapamune) | mTOR | LAM (2015) | Pfizer |
The discovery and use of BRAF inhibitors opened up an avenue for the development of inhibitors of MEK1/2 and ERK, downstream targets of RAS/RAF/MEK/ERK signaling. Three MEK1/2 inhibitors have been approved in combination with BRAF inhibitors for the treatment of unresectable or metastatic melanoma harboring BRAF V600E/K mutation including reversible allosteric inhibitors trametinib and binimetinib as well as cobimetinib, a highly selective inhibitor that restrains the catalytic activity of MEK1/2 (Table 3); trametinib is also administered as a monotherapy.341 The FDA-approved combination regimens of BRAF and MEK inhibitors (vemurafenib plus cobimetinib, dabrafenib plus trametinib, and encorafenib plus binimetinib) can achieve a longer PFS in patients with melanoma compared with BRAF inhibitor monotherapy in clinical trials, and encorafenib-binimetinib combination had the best toxicity profile.342 The combination of dabrafenib and trametinib has also been approved for the treatment of NSCLC or anaplastic thyroid cancer (ATC) patients harboring BRAF V600E mutation. Moreover, the MEK inhibitor binimetinib (as monotherapy or in combination therapy) has been reported to be effective for patients with NRAS mutant melanoma.343 Recently, the FDA-approved a novel MEK1/2 inhibitor named selumetinib. It is a highly selective allosteric inhibitor of MEK1/2 and was initially assessed in patients with metastatic uveal melanoma. It has been granted orphan drug status as adjuvant treatment for thyroid cancer and as treatment for neurofibromatosis type 1. Based on the results of the phase II SPRINT trial, selumetinib was approved for pediatric patients with neurofibromatosis type 1 and symptomatic, inoperable plexiform neurofibromas in April 2020 (Table 3).344 Currently, no ERK inhibitor has been approved for clinical use.
The success of RAS/MAPK signaling inhibitors in the treatment of melanoma has promoted the further development of such inhibitors, and a variety of novel inhibitors have entered clinical trials.345,346 Typically, pimasertib (AS703026) is a highly selective ATP non-competitive allosteric inhibitor of MEK1/2. A phase II trial comparing the combination of pimasertib with SAR245409 (PI3K inhibitor) to pimasertib alone in patients with recurrent unresectable borderline or low-grade ovarian cancer has been completed (NCT01936363). Response to pimasertib alone (ORR: 12%) was as effective as the combination, suggesting that MEK inhibition could be used as an alternative treatment method to cytotoxic chemotherapy in this population.347 RO5126766 is a dual RAF/MEK allosteric inhibitor with a novel structure based on coumarin skeleton. A phase I clinical trial (NCT02407509) is conducted to assess RO5126766 alone or together with everolimus on patients with solid tumors or MM harboring BRAF, NRAS, and/or KRAS mutation;348 the completion date of this trial is targeted for June 2020. The pyrazole amino-pyrimidine derivative ravoxertinib (GDC-0994) is a selective ERK1/2 inhibitor with an IC50 in the subnanomolar range. Preclinical studies have demonstrated that ravoxertinib had good PD and PK properties. Interestingly, it also showed strong antitumor activity in nude mice harboring KRAS-mutant xenografts.349 The efficacy and safety of ravoxertinib in combination with cobimetinib in the treatment of locally advanced or metastatic solid tumors were evaluated in a phase I study (NCT02457793), but the data are not yet available.
The clinical application of BRAF and MEK inhibitors has changed the prospect of melanoma treatment, especially the combination therapy with both agents. Treatment with BRAF inhibitors is often accompanied by paradoxical MAP kinase reactivation that limits the clinical response.350 The combination with MEK inhibitors alleviates this situation to some extent, thereby significantly improving the response rate and survival of patients with melanoma. However, the efficacy of this therapy is limited by acquired resistance caused by gene mutation and bypass activation.351 Some novel combinations of targeted therapy are expected to improve efficacy and overcome drug resistance, such as the combination with ERK inhibitors, CDK4/6 inhibitors, or inhibitors of the PI3K/AKT/mTOR pathway.352 In addition, resistance to BRAF and MEK inhibitors is driven partly by immune-mediated mechanisms; therefore, combined targeted therapy and immunotherapy have become the focus in clinical melanoma treatment in recent years.351 An array of such trials is under assessment for toxicity, efficacy, and treatment sequence to support the continuous progress of individualized treatment of melanoma.351,352
CDK inhibitors
Cell cycle abnormalities result in uncontrolled cell proliferation and have been considered one of the important hallmarks of cancer.353 Cyclin-dependent kinases (CDKs) are critical enzymes regulating cell cycle progression and require cyclin proteins for activation and downstream phosphorylation.354 To date, at least 20 CDKs and 29 cyclins have been identified in humans.355 Among them, CDK4 and CDK6 are necessary for regulating growth signaling and driving the transition of the cell cycle from G1 to S phase. During the process of regulation, CDK4 and CDK6 are activated by D-type cyclins, which induce the phosphorylation of tumor suppressor retinoblastoma protein 1 (RB1) early in the G1 phase. This leads to the inactivation of RB and release of E2F transcription factors, thereby promoting the transcription of target genes related to cell cycle progression.356–358 Given the critical role of the CDK4/6-RB1 axis in mediating cellular proliferation and tumorigenesis, inhibition of CDK4/6 is a promising strategy for cancer therapy that can induce cell cycle arrest in G1 phase and result in decreased cell viability.359 Notably, CDK4 and CDK6 are functionally identical in their biological effects; therefore, dual inhibition of CDK4 and CDK6 is essential because of the compensatory effects. Cyclin D, the catalyst for CDK4/6, is a major transcriptional target of the estrogen receptor (ER). Estrogen binding to the ER initiates cyclin D transcription, followed by activation of the CDK4/6-RB1 pathway.360–362 Hence, dysregulation of the CDK4/6-RB1 pathway is a significant feature of hormone receptor (HR)-positive breast cancers.363
In the past three decades, great progress has been made in the development of CDK inhibitors.364 However, many early developed non-selective and pan-CDK inhibitors (e.g., flvopiridol, seliciclib, UCN-01) have been discontinued due to their limited clinical efficacy or serious side effects. To date, three CDK inhibitors are clinically available: palbociclib,365 ribociclib,366 and abemaciclib (Table 3).367 All of them have similar chemical structures and are orally selective reversible inhibitors specifically targeting CDK4/6. They are approved for the treatment of metastatic HR-positive, HER2-negative breast cancer in combination with specific endocrine therapies.368 In contrast, palbociclib and ribociclib are used in combination with non-steroidal aromatase inhibitors (e.g., letrozole) in postmenopausal women with HR-positive HER2-negative metastatic breast cancer,369 and palbociclib is also combined with fulvestrant for breast cancer patients with disease progression following endocrine therapy.370 Abemaciclib shares an indication with palbociclib for use in combination with fulvestrant in HR-positive HER2-negative breast cancer progressing after endocrine therapy.371 In particular, it is also the only CDK4/6 inhibitor approved as monotherapy for HR-positive HER2-negative metastatic breast cancer pretreated with endocrine therapy and chemotherapy.372 Despite being approved only for specific breast cancer, the three approved CDK4/6 inhibitors have also been assessed in other solid tumors and hematologic malignancies, such as lung cancer, prostate cancer, melanoma, glioblastoma, and myelofibrosis, and have shown promising results in preclinical studies or clinical trials of some malignancies.373,374 Nevertheless, further clinical research is needed to confirm their efficacies.
Additionally, several newly developed CDK4/6 inhibitors have entered clinical trials. Trilaciclib (G1T28) inhibits CDK4 and CDK6 with IC50 values of 1 and 4 nM, respectively. It has been evaluated clinically for the prevention of chemotherapy-induced myelosuppression in triple-negative breast cancer (TNBC) and SCLC.375 The pyridopyrimidine derivative PF-06873600 is potent against CDK2/4/6 and can overcome palbociclib resistance in preclinical studies. Phase II trials of PF-06873600 in the treatment of metastatic breast cancer and other gynecological cancers are ongoing (NCT03519178). SHR-6390 conquered resistance to tamoxifen or trastuzumab, and its combination with endocrine therapy had significant synergistic effects in breast cancer.376 BPI-16350, FCN-437, and XZP-3287 can effectively penetrate the blood–brain barrier. These CDK4/6 inhibitors are currently being studied in phase I/II trials (NCT03791112, NCT04488107, and NCT04539496).364 A variety of reversible ATP-competitive inhibitors of other CDK isoforms have been evaluated in the clinic in combination with standard-of-care agents or as monotherapy, such as the CDK2 inhibitors inditinib (AGM-130)377 and FN-1501,378 the selective CDK7 inhibitor ICEC0942,379 the CDK9 inhibitors BAY-1251152,364 and CYC-065, a second-generation inhibitor of CDK2/5/9. Meanwhile, with the development of modern biotechnology, non-classical CDK inhibitors (allosteric inhibitors, covalent inhibitors, and PROTACS) have laid the foundation for the discovery of a novel generation of selective CDK inhibitors. Notably, SY-1365 is an ATP-competitive covalent inhibitor of CDK7 and is the first irreversible CDK inhibitor entering the clinical study.380 However, the development of this drug was discontinued in September 2019 by Syros Pharmaceuticals due to poor clinical data, which showed that sustaining CDK7 target coverage levels to enhance clinical activity would require more frequent doses or a higher dose, and this may lead to an overly burdensome dosing schedule for patients.364
Attention should be paid to the adverse events related to the approved CDK4/6 inhibitors. Hematological toxicities such as neutropaenia are often observed in the clinical use of palbociclib and ribociclib,359 while gastrointestinal toxicities, especially diarrhea, are common in abemaciclib treatment.368 Most adverse reactions are normally easily controlled by standard supportive care and dose adjustments. In September 2019, the FDA issued a drug safety newsletter and warned that palbociclib, ribociclib, and abemaciclib used to treat advanced breast cancer could trigger rare but serious pneumonia called interstitial lung disease.381 This risk warning information must be added to the drug labels and patient instructions of all approved CDK4/6 inhibitors.382 Moreover, understanding the mechanisms of resistance to CDK4/6 inhibitors is another issue that needs to be considered. RB1 is the phosphorylation target of CDK4/6; therefore, loss of RB1 expression is one of the potential mechanisms leading to CDK4/6 inhibitor resistance. RB1 mutations have been detected in the ctDNA of breast cancer patients resistant to CDK4/6 inhibitors, with an estimated frequency of 5%.383 Ectopic overexpression of cyclin E1 (CCNE1) and cyclin E2 (CCNE2) can also result in resistance to antioestrogen and palbociclib monotherapy through activation of CDK2.384 In addition to cell cycle alterations, overexpression or mutation in upstream proteins, including AKT1, KRAS/HRAS/NRAS, ERBB2, and FGFR2, have also been observed as potential resistance mechanisms. Given these findings, exploring rational combination treatment strategies may be efficacious to conquer CDK inhibitor resistance.385
PI3K/AKT/mTOR inhibitors
The phosphatidylinositol 3-kinase (PI3K)/V-AKT murine thymoma viral oncogene homolog (AKT)/mammalian target of rapamycin (mTOR) signaling pathway has an important role in cell growth, proliferation, survival, apoptosis, and motility and is frequently activated in human cancer.353 PI3Ks are a family of lipid kinases that catalyze the phosphorylation of phosphatidylinositol D3. According to the structural characteristics of the subunits and substrates, PI3Ks can be divided into three classes. Of these, class I PI3K is the major isoform implicated in cancer and can be further subdivided into class IA and class IB, which are activated by RTKs and GPCRs, respectively. Class IA consists of PI3Kα, PI3Kβ, and PI3Kδ encoded by PIK3CA, PIK3CB, and PIK3CD genes, respectively. Class IB comprises only the PI3Kγ subtype encoded by PIK3CG.386,387 PI3K/AKT/mTOR pathway is activated by various mechanisms in oncogenesis and progression. PIK3CA gene is frequently dysregulated in multiple cancers, both by point mutations and amplification. The tumor suppressor PTEN negatively regulates the PI3K pathway by dephosphorylating PIP3 to PIP2. It is also mutated frequently and results in loss of function in human cancers, which upregulates PIP3 levels and leads to constitutive activation of AKT and downstream components. Moreover, as the immediate downstream effector of PI3K, amplification and activating mutations of AKT are often observed in solid tumors.388 Hyperactivation of PI3K/AKT/mTOR signaling is not only common in a variety of tumors but also closely related to drug resistance (e.g., resistance mechanism of EGFR inhibitors); thus, this pathway has become an attractive target for developing antitumor targeted drugs. Many small-molecule inhibitors of PI3K, AKT, and mTOR have been developed in the past few years. However, only several PI3K and mTOR inhibitors have been approved for cancer treatment.
The early developed PI3K inhibitors are mostly pan-PI3K inhibitors that are capable of binding to all class I PI3Ks, such as wortmannin and LY294002. However, due to their poor PK properties, they are not ultimately approved for clinical use. The second-generation isoform-selective PI3K inhibitors are highly selective for the four isoforms of the class I PI3K catalytic subunit p110 (α, β, γ, and δ). As indicated in Table 3, idelalisib is the first approved selective PI3Kδ inhibitor based on its efficacy in the treatment of relapsed or refractory CLL patients. It is also recommended for the treatment of lymphocytic lymphoma patients who have received at least two prior systemic therapies or patients with relapsed follicular B-cell NHL.389 Copanlisib, approved in September 2017, is a pan-PI3K inhibitor with IC50 values of 0.5, 3.7, 6.4, and 0.7 nM against class I PI3K-α, β, γ, and δ isoforms, respectively.390 It is used clinically for the treatment of adult patients with relapsed follicular lymphoma who have received at least two prior systemic therapies. Duvelisib is an oral dual PI3Kγ and PI3Kδ inhibitor with IC50 values of 27 and 2.5 nM, respectively.391 It prevents the activation of PI3K-γ and δ isoforms by competitively and reversibly binding to the ATP-binding pocket of the p110 subunit.392 The FDA has approved duvelisib for the treatment of adult patients with relapsed or refractory CLL, SLL, and follicular lymphoma after at least two prior therapies. The newly approved alpelisib is a selective inhibitor targeting the α-isoform of class I PI3K, with an in vitro IC50 of 4.6 nM. It is indicated in combination with fulvestrant for the treatment of postmenopausal women and men with HR-positive, HER2-negative, PIK3CA-mutated, advanced, or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.393 This is also the first PI3K inhibitor approved for clinical treatment of breast cancer. In addition, there are also a variety of PI3K inhibitors undergoing clinical evaluation, such as the pan-class I PI3K inhibitors XL147 and ZSTK474, PI3Kγ inhibitor IPI-549, and PI3Kα inhibitor serabelisib.394–397 Some of them have progressed to phase II or III trials and have good prospects for approval. In addition, the promising efficacy of PI3K inhibitors raised the question of whether the combined inhibition of multiple pathway components could further improve the efficacy without excessive side effects. Developing dual PI3K/mTOR inhibitors is a predominant strategy due to the high homology between the ATP-binding domain of p110 and the catalytic site of mTOR. Several dual PI3K/mTOR inhibitors have entered clinical trials, such as bimiralisib, dactolisib (BEZ235), GDC-0084 (RG7666), and gedatolisib (PKI-587/PF-05212384).398–400 Current clinical trials focus on combining them with a range of other antitumor agents. Notably, GDC-0084 is orally administered and can penetrate the BBB. It is currently specifically developed for patients with glioma or brain metastases from solid tumors.
Compared with PI3K and mTOR inhibitors, the development of AKT inhibitors is relatively slow. The obtained results from numerous preclinical studies revealed that AKT inhibitors are potent for tumors with PIK3CA mutations or loss of PTEN function.386 However, most of these agents displayed limited clinical effects in patient settings, especially as monotherapy. Only several AKT inhibitors, including ATP-competitive inhibitors and allosteric inhibitors, have progressed to clinical trials. The ATP-competitive inhibitors ipatasertib, capivasertib, afuresertib, and uprosertib are all highly selective pan-AKT inhibitors and can prevent AKT activation and enhance the antitumor activity of chemotherapeutic drugs.401,402 A phase III trial (NCT03072238) of ipatasertib is being conducted to evaluate the efficacy of its combination with abiraterone and prednisone/prednisolone in adult male patients with metastatic castration-resistant prostate cancer (CRPC). Three other phase III trials related to ipatasertib combination are actively recruiting patients with HR-positive and HER2-negative locally advanced unresectable or metastatic breast cancer (NCT04060862, NCT04177108, and NCT03337724). The efficacy of capivasertib (AZD5363) combined with paclitaxel as a first-line treatment for locally advanced or metastatic TNBC patients is under evaluation in a phase III trial (NCT03997123).403 Afuresertib (GSK2110183) monotherapy showed satisfactory safety and clinical activity against hematological malignancies, including MM.404 Uprosertib (GSK2141795) was used for relapsed or refractory MM either alone or in combination with trametinib in phase I and II clinical trials (NCT01989598, NCT01979523).405 In addition, allosteric AKT inhibitors are represented by MK-2206. Several clinical trials are ongoing to test MK-2206 monotherapy or in combination with targeted therapy or chemotherapy for metastatic breast cancer and in combination with lapatinib and trastuzumab for HER2-positive breast cancer (NCT01277757, NCT01245205, and NCT01235897). Notably, there is currently an AKT inhibitor approved for marketing, named miltefosine. However, it is used clinically for the treatment of visceral and cutaneous leishmaniasis but not for malignancies.
mTOR inhibitors can be divided into two categories: rapamycin analogs (rapalogs) and ATP-competitive inhibitors. The former is the first-generation mTOR inhibitor, which forms a complex with FK506 binding protein 12 (FKBP12) and inhibits the activity of only mTORC1 but not mTORC2.406 They are also the first compounds developed to target the PI3K/mTOR pathway. Currently, multiple rapalogs, such as sirolimus (rapamycin), temsirolimus, and everolimus, have been approved for the treatment of various cancers, including RCC, breast cancer, and pancreatic cancer (Table 3). Of these, rapamycin was initially approved as an immunosuppressant for transplant patients, and its indications were subsequently extended to lymphangioleiomyomatosis (LAM).407 Although patients can benefit from such medications, drug resistance caused by mTORC2-mediated negative feedback limits their clinical use. Second-generation mTOR inhibitors are designed as ATP-competitive inhibitors, which can simultaneously suppress the activity of both mTORC1 and mTORC2. At present, no such inhibitors have received approval, but some of them are under clinical evaluation, such as sapanisertib (TAK-228), vistausertib (AZD2014), CC-223, BI860585, DS-3078a, ME-344, GDC-0349, OSI-027, and P529.406 Representatively, sapanisertib (TAK-228), formerly known as INK128 or MLN0128, is an orally effective mTOR inhibitor with an IC50 of 1 nM.408 It is being evaluated for the treatment of multiple cancers in several phase II trials (NCT02484430, NCT03047213, and NCT02893930). Vistusertib is a selective mTOR inhibitor with an IC50 of 2.8 nM. In preclinical studies, vistusertib exhibited a broad spectrum of antitumor activity.409 It is undergoing evaluation in a phase II clinical trial for the treatment of recurrent grade II–III meningiomas (NCT03071874).
Isoform-specific inhibitors have fewer off-target effects and side effects than pan-PI3K, pan-AKT, and dual PI3K/mTOR inhibitors. However, due to their limited therapeutic efficacy caused by compensation effects or various mutations of the PI3K/AKT/mTOR pathway, most clinical trials still tend to use pan-inhibitors. Therefore, clarifying the mutant types via molecular pathological diagnosis in advance can improve the clinical application of specific inhibitors. On the other hand, the clinical efficacy using only PI3K/AKT/mTOR inhibitors were shown to have modest effects for patients in actual treatment. More importantly, PI3K/AKT/mTOR cascade exchanges crosstalk with many other signaling pathways, such as Wnt and MAPK signaling. Hence, using such inhibitors is prone to negative feedback regulation, resulting in resistance. These problems highlight the limitations of PI3K/AKT/mTOR inhibitors as monotherapy in malignancies. Currently, efforts have focused on combination therapy, including inhibition of parallel pathways as well as targeted therapy combined with cytotoxic drugs,388 and the results of most clinical trials are promising. Moreover, treatment with PI3K pathway inhibitors could also induce adverse effects, even though isoform-specific inhibitors are no exception. For example, the PI3Kδ inhibitor idelalisib was shipped with four black-frame warnings, suggesting fatal and severe liver toxicity, diarrhea and colitis, pneumonia, and intestinal perforation.410 Other toxicities reported in clinical trials of the PI3K/AKT/mTOR inhibitors include immunosuppression, hypoglycemic, cardiac toxicity, neuropsychiatric effects, cutaneous reactions, nausea, mouth ulcers, constipation, etc. A better understanding of the mechanism of side effects caused by such drugs and their management might advance novel PI3K/AKT/mTOR inhibitors from clinical trials to the bedside, with new treatment options for patients.
We in the above summarize the anti-cancer kinase inhibitors approved by the US FDA and NMPA of China. To date, a total of 70 kinase inhibitors have been approved to use in the clinic, which accounts for almost 80% of all approved small-molecule targeted anti-cancer drugs (89 in total). Despite this great achievement, these kinase inhibitors just act on a small number of kinase targets (<30 kinases). Many kinases that are thought of as potential anti-cancer targets still lack inhibitors or at least potent and selective inhibitors. Therefore, drug discovery targeting kinases still has a lot of room for development. Another hot topic of debate regarding kinase inhibitors is whether single-target drugs or multitarget drugs have more advantages. Target-specific kinase inhibitors generally have low toxicity but are easy to develop resistance. While multikinase inhibitors often have high side effects, but have advantages in anti-cancer efficacy and overcoming drug resistance.
Epigenetic inhibitors
Epigenetics is a branch of genetics that studies the heritable changes of gene expression without changing the nucleotide sequence of genes. It is strictly regulated by a variety of chemical modifying enzymes and recognition proteins, which are often called “writers”, “erasers”, and “readers”.411,412 The writers refer to enzymes that transfer chemical groups to DNA or histones, which include DNA methyltransferases (DNMTs), histone acetyltransferases (HATs), and histone lysine methyltransferases (KMTs). The erasers remove post-translational modifications, and include histone deacetylases (HDACs) and histone lysine demethylases (KDMs). The readers are proteins that can recognize the modified histones or DNA, such as methyl-binding domain proteins, and bromodomain and extra-terminal (BET) family proteins (Fig. 3). Abnormal epigenetic regulation is also closely related to various diseases including tumor, immune diseases, and many rare diseases. Though numerous epigenetic regulatory proteins have been identified as potential disease targets, only fewer epigenetic drugs are approved for clinical use at present.
Fig. 3.
Commonly altered epigenetic regulatory proteins implicated in cancer. Gene silencing in mammalian cells is usually caused by methylation of DNA CpG islands as well as hypermethylation or hypoacetylation of histones. The writers (DNMTs, HATs, and HMTs) refer to enzymes that transfer chemical groups to DNA or histones; the erasers (HDACs and KDMs) are enzymes responsible for removing chemical groups from histones; the proteins (MBDs and BET family proteins) that can recognize the methyl-CpGs and modified histones are readers. Mutated IDH1/2 catalyzes the reduction of α-KG to 2-HG, which inhibits the activity of TET and lysine demethylases, resulting in DNA hypermethylation and increased histone lysine methylation. Figure created with BioRender.com
EZH2 Inhibitors
Enhancer of zeste homolog 2 (EZH2), a histone methyltransferase, functions as a catalytic subunit of the polycomb repressor complex 2 (PRC2), which also comprises other members including embryonic ectoderm development (EED), suppressor of zeste 12 (SUZ12), and histone-binding proteins RbAp46/48.413 PRC2 is one of the two core complexes of polycomb group proteins (PcGs), and is responsible for transferring methyl groups from S-adenosyl-L-methionine (SAM) to lysine 27 on histone H3 (H3K27) through its C-terminal SET domain, resulting in chromatin compaction and transcriptional silencing of target genes. As the central component of PRC2, EZH2 is involved in numerous epigenetic modifications that are associated with cell proliferation, differentiation, survival, adhesion, and DNA damage repair.414 Dysfunction of EZH2 is closely related to tumorigenesis and progression. Accumulating evidence has confirmed that EZH2 is frequently mutated and abnormally overexpressed in various malignant tumors including prostate cancer,415,416 ovarian cancer,417 endometrial carcinoma,418 breast cancer,419 melanoma as well as hematological malignancies,420 such as NHL, B-cell lymphoma, and T-cell ALL.421–424 It promotes tumorigenesis mainly through three mechanisms: PRC2-dependent H3K27 methylation, PRC2-dependent non-histone protein methylation, and PRC2-independent coactivator of transcriptional factors. Given the evidence for EZH2 enzymatic gain of function being a cancer driver, inhibition of EZH2 has been thought of as a novel and promising approach for cancer therapy.413
The first reported small-molecule EZH2 inhibitor is 3-deazaneplanocin A (DZNep), a cyclopentanyl analog of 3-deazaadenosine. This compound can potently inhibit the S-adenosyl-l-homocysteine (SAH) hydrolase activity and induce the increase of cellular 5-adensylhomocystein levels, thus suppressing the activity of global S-adenosyl-l-methionine (SAM)-dependent histone lysine methyltransferase, including EZH2-mediated histone methylation. Therefore, DZNep is a non-specific EZH2 inhibitor.425 Although treatment with DZNep showed significant antitumor activity in various preclinical studies, the poor PK profile and safety encouraged further development of more potent and selective EZH2 inhibitors. Since 2012, multiple SAM-competitive EZH2 inhibitors have been reported, including EI1, EPZ005687, GSK126, GSK343, UNC1999, tazemetostat (EPZ6438), SHR2554, CPI-1205, DS-3201, PF-06821497, and HH2853 (NCT04390737).413 These compounds display better selectivity for EZH2 or EZH1 compared with other methyltransferases, and most of them bind to both wild-type and mutant EZH2, particularly Y641 and Y646 mutations.426,427 Currently, several of these inhibitors (e.g., tazemetostat, SHR2554, CPI-1205, DS-3201, PF-06821497, and HH2853) have entered clinical trials to evaluate their clinical efficacy and safety in a variety of solid tumors or hematological malignancies. Among them, tazemetostat developed by Epizyme and Eisai is an oral competitive inhibitor of the SAM pocket of the EZH2 SET domain, and received accelerated approval in January 2020 in the USA for the treatment of adults and adolescents aged ≥16 years with locally advanced or metastatic epithelioid sarcoma not eligible for complete resection (Table 4). Tazemetostat is the first approved EZH2 inhibitor and also the first therapy specifically for the treatment of epithelioid sarcoma.
Table 4.
Properties of approved small-molecule inhibitors of epigenetic targets
| Chemical structure | Name | Targets | Approved indications (year) | Corporation |
|---|---|---|---|---|
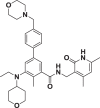 |
Tazemetostat (Tazverik) | EZH2 | Epithelioid sarcoma (2020) | Epizyme/Eisai |
 |
Vorinostat (Zolinza) | HDAC1/2/3/6 | CTCL (2006) | Merck |
 |
Romidepsin (Istodax) | HDACs |
CTCL (2010) PTCL (2011) |
Celgene |
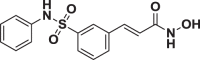 |
Belinostat (Beleodaq) | HDAC1/2 | PTCL (2014) | Onxeo/Spectrum |
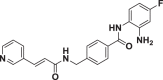 |
Tucidinostat (Epidaza) | HDAC1/2/3/10 | PTCL (2015) | Chipscreen Biosciences |
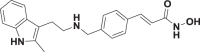 |
Panobinostat (Farydak) | HDACs | MM (2015) | Secura Bio |
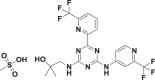 |
Enasidenib (Idhifa) | IDH2 | AML (2017) | Agios/Celgene |
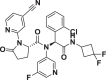 |
Ivosidenib (Tibsovo) | IDH1 | AML (2018) | Agios |
In addition to the highly selective SAM-competitive inhibitors, EZH2 can also be inhibited by disrupting its interaction with other PRC2 subunits. EED226 (MAK683) is a potent and selective PRC2 allosteric antagonist, which directly binds to the H3K27me3 pocket of EED and induces a conformational change, therefore disrupting EZH2-EED interaction and resulting in loss of PRC2 activity.428 EED226 shows similar activity to SAM-competitive inhibitors in blocking H3K27 methylation of PRC2 target genes and inducing regression of human lymphoma xenograft tumors. Interestingly, EED226 could also potently inhibit the activity of PRC2 containing a mutant EZH2 protein resistant to SAM-competitive inhibitors.428 A phase I trial (NCT02900651) is ongoing to evaluate the safety and efficacy of EED226 in patients with advanced malignancies, such as DLBCL, nasopharyngeal carcinoma (NPC) or other advanced solid tumors for whom no further effective standard treatment is available. Besides, some other small-molecule inhibitors, such as A-395,429 BR-001, and UNC6852, were also reported as EED inhibitors that inhibit the interaction between EZH2 and EED and destabilize PRC2 complex,430,431 thereby exerting antitumor activity. Moreover, Wang et al. developed a unique strategy to target EZH2 by protein degradation.432 They identified a gambogenic acid (GNA) derivative GNA002 that could specifically and covalently bind to Cys668 within the EZH2-SET domain and trigger EZH2 degradation through COOH terminus of Hsp70-interacting protein (CHIP)-mediated ubiquitination. In this study, GNA002 significantly suppressed H3K27Me3 and effectively reactivated PRC2-silenced tumor suppressor genes, and could inhibit tumor growth in an EZH2-dependent manner.432
The efficacy of EZH2 inhibitors is limited by both primary and acquired resistance, which is the main challenge for the clinical use of EZH2 inhibitors. Primary resistance is often due to EZH2 resistant mutations, differences in the tumor microenvironment, heterogeneity of tumor tissue structure (influencing PKs and drug delivery), or activation of prosurvival pathways as reported in DLBCL and SWI/SNF mutant cancer cells.433,434 Huang et al. profiled global post-translational histone modification changes across a large panel of cancer cell lines with various sensitivities to EZH2 inhibitors, and found that oncogenic transcriptional reprogramming mediated by MLL1’s interaction with the p300/CBP complex directed H3K27me loss to reciprocal H3K27ac gain and restricted response to EZH2 inhibition.435 This resistance could be effectively reversed by concurrent inhibition of H3K27me and H3K27ac with a combination of EZH2 and BRD4 inhibitors. Acquired resistance is usually because of multiple secondary mutations of EZH2, such as Y641F, C663Y, E720G, Y726F, Y111L, and Y661D.433,434,436,437 Combination therapy is thought as the most efficient strategy to improve the limited effectiveness of EZH2 inhibitors.438 For example, the approved drug tazemetostat is currently assessed clinically in several combined treatments for a variety of malignant tumors. Exploring various effective combination strategies, including combination with chemotherapy, immunotherapy, kinase targeted therapy, and metabolic regulators, is a promising direction in the near future.414,439,440
HDAC inhibitors
Histone deacetylases (HDACs) are important epigenetic regulators that remove the acetyl groups from the N-acetylated lysine residues of histones and various non-histone substrates. To date, 18 HDACs have been identified in mammals and are characterized into 4 subfamilies: class I HDACs (HDACs 1, 2, 3, 8), class II HDACs (class IIa: HDACs 4, 5, 7, 9; class IIb: HDACs 6, 10), class III HDACs (Sirt1-7) and class IV HDAC (HDAC11).441,442 Class I, II, and IV HDACs are Zn2+-dependent histone deacetylases and can be restrained by inhibitors (such as TSA and SAHA) that occupy the catalytic core of the zinc-binding site. Class III HDACs require nicotinamide adenine dinucleotide (NAD+) for their activity.441–443 Aberrant upregulation of HDACs has been reported in various types of cancers. Such changes alter the transcription of oncogenes and tumor suppressor genes, which are closely associated with cell proliferation, apoptosis, differentiation, migration, and cancer angiogenesis.443,444 Inhibition of HDACs has proven to be an effective strategy for cancer therapy. To date, a variety of HDAC inhibitors have been approved by the FDA (Table 4) or are undergoing clinical trials.
Based on chemical structural differences, HDAC inhibitors can be divided into four categories: hydroxamates, cyclic tetrapeptides, benzamides, and aliphatic acids.445 Hydroxamide HDAC inhibitors inhibit HDAC activity through coordination with Zn2+. TSA was the first natural hydroxamate HDAC inhibitor. Its structural analog vorinostat (SAHA) is a pan-inhibitor of classical classes of HDACs (I, II, and IV) and was approved in October 2006 for the treatment of cutaneous T-cell lymphoma (CTCL) (Table 4).446 Belinostat and panobinostat are two other hydroxamate pan-HDAC inhibitors that are approved for the treatment of relapsed or refractory peripheral T-cell lymphomas (PTCL) and drug-resistant MM, respectively.447,448 Both of them are derivatives of M-carboxycinnamic acid bishydroxamate. Cyclic tetrapeptides are a class of HDAC inhibitors with complex structures. Romidepsin isolated from Chromobacterium violaceum is the only such inhibitor granted US FDA approval. Its indications were initially only CTCL and expanded to PTCL in November 2011, and the objective response rate was 34% in CTCL patients and 25% in patients with PTCL.449 Moreover, tucidinostat (chidamide), containing pyridine and N-(2-amino-5-fluorophenyl)-benzamide groups, is a benzamide inhibitor of HDAC1/2/3/10. It was developed wholly in China and approved for the treatment of refractory or relapsed PTCL in 2015 by the NMPA.450
Aliphatic acids, such as valproic acid and phenylbutyrate, show relatively weak inhibitory activity against class I and class II HDACs, and both of these aliphatic acid HDAC inhibitors have already been approved for some non-oncological uses in the clinic and are recently under clinical evaluation for cancer therapy.443 Sirtuin (class III HDAC) inhibitors include the allosteric non-competitive pan-inhibitor nicotinamide and Sirt-specific inhibitors such as EX-527, sirtinol, and cambinol.451 Although Sirt inhibitors have been reported to be useful for cancer treatment, so far no such inhibitors have been approved for clinical use. Moreover, more than 10 other HDAC inhibitors have undergone or are undergoing clinical trials as monotherapy or in combination therapy in patients with hematologic malignancies or solid tumors.452,453 For example, the benzamide inhibitor entinostat is currently assessed in phase III trials (NCT02115282 and NCT03538171) for the clinical benefit in patients with HR-positive, HER2-negative, locally advanced, or metastatic breast cancer. A phase I dose-escalation multicentre trial (NCT00741234) demonstrated that the hydroxamate HDAC inhibitor pracinostat was safe, with modest single-agent activity in patients with advanced hematological malignancies.454
Currently, the clinical activity of HDAC inhibitors as monotherapy is largely restricted to hematological malignancies, including lymphomas, leukemia, and MM. In solid tumors, HDAC inhibitors just showed limited single-agent activity, which may be attributed to the non-specific blocking of angiogenesis and inflammation as well as the poor PK properties of some agents.443,455 Inhibition of tumor angiogenesis hinders drug delivery in solid tumors. The anti-inflammatory effect may induce apoptosis of tumor-fighting immune cells. Another obstacle limiting the clinical use of HDAC inhibitors is their side effects. The common side effects associated with vorinostat, belinostat, and romidepsin were nausea, anorexia, fatigue, and vomiting, which are mostly manageable, but some agents may cause more serious toxicities.455 Currently, major efforts to overcome these obstacles are focused on the development of small-molecule isoforms or class-selective HDAC inhibitors. Selective inhibitors can also be used as chemical probes to explore the epigenetic effects and biological processes induced by HDACs in cancer cells. Additionally, HDAC inhibitors are used in combination with other antitumor drugs to optimize their efficacy and conquer drug toxicity and resistance. Many combination therapy regimens have been used in clinical oncology treatment.452,456,457 Furthermore, some novel combination strategies, such as combining HDAC inhibitors with epigenetic-targeted drugs, proteasome inhibitors, or immunotherapy, have also exhibited good therapeutic effects in preclinical studies.453,458–460
IDH1/2 inhibitors
Isocitrate dehydrogenases (IDHs) include three subtypes (IDH1, IDH2, and IDH3) and are key enzymes that catalyze the conversion of isocitrate to α-ketoglutarate (α-KG) using nicotinamide adenine dinucleotide phosphate (NADP+) or NAD+ as a cofactor in the tricarboxylic acid (TCA) cycle.461 NADP-dependent IDH1 and IDH2 are homodimeric isoenzymes with 70% sequence similarity and almost the same protein structure, whereas IDH3 is a NAD-dependent enzyme that has a unique sequence.462,463 IDH1/2 are mutated in several types of tumors, including AML, glioma, myelodysplastic syndrome (MDS), myeloproliferative neoplasms, chondrosarcoma, and cholangiocarcinoma.463–467 Mutations in IDH3 are rarely reported in cancer. IDH1 R132 H/C, IDH2 R140Q, and R172K are the main identified mutants of IDH1/2, and mutant IDH1/2 (mutIDH1/2) loses its normal catalytic function and instead catalyzes the reduction of α-KG to the oncometabolite product 2-hydroxyglutarate (2-HG).468,469 The accumulation of 2-HG competitively inhibits the α-KG-dependent dioxygenases involved in epigenetic remodeling and DNA repair, such as JMJD-containing histone lysine demethylases (KDMs) and the ten-eleven translocation (TET) family of 5-methylcytosine hydroxylases (DNA demethylases) (Fig. 3), thereby promoting oncogenesis through transcriptional dysregulation and impairing normal cellular differentiation.470 Thus, targeted inhibition of mutated IDH1/2 can reduce the serum 2-HG level and may be therapeutically beneficial for malignancies with IDH1 or IDH2 mutations.471,472
Two IDH1/2 inhibitors have been approved for AML therapy (Table 4). Enasidenib (AG-221) is an oral selective inhibitor of mutIDH2 with IC50 values of 4.0 nM on IDH2 R140Q and 340 nM on wild-type IDH2.471 The precursor form of enasidenib was initially screened by Agios Pharmaceuticals to find potent inhibitors of the IDH R140Q mutant, the most common form of mutIDH2 in AML, and then licensed to Celgene for further development.472,473 Enasidenib binds to IDH2 R140Q at an allosteric site within the heterodimer interface of the enzyme, which forces mutIDH2 to form an open conformation with no catalytic activity.474,475 It also showed inhibitory activity on IDH2 R172K, and its clinical efficacy was stronger in patients harboring the IDH2 R172K mutation than in patients with the IDH1 R140Q mutation (ORR: 53.3% vs. 35.4%; CR: 24.4% vs. 17.7%).476 In 2017, enasidenib received FDA approval for the treatment of IDH2-mutated relapsed/refractory AML.473 Ivosidenib (AG-120) is a reversible allosteric mutIDH1 inhibitor optimized from AGI-5198; the development of the latter has been discontinued due to its poor PKs.477 Ivosidenib was also developed by Agios and Celgene Pharmaceuticals and prevents the formation of catalytically active sites by binding with the cofactor (magnesium ion) of IDH1.478 It is highly selective for IDH1 R132 mutants but has little inhibitory activity against wild-type or mutant IDH2.479 The successes of preclinical studies and phase I trials earned ivosidenib orphan drug designation for glioma treatment, and it was first approved by the FDA on 20 July 2018 for adult relapsed/refractory AML patients with mutIDH1.480 In addition to AML indications, several clinical trials evaluating the efficacy and safety of enasidenib and ivosidenib in other tumors, such as glioma, T-cell lymphoma, MDS, or cholangiocarcinoma, are ongoing.481,482 Some of these studies may support the future expansion of these two drugs.
In the past few years, a variety of other IDH inhibitors have also been developed, and several of them have entered clinical trials.483 Typically, vorasidenib (AG-881) is an orally available pan-IDH inhibitor with IC50 ranges of 0.04–130 nM against various IDH1 R132, IDH2 R140, and R172 mutations. It could easily penetrate the BBB in preclinical studies, indicating the potential for glioma therapy. The drug is undergoing clinical investigation in hematologic malignancies and solid tumors including glioma. BAY-1436032 developed by Bayer is a specific allosteric inhibitor of mutIDH1, which is potent against all reported IDH R132 mutants. It could also efficaciously pass through the BBB and exert antitumor effects in glioma and AML animal models with IDH R132 mutations.484 Two open-label phase I trials of BAY-1436032 are ongoing to evaluate its PDs, safety, tolerability, and preliminary efficacy in patients with AML (NCT03127735) and solid tumors (NCT02746081), but the initial results have not been announced. Clinical trials of other mutIDH1 inhibitors (e.g., FT-2102, DS-1001b, and IDH305) in hematological malignancies or glioma are also underway.485,486 Notably, the development of IDH305 has been halted due to its dose-limiting adverse events observed in clinical studies, such as transaminitis and hyperbilirubinaemia.487
Although two IDH inhibitors have been approved for AML therapy and showed efficacy and safety in clinical trials of other solid tumors, including glioma, some issues still need to be resolved. Acquired resistance caused by mutIDH isoform switching was detected after treatment with specific IDH inhibitors, such as the IDH2 R140Q mutation after ivosidenib treatment and IDH1 R132C or R132H mutations after enasidenib treatment.488 Moreover, IDH2 secondary mutations occurring at the glutamine 316 site (Q316E mutation) or isoleucine 319 site (I319M mutation) after enasidenib treatment will change the conformation of the IDH2 trans-dimer interface and result in resistance to enasidenib.489 The pan-IDH inhibitor AG-881 has been reported to be effective for some mutIDH isoform switching-induced resistance.490 Nevertheless, there is still a demand to develop novel pan- or specific IDH inhibitors, especially mutIDH2 inhibitors, which were all previously optimized from the scaffolds of enasidenib, to conquer special-site mutations. In addition, due to the complex global epigenetic alterations in oncogenesis, IDH inhibitor monotherapy may be insufficient to treat IDH-mutated malignancies, in particular in glioma; this is also observed in clinical trials. Therefore, reasonable combination therapy with other antitumor agents, especially histone-modifying drugs or BET inhibitors, may be therapeutically beneficial for IDH-mutated patients. Interestingly, Fujiwara H et al. reported that AML patients with IDH mutations had a 14-fold higher response rate to BET inhibitors than common patients.491 The combination strategy of IDH inhibitors with the DNMT inhibitor azacitidine in AML treatment has also been evaluated in several clinical trials, and the preliminary results are encouraging.492,493
In addition to EZH2, HDAC, and IDH inhibitors, targeting DNMT is also a potential strategy for cancer therapy. Two DNMT inhibitors (azacytidine and decitabine) cxvdv have been approved for the treatment of hematologic malignancies, particularly AML and MDS.494–496 These cytosine analogs can incorporate into the DNA or RNA backbone to replace C-5 of cytosine with N-5 and interfere with the methylation as well as induce DNMTs degradation. Moreover, agents for other epigenetic targets are also in development. DOT1L methyltransferase inhibitor (e.g., pinometostat), BET inhibitors (e.g., birabresib, molibresib, ZEN003694, PLX51107), and lysine-specific demethylase 1 (LSD1) inhibitors (e.g., iadademstat, INCB059872, CC-90011) have progressed to different stages of clinical trials.412,497,498 Despite the availability of more and more epigenetic agents, there are still many problems for epigenetic therapies. Epigenetic alterations are widely distributed across normal and cancer cells. Even in the cancer cells, these changes may occur early as foundational mutations or arise late and drive clonal subgroups. Furthermore, the sensitivity to certain epigenetic regulation is discrepant in different cancers. These factors are the root causes of drug resistance and side effects. Deeper understanding of the mechanisms and difference of epigenetic alterations in different cancers may contribute to further development and optimization of epigenetic therapies. Normalization of the epigenome can sensitize cancer chemotherapy, molecular targeted therapy, and immunotherapy. Combination therapies are evaluated clinically to broaden the response rates among patients with hematologic malignancies and to expand the indication to solid tumors. However, the current results are not satisfactory. Thus far, only the combination of panobinostat, dexamethasone, and bortezomib has received FDA approval.499 The hope is still the deeper epigenetic insights, which may eventually guide the development of epigenetic agents and the relevant combinations.
BCL-2 inhibitors
The B-cell lymphoma 2 (BCL-2) family of proteins consists of more than 20 members that regulate the intrinsic apoptosis pathway, and fall into three subfamilies (anti-apoptotic proteins, pro-apoptotic proteins, and the cell death mediators) based on their structure and function.500 Anti-apoptotic proteins such as BCL-2 and the closely related proteins BCL-XL, BCL-W, MCL-1, and A1/BFL-1 have four tandem BCL-2 homology (BH) domains and promote cell survival.500,501 While the multi-region pro-apoptotic effector proteins (BAX and BAK) and BCL-2 homology 3 (BH3)-only pro-apoptotic proteins (BIM, PUMA, BAD, or NOXA) promote cell apoptosis upon diverse cellular stresses by the alternation of mitochondrial outer membrane permeabilization and release of cytochrome C from the mitochondria (Fig. 4).501,502 The interaction between the anti-apoptotic and pro-apoptotic BCL-2 family of proteins regulates the apoptotic state of cells. Dysregulation of the apoptosis pathway is common in malignant tumors, especially in hematologic malignancies.503–505 BCL-2 was first discovered as an inhibitor of cellular apoptosis, and the in-depth understanding of this target promotes the development and application of BCL-2 inhibitors in cancer treatment.506,507
Fig. 4.
Schematic illustration of extrinsic and intrinsic pathways of apoptosis. In healthy cells, anti-apoptotic BCL-2 proteins (BCL-2, BCL-XL, BCL-W, MCL-1, and A1/BFL-1) bind to and inhibit activators (BH3-only proteins) and effectors (BAX and BAK). Treatment with BCL-2 inhibitors releases the inhibitory effects of anti-apoptotic BCL-2 proteins on activators and effectors. The subsequent activation and oligomerization of the pro-apoptotic proteins BAK and BAX result in the formation of mitochondrial outer membrane permeabilization (MOMP) and the release of cytochrome C as well as a second mitochondria-derived activator of caspase (SMAC) from the mitochondria. Cytochrome C can form a complex with procaspase 9 and apoptosis protease-activating factor 1 (APAF1), thereby activating caspase 9. Caspase 9 then activates procaspase 3 and procaspase 7, resulting in cell apoptosis. Figure created with BioRender.com
Several early efforts have been made to target the anti-apoptotic BCL-2 family members including antisense oligonucleotide drug oblimersen, the natural product gossypol, and its synthetic derivatives.508 A major breakthrough in targeting BCL-2 is the development of small-molecule BH3 mimetics ABT-737 and ABT-263 (navitoclax).509,510 These agents can mimic the physiologic inhibitors of anti-apoptotic BCL-2 and its relatives, thereby promoting apoptosis. ABT-737 is the first synthetic BH3 mimetic discovered by structure-activity relationship and nuclear magnetic resonance screening. It binds to BCL-2, BCL-XL, and BCL-W with high affinity, and exhibits 500-fold weaker affinity for MCL1 and BFL-1/A1.509 However, ABT-737 has poor bioavailability and requires continuous parenteral administration, which hindered its clinical development. Navitoclax is a structurally related molecule of ABT-737 with high oral bioavailability (~50% in dogs) and entered clinical trials in 2006.511 Although the efficacy of navitoclax was observed in preclinical and clinical studies, dose-dependent thrombocytopenia caused by BCL-XL suppression is the major obstacle restricting the clinical application of navitoclax.510 Initial efforts on the BH3 mimetics ABT-737 and navitoclax facilitated the successful development of ABT-199 (venetoclax), a highly selective BH3 mimetic with greater affinity for BCL-2 but a much lower affinity for BCL-XL and BCL-W.512 In a single-arm phase II trial of venetoclax monotherapy, 79.4% of patients with CLL with the 17p deletion achieved an objective response over a median of 12 months among 107 enrolled patients, and complete remission was achieved in a median of 8.2 months (NCT01889186).513 Due to its remarkable efficacy and safety, the FDA-approved venetoclax for the treatment of CLL patients with 17p deletion in April 2016 (Table 5). This indication was subsequently expanded in June 2018 to include patients with CLL or SLL, with or without 17p deletion, who have received at least one prior therapy. Moreover, in November 2018, venetoclax was approved in combination with standard chemotherapy agents, including azacitidine, decitabine, and low-dose cytarabine, for the treatment of newly diagnosed AML in adults who are 75 years of age or older, or who have comorbidities that preclude the use of intensive induction chemotherapy; this approval was based on the impressive results of two clinical studies conducted in this population.514,515 As a BCL-2-specific inhibitor, venetoclax is also under assessment for the treatment of many other malignancies in the clinic, including solid tumors, and most of the clinical trials have tested its role in combination therapies.516
Table 5.
Properties of approved small-molecule inhibitors of BCL-2, hedgehog pathway, proteasome, and PARP
| Chemical structure | Name | Targets | Approved indications (year) | Corporation |
|---|---|---|---|---|
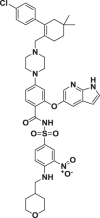 |
Venetoclax (Venclyxto) | BCL-2 |
CLL (2016) SLL (2018) AML (2018) |
Abbvie/Genentech |
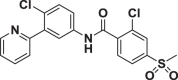 |
Vismodegib (Erivedge) | SMO | BCC (2012) | Genentech |
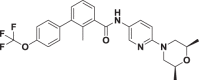 |
Sonidegib (Odomzo) | SMO | BCC (2015) | Novartis |
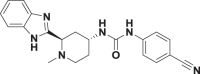 |
Glasdegib (Daurismo) | SMO | AML (2018) | Pfizer |
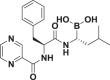 |
Bortezomib (Velcade) | Proteasome |
MM (2003) MCL (2006) |
Millennium |
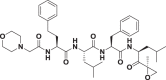 |
Carfilzomib (Kyprolis) | Proteasome | MM (2012) | Onyx |
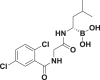 |
Ixazomib (Ninlaro) | Proteasome | MM (2015) | Takeda |
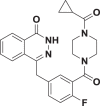 |
Olaparib (Lynparza) | PARP1/2/3 |
Ovarian cancer (2014) FTC (2015) Breast cancer (2018) Pancreatic adenocarcinoma (2019) Prostate cancer (2020) |
AstraZeneca |
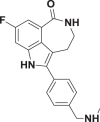 |
Rucaparib (Rubraca) | PARP1/2/3 |
Ovarian cancer (2016) FTC (2016) PPC (2018) Prostate cancer (2020) |
Clovis |
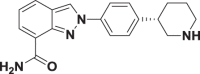 |
Niraparib (Zejula) | PARP1/2 |
Ovarian cancer (2017) FTC (2017) PPC (2017) |
Tesaro/Takeda/Janssen |
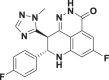 |
Talazoparib (Talzenna) | PARP1/2 | Breast cancer (2018) | Pfizer |
S55746 (also known as BCL201 or Servier-1) is another orally bioavailable small-molecule BCL-2 inhibitor being studied in clinical trials. It is a BH3 mimetic derived from tetrahydroisoquinoline amide-substituted phenyl pyrazoles, and shows high affinity and selectivity for BCL-2 with a Ki value of 1.3 nM.517 The results of a phase I study (NCT02920697) indicated the efficacy and safety of S55746 in patients with B-cell NHL. In addition to the BCL-2 inhibitor, several inhibitors targeting MCL-1 or BCL-XL are also being evaluated in clinical trials. AMG-176 was the first selective MCL-1 inhibitor to be evaluated in the clinic, and is currently being assessed in a phase I study for efficacy, tolerability, and PK properties in patients with relapsed/refractory MM or AML (NCT02675452).518 S64315 (MIK665), another MCL-1 inhibitor derived from S63845, can induce apoptosis in diverse hematological malignancies in a Bax/Bak-dependent manner.519 It is undergoing evaluation in several phase I clinical trials in patients with relapsed/refractory AML or MDS (NCT02979366), and relapsed/refractory MM or lymphoma (NCT02992483). Two other MCL-1 inhibitors AZD-5991520 and AMG-176521 have also entered clinical trials to evaluate their safety, PKs, and antitumor response in patients with hematological malignancies (NCT03218683 and NCT02675452). Neither efficacy nor safety data of MCL-1 inhibitors are currently available. In addition, since BCL-XL overexpression is closely related to the oncogenesis of various solid tumors, targeting BCL-XL is a promising therapeutic strategy for some malignancies, although thrombocytopenia induced by BCL-XL inhibition remains a concern. Multiple BCL-XL inhibitors such as WEHI-539 and A-1331852 have been developed, and exhibit significant monotherapy activity in preclinical studies carried out in solid tumors.522 As the time of this review, none of the selective BCL-XL inhibitors have entered clinical trials.
The success of BCL-2 family inhibitors in the treatment of hematological malignancies has broken new ground in small-molecule targeted therapy by confirming that protein–protein interactions can also be efficiently and specifically targeted. Despite favorable outcomes, challenges for these inhibitors remain, including identifying reliable biomarkers of response and elucidating resistant mechanisms to BCL-2 inhibition. A study conducted using samples from patients with venetoclax-treated CLL found that neither a cell sensitivity test in vitro nor TP53 status was a predictor of the antitumor response to venetoclax.523 BH3 profiling, an in vitro assay to determine the dependence of tumor on individual anti-apoptotic protein, was reported as a potential solution to this problem.523 However, due to the diversity of factors influencing the efficacy of BCL-2 inhibitors, the reliability of this finding still requires validation by systematic clinical evaluation. As has been demonstrated repeatedly, cancer cells eventually become resistant to targeted therapies, and BCL-2 apoptotic pathway-dependent malignancies are no exception. The resistance mechanisms involve upregulation of alternative anti-apoptotic BCL-2 family members such as BCL-XL and MCL-1, mutation or phosphorylation of BCL-2, and loss-of-function mutations of pro-apoptotic proteins BAX and BAK.524 At present, combination therapy either with diverse anti-apoptotic BCL-2 family inhibitors or with BCL-2 family inhibitors and conventional chemotherapy is the main strategy to overcome resistance. There are considerable clinical studies focusing on these combined effects.524,525 How to choose synergistic drugs and avoid the possible side effects are new challenges for researchers. As data from multiple phase I trials of combination therapy become available, close and critical assessments are needed.
Hedgehog pathway inhibitors
The hedgehog (HH) signaling pathway is highly conserved and has an important role in embryonic development and tissue regeneration. The HH pathway can be divided into canonical and noncanonical pathways. Activation of the canonical HH pathway is initiated by the release of HH ligands (Desert-DHH, Indian-IHH, and Sonic-SHH), and these ligands can bind and suppress the 12-pass transmembrane receptor Patched-1 (PTCH1).526 In the absence of HH ligands, PTCH1 constitutively inhibits HH signaling by suppressing the transmembrane transducer Smoothened (SMO). Upon ligand binding, PTCH1 is internalized from the cell membrane and degraded, which results in the release of SMO to the primary cilium and phosphorylation at the cytoplasmic end. Active SMO promotes the activation of the glioma-associated oncogene (GLI) transcription factors GLI1, GLI2, and GLI3 and then induces the expression of target genes related to cell proliferation, survival, and differentiation (Fig. 5).526 HH signaling can be abnormally activated through various mechanisms, such as HH ligand upregulation and PTCH1 or SMO mutations. Accumulating evidence has suggested that aberrant activation of the HH pathway is closely related to the oncogenesis and progression of a variety of tumors, including solid carcinomas and hematological tumors, as well as self-renewal of cancer stem cells (CSCs).527,528 Therefore, the HH pathway has emerged as an attractive target for cancer therapy.
Fig. 5.
Canonical SMO-dependent hedgehog (HH) signaling pathway. Unliganded PTCH1 prevents the ciliary translocation of SMO effector protein. GLI2 and GLI3 proteins are sequestered in the cytoplasm by SUFU and phosphorylated by protein kinases, thereby preventing HH target-gene transcription. HH ligands binding triggers endocytic internalization of PTCH1, which results in the accumulation and activation of SMO. Active SMO relieves SUFU-mediated inhibition of GLI2 and GLI3. Activator forms of GLI (GLI1A/GLI2A/GLI3A) translocate into the nucleus and initiate the transcription of target genes. Figure created with BioRender.com
To date, three HH pathway inhibitors have been approved by the FDA for clinical oncology treatment (Table 5). Among them, both vismodegib and sonidegib are oral SMO inhibitors and are used for the treatment of locally advanced, unresectable, or metastatic basal cell carcinoma (mBCC).529,530 The results of an open-label trial (NCT01327053) for vismodegib showed a response rate of 68.5% in 1119 locally advanced BCCs (laBCCs) and 36.9% in 96 mBCC patients. The median PFS in laBCC and mBCC was 13.1 and 23.2 months, respectively.531 Moreover, in a randomized trial of sonidegib for 66 laBCCs and 13 mBCCs (NCT00833417), the 200 mg/day treatment group had response rates of 57.6% and 7.7%, respectively. The disease control rate, including stable disease, was 91.9% in laBCC and 92.3% in mBCC.532 In addition to BCC, many clinical trials have been performed to evaluate the efficacy of these two agents in the treatment of various tumors, including rare tumors.533–536 Glasdegib is also a selective HH pathway inhibitor that binds to SMO. In 2018, it was approved by the FDA in combination with low-dose cytarabine chemotherapy for newly diagnosed AML patients who were older than 75 years or unable to receive intensive chemotherapy due to chronic health problems and diseases.537 This is also the first HH pathway inhibitor approved to treat AML.
Several other SMO inhibitors have also entered clinical trials to evaluate their therapeutic effects on BCC, pancreatic cancer, colon cancer, and breast cancer.538 However, clinical studies of some drugs, such as saridegib (a cyclopamine analog), TAK-441, IPI-926, and CUR-61414, have been terminated due to detrimental effects and lack of response.539 It is worth mentioning that the FDA-approved antifungal drug itraconazole was found to have an inhibitory effect against the HH pathway by antagonizing SMO. It has been reported that the clinical benefit of high-dose itraconazole in prostate cancer was mainly attributed to HH signaling inhibition rather than an anti-androgen effect.540 Moreover, vitamin D3 could also bind SMO with high affinity and is currently in phase I or phase III clinical trials as a neoadjuvant for the treatment of BCC, pancreatic cancer, CLL, and NHL.539
Inhibition of GLI-mediated transcription is an alternative strategy for developing HH signaling inhibitors, and this strategy has the potential to overcome acquired resistance of the approved SMO inhibitors. Currently, there are many reports on GLI inhibitors, but most of them are in the preclinical stage. Arsenic trioxide (ATO), a well-known agent approved by the FDA for acute promyelocytic leukemia treatment, is also a GLI inhibitor. Mechanistically, ATO blocks GLI2 accumulation and thus inhibits the transcriptional activation of GLI target genes. In a preclinical study, treatment with ATO or its combination with itraconazole effectively inhibited the growth of medulloblastoma harboring the SMO D477G mutation (or SMO D473H in humans) that is resistant to SMO inhibitors.541 In a clinical study conducted in patients with refractory mBCC, combined treatment with ATO and itraconazole induced stable disease in 3 of 4 patients.542 The hexahydropyrimidine derivatives GANT58 and GANT61 screened by constructed cells are selective GLI inhibitors.543 They could interfere with GLI1/2-mediated transcriptional activity by blocking their DNA binding and restraining the growth of multiple tumors in a GLI-dependent manner. However, there has been no clinical study of these drugs due to their restricted pharmacological applicability.
Although the approved HH pathway inhibitors (SMO inhibitors) have shown significant antineoplastic activity in a variety of tumors, especially BCC, the diseases relapse and progress within several months due to acquired resistance. The resistance mechanisms involve SMO mutations, such as SMO D473H, activation of HH signaling downstream of SMO, and activation of compensatory signaling pathways.544 As mentioned above, GLI inhibitors have the potential to conquer resistance to SMO inhibitors. Moreover, novel therapeutics targeting HH signaling has also been reported. LEQ-506 is an SMO inhibitor undergoing phase I clinical studies in some advanced solid tumors (NCT01106508). It could suppress the proliferation of medulloblastoma cells harboring the SMO D473H mutation, which is derived from a patient who relapsed after an initial response to vismodegib treatment.545 The SMO inhibitor taladegib can bind to and inhibit SMO D473H activity. A phase I trial (NCT01919398) assessed the efficacy of taladegib in several advanced solid tumors and suggested that it is effective only in BCC with an estimated ORR of 46.8%.546 This clinical trial also indicated that taladegib could benefit patients who were resistant to SMO inhibitors. As the compensatory upregulation of RAS/MAPK and PI3K-mTOR signaling pathways contributes to the mechanism of SMO-independent GLI regulation and resistance to SMO inhibitors, the combined use of HH signaling inhibitors and PI3K or MAPK pathway inhibitors may be a potential strategy to overcome the current resistance to SMO inhibitors.528,547
Proteasome inhibitors
Proteasomes are large multicatalytic enzyme complexes that are expressed in the nucleus and cytoplasm of all eukaryotic cells and are responsible for more than 80% protein degradation in human cells.548 The ubiquitin–proteasome system (UPS) has an important role in maintaining cellular protein homeostasis and regulating numerous biological processes, such as cell survival, signal transduction, DNA repair, and antigen presentation.549 Most misfolded, unassembled, or damaged proteins that could otherwise form potentially toxic aggregates are degraded via UPS, in which proteins are tagged by ubiquitin and then recognized and degraded into small peptides by the proteasome complex (Fig. 6). Structurally, all proteasomes contain a common core, referred to as the 20S proteasome. The 20S core consists of a cylinder made of four stacked rings: 2 identical outer α-rings and 2 identical inner β-rings, each containing 7 distinct but related subunits.548,550 The specificity of the 20S proteasome for substrate action depends on the peptide bond on the N2 terminal threonine residue of the β1, β2, β5 subunits. Dysfunction of the UPS is related to multiple human diseases, such as cancers, autoimmune diseases, and genetic diseases;550,551 thus, much work has been conducted by targeting the UPS as a potential treatment strategy.
Fig. 6.
Proteasome inhibition acts through multiple mechanisms to induce cell apoptosis. Proteasome inhibition leads to NF-κB deactivation, thereby downregulating multiple pro-neoplastic pathways associated with cell proliferation, invasion, metastasis, and angiogenesis. Inhibition of proteasome activates the JNK signaling pathway and results in programmed cell death via caspase 3 and 7. Additionally, proteasome inhibition can indirectly cause apoptosis by preventing the degradation of pro-apoptotic family proteins such as BAX, BID, BIK, and BIM as well as NOXA. Inhibition of proteasome prevents the degradation of ubiquitinated proteins, which can increase endoplasmic reticulum (ER) stress and activate the UPR, cell cycle arrest, and subsequent apoptosis. Figure created with BioRender.com
Multiple myeloma (MM) cells produce excessive paraproteins, and their growth is dependent on proteasome-regulated signaling pathways. Therefore, MM cells are particularly susceptible to proteasome inhibition, and proteasome inhibitors (PIs) have become the backbone of MM clinical therapy.552 Bortezomib is the first approved PI for the treatment of relapsed or refractory MM (Table 5). It is a peptide boronic acid and reversibly acts on the β5 catalytic subunit of the proteasome.553 The clinical application of bortezomib significantly improves the long-term outcomes for MM patients. Moreover, Bortezomib has also been approved for the treatment of MCL. Currently, there are more than 200 clinical trials related to bortezomib that focus on its combination with other agents, efficacy in other cancers, and even other noncancer applications such as graft-versus-host disease.554 However, bortezomib treatment has several limitations including primary resistance in MM and MCL patients, relapse in many initially-responding patients, and induction of dose-limiting peripheral neuropathy (PN).554,555 To conquer these limitations, the second-generation PIs have been developed, and many of them are derived from synthetic and natural products.552,556 Carfilzomib approved by the FDA in 2012 is a second-generation PI and is derived from the natural product epoxomicin (Table 5).557 Unlike bortezomib, carfilzomib is an irreversible inhibitor that contains an epoxyketone warhead, which could covalently bind to the N-terminal threonine-containing active sites of the 20S proteasome.558 The irreversible nature of carfilzomib contributes to its efficacy even in MM patients relapsed from or refractory to bortezomib. In addition, the carfilzomib-containing regimens exhibit significantly reduced peripheral neurotoxicity, while cardiovascular events were observed in MM patients treated with carfilzomib.557 Another second-generation PI ixazomib was approved in 2015 in combination with lenalidomide and dexamethasone for MM patients who have received at least one prior therapy (Table 5).559 Ixazomib is an N-capped dipeptidyl leucine boronic acid that reversibly inhibits the CT-L proteolytic (β5) site of the 20S proteasome.560 Ixazomib shares the same pharmacophore boronic acid residue with bortezomib, however, the elimination half-life of the former is much shorter than that of the latter (18 vs. 110 min), which may contribute to the improved safety profiles of xazomib over bortezomib.559,560 Patients resistant to bortezomib can still benefit from ixazomib treatment. Moreover, it is worth mentioning that both bortezomib and carfilzomib require parenteral (intravenous or subcutaneous) administration, whereas ixazomib is the first oral PI and is a prodrug.561 Ixazomib is rapidly hydrolyzed to the active form (MLN2238) under physiological conditions.
Following the clinical success of the PIs, a number of novel PIs have been developed in recent years but only three of them are currently being evaluated in clinical trials. Marizomib (Salinosporamide A) is a naturally occurring β-lactone-γ-lactam bicyclic compound isolated from Salinispora bacteria. It can bind to 3 major catalytic sites on β5, β1, and β2 of proteasome irreversibly and inhibit proteasome activity at nanomolar concentrations.562 The efficacy of marizomib in relapse or refractory MM and lymphoma is evaluated in phase I/II trials (NCT00461045, NCT00396864). Moreover, marizomib is reported to have CNS adverse events, which suggests that it can penetrate the BBB.563 Therefore, it is currently under evaluation in a phase III clinical trial for malignant glioblastoma treatment (NCT03345095). Oprozomib is a structural analog of carfilzomib, with a peptide-like backbone and an epoxyketone warhead.564 The development of oprozomib aims to find an oral PI by chemical modification of carfilzomib. Currently, it is assessed in clinical trials for the treatment of refractory MM or relapsed MM after receiving bortezomib- or carfilzomib-based therapies (NCT02939183). Another reversible PI under clinical assessment is delanzomib, an orally bioavailable structural homolog of bortezomib with a peptide-like backbone and a boronate warhead.565 It is assessed in several phase I/II clinical trials for MM, lymphoma, and solid tumors.566,567 Compared with BTZ, delanzomib showed slightly reduced efficacy against MM and some solid tumor cells, but had higher selectivity to cancer cells over normal cells, which means better drug safety. A phase I study of delanzomib also exhibits that it has a favorable safety profile with limited PN.566
While emerging as an important treatment strategy for MM, PIs showed weak efficacy against solid cancers. The lack of clinical benefits of PIs in treating solid cancers was speculated to be attributed to their short elimination time and poor distribution to the proteasome target located in solid cancers.554,568 Moreover, drug resistance has also been a major hurdle for PIs. As mentioned above, the next-generation PIs with improved PK/PD characteristics may promote the clinical efficacy of MM patients as well as those who are resistant to existing PI treatment, and extend treatment benefits to solid cancers. Meanwhile, combined proteasome inhibition with other active agents, such as BCL-2 inhibitor, HDAC inhibitor, immunomodulatory agents, and some other inhibitors of signaling pathways related to resistant mechanisms, is also a strategy to conquer resistance to PIs.569 These combination therapies are being assessed in many preclinical and clinical studies.
PARP inhibitors
Genomic instability is one of the typical characteristics of tumor cells. To maintain genomic integrity, tumor cells have multiple mechanisms to repair DNA lesions, such as the repair pathways of DNA double-strand breaks (DSBs) and single-strand breaks (SSBs). Among them, the former includes homologous recombination and non-homologous end joining (NHEJ), while the latter includes base excision repair (BER), nucleotide excision repair (NER), and mismatch repair (MMR).570 Poly (ADP-ribose) polymerases (PARPs) are a group of multifunctional post-translational modification enzymes that engage in a diverse set of cellular processes, including DNA repair, transcription, mitosis, and cell cycle regulation.571 To date, 18 members have been identified in PARP family proteins, among which PARP1 is the best-studied PARP member and has an important role in the repair of DNA SSBs. Once DNA SSBs occur, PARP1 binds to damaged DNA through N-terminal zinc finger domains, allowing its cofactor β-nicotinamide adenine dinucleotide (β-NAD) to bind to the active site of the enzyme and activating the catalytic function of the ADP-ribosyltransferase catalytic domain. PARP1 then catalyzes the transfer of PAR chains to the target proteins (PARylation) in the vicinity of the DNA breaks, which promotes chromatin remodeling and the recruitment of a series of DNA repair effectors and completes the DNA repair process (Fig. 7).570,571 PARP1 autoPARylation eventually causes its dissociation from DNA damage and restores its autoinhibitory status. Inhibition of another DNA repair pathway in cancer cells with defective DNA repair mechanisms may create a “synergistic lethal” effect; this theory was first proposed by Theodosius Dobzhansky et al.572 Breast cancer susceptibility genes BRCA1 and BRCA2 are two key tumor suppressors that repair DNA DSBs. Mutations in BRCA1 and BRCA2 are susceptible to breast and ovarian cancers, and DSBs are not easily repaired in BRCA-mutant tumor cells.573 Therefore, PARP inhibition in BRCA-mutant cancers can induce synthetic lethality due to the simultaneous blockade of both DSB and SSB repair pathways (Fig. 7).
Fig. 7.
Molecular process of DNA damage repair related to PARP and the mechanism of action of PARP inhibitors. Endogenous single-strand breaks (SSB) are repaired mostly by PARP-dependent base excision repair (BER) pathway. PARP inhibitors suppress the repair of SSB and the unrepaired SSB can be converted to double-strand breaks (DSB) that are toxic to cells. Homologous recombination (HR) is the major pathway to repair DSB. However, the DSB in BRCA1/2 mutant cells cannot be repaired through HR, thus resulting in genomic instability and cell death. Figure created with BioRender.com
Nicotinamide, the cofactor of PARPs, which competes with NAD for the catalytic pocket of PARPs, is the first identified PARP inhibitor.574 Currently, four PARP inhibitors with nicotinamide pharmacophores (olaparib, rucaparib, niraparib, and talazoparib) have been approved by the FDA or the EMA (Table 5).575–578 All PARP inhibitors have the ability to inhibit the catalytic activity of PARPs. However, this mechanism cannot fully explain the antitumor activity of PARP inhibitors. They can also trap PARP in a non-effective state at chromatin, and such binding to the PARP–chromatin complex will produce more effective cytotoxicity.579 Based on in-depth research on the mechanisms of action of PARP inhibitors and the results of clinical trials, indications of PARP inhibitors have been continuously updated since the first inhibitor olaparib was approved in 2014. Olaparib was originally approved for patients with deleterious or suspected deleterious germline BRCA-mutant advanced ovarian cancer who had undergone three or more prior lines of chemotherapy, followed by rucaparib in 2016, and niraparib in 2017.575,576 Later, in 2017 and 2018, olaparib, niraparib, and rucaparib were approved as maintenance therapies in recurrent platinum-sensitive cancers, including epithelial ovarian cancer, fallopian tube cancer (FTC), or primary peritoneal cancer (PPC), regardless of BRCA status.575–577 Furthermore, olaparib became a first-line maintenance treatment in germline or somatic BRCA-mutated cancer patients who responded to platinum-based chemotherapy according to the data of clinical trial (NCT01874353).580 In 2018, the FDA successively approved olaparib and talazoparib for BRCA-mutated HER2-negative locally advanced or metastatic breast cancer. In addition, niraparib expanded the indications for the treatment of advanced ovarian cancer, FTC, and PPC associated with homologous recombination deficiency positive status in 2019.581
At present, a large number of clinical trials related to olaparib, rucaparib, niraparib, or talazoparib alone or in combination are still underway to identify responding patients beyond the ovarian or breast cancer population. Additionally, several other PARP inhibitors, such as pamiparib, veliparib, INO-1001, E7449, iniparib, AZD2461, amelparib, IMP4297, RBN-2397, and fluzoparib, have also entered clinical trials and are in different stages.582,583 Among them, veliparib (ABT-888) is an orally bioavailable PARP inhibitor developed by Abbvie that can cross the blood–brain barrier.584 Its clinical efficacy has been evaluated in multiple solid tumors. A phase III trial of veliparib with first-line chemotherapy showed an increased PFS in high-grade serous ovarian carcinoma compared with paclitaxel and carboplatin alone (NCT02470585). However, the clinical outcomes could not be significantly improved by veliparib as monotherapy in patients with ovarian or pancreatic cancer as well as by its combination with carboplatin/paclitaxel in TNBC.585 To date, veliparib has not been approved by any agency.
The efficacy of PARP inhibitors in cancer treatment is not restricted to BRCA1/2 mutant sufferers. In the treatment of ovarian cancers, 40–70% of BRCA1/2 mutated patients failed to respond to PARP inhibitors.586 Platinum sensitivity is also reported as a prospective indicator for predicting the response to these inhibitors.587 Nevertheless, these two predictors still cannot completely cover the tumor types suitable for PARP inhibitor treatment. This is one of the problems that need further research in PARP inhibition therapy. In addition, with the widespread use of PARP inhibitors in the clinic, the issue of acquired resistance has emerged frequently. Mechanisms of resistance are multifactorial, mainly involving the restoration of HR function caused by secondary mutations of homologous recombination repair genes (BRCA1/2, RAD51C/D, PALB2) and defects in NHEJ as well as loss of 53BP1, stabilization of replication forks, PARP1 mutations, and drug efflux.588 Combined therapy is increasingly explored as a means to enhance the efficacy and overcome drug resistance to PARP inhibitors. Many studies have shown that simultaneously targeting PARPs and cell cycle checkpoints (ATR, CHK1, and WEE1) can overcome the resistance caused by restored replication fork stabilization.589,590 A phase II study of olaparib plus ATR/WEE1 inhibitor vs. olaparib monotherapy is currently underway in TNBC patients (NCT04191135).591 Moreover, it should be noted that combination therapy may also increase the risk of side effects. Severe myelosuppression was observed in several phase I trials of PARP inhibitors combined with chemotherapy drugs.592 It is still of great importance to better understand the predictors and resistance mechanisms of PARP inhibitors and ultimately improve diagnosis, treatment strategy, and outcome.
Concluding remarks: challenges and future perspectives
With the evolution of modern molecular biology and the application of some advanced technologies such as computer-aided drug design, structure biology, and combinatorial chemistry, small-molecule targeted anti-cancer drugs have entered a rapid development stage. To date, 89 small-molecule targeted drugs have been approved by the FDA and/or NMPA to treat various cancers (Fig. 1). Thousands of targeted agents are undergoing clinical trials for cancer treatment (Supplementary Fig. S1). Among them, a large number of promising agents have advanced to phase III trials (Supplementary Tables S2–S5). According to the prediction of the Business Research Company, the global anti-cancer drug market size will reach 200 billion dollars in 2021, among which targeted drugs are the “main force”. Despite the significant progress achieved, there are still some challenges that small-molecule targeted anti-cancer drugs face.
The first major challenge is drug resistance. Almost all targeted anti-cancer drugs come across resistance after a period of time of clinical use. Drug resistance has been linked to many mechanisms, including gene mutation, amplification, CSCs, efflux transporters, apoptosis dysregulation, and autophagy, etc (Fig. 8).593–599 Gene mutation is the main reason leading to anti-cancer drug resistance. There are two different views regarding drug-resistant gene mutations. One is that the gene mutations are induced by drugs. The other one is that the drug-resistant mutations have already existed. In the early stage of treatment, cancer cells with drug-sensitive mutations dominate and suppress the proliferation of cells containing drug-resistant mutations. After cells with sensitive mutations are killed, the resistant mutant cells become the mainstream and show resistance. Amplification of other genes is another common reason for anti-cancer drug resistance. For example, MET amplification accounts for about 20% of EGFR inhibitor-resistant cases.46 CSCs are also thought to be an important reason for drug resistance and recurrence.595,596 The CSC theory proposes that the different cells within a tumor, as well as metastasis deriving from it, originate from a single subpopulation of cells with self-renewal and differentiation capacities, which are similar to stem cells. Overexpression of efflux transporters, such as multidrug resistance transporter proteins, especially P-glycoprotein, which renders the resistance to chemotherapeutic drugs, also has a role in the targeted anti-cancer drug resistance.597,598 In addition to these causes, apoptosis dysregulation and autophagy also could be responsible for anti-cancer drug resistance.593,599
Fig. 8.
Mechanisms and insights in drug resistance of small-molecule targeted anti-cancer agents. Figure created with BioRender.com
Low efficiency is another major challenge for targeted anti-cancer drugs. As mentioned many times before, targeted anti-cancer drugs are effective only in a limited number of patients. For example, less than 20% of patients with NSCLC are sensitive to EGFR inhibitors (e.g., gefitinib and erlotinib). Patients that are sensitive to these EGFR inhibitors are found to harbor EGFR-activating mutations (for example, exon 19 deletion or exon 21 L858R point mutations).74 TRK inhibitors larotrectinib and entrectinib have been approved for the treatment of patients with NTRK gene rearrangements, regardless of cancer type and patient age. These rearrangements can be found at high frequencies (up to 90%) in certain types of cancer, such as infantile fibrosarcoma (a rare disease), but the incidence is only 1% of all malignancies.225 These highlight the importance of the identification of predictive biomarkers for response to targeted anti-cancer drugs.
Currently, in order to deal with the major challenges of targeted anti-cancer drugs, many strategies have been applied, such as new generation anti-cancer drugs against drug resistance mutations, multitarget drugs, combination therapy, and drugs targeting CSCs.594,600,601 In addition to these, several new research trends in this area deserve attention.
The first one is the drug discovery against new type cancer targets. For example, in the past years, some new epigenetic regulatory proteins have garnered increasing attention, such as RNA m6A methylation-related proteins (METTL3/14, FTO, ALKBH5, WTAP, and YTHDFs).602,603 microRNAs (miRNAs) are another new type of cancer targets, which are frequently dysregulated in cancers and may serve as promising targets for cancer therapy. Currently, some effects have been paid to discover small-molecule inhibitors against miRNAs, such as miR-21 inhibitor AC1MMYR2 (also known as NSC211332), Lin28-let-7 inhibitors 6-hydroxy-DL-DOPA, SB/ZW/0065, and KCB3602.604
Furthermore, some proteins that are previously thought undruggable may also be attractive anti-cancer targets. A typical example is KRAS, a most frequently mutated isoform of RAS proto-oncogene, which has a predominant role in driving the initiation and progression of cancers.605,606 Decades of efforts failed to target KRAS with small molecules due to the high binding affinity of the intrinsic ligand GTP to KRAS, the relatively small size and smooth surface of KRAS, and the high flexibility of KRAS switch regions.607–609 However, this dilemma has begun to change recently. A variety of novel small molecules that directly target KRAS are being developed, including covalent allosteric inhibitors for KRAS G12C mutant, protein–protein interaction inhibitors that bind in the switch I/II pocket or the A59 site, and GTP-competitive inhibitors targeting the nucleotide-binding site.609,610 To date, four KRAS G12C covalent inhibitors (AMG510, JNJ-74699157, MRTX849, and GDC-6036) with similar allosteric mechanisms have advanced to clinical trials.611,612 In addition to KRAS, other types of such previously undruggable cancer targets include MYC,613 phosphatases,614 and protein–protein interactions,615,616 etc.
The second one is the combination of small-molecule targeted drugs with immunotherapy such as PD-1 antibody. Lenvatinib plus pembrolizumab, a PD-1 antibody, was designated as a breakthrough therapy in 2018 by the FDA for patients with advanced or metastatic RCC. The ORR was 63.3% for all RCC patients receiving this combination therapy, and 83.3% in the first-line treatment setting.617 Inspiringly, the combination of pembrolizumab with another small-molecule anti-angiogenesis agent axitinib has been approved for the treatment of patients with advanced RCC.618,619 This is also the first combination therapy of PD-1 antibody plus targeted drugs approved by the FDA for the first-line treatment of advanced RCC.
The third one is antibody-drug conjugate (ADC) drugs. Though the first ADC drug (Mylotarg, Pfizer) suffered setback in 2010 due to the limitations of coupling technology, targeting, and effectiveness, people did not lose confidence in ADC drugs.620,621 With the improvement of antibody-conjugate technology, ten ADC drugs such as polatuzumab vedotin-piiq (Polivy), enfortumab vedotin-ejfv (Padcev), fam-trastuzumab deruxtecan-nxki (Enhertu), sacituzumab govitecan-hziy (Trodelvy), and belantamab mafodotin-blmf (Blenrep) have been approved by the FDA in the past decade.622–624 ADC drugs will be an important development direction in the future.
The fourth one is PROTAC, which employs small molecules that recruit target proteins for ubiquitination and removal by the proteasome.625 PROTAC technology is different from the traditional “target occupying” inhibitor therapy. It reduces the activity of target proteins by catalyzing the degradation of target proteins. Due to the slow biosynthesis of target proteins, this method can greatly slow down the recovery of target protein activity. PROTAC is a rapidly emerging alternative therapeutic strategy with the potential to address many of the challenges currently faced in modern drug development programs. Currently, two drugs (ARV-110 and ARV-471) designed by PROTAC technology have entered clinical trials.625 Both of them are developed by Arvinas and approved by the FDA in 2019 for phase I clinical trials. Among them, ARV-110 specifically binds to androgen receptor (AR) and mediates AR degradation, and is used for the treatment of patients with metastatic CRPC.626 ARV-471 is an ER protein degrading agent. In preclinical studies, ARV-471 led to almost completely ER degradation in tumor cells and exhibited strong growth suppression in multiple ER-driven xenograft models.627 It is now evaluated clinically for the treatment of patients with locally advanced or metastatic ER+/HER2- breast cancer.
The fifth one is synthetic lethality, which means that loss of function of either of the two genes individually has little effect on cancer cell viability, but the inactivation of both genes simultaneously by gene mutation/deletion or pharmacological inhibition leads to cell death.628 Normal cells are usually spared the effect of synthetic lethality due to the lack of the fixed genetic alteration. The concept of synthetic lethality has been used in the development of targeted therapy for many years. The most popular examples are the synthetic lethal interaction of combined BCL-XL and MEK inhibition in KRAS-mutant cancer models as well as the successful clinical application of PARP inhibitors in BRCA-mutant ovarian cancer.574,629 Although it is not a novel idea, synthetic lethality provides an alternative treatment strategy for some known genetic drivers of cancer that cannot be directly targeted owing to their molecular structure (undruggable oncogenes) or because they result in a functional loss (tumor suppressor genes). Therefore, synthetic lethality has promising potential to drive the discovery of new anti-cancer targets and subsequently the development of effective medicines or combination strategies that are still needed for most cancers.
In summary, small-molecule targeted drugs will continue to be the mainstream in cancer treatment because of their unique advantages, despite the competition from macromolecule drugs. With an in-depth understanding of tumor pathology and the evolution of new drug research and development technology, we believe that more new small-molecule anti-cancer drugs that target novel genes or the mechanism of action will be developed in the near future. It is also expected that some new areas such as the combination of small-molecule targeted drugs with tumor immunotherapy, ADC, and PROTAC will gain significant development in the next decade.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81930125, 81702286, 81773633, and 21772130), the Research Project of Science and Technology Department of Sichuan Province (2021YJ0134, 2019YFS0514, and 2020YFS0006), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18001), and partially by the fast-track grants from West China Hospital, Sichuan University (HX-2019-nCoV-053), and the Open Project of Radiation Oncology Key Laboratory of Sichuan Province (2020FSZLX04).
Competing interests
Shengyong Yang is the editorial board member of Signal Transduction and Targeted Therapy, but he has not been involved in the process of manuscript handling. The remaining authors declare no competing interests.
Footnotes
These authors contributed equally: Lei Zhong, Yueshan Li, Liang Xiong, Wenjing Wang
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-021-00572-w.
References
- 1.Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395:1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 2.Savage DG, Antman KH. Imatinib mesylate–a new oral targeted therapy. N. Engl. J. Med. 2002;346:683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 3.Wilkes GM. Targeted therapy: attacking cancer with molecular and immunological targeted agents. Asia Pac. J. Oncol. Nurs. 2018;5:137–155. doi: 10.4103/apjon.apjon_79_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur. J. Pharm. 2018;834:188–196. doi: 10.1016/j.ejphar.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Ardito F, et al. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (review) Int J. Mol. Med. 2017;40:271–280. doi: 10.3892/ijmm.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson LJ, et al. New perspectives, opportunities, and challenges in exploring the human protein kinome. Cancer Res. 2018;78:15–29. doi: 10.1158/0008-5472.CAN-17-2291. [DOI] [PubMed] [Google Scholar]
- 7.Roskoski R., Jr. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharm. Res. 2016;103:26–48. doi: 10.1016/j.phrs.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1995;267:316–317. doi: 10.1126/science.267.5196.316-b. [DOI] [PubMed] [Google Scholar]
- 9.Iwahara T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 10.McManus DT, et al. ALK-positive diffuse large B-cell lymphoma of the stomach associated with a clathrin-ALK rearrangement. Hum. Pathol. 2004;35:1285–1288. doi: 10.1016/j.humpath.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Cessna MH, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod. Pathol. 2002;15:931–938. doi: 10.1097/01.MP.0000026615.04130.1F. [DOI] [PubMed] [Google Scholar]
- 12.Soda M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 13.Waggott W, et al. Detection of NPM-ALK DNA rearrangement in CD30 positive anaplastic large cell lymphoma. Br. J. Haematol. 1995;89:905–907. doi: 10.1111/j.1365-2141.1995.tb08434.x. [DOI] [PubMed] [Google Scholar]
- 14.Trinei M, et al. A new variant anaplastic lymphoma kinase (ALK)-fusion protein (ATIC-ALK) in a case of ALK-positive anaplastic large cell lymphoma. Cancer Res. 2000;60:793–798. [PubMed] [Google Scholar]
- 15.Ma Z, et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 16.Kong X, et al. Drug discovery targeting anaplastic lymphoma kinase (ALK) J. Med. Chem. 2019;62:10927–10954. doi: 10.1021/acs.jmedchem.9b00446. [DOI] [PubMed] [Google Scholar]
- 17.Cui JJ, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK) J. Med. Chem. 2011;54:6342–6363. doi: 10.1021/jm2007613. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AT, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 19.Solomon BJ, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–6060. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa DB, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsilje TH, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J. Med. Chem. 2013;56:5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita K, et al. 9-substituted 6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazoles as highly selective and potent anaplastic lymphoma kinase inhibitors. J. Med. Chem. 2011;54:6286–6294. doi: 10.1021/jm200652u. [DOI] [PubMed] [Google Scholar]
- 24.Huang WS, et al. Discovery of brigatinib (AP26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J. Med. Chem. 2016;59:4948–4964. doi: 10.1021/acs.jmedchem.6b00306. [DOI] [PubMed] [Google Scholar]
- 25.Friboulet L, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markham A. Brigatinib: first global approval. Drugs. 2017;77:1131–1135. doi: 10.1007/s40265-017-0776-3. [DOI] [PubMed] [Google Scholar]
- 27.Qian M, Zhu B, Wang X, Liebman M. Drug resistance in ALK-positiveNon-small cell lungcancer patients. Semin. Cell Dev. Biol. 2017;64:150–157. doi: 10.1016/j.semcdb.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Basit S, Ashraf Z, Lee K, Latif M. First macrocyclic 3(rd)-generation ALK inhibitor for treatment of ALK/ROS1 cancer: clinical and designing strategy update of lorlatinib. Eur. J. Med. Chem. 2017;134:348–356. doi: 10.1016/j.ejmech.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Syed YY. Lorlatinib: first global approval. Drugs. 2019;79:93–98. doi: 10.1007/s40265-018-1041-0. [DOI] [PubMed] [Google Scholar]
- 30.Guan J, et al. The ALK inhibitor PF-06463922 is effective as a single agent in neuroblastoma driven by expression of ALK and MYCN. Dis. Model Mech. 2016;9:941–952. doi: 10.1242/dmm.024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drilon A, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1) Cancer Discov. 2017;7:400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachdev J, et al. 506 Phase (Ph) 1/2a study of TSR-011, a potent inhibitor of ALK and TRK, in advanced solid tumors including crizotinib-resistant ALK positive non-small cell lung cancer. Eur. J. Cancer. 2014;50:165. doi: 10.1016/S0959-8049(14)70632-X. [DOI] [Google Scholar]
- 33.Drilon A, et al. Repotrectinib (TPX-0005) Is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- 34.Menichincheri M, et al. Discovery of entrectinib: a new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (Pan-TRKs) inhibitor. J. Med. Chem. 2016;59:3392–3408. doi: 10.1021/acs.jmedchem.6b00064. [DOI] [PubMed] [Google Scholar]
- 35.Infante JR, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J. Clin. Oncol. 2012;30:1527–1533. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
- 36.Shaw AT, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N. Engl. J. Med. 2016;374:54–61. doi: 10.1056/NEJMoa1508887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, et al. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010;70:9827–9836. doi: 10.1158/0008-5472.CAN-10-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roskoski R., Jr. Anaplastic lymphoma kinase (ALK) inhibitors in the treatment of ALK-driven lung cancers. Pharm. Res. 2017;117:343–356. doi: 10.1016/j.phrs.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Courtin A, et al. Emergence of resistance to tyrosine kinase inhibitors in non-small-cell lung cancer can be delayed by an upfront combination with the HSP90 inhibitor onalespib. Br. J. Cancer. 2016;115:1069–1077. doi: 10.1038/bjc.2016.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, et al. Proteolysis targeting chimeras (PROTACs) of anaplastic lymphoma kinase (ALK) Eur. J. Med. Chem. 2018;151:304–314. doi: 10.1016/j.ejmech.2018.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park M, et al. Mechanism of met oncogene activation. Cell. 1986;45:895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- 42.Baldanzi G, Graziani A. Physiological signaling and structure of the HGF receptor MET. Biomedicines. 2014;3:1–31. doi: 10.3390/biomedicines3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes O, et al. Insights into the structure/function of hepatocyte growth factor/scatter factor from studies with individual domains. J. Mol. Biol. 2007;367:395–408. doi: 10.1016/j.jmb.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 44.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011;3:S7–s19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumenschein GR, Jr., Mills GB, Gonzalez-Angulo AM. Targeting the hepatocyte growth factor-cMET axis in cancer therapy. J. Clin. Oncol. 2012;30:3287–3296. doi: 10.1200/JCO.2011.40.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turke AB, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frampton GM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 48.Garajova I, Giovannetti E, Biasco G, Peters GJ. c-Met as a Target for Personalized Therapy. Transl. Oncogenomics. 2015;7:13–31. doi: 10.4137/TOG.S30534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heigener DF, Reck M. Crizotinib. Recent Results Cancer Res. 2018;211:57–65. doi: 10.1007/978-3-319-91442-8_4. [DOI] [PubMed] [Google Scholar]
- 50.Choueiri TK, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin. Cancer Res. 2011;17:7127–7138. doi: 10.1158/1078-0432.CCR-11-1157. [DOI] [PubMed] [Google Scholar]
- 52.Wolf J, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N. Engl. J. Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 53.Wu YL, et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J. Clin. Oncol. 2018;36:3101–3109. doi: 10.1200/JCO.2018.77.7326. [DOI] [PubMed] [Google Scholar]
- 54.Lara MS, et al. Preclinical evaluation of MET inhibitor INC-280 with or without the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung cancer. Clin. Lung Cancer. 2017;18:281–285. doi: 10.1016/j.cllc.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bladt F, et al. EMD 1214063 and EMD 1204831 constitute a new class of potent and highly selective c-Met inhibitors. Clin. Cancer Res. 2013;19:2941–2951. doi: 10.1158/1078-0432.CCR-12-3247. [DOI] [PubMed] [Google Scholar]
- 56.Friese-Hamim M, et al. The selective c-Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c-Met activation in NSCLC models. Am. J. Cancer Res. 2017;7:962–972. [PMC free article] [PubMed] [Google Scholar]
- 57.Markham A. Tepotinib: first approval. Drugs. 2020;80:829–833. doi: 10.1007/s40265-020-01317-9. [DOI] [PubMed] [Google Scholar]
- 58.Leighl NB, et al. A phase I study of foretinib plus erlotinib in patients with previously treated advanced non-small cell lung cancer: Canadian cancer trials group IND.196. Oncotarget. 2017;8:69651–69662. doi: 10.18632/oncotarget.18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engstrom LD, et al. Glesatinib exhibits antitumor activity in lung cancer models and patients harboring MET exon 14 mutations and overcomes mutation-mediated resistance to type I MET inhibitors in nonclinical models. Clin. Cancer Res. 2017;23:6661–6672. doi: 10.1158/1078-0432.CCR-17-1192. [DOI] [PubMed] [Google Scholar]
- 60.Miranda, O., Farooqui, M. & Siegfried, J. M. Status of agents targeting the HGF/c-met axis in lung cancer. Cancers10, 280 (2018). [DOI] [PMC free article] [PubMed]
- 61.Rodon J, et al. First-in-human phase I study of oral S49076, a unique MET/AXL/FGFR inhibitor, in advanced solid tumours. Eur. J. Cancer. 2017;81:142–150. doi: 10.1016/j.ejca.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Sequist LV, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J. Clin. Oncol. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 63.Yang J-J, et al. Preliminary results of a phase Ib trial of savolitinib combined with gefitinib in EGFR-mutant lung cancer. J. Clin. Oncol. 2016;34:e20559–e20559. doi: 10.1200/JCO.2016.34.15_suppl.e20559. [DOI] [Google Scholar]
- 64.Egile C, et al. The selective intravenous inhibitor of the MET tyrosine kinase SAR125844 inhibits tumor growth in MET-amplified cancer. Mol. Cancer Ther. 2015;14:384–394. doi: 10.1158/1535-7163.MCT-14-0428. [DOI] [PubMed] [Google Scholar]
- 65.Shitara K, et al. Phase I dose-escalation study of the c-Met tyrosine kinase inhibitor SAR125844 in Asian patients with advanced solid tumors, including patients with MET-amplified gastric cancer. Oncotarget. 2017;8:79546–79555. doi: 10.18632/oncotarget.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parikh PK, Ghate MD. Recent advances in the discovery of small molecule c-Met Kinase inhibitors. Eur. J. Med. Chem. 2018;143:1103–1138. doi: 10.1016/j.ejmech.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 67.Bradley CA, et al. Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat. Rev. Clin. Oncol. 2017;14:562–576. doi: 10.1038/nrclinonc.2017.40. [DOI] [PubMed] [Google Scholar]
- 68.Salgia R. MET in lung cancer: biomarker selection based on scientific rationale. Mol. Cancer Ther. 2017;16:555–565. doi: 10.1158/1535-7163.MCT-16-0472. [DOI] [PubMed] [Google Scholar]
- 69.Qi J, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res. 2011;71:1081–1091. doi: 10.1158/0008-5472.CAN-10-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cepero V, et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 2010;70:7580–7590. doi: 10.1158/0008-5472.CAN-10-0436. [DOI] [PubMed] [Google Scholar]
- 71.Threadgill DW, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 72.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 73.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol./Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-I. [DOI] [PubMed] [Google Scholar]
- 74.Metro G, et al. Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC) Rev. Recent Clin. trials. 2006;1:1–13. doi: 10.2174/157488706775246157. [DOI] [PubMed] [Google Scholar]
- 75.Roskoski R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharm. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Gu A, et al. Efficacy and safety evaluation of icotinib in patients with advanced non-small cell lung cancer. Chin. J. Cancer Res. 2013;25:90–94. doi: 10.3978/j.issn.1000-9604.2012.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 78.Kulke MH, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J. Clin. Oncol. 2007;25:4787–4792. doi: 10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 79.Maione P, et al. Overcoming resistance to targeted therapies in NSCLC: current approaches and clinical application. Ther. Adv. Med. Oncol. 2015;7:263–273. doi: 10.1177/1758834015595048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller VA, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13:528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 81.Lavacchi D, Mazzoni F, Giaccone G. Clinical evaluation of dacomitinib for the treatment of metastatic non-small cell lung cancer (NSCLC): current perspectives. Drug Des. Dev. Ther. 2019;13:3187–3198. doi: 10.2147/DDDT.S194231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schrank, Z. et al. Current molecular-targeted therapies in NSCLC and their mechanism of resistance. Cancers10, 224 (2018). [DOI] [PMC free article] [PubMed]
- 83.Yver A. Osimertinib (AZD9291)-a science-driven, collaborative approach to rapid drug design and development. Ann. Oncol. 2016;27:1165–1170. doi: 10.1093/annonc/mdw129. [DOI] [PubMed] [Google Scholar]
- 84.Yang, J. C. et al. Safety, efficacy, and pharmacokinetics of almonertinib (HS-10296) in pretreated patients with EGFR-mutated advanced NSCLC: a multicenter, open-label, phase 1 trial. J. Thorac. Oncol. 15, 1907–1918 (2020). [DOI] [PubMed]
- 85.Paul B, Trovato JA, Thompson J. Lapatinib: a dual tyrosine kinase inhibitor for metastatic breast cancer. Am. J. Health Syst. Pharm. 2008;65:1703–1710. doi: 10.2146/ajhp070646. [DOI] [PubMed] [Google Scholar]
- 86.Deeks ED. Neratinib: first global approval. Drugs. 2017;77:1695–1704. doi: 10.1007/s40265-017-0811-4. [DOI] [PubMed] [Google Scholar]
- 87.Murthy RK, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 88.Kim ES. Olmutinib: first global approval. Drugs. 2016;76:1153–1157. doi: 10.1007/s40265-016-0606-z. [DOI] [PubMed] [Google Scholar]
- 89.Roskoski R., Jr. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharm. Res. 2019;139:395–411. doi: 10.1016/j.phrs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 90.Kim DW, et al. Safety, tolerability, and anti-tumor activity of olmutinib in non-small cell lung cancer with T790M mutation: a single arm, open label, phase 1/2 trial. Lung Cancer. 2019;135:66–72. doi: 10.1016/j.lungcan.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Ma Y, et al. First-in-human phase I study of AC0010, a mutant-selective EGFR inhibitor in non-small cell lung cancer: safety, efficacy, and potential mechanism of resistance. J. Thorac. Oncol. 2018;13:968–977. doi: 10.1016/j.jtho.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 92.Erlichman C, et al. Phase I study of EKB-569, an irreversible inhibitor of the epidermal growth factor receptor, in patients with advanced solid tumors. J. Clin. Oncol. 2006;24:2252–2260. doi: 10.1200/JCO.2005.01.8960. [DOI] [PubMed] [Google Scholar]
- 93.Thress KS, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015;21:560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Z, et al. Durable clinical response of lung adenocarcinoma harboring EGFR 19Del/T790M/in trans-C797S to combination therapy of first- and third-generation EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 2019;14:e157–e159. doi: 10.1016/j.jtho.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 95.Jia Y, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534:129–132. doi: 10.1038/nature17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu X, et al. Discovery of JND3229 as a new EGFR(C797S) mutant inhibitor with in vivo monodrug efficacy. ACS Med. Chem. Lett. 2018;9:1123–1127. doi: 10.1021/acsmedchemlett.8b00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen, J. et al. Structure-based design of 5-methylpyrimidopyridone derivatives as new wild-type sparing inhibitors of the epidermal growth factor receptor triple mutant (EGFR(L858R/T790M/C797S). J. Med. Chem.62, 7302–7308 (2019). [DOI] [PubMed]
- 98.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 99.Kiyoi H, et al. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21:2555–2563. doi: 10.1038/sj.onc.1205332. [DOI] [PubMed] [Google Scholar]
- 100.Quentmeier H, Reinhardt J, Zaborski M, Drexler HG. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia. 2003;17:120–124. doi: 10.1038/sj.leu.2402740. [DOI] [PubMed] [Google Scholar]
- 101.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 102.Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312. doi: 10.1038/s41375-018-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Auclair D, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 104.Stone RM, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia JS, Stone RM. The development of FLT3 inhibitors in acute myeloid leukemia. Hematol. Oncol. Clin. North Am. 2017;31:663–680. doi: 10.1016/j.hoc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Knapper S. The clinical development of FLT3 inhibitors in acute myeloid leukemia. Expert Opin. Investig. Drugs. 2011;20:1377–1395. doi: 10.1517/13543784.2011.611802. [DOI] [PubMed] [Google Scholar]
- 107.Baldi GG, Gronchi A, Stacchiotti S. Pexidartinib for the treatment of adult symptomatic patients with tenosynovial giant cell tumors. Expert Rev. Clin. Pharm. 2020;13:571–576. doi: 10.1080/17512433.2020.1771179. [DOI] [PubMed] [Google Scholar]
- 108.Mori M, et al. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Invest. N. Drugs. 2017;35:556–565. doi: 10.1007/s10637-017-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zarrinkar PP, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cortes J, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018;19:889–903. doi: 10.1016/S1470-2045(18)30240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galanis A, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. 2014;123:94–100. doi: 10.1182/blood-2013-10-529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao ZX, et al. SKLB1028, a novel oral multikinase inhibitor of EGFR, FLT3 and Abl, displays exceptional activity in models of FLT3-driven AML and considerable potency in models of CML harboring Abl mutants. Leukemia. 2012;26:1892–1895. doi: 10.1038/leu.2012.67. [DOI] [PubMed] [Google Scholar]
- 113.Sutamtewagul G, Vigil CE. Clinical use of FLT3 inhibitors in acute myeloid leukemia. Onco Targets Ther. 2018;11:7041–7052. doi: 10.2147/OTT.S171640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim SH, Dubielecka PM, Raghunathan VM. Molecular targeting in acute myeloid leukemia. J. Transl. Med. 2017;15:183. doi: 10.1186/s12967-017-1281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller GD, Bruno BJ, Lim CS. Resistant mutations in CML and Ph(+)ALL - role of ponatinib. Biologics. 2014;8:243–254. doi: 10.2147/BTT.S50734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Elshoury A, Przespolewski A, Baron J, Wang ES. Advancing treatment of acute myeloid leukemia: the future of FLT3 inhibitors. Expert Rev. Anticancer Ther. 2019;19:273–286. doi: 10.1080/14737140.2019.1573679. [DOI] [PubMed] [Google Scholar]
- 117.Smith CC, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sung L, et al. Predictors and short-term outcomes of hyperleukocytosis in children with acute myeloid leukemia: a report from the Children’s Oncology Group. Haematologica. 2012;97:1770–1773. doi: 10.3324/haematol.2012.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin WH, et al. Evaluation of the antitumor effects of BPR1J-340, a potent and selective FLT3 inhibitor, alone or in combination with an HDAC inhibitor, vorinostat, in AML cancer. PLoS ONE. 2014;9:e83160. doi: 10.1371/journal.pone.0083160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Larrosa-Garcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol. Cancer Ther. 2017;16:991–1001. doi: 10.1158/1535-7163.MCT-16-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao T, et al. The FLT3-ITD mutation and the expression of its downstream signaling intermediates STAT5 and Pim-1 are positively correlated with CXCR4 expression in patients with acute myeloid leukemia. Sci. Rep. 2019;9:12209. doi: 10.1038/s41598-019-48687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Uras IZ, et al. Palbociclib treatment of FLT3-ITD+ AML cells uncovers a kinase-dependent transcriptional regulation of FLT3 and PIM1 by CDK6. Blood. 2016;127:2890–2902. doi: 10.1182/blood-2015-11-683581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sandhofer N, et al. Dual PI3K/mTOR inhibition shows antileukemic activity in MLL-rearranged acute myeloid leukemia. Leukemia. 2015;29:828–838. doi: 10.1038/leu.2014.305. [DOI] [PubMed] [Google Scholar]
- 124.Yamaura T, et al. A novel irreversible FLT3 inhibitor, FF-10101, shows excellent efficacy against AML cells with FLT3 mutations. Blood. 2018;131:426–438. doi: 10.1182/blood-2017-05-786657. [DOI] [PubMed] [Google Scholar]
- 125.Chen CT, et al. Identification of a potent 5-phenyl-thiazol-2-ylamine-based inhibitor of FLT3 with activity against drug resistance-conferring point mutations. Eur. J. Med. Chem. 2015;100:151–161. doi: 10.1016/j.ejmech.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 126.Smith CC, et al. Characterizing and overriding the structural mechanism of the quizartinib-resistant FLT3 “Gatekeeper” F691L mutation with PLX3397. Cancer Discov. 2015;5:668–679. doi: 10.1158/2159-8290.CD-15-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu B, et al. MZH29 is a novel potent inhibitor that overcomes drug resistance FLT3 mutations in acute myeloid leukemia. Leukemia. 2017;31:913–921. doi: 10.1038/leu.2016.297. [DOI] [PubMed] [Google Scholar]
- 128.Park I. Angiogenesis and microsatellite alterations in oral cavity and oropharynx cancer. Otolaryngol. Head Neck Surg. 2003;129:P161. doi: 10.1016/S0194-5998(03)00974-4. [DOI] [Google Scholar]
- 129.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 130.Klagsbrun M, Moses MA. Molecular angiogenesis. Chem. Biol. 1999;6:R217–R224. doi: 10.1016/S1074-5521(99)80081-7. [DOI] [PubMed] [Google Scholar]
- 131.Yadav L, et al. Tumour angiogenesis and angiogenic inhibitors: a review. J. Clin. Diagn. Res. 2015;9:Xe01–xe05. doi: 10.7860/JCDR/2015/12016.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang WH, Xu J, Mu JB, Xie J. Revision of the concept of anti-angiogenesis and its applications in tumor treatment. Chronic Dis. Transl. Med. 2017;3:33–40. doi: 10.1016/j.cdtm.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Folkman J, et al. Tumor angiogenesis - therapeutic implications. N. Engl. J. Med. 1971;285:1182. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- 134.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 135.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/S0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 136.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 137.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 138.Zheng X, et al. The regulation of cytokine signaling by retinal determination gene network pathway in cancer. Onco Targets Ther. 2018;11:6479–6487. doi: 10.2147/OTT.S176113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leung DW, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 140.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 141.Chen PH, Chen X, He X. Platelet-derived growth factors and their receptors: structural and functional perspectives. Biochim. Biophys. Acta. 2013;1834:2176–2186. doi: 10.1016/j.bbapap.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 143.Rak J, Yu JL, Klement G, Kerbel RS. Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J. Investig. Dermatol Symp. Proc. 2000;5:24–33. doi: 10.1046/j.1087-0024.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 144.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 145.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur. J. Cancer. 2006;42:3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 146.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 147.Wilhelm SM, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 149.Wilhelm S, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 150.Woo HY, Heo J. Sorafenib in liver cancer. Expert Opin. Pharmacother. 2012;13:1059–1067. doi: 10.1517/14656566.2012.679930. [DOI] [PubMed] [Google Scholar]
- 151.Escudier B, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 152.Brose MS, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Motzer RJ, Escudier B, Gannon A, Figlin RA. Sunitinib: ten years of successful clinical use and study in advanced renal cell carcinoma. Oncologist. 2017;22:41–52. doi: 10.1634/theoncologist.2016-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van der Graaf WTA, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 155.Keating GM. Axitinib: a review in advanced renal cell carcinoma. Drugs. 2015;75:1903–1913. doi: 10.1007/s40265-015-0483-x. [DOI] [PubMed] [Google Scholar]
- 156.Elisei R, et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Escudier B, et al. The role of tivozanib in advanced renal cell carcinoma therapy. Expert Rev. Anticancer Ther. 2018;18:1113–1124. doi: 10.1080/14737140.2018.1508348. [DOI] [PubMed] [Google Scholar]
- 158.Schenone S, Brullo C, Botta M. Small molecules ATP-competitive inhibitors of FLT3: a chemical overview. Curr. Med. Chem. 2008;15:3113–3132. doi: 10.2174/092986708786848613. [DOI] [PubMed] [Google Scholar]
- 159.Roskoski R., Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem. Biophys. Res. Commun. 2007;356:323–328. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- 160.Morabito A, et al. Vandetanib: an overview of its clinical development in NSCLC and other tumors. Drugs Today. 2010;46:683–698. doi: 10.1358/dot.2010.46.9.1516989. [DOI] [PubMed] [Google Scholar]
- 161.Yoh K, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir. Med. 2017;5:42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y, et al. XL-184, a MET, VEGFR-2 and RET kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and NSCLC. IDrugs Investig. Drugs J. 2010;13:112–121. [PMC free article] [PubMed] [Google Scholar]
- 163.Markham A. Selpercatinib: first approval. Drugs. 2020;80:1119–1124. doi: 10.1007/s40265-020-01343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Markham A. Pralsetinib: first approval. Drugs. 2020;80:1865–1870. doi: 10.1007/s40265-020-01427-4. [DOI] [PubMed] [Google Scholar]
- 165.Wilhelm SM, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J. Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 166.Strumberg D, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br. J. Cancer. 2012;106:1722–1727. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Demetri GD, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dhillon S. Nintedanib: a review of its use as second-line treatment in adults with advanced non-small cell lung cancer of adenocarcinoma histology. Target Oncol. 2015;10:303–310. doi: 10.1007/s11523-015-0367-8. [DOI] [PubMed] [Google Scholar]
- 169.Keating GM. Nintedanib: a review of its use in patients with idiopathic pulmonary fibrosis. Drugs. 2015;75:1131–1140. doi: 10.1007/s40265-015-0418-6. [DOI] [PubMed] [Google Scholar]
- 170.Alshangiti A, Chandhoke G, Ellis PM. Antiangiogenic therapies in non-small-cell lung cancer. Curr. Oncol. 2018;25:S45–S58. doi: 10.3747/co.25.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Aoyama T, Yoshikawa T. Targeted therapy: apatinib - new third-line option for refractory gastric or GEJ cancer. Nat. Rev. Clin. Oncol. 2016;13:268–270. doi: 10.1038/nrclinonc.2016.53. [DOI] [PubMed] [Google Scholar]
- 172.Syed YY. Anlotinib: first global approval. Drugs. 2018;78:1057–1062. doi: 10.1007/s40265-018-0939-x. [DOI] [PubMed] [Google Scholar]
- 173.Han B, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302) Br. J. Cancer. 2018;118:654–661. doi: 10.1038/bjc.2017.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shirley M. Fruquintinib: first global approval. Drugs. 2018;78:1757–1761. doi: 10.1007/s40265-018-0998-z. [DOI] [PubMed] [Google Scholar]
- 175.Li J, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486–2496. doi: 10.1001/jama.2018.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen Z, Jiang L. The clinical application of fruquintinib on colorectal cancer. Expert Rev. Clin. Pharm. 2019;12:713–721. doi: 10.1080/17512433.2019.1630272. [DOI] [PubMed] [Google Scholar]
- 177.Perera TPS, et al. Discovery and pharmacological characterization of JNJ-42756493 (Erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol. Cancer Ther. 2017;16:1010–1020. doi: 10.1158/1535-7163.MCT-16-0589. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Y, et al. Constitutive activating mutation of the FGFR3b in oral squamous cell carcinomas. Int J. Cancer. 2005;117:166–168. doi: 10.1002/ijc.21145. [DOI] [PubMed] [Google Scholar]
- 179.Hoy SM. Pemigatinib: first approval. Drugs. 2020;80:923–929. doi: 10.1007/s40265-020-01330-y. [DOI] [PubMed] [Google Scholar]
- 180.Okamoto I, et al. Comparison of carboplatin plus pemetrexed followed by maintenance pemetrexed with docetaxel monotherapy in elderly patients with advanced nonsquamous non-small cell lung cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:e196828. doi: 10.1001/jamaoncol.2019.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dhillon S. Avapritinib: first approval. Drugs. 2020;80:433–439. doi: 10.1007/s40265-020-01275-2. [DOI] [PubMed] [Google Scholar]
- 182.Kasireddy V, von Mehren M. Emerging drugs for the treatment of gastrointestinal stromal tumour. Expert Opin. Emerg. Drugs. 2017;22:317–329. doi: 10.1080/14728214.2017.1411479. [DOI] [PubMed] [Google Scholar]
- 183.Heinrich MC, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2020;21:935–946. doi: 10.1016/S1470-2045(20)30269-2. [DOI] [PubMed] [Google Scholar]
- 184.Saleh, N. Avapritinib approved for GIST subgroup. Cancer Discov. 10, 334 (2020). [DOI] [PubMed]
- 185.Dhillon S. Ripretinib: first approval. Drugs. 2020;80:1133–1138. doi: 10.1007/s40265-020-01348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Batchelor TT, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu JF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Renhowe PA, et al. Design, structure-activity relationships and in vivo characterization of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones: a novel class of receptor tyrosine kinase inhibitors. J. Med. Chem. 2009;52:278–292. doi: 10.1021/jm800790t. [DOI] [PubMed] [Google Scholar]
- 189.Kim KB, et al. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin. Cancer Res. 2011;17:7451–7461. doi: 10.1158/1078-0432.CCR-11-1747. [DOI] [PubMed] [Google Scholar]
- 190.Raghav KP, Blumenschein GR. Motesanib and advanced NSCLC: experiences and expectations. Expert Opin. Investig. Drugs. 2011;20:859–869. doi: 10.1517/13543784.2011.579103. [DOI] [PubMed] [Google Scholar]
- 191.Kubota K, et al. Phase III, randomized, placebo-controlled, double-blind trial of motesanib (AMG-706) in combination with paclitaxel and carboplatin in East Asian patients with advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2017;35:3662–3670. doi: 10.1200/JCO.2017.72.7297. [DOI] [PubMed] [Google Scholar]
- 192.Schlumberger MJ, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J. Clin. Oncol. 2009;27:3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 193.Bass MB, et al. Biomarkers as predictors of response to treatment with motesanib in patients with progressive advanced thyroid cancer. J. Clin. Endocrinol. Metab. 2010;95:5018–5027. doi: 10.1210/jc.2010-0947. [DOI] [PubMed] [Google Scholar]
- 194.Sherman SI, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N. Engl. J. Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 195.Xu JM, et al. Sulfatinib, a novel kinase inhibitor, in patients with advanced solid tumors: results from a phase I study. Oncotarget. 2017;8:42076–42086. doi: 10.18632/oncotarget.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu J, et al. Surufatinib in advanced well-differentiated neuroendocrine tumors: A Multicenter, Single-arm, Open-label, Phase Ib/II Trial. Clin. Cancer Res. 2019;25:3486–3494. doi: 10.1158/1078-0432.CCR-18-2994. [DOI] [PubMed] [Google Scholar]
- 197.Xu, J. et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol.21, 1500–1512 (2020). [DOI] [PubMed]
- 198.Heinrich MC, et al. Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin. Cancer Res. 2012;18:4375–4384. doi: 10.1158/1078-0432.CCR-12-0625. [DOI] [PubMed] [Google Scholar]
- 199.Indio, V. et al. Integrated molecular characterization of gastrointestinal stromal tumors (GIST) harboring the rare D842V mutation in PDGFRA gene. Int. J. Mol. Sci. 19, 732 (2018). [DOI] [PMC free article] [PubMed]
- 200.Jetani H, et al. CAR T-cells targeting FLT3 have potent activity against FLT3(-)ITD(+) AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018;32:1168–1179. doi: 10.1038/s41375-018-0009-0. [DOI] [PubMed] [Google Scholar]
- 201.Guffanti F, et al. In vitro and in vivo activity of lucitanib in FGFR1/2 amplified or mutated cancer models. Neoplasia. 2017;19:35–42. doi: 10.1016/j.neo.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cai ZW, et al. Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215) J. Med. Chem. 2008;51:1976–1980. doi: 10.1021/jm7013309. [DOI] [PubMed] [Google Scholar]
- 203.Johnson PJ, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 204.Kudo M, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60:1697–1707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]
- 205.Tucker JA, et al. Structural insights into FGFR kinase isoform selectivity: diverse binding modes of AZD4547 and ponatinib in complex with FGFR1 and FGFR4. Structure. 2014;22:1764–1774. doi: 10.1016/j.str.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 206.Felix NS, et al. Effects of the FGF receptor-1 inhibitor, infigratinib, with or without sildenafil, in experimental pulmonary arterial hypertension. Br. J. Pharm. 2019;176:4462–4473. doi: 10.1111/bph.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Makawita S, et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: the PROOF 301 trial. Future Oncol. 2020;16:2375–2384. doi: 10.2217/fon-2020-0299. [DOI] [PubMed] [Google Scholar]
- 208.Lahn M, Kloeker S, Berry BS. TGF-beta inhibitors for the treatment of cancer. Expert Opin. Investig. Drugs. 2005;14:629–643. doi: 10.1517/13543784.14.6.629. [DOI] [PubMed] [Google Scholar]
- 209.Herbertz S, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Dev. Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jung SY, et al. Population pharmacokinetics of vactosertib, a new TGF-beta receptor type Iota inhibitor, in patients with advanced solid tumors. Cancer Chemother. Pharm. 2020;85:173–183. doi: 10.1007/s00280-019-03979-z. [DOI] [PubMed] [Google Scholar]
- 211.Xu G, et al. Synthesis and biological evaluation of 4-(pyridin-4-oxy)-3-(3,3-difluorocyclobutyl)-pyrazole derivatives as novel potent transforming growth factor-beta type 1 receptor inhibitors. Eur. J. Med. Chem. 2020;198:112354. doi: 10.1016/j.ejmech.2020.112354. [DOI] [PubMed] [Google Scholar]
- 212.Yingling JM, et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-β receptor type I inhibitor. Oncotarget. 2018;9:6659–6677. doi: 10.18632/oncotarget.23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Santini V, et al. Phase II study of the ALK5 inhibitor galunisertib in very low-, low-, and intermediate-risk myelodysplastic syndromes. Clin. Cancer Res. 2019;25:6976–6985. doi: 10.1158/1078-0432.CCR-19-1338. [DOI] [PubMed] [Google Scholar]
- 214.Holmgaard RB, et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J. Immunother. Cancer. 2018;6:47. doi: 10.1186/s40425-018-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Teleanu, R. I., Chircov, C., Grumezescu, A. M. & Teleanu, D. M. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J. Clin. Med. 9, 84 (2019). [DOI] [PMC free article] [PubMed]
- 216.Rajabi, M. & Mousa, S. A. The role of angiogenesis in cancer treatment. Biomedicines. 5, 34 (2017). [DOI] [PMC free article] [PubMed]
- 217.Russo M, Giavazzi R. Anti-angiogenesis for cancer: current status and prospects. Thromb. Res. 2018;164:S3–S6. doi: 10.1016/j.thromres.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 218.Mauceri HJ, et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 219.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J. Natl Cancer Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 220.Giuliano S, Pagès G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie. 2013;95:1110–1119. doi: 10.1016/j.biochi.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 221.Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat. Rev. Nephrol. 2017;13:496–511. doi: 10.1038/nrneph.2017.82. [DOI] [PubMed] [Google Scholar]
- 222.Sheng X, et al. Axitinib in combination with toripalimab, a humanized immunoglobulin G(4) monoclonal antibody against programmed cell death-1, in patients with metastatic mucosal melanoma: An Open-Label Phase IB Trial. J. Clin. Oncol. 2019;37:2987–2999. doi: 10.1200/JCO.19.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu X, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J. Hematol. Oncol. 2017;10:153. doi: 10.1186/s13045-017-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rini BI, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin. Cancer Res. 2011;17:3841–3849. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]
- 225.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Scott-Solomon E, Kuruvilla R. Mechanisms of neurotrophin trafficking via Trk receptors. Mol. Cell. Neurosci. 2018;91:25–33. doi: 10.1016/j.mcn.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 2001;169:107–114. doi: 10.1016/S0304-3835(01)00530-4. [DOI] [PubMed] [Google Scholar]
- 228.Bertrand T, et al. The crystal structures of TrkA and TrkB suggest key regions for achieving selective inhibition. J. Mol. Biol. 2012;423:439–453. doi: 10.1016/j.jmb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 229.Drilon A, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Doebele RC, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ardini E, et al. Entrectinib, a pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol. Cancer Ther. 2016;15:628–639. doi: 10.1158/1535-7163.MCT-15-0758. [DOI] [PubMed] [Google Scholar]
- 232.Federman N, McDermott R. Larotrectinib, a highly selective tropomyosin receptor kinase (TRK) inhibitor for the treatment of TRK fusion cancer. Expert Rev. Clin. Pharm. 2019;12:931–939. doi: 10.1080/17512433.2019.1661775. [DOI] [PubMed] [Google Scholar]
- 233.Grüllich C. Cabozantinib: multi-kinase Inhibitor of MET, AXL, RET, and VEGFR2. Recent Results Cancer Res. 2018;211:67–75. doi: 10.1007/978-3-319-91442-8_5. [DOI] [PubMed] [Google Scholar]
- 234.Konicek BW, et al. Merestinib (LY2801653) inhibits neurotrophic receptor kinase (NTRK) and suppresses growth of NTRK fusion bearing tumors. Oncotarget. 2018;9:13796–13806. doi: 10.18632/oncotarget.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lin CC, et al. A phase 1, open-label, dose-escalation trial of oral TSR-011 in patients with advanced solid tumours and lymphomas. Br. J. Cancer. 2019;121:131–138. doi: 10.1038/s41416-019-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang, Y. et al. Sitravatinib, a tyrosine kinase inhibitor, inhibits the transport function of ABCG2 and restores sensitivity to chemotherapy-resistant cancer cells in vitro. Front. Oncol. 10, 700 (2020). [DOI] [PMC free article] [PubMed]
- 237.Piao Y, et al. Novel MET/TIE2/VEGFR2 inhibitor altiratinib inhibits tumor growth and invasiveness in bevacizumab-resistant glioblastoma mouse models. Neuro-Oncol. 2016;18:1230–1241. doi: 10.1093/neuonc/now030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Katayama R, et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat. Commun. 2019;10:3604. doi: 10.1038/s41467-019-11496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Khotskaya YB, et al. Targeting TRK family proteins in cancer. Pharmacol. Therap. 2017;173:58–66. doi: 10.1016/j.pharmthera.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 240.Russo M, et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discov. 2016;6:36–44. doi: 10.1158/2159-8290.CD-15-0940. [DOI] [PubMed] [Google Scholar]
- 241.Drilon A, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior trk kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017;7:963–972. doi: 10.1158/2159-8290.CD-17-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 2019;30:viii23–viii30. doi: 10.1093/annonc/mdz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113:1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Redaelli A, et al. Clinical and epidemiologic burden of chronic myelogenous leukemia. Expert Rev. Anticancer Ther. 2004;4:85–96. doi: 10.1586/14737140.4.1.85. [DOI] [PubMed] [Google Scholar]
- 245.Kurzrock R, Kantarjian HM, Druker BJ, Talpaz M. Philadelphia chromosome-positive leukemias: from basic mechanisms to molecular therapeutics. Ann. Intern. Med. 2003;138:819–830. doi: 10.7326/0003-4819-138-10-200305200-00010. [DOI] [PubMed] [Google Scholar]
- 246.Klein F, et al. The BCR-ABL1 kinase bypasses selection for the expression of a pre-B cell receptor in pre-B acute lymphoblastic leukemia cells. J. Exp. Med. 2004;199:673–685. doi: 10.1084/jem.20031637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 248.Druker BJ, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 249.Hochhaus A, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 250.Hantschel O, Grebien F, Superti-Furga G. The growing arsenal of ATP-competitive and allosteric inhibitors of BCR-ABL. Cancer Res. 2012;72:4890–4895. doi: 10.1158/0008-5472.CAN-12-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/S0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 252.Jabbour E, Cortes J, Kantarjian H. Treatment selection after imatinib resistance in chronic myeloid leukemia. Target Oncol. 2009;4:3–10. doi: 10.1007/s11523-008-0100-y. [DOI] [PubMed] [Google Scholar]
- 253.Weisberg E, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 254.Puttini M, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 255.Sawyers CL. Even better kinase inhibitors for chronic myeloid leukemia. N. Engl. J. Med. 2010;362:2314–2315. doi: 10.1056/NEJMe1004430. [DOI] [PubMed] [Google Scholar]
- 256.O’Hare T, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 257.O’Hare T, et al. Targeting the BCR-ABL signaling pathway in therapy-resistant Philadelphia chromosome-positive leukemia. Clin. Cancer Res. 2011;17:212–221. doi: 10.1158/1078-0432.CCR-09-3314. [DOI] [PubMed] [Google Scholar]
- 258.O’Hare T, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wylie AA, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–737. doi: 10.1038/nature21702. [DOI] [PubMed] [Google Scholar]
- 260.Eide CA, et al. The ABL switch control inhibitor DCC-2036 is active against the chronic myeloid leukemia mutant BCR-ABLT315I and exhibits a narrow resistance profile. Cancer Res. 2011;71:3189–3195. doi: 10.1158/0008-5472.CAN-10-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yokota A, et al. INNO-406, a novel BCR-ABL/Lyn dual tyrosine kinase inhibitor, suppresses the growth of Ph+ leukemia cells in the central nervous system, and cyclosporine A augments its in vivo activity. Blood. 2007;109:306–314. doi: 10.1182/blood-2006-03-013250. [DOI] [PubMed] [Google Scholar]
- 262.Zabriskie MS, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. 2014;26:428–442. doi: 10.1016/j.ccr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat. Rev. Cancer. 2012;12:513–526. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- 264.Gibbons DL, et al. Molecular dynamics reveal BCR-ABL1 polymutants as a unique mechanism of resistance to PAN-BCR-ABL1 kinase inhibitor therapy. Proc. Natl Acad. Sci. USA. 2014;111:3550–3555. doi: 10.1073/pnas.1321173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Alvandi F, et al. U.S. Food and Drug Administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist. 2014;19:94–99. doi: 10.1634/theoncologist.2013-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Khoury HJ, et al. Omacetaxine mepesuccinate in patients with advanced chronic myeloid leukemia with resistance or intolerance to tyrosine kinase inhibitors. Leuk. Lymphoma. 2015;56:120–127. doi: 10.3109/10428194.2014.889826. [DOI] [PubMed] [Google Scholar]
- 267.Ogbogu PU, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J. Allergy Clin. Immunol. 2009;124:1319–1325.e1313. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Montemurro M, et al. Long-term outcome of dasatinib first-line treatment in gastrointestinal stromal tumor: a multicenter, 2-stage phase 2 trial (Swiss Group for Clinical Cancer Research 56/07) Cancer. 2018;124:1449–1454. doi: 10.1002/cncr.31234. [DOI] [PubMed] [Google Scholar]
- 269.Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J. Hematol. Oncol. 2018;11:84. doi: 10.1186/s13045-018-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tanaka S, Baba Y. B cell receptor signaling. Adv. Exp. Med. Biol. 2020;1254:23–36. doi: 10.1007/978-981-15-3532-1_2. [DOI] [PubMed] [Google Scholar]
- 271.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat. Rev. Cancer. 2014;14:219–232. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 272.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nat. Rev. Immunol. 2005;5:284–295. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 273.Khan WN, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 274.Ma L, et al. Discovery and characterization of LY2784544, a small-molecule tyrosine kinase inhibitor of JAK2V617F. Blood Cancer J. 2013;3:e109. doi: 10.1038/bcj.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Davids MS, Brown JR. Ibrutinib: a first in class covalent inhibitor of Bruton’s tyrosine kinase. Future Oncol. 2014;10:957–967. doi: 10.2217/fon.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Skarzynski M, et al. Interactions between ibrutinib and anti-CD20 antibodies: competing effects on the outcome of combination therapy. Clin. Cancer Res. 2016;22:86–95. doi: 10.1158/1078-0432.CCR-15-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Akinleye A, et al. Ibrutinib and novel BTK inhibitors in clinical development. J. Hematol. Oncol. 2013;6:59. doi: 10.1186/1756-8722-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wang ML, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Byrd JC, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Noy A, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129:2224–2232. doi: 10.1182/blood-2016-10-747345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mato AR, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103:874–879. doi: 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rajasekaran, N. et al. Three BTK-specific inhibitors, in contrast to ibrutinib, do not antagonize rituximab-dependent NK-cell mediated cytotoxicity. Blood. 124, 3118 (2014).
- 283.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J. Hematol. Oncol. 2016;9:21. doi: 10.1186/s13045-016-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wu J, Liu C, Tsui ST, Liu D. Second-generation inhibitors of Bruton tyrosine kinase. J. Hematol. Oncol. 2016;9:80. doi: 10.1186/s13045-016-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Herman SEM, et al. The Bruton tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin. Cancer Res. 2017;23:2831–2841. doi: 10.1158/1078-0432.CCR-16-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Guo Y, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J. Med. Chem. 2019;62:7923–7940. doi: 10.1021/acs.jmedchem.9b00687. [DOI] [PubMed] [Google Scholar]
- 287.Syed YY. Zanubrutinib: first approval. Drugs. 2020;80:91–97. doi: 10.1007/s40265-019-01252-4. [DOI] [PubMed] [Google Scholar]
- 288.Blum KA. B-cell receptor pathway modulators in NHL. Hematol. Am. Soc. Hematol. Educ. Program. 2015;2015:82–91. doi: 10.1182/asheducation-2015.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Naymagon L, Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J. Hematol. Oncol. 2016;9:52. doi: 10.1186/s13045-016-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J. Immunother. Cancer. 2016;4:40. doi: 10.1186/s40425-016-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Burger JA. Bruton’s tyrosine kinase (BTK) inhibitors in clinical trials. Curr. Hematol. Malig. Rep. 2014;9:44–49. doi: 10.1007/s11899-013-0188-8. [DOI] [PubMed] [Google Scholar]
- 292.Walter HS, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–419. doi: 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Danilov AV, et al. Phase Ib study of tirabrutinib in combination with idelalisib or entospletinib in previously treated chronic lymphocytic leukemia. Clin. Cancer Res. 2020;26:2810–2818. doi: 10.1158/1078-0432.CCR-19-3504. [DOI] [PubMed] [Google Scholar]
- 294.Evans EK, et al. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. J. Pharm. Exp. Ther. 2013;346:219–228. doi: 10.1124/jpet.113.203489. [DOI] [PubMed] [Google Scholar]
- 295.Abdelhameed AS, Attwa MW, Al-Shaklia NS, Kadi AA. A highly sensitive LC-MS/MS method to determine novel Bruton’s tyrosine kinase inhibitor spebrutinib: application to metabolic stability evaluation. R. Soc. Open Sci. 2019;6:190434. doi: 10.1098/rsos.190434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Brown JR, et al. Phase I study of single-agent CC-292, a highly selective Bruton’s tyrosine kinase inhibitor, in relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2016;101:e295–e298. doi: 10.3324/haematol.2015.140806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Haselmayer P, et al. Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models. J. Immunol. 2019;202:2888–2906. doi: 10.4049/jimmunol.1800583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Watterson SH, et al. Discovery of Branebrutinib (BMS-986195): a strategy for identifying a highly potent and selective covalent inhibitor providing rapid in vivo inactivation of Bruton’s tyrosine kinase (BTK) J. Med. Chem. 2019;62:3228–3250. doi: 10.1021/acs.jmedchem.9b00167. [DOI] [PubMed] [Google Scholar]
- 299.Park JK, et al. HM71224, a novel Bruton’s tyrosine kinase inhibitor, suppresses B cell and monocyte activation and ameliorates arthritis in a mouse model: a potential drug for rheumatoid arthritis. Arthritis Res. Ther. 2016;18:91. doi: 10.1186/s13075-016-0988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhang Z, et al. Targeting Bruton’s tyrosine kinase for the treatment of B cell associated malignancies and autoimmune diseases: preclinical and clinical developments of small molecule inhibitors. Arch. Pharm. 2018;351:e1700369. doi: 10.1002/ardp.201700369. [DOI] [PubMed] [Google Scholar]
- 301.Kim HO. Development of BTK inhibitors for the treatment of B-cell malignancies. Arch. Pharm. Res. 2019;42:171–181. doi: 10.1007/s12272-019-01124-1. [DOI] [PubMed] [Google Scholar]
- 302.Thompson PA, Burger JA. Bruton’s tyrosine kinase inhibitors: first and second generation agents for patients with chronic lymphocytic leukemia (CLL) Expert Opin. Investig. Drugs. 2018;27:31–42. doi: 10.1080/13543784.2018.1404027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Brandhuber B, et al. LOXO-305, a next generation reversible BTK inhibitor, for overcoming acquired resistance to irreversible BTK inhibitors. Clin. Lymphoma Myeloma Leuk. 2018;18:S216. doi: 10.1016/j.clml.2018.07.081. [DOI] [Google Scholar]
- 304.Erickson RI, et al. Bruton’s tyrosine kinase small molecule inhibitors induce a distinct pancreatic toxicity in rats. J. Pharm. Exp. Ther. 2017;360:226–238. doi: 10.1124/jpet.116.236224. [DOI] [PubMed] [Google Scholar]
- 305.Eathiraj S, et al. Targeting ibrutinib-resistant BTK-C481S mutation with ARQ 531, a reversible non-covalent inhibitor of BTK. Clin. Lymphoma Myeloma Leuk. 2016;16:S47–S48. doi: 10.1016/j.clml.2016.07.068. [DOI] [Google Scholar]
- 306.Woyach JA, et al. BTK(C481S)-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J. Clin. Oncol. 2017;35:1437–1443. doi: 10.1200/JCO.2016.70.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Serra López-Matencio JM, Morell Baladrón A, Castañeda S. JAK-STAT inhibitors for the treatment of immunomediated diseases. Med. Clín. 2019;152:353–360. doi: 10.1016/j.medcli.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 308.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Cacalano NA, et al. Autosomal SCID caused by a point mutation in the N-terminus of Jak3: mapping of the Jak3-receptor interaction domain. EMBO J. 1999;18:1549–1558. doi: 10.1093/emboj/18.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Banerjee S, et al. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.O’Shea JJ, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bryan MC, Rajapaksa NS. Kinase inhibitors for the treatment of immunological disorders: recent advances. J. Med. Chem. 2018;61:9030–9058. doi: 10.1021/acs.jmedchem.8b00667. [DOI] [PubMed] [Google Scholar]
- 313.Schwartz DM, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017;16:843–862. doi: 10.1038/nrd.2017.201. [DOI] [PubMed] [Google Scholar]
- 314.Strand V, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res. Ther. 2015;17:362. doi: 10.1186/s13075-015-0880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nat. Rev. Drug Discov. 2012;11:103–104. doi: 10.1038/nrd3652. [DOI] [PubMed] [Google Scholar]
- 316.Jamieson C, et al. Effect of treatment with a JAK2-selective inhibitor, fedratinib, on bone marrow fibrosis in patients with myelofibrosis. J. Transl. Med. 2015;13:294. doi: 10.1186/s12967-015-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhang FQ, et al. JAK2 inhibitor TG101348 overcomes erlotinib-resistance in non-small cell lung carcinoma cells with mutated EGF receptor. Oncotarget. 2015;6:14329–14343. doi: 10.18632/oncotarget.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Gremese E, Ferraccioli G. Tofacitinib for rheumatoid arthritis. Lancet. 2013;381:1812. doi: 10.1016/S0140-6736(13)61114-9. [DOI] [PubMed] [Google Scholar]
- 319.Sanchez GAM, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J. Clin. Invest. 2018;128:3041–3052. doi: 10.1172/JCI98814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Verstovsek S, et al. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin. Cancer Res. 2008;14:788–796. doi: 10.1158/1078-0432.CCR-07-0524. [DOI] [PubMed] [Google Scholar]
- 321.Hexner EO, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Mascarenhas J, et al. Phase II trial of Lestaurtinib, a JAK2 inhibitor, in patients with myelofibrosis. Leuk. Lymphoma. 2019;60:1343–1345. doi: 10.1080/10428194.2018.1532509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mascarenhas JO, et al. Primary analysis of a phase II open-label trial of INCB039110, a selective JAK1 inhibitor, in patients with myelofibrosis. Haematologica. 2017;102:327–335. doi: 10.3324/haematol.2016.151126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yang EG, et al. Design and synthesis of Janus kinase 2 (JAK2) and histone deacetlyase (HDAC) bispecific inhibitors based on pacritinib and evidence of dual pathway inhibition in hematological cell lines. J. Med. Chem. 2016;59:8233–8262. doi: 10.1021/acs.jmedchem.6b00157. [DOI] [PubMed] [Google Scholar]
- 325.Mesa RA, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4:e225–e236. doi: 10.1016/S2352-3026(17)30027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J. Inflamm. Res. 2020;13:519–531. doi: 10.2147/JIR.S219586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Xu P, et al. Janus kinases (JAKs): The efficient therapeutic targets for autoimmune diseases and myeloproliferative disorders. Eur. J. Med. Chem. 2020;192:112155. doi: 10.1016/j.ejmech.2020.112155. [DOI] [PubMed] [Google Scholar]
- 328.Chan LC, et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J. Clin. Invest. 2019;129:3324–3338. doi: 10.1172/JCI126022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Dai Z, Zeng W, Christiano AM. 1098 Efficacy of selective next-generation JAK inhibitors in the treatment of alopecia areata. J. Investig. Dermatol. 2018;138:S186. doi: 10.1016/j.jid.2018.03.1111. [DOI] [Google Scholar]
- 330.Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin. Investig. Drugs. 2014;23:1067–1077. doi: 10.1517/13543784.2014.918604. [DOI] [PubMed] [Google Scholar]
- 331.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Degirmenci, U., Wang, M. & Hu, J. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells. 9, 198 (2020). [DOI] [PMC free article] [PubMed]
- 333.Flaherty KT, McArthur G. BRAF, a target in melanoma: implications for solid tumor drug development. Cancer. 2010;116:4902–4913. doi: 10.1002/cncr.25261. [DOI] [PubMed] [Google Scholar]
- 334.Long GV, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 335.Ostrem JM, et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Liu T, Wang Z, Guo P, Ding N. Electrostatic mechanism of V600E mutation-induced B-Raf constitutive activation in colorectal cancer: molecular implications for the selectivity difference between type-I and type-II inhibitors. Eur. Biophys. J. 2019;48:73–82. doi: 10.1007/s00249-018-1334-y. [DOI] [PubMed] [Google Scholar]
- 337.Tsai J, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl Acad. Sci. USA. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Hauschild A, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 339.Li Z, et al. Encorafenib (LGX818), a potent BRAF inhibitor, induces senescence accompanied by autophagy in BRAFV600E melanoma cells. Cancer Lett. 2016;370:332–344. doi: 10.1016/j.canlet.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 340.Molnar E, et al. Pan-RAF and MEK vertical inhibition enhances therapeutic response in non-V600 BRAF mutant cells. BMC Cancer. 2018;18:542. doi: 10.1186/s12885-018-4455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Saro S, Diwakar D. MEK inhibitors for the treatment of NRAS mutant melanoma. Drug Des. Devel. Ther. 2018;12:2553–2565. doi: 10.2147/DDDT.S131721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Roskoski R., Jr. Targeting oncogenic Raf protein-serine/threonine kinases in human cancers. Pharm. Res. 2018;135:239–258. doi: 10.1016/j.phrs.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 343.Khunger A, Khunger M, Velcheti V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: clinical evidence and experience. Ther. Adv. Respir. Dis. 2018;12:1753466618767611. doi: 10.1177/1753466618767611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Markham A, Keam SJ. Selumetinib: first approval. Drugs. 2020;80:931–937. doi: 10.1007/s40265-020-01331-x. [DOI] [PubMed] [Google Scholar]
- 345.Luebker SA, Koepsell SA. Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front. Oncol. 2019;9:268. doi: 10.3389/fonc.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Al-Olabi L, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Invest. 2018;128:1496–1508. doi: 10.1172/JCI98589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Arend RC, et al. EMR 20006-012: a phase II randomized double-blind placebo controlled trial comparing the combination of pimasertib (MEK inhibitor) with SAR245409 (PI3K inhibitor) to pimasertib alone in patients with previously treated unresectable borderline or low grade ovarian cancer. Gynecol. Oncol. 2020;156:301–307. doi: 10.1016/j.ygyno.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 348.Chenard-Poirier M, et al. Results from the biomarker-driven basket trial of RO5126766 (CH5127566), a potent RAF/MEK inhibitor, in RAS- or RAF-mutated malignancies including multiple myeloma. J. Clin. Oncol. 2017;35:2506–2506. doi: 10.1200/JCO.2017.35.15_suppl.2506. [DOI] [Google Scholar]
- 349.Blake JF, et al. Discovery of (S)-1-(1-(4-Chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-y l)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) Inhibitor in Early Clinical Development. J. Med. Chem. 2016;59:5650–5660. doi: 10.1021/acs.jmedchem.6b00389. [DOI] [PubMed] [Google Scholar]
- 350.Okimoto RA, et al. Preclinical efficacy of a RAF inhibitor that evades paradoxical MAPK pathway activation in protein kinase BRAF-mutant lung cancer. Proc. Natl Acad. Sci. USA. 2016;113:13456–13461. doi: 10.1073/pnas.1610456113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Pelster MS, Amaria RN. Combined targeted therapy and immunotherapy in melanoma: a review of the impact on the tumor microenvironment and outcomes of early clinical trials. Ther. Adv. Med. Oncol. 2019;11:1758835919830826. doi: 10.1177/1758835919830826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kakadia S, et al. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther. 2018;11:7095–7107. doi: 10.2147/OTT.S182721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 354.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 355.Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 356.O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 357.Chen P, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol. Cancer Ther. 2016;15:2273–2281. doi: 10.1158/1535-7163.MCT-16-0300. [DOI] [PubMed] [Google Scholar]
- 358.Anders L, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Choi YJ, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sawai CM, et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell. 2012;22:452–465. doi: 10.1016/j.ccr.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ameratunga M, Kipps E, Okines AFC, Lopez JS. To cycle or fight-CDK4/6 inhibitors at the crossroads of anticancer immunity. Clin. Cancer Res. 2019;25:21–28. doi: 10.1158/1078-0432.CCR-18-1999. [DOI] [PubMed] [Google Scholar]
- 363.Chen X, et al. Latest overview of the cyclin-dependent kinases 4/6 inhibitors in breast cancer: the past, the present and the future. J. Cancer. 2019;10:6608–6617. doi: 10.7150/jca.33079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sanchez-Martinez C, Lallena MJ, Sanfeliciano SG, de Dios A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: recent advances (2015-2019) Bioorg. Med. Chem. Lett. 2019;29:126637. doi: 10.1016/j.bmcl.2019.126637. [DOI] [PubMed] [Google Scholar]
- 365.Laderian B, Fojo T. CDK4/6 Inhibition as a therapeutic strategy in breast cancer: palbociclib, ribociclib, and abemaciclib. Semin. Oncol. 2017;44:395–403. doi: 10.1053/j.seminoncol.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 366.Infante JR, et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2016;22:5696–5705. doi: 10.1158/1078-0432.CCR-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Patnaik A, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6:740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 368.Spring LM, Zangardi ML, Moy B, Bardia A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. Oncologist. 2017;22:1039–1048. doi: 10.1634/theoncologist.2017-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 370.Cristofanilli M, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 371.Sledge GW, Jr., et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 372.Barroso-Sousa R, Shapiro GI, Tolaney SM. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care. 2016;11:167–173. doi: 10.1159/000447284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Goldman JW, et al. Treatment Rationale and Study Design for the JUNIPER Study: a randomized phase III study of abemaciclib with best supportive care versus erlotinib with best supportive care in patients with stage IV non-small-cell lung cancer with a detectable KRAS mutation whose disease has progressed after platinum-based chemotherapy. Clin. Lung Cancer. 2016;17:80–84. doi: 10.1016/j.cllc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 374.Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin. Cancer Res. 2017;23:3251–3262. doi: 10.1158/1078-0432.CCR-16-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.He, S. et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Science Transl. Med.9, eaal3986 (2017). [DOI] [PMC free article] [PubMed]
- 376.Wang J, et al. Critical roles of conventional dendritic cells in promoting T cell-dependent hepatitis through regulating natural killer T cells. Clin. Exp. Immunol. 2017;188:127–137. doi: 10.1111/cei.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kim WS, et al. 5’-OH-5-nitro-indirubin oxime (AGM130), an indirubin derivative, induces apoptosis of Imatinib-resistant chronic myeloid leukemia cells. Leuk. Res. 2013;37:427–433. doi: 10.1016/j.leukres.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 378.Wang Y, et al. Discovery of 4-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-N-(4-((4-methylpiperazin-1-yl)methyl)p henyl)-1H-pyrazole-3-carboxamide (FN-1501), an FLT3- and CDK-kinase inhibitor with potentially high efficiency against acute myelocytic leukemia. J. Med Chem. 2018;61:1499–1518. doi: 10.1021/acs.jmedchem.7b01261. [DOI] [PubMed] [Google Scholar]
- 379.Hazel P, et al. Corrigendum: inhibitor selectivity for cyclin-dependent kinase7: a structural, thermodynamic, and modelling study. ChemMedChem. 2018;13:207. doi: 10.1002/cmdc.201700826. [DOI] [PubMed] [Google Scholar]
- 380.Hu S, et al. Discovery and characterization of SY-1365, a selective, covalent inhibitor of CDK7. Cancer Res. 2019;79:3479–3491. doi: 10.1158/0008-5472.CAN-19-0119. [DOI] [PubMed] [Google Scholar]
- 381.Sielecki TM, Boylan JF, Benfield PA, Trainor GL. Cyclin-dependent kinase inhibitors: useful targets in cell cycle regulation. J. Med. Chem. 2000;43:1–18. doi: 10.1021/jm990256j. [DOI] [PubMed] [Google Scholar]
- 382.Condorelli R, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 2018;29:640–645. doi: 10.1093/annonc/mdx784. [DOI] [PubMed] [Google Scholar]
- 383.Herrera-Abreu MT, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Caldon CE, et al. Cyclin E2 overexpression is associated with endocrine resistance but not insensitivity to CDK2 inhibition in human breast cancer cells. Mol. Cancer Ther. 2012;11:1488–1499. doi: 10.1158/1535-7163.MCT-11-0963. [DOI] [PubMed] [Google Scholar]
- 385.Spring LM, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395:817–827. doi: 10.1016/S0140-6736(20)30165-3. [DOI] [PubMed] [Google Scholar]
- 386.Bilanges B, Posor Y, Vanhaesebroeck B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 2019;20:515–534. doi: 10.1038/s41580-019-0129-z. [DOI] [PubMed] [Google Scholar]
- 387.Jean S, Kiger AA. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014;127:923–928. doi: 10.1242/jcs.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Fruman DA, et al. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Fresno Vara JA, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 390.Liu N, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 391.Vangapandu HV, Jain N, Gandhi V. Duvelisib: a phosphoinositide-3 kinase delta/gamma inhibitor for chronic lymphocytic leukemia. Expert Opin. Investig. Drugs. 2017;26:625–632. doi: 10.1080/13543784.2017.1312338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Vangapandu HV, et al. B-cell receptor signaling regulates metabolism in chronic lymphocytic leukemia. Mol. Cancer Res. 2017;15:1692–1703. doi: 10.1158/1541-7786.MCR-17-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.James A, et al. Absorption, distribution, metabolism, and excretion of [(14)C]BYL719 (alpelisib) in healthy male volunteers. Cancer Chemother. Pharm. 2015;76:751–760. doi: 10.1007/s00280-015-2842-4. [DOI] [PubMed] [Google Scholar]
- 394.Foster P, et al. The selective PI3K inhibitor XL147 (SAR245408) inhibits tumor growth and survival and potentiates the activity of chemotherapeutic agents in preclinical tumor models. Mol. Cancer Ther. 2015;14:931–940. doi: 10.1158/1535-7163.MCT-14-0833. [DOI] [PubMed] [Google Scholar]
- 395.Yaguchi S, et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J. Natl Cancer Inst. 2006;98:545–556. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- 396.Evans CA, et al. Discovery of a selective phosphoinositide-3-kinase (PI3K)-gamma inhibitor (IPI-549) as an immuno-oncology clinical candidate. ACS Med. Chem. Lett. 2016;7:862–867. doi: 10.1021/acsmedchemlett.6b00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Juric D, et al. A first-in-human, phase I, dose-escalation study of TAK-117, a selective PI3Kalpha isoform inhibitor, in patients with advanced solid malignancies. Clin. Cancer Res. 2017;23:5015–5023. doi: 10.1158/1078-0432.CCR-16-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Serra V, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 399.Chang KY, et al. Novel phosphoinositide 3-kinase/mTOR dual inhibitor, NVP-BGT226, displays potent growth-inhibitory activity against human head and neck cancer cells in vitro and in vivo. Clin. Cancer Res. 2011;17:7116–7126. doi: 10.1158/1078-0432.CCR-11-0796. [DOI] [PubMed] [Google Scholar]
- 400.Lin J, et al. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin. Cancer Res. 2013;19:1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 401.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 402.McKenna M, McGarrigle S, Pidgeon GP. The next generation of PI3K-Akt-mTOR pathway inhibitors in breast cancer cohorts. Biochim. Biophys. Rev. Cancer. 2018;1870:185–197. doi: 10.1016/j.bbcan.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 403.Schmid P, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: The PAKT Trial. J. Clin. Oncol. 2020;38:423–433. doi: 10.1200/JCO.19.00368. [DOI] [PubMed] [Google Scholar]
- 404.Spencer A, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014;124:2190–2195. doi: 10.1182/blood-2014-03-559963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Tolcher AW, et al. Phase I dose-escalation trial of the oral AKT inhibitor uprosertib in combination with the oral MEK1/MEK2 inhibitor trametinib in patients with solid tumors. Cancer Chemother. Pharm. 2020;85:673–683. doi: 10.1007/s00280-020-04038-8. [DOI] [PubMed] [Google Scholar]
- 406.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 407.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Hsieh AC, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Basu B, et al. First-in-human pharmacokinetic and pharmacodynamic study of the dual m-TORC 1/2 inhibitor AZD2014. Clin. Cancer Res. 2015;21:3412–3419. doi: 10.1158/1078-0432.CCR-14-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Mosedale M, et al. Identification of candidate risk factor genes for human idelalisib toxicity using a collaborative cross approach. Toxicol. Sci. 2019;172:265–278. doi: 10.1093/toxsci/kfz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Tarakhovsky A. Tools and landscapes of epigenetics. Nat. Immunol. 2010;11:565–568. doi: 10.1038/ni0710-565. [DOI] [PubMed] [Google Scholar]
- 412.Cheng Y, et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct. Target Ther. 2019;4:62. doi: 10.1038/s41392-019-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J. Hematol. Oncol. 2020;13:104. doi: 10.1186/s13045-020-00937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yang YX, et al. Therapeutic potential of enhancer of zeste homolog 2 in autoimmune diseases. Expert Opin. Ther. Targets. 2019;23:1015–1030. doi: 10.1080/14728222.2019.1696309. [DOI] [PubMed] [Google Scholar]
- 415.Wu X, et al. Increased EZH2 expression in prostate cancer is associated with metastatic recurrence following external beam radiotherapy. Prostate. 2019;79:1079–1089. doi: 10.1002/pros.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Bai Y, et al. Inhibition of enhancer of zeste homolog 2 (EZH2) overcomes enzalutamide resistance in castration-resistant prostate cancer. J. Biol. Chem. 2019;294:9911–9923. doi: 10.1074/jbc.RA119.008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Jones BA, Varambally S, Arend RC. Histone methyltransferase EZH2: A Therapeutic Target For Ovarian Cancer. Mol. Cancer Ther. 2018;17:591–602. doi: 10.1158/1535-7163.MCT-17-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Jia N, et al. Enhancer of zeste homolog 2 is involved in the proliferation of endometrial carcinoma. Oncol. Lett. 2014;8:2049–2054. doi: 10.3892/ol.2014.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Puppe J, et al. EZH2 is overexpressed in BRCA1-like breast tumors and predictive for sensitivity to high-dose platinum-based chemotherapy. Clin. Cancer Res. 2019;25:4351–4362. doi: 10.1158/1078-0432.CCR-18-4024. [DOI] [PubMed] [Google Scholar]
- 420.Mahmoud F, et al. Role of EZH2 histone methyltrasferase in melanoma progression and metastasis. Cancer Biol. Ther. 2016;17:579–591. doi: 10.1080/15384047.2016.1167291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Sashida G, Iwama A. Multifaceted role of the polycomb-group gene EZH2 in hematological malignancies. Int J. Hematol. 2017;105:23–30. doi: 10.1007/s12185-016-2124-x. [DOI] [PubMed] [Google Scholar]
- 422.Italiano A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19:649–659. doi: 10.1016/S1470-2045(18)30145-1. [DOI] [PubMed] [Google Scholar]
- 423.Lue JK, Amengual JE. Emerging EZH2 inhibitors and their application in lymphoma. Curr. Hematol. Malig. Rep. 2018;13:369–382. doi: 10.1007/s11899-018-0466-6. [DOI] [PubMed] [Google Scholar]
- 424.Danis E, et al. Ezh2 controls an early hematopoietic program and growth and survival signaling in early T cell precursor acute lymphoblastic leukemia. Cell Rep. 2016;14:1953–1965. doi: 10.1016/j.celrep.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Miranda TB, et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol. Cancer Ther. 2009;8:1579–1588. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Yap DB, et al. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood. 2011;117:2451–2459. doi: 10.1182/blood-2010-11-321208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Bodor C, et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013;122:3165–3168. doi: 10.1182/blood-2013-04-496893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Qi W, et al. An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED. Nat. Chem. Biol. 2017;13:381–388. doi: 10.1038/nchembio.2304. [DOI] [PubMed] [Google Scholar]
- 429.He Y, et al. The EED protein-protein interaction inhibitor A-395 inactivates the PRC2 complex. Nat. Chem. Biol. 2017;13:389–395. doi: 10.1038/nchembio.2306. [DOI] [PubMed] [Google Scholar]
- 430.Dong H, et al. An allosteric PRC2 inhibitor targeting EED suppresses tumor progression by modulating the immune response. Cancer Res. 2019;79:5587–5596. doi: 10.1158/0008-5472.CAN-19-0428. [DOI] [PubMed] [Google Scholar]
- 431.Potjewyd F, et al. Degradation of polycomb repressive complex 2 with an EED-targeted bivalent chemical degrader. Cell Chem. Biol. 2020;27:47–56.e15. doi: 10.1016/j.chembiol.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Wang X, et al. A covalently bound inhibitor triggers EZH2 degradation through CHIP-mediated ubiquitination. EMBO J. 2017;36:1243–1260. doi: 10.15252/embj.201694058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Bisserier M, Wajapeyee N. Mechanisms of resistance to EZH2 inhibitors in diffuse large B-cell lymphomas. Blood. 2018;131:2125–2137. doi: 10.1182/blood-2017-08-804344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Wu S, et al. SWI/SNF catalytic subunits’ switch drives resistance to EZH2 inhibitors in ARID1A-mutated cells. Nat. Commun. 2018;9:4116. doi: 10.1038/s41467-018-06656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Huang X, et al. Targeting epigenetic crosstalk as a therapeutic strategy for EZH2-aberrant solid tumors. Cell. 2018;175:186–199 e119. doi: 10.1016/j.cell.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 436.Gibaja V, et al. Development of secondary mutations in wild-type and mutant EZH2 alleles cooperates to confer resistance to EZH2 inhibitors. Oncogene. 2016;35:558–566. doi: 10.1038/onc.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Tremblay-LeMay R, Rastgoo N, Pourabdollah M, Chang H. EZH2 as a therapeutic target for multiple myeloma and other haematological malignancies. Biomark. Res. 2018;6:34. doi: 10.1186/s40364-018-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zhang Y, et al. Combination of EZH2 inhibitor and BET inhibitor for treatment of diffuse intrinsic pontine glioma. Cell Biosci. 2017;7:56. doi: 10.1186/s13578-017-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Lue JK, et al. Precision targeting with EZH2 and HDAC inhibitors in epigenetically dysregulated lymphomas. Clin. Cancer Res. 2019;25:5271–5283. doi: 10.1158/1078-0432.CCR-18-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Richart L, Margueron R. Drugging histone methyltransferases in cancer. Curr. Opin. Chem. Biol. 2020;56:51–62. doi: 10.1016/j.cbpa.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 441.Park SY, Kim JS. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020;52:204–212. doi: 10.1038/s12276-020-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin. Epigenet. 2012;4:5. doi: 10.1186/1868-7083-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Li, Y. & Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 6, a026831 (2016). [DOI] [PMC free article] [PubMed]
- 444.Eckschlager, T., Plch, J., Stiborova, M. & Hrabeta, J. Histone deacetylase inhibitors as anticancer drugs. Int. J. Mol. Sci. 18, 1414 (2017). [DOI] [PMC free article] [PubMed]
- 445.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 446.Mann BS, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin. Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 447.Lee HZ, et al. FDA approval: belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin. Cancer Res. 2015;21:2666–2670. doi: 10.1158/1078-0432.CCR-14-3119. [DOI] [PubMed] [Google Scholar]
- 448.Yee AJ, Raje NS. Panobinostat and multiple myeloma in 2018. Oncologist. 2018;23:516–517. doi: 10.1634/theoncologist.2017-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Piekarz RL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Xu Y, Zhang P, Liu Y. Chidamide tablets: HDAC inhibition to treat lymphoma. Drugs Today. 2017;53:167–176. doi: 10.1358/dot.2017.53.3.2595452. [DOI] [PubMed] [Google Scholar]
- 451.Cea M, et al. Synergistic interactions between HDAC and sirtuin inhibitors in human leukemia cells. PLoS ONE. 2011;6:e22739. doi: 10.1371/journal.pone.0022739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Suraweera A, O’Byrne KJ, Richard DJ. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front. Oncol. 2018;8:92. doi: 10.3389/fonc.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Pan D, Lu X. New therapeutic avenue of epigenetic modulations in cancer. Transl. Breast Cancer Res. 2020;1:2–2. doi: 10.21037/tbcr.2020.03.03. [DOI] [Google Scholar]
- 454.Abaza YM, et al. Phase 1 dose escalation multicenter trial of pracinostat alone and in combination with azacitidine in patients with advanced hematologic malignancies. Cancer. 2017;123:4851–4859. doi: 10.1002/cncr.30949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Qin HT, Li HQ, Liu F. Selective histone deacetylase small molecule inhibitors: recent progress and perspectives. Expert Opin. Ther. Pat. 2017;27:621–636. doi: 10.1080/13543776.2017.1276565. [DOI] [PubMed] [Google Scholar]
- 456.Kyaw MTH, et al. The HDAC inhibitor, SAHA, combined with cisplatin synergistically induces apoptosis in alpha-fetoprotein-producing hepatoid adenocarcinoma cells. Acta Histochem. Cytochem. 2019;52:1–8. doi: 10.1267/ahc.18044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Vitkeviciene A, et al. HDAC and HMT inhibitors in combination with conventional therapy: a novel treatment option for acute promyelocytic leukemia. J. Oncol. 2019;2019:6179573. doi: 10.1155/2019/6179573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Gray J, Cubitt CL, Zhang S, Chiappori A. Combination of HDAC and topoisomerase inhibitors in small cell lung cancer. Cancer Biol. Ther. 2012;13:614–622. doi: 10.4161/cbt.19848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Zhang Y, et al. Combined HDAC and bromodomain protein inhibition reprograms tumor cell metabolism and elicits synthetic lethality in glioblastoma. Clin. Cancer Res. 2018;24:3941–3954. doi: 10.1158/1078-0432.CCR-18-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Banik, D., Moufarrij, S. & Villagra, A. Immunoepigenetics combination therapies: an overview of the role of HDACs in cancer immunotherapy. Int. J. Mol. Sci. 20, 2241 (2019). [DOI] [PMC free article] [PubMed]
- 461.Stoddard BL, Dean A, Koshland DE., Jr. Structure of isocitrate dehydrogenase with isocitrate, nicotinamide adenine dinucleotide phosphate, and calcium at 2.5-A resolution: a pseudo-Michaelis ternary complex. Biochemistry. 1993;32:9310–9316. doi: 10.1021/bi00087a008. [DOI] [PubMed] [Google Scholar]
- 462.Xu X, et al. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J. Biol. Chem. 2004;279:33946–33957. doi: 10.1074/jbc.M404298200. [DOI] [PubMed] [Google Scholar]
- 463.Henderson NS. Isozymes of isocitrate dehydrogenase: subunit structure and intracellular location. J. Exp. Zool. 1965;158:263–273. doi: 10.1002/jez.1401580303. [DOI] [PubMed] [Google Scholar]
- 464.Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin. Cancer Res. 2012;18:5562–5571. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Amary MF, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 466.Ganguly BB, Kadam NN. Mutations of myelodysplastic syndromes (MDS): an update. Mutat. Res Rev. Mutat. Res. 2016;769:47–62. doi: 10.1016/j.mrrev.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 467.Farshidfar F, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Gross S, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J. Exp. Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Ward PS, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Prensner JR, Chinnaiyan AM. Metabolism unhinged: IDH mutations in cancer. Nat. Med. 2011;17:291–293. doi: 10.1038/nm0311-291. [DOI] [PubMed] [Google Scholar]
- 472.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends Mol. Med. 2010;16:387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 473.Kim ES. Enasidenib: first global approval. Drugs. 2017;77:1705–1711. doi: 10.1007/s40265-017-0813-2. [DOI] [PubMed] [Google Scholar]
- 474.Stein EMEnasidenib. a targeted inhibitor of mutant IDH2 proteins for treatment of relapsed or refractory acute myeloid leukemia. Future Oncol. 2018;14:23–40. doi: 10.2217/fon-2017-0392. [DOI] [PubMed] [Google Scholar]
- 475.Yen K, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7:478–493. doi: 10.1158/2159-8290.CD-16-1034. [DOI] [PubMed] [Google Scholar]
- 476.Galkin M, Jonas BA. Enasidenib in the treatment of relapsed/refractory acute myeloid leukemia: an evidence-based review of its place in therapy. Core Evid. 2019;14:3–17. doi: 10.2147/CE.S172912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Popovici-Muller J, et al. Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med. Chem. Lett. 2018;9:300–305. doi: 10.1021/acsmedchemlett.7b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.DiNardo CD, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med. 2018;378:2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 479.Norsworthy KJ, et al. FDA approval summary: ivosidenib for relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase-1 mutation. Clin. Cancer Res. 2019;25:3205–3209. doi: 10.1158/1078-0432.CCR-18-3749. [DOI] [PubMed] [Google Scholar]
- 480.Duggan S. Caplacizumab: first global approval. Drugs. 2018;78:1639–1642. doi: 10.1007/s40265-018-0989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Feliciano P. PAK inhibitor in fragile X. Nat. Genet. 2013;45:477–477. [Google Scholar]
- 482.de Botton S. Targeting isocitrate dehydrogenase (IDH)1 and IDH2 mutations clinical results in advanced hematologic malignancies. Ann. Oncol. 2015;26:ii15. doi: 10.1093/annonc/mdv089.3. [DOI] [Google Scholar]
- 483.Ma T, et al. Inhibitors of mutant isocitrate dehydrogenases 1 and 2 (mIDH1/2): an update and perspective. J. Med. Chem. 2018;61:8981–9003. doi: 10.1021/acs.jmedchem.8b00159. [DOI] [PubMed] [Google Scholar]
- 484.Chaturvedi A, et al. Pan-mutant-IDH1 inhibitor BAY1436032 is highly effective against human IDH1 mutant acute myeloid leukemia in vivo. Leukemia. 2017;31:2020–2028. doi: 10.1038/leu.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Caravella JA, et al. Structure-based design and identification of FT-2102 (olutasidenib), a potent mutant-selective IDH1 inhibitor. J. Med. Chem. 2020;63:1612–1623. doi: 10.1021/acs.jmedchem.9b01423. [DOI] [PubMed] [Google Scholar]
- 486.Cho YS, et al. Discovery and evaluation of clinical candidate IDH305, a brain penetrant mutant IDH1 inhibitor. ACS Med. Chem. Lett. 2017;8:1116–1121. doi: 10.1021/acsmedchemlett.7b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.DiNardo CD, Stein EM. SOHO state of the art update and next questions: IDH therapeutic targeting in AML. Clin. Lymphoma Myeloma Leuk. 2018;18:769–772. doi: 10.1016/j.clml.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 488.Harding JJ, et al. Isoform switching as a mechanism of acquired resistance to mutant isocitrate dehydrogenase inhibition. Cancer Discov. 2018;8:1540–1547. doi: 10.1158/2159-8290.CD-18-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Intlekofer AM, et al. Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations. Nature. 2018;559:125–129. doi: 10.1038/s41586-018-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Medeiros BC, et al. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;31:272–281. doi: 10.1038/leu.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Fujiwara H, et al. Isocitrate dehydrogenase 1 mutation sensitizes intrahepatic cholangiocarcinoma to the BET inhibitor JQ1. Cancer Sci. 2018;109:3602–3610. doi: 10.1111/cas.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.DiNardo CD, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Chaturvedi, A. et al. Synergistic activity of IDH1 inhibitor BAY1436032 with azacitidine in IDH1 mutant acute myeloid leukemia. Haematologica106, 565–573 (2020). [DOI] [PMC free article] [PubMed]
- 494.Kantarjian H, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 495.Silverman LR, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J. Clin. Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 496.Olino K, Park T, Ahuja N. Exposing hidden targets: combining epigenetic and immunotherapy to overcome cancer resistance. Semin. Cancer Biol. 2020;65:114–122. doi: 10.1016/j.semcancer.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 497.Bates SE. Epigenetic therapies for cancer. N. Engl. J. Med. 2020;383:650–663. doi: 10.1056/NEJMra1805035. [DOI] [PubMed] [Google Scholar]
- 498.Ghasemi S. Cancer’s epigenetic drugs: where are they in the cancer medicines? Pharmacogenomics J. 2020;20:367–379. doi: 10.1038/s41397-019-0138-5. [DOI] [PubMed] [Google Scholar]
- 499.Laubach JP, Moreau P, San-Miguel JF, Richardson PG. Panobinostat for the treatment of multiple myeloma. Clin. Cancer Res. 2015;21:4767–4773. doi: 10.1158/1078-0432.CCR-15-0530. [DOI] [PubMed] [Google Scholar]
- 500.Knight T, et al. A delicate balance - The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem. Pharm. 2019;162:250–261. doi: 10.1016/j.bcp.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 501.Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Huang K, et al. BH3-only proteins target BCL-xL/MCL-1, not BAX/BAK, to initiate apoptosis. Cell Res. 2019;29:942–952. doi: 10.1038/s41422-019-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J. Gastroenterol. 2009;15:513–520. doi: 10.3748/wjg.15.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Schattenberg JM, Schuchmann M, Galle PR. Cell death and hepatocarcinogenesis: dysregulation of apoptosis signaling pathways. J. Gastroenterol. Hepatol. 2011;26:213–219. doi: 10.1111/j.1440-1746.2010.06582.x. [DOI] [PubMed] [Google Scholar]
- 505.Perini GF, et al. BCL-2 as therapeutic target for hematological malignancies. J. Hematol. Oncol. 2018;11:65. doi: 10.1186/s13045-018-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017;16:273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 507.Zhu Y, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Lampson BL, Davids MS. The development and current use of BCL-2 inhibitors for the treatment of chronic lymphocytic leukemia. Curr. Hematol. Malig. Rep. 2017;12:11–19. doi: 10.1007/s11899-017-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Anderson MA, Huang D, Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin. Hematol. 2014;51:219–227. doi: 10.1053/j.seminhematol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 510.Valentin R, Grabow S, Davids MS. The rise of apoptosis: targeting apoptosis in hematologic malignancies. Blood. 2018;132:1248–1264. doi: 10.1182/blood-2018-02-791350. [DOI] [PubMed] [Google Scholar]
- 511.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 512.Stilgenbauer S, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 513.Deeks ED. Venetoclax: first global approval. Drugs. 2016;76:979–987. doi: 10.1007/s40265-016-0596-x. [DOI] [PubMed] [Google Scholar]
- 514.Guerra VA, DiNardo C, Konopleva M. Venetoclax-based therapies for acute myeloid leukemia. Best Pr. Res. Clin. Haematol. 2019;32:145–153. doi: 10.1016/j.beha.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Caenepeel S, et al. AMG 176, a selective MCL1 inhibitor, is effective in hematologic cancer models alone and in combination with established therapies. Cancer Discov. 2018;8:1582–1597. doi: 10.1158/2159-8290.CD-18-0387. [DOI] [PubMed] [Google Scholar]
- 516.Casara P, et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget. 2018;9:20075–20088. doi: 10.18632/oncotarget.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.McBride A, et al. The role of inhibition of apoptosis in acute leukemias and myelodysplastic syndrome. Front. Oncol. 2019;9:192. doi: 10.3389/fonc.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Tron AE, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 2018;9:5341. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Yalniz FF, Wierda WG. Targeting BCL2 in chronic lymphocytic leukemia and other hematologic malignancies. Drugs. 2019;79:1287–1304. doi: 10.1007/s40265-019-01163-4. [DOI] [PubMed] [Google Scholar]
- 520.Yang S, et al. The chemical biology of apoptosis: revisited after 17 years. Eur. J. Med. Chem. 2019;177:63–75. doi: 10.1016/j.ejmech.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 521.Aldoss I, et al. Venetoclax and hypomethylating agents in TP53-mutated acute myeloid leukaemia. Br. J. Haematol. 2019;187:e45–e48. doi: 10.1111/bjh.16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Villalobos-Ortiz M, et al. BH3 profiling discriminates on-target small molecule BH3 mimetics from putative mimetics. Cell Death Differ. 2020;27:999–1007. doi: 10.1038/s41418-019-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Thomas S, et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets. 2013;17:61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Tahir SK, et al. Potential mechanisms of resistance to venetoclax and strategies to circumvent it. BMC Cancer. 2017;17:399. doi: 10.1186/s12885-017-3383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Lok SW, et al. A phase Ib dose-escalation and expansion study of the BCL2 inhibitor venetoclax combined with tamoxifen in ER and BCL2-positive metastatic breast cancer. Cancer Discov. 2019;9:354–369. doi: 10.1158/2159-8290.CD-18-1151. [DOI] [PubMed] [Google Scholar]
- 526.Pak E, Segal RA. Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev. Cell. 2016;38:333–344. doi: 10.1016/j.devcel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Tukachinsky H, Petrov K, Watanabe M, Salic A. Mechanism of inhibition of the tumor suppressor Patched by Sonic Hedgehog. Proc. Natl Acad. Sci. USA. 2016;113:E5866–e5875. doi: 10.1073/pnas.1606719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat. Rev. Drug Discov. 2012;11:437–438. doi: 10.1038/nrd3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Jain S, Song R, Xie J. Sonidegib: mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017;10:1645–1653. doi: 10.2147/OTT.S130910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Basset-Seguin N, et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer. 2017;86:334–348. doi: 10.1016/j.ejca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 531.Lear JT, et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J. Eur. Acad. Dermatol Venereol. 2018;32:372–381. doi: 10.1111/jdv.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Sekulic A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.LoRusso PM, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin. Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Stathis A, et al. Phase I trial of the oral smoothened inhibitor sonidegib in combination with paclitaxel in patients with advanced solid tumors. Invest. N. Drugs. 2017;35:766–772. doi: 10.1007/s10637-017-0454-z. [DOI] [PubMed] [Google Scholar]
- 535.Li Y, Song Q, Day BW, Phase I. and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: a systemic review and meta-analysis. Acta Neuropathol. Commun. 2019;7:123. doi: 10.1186/s40478-019-0773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Jamieson C, Martinelli G, Papayannidis C, Cortes JE. Hedgehog pathway inhibitors: a new therapeutic class for the treatment of acute myeloid leukemia. Blood Cancer Discov. 2020;1:134–145. doi: 10.1158/2643-3230.BCD-20-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Xie, H., Paradise, B. D., Ma, W. W. & Fernandez-Zapico, M. E. Recent advances in the clinical targeting of Hedgehog/GLI signaling in cancer. Cells. 8, 394 (2019). [DOI] [PMC free article] [PubMed]
- 538.Pietrobono, S. & Stecca, B. Targeting the oncoprotein smoothened by small molecules: focus on novel acylguanidine derivatives as potent smoothened inhibitors. Cells7, 272 (2018). [DOI] [PMC free article] [PubMed]
- 539.Suzman DL, Antonarakis ES. Clinical implications of hedgehog pathway signaling in prostate cancer. Cancers. 2015;7:1983–1993. doi: 10.3390/cancers7040871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Cortes JE, Gutzmer R, Kieran MW, Solomon JA. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat. Rev. 2019;76:41–50. doi: 10.1016/j.ctrv.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 541.Girardi, D., Barrichello, A., Fernandes, G. & Pereira, A. Targeting the Hedgehog pathway in cancer: current evidence and future perspectives. Cells8, 153 (2019). [DOI] [PMC free article] [PubMed]
- 542.Bhateja, P., Cherian, M., Majumder, S. & Ramaswamy, B. The Hedgehog signaling pathway: a viable target in breast cancer? Cancers11, 1126 (2019). [DOI] [PMC free article] [PubMed]
- 543.Kim J, et al. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc. Natl Acad. Sci. USA. 2010;107:13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Liu, X. et al. Development of hedgehog pathway inhibitors by epigenetically targeting GLI through BET bromodomain for the treatment of medulloblastoma. Acta Pharm. Sin. B11, 488–504 (2020). [DOI] [PMC free article] [PubMed]
- 545.Tu J, et al. Molecular modeling study on resistance of WT/D473H SMO to antagonists LDE-225 and LEQ-506. Pharm. Res. 2018;129:491–499. doi: 10.1016/j.phrs.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 546.Ueno H, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest. N. Drugs. 2018;36:647–656. doi: 10.1007/s10637-017-0544-y. [DOI] [PubMed] [Google Scholar]
- 547.Dong X, Wang C, Chen Z, Zhao W. Overcoming the resistance mechanisms of smoothened inhibitors. Drug Discov. Today. 2018;23:704–710. doi: 10.1016/j.drudis.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 548.Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 2003;29:3–9. doi: 10.1016/S0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 549.Burger AM, Seth AK. The ubiquitin-mediated protein degradation pathway in cancer: therapeutic implications. Eur. J. Cancer. 2004;40:2217–2229. doi: 10.1016/j.ejca.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 550.Kodroń, A., Mussulini, B. H., Pilecka, I. & Chacińska, A. The ubiquitin-proteasome system and its crosstalk with mitochondria as therapeutic targets in medicine. Pharmacol Res.163, 105248 (2020). [DOI] [PubMed]
- 551.Adams J. The proteasome: a suitable antineoplastic target. Nat. Rev. Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 552.Gandolfi S, et al. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36:561–584. doi: 10.1007/s10555-017-9707-8. [DOI] [PubMed] [Google Scholar]
- 553.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Investig. 2004;22:304–311. doi: 10.1081/CNV-120030218. [DOI] [PubMed] [Google Scholar]
- 554.Fricker LD. Proteasome inhibitor drugs. Annu. Rev. Pharmacol. Toxicol. 2020;60:457–476. doi: 10.1146/annurev-pharmtox-010919-023603. [DOI] [PubMed] [Google Scholar]
- 555.Dimopoulos MA, Richardson PG, Moreau P, Anderson KC. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat. Rev. Clin. Oncol. 2015;12:42–54. doi: 10.1038/nrclinonc.2014.200. [DOI] [PubMed] [Google Scholar]
- 556.Dick LR, Fleming PE. Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov. Today. 2010;15:243–249. doi: 10.1016/j.drudis.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 557.Herndon TM, et al. U.s. Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res. 2013;19:4559–4563. doi: 10.1158/1078-0432.CCR-13-0755. [DOI] [PubMed] [Google Scholar]
- 558.Demo SD, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 559.Zanwar S, Abeykoon JP, Kapoor P. Ixazomib: a novel drug for multiple myeloma. Expert Rev. Hematol. 2018;11:761–771. doi: 10.1080/17474086.2018.1518129. [DOI] [PubMed] [Google Scholar]
- 560.Kupperman E, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 561.Xie J, et al. Ixazomib - the first oral proteasome inhibitor. Leuk. Lymphoma. 2019;60:610–618. doi: 10.1080/10428194.2018.1523398. [DOI] [PubMed] [Google Scholar]
- 562.Groll M, Huber R, Potts BC. Crystal structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J. Am. Chem. Soc. 2006;128:5136–5141. doi: 10.1021/ja058320b. [DOI] [PubMed] [Google Scholar]
- 563.Ma L, Diao A. Marizomib, a potent second generation proteasome inhibitor from natural origin. Anti-Cancer Agents Med. Chem. 2015;15:298–306. doi: 10.2174/1871520614666141114202606. [DOI] [PubMed] [Google Scholar]
- 564.Zhou HJ, et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047) J. Med. Chem. 2009;52:3028–3038. doi: 10.1021/jm801329v. [DOI] [PubMed] [Google Scholar]
- 565.Piva R, et al. CEP-18770: a novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood. 2008;111:2765–2775. doi: 10.1182/blood-2007-07-100651. [DOI] [PubMed] [Google Scholar]
- 566.Gallerani E, et al. A first in human phase I study of the proteasome inhibitor CEP-18770 in patients with advanced solid tumours and multiple myeloma. Eur. J. Cancer. 2013;49:290–296. doi: 10.1016/j.ejca.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 567.Vogl DT, et al. Phase I/II study of the novel proteasome inhibitor delanzomib (CEP-18770) for relapsed and refractory multiple myeloma. Leuk. Lymphoma. 2017;58:1872–1879. doi: 10.1080/10428194.2016.1263842. [DOI] [PubMed] [Google Scholar]
- 568.Park JE, et al. Next-generation proteasome inhibitors for cancer therapy. Transl. Res. 2018;198:1–16. doi: 10.1016/j.trsl.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Dolloff NGEmerging. Therapeutic strategies for overcoming proteasome inhibitor resistance. Adv. Cancer Res. 2015;127:191–226. doi: 10.1016/bs.acr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 570.Caldecott KW. Protein ADP-ribosylation and the cellular response to DNA strand breaks. DNA Repair. 2014;19:108–113. doi: 10.1016/j.dnarep.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 571.Vyas S, Chang P. New PARP targets for cancer therapy. Nat. Rev. Cancer. 2014;14:502–509. doi: 10.1038/nrc3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Dobzhansky T. Genetics of natural populations; recombination and variability in populations of Drosophila pseudoobscura. Genetics. 1946;31:269–290. doi: 10.1093/genetics/31.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.King MC, Marks JH, Mandell JB, New York Breast Cancer Study, Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 574.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Kim G, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin. Cancer Res. 2015;21:4257–4261. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 576.Dockery LE, Gunderson CC, Moore KN. Rucaparib: the past, present, and future of a newly approved PARP inhibitor for ovarian cancer. Onco Targets Ther. 2017;10:3029–3037. doi: 10.2147/OTT.S114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Essel KG, Moore KN. Niraparib for the treatment of ovarian cancer. Expert Rev. Anticancer Ther. 2018;18:727–733. doi: 10.1080/14737140.2018.1490180. [DOI] [PubMed] [Google Scholar]
- 578.Hoy SM. Talazoparib: first global approval. Drugs. 2018;78:1939–1946. doi: 10.1007/s40265-018-1026-z. [DOI] [PubMed] [Google Scholar]
- 579.Strom CE, et al. Poly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediate. Nucleic Acids Res. 2011;39:3166–3175. doi: 10.1093/nar/gkq1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Pujade-Lauraine E, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 581.Konstantinopoulos, P. A. et al. Single-Arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 5, 1141–1149 (2019). [DOI] [PMC free article] [PubMed]
- 582.Sachdev E, et al. PARP inhibition in cancer: an update on clinical development. Target Oncol. 2019;14:657–679. doi: 10.1007/s11523-019-00680-2. [DOI] [PubMed] [Google Scholar]
- 583.Mateo J, et al. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019;30:1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Donawho CK, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin. Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 585.Coleman RL, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 2019;381:2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Moore K, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 587.Jiang X, et al. PARP inhibitors in ovarian cancer: Sensitivity prediction and resistance mechanisms. J. Cell Mol. Med. 2019;23:2303–2313. doi: 10.1111/jcmm.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.D’Andrea AD. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair. 2018;71:172–176. doi: 10.1016/j.dnarep.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 589.Haynes B, Murai J, Lee JM. Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition. Cancer Treat. Rev. 2018;71:1–7. doi: 10.1016/j.ctrv.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Carrassa L, Colombo I, Damia G, Bertoni F. Targeting the DNA damage response for patients with lymphoma: Preclinical and clinical evidences. Cancer Treat. Rev. 2020;90:102090. doi: 10.1016/j.ctrv.2020.102090. [DOI] [PubMed] [Google Scholar]
- 591.Burgess, B. T. et al. Olaparib combined with an ATR or Chk1 inhibitor as a treatment strategy for acquired olaparib-resistant BRCA1 mutant ovarian cells. Diagnostics10, 121 (2020). [DOI] [PMC free article] [PubMed]
- 592.Awada A, et al. An open-label, dose-escalation study to evaluate the safety and pharmacokinetics of CEP-9722 (a PARP-1 and PARP-2 inhibitor) in combination with gemcitabine and cisplatin in patients with advanced solid tumors. Anticancer Drugs. 2016;27:342–348. doi: 10.1097/CAD.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 593.Schram AM, Chang MT, Jonsson P, Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 2017;14:735–748. doi: 10.1038/nrclinonc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Pottier, C. et al. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers12, 731 (2020). [DOI] [PMC free article] [PubMed]
- 595.Gasch C, Ffrench B, O’Leary JJ, Gallagher MF. Catching moving targets: cancer stem cell hierarchies, therapy-resistance & considerations for clinical intervention. Mol. Cancer. 2017;16:43. doi: 10.1186/s12943-017-0601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. doi: 10.1016/j.lfs.2019.116781. [DOI] [PubMed] [Google Scholar]
- 597.Erin N, Grahovac J, Brozovic A, Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updat. 2020;53:100715. doi: 10.1016/j.drup.2020.100715. [DOI] [PubMed] [Google Scholar]
- 598.Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Mele L, et al. The role of autophagy in resistance to targeted therapies. Cancer Treat. Rev. 2020;88:102043. doi: 10.1016/j.ctrv.2020.102043. [DOI] [PubMed] [Google Scholar]
- 600.Hussain S, et al. Cancer drug resistance: a fleet to conquer. J. Cell Biochem. 2019;120:14213–14225. doi: 10.1002/jcb.28782. [DOI] [PubMed] [Google Scholar]
- 601.Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 2020;19:39–56. doi: 10.1038/s41573-019-0044-1. [DOI] [PubMed] [Google Scholar]
- 602.Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Meyer KD, Jaffrey SR. Rethinking m(6)A readers, writers, and erasers. Annu Rev. Cell Dev. Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Van Meter EN, Onyango JA, Teske KA. A review of currently identified small molecule modulators of microRNA function. Eur. J. Med. Chem. 2020;188:112008. doi: 10.1016/j.ejmech.2019.112008. [DOI] [PubMed] [Google Scholar]
- 605.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat. Rev. Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Jancik S, Drabek J, Radzioch D, Hajduch M. Clinical relevance of KRAS in human cancers. J. Biomed. Biotechnol. 2010;2010:150960. doi: 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Cox AD, et al. Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Cox AD, Der CJ, Philips MR. Targeting RAS membrane association: back to the future for anti-RAS drug discovery? Clin. Cancer Res. 2015;21:1819–1827. doi: 10.1158/1078-0432.CCR-14-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Papke B, Der CJ. Drugging RAS: know the enemy. Science. 2017;355:1158–1163. doi: 10.1126/science.aam7622. [DOI] [PubMed] [Google Scholar]
- 610.Chen H, et al. Small-molecule inhibitors directly targeting KRAS as anticancer therapeutics. J. Med. Chem. 2020;63:14404–14424. doi: 10.1021/acs.jmedchem.0c01312. [DOI] [PubMed] [Google Scholar]
- 611.Nagasaka M, et al. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat. Rev. 2020;84:101974. doi: 10.1016/j.ctrv.2020.101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Liu P, Wang Y, Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm. Sin. B. 2019;9:871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Bayliss R, Burgess SG, Leen E, Richards MW. A moving target: structure and disorder in pursuit of Myc inhibitors. Biochem. Soc. Trans. 2017;45:709–717. doi: 10.1042/BST20160328. [DOI] [PubMed] [Google Scholar]
- 614.Krzyzosiak A, et al. Target-based discovery of an inhibitor of the regulatory phosphatase PPP1R15B. Cell. 2018;174:1216–1228.e1219. doi: 10.1016/j.cell.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Kieffer C, Jourdan JP, Jouanne M, Voisin-Chiret AS. Noncellular screening for the discovery of protein-protein interaction modulators. Drug Discov. Today. 2020;25:1592–1603. doi: 10.1016/j.drudis.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 616.Mabonga L, Kappo AP. Protein-protein interaction modulators: advances, successes and remaining challenges. Biophys. Rev. 2019;11:559–581. doi: 10.1007/s12551-019-00570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Taylor MH, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J. Clin. Oncol. 2020;38:1154–1163. doi: 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Chau V, Bilusic M. Pembrolizumab in combination with axitinib as first-line treatment for patients with renal cell carcinoma (RCC): evidence to date. Cancer Manag. Res. 2020;12:7321–7330. doi: 10.2147/CMAR.S216605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Rini BI, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 620.Schapira L. Simple rules can improve prognostic accuracy. J. Clin. Oncol. 2011;29:347–349. doi: 10.1200/JCO.2010.32.9086. [DOI] [PubMed] [Google Scholar]
- 621.Beck A, et al. The next generation of antibody-drug conjugates comes of age. Discov. Med. 2010;10:329–339. [PubMed] [Google Scholar]
- 622.Thomas A, Teicher BA, Hassan R. Antibody–drug conjugates for cancer therapy. Lancet Oncol. 2016;17:e254–e262. doi: 10.1016/S1470-2045(16)30030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Yaghoubi S, et al. Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J. Cell Physiol. 2020;235:31–64. doi: 10.1002/jcp.28967. [DOI] [PubMed] [Google Scholar]
- 624.Torre, B. G. & Albericio, F. An analysis of FDA drug approvals from the perspective of molecules. Molecules26, 627 (2021). [DOI] [PMC free article] [PubMed]
- 625.An S, Fu L. Small-molecule PROTACs: an emerging and promising approach for the development of targeted therapy drugs. EBioMedicine. 2018;36:553–562. doi: 10.1016/j.ebiom.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Wang Y, et al. Degradation of proteins by PROTACs and other strategies. Acta Pharm. Sin. B. 2020;10:207–238. doi: 10.1016/j.apsb.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Lin X, Xiang H, Luo G. Targeting estrogen receptor alpha for degradation with PROTACs: A promising approach to overcome endocrine resistance. Eur. J. Med. Chem. 2020;206:112689. doi: 10.1016/j.ejmech.2020.112689. [DOI] [PubMed] [Google Scholar]
- 628.Huang A, Garraway LA, Ashworth A, Weber B. Synthetic lethality as an engine for cancer drug target discovery. Nat. Rev. Drug Discov. 2020;19:23–38. doi: 10.1038/s41573-019-0046-z. [DOI] [PubMed] [Google Scholar]
- 629.Corcoran RB, et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23:121–128. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.