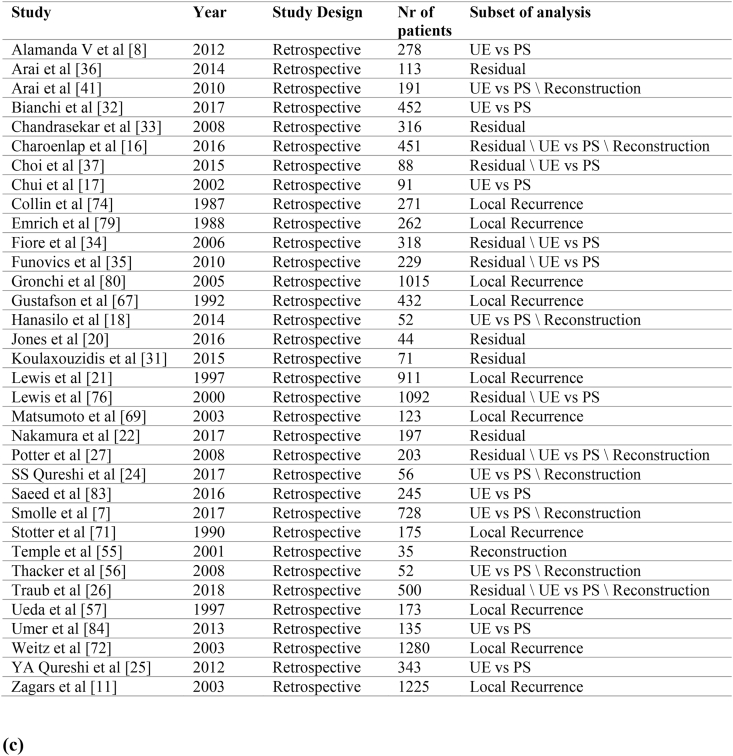
Abstract
In Soft Tissue Sarcomas (STS) referral centre many patients have already had an incomplete tumour resection. In the majority of specimen, tumoral residual is detected and linked to a worsen prognosis. Systematic surgical re-resection of the scar tissue area is often performed. Some authors suggested to postpone re-resections until a clinically evident local recurrence is detected. A searching strategy was applied to Pubmed-Central and Ovid Medline. Odds ratio (OR) for local recurrence (LR), distant metastasis (MTS) or overall survival (OS) were calculated comparing patients who had tumour residual to people who hadn't. OR of local recurrences, distant metastasis and OS were calculated in planned vs unplanned-excisions groups. OR to develop a metastasis and OS after a local recurrences were calculated. Residual tumour led to an OR for LR of 3,56, OR of MTS was 3,42; OR of decreased OS was 3,42. Having a LR lead to a OR of 1,55 for MTS and to a OR of decreased OS of 2,32. Patients who underwent a re-excision compared to planned surgery did not have an increased OR of LR and had an OR to develop a MTS of 0,56. Our data confirm that there is a strong correlation between local recurrences, distant relapses and overall survival. Although there is a selection bias; this analysis highlights the optimal oncological outcome in patients who underwent re-resection. The rationale for systematic re-resection after unplanned excision of soft tissue sarcomas is very strong and this treatment remains the gold standard of care in these patients.
Keywords: Sarcomas, Unplanned excision, Re-Excisions, Re-resections
1. Introduction
Soft tissue sarcomas (STS) are rare neoplasms as they represent 1% of all malignancies.1, 2, 3 Overall survival, is generally reported to be 50% at 5 years; STS are coupled with high mortality rate considering that they account for 2% of all tumour deaths.2, 3, 4 Unplanned excisions (UE) are defined as tumour excisions without an appropriate diagnosis, preoperative imaging or planning.5 Although in recent years, several educational strategies have been implemented, it is still very common to deal with patients who have been inadequately treated. In fact, referral sarcoma centres still report up to 18–66% of their patients to come to attention after unplanned excisions in another hospital.1,6, 7, 8, 9, 10, 11, 12, 13, 14, 15 These patients are most often initially affected by small lesions (<5 cm) superficial to the subcutaneous fascia that are mistaken for benign tumours. Surgeons are likely to inadequately excise these masses given the fact that generally out of 300 superficial and small neoplasms, 299 are benign and 1 is malign.1 When a re-excision is performed, the original surgical bed and scar tissue has to be removed with healthy tissue around. Tumour Residuals in tumour bed excisions have been reported in 35–91% of the specimens10,11,14,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 and accordingly to several authors it is linked with worse prognosis.9,10,16,22,27,31, 32, 33, 34, 35, 36, 37 At the present time it is not possible by imaging workup to distinguish before the re-excision procedure and specimen analysis, in which patients residual tumour could be found in the excised scar.11,33,38, 39, 40 For these reasons, systematic re-resection of the scar tissue area, coupled or not with adjuvant therapies is the gold standard of care in patients who underwent UE.3,5,11,17,21,23,30,31,33,41, 42, 43, 44, 45, 46, 47, 48 Nevertheless, some authors have recently suggested to postpone re-excisions until a clinically evident local recurrence is detected. This practice was introduced in some referral centres based upon the assumption that local control do not have influence on overall survival in patients affected by soft tissue sarcomas.49 Recently, a wait and see approach was reported to be a viable alternative in selected patients to systematic re-excision after unplanned surgeries.50 This systematic review aimed at studying the rationale and the outcomes of Re-Excisions surgeries. In addition, it was studied to what degree a local recurrence could be linked with mortality and risk to develop distant relapses. The authors tried to answer the following questions: what are the oncological outcomes of patients treated with Re-resection after UE compared to those ones initially treated with planned surgeries? What is the impact of residual tumour on mortality, local and distant relapses? What is the impact of a local recurrence on mortality and risk to develop a distant metastasis? What is the risk to undergo a plastic reconstructive surgery after re-excisions?
2. Methods
2.1. Literature search
A literature search was performed, following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines.51 Ovid MEDLINE and Pubmed central databases were included in the search strategy. Included studies were published from January 1986 to December 2018. Search strategy is displayed in detail in Fig. 1. Two Orthopaedic Surgeons with experience in Orthopaedic Oncology reviewed the articles. In case of overlapping populations, the larger one or the most detailed one was included in the analysis.
Fig. 1.
(a, b, c). Literature Search Strategy.Search flow diagram. Studies included in Metanalysis.
2.2. Data collection
From each study, the authors collected study design, clinical and epidemiological data and outcomes. In particular, authors extracted data from each article about: clinical scenario (re-excision or planned resection), preoperative dimensions, preoperative margins, tumour grade and subtype, tumour site, tumour location in relation to subcutaneous fascia, presence of residual tumour in the excision bed, post-operative surgical margins of re-excisions or planned resections, therapeutic management (adjuvant or neo-adjuvant chemotherapy or radiotherapy) and length of follow-up. Local Recurrence Free Survivals (LRFS), Metastasis Free Survivals (MFS), Overall Survivals (OS) and the need of plastic surgery reconstructions were the endpoints considered for the analysis. Residual tumour group was taken into account including patients who were assessed to have microscopic residual disease, macroscopic residual disease or non-specified residual disease on the base of different way of reporting the residual disease in different studies.
2.3. Outcomes
The aim of the current study was to assess the Odds Ratio (OR) of local recurrence and distant metastasis comparing patients who underwent a re-excision after an unplanned excision to patients who underwent a planned resection in the sarcoma referral centres among the included studies. Secondary analyses were performed to assess OR of mortality, local and distant recurrence comparing patients who had developed local recurrence to patients who had not. Mortality, local and distant recurrence OR were calculated considering if there were or not tumour residual in the excision bed. Finally, the OR to undergo a plastic reconstruction surgery for soft tissue gaps was analysed comparing patients who underwent re-excision after unplanned excision to patients who underwent planned resection.
2.4. Statistical analysis
Der Simonian and Laird method for random-effects model was applied to calculate OR of the prognostic factors analysed on overall survival and risk to develop local and distant metastases. When in the paper OR was not reported, the relative estimated value was derived from other raw data as Tierney et al. described.52 The metanalysis was graphically shown by forest plots. In order to evaluate heterogeneity and bias, meta-regression and funnel plot, in addition to Egger's linear test, were analysed. Differences were considered significant at p < 0.05. Statistical analyses were performed with SPSS version 23 (SPSS Inc., SPSS® Chicago, IL, USA), ProMeta version 3 (Internovi, Cesena, Italy).
3. Results
3.1. Literature review
In Fig. 1 are summarized the studies included in Metanalysis. The search flow diagram is detailed in Fig. 1. Thirty-two studies were included in our systematic review with a total of 10,444 patients.
3.2. Metanalysis
3.2.1. Re-excision VS planned surgeries
The Odds Ratio to develop a local recurrence in 5 years period of Re-Excision group was not significantly higher towards planned surgery group (OR = 1.36; 95% CI 0.94–1.96; p:0.1). Local recurrence free survival (LRFS) analysis was conducted on 6238 patients (Fig. 2).
Fig. 2.
Forest plot of 5 years local recurrence free survival of re-excision and planned excision groups.
The risk to develop a distant metastasis in a 5 years period was lower in patients who were treated by Re-excision compared to planned surgery (OR = 0.56; 95% CI 0.46–0.69; p < 0.001). This analysis involved 5689 patients (Fig. 3).
Fig. 3.
Forest plot of 5 years metastasis free survival of re-excision and planned excision groups.
3.3. local recurrence
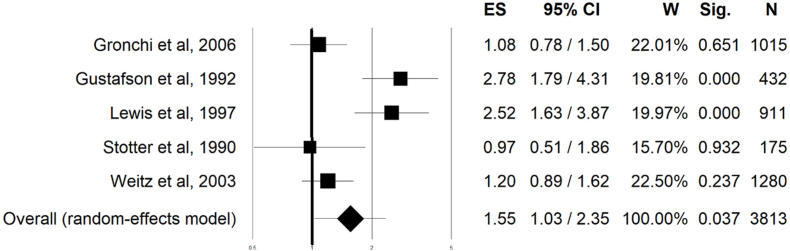
Patients who experienced a first local recurrence had a subsequent higher risk to develop a distant metastasis (OR = 1.55; 95% CI 1.03–2.35; p: 0.03). This analysis that involved 3813 patients (Fig. 4).
Fig. 4.
Forest plot of metastasis free survival of patients who had local recurrence and who did not have any local recurrence.
The occurrence of a local relapse was also linked to decreased overall survival (OR = 2.32; 95% CI 1.50–3.58; p < 0.001). This analysis was conducted on 4531 patients and relative forest plot is shown in Fig. 5.
Fig. 5.
Forest plot of overall survival of patients who had local recurrence and who did not have any local recurrence.
3.4. Tumour residual
The Odds Ratio to develop a local recurrence in a 5 years period was significantly in disfavour of patients in which a tumour residual was found in the excision bed (OR = 3.36; 95% CI 1.97–6.44; p < 0.001). LRFS analysis that was conducted on 1708 patients. Patients with residual tumour also faced higher risk to develop a distant metastasis (OR = 3.42; 95% CI 2.54–5.01; p < 0.001). Tumour residual was also linked to lower overall survival (OR = 2.26; 95% CI 1.63–3.14; p < 0.001), this analysis included 1669 patients.
3.5. Plastic reconstruction surgery
Re-excision were linked to a higher risk of plastic surgery reconstructions compared to planned surgeries (OR = 2.49; 95% CI 1.45–4.30; p < 0.001), this analysis included 2268 patients.
3.6. Bias
Meta-regression for publication years did not show significant variation of the effect size, nor publication bias arose from the Funnel plot, followed by Eggers’ linear regression for any of the analysis included in the study.
4. Discussion
Unplanned excisions of sarcoma are still representing a serious issue in the field of Orthopaedic Oncology. It is generally recommended to refer to a primary centre when dealing with a soft tissue mass of bigger sizes than 5 cm, a subfascial or rapidly growing one. Nevertheless, it is still common to deal with patients treated with ‘piecemeal’ or marginal excisions of small, superficial lesions which turn out to be sarcomas. The prevalence of benign soft tissue mass compared is tremendously higher than the malign counterpart, that could explain why educational strategies that have been implemented during time are still not successful. Furthermore, the histologic reports from tertiary centres after unplanned excisions usually lack details about macroscopic examination of the specimen and quality of surgical margins. Very often, even after revision of the samples in primary centres, doubts still remain about histologic diagnosis and UE surgical margins. Since Giuliano proposed the term ‘Unplanned excisions’ to describe a variety of clinical scenario characterized by a lack of preoperative diagnostic assessments or planning before excision procedures, several studies proved the efficacy of systematic tumour bed excisions, the so called ‘re-excision’ procedures.32 Systematic Re-Excisions are still representing the gold standard of treatment in patients who underwent unplanned resections of sarcoma. In this setting, these patients undergo a tumour bed excision generally within 3 months53 from the inadequate surgery even if there aren't any radiological or clinical evidences of local recurrences. Nevertheless, recently some authors have proposed a ‘wait and see approach’. Bonvalot et al.49 hypothesized that local control have no impact on mortality. In this study a retrospective analysis of STS patients was performed. On Univariate Analysis, Local Recurrence was a negative prognostic factor for overall survival (HR:1.7 CI 95%: 1–2.8 p:0.04). However, Local Recurrence didn't impact Overall Survival on multivariate analysis (HR:1.5 CI 95%: 0.9–2.6 p:0.2). Furthermore, scar tissue excision often causes a large soft tissues defect because anatomical plans are jeopardized after the first inadequate surgery and it is often needed to remove a wide area of healthy tissue54 to excise with sufficient confidence all hypothetical tumour residuals.22 As a consequence, other authors have described the outcomes of the ‘wait and see’ approach.50
The current study has some limitations. First of all, the vast majority of the included studies had a retrospective design. Given the rarity of STS, retrospective analysis of prospectively collected data from one or more referral institutions are the main source of information about prognostic factors and outcomes in STS. For this reason the authors advise to take with caution the results of the current study as there could be some bias related to the retrospective nature of raw data included in our analysis. In addition, when the authors analysed re-resection related outcomes, studies in which there were only patients treated with re-excision without a control group were excluded from the current analysis. For this reason, some information about outcomes in re-resection patients could be lost. In addition, residual tumour groups have been built coupling microscopic and macroscopic residual compared to no residual. Therefore, hypothetical prognostic differences in outcomes between microscopic and macroscopic residuals were not detected. The negative effect of macroscopic residuals on the outcomes may have been mitigated by the patients with microscopic residuals. Finally, all the studies included in metanalysis are based on retrospective series in which several different histotypes are mixed together. Future studies should outline the differences in prognosis between macro vs microscopic residuals and a separate interim analysis of major histotypes.
Re-excisions are usually coupled with higher rates of plastic reconstructive surgery when compared to planned surgery.7,16,18,26,27,48,55,56 Only few authors reported higher rates of reconstructions in planned surgery groups.24 Removing scar tissue with all hypothetical contamination is a very demanding procedure. Anatomical plans and tissue architecture had been jeopardized by previous inadequate surgery. The extension of tissue removal is difficult to assess and in order to achieve safe margins very wide excisions are often required. Soft tissue reconstructions are mandatory when large tissue gaps make a primary closure impossible. Our metanalysis quantifies the risk to undergo plastic surgery after re-excision procedures as 2,49 times higher compared to planned surgeries.
When an inadequate sarcoma surgery had been performed, the relative histologic report is often lacking details on surgical margins. Even when a negative margins is detailed in UE pathology report, after re-excision, tumour residual is often detected.14,17 Specimen revision by primary centre Pathologist is most of the time performed but even after the revision, doubts still remain about surgical margins and adequacy of the first procedure. In this scenario it is very difficult to make prevision about residual tumours in absence of any radiological or clinical evidence of local recurrence. This is one of the reasons why many centres perform a systematic re-excision of the scar tissue. At the present time there is no way to predict in advance when a tumour residual will be found in the excision bed. In this subset of patients, micro or macroscopic tumour residual is often detected. In this systematic review, the authors included 28 studies in which there were clearly stated how many patients had tumour residual out of the total patients who underwent re-excisions. The analysis included 2543 patients who underwent re-resections and among them 1486 had tumour residual (58,4% of the cases; min 35%-max 91%). Several authors reported tumour residual as a negative prognostic factor in mortality, local recurrence and distant metastasis survival.9,20,22,27,31, 32, 33, 34, 35,37 The current analysis showed an OR of 3,42 to develop a distant metastasis and 2,26 of death in patients where a tumour residual (micro or macroscopic) was found compared to patients in which there wasn't any tumour residual. It seems likely that although tumour residual could be just another sign of the inner biological tumour aggressiveness, it could significantly affect the outcomes. As several authors suggest, and this meta-analysis confirms, having residual cells or tissue is an important prognostic factor on mortality and distant relapses.
Bonvalot and other authors49 suggest a ‘wait and see approach’ after unplanned excisions; they postpone surgical re-excision until a detectable local recurrence become evident. As previously stated, the rationale behind this management lies in their attitude about the relationship between local recurrence and prognosis. The impact of local recurrences on mortality and on distant metastasis is a long debated topic. Some authors suggest that there is no causal correlation between LR and systemic prognosis as they just reflect the inner biological aggressiveness of the neoplasm.29,49,57, 58, 59, 60, 61, 62, 63, 64, 65 Some other authors, instead, suggest that Local Recurrences have a direct impact on distant metastasis and overall survival.59,66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80 With retrospective analyses it is very difficult to prove any cause-effect relationships and most authors must rely on statistical relationships among risk factors and prognosis. This is true for each prognostic factor studied on retrospective data. As stated before, most of the studies in STS are retrospective analyses of prospectively collected data. To shed a light on this topic, the authors included in this metanalysis, studies in which there were clear comparison in outcomes between patients who had a LR to patients who didn't have it. The analysed outcomes consisted in the risk to develop a distant metastasis and mortality. Although it is impossible to directly prove a cause-effect mechanism, the analysis showed an OR of 1,55 to have a distant metastasis and 2,32 to die after having a first local recurrence. Furthermore, to justify their ‘wait and see’ approach, Bonvalot et al. rely on a multivariate analysis that didn't show any statistic significant effect of LR on Overall Survival. Nevertheless, even in their study, the authors showed that when a LR occurred, a negative trend about mortality was seen (on univariate analysis LR was a negative prognostic factor on Overall Survival). The current metanalysis, based on 4831 patients, showed a clear connection between LR and Overall Survival, therefore the authors have some concern about the theoretical basis on which the ‘wait and see’ approach was developed.
Finally, a metanalysis about outcomes after systematic re-excision compared to planned surgeries was conducted. Several authors have reported that this comparison has in itself a confounding bias. Patients who undergo re-excision surgeries have significatively smaller and more superficial lesions than the counterpart of planned surgeries.7,8,18,21,34,37,81 This happens because surgeons are more likely to mistake a small and superficial mass for a benign one compared to a bigger and deeper one that could raise more suspects about its malignancy. Given this limitation, the metanalysis conducted on more than 5000 patients showed how there were no increase in the risk of developing a local or distant recurrence in re-excision procedures vs planned excisions. The OR for distant metastasis was 0,56, showing that after a re-excision there was less risk to develop a distant metastasis compared with planned surgeries. This finding could be partly explained by the above mentioned inclusion bias. At least, after re-excision surgery the majority of patients have a positive outcome. Surprisingly, although authors have reported contrasting outcomes on local recurrence free survival,7,10,18,21,24, 25, 26, 27,34,35,48,56,82, 83, 84 this metanalysis didn't show any significant difference between re-excision and planned surgery groups. This result is in favour of the current strategy to perform systematic re-excisions. In fact, as the authors showed, the very high proportion of patients who will have tumour residual in their excision bed, if not treated with re-excision, would eventually develop a local recurrence. In our analysis, 58% of re-excision patients had a tumour residual, so it can be suggested a local recurrence rate of at least 50% after unplanned excision in non-treated patients. In planned surgery, when tumour margins are free, the local recurrence rates at five years are generally reported between 4 and 20%.3,6,70,85,86 This metanalysis showed how well re-excision surgery lowered the incidence of local recurrence in patients treated with inadequate surgeries.
Summarizing, systematic re-excision surgery after unplanned excisions results in excellent oncological outcomes although it is often burdened with large soft tissue removal and the need of plastic reconstructive surgery. The rationale of systemic re-resections is strong as it is not possible to stratify patients in regards to likelihood to have a tumour residual. Residual is frequently diagnosed in excision beds after specimens analysis and has an impact on local, distant relapse and mortality.
5. Conclusion
Systematic re-excision after unplanned excisions of soft tissue sarcoma is the gold standard management at the present day. The rationale of a ‘wait and see’ approach appear less strong than the one behind systematic re-excision and could be risky. A wait and see approach could be justified only in case of unplanned excisions performed in tertiary centres when a low grade tumour is diagnosed and there is a reliable pathologic examination which guarantees an adequate surgery with appropriate margins.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Declaration of competing interest
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2021.05.022.
Contributor Information
Federico Sacchetti, Email: federico.sacchetti1989@gmail.com.
Andac Celasun Alsina, Email: andacalsina@gmail.com.
Riccardo Morganti, Email: r.morganti@med.unipi.it.
Matteo Innocenti, Email: innocenti.matteo11@gmail.com.
Lorenzo Andreani, Email: l.andreani@hotmail.it.
Francesco Muratori, Email: fmuratori75@gmail.com.
Guido Scoccianti, Email: guido.scoccianti@gmail.com.
Francesca Totti, Email: totti.francesca@live.it.
Domenico Andrea Campanacci, Email: campanaccid@gmail.com.
Rodolfo Capanna, Email: rodolfo.capanna@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tedesco N.S., Henshaw R.M. Unplanned resection of sarcoma. J Am Acad Orthop Surg. 2016;24(3):150–159. doi: 10.5435/JAAOS-D-15-00074. [DOI] [PubMed] [Google Scholar]
- 2.Corey R.M., Swett K., Ward W.G. Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med. 2014;3(5):1404–1415. doi: 10.1002/cam4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehren M Von, Randall R.L., Benjamin R.S. Soft tissue sarcoma, version 2.2018: clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw. 2018;16(5):536–563. doi: 10.6004/jnccn.2018.0025. [DOI] [PubMed] [Google Scholar]
- 4.Eilber F.C., Brennan M.F., Riedel E., Alektiar K.M., Antonescu C.R., Singer S. Prognostic factors for survival in patients with locally recurrent extremity soft tissue sarcomas. Ann Surg Oncol. 2005;12(3):228–236. doi: 10.1245/ASO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano A.E., Eilber F.R. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol. 1985;3(10):1344–1348. doi: 10.1200/JCO.1985.3.10.1344. [DOI] [PubMed] [Google Scholar]
- 6.Ray-Coquard I., Thiesse P., Ranchère-Vince D. Conformity to clinical practice guidelines, multidisciplinary management and outcome of treatment for soft tissue sarcomas. Ann Oncol. 2004;15(2):307–315. doi: 10.1093/annonc/mdh058. [DOI] [PubMed] [Google Scholar]
- 7.Smolle M.A., Tunn P.U., Goldenitsch E. The prognostic impact of unplanned excisions in a cohort of 728 soft tissue sarcoma patients: a multicentre study. Ann Surg Oncol. 2017;24(6):1596–1605. doi: 10.1245/s10434-017-5776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alamanda V.K., Delisca G.O., Archer K.R., Song Y., Schwartz H.S., Holt G.E. Incomplete excisions of extremity soft tissue sarcomas are unaffected by insurance status or distance from a sarcoma center. J Surg Oncol. 2013;108(7):477–480. doi: 10.1002/jso.23427. [DOI] [PubMed] [Google Scholar]
- 9.Davis A.M., Kandel R.A., Wunder J.S. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66(2):81–87. doi: 10.1002/(SICI)1096-9098(199710)66:2<81::AID-JSO2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Rehders A., Stoecklein N.H., Poremba C., Alexander A., Knoefel W.T., Peiper M. Reexcision of soft tissue sarcoma: sufficient local control but increased rate of metastasis. World J Surg. 2009;33(12):2599–2605. doi: 10.1007/s00268-009-0262-5. [DOI] [PubMed] [Google Scholar]
- 11.Zagars G.K., Ballo M.T., Pisters P.W.T., Pollock R.E., Patel S.R., Benjamin R.S. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer. 2003;97(10):2544–2553. doi: 10.1002/cncr.11367. [DOI] [PubMed] [Google Scholar]
- 12.Clasby R., Tilling K., Smith M.A., Fletcher C.D.M. Variable management of soft tissue sarcoma: regional audit with implications for specialist care. Br J Surg. 1997;84(12):1692–1696. doi: 10.1002/bjs.1800841213. [DOI] [PubMed] [Google Scholar]
- 13.Collin C., Hajdu S.I., Godbold J., Friedrich C., Brennan M.F. Localized operable soft tissue sarcoma of the upper extremity: presentation, management, and factors affecting local recurrence in 108 patients. Ann Surg. 1987;205(4):331–339. doi: 10.1097/00000658-198704000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodlad J.R., Fletcher C.D.M., Smith M.A. Surgical resection of primary soft-tissue sarcoma. J Bone Joint Surg Br. 1996;78-B(4):658–661. doi: 10.1302/0301-620x.78b4.0780658. [DOI] [PubMed] [Google Scholar]
- 15.Kang S., Kim H.S., Han I. Unplanned excision of extremity soft tissue sarcoma in Korea: a nationwide study based on a claims registry. PloS One. 2015;10(8):1–12. doi: 10.1371/journal.pone.0134354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charoenlap C., Imanishi J., Tanaka T. Outcomes of unplanned sarcoma excision: impact of residual disease. Cancer Med. 2016;5(6):980–988. doi: 10.1002/cam4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chui C.H., Spunt S.L., Liu T. Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg. 2002;37(10):1424–1429. doi: 10.1053/jpsu.2002.35405. [DOI] [PubMed] [Google Scholar]
- 18.Hanasilo C.E.H., Casadei M.S., Auletta L., Amstalden E.M.I., Matte S.R.F., Etchebehere M. Comparative study of planned and unplanned excisions for the treatment of soft tissue sarcoma of the extremities. Clinics. 2014;69(9):579–584. doi: 10.6061/clinics/2014(09)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scoccianti G., Innocenti M., Frenos F. Re-excision after unplanned excision of soft tissue sarcomas: long-term results. Surg Oncol. 2020;34:212–217. doi: 10.1016/j.suronc.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Jones D.A., Shideman C., Yuan J. Management of unplanned excision for soft-tissue sarcoma with preoperative radiotherapy followed by definitive resection. Am J Clin Oncol Cancer Clin Trials. 2016;39(6):586–592. doi: 10.1097/COC.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 21.Lewis J.J., Leung D., Espat J., Woodruff J.M., Brennan M.F. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231(5):655–663. doi: 10.1097/00000658-200005000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T., Kawai A., Sudo A. Analysis of the patients with soft tissue sarcoma who received additional excision after unplanned excision: report from the Bone and Soft Tissue Tumor Registry in Japan. Jpn J Clin Oncol. 2017;47(11):1055–1059. doi: 10.1093/jjco/hyx123. [DOI] [PubMed] [Google Scholar]
- 23.Zornig Mp C., S S. Re-excision of soft tissue sarcoma after inadequate initial operation (Br J Surg 1995; 82: 278-9) [2] Br J Surg. 1995;88(10):1417. doi: 10.1046/j.0007-1323.2001.1920.x. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi S.S., Prabhu A., Bhagat M. Re-excision after unplanned resection of nonmetastatic nonrhabdomyosarcoma soft tissue sarcoma in children: comparison with planned excision. J Pediatr Surg. 2017;52(8):1340–1343. doi: 10.1016/j.jpedsurg.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi Y.A., Huddy J.R., Miller J.D., Strauss D.C., Thomas J.M., Hayes A.J. Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol. 2012;19(3):871–877. doi: 10.1245/s10434-011-1876-z. [DOI] [PubMed] [Google Scholar]
- 26.Traub F., Griffin A.M., Wunder J.S., Ferguson P.C. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer. 2018;124(19):3868–3875. doi: 10.1002/cncr.31648. [DOI] [PubMed] [Google Scholar]
- 27.Potter B.K., Adams S.C., Pitcher J.D., Temple H.T. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res. 2008;466(12):3093–3100. doi: 10.1007/s11999-008-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stojadinovic A., Leung D.H.Y., Hoos A., Jaques D.P., Lewis J.J., Brennan M.F. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235(3):424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trovik C.S., Bauer H.C.F., Alvegård T.A. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Canc. 2000;36(6):710–716. doi: 10.1016/S0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 30.Pretell-Mazzini J., Barton M.D., Conway S.A., Temple H.T. Current concepts review unplanned excision of soft-tissue sarcomas: current concepts for management and prognosis. J Bone Jt Surg - Am. 2015;97(7):597–603. doi: 10.2106/JBJS.N.00649. [DOI] [PubMed] [Google Scholar]
- 31.Koulaxouzidis G., Schwarzkopf E., Bannasch H., Stark G.B. Is revisional surgery mandatory when an unexpected sarcoma diagnosis is made following primary surgery? World J Surg Oncol. 2015;13(1):1–10. doi: 10.1186/s12957-015-0719-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianchi G., Sambri A., Cammelli S. Impact of residual disease after “unplanned excision” of primary localized adult soft tissue sarcoma of the extremities: evaluation of 452 cases at a single Institution. Musculoskelet Surg. 2017;101(3):243–248. doi: 10.1007/s12306-017-0475-y. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekar C.R., Wafa H., Grimer R.J., Carter S.R., Tillman R.M., Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Jt Surg - Ser B. 2008;90(2):203–208. doi: 10.1302/0301-620X.90B2.19760. [DOI] [PubMed] [Google Scholar]
- 34.Fiore M., Casali P.G., Miceli R. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13(1):110–117. doi: 10.1245/ASO.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Funovics P.T., Vaselic S., Panotopoulos J., Kotz R.I., Dominkus M. The impact of re-excision of inadequately resected soft tissue sarcomas on surgical therapy, results, and prognosis: a single institution experience with 682 patients. J Surg Oncol. 2010;102(6):626–633. doi: 10.1002/jso.21639. [DOI] [PubMed] [Google Scholar]
- 36.Arai E., Sugiura H., Tsukushi S. Residual tumor after unplanned excision reflects clinical aggressiveness for soft tissue sarcomas. Tumor Biol. 2014;35(8):8043–8049. doi: 10.1007/s13277-014-2043-5. [DOI] [PubMed] [Google Scholar]
- 37.Choi E.S., Han I., Cho H.S., Kang H.G., Kim J.H., Kim H.S. Distinct clinical characteristics of unplanned excision in synovial sarcoma. CiOS Clin Orthop Surg. 2015;7(2):254–260. doi: 10.4055/cios.2015.7.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noria S., Davis A., Kandel R. Residual disease following unplanned excision of a soft-tissue sarcoma of an extremity. J Bone Jt Surg - Ser A. 1996;78(5):650–655. doi: 10.2106/00004623-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Davies A.M., Mehr A., Parsonage S., Evans N., Grimer R.J., Pynsent P.B. MR imaging in the assessment of residual tumour following inadequate primary excision of soft tissue sarcomas. Eur Radiol. 2004;14(3):506–513. doi: 10.1007/s00330-003-2023-4. [DOI] [PubMed] [Google Scholar]
- 40.Gingrich A.A., Elias A., Michael Lee C.Y. Predictors of residual disease after unplanned excision of soft tissue sarcomas. J Surg Res. 2017;208:26–32. doi: 10.1016/j.jss.2016.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cecchetto G., Guglielmi M., Inserra A. Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int. 2001;17(7):532–534. doi: 10.1007/s003830100580. [DOI] [PubMed] [Google Scholar]
- 42.Harati K., Lange K., Goertz O. A single-institutional review of 68 patients with dermatofibrosarcoma protuberans: wide re-excision after inadequate previous surgery results in a high rate of local control. World J Surg Oncol. 2017;15(1):1–9. doi: 10.1186/s12957-016-1075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang S., Han I., Lee S.A., Cho H.S., Kim H.S. Unplanned excision of soft tissue sarcoma: the impact of the referring hospital. Surg Oncol. 2013;22(2):e17–e22. doi: 10.1016/j.suronc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Kepka L., Suit H.D., Goldberg S.I. Results of radiation therapy performed after unplanned surgery (without re-excision) for soft tissue sarcomas. J Surg Oncol. 2005;92(1):39–45. doi: 10.1002/jso.20351. [DOI] [PubMed] [Google Scholar]
- 45.Manoso M.W., Frassica D.A., Deune E.G., Frassica F.J. Outcomes of re-excision after unplanned excisions of soft-tissue sarcomas. J Surg Oncol. 2005;91(3):153–158. doi: 10.1002/jso.20323. [DOI] [PubMed] [Google Scholar]
- 46.Morii T., Yabe H., Morioka H., Anazawa U., Suzuki Y., Toyama Y. Clinical significance of additional wide resection for unplanned resection of high grade soft tissue sarcoma. Open Orthop J. 2008;2(1):126–129. doi: 10.2174/1874325000802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugiura H., Takahashi M., Katagiri H. Additional wide resection of malignant soft tissue tumors. Clin Orthop Relat Res. 2002;394:201–210. doi: 10.1097/00003086-200201000-00024. [DOI] [PubMed] [Google Scholar]
- 48.Arai E., Nishida Y., Tsukushi S., Wasa J., Ishiguro N. Clinical and treatment outcomes of planned and unplanned excisions of soft tissue sarcomas. Clin Orthop Relat Res. 2010;468(11):3028–3034. doi: 10.1007/s11999-010-1392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonvalot S., Levy A., Terrier P. Primary extremity soft tissue sarcomas: does local control impact survival? Ann Surg Oncol. 2017;24(1):194–201. doi: 10.1245/s10434-016-5462-2. [DOI] [PubMed] [Google Scholar]
- 50.Decanter G., Stoeckle E., Honore C. Watch and wait approach for Re-excision after unplanned yet macroscopically complete excision of extremity and superficial truncal soft tissue sarcoma is safe and does not affect metastatic risk or amputation rate. Ann Surg Oncol. 2019;26(11):3526–3534. doi: 10.1245/s10434-019-07494-6. [DOI] [PubMed] [Google Scholar]
- 51.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:1–16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han I., Kang H.G., Kang S.C., Choi J.R., Kim H.S. Does delayed reexcision affect outcome after unplanned excision for soft tissue sarcoma? Clin Orthop Relat Res. 2011;469(3):877–883. doi: 10.1007/s11999-010-1642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enneking W.F., Spanier S.S., Malawer M.M. The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer. 1981;47(5):1005–1022. doi: 10.1002/1097-0142(19810301)47:5<1005::AID-CNCR2820470532>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 55.Temple H.T., Worman D.S., Mnaymneh W.A. Unplanned surgical excision of tumors of the foot and ankle. Cancer Control. 2001;8(3):262–268. doi: 10.1177/107327480100800306. [DOI] [PubMed] [Google Scholar]
- 56.Thacker M.M., Potter B.K., Pitcher J.D., Temple H.T. Soft tissue sarcomas of the foot and ankle: impact of unplanned excision, limb salvage, and multimodality therapy. Foot Ankle Int. 2008;29(7):690–698. doi: 10.3113/FAI.2008.0690. [DOI] [PubMed] [Google Scholar]
- 57.Ueda T., Yoshikawa H., Mori S. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J Bone Jt Surg - Ser B. 1997;79(4):553–557. doi: 10.1302/0301-620X.79B4.7487. [DOI] [PubMed] [Google Scholar]
- 58.Brennan M.F. The enigma of local recurrence. The Society of Surgical Oncology. Ann Surg Oncol. 1997;4(1):1–12. doi: 10.1007/BF02316804. http://www.ncbi.nlm.nih.gov/pubmed/8985511 [DOI] [PubMed] [Google Scholar]
- 59.Espat N.J., Lewis J.J. The biological significance of failure at the primary site on ultimate survival in soft tissue sarcoma. Semin Radiat Oncol. 1999;9(4):369–377. doi: 10.1016/S1053-4296(99)80031-9. [DOI] [PubMed] [Google Scholar]
- 60.Gronchi A., Miceli R., Fiore M. Extremity soft tissue sarcoma: adding to the prognostic meaning of local failure. Ann Surg Oncol. 2007;14(5):1583–1590. doi: 10.1245/s10434-006-9325-0. [DOI] [PubMed] [Google Scholar]
- 61.Gustafson P., Rooser B., Rydholm A. Local recurrence is of minor importance for metastases in soft tissue sarcoma. Acta Orthop Scand Suppl. 1991;62(246):68. doi: 10.1002/1097-0142(19910415)67:8<2083::aid-cncr2820670813>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 62.Harati K., Kolbenschlag J., Bohm J. Long-term outcomes of patients with soft tissue sarcoma of the chest wall: analysis of the prognostic significance of microscopic margins. Oncol Lett. 2018;15(2):2179–2187. doi: 10.3892/ol.2017.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potter D.A., Kinsella T., Glatstein E. High‐grade soft tissue sarcomas of the extremities. Cancer. 1986;58(1):190–205. doi: 10.1002/1097-0142(19860701)58:1<190::AID-CNCR2820580133>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 64.Stojadinovic A., Jaques D.P., Leung D.H.Y., Healey J.H., Brennan M.F. Amputation for recurrent soft tissue sarcoma of the extremity: indications and outcome. Ann Surg Oncol. 2001;8(6):509–518. doi: 10.1245/aso.2001.8.6.509. [DOI] [PubMed] [Google Scholar]
- 65.Sugiura H., Tsukushi S., Yoshida M., Nishida Y. What is the success of repeat surgical treatment of a local recurrence after initial wide resection of soft tissue sarcomas? Clin Orthop Relat Res. 2018;476(9):1791–1800. doi: 10.1007/s11999.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abatzoglou S., Turcotte R.E., Adoubali A., Isler M.H., Roberge D. Local recurrence after initial multidisciplinary management of soft tissue sarcoma: is there a way out? Clin Orthop Relat Res. 2010;468(11):3012–3018. doi: 10.1007/s11999-010-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gustafson P, Dreinh E, Rydholm A. Metastasis, Free Recurrence Survival of After Soft-Tissue. :658-660.
- 68.Karakousis C.P. Local recurrence and survival in soft-tissue sarcomas. Ann Surg Oncol. 1996;3(3):255–260. doi: 10.1007/BF02306280. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto S., Ahmed A.R., Kawaguchi N., Manabe J., Matsushita Y. Results of surgery for malignant fibrous histiocytomas of soft tissue. Int J Clin Oncol. 2003;8(2):104–109. doi: 10.1007/s101470300018. [DOI] [PubMed] [Google Scholar]
- 70.Novais E.N., Demiralp B., Alderete J., Larson M.C., Rose P.S., Sim F.H. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res. 2010;468(11):3003–3011. doi: 10.1007/s11999-010-1471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stotter A.T., A'Hern R.P., Fisher C., Mott A.F., Fallowfield M.E., Westbury G. The influence of local recurrence of extremity soft tissue sarcoma on metastasis and survival. Cancer. 1990;65(5):1119–1129. doi: 10.1002/1097-0142(19900301)65:5<1119::AID-CNCR2820650515>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 72.Weitz J., Antonescu C.R., Brennan M.F. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21(14):2719–2725. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 73.Billingsley K.G., Lewis J.J., Leung D.H.Y. Multifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcoma BACKGROUND. Despite optimal multimodality limb-sparing therapy for extremity. 1999:389–395. [PubMed] [Google Scholar]
- 74.Collin Godbold, Hajdu B. Localized extremity soft tissue sarcoma: an analysis of factors affecting survival. J Clin Oncol. 1987;5(4):601–612. doi: 10.1200/JCO.1987.5.4.601. [DOI] [PubMed] [Google Scholar]
- 75.Daigeler A., Zmarsly I., Hirsch T. Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br J Canc. 2014;110(6):1456–1464. doi: 10.1038/bjc.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis J.J., Leung D., Heslin M., Woodruff J.M., Brennan M.F. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15(2):646–652. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 77.Pisters P.W., Leung D.H., Woodruff J., Shi W., Brennan M.F. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 78.DeLaney T.F., Kepka L., Goldberg S.I. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67(5):1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 79.Emrich L.J., Ruka W., Driscoll D.L., Karakousis C.P. The effect of local recurrence on survival time in adult high-grade soft tissue sarcomas. J Clin Epidemiol. 1989;42(2):105–110. doi: 10.1016/0895-4356(89)90083-8. [DOI] [PubMed] [Google Scholar]
- 80.Gronchi A., Casali P.G., Mariani L. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol. 2005;23(1):96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- 81.Dyrop H.B., Safwat A., Vedsted P. Characteristics of 64 sarcoma patients referred to a sarcoma center after unplanned excision. J Surg Oncol. 2016;113(2):235–239. doi: 10.1002/jso.24137. [DOI] [PubMed] [Google Scholar]
- 82.Alamanda V.K., Crosby S.N., Archer K.R., Song Y., Schwartz H.S., Holt G.E. Primary excision compared with re-excision of extremity soft tissue sarcomas-is anything new? J Surg Oncol. 2012;105(7):662–667. doi: 10.1002/jso.23021. [DOI] [PubMed] [Google Scholar]
- 83.Saeed H., King D.M., Johnstone C.A. Preoperative radiation therapy followed by reexcision may improve local control and progression-free survival in unplanned excisions of soft tissue sarcomas of the extremity and chest-wall. Int J Surg Oncol. 2016 doi: 10.1155/2016/5963167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Umer H.M., Umer M., Qadir I., Abbasi N., Masood N. Impact of unplanned excision on prognosis of patients with extremity soft tissue sarcoma. Sarcoma. 2013 doi: 10.1155/2013/498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bauer H.C.F., Trovik C.S., Alvegård T.A. Monitoring referral and treatment in soft tissue sarcoma: study based on 1,851 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand. 2001;72(2):150–159. doi: 10.1080/000164701317323408. [DOI] [PubMed] [Google Scholar]
- 86.Herbert S.H., Corn B.W., Solin L.J. Limb‐preserving treatment for soft tissue sarcomas of the extremities. The significance of surgical margins. Cancer. 1993;72(4):1230–1238. doi: 10.1002/1097-0142(19930815)72:4<1230::AID-CNCR2820720416>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.