Highlights
-
•
Advances in 3D bioprinting have tremendous potential in therapeutic development for multiple cardiovascular applications.
-
•
3-dimensional bioprinting is moving toward in vivo studies to evaluate printed construct functionality and safety.
-
•
Bioprinting techniques predominantly use extrusion-based, inkjet, and light-based printing.
-
•
Bioinks are composed of cells and matrix material and consist of both scaffold-based and scaffold-free inks.
Key Words: bioink, bioprinting, cardiovascular disease, 3-dimensional, tissue engineering, regenerative medicine
Abbreviations and Acronyms: 3D, 3-dimensional; ECM, extracellular matrix; hCPC, human cardiac-derived progenitor cell(s); hiPSC, human induced-pluripotent stem cell(s); HUVEC, human umbilical vein endothelial cell(s); MSC, mesenchymal stem cell(s); UV, ultraviolet
Summary
Three-dimensional (3D) bioprinting may overcome challenges in tissue engineering. Unlike conventional tissue engineering approaches, 3D bioprinting has a proven ability to support vascularization of larger scale constructs and has been used for several cardiovascular applications. An overview of 3D bioprinting techniques, in vivo translation, and challenges are described.
Central Illustration
Recent advances in tissue engineering have direct applications for patient-specific therapies in cardiovascular disease (1, 2, 3). Whereas the human body generally has regenerative qualities, cardiac tissue has little regenerative capacity. Hence, end-stage heart failure has no definitive treatment other than heart transplantation. Tissue engineering can theoretically address these limitations by reproducing tissues that possess properties of native tissues and mimic their function, which may serve to replace dysfunctional tissues. Generally, tissue engineering involves the use of cells in combination with matrix material to generate 3-dimensional (3D) tissue constructs. However, key challenges in traditional tissue engineering limit the reliability and functionality of printed tissue models. Conventional methods of tissue engineering rely on seeding of cells onto scaffolds (4, 5, 6). This is usually associated with inhomogeneous distribution of cells in the scaffold with subsequent problems and failure. More recently, it has become possible to precisely control the type and location of cells used in the engineered constructs, and this may increase the potential of their function and success. Other challenges in tissue engineering include the loss of structural integrity with larger constructs and difficulty vascularizing thicker printed tissues (1,7).
As a potential solution, 3D bioprinting (Central Illustration) may overcome some of the aforementioned challenges associated with tissue engineering. Unlike conventional tissue engineering approaches, 3D bioprinting has a proven ability to support vascularization of larger scale constructs (7,8) and has been used for several cardiovascular applications. For example, aortic valves and conduits (7,9,10) have been constructed for treatment of aortic valve disease and aortic bypass procedures. Vascular 3D bioprinting has also been used to create grafts with enhanced biocompatibility and structural stability that are superior to traditional tissue engineering methods (11,12). Cardiac patches have also been constructed for preservation of cardiac tissue function in the setting of ischemic events (7,13, 14, 15, 16, 17, 18, 19, 20, 21). Active research efforts in 3D bioprinting have focused on ways to engineer 3D structures with cellular components that mimic native cardiac tissue structure and function. The ultimate goal of cardiovascular 3D bioprinting is to use engineered tissues as replacement for damaged tissues in patients.
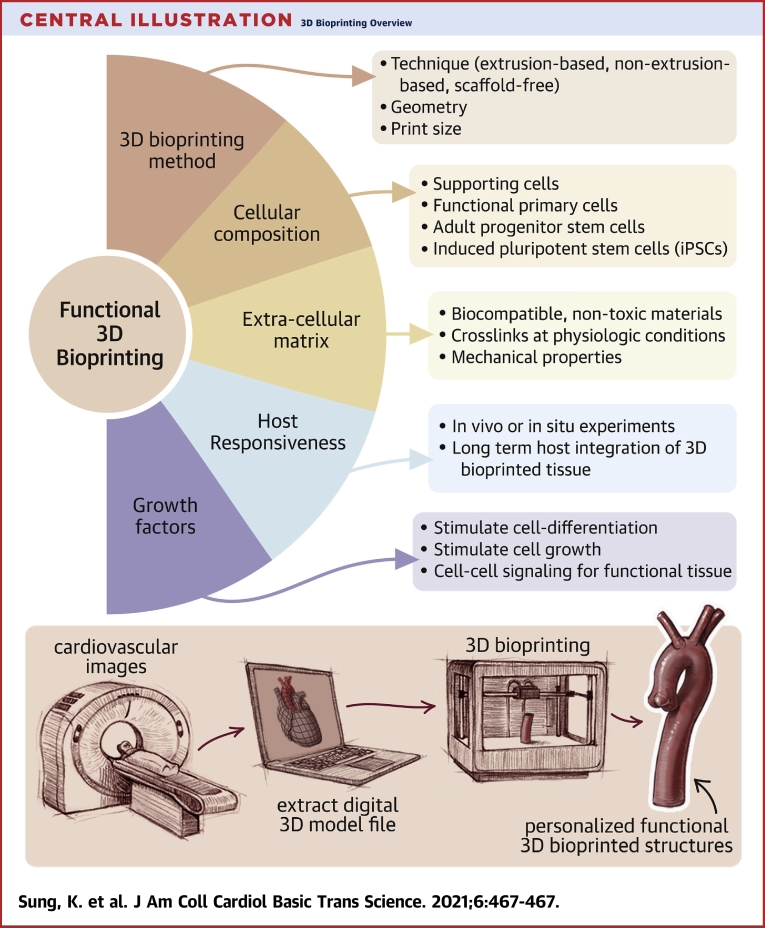
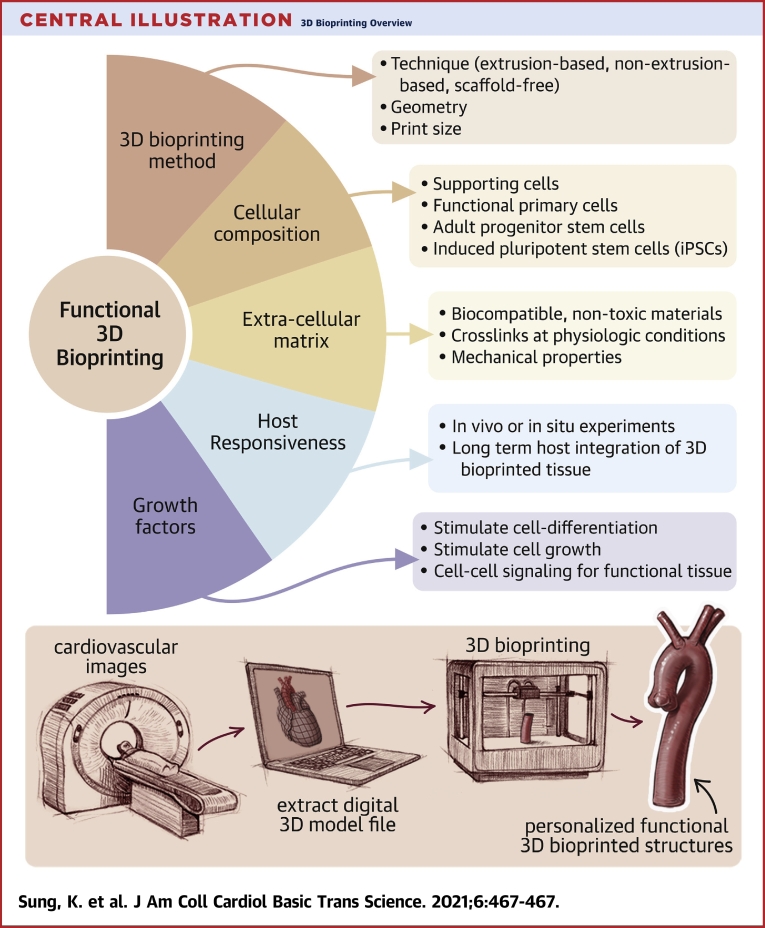
Central Illustration.
3D Bioprinting Overview
(Upper panel) Several key components and considerations are needed when designing a 3-dimensional (3D) bioprinting method. (Lower panel) A sample workflow of incorporating clinical imaging into personalized 3D bioprinting is provided. 3D digital prototype models of organs or tissues are generated from image DICOMs (Digital Imaging and Communications in Medicine) and are used in 3D bioprinting for personalized printed structures. DICOMs are universal file types that encode image data acquired from modalities such as computed tomography, magnetic resonance, and ultrasound.
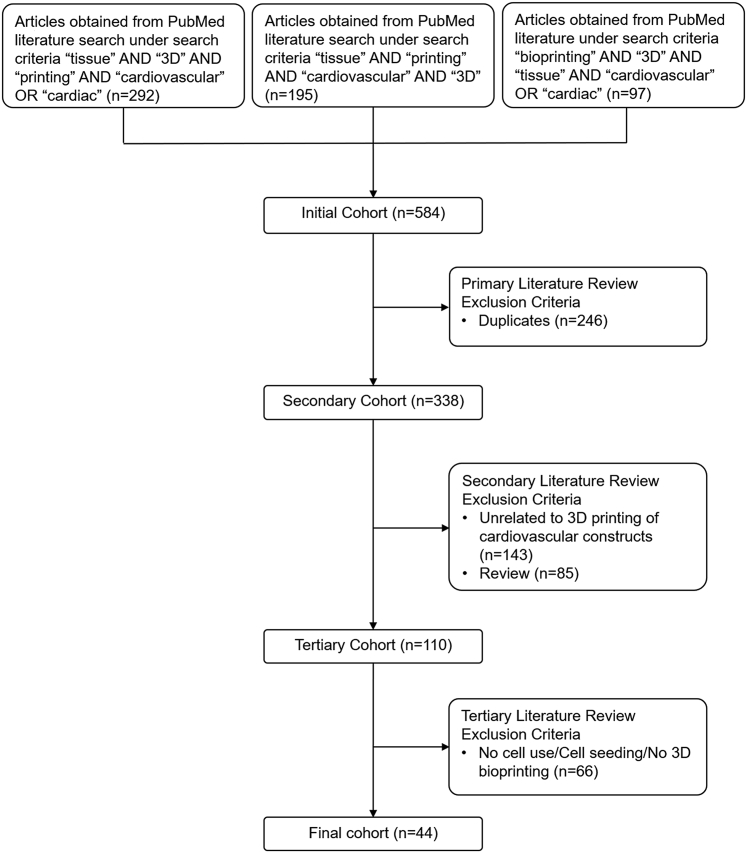
Readers interested in a comprehensive overview of cardiovascular tissue engineering techniques are directed to several excellent published papers on this general topic (1,22,23). In this review, we will focus on providing an overview of cardiovascular 3D bioprinting techniques, their potential applications, and current limitations applications. The systematic publications review was conducted in accordance with recommendations set forth by the PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-Analyses) guidelines (24). Figure 1 provides a summary of the search process using PubMed and outlines all exclusion criteria. The final set of publications consisted of 44 original research studies focusing on 3D bioprinting of cardiovascular constructs. A summary of recent work on 3D cardiovascular bioprinting discussed in this review is presented in Table 1.
Figure 1.
Publications Search
We identified 44 original research papers related to 3-dimensional (3D) bioprinting of cardiovascular constructs. The summary chart was adapted from the PRISMA (Preferred Reporting Systems for Systematic Reviews and Meta-Analyses) (24).
Table 1.
Cardiovascular 3D Bioprinting Projects, Their Printing Techniques, and Bioink Preparation
| 3D Bioprinting Technique | 3D-Bioprinted Construct | Research Goal | Cross-linking Agent | Scaffold Biomaterial | Cell Type | Ref. # |
|---|---|---|---|---|---|---|
| Digital light processing-based: microscale continuous optical printing | Cardiac tissue | Development of a microscale continuous optical bioprinting method to create pre-vascularized tissue and test in vivo | LAP Methacrylate anhydride |
GelMA HA HA glycidyl methacrylate |
HUVEC C3H/10T1/2 HepG2 |
(33) |
| Digital light processing-based | Cardiac tissue | Evaluate whether 3D-bioprinted cardiac tissue can be paced by induction of pacemaker cardiomyocyte fate by the Wnt signaling pathway | LAP | GelMA | Human embryonic stem cell–derived cardiomyocytes | (57) |
| Digital light processing-based: microcontinuous optical printing | Cardiac microtissue | Creation of cardiac tissue in vitro that mimics ventricular myocardial tissue | LAP | GelMA PEGDA HA glycidyl methacrylate |
Neonatal mouse ventricular cardiomyocytes | (58) |
| Digital light processing-based: microcontinuous optical printing | Cardiac tissue | Creation of cardiac scaffolds in vitro for drug screening | LAP | GelMA PEGDA HA glycidyl methacrylate |
Human embryonic stem cell–derived cardiomyocytes | (59) |
| Extrusion-based | Aortic valve | Creation of a growth-sustainable and functional multilayered valve replacement | Eosin Y | GelMA PEGDA PCL Methacrylic acid |
Human induced MSCs | (61) |
| Extrusion-based | Aortic valve conduit | Construction of heart valve conduits using photo–cross-linked hybrid hydrogels | Methacrylic anhydride Irgacure 2959 |
Methacrylated HA GelMA |
HAVICs | (9) |
| Extrusion-based | Aortic valve conduit | Construction of heart valve conduits using hybrid hydrogels and dual cell types | CaCl2 | Alginate Gelatin |
Porcine aortic valve interstitial cells Human aortic root smooth muscle cells |
(10) |
| Extrusion-based | Blood vessel | Printing perfusable vascular conduits using a coaxial nozzle system with focus on thick tissue printing applications | CaCl2 | Sodium alginate powder | Human umbilical vein smooth muscle cells | (32) |
| Extrusion-based | Blood vessel | Development of a multinozzle multichannel temperature deposition, and manufacturing printing method to create a blood vessel-like structure | Alginate Gelatin |
Not specified | (63) | |
| Extrusion-based | Blood vessel | Use of a multilayered coaxial extrusion system and a blend bioink to create perfusable vascular constructs | CaCl2 Irgacure 2959 |
GelMA 4-arm PEG tetra-acrylate Alginate |
Human MSCs HUVECs |
(40) |
| Extrusion-based | Blood vessel | Creation of small multilayered blood vessels that can maintain patency and endothelialize in vivo | CaCl2 | PCL Sodium alginate |
Mongrel dog autologous MSCs | (34) |
| Extrusion-based | Cardiac patch | Creation of a nanoreinforced hybrid cardiac patch for application to myocardial infarct areas | Irgacure CaCl2 |
Alginate Methacrylated collagen |
HCAECs from neonatal atrial appendage | (13) |
| Extrusion-based | Cardiac patch | Creation of a customizable cardiac patch using hCPCs for in vivo testing in myocardial infarct rats | Thiol-reactive | Gelatin HA |
Fetal hCPCs | (14) |
| Extrusion-based | Cardiac patch | Creation of a pre-vascularized cardiac patch to promote faster vascularization following patch implantation in vivo | Vitamin B2 Thermal gelation |
Korea domestic pig heart tissue-derived dECM | hCPCs Turbinate-tissue-derived human MSCs |
(15) |
| Extrusion-based | Cardiac patch | Application of a potentially patient tissue-specific cardiac patch, composed of decellularized cardiac ECM and pediatric cardiac progenitor cells, to damaged myocardium to release paracrine signals | Eosin Y | GelMA Porcine ventricular dECM |
hCPCs | (20) |
| Extrusion-based | Cardiac patch | Investigation of the effect of bioprinting patterns on the modulus, impedance, and porosity of 3D bioprinted cardiac implants | CaCl2 | Sodium alginate powder | HCAECs | (16) |
| Extrusion-based | Cardiac patch: left ventricle | Creation of thick, vascularized, patient-specific cardiac patch | Thermal gelation CaCO3 (printing support bath) |
Cardiac tissue bioink: human omental tissue dECM Printing support bath: sodium alginate Sacrificial ink: porcine skin gelatin |
Cardiac tissue bioink: iPSC-derived cardiomyocytes or neonatal Sprague-Dawley rat cardiac cells Sacrificial bioink: iPSC-derived endothelial cells or HUVECs + human neonatal dermal fibroblasts |
(18) |
| Extrusion-based | Cardiac tissue | Creation of vascularized cardiac tissue | Irgacure 2959 CaCl2 |
Alginate PEG monoacrylate-fibrinogen |
HUVEC Human iPSC-derived cardiomyocytes |
(54) |
| Extrusion-based | Cardiac tissue | Creation of endothelialized myocardial tissue through printing of HUVEC-laden bioink and subsequent scaffold-seeding with cardiomyocytes | Irgacure 2959 CaCl2 |
GelMA Alginate |
HUVECs | (55) |
| Extrusion-based | Cardiac tissue | Construction of cardiac tissue in vitro and maintenance of a cardiomyocyte progenitor cell commitment to cardiac cell fate | CaCl2 | Sodium alginate | Fetal hCPCs | (88) |
| Extrusion-based | Cardiac tissue | Evaluation of cardiomyocyte maturation in different bioink compositions and concentrations to assess the impact of differential microenvironments on cardiomyocyte functionality | Thermal gelation | Korean domestic pig left ventricular tissue-derived dECM COLTRIX PEVA |
Sprague-Dawley neonatal rat primary cardiomyocytes | (49) |
| Extrusion-based | Cardiac tissue | Enhancement of dECM bioink mechanical properties to improve printability and post-printing cardiac progenitor cell function | Vitamin B2 Thermal gelation |
Domestic pig left ventricular dECM riboflavin | hCPCs | (29) |
| Extrusion-based | Cardiac tissue | Development of a method to create thick vascularized tissues by coprinting bioinks | Cell-laden ink: thrombin, transglutaminase, thermal gelation | Silicone ink: silicone elastomer Fugitive ink: Pluronic F127 Cell-laden ink: gelatin, fibrinogen |
Human MSCs Human neonatal dermal fibroblasts |
(66) |
| Extrusion-based | Cardiac tissue | Incorporation of gold nanorods in bioink to improve conduction and electrical coupling in the printed cardiac constructs | Gold nanorod-incorporated GelMA Alginate |
Neonatal Sprague-Dawley rat ventricular cardiac cells Cardiac fibroblasts |
(89) | |
| Extrusion-based | Cardiac tissue | Development of a bioink capable of: 1) printing cardiac tissue that mimics myocardial tissue; and 2) coupling cardiomyocytes and cardiac fibroblasts | Thrombin CaCl2 Visible light |
Fibrinogen Furfuryl-gelatin |
Human iPSC-derived cardiomyocytes Human cardiomyocytes Human cardiac fibroblasts |
(90) |
| Extrusion-based | Cardiac tissue | Use of microextrusion to transfect cells in situ for gene therapy-induced cardiac repair | Polyurethane | Human umbilical cord-derived MSCs | (91) | |
| Extrusion-based | Cardiac tissue | Combine reverse engineering and 3D bioprinting to create hollow microfluid channel networks that can be sized and shaped | CaCl2 Thrombin |
Sodium alginate Agarose Platelet-rich plasma |
H9c2 rat cardiac myoblasts HUVECs |
(92) |
| Extrusion-based | Cardiac tissue | Evaluate the effects of extrusion-based bioprinting on cardiac myocytes and fibroblasts | Biokey | GelMA | Neonatal Sprague-Dawley rat cardiac myocytes Neonatal Sprague-Dawley rat cardiac fibroblasts |
(93) |
| Extrusion-based | Cardiac tissue: left ventricle | Use freeform reversible embedding of suspending hydrogels to bioprint a model of the left ventricle | Thrombin | Collagen type I Gelatin Pluronic F-127 Gum arabic Fibrinogen |
Human embryonic stem cell–derived cardiomyocytes Cardiac fibroblasts |
(94) |
| Extrusion-based | Chambered heart organoid | Development of large-scale, complex-structure cardiac muscle with pump function by 3D bioprinting chambered organizations | LAP | GelMA Collagen methacrylate Fibronectin Laminin-11 |
Human iPSCs | (60) |
| Extrusion-based | Composite hydrogel | Testing the effect of different photo–cross-linking variables on a 3D-bioprinted hydrogel encapsulated with cardiac cells | Irgacure 2959 VA086 |
GelMA PEGDA Alginate |
Adipose-derived human MSCs HAVICs Human aortic valve sinus smooth muscle cells |
(56) |
| Extrusion-based | Vessel-on-a-chip | Creation of a vessel-on-a-chip model using 3D bioprinting | Ultraviolet light | GelMA Methacrylate anhydride |
Human aortic endothelial cells CRL1999 human aortic smooth muscle cells NIH/3T3 fibroblasts |
(95) |
| Inkjet: drop-on-demand | Cardiac tissue | Development of computer models to create scaffold-free aortic tissue | NovoGel hydrogel | Primary mouse embryonic fibroblasts from C57BL/6 strain | (12) | |
| Inkjet: drop-on-demand | Hydrogel blend | Investigation of whether creating a cell-encapsulated hydrogel blend offers better compatibility following 3D bioprinting | Agarose Collagen type I |
Human umbilical artery smooth muscle cells | (96) | |
| Inkjet: drop-on-demand | Blood vessel | Construction of an in vitro vascular channel | Porcine skin gelatin Rat tail collagen type I |
HUVECs | (3) | |
| Inkjet: drop-on-demand; liquid support-based | Vascular-like structures | Freeform inkjet printing of cellular structure with bifurcations | CaCl2 | Sodium alginate | NIH/3T3 mouse fibroblasts | (97) |
| Inkjet: drop-on-demand; thermal-assisted | Blood vessel | Construction of microvasculature | Thrombin Ca2+ |
Fibrinogen | Human microvascular endothelial cells | (30) |
| Laser-assisted | Cardiac patch | Use of a laser-induced–forward-transfer technique to print cells onto a polyester urethane urea cardiac patch for use in myocardial infarct treatment | Gold BD Matrigel matrix |
HUVECs Human MSCs |
(17) | |
| Laser-assisted | Cardiac tissue | Development of a digital light processing-based scanning and continuous 3D bioprinter to print dECM-based heart tissue | LAP Methacrylic anhydride |
GelMA Yorkshire pig heart and liver dECM |
Human iPSC cardiomyocytes | (48) |
| Microscopic painting needle method | Cardiac tissue | Use of the painting needle method to create cardiac tissue | Thrombin | Fibronectin Gelatin HA |
Human iPSC cardiomyocytes Human cardiac fibroblasts |
(98) |
| Multicellular spheroid formation | Cardiac patch | Scaffold-free creation of a cardiac patch | Human iPSC cardiomyocytes human adult ventricular cardiac fibroblasts HUVEC |
(21) | ||
| Multicellular spheroid formation | Cardiac patch | Evaluate scaffold-free cardiac patch regeneration in vivo | Human iPSC cardiomyocytes Human adult ventricular fibroblasts HUVECs |
(19) | ||
| Multicellular spheroid formation | Cardiac tissue | Development of a novel scaffold-free bioprinting method to conduct cardiac tissue for use as a cardiac pump | Human iPSC cardiomyocytes HUVECs Human normal dermal fibroblasts |
(52) | ||
| Multicellular spheroid formation | Cardiac tissue | Development of a novel method to create scaffold-free cardiac tubular tissue and evaluate in vivo in rat aortae | HUVECs Human aortic smooth muscle cells Normal human dermal fibroblasts |
(2) |
3D = 3-dimensional; BD = Becton Dickinson (Franklin Lakes, New Jersey); COLTRIX = type-1 atelo-collagen; dECM = decellularized extracellular matrix; ECM = extracellular matrix; GelMA = gelatin methacryloyl/methacrylate; HA = hyaluronic acid; HAVIC = human aortic valve interstitial cell(s); HCAEC = human coronary artery endothelial cell(s); hCPC = human cardiac-derived progenitor cell(s); HUVEC = human umbilical vein endothelial cell(s); iPSC = induced-pluripotent stem cell(s); LAP = lithium phenyl-2,4,6-trimethylbenzoylphosphinate photoinitiator; MSC = mesenchymal stem cell(s); PCL = polycapractolone; PEG = polyethylene glycol; PEGDA = polyethylene glycol diacrylate; PEVA = polyethylene vinyl acetate.
3D Bioprinting Principles and Techniques
Three-dimensional bioprinting design requires synergy of multiple components including assessment of the desired function, scaffolding components, cellular composition, and 3D printing techniques (1,25, 26, 27, 28). These considerations are summarized in our Central Illustration. Each of these components introduces challenges relevant to cardiovascular tissue engineering. The printing techniques must be suitable for the relevant bioinks utilized, and the printing methods must be able to print at the necessary scale and correct geometry of the final application (13,29). Adequate nutrient and oxygen diffusion are necessary to create meaningful functional tissues, and these processes are often limited by scaffold thickness (30, 31, 32). Nuances in cellular composition, assurance of cell viability in engineered tissues, and close mimicry of function that resemble native tissues also pose additional obstacles (9,20,31). Whereas some 3D-bioprinted products have been tested in animal models, further testing and development are needed before these concepts can be applied to clinical trials (2,14,15,19,33,34). In the following paragraphs we summarize and discuss types of bioinks and techniques for 3D bioprinting.
Types of bioinks
Bioinks are mainly divided into scaffold-based and scaffold-free bioinks depending on the printing technique used. Bioink preparation for tissue engineering consists of multiple steps requiring optimization. First, a solution suitable for printing with appropriate biocompatibility is produced to maximize cell viability. Second, a specific cell type or a combination of cell types is chosen for use in the bioink. Finally, additional cross-linking materials are incorporated to modulate the mechanical properties of the final printed structure. The general preparation of bioinks involves first making a hydrogel slurry with biocompatible materials. Prior to printing, the desired cells are reconstituted into the hydrogel slurry. The cell-hydrogel slurry is then loaded into sterile cartridges for 3D bioprinting.
Cell types
Several cell types have been used in bioinks to create vessels and myocardial tissue and the manufacturing workflow divides cell types into autologous (patient-specific donor) and allogeneic (universal donor) therapeutics. The decision about cell lines often depends on the desired function of bioprinted structures. Among human cell lines, human umbilical vein endothelial cells (HUVECs) (n = 12), vascular smooth muscle cells (n = 6), and fibroblasts (n = 13) are commonly used in bioinks (Table 1). HUVECs allow for endothelial layer formation (35), whereas smooth muscle cells and fibroblasts are pertinent to creation of multilayer and well-supported vasculature. Fibroblasts contribute to formation of cell-based extracellular matrix (ECM).
Relative to stem cells, adult cell types or terminally differentiated cells have limited proliferative capabilities whereas certain cell types, such as cardiomyocytes, may be difficult to isolate without invasive procedures (36). Therefore, several attempts have been made to use stems cells such as mesenchymal stem cells (MSCs) (n = 8), human induced-pluripotent stem cell (hiPSC) cardiomyocytes (n = 9), and human cardiac-derived progenitor cells (hCPCs) (n = 5) in cardiovascular 3D bioprinting (Table 1). A large number of published reports (25,37, 38, 39) have focused on the induction, treatment, and differentiation of MSCs integral to cardiovascular engineering. The use of stem cells poses many advantages. Relative to differentiated cell types, stem cells can be induced to form various cell types and have self-renewing properties (36). MSCs have been known to secrete factors such as vascular endothelial growth factor and can differentiate into cells with vascular properties, including smooth muscle cell–like properties (40), depending on the growth conditions. Growth and culture of MSCs are also less costly and time-consuming than the growth and culture of hiPSCs. Use of iPSCs in bioinks does confer several advantages. hiPSCs have greater self-renewal properties than do adult stem cells such as mesenchymal cells. Because hiPSCs be generated from all somatic cells, they can also be produced in a less-invasive manner and at larger scale. The pluripotency of hiPSCs also allows them to proliferate and differentiate to a greater extent than multipotent MSCs and hCPCs can. Although hiPSCs are less likely to invoke immune response because they can be generated directly from a patient’s own somatic cells, they cannot, at this time, be directly used in the context of patient care (36). Furthermore, hiPSC usage includes the risk of teratoma formation (41). In addition, our current knowledge of hiPSCs has centered on ventricular cardiomyocyte fate. Therefore, use of hiPSC-derived cardiomyocytes for atrial or nodal tissue is less well-characterized (1). Relative to hCPCs, which are already committed to cardiac cell fate, hiPSC-derived cardiomyocytes tend to mature heterogeneously (1), and their differentiation into cardiac cells is more limited (42). hCPCs, therefore, can produce higher yield of cells with greater cardiogenic potential. Isolation of autologous hCPCs is an invasive process, however, and more challenging due to the limited abundance of hCPCs in the heart (43).
Three-dimensional bioprinting with autologous cell types such as patient-specific hiPSCs is highly advantageous due to its evasion of immune rejection, but direct clinical use is not possible at this juncture, and cost may be prohibitive. Compared with autologous methods, allogeneic (universal or off-the-shelf) approaches are significantly less costly and time-intensive and, therefore, demonstrate greater feasibility in terms of translation to patient care. There remain translational barriers in tissue engineering of autologous versus allogeneic cell types and bioprinting, which relate to product commercialization, scaling-up of manufactured products, and infrastructure for manufacturing. Potential solutions to overcome these barriers in manufacturing are well summarized by Hunsberger et al. (44).
Scaffold-based bioinks
Scaffold-based bioinks are composed of cells encapsulated in hydrogels that contain biomaterials such as collagen, fibrin, alginate, chitosan, gelatin, and polyethylene glycol diacrylate (Figure 2A). These biomaterials are typically used in extrusion-based printing techniques given their viscosity, cross-linking conditions, and cytocompatibility. Scaffold materials can be classically categorized into natural and synthetic materials. Natural materials include collagen, gelatin, fibrin, and chitosan and can be procured from both human and animal tissues. Synthetic materials include biomaterial polymers such as polyethylene glycol diacrylate and polyethylene glycol. Other synthetic materials include natural materials chemically modified to include synthetic materials or additional chemical binding groups.
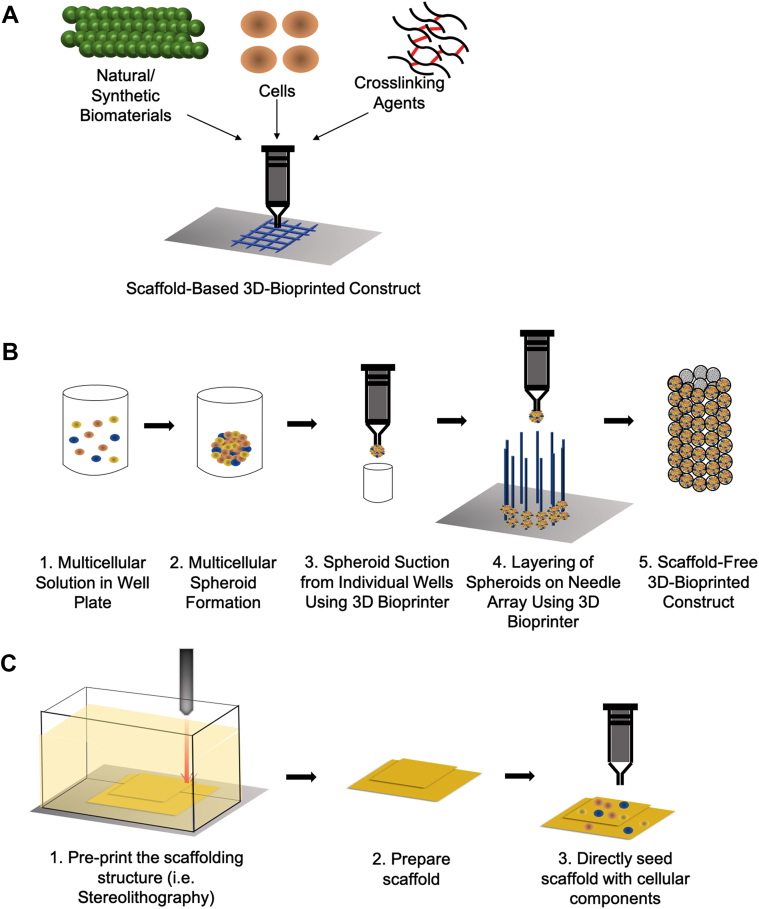
Figure 2.
Steps Involved in Extrusion-Based Printing, Scaffold-Free Printing, Non-Extrusion–Based Printing
(A) Traditional extrusion-based printing uses a formulated bioink that gets deposited by a cartridge in a layer-by-layer fashion to form a 3-dimensional (3D) structure. (B) Scaffold-free printing is a new generation of 3D bioprinting that requires culturing cells. The cells eventually clump and produce native extracellular matrix, forming spheroids. The spheroids may then be placed one-by-one onto a temporary support beams until the spheroids integrate with each other. The temporary support beams may then be removed forming the final structure. (C) Non-extrusion–based printing such as stereolithography uses light to cure a biomaterial into its desired structure. Because many non-extrusion–based printing techniques induce harsh conditions, cells are often seeded onto a pre-printed scaffold.
The majority of studies in our publications review used natural biomaterials with the most common being gelatin methacrylate (n = 14), gelatin (n = 9), and alginate (n = 15) (Table 1). Some of the key benefits of using hydrogels made of natural biomaterials, compared with synthetic materials, include good biocompatibility, support of cell viability via cross-linking in milder conditions, and relatively lower immunogenic properties. Unlike synthetic biomaterials such as polyethylene glycol diacrylate, many natural biomaterials, including collagen, fibrin, alginate, and chitosan, contain signaling molecules for cell adhesion (45). Compared with traditional scaffold cell-seeding methods, cell encapsulation in hydrogels supports cell adhesion in a 3D environment, allows for increased homogeneity in cell distribution, facilitates cell communication, and supports cell migration (45,46).
Several groups have developed additional formulations for bioinks by either increasing cell viability through addition of cell factors or through printing in an environment that better mimics native tissue (20,29). For example, Jang et al. (15,29) decellularized a porcine heart, morcellized the remaining ECM, and filtered the solution to obtain the protein building blocks of natural cardiac ECM. Addition of this slurry to the bioink resulted in no adverse reduction in cardiomyocyte viability and resulted in greater differentiation as assessed by various gene markers (29). Decellularized ECM has been used as scaffold components in studies with cardiovascular application (15,18,20,29,47, 48, 49) because intrinsic native signaling factors and structural maturity enable closer resemblance to native ECM relative to other scaffolding materials such as natural biomaterials, synthetic biomaterials, and cell-based ECMs. Cell-based ECMs are advantageous in terms of scalability, but they may not mimic surrounding ECM in vitro or in vivo as well as more complex tissue- or organ-derived ECMs would (47). In addition to biomaterials, factors that prevent cell aggregation (such as ethylenediamine tetraacetic acid), growth factors (such as vascular endothelial growth factor), and other factors that promote cell survival and biocompatibility are often added to bioinks. During cell incubation on endothelial cell growth media (14,50) and prior to bioink solution creation, growth factors can be added during the cell culture phase of bioink production. Alternatively, factors such as vascular endothelial growth factor can be directly added to the bioink solutions (51).
Scaffold-free bioinks
Scaffold-free bioinks consist solely of multicellular aggregates known as tissue spheroids (Figure 2B). Attempts have been made to construct cardiac patches (19,21) and cardiac tubular constructs (2,52) composed of various cell combinations using scaffold-free 3D bioprinting approaches. Scaffold-free approaches are more limited due to the lack of mechanical support provided to the cells by biomaterials.
Cross-linking agents
Cross-linking agents are used to create physical or chemical links within a hydrogel to preserve hydrogel mechanical strength and cell viability. Photo–cross-linking, thermal cross-linking, chemical cross-linking (53), and physical cross-linking (45) methods have been used to cross-link bioinks. The photoinitiators used for photo–cross-linking in the studies we reviewed included Irgacure 2959 (n = 6) (9,13,40,54, 55, 56), lithium phenyl-2,4,6 trimethylbenzoylphosphinate (n = 6) (33,48,57, 58, 59, 60), VA086 (56), and eosin Y (20,61). CaCl2 (n = 12) was also commonly used as a cross-linking agent to facilitate ionic interactions within hydrogels (Table 1). The main disadvantage of cross-linking is its association with cell cytotoxicity due to the impact of chemicals or light on cell function.
Bioink characterization
After they are bioprinted, the properties of cells and hydrogels are determined by several factors such as viability, vascularization, and cell functionality. Cell viability is often assessed with fluorescence-based live/dead assays that use Hoescht and calcein-AM staining (13,20) or with metabolic activity assays such as 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assays (9,10). High dispensing pressure and low nozzle diameter in extrusion-based systems (62) can affect cell viability. Cross-linking agents can negatively affect cell viability as well. Vascularization is assessed through measurement of perfusion and endothelialization. To monitor cell functionality after cell printing, cell differentiation and gene expression are evaluated.
Three-dimensional bioprinting techniques
Many 3D bioprinting techniques are now commonplace in tissue engineering, including stereolithography, extrusion-based printing, fused filament extraction, and selective laser sintering technologies (Figure 3). The challenges of applying 3D printing techniques to tissue engineering include developing methods suitable with biocompatible materials and under conditions that do not affect cell viability. In cardiovascular 3D bioprinting, scaffold formulation is paramount and scaffold mechanical stiffness, direction of fiber alignment, and chemical composition all play key roles (16,17,27,54).
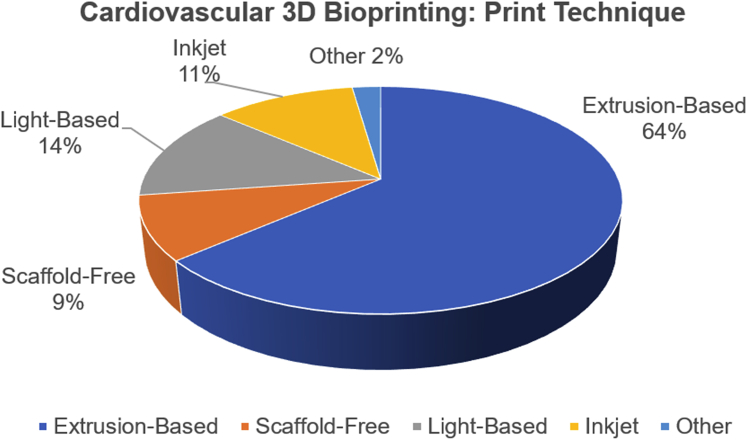
Figure 3.
Distribution of Cardiovascular 3D Bioprinting Methods
Research groups have predominantly opted for extrusion-based methods due to the technique’s favorable conditions such as maintaining cell viability and flexibility in handling different biocompatible materials. 3D = 3-dimensional.
By convention, 3D printing refers to the construction of a scaffolding structure that provide cells with temporary support. Stem cells are then seeded directly onto the scaffold and permeate the scaffold through diffusion. The scaffold itself degrades over time as the cells secrete native extracellular matrix that supports the initial printed structure. These traditional methods, however, have a few key limitations. One limitation is the time required for cells to diffuse through the scaffold to a viable engineered tissue with appreciable thickness. It is also well described in tissue engineering that cell viability decreases substantially in scaffolds with thickness >100 to 200 μm due to insufficient nutrient diffusion. Common workarounds include printing microchannels to simulate vascularization (31) as well as directly printing vessel structures with endothelial cells (63). Three-dimensional bioprinting with bioinks whereby cells are directly mixed into the solutions are also used to create the scaffolds.
Extrusion-based methods
Much of the original work in 3D bioprinting used extrusion-based methods that confer several advantages (Figure 2). These methods used nozzles that can handle a variety of soft, biocompatible materials such as polymers, gels, and colloids. They also do not require intense radiation (e.g., ultraviolet [UV], light) or heat to print the desired scaffold architecture. These advantages allow for preservation of construct shape during the printing process, without placing cells through conditions that may risk decreasing cell viability (64). Furthermore, extrusion-based methods can incorporate dedicated cartridges to house the bioinks previously discussed.
Extrusion-based methods are divided into single- or multiple-cartridge systems. Single-cartridge extrusion-based printing involves a single-mix-component bioink containing both cells and ECM materials (20). The single-cartridge technique is advantageous because it requires production of only 1 bioink to create scaffolds with infused cells throughout the 3D-printed structure. However, because cells and scaffold biomaterials are combined into a single bioink, the scaffold biomaterial must also have chemical properties and solidify in conditions appropriate for cell viability, which limits the variation of the final scaffold composition (54). In contrast, multicartridge extrusion-based printing techniques provide additional flexibility in the types of materials and bioinks that can be used for printing. The versatility afforded by multicartridge systems offers the advantage of including ECM, which is common for complex tissue systems. In a multicartridge system, individual cartridges may hold different bioinks, each containing scaffold materials with varying cellular composition (65). Dedicated gel or scaffold solution cartridges along with material-bioink cartridges can offer additional advantages (66), such as heating of the scaffold polymer solutions at higher temperatures, which results in smoother extrusion. The scaffold cartridge also enables the use of polymers that would otherwise be too viscous to print at lower temperatures. These attributes promote versatility in print design, such as printing heterogeneous structures with different cell types or porous scaffold designs that can be filled with bioink (65). These techniques show promise in cardiac tissue engineering, where the scaffold alignment and structure play a crucial role in generating synchronized contraction similar to native cardiac tissue (20).
Non-extrusion–based methods
There are a wide variety of non-extrusion–based 3D bioprinting methods, which include inkjet printing, light-based processes, and electrospinning. The inkjet printing method typically involves a droplet-by-droplet deposition of bioink delivered through a cartridge. The main difference is the inkjet method deposits droplets individually rather than in a continuous stream of material. Because the droplet method requires specific bioink viscosities to deposit consistent droplets, there are more constraints that limit its versatility relative to extrusion-based methods (67).
Other 3D bioprinting methods such as laser-based (stereolithography) (Figure 2C) or electrospinning are harsher for cell viability, but provide greater flexibility in printing complex scaffold structures, such as valves, at high speeds (68,69). The laser-based printing methods typically use a UV light source to cure biomaterials into the desired geometries. Electrospinning uses a high voltage to spin biomaterials into a fine weave that can be later seeded with cells. In the past, these methods could not directly print bioinks into the scaffold due to the harsh environmental conditions of the printing process. More recently, advances in fabrication techniques have enabled higher percentages of cell viability to achieve using electrospinning (70) and stereolithography 3D printing (71,72). These newer fabrication methods are still not as prevalent relative to extrusion-based and inkjet printing processing.
Scaffold-free bioprinting
Finally, a novel category of 3D bioprinting known as scaffold-free bioprinting (2,19,21,52,73,74) has emerged in the last decade. Scaffold-free bioprinting arose due to multiple limitations inherent with scaffold production One such limitation is the requirement for cross-linking or solidifying processes, which are necessary steps for effective scaffold function but often limit cell viability due to their use of heat or UV radiation. The goal of scaffold-free bioprinting is to print structures that use purely cell-based bioinks without the need for ECM materials. Use of scaffold-free bioinks has theoretical advantages because it allows cells to secrete their own “native” ECM, which may enable more direct cell-cell communication (75). This latter step is especially important for cardiac cellular contraction and synchronization of electrical signaling as discussed in previous sections. Recent work with scaffold-free cultures of human adipose-derived stem cells has demonstrated improved differentiation potential of stem cells (76,77). Using scaffold-free cultures of genetically engineered cardiac stem cells, Jeong et al. (78) demonstrated an association between increases in paracrine factors and cardioprotective properties in murine acute myocardial infarction models. Finally, some early data suggest cells may exhibit some degree of self-organization post-printing (79). Taken together, scaffold-free bioinks may provide the most natural environment for cellular differentiation and proliferation. Additional studies are needed to understand the mechanisms underlying the described results when scaffold-free bioprinting is used.
Major scaffold-free bioprinting techniques fall under 2 categories: 1) spheroid droplet method; and 2) magnetic suspension method. The spheroid droplet method is compatible with cardiac cells. Cardiac cells are seeded with a mixture of endothelial cells and fibroblasts in 96-well plates. The mixture is cultured in appropriate culture media until the cells aggregate into individual spheroids. These spheroids are then collected and printed in a droplet-by-droplet method (Kenzan method) (21,74) or layer-by-layer method (LaBarge) (73). The droplet-by-droplet Kenzan method (74) uses a 3D printer that deposits individual spheroids onto a microarray of tiny needles. The printer skewers spheroids individually onto the tiny needles, providing the spheroids with initial structural support. The entire construct is incubated for 72 h to allow for spheroid aggregation as cells begin to secrete native ECM. Once the construct has successfully aggregated, the 3D-printed structure is removed from the microarray. The droplet-by-droplet method has found increasing interest over time, with other groups (21) developing a modified version of the method. The Kenzan method has been commercialized by companies such as Amuza in the United States. The layer-by-layer method developed by LaBarge et al. (73) sought to improve printing speed. This method utilizes a print head with a vacuum to hold a sheet of spheroids that is subsequently deposited onto a needle microarray similar to the droplet-by-droplet method. The LaBarge method is fairly new and will require further development. Its ability to print larger scale structures and the resolution needed for cardiac applications will require additional investigations (73).
In the magnetic suspension method (80), a solution of magnetic nanoparticles is deposited into a cell culture and cells are allowed to take up the particles. An external magnetic field can then suspend or cause the cells to hold different structures until the cells secrete ECM to support their own shapes (80). Tseng et al. (81) used the magnetic suspension method to 3D bioprint cylindrical structures with aortic smooth muscle cells. Their work produced a structure with contractile properties that was responsive to clinically used vasoactive drugs such as verapamil and phenylephrine. Currently, a major drawback of this printing method is the limitation in magnet shape (81). Magnetic suspension will require further development to enable printing of more complicated structures.
In Vivo Studies and Validation
Several groups have conducted translational research to bring 3D bioprinting technologies closer to cardiac clinical applications. Attempts have been made to determine in vivo efficacy of 3D-bioprinted cardiovascular constructs. However, because cardiovascular 3D bioprinting remains a new tissue engineering discipline, no clinical trials for cardiovascular bioprinted therapeutics have been conducted.
For translational work, 3D bioprinting has been applied for tissue replacement in myocardial infarction and vascular pathologies. Gaebel et al. (17) printed cardiac patches seeded with human MSCs and HUVECs that were implanted into Rowett Nude rats with induced myocardial infarction. Improved left ventricular function, increased wall thickness, and increased capillary density were observed in rats implanted with the cardiac patches treated with the cardiac cell lines. Similar results were obtained using a 3D-bioprinted human cardiac-derived cardiomyocyte progenitor cell cardiac patch following implantation into mice infarct ventricular walls (14).
For vascular pathologies, compared with acellular control grafts, 3D-bioprinted cell-laden vascular grafts transplanted into canine carotid and femoral arteries have also been shown to have less inflammation and thromboses (34). Other groups have shown endothelialization in murine abdominal aortas following implantation of tubular tissues that were printed using a scaffold-free approach (2). Their translational work suggests potential use of 3D bioprinting in therapeutic vascular repair for conditions such as abdominal aortic aneurysms or even revascularization of coronary arteries.
Other studies have also investigated in vivo functionality of scaffold-free printing methods. While the field of scaffold-free printing is relatively new, individual groups such as Ong et al. (21) have tried to implant cardiac patches from scaffold-independent printing methods into murine hearts. Their work demonstrated cell viability and signs suggestive of engraftment into the native murine cardiac tissue. In female Lewis nude rat models with myocardial infarction that were treated with 3D-bioprinted cardiac patches relative to control rats, Yeung et al. (19) demonstrated increased survival rate, average vessel count, left ventricular ejection fraction, and cardiac output. Together, these studies further support early promise for translating scaffold-free bioprinting to relevant clinical applications.
Current Challenges and Future Directions
There are several barriers to 3D bioprinting of cardiovascular structures. Prior to cell printing, the major concern is scalability. Bioinks require the use of cell lines, stem cells, and/or progenitor cells, but production of these cell types in quantities capable of large tissue generation remains a challenge. Culture-based production of these cells is insufficient to make large-scale tissues (1). The most significant barriers in 3D bioprinting occur during and after the printing process. During printing, the main challenges lie in developing printing technology that maintains cell viability while minimizing printing complications such as nozzle clogging and maximizing parameters of print efficacy including print resolution and speed. After cell printing, issues may arise with cell survivability, biocompatibility, and maturation in host tissues.
There are several advantages and disadvantages to the main types of 3D bioprinting methods: extrusion, inkjet, and laser-assisted. Printing speeds, printing resolutions, cell deposition concentration, and control of cell placement during deposition differ between the various techniques. Common concerns with extrusion-based, inkjet bioprinting, and other nozzle-based bioprinting methods include cell clogging in the nozzle and mechanical stress on the cells during printing (33,82). Laser-assisted printing offers improved effects on cell viability, however, it is more costly, more time-consuming, and poses challenges with controlling cell directionality (82,83). One alternative solution includes combining bioprinting techniques to reduce these problems while maintaining cell viability. For example, combining extrusion printing with digital light processing and layer-by-layer UV photocuring to create soft tissues has recently been described (84).
Huang et al. (1) addressed several current challenges with cardiovascular tissue engineering that must be resolved. Issues such as cell maturation, vascularization, and integration into host tissues can influence cell viability after printing and require additional attention. During printing, cells undergo several mechanical changes, which affect their integration into host tissues (16). Moreover, cardiomyocyte progenitor cells and hiPSC-derived cardiomyocytes must be able to differentiate into cardiomyocytes with proper morphology and standard electrical function to integrate electromechanically into host tissues. Similarly, vascular tissues must retain their functional and structural properties. After maturation, bioprinted cardiovascular cells must also integrate into host tissues without causing an immune response (85). Lastly, printed constructs must be perfusable and be capable of anastomosing with host vasculature. While progress has been made in vascularization of cardiac tissues (3,30,32,40,63), the majority of this work has been conducted in vitro, which may not be fully representative of in vivo environments. The majority of bioprinted vasculature thus far, has been constructed using cell-scaffold combination to create tubular structures capable of blood flow. In contrast, maintaining microvasculature structural integrity is highly complex due the small-scale nature of capillary networks and requires further improvement in printing techniques (86). Future work will need to further investigate creation of multilayered structures that resemble native complex vascular microenvironments.
Although attractive, scaffold use has its limitations. Synthetic hydrogels exhibit remarkable mechanical strength but lack the bioactive properties of natural hydrogels that are integral to biocompatibility after printing (45). Several combinations of synthetic and natural biomaterials are available for use in bioinks to balance hydrogel strength and biocompatibility (87). Gelatin methacryloyl, for example, combines gelatin and synthetic methacryloyl to create a hydrogel with the advantageous properties of both synthetic and natural biomaterials and was included in 15 studies that we reviewed. Attempts have also been made to construct scaffold-free bioinks, which rely on self-assembly through cell-cell adhesion and natural production of ECM. These approaches, however, remain limited due to the challenges of maintaining cell viability and control of construct shape without scaffold mechanical support. Lastly, dissolvable, transient hydrogels have also been used for creation of sacrificial or fugitive bioinks. These latter bioinks contain biomaterials that provide initial structural cell support and are degraded after completion of cell assembly (66,75).
Conclusions
Innovation in cardiovascular research and therapeutics have made tremendous strides in the last 20 years. A relatively new subdiscipline of tissue engineering called 3D bioprinting has shown promise as a tool in basic science research, as well as therapeutic applications such as vascular grafts and myocardial tissue replacement. Using traditional extrusion-based 3D bioprinting techniques and various compositions of cell-loaded bioinks, researchers have printed robust structures with coherent contractile properties. Recent development of spheroid-based printing removed the need for scaffolds altogether, using only spheroids composed of cell aggregates secreting native ECM. Although the field is relatively young, recent in vivo work shows promise for 3D bioprinting techniques to be translated to the bedside.
Funding Support and Author Disclosures
Dr. Ashammakhi has received grant support from the National Institutes of Health (UG3TR003148) and the American Heart Association (18TPA34230036, 442611-NU-80922). Dr. Nguyen has received grant support from the American Heart Association (18TPA34170049); the National Heart, Lung, and Blood Institute (R01HL148182); and the Veterans Health Administration (VA-MERIT, I01-CX001901). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Huang N.F., Serpooshan V., Morris V.B. Big bottlenecks in cardiovascular tissue engineering. Commun Biol. 2018;1:199. doi: 10.1038/s42003-018-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh M., Nakayama K., Noguchi R. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee V.K., Kim D.Y., Ngo H. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014;35:8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinand C., Jian W.X., Peretti G.M., Bonassar L.J., Gill T.J. Conditions affecting cell seeding onto three-dimensional scaffolds for cellular-based biodegradable implants. J Biomed Mater Res B Appl Biomater. 2009;91:80–87. doi: 10.1002/jbm.b.31376. [DOI] [PubMed] [Google Scholar]
- 5.Radisic M., Euloth M., Yang L., Langer R., Freed L.E., Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 6.Dai W., Dong J., Chen G., Uemura T. Application of low-pressure cell seeding system in tissue engineering. Biosci Trends. 2009;3:216–219. [PubMed] [Google Scholar]
- 7.Pountos I., Tellisi N., Ashammakhi N. Springer International Publishing; New York: 2019. Potential Clinical Applications of Three-Dimensional Bioprinting: 3D Bioprinting in Medicine; pp. 101–125. [Google Scholar]
- 8.Kang H.W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 9.Duan B., Kapetanovic E., Hockaday L.A., Butcher J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014;10:1836–1846. doi: 10.1016/j.actbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan B., Hockaday L.A., Kang K.H., Butcher J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101 A:1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marga F., Jakab K., Khatiwala C. Toward engineering functional organ modules by additive manufacturing. Biofabrication. 2012;4 doi: 10.1088/1758-5082/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 12.Kucukgul C., Ozler S.B., Inci I. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol Bioeng. 2015;112:811–821. doi: 10.1002/bit.25493. [DOI] [PubMed] [Google Scholar]
- 13.Izadifar M., Chapman D., Babyn P., Chen X., Kelly M.E. UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods. 2018;24:74–88. doi: 10.1089/ten.TEC.2017.0346. [DOI] [PubMed] [Google Scholar]
- 14.Gaetani R., Feyen D.A.M., Verhage V. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Jang J., Park H.J., Kim S.W. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Izadifar M., Babyn P., Kelly M.E., Chapman D., Chen X. Bioprinting pattern-dependent electrical/mechanical behavior of cardiac alginate implants: characterization and ex vivo phase-contrast microtomography assessment. Tissue Eng Part C Methods. 2017;23:548–564. doi: 10.1089/ten.TEC.2017.0222. [DOI] [PubMed] [Google Scholar]
- 17.Gaebel R., Ma N., Liu J. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32:9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 18.Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci (Weinh) 2019;6:1900344. doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung E., Fukunishi T., Bai Y. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J Tissue Eng Regen Med. 2019;13:2031–2039. doi: 10.1002/term.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bejleri D., Streeter B.W., Nachlas A.L.Y. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong C.S., Fukunishi T., Zhang H. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. 2017;7:4566. doi: 10.1038/s41598-017-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen A.H., Marsh P., Schmiess-Heine L. Cardiac tissue engineering: state-of-the-art methods and outlook. J Biol Eng. 2019;13:57. doi: 10.1186/s13036-019-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis M.W., Russell B. Cardiac tissue engineering. J Cardiovasc Nurs. 2009;24:87–92. doi: 10.1097/01.JCN.0000343562.06614.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh K., Ingber D.E. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv Drug Deliv Rev. 2007;59:1306–1318. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Vukicevic M., Mosadegh B., Min J.K., Little S.H. Cardiac 3D printing and its future directions. J Am Coll Cardiol Img. 2017;10:171–184. doi: 10.1016/j.jcmg.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Z., Lee S.Y. A systematic review of 3-D printing in cardiovascular and cerebrovascular diseases. Anatol J Cardiol. 2017;17:423–435. doi: 10.14744/AnatolJCardiol.2017.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Sabbagh A., Eleid M.F., Al-Hijji M. The various applications of 3D printing in cardiovascular diseases. Curr Cardiol Rep. 2018;20:47. doi: 10.1007/s11886-018-0992-9. [DOI] [PubMed] [Google Scholar]
- 29.Jang J., Kim T.G., Kim B.S., Kim S.W., Kwon S.M., Cho D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016;33:88–95. doi: 10.1016/j.actbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Cui X., Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 31.Pimentel C.R., Ko S.K., Caviglia C. Three-dimensional fabrication of thick and densely populated soft constructs with complex and actively perfused channel network. Acta Biomater. 2018;65:174–184. doi: 10.1016/j.actbio.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Yu Y., Akkouch A., Dababneh A., Dolati F., Ozbolat I.T. In vitro study of directly bioprinted perfusable vasculature conduits. Biomater Sci. 2015;3:134–143. doi: 10.1039/C4BM00234B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu W., Qu X., Zhu J. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials. 2017;124:106–115. doi: 10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang E.H., Kim J.H., Lee J.H., Kim D.H., Youn Y.N. Enhanced biocompatibility of multi-layered, 3D bio-printed artificial vessels composed of autologous mesenchymal stem cells. Polymers (Basel) 2020;12:538. doi: 10.3390/polym12030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong C.S., Yesantharao P., Huang C.Y. 3D bioprinting using stem cells. Pediatr Res. 2018;83:223–231. doi: 10.1038/pr.2017.252. [DOI] [PubMed] [Google Scholar]
- 36.Romanazzo S., Nemec S., Roohani I. iPSC bioprinting: Where are we at? Materials (Basel) 2019;12:2453. doi: 10.3390/ma12152453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 38.Cook D., Genever P. Springer; Dordrecht, the Netherlands: 2013. Regulation of Mesenchymal Stem Cell Differentiation; pp. 213–229. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda K. Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif Organs. 2001;25:187–193. doi: 10.1046/j.1525-1594.2001.025003187.x. [DOI] [PubMed] [Google Scholar]
- 40.Jia W., Gungor-Ozkerim P.S., Zhang Y.S. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afra S., Matin M.M. Potential of mesenchymal stem cells for bioengineered blood vessels in comparison with other eligible cell sources. Cell Tissue Res. 2020;380:1–13. doi: 10.1007/s00441-019-03161-0. [DOI] [PubMed] [Google Scholar]
- 42.Ye J., Yeghiazarians Y. Cardiac stem cell therapy: review of the native cardiac progenitor cells and future direction. J Cardiovasc Pharmacol. 2014;63:85–94. [Google Scholar]
- 43.Wang W.E., Chen X., Houser S.R., Zeng C. Potential of cardiac stem/progenitor cells and induced pluripotent stem cells for cardiac repair in ischaemic heart disease. Clin Sci. 2013;125:319–327. doi: 10.1042/CS20130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunsberger J., Harrysson O., Shirwaiker R. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Transl Med. 2015;4:130–135. doi: 10.5966/sctm.2014-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hospodiuk M., Dey M., Sosnoski D., Ozbolat I.T. The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv. 2017;35:217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Rider P., Kačarević Ž.P., Alkildani S., Retnasingh S., Barbeck M. Bioprinting of tissue engineering scaffolds. J Tissue Eng. 2018;9 doi: 10.1177/2041731418802090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dzobo K., Motaung K.S.C.M., Adesida A. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci. 2019;20:4628. doi: 10.3390/ijms20184628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu C., Ma X., Zhu W. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials. 2019;194:1–13. doi: 10.1016/j.biomaterials.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das S., Kim S.W., Choi Y.J. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. 2019;95:188–200. doi: 10.1016/j.actbio.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 50.Floren M., Bonani W., Dharmarajan A., Motta A., Migliaresi C., Tan W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomater. 2016;31:156–166. doi: 10.1016/j.actbio.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poldervaart M.T., Gremmels H., Van Deventer K. Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J Control Release. 2014;184:58–66. doi: 10.1016/j.jconrel.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Arai K., Murata D., Verissimo A.R. Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knowlton S., Yenilmez B., Anand S., Tasoglu S. Photocrosslinking-based bioprinting: examining crosslinking schemes. Bioprinting. 2017;5:10–18. [Google Scholar]
- 54.Maiullari F., Costantini M., Milan M. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci Rep. 2018;8:13532. doi: 10.1038/s41598-018-31848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y.S., Arneri A., Bersini S. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang L.H., Armstrong P.A., Lee L.J., Duan B., Kang K.H., Butcher J.T. Optimizing photo-encapsulation viability of heart valve cell types in 3D printable composite hydrogels. Ann Biomed Eng. 2017;45:360–377. doi: 10.1007/s10439-016-1619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren J., Han P., Ma X. Canonical Wnt5b signaling directs outlying Nkx2.5+ mesoderm into pacemaker cardiomyocytes. Dev Cell. 2019;50:729–743.e5. doi: 10.1016/j.devcel.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J., Miller K., Ma X. Direct 3D bioprinting of cardiac micro-tissues mimicking native myocardium. Biomaterials. 2020;256:120204. doi: 10.1016/j.biomaterials.2020.120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J., He J., Liu J. Rapid 3D bioprinting of in vitro cardiac tissue models using human embryonic stem cell-derived cardiomyocytes. Bioprinting. 2019;13 doi: 10.1016/j.bprint.2019.e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kupfer M.E., Lin W.-H., Ravikumar V. In situ expansion, differentiation and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ Res. 2020;127:207–224. doi: 10.1161/CIRCRESAHA.119.316155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nachlas A.L.Y., Li S., Streeter B.W. A multilayered valve leaflet promotes cell-laden collagen type I production and aortic valve hemodynamics. Biomaterials. 2020;240:119838. doi: 10.1016/j.biomaterials.2020.119838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nair K., Gandhi M., Khalil S. Characterization of cell viability during bioprinting processes. Biotechnol J. 2009;4:1168–1177. doi: 10.1002/biot.200900004. [DOI] [PubMed] [Google Scholar]
- 63.Liu H., Zhou H., Lan H., Liu F., Wang X. Multinozzle multichannel temperature deposition system for construction of a blood vessel. SLAS Technol. 2018;23:64–69. doi: 10.1177/2472630317712221. [DOI] [PubMed] [Google Scholar]
- 64.Gillispie G., Prim P., Copus J. Assessment methodologies for extrusion-based bioink printability. Biofabrication. 2020;12 doi: 10.1088/1758-5090/ab6f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pepper M.E., Groff R.E., Cass C.A.P., Mattimore J.P., Burg T., Burg K.J.L. A quantitative metric for pattern fidelity of bioprinted cocultures. Artif Organs. 2012;36:E151–E162. doi: 10.1111/j.1525-1594.2012.01460.x. [DOI] [PubMed] [Google Scholar]
- 66.Kolesky D.B., Homan K.A., Skylar-Scott M.A., Lewis J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu Z., Fu J., Lin H., He Y. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J Pharm Sci. 2020;15:529–557. doi: 10.1016/j.ajps.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saidy N.T., Wolf F., Bas O. Biologically inspired scaffolds for heart valve tissue engineering via melt electrowriting. Small. 2019;15:1900873. doi: 10.1002/smll.201900873. [DOI] [PubMed] [Google Scholar]
- 69.Sodian R., Loebe M., Hein A. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO J. 2002;48:12–16. doi: 10.1097/00002480-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Nosoudi N., Jacob A.O., Stultz S. Electrospinning live cells using gelatin and pullulan. Bioengineering. 2020;7:21. doi: 10.3390/bioengineering7010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huber B., Engelhardt S., Meyer W. Blood-vessel mimicking structures by stereolithographic fabrication of small porous tubes using cytocompatible polyacrylate elastomers, biofunctionalization and endothelialization. J Funct Biomater. 2016;7:11. doi: 10.3390/jfb7020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miri A.K., Nieto D., Iglesias L. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv Mater. 2018;30 doi: 10.1002/adma.201800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LaBarge W., Morales A., Pretorius D., Kahn-Krell A.M., Kannappan R., Zhang J. Scaffold-free bioprinter utilizing layer-by-layer printing of cellular spheroids. Micromachines. 2019;10:570. doi: 10.3390/mi10090570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moldovan N.I., Hibino N., Nakayama K. Principles of the Kenzan method for robotic cell spheroid-based three-dimensional bioprinting. Tissue Eng Part B Rev. 2017;23:237–244. doi: 10.1089/ten.TEB.2016.0322. [DOI] [PubMed] [Google Scholar]
- 75.Moldovan N.I. Progress in scaffold-free bioprinting for cardiovascular medicine. J Cell Mol Med. 2018:2964–2969. doi: 10.1111/jcmm.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y., Guan R., Lei H. Therapeutic potential of adipose-derived stem cells-based micro-tissues in a rat model of postprostatectomy erectile dysfunction. J Sex Med. 2014;11:2439–2448. doi: 10.1111/jsm.12636. [DOI] [PubMed] [Google Scholar]
- 77.Yipeng J., Yongde X., Yuanyi W. Microtissues enhance smooth muscle differentiation and cell viability of hADSCs for three dimensional bioprinting. Front Physiol. 2017;8:534. doi: 10.3389/fphys.2017.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeong H.S., Park C.Y., Kim J.H. Cardioprotective effects of genetically engineered cardiac stem cells by spheroid formation on ischemic cardiomyocytes. Mol Med. 2020;26:15. doi: 10.1186/s10020-019-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shafiee A., McCune M., Forgacs G., Kosztin I. Post-deposition bioink self-assembly: a quantitative study. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/4/045005. [DOI] [PubMed] [Google Scholar]
- 80.Souza G.R., Molina J.R., Raphael R.M. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291–296. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tseng H., Gage J.A., Haisler W.L. A high-throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci Rep. 2016;6:30640. doi: 10.1038/srep30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 83.Kačarević Ž.P., Rider P.M., Alkildani S. An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials (Basel) 2018;11:2199. doi: 10.3390/ma11112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhuang P., Ng W.L., An J., Chua C.K., Tan L.P. Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qasim M., Haq F., Kang M.H., Kim J.H. 3D printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int J Nanomedicine. 2019;14:1311–1333. doi: 10.2147/IJN.S189587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy S.V., De Coppi P., Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2020:370–380. doi: 10.1038/s41551-019-0471-7. [DOI] [PubMed] [Google Scholar]
- 87.Ashammakhi N., Ahadian S., Xu C. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater Today Bio. 2019;1:100008. doi: 10.1016/j.mtbio.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaetani R., Doevendans P.A., Metz C.H.G. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33:1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Zhu K., Shin S.R., van Kempen T. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv Funct Mater. 2017;27:1605352. doi: 10.1002/adfm.201605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anil Kumar S., Alonzo M., Allen S.C. A visible light-cross-linkable, fibrin-gelatin-based bioprinted construct with human cardiomyocytes and fibroblasts. ACS Biomater Sci Eng. 2019;5:4551–4563. doi: 10.1021/acsbiomaterials.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang N.C., Lee C.M., Hsu S.-H. Effective naked plasmid DNA delivery into stem cells by microextrusion-based transient-transfection system for in situ cardiac repair. Cytotherapy. 2020;22:70–81. doi: 10.1016/j.jcyt.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 92.Zou Q., Grottkau B.E., He Z. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater Sci Eng C Mater Biol Appl. 2020;108:110205. doi: 10.1016/j.msec.2019.110205. [DOI] [PubMed] [Google Scholar]
- 93.Koti P., Muselimyan N., Mirdamadi E., Asfour H., Sarvazyan N.A. Use of GelMA for 3D printing of cardiac myocytes and fibroblasts. J 3D Print Med. 2019;3:11–22. doi: 10.2217/3dp-2018-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee A., Hudson A.R., Shiwarski D.J. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 95.Abudupataer M., Chen N., Yan S. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed Microdevices. 2019;22:10. doi: 10.1007/s10544-019-0460-3. [DOI] [PubMed] [Google Scholar]
- 96.Köpf M., Campos D.F.D., Blaeser A., Sen K.S., Fischer H. A tailored three-dimensionally printable agarose-collagen blend allows encapsulation, spreading, and attachment of human umbilical artery smooth muscle cells. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/2/025011. [DOI] [PubMed] [Google Scholar]
- 97.Christensen K., Xu C., Chai W., Zhang Z., Fu J., Huang Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng. 2015;112:1047–1055. doi: 10.1002/bit.25501. [DOI] [PubMed] [Google Scholar]
- 98.Chikae S., Kubota A., Nakamura H. Three-dimensional bioprinting human cardiac tissue chips of using a painting needle method. Biotechnol Bioeng. 2019;116:3136–3142. doi: 10.1002/bit.27126. [DOI] [PubMed] [Google Scholar]