Abstract
Objective
Primary hypophysitis refers to the isolated inflammation of the pituitary gland not associated with other secondary causes. Among its histopathologic subtypes, xanthomatous is the rarest.
Methods
We describe a 22-year-old woman with xanthomatous hypophysitis (XH), its clinical progression over 8 years as well as the treatment effects of prednisolone and azathioprine. Our patient was first referred for severe short stature and delayed puberty at the age of 14 years.
Results
Investigations revealed multiple pituitary deficiencies. Magnetic resonance imaging showed a pituitary mass whereby a partial resection was performed. A full resection was not feasible due to the location of the mass. The histopathologic analysis of the tissue was consistent with XH. The results of secondary workout for neoplasm, infection, autoimmune, and inflammatory disorders were negative. After surgery, a progressive enlargement of the mass was observed. Two courses of prednisolone were administered with a significant reduction in the mass size. Azathioprine was added due to the unsustained effects of prednisolone when tapered off and the concern of steroid toxicity with continued use. No further increase in the mass size was noted after 6 months on azathioprine.
Conclusion
Glucocorticoid and immunotherapy are treatment options for XH; however, more cases are needed to better understand its pathogenesis and clinical progression.
Key words: pituitary mass, primary hypophysitis, suprasellar mass, xanthomatous hypophysitis
Abbreviations: AP, anterior-posterior; CC, cranio-caudal; DI, diabetes insipidus; LH, lymphocytic hypophysitis; MRI, magnetic resonance imaging; W, width; XH, xanthomatous hypophysitis
Introduction
Hypophysitis is an inflammatory condition of the pituitary gland that can mimic a neoplastic lesion.1, 2, 3, 4, 5, 6 Primary hypophysitis refers to the isolated inflammation of the pituitary gland not associated with other secondary causes. It is rare, with an estimated incidence of 1 in 7 to 9 million individuals and less than 100 cases reported in children.1, 2, 3, 4, 5, 6 The histopathologic subtypes include lymphocytic (most common), granulomatous, xanthomatous, plasmacytic, necrotizing, or a mixed picture.1, 2, 3, 4, 5, 6 Xanthomatous hypophysitis (XH) is the rarest subtype, with only 34 definite reported cases.7, 8, 9, 10, 11 Majority of the cases were young adult women (mean age, 39 years; range, 12-72 years) with only 1 reported case in a 12-year-old girl.7,8
The treatment options for hypophysitis include glucocorticoid, immunotherapy, surgery, and radiotherapy.1, 2, 3, 4, 5, 6 Unlike lymphocytic hypophysitis (LH), XH is rarely reported to be associated with autoimmunity, and response to glucocorticoid and immunotherapy is unknown.
Case Report
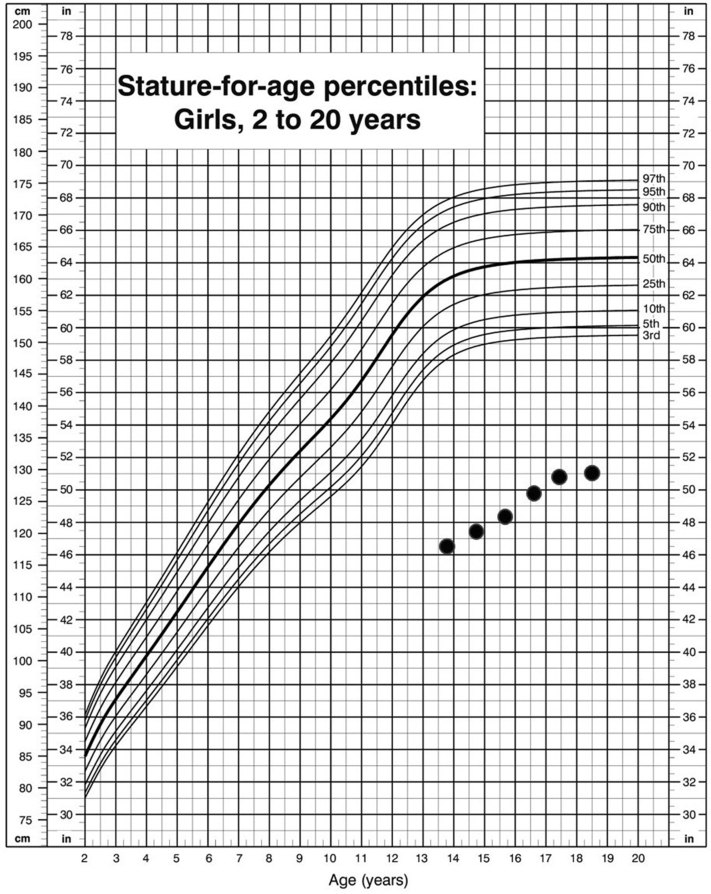
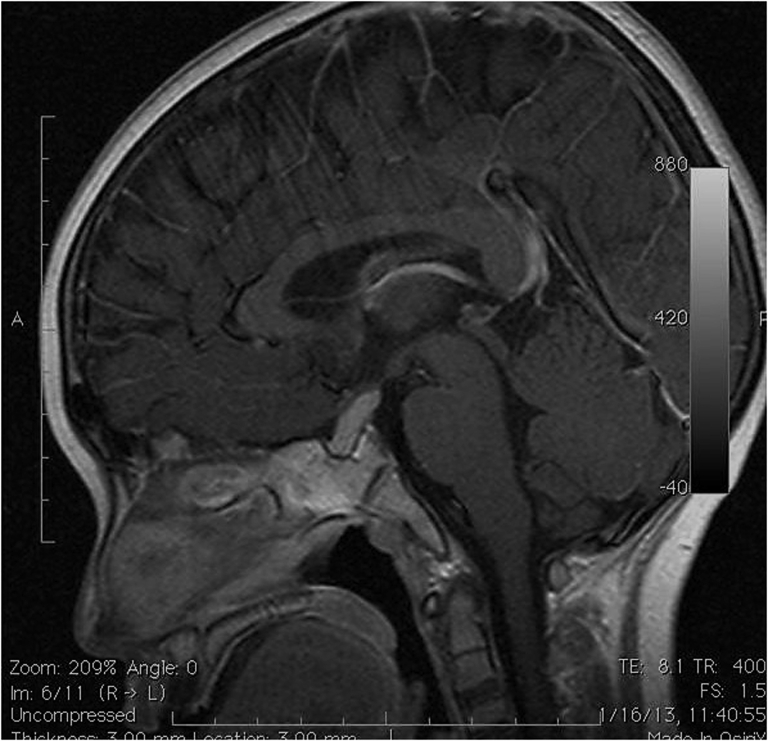
We present a 22-year-old woman with XH who was referred at the age of 14 years because of delayed puberty and poor growth (Fig. 1). She was prepubertal upon initial examination, with a height and weight of 120 cm (−6.12 standard deviation score) and 21.6 kg (−5.58 standard deviation score), respectively (refer to Fig. 1 for the growth chart). The findings of neurology, visual fields, and other systemic examinations were normal. Investigations revealed severe growth hormone deficiency, hypogonadotropic hypogonadism (insulin-like growth factor 1 <25 ng/mL; peak growth hormone, 0.47 ng/mL in insulin tolerance test; luteinizing hormone <0.2 mu/mL; and follicle-stimulating hormone, 2.7 mu/mL), and dyslipidemia (cholesterol, 7.9 mmol/L; low-density lipoprotein, 6.3 mmol/L; high-density lipoprotein, 1.9 mmol/L; and triglyceride, 0.81 mmol/L). Peak cortisol from the insulin tolerance test was optimal at 800 nmol/L. The results of thyroid function (free thyroxine, 13.6 pmol/L; thyroid-stimulating hormone, 2.27 mU/L), prolactin, complete blood count, liver and renal functions, electrolytes, calcium, magnesium, karyotype, antinuclear antibody, rheumatoid factor, thyroid antibodies, complement levels C3 and C4, erythrocyte sedimentation rate, and tumor markers (beta-hCG and alpha fetoprotein) were normal. Bone age was 9 years at a chronological age of 14 years. Magnetic resonance imaging (MRI) of the brain showed a homogeneously contrast-enhancing lobulated mass, measuring 0.6 × 1.3 × 1.7 cm (anterior-posterior [AP] × width [W] × cranio-caudal [CC]) arising from the pituitary stalk with extension to the sellar and suprasellar regions and abutting the optic chiasm (Fig. 2). She was started on pubertal induction and pravastatin. Growth hormone was not started in view of the MRI findings.
Fig. 1.
Growth chart of the patient.
Fig. 2.
Sagittal T1-weighted MRI image shows a homogenous contrast-enhancing lobulated suprasellar mass upon first presentation measuring 0.6 × 1.3 × 1.7 cm (AP × W × CC). AP = anterior-posterior; CC = cranio-caudal; MRI = magnetic resonance imaging; W = width.
She was treated for central hypothyroidism 2 years later at the age of 16 years (diagnosed from routine thyroid function monitoring) and diabetes insipidus (DI) at the age of 18 years. The diagnosis of DI was made when she was admitted for acute appendicitis. On day 2 after appendectomy, she had persistent polyuria (urine output range, 6-8 mL/kg/h) and hypernatremia (serum sodium range, 152-157 mmol/L). Paired samples confirmed the diagnosis of DI with an elevated serum osmolality of 322 mOsm/kg and a very low urine osmolality of 78 mOSm/kg. Her DI was controlled with a relatively low dose of oral desmopressin (25 μg twice daily). Prior to the acute event, she had no symptoms suggestive of DI, and the results of her serum sodium levels had been normal.
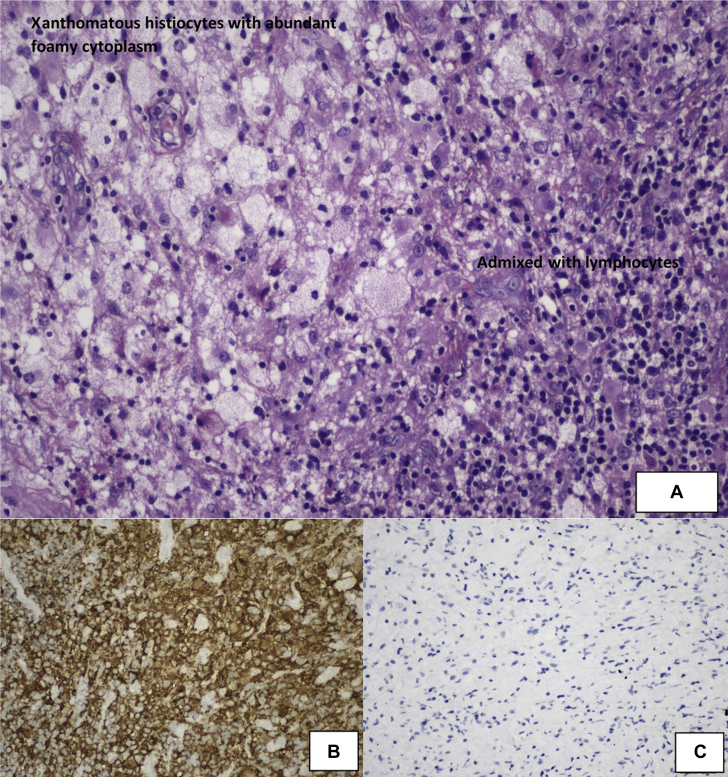
MRI was performed every 6th and 12th month from the initial presentation. Initially, there was no change in the mass size until 3 years later (at 17 years old), wherein it increased to 1.8 × 1.4 × 1.0 cm (AP × W × CC). She then underwent a partial resection via the transphenoidal approach. A complete resection was not performed due to the close proximity of the mass to the optic chiasm. The biopsied tissue was found to compose of fibrovascular tissues densely infiltrated by xanthomatous and histiocytic cells admixed with lymphocytes, plasma cells, eosinophils, and neutrophils (Fig. 3). No Langerhans cells, epitheliod granuloma, or malignant cells were seen. No infective organisms (fungal bodies and acid fast bacilli) were identified on special stains. The results of immunohistochemistry staining showed histiocytes that were immunoreactive to CD163 and CD68, negative for placental alkaline phosphatase and CD117 (markers for germinoma), and negative for S100 and CD1a (markers for Langerhans cell histiocytosis). The impression was a chronic inflammatory process with findings consistent with XH.
Fig. 3.
A, Histopathologic examination of the excised tissue shows a predominant infiltration of xanthomatous histiocytes with abundant foamy cytoplasm, which are admixed with lymphocytes, a few neutrophils, and eosinophils (hematoxylin-eosin; original magnification, x200). B, CD163 immunohistochemistry highlights the histiocytes (original magnification, x200). C, Absence of CD1a-positive histiocytes (original magnification, x200).
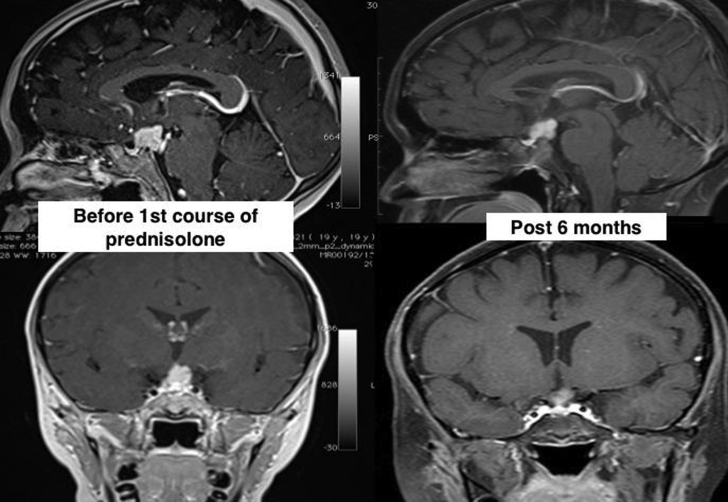
Postresection MRI over a 2-year period showed a continued increment of the mass size. A decision was made for a trial of steroids in view of the risks of surgery. Prednisolone 30 mg (1 mg/kg/day) was administered for 2 months and then gradually tapered off. MRI 6 months later showed a reduction in the mass size from 2.1 × 1.2 × 1.6 cm to 1.2 × 1.0 × 1.4 cm (AP × W × CC) (Fig. 4). While there was still a superior displacement of the optic chiasm, her vision remained normal.
Fig. 4.
Sagittal and coronal T1-weighted MRI images with contrast show comparison in the mass size before the first course of prednisolone (2.1 × 1.2 × 1.6 cm [AP × W × CC]) and the reduction in size after 6 months (1.2 × 1.0 × 1.4 cm [AP × W × CC]). AP = anterior-posterior; CC = cranio-caudal; MRI = magnetic resonance imaging; W = width.
The effect of steroid was unfortunately not sustained. Follow-up MRI 12 months after the withdrawal of prednisolone showed an increment in the mass size to 1.7 × 1.5 × 2.0 cm (AP × W × CC). A second course of prednisolone 35 mg (1 mg/kg/day) was subsequently started. Apart from a 3-kg weight gain, no other acute side effects of steroids were observed. A repeat MRI 3 weeks after showed a reduction in the mass size to 1.6 × 1.4 × 1.3 cm (AP × W × CC), with lesser compression onto the optic chiasm. Prednisolone was continued for a total of 2 months, then gradually tapered and shifted to a physiological dose of hydrocortisone 7 mg/m2/day (5 mg in the morning and 2.5 mg in the late afternoon).
Azathioprine was added during the prednisolone therapy as a steroid-sparing agent. It was started at 50 mg (1.6 mg/kg/day) for 2 weeks and then optimized to 75 mg once daily (2 mg/kg). The dose had to be reduced to 50 mg after 3 months because of lymphopenia (lowest acute lymphocytic count of 0.3 × 109/L), which improved after the dose reduction. Repeat MRI while on a 6-month azathioprine therapy did not show a further increment of the mass size.
Discussion
Etiology and Pathogenesis
The diagnosis of XH is made by the predominant presence of lipid-laden macrophages from the histopathologic examination of the pituitary tissue.7,8 Theories of its pathogenesis include an unknown initial precipitating event (possibly autoimmune, infectious, or localized endothelial dysfunction) that leads to the extravasation of macrophages into inflamed interstitial tissue.7,8 These macrophages phagocytosed the injured cell membranes, which contain phospholipids and cholesterol, and become characteristic lipid-laden xanthoma cells.
For all types of hypophysitis, it is essential to rule out an underlying systemic disease or secondary causes. Systemic diseases include inflammatory disorders and infections. Secondary causes can be due to a local lesion, such as a pituitary adenoma, craniopharygioma, Rathke cleft cyst, germinoma, or Langerhans cell histiocytosis.7, 8, 9, 10, 11 For the cystic forms of XH on MRI, differential diagnosis includes cystic adenoma or Rathke’s cleft cyst.11
Autoimmune and inflammatory markers as well as the 8-year follow-up did not reveal a secondary cause in our patient. The results of staining and immunohistochemical study of the biopsied tissue were negative for germinoma, Langerhans cell histiocytosis, and tuberculosis. Pituitary antibodies were not performed because these tests are not available in our laboratory. A high positivity of these antibodies suggest an autoimmune hypophysitis, although may also be present in a pituitary adenoma.2,3
Clinical Presentation
Hypophysitis may present as a neoplastic lesion.1, 2, 3, 4, 5, 6 It can present with symptoms of compression effects, pituitary deficiencies, or hyperprolactinemia. Gonadotrophin and growth hormone deficiencies were frequently reported in XH, whereas adrenocorticotropic hormone deficiency, hypothyroidism, and DI were less common.7, 8, 9, 10 This was similar in our patient who had gonadotrophin and growth hormone deficiency at presentation, followed by hypothyroidism and DI. Her adrenal function had remained normal for several years. She was started on a physiological dose of hydrocortisone after the completion of prednisolone in view of secondary adrenal suppression. Furthermore, visual symptoms are less commonly reported with XH.7, 8, 9, 10 The patient’s vision and visual field surveillance had remained normal over the years despite the close proximity of the mass to the optic chiasm.
The duration of symptoms of hypophysitis had been reported to be very variable. It may present acutely or over several years. The longest duration had been reported in XH.7, 8, 9, 10, 11 While our patient was first investigated at the age of 14 years, it was likely that the disease had been present much earlier considering the severe short stature at presentation and the significantly delayed bone age. The prognosis of XH is uncertain. Based on reports, it has ranged from recovery to partial resolution to no improvement.7, 8, 9, 10, 11
Management
There is no consensus on the treatment of hypophysitis owing to the wide spectrum of clinical presentation.1, 2, 3, 4, 5 The management consists of replacement of hormonal deficiencies and decreasing the mass size, in which the options include steroids, immunosuppressants, surgery, or radiotherapy. Surgery is indicated for compressive symptoms.1, 2, 3, 4, 5
Glucocorticoid is typically a first-line medical treatment, and the response has been reported to be favorable mostly in LH.1, 2, 3, 4, 5,12,13 The choice of steroid and dosage widely differs with experiences mostly reported in adult patients with LH. Types of steroids used include prednisolone/prednisone, dexamethasone, and methylprednisolone.1, 2, 3, 4, 5,12,13 We had chosen prednisolone due to its more frequent reported use in the literature. Also, our patient had chosen the oral route.
Immunosuppressants, most commonly azathioprine, were reported to be initiated if the steroid therapy failed or caused adverse effects. There had been a few case reports of success with LH.13, 14, 15 To our knowledge, there had been no published cases of XH treated with immunosuppressants. Similar to steroids, the degree of fibrosis, dose, and duration of azathioprine are factors that should be taken into account in assessing the treatment response.13, 14, 15 Our patient was started on azathioprine due to the unsustained effect of prednisolone. MRI after 6 months on azathioprine therapy did not show a further increase in the mass size. The halt in progression could be due to the effect of azathioprine; however, a long-term follow-up is still needed with a continued close surveillance.
Conclusion
We report a young woman with XH with a residual pituitary mass after surgery, which was progressively enlarging over the years. To our knowledge, there was only one case of XH that first presented in an adolescent prior to this. In our report, a reduction in the mass size was observed with prednisolone and no further progression with azathioprine. Steroids and immunotherapy are treatment options for XH; however, knowledge of its pathogenesis and longer follow-up of more cases are needed.
Acknowledgment
We thank Tan Sri Datuk Dr. Noor Hisham Abdullah Director General of Health Malaysia.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Honegger J., Buchfelder M., Schlaffer S. Treatment of primary hypophysitis in Germany. J Clin Endocrinol Metab. 2015;100(9):3460–3469. doi: 10.1210/jc.2015-2146. [DOI] [PubMed] [Google Scholar]
- 2.Angelousi A., Alexandraki K., Tsoli M., Kaltsas G., Kassi E. Hypophysitis (including IgG4 and immunotherapy) J Neuroendocrinol. 2020;110(9-10):822–835. doi: 10.1159/000506903. [DOI] [PubMed] [Google Scholar]
- 3.Gubbi S., Hannah-Shmouni F., Verbalis J.G., Koch C.A. Hypophysitis: an update on the novel forms, diagnosis and management of disorders of pituitary inflammation. Best Pract Res Clin Endocrinol Metab. 2019;33(6):101371. doi: 10.1016/j.beem.2019.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imga N.N., Yildirim A.E., Ozdemir B., Dilek B. Clinical and hormonal characteristics of patients with different types of hypophysitis: a single-centre experience. Arch Endocrinol Metab. 2019;63(1):47–52. doi: 10.20945/2359-3997000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gellner V., Kurschel S., Scarpatetti M., Mokry M. Lymphocytic hypophysitis in the pediatric population. Childs Nerv Syst. 2008;24(7):785–792. doi: 10.1007/s00381-007-0577-1. [DOI] [PubMed] [Google Scholar]
- 6.Gopal-Kothandapani J.S., Bagga V., Wharton S.B., Connolly D.J., Sinha S., Dimitri P.J. Xanthogranulomatous hypophysitis: a rare and often mistaken pituitary lesion. Endocrinol Diabetes Metab Case Rep. 2015;2015(1):140089. doi: 10.1530/EDM-14-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathkour M., Zeoli T., Werner C. Recurring primary xanthomatous hypophysitis behaving like pituitary adenoma: additional case and literature review. World Neurosurg. 2020;138:27–34. doi: 10.1016/j.wneu.2020.02.055. [DOI] [PubMed] [Google Scholar]
- 8.Bishoy H.A., Yan M., Li B. Xanthomatous hypophysitis. J Clin Neurosci. 2015;22(7):1091–1097. doi: 10.1016/j.jocn.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Joung J.Y., Jeong H., Cho Y. Steroid responsive xanthomatous hypophysitis associated with autoimmune thyroiditis: a case report. Endocrinol Metab. 2013;28(1):65–69. doi: 10.3803/EnM.2013.28.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aste L., Bellinzona M., Meleddu V., Farci G., Manieli C., Godanu U. Xanthomatous hypophysitis mimicking a pituitary adenoma: a case report and review of the literature. J Oncol. 2010;2010:195323. doi: 10.1155/2010/195323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan K., Asa S.L., Winer D., Gelareh Z., Gentili F., Mete O. Xanthomatous hypophysitis is associated with ruptured Rathke’s cleft cyst. Endocr Pathol. 2017;28(1):83–90. doi: 10.1007/s12022-017-9471-x. [DOI] [PubMed] [Google Scholar]
- 12.Kristof R.A., Van Roost D., KlingmuIler D., Springer W., Schramm J. Lymphocytic hypophysitis: non-invasive diagnosis and treatment by high dose methylprednisolone pulse therapy. J Neurol Neurosurg Psychiatry. 1999;67(3):398–402. doi: 10.1136/jnnp.67.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curto L., Torre M.L., Cotta O.R. Lymphocytic hypophysitis: differential diagnosis and effects of high-dose pulse steroids, followed by azathioprine, on the pituitary mass and endocrine abnormalities: report of a case and literature review. Sci World J. 2010;10:126–134. doi: 10.1100/tsw.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecube A., Francisco G., Rodriguez D. Lymphocytic hypophysitis successfully treated with azathioprine: first case report. J Neurol Neurosurg Psychiatry. 2003;74(11):1581–1583. doi: 10.1136/jnnp.74.11.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papanastasiou L., Pappa T., Tsiavos V. Azathioprine as an alternative treatment in primary hypophysitis. Pituitary. 2011;14(1):16–22. doi: 10.1007/s11102-010-0252-5. [DOI] [PubMed] [Google Scholar]