Abstract
Objective
We report a case series of 4 patients with type 1 diabetes who used hybrid closed-loop insulin pumps (Medtronic MiniMed 670 G) during hospitalization.
Methods
Clinical data and point-of-care glucose values are presented for each patient. Glucose values are shown graphically while in manual mode as well as in auto mode.
Results
The first case was a 30-year-old man admitted for pancreatitis. Mean point-of-care blood glucose was 165.7 mg/dL while in auto mode, without hypoglycemia, compared with 221 mg/dL while in manual mode. The second case was a 28-year-old woman who was admitted for a laparoscopic cholecystectomy. Mean point-of-care blood glucose in auto mode was 131.3 mg/dL, without hypoglycemia, compared with 117.6 mg/dL while in manual mode. The third case was a 46-year-old man admitted to the intensive care unit for influenzal pneumonia. Mean point-of-care blood glucose in auto mode was 159.1 mg/dL without hypoglycemia, compared with 218.5 mg/dL while in manual mode. The fourth case was a 60-year-old man who remained in auto mode throughout his hospitalization except for a period when he removed his pump for an endoscopic retrograde cholangiopancreatography and endoscopic ultrasound. His mean point-of-care blood glucose while in auto mode was 156.8 mg/dL without hypoglycemia.
Conclusion
These case reports support the use of hybrid closed-loop insulin-pump therapy in the inpatient setting to maintain inpatient glycemic targets and avoid hypoglycemia when part of an institution-sanctioned strategy for safe use of insulin pumps that includes point-of-care blood glucose monitoring.
Abbreviations: CGM, continuous glucose monitor; FDA, Food and Drug Administration; HCL, hybrid closed-loop; MARD, mean absolute relative difference; T1DM, type 1 diabetes mellitus
Introduction
Continuous subcutaneous insulin infusion commonly referred to as insulin-pump therapy, uses a portable electromechanical pump to continuously infuse insulin into the subcutaneous tissue at preselected rates. Despite limited data, insulin-pump therapy has been shown to be a safe and effective alternative to more traditional therapies.1 Currently approved by the US Food and Drug Administration (FDA) for outpatient treatment of both type 1 and type 2 diabetes, use of insulin-pump therapy has increased and is frequently encountered when patients are admitted to acute care settings.2,3 The American Diabetes Association and the American Association of Clinical Endocrinologists support the use of insulin-pump therapy in hospital settings when patient and clinical staff agree that continued use is safe and appropriate.
In 2016, the FDA approved a hybrid closed-loop (HCL) insulin-pump system, the Medtronic MiniMed 670 G.4 This technology utilizes an insulin pump programed with an algorithm to administer micro boluses of insulin for basal insulin requirements based upon glucose values transmitted from a continuous glucose monitor (CGM).5 Rather than preset rates, the basal rates automatically change in response to the CGM data. Bolus doses for meals and correction require manual entry. It can suspend insulin delivery if sensor data fall below or are predicted to fall below threshold values which reduces the risk of hypoglycemia. In 2019, the FDA cleared the Tandem Control-IQ HCL system.6 This system uses an algorithm to increase basal rates; deliver automated correction boluses; suspend before low blood glucose; and intensify basal rates.7
The mean absolute relative difference (MARD) is a common metric used to assess CGM accuracy. MARD is the mean of the absolute error between CGM readings and reference values. A low percentage (<10%) indicates that the CGM readings are close to the reference glucose value and a higher percentage indicates larger discrepancies between them.8 The Medtronic MiniMed 670 G uses the Medtronic Guardian sensor which has a MARD of 9.6%.9 The Tandem Control-IQ HCL system uses Dexcom G6 CGM which has a MARD of 9%.10
In HCL systems, basal rates are automatically adjusted and can only be evaluated with retrospective download of data. Use of HCL insulin pumps in the inpatient setting becomes a challenge for documenting basal rates according to standards defined by The Joint Commission Certification in Advanced Inpatient Diabetes Care.11 Generally, patients who choose to use HCL insulin pumps are savvy, so continued use requires a cooperative effort with the staff. Patients partner with nurses so that bolus doses can be entered into the electronic medical record by the nursing staff. Despite continued development and rising use of HCL insulin pumps, there are no guidelines or outcome data regarding the safety of HCL insulin pumps in the hospital setting where acute illness, medications and invasive procedures challenge glycemic management.
In an effort to investigate the safety and efficacy of HCL insulin pumps, we report 4 cases with different causes for admission. In each case, the patient continued using the HCL feature of the Medtronic MiniMed 670G insulin pump after meeting the institution’s criteria for insulin pump therapy. Safety and efficacy were defined in terms of number of hypoglycemic events throughout hospitalization as well as the mean and range of intermittent point-of-care blood glucose values compared to the glycemic target of 140 to 180 mg/dL.12
Case Series
Case 1 was a 30-year-old man with type 1 diabetes (T1DM) for 27 years and treated for 2 years using the HCL feature of his insulin pump. He was admitted with perforated appendicitis. Initially, the patient’s CGM sensor was removed prior to imaging testing and the insulin pump was in manual mode. During this time period, there was greater glycemic variability. The mean point-of-care blood glucose with insulin pump set in HCL mode was 165.7 mg/dL (range, 79-262 mg/dL) with no hypoglycemia. The mean point-of-care blood glucose in manual mode was 221 mg/dL (range, 179-264 mg/dL) with no hypoglycemia. Blood glucose values are shown in Fig. 1.
Fig. 1.
Glucose trends for for case 1.
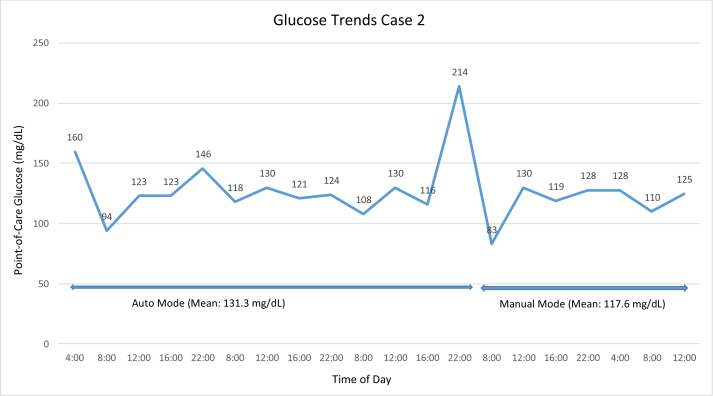
Case 2 was a 28-year-old woman with T1DM for 11 years who was admitted with gallstone pancreatitis 5 weeks postpartum. Imaging demonstrated gallbladder calculi, and she underwent a laparoscopic cholecystectomy. She used insulin-pump therapy throughout her pregnancy and transitioned to the HCL feature 1 week prior to the surgical admission. She transitioned from HCL to manual mode prior to magnetic resonance cholangiopancreatography. The insulin pump remained in manual mode with a temporary basal rate of 80% of her preprogrammed basal rates during surgery. She restarted the HCL feature when she could safely manipulate the pump. The mean point-of-care blood glucose in HCL mode was 131.3 mg/dL (range, 94-214 mg/dL) with no hypoglycemia. The mean point-of-care glucose in manual mode was 117.6 mg/dL (range, 83-130 mg/dL) with no hypoglycemia. Blood glucose values are shown in Fig. 2.
Fig. 2.
Glucose trends for case 2.
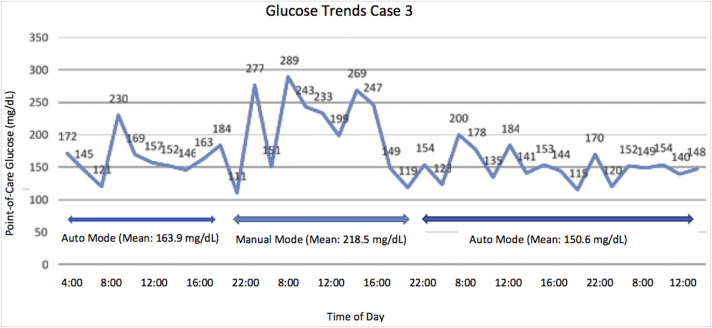
Case 3 was a 46-year-old man with chronic stage III kidney disease, hypothyroidism, and T1DM for 16 years and treated using HCL insulin therapy for 2 years. He was admitted to the intensive care unit for influenzal pneumonia. His blood glucose remained in the target range throughout his hospitalization with the exception of 1 day when his insulin pump was changed to manual mode to allow him to change the CGM site. The mean point-of-care blood glucose in HCL mode was 159.1 mg/dL (range, 115- 230 mg/dL) with no hypoglycemia. The mean point-of-care glucose in manual mode was 218.5 mg/dL with no hypoglycemia. Blood glucose values are shown in Fig. 3.
Fig. 3.
Glucose trends for case 3.
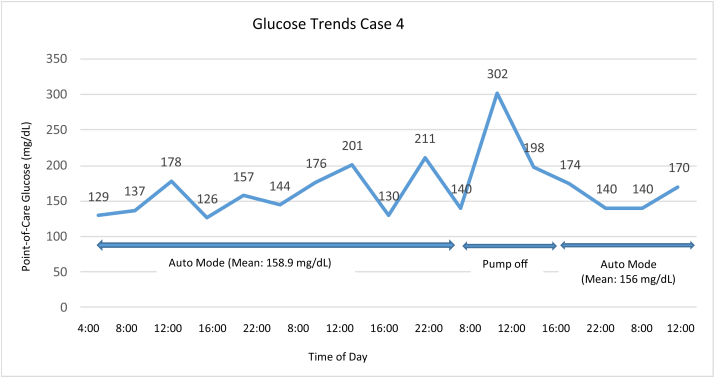
Case 4 was a 60-year-old man with T1DM for 7 years and treated using HCL therapy for 3 years. The patient had a history of metastatic pancreatic cancer and presented with recurrent fevers, nausea, and abdominal pain. He used the HCL feature throughout his hospital stay except when he briefly removed his insulin pump for a combined endoscopic retrograde cholangiopancreatography and endoscopic ultrasound. The mean point-of-care blood glucose in HCL mode was 156.8 mg/dL (range, 126 -302 mg/dL) with no hypoglycemia. Blood glucose values are shown in Fig. 4.
Fig. 4.
Glucose trends for case 4.
Discussion
The 4 cases presented demonstrate that use of a HCL system in the hospital is both safe and effective as demonstrated by an overwhelming majority of glucose values in target range and the absence of hypoglycemia. Despite the increasing use of insulin-pump therapy, there are currently no guidelines for use of an HCL system in the hospital setting. In an effort to investigate the safety and efficacy of HCL insulin pumps, we report 4 cases with different causes for admission where the patients used the HCL feature of their insulin pumps. They were treated at NYU Langone Health-Long Island which has an institution-sanctioned insulin-pump policy in line with insulin-pump standards and requirements of Joint Commission Certification. The policy requires all patients to sign an insulin-pump agreement and to have oversight by an endocrinologist. Required documentation includes the patient’s ability to safely manage the insulin pump, an order for insulin-pump therapy including basal rates and bolus doses, condition and location of insertions sites, and frequency and date of insertion site changes.11
The percentage of admitted patients using insulin pumps is unknown. The Type 1 Diabetes Exchange noted that use of pump therapy increased from 57% in 2010-2012 to 63% in 2016-2018.13 The use of insulin pumps is expected to continue to increase, as pump therapy has demonstrated improvements in several important parameters including increasing time spent in the target range and decreasing hypoglycemic episodes.13 Cook et al14 studied open-loop insulin-pump therapy in 253 hospital cases (136 unique patients; 82% T1DM). Of the 253 hospitalizations, 164 (65%) continued using open-loop pump therapy, 50 (20%) intermittently used pump therapy and 38 (15%) discontinued pump therapy. Mean glucose was not significantly different among those who remained compared with those who discontinued. However, episodes of hyperglycemia (>300 mg/dL) and hypoglycemia (<40 mg/dL) were significantly less common in those who continued insulin-pump therapy. In a small study, Lee et al15 demonstrated that continued use of insulin-pump therapy during hospitalization improved patient satisfaction scores. Beyond the obvious benefits of patient satisfaction, there are financial benefits, as patient satisfaction scores are linked to the Center for Medicare and Medicaid Services hospital payments.
To date, a few studies have addressed use of HCL insulin pumps to manage type 2 diabetes in the inpatient setting. Among inpatients with type 2 diabetes receiving noncritical care, the use of an automated, closed-loop insulin delivery system that is not commercially available resulted in significantly greater time-in-range (100-180 mg/dL) than conventional subcutaneous insulin therapy, without a higher risk of hypoglycemia.16 This study confirmed the results of the initial pilot study conducted without meal-time bolus dosing.17
Continuous Glucose Monitoring in the Inpatient Setting
It is important to distinguish the role of CGM data in HCL systems from using CGM data for inpatient subcutaneous insulin dosing. CGM data are generally not approved for insulin dosing in inpatient settings, so insulin dosing decisions are made based on the institution’s point-of-care blood glucose meter data.18 However, in HCL insulin-pump systems, CGM data are fed into the algorithm that drives the insulin-pump adjustments. As our cases demonstrate, the CGM data in tandem with the algorithm can support inpatient glycemic targets. An important shift occurred In April 2020, when both Dexcom and Abbott received feedback from the FDA that the agency would not object to use of CGM devices in hospitalized patients during COVID-19 related efforts, with the hope that CGM data could reduce hospital staff exposure and use of personal protective equipment.19,20 This has led to a number of accelerated clinical trial reports and publications providing guidance on the use of CGM in hospital settings.21 Fortmann et al22 reported on a subset of a large, statistically powered randomized controlled trial in an effort to provide immediate data to hospital systems implementing use of Dexcom G6. They reported the beneficial trends of real-time continuous glucose monitoring in a noncritical care setting including lower mean glucose and higher time-in-range compared with usual care in patients with type 2 diabetes. In a study that paired real-time CGM with a telemetry system that wirelessly transmitted CGM glucose values from the bedside to a centralized monitor and demonstrated decreased hypoglycemia in high-risk insulin-treated patients with type 2 diabetes, Singh et al23 halted their trial shortly after the interim analysis to allow for widespread dissemination in the context of the COVID-19 pandemic.
There is also evidence to suggest that use of CGM data by adult patients in noncritical care settings potentially detects both hypo-and hyperglycemia that would have been missed by intermittent point-of-care monitoring.18 However, there are no data concerning the accuracy of inpatient CGM data due to physiologic interferences in critical care settings such as edema.
Conclusion
We described 4 cases with different reasons for admission in which HCL insulin-pump therapy was used successfully. For those patients using HCL systems who meet the criteria to continue insulin-pump therapy, discontinuing the closed-loop mode no longer serves as an option that patients will tolerate. Until well-powered trials are conducted, these case reports support HCL insulin-pump therapy in the inpatient setting to maintain inpatient glycemic targets and avoid hypoglycemia when part of an institution-sanctioned strategy for safe use of insulin pumps that includes point-of-care blood glucose monitoring.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Yeh H.C., Brown T.T., Maruthur N. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–347. doi: 10.7326/0003-4819-157-5-201209040-00508. [DOI] [PubMed] [Google Scholar]
- 2.Sherr J., Tamborlane W.V. Past, present, and future of insulin pump therapy: better shot at diabetes control. Mt Sinai J Med. 2008;75(4):352–361. doi: 10.1002/msj.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umpierrez G.E., Klonoff D.C. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41(8):1579–1589. doi: 10.2337/dci18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration FDA approves first automated insulin delivery device for type 1 diabetes. https://www.fda.gov/news-events/press-announcements/fda-approves-first-automated-insulin-delivery-device-type-1-diabetes
- 5.Ly T.T., Roy A., Grosman B. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in Type 1 diabetes at diabetes camp. Diabetes Care. 2015;38(7):1205–1211. doi: 10.2337/dc14-3073. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration FDA authorizes first interoperable, automated insulin dosing controller designed to allow more choices for patients looking to customize their individual diabetes management system. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-automated-insulin-dosing-controller-designed-allow-more-choices
- 7.Brown S.A., Kovatchev B.P., Raghinaru D. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danne T., Nimri R., Battelino T. International consensus of use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen M.P., Garg S.K., Brazg R. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19(8):446–456. doi: 10.1089/dia.2017.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah V.N., Laffel L., Wadwa R.P., Garg S.K. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–433. doi: 10.1089/dia.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold P., Scheurer D., Dake A.W. Hospital guidelines for diabetes management and the Joint Commission-American Diabetes Association inpatient diabetes certification. Am J Med Sci. 2016;351(4):333–341. doi: 10.1016/j.amjms.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association 15. Diabetes care in the hospital: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S193–S202. doi: 10.2337/dc20-S015. [DOI] [PubMed] [Google Scholar]
- 13.Foster N.C., Beck R.W., Miller K.M. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook C.B., Beer K.A., Seifert K.M., Boyle M.E., Mackey P.A., Castro J.C. Transitioning insulin pump therapy from the outpatient to the inpatient setting: a review of 6 years’ experience with 253 cases. J Diabetes Sci Technol. 2012;6(5):995–1002. doi: 10.1177/193229681200600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee I.T., Liau Y.J., Lee W.J., Huang C.N., Sheu W.H.H. Continuous subcutaneous insulin infusion providing better glycemic control and quality of life in type 2 diabetic subjects hospitalized for marked hyperglycemia. J Eval Clin Pract. 2010;16(1):202–205. doi: 10.1111/j.1365-2753.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 16.Bally L., Thabit H., Hartnell S. Closed-loop insulin delivery for glycemic control in noncritical care. N Engl J Med. 2018;379(6):547–556. doi: 10.1056/NEJMoa1805233. [DOI] [PubMed] [Google Scholar]
- 17.Thabit H., Hartnell S., Allen J.M. Closed loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 2017;5(2):117–124. doi: 10.1016/S2213-8587(16)30280-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang M., Singh L.G., Spanakis E.K. Advancing the use of CGM devices in a non-ICU setting. J Diabetes Sci Technol. 2019;13(4):674–681. doi: 10.1177/1932296818821094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dexcom press release Dexcom continuous glucose monitoring systems to be temporarily offered to hospitals during COVID-19 emergency. https://www.dexcom.com/news/dexcome-cgm-hospital-covid19
- 20.Abbott press release Abbott’s Freestyle® Libre 14 day system now available in US for hospitalized patients with diabetes during COVID-19 pandemic. https://abbott.mediaroom.com/2020-04-08-Abbotts-FreeStyle-R-Libre-14-Day-System-Now-Available-in-U-S-for-Hospitalized-Patients-with-Diabetes-During-COVID-19-Pandemic
- 21.Galindo R., Aleppo G., Klonoff D.C. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol. 2020;14(4):822–832. doi: 10.1177/1932296820932903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortmann A.L., Bagsic S.R.S., Talavera L. Glucose as the fifth vital sign: a randomized controlled trial of continuous glucose monitoring in a non-ICU hospital setting. Diabetes Care. 2020;43(11):2873–2877. doi: 10.2337/dc20-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh L.G., Satyarengga M., Marcano I. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care. 2020;43(11):2736–2743. doi: 10.2337/dc20-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]