Abstract
Objective
To present radiofrequency ablation (RFA) of parathyroid adenomas as a safe and effective management strategy for primary hyperparathyroidism in patients who are not eligible for surgery or those who do not want surgery.
Methods
The diagnosis of primary hyperparathyroidism was confirmed by laboratory investigations. A bone density scan showed osteoporosis, which was an indication for the surgical treatment of primary hyperparathyroidism. Ultrasonography of the neck was done to localize the parathyroid adenoma, after which RFA was performed to shrink the adenoma. Laboratory investigations were performed 10 days, 6 months, and 12 months after the procedure. A literature review was also conducted, and other reports of primary hyperparathyroidism cases treated with RFA were identified.
Results
Biochemical cure of primary hyperparathyroidism was achieved by normalization of calcium levels, resolution of symptoms, elimination of complications, and decrease in the volume of the parathyroid adenoma.
Conclusion
RFA of parathyroid adenomas is a viable alternative to parathyroidectomy in patients who do not meet the criteria for surgery or do not wish to undergo surgery.
Key words: hyperparathyroidism, parathyroid adenoma, radiofrequency ablation
Abbreviations: D5W, dextrose 5% in water; iPTH, intact parathyroid hormone; PHPT, primary hyperparathyroidism; PTH, parathyroid hormone; RFA, radiofrequency ablation
Introduction
Primary hyperparathyroidism (PHPT) has traditionally been effectively treated with surgery, with minimally invasive parathyroidectomy preferred to open parathyroidectomy for single-gland disease.1 A select group of patients who are not eligible for surgery because of comorbidities and those who do not want surgery because of the risk of scarring or personal preference may benefit from an alternative approach.
Radiofrequency ablation (RFA) is a technique that uses an electrode with high-frequency alternating current to cause thermal injury and coagulative necrosis in a soft tissue. It has been used to ablate primary and metastatic tumors in the liver, lung, bone, and kidney, as well as in cardiac conduction pathways. RFA has been used in Korea and Italy for the treatment of benign thyroid nodules and more recently, has been gaining popularity in the U.S.
RFA of neck lesions is safer to perform using a transisthmic approach and hydrodissection.2 In the transisthmic approach, the electrode is inserted from the midline-to-lateral direction into the target nodule. This minimizes heat exposure to the recurrent laryngeal nerve and/or esophagus, prevents change in the position of the electrode because of talking or swallowing, and avoids leakage of a hot fluid into the perithyroidal area.3 Hydrodissection involves injecting cold dextrose 5% in water (D5W) between the target nodule and critical structures, creating a safety margin to isolate the nodule and prevent thermal injury to adjacent structures.2 RFA is generally safe and has a low complication rate (∼2.1%) in benign thyroid nodules.2 The major reported complications include nerve injuries, particularly the recurrent laryngeal nerve, and nodule rupture. The minor complications include hematomas, vomiting, skin burns, and pain.2,4 There are limited data regarding the complications when ablating parathyroid adenomas; however, hypocalcemia and voice hoarseness have been reported.5
We present the first reported case, to our knowledge, of PHPT treated with RFA in the U.S. Our objective was to demonstrate that RFA is a safe and effective alternative to treat parathyroid adenomas in patients who are not eligible for surgery or those who do not want surgery. This will add to the growing body of literature supporting the use of minimally invasive nonsurgical techniques for the treatment of endocrine disorders.
Case Report
A 67-year-old woman presented for further management of her PHPT. Her symptoms included fatigue, difficulty in swallowing, nausea, muscle cramps, anxiety, and shortness of breath. She did not have constipation, abdominal pain, nephrolithiasis, bone pain, or depression. Her laboratory workup, performed at Intermountain Laboratory, Utah, was significant for an intact parathyroid hormone (iPTH) level of 104 pg/mL (normal range: 22-94 pg/mL), 25-hydroxy D vitamin level of 64 ng/mL (normal range: 30-80 ng/mL), and serum calcium level of 11.5 mg/dL (normal range: 8.4-10.4 mg/dL). Dual-energy X-ray absorptiometry revealed that the patient had osteoporosis, with a T-score of −2.7 in the femoral neck. Forearm imaging was not performed. Therefore, she was started on oral bisphosphonates; however, she did not tolerate the medication and stopped it. The patient met the criteria for surgery for PHPT because she had osteoporosis and her serum calcium level was more than 1 mg/dL above the upper limit of the normal range. She had undergone 2 prior surgical consultations and refused surgical intervention after a discussion with the surgeons. The patient was offered an alternative option of RFA of her parathyroid adenoma, and after discussing the risks and benefits, she opted for this over surgery or medical management.
Preparation
A bedside neck ultrasound was performed in preparation for the procedure, and a left inferior parathyroid adenoma measuring 0.83 cm × 0.50 cm × 0.76 cm (volume: 0.16 cm3) was localized. This was confirmed by fine-needle aspiration with a parathyroid hormone (PTH) washout of >32 000 pg/mL (LabCorp, normal range: <10 pg/mL). The RFA procedure was performed at an outpatient surgery center. Informed consent was obtained, and standard aseptic techniques were used.
Procedure
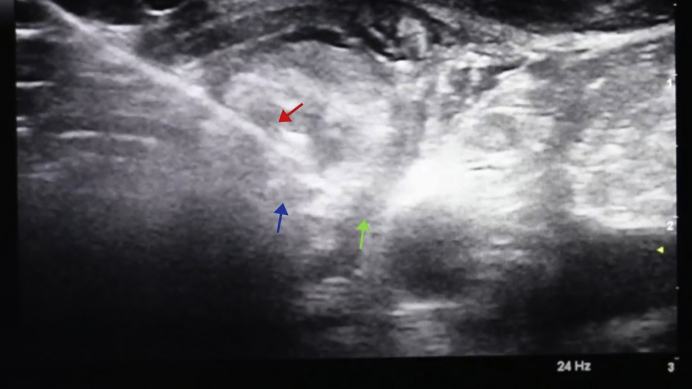
The parathyroid adenoma was again visualized in real time using ultrasound (Fig. 1); 2% lidocaine was used for local anesthesia. This was followed by hydrodissection to avoid damage to the recurrent laryngeal nerve; a total of 5 boluses of 10 mL of D5W were injected during the course of the ablation. Using the transisthmic approach, an 18-gauge RFA probe with a 0.5-cm active tip was introduced into the adenoma (Fig. 2), and ablation was started at 30 W of radiofrequency power. A total of 35 W of energy was delivered, and the total active ablation time was 1 minute. The patient tolerated the procedure well, and her vital signs remained within the normal range. Her voice remained normal, and voice strength was checked by verbalization both during and after the procedure. She was treated with enteric-coated ibuprofen for 3 days to minimize posttreatment inflammation and pain.
Fig. 1.
Neck ultrasonogram in sagittal view showing a yellow arrow indicating a hypoechoic nodule representing the left inferior parathyroid adenoma, measuring 0.76 cm in length, 0.50 cm in depth, and 0.83 cm in width, with a calculated volume of 0.167 cm3. A polar artery represented by the red and blue color Doppler at the inferior margin. Color Doppler also shows a red area inferior and medial to the parathyroid adenoma representing the common carotid artery.
Fig. 2.
Ultrasound-guided RFA procedure performed with visualization of the left parathyroid adenoma in transverse view. The green arrow indicates a hypoechoic area of separation (green arrow) created between the tracheoesophageal groove/carotid artery and parathyroid adenoma by hydrodissection (ie, injection of D5W into the space between the structures). The blue arrow indicates the left inferior parathyroid adenoma. The red arrow) indicates the RFA probe with its tip within the parathyroid adenoma. The entire length of the probe was visualized as a hyperechoic line using ultrasound (transisthmic approach, parallel to the plane of the transducer). The parathyroid tissue near the needle tip became hyperechoic as it was ablated. D5W = dextrose 5% in water; RFA = radiofrequency ablation.
Follow-up
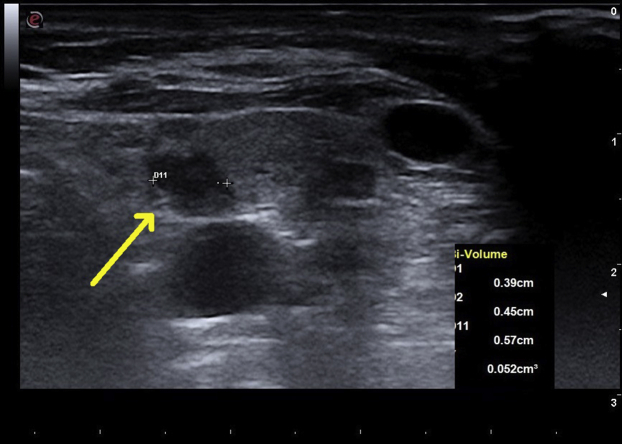
She was examined in clinic 10 days after RFA; she had no swallowing difficulty, breathing difficulty, cough, or hoarseness of voice. Both the calcium (10.1 mg/dL) and iPTH levels (85 pg/mL) were normalized. The ablated parathyroid adenoma was smaller, with ultrasonography showing decreased Doppler blood flow. However, a polar artery was still present. She felt well during her 6-month follow-up. Her laboratory study results indicated a serum calcium level of 10.2 mg/dL, iPTH level of 75 pg/mL, 25-hydroxy vitamin D level of 68 ng/mL, thyroid-stimulating hormone level of 1.63 mIU/L (normal range: 0.46-4.88 mIU/L), and free thyroxine level of 0.93 ng/dL (normal range: 0.75-1.5 ng/dL). A repeat ultrasound of her neck at this visit (Fig. 3) showed that the previously ablated parathyroid adenoma now measured 0.57 cm × 0.45 cm × 0.39 cm (volume: 0.05 cm3). Doppler blood flow observed using ultrasonography showed that the adenoma was now avascular, with the polar artery no longer visible. She was examined again 12 months after RFA and was noted to have a serum calcium level of 9.8 mg/dL and an iPTH level of 84 pg/mL. Ultrasonography showed that her adenoma now measured 0.54 cm × 0.24 cm × 0.47 cm (volume: 0.03 cm3) (Fig. 4). This represented a volume reduction of 80.7% compared with the volume seen in the initial neck ultrasonography, allowing for intraobserver variability of measurement, which was a significant decrease.
Fig. 3.
Neck ultrasonogram in sagittal view 6 months after RFA of the left inferior parathyroid adenoma. The yellow arrow indicates a hypoechoic nodule representing an adenoma now measuring 0.57 cm in length, 0.39 cm in depth, and 0.45 cm in width, with a calculated volume of 0.052 cm3. RFA = radiofrequency ablation.
Fig. 4.

Neck ultrasonogram in sagittal view 12 months after RFA of the left inferior parathyroid adenoma. The yellow arrow indicates a hypoechoic area representing an adenoma now measuring 0.54 cm in length, 0.24 cm in depth, and 0.47 cm in width, with a calculated volume of 0.032 cm3. RFA = radiofrequency ablation.
Discussion
RFA of parathyroid adenomas is a viable approach for treating PHPT, especially in patients who are not eligible for surgery. It is unlikely to replace surgery as a first-line treatment, but it is a reasonable choice for patients with symptomatic hypercalcemia who refuse or cannot tolerate surgery. Our case showed normalization of the serum calcium and iPTH levels (∼28% decrease) 6 months after the procedure, which was similar to biochemical cure after surgery (defined as normocalcemia persisting for more than 6 months postoperatively).6 Her calcium level remained normal 12 months after the procedure, and the adenoma continued to shrink.
Recurrent hyperparathyroidism (ie, normal PTH and calcium levels for at least 6 months postoperatively before experiencing recurrence) is far less common than persistent hyperparathyroidism (ie, failure of PTH and calcium levels to normalize after a procedure).6,7 The median time for the recurrence of PHPT is 40 months after parathyroidectomy.8 Therefore, a longer follow-up period is needed to determine if the recurrence rates are comparable with RFA even though having no persistent disease at 12 months in our case was promising. Similarly, there are no long-term data on whether RFA is equivalent to surgery in terms of outcomes regarding bone health, kidney disease, and nephrolithiasis. Larger studies are needed to evaluate these effects.
PHPT increases the risks of renal stones, cortical bone loss, and fractures.9 Although cinacalcet is approved for the medical management of PHPT, it only normalizes the serum calcium levels in ∼10% of patients and does not affect the rate of bone loss.9,10 Furthermore, it is expensive and has significant adverse effects. Bisphosphonates can improve bone mineral density in patients with PHPT, but it is unknown if they reduce the risk of fracture.9 Conversely, it has been suggested that they may be associated with an increased fracture risk in this population.11 Denosumab is associated with an increased bone mineral density in PHPT, but there are no data to indicate the ideal duration of therapy or whether it reduces the long-term fracture risk.12 Thus, surgical management is considered superior to the medical management of PHPT.13 In this situation, RFA has the potential to be a cheaper and more effective option for the treatment of PHPT in patients who otherwise meet the criteria for surgery but either cannot or will not undergo surgical management.
There are no randomized trials comparing RFA for hyperparathyroidism with surgery; however, case reports, notably all from outside the U.S., have been reported. A literature review was conducted by searching PubMed and the internet using the key words “radiofrequency ablation,” “primary hyperparathyroidism,” and “parathyroid adenoma,” which were not limited to the English language and did not have a time limit. Nine reports were identified that described 32 cases of PHPT treated with RFA (Table).5,14, 15, 16, 17, 18, 19, 20, 21 In these cases, RFA was performed because of either contraindication to surgery or the patient refusing surgery, and there was limited follow-up. Of note, in 1 case, RFA was performed inadvertently when a parathyroid adenoma was mistaken for a thyroid nodule; this was the only case where there was no improvement in biochemical profile.20 Twelve patients had decreases in the serum calcium and iPTH levels after RFA, and 19 had complete normalization of the serum calcium and iPTH levels.5,15,16 Only 3 patients had minor complications. RFA in all the cases was performed under local anesthesia. Generally, a perithyroidal lidocaine injection is recommended for pain control.2 This has several advantages, including the ability to monitor voice, and by extension, the recurrent laryngeal nerve, in real time; detect complications early and intervene if necessary. General anesthesia also carries a higher risk of complications in patients with significant comorbidities.
Table.
Cases of Parathyroid Adenomas Causing PHPT Treated with RFA Reported in the Literature
| Authors (year), country | Cases, n | Baseline elevated calcium, n | Operator experience, y | Complete response, na | Partial response, nb | No response, nc | Complications, n | Follow-up duration |
|---|---|---|---|---|---|---|---|---|
| Ha et al (2020), South Korea5 | 11d | 11 | 22, 19, and 14 | 7 | 4e | 0 | 1 (transient hypocalcemia) | 13.6 ± 18.7 (range: 3-69) mo |
| Korkusuz et al (2018), Germany14 | 9 | 8f | NR | 5 | 4 | 0 | 0 | ∼3 mo |
| Sormaz et al (2017), Turkey15 | 5g | 5 | NR | 3 | 2h | 0 | 1 (hypocalcemia) | ∼6 mo |
| Shenoy et al (2017), India16 | 1 | 1 | NR | 0 | 1i | 0 | 0 | <4 wk (total 2 y) |
| Sattarinezhad et al (2017), Iran17 | 1 | 1 | 12j | 1 | 0 | 0 | 0 | 1 y |
| Xu et al (2013), China19 | 2 | 2 | NR | 1 | 1k | 0 | 1 (transient hoarseness) | 2 mo |
| Kim et al (2013), South Korea20 | 1 | UNKl | NR | 0 | 0 | 1 | 0 | 4 y |
| Kim et al (2013), South Korea18 | 1 | 1 | NR | 1 | 0 | 0 | 0 | 20 mo |
| Hansler et al (2002), Germany21 | 1 | 1 | Noted to be experimental | 1m | 0 | 0 | 0 | 1 y |
Abbreviations: NR = not reported; PHPT = primary hyperparathyroidism; PTH = parathyroid hormone; RFA = radiofrequency ablation; UNK = unknown.
Number of patients with normal serum iPTH and calcium levels after RFA.
Number of patients with reduced serum PTH and/or calcium levels after RFA that still remained above the upper limit of the normal range.
Number of patients with unchanged hypercalcemia or further elevated serum iPTH and/or calcium levels after RFA.
Ten patients were treated with 1 RFA session, and 1 patient was treated with 2 sessions.
One patient underwent surgery because of an overlooked parathyroid adenoma on the contralateral side, an 2 patients were not fully treated because they refused to undergo additional treatment.
One patient did not have hypercalcemia, and the calcium level remained normal after the procedure.
One patient had recurrent PHPT after prior parathyroidectomy 6 years ago, and 1 patient underwent 2 RFA sessions 2 weeks apart because the patient was not fully treated in the first session, while the other 4 underwent 1 RFA session.
Two patients had normal serum calcium but elevated iPTH levels (initial PTH levels were 856 and 1575 pg/mL); the goal in 1 patient was to control hypercalcemia to stabilize for surgery, which was achieved; the other patient also underwent surgery 8 months after RFA because of concern for parathyroid malignancy (benign surgical pathology).
Patient was unable to tolerate second RFA session and was thus treated with percutaneous ethanol ablation a few weeks after RFA. The patient had normal serum iPTH and calcium levels at the end of 2-year follow-up.
Operator was reported to be experienced; experience was calculated from the date of RFA training listed on a research profile.
Patient had a normal calcium level and a down-trending iPTH level.
Serum iPTH and calcium levels from the time of the RFA procedure were not reported; subsequent levels 4 years later were elevated, with increased adenoma size.
Improvement in bone density was reported at 1 year.
There were no complications in our case, with low complication rates similar to those reported in the literature,5,14, 15, 16, 17, 18, 19, 20 suggesting that with appropriate training, this a relatively safe procedure. Operator experience likely plays a role in the complication rate. Techniques such as hydrodissection with D5W are effective in isolating the adenoma from vital structures, thus reducing the risk of damage to surrounding tissues. In addition, for parathyroid adenomas, a lower power output and smaller active tip size are recommended.
One of the risks of RFA of parathyroid adenomas, especially large ones, is the risk of ablating a parathyroid carcinoma because it is not always possible to differentiate an adenoma from a carcinoma prior to surgery. RFA is best performed in benign nodules because ablating a cancerous nodule leaves a positive margin and has a high risk of recurrence. If there is any suspicion of a parathyroid carcinoma based on suggestive clinical or ultrasonographic features, wide-excision surgical resection would be the best choice unless RFA is considered for palliative reasons.
The location of the parathyroid adenoma (inferior glands are more commonly present in aberrant locations) also influences the complication rate.22 A cuff of nonablated tissue posteriorly for an inferior parathyroid gland or anteriorly for a superior parathyroid gland should be left in order to avoid damage to the recurrent laryngeal nerve. The patient is asked to vocalize throughout the procedure to assess voice changes. Nerve monitoring is only possible under general anesthesia. Intrathyroidal parathyroid adenomas are rare, with reported rates of 0.7% to 6.7%.23 Their surgical management requires partial thyroidectomy, which is associated with the risks of hypothyroidism and vocal cord paralysis. RFA is a potentially safer alternative, with a lower risk of complications. RFA works best with a single adenoma that is easily visible on an ultrasonogram. Adenomas that are large enough to cause neck discomfort or cosmetic problems would be especially good candidates. However, patients who have multigland hyperplasia or syndromic hyperparathyroidism would likely respond better to the surgical approach of subtotal parathyroidectomy.
Conclusion
RFA of parathyroid adenomas is a viable alternative to parathyroidectomy for patients who do not meet the criteria for surgery or those who do not wish to undergo surgery. Future reports with long-term follow-up will be helpful in determining whether this procedure is as effective as surgery with regard to the recurrence of PHPT. Randomized controlled trials comparing RFA to surgery would be useful to determine if this procedure should be offered as a treatment option in patients with hyperparathyroidism who are eligible for surgery.
Acknowledgment
We thank Daniel Baek for his help with the figures and Dr. Naim Maalouf for his advice.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Laird A.M., Libutti S.K. Minimally invasive parathyroidectomy versus bilateral neck exploration for primary hyperparathyroidism. Surg Oncol Clin N Am. 2016;25(1):103–118. doi: 10.1016/j.soc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.H., Baek J.H., Lim H.K. 2017 thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655. doi: 10.3348/kjr.2018.19.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H.S., Baek J.H., Park A.W., Chung S.R., Choi Y.J., Lee J.H. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623. doi: 10.3348/kjr.2017.18.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J.F., Wu T., Hu K.P. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J. 2017;130(11):1361–1370. doi: 10.4103/0366-6999.206347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha E.J., Baek J.H., Baek S.M. Minimally invasive treatment for benign parathyroid lesions: treatment efficacy and safety based on nodule characteristics. Korean J Radiol. 2020;21(12):1388. doi: 10.3348/kjr.2020.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii H., Mihai R., Watkinson J.C., Kim D.S. Systematic review of cure and recurrence rates following minimally invasive parathyroidectomy. BJS Open. 2018;2(6):364–370. doi: 10.1002/bjs5.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udelsman R. Approach to the patient with persistent or recurrent primary hyperparathyroidism. J Clin Endocrinol Metab. 2011;96(10):2950–2958. doi: 10.1210/jc.2011-1010. [DOI] [PubMed] [Google Scholar]
- 8.Mazotas I.G., Yen T.W.F., Doffek K. Persistent/recurrent primary hyperparathyroidism: does the number of abnormal glands play a role? J Surg Res. 2020;246:335–341. doi: 10.1016/j.jss.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Insogna K.L. Primary hyperparathyroidism. N Engl J Med. 2018;379(11):1050–1059. doi: 10.1056/NEJMcp1714213. [DOI] [PubMed] [Google Scholar]
- 10.Ng C.H., Chin Y.H., Tan M.H.Q. Cinacalcet and primary hyperparathyroidism: systematic review and meta regression. Endocr Connect. 2020;9(7):724–735. doi: 10.1530/EC-20-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh M.W., Zhou H., Adams A.L. The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med. 2016;164(11):715–723. doi: 10.7326/M15-1232. [DOI] [PubMed] [Google Scholar]
- 12.Leere J.S., Karmisholt J., Robaczyk M. Denosumab and cinacalcet for primary hyperparathyroidism (DENOCINA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2020;8(5):407–417. doi: 10.1016/S2213-8587(20)30063-2. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm S.M., Wang T.S., Ruan D.T. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968. doi: 10.1001/jamasurg.2016.2310. [DOI] [PubMed] [Google Scholar]
- 14.Korkusuz H., Wolf T., Grunwald F. Feasibility of bipolar radiofrequency ablation in patients with parathyroid adenoma: a first evaluation. Int J Hyperthermia. 2018;34(5):639–643. doi: 10.1080/02656736.2018.1453552. [DOI] [PubMed] [Google Scholar]
- 15.Sormaz I.C., Poyanli A., Acar S. The results of ultrasonography-guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol. 2017;40(4):596–602. doi: 10.1007/s00270-016-1544-6. [DOI] [PubMed] [Google Scholar]
- 16.Shenoy M.T., Menon A.S., Nazar P.K. Radiofrequency ablation followed by percutaneous ethanol ablation leading to long-term remission of hyperparathyroidism. J Endocr Soc. 2017;1(6):676–680. doi: 10.1210/js.2017-00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattarinezhad A., Rasekhi A., Soveid M. Management of a parathyroid adenoma with radiofrequency ablation: a case report. Iran Red Crescent Med J. 2017;19(2) [Google Scholar]
- 18.Kim B.S., Eom T.I., Kang K.H., Park S.J. Radiofrequency ablation of parathyroid adenoma in primary hyperparathyroidism. J Med Ultrason (2001) 2014;41(2):239–243. doi: 10.1007/s10396-013-0501-0. [DOI] [PubMed] [Google Scholar]
- 19.Xu S.Y., Wang Y., Xie Q., Wu H.Y. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. Singapore Med J. 2013;54(7):e137–e140. doi: 10.11622/smedj.2013092. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.S., Choi B.H., Park J.R. Delayed surgery for parathyroid adenoma misdiagnosed as a thyroid nodule and treated with radiofrequency ablation. Endocrinol Metab (Seoul) 2013;28(3):231–235. doi: 10.3803/EnM.2013.28.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansler J., Harsch I.A., Strobel D., Hahn E.G., Becker D. Treatment of a solitary adenoma of the parathyroid gland with ultrasound-guided percutaneous radio-frequency-tissue-ablation (RFTA). Article in German. Ultraschall Med. 2002;23(3):202–206. doi: 10.1055/s-2002-33154. [DOI] [PubMed] [Google Scholar]
- 22.Taterra D., Wong L.M., Vikse J. The prevalence and anatomy of parathyroid glands: a meta-analysis with implications for parathyroid surgery. Langenbecks Arch Surg. 2019;404(1):63–70. doi: 10.1007/s00423-019-01751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman A., Politz D., Lopez J., Norman J. Intrathyroid parathyroid adenoma: incidence and location--the case against thyroid lobectomy. Otolaryngol Head Neck Surg. 2011;144(6):867–871. doi: 10.1177/0194599811400366. [DOI] [PubMed] [Google Scholar]