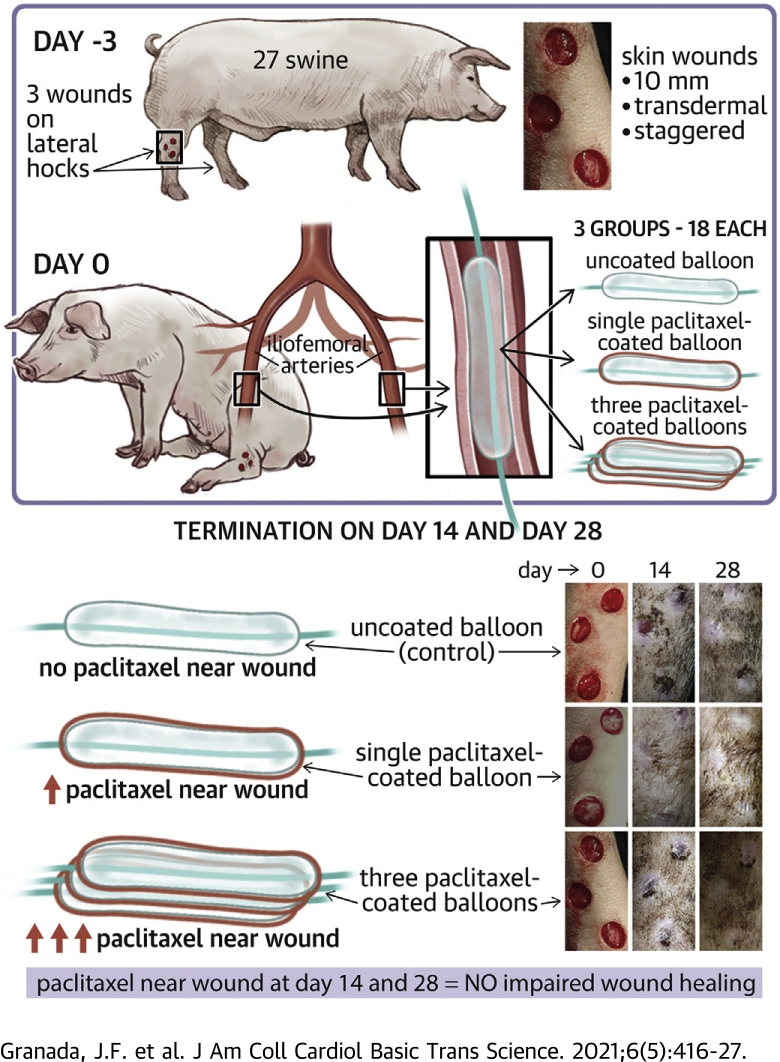
Visual Abstract
Key Words: histopathology, paclitaxel-coated balloon, swine, translational, wound healing
Abbreviations and Acronyms: BTK, below the knee; CLI, critical limb ischemia; PCB, paclitaxel coated balloon; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery
Highlights
-
•
PCBs are a clinically proven antirestenotic alternative to plain percutaneous transluminal angioplasty of superficial femoral arteries but their application in critical limb ischemia is inhibited by the concern that the downstream release of particulate paclitaxel may negatively impact distal lower limb’s circulation and its tissues already compromised by chronic ischemia.
-
•
To investigate this concern experimentally, we used an animal model of standardized distal limb wounds to determine the effect of downstream paclitaxel released during PCB treatment of superficial femoral arteries on distal wound healing process.
-
•
A clinically relevant concentration of paclitaxel in the vicinity of the wound did not impair the healing of preexisting distal cutaneous lesions in healthy swine even after multiple PCB deployments.
Summary
The authors evaluated the presence of paclitaxel and healing of distal hind limb wounds created in 27 swine using biopsy punches followed by paclitaxel-coated balloon (PCB) use in the iliofemoral arteries of healthy swine. After 14 and 28 days, no differences were seen in time course, appearance, and histopathology of wound healing between the single or triple PCB and uncoated balloon treatment despite clinically relevant paclitaxel concentrations in the skin adjacent to the healing wounds. Presence of paclitaxel downstream from the PCB treatment site does not impair the wound healing response of preexisting distal cutaneous lesions in healthy swine.
Paclitaxel-coated balloons (PCBs) have been shown to significantly reduce restenosis rates among patients undergoing superficial femoral artery (SFA) intervention in randomized controlled studies (1, 2, 3). The mechanism of action of PCBs involves the single transfer of paclitaxel particles onto the luminal surface during the process of arterial lesion dilatation (4). Fragmentation of the balloon coating typically occurs during this process and leads to shedding and downstream embolization of these particles (5, 6, 7). Downstream shedding of paclitaxel particles during delivery to the target site remains an inherent feature of clinically successful PCBs, warranting concerns about potential negative impact on distal lower limb’s circulation and its tissues already compromised by chronic ischemia. Accordingly, since the introduction of PCBs, downstream particle embolization during treatment has been clinically feared and its significance widely debated (8, 9, 10). Although large randomized controlled trials have shown amputation and thrombotic rates comparable to plain balloon angioplasty, they did not enroll subjects with distal limb ulceration (1, 2, 3). Therefore, the relationship of paclitaxel residence in downstream tissue to wound healing is still unknown, especially among patients with distal poor vascular runoff, leading to conflicting opinions about the safety of PCBs in critical limb ischemia (CLI) (11,12). To investigate this concern experimentally, we evaluated the presence and biological effects of paclitaxel on the distal hind limb wound healing following PCB (IN.PACT Admiral, Medtronic, Dublin, Ireland) use in the SFA of healthy swine.
Methods
Animal model development
All in-life procedures were conducted at PreClinical Research Services, Inc., Ft. Collins, Colorado), an AAALAC accredited and United States Department of Agriculture–registered facility. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee and conducted in compliance with the Guide for Care and Use of Laboratory Animals (13). A total of 27 female juvenile Yorkshire domestic swine weighing between 17.3 and 24.8 kg (approximately 2 months of age at time of treatment) were used in this study. Animals were singly housed to prevent disruption of bandaging and possible injury to surgical sites, as well as acclimated to a sling for 1 week before procedures to facilitate awake bandage changes and photography. The animals were fed swine diet once per day and provided tap water ad libitum.
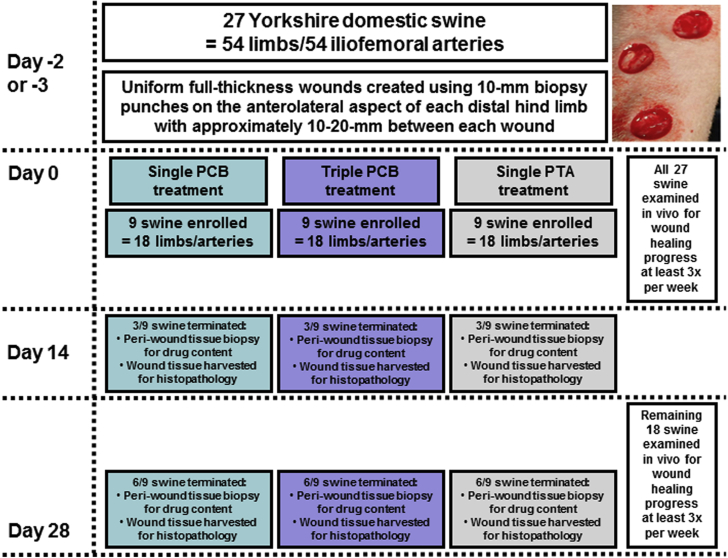
Figure 1 shows the key study design aspects and the timeline. Two to 3 days before PCB treatment, uniform full-thickness wounds were created under sedation (intramuscular administration of mixture of tiletamine-zolazepam 2 to 8.8 mg/kg of body weight and atropine 0.02 to 0.05 mg/kg of body weight and maintained by isoflurane inhalation up to 5%) using 10-mm biopsy punches. The wounds were placed in a staggered vertical pattern below the hock joint on the anterolateral aspect of each distal hind limb with approximately 10 to 20 mm between each wound. The wounds were bandaged with Tegaderm (3M, St. Paul, Minnesota) directly on the skin followed by a gauze pad and VetRap (Johnson & Johnson, New Brunswick, New Jersey) with Elasticon (Johnson & Johnson) over the proximal and distal ends of the VetRap to keep the bandaging secured. The bandaging was replaced on awake animals 3 times per week after wounding throughout the in-life duration of the study. Each animal received prophylactic antimicrobial and antibiotic medications (enrofloxacin 2.505 mg/kg by mouth) daily from day -4/-3 to day 7. Antiplatelet therapy was administered daily; 325 mg aspirin and 75 mg of clopidogrel from day -3 to -1 then 81 mg of aspirin and 75 mg of clopidogrel from day 0 for the remainder of the study. Animals received carprofen 4 mg/kg by mouth starting day -3/-2 through day 2/3 for pain management during surgical procedures.
Figure 1.
Study Flow Chart
Chart shows key study design aspects, methods and milestones. PCB = paclitaxel-coated balloon; PTA = percutaneous transluminal angioplasty.
Interventional treatment with PCB or percutaneous transluminal angioplasty
Animals were randomly allocated to 3 study arms and sacrificed at 14 days (n = 9) and 28 days (n = 18). The 3 study arms were: 1) single PCB treatment (PCB × 1, 3.5 μg/mm2, IN.PACT Admiral balloon catheters, 5.0/6.0 × 80 mm; Medtronic, Santa Rosa, California), 2) 3 overlapping PCB treatments (PCB × 3, 10.5 μg/mm2, IN.PACT Admiral balloon catheters, 5.0/6.0 × 80 mm; Medtronic), and 3) a percutaneous transluminal angioplasty (PTA) control (no drug coating, Admiral Extreme PTA catheter, 4.0/5.0/6.0 × 80 mm; Medtronic). Three animals from each arm were evaluated at the first time point (14 days), 6 animals from each arm were evaluated at the second time point (28 days). In the PCB × 3 arm, 3 sequential/different balloons were used consecutively and aligned to overlap an ∼100% margin of coverage by means of angiographic visualization using a digital fluoroscope and software. Two iliofemoral treatment sites were selected per animal; animals were limited to 1 study arm with bilateral treatment. Balloon inflations were performed for 1 min to reach a target vessel overstretch of 20% to 30% based on pretreatment qualitative vascular angiography of the target region using standard interventional techniques via carotid access. As such, there were 6 arteries treated in each study arm survived to 14 days (total of 18 sites in 9 animals) and 12 arteries in each study arm survived to 28 days (total of 36 sites in 18 animals).
Clinical observations and scoring of wounds
Wound healing was assessed over time by: 1) visual observation with sequential photography at time of creation and at each bandage change; and 2) histologic evaluation of re-epithelialization, granulation, inflammation, and collagen formation. Each bandage was changed a minimum of 3 times per week throughout the in-life study duration on awake animals while in a sling. The wound sites were photographed with a measuring device in view. Each wound was quantitatively scored in a blinded manner using a modified system of objective inflammation and cosmesis scoring parameters modified from published methods (Table 1) (14, 15, 16, 17).
Table 1.
Definitions and Range of Mean Scores of Inflammation and Cosmesis Parameters
| Erythema | Edema | Serous Discharge | Purulent Discharge | Step-Off Borders∗ | Contour Irregularity† | Margin Separation‡ | Excessive Distortion§ | |
|---|---|---|---|---|---|---|---|---|
| Dose | ||||||||
| PTA control | 0-0.7 | 0-0.6 | 0-0.6 | 0-1.3 | 0-0.5 | 0-0.5 | 0.4-4 | 0-0.2 |
| Nominal dose | 0-0.5 | 0-0.6 | 0-0.8 | 0-1.1 | 0-0.6 | 0-0.5 | 0.2-4 | 0-0.2 |
| High dose | 0-0.7 | 0-0.8 | 0-0.7 | 0-0.6 | 0-0.8 | 0-0.4 | 0.2-4 | 0 |
| Score | ||||||||
| 0 | No erythema | No edema | Wound is dry | No purulent exudate | Absence of appearance | Absence of appearance | Absence of appearance | Absence of appearance |
| 1 | Very slight (barely perceptible) | Very slight (barely perceptible) | Very slight (barely perceptible) | Small amount of purulent exudate – no color | Trace appearance | Trace appearance | Trace appearance | Trace appearance |
| 2 | Well defined erythema | Slight edema (edges of area well defined by definite raising) | Slight serous discharge | Moderate amount of purulent exudate – no color | Mild appearance | Mild appearance | Mild appearance | Mild appearance |
| 3 | Moderate to severe erythema | Moderate edema (raised approximately 1 mm) | Moderate serous discharge with blood-tinged fluid | Moderate amount of purulent exudate – red or green tinged color | Moderate appearance | Moderate appearance | Moderate appearance | Moderate appearance |
| 4 | Severe (beet redness) to slight eschar formation | Severe edema (raised more than 1 mm and extending beyond area of exposure) | Large volume of serous discharge with marked blood-tinged fluid | Purulent exudate including accumulation in subcutaneous tissues at wound margin – abscess formation | Severe appearance | Severe appearance | Severe appearance | Severe appearance |
PTA = percutaneous transluminal angioplasty.
Edges of the wound are not on the same plane as each other.
Wrinkling is apparent on skin surrounding the wound.
Gap between the sides of the wound (diameter or circular wound).
Apparent swelling, edema, and possibly infection.
Study termination and tissue sample collection
All animals survived to their scheduled termination time points of 14 or 28 days. At time of termination, while under general anesthesia, 3 full-thickness skin biopsy specimens per hind limb, 10 mm in diameter, were collected immediately adjacent to the healing wounds for a total of 6 biopsy specimens per animal and flash-frozen in liquid nitrogen for drug content analysis. Only local peri-wound tissue was analyzed for drug content. The animal was euthanized while still under general anesthesia using a pentobarbital-based solution of (88 mg/kg intravenously). The entire wounded area of each distal limb including approximately 2.5 cm of surrounding tissue was collected as 1 specimen with proximal end marked with suture. Tissue samples for histology traversed deep in the fascial plane and muscle so as not to disturb the wound or fibrosis below the wound. Each specimen was rinsed with 0.9% saline, pinned to a rigid surface, skin side away from the rigid surface, and submerged in container with 10% neutral buffered formalin at a volume of at least 20:1 formalin-to-tissue ratio, 1 specimen per container. Specimens were fixed in buffered formalin for at least 24 h before histological processing.
Drug content analysis
Porcine tissue samples were received from the animal testing facility frozen and were stored at ≤−65°C until preparation for analysis. The frozen tissue samples were weighed and suspended in buffer for processing. Following mincing, approximately 70-mg portions of tissue were then transferred into multiple 2-ml XXTuff reinforced micro-vials (Biospec Inc., Bartlesville, Ohio) containing zirconium oxide beads (Biospec Inc.). Individual tissue samples were processed using a Precellys 24-tissue homogenizer equipped with a Cryolys liquid nitrogen cooling system (Bertin Corp., Rockville, Maryland). The samples were placed in homogenizer and subjected to a pre-set method (5,500 rpm for 3 cycles × 75 s with 5 s wait). Once homogenized, each tissue suspension was transferred into appropriately sized vials and combined with the remaining buffer. The homogenized tissue samples were then stored in a -80oC freezer until analysis. Analysis was performed with an Agilent 1290 (Agilent Technologies, Santa Clara, California) with Phenomenex Luna phenyl-hexyl 3-μm 2 × 100-mm column and AB 4000 QTrap detector (Applied Biosystems, Foster City, California). Column temperature was maintained at 60oC and flow rate was 300 μl/min using a 5-μl injection volume. Frozen tissue samples were homogenized, and paclitaxel was quantified using liquid chromatography/tandem mass spectroscopy. The range of the assay was 0.1 ng/ml to 3,000 ng/ml and reported in nanograms per milligram of tissue.
Histopathology of wound healing
Each individual wound was bisected and embedded in paraffin and sectioned at approximately 5-μm thickness to produce 2 serial histological sections, 1 stained with hematoxylin and eosin and the second with Masson’s trichrome. The individual wounds were independently scored in a semiquantitative manner for re-epithelialization, fibroplasia, neovascularization, and inflammation (Table 2).
Table 2.
Scoring Scale (0–4) for Histopathologic Findings∗
| Re-epithelialization | |
| 0, no evidence of re-epithelialization | |
| 4, complete re-epithelialization (the entire wound surface was covered by a stratified squamous epithelium (epidermis) with keratin) | |
| Fibroplasia | |
| 0, no evidence of dermal collagen production | |
| 2, fibroplasia production of collagen to fill the wound defect was substantially present with amount of collagen and the density of the collagen bands clearly less than in the adjacent normal dermis | |
| 4, dermal collagen indistinguishable from the adjacent normal (nonwounded) dermis | |
| Neovascularization, inflammatory infiltrates, inflammation associated with hair fragments, and hemosiderin-containing macrophages | |
| 0, not present | |
| 1, minimal response | |
| 2, mild response | |
| 3, moderate response | |
| 4, marked response |
Thrombosis was not present in any sample and scored as 0.
Statistical analysis
Data are presented using means and SDs for inflammation and cosmesis scores, histological scores, and drug concentrations. Comparison of histological parameters was performed by Mann-Whitney test, while Student t-test was used for comparisons of drug concentrations. A p < 0.05 was considered statistically significant. Statistical analyses were performed using Microsoft Excel 2010 (Microsoft, Redmond, Washington) and GraphPad Prism version 7.03 (GraphPad Software Inc., San Diego, California).
Results
Clinical observations
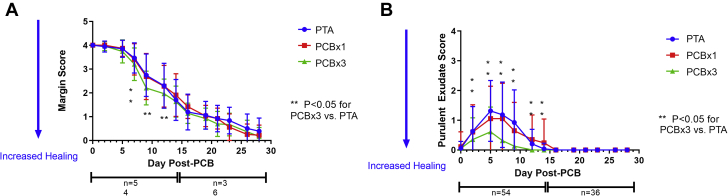
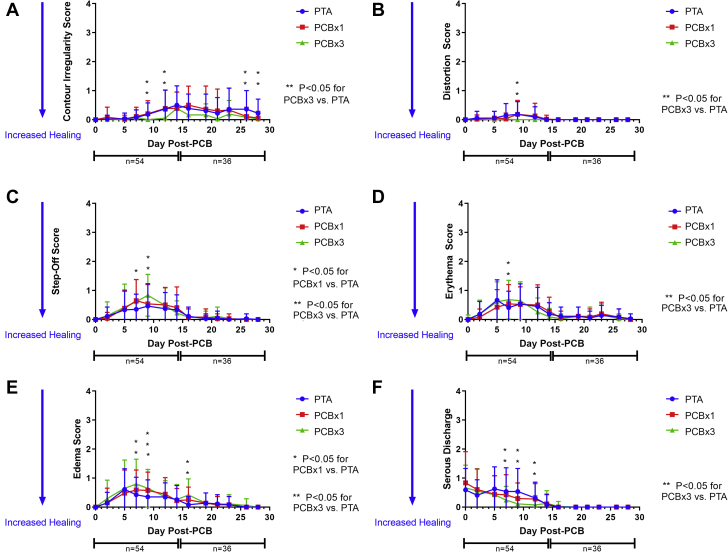
All animals survived to scheduled termination of either 14 or 28 days with expected weight gain and no adverse events. At the 28-day time point, all wounds in all 3 study arms epithelialized as assessed by visual examination (Figure 2). The ranges of inflammation and cosmesis scores indicated similar trends in healing parameters between both PCB and PTA control treatments. The most pronounced changes with evolution of the healing responses were observed in margin separation and purulent exudate scores (Figure 3) with lesser magnitude changes observed with the other inflammation and cosmesis parameters (Figure 4, Table 1). Margin separation scores indicated closely similar progression of wound resolution between all study arms (Figure 3) with no discernable inhibition of healing by PCB treatment, relative to the PTA control. Purulent exudate scores indicated an initial increase of inflammation during the first 2 weeks and resolving to 0 in all study arms by day 16 (Figure 3) with a consistent trend for lower scores observed in the PCB × 3 arm. Furthermore, margin separation scores and exudate scores of individual animals were examined over time to look for temporal variability across groups and consistency in distribution around the group means for any timepoint. Mean score on a per-animal basis were plotted at each time point as well as well as the individual animal scores at each time point (showing distribution around the mean score, represented by a line). These plots, shown in Supplemental Figures 1 and 2, demonstrated even distribution of scores around the mean with greatest variability occurring in the 7- to 14-day period, reflecting, as anticipated, normal variation in the healing response of wounds within individual animals. Lastly, to determine if position of the wounds impacted the resulting margin separation and purulent exudate scores, mean scores for each time point were plotted by position for each group (i.e., top, middle, or bottom wounds). The resulting plots show no consistent and obvious differences in healing rate based upon location of the wound in the leg (Supplemental Figure 3).
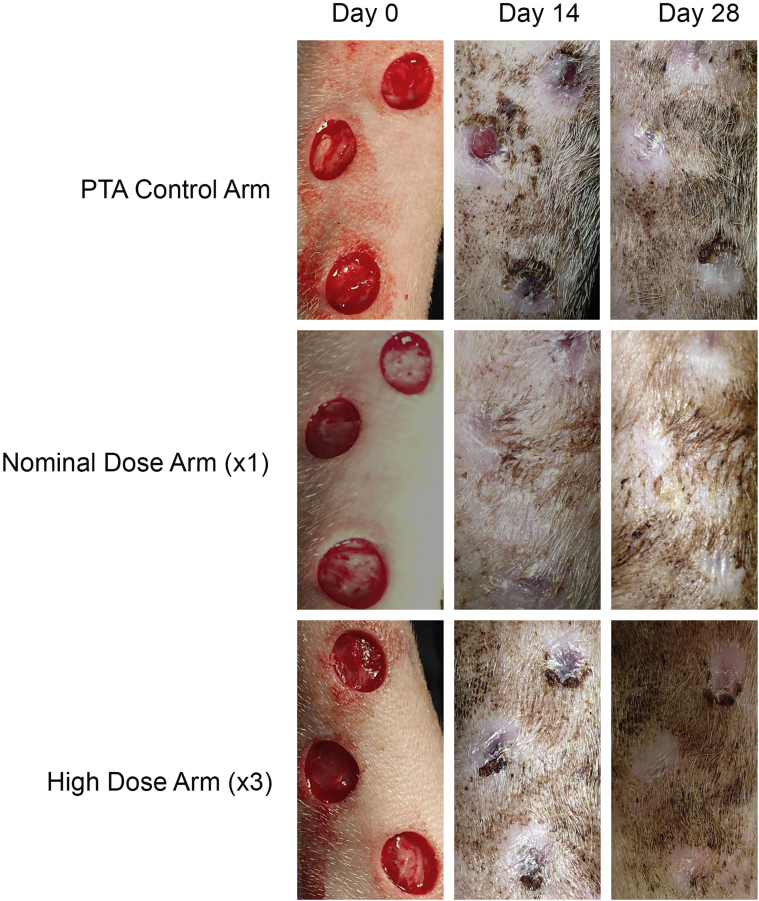
Figure 2.
Visual Assessment of Wound Healing Progression
Photographs showing representative examples of leg wounds from control and treatment arms over the course of the study. Granulation tissue was grossly well established in all arms after 14 days of healing with complete closure seen in most cases by day 28. Abbreviation as in Figure 1.
Figure 3.
Margin Separation Score and Purulent Exudate Score
(A) Margin separation score of all wounds by study arm. Mean scores ± SD are shown for 0 to 28 days post-treatment. The number (n) of monitored wounds decreased after 14 days due to termination of the first cohort of animals for histological and drug content sampling at the study midpoint. The observed rate of wound closure trended consistently across all 3 study arms, with scores decreasing with increased healing. (B) Purulent exudate score of all wounds by study arm. Mean scores ± SD are shown for 0 to 28 days post-treatment. The number (n) of scored wounds decreased after 14 days due to termination of the first cohort of animals for histological and drug content sampling. Scores for all arms trended in a similar manner with exudate largely resolved after 2 weeks, although scores for high-dose arm (PCB × 3) were consistently less than the other 2 arms. Abbreviations as in Figure 1.
Figure 4.
Inflammation and Cosmesis Parameter Scores
(A to F) Markers with maximum scores <1. Mean and SD shown for each timepoint. All arms followed the same wound resolution trends with regard to magnitude and time course. Distortion, step-off, erythema, edema and serous discharge were most apparent during the first 2 weeks after wounding, resolving during the following 2 weeks. Slight contour irregularity developed later during healing, but also showed similar trends across all arms. Abbreviations as in Figures 1 and 3.
Histological evaluation
The main histopathologic features were similar across all 3 study arms with no microscopic findings attributed to paclitaxel toxicity. At 14 days, 78% of wounds in the control arm, 50% of wounds in the nominal-dose (PCB × 1) arm, and 100% of wounds in the high-dose (PCB × 3) arm exhibited grade 3 or 4 re-epithelialization. In the underlying dermis, most wounds across all arms exhibited mild fibroplasia, scores being similar across all study arms. Production and orientation of dermal collagen was within expected limits. Mild neovascularization was observed throughout the wound defect.
At 28 days, 89% of wounds in the PTA control arm and 100% of wounds in the nominal- (PCB × 1) and high-dose (PCB × 3) arms exhibited grade 4 or complete re-epithelialization. The underlying dermis showed moderate fibroplasia, scores being similar across all study arms, with expected production of dermal collagen and minimal neovascularization. The amount and density of new collagen was less to that observed in the adjacent normal dermis; however, it was still more than the collagen observed at 14 days (Figure 5). Neovascularization was evident mostly in the superficial dermis indicating ongoing maturation of the collagen architecture and progression of wound healing compared to that at 14 days. Dermal inflammation was scored as mild to minimal in most wounds across all treatment arms (Figure 6).
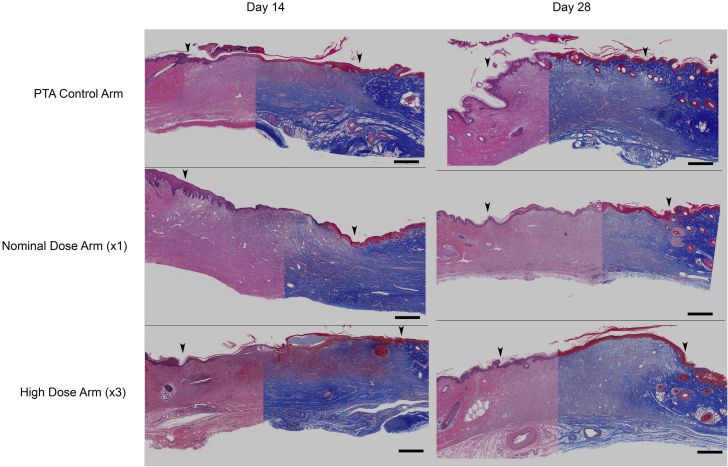
Figure 5.
Healing Response Histology
Composite images (Masson’s trichrome, right; hematoxylin and eosin stain, left) of PTA control arm, nominal-dose (PCB × 1) arm and high-dose (PCB × 3) arm at 14 and 28 days post-treatment, respectively (bar scale = 1 mm). Arrows indicate the approximate demarcation of the normal (nonwounded) skins to the left and right of the healing wound. Histological appearance of the healing responses across the study arms is similar; at 14 days, the dermal collagen is not as dense, and the collagen bundles are not as thick appearing in the center of the wound as in the flanking nonwounded skin areas and there is gradation of the collagen density along the wound border and from the base to the surface representing normal healing and progressive production of dermal collagen. At 28 days the wound surfaces are covered by keratinized epidermis and the wound defect is filled with mature granulation tissue. Abbreviations as in Figure 1.
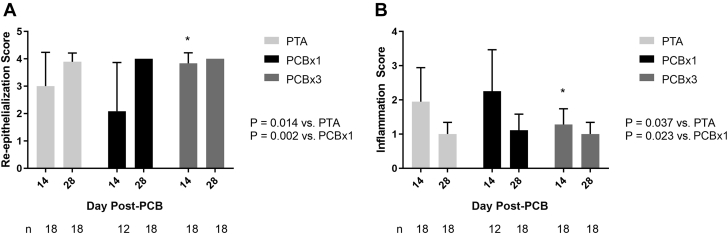
Figure 6.
Re-Epithelialization Score and Dermal Inflammation Score
(A) Re-epithelialization mean scores and SD by study arm. Numbers (n) of independent histological samples evaluated for epithelialization score are indicated. Significantly higher re-epithelialization score was found in the high-dose (PCB × 3) arm relative to both the PTA control and nominal-dose (PCB × 1) arms, although no significant differences were found between any arms at 28 days post-treatment (Mann-Whitney test). (B) Dermal inflammation mean scores and SD by study arm. Numbers (n) of independent histological samples evaluated for inflammation score indicated. Significantly lower inflammation score was found in the PCB × 3 arm relative to both the PTA control and PCB × 1 arms, although no significant differences were found between any arms at 28 days post-treatment (Mann-Whitney test). Abbreviations as in Figure 1.
Bioanalytical analysis of paclitaxel
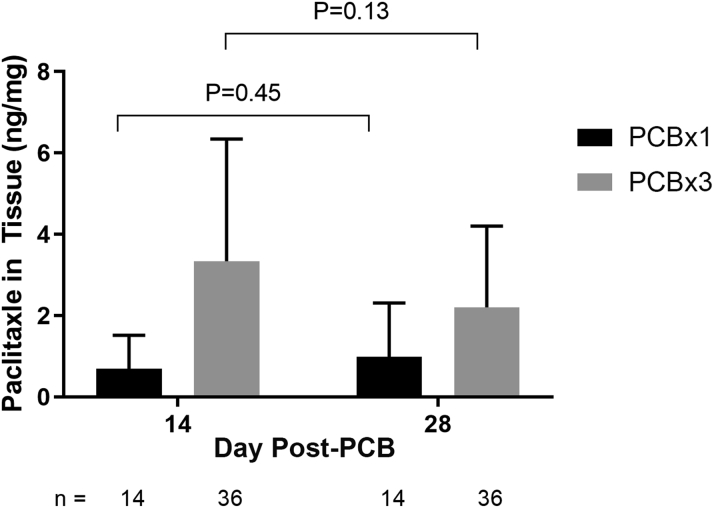
All biopsy samples obtained immediately adjacent to the wound in the nominal-dose (PCB × 1) and high-dose (PCB × 3) arms contained paclitaxel. The PCB × 3 arm had consistently higher concentrations of paclitaxel to that of the PCB × 1 arm at both time points (Figure 7). Drug concentrations in tissue showed no significant change from 14 to 28 days post treatment at either paclitaxel dose; 0.69 ng/mg and 0.98 ng/mg in the nominal-dose (PCB × 1) arm (p = 0.45) and 3.3 ng/mg and 2.2 ng/mg in the high-dose (PCB × 3) arm (p = 0.13) at 14 and 28 days, respectively.
Figure 7.
Paclitaxel Tissue Concentration
Mean amount of paclitaxel in full-thickness biopsy punch sample adjacent to wound. Mean drug concentration and SD are indicated for PCB × 1 and PCB × 3 study arms showing consistently greater drug concentrations with the high dose (PCB × 3) at both time points with no significant decline of drug concentrations from 14 to 28 days post-treatment in either arm. Abbreviations as in Figure 1.
Discussion
The major findings of this study include: 1) downstream paclitaxel embolization is a real phenomenon as confirmed by the presence of therapeutic paclitaxel tissue levels found around the healed wounds; 2) the presence of paclitaxel in the immediate vicinity of the lesions did not alter the dynamics of in vivo wound healing by any of the analytical parameters used in this study; and 3) histological evaluation of the wounds did not show evidence of paclitaxel toxicity even at a local dose 3 times higher than nominal.
Since the introduction of the PCB technology, paclitaxel particle embolization has been accepted as a trade-off for the clinical efficacy of the stent-free, single, but effective paclitaxel delivery for inhibition of restenosis. However, its occurrence has been feared by clinicians because of the concern that the downstream release of particulate paclitaxel may negatively impact the distal lower limb’s circulation and its tissues already compromised by chronic ischemia. Although the safety of PCB use in the SFA territory has been proven in large randomized controlled trials, most of these studies have excluded patients suffering of distal limb ulceration (1, 2, 3). For this reason, the concern has remained significant for more vulnerable patients such as those with below-the-knee (BTK) disease and/or CLI. Thus, the findings of the present study regarding the impact of resident paclitaxel on wound healing dynamics have important clinical implications for the PCB field.
An important finding of this study is the confirmation that paclitaxel particle embolization is a common phenomenon as evidenced by the relevant paclitaxel tissue levels around the healed wounds. The quantification of paclitaxel in the tissue adjacent to the healing wounds demonstrated drug content which should have been sufficient to produce inhibitory activity on proliferating cells (18). However, the methods generally used for determining drug content in tissue are unable to discriminate between soluble, bioavailable, sequestered, or solid-phase drug. In vivo studies have shown the presence of particulate drug deposited on the lumen of treated arteries by PCBs and the downstream deposition of microscopic drug particulates (5,6). Consequently, although the absolute drug content in downstream tissues may suggest the possibility that inhibition of wound healing may occur, the relatively slow rate of drug dissolution from the solid phase may limit the actual amount of bioavailable drug and its biological impact. As a result, normal wound healing could proceed in tissue with significant drug content if the amount of bioavailable drug is permissive within the wound itself. In the current study, significant paclitaxel content was detected throughout the entire time of the study in tissue in which full-thickness wounds successfully healed, supporting this hypothesis.
We used a well-characterized model and standardized analytical methods to evaluate wound healing dynamics (14, 15, 16, 17). The porcine model used to study wound healing provided highly reproducible wounds producing consistent granulation tissue responses and complete healing over the 28-day observation period. The most dramatic index of healing response in this study, margin separation score (Table 1), provided highly similar trends in rate of healing between all study arms with no indication of drug-induced inhibition of wound closure. Concurrently, resolution of gross wound–associated inflammation, scored as purulent discharge, trended for more rapid resolution of discharge in the high-dose (PCB × 3) arm, with no indication of any prolongation of inflammatory responses in the PCB-treated arms relative to the control. Likewise, resolution of all other inflammation and cosmesis parameters progressed in similar fashion across all three arms. Consequently, gross observation provided no suggestion for inhibition of the wound healing responses in the PCB-treated study arms.
Finally, histological evaluation of the wounds did not show evidence of paclitaxel-induced toxicity despite demonstrated residence of the drug in the wound vicinity. Histological scoring of re-epithelialization also failed to show any indication of drug-induced inhibition of healing; paradoxically, the high-dose (PCB × 3) arm showed significantly greater epithelialization at day 14 than the nominal-dose (PCB × 1) arm or the PTA control. This phenomenon was transient and no differences in epithelialization were detected between any arms at day 28. Histological scoring of inflammation also revealed that, the high-dose (PCB × 3) arm showed significantly less inflammation at day 14 than the nominal-dose (PCB × 1) arm or the PTA control. As previously, this observation was transient and inflammation scores were equivalent at day 28 but is in general agreement with the differences observed grossly in purulent exudate during the early phases of healing. Paclitaxel has been observed to inhibit leukocyte transmigration and chemotaxis as well as other inflammation associated responses, although the magnitude of differences in the current study are relatively small and have no long-term impact (19). The decreased inflammation was also associated with increased epithelialization at day 14, and while inflammation is generally considered to be an integral part of the wound healing process, chronic inflammation may also impede it (20). However, the transient nature of the observation and the convergence of the healing processes across the study arms at 28 days diminish the potential impact in a clinical setting.
One of the areas in which the use of PCB is somewhat controversial but highly needed is in BTK applications. The challenges of addressing revascularization in CLI patients are complicated by comorbidities and possible impact of local drug on the resolution of wound healing. Exposure of the tissues distal to the treatment site has not been identified as a significant problem in the treatment of the SFA. However, treatment of lesions in the BTK setting could potentially result in increased exposure of these tissues due to smaller local tissue volumes and hinder resolution of healing wounds. Consequently, understanding the impact of distal drug exposure on wound healing following PCB use could provide insight on the relative risks associated with such revascularization approaches.
Study limitations
First, the animals used in this study were normal, healthy, juvenile swine with excellent limb perfusion and run-off, optimizing clearance of drug from the treated limbs. Secondly, the rate of tissue repair in the wounded limbs was optimal and not compromised by hypoxia or other comorbidities that might impede wound healing. Thirdly, the healing responses observed in this study were accompanied by an elevated level of wound care with oral antibiotics and frequent dressing changes, decreasing the chance of infection and chronic inflammation. Consequently, actual wound healing in a clinical setting of obstructive atherosclerosis may be impacted by factors which are not easily or ethically reproduced experimentally. Lastly, the investigational focus of our study was on a high-level binary endpoint of complete cutaneous wound healing (or lack thereof). Although the structural (macro- and microscopic) indicators of wound healing were sufficient to evaluate such a rudimentary endpoint, we did not expand into any mechanistic evaluation (e.g., characterization of inflammatory cells, matrix metalloproteinase expression and enzymatic activity, and the role of interleukins). For example, a recent study showed that blockade of interleukin 6 signaling prevents paclitaxel-induced neuropathy, and another suggested that paclitaxel at low dose has the potential to treat liver fibrosis in rats possibly through activation of interleukin 10; these cytokines would be interesting research targets in the context of the present study (21,22).
Conclusions
This study shows that significant tissue drug content post-PCB use in the proximal arterial flow does not preclude the possibility of wound healing in downstream tissues. Therefore, the biological effect of particulate paclitaxel embolization on wound healing dynamics in vascular territories with small distribution volume and poor distal runoff merits further investigation to properly balance the relative risks and benefits.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: CLI is a severe condition affecting about 10% of peripheral artery disease patients, characterized by lower limb tissue loss and associated with high morbidity and mortality. PCBs are a clinically proven antirestenotic alternative to plain PTA of SFA but their application in CLI has been stymied by a concern that the downstream release of particulate paclitaxel may negatively impact distal lower limb’s circulation and its tissues already compromised by chronic ischemia. To investigate this concern experimentally, we utilized an animal model of standardized distal limb wounds to determine the effect of downstream paclitaxel released during PCB treatment of SFA on distal wound healing process. This preclinical study shows that even a clearly measurable concentration of paclitaxel in the vicinity of the wound does not impair the healing of preexisting distal cutaneous lesions in healthy swine even after multiple PCB deployments.
TRANSLATIONAL OUTLOOK: Even though the outcomes obtained in healthy animal limbs with excellent runoff and without any perfusion deficit are not directly translatable to severely diseased limbs with perfusion impairment, ischemia, and tissue loss, the lack of negative impact of clearly measurable concentration of paclitaxel in the vicinity of a skin wound on its healing encourage further clinical research of risks and benefits of PCB treatment in CLI patients and offers hope that such treatment option might be eventually proven safe in this subset of PAD patient population for whom prognosis is poor yet the therapeutic options remain limited.
Funding Support and Author Disclosures
Ms. Crookall, and Drs. Schulz-Jander, Tunev, and Melder are full-time employees of Medtronic PLC, the manufacturer of the IN.PACT Admiral product evaluated in the study. Dr. Granada is the CEO, and Dr. Kaluza is an employee of the Cardiovascular Research Foundation which receives research and educational grant support from Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Lindsey Rau, PhD, and Don Maul, DVM, MS, DACLAM, for their numerous contributions to the planning and execution and Kristen Nikula, DVM, PhD, DACVP, FIATP, for her histological evaluations.
Footnotes
Craig Emter, MD, served as Guest Editor for this paper.
Michael Bristow, MD, PhD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Laird J.A., Schneider P.A., Jaff M.R. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfield K., Jaff M.R., White C.J. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 3.Brodmann M., Werner M., Meyer D.R. Sustainable antirestenosis effect with a low-dose drug-coated balloon: the ILLUMENATE European randomized clinical trial 2-year results. J Am Coll Cardiol Intv. 2018;11:2357–2364. doi: 10.1016/j.jcin.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Cremers B., Speck U., Kaufels N. Drug-eluting balloon: very short-term exposure and overlapping. Thromb Haemost. 2009;101:201–206. [PubMed] [Google Scholar]
- 5.Gongora C.A., Shibuya M., Wessler J.D. Impact of paclitaxel dose on tissue pharmacokinetics and vascular healing: a comparative drug-coated balloon study in the familial hypercholesterolemic swine model of superficial femoral in-stent restenosis. J Am Coll Cardiol Intv. 2015;8:1115–1123. doi: 10.1016/j.jcin.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Yazdani S.K., Pacheco E., Nakano M. Vascular, downstream, and pharmacokinetic responses to treatment with a low dose drug-coated balloon in a swine femoral artery model. Catheter Cardiovasc Interv. 2014;83:132–140. doi: 10.1002/ccd.24995. [DOI] [PubMed] [Google Scholar]
- 7.Boitet A., Grassin-Delyle S., Louedec L. An experimental study of paclitaxel embolisation during drug coated balloon angioplasty. Eur J Vasc Endovasc Surg. 2019;57:578–586. doi: 10.1016/j.ejvs.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Kempin W., Kaule S., Reske T. In vitro evaluation of paclitaxel coatings for delivery via drug-coated balloons. Eur J Pharm Biopharm. 2015;96:322–328. doi: 10.1016/j.ejpb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Seidlitz A., Kotzan N., Nagel S. In vitro determination of drug transfer from drug-coated balloons. PloS One. 2013;8 doi: 10.1371/journal.pone.0083992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torii S., Jinnouchi H., Sakamoto A. Comparison of biologic effect and particulate embolization after femoral artery treatment with three drug-coated balloons in healthy swine model. J Vasc Interv Radiol. 2019;30:103–109. doi: 10.1016/j.jvir.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 11.Katsanos K., Spiliopoulos S., Kitrou P., Krokidis M., Paraskevopoulos I., Karnabatidis D. Risk of death and amputation with use of paclitaxel-coated balloons in the infrapopliteal arteries for treatment of critical limb ischemia: a systematic review and meta-analysis of randomized controlled trials. J Vasc Interv Radiol. 2020;31:202–212. doi: 10.1016/j.jvir.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Zeller T., Micari A., Scheinert D. The IN.PACT DEEP clinical drug-coated balloon trial: 5-year outcomes. J Am Coll Cardiol Intv. 2020;13:431–443. doi: 10.1016/j.jcin.2019.10.059. [DOI] [PubMed] [Google Scholar]
- 13.Guide for the Care and Use of Laboratory Animals / Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. 8th ed. National Academies Press; Washington, DC: 2011. https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf Available at: [Google Scholar]
- 14.Draize J.H., Woodard G., Calvery H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–390. [Google Scholar]
- 15.Quinn J.V., Wells G.A. An assessment of clinical wound evaluation scales. Acad Emerg Med. 1998;5:583–586. doi: 10.1111/j.1553-2712.1998.tb02465.x. [DOI] [PubMed] [Google Scholar]
- 16.Premarket Notification [510(k)] Submissions for Testing for Skin Sensitization To Chemicals In Natural Rubber Products. January 13, 1999. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/premarket-notification-510k-submissions-testing-skin-sensitization-chemicals-natural-rubber-products Available at: Accessed October 1, 2020.
- 17.Quinn J., Wells G., Sutcliffe T. Tissue adhesive versus suture wound repair at 1 year: randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann Emerg Med. 1998;32:645–649. doi: 10.1016/s0196-0644(98)70061-7. [DOI] [PubMed] [Google Scholar]
- 18.Axel D.I., Kunert W., Goggelmann C. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. doi: 10.1161/01.cir.96.2.636. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D., Yang R., Wang S., Dong Z. Paclitaxel: new uses for an old drug. Drug Des Devel Ther. 2014;8:279–284. doi: 10.2147/DDDT.S56801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diegelmann R.F., Evans M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 21.Huehnchen P., Muenzfeld H., Boehmerle W., Endres M. Blockade of IL-6 signaling prevents paclitaxel-induced neuropathy in C57Bl/6 mice. Cell Death Dis. 2020;11:45. doi: 10.1038/s41419-020-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharawy M.H., Abdel-Rahman N., Megahed N., El-Awady M.S. Paclitaxel alleviates liver fibrosis induced by bile duct ligation in rats: role of TGF-β1, IL-10 and c-Myc. Life Sci. 2018;211:245–251. doi: 10.1016/j.lfs.2018.09.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.