Abstract
Introduction
Liver cirrhosis is the ultimate condition of chronic liver diseases. Non-alcoholic steatohepatitis and fatty liver diseases are emerging in association with metabolic syndrome largely due to excess nutrition. Stromal cells of adipose tissue are enriched mesenchymal stem cells which are pluripotent and immunomodulatory, which are expected to be applied for repairing/regenerative therapy of the impaired organs.
Methods
We conducted the multi-institutional clinical trial (Japanese UMIN Clinical Trial Registry: UMIN000022601) of cell therapy using freshly isolated autologous adipose tissue-derived regenerative (stem) cells (ADRCs), which are obtained by the investigational trial device, adipose tissue dissociation device, for liver cirrhosis patients due to non-alcoholic steatohepatitis or fatty liver disease, to exploratory assess efficacy as well as safety of this trial. We completed treatment and 24 weeks follow-up for 7 patients.
Results
We observed that 6 out of 7 patients' serum albumin concentration was improved. As for prothrombin activity, 5 out of 7 patients showed improvement. No trial-related adverse events, which were serious or non-serious, was observed. Besides, no malfunction of the investigational trial device was encountered.
Conclusion
Thus, treatment with autologous ADRCs obtained with the investigational trial device in steatohepatitis-related cirrhosis was confirmed to be safely conductible and potentially promising for the retaining or improving the impaired hepatic reserve.
Keywords: Adipose tissue-derived regenerative (stem) cells, Adipose tissue dissociation device, Liver cirrhosis, Non-alcoholic steatohepatitis, Fatty liver diseases, Clinical trial
Abbreviations: MSCs, mesenchymal stem cells; ADRCs, adipose tissue derived regenerative (stem) cells; PMDA, Japan Pharmaceuticals and Medical Devices Agency; NASH, non-alcoholic steatohepatitis; NAFLD, nonalcoholic fatty liver disease
1. Introduction
Mesenchymal stem cells (MSCs) are somatic stem cells that are obtainable from miscellaneous organs, such as the umbilical cord, bone marrow, and adipose tissue [1]. Features of MSCs are pluripotency as well as immuno-modulatory capability [2,3], as such, they are recognized to be applicable for repairing/regenerating impaired organs. Especially, adipose tissue is an attractive and easy to obtain source of autologous stromal cells enriched with MSC [4]. Liver cirrhosis is the ultimate condition of chronic liver diseases; decompensated cirrhosis condition frequently leads to serious complications such as hepatic failure, rupture of gastroesophageal varices, obvious hepatic encephalopathy, ascites, jaundice, etc. which worsen the prognosis of cirrhotic patients [5]. Causes of chronic liver disease are chronic viral hepatitis, primary biliary cholangitis, alcoholic hepatitis, and non-alcoholic steatohepatitis, etc. [6]. Among these chronic liver diseases, non-alcoholic steatohepatitis, which is associated with metabolic syndrome due to excess of nutrition, is especially an emerging chronic liver disease in the developed countries [7], as treatments for chronic hepatitis B and C have been dramatically advanced [8,9]. Considering this background, treatments for non-alcoholic steatohepatitis and fatty liver diseases, especially in advanced condition, cirrhosis, should be developed.
We previously conducted the clinical study of the treatment by administration of autologous adipose tissue-derived stromal cells via hepatic artery for the liver cirrhosis patients and confirmed the safety of this treatment [10]. In the current study, we designed the investigator-initiated clinical trial protocol for treatment of liver cirrhosis due to non-alcoholic steatohepatitis or fatty liver disease by intrahepatic arterial infusion of autologous adipose tissue derived regenerative (stem) cells (ADRCs) for exploring safety and efficacy of this treatment [11]. We found that this treatment was safely conductible and potentially efficacious for retaining or improving liver function in steatohepatitis-related liver cirrhosis.
2. Methods
2.1. Objectives of the clinical trial
The clinical trial is aimed for exploratory assessment of safety and efficacy of treatment for liver cirrhosis using autologous adipose tissue-derived regenerative (stem) cells (ADRCs) which are obtained by adipose tissue dissociation device (Cytori Therapeutics Inc., San Diego, CA). By consultation with the Japan Pharmaceuticals and Medical Devices Agency (PMDA), the protocol for this trial was created, determining that the adipose tissue dissociation device was the investigational trial device. The completed clinical trial protocol was submitted to PMDA, as well as approved by the institutional review boards of Kanazawa University Hospital and Osaka Medical College Hospital. The clinical trial has been registered in the Japanese UMIN Clinical Trial Registry (UMIN000022601). All treatments procedures were conducted in accordance with the Declaration of Helsinki as well as International Conference on Harmonization Good Clinical Practice guidelines, and the written informed consent from each patient was obtained.
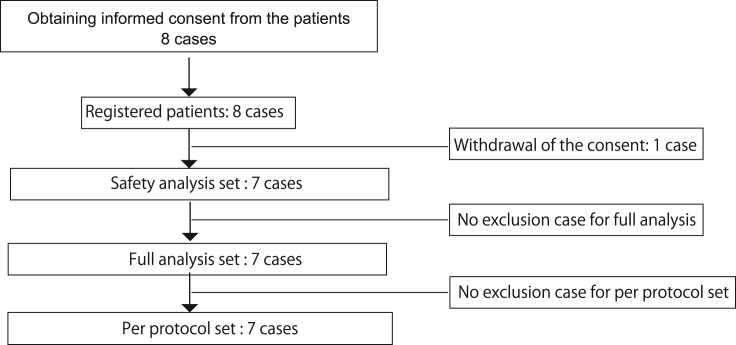
As for inclusion criteria, the objectives were liver cirrhosis patients, diagnosed by image or histology. Etiologies of liver cirrhosis were non-alcoholic steatohepatitis (NASH) or fatty liver disease. NASH and fatty liver disease criteria were defined by the followings: no other obvious liver injury etiologies, and association of conditions or complications which could cause fatty liver, i.e. obesity (especially visceral fat), metabolic syndrome, and diabetes mellitus. The different criteria for NASH and fatty liver disease was alcohol intake alone; less than equal 20 g/day of alcohol intake for NASH, and less than equal 70 g/day, and more than 20 g/day for fatty liver disease. Other inclusion criteria were as followings: age; more than equal 20 years and less than 80 years old, total bilirubin concentration; less than equal 3.0 mg/dL, platelet count; more than equal 5.0 × 104/μL, prothrombin activity; more than equal 70%, serum creatinine level; less than equal 1.5 mg/dL, serum albumin; less than equal 4.0 g/dL, at screening, and patients are able to provide written informed consent themselves. In addition to satisfying the inclusion criteria, the exclusion criteria were applied (Fig. 1). The target number of patients was 8 in this clinical trial.
Fig. 1.
Outline of patient's enrollment. Eight cases were registered and 7 cases completed treatment and 24 weeks' follow-up without deviation from the clinical trial protocol.
2.2. Treatment of the cirrhotic patients with autologous ADRCs, follow-up, and assessment
The subcutaneous adipose tissue was obtained from the buttock or abdomen by the tumescent liposuction method, which is the standard technique for cosmetic treatment. ADRCs contained in the obtained adipose tissue were isolated by the investigational trial device (identification code: Celution 800/IV, Cytori Therapeutics Inc. San Diego, CA). The freshly isolated cells were confirmed for viability which should be more than equal 70%. The 3.3 × 105/BW kg was administered via the hepatic artery using microcatheter IV (Asahi Intecc Co Ltd, Seto, Aichi, Japan). The follow-up period was 24 weeks after treatment. Endpoints for efficacy were serum albumin concentration and prothrombin activity. Endpoints for safety were adverse reactions and device malfunction. For other endpoints, the patients underwent liver biopsies prior to treatment and 24 weeks after treatment for histological assessment by nonalcoholic fatty liver disease (NAFLD) activity score and fibrosis [12].
2.3. Analysis of surface antigens expression
The residual obtained ADRCs after treatment usage were analyzed for surface antigens expression. They were analyzed by flow cytometry using BD Stemflow™ (Human MSC Analysis Kit; Becton, Dickinson and Company, Franklin Lakes, NJ), as well as with PE-labeled anti-CD31, PE-labeled anti-CD44 (Becton, Dickinson and Company), and PerCP™5.5-labeled anti-CD105 (Becton, Dickinson and Company) antibodies.
2.4. Statistical analysis of efficacy endpoints
Elevation of serum albumin concentration on 3 months after treatment compared to that of pre-treatment was defined as improvement. Improvement rate of cases was calculated for full analysis set, and test based on normal distribution approximation was performed with threshold of 3% and one-sided significance level of 5%. Confidential interval (C.I.) was calculated using Clopper-Pearson method. Prothrombin activity was also assessed in the same way.
3. Results
3.1. Endpoints assessment and ADRCs' surface antigens expression
We have completed the treatment of intrahepatic arterial infusion of autologous ADRCs and 24 weeks follow-up for 7 cirrhotic patients (Fig. 1) (3 male patients, and 4 female patients, age: 66.4 ± 5.6 years old, body weight: 80.17 ± 17.31 kg, Body Mass Index: 29.84 ± 5.23). Child-Pugh score [13] for assessing the severity of the 7 cirrhotic patients showed that 6 patients were in grade A and one patient was grade B (Supplemental Table 1). The 6 patients had non-alcoholic steatohepatitis, and 1 patient had fatty liver disease. These 7 patients were full analysis set as well per protocol set. The obtained adipose tissue volume was 258.6 mL in average (minimum: 165 mL, maximum: 340 mL), which provided a sufficient number of ADRCs for the administration, which was defined as 3.3 × 105 cells/BW kg. Viability of the obtained ADRCs by the adipose tissue dissociation device were over 91.8% (minimum: 87.6%, maximum: 94.5%) in all enrolled patients. Flow cytometry analysis of the obtained ADRCs for surface antigens expression showed that CD44 mesenchymal markers was highly expressed in 67.0% of ADRCs, while CD90 and CD105 expression were seen in 2.6% and 6.6% in average (Table 1).
Table 1.
Surface antigens expression of ADRCs.
| Registered number | CD34 (%) | CD44 (%) | CD45 (%) | CD90 (%) | CD105 (%) |
|---|---|---|---|---|---|
| SH-1 | 0.76 | 93.59 | 0.73 | 5.85 | 0 |
| SH-2 | 6.46 | 81.8 | 9.32 | 9.91 | 2.9 |
| SH-3 | 9.45 | 35.99 | 1.42 | 6.34 | 0.55 |
| SH-4 | 2.49 | 75.7 | 2.86 | 3.38 | 8.32 |
| SH-5 | 2.34 | 73.29 | 0.95 | 1.6 | 2.12 |
| SH-7 | 22 | 42.73 | 2.11 | 13.33 | 0.19 |
| SH-8 | 4.33 | 65.8 | 4.11 | 5.59 | 3.96 |
For all patients, we administered 3.3 × 105 cells/BW kg via the hepatic artery using a microcatheter. As for safety assessment, no adverse events related to this clinical trial were observed. In addition, no malfunction of the investigational trial device was encountered.
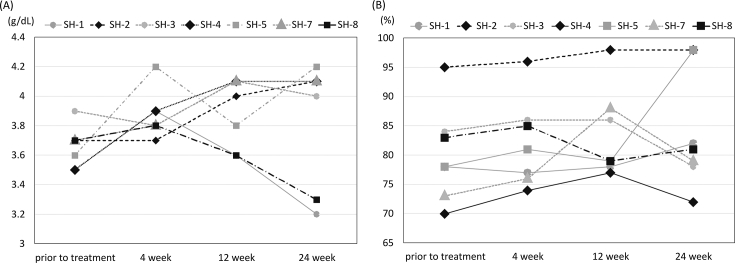
We assessed how many patients among full analysis set showed improvement of serum albumin concentration and prothrombin, which are both of the efficacy endpoints, on 3 months after treatment, compared to that of pre-treatment. We observed that 6 out of 7 patients' serum albumin concentration was improved (Fig. 2A, Supplemental Table 2); average improvement ratio of the treated patients was 85.7% (C.I. 47.9–99.3%). As for prothrombin activity, 5 out of 7 patients improved (improvement ratio 71.4%, C.I. 34.1–94.7%) (Fig. 2B, Supplemental Table 3). In terms of the severity grade of liver cirrhosis by Child-Pugh score classification, 24 weeks after treatment, all patients were in grade A (Supplemental Table 4).
Fig. 2.
Efficacy endpoints assessment during the trial observation period. Serum albumin concentration (A) and prothrombin activity (B) of each enrolled patient.
3.2. Assessment of the biopsied livers' histology and images of the abdominal CT or MRI
We also assessed the biopsied liver tissues which were obtained prior to and 24 weeks after treatment and assessed each parameters of NAFLD activity scoring system (Table 2). One patient reduced one point of hepatocyte steatosis, and one point of lobular inflammation score was reduced in one patient. Three patients' scores of hepatocyte ballooning reduced, while one patient's score increased. Fibrosis grade remained 4, cirrhotic conditions, 24 weeks after treatment (data not shown).
Table 2.
NAFLD activity scoring of the patients prior to and 24 weeks after treatment.
| Registration number | Hepatocyte steatosis |
Lobular inflammation |
Hepatocyte ballooning |
|||
|---|---|---|---|---|---|---|
| Prior to treatment | 24 week | Prior to treatment | 24 week | Prior to treatment | 24 week | |
| SH-1 | 2 | 2 | 2 | 2 | 2 | 2 |
| SH-2 | 1 | 1 | 1 | 1 | 0 | 1 |
| SH-3 | 1 | 1 | 2 | 1 | 1 | 0 |
| SH-4 | 1 | 1 | 1 | 0 | 1 | 1 |
| SH-5 | 1 | 1 | 2 | 2 | 2 | 1 |
| SH-7 | 0 | 0 | 1 | 1 | 0 | 0 |
| SH-8 | 3 | 2 | 1 | 1 | 2 | 1 |
As for the images of the abdominal CT or MRI before and 24 weeks after treatment, no apparent alteration of morphological improvement or deterioration was observed in each patient.
4. Discussion
We have conducted the multi-institutional clinical trial of autologous ADRCs treatment for liver cirrhosis patients due to non-alcoholic steatohepatitis or fatty liver disease. The investigational trial device in this trial was the adipose tissue dissociation device. No serious event which were not deniable for relation to this trial and no malfunction were observed. For assessment of serum albumin concentration, we observed that significant improvement of serum albumin concentration on week 12 after treatment compared to that of pre-treatment. Similarly, prothrombin activity was improved. We confirmed the safety and possible efficacy of this treatment.
Adipose tissue is attractive as a rich source of mesenchymal stem cells. Mesenchymal stem cells are pluripotent to differentiate into miscellaneous-lineage cells and are immunomodulatory to repair/restore the impaired organs [3,14,15]. Freshly isolated ADRCs from adipose tissues are heterogeneous population including not only MSCs, but also other type of cells [16,17]. Despite that, the advantage of unprocessed and not-manufactured cells enriched with MSCs from autologous tissues attracts much attention for practical clinical application of them for repairing/regenerative therapy [18,19], but details of MSCs are yet to be disclosed. Besides, other types of cells' effect are yet to be disclosed in the context of cells therapy, indeed, in mice, some cell fraction of adipose tissue-derived stromal cells are immune-suppressive M2-like macrophages, which are favorable for the treatment of inflammation-related impaired organs [16]. Details mechanisms of cells therapy, especially ADRCs, are principally more complex than the pharmaceutics, as they are considered to have pleiotropic effects with various biological effects. Thus, the future investigation of stromal cells of adipose tissue in details will shed light on repairing/restorative cells therapy.
The objective patients of the current clinical trial had liver cirrhosis due to NASH of fatty liver diseases. Although NASH pathology has been investigated and characterized by hepatocyte ballooning, appearance of hepatocyte steatosis, ballooning, lobular inflammatory cells, as well as pericellular fibrosis [12,20], NASH pathophysiological mechanisms, and how it ultimately develops cirrhosis are yet to be disclosed. Multiple hits theory for pathological development is proposed [21], reflecting its extreme complexity of pathology. Therefore, pharmaceutical development strategy has been not succeeded and established so far. Despite that this clinical trial was principally exploratory for safety and efficacy, administration of ADRCs into cirrhosis patients of NASH or fatty liver disease, serum albumin concentration and prothrombin activity were efficacy safety endpoints. The ratio of patients whose serum albumin concentration and prothrombin activity showed improvement after treatment was statistically significant. Despite of that, we observed that two patients showed decline of serum albumin on the 24 week compared to the 12 week. We could not find the specific reason for the decline in these two patients. We have confirmed that serum transferase activity, AST and ALT, were not exacerbated (data not shown). Furthermore, prothrombin activity was maintained, implying hepatic reserve was maintained. Although the liver fibrosis improvement was not the efficacy endpoint of this trial, it was not clearly shown to be improved, presumably in part due to short period of observation [22]. Treatment with autologous ADRCs obtained with the investigational trial device in steatohepatitis-related cirrhosis is potentially promising to maintain or improve the impaired hepatic reserve. Further studies and trials will show the possible autologous ADRCs therapeutic efficacy for NASH or fatty liver-related liver cirrhosis in future.
Discovery of somatic stem cells [4] and development of totipotent stem cells technology [[23], [24], [25], [26]] have been very attractive for the regenerative therapy. However, when considering the treatment of impaired organ, which is associated with persisting inflammation leading to destruction of tissue structure and malfunction, the simple potential of stem cells, capable to differentiate into miscellaneous cell types, may be insufficient for the repair and regeneration. In this sense, mesenchymal somatic stem cells are attractive since they are of beneficial inflammation modulatory effects [3,14]. Furthermore, autologous ADRCs which do not require manufacturing and processing cells are ideal for practical application in clinic.
Despite of that, for embodying the practical application of repairing/regenerative therapy using somatic stem cells, we should explore the clinical trial as well as non-clinical studies for disclosing the characteristics of mesenchymal stem cells as well as ADRCs. Further studies would pave the way of the potent capability of stromal cells of adipose tissues for repairing/regenerative therapy.
Author contributions
Design of the trial protocol: Y.S., Y.I., K.Y., T.M., S.K. Conducting the trial and follow up: Y.S., S.F., M.T., K.K., O.I., S.U., S.T., A.S., A.A., Y.T., K.H., S.K. Acquisition of data: S.Y., A.N., H. TBT, K.H. Drafting the manuscript: Y.S., S.F., K.K., A.N., Tar.Y., K.A., Tat.Y., E.M., M.H., T.W., K.H., S.K. All authors have approved the final article.
Declaration of competing interest
Y.S. and S.K. were scientific advisors to Cytori Therapeutics Inc.
Acknowledgments
We thank Dr. Nobuhisa Otani for his kind advice on liposuction. This work was supported by the Japan Agency of Medical Research and Development and the subsidy for clinical study of Kanazawa University Hospital.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2021.04.003.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Xu L., Liu Y., Sun Y., Wang B., Xiong Y., Lin W. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8:275. doi: 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porada C.D., Zanjani E.D., Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 3.Seki A., Sakai Y., Komura T., Nasti A., Yoshida K., Higashimoto M. Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology. 2013;58:1133–1142. doi: 10.1002/hep.26470. [DOI] [PubMed] [Google Scholar]
- 4.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborators G.B.D.C. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 8.Saab S., Le L., Saggi S., Sundaram V., Tong M.J. Toward the elimination of hepatitis C in the United States. Hepatology. 2018;67:2449–2459. doi: 10.1002/hep.29685. [DOI] [PubMed] [Google Scholar]
- 9.Lok A.S., McMahon B.J., Brown R.S., Jr., Wong J.B., Ahmed A.T., Farah W. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016;63:284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 10.Sakai Y., Takamura M., Seki A., Sunagozaka H., Terashima T., Komura T. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell. Regen Ther. 2017;6:52–64. doi: 10.1016/j.reth.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai Y., Fukunishi S., Takamura M., Inoue O., Takashima S., Usui S. Regenerative therapy for liver cirrhosis based on intrahepatic arterial infusion of autologous subcutaneous adipose tissue-derived regenerative (stem) cells: protocol for a confirmatory multicenter uncontrolled clinical trial. JMIR Res Protoc. 2020;9 doi: 10.2196/17904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunt E.M., Kleiner D.E., Wilson L.A., Belt P., Neuschwander-Tetri B.A., Network N.C.R. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 14.Higashimoto M., Sakai Y., Takamura M., Usui S., Nasti A., Yoshida K. Adipose tissue derived stromal stem cell therapy in murine ConA-derived hepatitis is dependent on myeloid-lineage and CD4+ T-cell suppression. Eur J Immunol. 2013;43:2956–2968. doi: 10.1002/eji.201343531. [DOI] [PubMed] [Google Scholar]
- 15.Yamato M., Sakai Y., Mochida H., Kawaguchi K., Takamura M., Usui S. Adipose tissue-derived stem cells prevent fibrosis in murine steatohepatitis by suppressing IL-17-mediated inflammation. J Gastroenterol Hepatol. 2019;34:1432–1440. doi: 10.1111/jgh.14647. [DOI] [PubMed] [Google Scholar]
- 16.Nasti A., Sakai Y., Seki A., Buffa G.B., Komura T., Mochida H. The CD45(+) fraction in murine adipose tissue derived stromal cells harbors immune-inhibitory inflammatory cells. Eur J Immunol. 2017;47:2163–2174. doi: 10.1002/eji.201646835. [DOI] [PubMed] [Google Scholar]
- 17.Gimble J.M., Katz A.J., Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito S., Kai Y., Masuda T., Tanaka F., Matsumoto T., Kamohara Y. Long-term outcome of adipose-derived regenerative cell-enriched autologous fat transplantation for reconstruction after breast-conserving surgery for Japanese women with breast cancer. Surg Today. 2017;47:1500–1511. doi: 10.1007/s00595-017-1544-4. [DOI] [PubMed] [Google Scholar]
- 19.Murohara T. Autologous adipose tissue as a new source of progenitor cells for therapeutic angiogenesis. J Cardiol. 2009;53:155–163. doi: 10.1016/j.jjcc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 21.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 22.Desmet V.J., Gerber M., Hoofnagle J.H., Manns M., Scheuer P.J. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 23.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 24.Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 25.Gurdon J.B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- 26.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.